Abstract
Purpose
The use of percutaneous cryoablation for T1b (4.1–7.0 cm) renal cell carcinoma, has not yet been widely adopted. The purpose of this study was to describe our experience in the cryoablation of stage T1b tumors with an emphasis on safety, technical results, and clinical outcomes.
Materials and Methods
A retrospective review of hospital records identified 37 patients who underwent cryoablation for T1b lesions from 2008 to 2018. Patient demographics, comorbidities, tumor characteristics, technical parameters, and outcomes were recorded and analyzed. Recurrence-free, overall, and cancer-specific survival rates were estimated using the Kaplan–Meier method.
Results
Thirty-seven patients (22 males, 15 females; mean age 66.5 ± 11.3) with 37 T1b tumors (mean diameter 47.3 ± 6.3 mm) were included. A median of 3 probes were used (range: 1–7). Angio-embolization was used in 3/37 (8.1%) and 2/37 patients (5.4%) required hydrodissection. The mean number of total cryoablation procedures for each patient was 1.5 (median 1; range: 1–4). Technical success was achieved in 88.2% of patients. Recurrence-free survival was 96.5%, 86.1%, and 62.6% at 1, 2, and 3 years respectively. Cancer-specific survival was 100% at 1, 2, and 3 years respectively. Overall survival was 96.7%, 91.8%, and 77.6% at 1, 2, and 3 years respectively. Complications classified as CIRSE grade 2 or higher occurred in 6/37 (16.2%) patients.
Conclusion
T1b cryoablation is associated with high rates of technical success, excellent cancer-specific survival, and an acceptable safety profile.
Level of Evidence
Level 4, Case Series.
Keywords: Ablation, Cryoablation, Renal cell carcinoma, Kidney
Introduction
Widespread reliance on cross-sectional imaging in the diagnosis of intra-abdominal pathology has led to a rise in the global incidence of localized renal cell carcinoma (RCC) in recent decades [1–4]. As such, an estimated 65,340 new cases of RCC were diagnosed in the United States in 2018 alone [5]. While partial nephrectomy (PN) remains the standard of care for patients with early-stage, non-centralized lesions, there is an increasing role for ablative techniques in the management of RCC, particularly in patients with multiple comorbidities or those who wish to avoid traditional extirpative surgery [6, 7]. Standard image-guided thermal ablation techniques include radiofrequency ablation (RFA), microwave ablation (MWA), and cryoablation, all of which have been shown to achieve good oncologic outcomes in patients with smaller, T1a tumors (≤ 4.0 cm) [8–12]. This is reflected in recent guidelines set by the National Comprehensive Cancer Network (NCCN) and American Urological Association (AUA), which present thermal ablation as a viable therapy for localized T1a tumors [6, 7].
Use of percutaneous ablation for larger tumors, such as T1b (4.1–7.0 cm), however, has not yet been adopted by clinical practice guidelines. This is, in part, due to concern that ablation of higher T stage RCC may be associated with a greater likelihood of recurrence [6, 7, 13–15]. Yet, early experience suggests percutaneous cryoablation may be an option for the treatment of T1b lesions in certain settings. In a subset analysis, cryoablation for T1b tumors achieved recurrence-free and metastasis-free survival comparable to that of PN [8]. Studies including both T1a and T1b lesions have shown rates of tumor recurrence at short-term follow-up are low and not necessarily related to tumor size [16, 17]. Furthermore, cryoablation of T1b tumors does not appear to be limited by technical feasibility [18]. In fact, compared to percutaneous RFA, cryoablation may attain a higher rate of tumor coverage by the ablative zone resulting in lower rates of retreatment [19]. Although complications following cryoablation of larger tumors remain a concern [16, 20], early experience suggests that ablation of T1b tumors is safe in the setting of thorough periprocedural care [18], a result which was corroborated in a recent case series [21]. Despite these promising results, further studies are necessary to establish the potential of percutaneous cryoablation in the treatment of stage T1b RCC. The purpose of this study was to describe our experience in the percutaneous cryoablation of stage T1b RCC with an emphasis on safety, technical results, and clinical outcomes.
Materials and Methods
Patient Population and Data Collection
This single-center retrospective review was approved by an Institutional Review Board (IRB) and was compliant with the Health Insurance Portability and Accountability Act (HIPAA). Patient informed consent was not required given the retrospective nature of this study. A review of our radiology information system (RIS) identified 37 adult patients with 37 RCC lesions measuring 4.1–7.0 cm who underwent percutaneous cryoablation from 2008 to 2018. Patient demographics (age, sex, race, and body mass index) and the presence of comorbid conditions were identified. Multiple comorbidities were summarized for each patient using the Charlson Comorbidity Index with age.
Tumor Classification
For all tumors, pre-operative multiplanar imaging was reviewed to evaluate anatomical features including size, location, tumor geometry, nearness to collecting system, and involvement of the renal sinus. Based on these characteristics, tumor complexity was graded according to both R.E.N.A.L. (radius, exophytic/endophytic properties, nearness to the collecting system, anterior/posterior, location relative to the polar lines) nephrometry scores [22] and PADUA scores (Preoperative Aspects and Dimensions Used for an Anatomical score) [23]. R.E.N.A.L. nephrometry scores were used to stratify tumors to low (score ≤ 6), intermediate (score 7–9), and high complexity groups (score > 9). PADUA scores were used similarly to stratify tumors to low (score 6–7), intermediate (score 8–9), and high complexity groups (score > 9).
Technical Aspects
Pre-procedural evaluation and planning was carried out by a combination of interventional radiologists and urologists with accompanying input from a multidisciplinary team when indicated. In all instances, percutaneous cryoablation was performed by a board-certified interventional radiologist. Routine prophylactic antibiotics were administered, and anticoagulation was held prior to the procedure according to published guidelines [24, 25]. Sites were prepared and draped in standard sterile fashion following mass localization and identification of the percutaneous route by preliminary computed tomography (CT) scans, cryoablation probes were advanced under CT guidance. All cryoprobes utilized in this series relied on rapidly-expanding, highly-pressurized argon gas to induce tumor freezing and subsequent necrosis. With cryoablation probes in place, patients typically underwent two freeze thaw cycles consisting of a 10-minute initial freeze with an 8-minute passive thaw followed by an additional 10-minute freeze followed by active thaw. Intermittent CT scans were performed during the freeze cycles in order to monitor the progression of the ablation. In this series, the need for pre-ablation angio-embolization was based on lesion size and/or the suggestion of a highly vascular RCC on pre-procedural imaging per review of the notes. In the instance that tissue displacement was required in order to avoid injury to adjacent structures either hydrodissection or pneumodissection was performed. Confirmatory biopsy was performed concurrently with cryoablation when possible [26].
The number and types of ablation probes required for each procedure was recorded. The mean number of total cryoablation procedures per patient was recorded. Follow-up consisted of a clinic visit approximately 2–3 weeks after the procedure to evaluate for early complications. In general, imaging of the ablation zone was performed 3 months after the procedure then again 9 months later (12 months post-procedure). Patients subsequently underwent yearly surveillance imaging. Follow-up imaging intervals were shortened if there was suspicion for persistent or new disease. Contrast-enhanced CT was the preferred modality for follow-up imaging, however the use of other modalities, such as magnetic resonance imaging (MRI) or contrast-enhanced ultrasound (CEUS), was dictated by specific patient characteristics such as allergies to iodinated contrast or compromised renal function. Post-procedural measures included technical success and local recurrence. Technical success was defined by coverage of the entire lesion with the ice ball and the absence of new contrast enhancement and/or tumor enlargement within 3 months of ablation. Staged ablation procedures were considered technically successful if planned at or before the index procedure, in which case technical success and local recurrence were measured from the last staged procedure. Local recurrence was defined as new contrast enhancement within the ablation zone or enlargement of the ablated tumor greater than or equal to 3 months after the procedure. Any complications that occurred from the time of the procedure until last known follow-up or death were identified. These were graded according to the Cardiovascular and Interventional Radiological Society of Europe (CIRSE) classification system for complications [27]. Other parameters such as reasons for interventional radiology referral and relevant laboratory values (pre- and post-procedural) were collected. Oncologic outcomes including recurrence-free, overall, and cancer-specific survival were assessed for all patients. Oncologic outcomes were measured from the index procedure if a single ablation session was performed or from the date of the last procedure if a staged ablation was performed.
Statistical Analysis
Continuous variables were summarized as means and standard deviations or medians with ranges. Categorical variables were expressed as frequencies and percentages. Univariate analyses were performed to assess whether tumor characteristics, patient demographics, comorbidities, or technical aspects (probe number, use of hydrodissection or embolization) correlated with observed outcomes. Due to small size, the Kruskal–Wallis test and Fisher’s exact test were used to compare continuous and categorical variables, respectively. Any variables with a p value ≤ 0.1 on univariate analysis were subsequently included in a multivariable logistic regression. Odds ratios (OR) with two-sided 95% confidence intervals (CI) were provided for any associations (p ≤ 0.05) from the stepwise selection method. Cancer-specific, recurrence-free, and overall survival were estimated at 1, 2, and 3 years using the Kaplan–Meier (KM) method. Time to event was defined from the date of procedure to the date of event or last follow-up. Subjects were censored if no event occurred or if lost to follow-up. A p-value of ≤ 0.05 was considered to indicate statistical significance. Statistical analyses were performed using the SAS software package, version 9.4 (SAS, Cary, North Carolina).
Results
Thirty-seven patients were treated with percutaneous cryoablation between 2008–2018. The decision to undergo cryoablation vs PN was based on poor surgical candidacy in 30/37 cases (81%) and patient preference in 7/37 cases (19%). Data regarding technical success and local recurrence were available for 34/37 (91.9%) patients. Detailed demographic and comorbidity data are summarized in Table 1. Tumor characteristics including renal nephrometry scores are presented in Table 2.
Table 1.
Summary of patient demographics (N = 37)
| Age | |
| Mean ± SD | 66.5 ± 11.3 |
| Median (min–max) | 65 (39.3–90.7) |
| Body mass index | |
| Mean ± SD | 34.8 ± 8.8 |
| Median (min–max) | 34 (15.5–54.3) |
| Comorbility index | |
| Mean ± SD | 7.1 ± 2.4 |
| Median (min–max) | 7 (2–12) |
| Sex n (%) | |
| Male | 22 (59.5) |
| Female | 15 (40.5) |
| Race n (%) | |
| White | 23 (62.2) |
| Black | 9 (24.3) |
| Asian | 2 (5.4) |
| Other | 3 (8.1) |
| Chronic kidney disease stage n (%) | |
| < 3 | 17 (45.9) |
| 3A | 8 (21.6) |
| 3B | 8 (21.6) |
| 4 | 1 (2.7) |
| 5 | 3 (8.1) |
| Coronary artery disease n (%) | |
| No | 25 (67.6) |
| Yes | 12 (32.4) |
| Diabetes mellitus n (%) | |
| No | 19 (51.4) |
| Yes | 18 (48.6) |
| Hypertension n (%) | |
| No | 8 (21.6) |
| Yes | 29 (78.4) |
| Heart failure n (%) | |
| No | 34 (91.9) |
| Yes | 3 (8.1) |
Table 2.
Summary of tumor characteristics (N = 37)
| Tumor size (mm) | |
| Mean ± SD | 47.3 ± 6.3 |
| Median (min–max) | 45 (41–67) |
| Total RENAL score | |
| Low n (%) | 10 (27.0) |
| Mean ± SD | 5.6 ± 0.5 |
| Median (min–max) | 6 (5–6) |
| Intermediate n (%) | 21 (56.8) |
| Mean ± SD | 8.1 ± 0.8 |
| Median (min–max) | 8 (7–9) |
| High n (%) | 6 (16.2) |
| Mean ± SD | 10.3 ± 0.5 |
| Median (min–max) | 10 (10–11) |
| Overall | |
| Mean ± SD | 7.8 ± 1.7 |
| Median (min–max) | 8 (5–11) |
| Total PADUA Score* | |
| Low n (%) | 3 (8.1) |
| Mean ± SD | 7 ± 0 |
| Median (min–max) | 7 (7–7) |
| Intermediate n (%) | 17 (45.9) |
| Mean ± SD | 8.5 ± 0.5 |
| Median (min–max) | 8 (8–9) |
| High n (%) | 17 (45.9) |
| Mean ± SD | 10.9 ± 1.0 |
| Median (min–max) | 11 (10–13) |
| Overall | |
| Mean ± SD | 9.5 ± 1.6 |
| Median (min–max) | 9 (7–13) |
| Stage n (%) | |
| T1b | 37 (100) |
| Anterior/posterior n (%) | |
| Anterior | 18 (48.6) |
| Posterior | 19 (51.4) |
| Histologic type n (%) | |
| Clear cell | 12 (32.4) |
| Papillary | 5 (13.5) |
| Unclassified | 6 (16.2) |
| No biopsy | 14 (37.8) |
Technical success was achieved in 88.2% (30/34) of patients (Fig. 1). Among the patients not achieving technical success, one underwent repeat cryoablation for tumor enhancement noted 2 months after the index procedure. Worsening enhancement was noted approximately 6 months after the second ablation and, due to the patient’s advanced age and comorbidities, a multidisciplinary decision was made to proceed with chemotherapy. The patient passed away due to respiratory failure 1 year later. A separate patient was noted to have residual enhancement on CEUS approximately 3-months after the index procedure. This patient underwent repeat ablation; however, the residual disease persisted after the second ablation. The patient subsequently decided to proceed with radical nephrectomy. A third patient was noted to have residual enhancement on CT 2 months after the index procedure and was treated with repeat cryoablation. Approximately 14 months after the second procedure, follow-up imaging showed persistent tumor with new renal vein thrombosis. Given the patient’s advanced age and poor surgical candidacy, a multidisciplinary decision was made to palliate the patient’s hematuria with trans-arterial embolization. This patient passed away 3 months after embolization. In a fourth patient, marginal enhancement was noted on follow-up CT. This was treated with repeat cryoablation and the patient has completed four months of follow-up without evidence of residual disease.
Fig. 1.
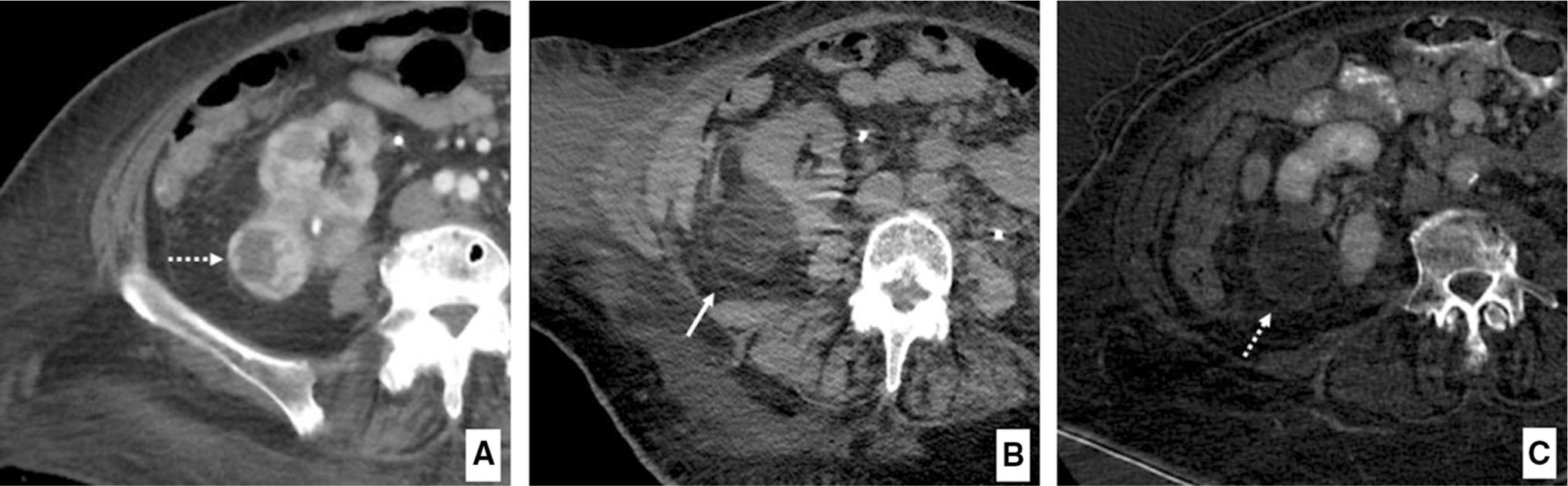
Example of technically successful T1b cryoablation as demonstrated by (A) pre-ablation axial contrast-enhanced CT image depicting exophytic T1b RCC (dashed arrow) of the right kidney (maximum diameter 46 mm) (B) ablation zone immediately post-procedure (solid white arrow) demonstrating ice ball with complete tumor coverage and appropriate margin (C) subtracted CT image showing ablation bed (dashed arrow) with no residual enhancement at 3-month follow-up
The median number of probes used was 3, (range 1–7). While it is not common practice to use a single probe for tumors ≥ 4 cm, this occurred in one instance as the patient was not able to tolerate additional probe insertion during the index procedure. Thus, the patient was scheduled for a second procedure as part of a staged ablation. The second ablation was technically successful and the patient has now undergone 33 months of follow-up with no evidence of residual or recurrent disease. Information regarding probe brand was available for 16/37 (43.2%) patients. Probes used included IceFORCE (n = 6, 2.1 mm, Galil Medical Inc., Arden Hills, Minnesota), combined IceFORCE and IcePearl probes (n = 4, 2.1 mm, Galil Medical Inc.), Endocare Perc-24 probes (n = 3, 2.4 mm, Endocare, Austin, Texas), IceSphere probes (n = 2, 1.5 mm, Galil Medical Inc.), and combined IceSphere and IceRod probes (n = 1, 1.5 mm, Galil Medical Inc.). In general, the decision to use specific cryoablation systems and probes was left to the discretion of individual providers. The mean number of total cryoablation procedures per patient was 1.5, (median 1; range 1–4). Pre-ablation biopsy was performed in 62.2% (n = 23) of patients (Table 2). Angio-embolization was used in 3 (8.1%) patients prior to ablation. Two patients (5.4%) required hydrodissection.
Mean follow-up was 26.4 ± 28 months (median: 16.4, range: 0.03–102.6). Among the 34 patients for whom local recurrence could be adequately assessed, 8 patients (23.5%) experienced recurrence, at a mean of 26.5 ± 14.9 months (median: 25.2, range: 4.4–53). Recurrence-free survival was 96.5%, 86.1%, and 62.6% at 1, 2, and 3 years respectively (Fig. 2). None of the evaluated clinical variables including total nephrometry scores or their component parts were associated with an increased risk of local recurrence (p > 0.05), and no variables met the significance threshold for inclusion in multivariable analysis. Cancer-specific survival was 100% at 1, 2, and 3 years respectively. Overall survival was 96.7%, 91.8%, and 77.6% at 1, 2, and 3 years respectively. Charlson Comorbidity Index was the only variable associated with overall survival on logistic regression analysis (OR = 1.55, 95% CI 1.01–2.39).
Fig. 2.
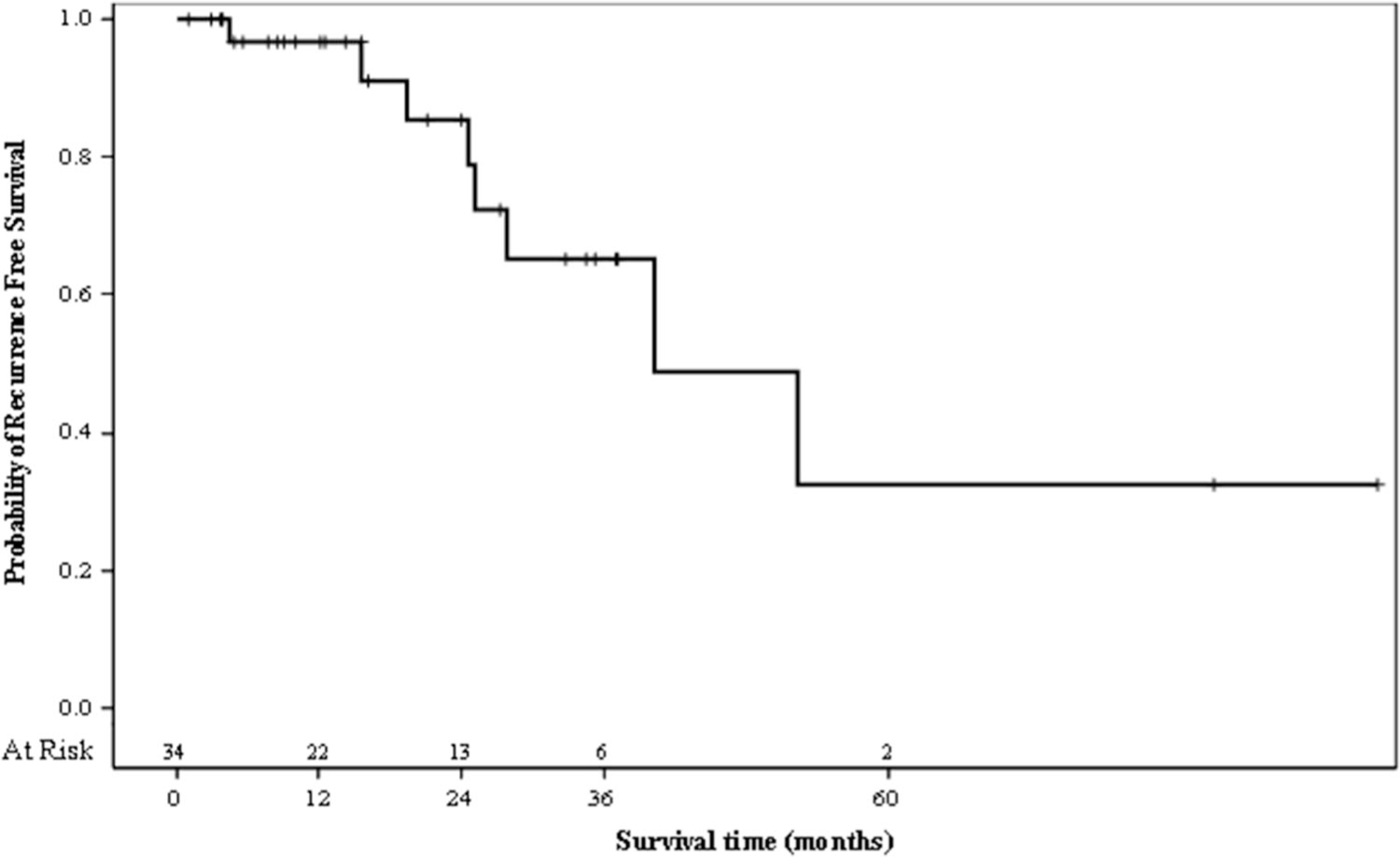
Kaplan Meier curve demonstrating recurrence-free survival of 100% at 0 months (at risk = 34), 96.5% at 12 months (at risk = 22), 86.1% at 24 months (at risk = 13), 62.6% at 36 months (at risk = 6), and 37.6% at 60 months (at risk = 2)
CIRSE grade 1 complications occurred in 11 patients (29.7%) including perinephric hematoma (n = 9) (Fig. 3), vasovagal episode (n = 1), and flank pain (n = 1). Complications classified as CIRSE grade ≥ 2 occurred in 6 (16.2%) patients (Table 3). One patient experienced post-procedural hematuria which resolved spontaneously following overnight observation (CIRSE grade 2). One patient developed a persistent abscess which was managed with percutaneous drainage (CIRSE grade 3). A single instance of pseudoaneurysm with arterio-venous fistula was recognized approximately 1 day after ablation and successfully treated with coil embolization (CIRSE grade 3). Two patients experienced short hospital stays of ≤ 48 h, one for urinary tract infection with altered mental status, and another for unexplained leukocytosis. Both resolved following management with appropriate antibiotics (CIRSE grade 3). One patient developed an abscess with colo-ureteral fistula approximately two weeks after the initial procedure, ultimately requiring surgical intervention (CIRSE grade 4). No significant change in creatinine was seen after ablation (p = 0.85). Among tested clinical variables including both total RENAL and PADUA scores and their component parts, only endophytic tumors (p = 0.005), involvement of the renal sinus (p = 0.045), and displacement or infiltration of the collecting system (p = 0.022) were associated with an increased risk of complications. On multivariable analysis, tumors with endophytic/mixed as opposed to exophytic location (OR = 12.76; 95% CI 2.10–77.4) and tumors involving the renal sinus (OR = 6.33; 95% CI 1.08–37.3) were independently associated with complications.
Fig. 3.
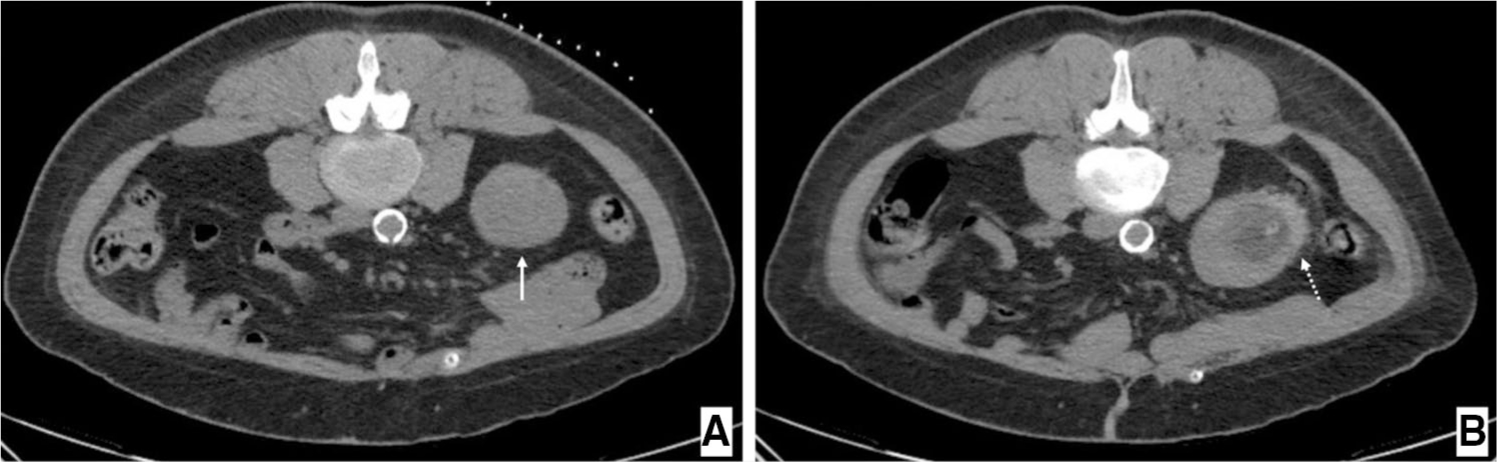
Axial CT images depicting (A) patient in prone position immediately prior to cryoablation of a 4.8 cm T1b renal tumor (solid white arrow) (B) ablation bed immediately after procedure surrounded by a small perinephric hematoma (dashed white arrow)
Table 3.
Complications and laboratory values, all patients (N = 37)
| Complications n (%) | |
| None | 20 (54.1) |
| ≤ 24 h | 14 (37.8) |
| ≤ 30 days | 2 (5.4) |
| > 30 days | 1 (2.7) |
| CIRSE Grade n (%) | |
| 1 | 11 (29.7) |
| 2 | 1 (2.7) |
| 3 | 4 (10.8) |
| 4 | 1 (2.7) |
| 5 | 0 |
| 6 | 0 |
| Creatinine before procedure | |
| Mean ± SD | 1.6 ± 1.3 |
| Median (min–max) | 1 (0.6–6.4) |
| INR before procedure | |
| Mean ± SD | 1.1 ± 0.3 |
| Median (min–max) | 1 (0.9–2.4) |
| Platelets before procedure | |
| Mean ± SD | 207.6 ± 84.8 |
| Median (min–max) | 189 (47–374) |
| GFR before procedure | |
| Mean ± SD | 50.2 ± 20.8 |
| Median (min–max) | 53 (8–126) |
| Creatinine after procedure | |
| Mean ± SD | 1.8 ± 1.7 |
| Median (min–max) | 1 (0.7–7.9) |
| GFR after procedure | |
| Mean ± SD | 47.6 ± 17.8 |
| Median (min–max) | 56 (6–70) |
Discussion
Cryoablation of T1b RCC presents unique technical challenges to the interventional radiologist [18]. Despite this, the present study indicates percutaneous cryoablation of these tumors can be achieved with a high rate of technical success (88.2%). Our results compare favorably to those of Hebbadj et al. [21] who reported the same measure (which authors termed “technical efficacy”) as 87.5% in a smaller series of 27 patients with T1b tumors. Moreover, Atwell et al. [18] also demonstrated excellent rates of technical success in their series of 46 T1b tumors treated with percutaneous cryoablation. These overall high rates of technical success may be explained by a number of factors. First, unlike other forms of thermal ablation, cryoablation permits real-time visualization of ice ball formation within the ablative zone. This enables the practitioner to monitor for complete coverage of the target zone with adequate margins [28]. Second, cryoablation also enables simultaneous use of multiple probes to create spherical, ellipsoid, or cylindrical ablation zones [29]. Once in place, probes work synergistically and can be manipulated independently to ensure large, irregular tumors are adequately treated while minimizing damage to nearby structures [30]. Taken together, these advantages suggest percutaneous cryoablation may be uniquely suited for the treatment of T1b tumors from a technical standpoint. To this end, a recent multi-center review comparing outcomes following RFA (n = 23) vs cryoablation (n = 23) for T1b tumors noted cryoablation was associated with better initial tumor coverage by the ablative zone and fewer instances of retreatment than RFA [19].
Current guidelines note that thermal ablation of larger tumors should be utilized with caution due to higher rates of local recurrence compared to partial nephrectomy. These guidelines, however, are based largely on experience with laparoscopic cryoablation and RFA rather than percutaneous cryoablation [12–14]. In one of the largest retrospective analyses of percutaneous cryoablation of T1b tumors to date, authors demonstrated favorable local tumor control rates with an estimated progression-free survival of 96.4% at 1-, 3- and 5-year follow-ups [18]. A more recent case series reported rates of local tumor control of 82.6% and 60.3% at 1 and 3 years respectively [21]. These authors defined local tumor control as absence of contrast enhancement and ablation area enlargement at imaging follow-up ≥ 6 months after the initial procedure. Similarly, 8 patients (23.5%) in our cohort experienced local recurrence with recurrence-free survival of 96.5%, 86.1%, and 62.6% at 1, 2, and 3 years respectively. The results of case series specific to T1b cryoablation, which now include a total of 110 patients, suggest that local recurrence following percutaneous cryoablation remains an important consideration in patients with T1b lesions. Despite this, the association between tumor size and local recurrence remains unclear [16, 17, 21, 31]. In our univariate analysis, none of the tested variables were associated with local recurrence, including tumor size. Nevertheless, local recurrence rates may not diminish the clinical utility of cryoablation for T1b tumors, given that repeat ablation following recurrence has been shown to be highly efficacious. For example, in a recent meta-analysis comparing thermal ablation to partial nephrectomy, any significant differences in recurrence free survival between surgery and thermal ablation disappeared when multiple ablations were considered [32]. Indeed, this may provide an explanation for the excellent cancer-specific survival noted in the present series (100% at 5 years) despite the rates of recurrence, as a majority of recurrences (63%) were treated with repeat ablation.
One major benefit of percutaneous cryoablation versus laparoscopic or traditional surgery appears to be a lower rate of complications [33]. It is important to assess whether these benefits persist in the treatment of larger tumors, as some studies have indicated complication rates increase with greater tumor size and number of probes used [16, 20]. In our cohort, we had an overall low rate of complications CIRSE grade 2 or above (16.2%). Complications were not associated with tumor size or the number of cryoablation probes on univariate analysis. The only variables associated with complications included: endophytic/mixed tumors (p = 0.005), involvement of renal sinus (p = 0.045) and displacement/infiltration of the collecting system (p = 0.022). On multivariable analysis, tumors with endophytic/mixed as opposed to exophytic location were greater than 12 times more likely to be associated with complications, OR = 12.7 (95% CI 2.10–77.4, p = 0.006). In addition, patients with tumors involving the renal sinus were over 6 times more likely to experience an adverse event, OR = 6.33 (1.08–37.3, p = 0.041). This result is similar to other studies which have identified centrally-located tumors to be associated with higher rates of complications, as these tumors may require more invasive probe placement and longer duration of freezing necessary to achieve tumor control [20, 34]. These findings may help guide patient selection and the need for close periprocedural monitoring in the future. Overall, the rate of clinically significant complications (CIRSE grade ≥ 2) observed in this study was similar to those reported by Hebbadj et al. and Atwell et al. in their experience with T1b cryoablation (11.1% and 15.2%, respectively). It should be noted that in both these instances major complications were reported according to Clavien-Dindo classification rather than the CIRSE classification [18, 21]. In the present study, CIRSE grade 1 complications, while not uncommon (29.7%), were largely limited to clinically insignificant perinephric hematomas (Fig. 3). Notably, a small degree of self-resolving perinephric bleeding is to be expected following cryoablation, and some authors do not consider it to be a true complication [18].
As the data presented here represents a decade of experience with percutaneous cryoablation of T1b RCC, it is important to acknowledge the ways in which practice patterns at our institution have changed over time. When this service line was first introduced, providers were chiefly concerned with the risk of complications associated with ablating larger lesions. This led to a conservative approach in which some providers preferred to stage ablations rather than use a large number of probes in the same session or employ adjunctive measures such as hydrodissection or embolization. We recognize that this is not a standard practice at other institutions. This factor contributed to the mean number of procedures performed in this cohort being > 1. Improved comfort and experience in treating larger lesions has enabled a more aggressive approach in our current practice. For example, in an aforementioned procedure performed earlier in our experience, a 4.2 cm RCC was treated in two separate ablation sessions with a single probe at each session. More recently, in 2018, a 6.7 cm RCC was treated using 7 probes in a single session. Regardless, it is possible that the more conservative approaches of our early practice, including the low utilization of displacement maneuvers intra-procedurally, could have resulted in under-sized ablation sizes thereby leading to recurrence. Despite this, recurrence rates were overall low, especially within the first two years after ablation, and similar to prior series. Nevertheless, the size and heterogeneity of the current cohort limits further analysis along these lines.
This study is not without limitations. First, probe brand was not systematically recorded within the electronic medical record, and therefore it was not possible to reliably assess the impact of specific probe type on measured outcomes. Second, the retrospective nature of this study did not allow us to control for important variables such as variations in technique that may exist between different providers at our institution or changing practice patterns over time. Third, in the literature specific to T1b ablation, definitions of technical success are discordant [18, 21]. Our definition aligns more closely with that offered by Atwell et al. although the present study allows for planned staged procedures to be considered technically successful [21]. This discordance highlights the need for outcomes to be classified in a more uniform manner as future experience with T1b RCC ablation is elaborated. Fourth, despite the fact that this study adds an additional 37 patients to the literature and is comparable in size to other studies that focused specifically on T1b RCC cryoablation [18, 21], it remains limited by small sample size with relatively few patients having follow-up > 60 mo. Fifth, this study was also limited by the fact that pre-procedural confirmatory biopsy was not performed universally, although it was carried out in a majority of patients (23/37, 62.2%). Standard practice at our institution involves placement of ablation probes prior to introduction of the biopsy needle. If bleeding is noted at this stage, the ablation may proceed without biopsy in order to minimize blood loss. Additionally, the use of multiple probes for large, irregular tumors may obscure biopsy needles on CT guidance, lowering the success rate of biopsy. Although histologic confirmation is in general an important component of interventional oncology, the optimal timing of percutaneous biopsy in the setting of thermal ablation has yet to be determined and remains an area of ongoing research [26].
Future directions include data collection in a multi-institutional, prospective fashion which would allow for more robust analysis of oncologic outcomes. Once sufficient data are available, a meta-analysis is likely to lend additional insight into the efficacy of percutaneous cryoablation in the treatment of higher T stage RCC. Ultimately, however, larger comparative effectiveness studies are needed in order to assess the relative merit of thermal ablation vs PN in the treatment of T1b RCC.
Conclusion
Percutaneous cryoablation is a viable option for T1b RCC with low rates of high grade complications. Local recurrence remains a concern in the cryoablation these tumors, however high rates of technical success may be achieved with excellent cancer-specific survival at 1, 2, and 3 years.
Acknowledgements
The authors would like to acknowledge the assistance of Dr. Yufeng Li, Ph.D, from the University of Alabama at Birmingham in performing the statistics.
Footnotes
Compliance with Ethical Standards
Conflict of interest Dr. Gunn is a consultant and speaker for BTG International, and Dr. Abdel Aal is a consultant for Abbott Laboratories, Baxter Intl., W.L. Gore & Associates, C.R. Bard Inc., Sirtex Medical, and Boston Scientific Corporation. None of the other authors report a potential conflict of interest associated with this work and there is no grant support associated with this work.
Ethical Approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study formal consent is not required. This study was approved by the Institutional Review Board (IRB) of the University of Alabama at Birmingham.
Human and Animal Rights This article does not contain any studies with animals performed by any of the authors.
Informed Consent For this type of study informed consent is not required.
References
- 1.Znaor A, Lortet-Tieulent J, Laversanne M, Jemal A, Bray F. International variations and trends in renal cell carcinoma incidence and mortality. Eur Urol. 2015;67(3):519–30. [DOI] [PubMed] [Google Scholar]
- 2.Sun M, Thuret R, Abdollah F, Lughezzani G, Schmitges J, Tian Z, et al. Age-adjusted incidence, mortality, and survival rates of stage-specific renal cell carcinoma in North America: a trend analysis. Eur Urol. 2011;59(1):135–41. [DOI] [PubMed] [Google Scholar]
- 3.Capitanio U, Montorsi F. Renal cancer. Lancet. 2016;387(10021):894–906. [DOI] [PubMed] [Google Scholar]
- 4.Hollingsworth JM, Miller DC, Daignault S, Hollenbeck BK. Rising incidence of small renal masses: a need to reassess treatment effect. J Natl Cancer Inst. 2006;98(18):1331–4. [DOI] [PubMed] [Google Scholar]
- 5.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA-Cancer J Clin. 2018;68(1):7–30. [DOI] [PubMed] [Google Scholar]
- 6.Kidney Cancer. In: NCCN Clinical Practice Guidelines in Oncology. National Comprehensive Cancer Network. 2018. https://www.nccn.org/professionals/physician_gls/pdf/kidney.pdf. Accessed 21 Dec 2018. [Google Scholar]
- 7.Campbell S, Uzzo RG, Allaf ME, Bass EB, Cadeddu JA, Chang A, et al. Renal mass and localized renal cancer: AUA guideline. J Urol. 2017;198(3):520–9. [DOI] [PubMed] [Google Scholar]
- 8.Thompson RH, Atwell T, Schmit G, Lohse CM, Kurup AN, Weisbrod A, et al. Comparison of partial nephrectomy and percutaneous ablation for cT1 renal masses. Eur Urol. 2015;67(2):252–9. [DOI] [PubMed] [Google Scholar]
- 9.Takaki H, Yamakado K, Soga N, Arima K, Nakatsuka A, Kashima M, et al. Midterm results of radiofrequency ablation versus nephrectomy for T1a renal cell carcinoma. Jpn J Radiol. 2010;28(6):460–8. [DOI] [PubMed] [Google Scholar]
- 10.Yang Y, Chen S, Chen F, Zhu K, Deng Q, Luo L, et al. Outcome of radiofrequency ablation over partial nephrectomy for small renal mass (< 4 cm): a systematic review and meta-analysis. Int J Clin Exp Med. 2015;8(11):20670. [PMC free article] [PubMed] [Google Scholar]
- 11.Dandrea D, Shariat SF, Klatte T. Update on ablative therapies of renal tumors. Curr Opin Urol. 2016;26(5):410–6. [DOI] [PubMed] [Google Scholar]
- 12.Moreland AJ, Ziemlewicz TJ, Best SL, Hinshaw JL, Lubner MG, Alexander ML, et al. High-powered microwave ablation of t1a renal cell carcinoma: safety and initial clinical evaluation. J Endourol. 2014;28(9):1046–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tanagho YS, Roytman TM, Bhayani SB, Kim EH, Benway BM, Gardner MW, et al. Laparoscopic cryoablation of renal masses: single-center long-term experience. Urology. 2012;80(2):307–14. [DOI] [PubMed] [Google Scholar]
- 14.Gervais DA, McGovern FJ, Arellano RS, McDougal WS, Mueller PR. Radiofrequency ablation of renal cell carcinoma: part 1, Indications, results, and role in patient management over a 6-year period and ablation of 100 tumors. AJR Am J Roentgenol. 2005;185(1):64–71. [DOI] [PubMed] [Google Scholar]
- 15.Best SL, Park SK, Yaacoub RF, Olweny EO, Tan YK, Trimmer C, et al. Long-term outcomes of renal tumor radio frequency ablation stratified by tumor diameter: size matters. J Urol. 2012;187(4):1183–9. [DOI] [PubMed] [Google Scholar]
- 16.Schmit GD, Thompson RH, Kurup AN, Weisbrod AJ, Carter RE, Callstrom MR, et al. Percutaneous cryoablation of solitary sporadic renal cell carcinomas. BJU Int. 2012;110(11b):E526–31. [DOI] [PubMed] [Google Scholar]
- 17.Schmit GD, Atwell TD, Callstrom MR, Farrell MA, Leibovich BC, Patterson DE, et al. Percutaneous cryoablation of renal masses ≥ 3 cm: efficacy and safety in treatment of 108 patients. J Endourol. 2010;24(8):1255–62. [DOI] [PubMed] [Google Scholar]
- 18.Atwell TD, Vlaminck JJ, Boorjian SA, Kurup AN, Callstrom MR, Weisbrod AJ, et al. Percutaneous cryoablation of stage T1b renal cell carcinoma: technique considerations, safety, and local tumor control. J Vasc Interv Radiol. 2015;26(6):792–9. [DOI] [PubMed] [Google Scholar]
- 19.Hasegawa T, Yamanaka T, Gobara H, Miyazaki M, Takaki H, Sato Y, et al. Radiofrequency ablation versus cryoablation for T1b renal cell carcinoma: a multi-center study. Jpn J Radiol. 2018;36(9):551–8. [DOI] [PubMed] [Google Scholar]
- 20.Atwell TD, Carter RE, Schmit GD, Carr CM, Boorjian SA, Curry TB, et al. Complications following 573 percutaneous renal radiofrequency and cryoablation procedures. J Vasc Interv Radiol. 2012;23(1):48–54. [DOI] [PubMed] [Google Scholar]
- 21.Hebbadj S, Cazzato RL, Garnon J, Shaygi B, Buy X, Tsoumakidou G, et al. Safety considerations and local tumor control following percutaneous image-guided cryoablation of T1b renal tumors. Cardiovac Intervent Radiol. 2018;41(3):449–58. [DOI] [PubMed] [Google Scholar]
- 22.Kutikov A, Uzzo RG. The RENAL nephrometry score: a comprehensive standardized system for quantitating renal tumor size, location and depth. J Urol. 2009;182(3):844–53. [DOI] [PubMed] [Google Scholar]
- 23.Ficarra V, Novara G, Secco S, Macchi V, Porzionato A, De Caro R, et al. Preoperative aspects and dimensions used for an anatomical (PADUA) classification of renal tumours in patients who are candidates for nephron-sparing surgery. Eur Urol. 2009;56(5):786–93. [DOI] [PubMed] [Google Scholar]
- 24.Venkatesan AM, Kundu S, Sacks D, Wallace MJ, Wojak JC, Rose SC, et al. Practice guideline for adult antibiotic prophylaxis during vascular and interventional radiology procedures. J Vasc Interv Radiol. 2010;21(11):1611–30. [DOI] [PubMed] [Google Scholar]
- 25.Patel IJ, Davidson JC, Nikolic B, Salazar GM, Schwartzberg MS, Walker TG, et al. Consensus guidelines for periprocedural management of coagulation status and hemostasis risk in percutaneous image-guided interventions. J Vasc Interv Radiol. 2012;23(6):727–36. [DOI] [PubMed] [Google Scholar]
- 26.Chung MS, Maxwell AW, Wang LJ, Mayo-Smith WW, Dupuy DE. Should renal mass biopsy be performed prior to or concomitantly with thermal ablation? J Vasc Interv Radiol. 2018;29(9):1240–4. [DOI] [PubMed] [Google Scholar]
- 27.Filippiadis DK, Binkert C, Pellerin O, Hoffmann RT, Krajina A, Pereira PL. Cirse quality assurance document and standards for classification of complications: the cirse classification system. Cardiovasc Interv Radiol. 2017;40(8):1141–6. [DOI] [PubMed] [Google Scholar]
- 28.Higgins LJ, Hong K. Renal ablation techniques: state of the art. AJR Am J Roentgenol. 2015;205(4):735–41. [DOI] [PubMed] [Google Scholar]
- 29.Patel N, King AJ, Breen DJ. Percutaneous image-guided cryoablation of small renal masses. Abdom Radiol (NY). 2016;41(4):754–66. [DOI] [PubMed] [Google Scholar]
- 30.Ahmed M, Brace CL, Lee FT Jr, Goldberg SN. Principles of and advances in percutaneous ablation. Radiology. 2011;258(2):351–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maxwell AW, Baird GL, Iannuccilli JD, Mayo-Smith WW, Dupuy DE. Renal cell carcinoma: comparison of RENAL nephrometry and PADUA scores with maximum tumor diameter for prediction of local recurrence after thermal ablation. Radiology. 2017;283(2):590–7. [DOI] [PubMed] [Google Scholar]
- 32.Pierorazio PM, Johnson MH, Patel HD, Sozio SM, Sharma R, Iyoha E, et al. Management of renal masses and localized renal cancer: systematic review and meta-analysis. J Urol. 2016;196(4):989–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taylor BL, Stavropoulos SW, Guzzo TJ. Ablative therapy for small renal masses. Urol Clin North Am. 2017;44(2):223–31. [DOI] [PubMed] [Google Scholar]
- 34.Schmit GD, Schenck LA, Thompson RH, Boorjian SA, Kurup AN, Weisbrod AJ, et al. Predicting renal cryoablation complications: new risk score based on tumor size and location and patient history. Radiology. 2014;272(3):903–10. [DOI] [PubMed] [Google Scholar]


