Abstract
Several studies have found an association of COVID-19 disease severity with Vitamin D deficiency and higher levels of anti-SARS-CoV-2 IgGs. The aim of this study was to determine whether levels of Vitamin D and “inflammatory state” influence the magnitude of anti-SARS-CoV-2 IgGs levels in COVID-19 patients. For this purpose, in 67 patients levels of anti-SARS-CoV-2 IgG were measured in week 4 whereas in 52 patients levels of Vitamin D were measured in week 1 after symptom onset. We found that low Vitamin D levels were significantly associated with age and disease severity whereas there was a trend without significance, towards negative correlation of Vitamin D with anti-SARS-CoV-2 IgG. Anti-SARS-CoV-2 IgG were significantly higher in older ages, patients with severe disease, diabetes and those who received corticosteroid and antibiotic therapy. There was a positive correlation of anti-SARS-CoV-2 IgG with IL-6, CRP, LDH, ESR and with percentages of granulocytes. In conclusion, Vitamin D and anti-SARS-CoV-2 IgG share common parameters associated with inflammatory state. However, even though Vitamin D protects against severe forms of COVID-19 it could not directly affect anti-SARS-CoV-2 IgG production.
Subject terms: Biomarkers, Medical research
Introduction
The coronavirus disease 2019 (COVID‑19) is caused by a new coronavirus which in addition to acute respiratory failure is associated with systemic disorders such as hyperinflammation, hypercoagulation and vasculitis1. Although many people exhibit mild ‘flu-like’ symptoms, in severe responses systemic changes have been attributed to the cytokine storm accompanying severe inflammatory syndrome2–4. Severe forms of COVID-19 have been linked with low levels of circulating 25-hydroxy Vitamin D (25[OH]D) as an expression of Vitamin D (Vit. D)5–10. Vit. D has immunomodulatory activity in response to invasion of bacterial and viral pathogens11,12 interacting with its receptor (VDR) in immune cells13–15. In several studies it was shown that severe inflammatory syndrome was accompanied with changes in hematological markers and increased several inflammatory markers such as CRP, LDH, ESR, ferritin etc.2,16–18. A recent study has shown that in the presence of Vit. D, IL-6 induces higher production of IL-10, a known anti-inflammatory cytokine which is expected to lead to the reduction of inflammatory markers such as CRP19. Several studies have shown inverse association between Vit. D and CRP levels20–22. Additionally, high levels of CRP were associated with lowering levels of Vit. D23 indicating that Vit. D is a negative acute phase reactant. Thus, Vit. D insufficiency could be the cause and effect of high CRP levels in COVID-19 patients.
Humoral and cellular immune responses, two wings of adaptive immunity, are crucial in clearing a variety of viral infections24, and have been implicated in recovered COVID-19 patients25,26. In several studies disease severity of COVID-19 was associated with higher levels of antibodies27–29 whereas asymptomatic patients minimally produced anti-SARS-CoV-2 IgGs which were poorly maintained28,30. Taking into account that Vit. D affects adaptive immune response, it may have an impact on serological response against SARS-CoV-2. The pathways on this impact could be through different molecules and cells31–33. Previous published data has demonstrated that Vit. D supplementation can boost antibody production after vaccination with Influenza virus14. Faniyi et al. showed that Vit. D deficiency (VDD) was an independent risk factor for COVID-19 seroconversion in healthcare workers34. Also, Kaufman et al. reported that COVID-19 positivity was inversely related to the patient’s Vit. D levels in the preceding 12 months35.
Knowing that till now there are a small number of reports on Vit. D effect in serological response in COVID-19 patients, the first aim of this study was to determine whether Vit. D and “inflammatory state” influence the magnitude of anti-SARS-CoV-2 IgG levels in COVID-19 patients. The second aim of the study was to analyze the change in Vit. D levels during the illness and the change in anti-SARS-CoV-2 IgG antibody levels after 3 months of disease onset.
Results
A total of 69 patients diagnosed with COVID-19 were enrolled in this study. The median age of patients was 59 years (44.5–67.0 years) of whom 53.6% were females and 46.4% males. Patient characteristics are presented in Table 1.
Table 1.
Patients characteristics.
| Patients (N = 69) | Patients with Vit. D-w1 (N = 52) | Patients with IgG-w4 (N = 67) | ||
|---|---|---|---|---|
| Sex N (%) | Female | 37 (53.6) | 26 (50.0) | 37 (55.2) |
| Male | 32 (46.4) | 26 (50.0) | 30 (44.8) | |
| Age | Median (IQR) | 59.00 (44.50–67.00) | 62.00 (51.50–70.00) | 59.00 (44.00–67.00) |
| Hospitalisation N (%) | Yes | 42 (60.9) | 39 (75.0) | 40 (59.7) |
| Disease severity N (%) | Mild | 16 (23.2) | 8 (15.4) | 16 (23.9) |
| Moderate | 24 (34.8) | 18 (34.6) | 24 (35.8) | |
| Severe | 25 (36.2) | 22 (42.3) | 25 (37.3) | |
| Critical | 4 (5.8) | 4 (7.7) | 2 (3.0) | |
| Comorbidities N (%) | Hypertension | 27 (39.1) | 22 (42.3) | 27 (40.3) |
| Diabetes Mellitus | 10 (14.5) | 9 (17.3) | 10 (14.9) | |
| Cancer | 5 (7.2) | 5 (9.6) | 4 (6.0) | |
| Hypothyreosis | 4 (5.8) | 2 (3.9) | 4 (6.0) | |
| Outcome N (%) | Recovery | 65 (94.2) | 48 (92.3) | 65 (97.0) |
| Death | 4 (5.8) | 4 (7.7) | 2 (3.0) | |
| Medications used during illness N (%) | Corticosteroids | 49 (71.0) | 43 (82.7) | 47 (70.1) |
| Antivirals | 29 (42.0) | 25 (48.1) | 28 (41.8) | |
| Antibiotics | 65 (94.2) | 51 (98.1) | 64 (95.5) | |
| Vit. D Groups N (%) | Deficient (≤ 20) | 17 (32.7) | 15 (22.4) | |
| Insufficent (21–29) | 17 (32.7) | 17 (25.4) | ||
| Sufficient (≥ 30) | 18 (34.6) | 18 (26.9) |
Age, disease severity and diabetes influence anti-SARS-CoV-2 IgG antibody levels
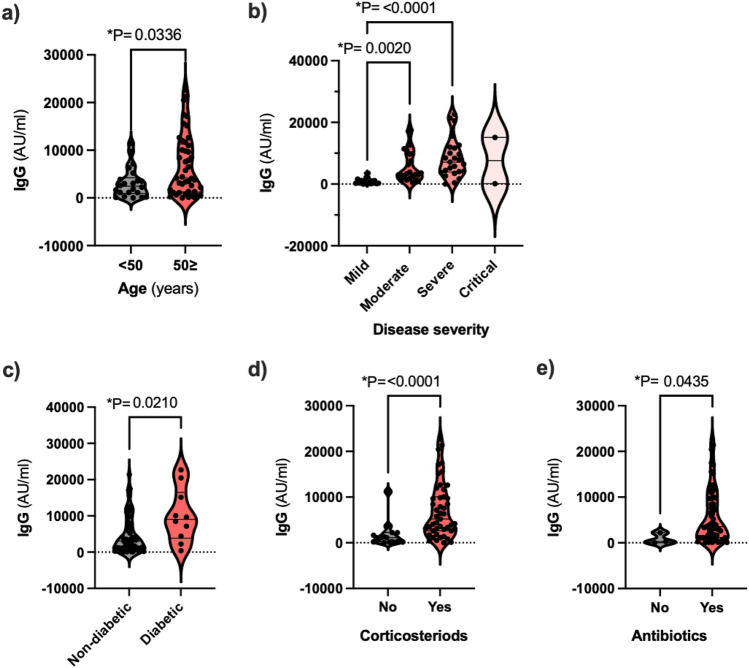
We investigated the factors that are likely to influence anti-SARS-CoV-2 IgG levels in COVID-19 patients. We found that age, but not gender, significantly influenced IgG levels in this group of patients. Specifically, patients aged > 50 were found to have significantly higher IgG levels than patients < 50 (Fig. 1). When grouping according to disease severity the distribution of IgG-w4 was different across categories of disease severity. Mean ranks of anti-SARS-CoV-2 IgG were significantly higher in patients with diabetes and those who received corticosteroid and antibiotic therapy (Fig. 1). IgG levels were also higher in patients with hypertension but this did not reach significance (p = 0.054).
Figure 1.
Distribution of anti-SARS-CoV-2 IgG across age groups, disease severity groups, patients with and without diabetes, corticosteroid and antibiotic administration. Levels of IgG were significantly higher in age group > 50, severe and critical patients, in diabetics and patients treated with corticosteroids and antibiotics.
Vit. D is associated with age and disease severity
Next, we sought to determine whether the magnitude of anti-SARS-CoV-2 IgG antibody level was related to Vit. D levels. In 65.4% of 52 patients, levels of Vit. D were below normal values. Gender did not influence Vit. D, however once again, age was significantly correlated with Vit. D. A significant difference in distribution of Vit. D was also found in different groups of patients according to disease severity and outcome (Fig. 2); all deceased patients were insufficient, 75% of them being VDD.
Figure 2.
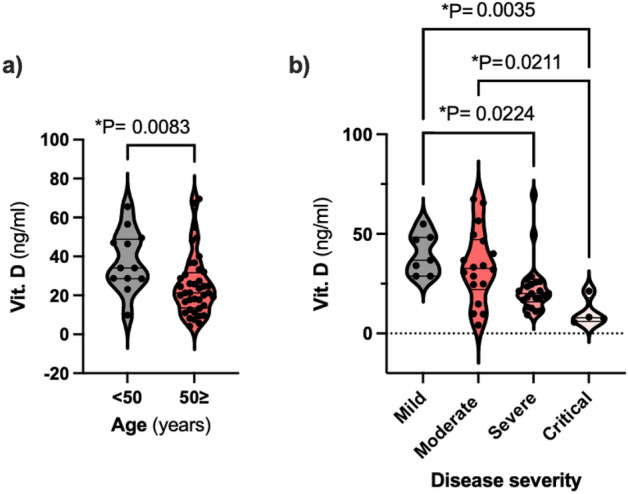
Distribution of Vit. D-w1 across age groups and disease severity groups. Levels of Vit. D were significantly higher in the age group < 50 and in mild/moderate patients.
Correlations between anti-SARS-CoV-2 IgG and Vit. D with other inflammatory and hematological parameters
To determine other factors that are likely to influence anti-SARS-CoV-2 IgG production and the relationship of Vit. D with these factors, we did a correlation analysis of anti-SARS-CoV-2 IgG and Vit. D with inflammatory and hematological markers in the first week post-symptom onset (PSO) and with the most changed values (maximum values of CRP, D-Dimer, LDH, ESR, percentages of granulocytes and minimum values of WBC count, platelet count, percentages of monocyte and percentages of lymphocytes) during the course of disease. Anti-SARS-CoV-2 IgG were positively correlated with IL-6, CRP, LDH, ESR and percentages of granulocytes determined in the first week PSO (Table 2). On the other hand, levels of IgG were weakly correlated with percentages of lymphocytes, but without significance. When analysed with the most changed values during the course of disease, we found stronger negative correlation between anti-SARS-CoV-2 with maximum values of CRP, LDH, ESR and percentages of granulocyte than with parameters found in the first week PSO. There are no maximum values of IL-6 for the fact that this parameter was determined only once, in the first week PSO. On the other hand, anti-SARS-CoV-2 resulted to be positively correlated with minimum values of WBC and negatively correlated with minimum values of percentages of lymphocytes and monocytes.
Table 2.
Mutual correlation between IgG-w4 and Vit. D-w1 and correlation between these parameters and inflammatory and hematological markers.
| IgG-w4 (N = 67) | Vit. D-w1 (N = 52) | |||||
|---|---|---|---|---|---|---|
| N | Spearman Rho | p value | N | Spearman Rho | p value | |
| Vit. D-w1 (ng/ml) | 50 | − 0.151 | 0.296 | |||
| IL6 (pg/ml) | 45 | 0.310 | 0.038 | 43 | − 0.361 | 0.017 |
| CRP-w1 (mg/l) | 52 | 0.515 | 0.000 | 51 | − 0.400 | 0.004 |
| Max. CRP (mg/l) | 63 | 0.539 | 0.000 | 51 | − 0.486 | 0.000 |
| D-Dimer-w1 (ng/ml) | 50 | − 0.018 | 0.903 | 49 | − 0.290 | 0.043 |
| Max. D-Dimer (ng/ml) | 59 | 0.224 | 0.089 | 49 | − 0.391 | 0.005 |
| LDH-w1 (U/l) | 25 | 0.515 | 0.008 | 25 | − 0.295 | 0.152 |
| Max. LDH (U/l) | 28 | 0.560 | 0.002 | 25 | − 0.097 | 0.645 |
| CK-w1 (U/l) | 20 | 0.242 | 0.304 | 21 | − 0.124 | 0.591 |
| Max. CK (U/l) | 20 | 0.232 | 0.326 | 21 | 0.160 | 0.487 |
| ESR-w1 (mm/h) | 42 | 0.436 | 0.004 | 40 | − 0.372 | 0.018 |
| Max. ESR (mm/h) | 47 | 0.552 | 0.000 | 40 | − 0.385 | 0.014 |
| WBC-w1 (× 109/l) | 57 | 0.241 | 0.071 | 52 | − 0.131 | 0.354 |
| Min. WBC (× 109/l) | 63 | 0.362 | 0.004 | 52 | − 0.322 | 0.020 |
| Granulocytes-w1 (%) | 54 | 0.310 | 0.023 | 49 | − 0.214 | 0.140 |
| Max. granulocytes (%) | 60 | 0.303 | 0.019 | 49 | − 0.214 | 0.140 |
| Lymphocytes-w1 (%) | 55 | − 0.236 | 0.083 | 50 | 0.159 | 0.271 |
| Min. lymphocytes (%) | 61 | − 0.266 | 0.038 | 50 | 0.169 | 0.240 |
| Monocytes-w1 (%) | 44 | − 0.250 | 0.101 | 39 | 0.120 | 0.466 |
| Min. monocytes (%) | 49 | − 0.284 | 0.048 | 39 | 0.122 | 0.461 |
| Platelet count-w1 | 56 | − 0.132 | 0.332 | 51 | 0.372 | 0.007 |
| Min. platelet count | 61 | − 0.123 | 0.346 | 51 | 0.415 | 0.002 |
Spearman correlation and significance.
When we analysed correlations between Vit. D and various inflammatory and hematological parameters, in the first week PSO we found a significant negative correlation between the Vit. D and IL-6, CRP, D-dimer, ESR and a significant positive correlation with platelet count. When we analysed correlation of Vit.D with the most changed laboratory parameters during the course of disease, stronger negative correlation was found between Vit. D and CRP, D-Dimer, ESR and platelet count than with these parameters in the first week PSO whereas a negative correlation was found also with minimum values of WBC count. There was a negative correlation of IgG-w4 with Vit. D-w1, however this did not reach statistical significance (p = 0.296) (Table 2).
Changes in Vit. D and anti-SARS-CoV-2 IgG levels over time
In order to estimate the change of Vit. D during illness and the change of anti-SARS-CoV-2 IgG after 3 months, we compared median differences in paired samples (Vit. D-w1 and Vit. D-w4; IgG-w4 and IgG-m4). In 66% of patients (N = 50) levels of Vit. D decreased during the illness although all patients were supplemented with Vit. D; hospitalised patients were supplemented with 4000 UI/ml, whereas ambulatory patients took no more than 1000 UI/ml. There were no significant differences in Vit. D decrease during illness between groups of patients by sex, age, disease severity and comorbidity (Table 3).
Table 3.
Vit. D level change (difference Vit. D-w1–Vit. D-w4) during illness and IgG level change 3 months PSO (difference IgG-w4–IgG-m4).
| Vitamin D change | IgG change | ||||
|---|---|---|---|---|---|
| Difference Vit. D-w1–Vit. D-w4 |
Statistics | Difference IgG-w4–IgG-m4 |
Statistics | ||
| Frequency N (%) | Increase | 17 (34.0) | 11 (64.7) | ||
| Decrease | 33 (66.0) | 7 (35.3) | |||
| Sex N (mean rank) | U = 266, p = 0.372 | U = 30, p = 0.763 | |||
| F | 26 (23.73) | 11 (9.27) | |||
| M | 24 (27.42) | 6 (8.50) | |||
| Age N (mean rank) | H(5) = 6.027, p = 0.304 | H(4) = 4.036, p = 0.401 | |||
| 0–29 | 5 (25.9) | 4 (8.50) | |||
| 30–39 | 3 (20.67) | 3 (10.00) | |||
| 40–49 | 4 (41.88) | 3 (8.67) | |||
| 50–59 | 10 (21.95) | 3 (4.67) | |||
| 60–69 | 14 (24.89) | 4 (12.25) | |||
| 70+ | 14 (24.86) | ||||
| Disease severity N (mean rank) | H(3) = 0.542, p = 0.910 | H(2) = 6.489, p = 0.039 | |||
| Mild | 8 (25.31) | 8 (6.63) | |||
| Moderate | 18 (24.00) | 6 (9.00) | |||
| Severe | 22 (26.30) | 3 (15.33) | |||
| Critical | 2 (31.00) | ||||
| Hospitalisation N (mean rank) | U = 194.5, p = 0.309 | U = 2, p = 0.017 | |||
| No | 13 (21.96) | 14 (7.64) | |||
| Yes | 37 (26.74) | 3 (15.33) | |||
| Difference between medians N (mean rank) | Z = − 2.129, p = 0.033 | Z = − 2.107, p = 0.035 | |||
| Negative Ranks | 33 (26.00) | 11 (11.0) | |||
| Positive Ranks | 17 (24.53) | 6 (5.33) | |||
U Mann–Whitney U test, H Kruskal–Wallis test, Z Wilcoxon Signed Ranks test.
The changes of IgG levels between two measurements were analysed in 17 patients. In 6 patients we found an increase of IgG levels, whereas in 11 we found a decrease of antibody levels. There was a significant difference in anti-SARS-CoV-2 decrease between groups of patients according to disease severity, with higher reduction of IgG levels in severe patients (Table 3).
Discussion
This is, to our knowledge, the first report analysing the Vit. D status and magnitude of anti-SARS-CoV-2 IgG antibody production in COVID-19 patients. Although in our study baseline levels of Vit. D were below normal values in 65.4% of patients, these values were in agreement with other publications36–38. We show that Vit. D distribution was affected by age and disease severity, but not sex. Similar findings were described in several other studies39–42. Ilie et al. also showed a negative correlation between Vit. D levels and COVID-19 cases and mortality8. A meta‐analysis with a total of 1368 COVID‐19 patients also showed that low Vit. D levels were significantly associated with poorer patient outcome and prognosis42. In contrast Hastie et al. found no link between Vit. D and risk of severe COVID-19 infection and mortality in 341,484 UK Biobank participants43.
Vit. D has well recognised immunoregulatory actions; it reduces production of pro-inflammatory cytokines (IL-6, IL-8 and IL-17) and increases anti-inflammatory cytokines (IL-10) leading to down-regulation of TH1 cells and up-regulation of TH2 cells14. It is therefore assumed that VDD could be a central factor in ‘cytokine storm’ seen in COVID-19 infection; patients with low concentrations of Vit. D (≤ 30 nmol/l or ≤ 12 ng/ml) have demonstrated significant, elevated markers of cytokine storm44. In this study we found a significant negative correlation between Vit. D levels and various inflammatory markers including IL-6, CRP, ESR and D-dimer. Despite observing trends, albeit not significant, Carpagnano et al. found higher levels of IL-6 in COVID patients with severe VDD45. IL-6 also plays a key role in cytokine storm and induces rise of CRP46, a well known inflammatory marker that also significantly increases in severe forms of COVID-1947,48. Since IL-6 bioactivity may change in the presence of Vit. D, CRP may be a more accurate indicator of pro-inflammatory cytokines than IL-646. Several studies, which are in line with our study, have shown an inverse association between Vit. D and CRP14,21.
In COVID-19, virus-specific B-cell mediated humoral immunity has been implicated and majority of patients seroconverted during recovery phase. In this study 97% of patients seroconverted with higher IgG levels in males than in females, but with no significant difference. The same results were found by Kutsuna et al. but they reported a significant difference for the fact that in that study significance was set at p = 0.132. Also Robbiani et al. reported higher anti-SARS-CoV-2 IgG in men than in women49. Age distribution of anti-SARS-CoV-2 IgG in this study was significantly different between the groups, with higher levels in patients > 50 years. This finding is in line with other studies50,51.
Our results confirm previous findings that clinical COVID-19 disease severity is associated with higher anti-SARS-CoV-2 serum-IgG antibodies27,32 although To et al. found that elevated antibody titers do not correlate with the severity of disease52. The exact immune mechanisms responsible for different IgG responses between different forms of disease are not known. Whereas Gozalbo-Rovira et al.18 reported that patients with severe forms of the disease could be exposed to higher and more perdurable viral burdens, Hoepel et al. suggest that worsening of disease during SARS-CoV-2 infection could be caused by antibodies53. Gao et al. found that moderate and severe symptomatic patients exhibited a significant increase in frequencies of B-cells compared to healthy controls54. This demonstrates that compared with potent SARS-CoV-2-specific B cell responses mounted in COVID-19 patients after moderate or severe illness, asymptomatic or mild symptomatic COVID-19 patients only induced weak and transient SARS-CoV-2-specific B cell responses54.
By analysing anti-SARS-CoV-2 IgG in relation to comorbidities, significantly different distribution was found among patients with and without diabetes but not with and without hypertension, results similar to Kutsuna et al.32. Esperança-Martins et al. found significantly lower levels of anti-SARS-CoV-2 IgG in cancer patients55, a finding we could not corroborate possibly due to the low number of cancer patients recruited in the study. With regard to therapies, our finding that corticosteroid use and IgG-w4 were associated were similar to findings by Kutsuna et al.32. This association may be a result of the use of corticosteroids in moderate and severe patients, which already showed a relationship with IgG levels.
In this study we sought to determine whether the magnitude of SARS-CoV-2 response was related to an inflammatory state. Correlation analyses show that anti-SARS-CoV-2 IgGs were significantly correlated with IL-6, CRP, ESR, LDH, lower WBC count, higher persentages of granulocytes, lower persentages of lymphocytes and lower persentages of monocytes. Gozalbo-Rovira et al. also found weak or very weak correlation of anti-RBD-IgG with inflammatory markers such as CRP, IL-6, D-Dimer and LDH18. Corrrelation between anti-SARS-CoV-2 antibodies and CRP levels were reported also by other authors32,56,57. Correlation of anti-SARS-CoV-2 antibodies with LDH levels found in first week PSO and maximum values during the disease are in line with Kutsuna et al.32. Increase in neutrophil count and decrease in the lymphocytes were more common in severe cases than in moderate cases of COVID-1958,59 and indicate the intensity of inflammatory response. In this study we found that the maximum values of granulocytes and minimum values of lymphocytes during the course of disease are in correlation with anti-SARS-Cov-2 IgG which are olso found to be higher in severe cases of disease who also showed higher inflammatory responses. The same was found by Gozalbo-Rovira et al.18.
A key question of this study was if baseline Vit. D levels may influence the serological response in patients with COVID-19. Similar to our findings, Yonghong et al. in 18,148 individuals found that SARS-CoV-2 seropositivity was not associated with having a Vit. D level less than 30 ng/ml before or during the pandemic independently of other risk factors38. Also Barassi et al. reported that there was no relationship between Vit. D and anti-SARS-CoV-2 IgG values60. Although Kaufman et al. found strong and inverse association between Vit. D and SARS-CoV-2 positivity, in contrast to our study they did not carry out quantitative analyses between these parameters35.
Prior studies reported that SARS-Cov-2 IgG levels decline during time post infection61,62 and the same was found in this study in which in 65% of patients with measured IgG-m4, levels of IgG decreased after three months. A significant difference in decrease of SARS-CoV-2 IgG was found between groups of patients according to disease severity, with higher reduction of IgG levels in severe patients. Our results are in line with the results of Kutsuna et al. in which in moderate and severely ill patients titers of IgG tended to decline 60 days PSO compared to mild cases32. Ma et al. analysed decline rate of IgG and predicted convalescent patients’ SARS-CoV-2 IgG to be undetectable approximately 273 days after hospital discharge63. This study has some limitations. First, the sample size of this study is modest and not all patients had all laboratory analyses completed making paired analysis difficult. The second limitation of the study was the collection of samples at different sites.
Conclusions
In summary, anti-SARS-CoV-2 IgGs and Vit. D shares common parameters associated with inflammatory state. This could lead us to suppose that Vit. D signaling, targeting several immune-mediated pathways, protects against severe forms of COVID-19 but it does not directly affect the anti-SARS-CoV-2 IgG production. However, further work needs to be completed to address whether Vit. D influences serological response in COVID-19 patients.
Methods
Study population
COVID-19 patients (n = 69), diagnosed by RT-PCR analysis of nasopharyngeal swab, were enrolled in this prospective study. Patients were either hospitalized at the University Clinical Center of Kosova (n = 42) or at the outpatients clinic (n = 27). Of these patients, 52 were recruited in the first week post-symptom onset (PSO); the remaining (n = 17) were recruited 3 weeks PSO. Fifty patients in this study group are also part of the research project "Association of Il-6 and other biomarkers of inflammation with outcome of Covid 19 patients: A study from Kosova”.
In an attempt to determine the relationship between the magnitude of anti-SARS-CoV-2 IgG levels with Vit. D and the “inflammatory state” of patients, we analysed anti-SARS-CoV-2 IgG in relation with Vit. D and inflammatory biomarkers. For anti-SARS-CoV-2 and Vit. D measurement blood was collected from the cubital vein. Serum was separated from whole blood (4 ml) collected in granules and clot activator tubes and stored at – 20 °C until measurement of Total 25(OH)D and anti-SARS-CoV-2 IgG antibodies.
Measurement of total serum 25(OH)D
Total serum 25[OH]D was quantified using an electrochemiluminescence assay, (Roche Cobase 411) according to the manufacturer’s instructions. Levels obtained were divided into three categories according to Endocrine Society Clinical Practice Guideline on Vitamin D: (1) deficient (≤ 20 ng/ml), (2) insufficient (21–29 ng/ml) and (3) sufficient (≥ 30 ng/ml). Vit. D was measured twice; in week 1 since symptom onset (Vit. D-w1; n = 52) and week 4 since symptom onset (Vit. D-w4; n = 50, two patients passed away without second measurement of Vit. D).
Measurement of anti-SARS-CoV-2 IgG antibodies
Anti-SARS-CoV-2 IgG antibodies levels were determined using the SARS-CoV-2 IgG II Quant assay, an quantitative chemiluminescent microparticle immunoassay (CMIA), (Abbott) according to the manufacturer’s instructions. The cut-off value for test positivity was 50.0 AU/ml with analytical measurement interval of 21–40,000 AU/ml. In 67 of 69 patients (as mentioned above, two passed away before week 4) anti-SARS-CoV-2 IgG antibodies were measured at week 4 since symptom onset (IgG-w4; n = 67). In 17 of these patients, IgG levels were also measured at 4 months since symptom onset (IgG-m4; n = 17).
Clinical parameters
Additional information was collected from recruited patients including, age, sex, illness severity, comorbidities, medications used to treat illness, clinical outcome, inflammatory markers (IL-6, CRP, D-dimer and LDH), hematological parameters [Erythrocyte Sedimantatio Rate (ESR), White Blood Cell count (WBC), percentages of granulocytes, lymphocytes and monocytes and platelet count] during acute phase of COVID-19.
Statistical analysis
Data were tested for normality by means of Kolmogorov–Smirnov and Shapiro–Wilk test. Categorical variables were presented as frequencies and percentages, whereas skewed continuous variables were expressed as medians and inter-quartile ranges. Chi-Square test was used to examine relationships between categorical variables. Due to non-normal distribution of data, comparisons across disease severity categories and age groups were conducted using Kruskal–Wallis test; Mann–Whitney U Test was used for comparisons accross two categorical independent goups (sex and hospitalisation); whereas comparison accross positive and negative ranks was carried out by using Wilcoxon Signed-Rank Test. Spearman’s rank correlation coefficient was used to gauge monotonic relationship between Vitamin D and IgG levels, as well as inflammatory and hematological markers. Statistical analyses were carried out with SPSS (version 25) and/or Graphpad Prism v9.0. All tests were 2-talied and P-values < 0.05 were considered significant.
Ethics declaration
This study was approved by the Ethics Committee at University Clinical Centre of Kosova (reference no 2548/2020). Written informed consent was obtained from all participants, in accordance with the Declaration of Helsinki.
Acknowledgements
The authors gratefully acknowledge the assistance of midwife Arlinda Fushtica, Dr. Luljeta Hasani and Dr. Albina Ponosheci-Biçaku for help in providing patients blood samples at the Infectious Diseases Clinic.
Author contributions
Designed the study: H.L.P. Collected data and blood samples: A.P., V.G. and G.B. Performed the experiment: A.K. and V.H. Analyzed the data: H.L.P. and S.N. Wrote the paper: H.L.P. and B.A.-S.
Funding
There was no funding attributed to the work presented in this manuscript. Consumable costs were self-funded by the first author.
Data availability
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Thomson TM, Toscano-Guerra E, Casis E, Paciucci R. C1 esterase inhibitor and the contact system in COVID-19. Br. J. Haematol. 2020;190(4):520–524. doi: 10.1111/bjh.16938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Santa Cruz A, et al. Interleukin-6 Is a biomarker for the development of fatal severe acute respiratory syndrome coronavirus 2 oxi. Front. Immunol. 2021;12:613422. doi: 10.3389/fimmu.2021.613422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Olbei M, et al. SARS-CoV-2 causes a different cytokine response compared to other cytokine storm-causing respiratory viruses in severely ill patients. Front. Immunol. 2021;12:629193. doi: 10.3389/fimmu.2021.629193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Darif D, Hammi I, Kihel A, El Idrissi SI, Guessous F, Akarid K. The pro-inflammatory cytokines in COVID-19 pathogenesis: What goes wrong? Microb. Pathog. 2021;153:104799. doi: 10.1016/j.micpath.2021.104799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ali N. Role of vitamin D in preventing of COVID-19 infection, progression and severity. J. Infect. Public Health. 2020;13(10):1373–1380. doi: 10.1016/j.jiph.2020.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahmed F. A network-based analysis reveals the mechanism underlying vitamin D in suppressing cytokine storm and virus in SARS-CoV-2 infection. Front. Immunol. 2020;11:590459. doi: 10.3389/fimmu.2020.590459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Honardoost M, Ghavideldarestani M, Khamseh ME. Role of Vitamin D in pathogenesis and severity of COVID-19 infection. Arch. Physiol. Biochem. 2020 doi: 10.1080/13813455.2020.1792505. [DOI] [PubMed] [Google Scholar]
- 8.Ilie PC, Stefanescu S, Smith L. The role of vitamin D in the prevention of coronavirus disease 2019 infection and mortality. Aging Clin. Exp. Res. 2020;32(7):1195–1198. doi: 10.1007/s40520-020-01570-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kalia V, Studzinski GP, Sarkar S. Role of Vitamin D in regulating COVID-19 severity—An immunological perspective. J. Leukoc. Biol. 2021;110(4):809–819. doi: 10.1002/JLB.4COVR1020-698R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yisak H, et al. Effects of Vitamin D on COVID-19 infection and prognosis: A systematic review. Risk Manag. Healthc. Policy. 2021;14:31–38. doi: 10.2147/RMHP.S291584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Charoenngam N, Holick MF. Immunologic effects of vitamin D on human health and disease. Nutrients. 2020;12(7):2097. doi: 10.3390/nu12072097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sassi F, Tamone C, D'Amelio P. Vitamin D: nutrient, hormone, and immunomodulator. Nutrients. 2018;10(11):1656. doi: 10.3390/nu10111656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chadha MK, et al. Effect of 25-hydroxyvitamin D status on serological response to influenza vaccine in prostate cancer patients. Prostate. 2011;71(4):368–372. doi: 10.1002/pros.21250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Daneshkhah A, Agrawal V, Eshein A, Subramanian H, Roy HK, Backman V. Evidence for possible association of vitamin D status with cytokine storm and unregulated inflammation in COVID-19 patients. Aging Clin. Exp. Res. 2020;32(10):2141–2158. doi: 10.1007/s40520-020-01677-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ebadi M, Montano-Loza AJ. Perspective: Improving vitamin D status in the management of COVID-19. Eur. J. Clin. Nutr. 2020;74(6):856–859. doi: 10.1038/s41430-020-0661-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Terpos E, et al. Hematological findings and complications of COVID-19. Am. J. Hematol. 2020;95(7):834–847. doi: 10.1002/ajh.25829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moutchia J, et al. Clinical laboratory parameters associated with severe or critical novel coronavirus disease 2019 (COVID-19): A systematic review and meta-analysis. PLoS One. 2020;15(10):e0239802. doi: 10.1371/journal.pone.0239802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gozalbo-Rovira R, et al. SARS-CoV-2 antibodies, serum inflammatory biomarkers and clinical severity of hospitalized COVID-19 patients. J. Clin. Virol. 2020;131:104611. doi: 10.1016/j.jcv.2020.104611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McGregor R, Chauss D, Freiwald T, et al. An autocrine vitamin D-driven Th1 shutdown program can be exploited for COVID-19. Preprint. bioRxiv. 2020 doi: 10.1101/2020.07.18.210161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mellenthin L, Wallaschofski H, Grotevendt A, Völzke H, Nauck M, Hannemann A. Association between serum vitamin D concentrations and inflammatory markers in the general adult population. Metabolism. 2014;63(8):1056–1062. doi: 10.1016/j.metabol.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 21.Palaniswamy S, et al. Could vitamin D reduce obesity-associated inflammation? Observational and Mendelian randomization study. Am. J. Clin. Nutr. 2020;111(5):1036–1047. doi: 10.1093/ajcn/nqaa056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bellia A, et al. Serum 25-hydroxyvitamin D levels are inversely associated with systemic inflammation in severe obese subjects. Intern. Emerg. Med. 2013;8(1):33–40. doi: 10.1007/s11739-011-0559-x. [DOI] [PubMed] [Google Scholar]
- 23.Ghashut RA, Talwar D, Kinsella J, Duncan A, McMillan DC. The effect of the systemic inflammatory response on plasma vitamin 25 (OH) D concentrations adjusted for albumin. PLoS One. 2014;9(3):e92614. doi: 10.1371/journal.pone.0092614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aoshi T, Koyama S, Kobiyama K, Akira S, Ishii KJ. Innate and adaptive immune responses to viral infection and vaccination. Curr. Opin. Virol. 2011;1(4):226–232. doi: 10.1016/j.coviro.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 25.Le Bert N, et al. SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature. 2020;584(7821):457–462. doi: 10.1038/s41586-020-2550-z. [DOI] [PubMed] [Google Scholar]
- 26.Ni L, Ye F, et al. Detection of SARS-CoV-2-specific humoral and cellular immunity in COVID-19 convalescent individuals. Immunity. 2020;52(6):971–977.e3. doi: 10.1016/j.immuni.2020.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao J, et al. Antibody responses to SARS-CoV-2 in patients with novel coronavirus disease 2019. Clin. Infect. Dis. 2020;71(16):2027–2034. doi: 10.1093/cid/ciaa344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Long QX, et al. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat. Med. 2020;26(8):1200–1204. doi: 10.1038/s41591-020-0965-6. [DOI] [PubMed] [Google Scholar]
- 29.Lee YL, et al. Dynamics of anti-SARS-Cov-2 IgM and IgG antibodies among COVID-19 patients. J. Infect. 2020;81(2):e55–e58. doi: 10.1016/j.jinf.2020.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ibarrondo FJ, et al. Rapid decay of anti-SARS-CoV-2 antibodies in persons with mild covid-19. N. Engl. J. Med. 2020;383(11):1085–1087. doi: 10.1056/NEJMc2025179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jego G, Palucka AK, Blanck JP, Chalouni C, Pascual V, Banchereau J. Plasmacytoid dendritic cells induce plasma cell differentiation through type I interferon and interleukin 6. Immunity. 2003;19(2):225–234. doi: 10.1016/s1074-7613(03)00208-5. [DOI] [PubMed] [Google Scholar]
- 32.Kutsuna S, et al. Factors associated with anti-SARS-CoV-2 IgG antibody production in patients convalescing from COVID-19. J. Infect. Chemother. 2021;27(6):808–813. doi: 10.1016/j.jiac.2021.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vinuesa CG, Linterman MA, Yu D, MacLennan IC. Follicular helper T cells. Annu. Rev. Immunol. 2016;34:335–368. doi: 10.1146/annurev-immunol-041015-055605. [DOI] [PubMed] [Google Scholar]
- 34.Faniyi AA, et al. Vitamin D status and seroconversion for COVID-19 in UK healthcare workers. Eur. Respir. J. 2021;57(4):2004234. doi: 10.1183/13993003.04234-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaufman HW, Niles JK, Kroll MH, Bi C, Holick MF. SARS-CoV-2 positivity rates associated with circulating 25-hydroxyvitamin D levels. PLoS One. 2020;15(9):e0239252. doi: 10.1371/journal.pone.0239252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Demir M, Demir F, Aygun H. Vitamin D deficiency is associated with COVID-19 positivity and severity of the disease. J. Med. Virol. 2021;93(5):2992–2999. doi: 10.1002/jmv.26832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kebapcilar AG, Kulaksizoglu M, Ipekci SH, et al. Relationship between mean platelet volume and low-grade systemic coagulation with vitamin D deficiency in primary ovarian insufficiency. Arch. Gynecol. Obstet. 2013;288(1):207–212. doi: 10.1007/s00404-013-2735-x. [DOI] [PubMed] [Google Scholar]
- 38.Li Y, Tong CH, Bare LA, Devlin JJ. Assessment of the association of vitamin D level with SARS-CoV-2 seropositivity among working-age adults. JAMA Netw. Open. 2021;4(5):e2111634. doi: 10.1001/jamanetworkopen.2021.11634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ghasemian R, Shamshirian A, Heydari K, et al. The role of vitamin D in the age of COVID-19: A systematic review and meta-analysis. Int. J. Clin. Pract. 2021;75(11):e14675. doi: 10.1111/ijcp.14675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jain A, Chaurasia R, Sengar NS, Singh M, Mahor S, Narain S. Analysis of vitamin D level among asymptomatic and critically ill COVID-19 patients and its correlation with inflammatory markers. Sci. Rep. 2020;10(1):20191. doi: 10.1038/s41598-020-77093-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kerget B, Kerget F, Kızıltunç A, et al. Evaluation of the relationship of serum vitamin D levels in COVID-19 patients with clinical course and prognosis. COVID-19 hastalarında serum vitamin D düzeyinin klinik seyir ve prognozla ilişkisinin değerlendirilmesi. Tuberk Toraks. 2020;68(3):227–235. doi: 10.5578/tt.70027. [DOI] [PubMed] [Google Scholar]
- 42.Munshi R, Hussein MH, Toraih EA, et al. Vitamin D insufficiency as a potential culprit in critical COVID-19 patients. J. Med. Virol. 2021;93(2):733–740. doi: 10.1002/jmv.26360. [DOI] [PubMed] [Google Scholar]
- 43.Hastie CE, Pell JP, Sattar N. Vitamin D and COVID-19 infection and mortality in UK Biobank. Eur. J. Nutr. 2021;60(1):545–548. doi: 10.1007/s00394-020-02372-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baktash V, et al. Vitamin D status and outcomes for hospitalised older patients with COVID-19. Postgrad. Med. J. 2021;97(1149):442–447. doi: 10.1136/postgradmedj-2020-138712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carpagnano GE, et al. Vitamin D deficiency as a predictor of poor prognosis in patients with acute respiratory failure due to COVID-19. J. Endocrinol. Investig. 2021;44(4):765–771. doi: 10.1007/s40618-020-01370-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Almerighi C, Sinistro A, Cavazza A, Ciaprini C, Rocchi G, Bergamini A. 1Alpha,25-dihydroxyvitamin D3 inhibits CD40L-induced pro-inflammatory and immunomodulatory activity in human monocytes. Cytokine. 2009;45(3):190–197. doi: 10.1016/j.cyto.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 47.Ali N. Elevated level of C-reactive protein may be an early marker to predict risk for severity of COVID-19. J. Med. Virol. 2020;92(11):2409–2411. doi: 10.1002/jmv.26097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tjendra Y, Al Mana AF, Espejo AP, et al. Predicting disease severity and outcome in COVID-19 patients: A review of multiple biomarkers. Arch. Pathol. Lab. Med. 2020;144(12):1465–1474. doi: 10.5858/arpa.2020-0471-SA. [DOI] [PubMed] [Google Scholar]
- 49.Robbiani DF, et al. Convergent antibody responses to SARS-CoV-2 in convalescent individuals. Nature. 2020;584(7821):437–442. doi: 10.1038/s41586-020-2456-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sholukh AM, Fiore-Gartland A, Ford ES, et al. Evaluation of SARS-CoV-2 neutralization assays for antibody monitoring in natural infection and vaccine trials. Preprint. medRxiv. 2020 doi: 10.1101/2020.12.07.20245431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li K, et al. Dynamic changes in anti-SARS-CoV-2 antibodies during SARS-CoV-2 infection and recovery from COVID-19. Nat. Commun. 2020;11(1):6044. doi: 10.1038/s41467-020-19943-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.To KK, et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: An observational cohort study. Lancet Infect. Dis. 2020;20(5):565–574. doi: 10.1016/S1473-3099(20)30196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hoepel W, et al. High titers and low fucosylation of early human anti-SARS-CoV-2 IgG promote inflammation by alveolar macrophages. Sci. Transl. Med. 2021;13(596):eabf8654. doi: 10.1126/scitranslmed.abf8654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gao L, et al. The dichotomous and incomplete adaptive immunity in COVID-19 patients with different disease severity. Signal Transduct. Target Ther. 2021;6(1):113. doi: 10.1038/s41392-021-00525-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Esperança-Martins M, et al. Humoral immune response of SARS-CoV-2-infected patients with cancer: Influencing factors and mechanisms. Oncologist. 2021;26(9):e1619–e1632. doi: 10.1002/onco.13828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Akter A, Ahmed T, Tauheed I, et al. Disease characteristics and serological responses in patients with differing severity of COVID-19 infection: A longitudinal cohort study in Dhaka, Bangladesh. PLoS Negl. Trop. Dis. 2022;16(1):e0010102. doi: 10.1371/journal.pntd.0010102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li X, Pang L, Yin Y, et al. Patient and clinical factors at admission affect the levels of neutralizing antibodies six months after recovering from COVID-19. Viruses. 2022;14(1):80. doi: 10.3390/v14010080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu Y, Huang X, Sun J, et al. Clinical characteristics and immune injury mechanisms in 71 patients with COVID-19. mSphere. 2020;5(4):e00362–e420. doi: 10.1128/mSphere.00362-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhao G, Su Y, Sun X, et al. A comparative study of the laboratory features of COVID-19 and other viral pneumonias in the recovery stage. J. Clin. Lab. Anal. 2020;34(10):e23483. doi: 10.1002/jcla.23483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Barassi A, et al. Vitamin D in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) patients with non-invasive ventilation support. Panminerva Med. 2021 doi: 10.23736/S0031-0808.21.04277-4. [DOI] [PubMed] [Google Scholar]
- 61.Figueiredo-Campos P, et al. Seroprevalence of anti-SARS-CoV-2 antibodies in COVID-19 patients and healthy volunteers up to 6 months post disease onset. Eur. J. Immunol. 2020;50(12):2025–2040. doi: 10.1002/eji.202048970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Marklund E, et al. Serum-IgG responses to SARS-CoV-2 after mild and severe COVID-19 infection and analysis of IgG non-responders. PLoS ONE. 2020;15(10):e0241104. doi: 10.1371/journal.pone.0241104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ma H, et al. Decline of SARS-CoV-2-specific IgG, IgM and IgA in convalescent COVID-19 patients within 100 days after hospital discharge. Sci. China Life Sci. 2021;64(3):482–485. doi: 10.1007/s11427-020-1805-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.