Abstract
Hepatitis B virus (HBV) infection represents a major public health problem infecting approximately 400 million people worldwide. Despite the availability of a preventive vaccine and anti-viral therapies, chronic HBV infection remains a major health issue because it increases the risk of developing liver cirrhosis and hepatocellular carcinoma (HCC). The lack of a relevant in vitro model for the study of the molecular mechanisms that drive HBV replication and latency, as well as HBV-related carcinogenesis, has been one of the major obstacles to the development of curative strategies. Here, we propose the use of human liver organoids as a platform for modeling HBV infection and related tumorigenesis. Human liver organoids can be seeded from both healthy and cirrhotic liver biopsies. They can be expanded in vitro when culturing in a medium containing a specific set of growth factors. When the culture medium is changed into a new medium containing growth factors that promote differentiation, organoids differentiate into functional hepatocytes, which makes them susceptible to infection with recombinant HBV. The novel in vitro primary model system described in this protocol can be utilized as a platform to study HBV pathogenesis and drug screening. Organoids generated from cirrhotic liver biopsies can be a potential tool for personalized medicine, and for modeling HCC and other liver diseases.
Graphic abstract:
Keywords: Healthy donor liver organoids, Patient-derived liver organoids, HBV-infection
Background
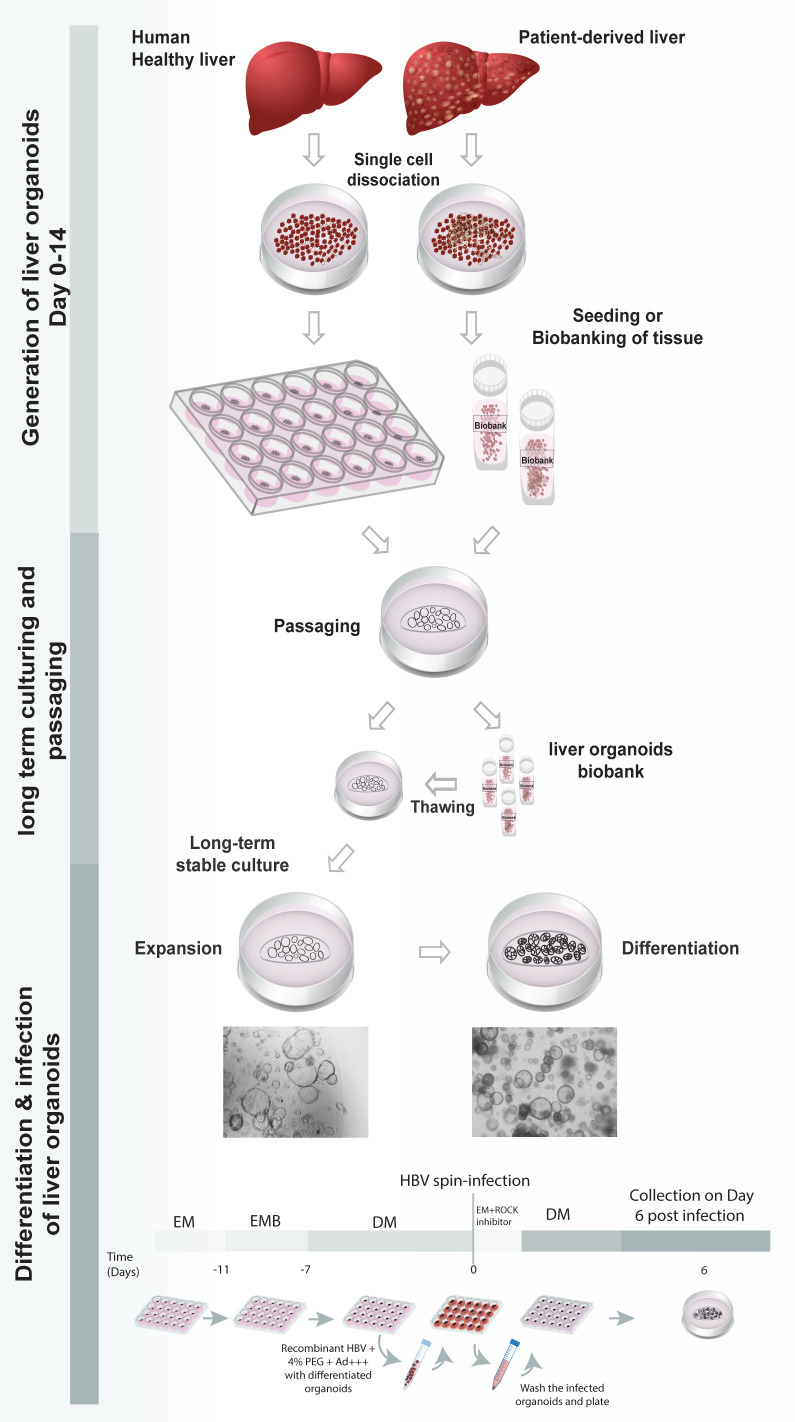
Organoid culture technology involves the generation of adult stem cell-derived, genetically-stable, in vitro, 3D-organ models of human origin. A primary liver culture system based on isolation and expansion of primary cells, that allows for the long-term expansion of liver cells as organoids, has already been established previously ( Huch et al., 2013 , 2015). They are generated from in vitro expansion of Lgr5+ adult stem cells present in the liver, by culturing them in a specified culture medium. Establishment of the liver organoids culture was previously reported by Broutier et al. (2016) . Liver organoids can be generated from both healthy and HBV-infected patient-derived liver tissue. In comparison with other in vitro cell hepatocytes models, liver organoids are expandable, biobankable and genetically stable. Recently, we have used liver organoids as a primary cell derived model to study HBV infection and associated tumorigenesis, as well as a personalized in vitro platform for anti-viral drug screening and drug induced toxicity (De Crignis et al., 2021 ). In our study, we used a modified version of the protocol by Broutier et al. (2016) for culturing and maintenance of liver organoids, where we introduced slight modifications to the isolation, as well as the expansion culture medium, to optimize liver organoid yield and viability. A detailed protocol for the generation, expansion, and differentiation of healthy and patient-derived liver organoids is described below. Differentiated liver organoids can be infected with recombinant HBV by spinoculation using concentrated virus.
Materials and Reagents
Sterile syringe filter, 0.1 µm (Merck Millipore, catalog number: SLVV033RS )
Sterile syringe filter, 0.2 μm (GE Healthcare, Whatman, catalog number: 6900-2502)
Sterile syringe (Omnifix, catalog numbers: 4616200V [20 mL]; 4616502F [50 mL])
Cell strainer 70 µm nylon (Falcon, catalog number: 352350)
Low retention sterile pipette tips with filter (Biotix, catalog numbers: M-0010-9FC [P10]; M-0020-9FC [P20]; M-0200-9FC [P200]; M-1000-9FC [P1000])
Pipette controller (Brand, catalog number: 26303)
Disposable sterile serological pipette with filter (VWR, catalog numbers: 89130-910 [10 mL]; 89130-890 [25 mL])
15 mL Falcon tubes (Greiner, catalog number: 188285)
50 mL Falcon tubes (Greiner, catalog number: 227285)
1.5 mL microcentrifuge tubes (Biotix, catalog number: MT-0150-BC)
24-well suspension plates (Greiner, catalog number: 662102)
48-well suspension plates (Greiner, catalog number: 677102)
60 mm Petri dish (Thermo Fisher Scientific, catalog number: 150462)
Disposable forceps (Servoprax, Mediware, catalog number: H7-301)
CryoTubeTM vials (Thermo Scientific, Nunc, catalog number: 366656)
Parafilm M (Sigma-Aldrich, catalog number: P7793)
Ethanol absolute (Sigma-Aldrich, Merck Millipore, catalog number: 1009832500)
DMEM (Dulbecco's Modified Eagle Medium) (Thermo Fisher Scientific, Gibco, catalog number: 41966029)
Fetal Bovine Serum (FBS) (Capricorn Scientific, catalog number: FBS-12A)
Penicillin-Streptomycin (10,000 U/mL) (Thermo Fisher Scientific, Gibco, catalog number: 15140122)
Belzer UW Cold Storage Solution (transfer medium) (Bridge to Life, BTLBUW-2000)
Advanced DMEM/F-12 (Thermo Fisher Scientific, Gibco, catalog number: 12634028)
HEPES, 1 M Buffer Solution in 0.85% NaCl (Lonza, catalog number: BE17-737E)
UltraGlutamine1 200 mM in 0.85% NaCl Solution (Lonza, catalog number: BE17- 605E/U1)
EBSS (Earle's Balanced Salt Solution) (Thermo Fisher Scientific, catalog number: 24010-043)
Collagenase D (Sigma-Aldrich, catalog number: 11088858001)
Recovery cell culture freezing medium (Invitrogen, catalog number: 12648010)
TrypLE Express (Thermo Fisher Scientific, Gibco, catalog number: 12604013)
Cultrex Reduced Growth Factor Basement Membrane Extract (BME), Type 2, Pathclear (R&D Systems, catalog number: 3533-010-02)
Bovine Serum Albumin (Sigma-Aldrich, catalog number: A7030)
Phosphate Buffered Saline (PBS) (Thermo Fisher Scientific, Gibco, catalog number: 10010023)
N2 Supplement (100×) (Thermo Fisher Scientific, Gibco, catalog number: 17502-048)
B27 Supplement (without vit A) (Thermo Fisher Scientific, Gibco, catalog number: 12587010)
N-acetyl Cysteine (Sigma-Aldrich, catalog number: A7250)
Recombinant Human EGF (PeproTech, catalog number: AF-100-15)
Recombinant [Leu15]-Gastrin I human (Sigma-Aldrich, catalog number: G9145)
Recombinant Human HGF (PeproTech, catalog number: 100-39H)
Primocin (Invivogen, catalog number: ant-pm-2)
Recombinant human R-spondin 3 (conditioned medium, house made) ( Broutier et al., 2016 )
Wnt 3A (conditioned medium, house made) ( Broutier et al., 2016 )
Recombinant Human FGF-10 (PeproTech, catalog number: 100-26)
Nicotinamide (Sigma-Aldrich, catalog number: N3376)
TGF-β inhibitor (A83.01) (Tocris, catalog number: 2939)
Forskolin (Sigma-Aldrich, catalog number: F3917)
Rho-kinase inhibitor (ROCK Inhibitor, Y-27632) (Sigma-Aldrich, catalog number: SCM075)
Recombinant Human Noggin (PeproTech, catalog number: 120-10C)
Recombinant human BMP7 (PeproTech, catalog number: 120-03P)
Recombinant human FGF19 (PeproTech, catalog number: 100-32)
DAPT (Sigma-Aldrich, catalog number: D5942)
Dexamethasone (Sigma-Aldrich, catalog number: D4902)
PEG-6000 (Millipore, catalog number: 528877)
70% ethanol solution (100 mL) (see Recipes)
DMEM +/+ (50 mL) (see Recipes)
Digestion solution (10 mL) (see Recipes)
Ad+++ culture medium (500 mL) (see Recipes)
0.1% BSA/PBS (10 mL) (see Recipes)
2× Human liver organoid basal medium (2× LBM) (50 mL) (see Recipes)
Human liver organoid Isolation medium (IM) (20 mL) (see Recipes)
Human liver organoid Expansion medium (EM) (20 mL) (see Recipes)
Human liver organoid Expansion medium without wnt3A supplemented with BMP7 (EMB) (20 mL) (see Recipes)
Human liver organoid Differentiation medium (DM) (20 mL) (see Recipes)
Equipment
Calibrated micropipette (VWR, Gilson, catalog numbers: 613-5945 [P10]; 613-5946 [P20]; 613-5948 [P200]; 613-5949 [P1000])
Biosafety cabinet (Clean Air by Baker, model: BioVanguard Biological Safety cabinet-Class ll)
Cell culture incubator with 5% CO2, 37°C (Panasonic, catalog number: MCO-170AICUVH-PE)
Bright field microscope (Leica DMIL microscope and a DFC420C camera)
Shaker (Thermo Scientific, model: 88881102)
Eppendorf Centrifuge 5810 R for 15 mL Falcon tubes and cell culture plates (Eppendorf, model: A-4-62)
Eppendorf Centrifuge 5417 R for 1.5 mL tubes (Eppendorf, model: F45-30-11)
Milli-Q® IQ 7000 Ultrapure Lab Water System
Water bath 37°C (Grant, VFP)
-80°C freezer (Panasonic, catalog number: MDF-794-PE)
Freezing container (NalgeneTM Cryo, catalog number: 5100-0001)
Refrigerator (Liebherr, catalog number: CP3523-22)
-20°C freezer (Bosch, GSD-3513)
Liquid nitrogen storage
Scalpels (Blade 10 or 11) (Swann Morton, catalog number: 233-5364 or 233-5363)
Scalpels (Blade 12D, double edge) (Swann Morton, catalog number: 233-5516)
Alcohol-resistant cryogenic scientific marker (LabTAG, catalog number: SM-1)
Cell counting chamber (Marienfeld, Bürker-Türk, catalog number: 1 630-1545)
Procedure
-
Dissociation of human liver hepatocytes and seeding of organoids
Safety instructions for working with human tissue:
While handling human tissue, work inside the biosafety cabinet.
-
Take precautions while working with human tissue (i.e., wear lab coats, gloves, sterilize the working space, etc.)
Critical Step:
Warm up a 24/48-well suspension plate in the incubator at 37°C.
-
Prepare all the buffers/solution required for the procedure prior to start.
-
After surgical excision, collect the sample immediately in a 15 mL or 50 mL Falcon tube, depending on the size of the biopsies and preserve in 5–10 mL of Belzer UW cold storage solution (commercial transfer medium provided by surgeons to transfer the biopsies) at 4°C until processing.
Optional step: Biopsies can be stored up to 48 h at 4°C. We recommend processing the tissue immediately after surgery.
Remove the basal medium and wash the tissue twice with 10 mL of DMEM +/+ (see Recipes) using a 10 mL pipette.
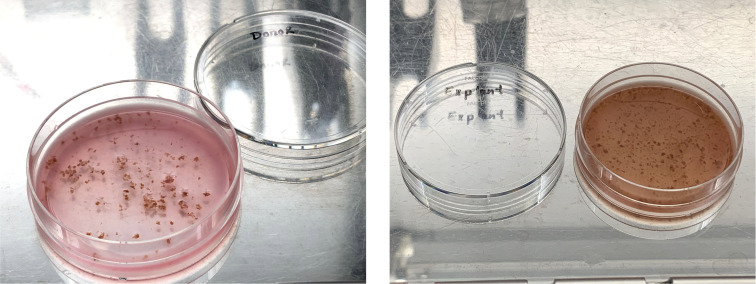
Place the tissue into a 60 mm Petri dish using sterile tweezers (Figure 1).
-
Add 1 2 mL of Ad+++ medium on top of the tissue and mince it into pieces of 0.5 1 mm3, using two scalpels (mince the tissue until it can be easily triturated with a p1000 pipette) (Figures 2 3).
Critical Step: Use a scalpel blade 12D and scalpel blade 10 or 11 to cut the tissue. Try to slice the tissue gently and vertically with the scalpels, instead of pressing or mashing the tissue at an angle with the side of the scalpels with force. Excessive force can cause cell rupture.
Figure 3. Minced liver tissue from donor (left) and patient (right).
Transfer the minced tissue with a P1000 pipette to a 15 mL Falcon tube and top up to 10 mL with ice-cold Ad+++. Gently pipette up and down with a 10 mL pipette, to wash the liver pieces.
-
Centrifuge at 100 × g and 4°C for 10 min.
Critical Step: For biobanking the tissue:
Divide the minced tissue into two after centrifugation and place half in a cryoTube containing 1 mL of freezing media.
Label with an alcohol-resistant marker and transfer the cryotube in the pre-cooled (4°C) freezing container.
Transfer the freezing container to -80°C.
Transfer the samples within 2 3 days from the -80°C freezer to liquid nitrogen, for long-term storage.
Organoids can be generated from frozen tissue, albeit with lower efficiency. Frozen tissue can also be used for genomic, transcriptomic, and proteomics studies.
To generate organoids from frozen minced tissue, take the sample out from the liquid nitrogen tank and transfer on dry ice. Take the vial and place it in a water bath (37°C) for 40 60 s (~90% of the sample is thawed) and immediately add 1 mL of Ad+++ dropwise to the vial. Transfer the volume to a 15 mL Falcon tube containing 10 mL of Ad+++. Continue further from step 6.
Discard the supernatant and add a sufficient volume (4 6 mL based on the size of the tissue, 4 mL for smaller tissue, and 6 mL for larger tissue) of pre-warmed digestion solution.
Homogenize the tissue gently with digestion solution by inverting the tube 5 8 times and pipetting up and down with a P1000 pipette.
-
Place the tube containing the homogenized sample horizontally in a rack and transfer it to a shaker incubator at 100 120 rpm and 37°C for 45 min 1 h (Figure 4).
Critical Step: Check every 15 min, to make sure not to over-digest the tissue. Whenever almost all the pieces of tissue are digested, start the next step immediately.
Resuspend the cells by pipetting up and down with a P1000 pipette (Figure 5).
Neutralize the digestion by adding 10 mL of cold Ad+++.
-
Pass the digested material through a 70 µm cell strainer (Figure 6) and collect the material in a 50 mL Falcon tube.
Optional step: If any partially digested tissue remains on the strainer, collect it with a p1000 pipette and incubate further with 1 mL of TrypLE at 37°C and 5% CO2 for 10–15 min. After incubation, follow step 12 again.
Optional step: If any debris or plastic pieces from the Petri dish are still present in the solution, repeat step 12 to remove these.
Add pre-warmed Ad+++ (37°C) to increase the volume to 25–30 mL.
Centrifuge at 200 × g and 4°C for 5 min.
Remove the supernatant. Resuspend the cell pellet in 10 mL of cold Ad+++ with a 10 mL pipette and transfer the resuspended cells to a 15 mL Falcon tube.
Centrifuge at 200 × g and 4°C for 5 min.
Remove the supernatant and resuspend the cell pellet in 2 mL of Ad+++. Count the cells and mix the appropriate number of cells with BME, which will polymerize and form a dome like structure (drop), to give the organoids mechanical support to grow in 3D.
Count the cells with a hemocytometer to seed approximately 10,000 15,000 cells/25 µL drop in a 48-well plate.
Centrifuge at 200 × g and 4°C for 5 min.
-
Resuspend the cells with the appropriate volume of Ad+++ and BME to make the drops (each drop contains 1/3 Ad+++ and 2/3 BME, i.e., 8.3 µL of Ad+++ plus 16.6 µL of BME per 25 µL-drop in a 48-well plate).
Critical Step: When homogenizing the BME/Ad+++ mixture and making the drops, it is important to avoid making any bubbles.
Critical Step: While handling the BME, always keep the BME on ice, and be quick when making the drops. The BME will start to solidify if removed from ice for longer than 1 min.
-
Take the pre-warmed plate from the incubator and start making the drops (Figure 7).
Note: The volume of a drop for each well of a 48-well plate is 25 µL. For a 24-well plate, both 3× 25 µL drops or 1× 50 µL drop can be used.
Incubate at 37°C and 5% CO2 for 30–45 min, and let the BME polymerize.
Add 250 µL (48-well plate) or 500 µL (24-well plate) of warm human liver organoid isolation medium (IM) in each well (Figure 8).
Critical step:
-
Add the medium gently on the side of the well. Avoid adding the medium directly on top of the drop.
During the first seven days, change the medium every 3–4 days and use IM.
After 1 week of seeding the organoids, change IM to human liver expansion medium.
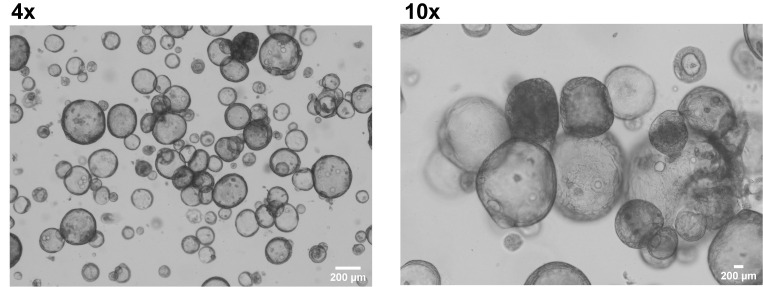
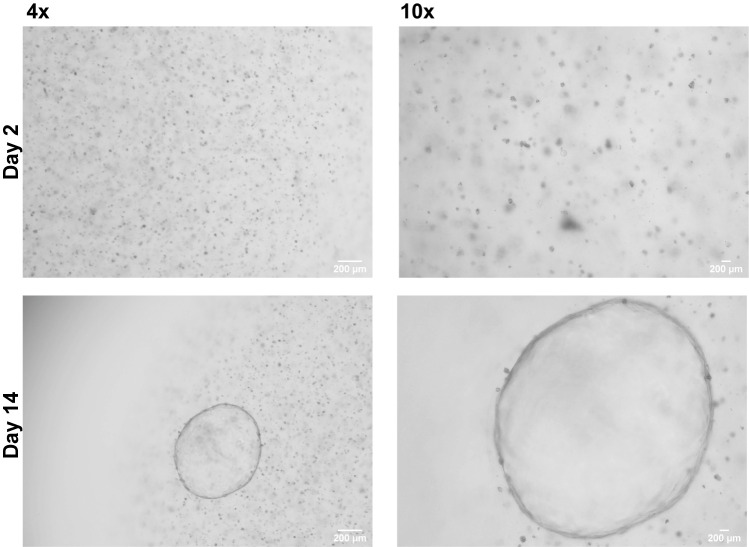
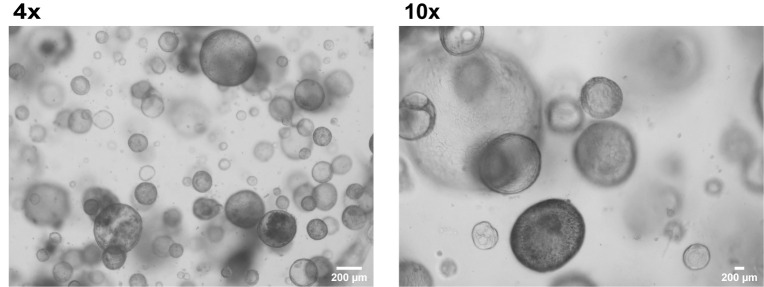
Organoids will be visible within 2–14 days post seeding. Daily monitoring using a bright field microscope is required to identify growing organoids (Figure 9).
-
Expansion and maintenance of organoids in culture
Split the organoids every 7–10 days according to organoid density and size (>500–1,000 µm). Here, the term splitting means digesting the organoids enzymatically and mechanically, and distributing the digested organoids into the required number of wells by means of mixing the digested organoids with BME/Ad+++ and making the drops. The general split ratio is 1:4–1:8, depending on growth rate of the donor organoid lines.
Critical step: Pre-warm the 24-well suspension plate
Prepare 1× 15 mL Falcon tube containing 10 mL of ice-cold Ad+++.
Discard the medium from each well.
To collect the drops, take 4 mL of ice-cold Ad+++ from the 15 mL Falcon tube and add 1 mL into each well containing organoids that need to be split.
Disrupt the drop containing organoids by scraping with P1000 pipette.
-
Collect the disrupted organoids and transfer to one 15 mL Falcon tube. A maximum of 4 wells can be collected in one 15 mL Falcon tube (e.g., if 8 wells are split, the organoids can be collected in two 15 mL Falcon tubes).
Note: Follow step 3–5 for each 4 wells of organoids.
Triturate 3–5 times with a 10 mL pipette.
Keep the Falcon tubes on ice for 20–45 min. Invert the Falcon tubes every 10 min, this can help the BME to dissolve efficiently.
Centrifuge at 200 × g and 4°C for 5 min.
Discard the supernatant.
-
Add 60–100 µL of TrypLE to the pellet of organoids. Digest the organoids mechanically at room temperature for 1–5 min, by pipetting with a P200 pipette.
Note: Check the size of the digested organoids under the microscope. The desired size depends on the experimental requirements. Make sure that the digested organoids are uniform in size.
Add 10 mL of ice-cold Ad+++ to stop the digestion and centrifuge at 200 × g and 4°C for 5 min.
Discard the supernatant completely.
Resuspend the digested organoid pellet with the appropriate amount of 1/3 Ad+++ and 2/3 BME (e.g., if splitting two wells 1:4, then 8 drops need to be made; if 6 drops are split 1:6, then 36 drops need to be made).
Make drops of appropriate volume based on the plate that is used.
Allow the drops to polymerize at 37°C and 5% CO2 for 30–45 min.
Add 250 µL (48-well plate) or 500 µL (24-well plate) of warm EM.
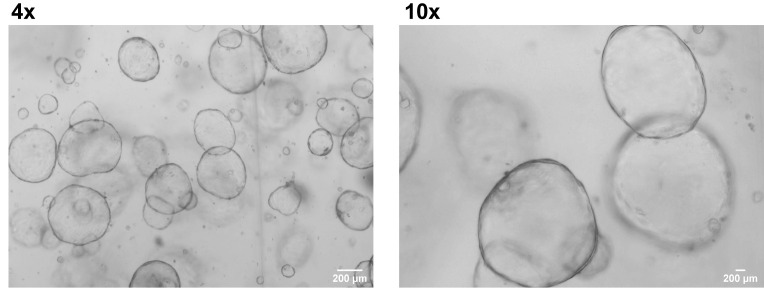
Change the medium every 2–3 days (Figure 10).
-
Hepatic differentiation of liver organoids
-
Culture liver organoids with EM-wnt3A+BMP7 for 3–5 days prior to differentiation, to allow the organoids to recover and completely reform after splitting.
Critical step: If the organoids are big, this might result in breaking of organoids during spin infection. Therefore, start differentiating the liver organoids when they are small (50–350 µm).
Wash the drops gently two times with 500 µL of warm Ad+++.
Add 250 µL (48-well plate) or 500 µL (24-well plate) of warm human liver organoid differentiation medium (DM).
Change the medium every 2–3 days. Differentiate the organoids for 7 days (Figure 11).
-
-
HBV infection
The infection of human liver organoids can be done either without the addition of PEG or with addition of 4% PEG, which plays a role in increasing the infection efficacy.
Safety instructions for working with recombinant HBV:
Handling HBV should only be done by HBV vaccinated personnel.
While handling HBV, work inside the biosafety cabinet
-
Take proper precautions while working with HBV (i.e., wear lab coat, gloves, sterilize the working space, etc.)
Remove the medium from each well.
Collect the organoids by disrupting the drops gently with a 10 mL pipette.
Pull together 3–4 wells of organoids into a 1× 15 mL Falcon tubes containing 10 mL ice cold Ad+++
Pipette up and down gently 3–5 times with a 10 mL pipette.
-
Incubate on ice for 50–60 min.
Critical step: Mix the organoids by inverting the flacon tubes every 5 min. This will help the BME to dissolve efficiently.
Centrifuge the differentiated organoids at 100 × g and 4°C for 10 min.
Discard the supernatant.
Thaw the pre-titered recombinant HBV stock on ice.
Centrifuge the virus at max speed and 4°C for 10 min.
Prepare 500 μL of Ad+++ containing 1–10 × 107 copies of HBV (or heat inactivated HBV as control) with or without 4% PEG. Resuspend a pellet of washed differentiated organoids corresponding to 3–4 wells (of 24-well plate) in the 500 μL virus mix.
Divide the volume over a 48 (250 μL/well) or 24-well plate (500 μL/well)
Seal the plate with parafilm (Figure 12).
Place the sealed plates in the pre-warmed (32°C) Eppendorf centrifuge (5810 R) and spin infect at 600 × g and 32°C for 60 min.
Remove the parafilm.
-
Triturate gently the organoids with a P1000 pipette.
Critical step: If the organoids are too big, snip the tip of the P1000 before triturating.
Incubate at 37°C and 5% CO2 for 5 h.
Collect the organoids in a 15 mL Falcon tube with a 10 mL pipette.
Centrifuge at 100 × g and 4°C for 10 min.
-
Discard the supernatant containing the virus.
Critical step: Virus can be recycled 2–3 times. It is recommended to check the virus titer before each subsequent infection.
Resuspend the organoids with 10 mL of ice-cold Ad+++.
Centrifuge at 100 × g and 4°C for 10 min.
Repeat steps 20–21 4–5 times.
Remove the supernatant completely.
Resuspend the pellet with an appropriate volume of 1/3 Ad+++ and 2/3 BME.
Make the drops in pre-warmed plate. Keep the number of wells the same as the number of wells that were collected for infection (i.e., if 12 wells of differentiated organoids are used for infection, the organoids can be seeded back into 12 wells post spin infection.)
Incubate the plate at 37°C and 5% CO2 for 30–45 min.
-
Add 250 µL (48-well plate) or 500 µL (24-well plate) of warm EM supplemented with 10 µM Rho-kinase inhibitor.
Figure 13. Hepatitis B virus (HBV)-infected liver organoids.
The next day, remove the medium of organoids.
Keep the medium to titer.
Gently wash the drops with 500 µL of pre-warmed Ad+++ four times.
Add 250 µL (48-well plate) or 500 µL (24-well plate) of pre-warmed DM.
Change the medium every 2–3 days depending on experiment.
Figure 1. Human liver biopsies from donor (left) and patient (right).
Figure 2. Mincing of liver biopsy.
Figure 4. Placement of minced tissue on the shaker inside the incubator.
Figure 5. Digested liver biopsy.
Figure 6. Straining of digested liver biopsy.
Figure 7. BME drops containing hepatocytes.
Figure 8. Adding the organoids culture medium.
Figure 9. Generation of organoids from liver biopsy.
Figure 10. Liver organoids in expansion medium.
Figure 11. Liver organoids in differentiation medium.
Figure 12. Parafilm sealed plate containing differentiated liver organoids and HBV.
Recipes
-
70% ethanol solution (100 mL)
70 mL of ethanol absolute
30 mL of Milli-Q treated Water
-
DMEM +/+ (50 mL)
49.45 mL of DMEM
500 µL of FBS
50 µL of Penicillin-Streptomycin
Filter with 0.2 µm filter
-
Digestion solution (10 mL)
2.5 mL of 10 mg/mL Collagenase D
7.49 mL of EBSS
10 µL of Rho-kinase inhibitor
Filter with 0.2 µm filter
Incubate at 37°C for 15–20 min
-
Ad+++ culture medium (500 mL)
485 mL of DMEM
5 mL of HEPES
5 mL of Ultraglutamine
5 mL of Penicillin-Streptomycin
-
0.1% BSA/PBS (10 mL)
10 mg of BSA
Top up to 10 mL with PBS
Filter with 0.2 µm filter
-
2× Human liver organoid basal medium (2× LBM) (50 mL)
1 mL of N2 supplement
2 mL of B27 supplement
250 µL of 500 mM N-acetyl Cysteine (NAC in water)
10 µL of 500 µg/mL hEGF in 0.1% BSA/PBS
10 µL of 100 µM Gastrin in PBS
50 µL of 50 µg/mL HGF in 0.1% BSA/PBS
200 µL of Primocin
46.48 mL of Ad+++
-
Human liver organoids isolation medium (IM) (20 mL)
10 mL of 2× LBM
4 mL of R-spondin 3 conditioned medium
2 mL of Wnt3A conditioned medium
20 µL of 100 µg/mL FGF10 in 0.1% BSA/PBS
200 µL of 1 M Nicotinamide in PBS
5 µL of 20 mM TGF-β inhibitor (A83.01) in DMSO
10 µL of 20 mM Forskolin in DMSO
20 µL of 10 mM Rho-kinase inhibitor in DMSO
20 µL 25 µg/mL Noggin in 0.1% BSA/PBS
3.725 mL of Ad+++
Filter with 0.1 µm filter
-
Human liver organoids Expansion medium (EM) (20 mL)
10 mL of 2× LBM
4 mL of R-spondin 3 conditioned medium
250 µL of Wnt3A conditioned medium
20 µL of 100 µg/mL FGF10 in 0.1% BSA/PBS
200 µL of 1 M Nicotinamide in PBS
5 µL of 20 mM TGF-β inhibitor (A83.01) in DMSO
10 µL of 20 mM Forskolin in DMSO
5.515 mL of Ad+++
Filter with 0.1 µm filter
-
Human liver organoids Expansion medium without wnt3A supplemented with BMP7 (EMB) (20 mL)
10 mL of 2× LBM
4 mL of R-spondin 3 conditioned medium
20 µL of 100 µg/mL FGF10 in 0.1% BSA/PBS
200 µL of 1M Nicotinamide in PBS
5 µL of 20mM TGF-β inhibitor (A83.01) in DMSO
10 µL of 20mM Forskolin in DMSO
20 µL of 25 µg/ml BMP7 in 0.1% BSA/PBS
5.745 mL of Ad+++
Filter with 0.1 µm filter
-
Human liver organoids Differentiation medium (DM) (20 mL)
10 mL of 2× LBM
20 µL of 25 µg/mL BMP7 in 0.1% BSA/PBS
20 µL of 100 µg/mL FGF19 in 0.1% BSA/PBS
2 µL of 5 mM TGF-β inhibitor (A83.01) in DMSO
20 µL of 10 mM DAPT in DMSO
2 µL of 30 mM Dexamethasone in DMSO
9.936 mL of Ad+++
Acknowledgments
The generation, maintenance and differentiation of human liver organoids part of the protocol has been adapted from previously published work ( Broutier et al., 2016 ).
Competing interests
The authors declare no conflict of interest.
Citation
Readers should cite both the Bio-protocol article and the original research article where this protocol was used.
Q&A
Post your question about this protocol in Q&A and get help from the authors of the protocol and some of its users.
References
- 1. Huch M., Dorrell C., Boj S. F., van Es J. H., Li V. S., van de Wetering M., Sato T., Hamer K., Sasaki N., Finegold M. J., et al.(2013). In vitro expansion of single Lgr5+ liver stem cells induced by Wnt-driven regeneration . Nature 494(7436): 247-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Huch M., Gehart H., van Boxtel R., Hamer K., Blokzijl F., Verstegen M. M., Ellis E., van Wenum M., Fuchs S. A., de Ligt J., et al.(2015). Long-term culture of genome-stable bipotent stem cells from adult human liver. Cell 160(1-2): 299-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Broutier L., Andersson-Rolf A., Hindley C. J., Boj S. F., Clevers H., Koo B. K. and Huch M.(2016). Culture and establishment of self-renewing human and mouse adult liver and pancreas 3D organoids and their genetic manipulation. Nat Protoc 11(9): 1724-1743. [DOI] [PubMed] [Google Scholar]
- 4. De Crignis E., Hossain T., Romal S., Carofiglio F., Moulos P., Khalid M. M., Rao S., Bazrafshan A., Verstegen M. M., Pourfarzad F., et al.(2021). Application of human liver organoids as a patient-derived primary model for HBV infection and related hepatocellular carcinoma. Elife 10: e60747. [DOI] [PMC free article] [PubMed] [Google Scholar]