Abstract
The lumen of blood vessels is covered by endothelial cells, which regulate their permeability to ions and solutes. Endothelial permeability depends on the vascular bed and cell phenotype, and is influenced by different disease states. Most characterization of endothelial permeability has been carried out using isolated cells in culture. While analysis of cultured cells is a valuable approach, it does not account for factors of the native cell environment. Building on Ussing chamber studies of intact tissue specimens, here we describe a method to measure the electrophysiological properties of intact arteriole and venule endothelia, including transendothelial electrical resistance (TEER) and ion permselectivity. As an example, vessels isolated from the mesentery were treated ex vivo, then mounted in a custom-made tissue cassette that enable their analysis by classical approaches with an Ussing chamber. This method enables a detailed analysis of electrophysiological vessel responses to stresses such as proinflammatory cytokines, in the context of an intact vessel.
Graphic abstract:
Keywords: Endothelium, Vasculature, Transendothelial resistance, TEER, Vein, Artery, Dilution potential
Background
The endothelial barrier regulates the passage of ions, water, and proteins across blood vessels. The vasculature is heterogeneous, with veins being generally more permeable than arteries (Crone and Christensen, 1981; Durán et al., 2008 ; Hilfenhaus et al., 2018 ). Several diseases, including sepsis, stroke, and hypertension are exacerbated by a failure in endothelial barrier function (Wang et al., 2002; Granger et al., 2010 ; Rosenberg, 2012; Schoknecht and Shalev, 2012). Thus, it is critically important to understand the molecular regulation of vascular function in general, and of the endothelium in particular.
Ussing chambers enable the most detailed analysis of the electrophysiological properties of barrier-forming cells (Clarke, 2009; Herrmann and Turner, 2016). Originally developed to measure sodium permeability across frog skin (Ussing and Zerahn, 1951; Lindemann, 2001), Ussing chambers have been used to measure ion permeability in systems ranging from epithelial cells cultured on Transwell permeable supports ( Molina et al., 2015 ) to intact tissues, such as the intestine ( Laukoetter et al., 2007 ). The vast majority of Ussing chamber measurements have been performed on epithelial cells. By contrast, Ussing chamber analysis of cultured endothelial cells is much more difficult, although there are some examples where the approach was used to measure endothelial ion channel activity ( Wigham et al., 2000 ; Hatou et al., 2010 ). The barrier function of cultured endothelial cells is generally characterized with other techniques, including cells cultured on Transwells with the EndOhm/ Millicell-ERS system, measuring flux with fluorescent tracers, and/or cells cultured on gold electrodes using electric cell-substrate impedance sensing (ECIS) ( Dubrovskyi et al., 2013 ; Adil and Somanath, 2021; Maier- Begandt et al., 2021 ).
Here, we describe a method for Ussing chamber analysis of the electrophysiological properties of semi-intact blood vessels, which have been dissected and longitudinally cut. This method can be applied to veins and arteries, and avoids difficulties encountered when measuring intact perfused blood vessels (Crone and Christensen, 1981). Several parameters can be measured, including transendothelial electrical resistance (TEER), short circuit current (Isc), and determination of relative ion permeability. Each of these measurements reflects both transcellular and paracellular ion transport, where transcellular ion transport is largely mediated by plasma membrane ion channels and the paracellular component is mediated through tight junctions (Anderson and van Itallie, 2009; Clarke, 2009). Together, the transcellular and paracellular pathways regulate tissue barrier function, a parameter that can be impacted by several pathophysiological conditions, such as sepsis ( Joffre et al., 2020 ).
The ability to analyze an intact vascular barrier enables electrophysiological analysis of the endothelium in a native context that complements and validates measurements done with cultured cells or isolated perfused vessels. Native tissues also have better tissue integrity than endothelial cells in culture, and are more amenable to Ussing chamber analysis. Here, we describe techniques needed for electrophysiological analysis of intact vessels, and how these were applied to analyze the effect of a proinflammatory cytokine called TNFα on vein barrier function and ion permselectivity.
Materials and Reagents
Mice (at least 10 weeks of age)
Isofluorane
Rapid flow bottle top filter, 0.2 μm (ThermoFisher, catalog number: 291-4520)
Parafilm (Bemis)
1 mL slip tip syringe (BD, catalog number: 309659)
Gel-Loading tips (Fisher, catalog number: 02-707-138 or Bio-Rad, catalog number: 223-9915)
Copper bell wire
35 mm tissue culture dish (BD, Falcon Easy Grip, catalog number: 353001)
1.5 mL tubes (Eppendorf, catalog number: 022363204)
50 mL conical centrifuge tubes (BD, catalog number: 352098)
Absolute ethanol
KCl (Sigma-Aldrich, catalog number: P3911)
Noble Agar, Ultrapure (ThermoFisher, catalog number: AAJ1090736)
Sodium hypochlorite, 5% (Clorox Chlorine Bleach)
NaCl (Sigma-Aldrich, catalog number: S9888)
CaCl2·2H2O (Sigma-Aldrich, catalog number: C3306)
MgCl2·6H2O (Sigma-Aldrich, catalog number: M2393)
Glucose (Sigma-Aldrich, catalog number: G7021)
HEPES (Sigma-Aldrich, catalog number: H4034)
Lysine-Cl (Sigma-Aldrich, catalog number: L8662)
Na Aspartate (Sigma-Aldrich, catalog number: A6683)
Mannitol (Sigma-Aldrich, catalog number: M9647)
Dow Corning High Vacuum Grease
Wet or dry 600 grit ultrafine sandpaper (3M, catalog number: 1077259)
Extra sharp uncoated single edge razor blade (GEM, catalog number: 121-2)
5% CO2/95% O2 medical-grade gas mixture
DMSO, tissue culture grade (Sigma-Aldrich, catalog number: D2650)
Recombinant murine TNFα (R&D Systems, catalog number: 410-MT)
3 M KCl (100 mL) (see Recipes)
Agar solution (100 mL in 3 M KCl) (see Recipes)
70% ethanol (see Recipes)
10 M NaOH solution (100 mL) (see Recipes)
1 M HEPES (100 mL) (see Recipes)
Ringer’s Saline (500 mL) (see Recipes)
Ringer’s Mannitol (500 mL) (see Recipes)
Diluted Ringer’s Saline/Mannitol (1:4) (see Recipes)
Ringer’s Lysine-Cl (500 mL) (see Recipes)
Ringer’s Na-Aspartate (500 mL) (see Recipes)
1,000× TNFα (50 μg/mL) (see Recipes)
Equipment
Mouse Dissecting Kit (World Precision Instruments, catalog number: MOUSEKIT)
Dissecting Pan, Aluminum, with Vinyl Dissecting Pad (Carolina Supply, catalog number: 629004)
Silicone Dissecting Pad Kit (World Precision Instruments, catalog number: 501986)
Olympus SZX12 Dissecting Microscope or equivalent
Dual-channel, self-contained Ussing chamber (Warner Instruments, catalog number: U2500)
Custom made plug-in cassette, slot size: 175 μm in width and 1,500 μm in length (Emory Department of Physics Machine Shop), external dimensions comparable to Warner Instruments cassette, catalog number: U9924C-05.
Ussing Electrode Set (2 Ag/AgCl pellets and 2 Ag wires) (ADInstruments, catalog number: U9975A)
Ussing Electrode Bridge Fitting Kit (12 Fittings & 6 Adapters) (ADInstruments catalog number: U9565SC)
Bunsen burner
500 mL Erlenmeyer flask connected to in-house vacuum line
Multimeter Ohmmeter/voltmeter (Kosmos FKV-1025 or equivalent)
Lab stand with a 3 prong clamp
Multichannel Voltage/Current Clamp controller (Physiological Instruments, catalog number: VCC-MC2)
Circulating water bath (Haake DC10 or equivalent)
Fisherbrand accumet AB315 pH meter with electrode (13636AB315A)
Nutating platform (VWR, catalog number: 82007-202)
Software
Acquire & Analyze software ver 2.3 (Physiological Instruments)
Excel (Microsoft)
Procedure
-
Ussing chamber electrode preparation
-
Chloriding silver wires
The barrel end of the fitting is female and threaded to accept male-threaded Ag/AgCl (voltage) electrodes, and Ag wire (current) electrodes. Note that Ag wire electrodes can also be used as voltage electrodes, but the AgCl pellet electrodes can only be used as voltage electrodes. Make plenty of spare electrodes, in case they fail during the course of an experiment.
Clean the wire electrode with 100% ethanol.
To remove old chloride coating, quickly pass the wire through a flame. Be careful not to melt the plastic fitting. A properly flamed wire will be bright silver in color.
To chloride the Ag wire, place ~20 mL of fresh full strength common household bleach (Clorox) into a 50 mL plastic tube and immerse the wire for 15 to 30 min, until a purple-gray color is observed. Rinse by dipping the wire into a tube with distilled water, and shake dry before electrode assembly. If the wire does not look uniformly gray/purple, or fails in the ohm-meter test, clean it using ultrafine sandpaper (600 grit), and re-process.
AgCl pellet electrodes do not require bleach treatment but, if they become corroded, gently sand them with ultrafine sandpaper, and then rinse with distilled water.
-
Bridge fittings and filling with agar
Prepare the agar bridge solution (see Recipes) so that it is melted and at ~65–75°C.
The electrode bridge fittings are modified gel loading micropipette tips with a Luer-taper that match the insertion ports (ports 2 and 3, in Figure 1) of the Ussing chamber.
Cut the top portion of the tip off, so that the adapter fitting fits snugly into the top of the tip (Figure 2).
Fill a 1 mL syringe with approximately 0.4 mL of melted agar solution.
Slowly inject the agar/KCl solution into the tip of a bridge fitting, making sure that the tip and fitting are completely filled and that no air bubbles form.
Attach the electrodes to the fittings and screw them into place. Allow the solution to cool and solidify within the fittings. The agar solution will properly encapsulate the Ag/AgCl pellet or Ag wire during solidification.
After cooling and solidifying, cut the bottom portion of the tips at a 45° angle, to expose the largest possible tip area to the membrane.
-
Testing electrodes
Connect the positive terminal (VΩmA) of the volt-ohm-meter to a copper wire in a 50 mL tube containing Ringer’s Saline buffer (Figure 3).
Connect the electrode to be tested to the common (com) terminal, which is then placed into the Ringer's Saline buffer. Place only the tip of the electrode under the surface of the buffer, without completely submerging the electrode.
Set the ohm-meter to 20KΩ. The resistance should settle to a positive value within a minute or so. If the reading is negative or shows a "1" with no decimal places, the electrode is faulty and should not be reassembled.
Keep spare electrodes in Ringer's Saline (at room temperature) when not in use during the experiment.
Disassemble the electrodes upon completion of the experiment. Pellet electrodes become easily corroded and can crumble, so it is important that they be cleaned, by thorough rinsing in distilled water, and carefully dried.
-
-
Isolation of mesenteric arterioles and venules
Anaesthetise mice of at least 10 weeks of age with carbon dioxide or isoflurane (2.5%) and sacrifice them by cervical dislocation. Remove the entire mesentery as soon as possible following euthanasia.
Pin the mouse on a dissecting board, and sterilize it using 70% ethanol in water.
Use surgical dissecting scissors to make an incision in the skin, starting just below the sternum. Continue cutting along the midline to the pelvis. Then make transverse cuts on both sides, and pin the skin to the dissecting board.
Use forceps to grasp the abdominal wall, and cut it open with surgical scissors.
Gently elevate the intestines out of the abdominal cavity, and close with a suture just above the rectum and below the stomach, to avoid leakage.
Cut the intestines just past the suture sites. Rinse with Ringer’s Saline buffer.
Transfer to a Petri dish containing an elastomer dissection pad wetted with cold Ringer’s Saline buffer and pin the ends of the jejunum to form a circle, spreading the jejunal arcade, allowing easier visualization and isolation of the individual vessel branches (Figure 4A).
-
Use a 10× dissecting microscope to identify the arterioles and venules within the tissue. Both have a clear lumen.
Because they are surrounded by vascular smooth muscle, arterioles are smooth walled and more rigid than venules. Lymphatics tend to be more opaque and have more frequent and prominent valves than venules (Figure 4B).
Vessel identity can be further verified using markers for arterioles (GJA5) or venules (CLDN11) by Q-RT-PCR or immunohistochemistry ( Buschmann et al., 2010 ; Maier- Begandt et al., 2021 ).
Remove irrelevant tissues to reveal the vessels of interest (Figure 4).
-
Remove 2nd and 3rd order arteries or veins 2–3 mm in length and with diameters of 100 μm or less, then cut longitudinally, and transfer to a 35 mm tissue culture dish containing Ringer’s Saline buffer.
At this point, the isolated vessels are ready for mounting into the Ussing chamber. Both veins and arteries can be analyzed using this approach.
-
Alternatively, vessels can be pretreated with different agents or cytokines prior to mounting in the Ussing chamber.
Place freshly isolated vessels into a 1.5 mL Eppendorf tube containing 800 μL of Ringer’s Saline buffer, containing either TNFα (50 ng/mL) or control vehicle 0.1% dimethyl sulfoxide (DMSO) pre-warmed to 37°C.
Incubate at 37°C for 30 min, while rocking on a nutating platform.
Mount the vessels into the Ussing chamber.
-
Ussing chamber assembly
-
Preliminary chamber preparation
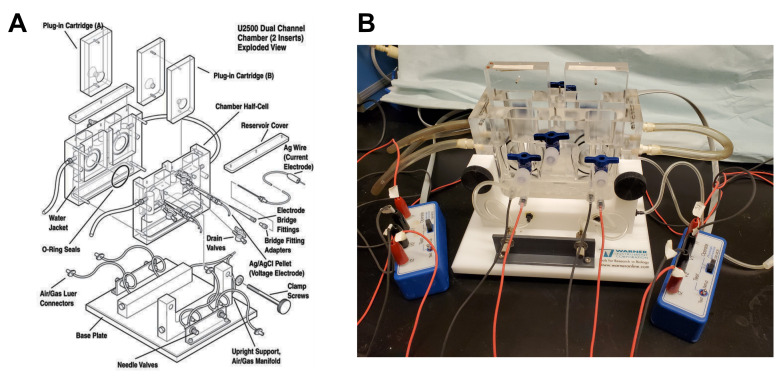
Make sure that you have enough Ringer's Saline buffer and that it is pre-warmed to 37°C. Approximately 36 mL of buffer will be required for initial filling of the chamber assembly. Additional volume will be required to replace fluid lost through normal evaporation, as well as any fluid removed for sampling or buffer exchange during the course of the experiment.
Lightly coat the O-ring seals on the inner chamber with vacuum grease (Figure 5).
Connect the water circulation source to the chamber, and turn on the circulation bath. Begin water circulation to bring the chamber to the desired operating temperature (usually 15–30 min).
Loosely assemble the two half-cells on the chamber base using the two clamp screws, so that there is sufficient clearance for insertion of the cartridge.
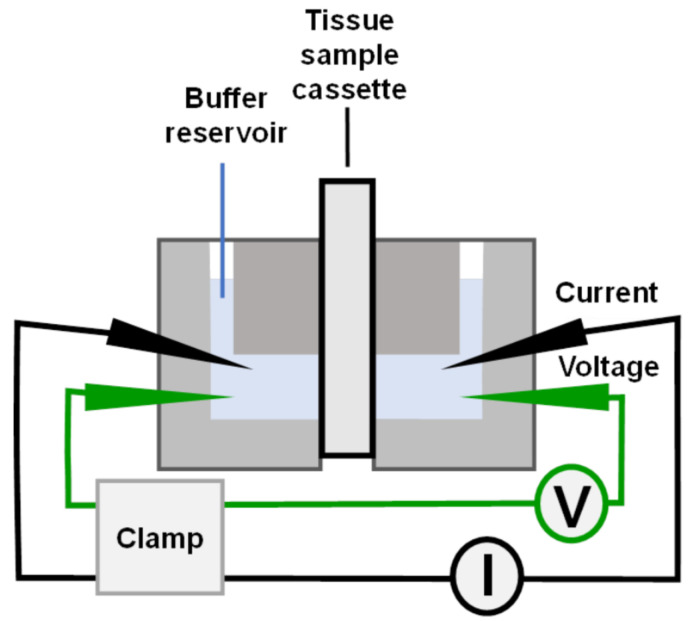
Insert all electrode bridge fittings into their respective ports. Push gently until they are seated against the internal stops. Make sure that the voltage/clamp apparatus is set to "zero", and connect the 2 mm pins of the electrodes directly to the headstage of the voltage clamp. Make sure that V1 and I1 are connected to the same side of the chamber, as well as V2 and I2 (see Figures 1 and 5).
Make sure that the 2-way stopcock valves in the drain ports are closed, by turning the handles to a position that is perpendicular to the valve body. After completion of the experiment, these ports provide a convenient way of draining the buffer from the chambers before separating the two halves.
-
Vessel mounting and chamber assembly
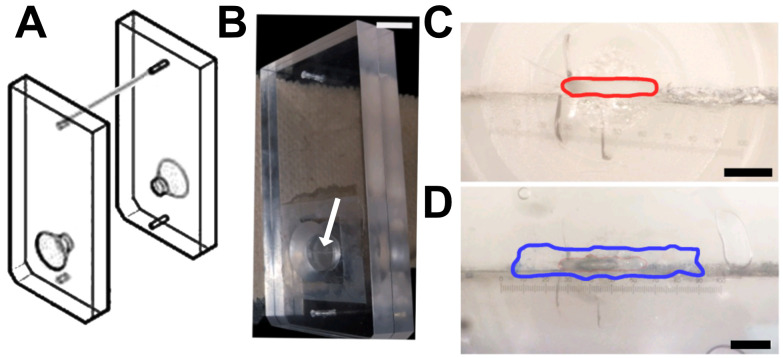
Orient a vessel between the two halves of a tissue cassette (Figure 6). Use a light coating of vacuum grease in areas surrounding the tissue, to provide a tight seal. Keep track of apical/basolateral orientation. Vessels are slightly curved. When placed on a surface with the concave orientation upwards, the apical direction is on top.
Seal the cassettes by surrounding them with a gasket made from parafilm. If the tissue cassettes leak or result in short circuit measurements, apply a thin film of vacuum grease on the inside surface before assembling them.
Place the cartridge inserts into the loosely assembled chamber halves (Figure 4). Note that the large hole is on the bottom and will align with the center of the chamber when inserted. After inserting the cartridges, securely tighten the screw clamps, but do not over-tighten. The clamps must be equally tightened for the O-rings to seat properly on the sides of the cartridge inserts. If misaligned or improperly tightened, the chamber will leak. Visually observe the size of the gaps on each side of the chamber halves, and adjust the clamp screws to make this gap spacing equal.
Equilibrate the chamber for 5–10 min with Ringer’s Saline solution at 37°C, using a temperature-controlled water bath. Solutions are aerated during the course of the experiment with a 5% CO2/95% O2 medical-grade gas mixture.
Connect the leads from the Ussing chamber to the Multichannel Voltage/Current Clamp controller.
-
-
Electrophysiological measurements
-
Calibration
Switch the FUNCTION to OPEN and switch the METER to voltage (V). The voltage reading reflects all of the asymmetries in the system. To compensate for this, if it does not equal zero, place the OFFSET switch in either the plus or minus position, and adjust the OFFSET control knob until the voltage reads 0.00 on the meter.
Set the fluid resistance compensation (FLUID RES COMP) to 0.0.
Switch the METER to current (I) and press and hold the PUSH TO ADJ test button. The current should be 60–68 μAmps on the meter. If it is not, adjust the FLUID RES COMP until it falls in range.
Set MODE to remote (REM), and control the voltage/current clamp apparatus with the Acquire & Analyze software.
Calibration only needs to be done once per Ussing chamber session, unless electrodes need to be changed.
-
TEER and Isc measurements
To measure voltage potential (mV), clamp the current to 0 μA and then collect 2 min of equilibrium data. A sustained consistent voltage measurement is an indication that the system and sample are working properly. If the system does not provide a voltage measurement, this is an indication that one or more electrodes are not functioning and should be replaced.
To measure resistance (Ohm), clamp (set) the voltage at 20 mV, period = 10.0 s, duration = 0.30 s, and polarity to plus (+). Collect data for at least 10 min, making sure the measurement has reached an equilibrium state. Multiply resistance times area to obtain transendothelial resistance (TEER; Ohm × cm2), as described below. TEER values should be greater than zero; if they are not, then the tissue sample is compromised and should be replaced.
To measure short circuit current (Isc; μA), clamp the voltage to 0 mV, then collect 10 min of equilibrium data.
-
Dilution potential measurements
Measure the voltage potential (mV) in Ringer’s Saline, by clamping the current to 0 μA, and collecting 10 min of equilibrium data.
Replace the Ringer’s Saline in the apical side of the chamber with pre-warmed Diluted Ringer’s Saline/Mannitol (1:4).
Measure the voltage potential (mV) with current clamped to 0 μA, and then collect 10 min of equilibrium data.
Calculate the Dilution Potential (VDP; mV) as described below.
-
Measurements in Ringer’s Lysine-Cl (optional)
Replace both chambers with pre-warmed Ringer’s Lysine-Cl.
Measure the voltage potential with current clamped to 0 μA, as described above.
-
Measurements in Ringer’s Na-Aspartate (optional)
Replace both chambers with pre-warmed Ringer’s Na-Aspartate.
Measure the voltage potential with current clamped to 0 μA, as described above.
-
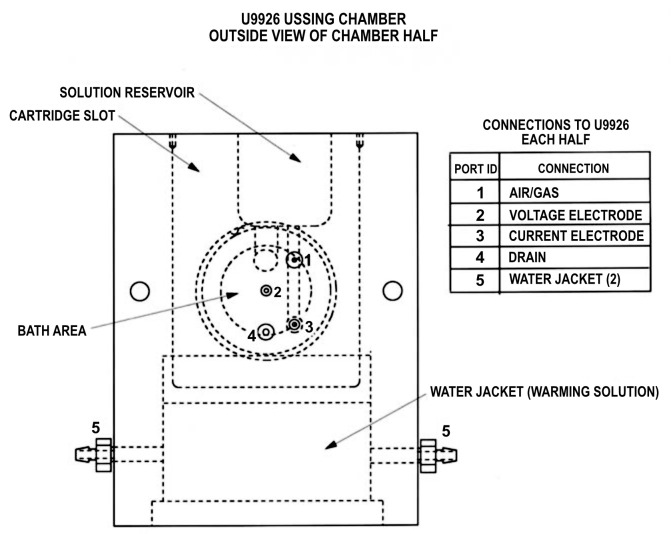
Figure 1. Ussing chamber port connections.
Used with permission from Warner Instruments, ©2022.
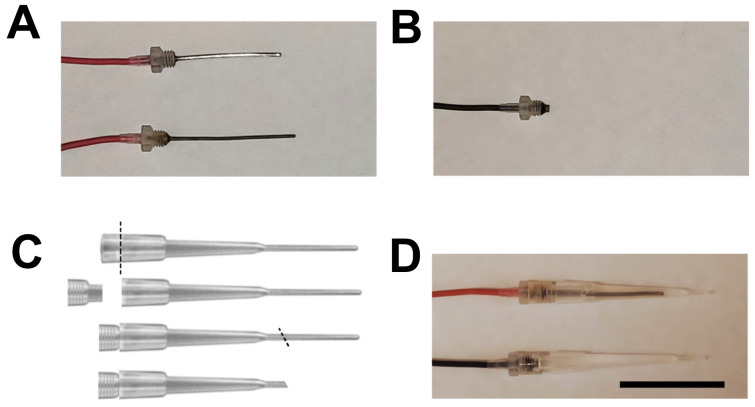
Figure 2. Electrode preparation.
A. Shown are two Ag wire electrodes, one is cleaned (top) the other is fully chlorided (bottom). B. Ag pellet electrode. C. Tip preparation. Diagonal lines indicate the position and orientation to cut the tips with a safety razor blade. The top is cut so that the tips fit well into the electrode adapters, the bottom is cut after the electrodes are filled by agar and assembled. D. Fully assembled electrodes. Scale bar: 3 cm.
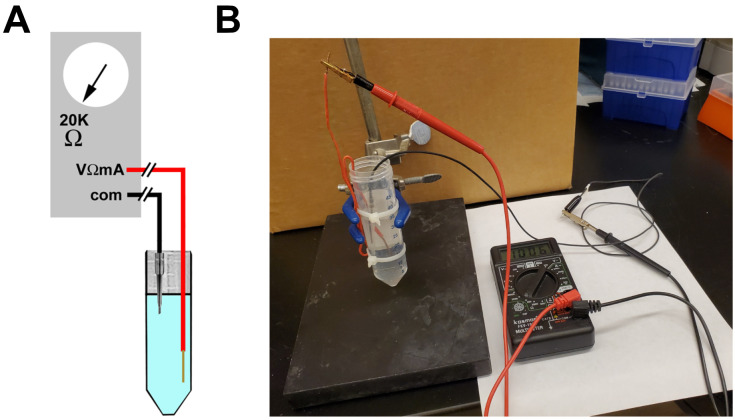
Figure 3. Testing electrodes.
A. Schematic of the electrode testing apparatus. The red wire is a copper wire connecting the Volt-Ohmmeter to the Ringer’s Saline, and the black wire contains an assembled bridge electrode. B. Photograph showing the apparatus and measurement of a functional electrode.
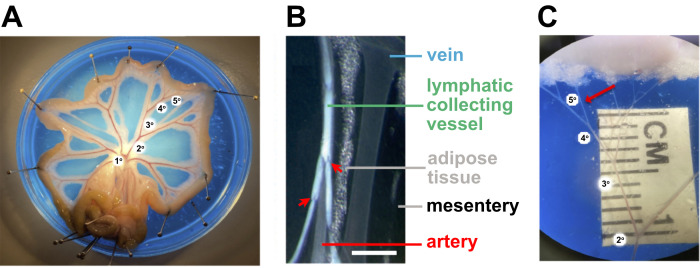
Figure 4. Isolation of small mesenteric arteries for vascular studies.
A. The isolated rat mesenteric bed is pinned out in a Petri dish containing colored silicon elastometer and filled with physiological salt solution. B. Magnified brightfield image showing distinctions between different classes of vessels and tissue in the mesentery. Scale bar: 1 mm. C. Magnified image illustrating the 5th order artery selected for cannulation after removal of the adipose tissue, veins, lymphatic vessels, and connective tissue. Figure 3A and 3C were provided by Perenkita Mendiola at the Dept. of Cell Biology and Physiology, Univ. of New Mexico, Albuquerque, NM, and used with permission ( Wenceslau et al., 2021 ). Figure 3B is used with permission from Sabine et al. (2018) .
Figure 5. Ussing chamber apparatus.
A. Exploded view, used with permission from Warner Instruments, ©2022. B. Photograph showing a fully assembled and connected Ussing chamber.
Figure 6. Tissue cassette designed for vessel analysis.
A. Diagram of a tissue cassette, used with permission from Warner Instruments, ©2022. The sample is placed within the two halves of the cassette. B. Arrow indicates the horizontal opening of the cassette in the photograph. Scale bar, 1 cm. C–D. Magnified image of the cassette showing the opening with an approximate area of 0.26 mm2 (red line; C), which can be completely covered with a longitudinally sectioned vessel (border of the vein marked in blue; D). Scale bars, 750 μm (C) and 650 μm (D). Numerical marks in C and D reflect eyepiece magnification. Figures B-D are from Maier-Begandt et al. (2021) , used with permission.
Data analysis
For all analysis, export the data from Acquire & Analyze to Excel. Measurements are most accurate when calculated as the average of 20–100 values, obtained when the sample has reached a steady state equilibrium.
Transendothelial electrical resistance (TEER; Ohm x cm2) is calculated by averaging multiple values collected over the time course and multiplied by the sample area (0.26 mm2 for the custom vessel tissue cassette). TEER is a measure of overall tissue resistance, reflecting the contributions of both the transcellular and paracellular ion transport pathways.
Short circuit current (Isc; μA) is directly obtained with voltage clamped at 0 mV. Isc represents the external current needed to clamp voltage potential to zero, and provides a measure of total ion movement due to active transport.
Voltage potential (V; mV) is directly obtained with current clamped at 0 μA.
Dilution potential (mV) is calculated by VDP = Vundil – V1:4 dil, where Vundil and V1:4 dil are voltage potential measurements obtained from undiluted Ringer’s Saline and Diluted Ringer’s Saline/Mannitol (1:4). The effect of an ion gradient on voltage potential is influenced by differences in permeability to cations and anions, and provides a method to measure relative ion permeability.
-
Calculations for ion permselectivity are based on an approach by Hou et al. (2005) , which was derived from the Goldman-Hodgkin-Katz equation. To calculate relative permeability (PNa/PCl) from dilution potential measurements, use the following equations:
-
ν = VDP/qkT
q=1, for NaCl; q = charge
kT= 8.617 × 10-2 mV/deg K × 310.1 deg K = 26.72 mV at T=37°C
k = Boltzmann constant; T = temperature
-
PNa/PCl = – (ε – eν)/(1 – ε·eν)
ε = 4 (dilution factor)
e = Euler’s number
-
-
Example experiment
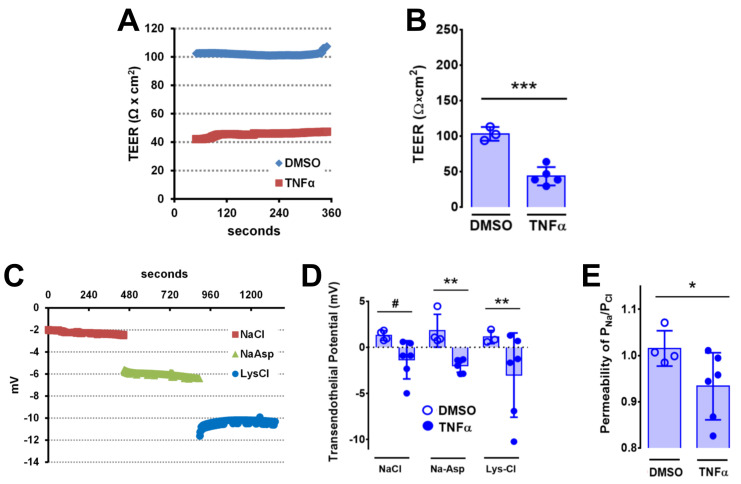
Veins were incubated in either 50 ng/mL TNFα or vehicle control at 37°C for 30 min prior to analysis. As shown in Figure 7, veins showed a ~2.4-fold decrease in TEER 30 min after TNFα treatment, consistent with an effect of this pro-inflammatory cytokine on vascular barrier function. Changes in vessel ion permselectivity due to TNFα were examined by measuring vessel transendothelial potential when the current was clamped to 0 mA. TNFα induced a change in vein polarity from 1.3 mV under control conditions of −1.1 mV in normal Ringer’s Saline buffer. Vein potential showed a comparable TNFα-dependent change in polarity when aspartate was substituted for Cl−, or lysine substituted for Na+, suggesting that the effect of TNFα on transendothelial potential involved changes in both Na+ and Cl− permeability. Dilution potential measurements showed that TNFα treatment specifically increased venous Cl− permeability, as reflected by a decrease in PNa/PCl. These changes in ion permeability suggest that the effect of TNFα on endothelial barrier function is not due to overt disruption of tight junctions creating a resistance-free path of diffusion. Determining whether these TNFα dependent changes in ion permeability are due to altered ion channel or claudin regulation may provide potential targets to prevent vascular leak in response to inflammation.
Ex vivo second and third order mesenteric veins from C57BL/6 mice were treated with vehicle (0.1% DMSO in PBS) or TNFα (50 ng/mL) for 30 min and mounted on Ussing chamber cassettes in physiologic Ringer’s solution. A–B. Transendothelial electrical resistance (TEER) was measured and calculated as described above, where A is an example trace (DMSO, blue; TNFα, red) and B represents aggregate data. *** P=0.0005 (B) by unpaired two-tailed t-test, n=3–5 venules. C. Example traces of an individual venule treated with TNFα (50 ng/mL) for 30 min and then measured in Ringer’s saline (NaCl, red), Ringer’s Na-Aspartate (NaAsp, green), and Ringer’s Lysine-Cl (LysCl, blue). D. Aggregate data demonstrating that veins show increased changes in ion permeability in response to TNFα. Open circles represent vehicle controls, filled circles represent vessels treated with TNFα (50 ng/mL). # P=0.064 and ** P=0.0093, analyzed by two-way ANOVA and Fisher’s LSD test. In both cases (D), n=5 venules for each treatment. E. Ion permselectivity was calculated as described above. * P=0.034 by one-way ANOVA Fisher’s LSD test, n=4–5 venules. Graphs in B, D, and E represent mean ± SD, and are reproduced from Maier-Begandt et al. (2021) , used with permission.
Figure 7. Effects of TNFα on vein electrophysiology.
Notes
A video is available showing dissection of mouse 4th order mesenteric arterioles by Jadeja et al. (2015) . The protocol described here is designed for Ussing chamber measurements using 2nd and 3rd order venules and arterioles.
Bridge electrodes need to be freshly made on the day of the experiment. The inability to get a reproducible current or voltage measurement is an indication that an electrode is failing. To plan for this possibility, it is best to make spare electrodes, so that they can be readily substituted.
Ringer’s solutions should be used within a month after making them. They should be stored refrigerated, and pre-warmed to 37°C before use.
Measurements using Ringer’s Na-Aspartate and Ringer’s Lysine-Cl ( Alexandre et al., 2005 ) provide a method to validate PNa/PCl determination, by replacing Cl– and Na+ with relatively non-permeable, comparably charged amino acids, to isolate the contributions to transendothelial current of Na+ and Cl–, respectively.
The protocol described here focuses on barrier function measurements and how they are impacted by TNFα. However, this approach may be readily adapted to other measurements. For instance, blood vessels from transgenic mice can be studied using this approach to define roles for specific proteins in endothelial barrier function. Since ion permeability is the combination of flux through the paracellular route (through tight junctions) and the transcellular route (though plasma membrane ion channels), pharmacologic agents that specifically inhibit ion channels can be used to account for transport through the transcellular route (MacAulay, 2021; Schultz et al., 1999 ; Verkman and Galietta, 2009).
Recipes
-
3 M KCl (100 mL)
22.37 g
-
Agar solution (100 mL in 3 M KCl)
Agar 3.3 g
To dissolve, the agar requires a double boiler, do not microwave directly. Instead, place 200 mL of water into a large beaker and microwave the water alone to near boiling. Place the beaker onto a hot plate (setting = ~7–8) and the bottle with agar into it with the cap slightly loosened. Allow to heat until the agar solution is uniformly melted, then reduce to a setting of 4–5, to allow the agar to partially cool. Note that this is not the same as agarose. Agar solution can be made in advance and stored at 4°C.
-
70% ethanol
Dliute 70 mL of absolute ethanol with 30 mL of H2O
-
10 M NaOH solution (100 mL)
40.0 g
-
1 M HEPES (100 mL)
Dissolve 23.8 g of HEPES in 80 mL of H2O
Adjust to pH 7.5 with 10 M NaOH solution
Dilute to 100 mL total volume
-
Ringer’s Saline (500 mL)
140 mM NaCl: 4.09 g
2 mM CaCl2·2H2O: 147 mg
1 mM MgCl2·6H2O: 102 mg
10 mM glucose: 901 mg
10 mM HEPES: 1.19 g
Adjust to pH 7.3 with 1 M HEPES, and then filter (0.2 μm). The solution can be made up to a month in advance, stored refrigerated, and pre-warmed to 37°C before use.
-
Ringer’s Mannitol (500 mL)
280 mM Mannitol: 25.5 g
2 mM CaCl2·2H2O: 147 mg
1 mM MgCl2·6H2O: 102 mg
10 mM glucose: 901 mg
10 mM HEPES: 1.19 g
Adjust to pH 7.3 with 1 M HEPES, and then filter (0.2 μm). The solution can be made up to a month in advance, stored refrigerated, and pre-warmed to 37°C before use.
-
Diluted Ringer’s Saline/Mannitol (1:4)
Mix 25 mL of Ringer’s Saline with 75 mL of Ringer’s Mannitol on the day of use. Pre-warm to 37°C before use.
-
Ringer’s Lysine-Cl (500 mL)
140 mM Lysine-HCl: 12.8 g
2 mM CaCl2·2H2O: 147 mg
1 mM MgCl2·6H2O: 102 mg
10 mM glucose: 901 mg
10 mM HEPES: 1.19 g
Adjust to pH 7.3 with 1 M HEPES, and then filter (0.2 μm). The solution can be made up to a month in advance, stored refrigerated, and pre-warmed to 37°C before use.
-
Ringer’s Na-Aspartate (500 mL)
140 mM Na-Aspartate: 12.1 g
2 mM CaCl2·2H2O: 147 mg
1 mM MgCl2·6H2O: 102 mg
10 mM glucose: 901 mg
10 mM HEPES: 1.19 g
Adjust to pH 7.3 with 1 M HEPES, and then filter (0.2 μm). The solution can be made up to a month in advance, stored refrigerated, and pre-warmed to 37°C before use.
-
1,000× TNFα (50 μg/mL)
Dissolve in DMSO and store refrigerated up to a month in advance. On the day of use, dilute into Ringer’s Saline and pre-warm to 37°C.
Acknowledgments
This work was supported by R01-HL137112 (to B.E.I. and M.K.), R01-AA025854 (to M.K.), R01-HL120840 (to B.E.I.) and DFG SFB914 A02 (to D.M.-B.). The protocol was used in Maier-Begandt et al. (2021) A venous-specific purinergic signaling cascade initiated by Pannexin 1 regulates TNFα-induced increases in endothelial permeability, Sci Signal 14(672):eaba2940.
Competing interests
The authors declare that we have no competing interests.
Ethics
Samples were from mice that were cared for under the provisions of the University of Virginia and Emory Animal Care and Use Committees following the National Institutes of Health (NIH) guidelines for the care and use of laboratory animals.
Citation
Readers should cite both the Bio-protocol article and the original research article where this protocol was used.
Q&A
Post your question about this protocol in Q&A and get help from the authors of the protocol and some of its users.
References
- 1. Adil M. S. and Somanath P. R.(2021). Endothelial Permeability Assays In Vitro . Methods Mol Biol 2367: 177-191. [DOI] [PubMed] [Google Scholar]
- 2. Alexandre M. D., Lu Q. and Chen Y. H.(2005). Overexpression of claudin-7 decreases the paracellular Cl- conductance and increases the paracellular Na+ conductance in LLC-PK1 cells . J Cell Sci 12): 2683-2693. [DOI] [PubMed] [Google Scholar]
- 3. Anderson J. M. and van Itallie C.(2009). Physiology and function of the tight junction. Cold Spring Harb Perspect Biol 1(2):a002584.
- 4. Buschmann I., Pries A., Styp-Rekowska B., Hillmeister P., Loufrani L., Henrion D., Shi Y., Duelsner A., Hoefer I., Gatzke N., et al.(2010). Pulsatile shear and Gja5 modulate arterial identity and remodeling events during flow-driven arteriogenesis. Development 137(13): 2187-2196. [DOI] [PubMed] [Google Scholar]
- 5. Clarke L. L.(2009). A guide to Ussing chamber studies of mouse intestine. Am J Physiol Gastrointest Liver Physiol 296(6): G1151-1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Crone C. and Christensen O.(1981). Electrical resistance of a capillary endothelium. J Gen Physiol 77(4): 349-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dubrovskyi O., Birukova A. A. and Birukov K. G.(2013). Measurement of local permeability at subcellular level in cell models of agonist- and ventilator-induced lung injury. Lab Invest 93(2): 254-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Durán W. N., Sánchez F. A. and Breslin J. W.(2008). Chapter 4- Microcirculatory Exchange Function. Tuma, R. F., Durán, W. N. and Ley, K.(Eds.). In: Microcirculation(Second Edition). Academic Press, 81-124. [Google Scholar]
- 9. Granger D. N., Rodrigues S. F., Yildirim A. and Senchenkova E. Y.(2010). Microvascular responses to cardiovascular risk factors. Microcirculation 17(3): 192-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hatou S., Yamada M., Akune Y., Mochizuki H., Shiraishi A., Joko T., Nishida T. and Tsubota K.(2010). Role of insulin in regulation of Na+-/K+-dependent ATPase activity and pump function in corneal endothelial cells . Invest Ophthalmol Vis Sci 51(8): 3935-3942. [DOI] [PubMed] [Google Scholar]
- 11. Herrmann J. R. and Turner J. R.(2016). Beyond Ussing's chambers: contemporary thoughts on integration of transepithelial transport. Am J Physiol Cell Physiol 310(6): C423-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hilfenhaus G., Nguyen D. P., Freshman J., Prajapati D., Ma F., Song D., Ziyad S., Cuadrado M., Pellegrini M., Bustelo X. R., et al.(2018). Vav3-induced cytoskeletal dynamics contribute to heterotypic properties of endothelial barriers. J Cell Biol 217(8): 2813-2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hou J., Paul D. L. and Goodenough D. A.(2005). Paracellin-1 and the modulation of ion selectivity of tight junctions. J Cell Sci 21): 5109-5118. [DOI] [PubMed] [Google Scholar]
- 14. Jadeja R. N., Rachakonda V., Bagi Z., Khurana S.(2015). Assessing Myogenic Response and Vasoactivity In Resistance Mesenteric Arteries Using Pressure Myography. J Vis Exp(101): e50997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Joffre J., Judith J., Ince C. and Ait-Oufella H.(2020). Endothelial Responses in Sepsis. Am J Respir Crit Care Med 202(3): 361-370. [DOI] [PubMed] [Google Scholar]
- 16. Laukoetter M. G., Nava P., Lee W. Y., Severson E. A., Capaldo C. T., Babbin B. A., Williams I. R., Koval M., Peatman E., Campbell J. A., et al.(2007). JAM-A regulates permeability and inflammation in the intestine in vivo . J Exp Med 204(13): 3067-3076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lindemann B.(2001) Hans Ussing, Experiments and Models. J Membr Biol 184(3):203-10. [DOI] [PubMed] [Google Scholar]
- 18. MacAulay N.(2021). Molecular mechanisms of brain water transport. Nat Rev Neurosci 22(6): 326-344. [DOI] [PubMed] [Google Scholar]
- 19. Maier-Begandt D., Comstra H. S., Molina S. A., Kruger N., Ruddiman C. A., Chen Y. L., Chen X., Biwer L. A., Johnstone S. R., Lohman A. W., et al.(2021). A venous-specific purinergic signaling cascade initiated by Pannexin 1 regulates TNFalpha-induced increases in endothelial permeability. Sci Signal 14(672): eaba2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Molina S. A., Stauffer B., Moriarty H. K., Kim A. H., McCarty N. A. and Koval M.(2015). Junctional abnormalities in human airway epithelial cells expressing F508del CFTR. Am J Physiol Lung Cell Mol Physiol 309(5): L475-487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rosenberg G. A.(2012). Neurological diseases in relation to the blood-brain barrier. J Cereb Blood Flow Metab 32(7): 1139-1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sabine A., Davis M. J., Bovay E. and Petrova T. V.(2018). Characterization of Mouse Mesenteric Lymphatic Valve Structure and Function. Methods Mol Biol 1846: 97-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schoknecht K. and Shalev H.(2012). Blood-brain barrier dysfunction in brain diseases: clinical experience. Epilepsia 6: 7–13.. [DOI] [PubMed] [Google Scholar]
- 24. Schultz B. D., Singh A. K., Devor D. C. and Bridges R. J.(1999). Pharmacology of CFTR chloride channel activity. Physiol Rev 79(1 Suppl): S109-144. [DOI] [PubMed] [Google Scholar]
- 25. Ussing H. H. and Zerahn K.(1951). Active transport of sodium as the source of electric current in the short-circuited isolated frog skin. Acta Physiol Scand 23(2-3): 110-127. [DOI] [PubMed] [Google Scholar]
- 26. Verkman A. S. and Galietta L. J.(2009). Chloride channels as drug targets. Nat Rev Drug Discov 8(2): 153-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang Y., Gu Y., Granger D. N., Roberts J. M. and Alexander J. S.(2002). Endothelial junctional protein redistribution and increased monolayer permeability in human umbilical vein endothelial cells isolated during preeclampsia. Am J Obstet Gynecol 186(2): 214-220. [DOI] [PubMed] [Google Scholar]
- 28. Wenceslau C. F., McCarthy C. G., Earley S., England S. K., Filosa J. A., Goulopoulou S., Gutterman D. D., Isakson B. E., Kanagy N. L., Martinez-Lemus L. A., Sonkusare S. K., et al.(2021). Guidelines for the measurement of vascular function and structure in isolated arteries and veins. Am J Physiol Heart Circ Physiol 321(1): H77-H111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wigham C. G., Turner H. C., Swan J. and Hodson S. A.(2000). Modulation of corneal endothelial hydration control mechanisms by Rolipram. Pflugers Arch 440(6): 866-870. [DOI] [PubMed] [Google Scholar]