Abstract
Introduction
There are increasing reports of COVID-19 related neurological complications which may be due to direct viral invasion, or immune mediated inflammatory diseases such as autoimmune encephalitis and ADEM (acute demyelinating encephalomyelitis). In this study, a systematic review is presented of the reported cases infected by the COVID-19 who were diagnosed with various forms of autoimmune encephalitis (AE).
Methods
The authors searched three databases including PubMed, Scopus, and Web of science for extracting original articles on coronavirus/ COVID-19 and AE.
Results
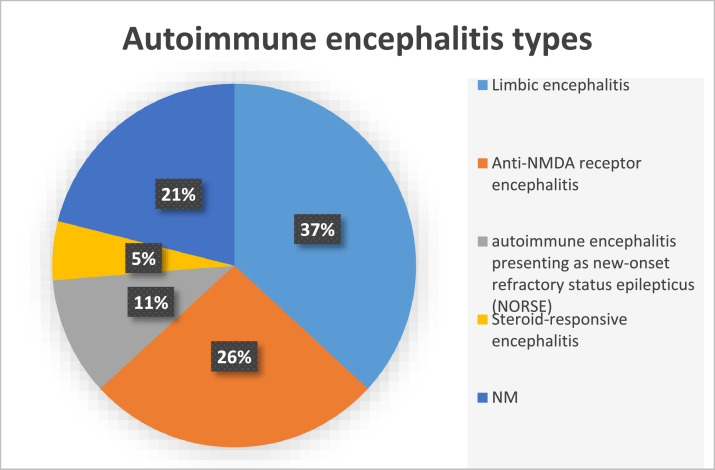
Eighteen articles were considered in this study, including 15 case reports, and three case series with a total of 81 patients. Among the studies, 19 cases were reported with AE including 7 (37%) cases of limbic encephalitis, 5 (26%) patients with anti-N-methyl-d-aspartate (NMDA) receptor encephalitis, 2 (11%) with AE presenting as new-onset refractory status epilepticus (NORSE), 1 (5%) case of steroid-responsive encephalitis, and 4 (21%) cases with an unknown type of AE.
Conclusion
Our systematic review revealed evidence on AE development in patients infected with the COVID-19. Clinicians should be aware of the possible diagnosis of AE when considering other neurological differential diagnosis in SARS-CoV-2 infected patients.
Keywords: COVID-19, Autoimmune encephalitis, Systematic review
1. Introduction
The novel coronavirus disease (COVID-19) appeared for the first time in Wuhan of China in December 2019 (Lu et al., 2020). Caused by the SARS-CoV-2 virus (severe acute respiratory syndrome coronavirus 2), it is a member of the family of coronaviruses infecting humans which is responsible for lower respiratory infection and can cause acute respiratory distress syndrome (ARDS) (Zhou et al., 2020; Chen et al., 2020).
According to previous reports, there are confirmed cases with neurological manifestations who developed the disease either via direct attack to the central nervous system (CNS) or via autoimmune processes (Sánchez-Morales et al., 2021a; Bhagat et al., 2021). New evidence has identified an association between the COVID-19 and systemic autoimmune diseases like autoimmune hemolytic anemia or one-organ-specific diseases like Guillain-Barre syndrome (Toscano et al., 2020; Lazarian et al., 2020). Several neurological complications have now been described: encephalitis, meningitis, and cerebrovascular diseases including ischemic stroke and hemorrhage, ADEM, encephalopathy, and myopathy (Liu et al., 2020a; Helms et al., 2020; Guilmot et al., 2021).
In the following, a systematic review can be found of the reported cases who were infected by the COVID-19, and diagnosed with various forms of autoimmune encephalitis (AE).
2. Methods
2.1. Design
This systematic review was conducted based on Preferred Reporting for Systematic Review and Meta-Analysis (PRISMA) (Moher et al., 2009).
2.2. Search strategy
We searched three databases of Pubmed, Scopus, and Web of science for extracting original articles on coronavirus/ COVID-19 and autoimmune encephalitis (AE) on the 1st of April. The search strategy consisted of keywords including “Autoimmune encephalitis”, “Limbic encephalitis”, “Anti NMDA”, “Anti VGKC”, “Anti LGI1”, “Anti CASPR2”, “Anti AMPA”, “Anti GAD65”, “Anti GABA”, “Anti GABAA”, “Anti GABAB”, “Anti D2”, “Anti Dopamine-2″, “Anti DPPX”, “Anti Glycine”, “Anti GlyR”, “Anti Glutamate”, “Anti mGluR1”, “Anti mGluR5”, “Anti amphiphysin”, “Anti Neuronal”, “Anti CV2”, “Anti Ta”, “Anti Purkinje”, “COVID-19″, “Sars-Cov-2″, and “coronavirus”.
2.3. Eligibility criteria
We included the studies have reported the AE related to COVID-19 infection. The studies with other types of encephalitis or unconfirmed cases of autoimmune encephalitis were excluded.
2.4. Study selection
Articles were independently selected by two investigators (M.B and M.R) following two steps. First, the title and abstract were screened. In the next step, the full texts of the selected studies were reviewed according to eligibility criteria. At the end of each step, any disagreements were resolved by discussing the issue with the third investigator (F.N).
2.5. Data extraction
The same reviewers (M.B and M.R) independently extracted the following data manually: author, year, country, study design, number of patients, mean age, sex, number of AE cases, type of AE, and symptoms.
2.6. Data syntheses and analyses
The quantitative data was represented as percentage or mean ± standard deviation. Studies and results were qualitatively compared.
3. Results
3.1. Eligible publications
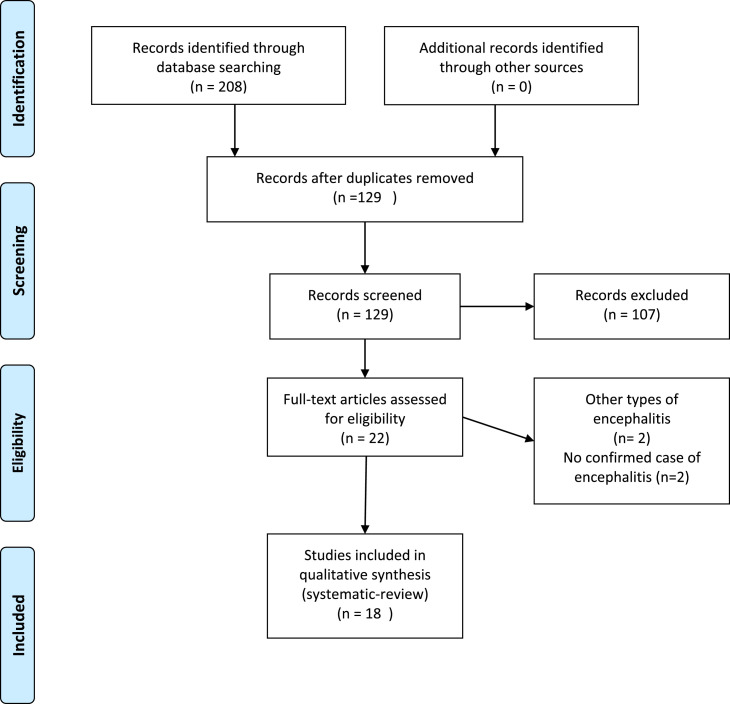
Our comprehensive literature search yielded a total of 208 articles. After removing duplicates and screening titles and abstracts, 22 reports remained; however, after full text reviews, four studies did not meet the inclusion criteria. Finally, qualitative synthesis was performed on18 studies (Fig. 1 ).
Fig. 1.
PRISMA flow diagram depicting the flow of information through the different phases of a systematic review.
3.2. Study characteristics
Among the included studies, there were 15 case report studies, two case series, and one cohort article with a total of 81 patients. 62.6% of these patients were men. Three studies were multicenter (Ayatollahi et al., 2020; Pilotto et al., 2020a; Moideen et al., 2020), six from Italy (Manganotti et al., 2021; Pilotto et al., 2021; Dono et al., 2021; Monti et al., 2020; Pizzanelli et al., 2021; Panariello et al., 2020), four from UK (Zambreanu et al., 2020a; Khoo et al., 2020a; Dhillon et al., 2021; Hosseini et al., 2020), two from USA (Bhagat et al., 2021; Burr et al., 2021), one from Mexico (Sánchez-Morales et al., 2021a), one from China (Wang et al., 2020a), and one from France (Grimaldi et al., 2020a).
3.3. Autoimmune encephalitis
Findings from the studies on the AE are summarized in Table 1 . Among the included studies, 19 cases were reported with AE, including 7 (37%) cases of limbic encephalitis, 5 (26%) patients with anti-N-methyl-d-aspartate (NMDA) receptor encephalitis, 2 (11%) with AE presenting as new-onset refractory status epilepticus (NORSE), 1 (5%) case of steroid-responsive encephalitis, and 4 (21%) cases with unknown type of AE (Table 1, Fig. 2 ).
Table 1.
Charcteristics and autoimmune encephaltis of included studies.
| Author | year | country | Study design | number of patients | age | gender | total number of encephalitis cases | SARS-CoV-2 laboratory results | Encephalitis laboratory results | type of autoimmune encephalitis | presenting signs |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ayatollahi | 2020 | Iran, Canada | case report | 1 | 18 | Female | 1 | SARS-CoV-2 rRT-PCR: positive, SARS-CoV-2 CSF PCR: negative, Lung CT scan: normal | MRI: normal, EEG: showed moderate intermittent nonepileptiform abnormality over the frontocentrotemporal regions | NM | drowsiness, confusion, urinary retention,generalized tonic-clonic seizure |
| Zambreanu | 2020 | UK | case report | 1 | 66 | Female | 1 | SARS-CoV-2 rRT-PCR: positive, SARS-CoV-2 IgG: negative, SARS-CoV-2 CSF PCR: negative | MRI: symmetrical T2 and FLAIR hyperintensities, Diffusion MRI: punctate bright signal on the B1000 map in the medial temporal lobes, thalami and fornices, Autoantibody testing of immunemediated encephalitis: negative | Limbic encephalitis | confusion, amnestic |
| Grimaldi | 2020 | France | case report | 1 | 72 | Male | 1 | Lung CT scan: peripheral bilateral ground-glass lesions and consolidative opacities, SARS-CoV-2 rRT-PCR: positive first then turned to negative, SARS-CoV-2 IgG and IgM: negative | EEG: symmetric diffuse background, MRI: normal, F-FDG PET: putaminal and cerebellum hypermetabolism associated with diffuse cortical hypometabolism, serum IgG isotype autoantibodies: High | NM | action tremor, ataxia, dysarthria and upper limbs dysmetria |
| Manganotti | 2021 | Italy | case report | 2 | 54 | Male | 1 | SARS-CoV-2 rRT-PCR: positive | EEG: generalized epileptic discharges with bilateral frontal high amplitude delta waves, MRI: normal | autoimmune encephalitis presenting as new-onset refractory status epilepticus (NORSE) | convulsive status epilepticus, generalized myoclonic jerks of axial muscles and face |
| Pilotto | 2020 | Italy | case series | 25 | 65/9 | 15 Male/10 Female | 2 | RT PCR: positive | NM | Limbic encephalitis | altered mental status, aphasia, seizure, focal motor deficits |
| Khoo | 2020 | UK | case report | 1 | 65 | Female | 1 | SARS-CoV-2 rRT-PCR: positive, Chest X-ray: bilateral peripheral pulmonary infiltrates | MRI: normal, CSF white cell count: normal, Autoantibody testing of immunemediated encephalitis: negative, EEG: normal, | NM | involuntary movements, only speak in native language, speaking difficulties and increasing confusion. |
| Pilotto | 2020 | Italy, UK, sweden | case report | 1 | 60 | Male | 1 | SARS-CoV-2 rRT-PCR: positive, Chest X-ray: moderate bilateral interstitial pneumonia, SARS-CoV-2 CSF PCR: negative | CSF: mild lymphocytic pleocytosis and moderate increase of CSF protein, EEG: generalized slowing with decreased reactivity to acoustic stimuli, MRI: normal, Autoantibody testing of immunemediated encephalitis: negative | Steroid-responsive encephalitis | severe akinetic syndrome, unable to carry out simple orders, palmomental and glabella reflexes, nuchal rigidity, |
| Wang | 2020 | China | case report | 1 | 68 | Male | 1 | Lung CT scan: groundglass shadows in both lungs, PCR: negative, SARS-CoV-2 IgG and IgM: negative and positive, respectivly, SARS-CoV-2 CSF IgG and IgM: positive, | Brain CT scan: lacunar lesions in the left basal ganglia region | NM | unable to walk, uroclepsia, coprolalia, and persecution delusion. |
| Burr | 2020 | USA | case report | 1 | 2 | Female | 1 | SARS-CoV-2 PCR: positive, SARS-CoV-2 CSF PCR: negative, SARS-CoV-2 IgG: positive | MRI: normal, serum and CSF NMDA receptor autoantibody: positive | Anti-NMDA receptor encephalitis | fever, fussiness, poor sleep, constipation, decreased oral intake, fussy, not talking, constant kicking and thrashing movements of arms and legs. |
| Dhillon | 2021 | UK | cohort | 29 | 68/9 | 16 Male/13 Female | 1 | SARS-CoV-2 CSF PCR: negative | MRI; Lesions in the limbic system, predominantly in the left amygdala and hippocampus with partial restricted diffusion, Autoantibody testing of immunemediated encephalitis: negative | Limbic encephalitis | acute-onset delirium, altered mental status, status epilepticus, cognitive impairment |
| Sánchez-Morales | 2021 | Mexico | case series | 10 | 11/8 | 5 Male/5 Female | 1 | SARS-CoV-2 CSF PCR: positive, Serum SARS-CoV-2 IgM and IgG: negative, CSF antibodies SARS-CoV-2 IgG: positive | NM | Anti-NMDA receptor encephalitis | Altered behavior and mental status, seizures, insomnia, orolingual dyskinesias |
| Dono | 2020 | Italy | case report | 1 | 81 | Male | 1 | Chest X-ray: normal, Lung CT scan: ground-glass pattern in both the inferior lobe segments, SARS-CoV-2 PCR: positive, SARS-CoV-2 CSF PCR: negative | EEG: normal, MRI: hyperintense lesions of the bilateral parietal cortex, left temporal cortex, and right cingulate cortex, Autoantibody testing of immunemediated encephalitis: negative | autoimmune encephalitis presenting as new-onset refractory status epilepticus (NORSE) | drowsy with Glasgow coma scale (GCS) of 5 (no eyes opening both to verbal and pain stimulation, no verbal response, and flexion of the upper limbs to pain), Contemporarily, frequent jerky myoclonic con- tractions of the abdomen and the right lower limb appeared. |
| Bhagat | 2020 | USA | case report | 1 | 54 | Male | 1 | SARS-CoV-2 rRT-PCR: positive, Chest X-ray: normal | MRI: hyperintensity on right posterior medial temporal lobe, Autoantibody testing of immunemediated encephalitis: negative, EEG: right posterior quadrant lateralized periodic discharges | Limbic encephalitis | nonradiating pressure type holocranial headache, diaphoresis, palpitation, nausea drowsy, oriented to time, place, and person with intact language, cranial nerves, motor, sensory, and cerebellar functions |
| Monti | 2020 | Italy | case report | 1 | 50 | Male | 1 | SARS-CoV-2 rRT-PCR: positive, Lung CT scan: normal | anti-NMDA receptors antibodies in CSF: positive, MRI: normal | Anti-NMDA receptor encephalitis | confabulations and delirious ideas, focal motor seizures with impaired awareness and oro-facial dyskinesia/automatisms, refractory status epilepticus |
| Hosseini | 2020 | UK | case report | 2 | 62/5 | Male | 1 | SARS-CoV-2 rRT-PCR: positive, SARS-CoV-2 CSF PCR: negative, Lung CT scan: bilateral pleural effusion | MRI: partial diffusion restriction in limbic system | Limbic encephalitis | dysphasia and impaired orientation, attention and memory. |
| Pizzanelli | 2021 | Italy | case report | 1 | 74 | Female | 1 | Lung CT scan: bilateral diffuse areas of ground-glass pattern with subpleural parenchymal consolidations, SARS-CoV-2 rRT-PCR: positive, SARS-CoV-2 CSF PCR: negative | Brain CT scan: normal, EEG: focal seizures with impaired awareness and oral automatisms, Autoantibody testing of immunemediated encephalitis in CSF and serum: negative, MRI: bilateral symmetric mesial temporal lobes hyperintensity and mild hippocampal thickness | Limbic encephalitis | mild confusion and a brief episode of non-responsivity with staring |
| Moideen | 2020 | India, UK | case report | 1 | 17 | Male | 1 | NM | MRI: normal, EEG: normal, anti-NMDA receptors antibodies in CSF: positive | Anti-NMDA receptor encephalitis | over-familiar attitude, increased psychomotor activity, and labile affect |
| Panariello | 2020 | Italy | case report | 1 | 23 | Male | 1 | Chest X-ray: bilateral ground glass opacities, Lung CT scan: patchy bi-basilar consolidations | Brain CT scan: normal, EEG: unstable non reactive to visual stimuli, anti-NMDA receptors antibodies in CSF: positive | Anti-NMDA receptor encephalitis | psychomotor agitation, anxiety, thought disorganization, persecutory delusions, auditory hallucinations, and global insomnia |
NM: Not Mentioned; NMDA: N-Methyl-D aspartate, SARS-CoV-2: severe acute respiratory syndrome coronavirus 2, rRT-PCR: Real-time reverse-transcription polymerase chain reaction analysis, CT scan: Computed tomography, MRI: Magnetic resonance imaging, PET: Positron emission tomography, F-FDG: Fludeoxyglucose, EEG: Electroencephalography.
Fig. 2.
Types of autoimmune encephalitis expressed as percentage.
A cohort study from UK reported one case of limbic encephalitis out of 29 COVID-19 cases (Dhillon et al., 2021). Also, there was a case series study reporting two patients with limbic encephalitis among 25 COVID-19 patients (Pilotto et al., 2021). The other cases of limbic encephalitis were reported in four case reports (Bhagat et al., 2021; Pizzanelli et al., 2021; Hosseini et al., 2020). The patients were more likely to develop acute-onset delirium, altered mental status, status epilepticus, cognitive impairment, aphasia, seizure, focal motor deficits, headache, diaphoresis, palpitation, nausea drowsy, mild confusion, and a brief episode of non-responsivity (Table 1). In limbic encephalitis, when measured, no cases of LGI1 or CASPR2 antibodies were found.
Five studies reported cases of Anti-NMDA receptor encephalitis. Among the cases, there was a two-year-old girl with symptoms such as fever, poor sleep, constipation, decreased oral intake, not talking, constant kicking, and thrashing movements of arms and legs (Burr et al., 2021). Furthermore, three other cases were patients aged 17, 23, and 50 years old with Anti-NMDA receptor encephalitis (Sánchez-Morales et al., 2021a; Moideen et al., 2020; Monti et al., 2020). It seems that this type of AE affects younger individuals infected with COVID-19. These patients were reported with altered behavior and mental status, seizures, insomnia, dyskinesia, refractory status epilepticus, over-familiar attitude, increased psychomotor activity, anxiety, and labile affect (Table 1).
Moreover, there were two cases with AE presenting as new-onset refractory status epilepticus (NORSE), both from Italy (Manganotti et al., 2021; Dono et al., 2021). The clinical symptoms including convulsive status epilepticus, and generalized myoclonic jerks of axial muscles and face were reported in these patients (Table 1).
A case report also described a patient with steroid responsive encephalitis after COVID 19 infection (Pilotto et al., 2020a). The case was unable to carry out simple orders and presented severe akinetic syndrome, palmomental and glabella reflexes, and nuchal rigidity.
Also, seven other studies reported AE in COVID-19 the patients without mentioning the exact type (Ayatollahi et al., 2020; Khoo et al., 2020a; Wang et al., 2020a; Grimaldi et al., 2020a). All the clinical symptoms of encephalitis in reported cases are detailed in Table 1.
4. Discussion
Different theories have been postulated to explain the pathophysiology of SARS-CoV-2 CNS invasion. SARS-CoV-2 reaches to CNS through systemic circulating system. The virus cannot easily cross the blood brain barrier (BBB) and invade the CNS (Achar and Ghosh, 2020). However, virus recruits three main strategies to cross the BBB. These mechanisms include transcellular migration, paracellular migration, and Trojan horse (Dahm et al., 2016).
There is another important mechanism explaining the development of AE in the COVID-19 infection. It has been demonstrated that cytokine-mediated neuroinflammation in response to SARS-CoV-2 infection plays an important role in the pathogenesis of AE (Dhillon et al., 2021). Severe case of COVID-19 infection induces overproduction of inflammatory cytokines, known as cytokine storm (Achar and Ghosh, 2020). Supportably, patients with a severe condition of COVID-19 show higher levels of inflammatory cytokines such as IL-2R, IL-6, IL-8, IL-10, and TNF-α compared to cases with non-severe COVID-19 condition (Liu et al., 2020b). Elevated IL-6 which is known as a common feature of the disease during the inflammatory phase can be a linking factor due to its role in facilitating autoantibody production in anti-NMDA-R encephalitis (Byun et al., 2016). Overproduction of inflammatory cytokines seems to affect BBB integrity, increase its permeability, and lead to viral transmission through BBB (Achar and Ghosh, 2020). Furthermore, it has been shown that SARS-CoV-2 induces production of anti-NMDA-R autoantibodies and causes AE (Sánchez-Morales et al., 2021b). Based on the reviewed articles in this study, hyperinflammation syndrome is a leading cause for the development of AE in the majority of COVID-19 patients. Moreover, the results indicate that the AE is mostly presented several days after respiratory symptoms, suggesting a trigger role for COVID-19 infection in autoimmune induced CNS disorders.
Based on the reviewed articles in this study, limbic encephalitis is the most frequent type of encephalitis among patients with AE, followed by anti-NMDA receptor encephalitis. This type of encephalitis is caused by autoantibodies that target NMDA receptors located in CNS. Infection-induced anti-NMDA-R encephalitis has been reported after herpes simplex virus (HSV1) infection (Monti et al., 2020). In a prospective study, AE was observed in about 27% of patients with HSV1 infection (Armangue et al., 2018). Among patients who developed AE after the COVID-19, only one case was reported with positive CSF PCR and there was one positive case for IgG and IgM in CSF for SARS-CoV-2 (Table 1). Based on this finding, the presence of undetectable virus in the CSF of COVID-19 patients with AE maybe due to transient presence of the virus in cerebrospinal fluid (CSF) or immunological response-induced CSF injury (Ayatollahi et al., 2020; Zambreanu et al., 2020b). Moreover, the absence of virus RNA is not associated with the severity of neurological disorders (Matschke et al., 2020). Positive autoimmune antibody and high level of proteins in CSF are applied as diagnostic criteria for AE (Wang et al., 2020b). However, antibody test could be negative in some patients (Khoo et al., 2020b). Studies have reported that corticosteroids and intravenous immunoglobulin are considered as first-line therapies for AE treatment (Manganotti et al., 2021; Khoo et al., 2020b; Pilotto et al., 2020b; Grimaldi et al., 2020b).
5. Conclusion
Our systematic review revealed evidence on the probability of developing AE in patients infected with the COVID-19. Clinicians should be aware of the possible diagnosis of AE when considering other neurological differential diagnosis in SARS-CoV-2 infected patients.
Declaration of Competing Interest
The authors declare there is no conflict of interest
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.msard.2022.103795.
Appendix. Supplementary materials
References
- Achar A., Ghosh C. COVID-19-associated neurological disorders: the potential route of CNS invasion and blood-brain relevance. Cells. 2020;9(11):2360. doi: 10.3390/cells9112360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armangue T., Spatola M., Vlagea A., Mattozzi S., Cárceles-Cordon M., Martinez-Heras E., et al. Frequency, symptoms, risk factors, and outcomes of autoimmune encephalitis after herpes simplex encephalitis: a prospective observational study and retrospective analysis. Lancet Neurol. 2018;17(9):760–772. doi: 10.1016/S1474-4422(18)30244-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayatollahi P., Tarazi A., Wennberg R. Possible autoimmune encephalitis with claustrum sign in case of acute SARS-CoV-2 infection. Can. J. Neurol. Sci. 2020;48(3):430–432. doi: 10.1017/cjn.2020.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhagat R., Kwiecinska B., Smith N., Peters M., Shafer C., Palade A., et al. New-onset seizure with possible limbic encephalitis in a patient With COVID-19 infection: a case report and review. J. Investig. Med. High Impact Case Rep. 2021;9 doi: 10.1177/2324709620986302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burr T., Barton C., Doll E., Lakhotia A., Sweeney M. N-methyl-d-aspartate receptor encephalitis associated with COVID-19 infection in a toddler. Pediatr. Neurol. 2021;114:75–76. doi: 10.1016/j.pediatrneurol.2020.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byun J.I., Lee S.T., Moon J., Jung K.H., Sunwoo J.S., Lim J.A., et al. Distinct intrathecal interleukin-17/interleukin-6 activation in anti-N-methyl-d-aspartate receptor encephalitis. J. Neuroimmunol. 2016;297:141–147. doi: 10.1016/j.jneuroim.2016.05.023. [DOI] [PubMed] [Google Scholar]
- Chen Y., Liu Q., Guo D. Emerging coronaviruses: genome structure, replication, and pathogenesis. J. Med. Virol. 2020;92(4):418–423. doi: 10.1002/jmv.25681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahm T., Rudolph H., Schwerk C., Schroten H., Tenenbaum T. Neuroinvasion and inflammation in viral central nervous system infections. Med. Inflamm. 2016;2016 doi: 10.1155/2016/8562805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhillon P.S., Dineen R.A., Morris H., Tanasescu R., Nikfekr E., Evans J., et al. Neurological disorders associated with COVID-19 hospital admissions: experience of a single tertiary healthcare center. Front. Neurol. 2021;12 doi: 10.3389/fneur.2021.640017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dono F., Carrarini C., Russo M., De Angelis M.V., Anzellotti F., Onofrj M., et al. New-onset refractory status epilepticus (NORSE) in post SARS-CoV-2 autoimmune encephalitis: a case report. Neurol. Sci. Off. J. Ital. Neurol. Soc. Ital. Soc. Clin. Neurophysiol. 2021;42(1):35–38. doi: 10.1007/s10072-020-04846-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimaldi S., Lagarde S., Harlé J.R., Boucraut J., Guedj E. Autoimmune encephalitis concomitant with SARS-CoV-2 infection: insight from (18)F-FDG PET imaging and neuronal autoantibodies. J. Nucl. Med. 2020;61(12):1726–1729. doi: 10.2967/jnumed.120.249292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimaldi S., Lagarde S., Harlé J.R., Boucraut J., Guedj E. Autoimmune encephalitis concomitant with SARS-CoV-2 infection: insight from 18F-FDG PET imaging and neuronal autoantibodies. J. Nucl. Med. 2020;61(12):1726–1729. doi: 10.2967/jnumed.120.249292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilmot A., Maldonado Slootjes S., Sellimi A., Bronchain M., Hanseeuw B., Belkhir L., et al. Immune-mediated neurological syndromes in SARS-CoV-2-infected patients. J. Neurol. 2021;268(3):751–757. doi: 10.1007/s00415-020-10108-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms J., Kremer S., Merdji H., Clere-Jehl R., Schenck M., Kummerlen C., et al. Neurologic features in severe SARS-CoV-2 infection. N. Engl. J. Med. 2020;382(23):2268–2270. doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseini A.A., Shetty A.K., Sprigg N., Auer D.P., Constantinescu C.S. Delirium as a presenting feature in COVID-19: neuroinvasive infection or autoimmune encephalopathy? Brain Behav. Immun. 2020;88:68–70. doi: 10.1016/j.bbi.2020.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoo A., McLoughlin B., Cheema S., Weil R.S., Lambert C., Manji H., et al. Postinfectious brainstem encephalitis associated with SARS-CoV-2. J. Neurolo. Neurosurg. Psychiatry. 2020;91(9):1013. doi: 10.1136/jnnp-2020-323816. [DOI] [PubMed] [Google Scholar]
- Khoo A., McLoughlin B., Cheema S., Weil R.S., Lambert C., Manji H., et al. Postinfectious brainstem encephalitis associated with SARS-CoV-2. J. Neurol. Neurosurg. Psychiatry. 2020;91(9):1013–1014. doi: 10.1136/jnnp-2020-323816. [DOI] [PubMed] [Google Scholar]
- Lazarian G., Quinquenel A., Bellal M., Siavellis J., Jacquy C., Re D., et al. Autoimmune haemolytic anaemia associated with COVID-19 infection. Br. J. Haematol. 2020;190(1):29–31. doi: 10.1111/bjh.16794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K., Pan M., Xiao Z., Xu X. Neurological manifestations of the coronavirus (SARS-CoV-2) pandemic 2019-2020. J. Neurol. Neurosurg. Psychiatr. 2020;91(6):669–670. doi: 10.1136/jnnp-2020-323177. [DOI] [PubMed] [Google Scholar]
- Liu J., Li S., Liu J., Liang B., Wang X., Wang H., et al. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine. 2020;55 doi: 10.1016/j.ebiom.2020.102763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H., Stratton C.W., Tang Y.W. Outbreak of pneumonia of unknown etiology in Wuhan, China: the mystery and the miracle. J. Med. Virol. 2020;92(4):401–402. doi: 10.1002/jmv.25678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manganotti P., Furlanis G., Ajčević M., Moras C., Bonzi L., Pesavento V., et al. Intravenous immunoglobulin response in new-onset refractory status epilepticus (NORSE) COVID-19 adult patients. J. Neurol. 2021;268(10):3569–3573. doi: 10.1007/s00415-021-10468-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matschke J., Lütgehetmann M., Hagel C., Sperhake J.P., Schröder A.S., Edler C., et al. Neuropathology of patients with COVID-19 in Germany: a post-mortem case series. Lancet Neurol. 2020;19(11):919–929. doi: 10.1016/S1474-4422(20)30308-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7) doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moideen S., Thomas R., Suresh Kumar P.N., Uvais N.A., Ul Haq Katshu M.Z. Psychosis in a patient with anti-NMDA-receptor antibodies experiencing significant stress related to COVID-19. Brain Behav. Immun. Health. 2020;7 doi: 10.1016/j.bbih.2020.100125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monti G., Giovannini G., Marudi A., Bedin R., Melegari A., Simone A.M., et al. Anti-NMDA receptor encephalitis presenting as new onset refractory status epilepticus in COVID-19. Seizure. 2020;81:18–20. doi: 10.1016/j.seizure.2020.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panariello A., Bassetti R., Radice A., Rossotti R., Puoti M., Corradin M., et al. Anti-NMDA receptor encephalitis in a psychiatric COVID-19 patient: a case report. Brain Behav. Immun. 2020;87:179–181. doi: 10.1016/j.bbi.2020.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilotto A., Odolini S., Masciocchi S., Comelli A., Volonghi I., Gazzina S., et al. Steroid-responsive encephalitis in coronavirus disease 2019. Ann. Neurol. 2020;88(2):423–427. doi: 10.1002/ana.25783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilotto A., Odolini S., Masciocchi S., Comelli A., Volonghi I., Gazzina S., et al. Steroid-responsive encephalitis in coronavirus disease 2019. Ann. Neurol. 2020;88(2):423–427. doi: 10.1002/ana.25783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilotto A., Masciocchi S., Volonghi I., Crabbio M., Magni E., De Giuli V., et al. Clinical presentation and outcomes of severe acute respiratory syndrome coronavirus 2-related encephalitis: the ENCOVID multicenter study. J. Infect. Dis. 2021;223(1):28–37. doi: 10.1093/infdis/jiaa609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzanelli C., Milano C., Canovetti S., Tagliaferri E., Turco F., Verdenelli S., et al. Autoimmune limbic encephalitis related to SARS-CoV-2 infection: case-report and review of the literature. Brain behav. Immun. Health. 2021;12 doi: 10.1016/j.bbih.2021.100210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Morales A.E., Urrutia-Osorio M., Camacho-Mendoza E., et al. Neurological manifestations temporally associated with SARS-CoV-2 infection in pediatric patients in Mexico. Childs Nerv. Syst. 2021;37(7):2305–2312. doi: 10.1007/s00381-021-05104-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Morales A.E., Urrutia-Osorio M., Camacho-Mendoza E., Rosales-Pedraza G., Dávila-Maldonado L., González-Duarte A., et al. Neurological manifestations temporally associated with SARS-CoV-2 infection in pediatric patients in Mexico. Child's Nerv. Syst. 2021:1–8. doi: 10.1007/s00381-021-05104-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toscano G., Palmerini F., Ravaglia S., Ruiz L., Invernizzi P., Cuzzoni M.G., et al. Guillain–barré syndrome associated with SARS-CoV-2. N. Engl. J. Med. 2020;382(26):2574–2576. doi: 10.1056/NEJMc2009191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M., Li T., Qiao F., Wang L., Li C., Gong Y. Coronavirus disease 2019 associated with aggressive neurological and mental abnormalities confirmed based on cerebrospinal fluid antibodies: a case report. Medicine. 2020;99(36):e21428. doi: 10.1097/MD.0000000000021428. (Baltimore) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M., Li T., Qiao F., Wang L., Li C., Gong Y. Coronavirus disease 2019 associated with aggressive neurological and mental abnormalities confirmed based on cerebrospinal fluid antibodies: a case report. Medicine. 2020;99(36) doi: 10.1097/MD.0000000000021428. (Baltimore) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambreanu L., Lightbody S., Bhandari M., Hoskote C., Kandil H., Houlihan C.F., et al. A case of limbic encephalitis associated with asymptomatic COVID-19 infection. J. Neurol. Neurosurg. Psychiatr. 2020;91(11):1229–1230. doi: 10.1136/jnnp-2020-323839. [DOI] [PubMed] [Google Scholar]
- Zambreanu L., Lightbody S., Bhandari M., Hoskote C., Kandil H., Houlihan C.F., et al. A case of limbic encephalitis associated with asymptomatic COVID-19 infection. J. Neurol. Neurosurg. Psychiatry. 2020;91(11):1229–1230. doi: 10.1136/jnnp-2020-323839. [DOI] [PubMed] [Google Scholar]
- Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.