Abstract
The retinal pigment epithelium (RPE) is a post-mitotic epithelial monolayer situated between the light-sensitive photoreceptors and the choriocapillaris. Given its vital functions for healthy vision, the RPE is a primary target for insults that result in blinding diseases, including age-related macular degeneration (AMD). One such function is the phagocytosis and digestion of shed photoreceptor outer segments. In the present study, we examined the process of trafficking of outer segment disk membranes in live cultures of primary mouse RPE, using high speed spinning disk confocal microscopy. This approach has enabled us to track phagosomes, and determine parameters of their motility, which are important for their efficient degradation.
Keywords: Live-cell imaging, Retinal pigment epithelium, Intracellular trafficking, Photoreceptor outer segment, Phagocytosis
100.1. Introduction
The retinal pigment epithelium (RPE) is a post-mitotic epithelial monolayer of cuboidal cells situated between the light-sensitive photoreceptors and the choriocapillaris (Bok 1993). The RPE performs numerous functions vital to the health of photoreceptors and thus to healthy vision. These functions include recycling of retinoids during the visual cycle, transport of nutrients from the blood to the photoreceptors, and secretion of growth factors, such as vascular endothelial growth factor (VEGF) and pigment epithelial-derived factor (PEDF) (Strauss 2005). One of the most critical functions performed by the RPE is the phagocytosis of photoreceptor outer segment (POS) tips (Young and Bok 1969), an event that occurs on a daily cycle (LaVail 1976).
The RPE is a professional phagocyte, internalizing and degrading approximately 10 % of each photoreceptor outer segment on a daily basis. Phagosomes containing POS membranes move from the apical region of the RPE towards the basal region (Herman and Steinberg 1982; Gibbs et al. 2003), fusing with degradative organelles such as endosomes and lysosomes along the way (Wavre-Shapton et al. 2014; Bosch et al. 1993). By-products that are not completely degraded tend to form constituents of aggregates, such as lipofuscin or sub-RPE deposits, common features associated with macular degeneration (Brunk and Terman 2002). Given the movement of phagosomes from the apical region, their motility is closely related with their degradation. In an early study, it was shown that colchicine, which disrupts microtubules, inhibited the translocation of phagosomes from the apical region (Herman and Steinberg 1982). More recently, the importance of actin-based motility was demonstrated in mice lacking MYO7A, an unconventional myosin. In those mice, phagosomes were retained longer in the apical region of the RPE, and were degraded more slowly (Gibbs et al. 2003). In the present report, we describe the use of live-cell imaging, using spinning disk confocal microscopy, to study the intracellular trafficking of POS-containing phagosomes within primary mouse RPE cells.
100.2. Isolation and Culture of Primary Mouse RPE
Primary mouse RPE were isolated as previously described (Gibbs et al. 2003). Intact eyes were enucleated from P10-P15 mice and washed 3–4 times by inversion with growth medium (Dulbecco’s modified Eagle’s medium (DMEM) with 4.5 g/L glucose, L-glutamine, and sodium pyruvate). The eyes were then incubated in a 2 % dispase solution for 45 min at 37° C. Following removal of the enzyme solution, the eyes were washed 3 times with growth medium containing 10 % fetal bovine serum (FBS) and 20 mM HEPES. The eyes were dissected into eyecups by making an incision along the ora serrata to remove the cornea, iris, lens, and ciliary body. Eyecups were then incubated in growth medium for 20 min at 37° C, as this facilitates the separation of the RPE from the retina and Bruch’s membrane. Sheets of RPE were gently scraped from Bruch’s membrane and collected in growth medium with 10 % FBS. The sheets were then washed 3 times with growth medium and twice with calcium- and magnesium-free Hank’s Balanced Salt Solution (HBSS). The cells were then briefly and gently triturated and plated on Lab-Tek chambered coverglass. Live-cell imaging experiments were carried out on 3–7 day old cultures.
100.3. Isolation and Labeling of Mouse POSs
Mouse POSs were isolated as previously described (Gibbs et al. 2003). Mouse retinas were collected under dim red light and homogenized in Ringer’s solution (130 mM NaCl, 3.6 mM KCl, 2.4 mM MgCl2, 1.2 mM CaCl2, 10 mM HEPES, and 0.02 mM EDTA). The homogenate was cleared by centrifugation for 30 s at 100 g, and then the supernatant was layered on top of a discontinuous Optiprep 8 %−10 %−15 % step gradient in Ringer’s solution and spun at 12,000 g for 20 min at 4° C. POSs were collected at the 10 %/15 % interface and diluted 3 times with Ringer’s solution. POSs were then pelleted by spinning the solution at 10,300 g for 10 min at 4° C. The POSs were then labeled by incubation with 0.1 mg Texas Red-X, succinimidyl ester or 5 % (v/v) Alexa Fluor 488 carboxylic acid, succinimidyl ester, mixed isomers in 1 mL 0.1 M NaHCO3, pH 8.3 for 1 h at 4° C. POSs were then washed with Ringer’s solution, resuspended in RPE growth medium, and counted using a haemocytometer to determine the yield.
100.4. Live Imaging Using Spinning Disk Confocal Microscopy
Figure 100.1a depicts a schematic diagram of the protocol used for live-cell imaging. We used C57BL/6J mice for both the RPE cells and the POSs. Cultured RPE cells were incubated with 1–5 × 106 fluorescently-labeled POSs in growth medium with 10 mM HEPES for 20 min at 37° C, washed extensively with growth medium, and then immediately imaged for a maximum of 1 h, using an Ultraview Spinning Disk Confocal Microscope system with a Zeiss Axiovert photomicroscope, including an environment chamber. Movies were acquired at 3 frames per second with the Volocity software (PerkinElmer), using a 63x oil immersion objective and a Hamamatsu EM-CCD camera (see supplementary video). Trajectories of phagosomes were analyzed using the Volocity software (Fig. 100.1b).
Fig. 100.1.
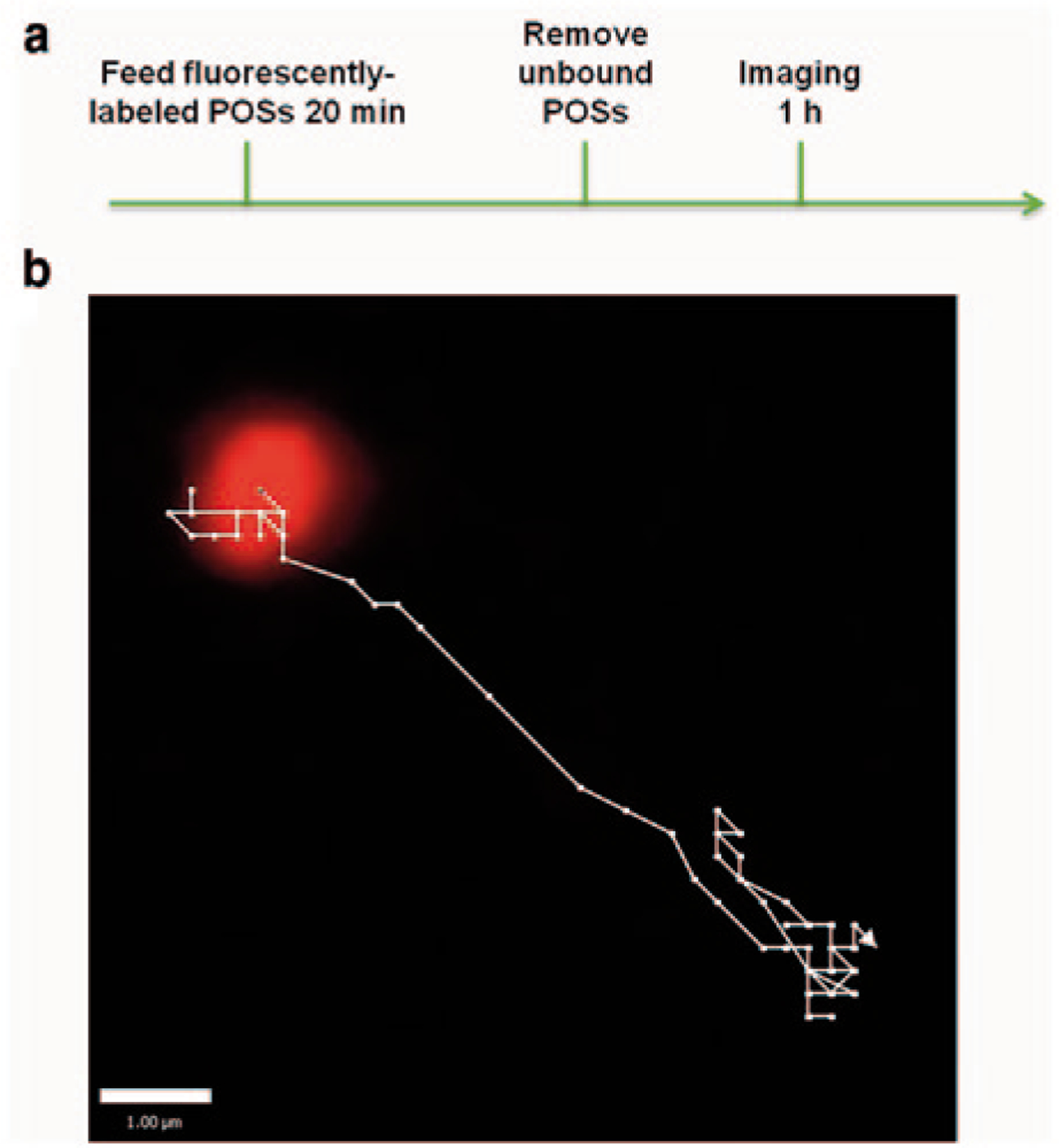
Method for monitoring phagocytosis of photoreceptor outer segments in live RPE. a Scheme of phagocytosis assay for live cultures of RPE, b Magnified view of a fluorescently-labeled phagosome and its trajectory inside a live RPE cell
Not all phagosomes were moving at a given time, however, the paths of those that were moving typically followed relatively straight lines, with back and forth movements along these lines. This motility is consistent with movements along microtubules, as cargos of plus- and minus-end directed microtubule motors. The paths can be analyzed to assess a variety of phagosome motility parameters. Speed and distance traveled represent two basic parameters. From analysis of the paths of phagosomes that traveled at least 3 μm in a 24-second interval, we found a mean speed of 1.2 ± 0.1 μm/s and a mean total distance traveled of 11.3 ± 1.9 μm, during the 24-sec interval. This speed is typical of transport by microtubule motors (Okada et al. 1995). Transport along actin filaments by myosins is typically many fold slower (Boal 2012), suggesting that the observed motility was dominated by the microtubule motors, kinesin and dynein.
100.5. Conclusions
Fluorescently-labeled POS phagosomes can be monitored in live RPE cells, using spinning disk confocal microscopy. Their motility can be determined by tracking their trajectories, thus providing a sensitive, real-time measurement of a critical parameter of RPE health—one, which we are finding in other studies, feeds directly into the efficiency of phagosome degradation, and the propensity for the accumulation of debris and consequent activation of downstream events, such as inflammation and oxidative stress.
Supplementary Material
Acknowledgements
We thank Barry Burgess for technical assistance. This study was supported by NIH R01 grant EY 07042 and P30 grant EY 00331.
Footnotes
The online version of this chapter 10.1007/978-3-319-17121-0_100 contains supplementary video material, which can be downloaded from: http://extra.springer.com.
References
- Boal D (2012) Mechanics of the cell. In: Boal D (eds) Dynamic filaments Cambridge University, Cambridge [Google Scholar]
- Bok D (1993) The retinal pigment epithelium: a versatile partner in vision. J Cell Sci 17:189–195 [DOI] [PubMed] [Google Scholar]
- Bosch E, Horwitz J, Bok D (1993) Phagocytosis of outer segments by retinal pigment epithelium: phagosome-lysosome interaction. J Histochem Cytochem 41:253–263 [DOI] [PubMed] [Google Scholar]
- Brunk UT, Terman A (2002) Lipofuscins: mechanisms of age-related accumulation and influence on cell function. Free Rad Biol Med 33:611–619 [DOI] [PubMed] [Google Scholar]
- Gibbs D, Kitamoto J, Williams DS (2003) Abnormal phagocytosis by retinal pigmented epithelium that lacks myosin VIIa, the usher syndrome 1B protein. Proc Natl Acad Sci U S A 100:6481–6486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman KG, Steinberg RH (1982) Phagosome movement and the diurnal pattern of phagocytosis in the tapetal retinal pigment epithelium of the opossum. Invest Ophthalmol Vis Sci 23: 277–290 [PubMed] [Google Scholar]
- LaVail MM (1976) Rod outer segment disk shedding in rat retina: relationship to cyclic lighting. Science 194:1071–1074 [DOI] [PubMed] [Google Scholar]
- Okada Y, Yamazaki H, Sekine-Aizawa Y (1995) The neuron-specific kinesin superfamily protein KIF1A is a unique monomeric motor for anterograde axonal transport of synaptic vesicle precursors. Cell 81:769–780 [DOI] [PubMed] [Google Scholar]
- Strauss O (2005) The retinal pigment epithelium in visual function. Physiol Rev 85:845–881 [DOI] [PubMed] [Google Scholar]
- Wavre-Shapton ST, Meschede IP, Seabra MC (2014) Phagosome maturation during endosomes interaction revealed by partial rhodopsin processing in retinal pigment epithelium. J Cell Sci 127:3852–3861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young RW, Bok D (1969) Participation of the retinal pigment epithelium in the rod outer segment renewal process. J Cell Biol 42:392–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


