Abstract
Purpose
It is thought that COVID-19 may cause hearing loss, but its effects on the hearing system are not clear. This study aimed to reveal the effects of COVID-19 on the auditory system by using various audiological measurement methods in individuals diagnosed with COVID-19.
Methods
Thirty individuals between the ages of 18–45, who were diagnosed with COVID-19 by PCR at least one month ago, and had no pre-COVID-19 hearing loss complaints, constituted the test group. Thirty individuals aged between 18 and 30 years and who had no history of hearing loss constituted the control group. Audiological evaluations of all participants were made with pure-tone audiometry, high-frequency audiometry, transient-evoked otoacoustic emission (TEOAE), distortion product otoacoustic emission (DPOAE), and auditory brainstem response (ABR) measurements.
Results
A significant difference was found between the groups at all high frequencies between 4 and 14 kHz (p < 0.05). TEOAE amplitudes at 1500 Hz, 2000 Hz and 4000 Hz frequencies and DPOAE amplitudes at 4003 Hz and higher frequencies were significantly lower in the test group (p < 0.05). While there was a significant difference between the I, III and V absolute latencies between the groups (p < 0.05), there was no significant difference between the I-III, III-V and I-V interpeak latencies (p > 0.05) as a result of the ABR test.
Conclusion
This study showed that COVID-19 can cause cochlear damage, especially at high frequencies. More studies are needed to determine the effects of COVID-19 on the auditory system.
Keywords: COVID-19, Hearing, Cochlear damage, OAE, ABR
1. Introduction
A new type of coronavirus (nCoV), named acute respiratory syndrome coronavirus-2 (SARS-CoV-2), emerged in Wuhan, China, in December 2019 [1]. The new coronavirus type is responsible for the COVID-19 outbreak. The World Health Organization officially declared the COVID-19 outbreak as an international human health emergency of concern on 30 January 2020 [2].
Coronaviruses are a large family of viruses with many members. Six strains of coronavirus were reported to infect humans before COVID-19. These viruses can cause damage to the respiratory, gastrointestinal, and nervous systems of humans. Severe acute respiratory syndrome (SARS) coronavirus (SARS-CoV), Middle East respiratory syndrome (MERS) coronavirus (MERS-CoV), and SARS-CoV-2 are the coronaviruses that have caused epidemics to date [3].
SARS-CoV-2 spreads in humans, usually between close contacts. Coughing, sneezing, speaking, or breathing of the person carrying the virus is sufficient for the virus to be transmitted to the other person especially if the distance between two people is <2 m. SARS-CoV-2 can be transmitted from person to person through respiratory droplets. SARS-CoV-2 particles can remain suspended in the air and the risk of infection increases, especially in poorly ventilated areas [4].
The most common symptoms accompanying COVID-19 are fever, dry cough, and fatigue [1]. However, some patients may also experience symptoms such as loss of smell and taste, nasal congestion, sore throat, headache, nausea, vomiting, feeling of imbalance, and tremor [1], [5]. COVID-19 symptoms may occur between 2 and 14 days after the entry of the infectious agent into the body, but this period has been reported to be 5 days on average [6], [7].
Many viral infections (e.g., cytomegalovirus, herpes simplex, rubella, hepatitis, mumps, and varicella zoster) cause sensorineural hearing loss [8]. Extensive damage can directly occur to inner ear structures, including hair cells, the corti organ, and the cochlear nerve because of these viral infections [8], [9]. The SARS-CoV-2 virus is thought to cause hearing loss like most viral infections. Various case reports have been published reporting that individuals with COVID-19 develop hearing loss simultaneously with or after the disease [10], [11], [12], [13].
Studies on audiological evaluation in COVID-19 patients are limited. Although it is thought that COVID-19 may cause hearing loss, its effects on the hearing system are not clearly known. This study aimed to reveal the effects of COVID-19 on the auditory system using various audiological measurement methods in individuals diagnosed with COVID-19.
2. Material and methods
The study was conducted in a Department of Audiology with the approval of the Ethics Committee of the University's Faculty of Medicine (Decision no: 2020/034). The informed consent form and detailed anamnesis were obtained from all participants. Thirty individuals (14 men and 16 women) who were diagnosed with COVID-19 by PCR at least one month ago constituted the test group. Participants between 18 and 45 years old (mean, 28.9 ± 8.8) were included in the study to minimize the possible effects of aging on the auditory system. Individuals with pre-COVID-19 hearing complaints, a known history of hearing loss, a history of noise exposure, a history of otological disease or surgery, a diagnosis of neurological disease, and additional disabilities were not included in the study. Thirty individuals (15 males and 15 females) aged between 18 and 30 years (mean, 23.1 ± 2.6) whose hearing thresholds of 15 dB HL or better in the frequencies of 250 Hz to 8000 Hz and who had no history of hearing loss constituted the control group.
Otoscopic examination and acoustic immitance measurements were performed on all participants to exclude possible middle ear pathology. The video otoscope device (Orlvision) was used for otoscopic examination. Tympanogram was obtained between +200 and − 400 daPa using 226-Hz probe tone at 85 dB SPL intensity level with tympanometer (Interacoustic Titan). This study excluded those whose otological examination was not normal and/or those whose tympanogram was not type A according to the Jerger classification.
Audiological evaluations of the participants were made with pure-tone audiometry, high-frequency audiometry, transient-evoked otoacoustic emission (TEOAE), distortion product otoacoustic emission (DPOAE), and auditory brainstem response (ABR) measurements. All audiological evaluations were made in quiet cabin rooms following the Health Technical Memorandum 2045 and International Organization of Standardization 8253 standards.
Pure-tone and high-frequency audiometry tests were performed using AC-40 clinical audiometer (Interacoustics) and HDA300 headphones (Sennheiser). Air conduction hearing thresholds were determined at frequencies of 250, 500, 1000, 2000, 4000, 6000, 8000, 10,000, 12,500, and 14,000 Hz using the modified Hughson–Westlake method. Bone conduction hearing thresholds were determined using a B-71 bone vibrator (Radioear) at frequencies of 0.5–4 kHz.
Otoacoustic emission assessments were performed using the Capella portable Otoacoustic Emission (OAE) device (Otometrics Madsen) OTOsuite software. Attention was focused to ensure that the wave repeatability and stimulus stability was >70% and >80%, respectively, in OAE measurements. In DPOAE and TEOAE measurements, the noise rejection level was 40 dB SPL and 520 click stimulus was taken above the noise level. DPOAE measurement was recorded at frequencies between 1000 and 8000 Hz, 4 pts/oct as octave, and DP-gram was performed graphically. The difference between L1 and L2 levels was kept at 10 dB SPL (L1 = 65 dB SPL and L2 = 55 dB SPL), and the f2/f1 ratio was determined as 1:22 in all measurements using the DP-gram test protocol. DPOAEs were measured with the microphone in the external ear canal at a 2f1-f2 frequency. In the control group, signal-to-noise ratio (SNR) values for each frequency were used as operating parameters, and those with SNR <6 dB were not included in the evaluation. TEOAE measurement was applied at a range of 1000–4000 Hz. The measurement was made using an alternating polarity, click stimulus, at 85 dB SPL in the nonlinear module. In the control group, the SNR values for each frequency were used as operating parameters and the stimulus/noise ratio was ≥6 dB. Amplitude (DP-SPL) values were used to evaluate TEOAE and DPOAE.
ABR measurements were made using the device Socrates (Hedera Biomedics). The ABR test was recorded at 80 dB nHL in alternating polarity, using 11.7 click stimulus per second, averaging up to 2000 sweeps. Bandpass filter range is adjusted to be at a high and low frequencies cutoff of 30 and 3000 Hz, respectively.
2.1. Statistical analysis
The SPPS 25 (released 2017, version 25.0; IBM Corp., IBM SPSS Statistics for Windows; Armonk, NY, USA) statistical package program was used to evaluate the data. The homogeneity of the variances, which is one of the prerequisites of the parametric tests, was checked with the Levene test. Normality assumption was checked with the Shapiro–Wilk test. Bivariate relations were analyzed using the independent t-test when the normality condition was met and the Mann–Whitney U test when not. A p < 0.05 level was considered statistically significant.
3. Results
The most common symptoms in the group with COVID-19 were joint pain (46.7%), fatigue (40%), dry cough (36.7%), loss of smell and taste (33.3%), headache (33.3%), and fever (33.3%). Although respiratory distress was observed in 3 (10%) of the patients, none of them received oxygen support. Dizziness occurred in 8 of the patients (26.7%). According to the information received from the patients about their hearing; 2 patients (6.7%) reported hearing loss during COVID-19 and 5 patients (16.7%) after COVID-19. In addition, 11 patients (33.3%) had tinnitus, 1 patient (3.3%) had ear pain, and 1 patient (3.3%) had ear fullness during COVID-19. Tinnitus persisted after COVID-19 in 8 patients. There is no hospitalized patient. While 16 (53.3%) of the patients used favipiravir and 4 (13.3%) hydroxychloroquine in the treatment of COVID-19, 10 (33.4%) did not use any medication.
3.1. Pure-tone audiometry and high-frequency audiometry
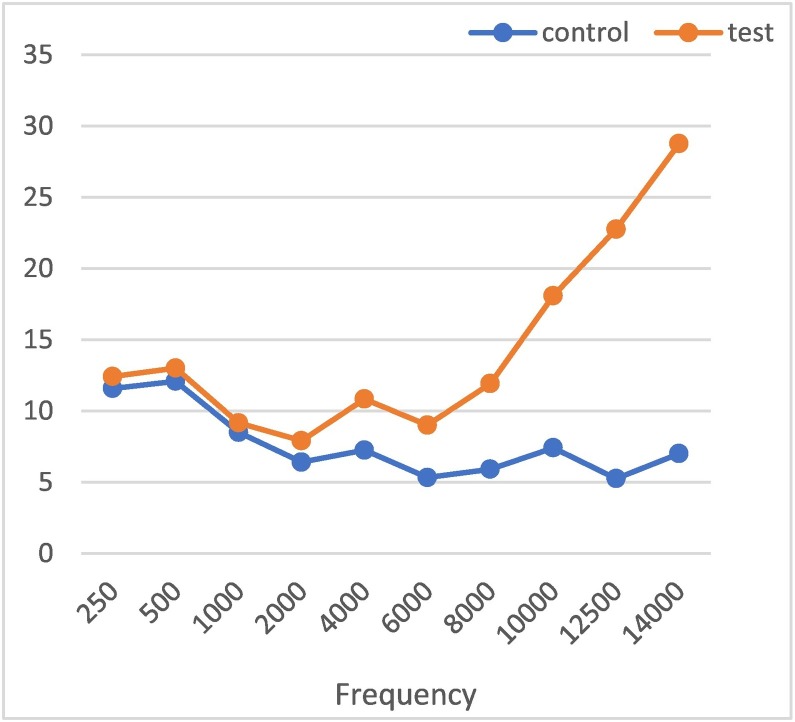
A significant difference was found between the groups at all high frequencies between 4 and 14 kHz when the test and control groups were evaluated according to the frequencies (p < 0.05). Fig. 1 shows that the hearing thresholds of the test group become progressively worse toward frequencies higher than 6 kHz.
Fig. 1.
Frequency graph of pure-tone and high-frequency audiometry measurements in the control and test groups.
3.2. TEOAE
TEOAE amplitudes obtained for each frequency in the control and test groups were compared. A significant difference was found between the groups at 1500 Hz, 2000 Hz and 4000 Hz frequencies (p < 0.05). This evaluation is shown in Table 1 .
Table 1.
Comparison of TEOAE amplitudes in control and test groups.
| Frequency | Group | Mean ± sd | p value |
|---|---|---|---|
| 1000 Hz | Control | 10.42 ± 4.27 | 0.421 |
| Test | 9.76 ± 4.66 | ||
| 1500 Hz | Control | 8.32 ± 4.88 | 0.009⁎ |
| Test | 5.89 ± 5.19 | ||
| 2000 Hz | Control | 5.14 ± 5.72 | 0.007⁎ |
| Test | 2.29 ± 5.60 | ||
| 3000 Hz | Control | 2.17 ± 5.51 | 0.060 |
| Test | −0.31 ± 8.54 | ||
| 4000 Hz | Control | 1.01 ± 4.96 | 0.001⁎ |
| Test | −2.26 ± 5.41 |
sd: standard deviation.
p < 0,05 there is a statistically significant difference between groups.
3.3. DPOAE
DPOAE amplitudes obtained for each frequency in the control and test groups were compared. A significant difference was found between the groups at 4003 Hz and higher frequencies (p < 0.05). This evaluation is shown in Table 2 .
Table 2.
Comparison of DPOAE amplitudes in control and test groups.
| Frequency | Group | Mean ± sd | p value |
|---|---|---|---|
| 996 Hz | Control | 11.34 ± 5.62 | 0.378 |
| Test | 9.60 ± 14.13 | ||
| 1191 Hz | Control | 13.20 ± 6.13 | 0.871 |
| Test | 13.52 ± 14.06 | ||
| 1416 Hz | Control | 14.19 ± 6.19 | 0.818 |
| Test | 14,82 ± 20.40 | ||
| 1679 Hz | Control | 12.74 ± 7.20 | 0.682 |
| Test | 11.65 ± 19.10 | ||
| 2001 Hz | Control | 11.83 ± 6.78 | 0.812 |
| Test | 12.57 ± 23.23 | ||
| 2382 Hz | Control | 10.92 ± 7.54 | 0.585 |
| Test | 12.87 ± 26.52 | ||
| 2832 Hz | Control | 8.98 ± 6.90 | 0.727 |
| Test | 9.96 ± 20.43 | ||
| 3359 Hz | Control | 4.84 ± 6.21 | 0.787 |
| Test | 4.11 ± 19.90 | ||
| 4003 Hz | Control | 2.07 ± 6.04 | 0.007⁎ |
| Test | −3.98 ± 15.99 | ||
| 4755 Hz | Control | 0.72 ± 6.96 | 0.010⁎ |
| Test | −3.94 ± 11.92 | ||
| 5654 Hz | Control | 1.75 ± 6.33 | 0.027⁎ |
| Test | −2.52 ± 13.37 | ||
| 6728 Hz | Control | 0.36 ± 5.90 | 0.000⁎ |
| Test | −6.62 ± 11.12 | ||
| 7998 Hz | Control | −0.22 ± 6.01 | 0.044⁎ |
| Test | −4.18 ± 13.81 |
sd: standard deviation.
p < 0,05 there is a statistically significant difference between groups.
3.4. ABR
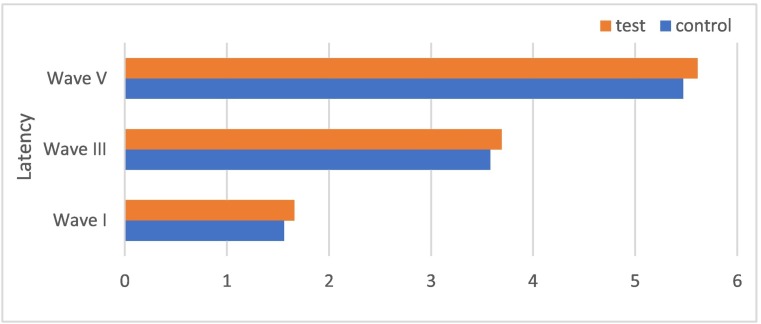
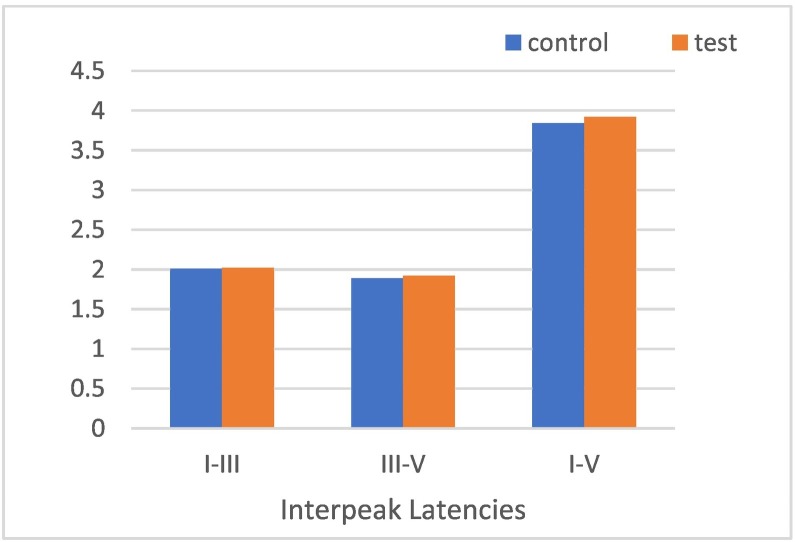
A significant difference between the groups in I, III, and V absolute latencies was found when the test and control groups were evaluated (p < 0.05). This assessment is presented in Fig. 2 I-III, III-V and I-V interpeak latencies were compared between the groups, and no significant difference was found in any of them (p > 0.05). This assessment is shown in Fig. 3 .
Fig. 2.
Measurement graph of I, III, and V absolute latencies in the control and test groups.
Fig. 3.
Comparison of I–III, III–V, and I-V interpeak latencies in the control and test groups.
4. Discussion
Many viral infections cause congenital or acquired, unilateral or bilateral, and mild to severe sensorineural hearing loss. Viral infections are typically thought to cause intracochlear damage [8]. However, some viral infections can affect the brainstem [14]. Former members of the coronavirus family (MERS and SARS) have had effects on the auditory system. Similarly, the SARS-CoV-2 virus may also cause hearing loss. SARS-CoV-2 may have direct neurological involvement or inner ear involvement due to hypercoagulation, which is common in recent COVID-19 patients [15].
A study reported that high-frequency pure-tone thresholds (4, 6, and 8 kHz) and TEOAE amplitudes were significantly worse in individuals with asymptomatic COVID-19 compared to the control group [16]. Similarly, another study found a significant difference in high-frequency pure-tone thresholds (4 and 8 kHz) between the COVID-19 positive group and the control groups [15]. No significant difference was found in this study between the two groups in terms of TEOAE measurements, but the test group's values were lower at all frequencies [15]. In another study, low (0.125, 0.25, 0.5, and 1 kHz), high (2, 4, 6, and 8 kHz), and extended high frequency (10, 12.5, 14, and 16 kHz) pure-tone thresholds were significantly reduced in patients with COVID-19 history [17]. The 4 kHz TEOAE SNR rate was significantly lower in patients with COVID-19 history, and no significant difference was observed between control and test groups in other frequencies and the DPOAE test [17]. When the literature is examined, it has been observed that COVID-19 may cause damage to the outer hair cells in the cochlea, especially at high frequencies. In our study, hearing thresholds were significantly worse at 4 kHz and higher frequencies in individuals diagnosed with COVID-19. In addition, TEOAE amplitudes at 1500 Hz, 2000 Hz and 4000 Hz frequencies and DPOAE amplitudes at 4003 Hz and higher frequencies were significantly lower in this group. The current findings support the view that COVID-19 causes damage to cochlear outer hair cells.
There are some theories on the effects of COVID-19 on the auditory system. COVID-19 may cause ischemic damage to the auditory structures by causing clot formation in the vessels feeding the auditory system because it enters the cell by binding to the ACE-2 receptor in the vessels. In addition, the ACE-2 receptor is abundant in the brain and brainstem, and therefore, the virus may affect the hearing centers in the brainstem [18], [19]. The virus development timeline from initial symptoms to moderate or severe complications (approximately 5 days) is long enough for the virus to enter and damage brainstem cranial nerves and nuclei [20].
The ABR test can be used to evaluate the effects of COVID-19 on the brainstem. In a study evaluating ABR findings in individuals diagnosed with COVID-19, no significant difference was found compared to the control group although longer I, III, and V absolute latencies were obtained in this group. Nevertheless, III–V interpeak latencies were significantly longer in the test group than in the control group [17]. In our study, I, III, and V absolute latencies were significantly prolonged in the test group, but no significant difference was found between the I–III, III–V, and I-V interpeak latencies between the groups. It is thought that the prolongations in I, III and V absolute latencies in the test group are due to cochlear damage at high frequencies rather than neural pathology and/or brainstem damage.
The effect of COVID-19 on the auditory system was interpreted in the present study by examining pure-tone audiometry, high-frequency audiometry, OAE, and ABR tests. In routine audiological evaluation, hearing thresholds are tested in the frequency range of 0.125–8 kHz. However, determining hearing thresholds at frequencies above 8 kHz can provide an early prediction of auditory damage [21], [22]. In this study, hearing thresholds were obtained outside the normal limits only at frequencies above 8 kHz, although hearing thresholds at 4 kHz and higher frequencies deteriorated significantly in individuals with COVID-19. Therefore, high frequency audiometry can be quite useful in evaluating the effects of viral infections such as COVID-19 on the auditory system.
In this study, the combined use of OAE and ABR tests enabled the sensorineural hearing loss to be differentiated from sensor/neural and evaluated whether the damage was at the brainstem level. According to the results of the present study, COVID-19 can damage outer hair cells, especially affecting the high-frequency region. It is thought that longer ABR absolute latencies in the COVID-19 group may be a result of cochlear damage at high frequencies, but a possible brainstem injury should not be ignored. Thus, more studies are needed to determine whether COVID-19 causes auditory brainstem involvement.
In this study, few patients reported hearing loss that may be associated with COVID-19. Although the comparison results of the audiological assessments in the test and control groups were significant, the differences were small. While COVID-19 appears to have effects on the auditory system, these effects are thought to be minor and the resulting symptoms are rare.
The lack of audiological test results of the individuals included in the present study before they had COVID-19 and ignoring the differences in the drugs used in the treatment are the limitations of the present study. Hearing loss in COVID-19 patients may be caused by viruses or may result from the ototoxic effect of the drugs used in the treatment. Chloroquine and hydroxychloroquine used in COVID-19 treatment may have temporary or permanent ototoxic effects (e.g., sensorineural hearing loss and tinnitus) [23]. The ototoxic effect of favipiravir, another drug used in COVID-19 treatment, is unknown. Thus, conducting studies evaluating the ototoxic effect of drugs used in COVID-19 treatment would be useful.
5. Conclusion
Hearing thresholds at high frequencies deteriorated, and OAE amplitudes decreased in individuals diagnosed with COVID-19. This study shows that COVID-19 can cause cochlear damage, especially at high frequencies. More studies with different age groups and larger sample sizes are needed to determine the effects of COVID-19 on the auditory system.
Author contributions
Conceptualization: Burak Öztürk, Hatice Kavruk, Ayşenur Aykul.
Formal analysis: Hatice Kavruk.
Supervision: Burak Öztürk.
Writing-original draft: Hatice Kavruk, Ayşenur Aykul.
Writing-review & editing: Burak Öztürk, Hatice Kavruk, Ayşenur Aykul.
Declaration of competing interest
None.
Acknowledgments
Acknowledgements
We would like to thanks, Audiologist Ümit Aktürk for his help in data collection.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang Z., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jin Y., Yang H., Ji W., Wu W., Chen S., Zhang W., Duan G. Virology, epidemiology, pathogenesis, and control of COVID-19. Viruses. 2020;12:372. doi: 10.3390/v12040372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu D., Wu T., Liu Q., Yang Z. The SARS-CoV-2 outbreak: what we know. Int J Infect Dis. 2020;94:44–48. doi: 10.1016/j.ijid.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention How does the virus spread. 2020. https://www.cdc.gov/coronavirus/2019-ncov/faq.html#:%20%20~:text=The%20virus%20that%20causes%20COVID,%20sings%2C%20talks%2C%20or%20breathes
- 5.Guan W., Ni Z., Hu Y., Liang W., Ou C., Zhong N. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rothan H.A., Byrareddy S.N. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J Autoimmun. 2020;109 doi: 10.1016/j.jaut.2020.102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lauer S.A., Grantz K.H., Bi Q., Jones F.K., Zheng Q., Meredith H.R., Azman A.S., Reich N.G., Lessler J. The incubation period of coronavirus disease 2019 (CoVID-19) from publicly reported confirmed cases: estimation and application. Ann Intern Med. 2020;172:577–582. doi: 10.7326/M20-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen B.E., Durstenfeld A., Roehm P.C. Viral causes of hearing loss: a review for hearing health professionals. Trends Hear. 2014;18:1–17. doi: 10.1177/2331216514541361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen X., Fu Y.Y., Zhang T.Y. Role of viral infection in sudden hearing loss. Journal of International Medical Research. 2019;47:2865–2872. doi: 10.1177/0300060519847860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sriwijitalai W., Wiwanitkit V. Hearing loss and COVID-19: a note. Am J Otolaryngol. 2020;41 doi: 10.1016/j.amjoto.2020.102473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Degen C., Lenarz T., Willenborg K. Acute profound sensorineural hearing loss after COVID-19 pneumonia. Mayo Clin Proc. 2020;95:1801–1803. doi: 10.1016/j.mayocp.2020.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lang B., Hintze J., Conlon B. Coronavirus disease 2019 and sudden sensorineural hearing loss. J Laryngol Otol. 2020;134:1–3. doi: 10.1017/S0022215120002145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chern A., Famuyide A.O., Moonis G., Lalwani A.K. Bilateral sudden sensorineural hearing loss and ıntralabyrinthine hemorrhage in a patient with COVID-19. Otol Neurotol. 2021;42:10–e14. doi: 10.1097/MAO.0000000000002860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jereb M., Lainscak M., Marin J., Popovic M. Herpes simplex virus infection limited to the brainstem. Wien Klin Wochenschr. 2005;117:495–499. doi: 10.1007/s00508-005-0324-0. [DOI] [PubMed] [Google Scholar]
- 15.Tan M., Cengiz D.U., Demir İ., Demirel S., Çolak S.C., Karakaş O., Bayındır T. Effects of Covid-19 on the audio-vestibular system. Am J Otolaryngol. 2022;43 doi: 10.1016/j.amjoto.2021.103173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mustafa M.W.M. Audiological profile of asymptomatic Covid-19 PCR-positive cases. Am J Otolaryngol. 2020;41 doi: 10.1016/j.amjoto.2020.102483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gedik Ö., Hüsam H., Başöz M., Tas N., Aksoy F. The effect of coronavirus disease 2019 on the hearing system. J Laryngol Otol. 2021;135:810–814. doi: 10.1017/S0022215121001961. [DOI] [PubMed] [Google Scholar]
- 18.Cure E., Cure M.C. COVID-19 may predispose to thrombosis by affecting both vascular endothelium and platelets. Clin Appl Thromb Hemost. 2020;26 doi: 10.1177/1076029620933945. 1076029620933945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yong S.J. Persistent brainstem dysfunction in long-COVID: a hypothesis. ACS Chem Nerosci. 2021;12:573–580. doi: 10.1021/acschemneuro.0c00793. [DOI] [PubMed] [Google Scholar]
- 20.Jafari Z., Kolb B.E., Mohajerani M.H. Hearing loss, tinnitus, and dizziness in COVID-19: a systematic review and meta-analysis. Can J Neurol Sci. 2021;12:1–33. doi: 10.1017/cjn.2021.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mehrparvar A.H., Mirmohammadi S.J., Ghoreyshi A., Mollasadeghi A., Loukzadeh Z. High-frequency audiometry: a means for early diagnosis of noise-induced hearing loss. Noise Health. 2011;13:402–406. doi: 10.4103/1463-1741.90295. [DOI] [PubMed] [Google Scholar]
- 22.Singh Chauhan R., Saxena R.K., Varshey S. The role of ultrahigh-frequency audiometry in the early detection of systemic drug-induced hearing loss. Ear Nose Throat J. 2011;90:218–222. doi: 10.1177/014556131109000506. [DOI] [PubMed] [Google Scholar]
- 23.Prayuenyong P., Kasbekar A.V., Baguley D.M. Clinical implications of chloroquine and hydroxychloroquine ototoxicity for COVID-19 treatment: a mini-review. Front Public Health. 2020;8:252. doi: 10.3389/fpubh.2020.00252. [DOI] [PMC free article] [PubMed] [Google Scholar]