Abstract
Background
There is a need for improved antibiotic formulations for the treatment of acute bacterial skin and soft structure infection (ABSSSI), especially with the rise of antimicrobial resistance among Gram-positive bacteria. A new formulation of oritavancin was developed to reduce intravenous infusion volume (from 1000 mL to 250 mL), shorten infusion time (from 3 hours to 1 hour), and provide pharmacies with flexibility in oritavancin preparation (from 5% dextrose in sterile water to either normal saline or 5% dextrose in sterile water) compared with the current formulation.
Methods
A total of 102 adult patients with a diagnosis of ABSSSI suspected or confirmed to be caused by a Gram-positive pathogen were randomized 1:1 to receive either the new formulation of oritavancin or the current formulation. After a single 1200-mg intravenous infusion of oritavancin, the relative area-under-the-curve exposure of the new formulation and current formulation groups were compared. Safety and tolerability of the new formulation were assessed for treatment-emergent adverse events, serious adverse events, and changes to laboratory parameters.
Results
The area under the curve for 0 hour to 72 hours postdose was very similar in the new formulation group compared with the current formulation group. No differences in treatment-emergent adverse events were observed between the current and new formulation groups, and all treatment-emergent adverse events were consistent with the known safety profile of the current formulation.
Conclusions
The new formulation of oritavancin with reduced volume and duration of intravenous infusion demonstrates a safety profile and pharmacokinetics similar to that of the original formulation.
Keywords: ABSSSI, oritavancin, pharmacokinetics, skin infections
A randomized, open-label, pharmacokinetics and safety study evaluated the relative exposure and safety of a new cyclodextrin-containing oritavancin formulation in subjects being treated for ABSSSI and demonstrated a favorable safety profile and similar pharmacokinetics compared with the current formulation.
Acute bacterial skin and soft structure infections (ABSSSIs) are common infections, encompassing cellulitis, major cutaneous abscesses, and wound infections, which involve the skin and subcutaneous tissues. Acute bacterial skin and soft structure infections are a common cause of morbidity and mortality [1, 2], requiring antimicrobial intervention and, occasionally, drainage for larger abscesses. Treatment is focused on complete eradication of the infection, because clinical complications of inadequately treated or untreated ABSSSIs may include local spread, secondary bacteremia with potential for distant metastatic foci of infection, and systemic effects of bacterial infection (ie, sepsis/septic shock and/or toxic shock). The organisms responsible for ABSSSIs, especially staphylococci and enterococci, are becoming increasingly resistant to available antibiotics, and many isolates are resistant to multiple antibiotics. Methicillin-resistant Staphylococcus aureus (MRSA) is a common cause of ABSSSIs, reaching an incidence of 60% to 74% in some large US teaching hospitals and is being reported with increasing frequency in outpatient infections [3]. Although recent data suggest a decline in MRSA as a proportion of all S aureus isolates [4], it remains a predominant pathogen among those with purulent ABSSSI [5].
Oritavancin diphosphate (oritavancin) is a semisynthetic, lipoglycopeptide antibiotic for the treatment of serious Gram-positive bacterial infections. Oritavancin has been approved by the US Food and Drug Administration (FDA) [6] and the European Medicines Agency [7] for the treatment of adult patients with ABSSSI caused or suspected to be caused by susceptible isolates of designated Gram-positive microorganisms. Streptococcus pyogenes, Streptococcus agalactiae, Streptococcus dysgalactiae, Streptococcus anginosus group (includes S. anginosus, S. intermedius, and S. constellatus), and vancomycin-susceptible isolates of Enterococcus faecalis; however, vancomycin-resistant enterococci ([VRE] E faecalis and E faecium) are usually also susceptible if the vancomycin-susceptible Enterococcus breakpoint is applied [8, 9]. The current approved formulation of oritavancin is a 1200-mg single intravenous (IV) infusion. It is packaged as 3 single-use vials, each containing lyophilized oritavancin (400 mg) and the inactive component, mannitol. The vials are reconstituted with sterile water for infusion (SWFI), further diluted in 5% dextrose in sterile water (D5W) for a total volume of 1000 mL, and infused IV over 3 hours [6].
The antibacterial activity of oritavancin is best correlated with the area under the concentration-time curve (AUC) in animal models of infection, and the key parameter for clinical efficacy in humans has favored AUC/minimum inhibitory concentration (MIC). However, Cmax further optimizes the pharmacokinetics (PK)/pharmacodynamics (PD) of oritavancin [10, 11]. Oritavancin demonstrates rapid in vitro bactericidal activity against clinically relevant Gram-positive pathogens including methicillin-sensitive S aureus, MRSA [12], and various streptococci [13]. Against VRE, oritavancin demonstrates in excess of 3-log kill within 48 to 72 hours, and it is attributed to its multiple mechanisms of action [14].
Oritavancin disrupts Gram-positive bacterial membrane integrity, leading to depolarization, permeabilization, and rapid cell death [15, 16]. It likely uses a mechanism distinct from daptomycin because oritavancin maintains potent activity against daptomycin-nonsusceptible strains [17, 18]. In addition, oritavancin inhibits the transglycosylation step of cell wall synthesis by binding to D-ala-D-ala stem termini, a mechanism shared with all glycopeptides and lipoglycopeptides [19–21]. Finally, oritavancin inhibits the transpeptidation step of cell wall synthesis by binding to the bridging segment, a secondary binding site that has not been demonstrated for vancomycin [8, 19, 20]. Consequently, oritavancin is effective against VRE [9, 17] and vancomycin-resistant S aureus [17, 22]. Oritavancin has shown in vitro potency (MIC90 ≤0.5 µg/mL) against enterococci carrying vanA, vanB, and vanC genes [9]. Compared with vancomycin, oritavancin is more potent against Clostridioides difficile, presenting additional therapeutic implications [23]. Unlike vancomycin, oritavancin adheres to C difficile endospores and may prevent vegetative outgrowth [24, 25].
A new oritavancin formulation ([NF] Kimyrsa) has been developed to simplify preparation of the solution for infusion; reduce the volume of the infusion (important for those at risk of fluid overload, including renally impaired patients and those with congestive heart failure); shorten the infusion time (lessening the burden for patients); and giving pharmacies the flexibility to prepare in D5W or normal saline (NS). The NF product was approved by the FDA on March 12, 2021 (https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2021/214155Orig1s000ltr.pdf) and utilizes the excipient 2-hydroxypropyl-β-cyclodextrin (HPβCD) as a solubility enhancer. When oritavancin is formulated in a 2:1 (w/w) ratio of HPβCD/oritavancin, its solubility in isotonic media at physiologic pH is significantly improved. This improved solubility prevents oritavancin precipitation at markedly higher concentrations, and with different infusates than the current formulation ([CF] Orbactiv). The NF is packaged as a single vial with lyophilized powder containing 1200 mg of oritavancin and inactive ingredients of 2400 mg of HPβCD, 800 mg of mannitol, and phosphoric acid or sodium hydroxide (to adjust pH 4.0 to 6.0). The vial should be reconstituted with SWFI, further diluted with NS or D5W for a total volume of 250 mL, and infused IV over 1 hour. The NF will deliver the same dose of oritavancin as CF and is administered for the same indication [26, 27]. This study assessed the relative PK and tolerability of the new oritavancin formulation versus the current approved formulation in patients with an ABSSSI.
MATERIALS AND METHODS
Study Design
A randomized, open-label, multicenter study was conducted at 3 centers to evaluate the relative exposure of a new oritavancin formulation versus the approved formulation in patients with ABSSSI (ClinicalTrials.gov Identifier NCT03873987 [https://clinicaltrials.gov]). Three analysis sets were defined: (1) the Intent-to-Treat (ITT) Analysis Set included all subjects randomized; (2) the Safety Analysis Set included all subjects who received any amount of IV oritavancin; and (3) the PK Analysis Set included all subjects who have received the full dose of oritavancin and have any valid samples measured for study drug levels.
The primary objective was to determine the relative AUC exposure of NF compared with the approved CF after a single 1200-mg IV infusion of oritavancin in adult patients with ABSSSI. The secondary objective was to evaluate the safety and tolerability of the NF by the incidence of treatment-emergent adverse events (TEAEs), laboratory parameters, vital signs, and physical examination. Patients were monitored for TEAEs from the time of study drug initiation to Day 15. Patients received their dose of oritavancin on Day 1 and were asked to return to the study center on Day 2, Day 4, Day 8, and Day 15 for collection of additional blood samples and procedure assessments. The subject’s total participation was approximately 17 days. The MedDRA Version 22.0 was used for coding adverse events (AEs). An AE could have been any unfavorable or unintended sign (including an abnormal laboratory finding), symptom, or disease temporally associated with the use of the medicinal product, related or not to the medicinal product. Adverse events of special interest (AESI) were defined as drug hypersensitivity/infusion-related reaction, pseudomembranous colitis/C difficile-associated diarrhea (CDAD), osteomyelitis, and hemolytic anemia.
The study randomly assigned patients in a 1:1 ratio to receive either NF or CF. Randomization was performed by the electronic data capture system once the subject was screened and confirmed to be eligible for participation. Patients randomized to CF received the approved dosing regimen in which SWFI was used for reconstitution and D5W was used for further dilution to a final volume of 1000 mL, infused over 3 hours. Patients randomized to NF received oritavancin reconstituted with SWFI, further diluted in NS to a final volume of 250 mL and infused over 1 hour.
The study protocol, informed consent form, patient recruitment material, and any other pertinent study-related documents were reviewed and approved by the Institutional Review Board for the investigational sites. Written informed consent was obtained from all patients before screening and before initiation of protocol-specified procedures. The study was conducted in accordance with the relevant articles of the Declaration of Helsinki, the International Council for Harmonization guidelines for Good Clinical Practice (GCP), and, as appropriate, the principles of GCP as outlined in the United States Code of Federal Regulations, Title 21.
Study Population
Patients were men or women 18 years of age and older who had given informed consent and had a diagnosis of ABSSSI suspected or confirmed to be caused by a Gram-positive pathogen. Female patients were not pregnant or breastfeeding and, if of childbearing potential, agreed to use at least 2 highly effective methods of birth control for the duration of the study until 60 days after study drug administration. Exclusion criteria included the following: infections associated with or in close proximity to a prosthetic device; severe sepsis or refractory shock; and known or suspected bacteremia at time of screening. Patients were excluded for ABSSSI due to or associated with any of the following: infections known to be caused by an organism resistant to oritavancin; infections suspected or documented to be caused by only Gram-negative pathogens; diabetic foot infections; concomitant infection at another site not including a secondary ABSSSI; infected burns; a primary infection secondary to a pre-existing skin disease with associated inflammatory changes; decubitus or chronic skin ulcer, or ischemic ulcer due to peripheral vascular disease; any evolving necrotizing process (eg, necrotizing fasciitis), gangrene, or infection suspected or proven to be caused by Clostridium species (although these are susceptible to oritavancin but were excluded for reasons of trial design); or catheter site infections. Patients were excluded from receiving treatment with an investigational medicinal product within 30 days or 5 half-lives, whichever was longer, before enrollment and for the duration of the study; current administration of anticoagulant therapy; known liver function tests ≥3 times the upper limit of normal (ULN) or total bilirubin ≥2 times ULN; any medical condition that in the judgment of the investigator might interfere with the PK, distribution, metabolism, or excretion of the study drug; any planned, major surgical procedure during the study period; known hypersensitivity to oritavancin, glycopeptides, or HPβCD; or previous use of oritavancin or anticipated need to use a long-acting glycopeptide during the study.
Minor surgical or bedside procedures (including incision and drainage, aspiration, lavage, etc) were allowed during the study. Use of additional antibiotics was minimized unless the patient failed treatment and required unexpected rescue antibiotics or additional antibiotics to treat a new infection during the study. Concomitant antibiotic coverage for a Gram-negative infection was allowed.
Bioanalytical Methodology
Plasma samples were analyzed for oritavancin concentration using a validated bioanalytical method. Plasma samples were extracted by protein precipitation, separated by high-performance liquid chromatography, and quantified using tandem mass spectrometry.
Pharmacokinetic Analysis
Samples for PK analysis were collected at predose and at the end of infusion (1 hour or 3 hours), and then at 3 hours (for 1-hour infusions), 6, 12, 24, 72, and 168 hours after the start of infusion.
Pharmacokinetic parameters were analyzed using noncompartmental analysis by Phoenix WinNonlin (v8.1 or higher; Certara USA Inc., Princeton, NJ). Pharmacokinetic parameters included the following for both groups: Cmax, Tmax, and AUC from time 0 to 72 hours postdose (AUC0-72) and 0 to 168 hours postdose (AUC0-168). Actual sample times were used for the calculation of AUC. Nominal sample times were used for the preparation of mean concentration-time profile plots. Details of additional PK analysis are described in the Supplementary Materials.
RESULTS
Demographics and Disposition of Patients
A total of 102 patients were enrolled; 52 patients were randomized to the CF group and 50 to the NF group. Overall, a total of 99 patients (97.1%) completed the study and 3 patients (2.9%) discontinued the study. Two patients in the CF group (3.8%) withdrew from the study because of drug hypersensitivity and the other patient discontinued because of poor venous access. One patient in the NF group (2.0%) was lost to follow-up.
Patient demographics and baseline characteristics are presented in Table 1. Overall, the frequency of ABSSSI types was cutaneous abscess (44.1%), cellulitis or erysipelas (29.4%), and wound infection (26.5%). The distribution of patients among the 3 ABSSSI types were similar between treatment groups. The treatment groups were balanced with regard to medical and surgical history.
Table 1.
Demographic and Baseline Characteristics
| Parameter | Current Formulation (3-Hour IV) (N = 52) |
New Formulation (1-Hour IV) (N = 50) |
Overall (N = 102) |
|---|---|---|---|
| Age (years) | |||
| Mean (SD) | 46.4 (12.1) | 42.1 (12.7) | 44.3 (12.6) |
| Median | 46.5 | 39.0 | 42.0 |
| Minimum, maximum | 23, 68 | 22, 70 | 22, 70 |
| Sex, n (%) | |||
| Male | 37 (71.2) | 30 (60.0) | 67 (65.7) |
| Female | 15 (28.8) | 20 (40.0) | 35 (34.3) |
| Height (cm) | |||
| Mean (SD) | 171.8 (10.0) | 172.8 (10.5) | 172.3 (10.2) |
| Median | 171.7 | 171.5 | 171.5 |
| Minimum, maximum | 152.4, 192.3 | 147.3, 196.9 | 147.3, 196.9 |
| Weight (kg) | |||
| Mean (SD) | 80.8 (19.5) | 88.7 (26.9) | 84.7 (23.7) |
| Median | 78.45 | 84.00 | 79.85 |
| Minimum, maximum | 51.2, 132.9 | 50.8, 178.3 | 50.8, 178.3 |
| BMI (kg/m2) | |||
| Mean (SD) | 27.3 (6.0) | 29.7 (8.5) | 28.5 (7.4) |
| Minimum, maximum | 18.5, 42.2 | 16.4, 56.4 | 16.4, 56.4 |
| Race, n (%) | |||
| American Indian or Alaska ative | 2 (3.8) | 1 (2.0) | 3 (2.9) |
| Black or African American | 5 (9.6) | 3 (6.0) | 8 (7.8) |
| White | 45 (86.5) | 46 (92.0) | 91 (89.2) |
| Ethnicity, n (%) | |||
| Hispanic or Latino | 21 (40.4) | 21 (42.0) | 42 (41.2) |
| Not Hispanic or Latino | 31 (59.6) | 29 (58.0) | 60 (58.8) |
Abbreviations: BMI, body mass index; IV, intravenous; SD, standard deviation.
All 102 enrolled patients were included in the safety population, whereas 100 patients were included in the PK analysis. The 2 patients excluded from the PK population did not complete the study drug infusion and did not have postbaseline PK samples.
Plasma Concentrations of Oritavancin 1200-Milligram Infusions
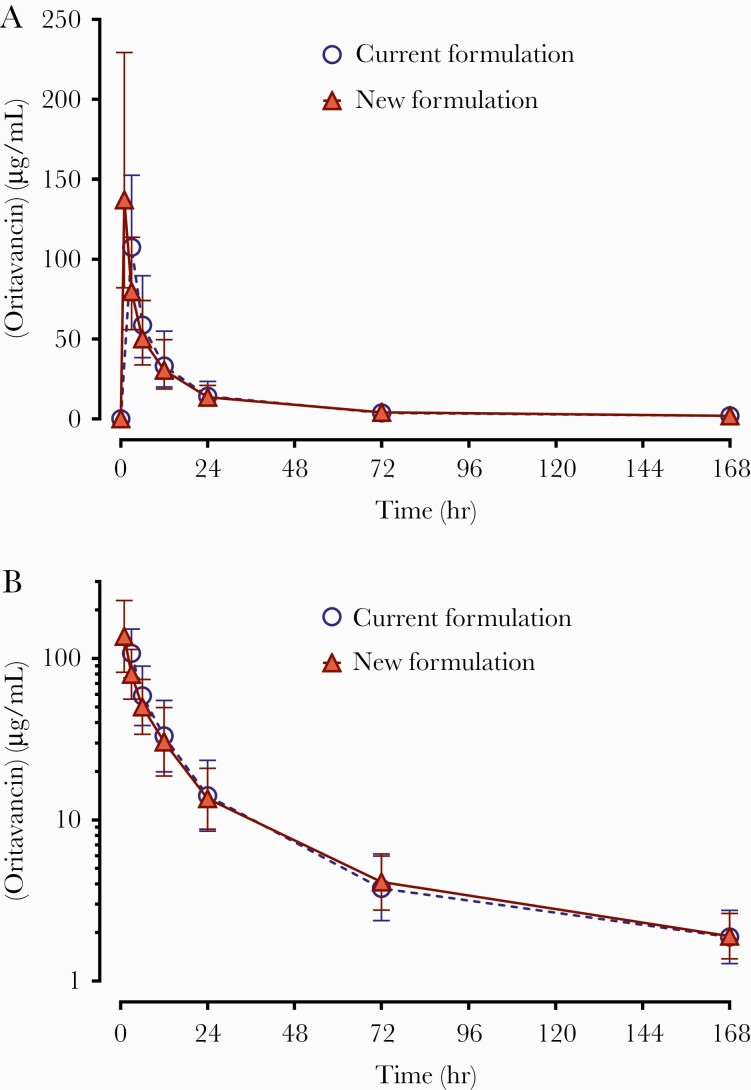
Geometric mean oritavancin plasma concentration-time profiles are presented in Figure 1. Nominal sample times were used in the calculation of means. The mean plasma drug concentration profiles were similar after administration of NF versus CF. Due to the shorter infusion time, the mean peak concentration of NF was higher than CF, and the time to reach maximum concentration was slightly faster with NF.
Figure 1.
Oritavancin plasma concentration (µg/mL) versus time (pharmacokinetic population) after a single 1200-mg intravenous infusion. Scaling: A, linear; B, semi-logarithmic. Symbols represent the geometric mean at each time point with +/- geometric standard deviation error bars.
Oritavancin Plasma Pharmacokinetic Parameters
Oritavancin PK parameters are presented in Table 2. The mean oritavancin Cmax was approximately 1.3-fold higher after administration of NF. Mean AUC0-72 and AUC0-168 were similar between NF and CF. Mean AUC0-72 was 1470 µg * h/mL for CF, compared with 1460 µg * h/mL for NF. Mean AUC0-168 was 1760 µg * h/mL for CF, relative to 1750 µg * h/mL for NF.
Table 2.
Oritavancin Mean (CV%) Plasma Pharmacokinetic Parameters (1200-mg Infusions)
| Parameter | Current Formulation | New Formulation |
|---|---|---|
| Dose (mg) | 1200 | 1200 |
| IV Infusion Duration | 3 hours | 1 hour |
| n | 50 | 50 |
| C max (µg/mL) | 112 (30.8) | 148 (29) |
| T max (hour)a | 3.29 (3.02–5.83) | 1.15 (0.90–3.17) |
| AUC0-72 (µg * h/mL) | 1470 (39.7) | 1460 (35.1) |
| AUC0-168 (µg * h/mL) | 1760 (41.4) | 1750 (35.0) |
Abbreviations: AUC, area under the concentration-time curve; CV, coefficient of variation; IV, intravenous.
Median (minimum-maximum).
For oritavancin, overall exposure as measured by AUC is the key PK parameter associated with PD efficacy. Analyses of relative exposure of the 2 formulations were conducted and the results shown in Table 3. As previously described, AUC results from certain patients were excluded for failure to meet acceptance criteria. The number of accepted results is indicated in Table 3 along with their least squares geometric means. The geometric mean ratios (GMRs) of the geometric means of AUC0-72 and AUC0-168 were within the range of 80% to 125% (110% for AUC0-72, 107% for AUC0-168). The lower limits of the 90% confidence interval (CI) of the GMR for both AUC0-72 and AUC0-168 were greater than 80% (95.0% and 92.3%, respectively). The upper limits of the 90% CI of the GMR for AUC0-72 and AUC0-168 were 126% and 123%, respectively.
Table 3.
Relative AUC Exposure Evaluation of Current vs New Formulations of Oritavancin
| AUC Parameter | Least Squares Geometric Mean | Ratio (%) of Geometric Means (Test/Reference) | 90% CI of the Ratio (Test/Reference) | |
|---|---|---|---|---|
| Reference | Test | |||
| AUC0-72 (µg * h/mL) | 1290 (n = 44) | 1410 (n = 43) | 110 | 95.0–126 |
| AUC0-168 (µg * h/mL) | 1580 (n = 42) | 1680 (n = 42) | 107 | 92.3–123 |
Abbreviations: AUC, area under the concentration-time curve; CI, confidence interval.
NOTE: Reference: Current formulation of oritavancin. Test: New formulation of oritavancin.
Safety Evaluation
The safety profile of oritavancin has been previously established in the phase 3 SOLO trials of CF [6, 10, 26]. Details of additional safety analysis are described in the Supplementary Materials.
In the current trial, a total of 31 (59.6%) patients in the CF group and 24 (48.0%) patients in the NF group reported at least 1 TEAE (Table 4). The most common TEAEs in the CF group versus the NF group were pruritus (7 patients vs 2 patients) and diarrhea (5 patients vs 3 patients). Compared with the CF group, urticaria was decreased in the NF group. However, mild or moderate chills and pyrexia were observed in 3 patients (6%) in the NF group versus 1 patient (1.9%) in the CF group during or shortly after completion of infusion.
Table 4.
Summary of Treatment-Emergent Adverse Events
| TEAE (Safety Analysis Set) | Current Formulation in 52 Patients (3-Hour IV) N (%) | New Formulation in 50 Patients (1-Hour IV) N (%) |
|---|---|---|
| Patients with at least 1 TEAE | 31 (59.6) | 24 (48.0) |
| Patients with a study drug-related TEAE | 20 (38.5) | 11 (22.0) |
| Patients with a severe or higher TEAE | 3 (5.8) | 1 (2.0) |
| Patients with a serious TEAE | 1 (1.9) | 2 (4.0) |
| Patients with a study drug-related serious TEAE | 0 (0.0) | 0 (0.0) |
| Patients with a TEAE leading to study drug discontinuation | 1 (1.9) | 0 (0.0) |
| Patients with a TEAE leading to study drug interruption | 3 (5.8) | 2 (4.0) |
| Patients with a TEAE of special interest (AESI) | 2 (3.8) | 2 (4.0) |
Abbreviations: AESI, adverse events of special interest; IV, intravenous; TEAE, treatment-emergent adverse event.
Twenty (38.5%) patients in the CF group and 11 (22.0%) patients in the NF group experienced at least 1 study-related TEAE that was considered related to a study drug. Although the majority of TEAEs for both groups were either mild or moderate, 3 (5.8%) patients in the CF group experienced 1 severe or higher TEAE each of hypersensitivity, cellulitis, and overdose (opiate), whereas 1 (2.0%) patient in the NF group experienced severe cellulitis.
No renal TEAEs were reported for any of the patients. A single patient had a history of chronic renal insufficiency (serum creatinine 2.5 mg/dL at baseline), which remained stable throughout the study. There was no evidence that oritavancin with HPβCD was associated with increased renal toxicity in this study.
A similar percentage of patients in the CF group (1.9% [1 patient]) and NF group (4.0% [2 patients]) had serious TEAEs that were not considered related to study drug. One (1.9%) patient in the CF group experienced a TEAE leading to study drug discontinuation, whereas 3 (5.8%) patients in the CF group and 2 (4.0%) patients in the NF group had a TEAE leading to study drug interruption.
Two patients in each treatment group experienced an AESI of hypersensitivity/infusion-related reaction. In the CF group, 1 patient with hypersensitivity/infusion-related reactions experienced pruritus and hives, which was considered treatment-related and resulted in drug interruption; the patient subsequently completed the infusion after diphenhydramine was given and completed the trial. The second patient in the CF group experienced an acute allergic reaction, which was considered treatment related. The patient did not complete study drug or the study due to the AE, and this AESI was scored as severe (grade 3). In the NF group, 1 patient experienced a hypersensitivity reaction several hours after the end of the study drug infusion. The investigator administered diphenhydramine and the event resolved by the following morning. The second patient in the NF group experienced a TEAE of infusion-related reaction (listed as Red Man Syndrome as a term) during the study treatment infusion that caused drug interruption of 13 minutes. The patient was given diphenhydramine, the infusion was restarted, and the event was considered resolved before the end of study treatment. Both events in the NF group were considered treatment related but did not result in study drug withdrawal. These 2 patients completed the trial. No pseudomembranous colitis/CDAD, osteomyelitis, or hemolytic anemia was reported in either the CF or NF group. Ultimately, there were no differences in AEs between the CF and NF groups, and all TEAEs were consistent with the known safety profile of the approved formulations of oritavancin.
DISCUSSION
This study compared the PK and tolerability of an NF versus CF of oritavancin. The PK profiles were evaluated after a single 1200-mg IV infusion using the NF infused over 1 hour compared with the CF infused over 3 hours in patients with ABSSSI. Oritavancin AUC0-72 is considered an appropriate exposure metric for the evaluation of PD effect [10]. In the primary relative exposure analysis, AUC0-72 was slightly higher in the NF group than the CF group, and the upper bound of the 80% to 125% 90% CI criteria was slightly exceeded (upper bound 126%). In contrast, 90% CI values for AUC0-168 fell within 80% to 125%. The results from the NF group indicate adequate exposure was achieved, similar to the CF group.
The AUC values for the entire PK population in the current study were compared with previous AUC results from 2 phase 3 studies in ABSSSI patients [28] and demonstrate similar exposures in both the NF and CF groups. The phase 3 studies estimated a mean (coefficient of variation) AUC0-72 of 1530 μg * h/mL (36.9%), with a median value of 1430 μg * h/mL. Although mean AUC0-72 was slightly lower in the present study compared with the phase 3 studies, the results indicate sufficient exposure is reached with NF to achieve the key PK parameter linked to efficacy. The results of this pharmacokinetic study have been summarized in the package insert [26] and were considered in the FDA approval.
Oritavancin is approved for the treatment of Gram-positive infections, including ABSSSIs. Studies have also demonstrated the efficacy and safety of oritavancin for the treatment of bacteremia and osteomyelitis, but these are unapproved uses for the drug and require further exploration [29–31]. In addition, because oritavancin is administered as a single dose for ABSSSI treatment, it is associated with reduced hospital admission rates and length of stay [32–34]. In particular, positive benefits were noted in cohorts that received oritavancin compared with cohorts that received vancomycin [35, 36].
Several reports examined the positive economic benefits of oritavancin use. Because oritavancin is administered as a single dose for the treatment of ABSSSI, it was found that hospital admission rates and length of stay were reduced in patients receiving oritavancin [32–34]. Positive benefits were noted particularly in cohorts that received oritavancin versus cohorts that received vancomycin [35, 36]. Kimyrsa may facilitate discharge from overwhelmed emergency rooms where bed turnover times are essential for care.
Both formulations of oritavancin were safe and well tolerated in ABSSSI patients, with no new safety signals identified. In addition, there was no evidence of an increase or change in TEAEs with decreased infusion time or higher Cmax between the CF and NF groups. Furthermore, the NF containing cyclodextrin HPβCD demonstrated no specific toxicity, including renal toxicity. Cyclodextrins improve drug delivery by affecting water solubility and bioavailability [37]. Older generation cyclodextrins concentrate in renal tubules and affect cell integrity; however, HPβCD has increased water solubility, decreased toxicity [38], and is used in telavancin, itraconazole, and diclofenac formulations. The total plasma clearance for HPβCD in all species tested is similar to the glomerular filtration rate, and 100% of a given dose is recovered in the urine within 6 to 12 hours after IV administration. Elimination half-life in humans is reported as 102 to 114 minutes [39]. The HPβCD is removed by hemodialysis, and no side effects were observed in participants receiving up to 24 grams of parenteral HPβCD daily for 15 days [40, 41]. Doses of up to 2.6 g/kg weekly have been used in children and young adults with Niemann-Pick disease [42]. Moreover, IV itraconazole containing 8 grams of HPβCD has been found to be safe [43]. One 1200-mg dose of IV oritavancin contains 2.4 grams of HPβCD [26]; hence, the toxicity risk remains low.
CONCLUSIONS
The NF can reduce the volume and duration of infusion compared with CF, thereby improving delivery to patients, especially those with congestive heart failure. In addition, NF can be administered using NS or D5W in the infusion solution, thereby increasing the flexibility to meet patient needs, especially those with unstable insulin-dependent diabetes. The NF exhibits very similar PK exposure in ABSSSI patients as CF, and it is expected to produce similar efficacy and safety as CF.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
Author contributions. R. K. H. provided substantial contribution to conception, design of the work; writing the initial draft of paper; acquisition, analysis, and interpretation of data; and development of tables and figures from study report. M. K., K. C. M., and K. S. contributed to critical revision of content and review of references. M. R. reviewed the of all work by authors and incorporating comments into final paper. All authors provided final approval of the paper and agreed to be accountable for the submitted work.
Disclaimer. M. K., K. C. M., and K. S. did not participate in designing and administering the study or data collection and analysis.
Financial support. This work was funded by Melinta Therapeutics.
Potential conflicts of interest. M. R. is currently an employee of Melinta Therapeutics, and R. K. H. is a consultant to Melinta Therapeutics (Morristown, NJ) at the time of study conduct and data collection. K. C. M. has served on an Advisory Board for Shionogi Inc. M. K. has served on an Advisory Board for Melinta, and research has been provided by Allergan/AbbVie in the form of drug supply. K. S. has been a consultant to Melinta Therapeutics. Editorial support was provided under the direction of the authors by Strategix, an affiliate of The Lynx Group, LLC. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Lipsky BA, Moran GJ, Napolitano LM, et al. Prospective, multicenter, observational study of complicated skin and soft tissue infections in hospitalized patients: clinical characteristics, medical treatment, and outcomes. BMC Infect Dis 2012; 12:227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Garau J, Ostermann H, Medina J, et al. Europe (2010-2011): assessment of clinical practice patterns and real-life effectiveness of antibiotics from the REACH study. Clin Microbiol Infect 2013; 19:E377–85. [DOI] [PubMed] [Google Scholar]
- 3. Klein EY, Sun L, Smith DL, Laxminarayan R.. The changing epidemiology of methicillin-resistant Staphylococcus aureus in the United States: a national observational study. Am J Epidemiol 2013; 177:666–74. [DOI] [PubMed] [Google Scholar]
- 4. Diekema J, Pfaller MA, Shortridge D, et al. Twenty-year trends in antimicrobial susceptibilities among Staphylococcus aureus from the SENTRY antimicrobial surveillance program. Open Forum Infect Dis 2019; 6:S47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tong SYC, Davis JS, Eichenberger E, Holland TL, Fowler VG Jr. Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin Microbiol Rev 2015; 28:603–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Orbactiv [package insert]. Lincolnshire, IL: Melinta Therapeutics, LLC; 2021. [Google Scholar]
- 7. European Medicines Agency, Tenkasi (previously Orbactiv); Summary of Product Characteristics. Available at: https://www.ema.europa.eu/en/documents/product-information/tenkasi-previously-orbactiv-epar-product-information_en.pdf. Accessed 8 January 2022.
- 8. Patti GJ, Kim SJ, Yu TY, et al. Vancomycin and oritavancin have different modes of action in Enterococcus faecium. J Mol Biol 2009; 392:1178–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mendes RE, Woosley LN, Farrell DJ, et al. Oritavancin activity against vancomycin-susceptible and vancomycin-resistant enterococci with molecularly characterized glycopeptide resistance genes recovered from bacteremic patients (2009–2010). Antimicrob Agents Chemother 2012; 56:1639–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bhavnani SM, Hammel JP, Rubino CM, et al. . Oritavancin pharmacokinetic-pharmacodynamic analyses for efficacy based on data from patients with acute bacterial skin and skin structure infections enrolled in SOLO I and II. Poster A-1309. 54th Interscience Conference on Antimicrobial Agents and Chemotherapy (Washington, DC). September 5–9, 2014.
- 11. Boylan CJ, Campanale K, Iversen PW, et al. Pharmacodynamics of oritavancin (LY–33328) in a neutropenic-mouse thigh model of Staphylococcus aureus infection. Antimicrob Agents Chemother 2003; 47:1700–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Belley A, Lalonde Seguin D, Arhin FF, Moeck G.. Comparative in vitro activities of oritavancin, dalbavancin, and vancomycin against methicillin-resistant Staphylococcus aureus isolates in a nondividing state. Antimicrob Agents Chemother 2016; 60:4342–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Biedenbach DJ, Arhin FF, Moeck G, et al. In vitro activity of oritavancin and comparator agents against staphylococci, streptococci and enterococci from clinical infections in Europe and North America, 2011-2014. Intern J Antimicrob Agents 2015; 46:674–81. [DOI] [PubMed] [Google Scholar]
- 14. McKay GA, Beaulieu S, Arhin FF, et al. Time-kill kinetics of oritavancin and comparator agents against Staphylococcus aureus, Enterococcus faecalis and Enterococcus faecium. J Antimicrob Chemother 2009; 63:1191–9. [DOI] [PubMed] [Google Scholar]
- 15. Belley A, Harris R, Beveridge T, Parr T Jr, Moeck G.. Ultrastructural effects of oritavancin on methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus. Antimicrob Agents Chemother 2009; 53:800–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Belley A, Mckay GA, Arhin FF, et al. Oritavancin disrupts membrane integrity of Staphylococcus aureus and vancomycin-resistant Enterococci to effect rapid bacterial killing. Antimicrob Agents Chemother 2010; 54:5369–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Arhin FF, Draghi DC, Pillar CM, Parr TR Jr, Moeck G, Sahm DF.. Comparative in vitro activity profile of oritavancin against recent Gram-positive clinical isolates. Antimicrob Agents Chemother 2009; 53:4762–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Saravolatz L, Pawlak J, Johnson L.. In vitro activity of ceftaroline against community-associated methicillin-resistant, vancomycin-intermediate, vancomycin-resistant, and daptomycin-nonsusceptible Staphylococcus aureus isolates. Antimicrob Agents Chemother 2010; 54:3027–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kim SJ, Cegelski L, Preobrazhenskaya M, Schaefer J.. Structures of Staphylococcus aureus cell-wall complexes with vancomycin, eremomycin, and chloroeremomycin derivatives by 13C{19F}and 15N{19F} rotational-echo double resonance. Biochemistry 2006; 45:5235–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kim SJ, Cegelski L, Stueber S, et al. Oritavancin exhibits dual mode of action to inhibit cell-wall biosynthesis in Staphylococcus aureus. J Mol Biol 2008; 377:281–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Allen NE. From vancomycin to oritavancin: the discovery and development of a novel lipoglycopeptide antibiotic. Anti-Infect Agents Med Chem 2010; 9:23–7. [Google Scholar]
- 22. Arhin FF, Sarmiento I, Parr TR Jr, Moeck G.. Comparative in vitro activity of oritavancin against Staphylococcus aureus strains that are resistant, intermediate or heteroresistant to vancomycin. J Antimicrob Chemother 2009; 64:868–70. [DOI] [PubMed] [Google Scholar]
- 23. O’Connor R, Baines SD, Freeman J, Wilcox MH.. In vitro susceptibility of genotypically distinct and clonal Clostridium difficile strains to oritavancin. J Antimicrob Chemother 2008; 62:762–5. [DOI] [PubMed] [Google Scholar]
- 24. Freeman, J, Marquis M., Crowther GS., et al. Oritavancin does not induce Clostridium difficile germination and toxin production in hamsters or a human gut model. J Antimicrob Chemother 2012; 67:2919–26. [DOI] [PubMed] [Google Scholar]
- 25. Chilton CH, Freeman J, Crowther GS, et al. Effectiveness of a short (4 day) course of oritavancin in the treatment of simulated Clostridium difficile infection using a human gut model. J Antimicrob Chemother 2012; 67:2434–7. [DOI] [PubMed] [Google Scholar]
- 26. Kimyrsa® (oritavancin) Full Prescribing Information [package insert]. Morristown, NJ: Melinta Therapeutics, LLC; 2021. [Google Scholar]
- 27. Griffith DC. Hydroxy Propyl-β-Cyclodextrin as an Excipient in the New Formulation of Orbactiv. San Diego, CA: The; Medicines Company; 2015. [Google Scholar]
- 28. Rubino CM, Bhavnani SM, Moeck G, Bellibas SE, Ambrose PG.. Population pharmacokinetic analysis for a single 1200-milligram dose of oritavancin using data from two pivotal phase 3 clinical trials. Antimicrob Agents Chemother 2015; 59:3365–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Redell M, Sierra-Hoffman M, Assi M, et al. The CHROME Study, a real-world experience of single- and multiple-dose oritavancin for treatment of Gram-positive infections. Open Forum Infect Dis 2019; 6:ofz479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Scoble PJ, Reilly J, Tillotson G.. Real-world use of oritavancin for the treatment of osteomyelitis. Drugs Real World Outcomes 2020; 7:46–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Van Hise NW, Chundi V, Didwania V, et al. Treatment of acute osteomyelitis with once-weekly oritavancin: a two-year, multicenter, retrospective study. Drugs Real World Outcomes 2020; 7:41–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Brownell LE, Adamsick ML, McCreary EK, et al. Clinical outcomes and economic impact of oritavancin for Gram-positive infections: a single academic medical center health system experience. Drugs Real World Outcomes 2020; 7:13–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Estrada S, Lodise TP, Tillotson GS, Delaportas D.. The real-world economic and clinical management of adult patients with skin and soft tissue infections (SSTIs) with oritavancin: data from two multicenter observational cohort studies. Drugs Real World Outcomes 2020; 7:6–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Whittaker C, Lodise TP, Nhan E, et al. Expediting discharge in hospitalized, adult patients with skin and soft tissue infections who received empiric vancomycin therapy with oritavancin: description of findings from an institutional pathway. Drugs Real World Outcomes 2020; 7:30–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Helton B, MacWhinnie A, Minor SB, et al. Early directed oritavancin therapy in the emergency department may lead to hospital avoidance compared to standard treatment for acute bacterial skin and skin structure infections: a real-world retrospective analysis. Drugs Real World Outcomes 2020; 7:20–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Williams B, Muklewicz J, Steuber TD, et al. . Comparison of inpatient standard-of-care to outpatient oritavancin therapy for patients with acute uncomplicated cellulitis. J Pharm Pract 2021. doi: 10.1177/08971900211021258. [DOI] [PubMed] [Google Scholar]
- 37. European Medicines Agency. EMA/CHMP/333892/2013 Committee for Human Medicinal Products (CHMP). Cyclodextrins used as excipients. Report published in support of the “Questions and answers on cyclodextrins used as excipients in medicinal products for human use” (EMA/CHMP/495747/2013). Available at: https://www.ema.europa.eu/en/documents/scientific-guideline/questions-answers-cyclodextrins-used-excipients-medicinal-products-human-use_en.pdf. Accessed 14 March 2022.
- 38. Gould S, Scott RC.. 2-Hydroxypropyl-beta-cyclodextrin (HP-beta-CD): a toxicology review. Food Chem Toxicol 2005; 43:1451–9. [DOI] [PubMed] [Google Scholar]
- 39. Stella VJ, He Q.. Cyclodextrins. Toxicol Pathol 2008; 36:30–42. [DOI] [PubMed] [Google Scholar]
- 40. Mohr JF, Finkel KW, Rex JH, et al. Pharmacokinetics of intravenous itraconazole in stable hemodialysis patients. Antimicrob Agents Chemother 2004; 48:3151–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Loftsson T, Jarho P, Masson M, Jarvinen T.. Cyclodextrins in drug delivery. Expert Opin Drug Deliv 2005; 2:335–51. [DOI] [PubMed] [Google Scholar]
- 42. Hastings C, Vieira C, Liu B, et al. Expanded access with intravenous hydroxypropyl-β-cyclodextrin to treat children and young adults with Niemann-Pick disease type C1: a case report analysis. Orphanet J Rare Dis 2019; 14:228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Buchanan CM, Buchanan NL, Edgar KJ, et al. Pharmacokinetics of itraconazole after intravenous and oral dosing of itraconazole‐cyclodextrin formulations. J Pharm Sci 2007; 96:3100–16. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.