Abstract
Naïve T and T memory cell subsets are closely related to immune response and can provide important information for the diagnosis and treatment of immunological and hematological disorders. Lymphocyte compartment undergoes dramatic changes during adulthood; age-related reference values derived from healthy individuals are crucial. However, extensively detailed reference values of peripheral blood lymphocytes in the whole spectrum of adulthood detected by multi-color flow cytometry on a single platform are rare. Three hundred and nine healthy adult volunteers were recruited from Tianjin in China. The absolute counts and percentages of CD3+CD4+ T cells, CD3+CD8+ T cells, naïve T cells (Tn), T memory stem cells (Tscm), central memory T cells (Tcm), effector memory T cells (Tem), and terminal effector T cells (Tte) were detected by flow cytometry with single platform technologies. Reference range of absolute counts and percentage of T lymphocyte subsets were formulated by different age and gender. The results showed that Tn and Tscm cells, which had stem cell properties, decreased with aging; while, Tcm and Tem increased with aging, which increased from 18 to 64 years old but presented no significant change over the 65 years old. Gender had an influence on the fluctuation of lymphocyte subsets, the absolute count of CD3+CD8+, CD8+Tcm, CD8+Tem in males were higher than those in females. The reference values of percentages and absolute numbers of naïve T and T memory cell subsets can help doctors to understand the immune state of patients and evaluate conditions of prognosis then adjust the treatment for patients. (Chinese Clinic Trial Registry number: ChiCTR-IOR-17014139.)
Keywords: naïve T cell, T memory cell subsets, reference range, changing regularity, flow cytometry
The absolute counts and percentages of CD3+CD4+ T cells, CD3+CD8+ T cells, naïve T cells (Tn), T memory stem cells (Tscm), central memory T cells (Tcm), effector memory T cells (Tem), terminal effector T cells (Tte) were detected by flow cytometry with single platform technologies. Reference range of absolute counts and percentage of T lymphocyte subsets were formulated by different age and gender. Gender and age had influence on the absolute counts of T lymphocyte subsets.
Graphical Abstract
Graphical Abstract.
Introduction
Long-lived memory T cells maintained long-time immunological memory in the host [1, 2]. The immune system gained a long-term ability of responding to a broad diverse spectrum of pathogens and tumor cell antigens through developing special lymphocyte differentiation programs to ensure the duration of a given antigen-specific immune response [3, 4]. Upon antigen stimulation, Tn entered distinct cell programs of development and differentiation and then produced Tscm, Tcm, and Tem [5]. The key mediator in this process was Tscm, a kind of multipotent progenitor that owned the capacity of self-renewing and could replenish more differentiated subsets of memory T cells, including Tcm, Tem, and Tte [6]. Tscm played a vital role in many physiological and pathological human processes owing to their extreme longevity and robust potential for immune reconstitution [7]. Some studies indicated that CD4+CD45RA+CD95+T cells from patients with lung cancer exhibiting stronger anti-tumor function possessed certain memory cell phenotypes which could help contribute to favorable prognostic factors of disease [8]. In infection disease, such as infected with Mycobacterium tuberculosis (M. tb), M. tb-specific Tscm would be produced in the host, which was also functional and could produce IL-2, IFN-γ, TNF-α upon antigen stimulation, and the percentages of Tscm were correlated positively with long-term Calmette-Guerin-specific CD4+ T cell proliferative potential after infant vaccination [9].
Detecting the changes of the circulating T lymphocyte subsets can be beneficial to monitor the onset and progression of the disease and determine optimal treatment [10]. Therefore, establishing a reference range for Tn and Tm lymphocyte subsets is crucial for analyzing the clinical status of the immune and its response. But there was a rare reference range of naïve T and T memory cell subsets provided by the single platform method, the need of precision reference range of naïve T and T memory cell subsets at different age stages prompted us to carry out this work. Flow cytometric analysis is a convenient and efficient method for studying immune status and has been widely used in clinical diagnosis and administrating of immune diseases associated with phenotypic and functional perturbations of lymphocyte subsets [11].
So far, almost all absolute numbers of lymphocyte subsets in the peripheral circulation have been detected by dual-platform technique in a traditional way which couples percentages of positive cell subsets determined by flow cytometry with the absolute lymphocyte count obtained by automated hematology analyzers [10, 12]. It was indicated that this conventional, universal technique was responsible for substantial differences in absolute lymphocyte counts reported by different laboratories [13, 14]. The more advanced method of a single platform, which was performed completely on the flow cytometer, had effectively increased the assay precision and allowed for greater uniformity of results between laboratories [13–15]. So, the percentages and absolute numbers of these subsets were tested by ten-color flow cytometer based on a single-platform technique.
We examined Tn and Tm subsets of 309 healthy volunteers, some regional data of lymphocyte phenotypes showed variations due to the influence of gender, age, ethnicity, and lifestyle differences [16], gender and age were the most common influence factors, therefore, we explored reference ranges based on age and gender.
Materials and methods
Clinical data
All the subjects were given informed consent in accordance with the Declaration of Helsinki and the clinical trial was approved by the hospital ethics committee (TYLL2017[K]002) and registered at the Chinese Clinic Trial Registry (ChiCT-R-IOR-17014139). A total of 309 healthy adult (171males and 138 females) volunteers ranging from 18 to 88 years old were recruited from Tianjin in China between 1 September 2019 and 1 July 2020. According to the aging definition by the National Health and Family Planning Commission of the People’s Republic of China, the subjects were classified into three age groups as follows: 18–44 years (n = 101); 45–65years (n = 106) and >65years (n = 102) [10].
Inclusion criteria
All the subjects were healthy without diseases related to abnormal of heart, brain, liver, kidney, hematology, immune system, and so on. Physical examination, blood routine examination, liver functions, renal functions, and blood glucose levels were normal.
Exclusion criteria
Except for the diseases including influenza, systemic infection, autoimmune diseases, connective tissue disease, HIV, abnormal tumor marker, or cancer that caused the abnormal of immune.
Reagents and instruments
The T lymphocyte subsets were analyzed using a lyse/no-wash procedure based on a single-platform technique by ten-color flow cytometry (BD FACS Canto II:U657338000541). The main reagents were Percp-cy-labeled mouse Anti-Human CD95 (cat: 561655), Bv421-labeled mouse Anti-Human CD62L (cat: 563862), PE-cy7-labeled mouse Anti-Human CD4 (cat: 663493), APC-labeled mouse Anti-Human CD8 (cat: 663524), APC-H7-labeled mouse Anti-Human CD3 (cat: 663490), V-500-C labeled-mouse Anti-Human CD45 (cat:662912), PE-labeled mouse Anti-Human CD45RO (cat:663530), FITC-labeled mouse Anti-Human CD45RA (cat: 662840), BD Multitest hemolysin (340503). The EDTA blood collecting tubes and trucount tubes (340334) were also from BD Biosciences.
Sample collection
Two milliliters of EDTA anticoagulated fresh peripheral blood were obtained from healthy adults, intensively mixed by turning upside-down immediately.
Cellular staining and analysis
The procedure was performed by the flow cytometer with single platform technique. The manipulation was done according to BD operating instruction. CD3+CD4+T cells, CD3+CD8+T cells, Tn, Tscm, Tcm, Tem, and Tte were identified according to published protocols, such as Tn(CD45RA+CD45RO-CD62L+CD95−), Tscm(CD45RA+CD45RO-CD62L+CD95+), Tcm(CD45RA−CD45RO+CD62L+CD95+), Tem(CD45RA−CD45RO+CD62L-CD95+), Tte(CD45RA+CD45RO-CD62L−CD95+) [7, 17–19].
-
(1)
For each sample, 20μl of PE-labelled mouse Anti-Human CD45RO and FITC-labelled mouse Anti-Human CD45RA reagents were respectively pipetted into the bottom of trucount tubes; then 5μl of Percp-cy-labelled mouse Anti-Human CD95, Bv421-labelled mouse Anti-Human CD62L, PE-cy7-labelled mouse Anti-Human CD4, APC-labelled mouse Anti-Human CD8, APC-H7-labelled mouse Anti-Human CD3, and V-500-C-labelled mouse Anti-Human CD45 were added into the bottom of trucount tubes respectively
-
(2)
Next, 50μl of well-mixed and anti-coagulated whole blood was pipetted into the bottom of every tube.
-
(3)
Then vortexed gently to mix, incubated for 15 min in dark at room temperature.
-
(4)
Finally, 400μl of 1×BD Multitest IMK kit lysing solution was pipetted into every tube. The solution was vortexed gently to mix and incubated for 15 min in dark at room temperature.
-
(5)
Samples were analyzed on the flow cytometer.
Statistical analysis
Statistical analysis was performed by SPSS software 25.0. Kolmogorov–Smirnov was used for the distribution test. Reference ranges were presented as the median together with the 5th and 95th percentile (90% confidence interval). Comparisons among three variables were performed using the Kruskal–Wallis test. Variables were grouped by gender comparing with Mann–Whitney U test for non-parametric data. Using a non-parametric Spearman’s rank correlation test to analyse the association between variables and age. Probability value was obtained from two-sided tests and P < 0.05 was considered statistically significant.
Result
Gating strategy
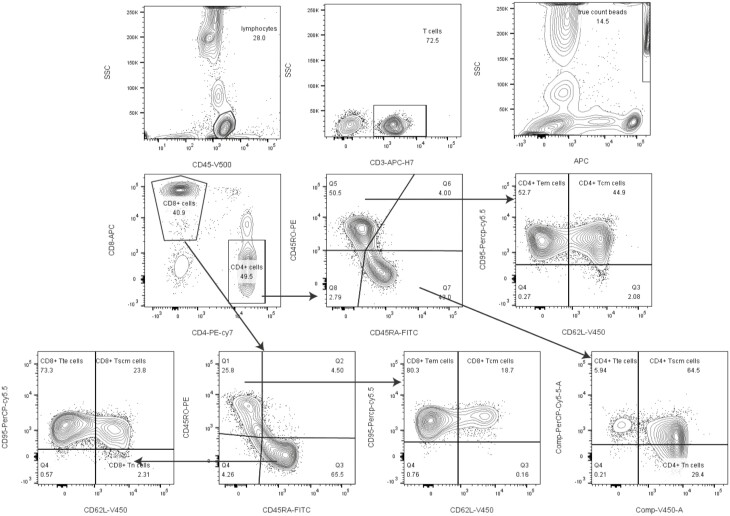
The gating strategy for naïve T and T memory cell subsets is shown in Fig. 1. Firstly, we gated lymphocyte identified by CD45 from leukocyte, then gated CD3+ T cells from lymphocyte. Tcm (CD95+ CD62L−) and Tem (CD95+CD62L+) were gated from CD3+CD4+ (CD45RO+ CD45RA−) and CD3+CD8+ (CD45RO+ CD45RA−) T subsets; Tscm (CD95+ CD62L+), Tn (CD95−CD62L+) and Tte (CD95+CD62L−) were gated from CD3+CD4+ (CD45RO−CD45RA+) and CD3+CD8+ (CD45RO−CD45RA+) T subsets.
Fig. 1.
Gating strategies. Firstly, we gated lymphocyte identified by CD45 from leukocyte, then gated CD3+ T cells from lymphocyte. T cell subsets populations Tcm (CD95+ CD62L−) and Tem (CD95+ CD62L+) were gated from CD3+CD4+ (CD45RO+ CD45RA−) and CD3+CD8+ (CD45RO+ CD45RA−)T subsets; Tscm (CD95+CD62L+), Tn (CD95−CD62L+) and Tte (CD95+CD62L−) were gated from CD3+CD4+ (CD45RO−CD45RA+) and CD3+CD8+ (CD45RO−CD45RA+)T subsets.
Reference range of T lymphocyte subsets in different age groups
The healthy volunteers were recruited for assessment of human T lymphocyte subsets including 171 males (55.33 %) and 138 females (44.67 %). Hundred and one (32.7%) were in 18–44 years group (56 males, 45 females, mean age 30.73 years), 106 (34.3%) belonged to 45–65 years group (56 males, 50 females, mean age 54.63 years) and 102 (33.0%) belonged to the over 65 years old group (59 males, 43 females, mean age 73.59 years). The Kolmogorov–Smirnov test demonstrated that absolute counts and percentages of each subsets were abnormal distribution among the three cohorts (P < 0.001). So we used the percentile method to determine the reference range of each parameter. The median and reference range of absolute counts and percentage for each group are shown in Table 1,2.
Table 1.
Reference range of absolute counts of T lymphocyte subsets in different age groups
| Parameters | All | (18–44) years | (45–65) years | >65years | |
|---|---|---|---|---|---|
| n = 309 | n = 101 | n = 106 | n = 102 | ||
| Age | Mean±SD | 50.52 ± 18.00 | 30.73 ± 6.52 | 54.63 ± 6.37 | 73.59 ± 6.60 |
| Sex | Male:female | 171:138 | 56:45 | 56:50 | 59:43 |
| Lymphocyte counts (cells/μl) | Median | 1766.39 | 1692.59 | 1791.59 | 1668.16 |
| Reference range Cl 90% | (1201.93–2509.45) | (1154.13–2222.62) | (1289.37–2569.88) | (1089.05–2858.61) | |
| CD3+ (cells/μl) | Median | 1139.49 | 1137.67 | 1203.23 | 1045.59 |
| Reference range Cl 90% | (744.15–1680.36) | (735.08–1581.36) | (750.31–1717.94) | (721.92–1791.96) | |
| CD3+CD4+ (cell/μl) | Median | 585.91 | 538.20 | 647.07 | 562.21 |
| Reference range Cl 90% | (364.51–960.9) | (361.84–908.7) | (360.52–964.2) | (365.22–1052.91) | |
| CD4+Tn (cells/μl) | Median | 25.11 | 34.38 | 23.06 | 14.9 |
| Reference range Cl 90% | (5.05–76.43) | (10.56–74.91) | (5.13–87.66) | (2.57–57.36) | |
| CD4+Tscm (cells/μl) | Median | 102.90 | 140.9 | 85.49 | 69.2 |
| Reference range Cl 90% | (19.94–306.54) | (37.85–333.11) | (15.10–281.59) | (9.41–280.89) | |
| CD4+Tcm (cells/μl) | Median | 154.85 | 141.74 | 183.84 | 155.81 |
| Reference range Cl 90% | (76.43–323.05) | (70.26–223.04) | (86.00–343.58) | (77.75–401.63) | |
| CD4+Tem (cells/μl) | Median | 116.59 | 100.20 | 132.72 | 123.77 |
| Reference range Cl 90% | (58.07–268.76) | (54.47–160.63) | (65.23–272.08) | (57.17–336.65) | |
| CD4+Tte (cells/μl) | Median | 2.41 | 2.24 | 2.58 | 2.94 |
| Reference range Cl 90% | (0.20–50.52) | (0.35–53.06) | (0.17–47.15) | (0.14–58.93) | |
| CD3+CD8+ (cell/μl) | Median | 423.88 | 449.64 | 404.97 | 323.01 |
| Reference range Cl 90% | (210.24–705.0) | (224.66–669.0) | (212.49–700.9) | (181.96–892.3) | |
| CD8+Tn (cells/μl) | Median | 12.42 | 27.5 | 10.49 | 3.01 |
| Reference range Cl 90% | (1.31–46.06) | (5.78–51.99) | (2.18–31.37) | (0.58–17.08) | |
| CD8+Tscm (cells/μl) | Median | 57.24 | 110.47 | 48.63 | 18.67 |
| Reference range Cl 90% | (6.10–212.35) | (30.89–261.21) | (11.21–109.95) | (3.39–99.07) | |
| CD8+Tcm (cells/μl) | Median | 36.15 | 31.18 | 36.70 | 37.61 |
| Reference range Cl 90% | (12.74–96.56) | (11.49–70.20) | (13.10–102.61) | (12.59–129.56) | |
| CD8+Tem (cells/μl) | Median | 85.75 | 94.33 | 83.44 | 75.19 |
| Reference range Cl 90% | (24.34–247.25) | (21.45–209.90) | (26.51–222.79) | (19.26–286.57) | |
| CD8+Tte (cells/μl) | Median | 53.23 | 47.93 | 57.55 | 38.05 |
| Reference range Cl 90% | (6.79–181.57) | (6.97–136.30) | (4.52–241.49) | (4.97–283.41) | |
Table 2.
Reference range of percentages of T lymphocyte subsets in different age groups
| Parameters | All | (18–44) years | (45–65) years | >65years | |
|---|---|---|---|---|---|
| n = 309 | n = 101 | n = 106 | n = 102 | ||
| Age | Mean±SD | 50.52 ± 18.00 | 30.73 ± 6.52 | 54.63 ± 6.37 | 73.59 ± 6.60 |
| Sex | Male:female | 171:138 | 56:45 | 56:50 | 59:43 |
| CD3+%lymphocyte cells | Median | 67.90% | 68.5% | 66.55% | 64.2% |
| Reference range Cl 90% | (49.58–79%) | (55.33–79.28%) | (50.06–78.53%) | (48.52%–81.26%) | |
| CD3+CD4+%CD3+ | Median | 52.8% | 50.4% | 53.95% | 57.05% |
| Reference range Cl 90% | (37%–68.34) | (34.6–65.46%) | (41.85%–69.49%) | (35.43%–71.17%) | |
| CD3+CD4+%lymphocyte cells | Median | 34.1% | 32.35% | 35.05% | 35.1% |
| Reference range Cl 90% | (23.86–47.86%) | (23.7–45.81%) | (25.64–48.49%) | (19.36–50.13%) | |
| CD4+Tn%CD3+CD4+ | Median | 4.3% | 6% | 3.45% | 2.6% |
| Reference range Cl 90% | (1–10.82%) | (2.14–11.62%) | (1.1–11.16%) | (0.52–7.09%) | |
| CD4+Tscm%CD3+CD4+ | Median | 17.6 | 26.35 | 13.55 | 11.4 |
| Reference range Cl 90% | (3.3–38.94%) | (8.34–41.30%) | (3.3–35.77%) | (2.02–30.55%) | |
| CD4+Tcm%CD3+CD4+ | Median | 27.00% | 26.1% | 28.1% | 26.45% |
| Reference range Cl 90% | (14.7–43.42%) | (11.41–38.13%) | (16.37–43.20%) | (14.81–52.9%) | |
| CD4+Tem%CD3+CD4+ | Median | 20.3% | 17.6% | 22.1% | 20.85% |
| Reference range Cl 90% | (10.48–40.2%) | (8.03–34.67%) | (10.88–42.27%) | (10.27–42.41) | |
| CD4+Tte%CD3+CD4+ | Median | 0.4% | 0.5% | 0.4% | 0.7% |
| Reference range Cl 90% | (0.01–9.06%) | (0.01–10.24%) | (0.01–8.58%) | (0.01–10.7%) | |
| CD3+CD8+%CD3+ | Median | 36.8% | 38% | 35.3% | 34.8% |
| Reference range Cl 90% | (23–51.44%) | (25.13–53.70%) | (22.98–48.7%) | (18.25–57.71%) | |
| CD3+CD8+%Lymphocyte cells | Median | 24.9% | 26.15% | 22.85% | 20.85% |
| Reference range Cl 90% | (13.1–37.42%) | (16.56–40.02%) | (13.38–35.17%) | (10.39–37.97%) | |
| CD8+Tn%CD3+CD8+ | Median | 3.2% | 6.45% | 2.5% | 0.8% |
| Reference range Cl 90% | (0.3–9.34%) | (2.05–10.33%) | (0.5–8.02%) | (0.11–4.75%) | |
| CD8+Tscm%CD3+CD8+ | Median | 13.7% | 28.05% | 11.6% | 6.1% |
| Reference range Cl 90% | (1.9–38.42%) | (8.30–48.90%) | (2.67–31.81%) | (1.3–21.86%) | |
| CD8+Tcm%CD3+CD8+ | Median | 8.9% | 7.4% | 8.8% | 12.15% |
| Reference range Cl 90% | (3.38–21.04%) | (2.73–15.05%) | (3.41–21.46%) | (4.91–27.45%) | |
| CD8+Tem%CD3+CD8+ | Median | 22.8% | 22.3% | 21.35% | 27.45% |
| Reference range Cl 90% | (6–48.8%) | (5.49–37.72%) | (6.94–49.84%) | (4.91–27.45%) | |
| CD8+Tte%CD3+CD8+ | Median | 12.2% | 10.5% | 13.85% | 10.6% |
| Reference range Cl 90% | (1.9–38.42%) | (2.00–30.62%) | (1.77–39.05%) | (1.47–45.85%) | |
The difference of absolute counts of T lymphocyte subsets in each age group
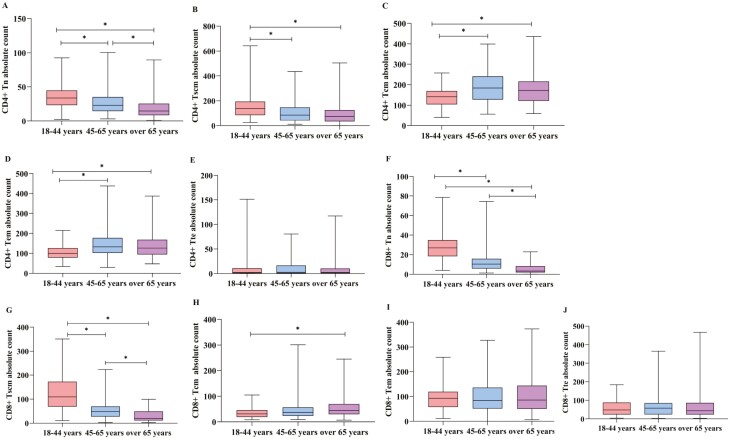
To analyze the absolute counts further, the data in Table 1 were performed by statistics. The absolute counts of CD4+Tn and Tscm decreased gradually with aging among the three groups, the median of CD4+Tn were 34.38 cells/μl, 23.06 cells/μl, and 14.9 cells/μl in each group with aging, respectively (P < 0.05, Fig. 2A), and the median of CD4+Tscm were 140.9 cells/μl, 85.49 cells/μl, and 69.2 cells/μl in every group respectively (P < 0.05, Fig. 2B). The same changes could be seen in CD8+Tn and CD8+Tscm cell populations (P < 0.05, Fig. 2F and G). The absolute counts of CD4+Tcm, CD4+Tem increased with aging (P < 0.05, Fig. 2C and D), but there was no obvious difference between the group of 45–64 years old and over 65 years old (P > 0.05, Fig. 2C and D). The absolute count of CD8+Tcm showed increased with aging which was also similar to CD4+Tcm (P < 0.05, Fig. 2H). The absolute counts of CD4+Tte, CD8+Tem, CD8+Tte did not change with aging (P > 0.05, Fig. 2E, I, and J).
Fig. 2.
Comparison of absolute counts of T lymphocyte subsets in different age groups. (A, B, F, G) showed the absolute count of CD4+Tn, CD4+Tscm, CD8+Tn, CD8+Tscm in group of 18–45 years were higher than those in group of 45–65years and over 65years (P < 0.05); (C, D) stated that the absolute counts of CD4+Tcm, CD4+Tem in groups of 45–64 years old and over 65 years old were higher than those in 18–45 years old (P < 0.05), but there were no difference between the group of 45–64 years old and over 65 years old (P > 0.05); (H) indicated that the absolute count of CD8+Tcm in the over 65 years old group was more than those in the 18–45 years old (P < 0.05); (E, I, J) demonstrated that the absolute counts of CD4+Tte, CD8+Tem, CD8+Tte did not changes with age (∗ represents significant differences).
Age-related T cell changes in distribution
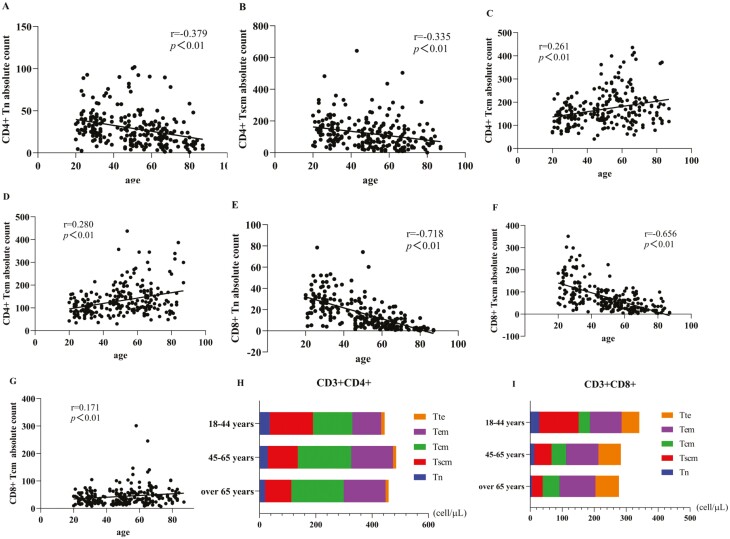
There was a weak negative correlation between age and the counts of CD4+Tn (r = −0.379, P < 0.01, Fig. 3A) and CD4+Tscm (r = −0.335, P < 0.01, Fig. 3B) which stated that the numbers of CD4+Tn and CD4+Tscm did not fluctuate much. A strong negative correlation between age and the counts of CD8+Tn (r = −0.718, P < 0.01, Fig. 3E) and CD8+Tscm r = −0.656, P < 0.01, Fig. 3F) suggested that the numbers of CD8+Tn and CD8+Tscm fluctuated significantly with aging. The weak positive correlation between age and the counts of CD4+Tcm (r = 0.261, P < 0.01, Fig. 3C), CD4+Tem (r = 0.280, P < 0.01, Fig. 3D), CD8+Tcm (r = 0.171, P < 0.01, Fig. 3G) were showed in our study. The cells which have stem cell properties, such as Tn, Tscm cells decreased with age. However, memory cell subsets, which have no stem cell properties such as Tcm, Tem cells increased with age (Fig. 3I and H).
Fig. 3.
Relationship between age and T lymphocyte subsets and changes in distribution. (A, B, E, F) showed a trend of decrease in CD4+Tn cell counts (r = −0.379, P < 0.01), CD4+Tscm cell counts (r = −0.335, P < 0.01), CD8+Tn cell counts (r = −0.718, P < 0.01), and CD8+ Tscm cell counts (r = −0.656, P < 0.01) with increased age. (C, D, G) indicated an increase trend with aging in CD4+Tcm (r=0.261, P < 0.01), CD4+Tem (r = 0.280, P < 0.01), CD8+Tcm (r = 0.171, P < 0.01). (I, H) represented that the Tn, Tscm cells which had stem cell properties decreased with aging; however, memory cell subsets, such as Tcm, Tem increased with aging (∗ represents significant differences).
Reference range of T lymphocyte subsets in different gender
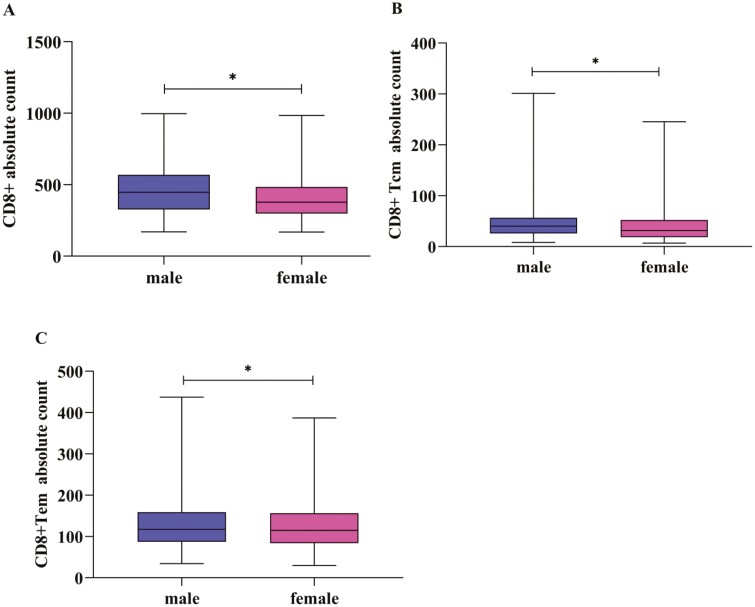
Gender has influences on the fluctuation of lymphocyte subsets [10]. So we established the reference range of lymphocyte subgroups according to gender(Tables 3 and 4). Compared parameters between genders, absolute counts of CD3+CD8+, CD8+Tcm, CD8+Tem in the male group were higher than those in the female group (P < 0.05, Fig. 4A–C).
Table 3.
Reference range of absolute counts of T lymphocyte subsets in different gender
| Parameters | Male | Female | |
|---|---|---|---|
| CD3+ (cells/μl) | Median | 1179.20 | 1097.76 |
| Reference range Cl 90% | (743.07–1671.83) | (744.05–1717.67) | |
| CD3+CD4+ (cells/μl) | Median | 592.17 | 579.8 |
| Reference range Cl 90% | (369.76–968.89) | (353.70–982.87) | |
| CD4+Tn (cells/μl) | Median | 24.93 | 25.46 |
| Reference range Cl 90% | (4.44–70.56) | (6.93–85.49) | |
| CD4+Tscm (cells/μl) | Median | 107.31 | 98.84 |
| Reference range Cl 90% | (23.63–326.29) | (12.80–268.27) | |
| CD4+Tcm (cells/μl) | Median | 156.55 | 145.2 |
| Reference range Cl 90% | (74.89–299.08) | (79.22–369.68) | |
| CD4+Tem (cells/μl) | Median | 117.46 | 114.84 |
| Reference range Cl 90% | (58.48–265.54) | (57.52–319.33) | |
| CD4+Tte (cells/μl) | Median | 2.46 | 2.19 |
| Reference range Cl 90% | (0.19–64.98) | (0.20–39.58) | |
| CD3+CD8+ (cells/μl) | Median | 447.28 | 378.38 |
| Reference range Cl 90% | (198.21–747.19) | (212.78–645.61) | |
| CD8+Tn (cell/μl) | Median | 12.59 | 12.01 |
| Reference range Cl 90% | (1.38–45.99) | (1.14–47.77) | |
| CD8+Tscm (cells/μl) | Median | 67.60 | 43.24 |
| Reference range Cl 90% | (6.33–212.74) | (6.10–192.84) | |
| CD8+Tcm (cells/μl) | Median | 40.10 | 31.37 |
| Reference range Cl 90% | (13.11–102.13) | (12.63–87.83) | |
| CD8+Tem (cells/μl) | Median | 91.12 | 72.71 |
| Reference range Cl 90% | (26.51–248.96) | (21.50–246.29) | |
| CD8+Tte (cells/μl) | Median | 54.32 | 43.63 |
| Reference range Cl 90% | (4.41–182.83) | (8.71–216.17) | |
Table 4.
Reference range of percentages of T lymphocyte subsets in different gender
| Parameters | Male | Female | |
|---|---|---|---|
| CD3+CD4+ % CD3+ | Median | 52.80% | 52.80% |
| Reference range Cl 90% | (36.94–68.43%) | (37.30–68.40%) | |
| CD4+Tn %CD4+ | Median | 4.10% | 4.50% |
| Reference range Cl 90% | (0.9–9.10%) | (1.55–12.25%) | |
| CD4+Tscm %CD4+ | Median | 18.30% | 16.90% |
| Reference range Cl 90% | (3.51–39.29%) | (2.4–38.55%) | |
| CD4+Tcm %CD4+ | Median | 27.15% | 26.10% |
| Reference range Cl 90% | (14.11–42.23%) | (15.65–46.9%) | |
| CD4+Tem %CD4+ | Median | 20.35% | 20.00% |
| Reference range Cl 90% | (10.44–40.07%) | (10.55–41.55%) | |
| CD4+Tte %CD4+ | Median | 0.50% | 0.40% |
| Reference range Cl 90% | (0.01–11.13%) | (0.01–8.1%) | |
| CD3+CD8+ %CD3+ | Median | 37.80% | 35.20% |
| Reference range Cl 90% | (23.18–53.17%) | (21.95–49.75%) | |
| CD8+Tn %CD8+ | Median | 3.25% | 3.10% |
| Reference range Cl 90% | (0.4–9.03%) | (0.3–9.65%) | |
| CD8+Tscm %CD8+ | Median | 15.15% | 12.70% |
| Reference range Cl 90% | (2.21–42.13%) | (1.4–41.85%) | |
| CD8+Tcm %CD8+ | Median | 9.10% | 8.40% |
| Reference range Cl 90% | (3.4–21.53%) | (2.95–20.9%) | |
| CD8+Tem %CD8+ | Median | 23.10% | 21.30% |
| Reference range Cl 90% | (6.34–48.8%) | (5.55–49.2%) | |
| CD8+Tte %CD8+ | Median | 12.40% | 10.90% |
| Reference range Cl 90% | (1.64–38.12%) | (2.55–42.35%) | |
Fig. 4.
Comparison of the absolute counts of T lymphocyte subsets in different gender. (A–C) showed that the absolute counts of CD3+CD8+, CD8+Tcm, CD8+Tem in the male group were higher than those in the female group (P<0.05) (∗ represents significant differences).
The difference of Tte in CD4+ and CD8+ cells
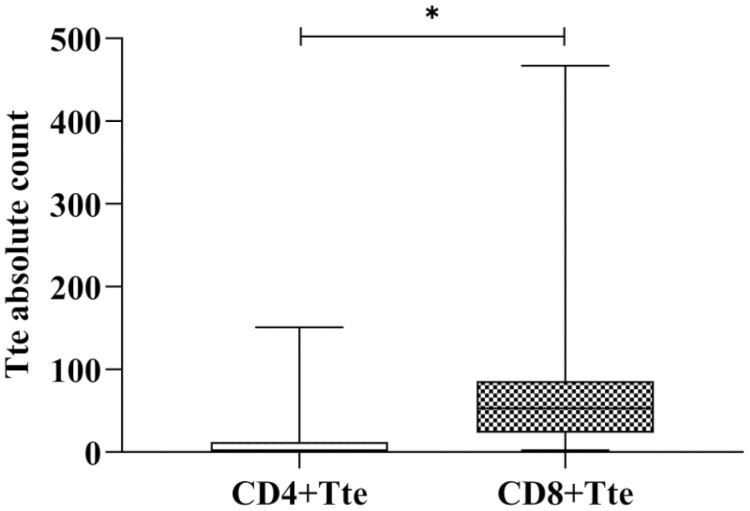
The analysis showed that the absolute counts of CD4+Tte and CD8+Tte were different, the absolute counts of Tte in CD8+ were higher than that in CD4+ (P < 0.05, Fig. 5).
Fig. 5.
The absolute count was different in CD3+CD4+Tte and CD3+CD8+Tte. The absolute of Tte in CD8+ were higher than that in CD4+ (∗ represents significant differences).
Discussion
Human T cells subsets were classified based on the expression of the surface receptor molecules: naïve and T memory cells could be identified by the expression of the CD45RA+ and CD45RO+ isoforms, respectively; Tcm and Tem were discriminated by lymphoid-homing molecules CCR7 and CD62L (L-selectin); Tscm cells had been described as a long-lived memory T cell population which expressed CD45RO−, CCR7+, CD45RA+, CD62L+, CD27+, CD28+, and IL-7Rα+, CD95+. CD95+ indicated that there were some cells with naïve markers that have memory properties and could be used to distinguish Tn from Tscm; CD45RA− was used to identify memory T cell population; CD62L+ stated that cells had limited effector functions, CD62L− represented mediating rapid effector functions [19–24]. So we identified the memory cells by phenotype of major clusters including Tn (CD45RA+CD45RO-CD62L+CD95−), Tscm(CD45RA+CD45RO-CD62L+CD95+), Tcm(CD45RA−CD45RO+CD62L+CD95+), Tem(CD45RA−CD45RO+CD62L−CD95+), Tte(CD45RA+CD45RO−CD62L−CD95+) [18].
In this study, we detected the percentages and absolute counts of T lymphoid subgroups by the single platform technique. There were two reasons: firstly, the precision and difference of detecting the percentages and absolute numbers of lymphocyte subset were different among laboratories. The single-platform was of more accuracy and consistency because of using the known total number of fluorescent microbeads as the standard internal parameters and adding fluorescent labelled antibodies into the trucount tubes, then applied acquisition and analysis software in the flow cytometry to gain accurate data according to the formula as follow.
Secondly, the clinical significance was different. The percentages of T lymphocyte subsets represented the proportion or composition of each subsets, indicating the development and differentiation of lymphocytes, while absolute counts suggested the proliferation capacity of lymphocytes characterized by the precise amount [12]. The percentage often did not accurately reflect the immune damage to the patients because they did not consider total, which might constantly change in these patients, especially those who were receiving anticancer therapies [12]. In our previous study, we had proved that comparing healthy controls to patients, absolute numbers of CD3+, CD3+CD4+, CD3+CD8+, B and NK cells decreased in the patients with the non-small cell lung cancer obviously, but the percentages of them were normal [25]. Therefore, it is crucial and urgent to detect both percentages and absolute numbers of lymphocyte subsets in the clinic, it will help us to know the changes in the patient’s immunologic function comprehensively, to analyze the clinical condition and predict the curative effect of patients for clinicians [26].
From the result, we could see stem-like cells, such as Tn, Tscm cells decrease with aging. Along with aging, thymus occurs changes that begin during childhood, including a reduction in thymic volume, loss of epithelial cells, increase in perivascular space, and replacement of thymic tissue by fat [27]. Thymus output declines with aging [28], which probably leads to the gradual decline in CD3+CD4+ and CD3+CD8+ naïve T cell numbers, although naïve T cell numbers decline less dramatically than thymocyte numbers [29]. In vitro, researchers had demonstrated that Tscm originated from Tn [24], so it is easier to understand why Tscm decreased with aging, the production of naïve T cells diminished with the involution of the thymus [30–32]. These cells have the ability not only to self-renew but also to differentiate into all subsets of memory and effector T cells [33, 34]. Combined with their longevity, the reserve of Tscm plasticity may play a central role in maintaining immunologic competence with aging [35]. The exhaustion of the Tn and Tscm reservoir suggests that the T-cell pool is a major target of the aging process and may define a parameter possibly related to the life span of humans [29].
In contrast to Tn and Tscm, the absolute counts of CD4+Tcm, CD4+Tem increased with aging, the counts of 45–64 years old and over 65 years old were higher than those in 18–45 years old (P < 0.05, Fig. 2C and D), but there was no difference between the group of 45 and 64 years old and over 65 years old (P > 0.05). The absolute count of CD8+Tcm in over 65 years old group was more than that in 18–45 years old (P < 0.05, Fig. 2G). Tcm and Tem increased with aging and keep less fluctuation over 65 years old [36]. With advancing age, the major goal of T cells shifts to mounting appropriate responses against novel infections and protecting the host against reinfection with common pathogens [37]. When a first stimulus triggers a first response [1], Tn encounters cognate antigen and expand clonally to generate effector cells that migrate to peripheral tissues and eliminate virus and malignant cells [38]. During this effector process, most effector cells become terminally differentiated, termed as short-lived effector cells, while a fraction of effector cells, termed as memory precursor effector cells acquired the ability to survive under the contraction stage of the immune response [22], and further differentiate into a heterogeneous pool of memory cells under optimal developmental conditions, when a second stimulus triggers a second response, more stronger, speedy, durative, and specificity occurs [22].
The majority of effector T cells contract rapidly and are not present in at a steady state, a population of Tte exhibiting CD45RA+CD62L− phenotypes can persist in circulation [39]. Tte cells are mostly present within the CD8+ T cell lineage, exhibiting high capacity for IFN-γ production and low proliferative capacity [40], CD4+Tte cells are rarely detected, some researchers found that the expansion of CD4+Tte cells with cytotoxic function occurs in individuals infected with Dengue virus and was associated with protection [41].
All in all, we should pay more attention to the percentages and absolute counts’ changes of T lymphocyte subsets in the clinic simultaneously, for it may give us more important references on treatment.
Conclusion
The reference values of percentages and absolute numbers of naïve T and T memory cell subsets can help doctors to understand the immune state of patients and to evaluate conditions of prognosis then adjust treatment for patients.
Acknowledgments
The technical guidance of this work was supported by Juan Du and Gong Cheng in BD Biosciences.
Glossary
Abbreviations
- Tn
naïve T cells
- Tm
T memory cells
- Tscm
T memory stem cells
- Tcm
central memory T cells
- Tem
effector memory T cells
- Tte
terminal effector T cells
- M. tb
Mycobacterium tuberculosis
Funding
The work was supported by the Specialized Research Fund for the Doctoral Program of Higher Education (No.20121210110010), Traditional Chinese Medicine and Western Medicine Research Project of Tianjin Health and Family Planning Commission (No. 2017003) and the Scientific Research Plan Project of Tianjin Education Commission (No. 2018KJ034).
Conflict of interests
The authors declare that they have no potential conflicts of interest.
Author contributions
J.Y. conceptualization, methodology. Y.X., A.L., and W.L. writing – original draft, formal analysis, writing – review and editing. Y.L., S.Y., G.Z., and Z.Z. data curation, validation. J.S. Y.J., X.L., Y.G., and H.C. resources.
Ethics approval and consent to participate
All clinical and ethical regulations were given the informed consent in accordance with Declaration of Helsinki and the clinical trial was approved by the First teaching hospital of Tianjin University of TCM ethics committee review (TYLL2017[K]002) and registered at the Chinese Clinic Trial Registry (ChiCTR-IOR-17014139).
Availability of data and materials
All data generated and analyzed during this study are available from the corresponding author in response to reasonable requests.
References
- 1. Pradeu T, Du Pasquier L.. Immunological memory: What’s in a name? Immunol Rev 2018, 283, 7–20. [DOI] [PubMed] [Google Scholar]
- 2. Macallan DC, Borghans JAM, Asquith B.. Human T cell memory: a dynamic view. Vaccines 2017, 5, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lauvau G, Soudja SM.. Mechanisms of memory T cell activation and effective immunity. Adv Exp Med Biol 2015, 850, 73–80. doi: 10.1007/978-3-319-15774-0_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chang JT, Wherry EJ, Goldrath AW.. Molecular regulation of effector and memory T cell differentiation. Nat Immunol 2014, 15, 1104–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Morrot A. Human stem memory T cells (TSCM) as critical players in the long-term persistence of immune responses. Ann Transl Med 2017, 5, 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ahmed R, Roger L, Costa Del Amo P, Miners KL, Jones RE, Boelen L, et al. Human stem cell-like memory t cells are maintained in a state of dynamic flux. Cell Rep 2016, 17, 2811–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gattinoni L, Speiser DE, Lichterfeld M, Bonini C.. T memory stem cells in health and disease. Nat Med 2017, 23, 18–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hong H, Gu Y, Sheng SY, Lu CG, Zou JY, Wu CY.. The distribution of human stem cell-like memory T cell in lung cancer. J Immunother 2016, 39, 233–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mpande CAM, Dintwe OB, Musvosvi M, Mabwe S, Bilek N, Hatherill M, et al.; SATVI Clinical Immunology Team . Functional, antigen-specific stem cell memory (TSCM) CD4+ T cells are induced by human mycobacterium tuberculosis infection. Front Immunol 2018, 9, 324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Qin L, Jing X, Qiu Z, Cao W, Jiao Y, Routy J-P, Li T, et al.. Aging of immune system: immune signature from peripheral blood lymphocyte subsets in 1068 healthy adults. Aging 2016, 8, 848–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jalla S, Sazawal S, Deb S, Black RE, Das SN, Sarkar A, et al.. Enumeration of lymphocyte subsets using flow cytometry: effect of storage before and after staining in a developing country setting. Indian J Clin Biochem 2004, 19, 95–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kuss I, Hathaway B, Ferris RL, Gooding W, Whiteside TL.. Decreased absolute counts of T lymphocyte subsets and their relation to disease in squamous cell carcinoma of the head and neck. Clin Cancer Res 2004, 10, 3755–62. [DOI] [PubMed] [Google Scholar]
- 13. Brando B, Barnett D, Janossy G, Mandy F, Autran B, Rothe G, et al. Cytofluorometric methods for assessing absolute numbers of cell subsets in blood. European Working Group on Clinical Cell Analysis. Cytometry 2000, 42, 327–46. [DOI] [PubMed] [Google Scholar]
- 14. O’Gorman MR, Nicholson JK.. Adoption of single-platform technologies for enumeration of absolute T-lymphocyte subsets in peripheral blood. Clin Diagn Lab Immunol 2000, 7, 333–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Reimann KA, O’Gorman MG, Spritzler J, Wilkening CL, Sabath DE, Helm K, et al. Multisite comparison of CD4 and CD8 T-lymphocyte counting by single-versus multiple-platform methodologies: evaluation of Beckman Coulter Flow Count fluorospheres and the tetraONE system. Clin Diag Lab Immunol 2000, 7, 344–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chng WJ, Tan GB, Kuperan P.. Establishment of adult peripheral blood lymphocyte subset reference range for an Asian population by single-platform flow cytometry: influence of age, sex, and race and comparison with other published studies. Clin Diagn Lab Immunol 2004, 11, 168–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lugli E, Gattinoni L, Roberto A, Mavilio D, Price DA, Restifo NP, et al. Identification, isolation and in vitro expansion of human and nonhuman primate T stem cell memory cells. Nat Protoc 2013, 8, 33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Oliveira G, Ruggiero E, Stanghellini MT, Cieri N, D’Agostino M, D’Agostino M, et al. Tracking genetically engineered lymphocytes long-term reveals the dynamics of T cell immunological memory. Sci Transl Med 2015, 7, 317ra198. [DOI] [PubMed] [Google Scholar]
- 19. Cieri N, Oliveira G, Greco R, Forcato M.. Generation of human memory stem T cells after haploidentical T-replete hematopoietic stem cell transplantation. Blood 2015, 125, 2865–74. [DOI] [PubMed] [Google Scholar]
- 20. Xu L, Zhang Y, Luo G, Li Y.. The roles of stem cell memory T cells in hematological malignancies. J Hematol Oncol 2015, 8, 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sallusto F, Lenig D, Förster R, Lipp M, Lanzavecchia A.. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 1999, 401, 708–12. [DOI] [PubMed] [Google Scholar]
- 22. Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R.. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat Immunol 2003, 4, 1191–8. [DOI] [PubMed] [Google Scholar]
- 23. Xu L, Yao D, Jiaxiong T, He Z, Yu Z, Chen J, et al.. Memory T cells skew toward terminal differentiation in the CD8+ T cell population in patients with acute myeloid leukemia. J Hematol Oncol 2018, 11, 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Biasco L, Scala S, Ricci LB, Dionisio F, Baricordi C, Calabria A, et al.. In vivo tracking of T cells in humans unveils decade-long survival and activity of genetically modified T memory stem cells. Sci Transl Med 2015, 7, 273ra13. [DOI] [PubMed] [Google Scholar]
- 25. Xia Y, Li W, Li Y, Yu J.. The clinical value of the changes of peripheral lymphocyte subsets absolute counts in patients with non-small cell lung cancer. Transl Oncol 2020, 13. doi: 10.1016/j.tranon.2020.100849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Karaman H, Karaman A, Erden A, Poyrazoglu OK, Karakukcu C, Tasdemir A.. Relationship between colonic polyp type and the neutrophil/ lymphocyte ratio as a biomarker. Asian Pac J Cancer Prev 2013, 14, 3159–61. [DOI] [PubMed] [Google Scholar]
- 27. Haynes BF, Markert ML, Sempowski GD, Patel DD, Hale LP.. The role of the thymus in immune reconstitution in aging, bone marrow transplantation, and HIV-1 infection. Annu Rev Immunol 2000, 18, 529–60. [DOI] [PubMed] [Google Scholar]
- 28. Sleinmann GG.Klaii SB, Muler-Hcrmclink H-K.. The involution of the ageing human thymic epithelium is independent of puberty. Scaml J Immunol 1985, 22, 563–75. [DOI] [PubMed] [Google Scholar]
- 29. Fagnoni FF, Vescovini R, Passeri G, Bologna G, Pedrazzoni M, Lavagetto G, et al.. Shortage of circulating naive CD8(+)T cells provides new insights on immunodeficiency in aging . Blood 2000, 95. doi: 10.1182/blood.V95.9.2860.009k35_2860_2868. [DOI] [PubMed] [Google Scholar]
- 30. Nikolich-Žugich J. The twilight of immunity: emerging concepts in aging of the immune system. Nat Immunol 2018, 19, 10–9. [DOI] [PubMed] [Google Scholar]
- 31. Goronzy JJ, Weyand CM.. Mechanisms underlying T cell ageing. Nat Rev Immunol 2019, 19, 573–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Thome JJ, Grinshpun B, Kumar BV, Kubota M, Ohmura Y, Lerner H, et al.. maintenance of human naive T cells through in situ homeostasis in lymphoid tissue sites. Sci Immunol 2016, 1. doi: 10.1126/sciimmunol.aah6506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Del Amo PC, Lahoz-Beneytez J, Boelen L, Ahmed R, Miners KL, Zhang Y.et al.. Human TSCM cell dynamics in vivo are compatible with long-lived immunological memory and stemness. PLoS Biol 2018, 16, e2005523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kared H, Tan SW, Lau MC.. Immunological history governs human stem cell memory CD4 heterogeneity via the Wnt signaling pathway. Nat Commun 2020, 11, 821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lugli E, Dominguez MH, Gattinoni L, Chattopadhyay PK, Bolton DL, Song K, et al.. Superior T memory stem cell persistence supports long-lived T cell memory. J Clin Invest 2013, 123, 594–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Naylor K, Li G, Vallejo AN.. The influence of age on T cell generation and TCR diversity. J Immunol 2005, 174, 7446–52. [DOI] [PubMed] [Google Scholar]
- 37. Davenport MP, Smith NL, Rudd BD.. Building a T cell compartment: how immune cell development shapes function. Nat Rev 2020, 20, 499–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lugli E, Galletti G, Boi SK, Youngblood BA.. Stem, effector, and hybrid states of memory CD8+ T cells. Trends Immunol 2020, 41, 17–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kumar BV, Connors T.. Human T cell development, localization, and function throughout life. Immunity 2018, 48, 202–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Larbi A, Fulop T.. From “truly naïve” to “exhausted senescent” T cells: when markers predict functionality. Cytometry A 2014, 85, 25–35. [DOI] [PubMed] [Google Scholar]
- 41. Weiskopf D, Bangs DJ, Sidney J, Kolla RV, De Silva AD, de Silva AM, et al.. Dengue virus infection elicits highly polarized CX3CR1+ cytotoxic CD4+ T cells associated with protective immunity. Proc Natl Acad Sci U S A 2015, 112, E4256–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated and analyzed during this study are available from the corresponding author in response to reasonable requests.