Abstract
Pheochromocytomas and paragangliomas (PHEOs/PGLs) represent diagnostically challenging and complex neuroendocrine tumors. Current biomarker tests for PHEOs/PGLs are technically complex or limited. We assessed the diagnostic utility of the NETest in patients with PHEOs/PGLs (n=81), including 10 pediatric patients, and age-/gender-matched controls (n=142) using a prospective case:control (1:2) analysis. mRNA was measured (qPCR) and results caled 0-100 (ULN<20). Receiver Operating Curve (ROC) and non-parametric (Mann-Whitney) were used for analyses (2-tailed). All data is presented as mean±SEM. NETest accuracy for PHEO/PGL diagnosis was 100%. PHEO/PGL scores were 70±3 versus 8.5±1 in controls (p<0.0001) and ROC analysis was 0.99±0.004 (p<0.0001). Diagnostic metrics were 94% accurate, 100% sensitive, and 92% specific. Imaging correlation with 68Ga-PET-SSA was 100%. NETest levels in PHEOs (n=26) were significantly (p<0.0001) elevated (83±4) versus 66±4 in PGLs (n=40) and mixed PHEOs/PGLs (n=5: 37±3). Cluster 2 tumors exhibited significantly (p=0.034) elevated NETest levels (n=4: 92±2) versus Cluster 1 tumors (n=35: 69±4). Regulatory pathway analysis identified elevated RAS-RAF, metastatic, pluripotential, neural and secretory gene cluster levels (p<0.05) in PHEOs compared to PGLs. Cluster 2 PPGLs exhibited elevated (p=0.046) levels of growth-factor signaling genes compared to cluster 1. The PHEOs/PGLs in the pediatric cohort (n=10) were all NETest-positive (81±8) and exhibited a gene expression profile spectrum analogous to adults. Overall, circulating NET transcript analysis identifies PHEOs/PGLs with 100% efficacy. Since the NETest is not affected by medications, postural position or activity levels, this test will likely have clinical utility in the diagnosis and management of PHEO/PGL patients.
Keywords: Biomarker, NETest, pheochromocytoma, paraganglioma, molecular assay, PCR
INTRODUCTION:
Pheochromocytomas (PHEOs) and paragangliomas (PGLs) are neural crest-derived tumors that are most commonly found in the adrenal glands. These tumors typically arise from chromaffin cells, which are a group of cells that have the ability to synthesize and secrete a variety of amines and peptides (including catecholamines, adrenocorticotropic hormone, chromogranins, neuropeptide Y, vasoactive intestinal polypeptide, synaptophysin, and many others) (Antonio et al., 2020). Such cells and their neoplastic counterparts are characterized by various mechanistic secretory drivers including hydroxylases, transporters (SLC18A1 and SLC18A2, also known as VMAT1 and VMAT2) and granin-associated secretory vesicle systems (Antonio et al., 2020, Eisenhofer et al., 2017). Certain cell function regulators are known to be widespread and common to other neuroendocrine cells, including neoplastic cells (Crona and Skogseid, 2016). Thus, it has been proposed to subcategorize PHEOs/PGLs into Cluster I and II within the neuroendocrine tumor (NET) grading classification system, as seen in the classification of gastroenteropancreatic (GEP) and bronchopulmonary (BP) tumors (Favier et al., 2015). NETs of the GEP and BP systems have a specific molecular signature that can be identified in blood thereby facilitating accurate (about 90%) diagnosis (Modlin et al., 2018, Öberg et al., 2020).
Although the majority of PHEOs/PGLs are sporadic (60%), about 40% are hereditary (autosomal dominant) and are commonly associated with multiple endocrine neoplasia type 2 (MEN2A or MEN2B), von Recklinghausen’s neurofibromatosis type 1 (NF-1), von Hippel–Lindau (VHL) syndrome or germline mutations in the succinate dehydrogenase (SDH) subunits (Greenberg et al., 2020, Bayley et al., 2020, Buffet et al., 2018, Fishbein et al., 2017). Somatic analyses have identified additional genetic mutations relevant to disease pathobiology (Greenberg et al., 2020, Dahia et al., 2020, Fishbein et al., 2017). Each of these mutations affects a specific metabolic pathway (Moog et al., 2020, Richter et al., 2019). The Cancer Genomic Atlas (TCGA) group proposed a comprehensive system to classify PHEO/PGL-susceptibility genes: A pseudohypoxia subtype (Cluster 1), a kinase-signaling subtype (Cluster 2) and Wnt3 signaling (Cluster 3). These integrative efforts provide a mechanistic basis for about 75% of PHEOs/PGLs (Fishbein et al., 2017). Since the NETest is about 95% accurate in the identification of MEN-I tumors (Perrier, 2018), it is probable, given the similar biological background of the neuroendocrine cell system, that the NETest may be effective in identifying chromaffin cell-based lesions in MEN-II tumors.
The clinical diagnosis of PHEO/PGL can be challenging. An accurate diagnosis relies heavily upon the biochemical identification of catecholamines and their metabolites, metanephrines, as a diagnostic gold standard (Eisenhofer et al., 2020). Nevertheless, such measurements have limitations, especially in patients taking various medications (Eisenhofer et al., 2003). Inaccuracies in biochemical diagnosis may also occur in cases of small tumors, episodic release of catecholamines, and by position (sitting vs. supine) (Eisenhofer et al., 2015). Certain PHEOs/PGLs, especially hereditary cases, differ considerably in the rates of catecholamine synthesis, turnover, release, and type of catecholamine and metabolite produced (Eisenhofer et al., 2017). Furthermore, the measurement of secretory analytes is limited in the information it provides about tumorigenesis and objective predictions of tumor malignancy or potential to metastasize. However, elevated 3-methoxytyramine concentrations have been noted to predict or detect metastatic PHEO/PGL in certain cases (Eisenhofer et al., 2012).
68Ga-DOTATATE PET/CT imaging is the standard of care used in the localization, staging, and theranostic management of PHEO/PGL (Taïeb et al., 2019b, Taïeb et al., 2019a). However, somatostatin receptor profiles (expression levels and types of receptors) vary in hereditary versus non-hereditary PHEO/PGL. Furthermore, these receptors may change or lose expression during disease evolution (although rare) (Leijon et al., 2019). Loss of receptor expression may result in only 35% of PGL detection in some studies (Han et al., 2019). Furthermore, small PHEOs/PGLs (<5 mm) may not be visible on imaging.
Patients with PHEO/PGL are often not diagnosed efficiently or are misdiagnosed completely as a result of non-specific clinical symptomatology and on a lesser extent, diagnostic limitations (Lenders et al., 2014b). For example, about 30% of patients with PHEO/PGL are asymptomatic or have only minor signs and symptoms of disease (Lenders et al., 2020). As a consequence, the PHEO/PGL diagnostic algorithm is driven by a “rule out” method of modern healthcare. The standard of care for a precise diagnosis involves repeated biochemical tests and assessment of at least two imaging modalities. There is a critical area of unmet need in this disease, especially in the era of genomics research. An accurate multianalyte molecular diagnostic tool would significantly advance this field. A liquid biopsy approach (e.g., using blood instead of tissue) could not only help identify a tumor, but it could also objectively quantify tumor biology and predict biological behavior.
The NETest is a multigenomic mRNA liquid biopsy that has proved to be an accurate in vitro diagnostic (IVD) for NETs of the GEP and BP systems (Kidd et al., 2015, Walenkamp et al., 2014, Li et al., 2013). It uses a 51-marker gene expression assay to identify neuroendocrine neoplasia mRNA using 1ml of blood (Modlin et al., 2013, Chen et al., 2018). The assay uses a gene expression score derived from gene mapping of about one quarter of a million tumor-related genes that objectively quantify NET-associated mRNAs. Specific genes are merged into 14 “omic clusters” that define biologically-relevant hallmarks of neoplasia (e.g., neural-genes, proliferation, and somatostatin receptors) (Kidd et al., 2020). Gene levels in blood correlate with gene expression at a tumoral tissue level. A number of reports and meta-analyses have confirmed its diagnostic and clinical utility in these tumors (Modlin et al., 2016, Pavel et al., 2017a, Oberg et al., 2020, van Treijen et al., 2020). Given that there are certain common biological similarities between NETs and PHEOs/PGLs, we undertook this study to evaluate whether neuroendocrine tumor transcript (NETest) levels in blood could accurately identify PHEO/PGL. We further sought to identify whether specific omic gene clusters would provide objective biological information that could be used to delineate tumor biology or used in clinical practice.
MATERIALS AND METHODS:
Study Design:
This is a prospective, age/gender matched case-control study. This study included 71 adult PHEO/PGL patients enrolled over a 12-month period (2/1/2019-1/31/2020) under the Eunice Kennedy Shriver NICHD protocol 00-CH-0093. Age- and gender-matched controls (2 controls:1 case) were available as comparators. The adult PHEO/PGL cohort was comprised of 40 PGLs (extra-adrenal location), 26 PHEOs (adrenal) and 5 mixed (including both PHEOs and PGLs. Mutation status was known in 82% of the samples (58/71).
Eighteen patients had no prior treatment. Fifty-three patients underwent several therapies. Overall, 50 (70%) had surgery, 16 received radiation, 8 received cold somatostatin analogues, 9 received chemotherapy, 2 received peptide receptor radionuclide therapy (PRRT), 2 underwent embolization and 2 received 131I- metaiodobenzylguanidine (MIBG) therapy. All except 1 patient had image-detectable disease. The matched control cohort was comprised of 93 healthy controls: 18 with benign disease (e.g., thyroiditis) and 31 with other neoplasms including lung, pancreatic and colon cancers (none of which were neuroendocrine tumors). Eight were receiving proton pump inhibitor (PPI) treatment, 6 were receiving chemotherapy and 3 were receiving metformin treatment.
The demographics of each group are included in Table 1. There were no differences in sex distribution (PHEO/PGL: M:F = 32:39 versus controls: 67:75) or age between the two groups (PHEO/PGL: median 40, range: 20-74; controls: median 42, range: 20-74).
Table 1.
Demographics: PHEOs/PGLs and controls
| PHEOs/PGLs | Controls | |
|---|---|---|
|
| ||
| No. | 71 | 142 |
|
| ||
| Age, years (median & range) | 40 (20-74) | 42 (20-74)† |
|
| ||
| Gender (M:F) | 32:39 | 67:75‡ |
|
| ||
| Type (%) | PGL: 40 (56%) | Healthy controls: 93 (65%) |
| PHEO: 26 (37%) | Benign diseases: 18 (13%) | |
| Mixed PHEO/PGL:5 (7%) | Other neoplasia: 31 (22%) | |
|
| ||
| Disease detectable | 70/71 (98.6%)ᶲ | N/A |
|
| ||
| Disease Extent | Local: 26 (37%) | N/A |
| Metastatic: 45 (63%) | ||
|
| ||
| Therapy | None: 18 (25%) | Chemotherapy: 6 (4%) |
| Surgery: 50 (70%) | Metformin 3 (2%) | |
| Embolization: 2 (3%) | PPI: 8 (6%) | |
| PRRT: 2 (3%) | ||
| Radiation: 16 (23%) | ||
| MIBG: 2 (3%) | ||
| Chemotherapy: 9 (13%) | ||
| Immunotherapy: 2 (3%) | ||
| SSA: 8 (11%) | ||
| Sunitinib: 1 (1%) | ||
| Temozolomide: 1 (1%) | ||
| TACE: 1 (1%) | ||
| Cryotherapy: 1 (1%) | ||
|
| ||
| Mutation status | EGLN1: 1 (1.4%) | N/A |
| FH: 1 (1.4%) | ||
| HIF2A: 1 (1.4%) | ||
| MAX: 1 (1.4%) | ||
| RET: 3 (4.2%) | ||
| RINT1: 1 (1.4%) | ||
| SH: 1 (1.4%) | ||
| SDHB: 25 (35.2%) | ||
| SDHC: 1 (1.4%) | ||
| SDHD: 3 (4.2%) | ||
| VHL: 1 (1.4%) | ||
| Negative: 19 (26.7%) | ||
| No data: 13 (18.3%) | ||
|
| ||
| Cluster assessment § | Cluster 1: 35 (49%) | N/A |
| Cluster 2: 4 (6%) | ||
| No cluster: 32 (45%) | ||
p=0.85 (Mann-Whitney U-test, 2 -tailed).
p=0.88 (Fisher’s exact test, 2-tailed)
One patient exhibited biochemical evidence of recurrence
Per TCGA
Metaiodobenzylguanidine = MIBG; PHEO = pheochromocytoma; PGL = paraganglioma; PPI = Proton pump inhibitors; PRRT = peptide receptor radionuclide therapy; SSA = somatostatin analogs; TACE: Transcatheter arterial chemoembolization; TCGA = The Cancer Genome Atlas
A separate cohort of 10 children with PHEO/PGL were also evaluated and tumor tissue was collected over the same time period. These included 3 PGLs, 5 PHEOs and 2 mixed tumor types. One patient had no prior treatment. Nine patients underwent several treatments. Eight (80%) had prior surgery, 3 received chemotherapy, 2 received somatostatin analogues, and 1 received radiotherapy. All had image-detectable disease. Demographics are included in Table 2.
Table 2.
Demographics: PHEOs/PGLs (minors: n=10)
| Age, years (median/range) | 13.5 (10-19) |
|
| |
| Gender (M:F) | 5:5 |
|
| |
| Tumor Type | PHEO: 5 (13%) |
| Mixed PHEO/PGL:2 (31%) | |
| PGL: 3 (56%) | |
|
| |
| Disease detectable | 10/10 (100%) |
|
| |
| Disease Extent | Local: 3 (30%) |
| Metastatic: 7 (70%) | |
|
| |
| Therapy | None: 1 (10%) |
| Surgery: 8 (80%) | |
| Radiation: 1 (10%) | |
| Chemotherapy: 3 (30%) | |
| SSA: 2 (20%) | |
|
| |
| Mutation status | HIF2A: 2 (20%) |
| SDHB: 4 (40%) | |
| VHL: 1 (10%) | |
| Negative: 2 (20%) | |
| No data: 1 (10%) | |
|
| |
| Cluster assessment § | Cluster 1: 7 (70%) |
| No cluster: 3 (20%) | |
Per TCGA
PHEO = pheochromocytoma; PGL = paraganglioma; SSA = somatostatin analogs; TCGA = The Cancer Genome Atlas
The primary outcome of the study focused on a PHEO/PGL histological diagnosis. Variables including clinical data (diagnosis, treatment status), molecular genetic assessment (mutation) and NETest measurements were evaluated.
Sample collection:
All samples were collected and analyzed according to standard IRB protocols (Western Institutional Review Board Ethics Protocol #20150174 and the Eunice Kennedy Shriver NICHD 00-CH-093 protocol) in accordance with the National Institutes of Health and the World Medical Association Declaration of Helsinki regarding ethical conduct of research involving human subjects. Peripheral whole blood samples (3 ml) were collected in EDTA tubes, mixed, and stored on ice (Pavel et al., 2017b). Samples were shipped to Wren Laboratories, a clinically certified laboratory (CL-0704, CLIA 07D2081388, NYSDOH PFI: 9138) for NETest measurement. All blood samples were de-identified.
NETest measurement:
Details of the PCR methodology, mathematical analysis and validation have been published (Pavel et al., 2017b, Filosso et al., 2018, Kidd et al., 2019). In brief, NET target transcripts (mRNA) are isolated from whole blood, real-time PCR is performed and gene expression is evaluated using multi-algorithmic artificial intelligence-based analyses (Pavel et al., 2017b, Filosso et al., 2018, Kidd et al., 2019). Final results are expressed as an activity index from 0-100 (Pavel et al., 2017b, Filosso et al., 2018, Kidd et al., 2019). Upper limit of normal is 20.
Statistical analysis:
All statistical analyses were performed using Prism 9.0 for Windows (GraphPad Software 9.0.0, La Jolla, CA, www.graphpad.com) and MedCalc Statistical Software version v19.6 (MedCalc Software bvba, Ostend, Belgium; www.medcalc.org; 2013).
Power analysis (PHEO/PGLs and controls, power 0.95 and α = 0.01) adequate to attain significant differences in NETest scores (using anticipated means of PHEO/PGL 40±20 versus a mean of 20 for controls (Peczkowska et al., 2017)) was calculated. Requirements were a minimum of 27 individuals in the PHEO/PGL group and 54 controls (total = 81). Overall, 213 subjects were evaluated including 71 subjects with PHEO/PGL.
We compared those with a positive biomarker score (NETest>20) to a negative score as a diagnostic method. We performed two separate analyses: Area under the receiver operating curve (AUROC) and diagnostic metric evaluation. A non-parametric ANOVA (Kruskal-Wallis) test was also performed. All data are presented as mean±SEM and where appropriate, [95% confidence intervals (CI)] are included. A p<0.05 was considered significant.
RESULTS:
PHEO/PGL Diagnosis:
In the matched cohort, the NETest score was significantly (p<0.0001) elevated in the PHEO/PGL group (70±3) compared to the control group (8±1) (Figure 1A). An AUROC analysis determined the AUC to be 0.99±0.004 [0.97-1.0] (Figure 1B). The z-statistic was particularly significant (126.1). The Youden J was 0.92.
Figure 1. NETest diagnostic metrics.
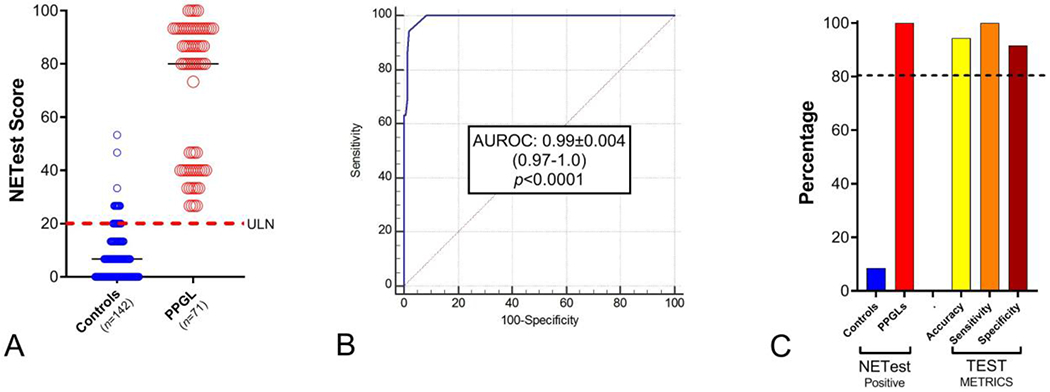
1A. NETest scores in non-PHEO/PGL (controls) and PHEO/PGL. Levels were significantly (p<0.0001) elevated in the PHEO/PGL group.
Dotted red line = upper limit of normal (ULN) for the NETest (score >20).
Black horizontal lines = median
1B. AUROC analysis: AUC = 0.99 (p<0.0001).
1C. NETest was positive in 100% of PHEO/PGL vs 8.5% controls. Metrics were >90% for PHEO/PGL diagnosis.
Dotted line = 80% cut-off per NIH for biomarker efficacy.
The NETest was positive in 71/71 (100%) PHEO/PGL samples (Figure 1C). Twelve (8.5%) of the controls (n=142) were positive. The NETest diagnostic metrics were accuracy: 94%, sensitivity: 100% and specificity: 92%. The “false positives” results (n=12) in the non-PHEO/PGL group included: 1 healthy control, 1 squamous cell cancer, 1 lung adenocarcinoma, 1 lung sarcoid, 2 Hashimoto’s thyroiditis, 3 polycystic ovary syndromes and 3 pancreatic cancers. Drug use (e.g., proton pump inhibitors or metformin) was irrelevant for scoring purposes.
Relationship to clinical parameters:
Seventy of 71 (98.6%) of PHEO/PGL patients exhibited image-detectable (68Ga-DOTATATE PET/CT) disease. One patient exhibited elevated biochemistry suggestive of residual PHEO/PGL. The NETest was positive in 100% of these samples. Thus, the NETest exhibited 100% image concordance with disease and could detect residual, image-negative disease (biochemical recurrence).
NETest scores were found to be positive in all tumors with (n=45: 71±4) and without (n=26: 68±5) metastases. An evaluation of disease status (stable vs. progressive disease) identified no significant differences in NETest scores between stable (n=27: 66±5) and progressive (n=44: 73±4, p=0.23). No significant differences were noted between treated (n=53, 69±4) and untreated (n=18, 72±6). All treated and non-treated groups were NETest-positive. Therefore, the NETest accurately identifies PHEO/PGL disease irrespective of prior therapeutic intervention.
NETest and tumor type:
The PHEO/PGL cohort was comprised of 40 PGLs, 26 PHEOs and 5 mixed tumor types. NETest scores were significantly (KW statistic: 17.3, p<0.0001) elevated in PHEOs (83±4) versus either PGLs (66±4) or mixed tumors (37±3). NETest scores were significantly different between each of the three groups. (Figure 2A). An AUROC analysis (PHEOs versus PGLs + mixed) identified an AUC of 0.76±0.06 [0.65-0.86] (Figure 2B). The z-statistic was particularly significant (4.23). The Youden J was 0.54.
Figure 2. NETest and tumor type.

2A. NETest scores in PGLs versus PHEOs versus mixed tumors. Levels were significantly elevated in PHEOs versus PGLs (p=0.0032) and versus mixed tumors (p=0.0016). No significant difference was found between PGLs and mixed tumors (p=0.20).
Dotted red line = upper limit of normal (ULN) for the NETest (score >20).
Black horizontal lines = median
2B. AUROC analysis differentiated PHEOs from PGLs and mixed tumors with an AUC of 0.76 (p<0.0001).
Relationship to mutation status:
The mutation status was determined in 82% of samples (n=58). Nineteen of the 58 cases (33%) were mutation-negative. Twenty-nine (50%) exhibited mutations in the SDHx cluster (predominantly SDHB: n=25). The remaining samples exhibited individual mutations in PHEO/PGL-associated genes. Cluster 1-associated mutations (VHL, FH, SDH, and ENGL1) were associated with significantly (p=0.034) lower NETest scores (69±4) than those with Cluster 2-associated mutations (RET, MAX, NF1) (92±2, Figure 3A).
Figure 3. NETest, molecular (omic) clusters and mutation status.
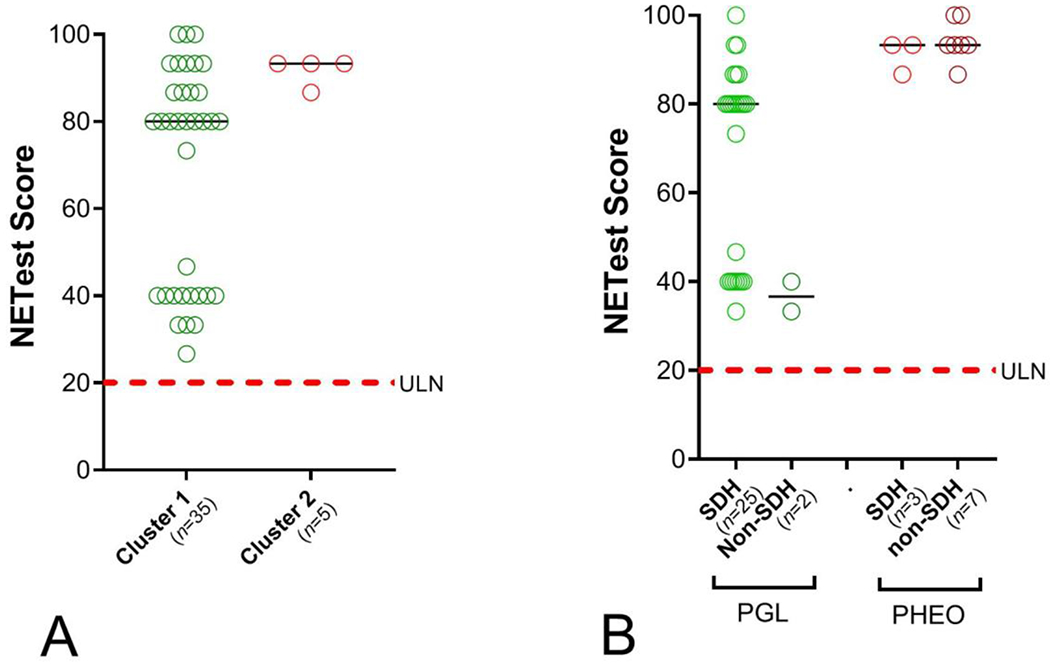
3A. NETest scores in Cluster 1 vs. Cluster 2 PHEOs/PGLs. Levels were significantly elevated in Cluster 2 compared to Cluster 1 PHEOs/PGLs(p=0.034).
3B. NETest scores in SDH mutations and non-SDH mutations in either PGLs or PHEOs. An association was identified between mutational status and NETest levels although PGLs with SDH mutations had significantly lower scores than PHEOs with SDH mutations (p=0.02).
Dotted red line = upper limit of normal (ULN) for the NETest (score >20).
Black horizontal line = median
In an assessment of mutational status in PGLs and PHEOs, PGLs exhibited the majority of SDHx mutations (25/28: 89%). Such mutations were only detected in 15% (n=3) of PHEOs (Chi2=12.3, p=0.0004). NETest levels in PGLs with SDHx mutations were significantly (p=0.02) lower (68±4) than in PHEOs (91±2) (Figure 3B).
Omic evaluation:
An evaluation of the 14 omes that comprise the NET signature (Kidd et al., 2020) identified elevated expression (p<0.05) of the growth-factor signalome (growth factor mediated signaling), metastasome (regulation of metastasis), NEDome (neuroendocrine cancer-associated genes – poor prognosis) (Chen et al., 2018), neurome (neural-related genes), plurome (genes associated with pluripotency) and secretome in PHEOs compared to PGLs (Figure 4A).
Figure 4. Omic gene expression in PGLs and PHEOs.

4A. Omic gene expression in PGLs and PHEOs. Elevated expression of the GF-signalome (p=0.018), metastasome (p=0.018), neurome (p=0.05), NEDome (p=0.025), plurome (p=0.027) and secretome (p=0.017) was identified in PHEOs versus PGLs.
4B. Cluster 1 versus Cluster 2 PHEOs/PGLs Raf-Ras signaling growth factor signaling) gene expression (ARAF, BRAF, KRAS and RAF-1). Levels were significantly elevated in Cluster 2 tumors (p=0.046).
Red horizontal line = median
In cluster 2, omic evaluation identified that the growth factor signalome (genes involved in RAF-Ras signaling) was significantly (p=0.046) overexpressed (Figure 4B) compared to cluster 1. No other omic clusters were significantly altered.
Evaluation of NETest in minors (n=10):
The NETest was elevated (81±8) in all 10 pediatric patient samples and was concordant with image-detectable disease in each case. Levels were similar between PGLs (n=3: 75±21), PHEOs (n=5: 81±11) and mixed tumors (n=2: 87±7). All tumors with mutations (n=7) exhibited a Cluster 1 phenotype (NETest score: 84±8).
DISCUSSION:
In this prospective study, we demonstrated that the NETest, a blood-based (liquid biopsy) multigenomic assay, was effective (accuracy>99%) as an IVD measure for PHEOs/PGLs. The NETest scores correlated with 68Ga-DOTATATE PET/CT detection of disease (100%) and detected a case of biochemical recurrence (image-negative). This suggests that the NETest may have value as a clinical surrogate for imaging or as an adjunct in instances where interpretation of imaging or general PHEO/PGL diagnosis is equivocal. The NETest scores were higher in PHEOs versus PGLs, providing biological evidence for the differences observed in clinical behavior of adrenal-derived tumors versus extra-adrenal-derived tumors. The detectable blood NET signature was effective (i.e., positive) irrespective of mutational status. The NETest signature also correlated with molecular pathway analyses. NETest scores were elevated in the Cluster 2/growth factor-chromatin remodeling phenotype compared to the pseudohypoxia phenotype (Cluster 1) (Fishbein et al., 2017). This information further supports the proposition that there is a biological or genomic difference between the two groups. The exact clinical application of these findings has yet to be determined.
The primary strength of this study is its ability to demonstrate that a multigenomic, NET-specific signature in blood can accurately diagnose PHEOs/PGLs. In addition, this study is prospective, it exhibits substantial power by having 1:2 age and gender matched controls and it contains a large number of PHEO/PGL patients (~3x the amount required for power analysis). Another strength of this study is its correlation with molecular genetic analyses (available in 82%). We also examined whether the assay was effective in children with PHEOs/PGLs, and we found that all pediatric patients with 68Ga-DOTATATE PET/CT detectable disease were also NETest-positive. A weakness of this study is the absence of matched, standard biomarker measurements. The goal of the study was to investigate if biologic and genetic markers of neuroendocrine neoplasms could identify PHEOs/PGLs (rather than secretory markers). We and other researchers have previously demonstrated that secretory markers of NETs, such as Chromogranin A (CgA), are ineffective in the diagnosis of PHEO/PGL (Malczewska et al., 2020, Sansone et al., 2019, Marotta et al., 2018).
The NETest scores were positive (accurately detected PHEOs/PGLs) irrespective of prior therapy including chemotherapy, radiotherapy, surgery and current clinical status (stable or progressive disease). Prior studies have shown that effective therapies, such as peptide receptor radionuclide therapy (PRRT), may reduce NETest scores (Oberg et al., 2020, Bodei et al., 2019). Surgery is particularly effective in decreasing NETest levels since there is a physical removal of the tumoral mRNA signal in the blood (Partelli et al., 2020, Modlin et al., 2016). Post-operatively, elevated NETest scores provide early evidence of incomplete tumor removal and predict recurrence with a greater than 90% accuracy within one month of surgery (Modlin et al., 2021a). In the current study, we did not specifically address the effect of treatment on PPGLs. However, all patients had elevated NETest scores, which is consistent with presence of active disease. This was corroborated by the observation that all exhibited positive 68Ga-DOTATATE scans except one. This patient who was considered surgically “cured”, exhibited biochemical evidence (catecholamine analysis) of recurrence and was in add NETest-positive. Follow-up remains in place for this individual. Modlin et al., demonstrated in a series of 1,684 NETs, that the NETest is most accurate for PPGL identification amongst the different NET disease types (PPGLs: 98%, GEP-NETs: 94%, BPNETs: 88%) (Modlin et al., 2021b). In previous studies of PPGLs, the NETest identified disease and it is likely that further evaluation of this issue will provide specific data of the accuracy of the NETest in the identification of residual disease (Peczkowska et al., 2017). The current data, however, suggest the NETest may have utility in the assessment of the effect of different therapies including surgery on the management of PPGLs.
This study also indicates that PHEOs/PGLs may effectively secrete molecular markers that can be detected in the blood. There have been only a few other studies that have evaluated circulating levels of molecular markers in PHEOs/PGLs. For example, exosomal dsDNA has been reported to correlate with PHEO/PGL gene levels (Wang et al., 2018). Prospective studies evaluating the treatment effects of surgery and PRRT may be useful in assessing if the NETest can be used to monitor treatment efficacy in PHEOs/PGLs.
The present results indicate that the NETest may have clinical utility in making a PHEO/PGL diagnosis irrespective of the secretory functionality of the tumor. Lesions less than 5 mm cannot be detected by current analytical (biomarker and imaging) methods (Antonio et al., 2020). Some PHEO/PGLs are non-functional, which means that they do not produce any catecholamines. Similarly, some PHEOs/PGLs produce only dopamine, which is not commonly measured (along with its 0-methylated metabolite, methoxytyramine (Eisenhofer et al., 2020)). In the United States, methoxytyramine assays are not currently available for the clinical assessment of these tumors. While diagnostically very useful, the measurement of catecholamines can be affected by a series of factors including the size of the tumor, the episodic secretion of catecholamines and the concomitant administration of various medications (e.g. antihypertensives) (van Berkel et al., 2014). Moreover, the conditions under which blood or urine samples are collected are critical to the reliability and interpretation of test results (Darr et al., 2014, van Berkel et al., 2014). In contrast, the NETest measures the molecular biological signature of neuroendocrine neoplasms and identifies neoplastic biology, irrespective of secretory status (Kidd et al., 2015). Similarly, specificity is critical given the diverse agents and diseases that can alter catecholamine secretion (van Berkel et al., 2014, Eisenhofer and Lenders, 2018). The molecular identification of the genes specific to neural and endocrine neoplasms obviate this issue. Furthermore, the NETest does not require fasting or cessation of medications. Similarly, the NETest does not require that patients be in resting conditions and a supine position for sample collection. In contrast, exercise, posture, food, stress, hypoglycemia and certain medications can alter the production, secretion or metabolism of catecholamines and their metabolites (Lenders et al., 2014a, Darr et al., 2014), making standard sample collection susceptible to error. In the current study, medications, food consumption, stress, posture, and exercise had no impact on NETest scores. This confirms earlier observations (Modlin et al., 2014a, Modlin et al., 2014b) and highlights the potential clinical utility of the NETest assay in PHEO/PGL patients. Nevertheless, a future study is needed to further assess the NETest against standard diagnostic approaches in patients who are suspected of having a PHEO/PGL.
We observed that NETest scores were higher in PHEOs than PGLs. This result was independent of the mutational status (same mutation but lower scores in PGLs). NETest levels in PGLs with SDHx mutations were significantly (p=0.02) lower (68±4) than in PHEOs with the same mutation (91±2). Moreover, we observed that non-SDHx mutations (or no detected mutations) were associated with similar NETest scores (62±8) to tumors with SDHx mutations (37±3). Similarly, there were no significant differences in NETest levels in PHEOs. These results suggest that NETest levels could be related more to the cell of origin compared to mutational status. Although both adrenal and extra-adrenal tumors displayed similar histopathological characteristics and are considered to be derived from chromaffin cells, such analyses have not evaluated genomic cell differences. Thus, the possibility exists that all chromaffin cells may not be equal. These two related cell types may arise from one common or two separate cell lineages of delaminating neural crest cells and hence exhibit different genomic profiles (Huber et al., 2009). This proposal is, to some extent, supported by an evaluation conducted by The Cancer Genome Atlas (TCGA) study of more than 10,000 tumors which identified several different patterns of expression within the PHEO/PGL cohort (Chen et al., 2018). One explanation may be transcriptomic heterogeneity due to embryologic origin. The alternative is that gene expression heterogeneity may reflect differences in transcriptional regulation that are site-, mutation- or migration route-specific. Our data suggest the former. However, this observation may reflect a specific referral bias of patients with complex or advanced disease to the National Cancer Institute (NCI). A larger, prospective study is needed to address this issue.
The NETest was developed to specifically define and identify neuroendocrine biology (Kidd et al., 2015) as described by Hanahan in the hallmarks of neoplasia (Hanahan and Weinberg, 2011). The NETest score is comprised of 14 different “omes” including the apoptome, epigenome, fibrosome, growth-factor signalome (RAS-RAF signaling), inflammasome, metabolome, metastasome, NEDome, neurome, plurome proliferome, secretome, SSTRome and the TFome (Figure 5) (Kidd et al., 2020). It was proven that the NET signature assay accurately identified PHEOs/PGLs (92% specific), which share several of the same “hallmarks” as NETs. Indeed, this study supports a previous pilot study that concluded the NETest may be able to detect PHEOs/PGLs (Peczkowska et al., 2017).
Figure 5: NETest and “omic clusters.”.

The NETest is a gene expression assay that measures 51 NET marker mRNAs in blood using real-time PCR (left ellipse). The assay utilizes a multi-algorithmic analysis to quantify tumor-related expression of gene clusters (right ellipse). NETest output is a score scaled 0-100 that represents the risk of NET disease. A normal score is ≤20, stable disease is 21-40 and progressive disease is 41-100. The assay has >90% sensitivity and specificity for a NET diagnosis. The 51 NETest genes are comprised of “14 omes.” These include the apoptome (apoptosis: n=3 genes), epigenome (epigenetic regulation: n=7), fibrosome (fibrosis: n=4 genes), growth factor signalome (RAS-RAF signaling: n=4), inflammasome (inflammatory response: n=4), metabolome (metabolic function: n=3), metastasome (metastatic progression: n=7), NEDome (neuroendocrine-like differentiation in cancers: n=21), neurome (neural-related genes: n=17), plurome (pluripotency: n=2), proliferome (regulation of proliferation: n=4), secretome (secretion: n=4), SSTRome (somatostatin receptor expression: n=4) and Tfome (transcriptional regulatory factors: n=3). Omic cluster analysis provides biological information that can be used to characterize the clinical behavior of an individual tumor.
In this study, we identified six omic clusters that were elevated in PHEOs compared to PGLs. These clusters included growth factor signaling, metastasis, secretion, neural gene expression, pluripotency and the NEDome. Most individual omic comprised clusters are made up of 3 to 8 individual genes that define a mechanistic regulatory function that represents a specific hallmark of neoplasia (Kidd et al., 2020). The NEDome (neuroendocrine differentiation cluster) is an aggregation of 21 genes which provide a signature that defines a negative prognostic outcome in a number of cancers including prostate, melanoma, cervical, ovarian, gastric and head and neck (Chen et al., 2018). Our data, although based on a selected group of tumors, suggest that there may be molecular genomic differences between the two tumor groups, which might further enable stratification of their clinical behavior. Thus, it may be possible to further delineate PHEOs and PGLs to better define their future clinical behavior by omic analysis. Such strategies have been effective in lung and prostate cancers (Chen et al., 2017, Kaur et al., 2020, Puca et al., 2019). This information may have clinical value in detecting lesions with invasive or metastatic potential, differentiating the tumor secretory profiles, determining how to stratify imaging and predicting prognosis. Clearly, larger, prospective studies are required to refine such speculation, but this strategy may be useful given the paucity of other methods to acquire such information.
NETest levels were also correlated with molecular biological clustering of PHEOs/PGLs and were decreased in those with a “pseudohypoxic” phenotype. This phenotype is associated with mitochondrial-related mutations which were more common in our PGL cohort. Such mutations would alter metabolic activity and growth-regulatory responses (Fishbein et al., 2017). We presume that the lower NETest values may reflect this genetic phenomenon. In contrast, mutations in epigenetic and chromatin remodeling (as encapsulated in “Cluster 2”) may drive a higher NETest score. When we further examined the “omic” clusters in the NETest, we identified an upregulation of gene expression involved in the growth factor signaling (RAS-RAF) pathway. Further investigation of the observed gene expression levels (B-raf or K-ras) may provide insight into whether the NETest will have additional value in defining PHEO/PGL pathobiology.
We did, however, identify “false” positive results in tumors known to exhibit neuroendocrine-like differentiation (NELD) (Chen et al., 2018). This observation has been recently described in which up to 30% of diverse neoplasms (lung, prostate and breast) exhibited NELD (Chen et al., 2018). This information may be relevant when assessing, for example, a gut lesion as a NET or an adenocarcinoma with NELD features. However, it would be rare to have a differential diagnosis between lung or prostate cancer and PHEO. Given the emerging issue of NELD in other neoplastic conditions, it is likely that larger PHEO/PGL studies will demonstrate that NETest specificity for PHEOs/PGLs is greater than the 92% demonstrated in this study.
Overall, the present data confirm that the NETest is an effective IVD for PHEO/PGL. A blood-based tool that has the ability to measure tumor transcripts in real-time will likely have clinical utility. The data derived from this study provide rationale for further investigating biologically relevant gene expression in PHEOs/PGLs, which could provide additional insights into the underlying molecular pathways and drivers of a specific tumor. The results of this study and future studies may be used to better define an individual tumor and facilitate the development of personalized management protocols.
Funding
NETest were provided pro bono by Wren Laboratories. All other study costs were borne by the individual institutes involved.
Footnotes
Declaration of interest
MK is an employee of Wren Laboratories
IMM has consulted for Novartis, Nycomed, Gradalis, Ipsen, AAA, Astra Zeneca and Clifton Life Sciences.
REFERENCES:
- ANTONIO K, VALDEZ MMN, MERCADO-ASIS L, TAÏEB D & PACAK K 2020. Pheochromocytoma/paraganglioma: recent updates in genetics, biochemistry, immunohistochemistry, metabolomics, imaging and therapeutic options. Gland Surg, 9, 105–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAYLEY JP, BAUSCH B, RIJKEN JA, VAN HULSTEIJN LT, JANSEN JC, ASCHER D, PIRES DEV, HES FJ, HENSEN EF, CORSSMIT EPM, DEVILEE P & NEUMANN HPH 2020. Variant type is associated with disease characteristics in SDHB, SDHC and SDHD-linked phaeochromocytoma-paraganglioma. J Med Genet, 57, 96–103. [DOI] [PubMed] [Google Scholar]
- BODEI L, KIDD MS, SINGH A, VAN DER ZWAN WA, SEVERI S, DROZDOV IA, MALCZEWSKA A, BAUM RP, KWEKKEBOOM DJ, PAGANELLI G, KRENNING EP & MODLIN IM 2019. PRRT neuroendocrine tumor response monitored using circulating transcript analysis: the NETest. Eur J Nucl Med Mol Imaging, 14, 019–04601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUFFET A, MORIN A, CASTRO-VEGA LJ, HABAROU F, LUSSEY-LEPOUTRE C, LETOUZÉ E, LEFEBVRE H, GUILHEM I, HAISSAGUERRE M, RAINGEARD I, PADILLA-GIROLA M, TRAN T, TCHARA L, BERTHERAT J, AMAR L, OTTOLENGHI C, BURNICHON N, GIMENEZ-ROQUEPLO AP & FAVIER J 2018. Germline Mutations in the Mitochondrial 2-Oxoglutarate/Malate Carrier SLC25A11 Gene Confer a Predisposition to Metastatic Paragangliomas. Cancer Res, 78, 1914–1922. [DOI] [PubMed] [Google Scholar]
- CHEN F, ZHANG Y, GIBBONS DL, DENEEN B, KWIATKOWSKI DJ, ITTMANN M & CREIGHTON CJ 2018. Pan-Cancer Molecular Classes Transcending Tumor Lineage Across 32 Cancer Types, Multiple Data Platforms, and over 10,000 Cases. Clin Cancer Res., 24, 2182–2193. doi: 10.1158/1078-0432.CCR-17-3378. Epub 2018 Feb 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEN F, ZHANG Y, PARRA E, RODRIGUEZ J, BEHRENS C, AKBANI R, LU Y, KURIE JM, GIBBONS DL, MILLS GB, WISTUBA II & CREIGHTON CJ 2017. Multiplatform-based molecular subtypes of non-small-cell lung cancer. Oncogene., 36, 1384–1393. doi: 10.1038/onc.2016.303. Epub 2016 Oct 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRONA J & SKOGSEID B 2016. GEP- NETS UPDATE: Genetics of neuroendocrine tumors. Eur J Endocrinol, 174, R275–90. [DOI] [PubMed] [Google Scholar]
- DAHIA PLM, CLIFTON-BLIGH R, GIMENEZ-ROQUEPLO AP, ROBLEDO M & JIMENEZ C 2020. HEREDITARY ENDOCRINE TUMOURS: CURRENT STATE-OF-THE-ART AND RESEARCH OPPORTUNITIES: Metastatic pheochromocytomas and paragangliomas: proceedings of the MEN2019 workshop. Endocr Relat Cancer, 27, T41–t52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DARR R, PAMPORAKI C, PEITZSCH M, MIEHLE K, PREJBISZ A, PECZKOWSKA M, WEISMANN D, BEUSCHLEIN F, SINNOTT R, BORNSTEIN SR, NEUMANN HP, JANUSZEWICZ A, LENDERS J & EISENHOFER G 2014. Biochemical diagnosis of phaeochromocytoma using plasma-free normetanephrine, metanephrine and methoxytyramine: importance of supine sampling under fasting conditions. Clin Endocrinol (Oxf). 80, 478–86. doi: 10.1111/cen.12327. Epub 2013 Oct 17. [DOI] [PubMed] [Google Scholar]
- EISENHOFER G, DÄRR R, PAMPORAKI C, PEITZSCH M, BORNSTEIN S & LENDERS JW 2015. Supine or Sitting? Economic and other considerations for use of plasma metanephrines for diagnosis of phaeochromocytoma. Clin Endocrinol (Oxf), 82, 463–4. [DOI] [PubMed] [Google Scholar]
- EISENHOFER G, DEUTSCHBEIN T, CONSTANTINESCU G, LANGTON K, PAMPORAKI C, CALSINA B, MONTEAGUDO M, PEITZSCH M, FLIEDNER S, TIMMERS H, BECHMANN N, FANKHAUSER M, NÖLTING S, BEUSCHLEIN F, STELL A, FASSNACHT M, PREJBISZ A, LENDERS JWM & ROBLEDO M 2020. Plasma metanephrines and prospective prediction of tumor location, size and mutation type in patients with pheochromocytoma and paraganglioma. Clin Chem Lab Med, 59, 353–363. [DOI] [PubMed] [Google Scholar]
- EISENHOFER G, GOLDSTEIN DS, WALTHER MM, FRIBERG P, LENDERS JW, KEISER HR & PACAK K 2003. Biochemical diagnosis of pheochromocytoma: how to distinguish true- from false-positive test results. J Clin Endocrinol Metab, 88, 2656–66. [DOI] [PubMed] [Google Scholar]
- EISENHOFER G, KLINK B, RICHTER S, LENDERS JW & ROBLEDO M 2017. Metabologenomics of Phaeochromocytoma and Paraganglioma: An Integrated Approach for Personalised Biochemical and Genetic Testing. Clin Biochem Rev, 38, 69–100. [PMC free article] [PubMed] [Google Scholar]
- EISENHOFER G, LENDERS JW, SIEGERT G, BORNSTEIN SR, FRIBERG P, MILOSEVIC D, MANNELLI M, LINEHAN WM, ADAMS K, TIMMERS HJ & PACAK K 2012. Plasma methoxytyramine: a novel biomarker of metastatic pheochromocytoma and paraganglioma in relation to established risk factors of tumour size, location and SDHB mutation status. Eur J Cancer, 48, 1739–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EISENHOFER G & LENDERS JWM 2018. Biochemical Diagnosis of Pheochromocytoma, a Rediscovered Catecholamine-Metabolizing Tumor. Clin Chem, 64, 1780–1781. [DOI] [PubMed] [Google Scholar]
- FAVIER J, AMAR L & GIMENEZ-ROQUEPLO AP 2015. Paraganglioma and phaeochromocytoma: from genetics to personalized medicine. Nat Rev Endocrinol., 11, 101–11. doi: 10.1038/nrendo.2014.188. Epub 2014 Nov 11. [DOI] [PubMed] [Google Scholar]
- FILOSSO P, KIDD M, ROFFINELLA M, LEWCZUK A, CHUNG K-M, KOLASINSKA-CWIKLA A, CWIKLA J, LOWCZAK A, DOBOSZYNSKA A, MALCZEWSKA A, CATALANO M, ZUNINO V, BOITA M, ARVAT E, CRISTOFORI R, GUERRERA F, OLIARO A, TESSELAAR M, BUIKHUISEN W, KOS-KUDLA B, PAPOTTI M, BODEI L, DROZDOV I & MODLIN I 2018. The utility of blood neuroendocrine gene transcript measurement in the diagnosis of bronchopulmonary neuroendocrine tumors (BPNET) and as a tool to evaluate surgical resection and disease progression. European J Cardiothoracic Surgery, 53, 631–9. [DOI] [PubMed] [Google Scholar]
- FISHBEIN L, LESHCHINER I, WALTER V, DANILOVA L, ROBERTSON AG, JOHNSON AR, LICHTENBERG TM, MURRAY BA, GHAYEE HK, ELSE T, LING S, JEFFERYS SR, DE CUBAS AA, WENZ B, KORPERSHOEK E, AMELIO AL, MAKOWSKI L, RATHMELL WK, GIMENEZ-ROQUEPLO AP, GIORDANO TJ, ASA SL, TISCHLER AS, PACAK K, NATHANSON KL & WILKERSON MD 2017. Comprehensive Molecular Characterization of Pheochromocytoma and Paraganglioma. Cancer Cell, 31, 181–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREENBERG SE, JACOBS MF, WACHTEL H, ANSON A, BUCHMANN L, COHEN DL, BONANNI M, BENNETT B, NAUMER A, SCHAEFER AM, KOHLMANN W, NATHANSON KL, ELSE T & FISHBEIN L 2020. Tumor detection rates in screening of individuals with SDHx-related hereditary paraganglioma-pheochromocytoma syndrome. Genet Med, 22, 2101–2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAN S, SUH CH, WOO S, KIM YJ & LEE JJ 2019. Performance of (68)Ga-DOTA-Conjugated Somatostatin Receptor-Targeting Peptide PET in Detection of Pheochromocytoma and Paraganglioma: A Systematic Review and Metaanalysis. J Nucl Med, 60, 369–376. [DOI] [PubMed] [Google Scholar]
- HANAHAN D & WEINBERG RA 2011. Hallmarks of cancer: the next generation. Cell., 144, 646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- HUBER K, KALCHEIM C & UNSICKER K 2009. The development of the chromaffin cell lineage from the neural crest. Auton Neurosci, 151, 10–6. [DOI] [PubMed] [Google Scholar]
- KAUR H, SAMARSKA I, LU J, FAISAL F, MAUGHAN BL, MURALI S, ASRANI K, ALSHALALFA M, ANTONARAKIS ES, EPSTEIN JI, JOSHU CE, SCHAEFFER EM, MOSQUERA JM & LOTAN TL 2020. Neuroendocrine differentiation in usual-type prostatic adenocarcinoma: Molecular characterization and clinical significance. Prostate, 80, 1012–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIDD M, DROZDOV I & MODLIN I 2015. Blood and tissue neuroendocrine tumor gene cluster analysis correlate, define hallmarks and predict disease status. Endocr Relat Cancer., 22, 561–75. doi: 10.1530/ERC-15-0092. Epub 2015 Jun 2. [DOI] [PubMed] [Google Scholar]
- KIDD M, DROZDOV IA, MATAR S, GURUNLIAN N, FERRANTI NJ, MALCZEWSKA A, BENNETT P, BODEI L & MODLIN IM 2019. Utility of a ready-to-use PCR system for neuroendocrine tumor diagnosis. PLoS One., 14, e0218592. doi: 10.1371/journal.pone.0218592. eCollection 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIDD M, KITZ A, DROZDOV I & MODLIN I 2020. Neuroendocrine Tumor Omic Gene Cluster Analysis Amplifies the Prognostic Accuracy of the NETest. Neuroendocrinology, May 11. doi: 10.1159/000508573. [DOI] [PubMed] [Google Scholar]
- LEIJON H, REMES S, HAGSTRÖM J, LOUHIMO J, MÄENPÄÄ H, SCHALIN-JÄNTTI C, MIETTINEN M, HAGLUND C & AROLA J 2019. Variable somatostatin receptor subtype expression in 151 primary pheochromocytomas and paragangliomas. Hum Pathol, 86, 66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LENDERS JW, DUH QY, EISENHOFER G, GIMENEZ-ROQUEPLO AP, GREBE SK, MURAD MH, NARUSE M, PACAK K & YOUNG WF JR. 2014a. Pheochromocytoma and paraganglioma: an endocrine society clinical practice guideline. J Clin Endocrinol Metab, 99, 1915–42. [DOI] [PubMed] [Google Scholar]
- LENDERS JW, DUH QY, EISENHOFER G, GIMENEZ-ROQUEPLO AP, GREBE SK, MURAD MH, NARUSE M, PACAK K & YOUNG WF JR. 2014b. Pheochromocytoma and paraganglioma: an endocrine society clinical practice guideline. J Clin Endocrinol Metab., 99, 1915–42. doi: 10.1210/jc.2014-1498. [DOI] [PubMed] [Google Scholar]
- LENDERS JWM, KERSTENS MN, AMAR L, PREJBISZ A, ROBLEDO M, TAIEB D, PACAK K, CRONA J, ZELINKA T, MANNELLI M, DEUTSCHBEIN T, TIMMERS H, CASTINETTI F, DRALLE H, WIDIMSKÝ J, GIMENEZ-ROQUEPLO AP & EISENHOFER G 2020. Genetics, diagnosis, management and future directions of research of phaeochromocytoma and paraganglioma: a position statement and consensus of the Working Group on Endocrine Hypertension of the European Society of Hypertension. J Hypertens, 38, 1443–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI SC, ESSAGHIR A, MARTIJN C, LLOYD RV, DEMOULIN JB, OBERG K & GIANDOMENICO V 2013. Global microRNA profiling of well-differentiated small intestinal neuroendocrine tumors. Mod Pathol., 26, 685–96. doi: 10.1038/modpathol.2012.216. Epub 2013 Jan 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MALCZEWSKA A, OBERG KE & KOS-KUDŁA B 2020. NETest is superior to chromogranin A in neuroendocrine neoplasia: a prospective ENETS CoE analysis. Endocr Connect. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAROTTA V, ZATELLI MC, SCIAMMARELLA C, AMBROSIO MR, BONDANELLI M, COLAO A & FAGGIANO A 2018. Chromogranin A as circulating marker for diagnosis and management of neuroendocrine neoplasms: more flaws than fame. Endocr Relat Cancer., 25, R11–R29. doi: 10.1530/ERC-17-0269. Epub 2017 Oct 24. [DOI] [PubMed] [Google Scholar]
- MODLIN I, DROZDOV I & KIDD M 2013. The Identification of gut neuroendocrine tumor disease by multiple synchronous transcript analysis in blood. Plos One, e63364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MODLIN I, DROZDOV I & KIDD M 2014a. Gut Neuroendocrine Tumor Blood qPCR Fingerprint Assay: Characteristics and Reproducibility. Clinical Chemistry, 52, 419–29. [DOI] [PubMed] [Google Scholar]
- MODLIN I, KIDD M, OBERG K, FALCONI M, FILOSSO P, FRILLING A, MALCZEWSKA A, SALEM R, TOUMPANAKIS C, LASKARATOS F-L, PARTELLI S, ROFFINELLA M, VON ARX C, KOS-KUDLA B, BODEI L, DROZDOV I & KITZ A 2021a. Early Identification of Residual Disease after Neuroendocrine Tumor Resection using a liquid biopsy multigenomic mRNA signature (NETest). Annals of Surgical Oncology, May 18. doi: 10.1245/s10434-021-10021-1. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- MODLIN IM, ASLANIAN H, BODEI L, DROZDOV I & KIDD M 2014b. A PCR blood test outperforms chromogranin A in carcinoid detection and is unaffected by PPIs. Endocr Connect, 14, 14-0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MODLIN IM, FRILLING A, SALEM RR, ALAIMO D, DRYMOUSIS P, WASAN HS, CALLAHAN S, FAIZ O, WENG L, TEIXEIRA N, BODEI L, DROZDOV I & KIDD M 2016. Blood measurement of neuroendocrine gene transcripts defines the effectiveness of operative resection and ablation strategies. Surgery., 159, 336–47. doi: 10.1016/j.surg.2015.06.056. Epub 2015 Oct 9. [DOI] [PubMed] [Google Scholar]
- MODLIN IM, KIDD M, FALCONI M, FILOSSO P, FRILLING A, MALCZEWSKA A, TOUMPANAKIS C, VALK G, PACAK K, BODEI L & OBERG K 2021b. A Multigenomic Liquid Biopsy Biomarker for Neuroendocrine Tumor Disease outperforms CgA and has Surgical and Clinical Utility Annals of Oncology, (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- MODLIN IM, KIDD M, MALCZEWSKA A, DROZDOV I, BODEI L, MATAR S & CHUNG KM 2018. The NETest: The Clinical Utility of Multigene Blood Analysis in the Diagnosis and Management of Neuroendocrine Tumors. Endocrinol Metab Clin North Am, 47, 485–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOOG S, LUSSEY-LEPOUTRE C & FAVIER J 2020. Epigenetic and metabolic reprogramming of SDH-deficient paragangliomas. Endocr Relat Cancer, 27, R451–r463. [DOI] [PubMed] [Google Scholar]
- OBERG K, CALIFANO A, STROSBERG JR, MA S, PAPE U, BODEI L, KALTSAS G, TOUMPANAKIS C, GOLDENRING JR, FRILLING A & PAULSON S 2020. A meta-analysis of the accuracy of a neuroendocrine tumor mRNA genomic biomarker (NETest) in blood. Ann Oncol., 31, 202–212. doi: 10.1016/j.annonc.2019.11.003. Epub 2019 Dec 20. [DOI] [PubMed] [Google Scholar]
- ÖBERG K, CALIFANO A, STROSBERG JR, MA S, PAPE U, BODEI L, KALTSAS G, TOUMPANAKIS C, GOLDENRING JR, FRILLING A & PAULSON S 2020. A meta-analysis of the accuracy of a neuroendocrine tumor mRNA genomic biomarker (NETest) in blood. Ann Oncol, 31, 202–212. [DOI] [PubMed] [Google Scholar]
- PARTELLI S, ANDREASI V, MUFFATTI F, SCHIAVO LENA M & FALCONI M 2020. Circulating Neuroendocrine Gene Transcripts (NETest): A Postoperative Strategy for Early Identification of the Efficacy of Radical Surgery for Pancreatic Neuroendocrine Tumors. Ann Surg Oncol, 6, 020-08425. [DOI] [PubMed] [Google Scholar]
- PAVEL M, JANN H, PRASAD V, DROZDOV I, MODLIN IM & KIDD M 2017a. NET Blood Transcript Analysis defines the Crossing of the Clinical Rubicon: When Stable Disease becomes Progressive. Neuroendocrinology, 104, 170–82. [DOI] [PubMed] [Google Scholar]
- PAVEL M, JANN H, PRASAD V, DROZDOV I, MODLIN IM & KIDD M 2017b. NET Blood Transcript Analysis Defines the Crossing of the Clinical Rubicon: When Stable Disease Becomes Progressive. Neuroendocrinology, 104, 170–182. [DOI] [PubMed] [Google Scholar]
- PECZKOWSKA M, CWIKLA J, KIDD M, LEWCZUK A, KOLASINSKA-CWIKLA A, NIEC D, MICHALOWSKA I, PREJBISZ A, JANUSZEWICZ A, CHIARELLI J, BODEI L & MODLIN I 2017. The clinical utility of circulating neuroendocrine gene transcript analysis in well-differentiated paragangliomas and pheochromocytomas. Eur J Endocrinol., 176, 143–157. Epub 2016 Nov 9. [DOI] [PubMed] [Google Scholar]
- PERRIER ND 2018. From Initial Description by Wermer to Present-Day MEN1: What have We Learned? World J Surg, 42, 1031–1035. [DOI] [PubMed] [Google Scholar]
- PUCA L, VLACHOSTERGIOS PJ & BELTRAN H 2019. Neuroendocrine Differentiation in Prostate Cancer: Emerging Biology, Models, and Therapies. Cold Spring Harb Perspect Med., 9(2). cshperspect.a030593. doi: 10.1101/cshperspect.a030593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RICHTER S, GIELDON L, PANG Y, PEITZSCH M, HUYNH T, LETON R, VIANA B, ERCOLINO T, MANGELIS A, RAPIZZI E, MENSCHIKOWSKI M, AUST D, KROISS M, BEUSCHLEIN F, GUDZIOL V, TIMMERS HJ, LENDERS J, MANNELLI M, CASCON A, PACAK K, ROBLEDO M, EISENHOFER G & KLINK B 2019. Metabolome-guided genomics to identify pathogenic variants in isocitrate dehydrogenase, fumarate hydratase, and succinate dehydrogenase genes in pheochromocytoma and paraganglioma. Genet Med, 21, 705–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANSONE A, LAURETTA R, VOTTARI S, CHIEFARI A, BARNABEI A, ROMANELLI F & APPETECCHIA M 2019. Specific and Non-Specific Biomarkers in Neuroendocrine Gastroenteropancreatic Tumors. Cancers (Basel). 11, 1113. doi: 10.3390/cancers11081113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAÏEB D, HICKS RJ, HINDIÉ E, GUILLET BA, AVRAM A, GHEDINI P, TIMMERS HJ, SCOTT AT, ELOJEIMY S, RUBELLO D, VIRGOLINI IJ, FANTI S, BALOGOVA S, PANDIT-TASKAR N & PACAK K 2019a. European Association of Nuclear Medicine Practice Guideline/Society of Nuclear Medicine and Molecular Imaging Procedure Standard 2019 for radionuclide imaging of phaeochromocytoma and paraganglioma. Eur J Nucl Med Mol Imaging, 46, 2112–2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAÏEB D, JHA A, TREGLIA G & PACAK K 2019b. Molecular imaging and radionuclide therapy of pheochromocytoma and paraganglioma in the era of genomic characterization of disease subgroups. Endocr Relat Cancer, 26, R627–r652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN BERKEL A, LENDERS JW & TIMMERS HJ 2014. Diagnosis of endocrine disease: Biochemical diagnosis of phaeochromocytoma and paraganglioma. Eur J Endocrinol, 170, R109–19. [DOI] [PubMed] [Google Scholar]
- VAN TREIJEN MJC, VAN DER ZEE D, HEERES BC, STAAL FCR, VRIENS MR, SAVEUR LJ, VERBEEK WHM, KORSE CM, MAAS M, VALK GD & TESSELAAR M 2020. Blood Molecular Genomic analysis predicts the disease course of GEP NET patients: a validation study of the predictive value of the NETest®. Neuroendocrinology, 3, 000509091. [DOI] [PubMed] [Google Scholar]
- WALENKAMP A, CRESPO G, FIERRO MAYA F, FOSSMARK R, IGAZ P, RINKE A, TAMAGNO G, VITALE G, OBERG K & MEYER T 2014. Hallmarks of gastrointestinal neuroendocrine tumours: implications for treatment. Endocr Relat Cancer., 21, R445–60. doi: 10.1530/ERC-14-0106. [DOI] [PubMed] [Google Scholar]
- WANG L, LI Y, GUAN X, ZHAO J, SHEN L & LIU J 2018. Exosomal double-stranded DNA as a biomarker for the diagnosis and preoperative assessment of pheochromocytoma and paraganglioma. Mol Cancer, 17, 128. [DOI] [PMC free article] [PubMed] [Google Scholar]


