Abstract
Aims:
This study was designed to assess links between lifetime levels of marijuana use and accelerated epigenetic aging.
Design:
Prospective longitudinal study, following participants annually from age 13 to age 30.
Setting and Participants:
A community sample of 154 participants recruited from a small city in the Southeastern United States.
Measurements:
Participants completed annual assessments of marijuana use from age 13 to age 29 and provided blood samples that yielded two indices of epigenetic aging (DNAmGrimAge and DunedinPoAm) at age 30. Additional covariates examined included history of cigarette smoking, anxiety and depressive symptoms, childhood illness, gender, adolescent-era family income, and racial/ethnic minority status.
Findings:
Lifetime marijuana use predicted accelerated epigenetic aging, with effects remaining even after covarying cell counts, demographic factors and chronological age (β’s = .32 & .27, p’s < .001, 95% CI’s = .21–.43 & .16–.39 for DNAmGrimAge and DunedinPoAm, respectively). Predictions remained after accounting for cigarette smoking (β’s = .25 & .21, respectively, p’s < .001, 95% CI’s =.14 – .37 & .09 – .32 for DNAmGrimAge and DunedinPoAm, respectively). A dose-response effect was observed and there was also evidence that effects were dependent upon recency of use. Effects of marijuana use appeared to be fully mediated by hypomethylation of a site linked to effects of hydrocarbon inhalation (cg05575921).
Conclusions:
Marijuana use predicted epigenetic changes linked to accelerated aging, with evidence suggesting that effects may be primarily due to hydrocarbon inhalation among marijuana smokers. Further research is warranted to explore mechanisms underlying this linkage.
Over the past fifteen years, debates around the status of marijuana (Cannabis sativa) have increased significantly in the United States and elsewhere as the movement to legalize its possession and use has gained steam (Caulkins, Kilmer, & Kleiman, 2016). As marijuana use becomes legal in more places and its use becomes more common (SAMSHA, 2020), increasing our understanding of its potential long-term physical effects becomes critical. Evidence to date regarding negative physical health effects of marijuana use has been inconsistent. Studies have implicated marijuana use in a range of physical and mental impairments, including bronchitis, emphysema, immune function, oral health problems, depression, and psychosis (Benson & Bentley, 1995; Cho, Hirsch, & Johnstone, 2005; Han et al., 2018; Owen, Sutter, & Albertson, 2014; Pletcher et al., 2012). Yet, several long-term studies have found no links to overall cardiovascular or mortality risk (Reis et al., 2017; Sidney, Beck, Tekawa, Quesenberry, & Friedman, 1997).
An important limitation of many of these studies has been their reliance upon retrospective reports of marijuana use. Yet, one of the few studies to utilize prospective data on marijuana use over a long period of time also found no significant links of marijuana use to physical health problems through the mid-thirties (Bechtold, Simpson, White, & Pardini, 2015). The study authors noted, however, that in mid-adulthood the low base rates of many negative health outcome markers limited the power of the study to detect effects. This low base rate problem highlights one of the most important limitations of much of marijuana outcome research: The long latency to onset of many health problems makes any prospective study of the health effects of marijuana use highly challenging.
The development of epigenetic algorithms to track overall biological aging processes, however, has the potential to begin to address these limits (Lu et al., 2019). Recent advances now make it possible to assess DNA methylation markers of physical deterioration that can be tracked well prior to the onset of any actual disease. Early epigenetic aging algorithms—yielding measures of ‗epigenetic age’ based on patterns of DNA methylation within the epigenome—have been found to yield estimates that correlate strongly with chronological age and add value in predicting future mortality (Horvath, 2013). By covarying out actual chronological age, these measures produce an indicator of epigenetic age acceleration—the degree to which an individual’s epigenome suggests that they have aged faster (or slower) than their chronological age would indicate.
Further research, however, found that these early measures were often driven more by naturally unfolding biological processes than by external behavioral or environmental factors (Palma-Gudiel, Fananas, Horvath, & Zannas, 2020). This in turn led to the development of second and third generation ‗epigenetic clocks’ that were specifically designed both to correlate with actual levels of physiological deterioration and also to be sensitive to environmental factors. One such clock, DNAmGrimAge, was designed to capture aspects of epigenetic aging that were most strongly correlated with actual indicators of physiological deterioration, and has been linked to a broad range of health indicators (Lu et al., 2019). A second clock, DunedinPoAm (Dunedin Pace of Aging methylation), was designed to capture the pace at which aging is currently occurring by identifying changes in the epigenome that predicted change in health-linked biomarkers over a seven-year period in mid adulthood (Belsky et al., 2020). This measure has been found to strongly predict future health outcomes in samples other than the norming sample, and even to demonstrate sensitivity to effects of behavioral interventions designed to slow the aging process (Belsky et al., 2020).
There are multiple reasons to hypothesize links from marijuana use to epigenetic age acceleration. The broad range of connections between marijuana use and the markers of physical and mental impairment cited above certainly make a potential connection to broader aging processes quite plausible. In addition, marijuana use has already been linked to a range of changes in the epigenome (Markunas et al., 2020; Szutorisz & Hurd, 2016). Although the changes observed thus far are not necessarily reflections of aging or biological deterioration, they do indicate the potential of marijuana use to impact the epigenome. Finally, marijuana use has also been linked to vascular aging, which is a significant correlate of biological aging (Reece, Norman, & Hulse, 2016). To date, however, no research has directly examined links of marijuana use to epigenetic markers of the biological aging process.
Assessing the overall link of lifetime marijuana use to epigenetic age acceleration is not, of course, sufficient to establish a causal relation of use to aging. Assessment of several corollary issues can, however, begin to rule out potential non-causal explanations of such a link. For example, one concern in assessing marijuana use as a predictor of epigenetic aging is the possibility that observation of the effects of marijuana use will be confounded by cigarette smoking behavior, which has been strongly related to both epigenetic and biological aging and which has also been found to be correlated with marijuana use (Hindocha et al., 2015; Joehanes et al., 2016; Norman et al., 2012). Likewise, a range of additional factors have at least the potential to confound observed links between marijuana use and aging, including gender, family income, education level, personality traits, hard drug use, childhood physical illness, and lifetime histories of anxiety and depressive symptoms. All of these potential confounds were considered in this study.
To the extent marijuana use is linked to epigenetic aging, several questions about the nature of this link also warrant consideration. First, the presence of a causal link would imply the likely presence of a dose-response relationship between the level of marijuana use and aging when looking just within the population of marijuana users. A related question regards the potential impact of the timing of any observed links. A substantial number of early marijuana users later desist from use (Bechtold et al., 2015; Brook, Zhang, & Brook, 2011), and cessation of marijuana smoking by marijuana-only smokers has been associated with resolution of preexisting symptoms of chronic bronchitis (Tashkin, Simmons, & Tseng, 2012). Similarly, cessation of cigarette smoking has been linked to many physiological benefits (Cho et al., 2005). This suggests the possibility that early marijuana use that later decreases or ceases may be less impactful than continued or more recent use. Whether and how the distribution and timing of marijuana use across the lifetime is linked to broader markers of epigenetic aging has never been examined, however.
A final important question is as to the potential mechanisms underlying any observed links between marijuana use and aging. Given that many of the potential deleterious effects of marijuana use are pulmonary in nature (Moore, Augustson, Moser, & Budney, 2005; Tashkin, 2013), it is reasonable to ask whether effects of use potentially reflect the inhalation of a smoked substance (vs. the ingestion of THC, the active ingredient in marijuana). Marijuana smoke is typically inhaled deeply and contains high levels of polycyclic aromatic hydrocarbon procarcinogens (e.g., benzopyrene and benzanthracene) even relative to cigarettes (Sidney et al., 1997). One epigenetic site (cg05575921), located within a putative enhancer of the aryl hydrocarbon receptor repressor (AHRR) gene, has particular promise for exploring potential epigenetic effects of marijuana use. Hypomethylation at this site has been robustly linked to smoked (but not non-smoked) tobacco use (Andersen et al., 2021; Andersen, Philibert, Gibbons, Simons, & Long, 2017). Further, although the link to tobacco use has been strong enough that hypomethylation of this site has been proposed as a proxy for tobacco use (Andersen et al., 2021), more recent research indicates that it is actually quite sensitive to inhalation of fine particulate matter (PM2.5) even among non-smokers (Tantoh et al., 2019). Additionally, cg05575921 methylation is significantly associated with AHRR gene expression in monocytes and mediates the effect of cigarette smoking increasing AHRR expression, indicating a functional role for this site in mediating the biological effects of smoking (Reynolds et al., 2015). This site thus provides an excellent avenue for testing the proposition that links of marijuana use to epigenetic aging may be mediated via epigenetic effects of smoke inhalation.
To address each of these issues, this seventeen-year, multi-method, prospective study utilized a diverse community sample to examine links between lifetime average marijuana use from 13 to 29, and two measures of epigenetic age acceleration assessed at age 30. The study considered the following specific hypotheses:
Lifetime marijuana use will be linked to epigenetic age acceleration and this link will exist over and above history of cigarette use.
There will be evidence of a dose-response relationship within the group of marijuana users from levels of lifetime use to epigenetic age acceleration.
Recent marijuana use will be more strongly linked to epigenetic age acceleration than use at earlier periods.
Links of marijuana use to epigenetic aging will be at least partially mediated via hypomethylation at site cg05575921, as an indication that effects may be in part due to effects of smoke inhalation when ingesting marijuana.
Links to epigenetic aging will exist over and above a range of potential behavioral and personality confounders, including education level, personality traits and history of use of harder drugs, childhood physical illness, anxiety, and depressive symptoms.
Note that because these hypotheses were not envisioned at the outset of the study, they were not pre-registered and results should be considered exploratory.
Methods
Participants
This report is drawn from a larger longitudinal investigation of the long-term outcomes of adolescent social development in familial and peer contexts. The final sample of participants for analyses included 154 participants of 184 originally assessed at age 13 and for whom epigenetic data was obtained at age 30 (M = 29.70, SD = 2.16; 84% retention). Adolescents were recruited from the 7th and 8th grades of a public middle school drawing from suburban and urban populations in the Southeastern United States. The final sample was racially/ethnically and socioeconomically diverse as seen in Table 1. Prior to turning 18, participants provided informed assent before each interview session, and parents provided informed consent for their adolescents. After age 18, all participants provided informed consent for each interview session.
Table 1.
Means and Standard Deviations of Primary Measures and Demographic Variables
| Mean | s.d. | |
|---|---|---|
| Naïve CD8+ T cells | 285.8 | 44.3 |
| CD8+ CD28- CD45 RA- T cells | 3.28 | 3.13 |
| Plasmablasts | 1.94 | 0.191 |
| Naïve CD4+ T cells | 770.3 | 95.9 |
| Natural Killer cells | 0.194 | 0.032 |
| Monocytes | 0.058 | 0.269 |
| Granulocytes | 0.634 | 0.104 |
| Chronological Age | 29.7 | 2.176 |
| DNAmGrimAge | 37.6 | 4.95 |
| DunedinPoAm | 1.00 | 0.08 |
| Lifetime Cigarette Use | 2.49 | 4.17 |
| Average Days/Month Lifetime Marijuana Use | 2.68 | 4.25 |
| Average Marijuana Use Last 4 Years | 3.39 | 6.51 |
| Average Marijuana Use 5 – 8 Years Prior | 3.42 | 6.16 |
| Average Marijuana Use 9–12 Years Prior | 3.35 | 5.82 |
| Family Income in Adolescence | $45,400 | $24,400 |
| % | % | |
| Participant Gender | ||
| Male | 67 | 44% |
| Female | 87 | 56% |
| Race/Ethnicity | ||
| White | 86 | 56% |
| Black/African-American | 48 | 31% |
| Asian | 2 | 1% |
| Hispanic | 1 | <1% |
| American Indian | 1 | <1% |
| Mixed race or other groups | 14 | 9% |
Confidentiality was assured to all study participants and adolescents were told that their parents would not be informed of answers they provided. Data were protected by a Confidentiality Certificate issued by the U.S. Department of Health and Human Services.
Measures
Epigenetic Age (Age 30). Eight and a half milliliters of whole blood were drawn into a PAXgene Blood DNA Tube (PreAnalytiX, Hombrechtikon, Switzerland). Samples were stored at −20°C for short-term storage (up to 3 months) then transferred to −80°C for long-term storage. DNA was extracted using the PAXgene Blood DNA kit (PreAnalytiX, Hombrechtikon, Switzerland) according to manufacturer instructions. DNA concentration was determined by Quant-iT™ PicoGreen® dsDNA reagent (Thermofisher Scientific, Waltham, MA, USA) per manufacturer’s instruction. Fluorescence was detected using a Tecan Infinite M200 Pro microplate reader (Tecan, Switzerland). 500 ng of DNA was bisulfite treated using a Zymo EZ DNA Methylation kit (Zymo Research, Irvine, CA) using PCR conditions for Illumina’s Infinium Methylation assay. Four uL of bisulfite converted DNA was used to measure DNA methylation using the Illumina Infinium MethylationEPIC BeadChips according to manufacturer’s protocol. Preprocessing and quality control were performed using previously described methods (Abdulrahim et al., 2019). All samples passed Illumina quality controls.
Unnormalized betas were filtered to include CpGs specified by Horvath as necessary for calculation of various clocks. The betas were uploaded to Horvath’s online DNA methylation age calculator (htpps://dnamage.genetics.ucla.edu), which provides measures of Horvath’s multi-tissue age estimator (Horvath, 2013), DNA methylation GrimAge (Lu et al., 2019), and cell type abundance. A sample annotation file was included. The options to normalize data and apply advanced analysis were selected. Dunedin Pace of Aging Methylation (Belsky et al., 2020) was calculated in R statistical software using code available from https://github.com/danbelsky/DunedinPoAm38.
History of Marijuana Use (Annual Assessments, ages 13 – 29). Marijuana use was assessed annually using the Monitoring the Future surveys (Johnston, O’Malley, & Bachman, 1987). Participants indicated the number of times in the past 30 days that they had smoked marijuana on a scale ranging from “none” to “1–2 times” to “3–5 times” to “6–9 times” to “10 or more”. These were recoded as average monthly times of use as 0, 1.5, 4, 7.5 and 20, respectively. Scores were averaged across years to obtain a measure of lifetime use. In addition, scores were also averaged across consecutive four-year periods, to obtain measures of use in the most recent four years, use 5 to 8 years prior to the epigenetic assessment, and use 9 to 12 years prior to the epigenetic assessment.
Lifetime History of Cigarette Smoking (Multiple waves, ages 13 – 30). Level of smoking was assessed annually each year from age 13 to 18 as packs smoked per day. At age 30, data were also obtained regarding year at which smoking began, year at which smoking ceased (if applicable) and average amount smoked. These two types of data were then harmonized; where data were inconsistent, the higher level of reported smoking was used. The final score is calculated as the product of the number of years smoked X the average number of packs smoked each year.
Lifetime History of Alcohol Use (Ages 13–29) was assessed in the same format as marijuana use, above.
Lifetime History of Hard Drug Use (Ages 13–29) was also assessed in the same format as marijuana use, above, regarding any use of hallucinogens, tranquilizers, barbiturates, heroin, amphetamines, inhalants, cocaine and any other illicit drugs.
Highest Education Level (Age 30). Education level was reported on a scale ranging from 1 - Eighth grade or less, to 8 – Post-college degree.
History of Serious Illness Prior to Age 18. During the adult phase of the study, participants reported on presence vs. absence of 43 distinct significant health problems first experienced prior to age 18 and which lead at some point to a hospitalization. The total number of such health problems was used as a marker of potential pre-existing health difficulties (Allen, Uchino, & Hafen, 2015).
Lifetime History of Depressive Symptoms (Ages 13–29). Depressive symptoms were assessed annually at ages 13 to 17 using the Child Depression Inventory (CDI) (Kovacs & Beck, 1977) and from 18 to 29 using the Beck Depression Inventory. Internal consistency for both measures was good (Cronbach’s α’s ranged from .84 to .90). Scores were standardized and averaged to yield a measure of lifetime depressive symptoms.
Lifetime History of Anxiety Symptoms (Age 13–29). Anxiety was assessed annually at ages 15–17 using the Beck Anxiety Inventory (BAI), and from 18–29 anxiety was assessed with the trait anxiety scale from the State-Trait Anxiety Inventory (Spielberger, Sydeman, Owen, & Marsh, 1999). Internal consistency was strong across both measures (Cronbach’s α’s = .89 and .94). Scores were standardized and averaged to yield a measure of lifetime anxiety symptoms.
Big Five Personality Traits (Ages 24, 27, 30). Personality traits were assessed with the International Personality Item Pool (Goldberg et al., 2006), tapping constructs of extraversion, agreeableness, conscientiousness, emotional stability, and imagination. Internal consistency for the scales ranged from Cronbach’s α = .74 to .89. Scores were averaged across assessment periods.
Statistical Analyses
Attrition analyses revealed no differences on any baseline measures between the 154 participants in the final sample and the 30 excluded because they lacked epigenetic data. In terms of missing data for primary measures, no participant was missing lifetime marijuana or cigarette use data. On average participants had available marijuana use data for 87% of the annual assessment points that went into calculating lifetime use. Between one and three participants were missing data for the various 4-year interval marijuana use assessments; these were handled using full information maximum likelihood techniques as described below.
For primary analyses, SAS PROC CALIS (version 9.4, SAS Institute, Cary, NC) was employed using full information maximum likelihood handling of missing data. Analyses began by regressing the epigenetic aging measure on blood cell counts, followed by chronological age, and participant demographic characteristics. Blood cell counts were estimated using the Horvath method for naïve CD8+ T cells, CD8+ CD28- CD45RA- T cells, plasmablasts (B cells) and naïve CD4+ T cells (Horvath & Levine, 2015). The Houseman method was used to estimate natural killer cells, monocytes, and granulocytes (Houseman et al., 2012). By examining predictors of epigenetic age after accounting for blood cell counts and chronological age, the result is a prediction of epigenetic age acceleration (e.g., the extent to which a participant has epigenetically aged faster than their chronological age would suggest).
Results
Preliminary Analyses
Means and standard deviations for all primary variables used in the study are presented in Table 1. Marijuana use and epigenetic aging scores were examined for distributional properties and skewness and kurtosis and were both within acceptable levels (i.e., less than 2). DNAmGrimAge and DunedinPoAm were correlated at r = .72; both measures were correlated with methylation at site cg05575921 (r’s = −.75 and −.65 for DNAmGrimage and DunedinPoAm, respectively; all p’s < .001).
Primary Analyses
Hypothesis 1: Lifetime marijuana use will be linked to epigenetic age acceleration and this link will exist over and above history of cigarette use.
Analyses first examined the effect of lifetime marijuana use. As shown in the first two columns of results in Table 2, there was a highly significant effect of marijuana use in predicting accelerated epigenetic aging, whether this was assessed via the DNAmGrimAge or the DunedinPoAm measure. As shown in the second set of columns in Table 2, although cigarette smoking was a significant predictor of accelerated epigenetic aging, even after accounting for this effect, lifetime marijuana use remained a significant predictor of both measures of epigenetic age acceleration.
Table 2.
Predicting Epigenetic Age Acceleration from Marijuana Use (with and without considering lifetime cigarette smoking history)
| Epigenetic Age Effects without covarying smoking |
Epigenetic Age Effects covarying smoking |
|||
|---|---|---|---|---|
| DNAmGrimAge | DunedinPoAm | DNAmGrimAge | DunedinPoAm | |
|
| ||||
| β | β | β | β | |
| Blood Cell Counts | ||||
| Naïve CD8+ T cells | −.35*** | −.33*** | −.28*** | −.26*** |
| CD8+ CD28-CD45 RA- T cells | .14 | .20** | .09 | .15* |
| Plasmablasts | −.12 | .20 | −.098 | .23* |
| Naïve CD4+ T cells | .26** | .30*** | .20 | .24** |
| Natural Killer cells | −.06 | .00 | −.04 | .02 |
| Monocytes | −.00 | −.10 | −.02 | −.07 |
| Granulocytes | .28* | .17 | .26* | .16 |
| Chronological Age | .41*** | .07 | .36*** | .03 |
| Demographic Characteristics | ||||
| Gender (Male = 1, Female = 2) | −.07 | .13* | .14* | |
| Racial/Ethnic Minority MembershipS | .30*** | .42*** | .31*** | .42*** |
| Family of Origin Income | −.11 | −.12 | −.06 −.09 |
−.11 |
| Lifetime Cigarette Use | -- | -- | .23*** | .22*** |
| Lifetime Marijuana Use | .32*** | .27*** | .25*** | .21*** |
Note.
p < .001.
p < .01.
p < .05.
β weights are from the full model.
Hypothesis 2: There will be evidence of a dose-response relationship within the group of marijuana users to epigenetic age acceleration.
Analyses next examined whether levels of marijuana use would predict epigenetic aging if assessed just within the group of individuals who had ever used marijuana. Table 3 presents results for predictions to epigenetic outcomes, while also accounting for cigarette smoking, among the 113 individuals who reported ever having smoked marijuana. Results indicate that within this group, a strong link from amount of marijuana use to both measures of epigenetic age acceleration was observed.
Table 3.
Dose Effects Predicting Epigenetic Age Acceleration from Marijuana Use among users only
| Epigenetic Age Measure | ||
|---|---|---|
| DNAmGrimAge | DunedinPoAm | |
| β | β | |
| Blood Cell Counts | ||
| Naïve CD8+ T cells | −.20* | −.14 |
| CD8+ CD28- CD45 RA- T cells | .14 | .23** |
| Plasmablasts | −.11 | .20* |
| Naïve CD4+ T cells | .18* | .14 |
| Natural Killer cells | .02 | .05 |
| Monocytes | −.03 | −.14* |
| Granulocytes | .33** | .15 |
| Chronological Age | .39*** | .03 |
| Demographic Characteristics | ||
| Gender (Male = 1, Female = 2) | −.06 | .16* |
| Racial/Ethnic Minority Membership | .32*** | .48*** |
| Family of Origin Income | −.10 | −.08 |
| Lifetime Cigarette Use | .19** | .22*** |
| Lifetime Marijuana Use | .29*** | .29*** |
Note.
p < .001.
p < .01.
p < .05.
β weights are from the full model. N = 113
Hypothesis 3: Recent marijuana use will be more strongly linked to epigenetic age acceleration than use at earlier periods.
Analyses next sought to assess a potential recency effect, in which marijuana use would be more strongly associated with epigenetic aging if use was recent rather than distal. Predictions from average levels of use for the most recent four years were compared to predictions from the prior four years, and from the four years prior to that. As before, cell counts, chronological age, demographic variables, and cigarette smoking were all covaried, although for simplicity of presentation, only the final β weights for marijuana use are shown in Figure 1. In addition, models also accounted for autoregressive effects between marijuana use at various time points. Results revealed that only the most recent period of marijuana use was linked to epigenetic aging (and links were actually stronger for this recent use than for lifetime use, as reported in Table 2). Follow-up analyses revealed that the difference in the magnitude of predictions for the most recent four-year period as compared to the two prior periods was significant (χ2(2) = 9.14, p = .01, and χ2(2) = 10.06, p = .01 for DNAmGrimAge and DunedinPoAm respectively). In addition, examination of the correlations between each four-year period and lifetime levels of use indicates that the recency effect observed was not likely due to the most recent use being simply the best proxy for lifetime use, as the correlations to lifetime use were .85, .91, and .77 for use in the most recent four-years, 5–8 years prior, and 9–12 years prior, respectively (all p’s < .001) (e.g., the 5–8 years prior period actually was more strongly correlated to lifetime use, yet less strongly related to epigenetic aging).
Figure 1 -. Relation of Timing of Marijuana Use to Epigenetic Aging.
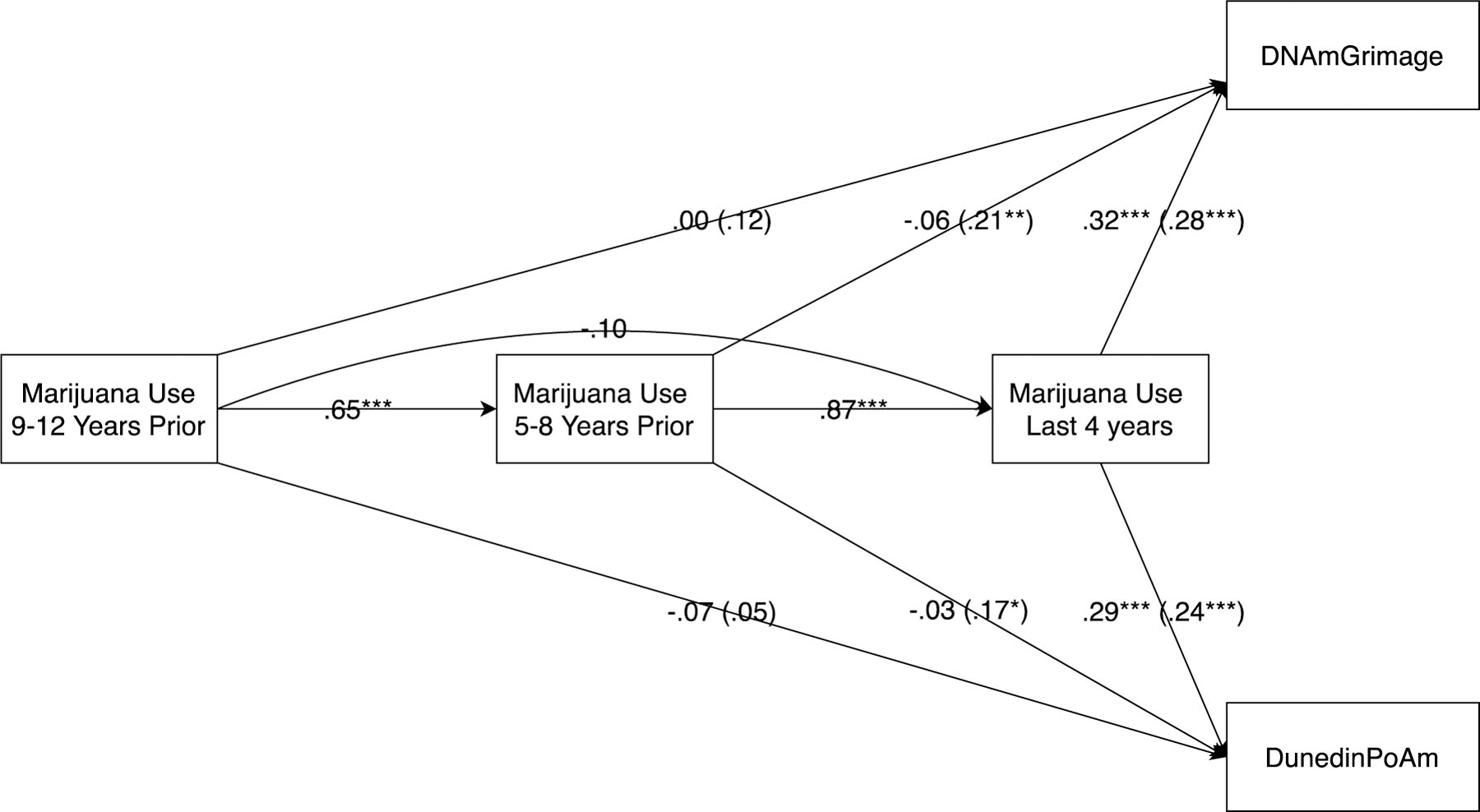
Note: Standardized estimates above are after accounting for cell counts, chronological age, demographic factors, lifetime history of cigarette smoking (not shown) and other variables shown in model. Estimates in parentheses are from marijuana use at a given time point without considering/accounting for use at other time points.
In parentheses, Figure 1 also shows the direct links (i.e., not accounting for marijuana use at other time periods) between marijuana use during a given period and epigenetic aging. These estimates provide an indication of what could be predicted of epigenetic aging knowing only about the level of use during a single period. These results also suggest a recency effect for prediction from marijuana use to both of the assessed markers of epigenetic aging.
Hypothesis 4: Links of marijuana use to epigenetic aging will be at least partially mediated via hypomethylation at site cg05575921, as an indication that effects may be in part due to effects of smoke inhalation when ingesting marijuana.
Analyses assessed the link between: a) lifetime marijuana use and the methylation status of cg05575921, b) methylation of cg05575921 and the two epigenetic aging outcomes, and c) marijuana use and epigenetic aging after accounting for methylation of cg05575921. Results, shown in Figure 2, reflect full mediation of effects of marijuana use via cg05575921 hypomethylation. After accounting for cg05575921, effects of marijuana use became non-significant (βs = .02 and .03 for predictions of DNAmGrimAge and DunedinPoAm, respectively, p’s > .60). Formal tests of mediation using a bootstrapping approach via the PROCESS macro in SAS (Hayes, 2019) indicated that the mediated pathways from marijuana use to both DNAmGrimAge and DunedinPoAm were significant (standardized indirect effects = .31, 95% CI [.19, .43] and .24, 95% CI [.15, .35], respectively).
Figure 2 – Mediating Role of cg05575921 in link between Marijuana Use and Epigenetic Aging.
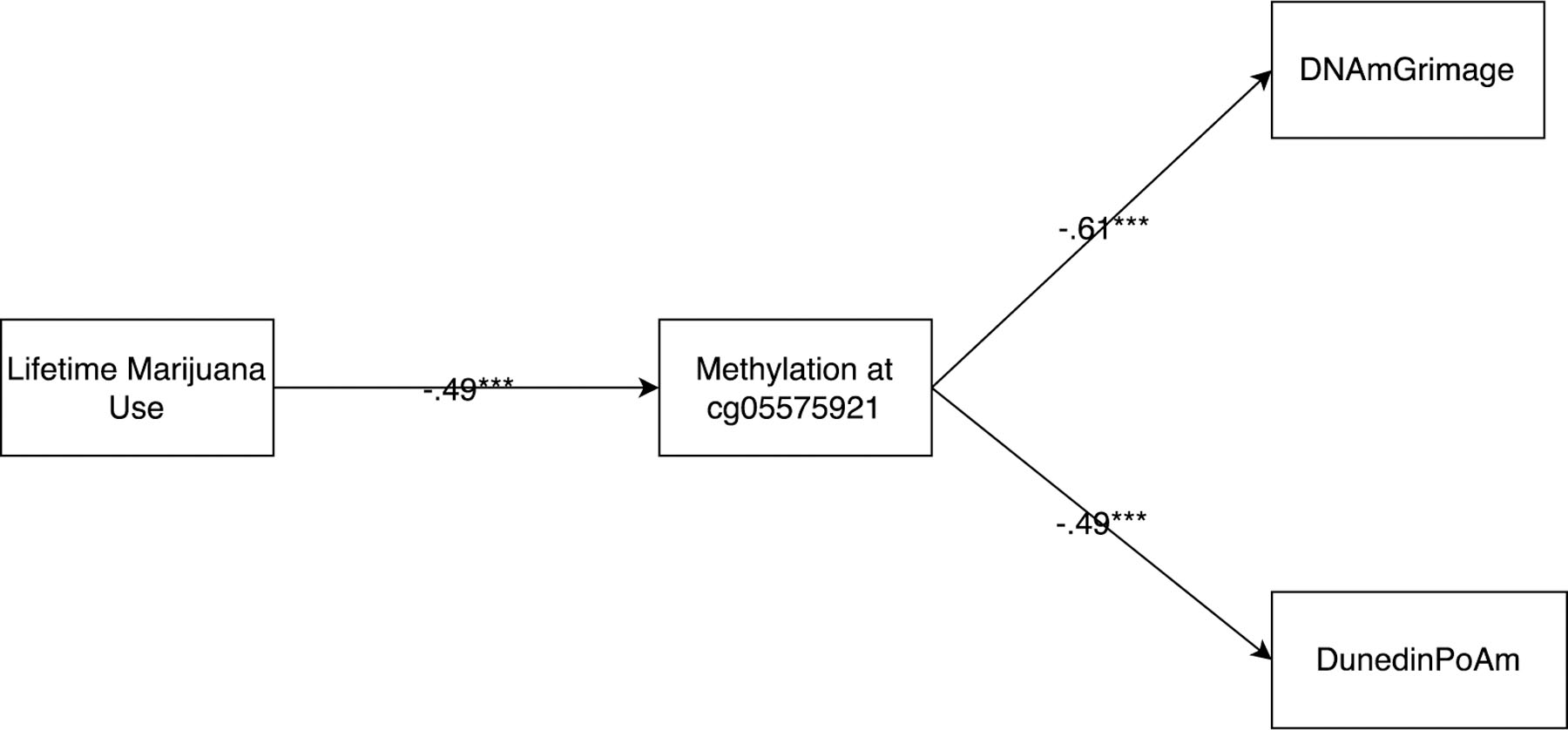
Note: Standardized estimates above are after accounting for cell counts, chronological age, and demographic factors.
To further rule out the possibility that apparent effects of marijuana use were confounded by cigarette smoking, we examined the link of marijuana use to cg05575921 for just the subsample of participants who reported never having smoked cigarettes. The resulting estimate (β = −.60, p < .001) is consistent with the explanation that the link of marijuana use to hypomethylation at this site exists independently of history of cigarette smoking. Further, when both cigarette smoking and marijuana use were examined together to predict methylation at this site, marijuana use not only added to predictions from cigarette smoking, it was and is of comparable magnitude to the effects of cigarette smoking (βMarijuanaUse = −.40, βCigaretteSmoking = −.32, both p’s < .001). This is consistent with the interpretation that lifetime history of marijuana use was affecting this hydrocarbon receptor repressor site to a similar degree as lifetime history of cigarette smoking.
Hypothesis 5: Links to epigenetic aging will exist over and above a range of potential behavioral and personality confounders, including education level, personality traits and history of use of harder drugs, childhood physical illness, anxiety, and depressive symptoms.
All of these potential confounders were added simultaneously to the model predicting epigenetic aging, and when added, the relation of lifetime marijuana use to epigenetic aging outcomes was virtually unchanged from results shown in Table 2 (βMarijuanaUse = .31 and .27 for predictions to DNAmGrimAge and DunedinPoAm respectively, both p’s < .001).
Discussion
This study found a substantial link between lifetime levels of marijuana use and two different measures of epigenetic aging, assessed at age 30. This link remained even after accounting for the effects of lifetime cigarette smoking history. Further, the predictive link from lifetime marijuana use to epigenetic age acceleration was of similar magnitude to the observed effect of cigarette smoking. Follow-up analyses suggest these links are likely to be mediated by the epigenetic effects of smoke inhalation among marijuana users. Strengths of the study include collection of marijuana usage data contemporaneously on an annual basis over a 17-year period, use of a diverse community sample, use of recently developed and well-validated epigenetic measures of age acceleration, and consideration of a wide array of potential confounding factors.
Prior to considering the implications of these findings, an important limitation to note is that even prospective longitudinal data is not sufficient to establish a causal link between marijuana use and epigenetic aging. For example, this study cannot determine whether the epigenetic links observed were effects of marijuana use, predisposing factors for this use, or even whether marijuana use and epigenetic changes were both driven by other unmeasured factors. This study did, however, seek to rule out several alternative explanations for the observed links and to test other hypotheses that a causal model would generate. For example, the link between marijuana use and epigenetic age acceleration was not found to be an artifact of prior health problems, a range of family income, gender, personality traits, nor lifetime history of anxiety and depressive symptoms—all of which could potentially have driven both aging and marijuana use. In addition, there was a dose-effect relation observed such that just within the population of marijuana users, higher levels of lifetime use were linked to greater epigenetic age acceleration. These findings are all consistent with, though cannot conclusively establish, a causal role of marijuana use in epigenetic aging.
Follow-up analyses provided evidence that links to epigenetic aging were dependent upon the recency of marijuana use, with more recent use strongly linked to age acceleration and with this effect fading for use in the more distant past. Related analyses suggested that this fade out was not simply a reflection of more recent use being more strongly related to overall use. These findings potentially help explain the lack of consistent findings linking marijuana use to longer-term markers of mortality and morbidity. If use creates impairment, but the impairment begins to subside over time if use decreases, this would suggest that the potential effects of marijuana use on the epigenome were less likely to be durable and cumulative in the longer-term. Notably, this pattern of decreasing morbidity following cessation or reduction in use has also been observed in at least some areas with respect to both marijuana use and cigarette smoking (Cho et al., 2005; Tashkin et al., 2012). An alternative explanation is that marijuana use may have had greater effects among users who were older (given that recency of use and age were of necessity confounded in this study). It is also possible that recent increases in the potency of marijuana available for consumption could at least partially account for these effects.
This study also found that the link between marijuana use and epigenetic aging was statistically mediated via hypomethylation at site cg05575921. This site is in a hydrocarbon receptor repressor gene, and hypomethylation of this site has also been linked, even among non-smokers, to exposure to fine particulate matter (PM2.5) such as that produced by car exhausts, wood burning and factory smoke (Tantoh et al., 2019). This mediational finding is thus consistent with the explanation that the epigenetic aging effects observed reflect effects of marijuana smoke inhalation (as opposed to ingestion of THC). This is consistent with the large body of findings regarding effects of marijuana use that are bronchial in nature (Tashkin et al., 2012). It also may help to further explain the dropoff in links to epigenetic aging for marijuana use in the more distal past, given the prior finding that hypomethylation of cg05575921 begins to revert among smokers upon their cessation from smoking (Philibert et al., 2020). As a methodological note, these findings also suggest that research use of cg05575921 to assess cigarette smoking among populations with significant marijuana use may require particular care, as marijuana use appears to render this site as less than a pure marker of cigarette smoking history.
If marijuana use effects are primarily a reflection of links to this site and associated physical effects, this might help explain why effects were so clear in this study, even though long-term studies of marijuana use have found only inconsistent evidence of significant physical harm: It may be that marijuana does have significant epigenetic aging effects, but that these are readily reversible when use ceases. In contrast, some aspects of cigarette use may display this characteristic, but many others may not. This would potentially explain the general lack of findings of long-term effects of marijuana use. For example, two of the strongest studies of these effects followed individuals well into middle age, but asked about lifetime levels of use (Reis et al., 2017; Sidney et al., 1997). Yet, given that levels of use decline with age, it may be that effects of more distal use were no longer present. On the one hand, these findings are consistent with a lack of effects of lifetime levels of use (presuming usage levels generally decline substantially with age), but they also suggest the possibility that potential deleterious effects of use may exist and may continue for as long as use continues. Further research is clearly needed to begin to parse out these possibilities.
In addition to the limitations noted above, several additional limitations also warrant consideration. Although this study identifies links from marijuana use to two broad epigenetic aging measures, further work exploring links of use to epigenetic changes at specific sites will be needed to more fully flesh out potential mechanisms by which these links come to exist (Szutorisz & Hurd, 2016). In addition, marijuana use was assessed only in terms of number of times of use, hence information about effects of quantity, quality, and potency could not be considered. Further, this study did not distinguish between whether use was via smoking vs. other forms of ingestion. Similarly, cigarette use was measured less frequently than marijuana use and reduced precision in this measurement could have reduced the relative apparent contribution of cigarette smoking in analyses. Finally, it should be recognized that health outcomes of marijuana use are only one aspect of debates regarding legalization of use, and this study was clearly not in a position to address the advisability of such legalization efforts.
Notwithstanding these limitations, this study raises important questions about broad health implications of marijuana use. It also provides insights regarding the potential role of the timing of such use and of links to epigenetic changes that may be linked to inhalation of smoke. To the extent that marijuana use is linked to markers of broad aging processes, research is now needed to further clarify the nature and extent of this linkage.
Highlights.
Lifetime levels of marijuana use predicted accelerated epigenetic aging.
Predictions to epigenetic aging from lifetime marijuana use remained significant even after accounting for cigarette smoking and a wide range of potential confounding variables.
A dose-response effect was observed within the population of marijuana users, and there was also evidence that effects were dependent upon recency of use, with more recent use being associated with greater epigenetic aging.
Acknowledgements:
This study was supported by grants from the National Institute of Child Health and Human Development and the National Institute of Mental Health (5R37HD058305-23, R01HD058305-16A1, R01-MH58066). We thank the Duke Molecular Physiology Institute Molecular Genomics Core for processing Illumina DNA methylation arrays.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest No conflict declared
Contributor Information
Joseph P. Allen, University of Virginia
Joshua S. Danoff, University of Virginia
Meghan A. Costello, University of Virginia
Gabrielle L. Hunt, University of Virginia
Amanda F. Hellwig, University of Virginia
Kathleen M. Krol, University of Virginia
Simon G. Gregory, Duke University
Stephanie N. Giamberardino, Duke University
Karen Sugden, Duke University.
Jessica J. Connelly, University of Virginia
References
- Abdulrahim JW, Kwee LC, Grass E, Siegler IC, Williams R, Karra R, … Shah SH (2019). Epigenome-Wide Association Study for All-Cause Mortality in a Cardiovascular Cohort Identifies Differential Methylation in Castor Zinc Finger 1 (CASZ 1). Journal of the American Heart Association, 8(21), e013228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen JP, Uchino BN, & Hafen CA (2015). Running with the pack: Teen peer-relationship qualities as predictors of adult physical health. Psychological Science, 26(10), 1574–1583. doi: 10.1177/0956797615594118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen A, Reimer R, Dawes K, Becker A, Hutchens N, Miller S, … D Long, J. (2021). DNA methylation differentiates smoking from vaping and non-combustible tobacco use. Epigenetics, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen AM, Philibert RA, Gibbons FX, Simons RL, & Long J (2017). Accuracy and utility of an epigenetic biomarker for smoking in populations with varying rates of false self-report. Am J Med Genet B Neuropsychiatr Genet, 174(6), 641–650. doi: 10.1002/ajmg.b.32555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechtold J, Simpson T, White HR, & Pardini D (2015). Chronic adolescent marijuana use as a risk factor for physical and mental health problems in young adult men. Psychol Addict Behav, 29(3), 552–563. doi: 10.1037/adb0000103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky DW, Caspi A, Arseneault L, Baccarelli A, Corcoran DL, Gao X, … Moffitt TE (2020). Quantification of the pace of biological aging in humans through a blood test, the DunedinPoAm DNA methylation algorithm. Elife, 9. doi: 10.7554/eLife.54870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson MK, & Bentley AM (1995). Lung disease induced by drug addiction. Thorax, 50(11), 1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook JS, Zhang C, & Brook DW (2011). Developmental trajectories of marijuana use from adolescence to adulthood: Personal predictors. Archives of pediatrics & adolescent medicine, 165(1), 55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caulkins JP, Kilmer B, & Kleiman MA (2016). Marijuana legalization: What everyone needs to know®: Oxford University Press. [Google Scholar]
- Cho C, Hirsch R, & Johnstone S (2005). General and oral health implications of cannabis use. Australian Dental Journal, 50(2), 70–74. [DOI] [PubMed] [Google Scholar]
- Goldberg LR, Johnson JA, Eber HW, Hogan R, Ashton MC, Cloninger CR, & Gough HG (2006). The international personality item pool and the future of publicdomain personality measures. Journal of Research in Personality, 40(1), 84–96. doi: 10.1016/j.jrp.2005.08.007 [DOI] [Google Scholar]
- Han LK, Aghajani M, Clark SL, Chan RF, Hattab MW, Shabalin AA, … Jansen R (2018). Epigenetic aging in major depressive disorder. American Journal of Psychiatry, 175(8), 774–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AF (2019). Introduction to Mediation, Moderation, and Conditional Process Analysis, Second Edition : A Regression-Based Approach. ProQuest Ebook Central; (http://ebookcentral.proquest.com/lib/uva/detail.action?docID=5109647): Guilford. [Google Scholar]
- Hindocha C, Shaban ND, Freeman TP, Das RK, Gale G, Schafer G, … Curran HV (2015). Associations between cigarette smoking and cannabis dependence: a longitudinal study of young cannabis users in the United Kingdom. Drug and Alcohol Dependence, 148, 165–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath S (2013). DNA methylation age of human tissues and cell types. Genome biology, 14(10), 3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath S, & Levine AJ (2015). HIV-1 infection accelerates age according to the epigenetic clock. The Journal of infectious diseases, 212(10), 1563–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houseman EA, Accomando WP, Koestler DC, Christensen BC, Marsit CJ, Nelson HH, … Kelsey KT (2012). DNA methylation arrays as surrogate measures of cell mixture distribution. BMC bioinformatics, 13(1), 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joehanes R, Just AC, Marioni RE, Pilling LC, Reynolds LM, Mandaviya PR, … Aslibekyan S (2016). Epigenetic signatures of cigarette smoking. Circulation: cardiovascular genetics, 9(5), 436–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, & Bachman JG (1987). Psychotherapeutic, licit, and illicit use of drugs among adolescents: An epidemiological perspective. Journal of Adolescent Health Care, 8(1), 36–51. [DOI] [PubMed] [Google Scholar]
- Kovacs M, & Beck AT (1977). An empirical clinical approach toward a definition of childhood depression/. New York: Raven Press. [Google Scholar]
- Lu AT, Quach A, Wilson JG, Reiner AP, Aviv A, Raj K, … Stewart JD (2019). DNA methylation GrimAge strongly predicts lifespan and healthspan. Aging (Albany NY), 11(2), 303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markunas CA, Hancock DB, Xu Z, Quach BC, Fang F, Sandler DP, … Taylor JA (2020). Epigenome-wide analysis uncovers a blood-based DNA methylation biomarker of lifetime cannabis use. Am J Med Genet B Neuropsychiatr Genet. doi: 10.1002/ajmg.b.32813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore BA, Augustson EM, Moser RP, & Budney AJ (2005). Respiratory effects of marijuana and tobacco use in a US sample. Journal of General Internal Medicine, 20(1), 33–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman RE, Byambaa M, De R, Butchart A, Scott J, & Vos T (2012). The long-term health consequences of child physical abuse, emotional abuse, and neglect: a systematic review and meta-analysis. PLoS Med, 9(11), e1001349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen KP, Sutter ME, & Albertson TE (2014). Marijuana: respiratory tract effects. Clinical reviews in allergy & immunology, 46(1), 65–81. [DOI] [PubMed] [Google Scholar]
- Palma-Gudiel H, Fananas L, Horvath S, & Zannas AS (2020). Psychosocial stress and epigenetic aging. Int Rev Neurobiol, 150, 107–128. doi: 10.1016/bs.irn.2019.10.020 [DOI] [PubMed] [Google Scholar]
- Philibert R, Mills JA, Long JD, Salisbury SE, Comellas A, Gerke A, … Hoffman EA (2020). The Reversion of cg05575921 Methylation in Smoking Cessation: A Potential Tool for Incentivizing Healthy Aging. Genes, 11(12), 1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pletcher MJ, Vittinghoff E, Kalhan R, Richman J, Safford M, Sidney S, … Kertesz S (2012). Association between marijuana exposure and pulmonary function over 20 years. JAMA, 307(2), 173–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reece AS, Norman A, & Hulse GK (2016). Cannabis exposure as an interactive cardiovascular risk factor and accelerant of organismal ageing: a longitudinal study. BMJ Open, 6(11), e011891. doi: 10.1136/bmjopen-2016-011891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis JP, Auer R, Bancks MP, Goff DC Jr, Lewis CE, Pletcher MJ, … Sidney S (2017). Cumulative lifetime marijuana use and incident cardiovascular disease in middle age: the Coronary Artery Risk Development in Young Adults (CARDIA) study. American Journal of Public Health, 107(4), 601–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds LM, Wan M, Ding J, Taylor JR, Lohman K, Su D, … Pittman GS (2015). DNA methylation of the aryl hydrocarbon receptor repressor associations with cigarette smoking and subclinical atherosclerosis. Circulation: cardiovascular genetics, 8(5), 707–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAMSHA. (2020). Key substance use and mental health indicators in the United States: Results from the 2019 National Survey on Drug Use and Health. Washington, DC: SAMSHA. [Google Scholar]
- Sidney S, Beck JE, Tekawa IS, Quesenberry CP, & Friedman GD (1997). Marijuana use and mortality. American Journal of Public Health, 87(4), 585–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD, Sydeman SJ, Owen AE, & Marsh BJ (1999). Measuring anxiety and anger with the State-Trait Anxiety Inventory (STAI) and the State-Trait Anger Expression Inventory (STAXI). In Maruish ME (Ed.), The use of psychological testing for treatment planning and outcomes assessment (2nd ed., pp. 993–1021). Mahwak, NJ, US: Lawrence Erlbaum Associates Publishers. [Google Scholar]
- Szutorisz H, & Hurd YL (2016). Epigenetic Effects of Cannabis Exposure. Biol Psychiatry, 79(7), 586–594. doi: 10.1016/j.biopsych.2015.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tantoh DM, Lee K-J, Nfor ON, Liaw Y-C, Lin C, Chu H-W, … Ho C-C (2019). Methylation at cg05575921 of a smoking-related gene (AHRR) in non-smoking Taiwanese adults residing in areas with different PM 2.5 concentrations. Clinical epigenetics, 11(1), 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashkin DP (2013). Effects of marijuana smoking on the lung. Ann Am Thorac Soc, 10(3), 239–247. doi: 10.1513/AnnalsATS.201212-127FR [DOI] [PubMed] [Google Scholar]
- Tashkin DP, Simmons MS, & Tseng C-H (2012). Impact of changes in regular use of marijuana and/or tobacco on chronic bronchitis. COPD: Journal of Chronic Obstructive Pulmonary Disease, 9(4), 367–374. [DOI] [PubMed] [Google Scholar]


