Dear Editor,
Acute primary angle-closure glaucoma (PACG) is an important cause of blindness in East Asia.1 It is estimated that the overall prevalence of PACG will increase from 1.44% to 2.01% from 2020 to 2050.2 Acute PACG is typically related to increased high intraocular pressure (IOP), with symptoms including red eye, blurred vision, nausea, vomiting, and headache. Delay in timely IOP-lowering treatment can result in permanent optic nerve damage and vision loss.3 Axial hyperopia is the main ocular risk factor for development of primary closure of the anterior chamber angle, while its systemic risk factors include older age, east Asian ethnic origin, and female sex.4 Although generally deemed to be a bilateral condition, PACG often occurs unilaterally, and bilateral simultaneous onset is very rare. For patients with unilateral onset, even if the effect of treatment is not satisfactory, relatively good outcomes can be achieved by carrying out early intervention on the fellow eye. However, for bilateral cases, the condition is usually severe and often comes with a poor prognosis. Therefore, appropriate preventive measures are critical. Here, we present a case of bilateral simultaneous acute PACG related to hyperopia.
A 58-year-old Chinese woman presented with intermittent headaches, nausea, vomiting, and acute pain in both eyes for 1 month, which had worsened recently. The patient reported no history of taking anticholinergic and sympathomimetic drugs, nor was any other cause identified. The vital signs included a body temperature of 37.4°C, blood pressure 150/96 mmHg, and a pulse 90 beats/min. Visual acuities were CF in 50 cm in both eyes and IOP was 51 and 53 mmHg in the right and left eye, respectively. Slit lamp examination revealed conjunctiva hyperemia, corneal edema, mid-dilated pupils, and shallow anterior chambers peripherally and centrally (Fig. 1). Fundus examination had a hazy view. B-scan ultrasonography demonstrated that retinas were attached in both eyes. Initial treatments included systemic acetazolamide 250 mg twice a day, pilocarpine 2% four times a day, brinzolamide 1.0% twice-a-day, and fixed-combination eye drops containing timolol maleate 0.5% and brimonidine tartrate 0.2% twice-a-day. A few hours later, the IOPs reduced to 28 OD and 32 OS mmHg respectively. The patient underwent a laser peripheral iridotomy and was sent home with prednisolone acetate eye drops to be applied four times a day, acetazolamide 500 mg every morning and 250 mg every night, and continued the same eye medications. In a follow-up visit 5 days later (Fig. 2), the IOPs were reduced to 11 and 14 mmHg, respectively. The best corrected visual acuities (BCVAs) were 0.1 OD and 0.4 OS. The patient had a refraction status of +4.50 diopter −1.00 × 160°, OD, and +3.00 diopter −0.25 × 57°, OS. A slit lamp examination revealed hazy corneas with edema and descemet membrane folds, pigment deposits on the anterior lens surface, anterior chambers were deep and quiet, OU. Fundus examination showed some disc hyperemia, OU. Three weeks later, the BCVAs were 0.4 and 0.6, respectively. IOPs were normal at 15 and 14 mmHg, respectively. Slit lamp examination revealed transparent corneas, deep and quiet anterior chambers (Fig. 3). The patient was advised to continue anti-glaucoma medications and return for a follow-up in 1 month.
Figure 1.
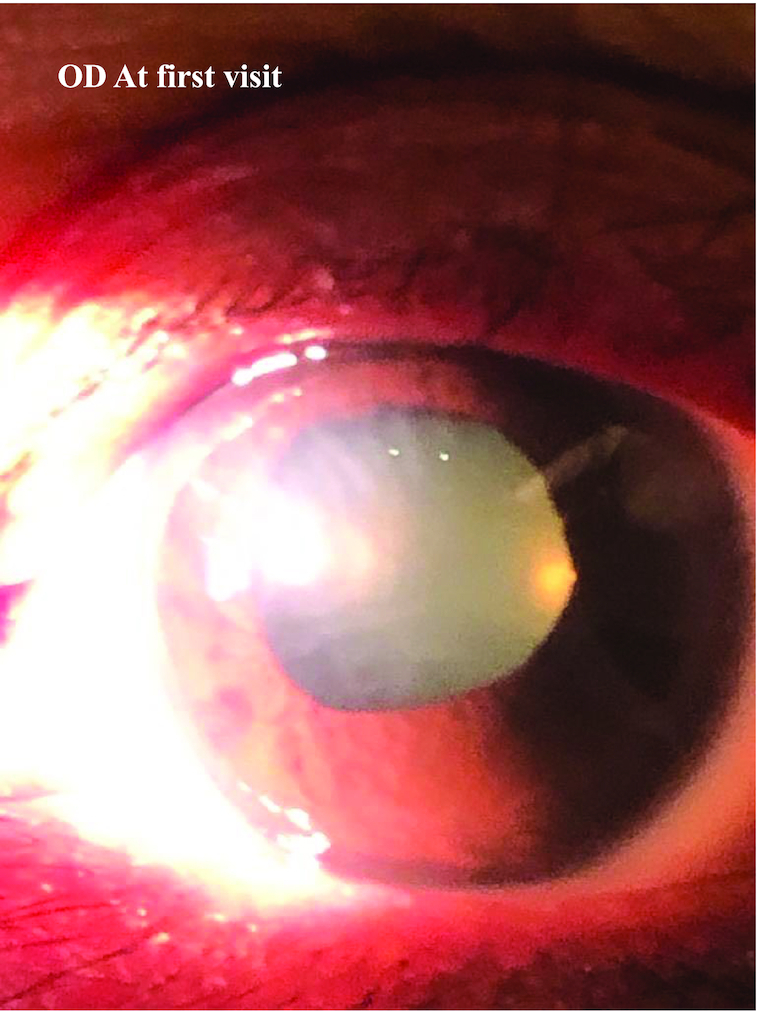
Slit-lamp photograph of the right eye at presentation showing conjunctiva hyperemia and corneal edema.
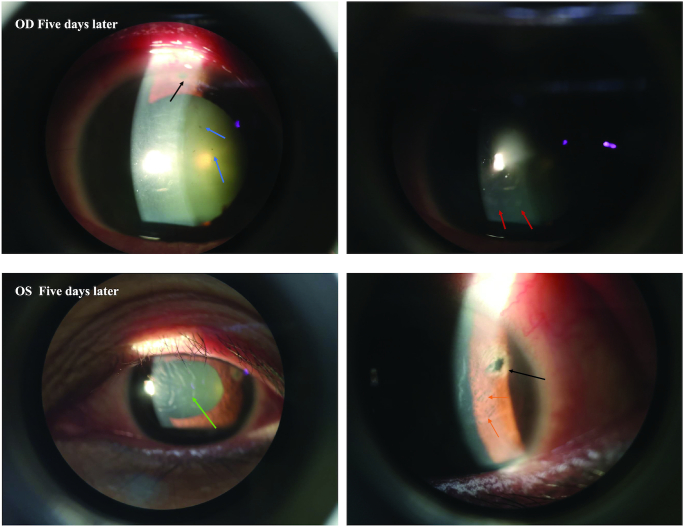
Figure 2.
(Top) Slit-lamp photographs of the right eyes on the fifth day of the presentation showing ciliary congestion and a hazy cornea with edema. Note the iridotomy site (black arrows), pigmentation on the posterior corneal surface (blue arrows), and glaucomflecken (red arrows). (Bottom) Slit-lamp photographs of the left eye on Day 5 of the presentation showing a hazy cornea with edema, descemet membrane folds (green arrows), iridotomy (black arrows), and patches of iris atrophy (orange arrows).
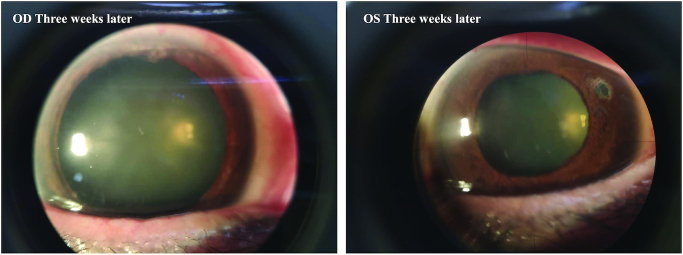
Figure 3.
Slit-lamp photographs at a 3-week visit show a clear cornea. Left panel, OD; right panel, OS.
Acute primary angle closure (APAC) attacks usually lead to more severe consequences in Asians compared with Caucasians, and such race differences may be explained by their anatomic differences. Chinese patients usually have a thinner ciliary body and a more anteriorly positioned lens.5 Previously, it was reported that Asian APAC patients usually have poor outcomes after treatment. However, in a recent study, successful treatment of APAC with laser peripheral iridotomy alone was achieved in 29 out of 36 Asian eyes (80.6%), while PACG developed in only nine eyes (21.4%), thereby needing further treatment, and the average time for patients with APAC post-treatment to progress to PACG was 11.9 months.6
The primary cause of PACG is IOP elevation from an obstruction of aqueous humor outflow at the angle caused by mechanical occlusion by iris tissue. A large number of studies have revealed that PACG is characterized by a shallow anterior chamber, narrow angle, anteriorly positioned lens, short ocular axis, and hyperopia. In an eye with a shallow anterior chamber and narrow angle, mechanical occlusion of the angle will occur under the influence of certain risk factors, leading to an acute attack. In bilateral simultaneous onset cases, both eyes must have the same anatomical characteristics before the acute attack, developing acute PACG simultaneously under the external risk factors. Therefore, bilateral simultaneous onset is relatively rare, whereas unilateral cases are much more common. In the current case, the patient was hyperopic in both eyes and possessed the anatomical characteristics essential for bilateral simultaneous onset.
Bilateral simultaneous onset is hard to prevent, and the condition is usually severe. Vision can be greatly compromised as a result of severe damage to the ocular tissue from the high IOP. Acute angle-closure should be immediately attended to, as the vision loss caused may progress rapidly and is often irreversible. Development of glaucoma could be prevented if the acute angle closure was identified at an early stage and appropriate interventions were adopted in a timely manner. Detection of hyperopia is also conducive to identifying patients with increased risk of acute angle closure. Hence, special attention should be given to patients with positive prescription for their spectacles, considering that most acute angle closure patients are hyperopic. It is crucial that IOP is lowered as quickly and by as much as possible after the onset of APAC, so as to minimize the damage to the optic nerve, after which, the pupil block should be eliminated with the aim of lowering the recurrence risk as well as the risk of developing into chronic PACG.7
Acknowledgements
This work was supported in part by the National Natural Science Foundation of China (NSFC, Grant No. 81700882).
Contributor Information
Charlotte L Zhang, Beijing Institute of Ophthalmology, Beijing Tongren Hospital, Capital Medical University, Beijing 100005, China.
Wico L Lai, University Hospital and Faculty of Medicine, Macau University of Science and Technology, Macao, China.
Ian Ziyar, University Hospital and Faculty of Medicine, Macau University of Science and Technology, Macao, China.
Laurance Lai Yuen Lau, University Hospital and Faculty of Medicine, Macau University of Science and Technology, Macao, China.
Jie Xu, Beijing Institute of Ophthalmology, Beijing Tongren Hospital, Capital Medical University, Beijing 100005, China.
Author contributions
C.L.Z, W.L.L, I.Z. and L.L. collected clinical information. C.L.Z, W.L.L and J.X. wrote and revised the manuscript, W.L.L. diagnosed, managed the case, J.X. supervised the project. All authors reviewed and approved the final version of the manuscript.
Conflict of interest statement
None declared.
References
- 1. Chan EW, Li X, Tham YC, et al. Glaucoma in Asia: regional prevalence variations and future projections. Br J Ophthalmol. 2016;100:78–85. doi:10.1136/bjophthalmol-2014-306102. [DOI] [PubMed] [Google Scholar]
- 2. Song P, Wang J, Bucan K, et al. National and subnational prevalence and burden of glaucoma in China: A systematic analysis. J Glob Health. 2017;7:020705. doi:10.7189/jogh.07.020705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhang K, Zhang L, Weinreb RN. Ophthalmic drug discovery: novel targets and mechanisms for retinal diseases and glaucoma. Nat Rev Drug Discov. 2012;11:541– 59. doi:10.1038/nrd3745. [DOI] [PubMed] [Google Scholar]
- 4. Jonas JB, Aung T, Bourne RR, et al. Glaucoma. Lancet. 2017;390:2183– 2193. doi:10.1016/S0140-6736(17)31469-1. [DOI] [PubMed] [Google Scholar]
- 5. He N, Wu L, Qi M,et al. ,et alComparison of ciliary body anatomy between American Caucasians and ethnic Chinese using ultrasound biomicroscopy. Curr Eye Res. 2016;41:485– 91. doi:10.3109/02713683.2015.1024869. [DOI] [PubMed] [Google Scholar]
- 6. Tan AM, Loon SC, Chew PT. Outcomes following acute primary angle closure in an Asian population. Clin Exp Ophthalmol. 2009;37:467– 72. doi:10.1111/j.1442-9071.2009.02060.x. [DOI] [PubMed] [Google Scholar]
- 7. Chan PP, Pang JC, Tham CC. Acute primary angle closure-treatment strategies, evidences and economical considerations. Eye (Lond). 2019;33:110– 19. doi:10.1038/s41433-018-0278-x. [DOI] [PMC free article] [PubMed] [Google Scholar]