Editor's note
A commentary on “Combination of anti-PD-1 antibody with P-GEMOX as a potentially effective immunochemotherapy for advanced natural killer/T cell lymphoma”.
Keywords: immune checkpoint inhibitor, chemoimmunotherapy, PD-1, nasal lymphoma
Extranodal natural killer/T-cell lymphoma (ENKTL) is an uncommon and aggressive subtype of non-Hodgkin lymphoma with a geographic distribution unique to East Asia and Latin America.1 This disease is often characterized by extranodal involvement of the midline nasal areas and is invariably associated with Epstein-Barr virus (EBV) infection of the neoplastic lymphoid cells.2 Over the past decade, treatment for ENKTL has made notable progress with the introduction of L-asparaginase-based chemotherapy regimens, including SMILE (dexamethasone, methotrexate, ifosfamide, L-asparaginase, and etoposide), P-GEMOX (pegaspargase, gemcitabine and oxaliplatin), DDGP (dexamethasone, cisplatin, gemcitabine and pegaspargase), and AspaMetDex (pegaspargase, methotrexate, and dexamethasone). When combined with radiotherapy, a 3-year overall survival rate of 70% can be achieved in localized ENKTL. However, a patient diagnosed with advanced ENKTL today is still expected to suffer from a high relapse rate of approximately 70%, despite having received at least one of these contemporary chemotherapy regimens and having overcome the severe treatment-related toxicities that typically ensue.3–6 Emboldened by the dim clinical outlook for such patients who relapse or become refractory to current therapies, new treatment options, such as immunotherapy, are being explored to improve survival outcomes.
Up-regulation of programmed death-ligand 1 (PD-L1) expression by genomic alterations has provided a molecular basis for targeting the PD-1/PD-L1 immune checkpoint axis.7 Antibodies targeting PD-1 have demonstrated promising results. In an interim report of a phase II prospective study, use of sintilimab achieved an overall response rate of 68% in 28 patients with relapsed or refractory ENKTL.8 Given these encouraging results, it is imperative that anti-PD-1 therapy be fast-tracked to frontline treatment of ENKTL.
The clinical investigation published in Signal Transduction and Targeted Therapy by Cai et al.9 described the first combined usage of anti-PD-1 antibody with P-GEMOX as first-line treatment for patients with advanced ENKTL. The objective response rate was 88.9% (8/9), and the complete response rate was 77.8%. While the study concept and early results are interesting, the small numbers of patients included, short follow-up duration, and non-randomized design impose a limit to its clinical applicability at this point in time, as the authors have rightly acknowledged. Nonetheless, this study provided the initial proof-of-concept that such a combination is feasible and safe. Notably, treatment-related adverse effects were manageable and included mainly reversible cytopenia, as well as a single case of grade 2 hypothyroidism.
Intriguingly, the authors hypothesized that P-GEMOX may exert an immunostimulatory effect on the tumor microenvironment, favoring a potential synergistic effect with anti-PD-1 checkpoint immunotherapy. The actual mechanism will certainly require serial sampling of the tumors before and after treatment, and direct examination of the immune milieu for confirmation. In addition, the authors attempted to correlate PD-L1 immunohistochemical expression and genomic alterations with the observed treatment responses. However, the mixed use of four types of anti-PD-1 antibodies in this study may hinder identification of a definitive predictive biomarker using PD-L1 immunohistochemistry from a single 22C3 antibody clone, as has been the larger experience in the solid tumor field. Recently, our group conducted a systematic multi-center clinicopathologic and genetic analysis of 19 patients with relapsed or refractory ENKTL treated with pembrolizumab.10 We identified cryptic rearrangements of the PD-L1 gene disrupting the 3′-UTR as a strong positive predictor of response to pembrolizumab. On the other hand, PD-L1 immunohistochemistry using the SP263 antibody clone showed that PD-L1 was expressed in almost all cases, consistent with the findings by Cai et al., and is therefore not an ideal biomarker for checkpoint immunotherapy in ENKTL.
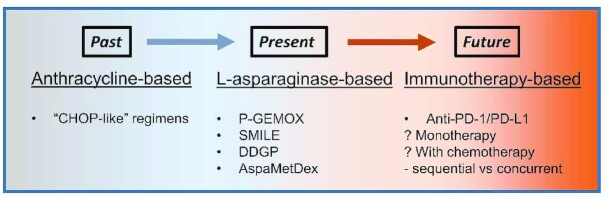
Where should we go from here? In the past few decades, the treatment of ENKTL has steadily evolved from anthracycline-based to L-asparaginase-based regimens (Fig. 1). The potential utility of checkpoint immunotherapy in combination with L-asparaginase-based chemotherapy in upfront treatment of ENKTL has certainly been placed in the spotlight. Closer inspection, however, reveals several issues that must be addressed before its wider adoption as a standard treatment modality. As witnessed in the study by Cai et al., within the short follow-up of 10.6 months, three of the nine patients had already experienced disease progression, suggesting an intrinsic limitation of this combined chemoimmunotherapy regimen. This highlights the need to further optimize the inclusion of specific chemotherapy agents and/or checkpoint inhibitors used, either alone or in combination, as well as their ideal sequencing. Perhaps most importantly, with the goal of achieving precision medicine, a genomic study on a larger treatment cohort is warranted to define the predictive biomarker landscape in ENKTL.
Figure 1.
Evolution of front-line therapies for advanced extranodal natural killer/T-cell lymphoma.
In the future, we envision that first-line treatment of ENKTL will enter a new era using immunotherapy-based regimens, with the hope of inducing durable remissions and prolonged survival in these patients. Randomized controlled trials evaluating checkpoint immunotherapy either alone or in combination with standard L-asparaginase-based regimens are urgently needed to achieve this goal.
Contributor Information
Jason Yongsheng Chan, Division of Medical Oncology, National Cancer Centre Singapore, 169610, Singapore; SingHealth Duke-NUS Blood Cancer Centre, 169610, Singapore.
Jing Quan Lim, Lymphoma Genomic Translational Research Laboratory, Division of Cellular and Molecular Research, National Cancer Centre Singapore, 169610, Singapore; Duke-NUS Medical School, 169857, Singapore.
Choon Kiat Ong, Lymphoma Genomic Translational Research Laboratory, Division of Cellular and Molecular Research, National Cancer Centre Singapore, 169610, Singapore; Duke-NUS Medical School, 169857, Singapore; Genome Institute of Singapore, 138672, Singapore.
Conflict of interest
None declared.
References
- 1. Aviles A. Nasal NK/T-cell lymphoma. A comparative analysis of a Mexican population with the other populations of Latin-America. Mediterr J Hematol Infect Dis. 2015;7:e2015052. doi:10.4084/MJHID.2015.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chan JKC, Quintanilla-Martinez L, Ferry JA, et al. In WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th ed. Swerdlow SH, Campo E, Harris NL. et al.(eds.). IARC Press, Lyon, 2008:; 285– 8. [Google Scholar]
- 3. Kwong YL, Kim WS, Lim ST, et al. SMILE for natural killer/T-cell lymphoma: analysis of safety and efficacy from the Asia Lymphoma Study Group. Blood. 2012;120:2973– 80. doi:10.1182/blood-2012-05-431460. [DOI] [PubMed] [Google Scholar]
- 4. Huang H-q, Yan G, Su H, et al. Clinical outcome of an multicentre, randomized, phase II clinical trial for patients with extranodal NK/T cell lymphoma treated by P-Gemox or aspametdex. Blood. 2019;134:1569. doi:10.1182/blood-2019-123478. [Google Scholar]
- 5. Li X, Cui Y, Sun Z, et al. DDGP versus SMILE in newly diagnosed advanced natural killer/T-Cell lymphoma: A randomized controlled, multicenter, open-label study in China. Clin Cancer Res. 2016;22:5223– 8. doi:10.1158/1078-0432.CCR-16-0153. [DOI] [PubMed] [Google Scholar]
- 6. Jaccard A, Gachard N, Marin B, et al. Efficacy of L-asparaginase with methotrexate and dexamethasone (AspaMetDex regimen) in patients with refractory or relapsing extranodal NK/T-cell lymphoma, a phase 2 study. Blood. 2011;117:1834– 9. doi:10.1182/blood-2010-09-307454. [DOI] [PubMed] [Google Scholar]
- 7. Song TL, Nairismägi M-L, Laurensia Y, et al. Oncogenic activation of STAT3 pathway drives PD-L1 expression in natural killer/T cell lymphoma. Blood. 2018;132:1146–58. doi:10.1182/blood-2018-01-829424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Li J, Tao R, Fan L, et al. Sintilimab for relapsed/refractory (r/r) extranodal NK/T cell lymphoma (ENKTL): Extended follow-up on the multicenter, single-arm phase II trail (ORIENT-4). J Clin Oncol. 2020;38:8050. doi:10.1200/JCO.2020.38.15_suppl.8050. [Google Scholar]
- 9. Cai J, Liu P, Huang H, et al. Combination of anti-PD-1 antibody with P-GEMOX as a potentially effective immunochemotherapy for advanced natural killer/T cell lymphoma. Signal Transduct Target Ther. 2020;5:289. doi:10.1038/s41392-020-00331-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lim JQ, Huang D, Tang T, et al. Whole-genome sequencing identifies responders to Pembrolizumab in relapse/refractory natural-killer/T cell lymphoma. Leukemia. 2020;34:3413–9. doi:10.1038/s41375-020-1000-0. [DOI] [PMC free article] [PubMed] [Google Scholar]