Abstract
We report the case of a 90-year-old female patient who was suffering from c-ros oncogene 1 (ros-1) rearrangement adenocarcinoma and breast cancer. After about 14 months of a reduced dose of crizotinib treatment, she had a stable disease according to the Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST 1.1). This patient’s case demonstrates that ros-1 rearrangements are not limited to patients of young age. In addition, this case indicates that crizotinib, as second-line, or even first-line, treatment may be effective and manageable in elderly patients. Furthermore, for elderly patients carrying a ros1 fusion, a reduced dose of crizotinib may be efficacious rather than a resistance factor. Based on our findings, we recommend that elderly patients with advanced lung adenocarcinoma should be considered for inclusion in molecular screening for ros-1 translocation, especially for never-smokers negative for epidermal growth factor receptor (egfr) mutation and the fusion between echinoderm microtubule associated protein-like 4 (EML4) and anaplastic lymphoma kinase (ALK). This deserves attention because the population is aging, with increasing incidence and morbidity of multiple primary malignant tumors. Neglect of breast nodules at the onset is one of the limitations of our case, as combination of primary lung cancer with breast cancer is common. Above all, use of antiestrogens before and after the diagnosis of non-small-cell lung cancer is related to a reduced risk of lung cancer mortality. Therefore, careful attention should always be paid to these cases.
Keywords: elderly patients, lung cancer, breast carcinoma, ros1 rearrangement, crizotinib
Introduction
In recent years, the field of precision medical treatment of cancer has evolved rapidly, leading to a major paradigm shift in oncology. Chromosomal rearrangements involving the ros1 receptor tyrosine kinase gene form a distinct molecular subset of non-small-cell lung cancer (NSCLC), which is predominantly found in adenocarcinomas, patients of younger age, and never-smokers or light smokers.1,2 Therapies that target rare mutation lung cancer subtypes have been approved by the US Food and Drug Administration (FDA), including crizotinib targeting of the ros1 kinase domain, which was described for the first time in a series of lung cancer patients in 2012.2
In a previous report, 72% of non-Asian patients, with a median age of 49.8 years, had ros1 translocations in 1073 cancer screening cases. The age of these patients was significantly lower than that of ros1-negative patients (mean age: 62.3 years), and also lower than that of patients with ALK translocations (mean age: 51.6 years).2 That is to say, it is a rare subtype, and more common in younger patients. However, in old previous clinical reports on ros1 alterations in lung cancer, the oldest patient with ros1-positive lung cancer was 90 years.3
In the EUROS1 cohort, researchers regarded poor health and a low dose of crizotinib as potential resistance factors.4 However, elderly patients often suffer from concomitant diseases and poor health status, which do not allow for conventional treatment. In addition, elderly patients who carry therapeutically tractable aberrations always require a dose reduction as a result of intolerable adverse reactions. Lung cancer and breast cancer are the most common malignancies in females. Analysis results from a study registered at the Geneva Cancer Registry indicated that breast cancer patients receiving antiestrogen therapy have a lower risk of dying from lung cancer.5
Case presentation
A 90-year-old never-smoker female patient was diagnosed with left lung adenocarcinoma at an outside institution in June 2017 and was treated with first-line general chemotherapy to which there was no response. Thoracentesis was carried out and intrathoracic instillation of cisplatin prevented re-accumulation of pleural effusion, thus dyspnea was improved because of early reduction of pleural effusion. Unfortunately, the pleural effusion soon increased and the toxicity of the local and systemic chemotherapy led to obvious symptoms. As a result of progressively worsening symptoms and dyspnea, the patient went to an outpatient clinic at West China Hospital Sichuan University. Computed tomography (CT) scan results at the outside institution showed a tumor mass of 16-mm maximum size in the superior lobe of the left lung (Fig. 1a), ipsilateral massive pleural effusion (Fig. 1b), and multiple ipsilateral breast nodules (Fig. 1c) but without lymph node involvement.
Figure 1 .

Computed tomography scan obtained at the outside institution showed a tumor mass of 16-mm maximum size in the superior lobe of the left lung (a), ipsilateral massive pleural effusion (b), and multiple ipsilateral breast nodules (c).
She underwent an additional pathological examination at our hospital with use of a pleural fluid specimen taken at the outside institution, and was found to be ros1-positive using ros1 immunohistochemistry (Fig. 2a) confirmed by fluorescent in situ hybridization. However, at the outset, breast nodules were ignored. Adenocarcinoma cells were found from histological examination of the pleural fluid. Immunohistochemical detection of NapsinA (Fig. 2b) and thyroid transcription factor-1 were positive (Fig. 2c), and PD-L1 was >50% (Fig. 2d), but detection was negative for ALK-V. Therefore, diagnosis of adenocarcinoma with a ros1 translocation could be established, which was most likely from the lung.
Figure 2 .
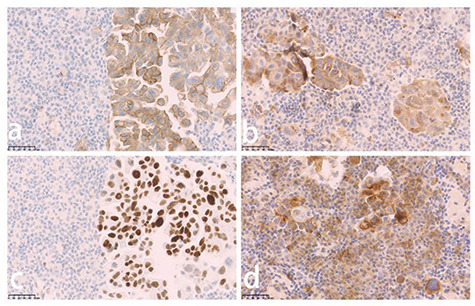
Immunohistopathological analysis of a pleural fluid specimen from the outside institution demonstrated positive staining for ros-1 (a), NapsinA (b), transcription factor-1 (TTF-1) (c), and PD-L1 (d).
In July 2017, the patient was started on crizotinib (250 mg once per day) as a result of an adverse effect (increased alanine aminotransferase, and aspartate aminotransferase levels) and PS has been defined in this place. In <1 month, she noted a significant improvement in symptoms, and by 2 months her dyspnea had resolved. Two months after initiation of the crizotinib treatment, the first tumor evaluation was made at West China Hospital Sichuan University with CT scans. The pleural effusion had decreased significantly and the primary lesion in the lung and breast showed no relevant changes (Fig. 3a,d). Re-evaluation scans at 4 months, 6 months, 10 months, and 12 months also showed no relevant changes in the lung tumor (Fig. 3b,c) and breast carcinoma (Fig. 3e). That is, the patient was judged to have stable disease after 2 months of crizotinib treatment, and the response lasted for 10 months. However, despite the initial response to crizotinib, the tumor became resistant to it and disease progressed in the pleura (Fig. 4a‑f). The chest CT scan reviewed 12 months after initiation of reduced dose crizotinib showed that the pleural nodule in the left had increased in size compared with the image taken 2 months after initiation of treatment. However, standard second-line targeted treatment options were unavailable at that time, so the patient continued on crizotinib treatment beyond disease progression and re-escalation was attempted. After a month, there was no response in the pleural nodule, and it was noteworthy that the nodules of the left mammary gland had increased and enlarged (Fig. 3f). A core needle biopsy was taken on a left breast nodule. Immunohistochemistry revealed that she had a primary breast carcinoma with P63 (−), cytokeratin (CK) 5/6 (−), E-C (+), estrogen receptor (ER) (+, strong, 90%) (Fig. 5a), progesterone receptor (PR) (+, strong, 90%) (Fig. 5b), human epidermal growth factor receptor (Her)-2 (2+) (Fig. 5c), cytokeratin (CK) 7 (partly+) (Fig. 5d), GATA-3 (+), GCDFP-15 (+), TTF-1 (−) (Fig. 5e), and MIB-1 (5%). Subsequent treatment of the patient took the form of hormone therapy with aromatase inhibitors, but RECIST evaluation showed progressive disease after 2 months of initiation. The overall survival calculated from the date of lung cancer diagnosis to the date of death was 14 months. The reason why our patient die is hepatic insufficiency, renal insufficiency and severe edema all over the body, which may be caused by side effects of drugs and the terminal stage of cancer. Later, a breast puncture specimen was screened using ros1 immunohistochemistry, and this showed faint cytoplasmic staining (Fig. 5f). Fluorescence in situ hybridization test showed the specimen to be ros1 mutation-negative (Fig. 6).
Figure 3 .
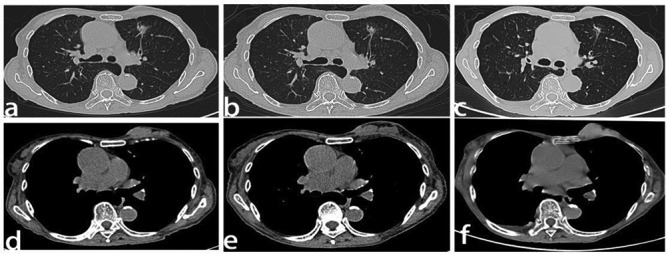
Computed tomography scan and response evaluation of the primary lesions after initiation of reduced dose crizotinib. (a,d) The primary lesion in the superior lobe of the left lung and in the breast 2 months after initiation of reduced dose crizotinib. (b,e) Re-evaluation scans at 6 months showed no relevant changes in the lung tumor and breast carcinoma. (c) The primary lesion in the superior lobe of the left lung 12 months after initiation of reduced dose crizotinib. (f) The primary lesion in the breast 12 months after initiation of reduced dose crizotinib and 1 month after normal dose crizotinib. Line 1: thin layer high resolution scanning; Line 2: mediastinal window.
Figure 4 .
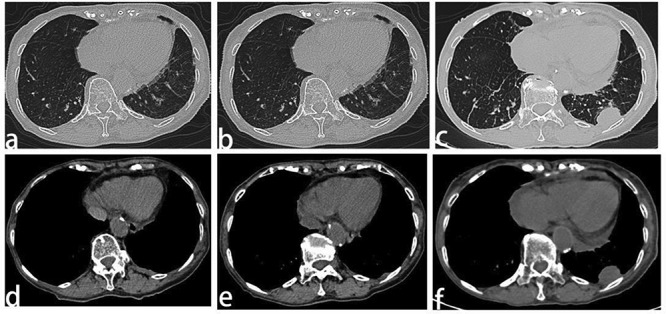
Computed tomography scan and response evaluation of the left pleura nodule 2 months (a,b), 6 months (c,d), and 12 months (e,f) after initiation of reduced dose crizotinib. Line 1: thin layer high resolution scanning; Line 2: mediastinal window.
Figure 5 .
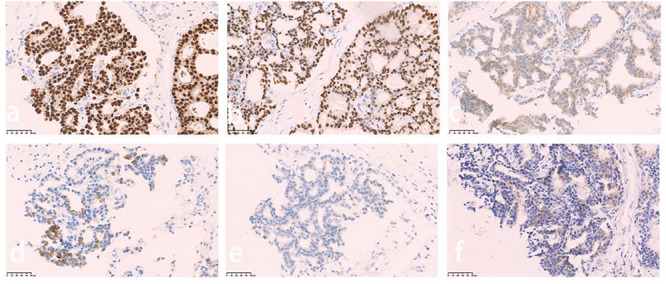
Immunohistopathological analysis of the breast lesion demonstrated positive staining for estrogen receptor (ER) (a), progesterone receptor (PR) (b), and human epidermal growth factor receptor (Her)-2 (C-erbB-2) (c), partly positive staining for cytokeratin (CK) 7 (d), and faint positive staining for ros-1 (f), but negative staining for transcription factor-1 (TTF-1) (e).
Figure 6 .

The breast puncture specimen was ros1 mutation-negative assessed with fluorescence in situ hybridization. Scale bar: 25 μm and 5 μm.
Discussion
c-ros oncogene 1 (ros1, located at 6q22), a receptor tyrosine kinase, was identified as a potential driver of NSCLC in 2007.6 About 1% of lung adenocarcinomas involve ros1 rearrangement, considered to be one of the newest molecular targets in NSCLC.6 The first large sample study carried out by Bergethon et al. indicated that the frequency of ros1 rearrangement in the general population with NSCLC was 1.7% (18 of 1073), mainly in patients with adenocarcinomas, patients of younger age, or never-smokers.2 Because of this, molecular screening for ros1 translocations is always restricted to patients of young age.
In the EUROS1 cohort, when crizotinib was used to treat lung cancer in patients with a ros1 rearrangement, a remarkable, objective reaction was seen in 24 cases, including complete remission in five cases (total effective rate, 80%; the rate of disease control, 86.7%). The median progression-free survival (PFS) was 9.1 months, with a PFS rate of 12 months seen in 44% of patients.4 Although crizotinib approved by US FDA for ros1-positive metastatic NSCLC on March 11, 2016, on the basis of a single-arm, multicenter study (N = 50),7 age-specific outcome subgroup analyses were not reported. Hence, the potency of crizotinib in elderly patients or those with poor PS has been defined in this place has yet to be determined. Dedicated trials in elderly or poor PS patients would be challenging, as this is a rare subtype, and more common in younger patients.8 In addition, in the EUROS1 cohort, a low dose of crizotinib was identified as a potential resistance factor.4
In previous clinical trials or case reports of ros1 rearrangements, most of the patients with ros1-rearranged NSCLC were of younger age. Preliminary results of the METROS trial are available, which is a multicenter prospective phase II study designed to assess the efficacy, safety, and tolerability of crizotinib in pretreated metastatic NSCLC with met amplification or met exon 14 mutation or ros1 rearrangement. This study included patients with ros1-rearrangements of age up to 77 years, similar to the maximum age of the patients treated with crizotinib in ros1-rearranged NSCLC.7,9 The EUROS1 cohort, crizotinib therapy for advanced lung adenocarcinoma and a ros1 rearrangement, included patients who were diagnosed at a median age of 50.5 years (range, 34–78 years).4 In a previous study of the clinicopathological characteristics and outcomes of patients with ros1-rearranged lung adenocarcinoma without egfr, k-ras mutations, and ALK rearrangements, the median age of the five ros1-positive patients was 53 years (range: 41–62).10 In another study to test the frequency and impact of ros1 rearrangement on clinical outcomes in never-smokers with lung adenocarcinoma, the median age of five ros1-positive patients was 55 years (range: 30–68).11 In addition, a phase II study of ceritinib in 32 patients with previously treated ros-1 mutated NSCLC included patients aged up to 79 years.12 In a previous report, 72% of non-Asians, with a median age of 49.8 years (range: 32–79), had ros1 fusions in 1073 cancer screening cases.2 A phase II study of crizotinib in 127 East Asian patients with ros1-positive advanced NSCLC included patients aged up to 79.7 years.13 The age of patients included in all these studies was generally younger than 80 years; however, there is one preliminary reported case in which the oldest patient with ros1-positive lung cancer was 90 years old.3 To the best of our knowledge, the present case report of a 90-year-old female with ros1-positive primary tumor of lung cancer combined with breast cancer, is unprecedented in clinical practice.
Results from a single institution indicate that 26 255 cancer patients admitted to the Institute of Oncology of the University of Istanbul between January 1987 and January 1997 were included in analysis, of which 271 (1%) had multiple primary malignancies.14 Multiple tumors of a patient were classified as synchronic tumors if they were diagnosed within six-month intervals; otherwise, they were classified as metachronous tumors.15 In the present case, it is difficult to distinguish whether the tumor is synchronous or metachronous because the onset of the breast cancer was ignored. In our clinical practice, breast cancer and lung cancer are the most prevalent cancers, and the literature indicates that associations between head/neck cancer and lung cancer, breast cancer and breast cancer are the most frequent.14,16 Data from multiple primary malignant neoplasms demonstrated that the leading primary tumor site and secondary tumor site were both the breast among females, and for secondary tumors the lung was the most frequent for males.16 Results from a study with data from the Surveillance, Epidemiology, and End Results program (1975–2001, 756 467 people in the United States) suggested that the percentage of female patients with first primary tumor in the lung and bronchus and second or later tumor in the breast was 27%, but the prevalence of primary bronchial pulmonary carcinoma in patients with a history of breast cancer was 5%.17 A review of breast cancer patients with pulmonary nodules in 2012,18 stated that 11.5%–48.1% had primary lung cancer. Data from a study in our country indicated that the prevalence of primary lung cancer was 49.3% in patients with a history of breast cancer.19 However, to our knowledge, an elderly female patient with a ros1 rearrangement lung cancer and breast carcinoma has not been reported previously.
The prevalence of multiple primary cancers remains high. This is a result of, on the one hand, prolonged population survival, and, on the other hand, significant advances in treatments and medical equipment.20–22 However, general principles and possible therapeutic management strategies for patients with multiple primary tumors are scarcely discussed, far less the aging population.20 So, how should the treating physician deal with two independent malignancies implicating different organs (lung and breast) occurring in the same elderly patient at the same time? The literature involving targeted treatment of associations of two primary malignancies in the aged patient population, lung cancer and breast cancer in a single aged patient, was reviewed, comprising a PubMed database search of reported literature in English. The search was implemented using different combinations of search keywords including “(multiple primary malignancies OR multiple primary cancers OR multiple concurrent cancers OR multiple primary carcinomas) AND (lung carcinoma OR lung cancer OR bronchiogenic cancer OR bronchiogenic OR lung adenocarcinoma) AND (breast carcinoma OR breast cancer) AND (old people OR the aged OR elderly people OR senior citizens OR old folks) AND (targeted therapy OR precise treatment)”. The results are as follows: if two current active malignancies are detected at an early stage, cancer treatment, whether radical surgical resection separately or curative radiation/chemoradiation therapy covering both carcinomas, could be performed actively on the premise of a patient’s requirements and tolerance.21,23,24 However, if at a later stage of combination of two primary cancers with a severe prognosis, it is often challenging to select treatment approaches. This is mostly not based on evidence from the literature as only case reports are accessible for most clinical situations. In clinical trials, the vast majority of patients with active secondary malignancy or aging patients are excluded. In the real-world examples of clinical situations, treatment approaches are the result of individual decisions.20,25 Proposed treatment approaches should involve a specific strategy for minimal suffering, in which both tumors are likely to respond.20 In daily clinical practice, it is critical to first treat the most unpleasant and life-threatening aspects of the conditions, which may vary from one patient to another, as in cases of multiple primaries.26 Whether treating localized disease or disease at an advanced, it is important that multidisciplinary team meetings are held to form a consensus on the therapeutic strategy, particularly when discussing treatment of elderly patients because of the higher likelihood of comorbidities, higher polypharmacy interactions, and aged organ dysfunction.20,25 With the progress and wider accessibility of genetic evaluation and testing, targeted therapies with greater antitumor efficacy and less toxicity compared with conventional chemotherapy also should be taken into consideration, especially in aged patients.26 With respect to our patient, not only does targeted therapy maximize the therapy benefits but it also minimizes the treatment risk, and its benefits have exceeded expectations. Targeted drugs should also be given with dose adaptation in elderly patients based on a case-by-case basis, but specific studies are lacking.27
Neglect of mammary nodules at the onset of our case may be the one blemish on an otherwise resounding treatment success. A study of 2320 women with NSCLC found that use of antiestrogen significantly reduced lung cancer mortality before and after a lung cancer diagnosis. This is consistent with results from a study registered at the Geneva Cancer Registry, including 6655 women.4,28 Early attention, early puncture, and early antiestrogen treatment may improve the prognosis of our patient and reduce the risk of death from lung cancer.
Conclusion
The case we report highlights the potential role of reduced-dose crizotinib treatment in management of ros1-positive elderly patients with lung adenocarcinomas, particularly in those who have concomitant diseases and poor health status. Consequently, it is crucial that screening is performed for these mutations in elderly patients. This case supports the efficacy of a dose reduction of crizotinib in senior patients with ros1-rearranged NSCLC. Furthermore, antiestrogen use may play a key role in the outcomes of patients with NSCLC.
Acknowledgements
The authors thank all the staff who provided care to the patient and editorial support.
Funding
This work was supported by the National Science and Technology Major Project (Grant No. 2017ZX09304023).
Conflict of interest statement
None declared.
References
- 1. Kim MH, Shim HS, Kang DR, et al. Clinical and prognostic implications of ALK and ROS1 rearrangements in never-smokers with surgically resected lung adenocarcinoma. Lung Cancer 2014;83:389–95. doi: 10.1016/j.lungcan.2014.01.003 [DOI] [PubMed] [Google Scholar]
- 2. Bergethon K, Shaw AT, Ou SH, et al. ROS1 rearrangements define a unique molecular class of lung cancers. J Clin Oncol 2012;30:863–70. doi: 10.1200/JCO.2011.35.6345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Overbeck TR, Schmitz K, Engelke C, et al. Partial response to first-line crizotinib in an elderly male patient with ROS1 translocation-positive lung cancer. Case Rep Oncol 2016;9:158–63. doi: 10.1159/000444745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mazieres J, Zalcman G, Crino L, et al. Crizotinib therapy for advanced lung adenocarcinoma and a ROS1 rearrangement: Results from the EUROS1 cohort. J Clin Oncol 2015;33:992–9. doi: 10.1200/JCO.2014.58.3302 [DOI] [PubMed] [Google Scholar]
- 5. Bouchardy C, Benhamou S, Schaffar R, et al. Lung cancer mortality risk among breast cancer patients treated with anti-estrogens. Cancer 2011, 117:1288–95. doi: 10.1002/cncr.25638 [DOI] [PubMed] [Google Scholar]
- 6. Rikova K, Guo A, Zeng Q, et al. Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell 2007;131:1190–203. doi: 10.1016/j.cell.2007.11.025 [DOI] [PubMed] [Google Scholar]
- 7. Shaw AT, Ou SH, Bang YJ, et al. Crizotinib in ROS1-rearranged non-small-cell lung cancer. N Engl J Med 2014;371:1963‑71. doi: 10.1056/NEJMoa1406766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Carmichael JA, Wing-San Mak D, O’Brien M. A review of recent advances in the treatment of elderly and poor performance NSCLC. Cancers (Basel)2018;10:236. doi: 10.3390/cancers10070236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Landi L, Chiari R, Delmonte A, et al. A07Crizotinib in ROS1 rearranged or MET deregulated non-small-cell lung cancer (NSCLC): Preliminary results of the METROS trial. Ann Oncol 2016;27(suppl_4):iv5-iv5. doi: 10.1093/annonc/mdw332.07 [DOI] [Google Scholar]
- 10.Wu S, Wang J, Zhou L, et al. Clinicopathological characteristics and outcomes of ROS1-rearranged patients with lung adenocarcinoma without EGFR, KRAS mutations and ALK rearrangements. Thorac Cancer 2015;6:413–20. doi: 10.1111/1759-7714.12191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kim H R, Lim S M, Kim H J, et al. The frequency and impact of ROS1 rearrangement on clinical outcomes in never smokers with lung adenocarcinoma. Ann Oncol 2013;24:2364–70. doi: 10.1093/annonc/mdt220 [DOI] [PubMed] [Google Scholar]
- 12. Lim S M, Kim H R, Lee J S, et al. Open-label, Multicenter, phase II study of ceritinib in patients with non-small-cell lung cancer harboring ROS1 rearrangement. J Clin Oncol 2017;35:2613. doi: 10.1200/JCO.2016.71.3701 [DOI] [PubMed] [Google Scholar]
- 13. Wu Y L, Yang C H, Kim D W, et al. Phase II study of crizotinib in east Asian patients with ROS1-positive advanced non-small-cell lung cancer. J Clin Oncol 2018;36:JCO2017755587. doi: 10.1200/JCO.2017.75.5587 [DOI] [PubMed] [Google Scholar]
- 14. Aydiner A, Karadeniz A, Uygun K, et al. Multiple primary neoplasms at a single institution: Differences between synchronous and metachronous neoplasms. Am J Clin Oncol 2000;23:364–70. PMID: 10955865 [DOI] [PubMed] [Google Scholar]
- 15. Hajdu S I, Hajdu E O.. Multiple primary malignant tumors. J Am Geriatr Soc 1968;16:11. doi: 10.1111/j.1532-5415.1968.tb03965.x [DOI] [PubMed] [Google Scholar]
- 16. Gursel B, Meydan D, Ozbek N, et al. Multiple primary malignant neoplasms from the Black Sea region of Turkey. J Int Med Res 2011;39:667–74. doi: 10.1177/147323001103900237 [DOI] [PubMed] [Google Scholar]
- 17. Loehrer PJ. Multiple cancer prevalence: A growing challenge in long-term survivorship. Yearbook of Oncology 2008;2008:368–9. [DOI] [PubMed] [Google Scholar]
- 18. Rashid OM, Takabe K.. The evolution of the role of surgery in the management of breast cancer lung metastasis. J Thoracic Dis 2012;4:420–24. doi: 10.3978/j.issn.2072-1439.2012.07.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Song Z, Ye T, Ma L, et al. Surgical outcomes of isolated malignant pulmonary nodules in patients with a history of breast cancer. Ann Surg Oncol 2017;24:3748–53. doi: 10.1245/s10434-017-6067-0 [DOI] [PubMed] [Google Scholar]
- 20. Vogt A, Schmid S, Heinimann K, et al. Multiple primary tumours: Challenges and approaches, a review. ESMO Open 2017;2:e000172. doi: 10.1136/esmoopen-2017-000172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Heroiu Cataloiu AD, Danciu CE, Popescu CR. Multiple cancers of the head and neck. Maedica (Buchar) 2013;8:80–5. PMC3749768 [PMC free article] [PubMed] [Google Scholar]
- 22. Herrmann C, Cerny T, Savidan A, et al. Cancer survivors in Switzerland: A rapidly growing population to care for. BMC Cancer 2013;13:287. doi: 10.1186/1471-2407-13-287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhang Z, Gao S, Mao Y, et al. Surgical outcomes of synchronous multiple primary non-small cell lung cancers. Sci Rep 2016;6:23252. doi: 10.1038/srep23252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Du BC, Jun L, Jing C, et al. Diagnosis and treatment of breast cancer combined with primary lung cancer. Medical Recapitulate 2013;50:265–66. [Google Scholar]
- 25. Klairmont M, Kopkash K, Favuzza J, et al. Four synchronous primary malignancies of the breast, lung, colon and esophagus. Anticancer Res 2015;35:6159–62. PMID: 26504043 [PubMed] [Google Scholar]
- 26. Zhang Y, Ge Y, Wu X, Liu S. Clinical treatment of advanced synchronous triple primary malignancies: Comprehensive treatment based on targeted therapy. Onco Targets Ther 2019;12:2421–30. doi: 10.2147/OTT.S200625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Leduc C, Antoni D, Charloux A, et al. Comorbidities in the management of patients with lung cancer. Eur Respir J 2017;49: 1601721. doi: 10.1183/13993003.01721-2016 [DOI] [PubMed] [Google Scholar]
- 28. Lother S A, Harding G A, Musto G, et al. Antiestrogen use and survival of women with non-small cell lung cancer in Manitoba, Canada. Horm Cancer 2013;4:270–6. doi: 10.1007/s12672-013-0149-7 [DOI] [PMC free article] [PubMed] [Google Scholar]


