Abstract
Background
Data conflict on whether vaccination decreases severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) viral load. The objective of this analysis was to compare baseline viral load and symptoms between vaccinated and unvaccinated adults enrolled in a randomized trial of outpatient coronavirus disease 2019 (COVID-19) treatment.
Methods
Baseline data from the first 433 sequential participants enrolling into the COVID-OUT trial were analyzed. Adults aged 30–85 with a body mass index (BMI) ≥25 kg/m2 were eligible within 3 days of a positive SARS-CoV-2 test and <7 days of symptoms. Log10 polymerase chain reaction viral loads were normalized to human RNase P by vaccination status, by time from vaccination, and by symptoms.
Results
Two hundred seventy-four participants with known vaccination status contributed optional nasal swabs for viral load measurement: median age, 46 years; median (interquartile range) BMI 31.2 (27.4–36.4) kg/m2. Overall, 159 (58%) were women, and 217 (80%) were White. The mean relative log10 viral load for those vaccinated <6 months from the date of enrollment was 0.11 (95% CI, –0.48 to 0.71), which was significantly lower than the unvaccinated group (P = .01). Those vaccinated ≥6 months before enrollment did not differ from the unvaccinated with respect to viral load (mean, 0.99; 95% CI, –0.41 to 2.40; P = .85). The vaccinated group had fewer moderate/severe symptoms of subjective fever, chills, myalgias, nausea, and diarrhea (all P < .05).
Conclusions
These data suggest that vaccination within 6 months of infection is associated with a lower viral load, and vaccination was associated with a lower likelihood of having systemic symptoms.
Keywords: SARS-CoV-2, symptoms, vaccines, viral load
Breakthrough coronavirus disease 2019 (COVID-19) infections after vaccination do occur, and there are conflicting data on the influence of vaccination on the viral load of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). With the emergence of the Delta variant of SARS-COV-2, COVID-19 cases have dramatically increased worldwide, and breakthrough infections have occurred at increasing rates among those who were previously considered “fully vaccinated” (ie, having received 2 doses of the Moderna or Pfizer vaccines or 1 dose of the Johnson and Johnson vaccine).
Several investigations of breakthrough infections have reported similar viral loads between vaccinated and unvaccinated individuals. In the Provincetown, Massachusetts, outbreak, similar polymerase chain reaction (PCR) cycle threshold (Ct) values were observed among 84 not fully vaccinated (median PCR Ct, 21.54) and 127 vaccinated individuals (median Ct, 22.77) [1]. Similarly, in a 719-person Wisconsin study during July 2021, when specimens were collected at a mode of 2 days post–symptom onset, no difference in SARS-CoV-2 viral load was observed between vaccinated and unvaccinated individuals [2].
However, other investigations of breakthrough cases suggest that while initial viral loads are similar to the Delta variant, vaccinated persons have a more rapid decline in SARS-CoV-2 viral load. This was observed in Singapore among 71 vaccinated persons compared with 130 unvaccinated persons and in a study in California of 171 fully vaccinated and 198 unvaccinated individuals [3, 4].
The objective of this analysis was to assess for an association between prior vaccination on baseline viral load levels and symptoms among individuals enrolling in a nationwide randomized clinical trial testing early outpatient treatment for COVID-19. We hypothesized that the viral load from participants who had not been vaccinated would be higher compared with that of those who had been vaccinated and that those vaccinated within 6 months would have a lower viral load than those vaccinated >6 months ago. We further hypothesized that individuals who had been vaccinated would have less severe symptoms and that symptom severity would correlate with viral load.
METHODS
Study Design
This is an analysis of baseline data collected from patients who enrolled in a phase 3, double-blind, factorial randomized, placebo-controlled COVID-OUT trial of early outpatient COVID-19 treatment (ClinicalTrials.gov NCT04510194). The trial is fully remote, with no in-person visits. Patients received all study materials by FedEx or same-day courier, including a paper daily symptom log to fill out. Participants could opt into the collection of nasal swab samples at days 1, 5, and 10. As such, this cross-sectional study was necessarily limited to those participating in voluntary biospecimen collection.
Study Sample
The trial enrolled adults aged 30–85 years throughout the United States and is ongoing. Inclusion criteria included a positive test for SARS-CoV-2 within the past 3 days and no known history of confirmed SARS-CoV-2 infection. Participants for the trial were limited to those with a body mass index (BMI) ≥25 kg/m2 (or ≥23 kg/m2 for participants of Latinx or Asian race/ethnicity) by self-reported height and weight. Exclusion criteria included currently hospitalized; symptom onset ≥7 days (though asymptomatic individuals may enroll); immune-compromised state; history of unstable heart, liver, or kidney disease; use of insulin or sulfonylureas; other medication exclusions.
Laboratory Procedures
Participants opted into self-collection of nasal swabs then received the supplies for collection of anterior-nares nasal swabs. They received written instructions with pictures on how to collect the anterior midturbinate nasal swabs, as well as instructions over the phone from research coordinators. Once collected, the samples were placed in the participants’ refrigerators, and the research coordinators arranged for the samples to be returned to the lab via overnight FedEx. Just before being placed outside for FedEx pickup, participants placed the samples in a small Styrofoam cooler with activated instant cold packs, and then the Styrofoam was placed in a rigid cardboard box.
The nasal samples were placed into tubes that contained Smart Transport Medium. They were received, processed, and tested at the Advanced Research and Diagnostic Laboratory (ARDL), a Clinical Laboratory Improvement Amendments–certified lab at the University of Minnesota. Testing was performed with the HDPCR SARS-CoV-2 assay (ChromaCode) on QuantStudio 7 (Applied Biosystems) using a validated extractionless protocol, and results were captured with the StarLims Laboratory Information Management System (Abbott). The final result is derived from Ct values from 3 targets, N1, N2, and RNAse P. N1 and N2 are targets in the nucleocapsid protein, and RNAse P is used as the internal control target.
Viral load is calculated relative to the human RNAse P (RP): 2^[CTRP – (N1 + N2)/2]. This calculation normalizes the raw Ct value for the SARS-CoV-2 viral targets (N1 and N2) to the Ct value of the human RP internal sample control. This provides a normalized relative value of the amount of viral RNA compared with total human nucleic acid to compensate for sampling quality [5, 6]. When N1 and N2 were undetected, they were replaced with a value of 45, the maximum number of cycles.
Statistical Methods
We utilized standard descriptive statistics to summarize the distribution of baseline covariates by COVID-19 vaccination status. Normally distributed continuous variables were compared using the Student t test with unequal variances, whereas skewed variables were compared using the Wilcoxon rank-sum test. Categorical variables were compared using the Fisher exact test, and a quasi-Poisson regression model was used to compare counts while accounting for overdispersion. We computed the standardized mean difference to assess the covariate balance between COVID-19 vaccination status groups. Covariates with a standardized mean difference >0.10 were adjusted for in subsequent analyses to reduce the chance of a biased association due to potential confounding.
We dichotomized symptom severity as moderate or severe vs absent or mild for a 10-symptom daily log [7]. Participants were defined as having a loss of smell or taste if their sense of smell or taste was less than usual or completely absent. Participants were defined as having vomited or had diarrhea in the last 24 hours if they vomited at least once or had diarrhea at least 3 times in the last 24 hours, respectively. We compared the proportion of participants with COVID-19 symptoms at baseline by vaccination status and log10 viral load tertiles using the Fisher exact test. The latter was achieved by grouping participants into log10 viral load tertiles using baseline measurements for all study participants, regardless of whether they reported symptom data.
To assess the association between log10 viral load and vaccination status, we fit a linear regression model initially treating vaccination status as binary. We then dichotomized the vaccinated subgroup based on date of vaccination to create a trichotomous variable with the following categories: unvaccinated, vaccinated within 6 months of enrollment, and vaccinated at least 6 months before enrollment. We assumed that all vaccinated individuals randomized before July 15, 2021, were vaccinated within 6 months of enrollment due to when the coronavirus vaccines became available. A linear regression model regressing log10 viral load against the trichotomous predictor was used to assess the impact of the duration of antecedent vaccination on viral load. A sensitivity analysis looking at those vaccinated within 4 months vs ≥4 months was also completed.
Of further interest was whether the relationship between log10 viral load and vaccination status was impacted by the emergence of the Delta variant. To this end, we fit 2 additional linear regression models regressing log10 viral load against vaccination status after stratifying by the emergence of the Delta variant. Participants were considered to have joined the study before the emergence of the Delta variant if their randomization date was before June 19, 2021, and after the emergence of the Delta variant if their randomization date was June 19, 2021, or later. This date has been used by the Centers for Disease Control and Prevention to indicate pre-Delta vs post-Delta times [8]. Between June 19 and July 15, 2021, only 3 trial participants were enrolled; thus the assumption of dates for vaccination and Delta variant are relatively distinct.
All analyses were conducted using R, version 4.1.1, and we used 2-sided P values <.05 for statistical significance.
Patient Consent
This protocol was reviewed by the Food and Drug Administration, investigational new drug license #152439. Institutional review board (IRB) approval for this protocol was obtained from the Advarra Central IRB (protocol MET29324) in compliance with the International Conference on Harmonization Good Clinical Practice Guideline. Written consent was obtained from all participants. The independent data safety monitoring board approved the release of baseline data.
RESULTS
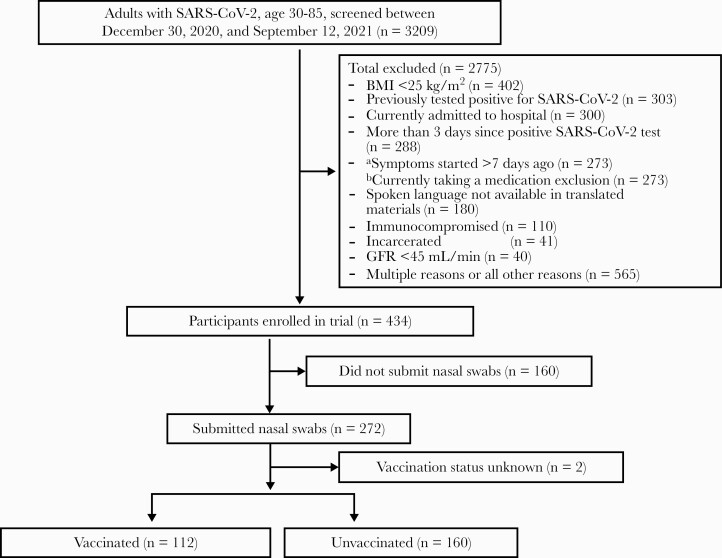
Among 434 sequential consented participants who had enrolled in the COVID-OUT trial through September 12, 2021, 274 agreed to provide optional self-collect nasal swabs for SARS-CoV-2 PCR and returned a specimen. Of these 274 participants, 272 provided vaccination status and were considered for analysis in this study. Of the 272, 112 (41%) were fully vaccinated, of whom 94 were vaccinated within 6 months of enrollment. Date of vaccination was available for 17 of the remaining 18 vaccinated patients, all of whom were vaccinated at least 6 months before enrollment (Figure 1). Supplementary Table 1 compares those who submitted nasal swabs with those who did not. Most notably, those who submitted swabs had more chronic medical conditions, had a higher mean BMI, and were more likely to have a loss of smell.
Figure 1.
Overview of the participants whose nasal swabs were included in the analysis. aSymptoms not required for participation. bMedication exclusion list: metformin, insulin, cimetidine, hydroxychloroquine, sulfonylurea, dolutegravir, dolutegravir, patiromir, ranalazine, tafenoquine, ivennectin, sodium picosulfate, lithium, valproate, fluvoxamine, rasagiline, selegiline, MAOis, linezolid, duloxetine, methylene blue, tizanidine, ramelteon, alosetron, agomelatine, bromopride, dapoxetine, tamsimelteon, thioridazine, urokinase, pimozide. Dose-dependent: SSRI, SNRI, tricyclic antidepressant, alprazolam, diazepam, theophylline, clozapine, olanzapine, NSAIDs, aspirin, warfarin, phenytoin, clopidrogrel, St. John’s wart, high-dose antipsychotic. Abbreviations: BMI, body mass index; CKD, chronic kidney disease; GFR, glomerular filtration rate; NSAIDs, nonsteroidal anti-inflammatory drugs; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SNRI, serotonin and norepinephrine reuptake inhibitor; SSRI, selective serotonin reuptake inhibitors.
The demographics for the overall study population and by vaccination status are provided in Table 1. The median age (interquartile range [IQR]) was 46 (38–53) years, and the median body mass index (IQR) was 31.2 (27.4–36.4) kg/m2. Overall, 159 (58%) were women, and 217 (80%) were White. The median PCR Ct at baseline (IQR) was 24.3 (19.8–30.6). Vaccinated participants had a higher median PCR Ct value (IQR) of 25.8 (20.6–31.2), reflective of a lower viral load than unvaccinated participants, who had a median PCR Ct value (IQR) of 23.1 (19.4–29.0). The mean log10 viral load for the overall study population (SD) was 0.78 (3.0); the log10 viral load was lower in the vaccinated group, 0.26 (2.6), compared with the unvaccinated group, 1.1 (3.2). The missingness for baseline symptoms was 24% as it was a secondary outcome. Among those for whom baseline COVID-19 symptoms were reported, the vaccinated group had fewer moderate or severe symptoms (mean [SD], 2.8 [2.3] symptoms) than the unvaccinated group (mean [SD], 3.4 [2.7] symptoms).
Table 1.
Demographic Characteristics, Comorbidities, and Baseline Symptoms
| Overall (n = 272) | Unvaccinated (n = 160) | Vaccinated (n = 112) | P Value | |
|---|---|---|---|---|
| Women, No. (%) | 159 (58) | 99 (62) | 60 (54) | .17 |
| Age, median [IQR], y | 46 [38–53] | 44 [38–51] | 47 [39–56] | .02 |
| BMI, median [IQR], kg/m2 | 31.2 [27.4–36.4] | 31.5 [27.8–36.0] | 31.0 [27.3–37.0] | .27 |
| Race, No. (%) | ||||
| Native American | 6 (2.2) | 2 (1.2) | 4 (3.6) | .39 |
| Asian | 6 (2.2) | 1 (0.6) | 5 (4.5) | .09 |
| Native Hawaiian or Pacific Islander | 2 (0.7) | 0 (0) | 2 (1.8) | .33 |
| Black or African American | 16 (5.9) | 11 (6.9) | 5 (4.5) | .57 |
| White | 217 (80) | 133 (83) | 84 (75) | .14 |
| Other/declined | 17 (6.2) | 8 (5.0) | 9 (8.0) | .45 |
| Insurance status, No. (%) | ||||
| Private | 170 (66) | 97 (63) | 73 (72) | <.01 |
| Public | 29 (11) | 23 (15) | 6 (5.9) | |
| Medicare | 24 (9.4) | 10 (6.5) | 14 (14) | |
| None | 33 (13) | 25 (16) | 8 (7.9) | |
| No. of high-risk comorbiditiesa (+SD) | 0.45 (+0.55) | 0.44 (+0.56) | 0.46 (+0.55) | .70 |
| Days from symptom onset to sample collection, median [IQR] | 5 [4–6] | 5 [4–6] | 5 [4–6] | .68 |
| No. of symptoms,b mean (+SD) | 3.2 (+2.5) | 3.4 (+2.7) | 2.8 (+2.3) | .08 |
| Vomited in the last 24 h, No. (%) | 21 (10) | 13 (10) | 8 (9.9) | 1.0 |
| Diarrhea in the last 24 h, No. (%) | 26 (13) | 23 (19) | 3 (3.7) | <.01 |
| Loss of taste, No. (%) | 124 (60) | 71 (56) | 53 (65) | .19 |
| Loss of smell, No. (%) | 122 (59) | 71 (56) | 51 (63) | .39 |
| No. of asymptomatic (%) | 24 (12) | 13 (10) | 11 (14) | .51 |
Abbreviations: BMI, body mass index; IQR, interquartile range.
Mean (+SD) of high-risk comorbidities, including diabetes mellitus, coronary artery disease, congestive heart failure, or obesity.
Moderate or severe value for symptoms: chills or shivering, cough, feeling hot or feverish, headache, fatigue, muscle or body ache, nausea, shortness of breath or difficulty breathing, sore throat, or stuffy or runny nose.
The cycle threshold mean and median were lower for the participants who were unvaccinated (Table 2). A higher percentage of unvaccinated individuals were in the lowest PCR cycle threshold group compared with vaccinated individuals (31% vs 21%).
Table 2.
PCR Cycle Threshold
| Overall | Unvaccinated | Vaccinated | P Value | |
|---|---|---|---|---|
| Overall, median [IQR] | 24.3 [19.8–30.6] | 23.1 [19.4–29.0] | 25.8 [20.6–31.2] | .02 |
| Overall, mean (+SD) | 25.8 (+7.6) | 25.0 (+7.5) | 27.1 (+7.6) | .03 |
| <20, No. (%) | 72 (26) | 49 (31) | 23 (21) | .25 |
| 20–<25, No. (%) | 76 (28) | 46 (29) | 30 (27) | |
| 25–<30, No. (%) | 50 (18.4) | 27 (17) | 23 (20.5) | |
| 30–<35, No. (%) | 34 (12.5) | 19 (12) | 15 (13) | |
| >35, No. (%) | 40 (15) | 19 (12) | 21 (19) | |
| Log10 viral load, mean (+SD) | 0.78 (+3.0) | 1.14 (+3.2) | 0.26 (+2.6) | .01 |
Abbreviations: IQR, interquartile range; PCR, polymerase chain reaction.
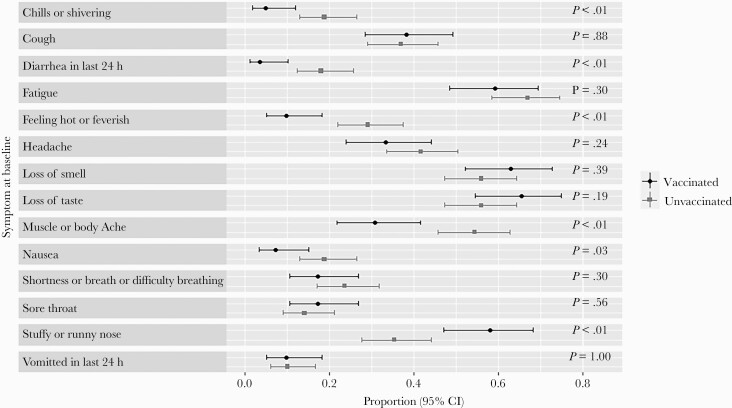
A larger proportion of unvaccinated vs vaccinated participants reported chills or shivering: 0.19 (24/127) vs 0.05 (4/81; P < .01); diarrhea in the last 24 hours: 0.18 (23/127) vs 0.04 (3/81; P < .01); feeling hot or feverish: 0.29 (37/127) vs 0.10 (8/81; P < .01); muscle or body aches: 0.54 (69/127) vs 0.31 (25/81; P < .01); and nausea: 0.19 (24/127) vs 0.07 (6/81; P = .03). Conversely, a larger proportion of vaccinated vs unvaccinated participants had a stuffy or runny nose: 0.58 (47/81) vs 0.35 (45/127; P < .01). The vaccinated and unvaccinated subgroups did not significantly differ with respect to the frequency of cough, headache, loss of smell, loss of taste, fatigue, shortness of breath or difficulty breathing, sore throat, and vomiting (Figure 2).
Figure 2.
COVID-19 symptoms by COVID-19 vaccination status. Proportions reflect the number of eligible patients with a moderate or severe symptom at baseline, as well as presence of symptoms for diarrhea in the last 24 hours, vomited in the last 24 hours, loss of smell, or loss of taste. Ninety-five percent Wilson score confidence intervals and P values for the differences in proportions are provided. Vaccinated participants had less frequent chills, diarrhea, subjective fever, myalgias, and nausea than unvaccinated participants. Abbreviation: COVID-19, coronavirus disease 2019.
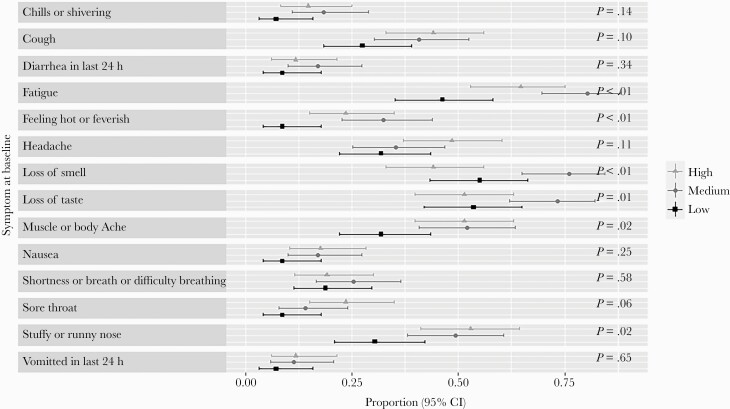
Figure 3 displays the proportion of participants with moderate or severe COVID-19 symptoms at baseline and associated 95% Wilson score CIs after dividing participants into the low viral load tertile (–10.3 to –0.32), medium viral load tertile (–0.32 to 1.95), and high viral load tertile (1.95 to 8.82). The proportion of participants with a given symptom significantly varied by viral load tertiles for subjective fever (P < .01), loss of smell (P < .01), loss of taste (P = .01), fatigue (P < .01), myalgia (P = .02), and stuffy or runny nose (P = .02), with the middle tertile containing the largest proportion of sick participants for each of these symptoms besides stuffy or runny nose. There were no significant differences between viral load tertile groups at baseline for the following symptoms: chills, cough, diarrhea, headache, nausea, dyspnea, sore throat, and vomiting.
Figure 3.
COVID-19 symptoms by log10 viral load tertile. Proportions reflect the number of eligible patients with a moderate or severe symptom at baseline, as well as presence of symptoms for diarrhea in the last 24 hours, vomited in the last 24 hours, loss of smell, or loss of taste. Ninety-five percent Wilson score confidence intervals and P values for the differences in proportions are provided. Abbreviation: COVID-19, coronavirus disease 2019.
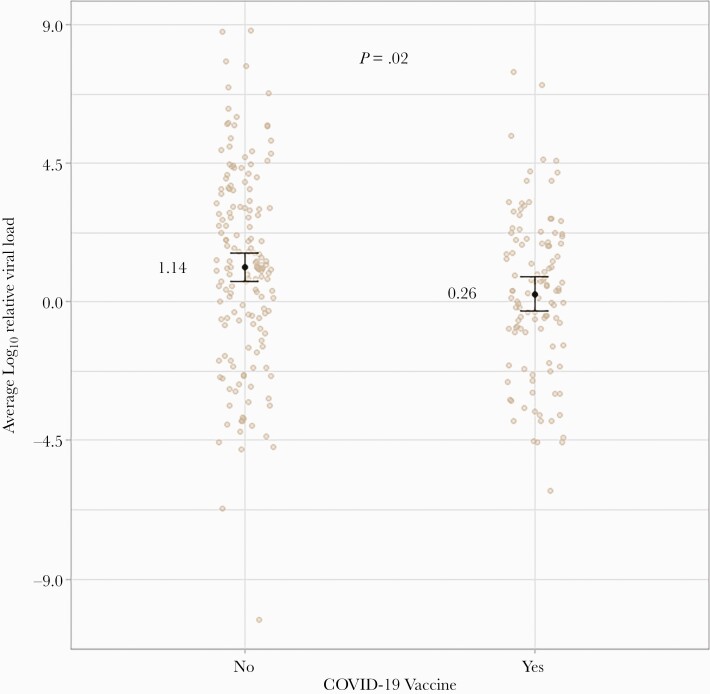
Log10 viral load values by COVID-19 vaccination status are presented in Figure 4. Each beige dot represents a unique observation, whereas error bars reflect average log10 viral load values and associated 95% CIs. The expected log10 viral load value for unvaccinated participants was 1.14 (95% CI, 0.68 to 1.60), which was significantly larger than the mean value of 0.26 (95% CI, –0.29 to 0.81) observed for those who received a vaccine (P = .02). The log10 viral load values also appear to be more dispersed for unvaccinated patients. In this study sample, log10 viral load values ranged from –6.14 to 7.48 for vaccinated and –10.3 to 8.82 for unvaccinated participants.
Figure 4.
SARS-CoV-2 log10 viral load value by COVID-19 vaccination status. The beige dots reflect each observation in the study sample, whereas error bars reflect average log10 viral load values and associated 95% confidence intervals. Random jittering was applied along the horizontal axis for visual clarity. Abbreviations: COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
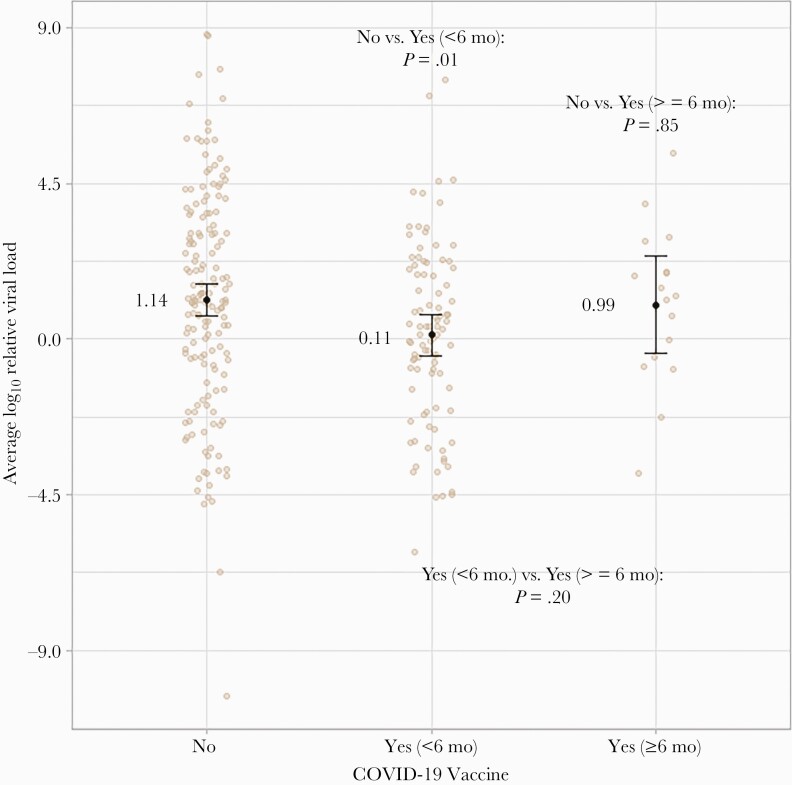
Figure 5 displays a similar plot for the trichotomous variable that considers vaccination duration before enrollment. Notably, the significant difference in average log10 viral load values between vaccinated and unvaccinated individuals found previously appears to be driven by those who were vaccinated within 6 months of enrollment. Whereas log10 viral load values were, on average, 1.03 units lower for participants who were vaccinated within 6 months (mean, 0.11; 95% CI, –0.48 to 0.71; P = .01) compared with the unvaccinated, this difference attenuated to –0.15 for participants who were vaccinated ≥6 months before enrollment (mean, 0.99; 95% CI, –0.41 to 2.4; P = .85). It is worth noting that the small sample size for the group of individuals vaccinated ≥6 months limited the power of the latter comparison. There was no difference in RNase between groups (Supplementary Figure 1). The sensitivity analysis using a cutoff of 4 months also found that those vaccinated within 4 months before enrollment (n = 39) had a lower viral load than those who were unvaccinated, but not those vaccinated ≥4 months before enrollment (n = 58) (Supplementary Figure 2).
Figure 5.
SARS-CoV-2 log10 viral load values by COVID-19 vaccination status and duration of antecedent vaccination. The beige dots reflect each observation in the study sample, whereas error bars reflect average log10 viral load values and associated 95% confidence intervals. Random jittering was applied along the horizontal axis for visual clarity. Abbreviations: COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
The relationship between log10 viral load and vaccination status after stratifying by June 19, 2021 (an assumed relation to the emergence of the Delta variant), is presented in Supplementary Figure 3. One hundred nineteen study participants were randomized before June 19, 2021, of whom 30 (25%) were vaccinated. Of the remaining 153 participants, 82 (54%) were vaccinated. Before the emergence of the Delta variant, vaccinated individuals had significantly lower log10 viral load values than unvaccinated individuals (vaccinated: mean, –0.44; 95% CI, –1.46 to 0.58; vs unvaccinated: mean: 1.22; 95% CI, 0.62 to 1.81; P < .01). However, this association between viral load and vaccination status was no longer significant after the Delta variant emerged (P = .28).
As a sensitivity analysis, we adjusted linear regression models for age, BMI, sex, and self-identified White vs non-White race. The results for the adjusted analyses are provided in Supplementary Tables 2 and 3 and do not meaningfully differ from the unadjusted analyses.
DISCUSSION
This primary data collection adds to growing data about viral load after vaccination against SARS-CoV-2 and about the timing of vaccination. In this sample of participants diagnosed with SARS-CoV-2 infection within 3 days, vaccination was associated with a lower viral load. This association with lower viral load is supported by epidemiologic data demonstrating that transmission rates are lower after vaccination [9]. This is a significant public health message for individuals who may be forgoing the vaccine because they feel they are not at risk for serious COVID-19 disease [10].
However, this beneficial reduction on viral load appears to no longer exist for individuals vaccinated ≥6 months before enrolling in the trial, though the small number vaccinated >6 months before enrollment limits interpretation, as does the fact that 6 months aligns with the emergence of the Delta variant. The sensitivity analysis showing that the association with lower viral load decreases with a cutoff of 4 months suggests that waning immunity is not only due to the Delta variant. Previous work also suggested that vaccine protection against infection waned over time after 12 weeks [11]. It may be appropriate to revisit what should be considered fully vaccinated, especially as recent data suggest that protection against infection and severe disease also decrease after 6 months [11, 12].
It is also notable that persons who had been vaccinated were less likely to have systemic symptoms (ie, chills, fever, body aches, or diarrhea). This association with a lower likelihood of having symptoms is similar to what has been found previously when looking at effectiveness of mRNA vaccines against the Delta variant [3, 13]. In our sample, vaccinated individuals were more likely to have a runny nose, perhaps indicating that the virus is less likely to invade beyond the upper respiratory tract in vaccinated individuals, though this is speculative. There was also no difference in cough between those who were vaccinated and those who were unvaccinated. Viral load correlated with the frequency of the presence of moderate/severe symptoms. While the numbers in each tertile were small, the middle tertile of viral load was more likely to have symptoms of neurologic involvement (eg, lack of taste and smell). Those in the lowest tertile were generally least likely to have symptoms. That the highest viral loads had fewer neurologic symptoms may reflect the lack of an immune response in this group.
Previous data suggesting that there was no difference in viral load between vaccinated and unvaccinated persons were from nasal swabs taken after 2 days of symptoms [2]. The mean days since symptom onset in our sample was 5 days. The fact that we observed a difference between vaccinated and unvaccinated may be consistent with other analyses showing that viral load degraded more quickly in vaccinated individuals [3, 4]. The effect of vaccination on viral load in our sample was less significant after June 19; more data will be needed to understand whether vaccines lower viral load in persons with the Delta variant. Other work does suggest that while vaccinated individuals can transmit the Delta variant to others, the Delta viral load does clear faster in persons who have been vaccinated [14]. Additionally, it is possible that viral particles in vaccinated individuals may be less able to replicate than those from unvaccinated individuals [15].
Limitations
There is likely a selection bias in who enters the study, such that individuals with more symptoms are more likely to enter the study. Thus, the vaccinated individuals may be those with a suboptimal vaccine response. Therefore, the difference in symptoms between vaccinated and unvaccinated individuals may be biased in this analysis. This comparison was among persons who submitted optional self-collected nasal swabs. There may be an unknown selection bias as to who opted into this substudy of the main trial. It could be possible that participants who had a higher degree of symptomatology were less likely to complete the steps for baseline nasal swab collection and submission to the study team. Subjects in this analysis were not randomized to being in the vaccinated vs unvaccinated group, and there may be unobserved confounding in comparing these groups. This data set was taken from individuals who enrolled before mid- September 2021. Thus, those who were vaccinated >6 months before their enrollment in the study were receiving the vaccine before it was widely available. This may be because they were higher risk individuals, because they were more likely to be in occupations that put them at risk of exposure to a higher viral load, or because they were more likely to have comorbidities. Alternatively, perhaps they valued preventive measures and thus received the vaccine as early as they could and may continue to avoid high exposure to the virus. These unobserved potential influences on viral load at baseline may bias both toward and away from the null hypothesis. Lastly, while efforts were made to standardize how the samples were collected, transport time via overnight FedEx, and the storage temperature during transport, it is possible that there was variation between participants in any or all of these variables. We are not aware of a reason why that variation would be systematically different between vaccinated and unvaccinated individuals.
CONCLUSIONS
In this cross-sectional analysis of baseline data collected from patients who enrolled in a phase 3 randomized trial of early outpatient treatment of SARS-CoV-2 infection, vaccinated individuals had a lower viral load and lower prevalence of systemic symptoms than those who had not been vaccinated. The effect on viral load was no longer present for persons vaccinated >6 months prior. The primary goal of being “fully vaccinated” is to prevent serious disease, which may still occur after 6 months. Future research should look at viral load beyond 6 months to understand whether the term “fully vaccinated” for viral load and transmission should define only those vaccinated within the previous 6 months. Vaccine- and booster-induced reduction in viral load may be an important component for achieving reduced coronavirus spread.
Supplementary Material
Acknowledgments
Financial support. The clinical trial is funded by The Rainwater Charitable Foundation, The Parsemus Foundation, Fast Grants, and UnitedHealth Group Research and Development. The trial receives support from the UMN Clinical and Translational Science Institute, UL1TR002494. Dr. Bramante was funded by the National Institutes of Health’s National Center for Advancing Translational Sciences, grants KL2TR002492 and UL1TR002494; and the National Institute of Digestive Diabetes and Kidney Diseases, grant K23DK124654-01A1.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health’s National Center for Advancing Translational Sciences.
Potential conflicts of interest. The authors declare no relevant conflicts of interest. For the trial, the fluvoxamine placebo tablets were donated by Apotex. The ivermectin and ivermectin placebo tablets were donated by Edenbridge. J.B.B.’s contracted consulting fees and travel support for contracted activities paid to the University of North Carolina by Adocia, AstraZeneca, Eli Lilly, Intarcia Therapeutics, MannKind, Novo Nordisk, Sanofi, Senseonics, and vTv Therapeutics; he reports grant support from AstraZeneca, Dexcom, Eli Lilly, Intarcia Therapeutics, Johnson & Johnson, Lexicon, NovaTarg, Novo Nordisk, Sanofi, Theracos, Tolerion, and vTv Therapeutics; he has received fees for consultation from Alkahest, Anji Pharmaceuticals, AstraZeneca, Boehringer Ingelheim, Cirius Therapeutics Inc., Dasman Diabetes Institute (Kuwait), US Department of Defense, Eli Lilly, Fortress Biotech, GentiBio, Glycadia, Glyscend, Janssen, Mellitus Health, Moderna, Pendulum Therapeutics, Praetego, Stability Health, Valo, and Zealand Pharma; he holds stock/options in Glyscend, Mellitus Health, Pendulum Therapeutics, PhaseBio, Praetego, and Stability Health; and he is supported by grants from the National Institutes of Health, Patient Centered Outcomes Research Institute, Juvenile Diabetes Research Foundation International, and the American Diabetes Association. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Brown CM, Vostok J, Johnson H, et al. Outbreak of SARS-CoV-2 infections, including COVID-19 vaccine breakthrough infections, associated with large public gatherings - Barnstable County, Massachusetts, July 2021. MMWR Morb Mortal Wkly Rep 2021; 70:1059–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Riemersma KK, Grogan BE, Kita-Yarbro A, et al. Shedding of infectious SARS-CoV-2 despite vaccination. medRxiv 2021.2007.2031.21261387 [Preprint]. 24 August 2021. Available at: 10.1101/2021.07.31.21261387. Accessed 15 September 2021. [DOI] [Google Scholar]
- 3. Chia PY, Xiang Ong SW, Chiew CJ, et al. Virological and serological kinetics of SARS-CoV-2 Delta variant vaccine-breakthrough infections: a multi-center cohort study. Clin Microbiol Infect. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Acharya CB, Schrom J, Mitchell AM, et al. No significant difference in viral load between vaccinated and unvaccinated, asymptomatic and symptomatic groups when infected with SARS-CoV-2 Delta variant. medRxiv 2021.2009.2028.21264262 [Preprint]. 29 September 2021. Available at: 10.1101/2021.09.28.21264262. Accessed 20 October 2021. [DOI] [Google Scholar]
- 5. Puskarich MA, Cummins NW, Ingraham NE, et al. A multi-center phase II randomized clinical trial of losartan on symptomatic outpatients with COVID-19. EClinicalMedicine 2021; 37:100957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nelson AC, Auch B, Schomaker M, et al. Analytical validation of a COVID-19 qRT-PCR detection assay using a 384-well format and three extraction methods. bioRxiv 2020.2004.2002.022186 [Preprint]. 5 April 2020. Available at: 10.1101/2020.04.02.022186. Accessed 21 November 2021. [DOI] [Google Scholar]
- 7. Assessing COVID-19-Related Symptoms in Outpatient Adult and Adolescent Subjects in Clinical Trials of Drugs and Biological Products for COVID-19 Prevention or Treatment Guidance for Industry. Available at: https://www.fda.gov/media/142143/download. Accessed 8 August 2020. [Google Scholar]
- 8. Delahoy MJ. Hospitalizations Associated with COVID-19 among children and adolescents — COVID-NET, 14 States, March 1, 2020 - Aug 14, 2021. 2021; 70:1255–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. de Gier B, Andeweg S, Joosten R, et al. Vaccine effectiveness against SARS-CoV-2 transmission and infections among household and other close contacts of confirmed cases, the Netherlands, February to May 2021. Euro Surveill 2021; 26:2100640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Johns Hopkins Bloomberg School of Public Health. I'm a healthy young person. Why should I get a COVID vaccine? 2021. Available at: https://publichealth.jhu.edu/2021/im-a-healthy-young-person-why-should-i-get-a-covid-vaccine. Accessed 19 November 2021. [Google Scholar]
- 11. Chemaitelly H, Tang P, Hasan MR, et al. Waning of BNT162b2 vaccine protection against SARS-CoV-2 infection in Qatar. N Engl J Med. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Goldberg Y, Mandel M, Bar-On YM, et al. Waning immunity after the BNT162b2 vaccine in Israel. N Engl J Med. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pouwels KB, Pritchard E, Matthews PC, et al. Effect of Delta variant on viral burden and vaccine effectiveness against new SARS-CoV-2 infections in the UK. Nat Med 2021; 27:2127–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Singanayagam A, Hakki S, Dunning J, et al. Community transmission and viral load kinetics of the SARS-CoV-2 Delta (B.1.617.2) variant in vaccinated and unvaccinated individuals in the UK: a prospective, longitudinal, cohort study. Lancet Infect Dis. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shamier MC, Tostmann A, Bogers S, et al. Virological characteristics of SARS-CoV-2 vaccine breakthrough infections in health care workers. medRxiv 2021.2008.2020.21262158 [Preprint]. 21 August 2021. Available at: 10.1101/2021.08.20.21262158. Accessed 2 January 2021. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.