Abstract
The postantifungal effect (PAFE) of fluconazole, MK-0991, LY303366, and amphotericin B was determined against isolates of Candida albicans and Cryptococcus neoformans. Concentrations ranging from 0.125 to 4 times the MIC were tested following exposure to the antifungal for 0.25 to 1 h. Combinations of azole and echinocandin antifungals (MK-0991 and LY303366) were tested against C. neoformans. Fluconazole displayed no measurable PAFE against Candida albicans or Cryptococcus neoformans, either alone or in combination with either echinocandin antifungal. MK-0991, LY303366, and amphotericin B displayed a prolonged PAFE of greater than 12 h against Candida spp. when tested at concentrations above the MIC for the organism and 0 to 2 h when tested at concentrations below the MIC for the organism.
As the frequency of fungal infections increases, there is a need for new antifungal agents and increased understanding of the pharmacodynamic properties of these agents. The echinocandin antifungals represent a new class of antifungal agents that inhibit fungal growth by inhibition of glucan synthase, an enzyme responsible for fungal cell wall formation. The activity of these agents has been described as either fungicidal or fungistatic, depending on the isolate and test conditions (1, 3). Amphotericin B (AMB), the mainstay of therapy for fungal infections, is described as having concentration-dependent fungicidal activity (8). In comparison, the azole antifungal agents are described as exhibiting concentration-independent fungistatic activity (8).
The postantifungal effect (PAFE) is the suppression of fungal growth that persists after limited exposure to an antifungal agent. This has been described for the azole antifungals and AMB, but not for the echinocandin antifungal agents (10, 12). The PAFE may have clinical relevance to the design of dosing regimens for new antifungal agents such as the echinocandins. Antimicrobials with long PAFEs may be given less frequently than antimicrobials with short PAFEs, which may require more frequent administration.
We sought to determine the PAFEs of echinocandin antifungals MK-0991 and LY303366 and compare them to the PAFEs of AMB and fluconazole (FLC). Secondarily, we sought to determine if the addition of an echinocandin antifungal would enhance the relatively short PAFE generally observed with FLC.
(This work was presented at the American College of Clinical Pharmacy Annual Meeting, Phoenix, Ariz., 9 to 12 November 1997.)
Stock solutions of AMB (Sigma Chemical Company, St. Louis, Mo.), FLC (Pfizer, Inc., New York, N.Y.), MK-0991 (Merck and Co., Inc., Rahway, N.J.), and LY303366 (Eli Lilly and Co., Indianapolis, Ind.) were prepared in RPMI 1640 medium (Sigma) buffered to a pH of 7.0 with 0.165 M morpholinopropanesulfonic acid (MOPS). All antifungals were solubilized with less than a 1% (vol/vol) final volume of dimethyl sulfoxide.
One clinical isolate, OY31.5, and one American Type Culture Collection isolate, 90028, of Candida albicans were selected for study based upon extensive experience with these isolates in our laboratory. Two clinical isolates of Cryptococcus neoformans were also selected for study because of our experience with the growth characteristics of these isolates. All isolates were obtained from the Molecular Epidemiology and Fungus Testing Laboratory, Department of Pathology, University of Iowa College of Medicine, Iowa City. Isolates were stored in sterile water, and each isolate was grown on potato dextrose agar and subcultured twice prior to testing.
MICs of FLC, MK-0991, LY303366, and AMB were determined by using broth microdilution methods according to National Committee for Clinical Laboratory Standards guidelines (M27-A) (11). The endpoint for the MICs of FLC, MK-0991, and LY303366 was 80% inhibition of growth compared to the control, determined by visual inspection (3, 5). The endpoint for the MIC of AMB was complete inhibition of visible growth (11). All MICs were measured in RPMI with MOPS medium (Sigma) and results were read after 48 h of incubation for C. albicans and 72 h of incubation for C. neoformans in a dark, moist chamber at 35°C.
The method used for determining the PAFE in C. albicans was based on the methods used for bacteria and our previous experience with antifungal time-kill methodology (2–4, 6–9). A fungal suspension was prepared by transfer of three to five colonies of test isolate from a 48-h-old culture plate into 9 ml of sterile water and adjusting it to a 0.5 McFarland turbidity standard. One milliliter of this fungal suspension was added to vials containing culture medium alone or with study drug at concentrations ranging from 0.125 to 4.0 times the MIC. Following an incubation period ranging from 0.25 h to 1.0 h, colony counts were conducted, and the study drug was removed by a process of three cycles of repeated centrifugation and washing with normal saline. After the final centrifugation, the fungal pellet was resuspended in 10 ml of warm medium, and colony counts were repeated at 2, 4, 6, 8, 10, 12, and 24 h after the final wash. A 100-μl sample was obtained from the culture vial and diluted with sterile water, and 30 μl was plated on potato dextrose agar for colony counting. The lower limit of accurately detectable CFU per milliliter by these methods is 50 (7). Procedures for C. neoformans were similar to those used for C. albicans, except the drugs were studied alone and in combination at only one drug concentration (FLC, two times the MIC; MK-0991, 80 μg/ml; LY303366, 80 μg/ml). A single drug concentration was selected because the echinocandin antifungals do not demonstrate appreciable activity alone in vitro against C. neoformans (MIC, >2 μg/ml). Since FLC did not demonstrate a PAFE against C. albicans at any concentration tested, a single concentration was selected for testing against C. neoformans. Isolates of C. neoformans were incubated at 30°C for optimal growth. All experiments were conducted in duplicate.
Plots of averaged colony counts (log10 CFU per milliliter) versus time were constructed and compared against a growth control. PAFE was calculated as the difference in time required for control and test isolates to grow 1 log10 following the final cell wash.
Both of the C. albicans isolates were susceptible to the AMB and FLC (Table 1). The C. neoformans isolates had MICs of 4.0 (susceptible) and >2.0 μg/ml for FLC and the echinocandin antifungals, respectively.
TABLE 1.
Results of susceptibility tests
| Isolate | MIC (μg/ml)
|
|||
|---|---|---|---|---|
| FLC | MK-0991 | LY303366 | AMB | |
| C. albicans 90028 | 0.25 | 0.03 | 0.015 | 1.0 |
| C. albicans OY31.5 | 0.25 | 0.03 | 0.015 | 0.5 |
| C. neoformans 625.012 | 4 | >2 | >2 | 1.0 |
| C. neoformans 1425.019 | 4 | >2 | >2 | 1.0 |
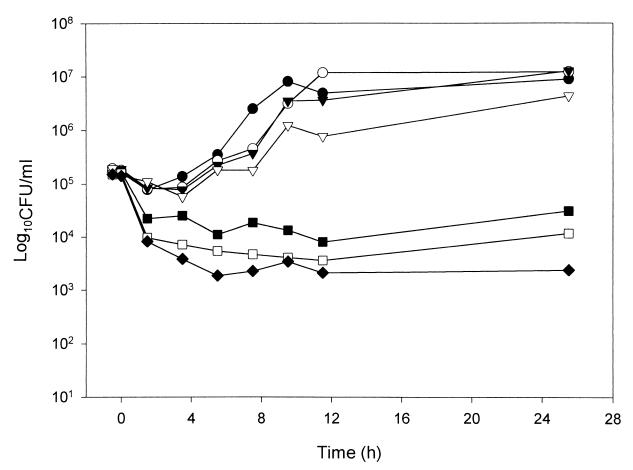
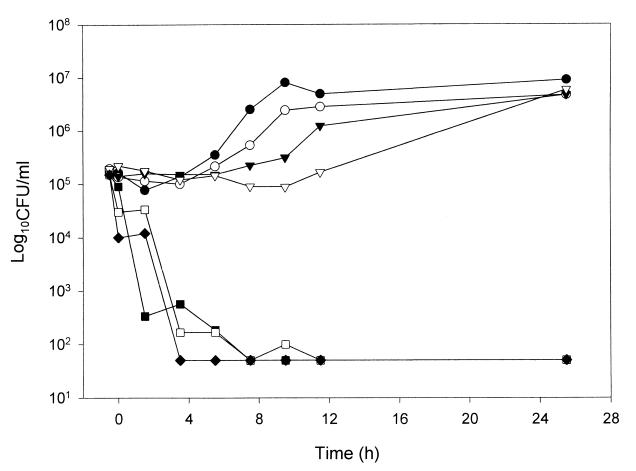
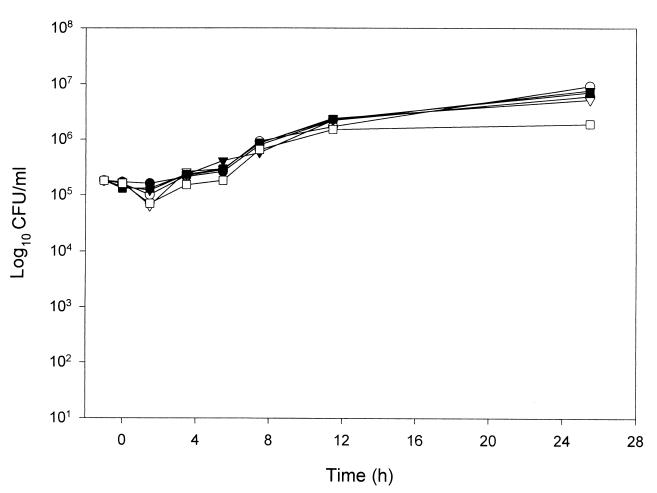
Yeast exposed to FLC for 1 h did not display any measurable PAFE when tested at concentrations of one to four times the MIC. As a result, concentrations below the MIC and shorter exposure times were not used with FLC. In contrast, MK-0991, LY303366, and AMB displayed a prolonged PAFE (>12 h) following exposure for 1 h at concentrations greater than or equal to the MIC of the organism (Table 2). At concentrations below the MIC, exposure to MK-0991 for 1 h resulted in no observable PAFE. In contrast, C. albicans exposed to LY303366 concentrations below the MIC displayed a prolonged PAFE of greater than 12 h following exposure for 1 h. For the 1-h exposure time and concentrations below the MIC, the PAFE of AMB ranged from 2 to >12 h. Concentrations above the MICs of MK-0991, LY303366, and AMB resulted in prolonged PAFEs of >12 h. In order to investigate the effect of exposure time on the observed PAFE, MK-0991, and AMB were also tested at 0.25- and 0.5-h exposure times. For yeast exposed to MK-0991 or AMB at concentrations above the MIC for 0.25 h there was a prolonged PAFE of >12 h. The PAFE observed for MK-0991 following an exposure time of 0.5 h was 0 to 2 h for concentrations below the MIC and >12 h for concentrations above the MIC (Fig. 1). AMB displayed no observable PAFE at the lowest concentration tested (0.125 times the MIC). Because AMB produced fungicidal activity at all other concentrations tested, the PAFE could not be determined (Fig. 2). FLC did not display a measurable PAFE against C. neoformans alone or in combination with the echinocandin antifungal agents (Fig. 3).
TABLE 2.
PAFE after 1.0 h of drug exposure time for concentrations ranging from 0.125 to 4.0 times the MIC for C. albicans OY31.5 and 90028
| Drug concn (multiple of MIC) | PAFE (h)
|
|||
|---|---|---|---|---|
| FLC | MK0991 | LY303366 | AMB | |
| C. albicans OY31.5 | ||||
| 0.125 | NTa | 0 | >12 | 2 |
| 0.25 | NT | 0 | >12 | 4 |
| 0.5 | NT | 0 | >12 | >12 |
| 1.0 | 0 | >12 | >12 | >12 |
| 2.0 | 0 | >12 | >12 | >12 |
| 4.0 | 0 | >12 | >12 | >12 |
| C. albicans 90028 | ||||
| 0.125 | NT | 0 | 0 | 2 |
| 0.25 | NT | 0 | >12 | >12 |
| 0.5 | NT | 0 | >12 | >12 |
| 1.0 | 0 | >12 | >12 | >12 |
| 2.0 | 0 | >12 | >12 | >12 |
| 4.0 | 0 | >12 | >12 | >12 |
NT, not tested.
FIG. 1.
PAFE of MK-0991 in C. albicans 90028 following 0.5 h of exposure to the drug at various concentrations. ●, control; ○, 0.125 times the MIC; ▾, 0.25 times the MIC; ▿, 0.5 times the MIC; ■, 1 times the MIC; □, 2 times the MIC; ⧫, 4 times the MIC.
FIG. 2.
PAFE of AMB in C. albicans 90028 following 0.5 h of drug exposure at various concentrations. ●, control; ○, 0.125 times the MIC; ▾, 0.25 times the MIC; ▿, 0.5 times the MIC; ■, 1 times the MIC; □, 2 times the MIC; ⧫, 4 times the MIC.
FIG. 3.
PAFE of FLC, MK-0991, and LY303366 alone and in combination in C. neoformans 1450. ●, control; ○, FLC; ▾, MK-0991; ▿, LY303366; ■, FLC plus MK-0991; □, FLC plus LY303366.
We have investigated the effect of concentration and exposure time on the PAFEs of three different classes of antifungal agents. FLC did not produce a measurable PAFE against C. albicans or C. neoformans regardless of concentration. This finding is consistent with previously published reports of the PAFEs of the azole antifungals, including FLC (10, 12). In contrast, the echinocandin antifungal MK-0991 displayed long PAFEs which were dependent on concentration. Concentrations below the MIC produced a very short PAFE (0 to 2 h) compared to concentrations above the MIC, which produced consistently prolonged PAFEs of >12 h. The prolonged PAFE observed was not affected by the exposure time, such that exposure for 0.25 h to the antifungal produced the same PAFE as exposure for 1 h. In comparison, LY303366, also an echinocandin antifungal, generally produced long PAFEs even at concentrations below the MIC for the organism, with the exception of C. albicans 90028, which at a concentration of 0.125 times the MIC produced no measurable PAFE. We did not test shorter exposure times with LY303366. It is possible that the variability in PAFE between these two compounds of the same antifungal class may result from imperfections in determining the MIC for an organism. That is, if the MIC used for determining the PAFE was 1 doubling dilution higher than the true MIC, then subinhibitory concentrations may actually be at or above the true MIC for the organisms, resulting in a longer observed PAFE. The addition of an echinocandin with FLC did not increase the PAFE against C. neoformans. AMB produced fungicidal activity at concentrations above the MIC regardless of the exposure time; therefore, it was not possible to calculate the PAFE under these conditions. The observed PAFE with AMB was shorter with concentrations below the MIC than with higher concentrations. This is consistent with the previously published reports of the PAFE of AMB (12).
This is the first published study of the PAFE produced by the echinocandin antifungal agents. Interestingly, the echinocandins and AMB, both of which have been described as exhibiting fungicidal activity, displayed PAFEs which were prolonged and dose dependent. In comparison, the azole antifungal FLC, which is fungistatic, does not produce a measurable PAFE against C. albicans or C. neoformans. The significant PAFEs observed with the echinocandin antifungal agents may have clinical relevance, but additional animal studies and human clinical trials are necessary to substantiate these observations.
REFERENCES
- 1.Bartizal K, Gill C J, Abruzzo G K, Flattery A M, Kong L, Scott P M, Smith J G, Leighton C E, Bouffard A, Dropinski J F, Balkovec J. In vitro preclinical evaluation studies with the echinocandin antifungal MK-0991 (L-743,872) Antimicrob Agents Chemother. 1997;41:2326–2332. doi: 10.1128/aac.41.11.2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Craig W A, Gudmundsson S. Postantibiotic effect. 4th ed. Baltimore, Md: Williams & Wilkins; 1996. [Google Scholar]
- 3.Ernst E J, Klepser M E, Ernst M E, Messer S A, Pfaller M A. In vitro pharmacodynamic properties of MK-0991 determined by time-kill methods. Diagn Microbiol Infect Dis. 1999;33:75–80. doi: 10.1016/s0732-8893(98)00130-8. [DOI] [PubMed] [Google Scholar]
- 4.Ernst M E, Klepser M E, Wolfe E J, Pfaller M A. Antifungal dynamics of LY 303366, an investigational echinocandin B analog, against Candida ssp. Diagn Microbiol Infect Dis. 1996;26:125–131. doi: 10.1016/s0732-8893(96)00202-7. [DOI] [PubMed] [Google Scholar]
- 5.Klepser M E, Ernst E J, Ernst M E, Messer S A, Pfaller M A. Evaluation of endpoints for antifungal susceptibility determinations with LY303366. Antimicrob Agents Chemother. 1998;42:1387–1391. doi: 10.1128/aac.42.6.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klepser M E, Ernst E J, Ernst M E, Pfaller M A. Growth medium effect on the antifungal activity of LY 303366. Diagn Microbiol Infect Dis. 1997;29:227–231. doi: 10.1016/s0732-8893(97)00144-2. [DOI] [PubMed] [Google Scholar]
- 7.Klepser M E, Ernst E J, Lewis R E, Ernst M E, Pfaller M A. Influence of test conditions on antifungal time-kill curve results: proposal for standardized methods. Antimicrob Agents Chemother. 1998;42:1207–1212. doi: 10.1128/aac.42.5.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klepser M E, Wolfe E J, Jones R N, Nightingale C H, Pfaller M A. Antifungal pharmacodynamic characteristics of fluconazole and amphotericin B tested against Candida albicans. Antimicrob Agents Chemother. 1997;41:1392–1395. doi: 10.1128/aac.41.6.1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klepser M E, Wolfe E J, Pfaller M A. Antifungal pharmacodynamic characteristics of fluconazole and amphotericin B against Cryptococcus neoformans. J Antimicrob Chemother. 1998;41:397–401. doi: 10.1093/jac/41.3.397. [DOI] [PubMed] [Google Scholar]
- 10.Minguez F, Chiu M L, Lima J E, Nique R, Prieto J. Activity of fluconazole: postantifungal effect, effects of low concentrations and of pretreatment on the susceptibility of Candida albicans to leucocytes. J Antimicrob Chemother. 1994;34:93–100. doi: 10.1093/jac/34.1.93. [DOI] [PubMed] [Google Scholar]
- 11.National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard M27-A. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 12.Turnidge J D, Gudmundsson S, Vogelman B, Craig W A. The postantibiotic effect of antifungal agents against common pathogenic yeasts. J Antimicrob Chemother. 1994;34:83–92. doi: 10.1093/jac/34.1.83. [DOI] [PubMed] [Google Scholar]