Abstract
Objective:
In late June 2020, a large outbreak of COVID-19 occurred at a sleep-away youth camp in Georgia affecting primarily persons ≤ 21 years. We conducted a retrospective cohort study among campers and staff (attendees) to determine the extent of the outbreak and assess factors contributing to transmission.
Methods:
Attendees were interviewed to ascertain demographic characteristics, known exposures to cases and community exposures, and mitigation measures before, during, and after attending camp. COVID-19 case status was determined for all camp attendees based on SARS-CoV-2 test results and reported symptoms. We calculated attack rates and instantaneous reproduction numbers, and sequenced SARS-CoV-2 viral genomes from the outbreak.
Results:
Among 627 attendees, median age was 15 years (interquartile range: 12–16 years); 56% (351/627) were female. The attack rate was 56% (351/627) among all attendees. Based on date of illness onset or first positive specimen collected, 12 cases were infected before arriving at camp, and 339 cases were camp-associated. Among 288 cases with available symptom information, 45 (16%) were asymptomatic. Despite cohorting, 50% of attendees reported direct contact with people outside their cabin cohort. On the first day of camp session, the instantaneous reproduction number was 10. Viral genomic diversity was low.
Conclusions:
Few introductions of SARS-CoV-2 into a youth congregate setting resulted in a large outbreak. Testing strategies should be combined with pre-arrival quarantine, routine symptom monitoring with appropriate isolation and quarantine, cohorting, social distancing, mask wearing, and enhanced disinfection and hand hygiene. Promotion of mitigation measures among younger populations is needed.
Table of Contents Summary:
A cohort study of attendees at a youth sleep-away camp with a COVID-19 outbreak highlights transmission dynamics of SARS-CoV-2 in youth congregate settings.
Introduction
Evidence for SARS-CoV-2 susceptibility and transmission dynamics among children is conflicting.1-5 School closures and stay-at-home orders early in the pandemic reduced contact among children, thereby limiting opportunities for transmission.6,7 Additionally, children more frequently experience asymptomatic and mild disease compared to adults8, which may result in less testing,9 further obscuring their role in transmission. A better understanding of transmission dynamics among children is needed to inform mitigation measures in youth-congregated settings.3,10
In June 2020, a large outbreak of COVID-19 occurred at a sleep-away youth camp in Georgia (eIntroduction),11 affecting primarily persons ≤21 years, despite the requirement of a negative SARS-CoV-2 nucleic acid amplification or antigen test (viral test) within 12 days of arrival. We conducted a retrospective cohort study and performed genetic sequencing of residual samples to determine the extent of the SARS-CoV-2 outbreak and assess factors contributing to transmission. We estimated effective case and instantaneous reproduction numbers.
Methods
Epidemiologic Investigation
All attendees of the camp during June 10-July 1, 2020 were eligible for inclusion in the retrospective cohort study. We categorized persons who attended staff orientation during June 17–20 as trainees if they only attended orientation, and as staff members if they also worked during the only camp session, held June 21–27. Campers only attended the camp session. The camp provided attendee contact information, age, sex, attendee type (trainee, staff, camper), and cabin. Based on contact information, we categorized attendees as residents of counties included in the metro Atlanta area, counties in Georgia not part of the metro Atlanta area, or out-of-state. During July 17-August 25, we contacted camp attendees for a phone interview; those we did not successfully reach after three attempts over different times of day, including evenings, and days of the week, including weekends, were considered non-respondents. We used a structured questionnaire to collect demographics, clinical characteristics, SARS-CoV-2 testing history, activities during camp, and known exposures to cases and community exposures 14 days before and 14 days after attending camp, mask use during camp attendance, and dates of arrival and departure from camp. We also reviewed pre-arrival laboratory results that were provided to the camp per Georgia executive order.12 We conducted a detailed interview with a senior staff member to assess mitigation measures adopted by the camp. For attendees who were Georgia residents, we obtained post-camp laboratory results by manually matching name and age, address, or phone number of attendees to known cases in the Georgia Department of Public Health (DPH) State Electronic Notifiable Disease Surveillance System (SENDSS) and collected symptom status and testing histories from state case investigations conducted during June–July. For out-of-state attendees, we contacted respective state health departments to obtain available information. In cases of discordant laboratory results or symptom reports between interviews and state case investigations, a positive test result or the presence of symptoms from either source superseded a negative test result or the absence of symptoms.
Main Outcomes
We classified camp attendees as COVID-19 cases, non-cases, or having an unknown case status using the Council of State and Territorial Epidemiologists (CSTE) definitions approved on August 5, 2020.13 Cases were defined as attendees who had a state- or self-reported positive viral test or met the CSTE clinical criteria without test information. Non-cases were defined as attendees who had a state- or self-reported negative viral test or had not been tested and did not meet the CSTE clinical criteria. Case status was unknown for attendees who we did not interview and were not identified in state case investigations. We defined the date of first positive specimen as the earliest specimen collection date, if available in the laboratory reports, or the earliest specimen collection date reported during the interview. We categorized cases as either community-associated or camp-associated. Cases with symptom onset or first positive specimen collection date, whichever was earliest, 10 days before until 2 days of arrival at camp were community-associated, and 3 days of arrival until 14 days after leaving camp were camp-associated.
Whole Genome Sequencing
For attendees who were Georgia residents, one commercial laboratory provided available residual specimens to the U.S. Centers for Disease Control and Prevention (CDC) for whole genome sequencing (WGS). Twenty-two specimens with cycle threshold values <32 by real-time reverse transcription polymerase chain reaction were selected for sequencing extraction. The nucleic acid was extracted and subjected to Illumina MiSeq sequencing following previously published protocols,14 and consensus sequences were generated with Minimap 2.17 and Samtools 1.9. We downloaded representative full-genome sequences on September 28, 2020, from GISAID and inferred phylogenetic relations using approximate maximum likelihood analyses implemented in TreeTime15 using the Nextstrain pipeline.16
Statistical Analyses
We tabulated demographic characteristics and exposures by case status and by attendee type. We calculated attack rates (AR) using two methods: 1) the proportion of attendees with COVID-19 among all attendees and 2) the proportion of attendees with COVID-19 among attendees excluding those with unknown case status. To estimate effective case and instantaneous reproduction numbers, we performed a probabilistic reconstruction of transmission chains, based on a serial interval distribution of illness onset among cases and time present at camp (eMethods). The effective case reproduction number is the average number of secondary cases per infectious case under observed conditions.17,18 The instantaneous reproduction number is the average number of secondary cases that each infectious case at time, t, would infect, if the conditions remained as they were at time, t (reflecting mitigation measures in place).19
For attendees aged 6–21 years with non-missing values for covariates of interest, we used unconditional generalized estimating equations to calculate unadjusted and adjusted risk ratios (RRs and aRRs) with 95% confidence intervals (CIs) for characteristics and exposures related to camp-associated case status. We conducted statistical analyses in SAS version 9.4 (SAS Institute) and R (version 4.0.2).
Ethical Considerations
This activity was reviewed by human subjects research advisors at CDC and DPH and was determined to not be human subjects research. For interviews with attendees younger than 18 years, we obtained parental or guardian permission and verbal assent from attendees.
Results
Camp Cohort
During June 10–July 1, 2020, 627 persons attended the camp, including 137 trainees, 127 staff, and 363 campers (Table 1). Trainee median age was 16 years (range = 14–20 years), and 61% (83/137) were female. Staff member median age was 17 years (range = 14–59 years), and 59% (75/127) were female. Camper median age was 12 years (range = 6–16 years), and 53% (193/363) were female. Most attendees were white (94%), non-Hispanic (96%), and metro Atlanta area residents (77%). Attendees spent a median of 6 days (range = 2–21 days) at camp. As part of the mitigation measures implemented by the camp (eResults), attendees were cohorted by cabin. During orientation, 137 trainees and 124 staff members stayed in 28 cabins with a median occupancy of 11 (range = 1–23 occupants). During the camp session, 127 staff and 363 campers stayed in 31 cabins with a median occupancy of 24 (range = 1–26 occupants); 98% of staff members stayed in the same cabin as during orientation.
Table 1.
Characteristics of Camp Attendees Overall and by Case Status
| Overall, No. (col %) | Known Case Statusa, No. (row %)b | ||
|---|---|---|---|
| (n = 627) | Case (n = 351) |
Not a case (n=211) |
|
| Age group (years) | |||
| 6–10 | 96 (15) | 54 (56) | 30 (31) |
| 11–14 | 197 (31) | 123 (62) | 60 (30) |
| 15–17 | 250 (40) | 127 (51) | 95 (38) |
| 18–21 | 75 (12) | 43 (57) | 23 (31) |
| 22–59 | 9 (1) | 4 (44) | 3 (33) |
| Sex | |||
| Male | 276 (44) | 164 (59) | 81 (29) |
| Female | 351 (56) | 187 (53) | 130 (37) |
| Racec | |||
| White | 465 (94) | 292 (63) | 173 (37) |
| Black | 0 | 0 | 0 |
| Other or Multiracial | 31 (6) | 27 (87) | 4 (13) |
| Ethnicityd | |||
| Hispanic or Latino | 20 (5) | 13 (65) | 7 (35) |
| Not Hispanic or Latino | 430 (96) | 266 (62) | 164 (38) |
| Residence | |||
| Metro Atlantae | 482 (77) | 274 (57) | 163 (34) |
| Non-Metro Atlanta, Georgia | 118 (19) | 64 (54) | 39 (33) |
| Out-of-state | 27 (4) | 13 (48) | 9 (33) |
| Attendee Type | |||
| Trainee | 137 (22) | 30 (22) | 83 (61) |
| Staff memberf | 127 (20) | 93 (73) | 23 (18) |
| Camper | 363 (58) | 228 (63) | 105 (29) |
| Length of stay (days) | |||
| ≤ 4 | 201 (32) | 73 (36) | 104 (52) |
| 5–6 | 299 (48) | 183 (61) | 85 (28) |
| ≥ 7 | 127 (20) | 95 (75) | 22 (17) |
An additional 65 attendees with an unknown case status are not shown.
Row percent was calculated using the overall number in each category as the denominator.
An additional 131 attendees with unknown race are not shown.
An additional 177 attendees with unknown ethnicity are not shown.
Metro-Atlanta defined as Fulton, DeKalb, Cobb, Douglas, Gwinnett, Clayton, Paulding, and Cherokee counties
Three staff members did not attend orientation.
Among 627 attendees, 598 (95%) provided negative pre-arrival laboratory results to the camp and 29 (5%) attendees (8 [6%] trainees, 11 [9%] staff members, and 10 [3%] campers) did not have record of pre-arrival tests. A total of 476 (80%) attendees had an available specimen collection date, with a mean time from specimen collection to arrival at camp of 6 days (range = 0–13 days).
Camp Attendee Cases and Clinical Characteristics
We identified 351 (56%) cases among camp attendees of which 340 (97%) had a positive viral test result and the remaining 11 (3%) reported no testing but had symptoms consistent with COVID-19. Among 211 (34%) attendees categorized as non-cases, 159 (75%) had a negative viral post-camp test result, and 52 (25%) reported no testing and no symptoms consistent with COVID-19. Case status was unknown for 65 (10%) attendees who were neither interviewed nor found in state reports.
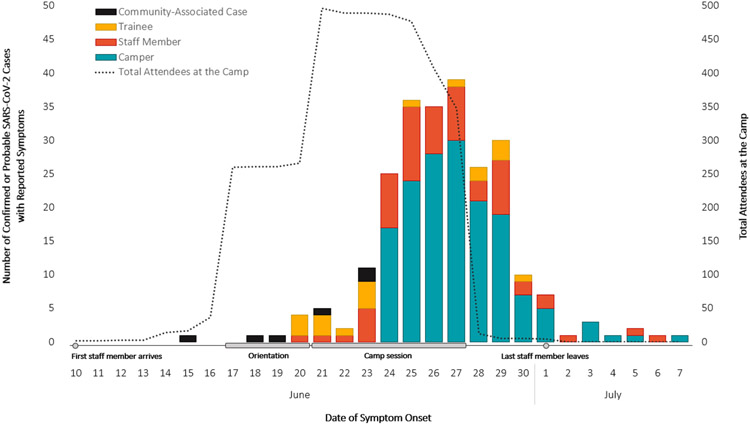
Of all 351 cases, 288 (82%) had symptom information available; 243 (84%) reported having symptoms, and 45 (16%) reported no symptoms. Most (74%) symptomatic cases reported developing symptoms by the last day of the camp session on June 27 (Figure 1). The most common symptoms included subjective or documented fever (56%), headache (52%), and fatigue (49%). Among cases with available information, 6% (16/258) had an underlying medical condition, 5% (12/259) sought medical care due to COVID-19 illness, and none were hospitalized.
Figure 1.
Epidemic Curve of Symptomatic Cases (n=242a) by Attendee Type, Number of Attendees at the Camp Over Timeb, and Key Events
aOne additional community-associated case was missing a symptom onset date and was excluded.
bSome trainees and staff (n=37) arrived at camp prior to orientation during June 10–16. Three staff arrived at camp on June 21 and did not attend orientation, and five campers and staff left during June 29–July 1.
Case Classification
Among 351 cases, 12 (3%) were categorized as community-associated cases. Negative pre-arrival laboratory test results were available for 11 community-associated cases; 1 was missing. Five cases (2 asymptomatic; 3 with missing symptom information) had a positive specimen collected a median of 7 days (range = 6–8 days) before arriving at camp but retested with a negative result a median of 3 days (range = 0–5 days) after their positive test (eFigure 1). Only negative results were supplied to the camp. Six cases with symptoms had symptom onset from 6 days prior to 2 days of arriving and had a positive specimen collected within 5–11 days of arriving at camp. One additional symptomatic community-associated case had a positive specimen collected within 2 days of arrival but symptom onset was missing.
There were 339 camp-associated cases; 328 (97%) had a positive viral test, and 11 (3%) were not tested but met the CSTE clinical case definition. Among the 279 camp-associated cases with available symptom information, 236 (85%) were symptomatic; 132 (56%) reported symptom onset date during camp and 104 (44%) after leaving camp. The median number of days from camp arrival to symptom onset was 7 days (range = 3–21).
Whole Genome Sequencing
Among 338 Georgia cases, 32 (9%) had available residual specimens. Full genome sequencing was successful in 22 (7%) isolates; all were clustered within 0–2 single nucleotide polymorphisms (SNPs) of another case isolate and were at least 6 SNPs from any other sequenced isolate available in the public database (eFigure 1). These findings indicate low viral genomic diversity, although cases with available sequences were from 10 different cabins, 2 were community-associated, and 20 camp-associated (eTable 1), with symptom onset dates between June 19–30 (n = 17).
Attack Rates
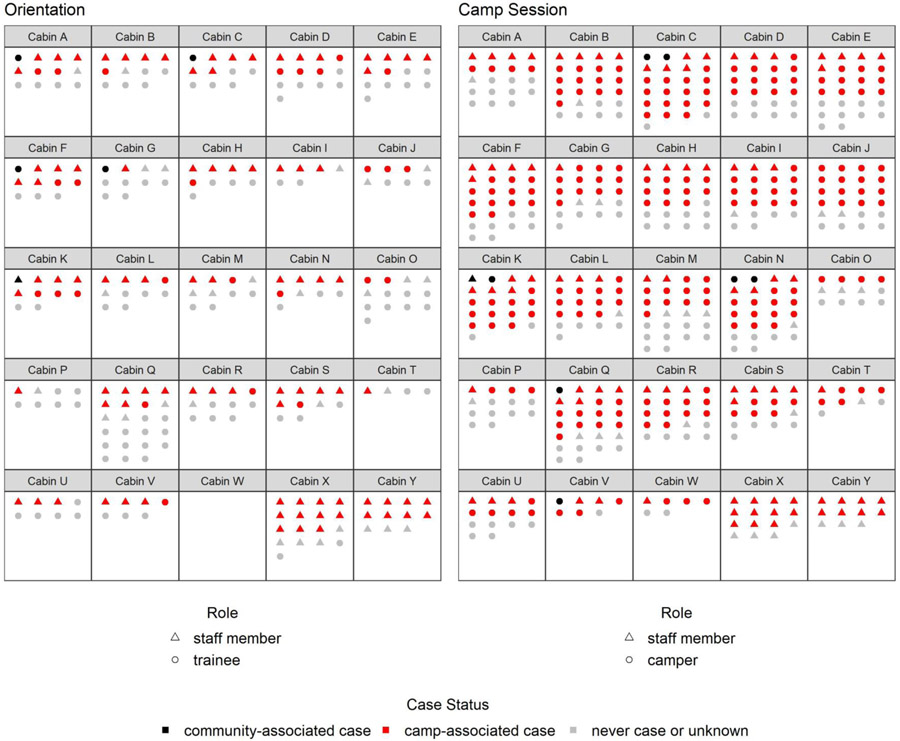
The overall AR was 56% (351/627) among all attendees; AR was 62% (351/562) excluding the 65 attendees with unknown case status. Across age groups, ARs ranged from 44% (4/9) among attendees aged 22–59 years to 62% (123/197) among those aged 11–14 years (Table 1). AR increased with increasing days spent at camp, up to 75% among attendees who spent ≥7 days at camp. Staff members had the highest attack rate (73%). Median cabin attack rate was 50% (interquartile range (IQR) = 35–59%) during orientation, and 67% (IQR = 54–72%) during the camp session; 94% (29/31) of cabins had ≥1 cases (Figure 2 and eVideo 1).
Figure 2.
Attack Ratesa by Cabinb During Orientation and Camp Session
aThe final case status is shown for each attendee. Staff members attended both orientation and the camp session, and their final case status is shown in both periods.
bSix cabins with three persons or less were not shown in this figure. Two of these cabins did not house any cases.
Reproduction Numbers
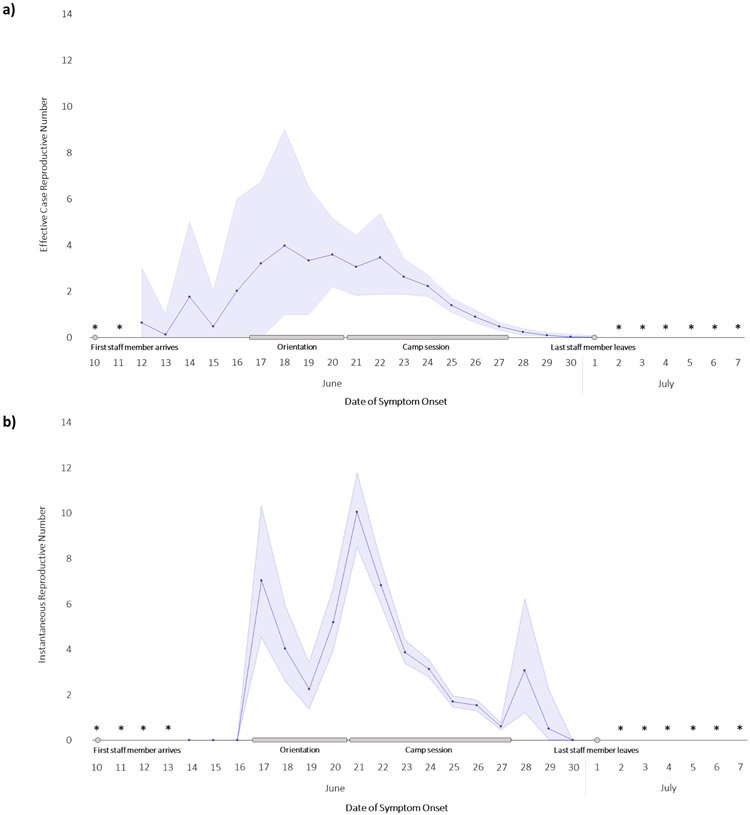
The mean effective case reproduction number ranged from 3.2 to 4.0 for cases with illness onset during orientation (June 17–20) and 0.1 to 3.5 for those during the camp session (June 21–28) (Figure 3a). For community-associated cases, the mean effective reproduction number was 2.0, and for camp-associated cases, it ranged from 0.8 among trainees to 1.3 among staff members. The instantaneous reproduction number was highest (10.1) on June 21 (Figure 3b), indicating a high probability of transmission from infectious cases at the beginning of the camp session when campers arrived.
Figure 3.
Case and Instantaneous Reproductive Numbers During Orientation and Camp Session
*Effective or instantaneous reproductive number could not be estimated for these dates.
Exposures and Activities Before, During, and After Camp
We interviewed 450 (70%) attendees to ascertain exposures and activities before, during, and after camp (eTable 2). Time spent at camp varied by attendee type as follows: 99% of trainees stayed ≤4 days onsite, 96% of staff stayed ≥7 days, and 81% of campers stayed 5–6 days at camp (Table 2). At the beginning of orientation, there were 5 community-associated cases in 5 separate cabins; 2 were symptomatic. At the beginning of the camp session, there were 10 cases across 7 cabins: 5 symptomatic community-associated cases, 3 asymptomatic community-associated cases, and 2 symptomatic camp-associated cases in staff members who stayed for the camp session. A total of 31 (23%) trainees, 23 (18%) staff members, and 100 (28%) campers stayed in a cabin with ≥1 case on the day they arrived at camp (Figure 2 and eVideo 1). The proportion of attendees who reported direct contact, such as hugging or kissing, or close contact, such as playing indoor sports or traveling in vehicles, with people outside their cabins was 88%. Approximately 15% of trainees and staff members reported always wearing a mask during camp, compared to 5% of campers. While singing and cheering were not individually assessed in interviews, a senior staff member described daily vigorous singing and cheering during the camp session. Community activities that could increase the risk for a SARS-CoV-2 exposure before camp, such as eating indoors at restaurants or attending gatherings with non-household members, were commonly reported (58% among staff members and 54% among campers), and 3 attendees reported a known exposure to a person who tested positive for SARS-CoV-2 before camp. While potential community exposures after camp were less commonly reported (2%), the proportion reporting known exposures, including exposures to other attendees who became sick with COVID-19, increased after camp (12% among staff and 6% among campers).
Table 2.
Characteristics, Exposures, and Behaviors Overall and by Camp Attendee Type
| Overalla, No. (col %) | Camp Attendee Type, No. (col %) | |||
|---|---|---|---|---|
| (n = 627) | Trainee (n = 137) |
Staff Member (n = 127) |
Camper (n = 363) |
|
| Age group (years) | ||||
| 6–10 | 96 (15) | 0 | 0 | 96 (26) |
| 11–14 | 197 (31) | 1 (1) | 1 (1) | 195 (54) |
| 15–17 | 250 (40) | 115 (84) | 63 (50) | 72 (20) |
| 18–21 | 75 (12) | 21 (15) | 54 (43) | 0 |
| 22–59 | 9 (1) | 0 | 9 (7) | 0 |
| Sex | ||||
| Male | 276 (44) | 54 (39) | 52 (41) | 170 (47) |
| Female | 351 (56) | 83 (61) | 75 (59) | 193 (53) |
| Length of stay (days) | ||||
| ≤ 4 | 201 (32) | 135 (99) | 1 (1) | 65 (18) |
| 5–6 | 299 (48) | 2 (1) | 4 (3) | 293 (81) |
| ≥ 7 | 127 (20) | 0 | 122 (96) | 5 (1) |
| Stayed in cabin with a case upon arrivalb | ||||
| Yes | 154 (25) | 31 (23) | 23 (18) | 100 (28) |
| No | 473 (75) | 106 (77) | 104 (82) | 263 (72) |
| Contact with people outside cabinc | ||||
| None | 37 (9) | 8 (9) | 3 (4) | 26 (10) |
| Outdoor sports only | 14 (3) | 2 (2) | 2 (3) | 10 (4) |
| Close contactd | 155 (38) | 22 (25) | 24 (32) | 109 (44) |
| Direct contacte | 204 (50) | 55 (63) | 45 (61) | 104 (42) |
| Mask use frequencyf | ||||
| Never | 133 (32) | 0 | 1 (1) | 132 (53) |
| Sometimes | 243 (59) | 75 (85) | 65 (86) | 103 (42) |
| Always | 36 (9) | 13 (15) | 10 (13) | 13 (5) |
| Exposures before attending camp g | ||||
| None | 195 (44) | 40 (43) | 33 (41) | 122 (45) |
| Community exposureh | 247 (56) | 53 (57) | 47 (58) | 147 (54) |
| Known exposurei | 3 (1) | 0 | 1 (1) | 2 (1) |
| Exposures after attending camp g | ||||
| None | 404 (91) | 80 (86) | 71 (88) | 253 (93) |
| Community exposureh | 7 (2) | 5 (5) | 0 | 2 (1) |
| Known exposurei | 34 (8) | 8 (9) | 10 (12) | 16 (6) |
Among attendees aged 6-21 years who provided exposure and behavior information during attendance at camp in interviews.
Defined as staying in a cabin with a community-associated case or a symptomatic camp-associated case on the day of arrival to camp.
An additional 217 attendees with unknown contact status with people not staying in the same cabin are not shown.
Defined as playing indoor sports or activities, traveling in vehicles, spending more than 15 minutes within 6 feet, having face-to-face contact within two feet, or spending any time within 6 feet while the other person was coughing or sneezing.
Defined as hugging or kissing the other person.
An additional 5 attendees with unknown mask use are not shown.
An additional 182 attendees with unknown exposure status before and after camp are not shown.
Defined as visiting, working, or volunteering in a healthcare setting, eating indoors at a restaurant, attending a gathering of any size with non-household members, using public transportation, or attending or working at a school or daycare.
Defined as close contact (within 6 feet for ≥ 15 minutes) with a person who tested positive for SARS-CoV-2, including other camp attendees
Multivariable Model
Among the 404 attendees aged 6–21 years with non-missing values for covariates of interest, staff members were 4.5 times as likely to become a camp-associated case compared to trainees (95% CI = 2.7–7.5), adjusting for age group, length of stay, staying in a cabin with a case when arriving at camp, and contact with people outside their cabin (eTable 3). Campers were 3.8 times as likely to become a camp-associated case compared to trainees (95% CI = 2.6–5.5), adjusting for the same covariates.
Discussion
This investigation demonstrates rapid, widespread SARS-CoV-2 transmission in a congregate setting with children, adolescents, and young adults. Relatively few community-associated cases were identified, but attack rates were as high as 73% among staff members in this sleep-away camp. In this cohort, which included >600 persons 6–21 years, a majority of whom were tested following a well-defined period of exposure, most cases were characterized by mild or asymptomatic illness, similar to previous, smaller studies characterizing SARS-CoV-2 infection among younger populations.8,20,21 Nearly half of symptomatic camp-associated cases reported symptoms that started after leaving camp, suggesting transmission from presymptomatic individuals contributed to this outbreak.22 Assuming cases with available sequences were representative of all cases, WGS results support the findings that few introductions resulted in widespread transmission.
In this outbreak, estimates of case reproduction number varied day to day and were as high as 4.0, demonstrating efficient transmission among children, adolescents, and young adults. The instantaneous case reproduction number peaked at 10.1 on June 21 (the first day of camp with an influx of susceptible individuals), indicating that the contact rate and intensity on that day, if sustained, would have resulted in 10 secondary cases per case among attendees. In the multivariable analysis, we found a higher risk of SARS-CoV-2 infection among staff and campers compared to trainees. During the camp session, when cabin occupancy increased, there were also more cases, either asymptomatic or presymptomatic, among attendees. Daily singing and cheering, which has contributed to previous outbreaks,23 might have increased transmission within cabin cohorts. Most attendees reported having direct or close contact with others outside their cabin, and only 9% reported wearing masks at all times, which likely led to increased transmission between different cabin cohorts. These findings underscore the importance of implementing layered mitigation strategies in settings where younger populations congregate.24,25
This investigation is subject to at least four limitations. First, the interviews were performed between 2–9 weeks after attendees’ last day at camp, subjecting responses to recall bias. Second, misclassification of case status and community- vs. camp-associated cases was possible because not all attendees were tested, and among those tested, there could be false-positive or false-negative results; a 56% attack rate among all attendees is likely an underestimate. Third, the effect of mask use could not be assessed; few campers reported wearing masks which were not required. Finally, the types of activities and intensity of contact among and within groups, mainly due to the sleeping arrangements in the camp setting, cannot be extrapolated to all settings that include children, adolescents, and young adults, although some similarities exist (e.g., high school students may participate in large-group indoor school activities, college students may interact with the surrounding community, including as counselors for young children in after-school programs).
Other youth-centric settings have also used pre-arrival testing to reduce transmission 26 In this outbreak, we found that testing within 12 days of arrival, without a mandatory 14-day quarantine was insufficient to prevent infected attendees from arriving at camp and infecting others. Most attendees were residents of the metro Atlanta area, which had a high incidence of COVID-19 in June 2020.27 Many attendees reported engaging in community activities before arriving at camp that could have increased their risk of exposure. These findings underscore the challenges of preventing outbreaks in areas with substantial community transmission.
Conclusion
Despite mitigation measures, including pre-arrival testing, relatively few introductions of SARS-CoV-2 into this congregate setting resulted in a large outbreak affecting >50% of attendees. Testing should not be used as the sole mitigation measure;28 instead, it should be used as one component of a layered mitigation approach combined with adherence to pre-arrival quarantine, routine symptom monitoring with appropriate isolation and quarantine, cohorting, social distancing, mask wearing, enhanced disinfection, and proper hand hygiene.25 Furthermore, it is important to emphasize appropriate isolation education and compliance for persons who test positive even in the absence of symptoms,29 particularly among younger adults who have been reported to have lower engagement in social mitigation behaviors.30,31 Targeted communication strategies about behavioral expectations for younger populations may be necessary to emphasize mitigation measures that should be adopted to avoid contracting and spreading COVID-19 to others in youth congregate settings.
Supplementary Material
What’s Known on This Subject.
COVID-19 outbreaks in adult congregate settings have fueled much of the pandemic, however the transmission dynamics of outbreaks in youth congregate settings are less understood.
What This Study Adds.
Few introductions of SARS-CoV-2 into a youth congregate setting, with substantial mixing of cohorts, combined with presymptomatic and asymptomatic transmission, resulted in a large outbreak with a 56% attack rate.
Acknowledgements:
Camp attendees and their household contacts
Georgia Department of Public Health: Luke Baertlein, Tiffany Baird, Aaron Blakney, Tom Campbell, Alicia Dunajcik, Amit Eichenbaum, Amanda Feldpausch, Pamela Logan, Amanda Mohammed, Stephanie O’Conner, Tonia Parrott, Haley Putnam, Zoe Schneider, Brandon Shih, Kat Topf, and Bill Williamson
Centers for Disease Control and Prevention: Ramika Archibald, Elizabeth Dietrich, Kathy Fowler, Leah Graziano, Chad Heilig, Margaret Honein, Mark Johnson, Scott Lee, Kelsey McDavid, Robert Montierth, Krista Queen, Joe Sexton, Anupama Shankar, and Robert Slaughter
Alabama Department of Public Health, Arkansas Department of Health, Colorado Department of Public Health and Environment, Florida Department of Health, Maryland Department of Health, North Carolina Division of Public Health, South Carolina Department of Health and Environmental Control, Tennessee Department of Health, Texas Department of State Health Services, and Ipsum Diagnostics.
Disclaimer:
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the U.S. Centers for Disease Control and Prevention.
Abbreviations:
- COVID-19
Coronavirus Disease 2019
- SENDSS
State Electronic Notifiable Disease Surveillance System
- CSTE
Council of State and Territorial Epidemiologists
- CDC
U.S. Centers for Disease Control and Prevention
- WGS
whole genome sequencing
- AR
attack rates
- RRs
unadjusted risk ratios
- aRRs
adjusted risk ratios
- CIs
confidence intervals
- SNPs
single nucleotide polymorphisms
Footnotes
Conflict of Interest Disclosures (including financial disclosures): The authors have no conflict of interest relevant to this article to disclose.
References
- 1.Viner RM, Mytton OT, Bonell C, et al. Susceptibility to SARS-CoV-2 Infection Among Children and Adolescents Compared With Adults: A Systematic Review and Meta-analysis. JAMA Pediatr. Published online September 25, 2020. doi: 10.1001/jamapediatrics.2020.4573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.James A High COVID-19 Attack Rate Among Attendees at Events at a Church — Arkansas, March 2020. MMWR Morb Mortal Wkly Rep. 2020;69. doi: 10.15585/mmwr.mm6920e2 [DOI] [PubMed] [Google Scholar]
- 3.Lopez AS. Transmission Dynamics of COVID-19 Outbreaks Associated with Child Care Facilities — Salt Lake City, Utah, April-July 2020. MMWR Morb Mortal Wkly Rep. 2020;69. doi: 10.15585/mmwr.mm6937e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Link-Gelles R Limited Secondary Transmission of SARS-CoV-2 in Child Care Programs — Rhode Island, June 1-July 31, 2020. MMWR Morb Mortal Wkly Rep. 2020;69. doi: 10.15585/mmwr.mm6934e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leeb RT. COVID-19 Trends Among School-Aged Children — United States, March 1–September 19, 2020. MMWR Morb Mortal Wkly Rep. 2020;69. doi: 10.15585/mmwr.mm6939e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schuchat A Public Health Response to the Initiation and Spread of Pandemic COVID-19 in the United States, February 24-April 21, 2020. MMWR Morb Mortal Wkly Rep. 2020;69. doi: 10.15585/mmwr.mm6918e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Auger KA, Shah SS, Richardson T, et al. Association Between Statewide School Closure and COVID-19 Incidence and Mortality in the US. JAMA. 2020;324(9):859–870. doi: 10.1001/jama.2020.14348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dong Y, Mo X, Hu Y, et al. Epidemiology of COVID-19 Among Children in China. Pediatrics. 2020;145(6). doi: 10.1542/peds.2020-0702 [DOI] [PubMed] [Google Scholar]
- 9.Greene DN, Jackson ML, Hillyard DR, Delgado JC, Schmidt RL. Decreasing median age of COVID-19 cases in the United States—Changing epidemiology or changing surveillance? PLoS ONE. 2020;15(10). doi: 10.1371/journal.pone.0240783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lipsitch M, Swerdlow DL, Finelli L. Defining the Epidemiology of Covid-19 — Studies Needed. N Engl J Med. Published online February 19, 2020. doi: 10.1056/NEJMp2002125 [DOI] [PubMed] [Google Scholar]
- 11.Szablewski CM. SARS-CoV-2 Transmission and Infection Among Attendees of an Overnight Camp — Georgia, June 2020. MMWR Morb Mortal Wkly Rep. 2020;69. doi: 10.15585/mmwr.mm6931e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.The State of Georgia. Providing Additional Guidance and Empowering a Healthy Georgia in Response to COVID-19-06.11.20.01.; 2020. https://gov.georgia.gov/executive-action/executive-orders/2020-executive-orders
- 13.CSTE Interim Position Statement: Update to COVID-19 Case Definition - Council of State and Territorial Epidemiologists. Accessed November 2, 2020. https://www.cste.org/news/520707/CSTE-Interim-Position-Statement-Update-to-COVID-19-Case-Definition.htm
- 14.Paden CR, Tao Y, Queen K, et al. Rapid, Sensitive, Full-Genome Sequencing of Severe Acute Respiratory Syndrome Coronavirus 2 - Volume 26, Number 10–October 2020. - Emerging Infectious Diseases journal - CDC. doi: 10.3201/eid2610.201800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saglulenko P et al. (2018) Treetime: maximum-likelihood phylodynamic analysis. Virus Evol 4. Vex042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hadfield J, Megill C, Bell SM, et al. Nextstrain: real-time tracking of pathogen evolution. Bioinformatics. 2018;34(23):4121–4123. doi: 10.1093/bioinformatics/bty407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wallinga J, Teunis P. Different Epidemic Curves for Severe Acute Respiratory Syndrome Reveal Similar Impacts of Control Measures. Am J Epidemiol. 2004;160(6):509–516. doi: 10.1093/aje/kwh255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He X, Lau EHY, Wu P, et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med. 2020;26(5):672–675. doi: 10.1038/s41591-020-0869-5 [DOI] [PubMed] [Google Scholar]
- 19.Cori A, Ferguson NM, Fraser C, Cauchemez S. A New Framework and Software to Estimate Time-Varying Reproduction Numbers During Epidemics. Am J Epidemiol. 2013; 178(9):1505–1512. doi: 10.1093/aje/kwt133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review. JAMA. 2020;324(8):782–793. doi: 10.1001/jama.2020.12839 [DOI] [PubMed] [Google Scholar]
- 21.Bai Y, Yao L, Wei T, et al. Presumed Asymptomatic Carrier Transmission of COVID-19. JAMA. 2020;323(14):1406–1407. doi: 10.1001/jama.2020.2565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wei WE. Presymptomatic Transmission of SARS-CoV-2 — Singapore, January 23-March 16, 2020. MMWR Morb Mortal Wkly Rep. 2020;69. doi: 10.15585/mmwr.mm6914e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hamner L High SARS-CoV-2 Attack Rate Following Exposure at a Choir Practice — Skagit County, Washington, March 2020. MMWR Morb Mortal Wkly Rep. 2020;69. doi: 10.15585/mmwr.mm6919e6 [DOI] [PubMed] [Google Scholar]
- 24.Blaisdell LL. Preventing and Mitigating SARS-CoV-2 Transmission — Four Overnight Camps, Maine, June-August 2020. MMWR Morb Mortal Wkly Rep. 2020;69. doi: 10.15585/mmwr.mm6935e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pray IW. COVID-19 Outbreak at an Overnight Summer School Retreat — Wisconsin, July-August 2020. MMWRMorb Mortal Wkly Rep. 2020;69. doi: 10.15585/mmwr.mm6943a4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walke HT, Honein MA, Redfield RR. Preventing and Responding to COVID-19 on College Campuses. JAMA. 2020;324(17):1727–1728. doi: 10.1001/jama.2020.20027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.COVID-19 Status Report. Georgia Department of Public Health. Accessed October 29, 2020. https://dph.georgia.gov/covid-19-daily-status-report [Google Scholar]
- 28.Van Pelt A, Glick HA, Yang W, Rubin D, Feldman M, Kimmel SE. Evaluation of COVID-19 Testing Strategies for Repopulating College and University Campuses: A Decision Tree Analysis. J Adolesc Health. Published online November 3, 2020. doi: 10.1016/j.jadohealth.2020.09.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Georgia Department of Public Health. Isolation Guidance. Georgia Department of Public Health. Accessed October 21, 2020. https://dph.georgia.gov/isolation-contact [Google Scholar]
- 30.Hutchins HJ. COVID-19 Mitigation Behaviors by Age Group — United States, April-June 2020. MMWR Morb Mortal Wkly Rep. 2020;69. doi: 10.15585/mmwr.mm6943e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boehmer TK. Changing Age Distribution of the COVID-19 Pandemic — United States, May-August 2020. MMWR Morb Mortal Wkly Rep. 2020;69. doi: 10.15585/mmwr.mm6939e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.