Abstract
Infantile hepatic hemangiomas (IHHs) are common benign tumors seen in the liver of infants. IHHs are true infantile hemangiomas (IHs) and have phases of proliferation and involution parallel to those of cutaneous IHs. The definition and classification of IHH are still confusing in the literature. The mechanisms during the pathogenesis of IHH have yet to be discovered. The clinical manifestations of IHH are heterogeneous. Although most IHH lesions are asymptomatic, some lesions can lead to severe complications, such as hypothyroidism, consumptive coagulopathy, and high-output congestive cardiac failure. Consequently, some patients can possibly encounter a fatal clinical condition. The heterogeneity of the lesions and the occurrence of disease-related comorbidities can make the treatment of IHH challenging. Oral propranolol is emerging as an effective systemic approach to IHH with obvious responses in tumor remission and symptom regression. However, the precise clinical characteristics and treatment strategies for patients with severe IHH have not yet been well established. Here, we summarize the epidemiology, pathogenic mechanism, clinical manifestations, diagnosis, and treatment of IHH. Recent updates and future perspectives for IHH will also be elaborated.
Keywords: infantile hepatic hemangioma, angiogenesis, vasculogenesis, screening, endothelial progenitor cells, glycolysis, consumptive hypothyroidism, treatment
Introduction
Hepatic hemangioma (HH) is a common benign hepatic tumor in infancy. Based on the guidance of the International Society for the Study of Vascular Anomalies (ISSVA), HHs are classified into three different subtypes: focal HH, multiple infantile HH (IHH), and diffuse IHH.1 Focal HH, also known as congenital HH (CHH), is the hepatic form of the congenital hemangioma of the skin. Fully grown at birth, CHH is distinct from IHH biologically. In contrast, IHH shares the same proliferation and regression phases as cutaneous infantile hemangioma (IH).2,3
Clinically, many multifocal IHHs are asymptomatic. However, some IHHs can result in severe complications, including congestive heart failure (CHF), hypothyroidism, and abdominal compartment syndrome (ACS).4,5 Under untreated/undetected conditions, multiple IHHs may continue proliferating and evolving into diffuse lesions. Diffuse IHHs are more likely to lead to death.6 The overall mortality rate in patients with IHH was 16%, with a rate of 38% within cases with diffuse IHH and 9% within cases with multifocal IHH.7 The precise pathogenesis of IHH remains to be fully understood, and the development of IHH is likely to be related to multiple molecular mechanisms. In this review, we will summarize the current knowledge of IHH and discuss the most recent and significant data concerning its pathogenesis, diagnosis, prognosis, and treatment.
Definition
Despite the notable progress that has been made in the understanding of vascular tumors and vascular malformations by the ISSVA, confusion regarding the terminology used to describe HH still persists. The term ‘hepatic hemangioendothelioma’ has inaccurately been used for patients with CHH or IHH.8–14 In fact, hepatic hemangioendothelioma is a histologic diagnosis. The term ‘hepatic hemangioendothelioma’ should not be used in the absence of a histologic examination. Besides, use of the term HH avoids confusion with epithelioid hemangioendothelioma, kaposiform hemangioendothelioma (KHE), and hemangioendothelioma not otherwise specified.
Additionally, it is noteworthy that CHH and IHH are vascular tumors that are unique to infancy. The term ‘hepatic hemangioma’ should not be used for any vascular malformation affecting the liver.15 In these scenarios, ‘hepatic hemangioma’ was commonly assigned erroneously to hepatic venous malformations, hepatic adenomas, hepatic arteriovenous malformations, and portal vein aneurysms. Notably, IHH in children is radically different from ‘hepatic hemangioma’ in adults since the latter is an essential venous malformation.16
Classification
Classification for HH continues to evolve as our knowledge of the natural history and biology of HH improve (Table 1). The distinctions between focal CHH and multifocal and diffuse IHH are not just semantic. In fact, CHHs are pathophysiologically and behaviorally distinct from IHHs.17,18 Generally present and fully formed at birth, CHHs are the hepatic equivalent of cutaneous congenital hemangiomas (Fig. 1). In contrast, IHHs belong to true IHs that undergo identical phases of proliferation and growth to cutaneous IHs.2,3 Positive staining for glucose transporter 1 (Glut-1) is one of the most specific histologic markers that can identify IHHs from other types of hepatic vascular anomalies (e.g. CHHs).19
Table 1.
Three different types of hepatic hemangiomas.
| Parameter | Congenital hepatic hemangioma | Multifocal infantile hepatic hemangioma | Diffuse infantile hepatic hemangioma |
|---|---|---|---|
| Age at onset | Prenatal | Postnatal | Postnatal |
| Growth pattens | Grow antenatally and fully formed at birth | Growth in the first weeks or months after birth | Rapid growth in the first weeks or months after birth |
| Symptoms and complications | Rarely associated with cutaneous IH. Most cases are asymptomatic. Some cases have mild anemia or thrombocytopenia. Few cases with high-output cardiac failure due to macrovascular shunting. Do not cause hypothyroidism | Frequently associated with cutaneous IH. Many cases are asymptomatic. Some cases with moderate hypothyroidism, high-output cardiac failure secondary to macrovascular shunting | Frequently associated with cutaneous IH. Almost all cases are symptomatic, with abdominal compartment syndrome, cardiac failure, and severe hypothyroidism |
| Image findings on MRI | A solitary, well-defined, spherical lesion; usually hypointense to liver on T1-weight image and hyperintense on T2-weight image; centripetal enhancement with gadolinium | Multiple homogeneous enhanced spherical lesions with intervening areas of normal hepatic parenchyma; hypointense to liver on T1-weight image and hyperintense on T2-weight image | Innumerable lesions nearly involve the whole liver |
| Pathological features | Negative for glucose transporter-1 | Positive for glucose transporter-1 | Positive for glucose transporter-1 |
| Management | Observation for asymptomatic patients; embolization or surgery for severe macrovascular shunting | Observation for asymptomatic patients; embolization or surgery for severe macrovascular shunting; propranolol, steroid, a combination of medical treatment; thyroid hormone replacement for cases with hypothyroidism; liver transplantation | Propranolol, steroid, or a combination of medical treatment; thyroid hormone replacement for cases with hypothyroidism; embolization for sever macrovascular shunting; liver transplantation |
Figure 1.
CHHs are the hepatic equivalent of cutaneous congenital hemangiomas, which arise in utero and are present and fully formed at birth. CHHs may have three distinct growth patterns: rapidly involuting congenital hemangiomas, which rapidly involute in infancy; partially involuting congenital hemangiomas; and noninvoluting congenital hemangiomas, which do not involute.81,86 (A) Prenatal T2-weighted MRI at 34 weeks gestational age demonstrates a CHH in the fetus. (B) Postnatal T2-weighted MRI at 5 days of age reveals a large hyperintense lesion in the liver. The lesion continued to involute over time with no treatment. (C) T2-weighted MRI at 2 years of age demonstrates nearly complete involution of the liver lesion.
According to the classification of IHH, multifocal IHHs are defined as individual spherical masses with intervening segments of normal liver parenchyma (Fig. 2); diffuse IHHs are defined as near-total replacement of normal liver parenchyma with innumerable tumors.2,3 In other words, the only difference between multifocal IHH and diffuse IHH is the degree of the liver replaced by tumor lesions (Fig. 3). Because IHH shares the same patterns of proliferation and regression as its more common cutaneous counterparts, for those untreated and/or undetected multifocal IHHs, it might continue to grow and have a trend to become diffuse lesions. A recent study demonstrated that NOGOB receptor-mediated RAS membrane accumulation in hemangioma-derived stem cells may contribute to this malignancy of IHH.20 There would be a phase in which the symptoms are parallel to diffuse lesions, but the hepatic parenchyma is not wholly occupied with hemangiomas (Fig. 4). We propose that there remains an ‘intermediate’ type of IHH between these two lesions. ‘Intermediate’ type of IHH might be the precursor of diffuse IHH. Several previous studies have found this phenomenon. In 2012, Kulungowski et al. first reported that two patients had the characteristics of both multifocal and diffuse IHH.21 Subsequently, Ji and colleagues also reported that patients presented a hepatic lesion type similar to both multifocal and diffuse IHH.22,23 Li et al. divided the multifocal type into countable and uncountable subgroups and found that the uncountable group was akin to the diffuse type in clinical characteristics and growth patents.24
Figure 2.
Multiple cutaneous IH with multifocal IHH. (A) Multiple cutaneous IH (IHs) in a 2-month-old female with multifocal IHH. (B) T2-weighted MRI shows multifocal hepatic lesions, which are hyperintense with intervening areas of normal hepatic parenchyma.
Figure 3.
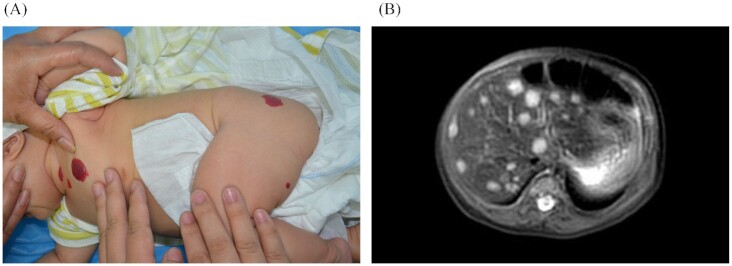
Diffuse IHH with severe complications. (A) A 1.5-month-old female with massive hepatomegaly, which caused severe respiratory compromise, high output cardiac failure, and abdominal compartment syndrome. (B) T2-weighted MRI shows massive liver involvement of innumerable lesions with near complete displacement of all liver parenchyma.
Figure 4.

‘Combined’ IHH. A representative T2-weighted MRI of ‘combined’ IHH. The IHHs were innumerable and coalesced, but the lesions did not entirely replace the hepatic parenchyma.
Epidemiology and demography
Since IHH is relatively rare and often neglected in the absence of symptoms, few studies have been reported on the incidence of IHH. The precise incidence of IHH is not known but likely occurs in 1 in 200 000 children per year.25 Clinically, asymptomatic IHH lesions are less likely to be identified or reported. The actual incidence of IHH is likely higher than that reported in the current published studies. However, the widespread use of noninvasive ultrasonography might increase the detection of asymptomatic IHH in infancy. Clinically, ∼90% of IHHs are diagnosed before the age of 6 months, and diffuse IHH is more likely to be diagnosed in early infancy.26 There is a significant female predominance in patients with IHH, with a ratio of 2:1.21
Risk factors
It has been reported that multiple cutaneous IHs may be associated with IHHs.27,28 Cutaneous IHs accompanied three-quarters of multifocal IHHs and more than half of diffuse IHHs.21 A recent study showed that increased numbers of cutaneous IHs were related to an increased risk of IHH.29 The mechanism behind this association is unknown. One interesting hypothesis is that an increasing number of circulating endothelial progenitor cells (EPCs) may prompt the development of IHs in the skin and liver.30
The pathogenesis of cutaneous IH has been proven to be related to maternal and perinatal factors, although little information is available for IHH. Maternal factors such as advanced maternal age, assisted reproductive technology, threatened abortion, gestational diabetes mellitus, preeclampsia, and progesterone use are related to cutaneous IH. Perinatal factors are also associated with IH, such as Caucasian sex, female sex, multiple gestations and so on.31–33
However, it is worth noting that the long-held conclusion that preterm birth and low birth weight (LBW) are risk factors has become controversial.31,34 A previous study identified that every 500 g decrease in birth weight can lead to an increase in the risk of IH by 40%.35 However, a recent nationwide longitudinal study in Japan identified that LBW and preterm birth were not significantly associated with IH.36 What we cannot deny is that with the development of medicine, more babies who would otherwise be preterm or LBW are not preterm or LBW due to various interventions, such as fetal preservation and lung maturation, making the picture of preterm birth and LBW more complicated today than it was a few decades ago. At present, there is still a lack of up-to-date mechanistic research.
In general, the risk factors for IH and IHH have been a research hotspot. With the gradual deepening understanding of the pathogenesis of IH, more potential risk factors have been discovered, which is conducive to exploring its pathogenesis. However, reliable studies with large sample sizes providing high-quality evidence are lacking.
Etiology and pathology
Placental abnormalities have a key aspect in IHH etiology
The pathogenesis of IHH is controversial and not fully elucidated. Hypoxia theory, cell implantation, and other theories have gradually been put forward.30,37 As one possible cell origin of IHH, the placenta is often considered to have a connection with IHH because they both express GLUT-1.38 There are histopathological similarities between placental chorangioma and hemangioma tissue, raising the possibility that chorangioma is the placental counterpart of cutaneous IH and IHH.39 Circulating EPCs may encounter ischemic tissue and the hypoxia-induced factors [vascular endothelial growth factor A (VEFG-A), hypoxia inducible factor 1α (HIF-1α)] required to stimulate their development in tissues (e.g. skin and liver).40 In fact, placental abnormalities (e.g. preeclampsia, placenta previa, retroplacental hematoma, and dilated vascular communications) are kinds of diseases involving abnormal placental morphology and function.32 The diseases encompassed are complicated, and the mechanisms behind them can be complex. It is interesting to further explore the connections.
Endothelial progenitor cells play a role in IHH progression
The initial clinical manifestation of the promontory mark of cutaneous IH as ‘area of low blood flow’ or ‘anemic nevi’ indicates tissue ischemia, which can stimulate neovascularization.41 In fact, EPCs were found to be present in proliferating IH and shared properties with cord blood EPCs and hemangioma-derived endothelial cells (HemECs).42,43 Hemangioma-derived EPCs (or hemangioma-derived stem cells) have the ability to produce GLUT-1-positive microvessels in nude mice.44,45 EPCs help tumor angiogenesis by differentiating into ECs and regulating preexisting ECs and other cell types (e.g. pericytes) with the production of paracrine and/or juxtacrine signals. These signaling pathways include VEGF/VEGF receptor (VEGFR)-2 and stromal cell-derived factor-1α (SDF-1α)/CXC motif chemokine receptor type 4 (CXCR4).46,47 Although they mainly exist in the bone marrow of adults, EPCs were also detected in fetal liver, umbilical cord blood, and peripheral blood. Interestingly, more circulating EPCs were detected in children with IH than in normal controls.48 These observations raise the intriguing possibility that EPCs can home to IHs with the stimulation of chemokine gradients (e.g. VEGF), which are formed in ischemic tissues and/or hemangioma precursors, where they can participate in IH vasculogenesis and angiogenesis (Fig. 5).37
Figure 5.
Angiogenesis in IH. Angiogenesis plays crucial roles in the development of IH. Angiogenic sprouts emerge from the newly formed vessels in response to proangiogenic cues, such as hypoxia-induced VEGF-A, epidermal growth factor (EGF), fibroblast growth factor (FGF), and HIF-1α.37 The IH site (hypoxic area) secretes cytokines (e.g. MMP9) that promote EPC homing.49 After homing, EPCs differentiate into ECs and promote both angiogenic signals and structural support to augment angiogenesis.43 During sprouting angiogenesis, the VEGF-A and Notch signaling pathways are implicated in the specification of tip and stalk cells in the vascular endothelium. VEGF-A stimulation, acting via VEGFR-2, increases Dll4 on endothelial cells, which in turn activates Notch receptors (e.g. Notch1) on neighbouring endothelial cells.99–101 Overexpression of platelet-derived growth factor (PDGF) within the hypoxic tumor site results in increased pericyte recruitment and coverage.102 (This figure was created by the authors on Biorender, https://biorender.com)
Estrogen and hypoxia in IHH: triggers of IHH
IHH is more common in females. Normally, females bear a higher level of estrogen. During labor, blood levels of estradiol in the mother may be >500 times the normal value. During the perinatal period, the significant increase in free estrogen levels may stimulate areas of hypoxic endothelium to induce IH development.37 Therefore, it is conceivable that higher estrogen level as well as higher sensitivity in females, are related to IHH. In fact, estrogens, erythropoietin, VEGF-A, SDF-1α, and HIF-1α have been demonstrated to induce the circulating EPCs.40,46,47 Estrogen and hypoxia work synergistically on the upregulation of matrix metalloproteinase (MMP-9) in HemECs.49 During postnatal vasculogenesis and angiogenesis, MMP-9 is a key mediator that can enhance the recruitment of EPCs to ischemic and hypoxic tissue. In addition, synergy between estrogen and hypoxia signaling leads to increased HemEC proliferation, which may explain the rapid proliferation of IH in the perinatal period.49
Glycolysis in IHH
Angiogenesis is widely accepted to contribute to the development of IH37. In conditions of growth factor stimulation, especially VEGF, EC sprouting angiogenesis starts with an EC phenotype changing from a quiescent state to an active state50. Therefore, a large amount of energy is required to support cell proliferation and migration during angiogenesis. Compared with healthy ECs, tumor ECs rely heavily on glycolysis for energy production even in the presence of O251. Metabolism-related biological processes (e.g. “glycogen biosynthetic process” and “respiratory electron transport chain”) and pathways, including “oxidative phosphorylation” and “pyruvate metabolism”, were found in proliferating hemangioma tissues52.
Glut-1, a diagnostic marker of IHH, is the main carrier that transports glucose into the cytoplasm for ATP production. A recent study demonstrated that several key enzymes in the glycolytic pathway, including Glut-1, hexokinase-2, and lactate dehydrogenase A, were highly expressed in HemECs compared with human umbilical vein ECs53. Additionally, inhibition of these glycolytic enzymes can suppress HemEC proliferation and migration53. In agreement, phosphofructokinase-1 (PFK-1) , the most important rate-limiting enzyme in the glycolytic pathway, was more highly expressed in proliferating IH tissue than in involuting IH tissue. Targeting the expression of PFK-1 by shRNA significantly inhibited HemEC proliferation and migration and induced HemEC cycle arrest52. These novel findings indicate that glycolysis may participate in the development of IHH (Fig. 6).
Figure 6.
Glycolysis promotes angiogenesis in IH. Glycolysis is defined as the conversion of glucose into pyruvate. In the presence of oxygen, glucose is finally converted into lactate, which is known as aerobic glycolysis or the “Warburg effect”. Several advantages exist for tumor endothelial cell reliance on glycolysis, including producing ATP faster, reducing reactive oxygen species production, providing more carbon skeletons for biosynthesis, and more adaptation to a hypoxic environment.103,104 Both the PI3K/Akt/mTOR pathway and HIF-1α pathway are involved in the pathogenesis of IH.49 The PI3K/Akt/mTOR pathway and HIF-1α pathway are key regulators of glycolysis.105 Upregulated VEGF/VEGFR-2 signaling in HemECs induced an autocrine signaling loop, which resulted in Akt activation.106 Activation of AKT can stabilize the glucose transporter Glut-1 at the plasma membrane, increasing aerobic glycolysis.105 Glycolysis promotes endothelial cell competitiveness for the tip position, thus promoting IH angiogenesis (This figure was created by the authors on Biorender, https://biorender.com). Abbreviations: G6P, Glucose-6-phosphatase; F6P, Fructose-6-phosphate; FBP, Fructose-1,6-bisphosphate; G3P, Glyceraldehyde-3-phosphate; 1,3BPG, 1,3-Bisphosphoglycerate; 3PG, 3-Phosphoglycerate; 2PG, 2-Phosphoglycerate; PEP, Phosphoenolpyruvate; Pyr, Pyruvate; Lac, Lactate; GLUT1, Glucose transporter 1; HK2, Hexokinase 2; PFK1, Phosphofructokinase 1; PKM2, Pyruvate kinase M2; LDHA, Lactate Dehydrogenase A; TCA, Tricarboxylic acid.
Clinical manifestations
Regarding clinical presentation, most cases of multifocal IHH are asymptomatic and are often found incidentally. Only a few patients (∼9.7%) detected by screening were symptomatic.29 However, when present, the clinical manifestations of IHH are nonspecific, and common symptoms are hepatomegaly, abdominal distention, malnutrition, and failure to thrive. Serious complications (e.g. hypothyroidism, CHF, and ACS) can occur in severe patients.3,10,54–62 If untreated and/or undetected, multifocal IHH may continue to grow rapidly and finally evolve into diffuse IHH.6 Therefore, patients with multifocal IHH should be regularly followed-up to monitor potential proliferation to diffuse lesions.
Evidence suggests that diffuse IHHs have more serious symptoms than multifocal IHHs. Most of these tumor lesions have a similar pattern, with progressive abdominal distention and hepatomegaly. It is convincing that patients suffering from diffuse IHH were associated with an increased risk of mortality. Abdominal distention, coagulopathy, and high-output cardiac failure were the major symptoms in patients with diffuse IHH.1,21
Complications
Complications in patients with IHH are not rare. Both multifocal and diffuse IHHs can potentially be associated with complications. The presence of complications and complication severity chiefly depend on the patient's age, tumor size, and tumor type. It is prudent for pediatric clinicians to remain alert for potential complications that may herald future morbidity or mortality.
Consumptive hypothyroidism
As a life-threatening complication of IHH, consumptive hypothyroidism is attributed to the overexpression of type 3 iodothyronine deiodinase in tumor lesions.62 Hypothyroidism in IHH differs from congenital hypothyroidism in clinical presentation and progression.56 Untreated consumptive hypothyroidism in cases with IHH can impair contractility, resulting in low cardiac output heart failure. In addition, hypothyroidism can also cause permanent neurologic damage. In a single-center retrospective study, the authors revealed that the incidence of clinical adverse events (e.g. hypothyroidism) in patients with HHs (including CHHs and IHHs) was low.63 However, CHHs do not express type 3 iodothyronine deiodinase, which inactivates the thyroid hormone, and therefore, they cannot cause hypothyroidism. In the largest prospective study to date, Ji et al. revealed that patients with cutaneous IH identified by screening had a 12.9% chance of having consumptive hypothyroidism.29 Diffuse lesions and large multifocal lesions were the risk factors of consumptive hypothyroidism. However, profound hypothyroidism (TSH > 50 μU/mL) occurred exclusively in diffuse IHH.2 Patients with diffuse IHH and large multifocal should be screened for consumptive hypothyroidism regularly due to its high risk.
Congestive heart failure
Macrovascular shunting (arteriovenous, arterioportal, or portovenous shunting) in patients with IHH can lead to a decrease in systemic blood volume and an increase in pulmonary blood volume, thus causing high-output cardiac failure. IHH associated with CHF is characterized by an early age of onset. The symptoms in these patients can be very severe.4,8,12,54 Compromised CHF is often an end-stage event that is usually fatal to infants. Therefore, when IHH with severe and complex conditions is detected for the first time, echocardiography is recommended to screen CHF as early as possible.64
Abdominal compartment syndrome
ACS is noted exclusively in diffuse IHH and is associated with morbidity in diffuse IHH. Diffuse IHHs have little liver parenchyma apparent between densely packed nodular hemangioma lesions throughout the involved liver. Cases with diffuse IHH usually present with abdominal distention and hepatomegaly, both of which can become massive.24 As a result, patients may develop ACS with compromised ventilation and poor inferior vena caval blood return to the heart. Respiratory distress and multiorgan failure can occur in patients with ACS.7,13,65
Thrombocytopenia and coagulopathy
Thrombocytopenia, coagulopathy, and anemia, may possibly coexist in patients with IHH.59,66 The thrombocytopenia and coagulopathy in IHH may be caused by the highly increased vascular bed and elevated shear stress within the tumor.67 Treatment-resistant coagulopathy has been revealed as a risk factor for mortality, especially if the coagulopathy is symptomatic. IHH with thrombocytopenia and coagulopathy presented significant deterioration among the patients who died.7,36 Moreover, Tatsuo et al. revealed that thrombocyte count and the prothrombin time improved after treatment in survivors, whereas these hematologic parameters deteriorated even after treatment in patients who had died.5
The thrombocytopenia and coagulopathy in IHH may overlap with the Kasabach–Merritt phenomenon (KMP) in KHE (or much less common in tufted hemangioma).68,69 KHE is a locally aggressive or borderline vascular tumor that never presents in the hepatic parenchyma.70 In addition, thrombocytopenia and coagulopathy in IHH are usually transient and milder than those seen in KHE with KMP.
Diagnosis
The radiologic characteristics of IHHs are well documented. US (with Doppler imaging), contrast-enhanced US, computed tomography (CT), and magnetic resonance imaging (MRI) provide different advantages in the detection and characterization of hepatic lesions in the pediatric population.57,61,71 Clinically, US is normally sufficient to make a confident diagnosis. US does not use radiation and is easily portable and available. The classic US appearance of IHH lesions is well-defined hypo- or hyper-echoic masses, multifocal or diffuse. Usually, IHH lesions do not present with central sparing, thrombosis, or necrosis. On contrast-enhanced US, IHH lesions showed hyperenhancement in the arterial phase.72 On MRI, IHH lesions are homogenously enhanced and are hypointense relative to the liver on T1-weighted images and hyperintense on T2-weighted images.73 The diagnosis of IHH can usually be made on typical imaging findings. Therefore, a biopsy of these lesions should be avoided if possible. However, for patients with atypical imaging and clinical findings, biopsy may be indicated. Appropriate clinical and radiographic examinations are crucial for diagnosis, delivery, and accurate treatment planning.
Differential diagnosis
The differential diagnosis of HHs includes hepatoblastoma, metastatic neuroblastoma, and focal nodular hyperplasia.74,75 Physiological serum levels of alpha-fetoprotein (AFP) are very high at birth. Subsequently, the serum levels of AFP taper exponentially to nearly normal adult levels by 8–9 months of age.76 AFP is an important serum biomarker for certain tumors (e.g. hepatoblastoma and teratoma) in children. Although patients with IHH and cutaneous IH have been reported with elevated serum levels of AFP, the serum levels of AFP are never as high as those in patients with hepatoblastoma.56,77 The serum levels of AFP should be assessed at diagnosis and thereafter during follow-up. Significantly high or rising serum levels of AFP should raise concerns about hepatoblastoma or malignancy change.
It is also vital to differentiate IHH from other less common malignant tumors of childhood, such as metastatic neuroblastoma, especially when there is a history of cutaneous IH.74,78,79 Both metastatic neuroblastoma and IHH can present with severe abdominal distension, hepatomegaly, respiratory distress, and high output heart failure. Even on MRI, both metastatic neuroblastoma and IHH have similar appearances, with T1-weighted hypointensity and T2-weighted hyperintensity. However, metastatic neuroblastoma is characterized by rapid filling and excretion, which are clearly different from the rapid filling and slow excretion of IHH. On contrast-enhanced US, IHH did not have marked washout or portal venous phase washout, both of which are characteristic of hepatic metastases. Therefore, contrast-enhanced US can be used to preliminarily exclude metastasis in infants with multiple hepatic lesions. In addition, the levels of 24-h urinary vanillylmandelic acid and homovanillic acid are elevated in metastatic neuroblastoma.57
Screening
Previous studies reported that there was an association between IHH and multiple cutaneous IHs.27,80 There was a tight relation between the number of IHH and the number of cutaneous IHs.29 In contrast to IHH, CHH has been shown to be not associated with cutaneous IHs. How many cutaneous IHs are the best screening threshold for IHH? Screening advice has changed over the past two decades. Screening for IHH in infants with 5 or more cutaneous IHs has been advocated by the clinical practice guidelines of the American Academy of Pediatrics.81,82 Screening cut-off points were set at 5–10 for the number of cutaneous IHs.27,28,64,83 However, these studies were weakened by not excluding CHHs. Most recently, a large multicenter prospective study including 1656 patients revealed that cases with <5 cutaneous IHs should not be ignored: abdominal ultrasonography should be considered for cases younger than 9 months of age who have 5 or more cutaneous IHs.29
It is noteworthy that IHH is not detectable at birth. Therefore, early neonatal US screening may miss IHH lesions, which may proliferate late in the first weeks of life. If US screening was performed before 1 month of age and the result was negative, the next US should be carried out at 2 months of age.29 Symptoms, such as hepatomegaly, may also suggest the presence of IHH in patients with or without multiple cutaneous IHs. Screening ultrasonographic examinations may allow for closer surveillance and earlier treatment to avoid life-threatening progression.64 However, a retrospective study suggested that routine US screening did not alter the clinical management and outcomes, although this study was weakened by having a small sample size.63
Management
It is now clear that the majority of multifocal IHHs can spontaneously involute, and therefore, these patients can be managed expectantly. Nevertheless, a subset of patients with multifocal IHHs have associated arteriovenous shunting and hypothyroidism that can cause high-output cardiac failure. The initiation of treatment for IHH should be based on an assessment of the patient's clinical, radiologic, and laboratory results. Because IHHs are biologically similar to cutaneous IHs, the involution of IHHs can be hastened by pharmacologic agents, such as corticosteroids and propranolol. In fact, medical therapies should be attempted initially in patients with IHH when treatment is indicated because pharmacologic agents have the advantages of lower cost, universal availability, ease of administration, and less invasiveness than embolization. Clinically, diffuse lesions exhibit a high risk for mortality due to serious complications.7 Consequently, active treatments should be immediately undertaken once the diagnosis of diffuse IHH is made. In severe cases, multimodality therapy or combination therapy should be considered as a part of the treatment regimen.54,84,85
Medical therapy
Previously, prednisone or prednisolone was the first-line medical therapy for multifocal and diffuse IHHs requiring systemic therapy. However, prednisone and prednisolone have undesired side effects. In addition, the possibility of treatment failure and corticosteroid resistance is also remarkable. Approximately 25%–33% of IHH patients do not show any response to corticosteroid therapy.2,23 Vincristine, interferon-α cyclophosphamide, and sirolimus have also been used to treat IHH.9,10,54,84 Nevertheless, the standard protocols for IHH are inadequate because responses to these pharmacotherapies are individually different and unpredictable.86 For patients with severe IHH (e.g. IHH associated with CHF and ACS), aggressive pharmacologic therapy, including a combination of corticosteroid plus propranolol or corticosteroid plus vincristine, is indicated.15
In the 14 years since 2008, when Leaute-Labreze and colleagues first reported that propranolol is effective in the management of problematic IHs,87 many studies on oral propranolol for IHHs have been published.9,11,13,22,65,88–92 However, no prospective study was found to support the efficacy of propranolol in the treatment of IHHs. In addition, due to the tremendous heterogeneity of IHHs, no validated scores are available to assess disease severity. Nonetheless, propranolol has become the preferred treatment for most clinicians who treat complicated IHHs. Previously, a smaller retrospective study demonstrated that oral propranolol was effective in both ceasing and decreasing proliferation of multifocal IHH and prompting more rapid involution of the lesions, an amazing finding not typically found in corticosteroid therapy.23 In cases with corticosteroid resistance, propranolol, but not interferon or vincristine, rapidly improved hematologic disorders (e.g. thrombocytopenia and coagulopathy).10 In a recent study by Macdonald et al., the authors revealed that propranolol was more effective than prednisolone in the treatment of HHs (both CHH and IHH).93 Remarkably, they found that the number of patients requiring surgical treatment dramatically decreased in the period of oral propranolol. However, information regarding differences in treatment responses among HH subtypes is lacking. The involution of CHHs is unlikely to be hastened by pharmacologic agents such as prednisolone and propranolol.18 In addition, no information is available for atenolol and nadolol in the treatment of IHH, although they have been shown to be potentially safer (atenolol) or faster (nadolol) than propranolol.94,95
Surgical intervention
Surgical interventions (e.g. hepatic artery ligation, embolization, and liver transplantation) were considered urgently as alternative treatments for patients in whom maximal tolerated pharmacotherapy failed to achieve satisfactory clinical responses.96,97 Currently, there is a scarcity of studies confirming the long-term beneficial effects of surgical intervention in IHH. On the basis of previous retrospective studies, embolization has been demonstrated to be an effective management of IHH, including in pharmacological treatment-resistant IHH. Embolization may also be effective when there is no time to allow for clinical improvement by pharmacological treatment.5 Although not curative, the rapid resolution of symptoms is a strong reason for adjunct embolization in patients with severe IHH.8 Remarkably, embolization has the potential to rapidly resolve CHF by excluding abnormal macrovascular shunting, especially in patients with CHH. However, embolization seemed to be less effective in pharmacological treatment-resistant cases with coagulation.5,10 There is no need for embolization in patients without macrovascular shunting (arteriovenous, arterioportal, or portovenous shunting) and a high-output state.
Most cases of IHH can be controlled with pharmacotherapies such as propranolol or by hepatic artery embolization. Liver transplantation is rarely necessary even in diffuse IHH. However, as mentioned above, uncontrolled IHHs (particularly diffuse subtypes) can lead to respiratory insufficiency and progressive heart failure with other concomitant symptoms of thrombocytopenia and coagulopathy. Therefore, in patients when pharmacotherapies and embolization are not effective or surgical interventions cannot be performed, total hepatectomy can be considered as soon as possible and performed followed by liver transplantation.97 However, neonatal IHH with early rapid decompensation may have a very complicated transplantation procedure, which may lead to a high mortality rate.25,98 Therefore, liver transplantation should be recommended only as the last rescue therapy after all other therapies have failed.
Conclusions and future directions
Although the incidence of IHHs is not high, they are important causes of morbidity and mortality in infancy. Prompt diagnosis, appropriate management, and careful follow-up are critical to improve the long-term outcomes of IHHs. The treatment of IHH continues to evolve and pediatric clinicians also have multiple treatment options. However, there are insufficient findings supporting the questions with respect to the demographic features, risk factors, etiology, clinical manifestations, associated complications, and treatment algorithms of IHH. Future epidemiological studies are required to better understand the natural course of IHHs. Intense research efforts are also needed to clarify the etiology of IHH and to develop clinical strategies to prevent serious complications. The recognition of molecular features and understanding the mechanism or mechanisms of IHH progression may promote the identification of new predictive and prognostic markers, as well as useful therapeutic targets for the management of this disease. This review also reinforces the idea that to prevent mortality and morbidity in severe cases, standard classifications, standard definitions, standard stratification criteria, standard use of treatments, and standard follow-up criteria of IHH need to be developed.
ACKNOWLEDGEMENTS
This work was supported by the Project of ‘0 to 1’ of Sichuan University (grant No. 2022SCUH0033), and the 1·3·5 Project for Disciplines of Excellence Clinical Research Incubation Project, West China Hospital of Sichuan University (grant Nos. 2019HXFH056, 2020HXFH048, and ZYJC21060).
Contributor Information
Xue Gong, Division of Oncology, Department of Pediatric Surgery, West China Hospital of Sichuan University, Chengdu 610041, China.
Yanan Li, Division of Oncology, Department of Pediatric Surgery, West China Hospital of Sichuan University, Chengdu 610041, China.
Kaiying Yang, Division of Oncology, Department of Pediatric Surgery, West China Hospital of Sichuan University, Chengdu 610041, China.
Siyuan Chen, Pediatric Intensive Care Unit, Department of Critical Care Medicine, West China Hospital of Sichuan University, Chengdu 610041, China.
Yi Ji, Division of Oncology, Department of Pediatric Surgery, West China Hospital of Sichuan University, Chengdu 610041, China.
Conflict of interest
The authors state no conflict of interest.
References
- 1. Hsi Dickie B, Fishman SJ, Azizkhan RG. Hepatic vascular tumors. Semin Pediatr Surg. 2014;23:168–72.. doi: 10.1053/j.sempedsurg.2014.06.018. [DOI] [PubMed] [Google Scholar]
- 2. Dickie B, Dasgupta R, Nair R, et al. Spectrum of hepatic hemangiomas: management and outcome. J Pediatr Surg. 2009;44:125–33.. doi: 10.1016/j.jpedsurg.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 3. Christison-Lagay ER, Burrows PE, Alomari A, et al. Hepatic hemangiomas: subtype classification and development of a clinical practice algorithm and registry. J Pediatr Surg. 2007;42:62–8.; discussion 67-68. doi: 10.1016/j.jpedsurg.2006.09.041. [DOI] [PubMed] [Google Scholar]
- 4. Butters CT, Nash M. Infantile Hepatic Hemangiomas. N Engl J Med. 2021;385:e10. doi: 10.1056/NEJMicm1907892. [DOI] [PubMed] [Google Scholar]
- 5. Kuroda T, Kumagai M, Nosaka S, et al. Critical infantile hepatic hemangioma: results of a nationwide survey by the Japanese Infantile Hepatic Hemangioma Study Group. J Pediatr Surg. 2011;46:2239–43.. doi: 10.1016/j.jpedsurg.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 6. Yang K, Feng L, Chen S, Ji Y. Progressive infantile hepatic hemangioma not responding to propranolol. J Dermatol. 2019;46:e275–6.. doi: 10.1111/1346-8138.14833. [DOI] [PubMed] [Google Scholar]
- 7. Rialon KL, Murillo R, Fevurly RD, et al. Risk factors for mortality in patients with multifocal and diffuse hepatic hemangiomas. J Pediatr Surg. 2015;50:837–41.. doi: 10.1016/j.jpedsurg.2014.09.056. [DOI] [PubMed] [Google Scholar]
- 8. Wang L, Song D, Wu C, Li J, Yin J, Guo L. Infantile hepatic hemangioendothelioma associated with pulmonary artery hypertension and cardiac insufficiency successfully treated with transcatheter arterial embolization and propranolol: A case report. Medicine (Baltimore). 2020;99:e20728. doi: 10.1097/MD.0000000000020728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tian R, Liang Y, Wang J, et al. Propranolol for infantile hepatic hemangioendothelioma: Clinical evaluation of drug efficacy and safety using a single-center patient cohort. Ann Hepatol. 2020;19:530–4.. doi: 10.1016/j.aohep.2020.04.008. [DOI] [PubMed] [Google Scholar]
- 10. Emad A, Fadel S, El Wakeel M, et al. Outcome of Children Treated for Infantile Hepatic Hemangioendothelioma. J Pediatr Hematol Oncol. 2020;42:126–30.. doi: 10.1097/MPH.0000000000001536. [DOI] [PubMed] [Google Scholar]
- 11. Maaloul I, Aloulou H, Hentati Y, et al. Infantile hepatic hemangioendothelioma successfully treated by low dose of propranolol. La Presse Médicale. 2017;46:454–6.. doi: 10.1016/j.lpm.2017.01.010. [DOI] [PubMed] [Google Scholar]
- 12. Wang T, Wang Y, Liang Y, Lu G. Infantile Hepatic Hemangioendothelioma Associated With Congestive Heart Failure: Two Case Reports With Different Outcomes. Medicine (Baltimore). 2015;94:e2344. doi: 10.1097/MD.0000000000002344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Avagyan S, Klein M, Kerkar N, et al. Propranolol as a first-line treatment for diffuse infantile hepatic hemangioendothelioma. Journal of Pediatric Gastroenterology & Nutrition. 2013;56:e17–20.. doi: 10.1097/MPG.0b013e31824e50b7. [DOI] [PubMed] [Google Scholar]
- 14. Sondhi V, Kurkure PA, Vora T, et al. Successful management of multi-focal hepatic infantile hemangioendothelioma using TACE/surgery followed by maintenance metronomic therapy. Case Reports. 2012;2012:bcr1220115456. doi: 10.1136/bcr.12.2011.5456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Iacobas I, Phung TL, Adams DM, et al. Guidance Document for Hepatic Hemangioma (Infantile and Congenital) Evaluation and Monitoring. J Pediatr. 2018;203:294–300.e2.. doi: 10.1016/j.jpeds.2018.08.012. [DOI] [PubMed] [Google Scholar]
- 16. Ji Y, Chen S, Yang K, et al. Kaposiform hemangioendothelioma: current knowledge and future perspectives. Orphanet J Rare Dis. 2020;15:39. doi: 10.1186/s13023-020-1320-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Aggarwal N, Tekes A, Bosemani T. Infantile Hepatic Hemangioma: Role of Dynamic Contrast-Enhanced Magnetic Resonance Angiography. J Pediatr. 2015;167:940–940.e1.. doi: 10.1016/j.jpeds.2015.06.068. [DOI] [PubMed] [Google Scholar]
- 18. Roebuck D, Sebire N, Lehmann E, et al. Rapidly involuting congenital haemangioma (RICH) of the liver. Pediatr Radiol. 2012;42:308–14.. doi: 10.1007/s00247-011-2268-z. [DOI] [PubMed] [Google Scholar]
- 19. North PE, Waner M, James CA, et al. Congenital nonprogressive hemangioma: a distinct clinicopathologic entity unlike infantile hemangioma. Arch Dermatol. 2001;137:1607–20.. doi: 10.1001/archderm.137.12.1607. [DOI] [PubMed] [Google Scholar]
- 20. Hu W, Liu Z, Salato V, et al. NOGOB receptor-mediated RAS signaling pathway is a target for suppressing proliferating hemangioma. JCI Insight. 2021;6:e142299. doi: 10.1172/jci.insight.142299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kulungowski AM, Alomari AI, Chawla A, et al. Lessons from a liver hemangioma registry: subtype classification. J Pediatr Surg. 2012;47:165–70.. doi: 10.1016/j.jpedsurg.2011.10.037. [DOI] [PubMed] [Google Scholar]
- 22. Yang K, Peng S, Chen L, et al. Efficacy of propranolol treatment in infantile hepatic haemangioma. J Paediatr Child Health. 2019;55:1194–200.. doi: 10.1111/jpc.14375. [DOI] [PubMed] [Google Scholar]
- 23. Ji Y, Chen S, Xiang B, et al. Clinical features and management of multifocal hepatic hemangiomas in children: a retrospective study. Sci Rep. 2016;6:31744. doi: 10.1038/srep31744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li K, Wang Z, Liu Y, et al. Fine clinical differences between patients with multifocal and diffuse hepatic hemangiomas. J Pediatr Surg. 2016;51:2086–90.. doi: 10.1016/j.jpedsurg.2016.09.045. [DOI] [PubMed] [Google Scholar]
- 25. Ernst L, Grabhorn E, Brinkert F, et al. Infantile Hepatic Hemangioma: Avoiding Unnecessary Invasive Procedures. Pediatric Gastroenterology, Hepatology & Nutrition. 2020;23:72–8.. doi: 10.5223/pghn.2020.23.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kuroda T, Hoshino K, Nosaka S, et al. Critical hepatic hemangioma in infants: recent nationwide survey in Japan. Pediatr Int. 2014;56:304–8.. doi: 10.1111/ped.12347. [DOI] [PubMed] [Google Scholar]
- 27. Horii KA, Drolet BA, Frieden IJ, et al. Prospective study of the frequency of hepatic hemangiomas in infants with multiple cutaneous infantile hemangiomas. Pediatr Dermatol. 2011;28:245–53.. doi: 10.1111/j.1525-1470.2011.01420.x. [DOI] [PubMed] [Google Scholar]
- 28. Hughes JA, Hill V, Patel K, et al. Cutaneous haemangioma: prevalence and sonographic characteristics of associated hepatic haemangioma. Clin Radiol. 2004;59:273–80.. doi: 10.1016/S0009-9260(03)00267-8. [DOI] [PubMed] [Google Scholar]
- 29. Ji Y, Chen S, Yang K, et al. Screening for infantile hepatic hemangioma in patients with cutaneous infantile hemangioma: A multicenter prospective study. J Am Acad Dermatol. 2021;84:1378–84.. doi: 10.1016/j.jaad.2020.11.062. [DOI] [PubMed] [Google Scholar]
- 30. Leaute-Labreze C, Harper JI, Hoeger PH. Infantile haemangioma. Lancet North Am Ed. 2017;390:85–94.. doi: 10.1016/S0140-6736(16)00645-0. [DOI] [PubMed] [Google Scholar]
- 31. Schoch JJ, Hunjan MK, Anderson KR, et al. Temporal trends in prenatal risk factors for the development of infantile hemangiomas. Pediatr Dermatol. 2018;35:787–91.. doi: 10.1111/pde.13659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hunjan MK, Schoch JJ, Anderson KR, et al. Prenatal Risk Factors for Infantile Hemangioma Development. J Invest Dermatol. 2017;137:954–7.. doi: 10.1016/j.jid.2016.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Munden A, Butschek R, Tom WL, et al. Prospective study of infantile haemangiomas: incidence, clinical characteristics and association with placental anomalies. Br J Dermatol. 2014;170:907–13.. doi: 10.1111/bjd.12804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Li J, Chen X, Zhao S, et al. Demographic and clinical characteristics and risk factors for infantile hemangioma: a Chinese case-control study. Arch Dermatol. 2011;147:1049–56.. doi: 10.1001/archdermatol.2011.122. [DOI] [PubMed] [Google Scholar]
- 35. Drolet BA, Swanson EA, Frieden IJ, et al. Infantile hemangiomas: an emerging health issue linked to an increased rate of low birth weight infants. J Pediatr. 2008;153:712–715.e1.. doi: 10.1016/j.jpeds.2008.05.043. [DOI] [PubMed] [Google Scholar]
- 36. Mizawa M, Matsumura K, Hamazaki K, et al. Infantile Hemangioma and the Risk Factors in a Japanese Population: A Nationwide Longitudinal Study-The Japan Environment and Children's Study. J Invest Dermatol. 2021;141:2745–2748.e2.. doi: 10.1016/j.jid.2021.05.011. [DOI] [PubMed] [Google Scholar]
- 37. Ji Y, Chen S, Li K, et al. Signaling pathways in the development of infantile hemangioma. J Hematol Oncol. 2014;7:13. doi: 10.1186/1756-8722-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pittman KM, Losken HW, Kleinman ME, et al. No evidence for maternal-fetal microchimerism in infantile hemangioma: a molecular genetic investigation. J Invest Dermatol. 2006;126:2533–8.. doi: 10.1038/sj.jid.5700516. [DOI] [PubMed] [Google Scholar]
- 39. Calicchio ML, Collins T, Kozakewich HP. Identification of signaling systems in proliferating and involuting phase infantile hemangiomas by genome-wide transcriptional profiling. Am J Pathol. 2009;174:1638–49.. doi: 10.2353/ajpath.2009.080517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. de la Puente P, Muz B, Azab F, Azab AK. Cell trafficking of endothelial progenitor cells in tumor progression. Clin Cancer Res. 2013;19:3360–8.. doi: 10.1158/1078-0432.CCR-13-0462. [DOI] [PubMed] [Google Scholar]
- 41. Tollefson MM, Frieden IJ. Early growth of infantile hemangiomas: what parents' photographs tell us. Pediatrics. 2012;130:e314–20.. doi: 10.1542/peds.2011-3683. [DOI] [PubMed] [Google Scholar]
- 42. Khan ZA, Melero-Martin JM, Wu X, et al. Endothelial progenitor cells from infantile hemangioma and umbilical cord blood display unique cellular responses to endostatin. Blood. 2006;108:915–21.. doi: 10.1182/blood-2006-03-006478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yu Y, Flint AF, Mulliken JB, et al. Endothelial progenitor cells in infantile hemangioma. Blood. 2004;103:1373–5.. doi: 10.1182/blood-2003-08-2859. [DOI] [PubMed] [Google Scholar]
- 44. Moisan F, Oucherif S, Kaulanjan-Checkmodine P, et al. Critical role of Aquaporin-1 and telocytes in infantile hemangioma response to propranolol beta blockade. Proc Natl Acad Sci. 2021;118:e2018690118. doi: 10.1073/pnas.2018690118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Khan ZA, Boscolo E, Picard A, et al. Multipotential stem cells recapitulate human infantile hemangioma in immunodeficient mice. J Clin Invest. 2008;118:2592–9.. doi: 10.1172/JCI33493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ceradini DJ, Kulkarni AR, Callaghan MJ, et al. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10:858–64.. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- 47. Yamaguchi J, Kusano KF, Masuo O, et al. Stromal cell-derived factor-1 effects on ex vivo expanded endothelial progenitor cell recruitment for ischemic neovascularization. Circulation. 2003;107:1322–8.. doi: 10.1161/01.cir.0000055313.77510.22. [DOI] [PubMed] [Google Scholar]
- 48. Kleinman ME, Tepper OM, Capla JM, et al. Increased circulating AC133+ CD34+ endothelial progenitor cells in children with hemangioma. Lymphat Res Biol. 2003;1:301–7.. doi: 10.1089/153968503322758102. [DOI] [PubMed] [Google Scholar]
- 49. Kleinman ME, Greives MR, Churgin SS, et al. Hypoxia-induced mediators of stem/progenitor cell trafficking are increased in children with hemangioma. Arterioscler Thromb Vasc Biol. 2007;27:2664–70.. doi: 10.1161/ATVBAHA.107.150284. [DOI] [PubMed] [Google Scholar]
- 50. Potente M, Carmeliet P. The Link Between Angiogenesis and Endothelial Metabolism. Annu Rev Physiol. 2017;79:43–66.. doi: 10.1146/annurev-physiol-021115-105134. [DOI] [PubMed] [Google Scholar]
- 51. Li X, Sun X, Carmeliet P. Hallmarks of Endothelial Cell Metabolism in Health and Disease. Cell Metab. 2019;30:414–33.. doi: 10.1016/j.cmet.2019.08.011. [DOI] [PubMed] [Google Scholar]
- 52. Yang K, Zhang X, Chen L, et al. Microarray expression profile of mRNAs and long noncoding RNAs and the potential role of PFK-1 in infantile hemangioma. Cell Division. 2021;16:1. doi: 10.1186/s13008-020-00069-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Chen J, Wu D, Dong Z, et al. The expression and role of glycolysis-associated molecules in infantile hemangioma. Life Sci. 2020;259:118215. doi: 10.1016/j.lfs.2020.118215. [DOI] [PubMed] [Google Scholar]
- 54. Speicher MV, Lim DM, Field AG, et al. An Unusual Case of Neonatal High-Output Heart Failure: Infantile Hepatic Hemangioma. J Emerg Med. 2021;60:107–11.. doi: 10.1016/j.jemermed.2020.10.008. [DOI] [PubMed] [Google Scholar]
- 55. Macchiaiolo M, Markowich AH, Diociaiuti A, et al. Diffuse infantile hepatic hemangiomas in a patient with Beckwith-Wiedemann syndrome: A new association?. American Journal of Medical Genetics Part A. 2020;182:1972–6.. doi: 10.1002/ajmg.a.61718. [DOI] [PubMed] [Google Scholar]
- 56. Simsek E, Demiral M, Gundogdu E. Severe consumptive hypothyroidism caused by multiple infantile hepatic haemangiomas. J Pediatr Endocrinol Metab. 2018;31:823–7.. doi: 10.1515/jpem-2018-0055. [DOI] [PubMed] [Google Scholar]
- 57. Gnarra M, Behr G, Kitajewski A, et al. History of the infantile hepatic hemangioma: From imaging to generating a differential diagnosis. World Journal of Clinical Pediatrics. 2016;5:273–80.. doi: 10.5409/wjcp.v5.i3.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Okuno T, Tokuriki S, Yoshino T, et al. Diffuse neonatal hemangiomatosis in a very low-birthweight infant treated with erythropoietin. Pediatr Int. 2015;57:e34–6.. doi: 10.1111/ped.12517. [DOI] [PubMed] [Google Scholar]
- 59. Glick ZR, Frieden IJ, Garzon MC, et al. Diffuse neonatal hemangiomatosis: an evidence-based review of case reports in the literature. J Am Acad Dermatol. 2012;67:898–903.. doi: 10.1016/j.jaad.2012.01.018. [DOI] [PubMed] [Google Scholar]
- 60. Metry DW, Hawrot A, Altman C, et al. Association of solitary, segmental hemangiomas of the skin with visceral hemangiomatosis. Arch Dermatol. 2004;140:591–6.. doi: 10.1001/archderm.140.5.591. [DOI] [PubMed] [Google Scholar]
- 61. Kassarjian A, Zurakowski D, Dubois J, et al. Infantile hepatic hemangiomas: clinical and imaging findings and their correlation with therapy. Am J Roentgenol. 2004;182:785–95.. doi: 10.2214/ajr.182.3.1820785. [DOI] [PubMed] [Google Scholar]
- 62. Huang SA, Tu HM, Harney JW, et al. Severe hypothyroidism caused by type 3 iodothyronine deiodinase in infantile hemangiomas. N Engl J Med. 2000;343:185–9.. doi: 10.1056/NEJM200007203430305. [DOI] [PubMed] [Google Scholar]
- 63. Mahon C, McHugh K, Alband N, et al. Routine liver ultrasound screening does not alter clinical management in a cohort study of multiple cutaneous infantile haemangioma. Br J Dermatol. 2021;184:340–1.. doi: 10.1111/bjd.19472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Rialon KL, Murillo R, Fevurly RD, et al. Impact of Screening for Hepatic Hemangiomas in Patients with Multiple Cutaneous Infantile Hemangiomas. Pediatr Dermatol. 2015;32:808–12.. doi: 10.1111/pde.12656. [DOI] [PubMed] [Google Scholar]
- 65. Mazereeuw-Hautier J, Hoeger PH, Benlahrech S, et al. Efficacy of propranolol in hepatic infantile hemangiomas with diffuse neonatal hemangiomatosis. J Pediatr. 2010;157:340–2.. doi: 10.1016/j.jpeds.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 66. Frieden IJ, Haggstrom AN, Drolet BA, et al. Infantile hemangiomas: current knowledge, future directions. Proceedings of a research workshop on infantile hemangiomas, April 7-9, 2005, Bethesda, Maryland, USA. Pediatr Dermatol. 2005;22:383–406.. doi: 10.1111/j.1525-1470.2005.00102.x. [DOI] [PubMed] [Google Scholar]
- 67. Ji Y, Chen S, Xu C, et al. The use of propranolol in the treatment of infantile haemangiomas: an update on potential mechanisms of action. Br J Dermatol. 2015;172:24–32.. doi: 10.1111/bjd.13388. [DOI] [PubMed] [Google Scholar]
- 68. Ji Y, Yang K, Peng S, et al. Kaposiform haemangioendothelioma: clinical features, complications and risk factors for Kasabach-Merritt phenomenon. Br J Dermatol. 2018;179:457–63.. doi: 10.1111/bjd.16601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Zhou J, Yang K, Dai S, et al. Clinical features and management of kaposiform hemangioendothelioma and tufted angioma: Similarities and differences. J Am Acad Dermatol. 2021. doi: 10.1016/j.jaad.2021.07.012. [DOI] [PubMed] [Google Scholar]
- 70. Ji Y, Chen S, Li L, et al. Kaposiform hemangioendothelioma without cutaneous involvement. J Cancer Res Clin Oncol. 2018;144:2475–84.. doi: 10.1007/s00432-018-2759-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Bosemani T, Puttgen KB, Huisman TA, et al. Multifocal infantile hepatic hemangiomas–imaging strategy and response to treatment after propranolol and steroids including review of the literature. Eur J Pediatr. 2012;171:1023–8.. doi: 10.1007/s00431-011-1671-7. [DOI] [PubMed] [Google Scholar]
- 72. El-Ali AM, McCormick A, Thakrar D, et al. Contrast-Enhanced Ultrasound of Congenital and Infantile Hemangiomas: Preliminary Results From a Case Series. Am J Roentgenol. 2020;214:658–64.. doi: 10.2214/AJR.19.22174. [DOI] [PubMed] [Google Scholar]
- 73. Feng ST, Chan T, Ching AS, et al. CT and MR imaging characteristics of infantile hepatic hemangioendothelioma. Eur J Radiol. 2010;76:e24–9.. doi: 10.1016/j.ejrad.2010.05.027. [DOI] [PubMed] [Google Scholar]
- 74. Nord KM, Kandel J, Lefkowitch JH, et al. Multiple cutaneous infantile hemangiomas associated with hepatic angiosarcoma: case report and review of the literature. Pediatrics. 2006;118:e907–13.. doi: 10.1542/peds.2006-0183. [DOI] [PubMed] [Google Scholar]
- 75. Ji Y, Chen S, Xiang B, et al. Clinical Features of Focal Nodular Hyperplasia of the Liver in Children. Journal of Pediatric Gastroenterology & Nutrition. 2016;62:813–8.. doi: 10.1097/MPG.0000000000001094. [DOI] [PubMed] [Google Scholar]
- 76. Wu JT, Book L, Sudar K. Serum alpha fetoprotein (AFP) levels in normal infants. Pediatr Res. 1981;15:50–2.. doi: 10.1203/00006450-198101000-00012. [DOI] [PubMed] [Google Scholar]
- 77. Itinteang T, Chibnall AM, Marsh R, et al. Elevated Serum Levels of Alpha-Fetoprotein in Patients with Infantile Hemangioma Are Not Derived from within the Tumor. Frontiers in Surgery. 2016;3:5. doi: 10.3389/fsurg.2016.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Karkoska K, Ricci K, VandenHeuvel K, et al. Metastatic neuroblastoma masquerading as infantile hemangioma in a 4-month-old child. Pediatr Blood Cancer. 2021;68:e28920. doi: 10.1002/pbc.28920. [DOI] [PubMed] [Google Scholar]
- 79. Grassia KL, Peterman CM, Iacobas I, et al. Clinical case series of pediatric hepatic angiosarcoma. Pediatr Blood Cancer. e26627, 2017;64. doi: 10.1002/pbc.26627. [DOI] [PubMed] [Google Scholar]
- 80. Horii KA, Drolet BA, Baselga E, et al. Risk of hepatic hemangiomas in infants with large hemangiomas. Arch Dermatol. 2010;146:201–3.. doi: 10.1001/archdermatol.2009.391. [DOI] [PubMed] [Google Scholar]
- 81. Krowchuk DP, Frieden IJ, Mancini AJ, et al. Clinical Practice Guideline for the Management of Infantile Hemangiomas. Pediatrics. 2019;143:e20183475. doi: 10.1542/peds.2018-3475. [DOI] [PubMed] [Google Scholar]
- 82. Darrow DH, Greene AK, Mancini AJ, et al. Diagnosis and Management of Infantile Hemangioma: Executive Summary. Pediatrics. 2015;136:786–91.. doi: 10.1542/peds.2015-2482. [DOI] [PubMed] [Google Scholar]
- 83. Vredenborg AD, Janmohamed SR, de Laat PC, et al. Multiple cutaneous infantile haemangiomas and the risk of internal haemangioma. Br J Dermatol. 2013;169:188–91.. doi: 10.1111/bjd.12229. [DOI] [PubMed] [Google Scholar]
- 84. Wasserman JD, Mahant S, Carcao M, et al. Vincristine for successful treatment of steroid-dependent infantile hemangiomas. Pediatrics. 2015;135:e1501–5.. doi: 10.1542/peds.2014-2542. [DOI] [PubMed] [Google Scholar]
- 85. Kowalska M, Debek W, Matuszczak E. Infantile Hemangiomas: An Update on Pathogenesis and Treatment. Journal of Clinical Medicine. 2021;10:4631. doi: 10.3390/jcm10204631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Zavras N, Dimopoulou A, Machairas N, et al. Infantile hepatic hemangioma: current state of the art, controversies, and perspectives. Eur J Pediatr. 2020;179:1–8.. doi: 10.1007/s00431-019-03504-7. [DOI] [PubMed] [Google Scholar]
- 87. Leaute-Labreze C, Dumas de la Roque E, Hubiche T, et al. Propranolol for severe hemangiomas of infancy. N Engl J Med. 2008;358:2649–51.. doi: 10.1056/NEJMc0708819. [DOI] [PubMed] [Google Scholar]
- 88. Varrasso G, Schiavetti A, Lanciotti S, et al. Propranolol as first-line treatment for life-threatening diffuse infantile hepatic hemangioma: A case report. Hepatology. 2017;66:283–5.. doi: 10.1002/hep.29028. [DOI] [PubMed] [Google Scholar]
- 89. Vergine G, Marsciani A, Pedini A, et al. Efficacy of propranolol treatment in thyroid dysfunction associated with severe infantile hepatic hemangioma. Hormone Research in Paediatrics. 2012;78:256–60.. doi: 10.1159/000337253. [DOI] [PubMed] [Google Scholar]
- 90. Tan ST, Itinteang T, Leadbitter P. Low-dose propranolol for multiple hepatic and cutaneous hemangiomas with deranged liver function. Pediatrics. 2011;127:e772–6.. doi: 10.1542/peds.2010-1703. [DOI] [PubMed] [Google Scholar]
- 91. Sciveres M, Marrone G, Pipitone S, et al. Successful first-line treatment with propranolol of multifocal infantile hepatic hemangioma with high-flow cardiac overload. Journal of Pediatric Gastroenterology & Nutrition. 2011;53:693–5.. doi: 10.1097/MPG.0b013e3182201a4e. [DOI] [PubMed] [Google Scholar]
- 92. Mhanna A, Franklin WH, Mancini AJ. Hepatic infantile hemangiomas treated with oral propranolol–a case series. Pediatr Dermatol. 2011;28:39–45.. doi: 10.1111/j.1525-1470.2010.01355.x. [DOI] [PubMed] [Google Scholar]
- 93. Macdonald A, Durkin N, Deganello A, et al. Historical and Contemporary Management of Infantile Hepatic Hemangioma: A 30-year Single-center Experience. Ann Surg. 2022;275:e250–5.. doi: 10.1097/SLA.0000000000003881. [DOI] [PubMed] [Google Scholar]
- 94. Ji Y, Chen S, Yang K, et al. Efficacy and Safety of Propranolol vs Atenolol in Infants With Problematic Infantile Hemangiomas: A Randomized Clinical Trial. JAMA. 2021;147:599–607.. doi: 10.1001/jamaoto.2021.0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Pope E, Lara-Corrales I, Sibbald C, et al. Noninferiority and Safety of Nadolol vs Propranolol in Infants With Infantile Hemangioma: A Randomized Clinical Trial. JAMA Pediatrics. 2022;176:34–41.. doi: 10.1001/jamapediatrics.2021.4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Sakamoto S, Kasahara M, Shigeta T, et al. Living donor liver transplantation for multiple intrahepatic portosystemic shunts after involution of infantile hepatic hemangiomas. J Pediatr Surg. 2011;46:1288–91.. doi: 10.1016/j.jpedsurg.2011.02.061. [DOI] [PubMed] [Google Scholar]
- 97. Draper H, Diamond IR, Temple M, John P, Ng V, Fecteau A. Multimodal management of endangering hepatic hemangioma: impact on transplant avoidance: a descriptive case series. J Pediatr Surg. 2008;43:120–5.; discussion 126. doi: 10.1016/j.jpedsurg.2007.09.030. [DOI] [PubMed] [Google Scholar]
- 98. Grabhorn E, Richter A, Fischer L, et al. Neonates with severe infantile hepatic hemangioendothelioma: limitations of liver transplantation. Pediatr Transplant. 2009;13:560–4.. doi: 10.1111/j.1399-3046.2008.01039.x. [DOI] [PubMed] [Google Scholar]
- 99. Wang Y, Singh AR, Zhao Y, et al. TRIM28 regulates sprouting angiogenesis through VEGFR-DLL4-Notch signaling circuit. FASEB J. 2020;34:14710–24.. doi: 10.1096/fj.202000186RRR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Margadant C. Positive and negative feedback mechanisms controlling tip/stalk cell identity during sprouting angiogenesis. Angiogenesis. 2020;23:75–7.. doi: 10.1007/s10456-020-09706-0. [DOI] [PubMed] [Google Scholar]
- 101. Ye X, Abou-Rayyah Y, Bischoff J, et al. Altered ratios of pro- and anti-angiogenic VEGF-A variants and pericyte expression of DLL4 disrupt vascular maturation in infantile haemangioma. J Pathol. 2016;239:139–51.. doi: 10.1002/path.4715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Boscolo E, Mulliken JB, Bischoff J. Pericytes from infantile hemangioma display proangiogenic properties and dysregulated angiopoietin-1. Arterioscler Thromb Vasc Biol. 2013;33:501–9.. doi: 10.1161/ATVBAHA.112.300929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Eelen G, de Zeeuw P, Treps L, et al. Endothelial Cell Metabolism. Physiol Rev. 2018;98:3–58.. doi: 10.1152/physrev.00001.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Polet F, Feron O. Endothelial cell metabolism and tumour angiogenesis: glucose and glutamine as essential fuels and lactate as the driving force. J Intern Med. 2013;273:156–65.. doi: 10.1111/joim.12016. [DOI] [PubMed] [Google Scholar]
- 105. Dai J, Zhou Q, Chen J, et al. Alpha-enolase regulates the malignant phenotype of pulmonary artery smooth muscle cells via the AMPK-Akt pathway. Nat Commun. 2018;9:3850. doi: 10.1038/s41467-018-06376-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Ji Y, Chen S, Li K, et al. Upregulated autocrine vascular endothelial growth factor (VEGF)/VEGF receptor-2 loop prevents apoptosis in haemangioma-derived endothelial cells. Br J Dermatol. 2014;170:78–86.. doi: 10.1111/bjd.12592. [DOI] [PubMed] [Google Scholar]