Abstract
Objective
The purpose of this study was to determine the pooled prevalence of vaccination willingness, unwillingness, and hesitancy among patients with multiple sclerosis.
Methods
Databases including PubMed, Scopus, EMBASE, Web of Science, and Google Scholar were searched. by two expert researchers, as well as references in the included studies, which were published before October 2021.
Results
Three hundred eighty articles were found in four data bases. One hundred eighty-two studies remained following deleting duplicates. Finally, ten studies remained for the meta-analysis. Totally, 5983 patients with MS were assessed. The pooled prevalence of willingness to vaccination among patients with MS was 76% (95% CI: 67–85%) (I2 = 98.4%, p < 0.001). Unwillingness pooled prevalence to vaccination among patients with MS was 2% (95% CI: 2–3%) (I2 = 97.9%, p < 0.001). Hesitancy pooled prevalence to vaccination among patients with MS was 0% (I2 = 98%, p < 0.001).
Conclusion
According to the findings of this systematic review and meta-analysis, more than two-thirds of patients with MS were willing to obtain COVID-19 vaccines.
Keywords: COVID-19, Vaccine, Multiple sclerosis
Introduction
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is responsible for COVID-19 infection and was first introduced in China and is now in the pandemic stage [1]. Patients with underlying disease, especially autoimmune disease, were considered at higher risk of the severe form of the disease [2]. A comprehensive review and meta-analysis assessed the pooled prevalence of COVID-19 infection and hospitalization in patients with MS to be 4% and 10%, respectively [3].
Vaccination against SARS-CoV-2 is the primary long-term strategy to stop this pandemic all over the world [4]. Concerns about vaccination were raised among patients with MS and their health care providers regarding disease exacerbation, side effects, and efficacy [5]. Willingness to get vaccines differs among nations, age groups, and genders [6]. Previous studies reported various rates of willingness regarding SARS-CoV-2 vaccination among patients with MS. So, we designed this systematic review and meta-analysis to estimate the pooled prevalence of vaccination willingness among MS patients.
Methods
Databases including PubMed, Scopus, EMBASE, Web of Science, and Google Scholar were searched by two expert researchers, as well as references of the included studies published before October 2021.
Our search strategy was:
((Sclerosis AND multiple) OR (sclerosis AND disseminated) OR “disseminated sclerosis” OR “multiple sclerosis” OR “acute fulminating” OR MS OR “Multiple sclerosis”) AND (“COVID 19 Vaccin*” OR (Vaccin* AND COVID-19) OR “COVID-19 Virus Vaccin*” OR (Vaccin* AND “COVID-19 Virus”) OR (“Virus Vaccin*” AND COVID-19) OR “COVID-19 Virus Vaccin*” OR “COVID 19 Virus Vaccin*” OR (“Virus Vaccin*” AND COVID-19) OR “COVID19 Virus Vaccin*” OR (Vaccin* AND “COVID19 Virus”) OR (“Virus Vaccin*” AND COVID19) OR “COVID19 Vaccin*” OR (Vaccin* AND COVID19) OR “SARS-CoV-2 Vaccin*” OR “SARS CoV 2 Vaccin*” OR (Vaccin* AND SARS-CoV-2) OR “SARS2 Vaccin*” OR (Vaccin* AND SARS2) OR “Coronavirus Disease 2019 Vaccin*” OR “Coronavirus Disease 2019 Virus Vaccin*” OR “Coronavirus Disease-19 Vaccin*” OR (Vaccin* AND “Coronavirus Disease-19”) OR “Coronavirus Disease 19 Vaccin*” OR “COVID 19 Vaccin*” OR (Vaccin* AND “COVID 19”) OR “2019-nCoV Vaccin*” OR “2019 nCoV Vaccin*” OR (Vaccin* AND 2019-nCoV) OR “2019 Novel Coronavirus Vaccin*” OR “2019-nCoV Vaccin*” OR “2019 nCoV Vaccin*” OR (Vaccin* AND 2019-nCoV) OR “COVID-19 Vaccin*” OR “SARS Coronavirus 2 Vaccin*”) AND (willingness OR accept* OR demand OR (vaccine* AND readiness) OR (vaccine* AND readiness) OR Hesita* OR (vaccine* AND hesita*) OR (vaccine* AND accept*) OR (vaccine* AND uptake*) OR (vaccine* AND adopt*) OR Attitude* OR decision-making OR (decision AND making) OR (health AND behaviour) OR “conspiracy theories” OR “conspiracy belie*” OR “obtain COVID-19 vaccin” OR (vaccine AND intention)).
Inclusion criteria
Our inclusion criteria were based on cross-sectional studies that reported the patients with MS who answered questions regarding COVID-19 vaccination. All answers were based on questions designed in the surveys.
Exclusion criteria
Our exclusion criteria were non-English studies, case reports, case–control, letters to the editor, and cross-sectional studies with no clear criteria.
We extracted data given the demographic data, article characteristics, the number of those willing to have vaccination, and the number of those who were hesitant or unwilling.
Risk of bias assessment
The Newcastle–Ottawa quality assessment scale (NOS) that was adapted for cross-sectional studies was investigated for the risk of bias [7].
Statistical analysis
We did all statistical analyses using STATA (Version 14.0; Stata Corp LP, College Station, TX, USA). We used random effects to estimate pooled prevalence. To determine heterogeneity, inconsistency (I2) was calculated.
Results
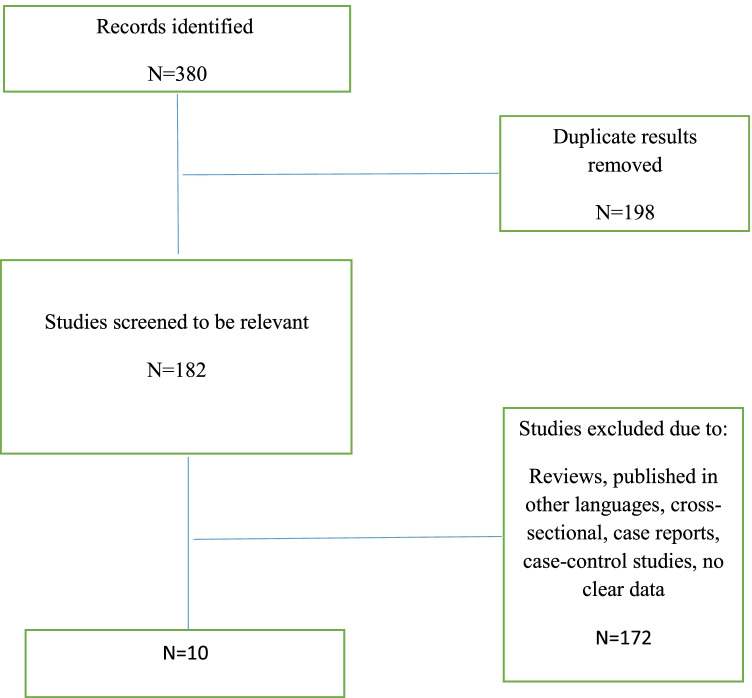
We found 380 studies in the databases; following eliminating duplicates, 182 studies remained. Finally, ten articles used for conducting meta-analysis (Fig. 1).
Fig. 1.
Flow chart summarizing the selection of eligible studies
Ten articles were included. Totally, 5983 MS patients were assessed.
The characteristics of the included studies are shown in Table 1.
Table 1.
Demographic information, and number of willingness, unwillingness, and hesitancy in patients with MS to get COVID-19 vaccines
| Author/country | Surveys | Sample size | Respondents by sex | Age (mean/SD) | MS subtype | Expanded Disability Status Scale (EDSS)/patient determined disease steps (PDDS) | Measure of willingness | Reasons of willingness | Reasons of unwillingness | Reasons of hesitancy | NOS quality assessment |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Xiang et al./Full text/USA/June 2021 [8] | Electronic | 401 |
Male: 88 Female: 312 Missing: 1 |
51.1 (13.5) |
Relapsing remitting: 290 Primary progressive: 30 Secondary progressive: 52 Unknown: 29 |
NR |
Willingness: 281 Unwillingness: 29 Hesitant: 91 |
NR | NR | NR | 7 out of 10 |
| Ehde et al./Full text/USA/July 2021 [9] | Electronic | 338 | NR | NR | NR | NR |
Willingness: 265 Unwillingness/ Hesitant: 73 |
NR |
-Efficacy: 18 -Short-term side effects: 19 -Long-term side effects: 23 -Process of vaccine approval: 20 -First one to receive vaccine: 16 -Health conditions/medical history: 20 -Religious beliefs: 5 -Others get vaccine first: 14 -Availability of vaccine: 5 - Dislike of needles: 4 -Want additional information: 18 -Not concern about COVID-19: 12 -More worried about vaccine than infection: 19 |
-Efficacy:31 -Short-term side effects:35 -Long-term side effects:46 -Process of vaccine approval:36 -First one to receive vaccine: 38 -Health conditions/medical history: 45 -Religious beliefs: 4 -Others get vaccine first: 34 -Availability of vaccine: 16 - Dislike of needles: 5 -Want additional information: 45 -not concern about COVID-19: 22 -More worried about vaccine than infection: 32 |
7 out of 10 |
| Uhr et al./Full text/[10]USA/June 2021 | Electronic | 701 |
Female: 553 Male: 148 |
-Willingness (n = 537): 56.5 (11.7) -Hesitant (n = 164): 51.94 (10.6) |
Relapsing remitting: 459 Progressive: 242 |
PDDS: 3(1–5) |
Willingness: 537 Unwillingness: 36 Hesitant: 128 |
-Important for health: 523/537 -Effectiveness: 524/537 - Important for the health of others: 531/537 -All vaccines offered by government are beneficial: 465/537 -More risks: 166/537 -Reliable and trustworthy resources: 431/537 -Protecting from getting disease: 522/537 -Doctor or health care provider recommends: 489/537 -side effects: 174/537 -No need vaccine to not common diseases: 27/537 |
-Important for health: 101/164 -Effectiveness: 112/164 -Important for the health of others: 122/164 -All vaccines offered by government are beneficial: 62/164 -More risks: 76/164 -Reliable and trustworthy resources: 49/164 -Protecting from getting disease: 100/164 -Doctor or health care provider recommends: 105/164 -Side effects: 114/164 -No need vaccine to not common diseases: 14/164 |
6 out of 10 | |
| Moniz Dionísio et al. /Full text/Portugal/June 2021 [11] | Telephone and electronic | 270 |
Female: 205 Male: 65 |
NR |
Clinically isolated syndrome: 4 Relapsing remitting: 168 Primary progressive: 18 Secondary progressive: 10 Unknown: 70 |
NR |
Willingness: 132 Unwillingness: 32 Hesitant: 106 |
NR | NR | NR | 6 out of 10 |
| Yap et al./Ireland/Full text/September 2021[12] | Clinical interview | 105 |
Female: 73 Male: 32 |
47.3 (12.8) |
Relapsing remitting: 74 Progressive: 31 |
EDDS: 2.0 (1–6) |
Willingness: 95 Hesitant: 10 |
-Vaccine was accessible: 84 | NR |
- Safety: 4 -Not enough realize about it: 2 -Not tested in MS: 1 -MS relapsing remitting: 1 -Will not work: 1 -Currently pregnant: 1 |
5 out of 10 |
| Serrazina et al./Full text/Portugal/March 2021[13] | Electronic | 256 |
Female: 187 Male: 69 |
45 [18–77] | NR | NR |
Willingness: 207 Unwillingness: 7 Hesitant: 42 |
NR | NR | NR | 7 out of 10 |
| Ehde et al./Full text/USA/January 2021 [5] | Electronic | 486 |
Female: 395 Male: 84 Non-binary: 2 Transgender: 1 Other/Prefer Not to Say/No answer: 4 |
55.7 (12.6) |
Clinically isolated syndrome: 5 Relapsing remitting: 316 Primary progressive: 48 Secondary Progressive: 80 Unknown: 5 |
NR |
Willingness: 411 Unwillingness: 75 |
NR | NR | NR | 6 out of 10 |
| Huang et al./Full text/UK/July 2021 [4] | Electronic | 3191 |
Female: 2346 Male: 845 |
NR |
Progressive: 1126 Relapsing remitting: 2065 |
NR |
Willingness: 3013 Unwillingness: 178 |
NR | NR | NR | 6 out of 10 |
| Ghadiri et al./Iran/May 2021 [14] | Electronic | 113 |
Female: 88 Male: 26 |
36 (9.61) |
Progressive: 23 Relapsing remitting: 91 |
EDSS: 2.99 (± 1.80) |
Willingness: 72/113 Unwillingness: 9/113 Hesitant: 32/113 |
-Vaccine is more effectiveness: 85/114 -Cause relapse in MS: 10/113 -Disease progression in MS:7/113 -Serious side effects: 20/114 -More side effects in MS patients: 16/112 -Preventing coronary heart disease in patients with MS is similar to health people: 58/114 -Vaccine should be a priority for MS patients: 78/114 |
-Vaccine is more effectiveness: 4/114 -Cause relapse in MS: 32/113 -Disease progression in MS: 39/113 -Serious side effects: 30/114 -More side effects in MS patients: 24/112 -Preventing coronary heart disease in patients with MS is similar to health people: 8/114 -Vaccine should be a priority for MS patients: 1/114 |
-Vaccine is more effectiveness: 25/114 -Cause relapse in MS: 71/113 -Disease progression in MS: 67/113 -Serious side effects: 64/114 -More side effects in MS patients: 72/112 -Preventing coronary heart disease in patients with MS is similar to health people: 48/114 -Vaccine should be a priority for MS patients: 35/114 |
7 out of 10 |
| Karlon et al./Israel/Abstract/October 2021 | Electronic | 122 | NR | NR | NR | NR |
Willingness: 86 Unwillingness: 7 Hesitant: 29 |
NR |
-disease worsening/MS Relapse: 14 -Safety of vaccine: 6 |
NR | 4 out of 10 |
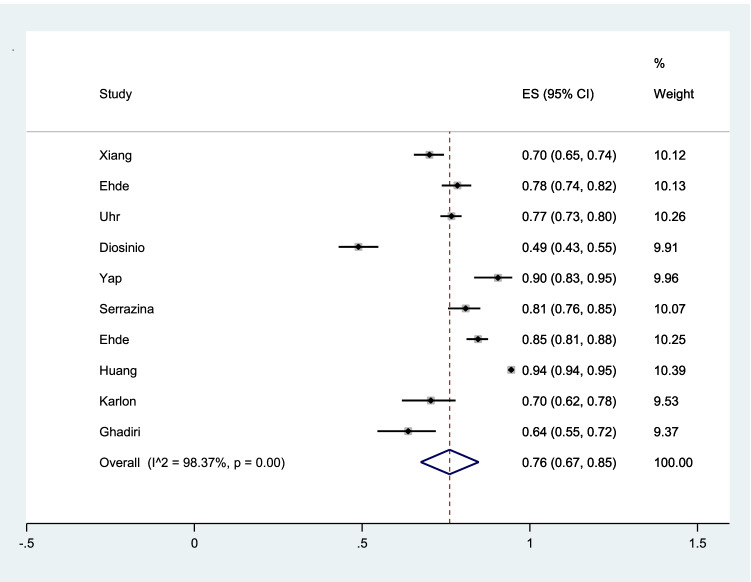
The pooled prevalence of willingness to vaccination among patients with MS was 76% (95% CI: 67–85%) (I2 = 98.4%, p < 0.001) (Fig. 2).
Fig. 2.
The pooled prevalence of willingness to vaccination among patients with MS
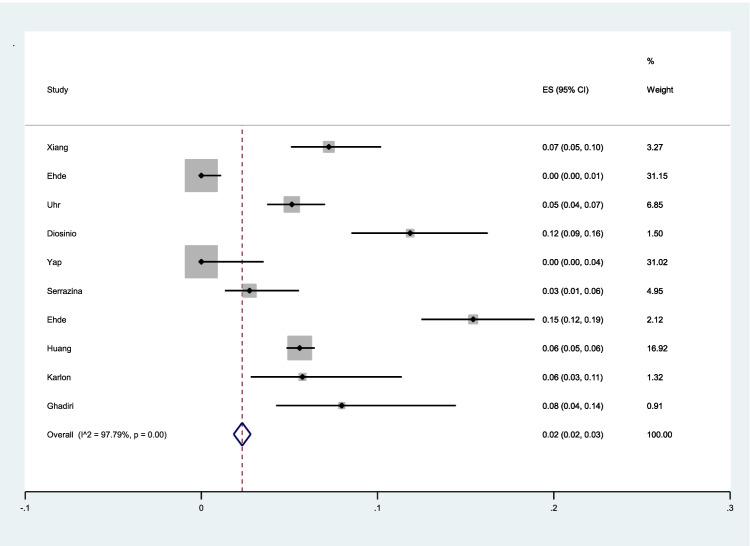
The pooled prevalence of unwillingness to vaccination among patients with MS was 2% (95% CI: 2–3%) (I2 = 97.9%, p < 0.001) (Fig. 3).
Fig. 3.
The pooled prevalence of unwillingness to vaccination among patients with MS
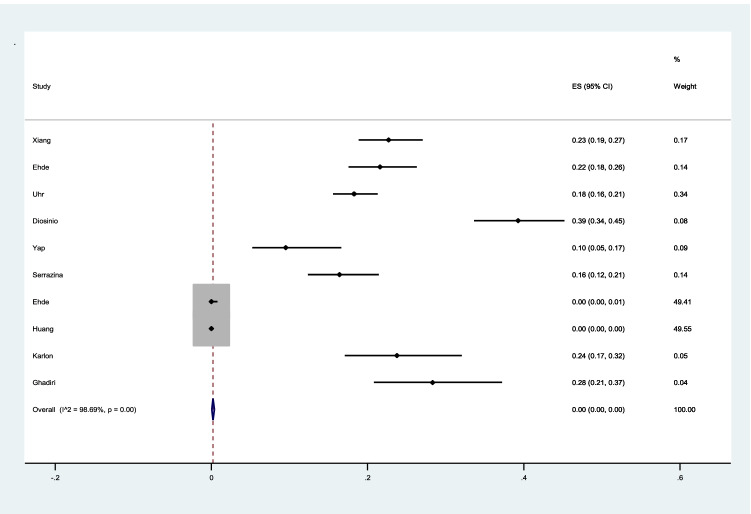
The pooled prevalence of hesitancy to vaccination among patients with MS was 0% (I2 = 98%, p < 0.001) (Fig. 4).
Fig. 4.
The pooled prevalence of hesitancy to vaccination among patients with MS
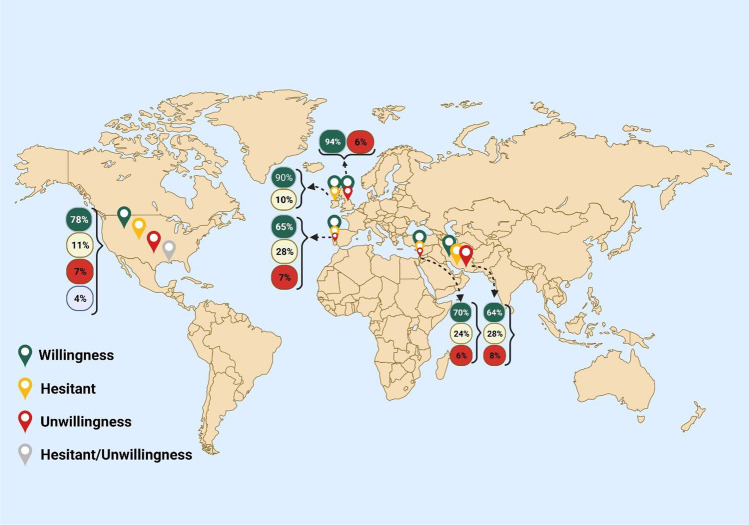
The percentage of willingness, unwillingness, and hesitancy in patients with MS to get COVID-19 vaccines is shown based on countries (Fig. 5).
Fig. 5.
All results of willingness, hesitancy, and unwillingness based on countries. Created with BioRender.com
Discussion
This study is the first systematic review and meta-analysis to assess the willingness, unwillingness, and hesitancy rates regarding COVID-19 vaccination among patients with MS.
The results show that the pooled willingness rate was 76%, ranging from 49 to 94%. The pooled prevalence of unwillingness was 2%, and the higher rate of unwillingness was reported by Ehde et al. [5]. They conducted a cross-sectional study enrolling 486 patients with MS in an online survey. Two-thirds of the participants were willing to get the COVID-19 vaccine, while 15% were unwilling. In their study, higher age and noticing a higher risk of contracting COVID-19 were two independent factors of predicting willingness.
In a survey by Huang et al. in the UK, 94% of patients with MS that were willing to get vaccines were higher than the general population (67%). Higher age, education level, and previous experience of influenza vaccine were associated with COVID-19 vaccination willingness. Female gender and no progressive type of the disease was associated with unwillingness in their survey [4].
Serrazina et al. reported willingness in 80% of 256 Portuguese patients with MS, while older patients and those with underlying diseases were more willing to obtain COVID-19 vaccines. Vaccination unwillingness was related to fewer concerns regarding COVID-19 infection and less impact of the disease on their lives [13]. In their study, the cases that wanted to postpone the vaccination were uncertain about the efficacy and safety of available vaccines.
It is shown that if patients had enough vaccination information, they were more willing to get the COVID-19 vaccination, and more hesitancy was related to more desire to get information. It was reported that if patients got their information from health care providers or National MS Societies, they trust more [5]. Previously, a physician’s advice was the most promoting factor of vaccination among the general population [15]. Vaccination of the MS population may prevent severe infection, hospitalization, disease exacerbation, and medication cessation, especially disease modifying therapies (DMTs) which are crucial for MS relief [16, 17]. So, providing enough trusty information by health care providers play an essential role in vaccination willingness among MS patients and preventing complications following no vaccination.
Changing attitude in hesitant and unwilling individuals to get COVID-19 vaccines has been important to have a successful vaccination. According to the Wang et al. study, which is performed among 1047 and 1000 individuals, respectively, in the first wave in February and the third wave on August–September 2020 in Hong Kong, participants who worked and received the influenza vaccine in the last year accepted more to get the vaccine in the first wave. Hesitancy was raised in the second wave. The reasons for hesitation were vaccine efficacy (63.2%), the vaccine is not unnecessary (24.5%), the short time to manufacture vaccines (4.8%), and concern about safety or side effects of the vaccines (4.6%) [18]. In another cross-sectional study conducted in China in March and November–December among 791 individuals, there was a 3% decrease in willingness to get vaccines due to concern about approval. Changing in intention was significant by attitude for the necessity, price, history, and physicians’ advice [18].
Regarding Raciborski et al.’s investigation between January and April 2021, hesitancy of getting vaccination in Poland was due to worry about side effects. In this study, younger men groups (18–34 years) declared more unwillingness to get the vaccine [19]. These investigations have not been conducted among patients with multiple sclerosis.
This study had some strengths. First, it included all published studies up to now. Second, we evaluated willingness, unwillingness, and hesitancy in patients with MS.
Conclusion
According to the findings of this systematic review and meta-analysis, more than two–thirds of patients with MS were willing to obtain COVID-19 vaccines.
Declarations
Ethical approval
Not applicable.
Conflict of interest
The authors declare no competing interests.
Research involving human participants and/or animals.
None.
Informed consent
Not applicable.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ghajarzadeh M, Bonavita S. Are patients with multiple sclerosis (MS) at higher risk of COVID-19 infection? Neurol Sci: Off J Ital Neurol Soc Ital Soc Clin Neurophysiol. 2020;41(9):2315–2316. doi: 10.1007/s10072-020-04570-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moghadasi AN, Mirmosayyeb O, Barzegar M, Sahraian MA, Ghajarzadeh M. The prevalence of COVID-19 infection in patients with multiple sclerosis (MS): a systematic review and meta-analysis. Neurol Sci: Off J Ital Neurol Soc Ital Soc Clin Neurophysiol. 2021;2021:1–7. doi: 10.1007/s10072-021-05373-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang Y, Rodgers WJ, Middleton RM, Baheerathan A, Tuite-Dalton KA, Ford DV, et al. Willingness to receive a COVID-19 vaccine in people with multiple sclerosis–UK MS Register survey. Mult Scler Relat Disord. 2021;55:103175. doi: 10.1016/j.msard.2021.103175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ehde DM, Roberts MK, Herring TE, Alschuler KN. Willingness to obtain COVID-19 vaccination in adults with multiple sclerosis in the United States. Mult Scler Relat Disord. 2021;49:102788. doi: 10.1016/j.msard.2021.102788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lazarus JV, Ratzan SC, Palayew A, Gostin LO, Larson HJ, Rabin K, et al. A global survey of potential acceptance of a COVID-19 vaccine. Nat Med. 2021;27(2):225–228. doi: 10.1038/s41591-020-1124-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Modesti PA, Reboldi G, Cappuccio FP, Agyemang C, Remuzzi G, Rapi S, et al. Panethnic differences in blood pressure in Europe: a systematic review and meta-analysis. PloS one. 2016;11(1):e0147601. doi: 10.1371/journal.pone.0147601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xiang XM, Hollen C, Yang Q, Brumbach BH, Spain RI, Wooliscroft L. COVID-19 vaccination willingness among people with multiple sclerosis. Mult Scler J-Exp Transl Clin. 2021;7(2):20552173211017159. doi: 10.1177/20552173211017159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ehde DM, Roberts MK, Humbert AT, Herring TE, Alschuler KN. COVID-19 vaccine hesitancy in adults with multiple sclerosis in the United States: a follow up survey during the initial vaccine rollout in 2021. Mult Scler Relat Disord. 2021;54:103163. doi: 10.1016/j.msard.2021.103163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Uhr L, Mateen FJ. COVID-19 vaccine hesitancy in multiple sclerosis: a cross-sectional survey. Mult Scler J 2021:13524585211030647. [DOI] [PubMed]
- 11.Dionísio JM, Santos M, Rodrigues AM, Rêgo A, Vítor J, Delgado S, et al. Opinions, beliefs and knowledge of people with multiple sclerosis on COVID-19 pandemic and vaccine. Mult Scler Relat Disord. 2021;54:103113. doi: 10.1016/j.msard.2021.103113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yap SM, Al Hinai M, Gaughan M, Callanan I, Kearney H, Tubridy N, et al. Vaccine hesitancy among people with multiple sclerosis. Mult Scler Relat Disord. 2021;56:103236. doi: 10.1016/j.msard.2021.103236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Serrazina F, Pinho AS, Cabral G, Salavisa M, Correia AS. Willingness to be vaccinated against COVID-19: an exploratory online survey in a Portuguese cohort of multiple sclerosis patients. Mult Scler Relat Disord. 2021;51:102880. doi: 10.1016/j.msard.2021.102880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghadiri F, Sahraian MA, Saeedi R, Moghadasi AN. Attitudes toward vaccination in patients with multiple sclerosis: a report from Iran. Mult Scler Relat Disord 2021 [DOI] [PMC free article] [PubMed]
- 15.Health TLCA. Vaccine hesitancy: a generation at risk. 2019. [DOI] [PubMed]
- 16.Reyes S, Ramsay M, Ladhani S, Amirthalingam G, Singh N, Cores C, et al. Protecting people with multiple sclerosis through vaccination. Pract Neurol. 2020;20(6):435–445. doi: 10.1136/practneurol-2020-002527. [DOI] [PubMed] [Google Scholar]
- 17.Riva A, Barcella V, Benatti SV, Capobianco M, Capra R, Cinque P, et al. Vaccinations in patients with multiple sclerosis: a Delphi consensus statement. Mult Scler J. 2021;27(3):347–359. doi: 10.1177/1352458520952310. [DOI] [PubMed] [Google Scholar]
- 18.Wang K, Wong EL-Y, Ho K-F, Cheung AW-L, Yau PS-Y, Dong D, et al. Change of willingness to accept COVID-19 vaccine and reasons of vaccine hesitancy of working people at different waves of local epidemic in Hong Kong, China: repeated cross-sectional surveys. Vaccines. 2021;9(1):62. doi: 10.3390/vaccines9010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raciborski F, Jankowski M, Gujski M, Pinkas J, Samel-Kowalik P. Changes in attitudes towards the COVID-19 vaccine and the willingness to get vaccinated among adults in Poland: analysis of serial, cross-sectional, representative surveys, January–April 2021. Vaccines. 2021;9(8):832. doi: 10.3390/vaccines9080832. [DOI] [PMC free article] [PubMed] [Google Scholar]