Abstract
We aimed to understand the contemporary changes in the characteristics and the determinants of outcomes among simultaneous liver-kidney transplantation (SLKT) recipients at 6 liver transplantation centers in the United States. We retrospectively enrolled SLKT recipients between 2002 and 2017 in the US Multicenter SLKT Consortium. We analyzed time-related trends in recipient characteristics and outcomes with linear regression and nonparametric methods. Clustered Cox regression determined the factors associated with 1-year and overall survival. We enrolled 572 patients. We found significant changes in the clinical characteristics of SLKT recipients: as compared with 2002, recipients in 2017 were older (59 versus 52 years; P < 0.001) and more likely to have chronic kidney disease (71% versus 33%; P < 0.001). There was a marked improvement in 1-year survival during the study period: 89% in 2002 versus 96% in 2017 (P < 0.001). We found that the drivers of 1-year mortality were SLKT year, hemodialysis at listing, donor distance, and delayed kidney allograft function. The drivers of overall mortality were an indication of acute kidney dysfunction, body mass index, hypertension, creatinine at SLKT, ventilation at SLKT, and donor quality. In this contemporary cohort of SLKT recipients, we highlight changes in the clinical characteristics of recipients. Further, we identify the determinants of 1-year and overall survival to highlight the variables that require the greatest attention to optimize outcomes.
The prevalence of chronic kidney disease (CKD) among liver transplantation candidates has increased more than 2-fold in the last decade, a cumulative manifestation of an aging population with a high burden of comorbidities (ie, diabetes mellitus and hypertension) coupled with an allocation system dependent on kidney dysfunction.(1–4) This rise in preliver transplantation CKD is a critical issue—patients with preliver transplantation kidney dysfunction are at a high risk of postliver transplantation CKD and postliver transplantation mortality.(1,2,5–7) To offset these trends, simultaneous liver-kidney transplantation (SLKT) utilization has increased by nearly 300% in the past decade (from 326 SLKTs in 2006 to 910 SLKTs in 2016).(3)
Despite this increase in utilization, there is little known about the drivers of outcomes among SLKT recipients. To date, studies evaluating outcomes after SLKT have been limited to either single-center or national data sets without the necessary granularity.(8–13) These studies report conflicting evidence regarding the benefit of SLKT, with the most optimistic studies demonstrating a 4-month increase in survival among transplantation candidates with chronic kidney dysfunction who underwent SLKT as compared with liver transplantation alone.(8,11,14) By contrast, others showed no significant survival benefit.(8–13)
Therefore, to better understand SLKT in the United States, we created the United States SLKT study (US-SLKT), a multicenter observational study structured to describe contemporary trends and inform the drivers of mortality among SLKT recipients.(15,16)
Patients and Methods
All patients undergoing SLKT between January 1, 2002, and December 31, 2017, at 6 transplantation centers (Columbia University; University of Michigan; Northwestern University; University of California, San Francisco; University of Washington; and Duke University) in 6 different United Network for Organ Sharing (UNOS) regions were retrospectively enrolled in the US-SLKT Consortium. This study was approved by the institutional review board at each of the participating centers. The University of Michigan is the data coordinating center.
STUDY DESIGN AND DEFINITIONS
Patients and Data Collection
We obtained detailed retrospective data on all SLKT recipients. We collected recipient data at the time of listing for SLKT and at the time of transplantation, as well as kidney and liver donor factors. The Model for End-Stage Liver Disease (MELD) score(17) was calculated and capped at 6 and 40, according to the current liver allocation policy. Because we included patients in this study before the implementation of Model for End-Stage Liver Disease–sodium (MELD-Na), we used the MELD score for this study. We grouped etiologies of cirrhosis into the following common diagnostic categories: hepatitis C virus, nonalcoholic fatty liver disease (including cryptogenic cirrhosis and nonalcoholic steatohepatitis [NASH]), alcohol-related cirrhosis, autoimmune etiologies (including primary biliary cirrhosis, primary sclerosing cholangitis, and autoimmune hepatitis), and other etiologies of cirrhosis (any other listing code that met inclusion criteria). We used laboratory MELD scores rather than allocation MELD scores for all descriptions of MELD score.
Etiology of Kidney Dysfunction
We ascertained the etiology of preliver transplantation kidney dysfunction at several time points. We defined kidney dysfunction as an estimated glomerular filtration rate (eGFR) of less than 60 mL/minute/1.73 m2 using the Modification of Diet in Renal Disease 4-item version (MDRD4) equation.(18) These time points were included at the time of listing for a liver transplantation, at the time of listing for a kidney transplantation, and at the time of SLKT. At each time point, we determined whether patients had acute kidney dysfunction (AKD), CKD, or indeterminate. We utilized the duration of kidney dysfunction that required SLKT to determine the group: AKD if less than 90 days, CKD if greater than or equal to 90 days, and indeterminate if the duration was unknown. We additionally captured the known time with an eGFR <60 mL/minute/1.73 m2 and an eGFR <25 mL/minute/1.73 m2. For those with AKD at the time of SLKT, we utilized the clinical documentation at SLKT to categorize AKD into the following clinical diagnoses: hepatorenal syndrome, acute tubular necrosis, other, and unknown. In other words, we attempted to analyze the information available in clinical practice—rather than retrospectively introduce an ascertainment bias. For those with CKD, we staged them according to current guidelines: Stage IV (eGFR <30 and ≥15 mL/minute/1.73 m2) or Stage V (eGFR <15 mL/minute/1.73 m2).(19)
Hemodialysis
At each time point, we determined the hemodialysis status and duration of hemodialysis. In all modeling, we assigned a serum creatinine of 4 mg/dL to all patients on hemodialysis, as done in the calculation of the MELD score. We defined delayed kidney allograft function (DGF) as the use of hemodialysis within 7 days of SLKT.(20)
Immunosuppression
The immunosuppression protocols among all 6 centers were similar. All of the centers use tacrolimus-based immunosuppression with mycophenolic acid and corticosteroids. Northwestern University has a stand-alone immunosuppression for SLKT. Their protocol consists of an induction phase with basiliximab (on day 0 and day 2 in addition to solumedrol for all patients) and a maintenance phase with tacrolimus and mycophenolic acid, followed by corticosteroid tapering which remains on 5 mg indefinitely. All other centers have an immunosuppression protocol for SLKT that was similar to the kidney transplantation–alone immunosuppression protocol. Induction with thymoglobulin, basiliximab, and dacluzimab was based on presence of panel reactive antibody and sensitization. The tacrolimus trough levels in all the centers were based on days after SLKT (days 0-90: 8-12 ng/mL; days 91-120: 6-10 ng/mL; and days >120: 4-8 ng/mL).
STATISTICAL APPROACH
We compared continuous variables between groups by Wilcoxon rank sum or Kruskal-Wallis test. We compared categorical variables between groups by the chi square test.
We tested statistical trends over time in 2 ways: (1) utilizing weighted linear regression models to evaluate the outcome (the clinical variable of interest) and the exposure (calendar year as a continuous variable); (2) performing nonparametric tests for trend. For a further description of bivariate relationships between clinical variables of interest and calendar year, we used the locally weighted scatter-plot smoothing (LOWESS) technique.
The primary outcome was early SLKT mortality, defined as death within 1 year of transplantation. The secondary outcome was overall survival, defined as death any time during follow-up. We utilized Cox regression analysis clustered on center to determine the factors associated with the primary and secondary outcomes. Unadjusted models were used to assess the association between covariables and the outcomes of interest. All covariables with a P < 0.2 in univariable analysis were considered for inclusion in multivariable models. Sequential backward selection was used to eliminate those not reaching significance of P < 0.05. Postkidney transplantation survival rates were estimated using the Kaplan-Meier method and compared using the log-rank test.
Significance
Two-sided P values less than 0.05 were considered statistically significant. We performed analyses using Stata 15.0 statistical software (StataCorp., College Station, TX).
Results
POPULATION CHARACTERISTICS
A total of 572 patients underwent SLKT during the study period. These patients had a median age of 57.7 years (interquartile range [IQR], 50.8-63.8) and 211 (37%) were female (Table 1). The most common etiologies of liver disease were hepatitis C virus in 188 (33%) and NASH in 110 (19%) patients. The median (IQR) MELD at listing was 28 (22-35) and the median (IQR) MELD at transplantation was 30 (25-36). Comorbidities were common: 239 (42%) patients had diabetes mellitus, 303 (53%) patients had hypertension, 434 (76%) patients had ascites, and 363 (64%) patients had hepatic encephalopathy.
TABLE 1.
Baseline Demographics of the 572 Patients Undergoing SLKT
| Characteristic | Value |
|---|---|
| Age, years | 57.7 (50.8-63.8) |
| Female sex | 211 (37) |
| Race | |
| Caucasian | 477 (83) |
| African American | 73 (13) |
| Other | 22 (4) |
| BMI, kg/m2 | 27.2 (23.5-32.2) |
| Year of transplantation | |
| 2002-2006 | 164 (29) |
| 2007-2010 | 130 (23) |
| 2011-2014 | 178 (31) |
| 2015-2017 | 100 (17) |
| Indication for SLKT | |
| AKD | 237 (41) |
| CKD | 303 (53) |
| Indeterminate | 32 (6) |
| Days with eGFR <60 mL/minute/1.73 m2 | 204 (60-672) |
| Days with eGFR <25 mL/minute/1.73 m2 | 67 (22-259) |
| Hemodialysis at listing | 224 (39) |
| Days on hemodialysis before SLKT | 13 (0-94) |
| Hepatitis C | 188 (33) |
| EtOH related | 132 (23) |
| NASH | 110 (19) |
| Diabetes mellitus | 239 (42) |
| Hypertension | 303 (53) |
| Coronary artery disease | 116 (20) |
| MELD at SLKT | 30 (25-36) |
| Serum creatinine at SLKT (mg/dL) | 3.0 (2.1-4.6) |
| Ascites | |
| None | 138 (24) |
| Mild | 74 (13) |
| Moderate | 180 (31) |
| Refractory | 180 (31) |
| Hepatic encephalopathy | 363 (63) |
| Hospitalization status | |
| Home | 171 (30) |
| Medical ward | 76 (13) |
| Intensive care unit | 325 (57) |
| Mechanical ventilation | 119 (21) |
| Hemodialysis at SLKT | 212 (37) |
| Cold ischemia time (min) | 360 (300-477) |
| Warm ischemia time (min) | 38 (23-60) |
| Donor distance | |
| Local | 522 (91) |
| Regional | 36 (6) |
| National | 14 (2) |
| Delayed graft function | 131 (23) |
NOTE: The data are expressed as median (IQR) or n (%).
The majority of patients (n = 472, 83%) had kidney dysfunction at the time of listing for liver transplantation. Of the 100 patients without kidney dysfunction at the time of listing for liver transplantation, 97 (97%) developed kidney dysfunction requiring SLKT while awaiting liver transplantation. The duration of kidney dysfunction was ascertainable in 538 (94%) patients. The median (IQR) duration with an eGFR less than 60 mL/minute/1.73 m2 was 204 (60-672) days, with an eGFR less than 25 mL/minute/1.73 m2 was 67 (22-259) days, and the median (IQR) time on hemodialysis was 13 (0-92) days. Of the 572 patients who underwent SLKT in this study, 237 (41%) had AKD, 303 (53%) had CKD, and 32 (6%) had an indeterminate duration of their kidney dysfunction (Table 1). Of the 572 patients who underwent SLKT in this study, a greater of proportion of patients with AKD were on mechanical ventilation at the time of transplantation (AKD: 36%; indeterminate: 13%; CKD: 7%; P < 0.001). Likewise, those with AKD had significantly higher MELD-Na (median [IQR]) scores (AKD: 38 [29-38]; indeterminate: 31 [26-36]; CKD: 29 [23-35]; P < 0.001). Of the 237 patients with AKD, 130 (55%) had a clinical diagnosis of hepatorenal syndrome, 78 (33%) had acute tubular necrosis, and 29 (12%) had an unknown diagnosis. Of these patients with AKD, 224 (39%) were on hemodialysis at the time of SLKT with a median (IQR) duration of 67 days (13-287).
TEMPORAL TRENDS AMONG SLKT RECIPIENTS
During the study period, there were several significant changes in the characteristics of patients undergoing SLKT. The median (IQR) age of SLKT increased from 52.2 (48.0-55.3) years in 2002 to 58.8 (54.1-65.4) years in 2017 (β = 0.24; P = 0.02; test for trend: P = 0.001). The percentage of patients with NASH increased from 17% in 2002 to 29% in 2017 (β = 0.38; P < 0.001; test for trend: P < 0.001). Among those with a diagnosis of CKD, the percentage of patients with NASH increased from 25% in 2003 to 35% in 2016 (β = 0.95; P < 0.001; test for trend: P < 0.001). The percentage of patients with diabetes mellitus increased from 33% in 2002 to 50% in 2017 (β = 0.32; P < 0.001; test for trend <0.001). Importantly, the indication (at the time of SLKT) for SLKT changed significantly during the study period—the percentage of patients listed with CKD increased significantly from 33% in 2003 to 75% in 2017 (β = 1.24; P < 0.001; test for trend: P < 0.001), whereas the percentage of patients with AKD decreased significantly from 28% in 2002 to 21% in 2017 (β = −0.30; P = 0.01; test for trend: P = 0.04). The percentage of patients on hemodialysis at the time of SLKT also increased from 28% in 2002 to 68% in 2017 (β = 2.7; P < 0.001; test for trend: P < 0.001). These trends are demonstrated through locally weighted scatterplots in Fig. 1
FIG. 1.
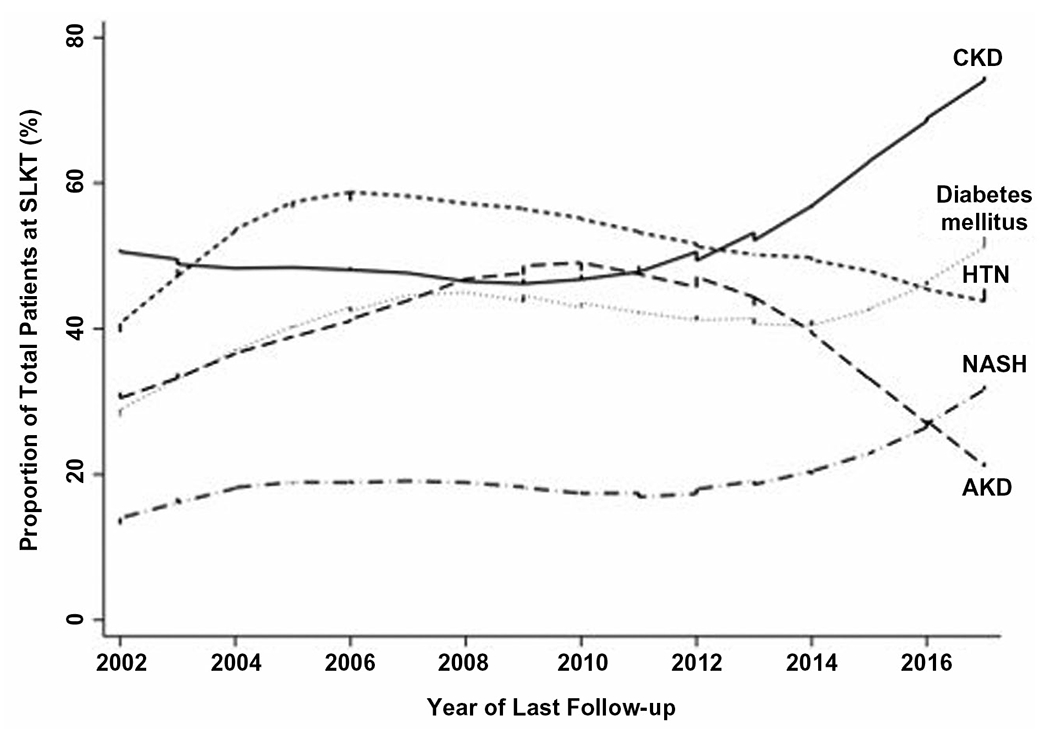
Correlation between proportion of total patients at SLKT and year of transplantation.
OUTCOMES FOLLOWING SLKT
Our cohort had a median follow-up of 5.3 (2.4-9.2) years. Of the 572 patients who underwent SLKT, 188 (33%) post-SLKT deaths were reported. Cumulative post-SLKT survival at 1 and 3 years was 92% (95% confidence interval [CI], 90%-94%) and 87% (95% CI, 84%-90%), respectively (Fig. 2). There was a significant improvement in 1-year mortality during the study period, decreasing from 11% in 2002 to 4% in 2017 (β = −0.85; P < 0.001; test for trend: P < 0.001).
FIG. 2.
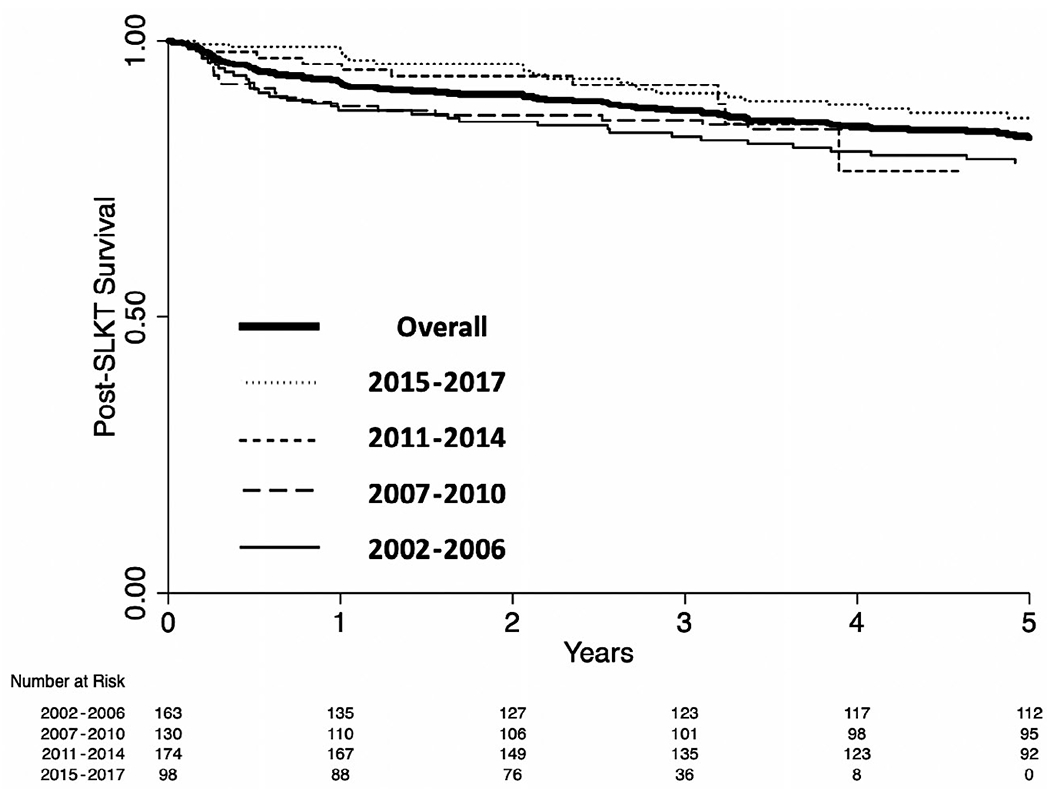
Kaplan-Meier curve for probability of overall post-SLKT mortality.
KIDNEY ALLOGRAFT OUTCOMES
Overall, 131 (31%) episodes of delayed graft function were reported. The proportion of patients with delayed graft function increased significantly during the study period—from 6% in 2002 to 43% in 2017 (β = 1.48; P < 0.001; test for trend: P < 0.001). A total of 120 (21%) SLKT recipients developed stage 4 or 5 CKD at the end of the follow-up period. The cumulative incidence of posttransplantation stage 4 or 5 CKD at 1, 3, 5, and 10 years was 10%, 12%, 16%, and 25%, respectively.
OUTCOMES 1 YEAR AFTER SLKT
In univariable Cox regression, the following factors were significantly associated with post-SLKT mortality: year of SLKT (0.88 per year from 2002; 95% CI, 0.82-0.95), hemodialysis at listing for SLKT (hazard ratio [HR], 1.63; 95% CI, 1.11-2.41), deceased donor (HR, 2.01; 95% CI, 1.15-3.49), national SLKT offer (as compared with local: HR, 6.60; 95% CI, 3.41-12.73), and delayed graft function (HR, 3.26; 95% CI, 1.26-8.46). In the final multivariable model clustered on institution, the following were associated with 1-year post-SLKT mortality: year of SLKT (adjusted HR [aHR], 0.85 per year from 2002; 95% CI, 0.78-0.92), hemodialysis at listing for SLKT (aHR, 1.84; 95% CI, 1.01-3.52), national SLKT offer (as compared with local: aHR, 4.76; 95% CI, 1.78-12.74), and delayed graft function (aHR, 3.14; 95% CI, 1.61-6.12; Table 2).
TABLE 2.
Cox Regression Analysis for 1-Year SLKT Mortality
| Univariable |
Multivariable |
|||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P Value | aHR | 95% CI | P Value | |
| Kidney diagnosis | ||||||
| Unknown | — | — | — | |||
| AKI | 1.31 | 0.30-5.67 | 0.72 | |||
| CKD | 1.35 | 0.32-5.74 | 0.69 | |||
| Age per year | 1.01 | 1.00-1.03 | 0.10 | |||
| Female sex | 1.22 | 0.91-1.63 | 0.18 | |||
| Race | ||||||
| Caucasian | — | — | — | |||
| African American | 0.91 | 0.36-2.33 | 0.85 | |||
| Other | — | — | — | |||
| BMI per 1 kg/m2 | 0.95 | 0.90-1.00 | 0.07 | |||
| Year of SLKT per year | 0.88 | 0.82-0.95 | 0.001 | 0.85 | 0.78-0.92 | <0.001 |
| Hemodialysis at listing | 1.63 | 1.11-2.41 | 0.01 | 1.84 | 1.01-3.52 | 0.04 |
| Hemodialysis duration per day | 1.00 | 1.00-1.00 | 0.36 | |||
| eGFR <60 mL/minute/1.73 m2 per day | 1.00 | 0.99-1.00 | 0.11 | |||
| eGFR <25 mL/minute/1.73 m2 per day | 1.00 | 1.00-1.00 | 0.16 | |||
| Hepatitis C | 1.83 | 0.94-3.57 | 0.08 | |||
| EtOH related | 1.22 | 0.86-1.55 | 0.10 | |||
| NASH | 0.83 | 0.36-1.93 | 0.67 | |||
| Diabetes mellitus | 1.20 | 0.64-2.24 | 0.56 | |||
| Hypertension | 1.11 | 0.83-1.48 | 0.47 | |||
| Coronary artery disease | 0.84 | 0.41-1.72 | 0.63 | |||
| MELD at SLKT per point | 1.03 | 0.99-1.07 | 0.14 | |||
| Serum creatinine at SLKT per 1 mg/dL | 0.93 | 0.79-1.10 | 0.41 | |||
| Ascites | ||||||
| None | — | — | — | |||
| Mild | 0.26 | 0.02-3.58 | 0.31 | |||
| Moderate | 1.41 | 0.50-3.95 | 0.52 | |||
| Refractory | 2.23 | 0.71-7.07 | 0.17 | |||
| Hepatic encephalopathy | 0.71 | 0.40-1.28 | 0.26 | |||
| Hospitalization status | ||||||
| Home | — | — | — | |||
| Medical ward | 1.27 | 0.68-2.37 | 0.45 | |||
| Intensive care unit | 0.87 | 0.40-1.90 | 0.73 | |||
| Mechanical ventilation | 0.20 | 0.05-0.74 | 0.02 | |||
| Hemodialysis at SLKT | 0.77 | 0.56-1.06 | 0.11 | |||
| Cold ischemia time per minute | 1.00 | 1.00-1.00 | 0.57 | |||
| Warm ischemia time per minute | 1.00 | 0.99-1.01 | 0.51 | |||
| Deceased donor | 2.01 | 1.15-3.49 | 0.01 | |||
| Donor distance | ||||||
| Local | — | — | — | — | — | — |
| Regional | 1.28 | 0.44-3.74 | 0.65 | 1.04 | 0.31-3.49 | 0.96 |
| National | 6.60 | 3.41-12.73 | <0.001 | 4.76 | 1.78-12.74 | 0.002 |
| Delayed graft function | 3.26 | 1.26-8.46 | 0.02 | 3.14 | 1.61-6.12 | 0.001 |
OVERALL SURVIVAL
In univariable Cox regression the following factors were significantly associated with post-SLKT mortality: as compared with those with an indeterminate kidney diagnosis, AKI (HR, 2.11; 95% CI, 1.73-2.58) and CKD (HR, 1.64; 95% CI, 1.12-2.41); presence of diabetes mellitus (HR, 1.36; 95% CI, 1.08-1.71); serum creatinine at the time of SLKT (HR, 0.89; 95% CI, 0.83-0.96); utilization of mechanical ventilation (HR, 1.70; 95% CI, 1.14-2.53); hepatic warm ischemia time (HR per minute, 1.01; 95% CI, 1.00-1.01); and national allograft (HR, 2.67, as compared with local offer; 95% CI, 1.52-4.69). In the final multivariable model clustered on institution, the following factors were significantly associated with overall post SLKT mortality: AKD as compared with indeterminate (aHR, 2.26; 95% CI, 1.44-3.54), body mass index (BMI; aHR, 1.00 per 1 kg/m2; 95% CI, 1.00-1.01), serum creatinine at SLKT (aHR, 0.90 per 1 mg/dL; 95% CI, 0.82-0.98), mechanical ventilation (aHR, 1.95; 95% CI, 1.59-2.38), hepatic warm ischemia time (aHR, 1.01 per minute; 95% CI, 1.00-1.01), national liver-kidney offer (as compared with local: aHR, 3.48; 95% CI, 2.09-5.78; Table 3).
TABLE 3.
Cox Regression Analysis for Overall SLKT Mortality
| Univariable |
Multivariable |
|||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P Value | aHR | 95% CI | P Value | |
| Kidney diagnosis | ||||||
| Unknown | — | — | — | — | — | — |
| AKI | 2.11 | 1.73-2.58 | <0.001 | 2.26 | 1.44-3.54 | <0.001 |
| CKD | 1.64 | 1.12-2.41 | 0.01 | 1.63 | 0.97-2.74 | 0.07 |
| Age per year | 1.01 | 1.00-1.03 | 0.10 | |||
| Female sex | 1.05 | 0.81-1.37 | 0.71 | |||
| Race | ||||||
| Caucasian | — | — | — | |||
| African American | 1.32 | 0.88-1.98 | 0.17 | |||
| Other | 0.61 | 0.25-1.50 | 0.28 | |||
| BMI per 1 kg/m2 | 1.00 | 1.00-1.00 | 0.16 | 1.00 | 1.00-1.01 | <0.001 |
| Year of admission | ||||||
| 2002-2006 | — | — | — | |||
| 2007-2010 | 1.42 | 1.01-1.98 | 0.04 | |||
| 2011-2014 | 1.48 | 0.44-4.95 | 0.53 | |||
| 2015-2017 | 1.06 | 0.33-3.42 | 0.92 | |||
| Hemodialysis at listing | 1.09 | 0.80-1.47 | 0.59 | |||
| Hemodialysis duration per day | 1.00 | 1.00-1.00 | 0.52 | |||
| eGFR <60 mL/minute/1.73 m2 per day | 1.00 | 0.99-1.00 | 0.40 | |||
| eGFR <25 mL/minute/1.73 m2 per day | 1.00 | 1.00-1.00 | 0.27 | |||
| Hepatitis C | 1.19 | 0.82-1.74 | 0.37 | |||
| EtOH related | 1.06 | 0.80-1.41 | 0.70 | |||
| NASH | 1.27 | 0.88-1.83 | 0.20 | |||
| Diabetes mellitus | 1.36 | 1.08-1.71 | 0.008 | |||
| Hypertension | 1.32 | 0.98-1.76 | 0.07 | |||
| Coronary artery disease | 1.33 | 0.97-1.83 | 0.08 | |||
| MELD at SLKT per point | 1.00 | 0.99-1.02 | 0.45 | |||
| Serum creatinine at SLKT per 1 mg/dL | 0.89 | 0.83-0.96 | 0.002 | 0.90 | 0.82-0.98 | 0.02 |
| Ascites | ||||||
| None | — | — | — | |||
| Mild | 0.73 | 0.49-1.10 | 0.13 | |||
| Moderate | 1.01 | 0.73-1.41 | 0.93 | |||
| Refractory | 1.17 | 0.82-1.66 | 0.39 | |||
| Hepatic encephalopathy | 1.10 | 0.87-1.39 | 0.41 | |||
| Hospitalization status | ||||||
| Home | — | — | — | |||
| Medical ward | 1.20 | 0.70-2.06 | 0.51 | |||
| Intensive care unit | 1.31 | 0.84-2.03 | 0.23 | |||
| Mechanical ventilation | 1.70 | 1.14-2.53 | 0.009 | 1.95 | 1.59-2.38 | <0.001 |
| Hemodialysis at SLKT | 0.77 | 0.56-1.06 | 0.11 | |||
| Cold ischemia time per minute | 1.00 | 1.00-1.00 | 0.37 | |||
| Warm ischemia time per minute | 1.01 | 1.00-1.01 | 0.02 | 1.01 | 1.00-1.01 | 0.01 |
| Donor distance | ||||||
| Local | — | — | — | — | — | — |
| Regional | 0.96 | 0.67-1.39 | 0.85 | 1.39 | 1.01-1.91 | 0.04 |
| National | 2.67 | 1.52-4.69 | 0.001 | 3.48 | 2.09-5.78 | <0.001 |
| Delayed graft function | 1.22 | 0.81-1.82 | 0.34 | |||
Discussion
The US Multicenter SLKT consortium includes 6 LT programs in 6 UNOS regions, providing a comprehensive assessment of SLKT in the United States. Prior studies either utilized the national database, without the necessary granularity, or were single center, limiting the generalizability of their findings. Our efforts included a characterization of the etiology and duration of kidney dysfunction before SLKT, a description of common comorbidities (eg, hypertension, diabetes mellitus), and an ascertainment of key kidney-related outcomes (eg, DGF). Herein, we more fully characterize the clinical characteristics and determinants of outcomes among 572 SLKT recipients.
We highlight that SLKT is a life-saving procedure with 1 and 3-year survival of 92% and 87%, respectively. We build on these findings to demonstrate that the demographics of SLKT are changing—SLKT recipients are getting older, with a higher likelihood of NASH and its associated comorbidities (ie, diabetes mellitus, coronary artery disease, and hypertension). The indication for SLKT is evolving with an increasing burden of CKD, as opposed to AKD, and the degree of kidney dysfunction at the time of SLKT is worsening, with a higher utilization of hemodialysis; therefore, special attention to these trends is needed as the criteria for SLKT continue to evolve. Despite these trends, we demonstrate that early (ie, 1-year) survival after SLKT is improving with a greater than 50% (11% in 2002 to 4% in 2017) reduction in 1-year mortality in recent years.
Next, to better understand why clinical outcomes are improving despite a higher prevalence of comorbid conditions among SLKT recipients, we determined the drivers of both short- and long-term mortality. Within the first year, we found that these included the year of transplantation, utilization of hemodialysis at listing for SLKT, the type of SLKT offer, and delayed graft function. These findings highlight that recipient clinical acuity (ie, hemodialysis status) and allograft quality are the main drivers of early post-SLKT mortality and, importantly, there have been significant improvements in early outcomes over the study period.
The factors identified to be associated with overall survival included AKD as opposed to indeterminant kidney dysfunction, serum creatinine and mechanical ventilation at the time of SLKT, hepatic warm ischemia time, national SLKT offer, BMI, and hypertension. For overall survival, the drivers not only include recipient clinical acuity (eg, AKD, mechanical ventilation) and allograft quality, but also expand to include causes of long-term posttransplantation complications—specifically, hypertension and BMI. We hypothesize that AKD, as opposed to indeterminant kidney dysfunction, may be associated with decreased survival, as these patients were more likely to have increased rates of multiorgan failure (eg, need for mechanical ventilation) and higher MELD-Na scores at transplantation. Collectively, these analyses highlight opportunities to optimize both early and late post-SLKT outcomes.
Our study has limitations. First, it is retrospective, and data are limited to those collected by the sites. The lack of missing data suggests clear effective documentation, but some variables, such as type of kidney dysfunction at the time of SLKT, were affected. Although the trends and associations described align with previously published data,(1,21) the increased granularity of our data, particularly with regard to variables not captured in national registries, highlights the value of multicenter studies structured to advance our understanding of SLKT. Second, the possibility that unmeasured center-related behaviors may be driving our findings remains. However, by completing all analyses clustered on center, we should be measuring the effects of captured variables on our outcomes of interest, limiting any residual confounding of center related practices. Third, we are unable to determine whether improvements in GFR, particularly in those with AKD and those with DGF, were driven by the transplanted kidney or improved function of the native kidneys. Finally, this study did not include those with kidney dysfunction who were considered for SLKT but underwent liver transplantation alone or died on the transplantation waiting list. The clinical decision to undergo liver alone or SLKT is complex, and prospective, protocolled studies are needed—we believe our multicenter, collaborative approach provides the framework to address this in the future. Despite these limitations, our study provides a comprehensive assessment of SLKT throughout the United States.
In conclusion, the characteristics of SLKT recipients have changed over time. Despite changing demographics, increasing age, comorbidities, and kidney DGF, survival has improved among SLKT recipients. Importantly, our evaluation of the evolving trends and the drivers of early and overall survival provide insights into the factors that require the most attention to refine current SLKT criteria and to optimize post-SLKT outcomes.
Acknowledgments
Lisa B. VanWagner is supported by a National Heart, Lung, and Blood Institute grant (K23 HL136891).
Lisa B. VanWagner received grants from W. L. Gore & Associates. Yuval A. Patel consults for Intercept.
Abbreviations:
- aHR
adjusted hazard ratio
- AKD
acute kidney dysfunction
- AKI
acute kidney injury
- BMI
body mass index
- CI
confidence interval
- CKD
chronic kidney disease
- DGF
delayed kidney allograft function
- eGFR
estimated glomerular filtration rate
- EtOH
ethyl alcohol
- HCC
hepatocellular carcinoma
- HR
hazard ratio
- HTN
hypertension
- IQR
interquartile range
- LOWESS
locally weighted scatter-plot smoothing
- MDRD4
Modification of Diet in Renal Disease 4-item version
- MELD
Model for End-Stage Liver Disease
- MELD-Na
Model for End-Stage Liver Disease–sodium
- NASH
nonalcoholic steatohepatitis
- SLKT
simultaneous liver-kidney transplantation
- UNOS
United Network for Organ Sharing
REFERENCES
- 1).Cullaro G, Verna EC, Lee BP, Lai JC. Chronic kidney disease in liver transplant candidates: a rising burden impacting post-liver transplant outcomes. Liver Transpl 2020;26:498–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2).Sharma P, Welch K, Eikstadt R, Marrero JA, Fontana RJ, Lok AS. Renal outcomes after liver transplantation in the model for end-stage liver disease era. Liver Transpl 2009;15:1142–1148. [DOI] [PubMed] [Google Scholar]
- 3).Formica RN, Aeder M, Boyle G, Kucheryavaya A, Stewart D, Hirose R, et al. Simultaneous liver-kidney allocation policy: a proposal to optimize appropriate utilization of scarce resources. Am J Transplant 2016;16:758–766. [DOI] [PubMed] [Google Scholar]
- 4).Sharma P, Schaubel DE, Guidinger MK, Goodrich NP, Ojo AO, Merion RM. Impact of MELD-based allocation on end-stage renal disease after liver transplantation. Am J Transplant 2011;11:2372–2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5).Northup PG, Argo CK, Bakhru MR, Schmitt TM, Berg CL, Rosner MH. Pretransplant predictors of recovery of renal function after liver transplantation. Liver Transpl 2010;16:440–446. [DOI] [PubMed] [Google Scholar]
- 6).Sharma P, Goodrich NP, Zhang M, Guidinger MK, Schaubel DE, Merion RM. Short-term pretransplant renal replacement therapy and renal nonrecovery after liver transplantation alone. Clin J Am Soc Nephrol 2013;8:1135–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7).Allen AM, Kim WR, Therneau TM, Larson JJ, Heimbach JK, Rule AD. Chronic kidney disease and associated mortality after liver transplantation—a time-dependent analysis using measured glomerular filtration rate. J Hepatol 2014;61:286–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8).Locke JE, Warren DS, Singer AL, Segev DL, Simpkins CE, Maley WR, et al. Declining outcomes in simultaneous liver-kidney transplantation in the MELD era: ineffective usage of renal allografts. Transplantation 2008;85:935–942. [DOI] [PubMed] [Google Scholar]
- 9).Fong T-L, Khemichian S, Shah T, Hutchinson IV, Cho YW. Combined liver-kidney transplantation is preferable to liver transplant alone for cirrhotic patients with renal failure. Transplant J 2012;94:411–416. [DOI] [PubMed] [Google Scholar]
- 10).Cullaro G, Hirose R, Lai JC. Changes in simultaneous liver kidney transplant allocation policy may impact post liver transplant outcomes. Transplantation 2019;103:959–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11).Sharma P, Shu X, Schaubel DE, Sung RS, Magee JC. Propensity score-based survival benefit of simultaneous liver-kidney transplant over liver transplant alone for recipients with pretransplant renal dysfunction. Liver Transpl 2016;22:71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12).Levitsky J, Baker T, Ahya SN, Levin ML, Friedewald J, Gallon L, et al. Outcomes and native renal recovery following simultaneous liver-kidney transplantation. Am J Transplant 2012; 12:2949–2957. [DOI] [PubMed] [Google Scholar]
- 13).Lunsford KE, Bodzin AS, Markovic D, Zarrinpar A, Kaldas FM, Gritsch HA, et al. Avoiding futility in simultaneous liver-kidney transplantation: analysis of 331 consecutive patients listed for dual organ replacement. Ann Surg 2017;265:1016–1024. [DOI] [PubMed] [Google Scholar]
- 14).Levitsky J, O’Leary JG, Asrani S, Sharma P, Fung J, Wiseman A, et al. Protecting the kidney in liver transplant recipients: practice-based recommendations from the American Society of Transplantation Liver and Intestine Community of Practice. Am J Transplant 2016;16:2532–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15).Sharma P, Sui Z, Zhang M, Magee JC, Barman P, Patel Y, et al. Renal outcomes after simultaneous Liver-Kidney Transplantation: results from the US multicenter Simultaneous Liver-Kidney Transplantation Consortium. Liver Transpl 2021;27:1144–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16).Asrani SK, Nadim MK. Chronic kidney disease after simultaneous liver-kidney transplantation: refining patient selection. Liver Transpl 2021;27:1092–1094. [DOI] [PubMed] [Google Scholar]
- 17).Kim WR, Biggins SW, Kremers WK, Wiesner RH, Kamath PS, Benson JT, et al. Hyponatremia and mortality among patients on the liver-transplant waiting list. N Engl J Med 2008;359:1018–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18).Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med 1999;130:461–470. [DOI] [PubMed] [Google Scholar]
- 19).Levey AS, Becker C, Inker LA. Glomerular filtration rate and albuminuria for detection and staging of acute and chronic kidney disease in adults: a systematic review. JAMA 2015;24:837–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20).Siedlecki A, Irish W, Brennan DC. Delayed graft function in the kidney transplant. Am J Transplant 2011;11:2279–2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21).Charlton MR, Burns JM, Pedersen RA, Watt KD, Heimbach JK, Dierkhising RA. Frequency and outcomes of liver transplantation for nonalcoholic steatohepatitis in the United States. Gastroenterology 2011;141:1249–1253. [DOI] [PubMed] [Google Scholar]


