Abstract
Unlike the adaptive immune system, the innate immune system has classically been characterized as being devoid of memory functions. However, recent research shows that innate myeloid and lymphoid cells have the ability to retain memory of prior pathogen exposure and become primed to elicit a robust, broad spectrum response to subsequent infection. This phenomenon has been termed innate immune memory or trained immunity. Innate immune memory is induced via activation of pattern recognition receptors and the actions of cytokines on hematopoietic progenitors and stem cells in bone marrow and innate leukocytes in the periphery. The trained phenotype is induced and sustained via epigenetic modifications that reprogram transcriptional patterns and metabolism. These modifications augment antimicrobial functions such as leukocyte expansion, chemotaxis, phagocytosis and microbial killing to facilitate an augmented host response to infection. Alternatively, innate immune memory may contribute to the pathogenesis of chronic diseases such as atherosclerosis and Alzheimer’s disease.
Introduction
Immunological memory is a hallmark of the adaptive immune system of vertebrates and the basis for modern vaccines (1, 2). B and T lymphocytes express a diverse repertoire of highly specific antigen receptors that are generated via gene rearrangement during lymphocyte development (3). Antigen recognition by naïve, antigen-specific lymphocytes elicits clonal expansion and generation of memory cells that persist for years and are poised to react quickly and robustly to future encounters with the same antigen (3, 4). In contrast, the innate immune system is classically characterized as being non-specific, generally devoid of memory functions and serving to provide a mechanism to rapidly respond to diverse pathogens and facilitate early containment of infection (5, 6). However, investigators recognized decades ago that ligands that activate the immune system independently of antigen receptors (i.e immunoglobulins, T cell receptor) have the capacity to elicit a memory response (7–10). More recent research shows that innate myeloid and lymphoid cells, as well as non-immune cells such as epithelial and endothelial cells, have the ability to retain memory of prior exposure to microbes and inflammatory mediators and become primed to elicit a heightened, broad spectrum response to subsequent infection (11, 12) (Figure 1). This phenomenon has been termed innate immune memory or trained immunity and is particularly crucial to the survival of organisms that lack an adaptive immune system (i.e. invertebrates and plants) but is also functional in the complex immune system of vertebrates (13, 14).
Figure 1:
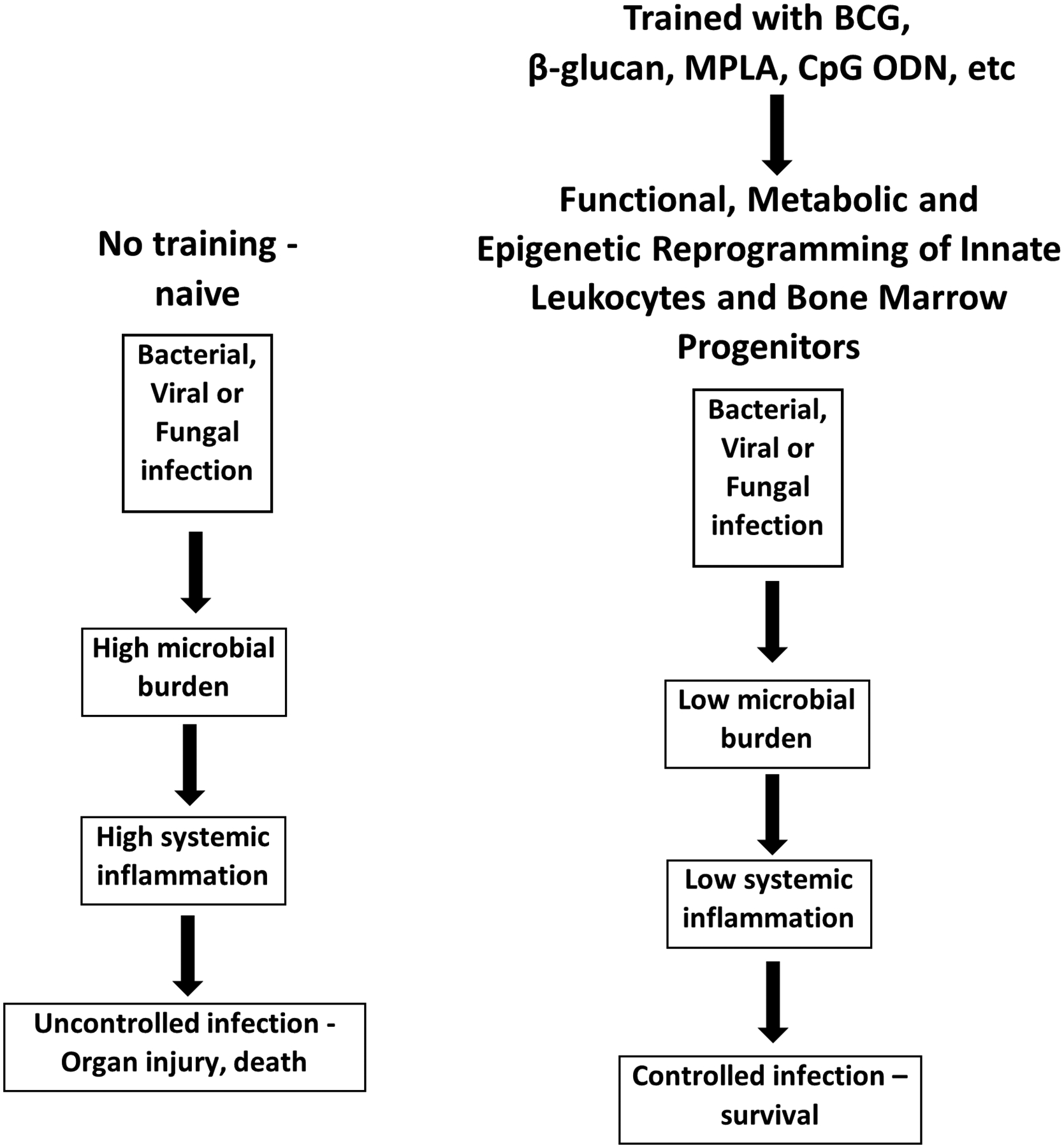
The biological basis of innate immune memory. Treatment with PAMPS such as β-glucan, MPLA or CpG ODN induces epigenetic, metabolic and functional reprogramming of the innate immune system resulting in augmented resistance to local and systemic infection.
Innate immune memory is induced by activation of pattern recognition receptors (PRR) and the actions of cytokines and hematopoietic factors on innate leukocytes and bone marrow progenitors and has the capacity to persist for weeks to months (15–18). The memory response can be induced by infection or treatment with microbe-derived pathogen-associated molecular patterns (PAMP) and is not specific to the inciting organism or PAMP. For example, treatment with the TLR4 ligands lipopolysaccharide (LPS) or monophosphoryl lipid A (MPLA), derived from Gram negative bacteria, confers resistance against Gram negative (P. aeruginosa), Gram positive (S. aureus) and fungal (C. albicans) pathogens as well as polymicrobial sepsis caused by cecal ligation and puncture (19–22). Peptidoglycan, a TLR2 ligand derived primarily from Gram positive bacteria, induces resistance to infection with Gram negative and Gram-positive pathogens (23, 24). β-glucan, a fungal cell wall constituent that activate dectin-1 and TLR2, induces resistance to infection with Gram-negative and Gram-positive bacterial and fungal pathogens (10, 16, 25).
The broad and non-specific nature of innate immune memory could have benefits in the clinical setting. Innate immune training agents such as MPLA and CpG ODN are already used clinically as vaccine adjuvants and are a major focus of current vaccine research (26–28). However, a multitude of organisms cause hospital- and community-acquired infections, and it is impossible to predict which pathogen will infect any given individual. Thus, the broad spectrum protection provided by innate immune training reagents may have application to treat or prevent opportunistic infections in critically ill, hospitalized and immunosuppressed patients. Vaccination of children with Bacillus Calmette-Guerin (BCG) not only provides targeted protection from infection with M. tuberculosis but protects from other respiratory pathogens and neonatal sepsis (29, 30). There is great interest in whether applying this strategy in other vulnerable populations could decrease the burden of infection. Recent commentaries have lauded the potential benefits of harnessing innate immune memory as a bridging strategy during pandemics, such as the current SARS-Cov-2 pandemic, to provide resistance to infection prior to development of vaccines (31). Innate immune training agents may also have benefit in the treatment of cancer as standalone drugs or in combination with other cancer therapies (32).
Trained immunity may play a role in the pathogenesis of some chronic diseases such as atherosclerosis and Alzheimer’s disease (33, 34). Bekkering and colleagues showed that treatment of human monocytes with oxLDL induced foam cell formation (35). Later work by Keating and colleagues showed that training of human monocyte-derived macrophages with oxLDL induced increased glycolysis and oxidative metabolism and enabled a pro-inflammatory phenotype (36). A link between innate immune training and Alzheimer’s disease has also been demonstrated, a phenomenon that may be mediated by training of microglia (37).
Most early work in the field of innate immune memory was performed using monocytes and macrophages. Evidence indicates that activation of innate myeloid cells with PAMPS induces epigenetic and metabolic reprogramming that facilitates a phenotype characterized by augmented antimicrobial functions such as chemotaxis, phagocytosis and microbial killing. Expansion and reprogramming of myeloid progenitors, including neutrophil precursors, in the bone marrow is also crucial for inducing and sustaining the trained phenotype (15, 38). This brief review provides an overview of the cellular and molecular alterations that initiate and sustain innate immune memory.
The Cellular Biology of Innate Immune Memory
Trained immunity can be induced in a multitude of innate leukocytes and in non-leukocyte populations (Figure 2). Early work on trained immunity focused on mature peripheral blood monocytes, which have a circulating lifespan of 1 to 7 days, depending on subset (39–41). Isolated monocytes were incubated with training agents, such as β-glucan or BCG, ex vivo for 24 hours and allowed to rest for 5 to 7 days to induce innate immune memory. Trained monocytes developed functional, epigenetic and metabolic alterations that are hallmarks of the trained phenotype (40–42). However, given the short lifespan of monocytes, questions arose as to how these findings translated to the in vivo setting where trained immunity persists for weeks to months. One possibility is that trained circulating monocytes migrate to sites of infection or distant organs in vivo where they differentiate into macrophages, which have a longer lifespan consistent with that of trained immunity (43). However, there is no published evidence to indicate that this occurs. In fact, work by Fensterheim and colleagues (21) showed that innate immune memory can be fully induced in CCR2−/− mice, which sequester monocytes in the bone marrow and prevent their migration to sites of infection or inflammation. Those findings suggest that migration of trained monocytes to tissues and subsequent differentiation into macrophages does not contribute to trained immunity in vivo. However, further work is needed to fully evaluate this cellular mechanism.
Figure 2:
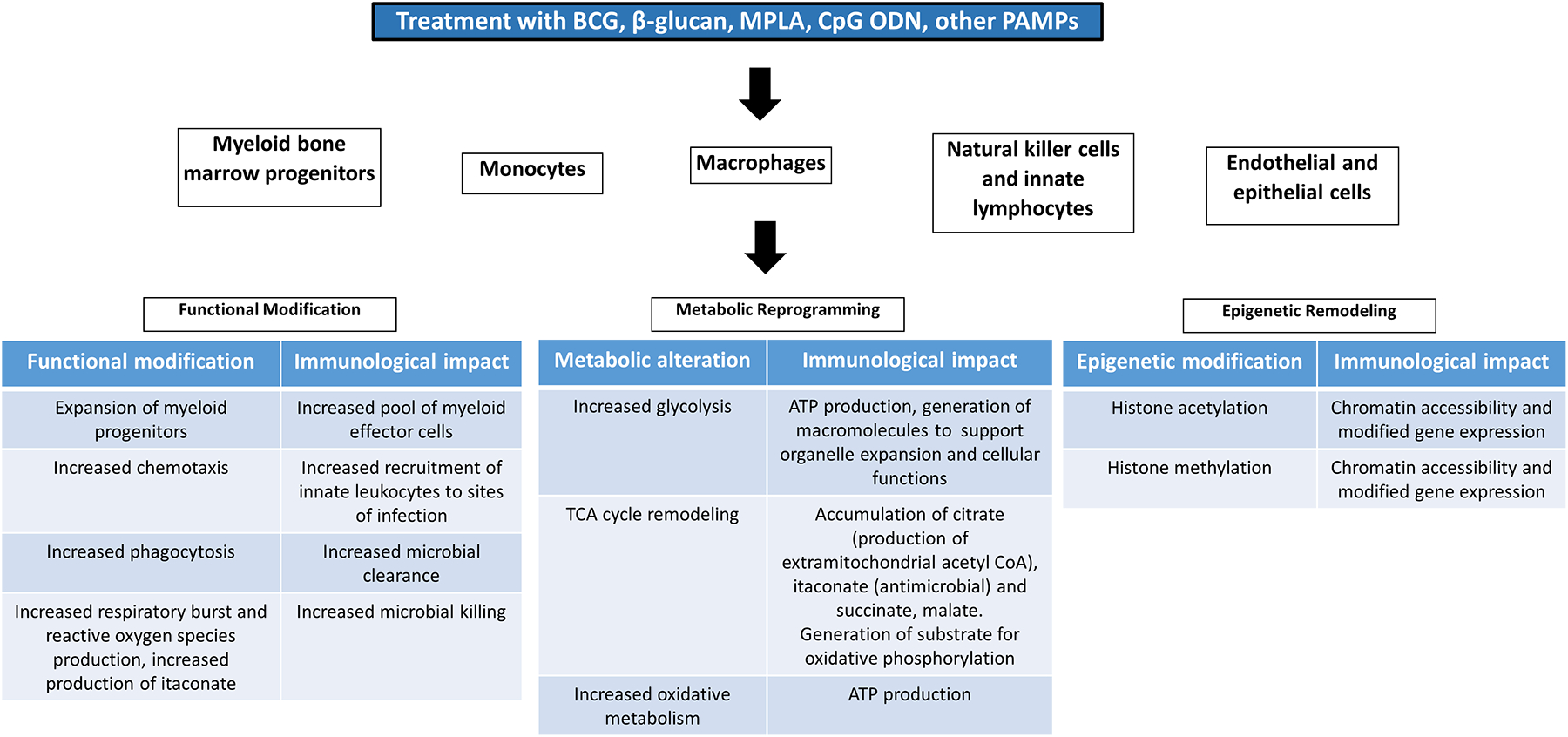
Functional, metabolic and epigenetic alterations driving innate immune memory. Training of innate leukocytes and non-leukocyte populations induces functional alterations that facilitate innate leukocyte progenitor expansion, leukocyte recruitment, phagocytosis and microbial killing. These alterations are enabled, in part, by epigenetic remodeling and metabolic reprogramming.
Later work provided evidence that myeloid-biased hematopoietic stem cells in the bone marrow can be trained and sustain the innate immune memory phenotype in vivo by providing a persistent supply of trained mature myeloid cells. Mitroulis and colleagues demonstrated that β-glucan induces a sustained increase in myeloid-biased hematopoietic progenitors that confers a protective response to subsequent inflammation- or chemotherapy-induced myeloablation (44). The observed myelopoietic response was associated with increased IL-1β and GM-CSF signaling and adaptations in glucose and cholesterol metabolism. Expansion of the bone marrow compartment by BCG vaccination has been reported by Cirovic et al (45). Progenitor expansion was associated with a specific epigenetic and transcriptional program that was transferred to peripheral blood monocytes up to 3 months after BCG treatment. Kalafati and colleagues (15) reported that β-glucan induces transcriptomic and epigenetic rewiring of granulopoiesis with associated augmentation of anti-tumor immunity, a process that was dependent on generation of reactive oxygen species. They further showed that the anti-tumor effect was conferred by transplantation of trained bone marrow progenitors into naïve mice. Likewise, Bohannon and colleagues showed that the TLR4 agonist MPLA induces expansion of myeloid progenitors in bone marrow resulting in augmented recruitment of neutrophils to sites of infection and concomitant enhancement of microbial clearance (38). The effect was ablated by G-CSF neutralization or neutrophil ablation. These findings are consistent with other studies showing that training with β-glucan or MPLA augments neutrophil and monocyte recruitment to sites of infection and enhances their phagocytic, respiratory burst and killing functions (16, 46).
Immune training of differentiated macrophages provides an alternative or complementary cellular mechanism driving trained immunity. Macrophages prominently express PRR and serve to initiate and orchestrate the host response to infection (47). Evidence indicates that a memory phenotype can be induced in tissue resident macrophages, which have a lifespan of months to years and the capacity to self-renew (48). Fensterheim and colleagues showed that treatment with MPLA induces macrophage expansion at sites of infection and that ablation of macrophages reverses the trained phenotype in vivo (21). The expansion of macrophages was independent of monocyte migration since the trained phenotype and macrophage expansion was observed in CCR2-deficient mice. Further studies by Stothers et al show that adoptive transfer of differentiated macrophages trained with β-glucan confers a level of resistance to infection with P. aeruginosa that mimics that of mice treated with β-glucan alone (16). Yao and colleagues (49) showed that memory is induced in alveolar macrophages via prior pathogen exposure, a response that required local T cell help. Sherwood et al showed that training of splenic, hepatic and peritoneal macrophages with β-glucan confers innate memory and an anti-tumor phenotype (50).
Taken together, research indicates that innate immune memory is mediated through training of myeloid precursors in the bone marrow and differentiated macrophages. However, innate lymphoid cells (i.e. natural killer cells and innate lymphoid cells), as well as nonimmune cells (i.e. endothelial cells, epithelial cells and fibroblasts) also have the capacity to develop memory under varying conditions (51–53). Many of the modifications induced in monocytes and macrophages are also likely to be induced in dendritic cells. Treatment of dendritic cells with training agents such as MPLA or CpG ODN augments antigen presentation and pro-inflammatory cytokine production and facilitates their effectiveness as vaccine adjuvants (54). Training of natural killer cells by prior viral exposure induces an antiviral phenotype that provides broad resistance to subsequent viral infection (52). Recent work by Larsen and colleagues shows that epidermal stem cells have the capacity to recall prior encounters with inflammatory mediators, a process that contributes to tissue homeostasis (53). Hato and colleagues (55) demonstrated that training with low dose LPS protects against sepsis-induced acute kidney injury, a process in which kidney proximal tubular cells and kidney-resident macrophages play important protective roles. That study underscores the importance of leukocyte and epithelial cell cross talk as a potential mechanism mediating organ protection after innate immune training.
Induction of Innate Immune Memory: Pattern Recognition Receptors and Signaling
Innate myeloid cells recognize pathogens via pattern recognition receptors (PRR) including toll-like receptors (TLR), nod-like receptors (NLR) and C-type lectin receptors including dectin-1, the β-glucan receptor (56–58). Activation of PRR triggers downstream signaling pathways leading to gene transcription and mobilization of cellular metabolism and antimicrobial functions (21, 39, 59). Numerous pathogen-associated molecular patterns, including microbe-derived proteins, carbohydrates, lipids and nucleic acids, serve as ligands for PRR and many are known to induce innate immune memory. In the 1950s, Landy and Pillemer noted that animals treated with the TLR4 ligand lipopolysaccharide (LPS) had augmented resistance to subsequent infection (60). Further work by DiLuzio, Williams and colleagues (10, 25, 61) established that treatment with β-glucan, a carbohydrate component of the fungal cell wall recognized by dectin-1, boosted host resistance to infection with a broad array of fungal and bacterial pathogens. Since then, a multitude of PAMPS have been recognized for their ability to induce innate immune memory and facilitate broad resistance to subsequent infection.
In addition to PAMPS, other mechanisms have been observed to induce innate immune memory. Weavers and colleagues (62) showed that phagocytosis of apoptotic cells, or efferocytosis, by macrophages induces innate immune memory and a more effective response to subsequent infection or injury. However, other investigators have reported that efferocytosis triggers a pro-resolving phenotype in macrophages that facilitates tissue repair and resolution of inflammation (63). Larsen and colleagues (53) showed that induction of skin inflammation with imiquimod induces a memory phenotype in epidermal stem cells that is similar to that induced in leukocytes by PAMPS. Several investigators showed that oxLDL induces a trained phenotype in endothelial cells and macrophages, with important implications in the pathogenesis of atherosclerosis (35, 64).
Numerous downstream signaling pathways are induced by activation of PRR. TLR initiate downstream signaling by recruiting the adaptor proteins MyD88 and/or TRIF (56). Activation of the MyD88-dependent pathway leads to downstream mobilization of the NF-κB, mitogen-associated protein kinase (MAPK) and phospho-inositide-3-kinase (PI3K) pathways (57, 65, 66). The transcription factor IRF3 is mobilized via activation of the TRIF-dependent pathway (67). Activation of NLR leads to mobilization of NF-κB and MAPK signaling and has varying impact on the PI3K/Akt pathway (68). Activation of dectin-1 and/or TLR2 by β-glucan induces NF-κB, MAPK and PI3K/Akt signaling (58). Of these signaling pathways, PI3K/Akt has been most extensively studied in the context of innate immune memory. Early work by Williams and colleagues (69) showed that activation of PI3K/Akt signaling by β-glucan reduced morbidity and mortality caused by subsequent sepsis and ischemia/reperfusion injury. Later work by Cheng et al (70) tied PI3K/Akt/mTOR/HIF-1α signaling to metabolic and epigenetic changes that are hallmarks of innate immune memory. Several other investigators have demonstrated that PI3K/Akt/mTOR/HIF-1α signaling is activated during acute inflammation and blockade of components of this pathway attenuate induction of the memory phenotype (46, 71–73). Based on these findings, PI3K/Akt/mTOR/HIF-1α signaling is considered an underlying mechanism driving innate immune memory, likely due to activation and orchestration of metabolic alterations that support the phenotype.
Recent research by Larsen and colleagues (53) showed that the coordinated activities of STAT3 and AP-1 regulate chromatin accessibility in a model of chronic skin inflammation that mimics trained immunity. They further demonstrated that numerous cell types exposed to inflammatory mediators showed enrichment for AP-1 footprints at memory domains and postulated that AP-1 serves as a universal mediator of innate immune memory. Work by Balic and colleagues (74) also implicated STAT3 as a factor mediating metabolic reprogramming during acute inflammation induced by TLR4 activation. These findings identify STAT3 and AP-1 as potential transcriptional regulators of the memory phenotype and open an intriguing new area of research.
Cytokines regulate the induction and maintenance of innate immune memory. Several studies have shown the ability of type I and II interferons to induce trained immunity independently or to augment the trained phenotype induced by PAMPs (75–78). Type I interferons have primarily been shown to possess the capacity to facilitate training in the hematopoietic compartment whereas interferon gamma (IFNγ) act on differentiated macrophages and other myeloid cells in the periphery (49, 76, 78, 79). Studies have also demonstrated contributions of IL-1β, GM-CSF and G-CSF to inducing hematopoietic stem cell expansion and memory in response to training agents such as BCG, β-glucan and MPLA (38, 80). In addition to direct virus recognition via Ly49 proteins and CD49, IL-12/15/18 play an important role in facilitating memory in NK cells (81). Cytokines, particularly those of the IL-1 family, also have the capacity to down regulate the memory response. Cavalli and colleagues reported that the anti-inflammatory cytokine IL-37 abrogates immunometabolic and epigenetic changes in trained myeloid cells and reverses protection from Candida infection conferred by β-glucan (82). De Graaf et al showed that IL-38 prevents trained immunity induced by β-glucan via its capacity to inhibit mTOR signaling (83).
Innate Immune Memory and Epigenetics
Gene expression is regulated by the accessibility of transcription factors, enhancers, repressors and RNA polymerase to promoter, enhancer and transcription start regions of the genome (84, 85). This accessibility is modulated by chromatin structure and under the control of epigenetic modifications including histone acetylation and methylation (86, 87). Epigenetic modifications associated with prior pathogen exposure have been described in plants and mammalian myeloid cells and evidence supports their role in sustaining the trained phenotype (88) (Figure 2). Kalafati and colleagues (15) reported increased chromatin accessibility in myeloid progenitors from mice trained with β-glucan at sites regulating granulocyte activation pathways. Similarly, Cirovic and colleagues (45) described changes in chromatin accessibility in human peripheral blood monocytes at 90 days after BCG vaccination with inflammation-associated loci being preferentially impacted. H3K27 acetylation (H3K27ac) at promoter sites and distal enhancer regions has been reported as the most dynamic epigenetic mark in monocytes trained with β-glucan and the location of the observed H3K27ac modifications correlated with transcriptional changes that characterize macrophage differentiation and training (40). Saeed and colleagues (40) further defined H3K4me1 as a memory mark associated with a faster and more robust response to restimulation. Likewise, H3K27ac and H3K4Me3 marks at promoter sites of genes regulating metabolism and inflammation have been described as prominent features of trained human monocytes (39, 70). Novakovic and colleagues (89) further described dynamic H3K27ac modifications in macrophages trained with β-glucan at gene clusters associated with leukocyte differentiation, activation, metabolism and phagocytosis and described replacement of repressive H3K4me3 marks with memory H3K4me1 marks. Later work by Rasid and colleagues (90) showed H3K4me1 to be an important memory mark in natural killer cells trained with LPS. Taken together, research indicates that H3KMe1 and H3K27ac marks are deposited during the early inflammatory response and are retained at memory domains in trained leukocytes.
Early in vitro studies showed that pan-methyltransferase inhibitors attenuate the trained phenotype induced by β-glucan or C. albicans, an observation that has been confirmed in later studies using a variety of training ligands and cell types (64, 70, 91). Keating and colleagues (92) reported that the Set7 lysine methyltransferase facilitates the induction of trained immunity in human peripheral blood monocytes and mouse bone marrow-derived macrophages by regulating expression of genes that control metabolic and inflammatory pathways, most notably H3K4Me1-mediated plasticity of genes regulating oxidative phosphorylation. Mourits and colleagues (93) reported that histone methyltransferase G9a is a negative regulator of trained immunity that is down-regulated in monocytes treated with BCG and accompanied by decreased H3K9me2 at pro-inflammatory gene promoters. G9a inhibition facilitated the induction of trained immunity by BCG, LPS and oxLDL. Research performed by Arts and colleagues (94) showed that accumulation of fumarate in trained monocytes induces epigenetic reprogramming by inhibiting KDM5 histone demethylases, which upregulates trimethylation of H3K4 in the promoter regions of genes regulating the inflammatory response. Less work has been done to determine the contributions of specific histone acetyltransferases and deacetylases to generation of trained immunity. However, Sun and colleagues (95) reported that chronic activation of NOD2 on monocyte-derived macrophages induced activation of histone deacetylase 1 (HDAC1) and HDAC3 in a Twist1- and Twist2-dependent manner to facilitate trained immunity. Further work is needed in this area.
Metabolic alterations that facilitate the availability of methyl and acetyl group donors are another mechanism mediating epigenetic regulation. Lauterbach and colleagues showed that LPS induces metabolic reprogramming in macrophages characterized by increased tricarboxylic acid (TCA) cycle flux, ATP-citrate lyase activity and extramitochondrial acetyl CoA generation (96). Induction of ATP-citrate lyase and accumulation of extramitochondrial acetyl CoA, a major intracellular acetyl group donor, was associated with LPS-induced histone acetylation and early gene expression. Work by Yu and colleagues (97) showed that LPS stimulates the pentose phosphate and serine synthesis pathways and one carbon metabolism to facilitate increased production of the methyl group donor S-adenosylmethonine (SAM). Increased SAM production was associated with histone methylation and expression of pro-inflammatory gene products. Although increased production of extracellular acetyl CoA and SAM appear to facilitate early gene expression after LPS exposure, it is unclear if these alterations facilitate the epigenetic and gene expression changes that drive trained immunity. Other epigenetic mechanisms that could facilitate innate immune memory include histone succinylation and lactylation. Zhang and colleagues demonstrated that LPS induces 28 distinct histone lactylation sites in macrophages that direct pro-inflammatory gene expression. However, the roles of histone succinylation and lactylation in trained immunity require further investigation.
Metabolic Basis of Innate Immune Memory
Reprogramming of cellular metabolism has been a consistent feature of the memory phenotype, most notably in monocytes and macrophages (Figure 2). Innate leukocytes respond to infection by mobilizing glycolysis to rapidly meet emerging energy requirements and to generate essential precursors for amino acids, nucleotides and fatty acids that are needed to mediate antimicrobial functions such as phagocytosis and killing (98, 99). Classically, this metabolic phenotype is characteristic of pro-inflammatory M1 macrophages. Alternatively, M2 macrophages, which facilitate tissue repair and homeostasis, predominantly utilize oxidative metabolism to sustain their ongoing functions (98). Cheng and colleagues (70) reported that training of human monocytes with β-glucan for 7 days induced a metabolic phenotype characterized by increase glucose consumption and glycolysis in parallel with decreased oxidative phosphorylation, a metabolic signature commonly referred to as the Warburg effect and characteristic of M1 macrophages. Other investigators have described the same metabolic shift in monocytes trained with BCG or oxLDL (100). Like monocytes, trained macrophages show a sustained increase in glycolysis (101). Yet, oxidative metabolism is also augmented in trained macrophages (101). The increase in oxidative metabolism is paralleled by increased tricarboxylic acid (TCA) cycle flux, mitochondrial mass and mitochondrial membrane potential. Similarly, Groh and colleagues (102) showed that monocytes trained with oxLDL developed a metabolic phenotype characterized by increased mitochondrial mass and membrane potential, TCA cycle flux and oxidative metabolism. Pharmacologic interference with mitochondrial function and oxidative metabolism alleviated key features of trained immunity induced by oxLDL, MPLA or β-glucan (101, 103).
Reprogramming of the TCA cycle is a common feature of trained innate leukocytes. Trained macrophages show increased flux of glucose through the TCA cycle and enhanced accumulation of TCA cycle intermediates, especially citrate, itaconate and succinate (21, 102). Fensterheim and colleagues showed a break in the TCA cycle distal to citrate with reestablishment of TCA cycle flux at α-ketoglutarate (αKG) in trained macrophages (101). The break at citrate was associated with increased production of itaconate and evidence of citrate transport out of the mitochondria with subsequent conversion to extramitochondrial acetyl CoA. Extramitochondrial acetyl CoA serves as a major acetyl group donor and its enhanced production in trained leukocytes likely contributes to histone acetylation (96). Reestablishment of the TCA cycle distal to isocitrate was dependent on glutamine anapleurosis. Arts and colleagues showed that glutaminolysis is essential for sustaining the trained phenotype, a finding that further supports the contribution of glutamine for sustaining TCA cycle reprogramming in innate leukocytes (94). Itaconate is a TCA cycle metabolite derived from cis-aconitate that is not part of the energy generating functions of the cycle (104). Several functions have been attributed to itaconate including inhibition of succinate dehydrogenase, attenuation of cytokine production and direct antimicrobial functions (104–106). The contribution of itaconate to trained immunity remains to be fully established. However, Dominiguez-Andres and colleagues reported that itaconate contributes to the development of immune tolerance and that training of monocytes with β-glucan inhibits Irg1, the enzyme catalyzing itaconate biosynthesis, to facilitate the induction of trained immunity (107). Other than citrate and itaconate, concentrations of succinate, malate and fumarate are increased in trained innate leukocytes (94, 101). Succinate and fumarate can stabilize HIF-1α and may play a role in stabilizing the metabolic features that are a hallmark of trained immunity (108). Fumarate has been shown to inhibit the activity of KDM5 histone demethylases and contribute to trained immunity by regulating epigenetic modifications (94). Succinate may also play a role in regulating the inflammatory response during innate immune training as succinate accumulation in innate leukocytes fuels a pro-inflammatory phenotype via succinate-induced stabilization of HIF-1α, augmentation of mitochondrial reactive oxygen species production and protein succinylation (109).
Alterations in cholesterol metabolism have also been reported in trained innate leukocytes (110). Inhibition of cholesterol biosynthesis using statins inhibited induction of innate immune memory by BCG, β-glucan and oxLDL in monocytes (111). Further analysis identified mevalonate accumulation as an important factor driving trained immunity. Changes in fatty acid metabolism may also contribute to the induction and maintenance of innate immune memory. Although inhibition of fatty acid biosynthesis during the training process did not impair the induction of innate immune memory, it did blunt the trained phenotype during restimulation suggesting that fatty acid biosynthesis plays a role in the innate memory response (112).
Conclusions
Innate immune memory can be defined as a set of sustained alterations in innate leukocyte function that supports a more robust response to downstream infections. This process is crucial for host defense in non-vertebrate organisms that depend on the innate immune system but is also functional in vertebrates. Recent research has identified cellular and molecular mechanisms that define and mediate innate immune memory. Functional, metabolic and epigenetic alterations have been described but further work is needed to fully understand the factors that induce and sustain innate immune memory. From a clinical perspective, understanding and application of innate immune memory has significant potential for preventing or treating infections in vulnerable populations and improving vaccine adjuvant development. On the other hand, a better understanding of the deleterious impact of innate immune memory may allow for novel treatment options for chronic inflammatory diseases such as atherosclerosis and Alzheimer’s disease.
Acknowledgments
This work was supported by the National Institutes of Health (NIH) Grants R01 GM119197 (E.R.S. and D.L.W.), R01 AI151210 (E.R.S.), R01 GM121711 (J.K.B), R35 GM141927 (J.K.B.), R01 GM083016 (D.L.W.), T32 GM108554 (N.K.P), F30 AI157036 (M.A.M.) and T32 GM007347 (Vanderbilt MSTP: C.L.S. and M.A.M.), the American Heart Association Grant 19PRE34430054 (C.L.S.), and Vanderbilt Faculty Research Scholars Award (N.K.P).
References
- 1.Kurtz J 2004. Memory in the innate and adaptive immune systems. Microbes Infect 6: 1410–1417. [DOI] [PubMed] [Google Scholar]
- 2.Bonilla FA, and Oettgen HC. 2010. Adaptive immunity. J Allergy Clin Immunol 125: S33–40. [DOI] [PubMed] [Google Scholar]
- 3.Litman GW, Anderson MK, and Rast JP. 1999. Evolution of antigen binding receptors. Annu Rev Immunol 17: 109–147. [DOI] [PubMed] [Google Scholar]
- 4.Yanagi Y, Caccia N, Kronenberg M, Chin B, Roder J, Rohel D, Kiyohara T, Lauzon R, Toyonaga B, Rosenthal K, and et al. 1985. Gene rearrangement in cells with natural killer activity and expression of the beta-chain of the T-cell antigen receptor. Nature 314: 631–633. [DOI] [PubMed] [Google Scholar]
- 5.Bohuslav J, Kravchenko VV, Parry GC, Erlich JH, Gerondakis S, Mackman N, and Ulevitch RJ. 1998. Regulation of an essential innate immune response by the p50 subunit of NF-kappaB. J Clin Invest 102: 1645–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Medzhitov R, and Janeway CA Jr. 1999. Innate immune induction of the adaptive immune response. Cold Spring Harb Symp Quant Biol 64: 429–435. [DOI] [PubMed] [Google Scholar]
- 7.Landy M, and Pillemer L. 1956. Increased resistance to infection and accompanying alteration in properidin levels following administration of bacterial lipopolysaccharides. J Exp Med 104: 383–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wooles WR, and Diluzio NR. 1963. Reticuloendothelial Function and the Immune Response. Science 142: 1078–1080. [DOI] [PubMed] [Google Scholar]
- 9.Browder W, Williams D, Sherwood E, McNamee R, Jones E, and DiLuzio N. 1987. Synergistic effect of nonspecific immunostimulation and antibiotics in experimental peritonitis. Surgery 102: 206–214. [PubMed] [Google Scholar]
- 10.Williams DL, Cook JA, Hoffmann EO, and Di Luzio NR. 1978. Protective effect of glucan in experimentally induced candidiasis. J Reticuloendothel Soc 23: 479–490. [PubMed] [Google Scholar]
- 11.Tercan H, Riksen NP, Joosten LAB, Netea MG, and Bekkering S. 2021. Trained Immunity: Long-Term Adaptation in Innate Immune Responses. Arterioscler Thromb Vasc Biol 41: 55–61. [DOI] [PubMed] [Google Scholar]
- 12.Divangahi M, Aaby P, Khader SA, Barreiro LB, Bekkering S, Chavakis T, van Crevel R, Curtis N, DiNardo AR, Dominguez-Andres J, Duivenvoorden R, Fanucchi S, Fayad Z, Fuchs E, Hamon M, Jeffrey KL, Khan N, Joosten LAB, Kaufmann E, Latz E, Matarese G, van der Meer JWM, Mhlanga M, Moorlag S, Mulder WJM, Naik S, Novakovic B, O’Neill L, Ochando J, Ozato K, Riksen NP, Sauerwein R, Sherwood ER, Schlitzer A, Schultze JL, Sieweke MH, Benn CS, Stunnenberg H, Sun J, van de Veerdonk FL, Weis S, Williams DL, Xavier R, and Netea MG. 2021. Trained immunity, tolerance, priming and differentiation: distinct immunological processes. Nat Immunol 22: 2–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Netea MG, Quintin J, and van der Meer JW. 2011. Trained immunity: a memory for innate host defense. Cell Host Microbe 9: 355–361. [DOI] [PubMed] [Google Scholar]
- 14.Gourbal B, Pinaud S, Beckers GJM, Van Der Meer JWM, Conrath U, and Netea MG. 2018. Innate immune memory: An evolutionary perspective. Immunol Rev 283: 21–40. [DOI] [PubMed] [Google Scholar]
- 15.Kalafati L, Kourtzelis I, Schulte-Schrepping J, Li X, Hatzioannou A, Grinenko T, Hagag E, Sinha A, Has C, Dietz S, de Jesus Domingues AM, Nati M, Sormendi S, Neuwirth A, Chatzigeorgiou A, Ziogas A, Lesche M, Dahl A, Henry I, Subramanian P, Wielockx B, Murray P, Mirtschink P, Chung KJ, Schultze JL, Netea MG, Hajishengallis G, Verginis P, Mitroulis I, and Chavakis T. 2020. Innate Immune Training of Granulopoiesis Promotes Anti-tumor Activity. Cell 183: 771–785 e712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stothers CL, Burelbach KR, Owen AM, Patil NK, McBride MA, Bohannon JK, Luan L, Hernandez A, Patil TK, Williams DL, and Sherwood ER. 2021. beta-Glucan Induces Distinct and Protective Innate Immune Memory in Differentiated Macrophages. J Immunol 207: 2785–2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cirovic B, de Bree LCJ, Groh L, Blok BA, Chan J, van der Velden W, Bremmers MEJ, van Crevel R, Handler K, Picelli S, Schulte-Schrepping J, Klee K, Oosting M, Koeken V, van Ingen J, Li Y, Benn CS, Schultze JL, Joosten LAB, Curtis N, Netea MG, and Schlitzer A. 2020. BCG Vaccination in Humans Elicits Trained Immunity via the Hematopoietic Progenitor Compartment. Cell Host Microbe 28: 322–334 e325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dominguez-Andres J, and Netea MG. 2019. Long-term reprogramming of the innate immune system. J Leukoc Biol 105: 329–338. [DOI] [PubMed] [Google Scholar]
- 19.Varma TK, Durham M, Murphey ED, Cui W, Huang Z, Lin CY, Toliver-Kinsky T, and Sherwood ER. 2005. Endotoxin priming improves clearance of Pseudomonas aeruginosa in wild-type and interleukin-10 knockout mice. Infect Immun 73: 7340–7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murphey ED, Fang G, Varma TK, and Sherwood ER. 2007. Improved bacterial clearance and decreased mortality can be induced by LPS tolerance and is not dependent upon IFN-gamma. Shock 27: 289–295. [DOI] [PubMed] [Google Scholar]
- 21.Fensterheim BA, Young JD, Luan L, Kleinbard RR, Stothers CL, Patil NK, McAtee-Pereira AG, Guo Y, Trenary I, Hernandez A, Fults JB, Williams DL, Sherwood ER, and Bohannon JK. 2018. The TLR4 Agonist Monophosphoryl Lipid A Drives Broad Resistance to Infection via Dynamic Reprogramming of Macrophage Metabolism. J Immunol 200: 3777–3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Romero CD, Varma TK, Hobbs JB, Reyes A, Driver B, and Sherwood ER. 2011. The Toll-like receptor 4 agonist monophosphoryl lipid a augments innate host resistance to systemic bacterial infection. Infect Immun 79: 3576–3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murphey ED, Fang G, and Sherwood ER. 2008. Pretreatment with the Gram-positive bacterial cell wall molecule peptidoglycan improves bacterial clearance and decreases inflammation and mortality in mice challenged with Staphylococcus aureus. Crit Care Med 36: 3067–3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murphey ED, and Sherwood ER. 2008. Pretreatment with the Gram-positive bacterial cell wall molecule peptidoglycan improves bacterial clearance and decreases inflammation and mortality in mice challenged with Pseudomonas aeruginosa. Microbes Infect 10: 1244–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Williams DL, Browder W, McNamee R, and Di Luzio NR. 1982. Glucan immunomodulation in experimental E. coli sepsis. Adv Exp Med Biol 155: 701–706. [DOI] [PubMed] [Google Scholar]
- 26.Martins KAO, Cooper CL, Stronsky SM, Norris SLW, Kwilas SA, Steffens JT, Benko JG, van Tongeren SA, and Bavari S. 2016. Adjuvant-enhanced CD4 T Cell Responses are Critical to Durable Vaccine Immunity. EBioMedicine 3: 67–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.James SF, Chahine EB, Sucher AJ, and Hanna C. 2018. Shingrix: The New Adjuvanted Recombinant Herpes Zoster Vaccine. Ann Pharmacother 52: 673–680. [DOI] [PubMed] [Google Scholar]
- 28.Didierlaurent AM, Morel S, Lockman L, Giannini SL, Bisteau M, Carlsen H, Kielland A, Vosters O, Vanderheyde N, Schiavetti F, Larocque D, Van Mechelen M, and Garcon N. 2009. AS04, an aluminum salt- and TLR4 agonist-based adjuvant system, induces a transient localized innate immune response leading to enhanced adaptive immunity. J Immunol 183: 6186–6197. [DOI] [PubMed] [Google Scholar]
- 29.de Castro MJ, Pardo-Seco J, and Martinon-Torres F. 2015. Nonspecific (Heterologous) Protection of Neonatal BCG Vaccination Against Hospitalization Due to Respiratory Infection and Sepsis. Clin Infect Dis 60: 1611–1619. [DOI] [PubMed] [Google Scholar]
- 30.Biering-Sorensen S, Jensen KJ, Monterio I, Ravn H, Aaby P, and Benn CS. 2018. Rapid Protective Effects of Early BCG on Neonatal Mortality Among Low Birth Weight Boys: Observations From Randomized Trials. J Infect Dis 217: 759–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Glisic S, Perovic VR, Sencanski M, Paessler S, and Veljkovic V. 2020. Biological Rationale for the Repurposing of BCG Vaccine against SARS-CoV-2. J Proteome Res 19: 4649–4654. [DOI] [PubMed] [Google Scholar]
- 32.Netea MG, Joosten LAB, and van der Meer JWM. 2017. Hypothesis: stimulation of trained immunity as adjunctive immunotherapy in cancer. J Leukoc Biol 102: 1323–1332. [DOI] [PubMed] [Google Scholar]
- 33.Flores-Gomez D, Bekkering S, Netea MG, and Riksen NP. 2021. Trained Immunity in Atherosclerotic Cardiovascular Disease. Arterioscler Thromb Vasc Biol 41: 62–69. [DOI] [PubMed] [Google Scholar]
- 34.De Sousa VL, Araujo SB, Antonio LM, Silva-Queiroz M, Colodeti LC, Soares C, Barros-Aragao F, Mota-Araujo HP, Alves VS, Coutinho-Silva R, Savio LEB, Ferreira ST, Da Costa R, Clarke JR, and Figueiredo CP. 2021. Innate immune memory mediates increased susceptibility to Alzheimer’s disease-like pathology in sepsis surviving mice. Brain Behav Immun 95: 287–298. [DOI] [PubMed] [Google Scholar]
- 35.Bekkering S, Quintin J, Joosten LA, van der Meer JW, Netea MG, and Riksen NP. 2014. Oxidized low-density lipoprotein induces long-term proinflammatory cytokine production and foam cell formation via epigenetic reprogramming of monocytes. Arterioscler Thromb Vasc Biol 34: 1731–1738. [DOI] [PubMed] [Google Scholar]
- 36.Keating ST, Groh L, Thiem K, Bekkering S, Li Y, Matzaraki V, van der Heijden C, van Puffelen JH, Lachmandas E, Jansen T, Oosting M, de Bree LCJ, Koeken V, Moorlag S, Mourits VP, van Diepen J, Strienstra R, Novakovic B, Stunnenberg HG, van Crevel R, Joosten LAB, Netea MG, and Riksen NP. 2020. Rewiring of glucose metabolism defines trained immunity induced by oxidized low-density lipoprotein. J Mol Med (Berl) 98: 819–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mizobuchi H, and Soma GI. 2021. Low-dose lipopolysaccharide as an immune regulator for homeostasis maintenance in the central nervous system through transformation to neuroprotective microglia. Neural Regen Res 16: 1928–1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bohannon JK, Luan L, Hernandez A, Afzal A, Guo Y, Patil NK, Fensterheim B, and Sherwood ER. 2016. Role of G-CSF in monophosphoryl lipid A-mediated augmentation of neutrophil functions after burn injury. J Leukoc Biol 99: 629–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Quintin J, Saeed S, Martens JH, Giamarellos-Bourboulis EJ, Ifrim DC, Logie C, Jacobs L, Jansen T, Kullberg BJ, Wijmenga C, Joosten LA, Xavier RJ, van der Meer JW, Stunnenberg HG, and Netea MG. 2012. Candida albicans infection affords protection against reinfection via functional reprogramming of monocytes. Cell Host Microbe 12: 223–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saeed S, Quintin J, Kerstens HH, Rao NA, Aghajanirefah A, Matarese F, Cheng SC, Ratter J, Berentsen K, van der Ent MA, Sharifi N, Janssen-Megens EM, Ter Huurne M, Mandoli A, van Schaik T, Ng A, Burden F, Downes K, Frontini M, Kumar V, Giamarellos-Bourboulis EJ, Ouwehand WH, van der Meer JW, Joosten LA, Wijmenga C, Martens JH, Xavier RJ, Logie C, Netea MG, and Stunnenberg HG. 2014. Epigenetic programming of monocyte-to-macrophage differentiation and trained innate immunity. Science 345: 1251086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Patel AA, Zhang Y, Fullerton JN, Boelen L, Rongvaux A, Maini AA, Bigley V, Flavell RA, Gilroy DW, Asquith B, Macallan D, and Yona S. 2017. The fate and lifespan of human monocyte subsets in steady state and systemic inflammation. J Exp Med 214: 1913–1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ifrim DC, Quintin J, Joosten LA, Jacobs C, Jansen T, Jacobs L, Gow NA, Williams DL, van der Meer JW, and Netea MG. 2014. Trained immunity or tolerance: opposing functional programs induced in human monocytes after engagement of various pattern recognition receptors. Clin Vaccine Immunol 21: 534–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parihar A, Eubank TD, and Doseff AI. 2010. Monocytes and macrophages regulate immunity through dynamic networks of survival and cell death. J Innate Immun 2: 204–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mitroulis I, Ruppova K, Wang B, Chen LS, Grzybek M, Grinenko T, Eugster A, Troullinaki M, Palladini A, Kourtzelis I, Chatzigeorgiou A, Schlitzer A, Beyer M, Joosten LAB, Isermann B, Lesche M, Petzold A, Simons K, Henry I, Dahl A, Schultze JL, Wielockx B, Zamboni N, Mirtschink P, Coskun U, Hajishengallis G, Netea MG, and Chavakis T. 2018. Modulation of Myelopoiesis Progenitors Is an Integral Component of Trained Immunity. Cell 172: 147–161 e112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cirovic B, de Bree LCJ, Groh L, Blok BA, Chan J, van der Velden W, Bremmers MEJ, van Crevel R, Handler K, Picelli S, Schulte-Schrepping J, Klee K, Oosting M, Koeken V, van Ingen J, Li Y, Benn CS, Schultze JL, Joosten LAB, Curtis N, Netea MG, and Schlitzer A. 2020. BCG Vaccination in Humans Elicits Trained Immunity via the Hematopoietic Progenitor Compartment. Cell Host Microbe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fensterheim BA, Guo Y, Sherwood ER, and Bohannon JK. 2017. The Cytokine Response to Lipopolysaccharide Does Not Predict the Host Response to Infection. J Immunol 198: 3264–3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Martinez FO, Sica A, Mantovani A, and Locati M. 2008. Macrophage activation and polarization. Front Biosci 13: 453–461. [DOI] [PubMed] [Google Scholar]
- 48.Franken L, Schiwon M, and Kurts C. 2016. Macrophages: sentinels and regulators of the immune system. Cell Microbiol 18: 475–487. [DOI] [PubMed] [Google Scholar]
- 49.Yao Y, Jeyanathan M, Haddadi S, Barra NG, Vaseghi-Shanjani M, Damjanovic D, Lai R, Afkhami S, Chen Y, Dvorkin-Gheva A, Robbins CS, Schertzer JD, and Xing Z. 2018. Induction of Autonomous Memory Alveolar Macrophages Requires T Cell Help and Is Critical to Trained Immunity. Cell 175: 1634–1650 e1617. [DOI] [PubMed] [Google Scholar]
- 50.Sherwood ER, Williams DL, and Di Luzio NR. 1986. Comparison of the in vitro cytolytic effect of hepatic, splenic and peritoneal macrophages from glucan-treated mice on sarcoma M5076. Methods Find Exp Clin Pharmacol 8: 157–161. [PubMed] [Google Scholar]
- 51.Kleinnijenhuis J, Quintin J, Preijers F, Joosten LA, Jacobs C, Xavier RJ, van der Meer JW, van Crevel R, and Netea MG. 2014. BCG-induced trained immunity in NK cells: Role for non-specific protection to infection. Clin Immunol 155: 213–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hammer Q, and Romagnani C. 2017. About Training and Memory: NK-Cell Adaptation to Viral Infections. Adv Immunol 133: 171–207. [DOI] [PubMed] [Google Scholar]
- 53.Larsen SB, Cowley CJ, Sajjath SM, Barrows D, Yang Y, Carroll TS, and Fuchs E. 2021. Establishment, maintenance, and recall of inflammatory memory. Cell Stem Cell 28: 1758–1774 e1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mata-Haro V, Cekic C, Martin M, Chilton PM, Casella CR, and Mitchell TC. 2007. The vaccine adjuvant monophosphoryl lipid A as a TRIF-biased agonist of TLR4. Science 316: 1628–1632. [DOI] [PubMed] [Google Scholar]
- 55.Hato T, Zollman A, Plotkin Z, El-Achkar TM, Maier BF, Pay SL, Dube S, Cabral P, Yoshimoto M, McClintick J, and Dagher PC. 2018. Endotoxin Preconditioning Reprograms S1 Tubules and Macrophages to Protect the Kidney. J Am Soc Nephrol 29: 104–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Akira S 2000. Toll-like receptors: lessons from knockout mice. Biochem Soc Trans 28: 551–556. [DOI] [PubMed] [Google Scholar]
- 57.Akira S, Hoshino K, and Kaisho T. 2000. The role of Toll-like receptors and MyD88 in innate immune responses. J Endotoxin Res 6: 383–387. [PubMed] [Google Scholar]
- 58.Brown GD, Taylor PR, Reid DM, Willment JA, Williams DL, Martinez-Pomares L, Wong SY, and Gordon S. 2002. Dectin-1 is a major beta-glucan receptor on macrophages. J Exp Med 196: 407–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hernandez A, Bohannon JK, Luan L, Fensterheim BA, Guo Y, Patil NK, McAdams C, Wang J, and Sherwood ER. 2016. The role of MyD88- and TRIF-dependent signaling in monophosphoryl lipid A-induced expansion and recruitment of innate immunocytes. J Leukoc Biol 100: 1311–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Landy M, and Pillemer L. 1956. Increased resistance to infection and accompanying alteration in properidin levels following administration of bacterial lipopolysaccharides. J Exp Med 104: 383–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Di Luzio NR, and Williams DL. 1978. Protective effect of glucan against systemic Staphylococcus aureus septicemia in normal and leukemic mice. Infect Immun 20: 804–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Weavers H, Evans IR, Martin P, and Wood W. 2016. Corpse Engulfment Generates a Molecular Memory that Primes the Macrophage Inflammatory Response. Cell 165: 1658–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Robb CT, Regan KH, Dorward DA, and Rossi AG. 2016. Key mechanisms governing resolution of lung inflammation. Semin Immunopathol 38: 425–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sohrabi Y, Lagache SMM, Voges VC, Semo D, Sonntag G, Hanemann I, Kahles F, Waltenberger J, and Findeisen HM. 2020. OxLDL-mediated immunologic memory in endothelial cells. J Mol Cell Cardiol 146: 121–132. [DOI] [PubMed] [Google Scholar]
- 65.Wesche H, Henzel WJ, Shillinglaw W, Li S, and Cao Z. 1997. MyD88: an adapter that recruits IRAK to the IL-1 receptor complex. Immunity 7: 837–847. [DOI] [PubMed] [Google Scholar]
- 66.Troutman TD, Hu W, Fulenchek S, Yamazaki T, Kurosaki T, Bazan JF, and Pasare C. 2012. Role for B-cell adapter for PI3K (BCAP) as a signaling adapter linking Toll-like receptors (TLRs) to serine/threonine kinases PI3K/Akt. Proc Natl Acad Sci U S A 109: 273–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Takeda K, and Akira S. 2004. TLR signaling pathways. Semin Immunol 16: 3–9. [DOI] [PubMed] [Google Scholar]
- 68.Costa A, Gupta R, Signorino G, Malara A, Cardile F, Biondo C, Midiri A, Galbo R, Trieu-Cuot P, Papasergi S, Teti G, Henneke P, Mancuso G, Golenbock DT, and Beninati C. 2012. Activation of the NLRP3 inflammasome by group B streptococci. J Immunol 188: 1953–1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Williams DL, Ozment-Skelton T, and Li C. 2006. Modulation of the phosphoinositide 3-kinase signaling pathway alters host response to sepsis, inflammation, and ischemia/reperfusion injury. Shock 25: 432–439. [DOI] [PubMed] [Google Scholar]
- 70.Cheng SC, Quintin J, Cramer RA, Shepardson KM, Saeed S, Kumar V, Giamarellos-Bourboulis EJ, Martens JH, Rao NA, Aghajanirefah A, Manjeri GR, Li Y, Ifrim DC, Arts RJ, van der Veer BM, Deen PM, Logie C, O’Neill LA, Willems P, van de Veerdonk FL, van der Meer JW, Ng A, Joosten LA, Wijmenga C, Stunnenberg HG, Xavier RJ, and Netea MG. 2014. mTOR- and HIF-1alpha-mediated aerobic glycolysis as metabolic basis for trained immunity. Science 345: 1250684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Luo L, Wall AA, Yeo JC, Condon ND, Norwood SJ, Schoenwaelder S, Chen KW, Jackson S, Jenkins BJ, Hartland EL, Schroder K, Collins BM, Sweet MJ, and Stow JL. 2014. Rab8a interacts directly with PI3Kgamma to modulate TLR4-driven PI3K and mTOR signalling. Nat Commun 5: 4407. [DOI] [PubMed] [Google Scholar]
- 72.Saz-Leal P, Del Fresno C, Brandi P, Martinez-Cano S, Dungan OM, Chisholm JD, Kerr WG, and Sancho D. 2018. Targeting SHIP-1 in Myeloid Cells Enhances Trained Immunity and Boosts Response to Infection. Cell Rep 25: 1118–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Watts BA 3rd, George T, Sherwood ER, and Good DW. 2017. Monophosphoryl lipid A induces protection against LPS in medullary thick ascending limb through a TLR4-TRIF-PI3K signaling pathway. Am J Physiol Renal Physiol 313: F103–F115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Balic JJ, Albargy H, Luu K, Kirby FJ, Jayasekara WSN, Mansell F, Garama DJ, De Nardo D, Baschuk N, Louis C, Humphries F, Fitzgerald K, Latz E, Gough DJ, and Mansell A. 2020. STAT3 serine phosphorylation is required for TLR4 metabolic reprogramming and IL-1beta expression. Nat Commun 11: 3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Leopold Wager CM, Hole CR, Campuzano A, Castro-Lopez N, Cai H, Caballero Van Dyke MC, Wozniak KL, Wang Y, and Wormley FL Jr. 2018. IFN-gamma immune priming of macrophages in vivo induces prolonged STAT1 binding and protection against Cryptococcus neoformans. PLoS Pathog 14: e1007358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Essers MA, Offner S, Blanco-Bose WE, Waibler Z, Kalinke U, Duchosal MA, and Trumpp A. 2009. IFNalpha activates dormant haematopoietic stem cells in vivo. Nature 458: 904–908. [DOI] [PubMed] [Google Scholar]
- 77.de Laval B, Maurizio J, Kandalla PK, Brisou G, Simonnet L, Huber C, Gimenez G, Matcovitch-Natan O, Reinhardt S, David E, Mildner A, Leutz A, Nadel B, Bordi C, Amit I, Sarrazin S, and Sieweke MH. 2020. C/EBPbeta-Dependent Epigenetic Memory Induces Trained Immunity in Hematopoietic Stem Cells. Cell Stem Cell 26: 657–674 e658. [DOI] [PubMed] [Google Scholar]
- 78.Kaufmann E, Sanz J, Dunn JL, Khan N, Mendonca LE, Pacis A, Tzelepis F, Pernet E, Dumaine A, Grenier JC, Mailhot-Leonard F, Ahmed E, Belle J, Besla R, Mazer B, King IL, Nijnik A, Robbins CS, Barreiro LB, and Divangahi M. 2018. BCG Educates Hematopoietic Stem Cells to Generate Protective Innate Immunity against Tuberculosis. Cell 172: 176–190 e119. [DOI] [PubMed] [Google Scholar]
- 79.Kamada R, Yang W, Zhang Y, Patel MC, Yang Y, Ouda R, Dey A, Wakabayashi Y, Sakaguchi K, Fujita T, Tamura T, Zhu J, and Ozato K. 2018. Interferon stimulation creates chromatin marks and establishes transcriptional memory. Proc Natl Acad Sci U S A 115: E9162–E9171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Moorlag S, Khan N, Novakovic B, Kaufmann E, Jansen T, van Crevel R, Divangahi M, and Netea MG. 2020. beta-Glucan Induces Protective Trained Immunity against Mycobacterium tuberculosis Infection: A Key Role for IL-1. Cell Rep 31: 107634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang X, Peng H, and Tian Z. 2019. Innate lymphoid cell memory. Cell Mol Immunol 16: 423–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cavalli G, Tengesdal IW, Gresnigt M, Nemkov T, Arts RJW, Dominguez-Andres J, Molteni R, Stefanoni D, Cantoni E, Cassina L, Giugliano S, Schraa K, Mills TS, Pietras EM, Eisenmensser EZ, Dagna L, Boletta A, D’Alessandro A, Joosten LAB, Netea MG, and Dinarello CA. 2021. The anti-inflammatory cytokine interleukin-37 is an inhibitor of trained immunity. Cell Rep 35: 108955. [DOI] [PubMed] [Google Scholar]
- 83.de Graaf DM, Teufel LU, van de Veerdonk FL, Joosten LAB, Netea MG, Dinarello CA, and Arts RJW. 2021. IL-38 prevents induction of trained immunity by inhibition of mTOR signaling. J Leukoc Biol 110: 907–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Love MI, Huska MR, Jurk M, Schopflin R, Starick SR, Schwahn K, Cooper SB, Yamamoto KR, Thomas-Chollier M, Vingron M, and Meijsing SH. 2017. Role of the chromatin landscape and sequence in determining cell type-specific genomic glucocorticoid receptor binding and gene regulation. Nucleic Acids Res 45: 1805–1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ramaker RC, Hardigan AA, Goh ST, Partridge EC, Wold B, Cooper SJ, and Myers RM. 2020. Dissecting the regulatory activity and sequence content of loci with exceptional numbers of transcription factor associations. Genome Res 30: 939–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dogan N, Wu W, Morrissey CS, Chen KB, Stonestrom A, Long M, Keller CA, Cheng Y, Jain D, Visel A, Pennacchio LA, Weiss MJ, Blobel GA, and Hardison RC. 2015. Occupancy by key transcription factors is a more accurate predictor of enhancer activity than histone modifications or chromatin accessibility. Epigenetics Chromatin 8: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Han H, Feng F, and Li H. 2021. Research advances on epigenetics and cancer metabolism. Zhejiang Da Xue Xue Bao Yi Xue Ban 50: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jaskiewicz M, Conrath U, and Peterhansel C. 2011. Chromatin modification acts as a memory for systemic acquired resistance in the plant stress response. EMBO Rep 12: 50–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Novakovic B, Habibi E, Wang SY, Arts RJW, Davar R, Megchelenbrink W, Kim B, Kuznetsova T, Kox M, Zwaag J, Matarese F, van Heeringen SJ, Janssen-Megens EM, Sharifi N, Wang C, Keramati F, Schoonenberg V, Flicek P, Clarke L, Pickkers P, Heath S, Gut I, Netea MG, Martens JHA, Logie C, and Stunnenberg HG. 2016. beta-Glucan Reverses the Epigenetic State of LPS-Induced Immunological Tolerance. Cell 167: 1354–1368 e1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rasid O, Chevalier C, Camarasa TM, Fitting C, Cavaillon JM, and Hamon MA. 2019. H3K4me1 Supports Memory-like NK Cells Induced by Systemic Inflammation. Cell Rep 29: 3933–3945 e3933. [DOI] [PubMed] [Google Scholar]
- 91.Quintin J, Saeed S, Martens JHA, Giamarellos-Bourboulis EJ, Ifrim DC, Logie C, Jacobs L, Jansen T, Kullberg BJ, Wijmenga C, Joosten LAB, Xavier RJ, van der Meer JWM, Stunnenberg HG, and Netea MG. 2012. Candida albicans infection affords protection against reinfection via functional reprogramming of monocytes. Cell Host Microbe 12: 223–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Keating ST, Groh L, van der Heijden C, Rodriguez H, Dos Santos JC, Fanucchi S, Okabe J, Kaipananickal H, van Puffelen JH, Helder L, Noz MP, Matzaraki V, Li Y, de Bree LCJ, Koeken V, Moorlag S, Mourits VP, Dominguez-Andres J, Oosting M, Bulthuis EP, Koopman WJH, Mhlanga M, El-Osta A, Joosten LAB, Netea MG, and Riksen NP. 2020. The Set7 Lysine Methyltransferase Regulates Plasticity in Oxidative Phosphorylation Necessary for Trained Immunity Induced by beta-Glucan. Cell Rep 31: 107548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mourits VP, van Puffelen JH, Novakovic B, Bruno M, Ferreira AV, Arts RJ, Groh L, Crisan TO, Zwaag J, Jentho E, Kox M, Pickkers P, van de Veerdonk FL, Weis S, Oosterwijk E, Vermeulen SH, Netea MG, and Joosten LA. 2021. Lysine methyltransferase G9a is an important modulator of trained immunity. Clin Transl Immunology 10: e1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Arts RJ, Novakovic B, Ter Horst R, Carvalho A, Bekkering S, Lachmandas E, Rodrigues F, Silvestre R, Cheng SC, Wang SY, Habibi E, Goncalves LG, Mesquita I, Cunha C, van Laarhoven A, van de Veerdonk FL, Williams DL, van der Meer JW, Logie C, O’Neill LA, Dinarello CA, Riksen NP, van Crevel R, Clish C, Notebaart RA, Joosten LA, Stunnenberg HG, Xavier RJ, and Netea MG. 2016. Glutaminolysis and Fumarate Accumulation Integrate Immunometabolic and Epigenetic Programs in Trained Immunity. Cell Metab 24: 807–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sun R, Hedl M, and Abraham C. 2019. Twist1 and Twist2 Induce Human Macrophage Memory upon Chronic Innate Receptor Treatment by HDAC-Mediated Deacetylation of Cytokine Promoters. J Immunol 202: 3297–3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lauterbach MA, Hanke JE, Serefidou M, Mangan MSJ, Kolbe CC, Hess T, Rothe M, Kaiser R, Hoss F, Gehlen J, Engels G, Kreutzenbeck M, Schmidt SV, Christ A, Imhof A, Hiller K, and Latz E. 2019. Toll-like Receptor Signaling Rewires Macrophage Metabolism and Promotes Histone Acetylation via ATP-Citrate Lyase. Immunity 51: 997–1011 e1017. [DOI] [PubMed] [Google Scholar]
- 97.Yu W, Wang Z, Zhang K, Chi Z, Xu T, Jiang D, Chen S, Li W, Yang X, Zhang X, Wu Y, and Wang D. 2019. One-Carbon Metabolism Supports S-Adenosylmethionine and Histone Methylation to Drive Inflammatory Macrophages. Mol Cell 75: 1147–1160 e1145. [DOI] [PubMed] [Google Scholar]
- 98.Galvan-Pena S, and O’Neill LA. 2014. Metabolic reprograming in macrophage polarization. Front Immunol 5: 420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dominguez-Andres J, Arts RJW, Ter Horst R, Gresnigt MS, Smeekens SP, Ratter JM, Lachmandas E, Boutens L, van de Veerdonk FL, Joosten LAB, Notebaart RA, Ardavin C, and Netea MG. 2017. Rewiring monocyte glucose metabolism via C-type lectin signaling protects against disseminated candidiasis. PLoS Pathog 13: e1006632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bekkering S, Blok BA, Joosten LA, Riksen NP, van Crevel R, and Netea MG. 2016. In Vitro Experimental Model of Trained Innate Immunity in Human Primary Monocytes. Clin Vaccine Immunol 23: 926–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fensterheim BA, Young JD, Luan L, Kleinbard RR, Stothers CL, Patil NK, McAtee-Pereira AG, Guo Y, Trenary I, Hernandez A, Fults JB, Williams DL, Sherwood ER, and Bohannon JK. 2018. The TLR4 Agonist Monophosphoryl Lipid A Drives Broad Resistance to Infection via Dynamic Reprogramming of Macrophage Metabolism. J Immunol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Groh LA, Ferreira AV, Helder L, van der Heijden C, Novakovic B, van de Westerlo E, Matzaraki V, Moorlag S, de Bree LC, Koeken V, Mourits VP, Keating ST, van Puffelen JH, Hoischen A, Joosten LAB, Netea MG, Koopman WJH, and Riksen NP. 2021. oxLDL-Induced Trained Immunity Is Dependent on Mitochondrial Metabolic Reprogramming. Immunometabolism 3: e210025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Groh L, Keating ST, Joosten LAB, Netea MG, and Riksen NP. 2018. Monocyte and macrophage immunometabolism in atherosclerosis. Semin Immunopathol 40: 203–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Luan HH, and Medzhitov R. 2016. Food Fight: Role of Itaconate and Other Metabolites in Antimicrobial Defense. Cell Metab 24: 379–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cordes T, Wallace M, Michelucci A, Divakaruni AS, Sapcariu SC, Sousa C, Koseki H, Cabrales P, Murphy AN, Hiller K, and Metallo CM. 2016. Immunoresponsive Gene 1 and Itaconate Inhibit Succinate Dehydrogenase to Modulate Intracellular Succinate Levels. J Biol Chem 291: 14274–14284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tang C, Wang X, Xie Y, Cai X, Yu N, Hu Y, and Zheng Z. 2018. 4-Octyl Itaconate Activates Nrf2 Signaling to Inhibit Pro-Inflammatory Cytokine Production in Peripheral Blood Mononuclear Cells of Systemic Lupus Erythematosus Patients. Cell Physiol Biochem 51: 979–990. [DOI] [PubMed] [Google Scholar]
- 107.Dominguez-Andres J, Novakovic B, Li Y, Scicluna BP, Gresnigt MS, Arts RJW, Oosting M, Moorlag S, Groh LA, Zwaag J, Koch RM, Ter Horst R, Joosten LAB, Wijmenga C, Michelucci A, van der Poll T, Kox M, Pickkers P, Kumar V, Stunnenberg H, and Netea MG. 2019. The Itaconate Pathway Is a Central Regulatory Node Linking Innate Immune Tolerance and Trained Immunity. Cell Metab 29: 211–220 e215. [DOI] [PubMed] [Google Scholar]
- 108.Riksen NP, and Netea MG. 2021. Immunometabolic control of trained immunity. Mol Aspects Med 77: 100897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mills EL, and O’Neill LA. 2016. Reprogramming mitochondrial metabolism in macrophages as an anti-inflammatory signal. Eur J Immunol 46: 13–21. [DOI] [PubMed] [Google Scholar]
- 110.Arts RJ, Joosten LA, and Netea MG. 2016. Immunometabolic circuits in trained immunity. Semin Immunol 28: 425–430. [DOI] [PubMed] [Google Scholar]
- 111.Bekkering S, Arts RJW, Novakovic B, Kourtzelis I, van der Heijden C, Li Y, Popa CD, Ter Horst R, van Tuijl J, Netea-Maier RT, van de Veerdonk FL, Chavakis T, Joosten LAB, van der Meer JWM, Stunnenberg H, Riksen NP, and Netea MG. 2018. Metabolic Induction of Trained Immunity through the Mevalonate Pathway. Cell 172: 135–146 e139. [DOI] [PubMed] [Google Scholar]
- 112.van der Heijden C, Keating ST, Groh L, Joosten LAB, Netea MG, and Riksen NP. 2020. Aldosterone induces trained immunity: the role of fatty acid synthesis. Cardiovasc Res 116: 317–328. [DOI] [PubMed] [Google Scholar]


