Abstract
Background
Despite modern therapeutic armamentariums, malaria remains a 21st century public health menace. The issue of combating malaria is the ever-growing resistance to high-tech medications in which novel phytomedicines are highly demanding, a rapidly expanding research avenue. In Ethiopian folklore medicine, Urtica simensis has been used to treat malaria by drinking its juice after the dry roots have been mashed and combined with water. Hitherto, no in vivo study has been reported in the literature so far. To substantiate this folkloric claim, the present work herein was done.
Methods
An acute oral toxicity study was conducted as per the standard protocol. To rule out, the extract's inherent potential effects on bodyweight, basal body To, and PCV changes were tracked for two weeks. A four-day suppressive model and a curative assay model were utilized to investigate the antimalarial activity of the plant. Percent parasitemia suppression, packed cell volume, mean survival date, bodyweight, and rectal body temperature were used to determine antimalarial activity.
Result
An acute toxicity study reveals that Urtica simensis was atoxic at a dose of 2000 mg/kg. It also affirms that U. simensis is free from intrinsic potential effects of interfering with bodyweight, temperature, and packed cell volume evolution. Both crude extract and its solvent fractions at all test doses exerted significant (P < 0.001) inhibition of parasitemia as compared to the control group. CF400 mg/kg provided the greatest chemosuppressive effect (79.24%). In a curative experiment, crude extract and CF were able to prevent the cardinal indications of P. berghei-induced malaria, such as weight loss, hypothermia, parasitemia, and anemia. Both crude extracts and their solvent fractions prolong survival dates.
Conclusion
The antimalarial activity of the crude extract and its solvent fractions was promising, confirming previous assertions. As a result, more research studies into chemical entities may be required.
1. Introduction
1.1. Background
Although malaria is a preventable and curable malady, it still remains a major public health menace. In 2019, there were an estimated 229 million malaria cases worldwide, with 409000 fatalities. In 2019, Africa accounted for more than 94% (215 million) of global cases and deaths (386,000). Due to a weakened immune system, children under the age of five and pregnant women are the most vulnerable [1]. Malaria infected 33.2 million pregnant women in Africa in 2019, accounting for 35% (11.6 million) of all infections in 2019. Children under the age of five will account for 67% of all malaria-related deaths worldwide in 2019 [2].
It is one of the top 10 causes of mortality in Ethiopia, accounting for 2% of all deaths [3]. The parasites Plasmodium falciparum and Plasmodium vivax are by far the most common and widely dispersed. P. vivax was responsible for only 2.3% of those cases [4]. Despite the fact that both malaria cases and deaths have decreased in recent years, progress is endangered by the emergence of insecticide-resistant mosquitoes, drug-resistant malaria parasites, and a lack of a successful and effective licensed malaria vaccine [5].
Since the dawn of time, mankind has employed medicinal plants to combat ailments such as malaria. According to the World Health Organization, about three-quarters of the world's population receives healthcare from plants and their extracts. Traditional medicine is used to meet some of the most basic healthcare needs in a number of African, Asian, and Latin American countries [6]. Over 80% of the population in Ethiopia relies on traditional medicine to cure a variety of illnesses because of the cultural acceptability of healers and the relative affordability of traditional medicine [6, 7]. Furthermore, botanicals account for more than a quarter of all modern pharmaceutical medications [8].
Many herbal plants around the world have antimalarial properties due to secondary metabolites such as tannins, alkaloids, saponins, flavonoids, steroids, and terpenoids [9]. Both quinine and artemisinin derivatives, now used to treat severe falciparum malaria, are natural products. In vitro and in vivo studies looking for novel antimalarial medicines of natural origin were inspired by the development of these two key treatments from natural sources [10].
Traditional herbalists use the plant without assessing and optimizing the amounts of physiologically active components. At a dose of 200 mg/kg, extract fractions from Withania somnifera had a considerable suppressive effect on malaria [11]. Similarly, crude extracts of C. myricoides, D. angustifolia, and A. debrana have potent anti-P. berghei properties in mice. These are only a few of the many therapeutic plants whose pharmacological effects have been approved [12].
Urtica simensis belongs to the family Urticaceae and genus Urtica. The Urticaceae family, also known as the nettle family, is made up of 48 genera and about 2000 species of plants. The tropical and subtropical regions are home to the majority of these plant species. Urtica is derived from the Latin word “urere,” which means “to burn.”
After having been stung by nettles, humans are generally able to recognize them [13, 14]. Medicinally, several Urtica species (particularly Urtica dioica L.) are used to cure a number of maladies. Urtica simensis is an indigenous nettle species found only in Ethiopia. The plant grows throughout the year at 1500–3500 m above sea level in Ethiopia's highlands, particularly in the North and South Gondar, North and South Wello, North Shewa, Wag Hamra, highlands of Sidama zone in the Southern region, and Arsi zone of Oromia region. It is mostly seen in the vicinity of dwellings. The plant is commonly known as “Nattle,” “sama” (Amharic) [15]. Urtica simensis is a dark green perennial wild plant that has been used for food in the past, especially during droughts. It has a lot of promise and can help with food security by meeting human nutritional needs [16, 17]. Urtica simensis is well known for its stinging hairs that grow beneath the stems and on the lower leaf surface. The plant is completely covered in stinging hairs. Herbal nettles are one meter tall, dioecious, erect, and nonbranched. The leaves are opposite and simple, with united stipules and interpetiolar lengths of 0.5–1 cm. The leaves are slightly cordate at the base, broad and sharp at the apex, and serrated at the border. Urtica simensis has unisexual, regular blooms, and the fruit is about 2 mm long [18].
Different portions of the U. simensis plant are used for the treatment of various illnesses and ailments, according to ethnobotanical studies on traditional medicinal usage of the plant in Ethiopia. To treat gonorrhea, the root and leaf parts of the U. simensis plant are powdered, mixed with water, and the filtrate is consumed. Washing the diseased area of the body once a day with a root and leaves infusion is also used to cure gonorrhea [15, 19]. The plant's leaves and young twigs, on the other hand, are crushed, and the resulting leaf juice is creamed with butter and applied topically to wound infections. In two varieties of preparation, the fresh leaves of U. simensis are used to treat gastritis. As a result, the leaves are roasted, ground into smaller bits, and the juice is consumed orally, or the fresh leaves can be prepared and eaten with “injera” [15, 20]. It was said to be used to treat acute stomach pain (by drinking the sap orally) and body edema (heat and put the leaf on the affected area topically) 16, a common cold (fresh root ground and taken orally) [21], Rh-factor, and heart failure (number 22) (fresh leaf steam vapor allowed to enter nasally and fumigated whole body) [22]. Furthermore, after being crushed and dried, the plant's root is mixed with fresh water and consumed along with a large amount of milk to treat malaria [23]. Its leaf extract possesses antibacterial, antifungal [24], antidiabetic [25], and cardioprotective [26] properties. The antimalarial activity of this plant's roots has not been scientifically confirmed. The purpose of this study is to test the antimalarial activity of both the crude extract and solvent fractions of Urtica simensis root. The study also aimed to shed light on the phytochemicals responsible for the plant's activity by examining the plant's phytochemical composition. The outcomes of this study may help to develop new antimalarial drugs that solve existing antimalarial drug concerns.
2. Materials and Methods
2.1. Materials
2.1.1. Drugs and Chemicals
The following drugs, chemicals, and instruments were used in the experiment during the study period: absolute methanol (ReAgentchem. Ltd., India), 0.9% normal saline (Cadila Pharmaceuticals, Bengaluru, India), distilled water (EPharm, Ethiopia), chloroform, ethyl acetate, n-butanol, examination glove, 0.5% trisodium citrate, 95% sulfuric acid (Fisher Scientific, UK), Mayer's reagent, Dragendorff's reagent, glacial acetic acid (Lobe Chemi, India), benzene (Nice Laboratory Reagent, Kerala, India), ammonia solution, FeCl3 (Super Tek Chemicals), 10% ammonium hydroxide (Rankem, Mumbai, India), 0.01 N sodium hydroxide (Central Drug House India), acetic anhydride and Mayer's reagent (May and Baker Ltd., England), dimethyl sulfoxide (DMSO), chloroquine 500 mg tablet, Giemsa stain solution, and syringe which is analytical graded (Addis Ababa Pharmaceutical and Chemical Suppliers).
2.1.2. Instruments, Apparatus, and Supplies
The mini-orbital shaker (MaxQ 2000, USA), rotary evaporator (rotary evaporator RE300, UK), digital weighing balance (Mettler Toledo, Switzerland), separatory funnel, lyophilizer (Wagtech Jouan Nordic DK-3450), deep freezer, light microscope (Olympus N-120A, Philippines), microscope slide (LANCONCO, Freeze Dry System, USA), grinder (KZ-III, Wuhan, China), Whatman filter paper (number 1) (Schleicher and Schuell Microscience GmbH, Germany), permanent marker, microhematocrit centrifuge (Hettich, Germany), microhematocrit reader, and digital thermometer were used in the experiment during the study period.
2.1.3. Experimental Animals
Healthy Swiss albino mice (aged 8–12 weeks, weighting 27–35 g, either sex) were used. They were housed in raised mesh bottom cages to prevent coprophagy in conventional laboratory settings (25.2°C, 12 h light and dark cycle). They were fed conventional pelleted feed and free access to water. They were acclimatized for 5 days before being divided into groups at random in each phase of the experiment. All procedures in this study were carried out in accordance with local and international ethical guidelines, including the Basel Declaration [27], ICLAS Ethical Guideline [28], and European Union directive [29].
2.2. Methods
2.2.1. Collection and Authentication of Plant Materials
The root of Urtica simensis was collected at Debre Tabor district, Amhara Regional state, about 655 km northwest of Addis Ababa in January 2020. Botanical identification and authentication were done by an expert taxonomist, Dr. Getinet Masresha, at Department of Biology College of Natural and Computational Sciences, University of Gondar, and a voucher specimen Wor3/2020 was kept there for future reference.
2.2.2. Preparation and Extraction of Plant Materials
The roots were first washed under flowing tap water to eliminate dirt or dust and then dried in the shade in the pharmacology laboratory. Using a grinder, it was mechanically chopped into coarse powder. Until extraction, the powder sample was weighed and stored in airtight containers.
2.2.3. Extraction Procedure for Crude Extract
This research made use of a 80% methanol because it can extract a wide variety of polar and moderately polar compounds [30]. The 80% methanol crude extract of root of U. simensis was prepared by the cold maceration technique. After weighing 1 kg of coarsely powdered root with a sensitive digital weighing balance, it was steeped in an 80% methanol Erlenmeyer conical flask. After that, the flask was shaken at 120 rpm for 72 h at room temperature with a mini-orbital shaker. A Whatman filter paper (No. 1) was used to refine the filtrate after it had been passed through two muslin cloth filter stages. A fresh solvent of 80% methanol was used to remacerate the leftover residue or marc twice for a total of six days to maximize production. After successive filtration, methanol was removed by a rotary evaporator set to 40°C and lyophilized to remove water. The % yield was computed by the following equation and kept at −20°C until used [31].
| (1) |
2.2.4. Fractionation of the Crude Extract
In a separatory funnel system, methanol extracts of the root of U. simensis underwent fractionation using a solvent-solvent partitioning technique using solvents of different polarities. Because, this process is critical as the initial step in separating chemicals from crude natural product extracts on a large scale [30]. It was done by initially measuring 85 g of crude extract, transferring it in to a clean Erlenmeyer conical flask containing 300 ml of distilled water, allowed to suspend, and then shaken to dissolve in solvent. After transferring the mixture to a separating funnel, 300 ml of chloroform was added. The chloroform fraction was collected in a separate flask. Furthermore, 300 ml of chloroform was added twice as portrayed above and finally concentrated and dried using a rotary evaporator and an oven, respectively. Ethyl acetate (300 ml) was poured into the marc thrice consecutively upon collecting the filtrate. Then, separated ethyl acetate filtrate was concentrated and dried using a rotary evaporator and an oven, respectively. Upon lyophilization, the leftover aqueous fraction was retrieved. Finally, the % yield was computed by the following equation:
| (2) |
2.2.5. Determination of Acute Oral Toxicity Potential
The median lethal dose of root extract of U. simensis was predicted in female mice, following the OECD guideline 425 with some modifications [32]. In brief, twelve animals of 8–12 weeks were used in experimentation. Then, they were arbitrarily divided into two groups. Following a 4 h fast, all animals were weighed, and doses were established depending on their physical weight. To do this, a limit dose of 2 g/kg extract dissolved in 2% tween 80% (TW80) was administered PO to a single female mouse and observed for gross toxicity and deaths within 24 h. Then, five other mice were sequentially treated and observed continuously for 2 weeks for toxicity patterns [33]. The other six animals of the control group were treated with TW80 10 ml/kg with identical scheme of administration. Likewise, bodyweight, rectal body temperature, and packed cell volume changes were recorded before treatment (baseline) and daily for two weeks.
2.3. In Vivo Antimalarial Probing
2.3.1. Parasite Inoculation
Ethiopian Health and Nutrition Research Institute (EHNRI) was the source of the chloroquine-sensitive Plasmodium berghei (ANKA) strain. This parasite enables us to produce a rodent model of malaria that mimics human malaria infection [34, 35]. The parasites were kept alive by serially transferring blood every week. Donors were albino mice which attains a 20–30% parasitemia level. Upon decapitation, their blood was drawn into a test tube. After dilution of blood with normal saline (0.9%), each mouse received 0.2 ml of diluted blood with 1 × 10 [7] Plasmodium berghei-infected red blood cells [36].
2.3.2. Grouping of Animals and Experimental Procedure
Female animals weighing 27–35 g were arbitrarily divided into five groups of 6 per group. Groups I–III received 100 mg/kg (US100), 200 mg/kg (US200), and 400 mg/kg (US400), respectively. Group IV and V received distilled water or TW80 (1 ml/100 g) and chloroquine phosphate 25 mg/kg (CQ25), respectively.
In a study of fractions, animals were randomly assigned into three treatment groups and two controls, six animals per group. Negative controls received the vehicle used for reconstitution. Treatment groups received 100 mg/kg, 200 mg/kg, and 400 mg/kg of corresponding fractions. Positive controls were treated with chloroquine. Models employed for this study were tested by Peter's test (4-day suppression test) and Rane's (curative) test [37].
2.3.3. Peter's Test
Peter' test was used to determine the treatment's schizonticidal activity using the approach provided by Peter et al. [38]. Three hours after infection, on day 0, treatment was initiated and then continued daily until day 3. Each animal had a sample taken from its tail after therapy was completed in order to measure parasitemia and the percentage of inhibition.
2.3.4. Curative Assay Model
The methodology described by Ryley and Peters was used to investigate the curative ability of the methanolic crude extract and the most active solvent fraction of U. simensis in Peter's test [39]. Mice were randomly divided into four groups 72 hours after infection and given the appropriate doses once daily for 4 days. The parasitemia level was assessed daily for five days employing a thin blood film dyed with Giemsa.
2.3.5. Mean Survival Time Determination
Over the course of 30 days (D0–D29), the average survival time (days) of mice was calculated arithmetically, as [40]
| (3) |
2.3.6. Packed Cell Volume Determination
To anticipate the effectiveness of the test extracts in halting hemolysis caused by rising parasitemic levels, the number of packed cells was analyzed. Each mouse's tail was scraped, and blood drawn into heparinized capillary tubes. After filling the capillary tubes with blood up to three-fourths of their volume, they were then sealed shut with sealing clay. Afterward, the sealed end of the tubes was placed in a microhematocrit centrifuge with 11000 rpm and spun for five minutes. Using a basic microhematocrit reader, we were able to determine PCV levels. The ratio of RBCs to plasma is assessed before and after parasite inoculation and therapy using the following formula:
| (4) |
2.3.7. Appraisal of Parasitemia Level
On day 4 for Peter's test and on days 3–7 for Rane's test, thin smears of blood were retrieved from the tail of individual animal, fixed for 15 min in pure methanol, and dyed for 15 min with 10% Geimsa stain at pH 7.2 for the smears on microscope slides (76 × 26 mm). Using distilled water, the stained slides were gently cleaned and allowed to air dry at room temperature. Each mouse had two stained slides analyzed using an Olympus microscope. The mean parasitemia was determined by considering the three separate fields on each slide, as shown in the graph [41].
| (5) |
Ultimately, the potential suppressive effect of the treatments was compared to that of infected but untreated groups, and parasitemia suppression was computed using the following equation.
| (6) |
2.3.8. Evaluation of Bodyweight and Rectal Temperature Flux
Bodyweight of animals was evaluated preinfection (day 0) and after treatment (day 4) in case of Peter's test. Furthermore, rectal To was evaluated by a rectal thermometer preinfection at day 0 and on day 4 after treatment. Bodyweight and rectal To were evaluated in day 3 and day 7 by using Rane's assay.
| (7) |
2.4. Preliminary Phytochemical Profiling
Standard assays were employed to conduct qualitative phytochemical studies on Urtica simensis root extracts [42–44].
2.5. Data Analysis
The study's findings are summarized as mean ± SEM. One-way analysis of variance (ANOVA) was performed followed by the Tukey HSD post hoc test for multiple comparisons between tests. Two-way analysis of variance was performed followed by Bonferroni's post hoc test for tests in outcome variable before and after treatment using IBM SPSS for window (Version 24.0) statistical package. P < 0.05 was considered statistically significant at a 95% confidence interval.
2.6. Data Quality Assurance
Data quality was maintained by categorizing experimental laboratory animals by a random sampling statistical technique, collecting data of all indicators blindly, maintaining and applying standard procedures consistently, and using scientifically labeled instruments.
3. Results
This study authenticated the percentage yield, phytochemical ingredients, the acute oral toxicity, and the antimalarial activity of 80% hydromethanolic crude extract and solvent fractions of roots of U. simensis against P. berghei in mice.
3.1. Extraction Yield of Hydromethanolic Crude Extract and Solvent Fractions
Traditionally, the roots of U. simensis are taken after being crushed and mixed with fresh water for the treatment of malaria. Since the role of water is as a vehicle, 80% MeOH was used so as to extract most of the constituents in the roots that might explain its activity. Generally, hydroalcohols are related with higher extract yields [45]. Accordingly, 80% methanol root extract of U. simensis afforded a dark bluish powder with percentage yield of 153 g (15.3% w/w) and stored in a refrigerator at 4°C until use (Table 1).
Table 1.
Quantity and quality of hydromethanolic crude extract and solvent fractions.
| Extracting solvent | Physical description of the extract | Yield (gm) | % yield (W/W) |
|---|---|---|---|
| 80% hydromethanol | Dark blue powder | 153 | 15.30 |
| Chloroform | Brown powder | 21.85 | 25.71 |
| Ethyl acetate | Gummy dark brown semi powder | 31.54 | 37.11 |
| Aqueous | Reddish brown powder | 31.01 | 35.31 |
3.2. Preliminary Phytochemical Profiling
The preliminary phytochemical screening of 80% hydromethanolic crude extract and solvent fractions of roots of U. simensis for the probable presence of different secondary metabolites are given in Table 2. These qualitative tests were done as per standard test protocols. These tests divulge the existence of terpenoids, tannins, saponins, flavonoids, alkaloids, saponins, and phenolic compounds.
Table 2.
Qualitative phytochemical profiling of hydromethanolic crude extract.
| Phytochemicals | Type of tests performed | Methanolic extract | Aqueous fraction | Ethylacetate fraction | Chloroform fraction |
|---|---|---|---|---|---|
| Alkaloids | Wagner's test | + | — | — | + |
| Phenolic compounds | Ferric chloride test | + | — | + | + |
| Glycosides | Keller–Killiani test | — | — | — | — |
| Plant steroids | Liebermann–Burchardt test | — | — | — | — |
| Anthraquinones | Borntrager's test | + | — | — | + |
| Terpenoids | Salkowski's test | + | + | + | − |
| Tannins | Braemer's test | + | + | + | + |
| Flavonoids | Alkaline reagent (NaOH) test | + | — | — | + |
| Saponins | Froth test | + | + | + | — |
+, present; —, not detected.
3.3. Acute Oral Toxicity Study
The acute toxicity study showed that the root extract caused no mortality at 2000 mg/kg within the first 24 h and for the next 2 weeks study period. Physical and behavioral observations of the experimental mice also revealed no visible signs of acute toxicity such as lacrimation, loss of appetite, tremors, sweating, piloerection, enuresis, salivation, seizure, trembling, and diarrhea. In light of this, the mean lethal dose of the candidate plant extract (LD50) was beyond 2000 mg/kg. Eventhough it lacks significant acute toxicity in mice at the dose level used in this experiment, in further studies, subacute toxicity needs to be executed. Based on OECD-425 guideline, if the limit dose (2000 mg/kg) is deemed atoxic, 1/10th of 2000 mg/kg (200 mg/kg) medium dose, 1/20th of 2000 mg/kg (200/2 = 100 mg/kg) low dose, and 1/5th of 2000 mg/kg (200 × 2 = 400 mg/kg) high dose can be applied in the study.
3.3.1. Effect of U. simensis on Bodyweight, Basal Body Temperature, and Packed Cell Volume of Normal Mice
In the next set of experiments, U. simensis at 2000 mg/kg did not bring neither bodyweight loss, basal body temperature reduction, nor hemolysis in test mice compared to control ones as well as to its baseline value. In brief, bodyweight in the test group has been increased further throughout the assay period in line with the control group, inferring that the extract does not affect bodyweight negatively by itself (Figure 1). While in case of basal body temperature and packed cell volume, there is no overt difference (P > 0.05) in between groups and before and after treatment periods in both test groups (Figures 2, 3).
Figure 1.
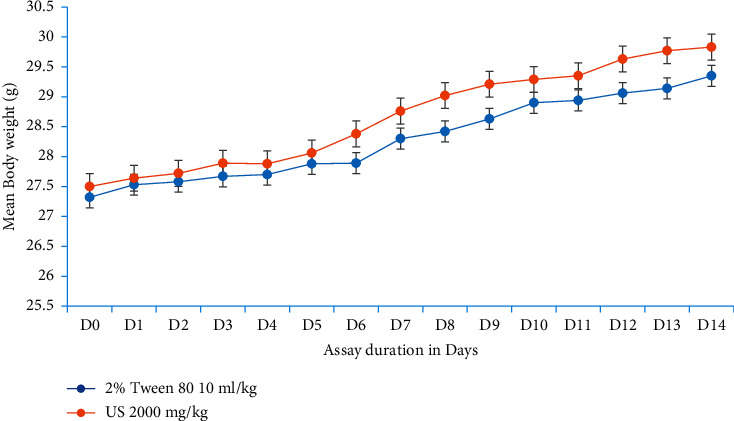
Evolution of mouse bodyweight over the course of an experiment. Data are expressed as mean ± SEM (n = 6). D, days; US, Urtica simensis.
Figure 2.
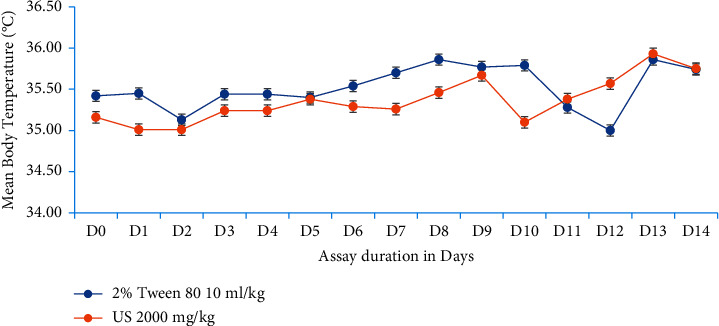
Progression of mouse basal body temperature with respect to experimentation duration. Data are expressed as mean ± SEM (n = 6). D, days; US, Urtica simensis.
Figure 3.
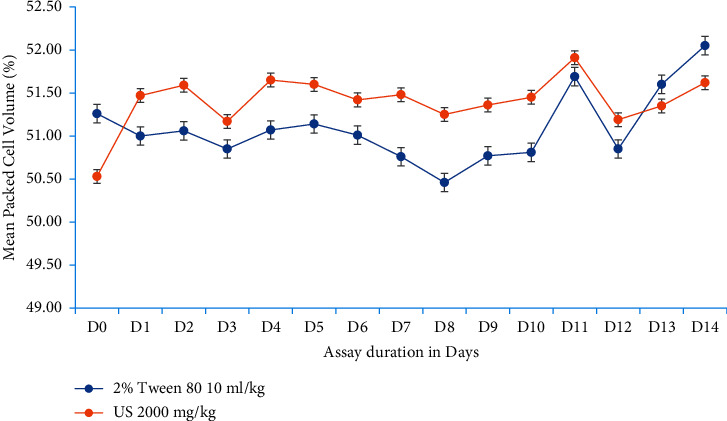
Progression of mouse packed cell volume with respect to experimentation duration. Data are expressed as mean ± SEM (n = 6). D, days; US, Urtica simensis.
3.4. In Vivo Antiplasmodial Efficacy Studies
3.4.1. Chemosuppressive Effect in the 4-Day Suppressive Test
The effect of hydromethanolic crude extract and its solvent fractions of root of U. simensis at different dose levels on parasitemia and mean survival time of mice infected with chloroquine-sensitive P. berghei are given in Table 3. Briefly, the results were expressed as mean percentage parasitemia, mean percentage chemosuppression, and mean survival time in days in comparison with the respective negative control groups.
Table 3.
Parasitemia and survival time of P. berghei-infected mice treated with crude extract and solvent fractions of U. simensis in the 4-day suppressive test.
| Extract | Animal group | % parasitemia level | % chemosuppression | Survival date |
|---|---|---|---|---|
| Crude extract | CON | 41.03 ± 0.95 | 00.00 ± 00.00b3 | 7.50 ± 0.59b3 |
| US100 | 18.37 ± 0.39 | 55.23 ± 00.00a3b3 | 8.21 ± 0.44b3 | |
| US200 | 13.13 ± 0.79 | 68.00 ± 00.00a3b3 | 11.00 ± 0.52a3b3 | |
| US400 | 9.24 ± 0.49 | 77.48 ± 00.00a3b3 | 14.33 ± 0.49a3b3 | |
| CQ25 | 00.00 ± 00.00 | 100.00 ± 00.00a3 | 30.00 ± 00.00a3 | |
|
| ||||
| Chloroform fraction | CON | 39.25 ± 2.34 | 00.00 ± 00.00b3 | 7.38 ± 0.42b3 |
| CF100 | 20.10 ± 0.94 | 48.79 ± 00a3b3 | 9.71 ± 0.31a1b3 | |
| CF200 | 13.95 ± 0.44 | 64.46 ± 00.00a3b3 | 12.37 ± 0.41a3b3 | |
| CF400 | 8.15 ± 0.51 | 79.24 ± 00.00a3b3 | 17.27 ± 0.98a3b3 | |
| CQ25 | 00.00 ± 00.00 | 100.00 ± 00.00a3 | 30.00 ± 00.00a3 | |
|
| ||||
| Ethylacetate fraction | CON | 42.36 ± 0.94 | 00.00 ± 00.00b3 | 5.23 ± 0.18b3 |
| EF100 | 26.55 ± 0.96 | 37.32 ± 00.00a3b3 | 7.57 ± 0.25a2b3 | |
| EF200 | 19.38 ± 0.53 | 54.25 ± 00.00a3b3 | 8.90 ± 0.27a3b3 | |
| EF400 | 14.85 ± 0.83 | 64.94 ± 00.00a3b3 | 12.83 ± 0.67a3b3 | |
| CQ25 | 00.00 ± 00.00 | 100 ± 00.00a3 | 30.00 ± 00.00a3 | |
|
| ||||
| Aqueous fraction | DW | 36.79 ± 2.01 | 00.00 ± 00.00b3 | 5.53 ± 0.10b3 |
| AF100 | 31.54 ± 0.55 | 14.27 ± 00.00a3b3 | 5.87 ± 0.06b3 | |
| AF200 | 23.20 ± 0.31 | 50.08 ± 00.00a3b3 | 8.74 ± 0.43a2b3 | |
| AF400 | 19.18 ± 0.60 | 58.73 ± 00.00a3b3 | 11.31 ± 1.02a3b3 | |
| CQ25 | 00.00 ± 00.00 | 100 ± 00.00a3 | 29.83 ± 0.11a3 | |
Data are expressed as mean ± SEM (n = 6). aCompared to negative control. bCompared to standard. 1P < 0.05; 2P < 0.01; 3P < 0.001. CON, negative control, received vehicle (2% Tween 80 10 ml/kg); DW, negative control, received distilled water 10 ml/kg; US, crude extract of Urtica simensis; CF, chloroform fraction; EF, ethylacetate fraction; AF, aqueous fraction; CQ, chloroquine. Numbers afterward letters in the second column refer to dose in mg/kg.
Blood smears from the untreated control on D4 (5th day after parasite inoculation) disclosed nil parasite reduction with a mean parasitemia of 41.03 ± 0.95, 39.25 ± 2.34, 42.36 ± 0.94, and 36.79 ± 2.01 parasites in blood assayed parallelly with crude extract, CF, EF, and AF, respectively. Crude extract produced a dose-dependent chemosuppressive effect when tested against early infection, with daily doses of 100, 200, and 400 mg/kg, causing 55.23%, 68.00%, and 77.48% chemosuppression 96 h postinfection, respectively. The standard drug, chloroquine, cleared the parasite (100% suppression) by day 4 when used at 25 mg/kg per identical condition. As given in Table 3, all three fractions significantly suppressed parasite count in the 4-day suppressive test. Of these, the aqueous fraction showed the lowest chemosuppression. All fractions at all doses exerted significant (p < 0.001) inhibition of parasitemia as compared to the control group. The highest chemosuppressive effect (79.24%) was offered by chloroform fraction, CF400. The reference agent, chloroquine, cleared parasitemia to undetectable state on the fifth day from a thin blood film with no observed mortality in the group after 30 days.
The mean survival rate of infected mice treated with the candidate plant crude extract significantly prolonged at all dose points (P < 0.001) of the extract except US100. However, this is deemed lower than the outstanding agent (chloroquine phosphate 25 mg/kg) that markedly (P < 0.0001) prolong survival of mice. Eventhough all solvent fractions were able to prolong life expectancy of mice at all dose points, the longest survival date (17.27 (±0.98) days) was recorded in CF400. On the contrary, AF100 failed to reach statistical significance in prolonging survival date. Moreover, group of mice treated with the standard agent was viable throughout the study period (30.00 ± 0.00 days), except positive control mice in AF (29.83 ± 0.11 days). Eventhough both crude extract and CF were able to prolong life of animals, it is markedly lower (P < 0.001) than the standard agent ensued.
3.4.2. Effect of U. simensis Crude Extract and Solvent Fractions on Packed Cell Volume in the Suppressive Test
As shown in Figure 4, in comparison to the control group, US200 and US400 significantly (P < 0.05) averted reduction in PCV in mice at all doses, but US100 fell short to reach the statistical significance which showed a significant reduction in PCV (−6.71 ± 6.60). Eventhough there was a decrease in PCV of crude extract treated animals, this reduction failed to reach the statistical significance between day 0 and day 4 compared to the vehicle-treated group. In the contrary, the vehicle-treated group of animals culminated a significant decrease in PCV on day 4 as compared to the baseline. The vehicle-treated group showed appreciable reduction in PCV 96 hours postinfection. In the contrary, the group of mice treated with the standard agent revealed an increase in volume packed cells (2.69 ± 7.83).
Figure 4.
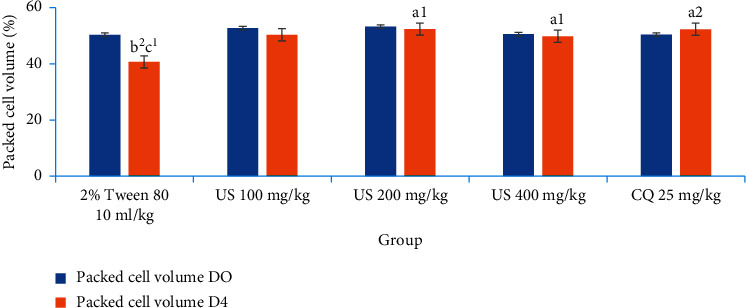
The effect of hydromethanolic crude root extract of U. simensis on packed cell volume of P. berghei-infected mice in the 4-day suppressive test. Data are expressed as mean ± SEM (n = 6). aCompared to negative control. bCompared to the standard. cCompared to baseline. 1P < 0.05; 2P < 0.01. US, Urtica simensis.
No apparent difference was observed among the three doses of the crude extract and solvent fractions in protecting the packed cell volume of the mice. The standard drug, in comparison with all doses of the crude extract, did not show notable differences in packed cell volume protection.
In congruent with crude extract, all solvent fractions ablated hemolysis in a dose-dependent manner (Figure 5). Eventhough there were some losses in PCV in the treatment groups (CE100 by 1.59 ± 4.72%, CF200 by 0.41 ± 2.56%, EF100 by 16.72 ± 4.66%, EF200 by 4.77 ± 4.14%, AF100 by 12.35 ± 2.47%, AF200 by 11.20 ± 1.71%,and AF400 by 5.23 ± 3.12%), these are incomparable (P > 0.05) to the PCV losses in the vehicle-treated group of mice (−20.07 ± 2.78%, −21.69 ± 8.82%, and −20.45 ± 5.53%), respectively. On the other side, group of animals treated with CF400 (1.2 6 ± 0.70%), EF400 (2.90 ± 3.38%), and standard treatment (3.72 ± 4.86%, 4.35 ± 2.96, and 4.05 ± 1.95, respectively) appreciate an increase in PCV. The standard drug, in comparison with all doses of the chloroform and ethyl acetate fraction, did not show notable differences in packed cell volume protection, but with AF indeed. Except negative controls, decrement and increment in PCV were insignificant compared to its baseline value.
Figure 5.
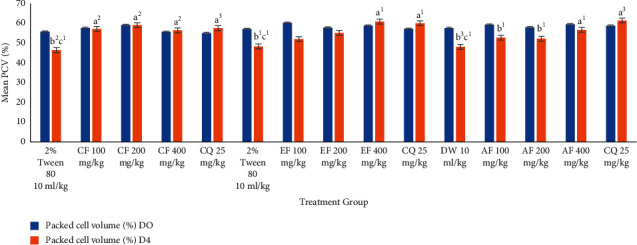
The effect of solvent fractions of root of U. simensis on packed cell volume of P. berghei-infected mice in the 4-day suppressive test. Data are expressed as mean ± SEM (n = 6). aCompared to negative control. bCompared to the standard. cCompared to baseline. 1P < 0.05; 2P < 0.01; 3P < 0.001. AF, aqueous fraction; CF, chloroform fraction; CQ, chloroquine; DW, distilled water; EF, ethylacetate fraction; US, Urtica simensis.
3.4.3. Effect of U. simensis Crude Extract and Solvent Fractions on Bodyweight and Temperature in the Suppressive Test
The effect of U. simensis on mouse bodyweight succeeding an infection was monitored, and the results conveyed that P. berghei infection inflicted a consequential bodyweight loss and basal body temperature in the infected but untreated group of mice compared to infected but treated animals (Table 4). Daily administration of U. simensis crude extract and chloroform fraction for four consecutive days significantly averted infected mice from bodyweight loss compared to negative control animals (Table 4), where bodyweight was contracted considerably postinfection. But neither AF nor EF was able to ablate bodyweight loss remarkably (P > 0.05) as compared to the negative control. Similarly, there was a significant variation in bodyweight between the baseline and at end of therapy. Both crude extract and CF's effect on bodyweight was in a dose-dependent approach; as the dose increases, its capacity to protect weight loss also increases in parallel. A group of mice was treated by US200, US400, CF400, and CQ25; in addition to preserve body weight at the hand, they can improve it by suppressing parasitemia efficiently.
Table 4.
Effect of Urtica simensis extract on bodyweight and rectal temperature of P. berghei-infected mice in Peter's test.
| Treatment group | Temperature | Weight | ||||
|---|---|---|---|---|---|---|
| D0 | D4 | % change in T | D0 | D4 | % change in Wt | |
| CON | 37.80 ± 0.15 | 33.86 ± 0.28 | −10.40 ± 0.93b3 | 33.67 ± 0.49 | 28.55 ± 0.19 | −15.21 ± 0.04b3 |
| US100 mg/kg | 37.89 ± 0.16 | 35.06 ± 0.40 | −7.47 ± 1.19b2 | 33.19 ± 0.48 | 32.70 ± 0.55 | −1.48 ± 1.40a2 |
| US200 mg/kg | 36.32 ± 0.38 | 33.92 ± 0.71 | −6.58 ± 2.06b2 | 33.82 ± 0.31 | 34.40 ± 0.27 | 1.71 ± 0.88a3 |
| US400 mg/kg | 38.44 ± 0.24 | 37.22 ± 0.97 | −3.19 ± 2.33a1 | 34.11 ± 0.21 | 35.67 ± 0.81 | 4.55 ± 2.02a3 |
| CQ25 mg/kg | 37.66 ± 0.14 | 38.26 ± 0.24 | 1.61 ± 0.75a3 | 34.23 ± 0.31 | 35.47 ± 0.53 | 3.65 ± 1.69a3 |
| CON | 37.7 ± 0.68 | 33.39 ± 1.28 | −11.44 ± 4.55b2 | 34.31 ± 0.31 | 30.10 ± 1.08 | −12.27 ± 3.83b3 |
| CF100 mg/kg | 37.23 ± 0.41 | 36.66 ± 0.79 | −1.51 ± 1.68a1 | 33.56 ± 0.30 | 32.28 ± 0.66 | −3.81 ± 1.67a1 |
| CF200 mg/kg | 37.24 ± 0.41 | 37.00 ± 0.54 | −0.64 ± 0.98a1 | 33.57 ± 0.62 | 32.93 ± 0.88 | −2.21 ± 2.72a1 |
| CF400 mg/kg | 37.08 ± 0.48 | 37.05 ± 0.53 | −0.08 ± 1.29a1 | 33.11 ± 0.74 | 33.71 ± 0.36 | 1.92 ± 2.03a3 |
| CQ25 mg/kg | 37.61 ± 0.49 | 37.81 ± 0.36 | 0.53 ± 0.77a2 | 34.25 ± 0.31 | 35.29 ± 0.32 | 3.04 ± 0.77a3 |
| CON | 36.78 ± 0.24 | 32.90 ± 0.85 | −10.54 ± 3.28b1 | 32.9 ± 0.76 | 30.78 ± 1.08 | −6.44 ± 1.60b2 |
| EF100 mg/kg | 36.79 ± 0.40 | 32.06 ± 0.66 | −12.79 ± 2.07b2 | 34.55 ± 0.40 | 35.59 ± 0.61 | −3.01 ± 3.07 |
| EF200 mg/kg | 36.18 ± 0.47 | 32.13 ± 0.61 | −11.19 ± 2.62b1 | 33.69 ± 0.55 | 29.8 ± 0.71 | −11.55 ± 1.65b3 |
| EF400 mg/kg | 33.87 ± 0.99 | 32.21 ± 0.76 | −4.90 ± 3.09 | 33.11 ± 0.67 | 30.34 ± 0.47 | −8.34 ± 2.18b1 |
| CQ25 mg/kg | 37.08 ± 0.52 | 37.34 ± 0.43 | 0.70 ± 0.47a1 | 33.53 ± 0.80 | 34.03 ± 1.01 | 1.48 ± 1.34a1 |
| DW10 ml/kg | 36.78 ± 0.26 | 34.10 ± 0.93 | −7.28 ± 3.08 | 32.87 ± 0.79 | 29.45 ± 1.50 | −10.40 ± 6.60b3 |
| AF100 mg/kg | 36.74 ± 0.33 | 33.28 ± 0.83 | −9.41 ± 1.94 | 33.96 ± 0.22 | 30.64 ± 0.53 | −9.77 ± 1.74b2 |
| AF200 mg/kg | 36.14 ± 0.50 | 33.49 ± 0.98 | −7.33 ± 2.91 | 33.64 ± 0.47 | 32.03 ± 0.40 | −5.11 ± 2.05b1 |
| AF400 mg/kg | 35.60 ± 0.89 | 33.71 ± 0.82 | −5.30 ± 2.12 | 33.53 ± 0.58 | 32.42 ± 0.63 | −4.78 ± 3.04 |
| CQ25 mg/kg | 36.81 ± 0.33 | 38.67 ± 0.52 | 5.05 ± 1.20a3 | 34.17 ± 0.36 | 34.89 ± 0.54 | 2.03 ± 0.57a1 |
Data are expressed as mean ± SEM (n = 6). aCompared to negative control. bCompared to the standard. 1P < 0.05; 2P < 0.01; 3P < 0.001. CON, negative control, received vehicle (2% Tween 80 10 ml/kg); DW, negative control, received distilled water 10 ml/kg; US, crude extract of Urtica simensis; CF, chloroform fraction; EF, ethylacetate fraction; AF, aqueous fraction; CQ, chloroquine.
As given in Table 4, the crude extract was able to significantly prevent the decrease in rectal temperature caused by P. berghei infection at a larger dose (US400, P < 0.05). But the lower and middle doses' capability to protect basal temperature loss fell short to reach a statistically significant level. Among all the three solvent fractions, only chloroform fraction was able to significantly avert the decrease in rectal temperature. All the three dose levels of the CF remarkably (P < 0.05) prevented the reduction in rectal temperature in relation to the control group. Furthermore, the reference drug also significantly (P < 0.01) prevented reduction in rectal body temperature in relation to the control group.
3.5. Antimalarial Curative Assay
Sanatory potential of the most active solvent fraction (chloroform fraction) and the hydromethanolic crude extract was evaluated in Rane's assay. The mean parasitemia in groups treated with hydromethanolic crude extract at US100 mg/kg ranged from 24.45 ± 1.07 to 18.82 ± 1.14, at US200 mg/kg, ranged from 27.42 ± 0.99 to 12.61 ± 0.74, and at US400 mg/kg, ranged from 27.67 ± 0.99 to 11.12 ± 0.60, while that of animals treated with chloroquine varied from 24.44 ± 0.62 to 0.26 ± 0.17. The mean parasitemia in the negative control group ranged from 25.08 ± 0.78 to 37.94 ± 1.30. All doses of crude extract demonstrated a significant mean percentage parasitemia difference when compared with the negative control group after the third dose (at day 5) (P < 0.001). But the activity of crude extract was significantly lower than the standard agent (Figure 6).
Figure 6.
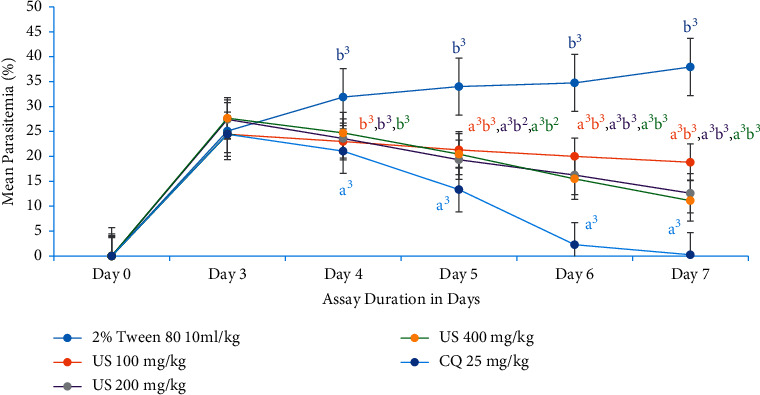
Evolution of parasitemia level of P. berghei-infected mice during treatment with hydromethanolic crude root extract of U. simensis in curative assay. Data are expressed as mean ± SEM (n = 6). aCompared to negative control. bCompared to the standard. 1P < 0.05; 2P < 0.01; 3P < 0.001. US, Urtica simensis; CQ, chloroquine.
The mean parasitemia in groups treated with chloroform fraction at CF100 mg/kg ranged from 29.74 ± 0.82 to 18.02 ± 1.16, at CF200 from 29.40 ± 1.30 to 12.98 ± 0.66, and at CF400 from 30.45 ± 1.15 to 10.25 ± 0.8, while that of animals treated with chloroquine varied from 27.15 ± 1.01 to 0.31 ± 0.10. In congruent with the crude extract, all doses of chloroform fraction revealed a significant mean percentage parasitemia difference when compared with the untreated control group after the third dose (at day 5) (P < 0.001). But the activity of both crude extract and chloroform fraction was significantly lower (day 5, P < 0.01; day 6, P < 0.001; day 7, P < 0.001) than the standard agent (Figure 7).
Figure 7.
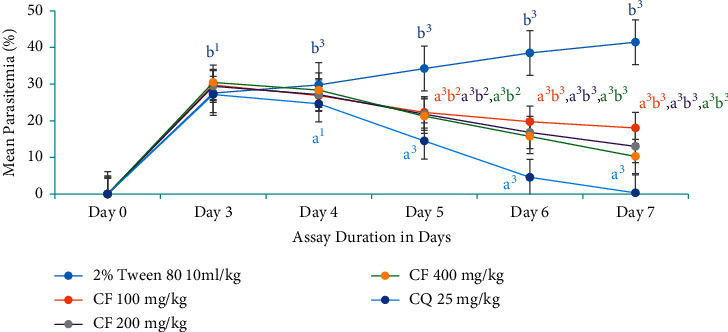
The effect of chloroform fraction of root of U. simensis on parasitemic level of P. berghei-infected mice in curative assay. Data are expressed as mean ± SEM (n = 6). aCompared to negative control. bCompared to the standard. 1P < 0.05; 2P < 0.01; 3P < 0.001. CF, chloroform fraction; CQ, chloroquine.
In vivo curative assay suppression outcomes for the hydromethanolic root extract of U. simensis using Plasmodium berghei-infected mice are shown in Figure 8. Crude extract of U. simensis roots decreased parasitemia level by 50.40%, 66.76%, and 70.69% for US100, US200 and, US400, respectively, compared to negative control mice. Similar to the crude extract, the result from chloroform fractions turned out that the parasitemia level was contracted by 56.5%, 68.67%, and 75.26% for CF100, CF200, and CF400, respectively, at day 7 compared to negative control mice which was reduced significantly (P < 0.001) as compared to vehicle-treated mice. Among all doses of crude extract and chloroform fractions, maximum inhibition (75.26%) of parasitemia levels was attained by 400 mg/kg dose of the chloroform fraction. The parasitemia inhibition seen with CQ25 was significantly (P < 0.001) higher than the crude extract and chloroform fraction. The positive control (chloroquine, 25 mg/kg) assayed in parallel with crude extract and CF reduced parasitemia by 99.31% and 99.25% with no observed mortality in the group after 30 days, respectively.
Figure 8.
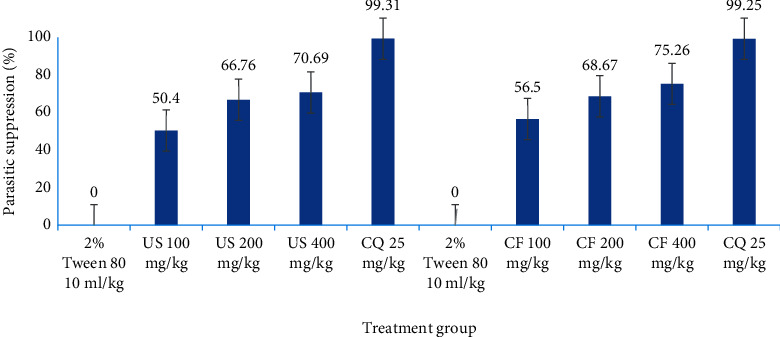
Parasitemic suppression capability of hydromethanolic crude root extract and chloroform fraction of U. simensis in P. berghei-infected mice in curative assay at day 7. Data are expressed as mean ± SEM (n = 6). US, crude extract of Urtica simensis; CF, chloroform fraction; CQ, chloroquine.
3.6. Effects of Crude Hydromethanolic Extract and Chloroform Fraction of U. simensis Roots on Mean Survival Time of Mice Infected with P. berghei in a Curative Assay
Mean survival time analysis demonstrated that mice which received all doses of crude (except US100 (P < 0.05) and chloroform fraction (except CF100 (P < 0.05)) lived longer (with significant value P < 0.0001) than the mice in negative control (Figure 9). The mice treated with low (CF100), medium (CF200), and high (US400) doses of crude extract lived for 7.59 ± 0.21, 10.48 ± 0.42, and 14.22 ± 0.43 days, respectively). The survival rate of mice treated by lower (CF100), middle (CF200), and larger (CF400) doses of chloroform fractions was 9.55 ± 0.30, 12.53 ± 0.32, and 16.69 ± 1.07 days which was markedly prolonged (P < 0.001) than mice in the negative control group (Figure 9). Moreover, the group of mice treated with the standard agent was alive throughout the monitoring period (30.00 ± 0.00) days. Eventhough both crude extract and CF were able to prolong life of animals, but it is markedly lower (P < 0.001) than the standard agent ensued.
Figure 9.

The effect of hydromethanolic crude root extract and chloroform fraction of U. simensis mean survival time in P. berghei-infected mice in a curative assay. Data are expressed as mean ± SEM (n = 6). aCompared to negative control. bCompared to the standard. 1P < 0.05; 3P < 0.001. CF, chloroform fraction; CQ, chloroquine; US, Urtica simensis.
3.7. Effects of Crude Hydromethanolic Extract and Chloroform Fraction of U. simensis Roots on Bodyweight and Temperature of Mice Infected with P. berghei in a Curative Assay
Group of mice treated with the middle (US200 mg/kg per day, P < 0.05) and highest dose (US400 mg/kg per day, P < 0.001) of crude extract prevented reduction in bodyweight loss due to parasitemia. Whereas, the lowest dose (US100 mg/kg) fell flat in this regard (Table 5). Chloroform fraction at all doses (P < 0.05, P < 0.01, and P < 0.001) was able to ablate weight loss due plasmodial infection as compared to the negative control group of mice. Both crude extract and chloroform fraction at all doses were able to preclude basal body temperature reduction except at lower respective doses.
Table 5.
Effects of crude hydromethanolic extract and chloroform fraction of U. simensis roots on bodyweight and temperature of mice infected with P. berghei in a curative assay.
| Treatment group | Temperature | Weight | ||||
|---|---|---|---|---|---|---|
| D3 | D7 | % change in T | D0 | D4 | % change in Wt | |
| CON | 37.57 ± 0.31 | 33.80 ± 0.36 | −10.02 ± 0.82b3 | 33.67 ± 0.50 | 30.04 ± 0.54 | −10.78 ± 2.11b2 |
| US100 mg/kg | 37.02 ± 0.57 | 35.03 ± 1.03 | −5.45 ± 1.75b1 | 31.14 ± 1.21 | 29.44 ± 1.26 | −5.45 ± 2.98b2 |
| US200 mg/kg | 37.44 ± 0.48 | 36.18 ± 0.95 | −3.41 ± 1.80a1 | 29.23 ± 1.21 | 28.41 ± 0.88 | −2.80 ± 2.46a1 |
| US400 mg/kg | 37.50 ± 0.79 | 37.82 ± 0.42 | 0.75 ± 1.53a2 | 29.63 ± 0.92 | 31.25 ± 1.07 | 5.46 ± 2.87a3 |
| CQ25 mg/kg | 37.03 ± 0.47 | 37.56 ± 0.09 | 1.49 ± 1.25a3 | 33.61 ± 0.56 | 34.49 ± 0.38 | 2.61 ± 2.42a2 |
| CON | 37.07 ± 0.68 | 35.57 ± 0.78 | −9.46 ± 0.80b3 | 29.83 ± 1.25 | 27.61 ± 0.97 | −7.44 ± 1.36b3 |
| CF100 mg/kg | 36.56 ± 0.73 | 34.67 ± 0.82 | −5.16 ± 1.20b2 | 29.82 ± 1.09 | 28.20 ± 1.24 | −5.43 ± 0.93b2 |
| CF200 mg/kg | 36.55 ± 0.69 | 35.29 ± 0.47 | −3.32 ± 1.85a1b1 | 29.74 ± 1.24 | 29.72 ± 1.20 | −0.06 ± 1.09a1 |
| CF400 mg/kg | 37.20 ± 0.42 | 36.64 ± 0.74 | −1.53 ± 1.21a2 | 29.33 ± 1.03 | 29.89 ± 1.09 | 1.90 ± 2.18a2 |
| CQ25 mg/kg | 37.60 ± 0.36 | 38.40 ± 0.49 | 2.14 ± 1.27a3 | 30.78 ± 1.11 | 31.62 ± 1.01 | 2.73 ± 1.15a3 |
Data are expressed as mean ± SEM (n = 6). aCompared to negative control. bCompared to the standard. 1P < 0.05; 2P < 0.01; 3P < 0.001. CON, negative control, received vehicle (2% Tween 80 10 ml/kg); US, crude extract of Urtica simensis; CF, chloroform fraction; CQ, chloroquine.
3.8. Effects of Crude Hydromethanolic Extract and Chloroform Fraction of U. simensis Roots on Packed Cell Volume of Mice Infected with P. berghei in a Curative Assay
Among hydromethanolic root crude extract doses, only the largest dose was able to prevent hemolysis as compared to the negative control group (P < 0.05). However, CF at all test doses was able to prevent hemolysis associated with high parasitemia (Figure 10) as compared to the respective negative control group appreciably (P < 0.001). But only negative controls showed a significant discrepancy from their baseline PCV (P < 0.05).
Figure 10.
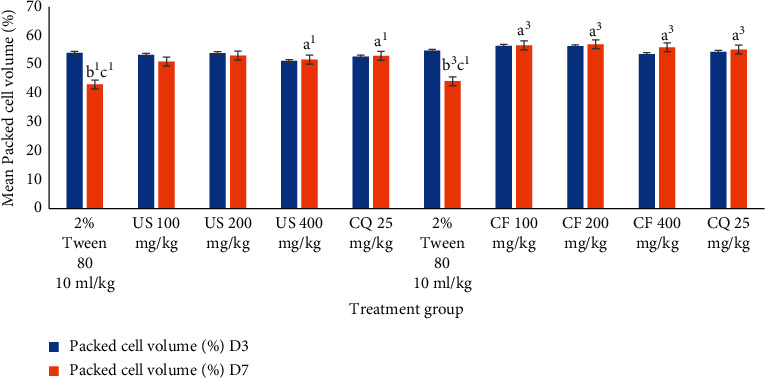
Effects of crude hydromethanolic extract and chloroform fraction of U. simensis roots on packed cell volume of mice infected with P. berghei in a curative assay. Data are expressed as mean ± SEM (n = 6). aCompared to negative control. bCompared to the standard. cCompared to baseline. 1P < 0.05; 3P < 0.001. CF, chloroform fraction; CQ, chloroquine; D3, third day of P. berghei inoculation; D7, last day of the experiment; US, Urtica simensis.
4. Discussion
Despite progress, malaria remains one of the world's most dangerous diseases. The malaria's global effect has forced a search for new antimalarial agents, fuelled by resistance to available and affordable antimalarial medications [46]. Antimalarial medications can be obtained from a variety of sources, with traditional medicinal herbs being one of the most reliable [47]. Artemisinin derivatives are a good illustration of how medicinal plants might help with antimalarial medication development [48]. Hitherto, U. simensis has not been studied for its antimalarial activity, eventhough used in Ethiopian folkloric medicine. As a result, the goal of this work is to evaluate the antimalarial potential of U. simensis extract and its solvent fractions as a new source for antimalarial treatment without jeopardizing its toxicological properties. Because herbal medicines are “natural,” they are frequently thought to be harmless; nonetheless, some products containing bioactive ingredients have the potential to induce side effects [49]. To that purpose, the current study focused on toxicity, which is a major problem with indigenous therapeutic preparations. From the acute toxicity assay of U. simensis root hydromethanolic extract in naive animals, the LD50 of U. simensis was found to be above 2000 mg/kg as no mortality or signs of toxic manifestation were observed, implying a wide safety margin and partly justifying the safety of the plant in traditional medicine as per OECD guideline no. 425 [32]. This safety profile was congruent with previous reports [26, 50]. Furthermore, it had no inherent ability to interfere with bodyweight, basal body temperature, or red blood cell evolution. In the present study, the antiplasmodial activity of the 80% hydroalcoholic extract of the root of U. simensis and its solvent fractions as well as their effect on the cardinal signs of P. berghei-infected mice such as bodyweight loss, hypothermia, and hemolytic anemia was investigated in rodents [51]. Standard in vivo antiplasmodial activity models such as Peter's test and Rane's assay models were employed. The typical preliminary paradigm for testing antiplasmodial effects in mice is Peter's test. Because of its capacity to establish a rodent model of malaria that is similar to human malaria infection, chloroquine-sensitive P. berghei ANKA was utilized in this work for inoculation and to predict antimalarial bioactivity [34, 35]. Despite the fact that chloroquine is no longer a first-line medicine for malaria therapy, it is employed in this study as a standard agent since the Plasmodium parasite used was chloroquine susceptible. Crude extract and CF at 100, 200, and 400 mg/kg inhibited parasitemia more effectively than ethylacetate and water fractions, and they were tested further in a curative assay.
In both early (14.27–79.24%) and established infections (50.40–75.26%), U. simensis reduced parasitemia, indicating possible suppressive and therapeutic benefits in malaria infection. To be deemed very good, good, or medium in terms of antimalarial efficacy in humans, an extract must inhibit parasitemia by at least 50% at doses of 100, 250, or 500 mg/kg/day [52]. This criterion places U. simensis in the top tier of antimalarial drug contenders.
Furthermore, the parasite inhibition displayed by 80% methanol extracts is equivalent to that observed in previous experiments using methanolic soybean seed extract (Glycine max) [53], ethanolic leaf and twig extract of Faurea speciosa [54], 80% methanolic root extract of Echinops hoehnelii [55], hydroethanolic stem bark extract of Myrianthus libericus [40], and aqueous fraction of Schinus molle seeds [56]. Nevertheless, the chemosuppression demonstrated by aqueous extract is less than that observed in a study using an aqueous extract of Schinus molle seeds [56]. The chloroform fraction inhibited parasitemia at a higher percentage than the crude extract and other solvent sections. This suggested that the possible potent active principles were soluble in the solvents accustomed for crude extract and solvent fractionation, and they are highly partitioned to a more semipolar to nonpolar solvent. Superficially, this might be due to the absence of alkaloids and flavonoids in AF and EF fractions. As a result, alkaloids, anthraquinones, phenol, tannins, and flavonoids found in this fraction may have attributed to the discovered antimalarial activity. Alkaloids [57], flavonoids [58], phenols [59], anthraquinones [60], and tannins [61] have all been implicated with antimalarial activity. This outcome is concordant with the efficacy of CF reported on CF of Brucea antidysenterica leaves [62], but higher with the low effect of CF reported on root of Silene macrosolen A. Rich (Caryophyllaceae) [63]. As evidenced by the chemosuppression observed during the early infection test, the extract shows blood schizonticidal action.
Additionally, the extract and fractions substantially increased the survival duration of animals in both protocols' dosage dependently, indicating that the overall reduction in parasitemia-related debilitating consequences in the treated groups may be attributed to the existence of metabolites. Anemia is one cardinal sign of malaria infection. The reduction in PCV that causes malarial anemia happens via an increase in the rate at which old red blood cells are destroyed and a decrease in hematopoiesis. Plasmodium enables to destroy both infected and uninfected red blood cells [64]. Mice treated with CF200 and CF400 totally redeemed the malaria-induced fall in PCV, in a comparable pattern to the reference medicines. The folklore applications of the herb in this regard are supported by this evidence, which lends validity to its antimalarial efficacy (Figures 5 and 10).
Rane's test is a typical antimalarial screening method for determining the effectiveness of extracts/drugs to cure existing infections. The parasitemia level in the infected control groups increased throughout the assay periods in this experiment. Regarding afflicted animals treated with the methanolic crude extract, chloroform fraction, and chloroquine phosphate, the mean percentage of parasitemia fell from the first day of therapy to its conclusion in an assay period in a dose-dependent way. This result unfolds that the involved extract is effective in schizonticidal activity even with limited doses to treat malarial infections.
In addition to having a strong suppressive effect on parasitemia, the crude extract and CF fraction increased the study mice's survival time at all test doses. Furthermore, CF (16.69 ± 1.07) has a longer survival duration than the crude extract (14.22 ± 0.43). This finding is consistent with earlier studies showing that animals with CF had a longer survival time [41].
Preliminary phytochemical screening may reveal some clues as to the type of the pharmacologically active substance in U. simensis.
There were alkaloids, triterpenoids, saponins, coumarins, and flavonoids identified in the U. simensis samples that were tested using established techniques. These kinds of chemicals have been shown to exhibit a curative effect against a variety of pathogens, which could support their historic use in the treatment of a variety of ailments, including malaria. Plant steroids, anthraquinones, and glycosides were found to be absent. This plant's phytochemical profile matches those of earlier studies [24, 26, 50]. The presence of secondary metabolites in medicinal plants has been associated to antiplasmodial effects in several investigations [12, 65–68].
Triterpenes' antiplasmodial effect is thought to be due to their modification of the cell membrane of nonparasitized erythrocytes, which prevents parasites from invading healthy RBCs [65, 66, 69]. U. simensis has also confirmed antioxidant activity which might explain its pharmacological activity and antimalarial activity [70].
Many antimalarial herbal medicines may have antiinfective effects not only by attacking the pathogen directly but also by activating the host's innate and adaptive defense mechanisms. The host's immune system is crucial in the total suppression or elimination of infections [71]. Furthermore, several plants have been shown to have antiplasmodial properties, either by increasing red blood cell oxidation or by limiting protein production [72]. Although the specific mechanism of action of this extract is unknown, it is possible that the plant functions through one or more of the routes indicated above, depending on the phytochemical content of the extract. Thus, the antiplasmodial activity discovered in this study could be attributed to a single or combination action of phytochemicals found in these crude extracts or fractionations, as shown by the results of this investigation.
5. Conclusion
In conclusion, the extract of U. simensis demonstrated antimalarial activities in vivo against murine malaria. Furthermore, this phytomedicine has been shown to have certain efficacies in terms of lowering cardinal symptoms including weight loss, temperature reduction, and hemolysis. As a result, traditional healers' use of this component of the plant for treating the aforementioned ailments has been substantiated.
5.1. Recommendation
More research studies on the plant's antimalarial activity on diverse Plasmodium species and animal models are needed
Bioassay guided isolation should be conducted to isolate the responsible active principle
Elucidating the structure and mechanics of the active principle is recommended
Detailed pharmacological and toxicological research studies are required to turn the active principles into lead compounds
Acknowledgments
The authors thank EPHI for supplying experimental animals and parasites, Dr. Getinet Masresha for his indispensable support by botanical identification of the candidate plant, and their friends for their constructive criticism and recommendations while working on this project. The authors owe a huge debt of gratitude to Debre Tabor University for inspiring them to create this research study.
Abbreviations
- ANOVA:
Analysis of variance
- EPHI:
Ethiopian Public Health Institute
- ICLAS:
International Council for Laboratory Animal Science
- LD50:
Median lethal dose
- OECD:
Organization for Economic Cooperation and Development
- PCV:
Packed cell volume
- SPSS:
Statistical Package for Social Sciences.
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Ethical Approval
Ethical clearance was requested and obtained from the Research and Ethical Review Committee of the College of Health Sciences, Debre Tabor University, reference number SOP11/172/21.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
Woretaw Sisay conceptualized and designed the study. Yared Andargie performed parasitemia surveillance and laboratory investigations. Mulugeta Molla entailed a variety of laboratory tests as well as animal work. All authors performed statistical analysis and prepared and proofread the article.
References
- 1.Waiganjo B., Moriasi G., Onyancha J., Elias N., Muregi F. Antiplasmodial and cytotoxic activities of extracts of selected medicinal plants used to treat malaria in Embu County, Kenya. Journal of Parasitology Research . 2020;2020:1–12. doi: 10.1155/2020/8871375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. World Malaria Report 2020: 20 Years of Global Progress and Challenges . Geneva: 2020. [Google Scholar]
- 3.Federal Democratic Republic of Ethiopia. Malaria . Ethiopia: Ministry of Health; 2016. [Google Scholar]
- 4.Tajbakhsh E., Kwenti T. E., Kheyri P., Nezaratizade S., Lindsay D. S., Khamesipour F. Antiplasmodial, antimalarial activities and toxicity of African medicinal plants: a systematic review of literature. Malaria Journal . 2021;20(1):1–50. doi: 10.1186/s12936-021-03866-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Datoo M. S., Natama M. H., Somé A., et al. Efficacy of a low-dose candidate malaria vaccine, R21 in adjuvant Matrix-M, with seasonal administration to children in Burkina Faso: a randomised controlled trial. Lancet . 2021;397(10287):1809–1818. doi: 10.1016/S0140-6736(21)00943-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kassaye K. D., Amberbir A., Getachew B., Mussema Y. A historical overview of traditional medicine practices and policy in Ethiopia. Ethiop J Heal Dev . 2006;20(2):127–134. [Google Scholar]
- 7.Bekele E. Study on Actual Situation of Medicinal Plants in Ethiopia: Prepared for Japan Association for International Collaboration of Agriculture and Forestry . Addis Ababa: JAICAF; 2007. [Google Scholar]
- 8.Bodeker G., Bhat K. K. S., Burley J., Vantomme P. Non Wood Forest Products: Medicinal Plants for Forest Conservation and Health Care . Rome: Gift Heal FAO; 2003. pp. 1–165. [Google Scholar]
- 9.Omara T. Antimalarial Plants Used across Kenyan Communities. Evidence-Based Complementary and Alternative Medicine . 2020;2020:1–31. doi: 10.1155/2020/4538602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Batista R., De Jesus Silva Júnior A., De Oliveira A. B., Batista R., Júnior S. J. A., Oliveira B. A. Plant-derived antimalarial agents, new leads and efficient phytomedicines, part II, non-alkaloidal natural products. Molecules . 2009;14(8):3037–3072. doi: 10.3390/molecules14083037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Teklemariam Z., Petros B., Mekonnen Y. Evaluation of anti-Plasmodium berghei activity of crude and column fractions of extracts from Withania somnifera. Turkish Journal of Biology . 2013;37(147–150) [Google Scholar]
- 12.Deressa T., Mekonnen Y., Animut A. In vivo anti-malarial activities of Clerodendrum myricoides, Dodonea angustifolia and Aloe debrana against Plasmodium berghei. Ethiop J Heal Dev . 2010;24(1):25–29. doi: 10.4314/ejhd.v24i1.62941. [DOI] [Google Scholar]
- 13.Gülçin I., Küfrevioğlu Ö. I., Oktay M., Büyükokuroğlu M. E. Antioxidant, antimicrobial, antiulcer and analgesic activities of nettle (Urtica dioica L.) Journal of Ethnopharmacology . 2004;90(2–3):205–215. doi: 10.1016/j.jep.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 14.Ramtin M., Massiha A., Khoshkholgh-Pahlaviani M. R. M., Issazadeh K., Assmar M., Zarrabi S. In vitro antimicrobial activity of Iris pseudacorus and Urtica dioica. Zahedan J Res Med Sci . 2014;16(35–59) [Google Scholar]
- 15.Alemayehu K., Asfaw Z., Kelbessa E. Ethnobotanical study of medicinal plants used by local communities of Minjar-Shenkora district, North Shewa zone of Amhara region, Ethiopia. J Med Plant Stud . 2015;3:1–11. [Google Scholar]
- 16.Eskedar G. A., Gulelat D. H., Getachew A. D. Nutritional profile of Samma (Urtica simensis Steudel) leaves grown in Ethiopia. IJSID . 2013;366:454–461. [Google Scholar]
- 17.Tura B. K., Kibebew W. H., Admassu A. M. Investigating the role of apiculture in watershed management and income improvement in Galessa protected area, Ethiopia. Agriculture, Forestry and Fisheries . 2014;3:380–385. [Google Scholar]
- 18.Negesse G. S., Lulekal E. Use and Conservation of Traditional Medicinal Plants in Alelitu Woreda North Shewa . Oromia Region, Ethiopia: 2019. [Google Scholar]
- 19.Kefalew A., Asfaw Z., Kelbessa E. Ethnobotany of medicinal plants in Ada’a district, east Shewa zone of Oromia regional state, Ethiopia. Journal of Ethnobiology and Ethnomedicine . 2015;11:1–28. doi: 10.1186/s13002-015-0014-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reta H., Asfaw Z., Kelbessa E. Contribution of traditional farmers for medicinal plant conservation on the farming site in Gozamin District, Amhara Region, Ethiopia. International Journal of Life Sciences . 2015;4(1):24–35. [Google Scholar]
- 21.Maryo M., Nemomissa S., Bekele T. An ethnobotanical study of medicinal plants of the Kembatta ethnic group in Enset-based agricultural landscape of Kembatta Tembaro (KT) Zone, Southern Ethiopia. Asian Journal of Plant Science & Research . 2015;5(7):42–61. [Google Scholar]
- 22.Enyew A., Asfaw Z., Kelbessa E., Nagappan R. Ethnobotanical study of traditional medicinal plants in and around Fiche district, Central Ethiopia. Current Research Journal of Biological Sciences . 2014;6(4):154–167. doi: 10.19026/crjbs.6.5515. [DOI] [Google Scholar]
- 23.Abera T., Ashebir R., Basha H., et al. Phytochemical-constituents, safety and efficacy of commonly used medicinal plants for the treatment of malaria in Ethiopia-a review. Pharm Pharmacol Int J . 2019;7(6):284–295. doi: 10.15406/ppij.2019.07.00266. [DOI] [Google Scholar]
- 24.Kassa F., Nedi T., Feleke A., Eguale T., Alemayehu H., Shibeshi W. In vitro antimicrobial activity of 80% methanol extract and solvent fractions of Urtica simensis Hochst. ex. A. Rich. (Urticaceae) leaves against pathogenic bacteria and fungi. Ethiopian Pharmaceutical Journal . 2021;36(2) doi: 10.4314/epj.v36i2.3. [DOI] [Google Scholar]
- 25.Tsegaye W., Asres K. U. K. Antidiabetic activity of samma (Urtica simensis Hochst. ex. A. Rich.) in streptozotocin-induced diabetic mice. Ethiopian Pharmaceutical Journal . 2008;27:75–82. doi: 10.4314/epj.v27i2.58265. [DOI] [Google Scholar]
- 26.Tesfaye B. A., Berhe A. H., Wondafrash D. Z., Berhe D. F. Cardioprotective effect of crude extract and solvent fractions of Urtica simensis leaves on cyclophosphamide-induced myocardial injury in rats. Journal of Experimental Pharmacology . 2021;13:147–160. doi: 10.2147/JEP.S270038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Declaration B Over I Basel Declaration a Call for More Trust. Transparency and Communication on Animal Research . 2010;1–2 [Google Scholar]
- 28.International Council for Laboratory Animal Science. ICLAS Ethical Guideline for Researchers . 2015. pp. 5–6. [Google Scholar]
- 29.European Union. Legislation for the Protection of Animals Used for Scientific Purposes . European Commission; 2010. pp. 1–12. [Google Scholar]
- 30.Sarker S. D., Latif Z., Gray A. I. In: Methods in Biothechnology: Natural Products Isolation . Second. Otsuka H, editor. New Jersey: Humana Press Inc.; 2006. pp. 269–273. [Google Scholar]
- 31.Zewdu W. S., Aragaw T. J. Evaluation of the anti-ulcer activity of hydromethanolic crude extract and solvent fractions of the root of Rumex nepalensis in rats. Journal of Experimental Pharmacology . 2020;12:325–337. doi: 10.2147/JEP.S258586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.OECD. Organization for Economic Cooperation and Development Guidline Test No. 425: Acute Oral Toxicity: Up-And-Down Procedure(UDP) Paris, France: OECD Publishing; 2008. pp. 1–21. [Google Scholar]
- 33.Seyfe A., Yohannes M., Getachew M. D. A. In vivo anti-plasmodial activity evaluation of the leaves of Balanites rotundifolia (Van Tiegh.) Blatter (Balanitaceae) against Plasmodium berghei. J Exp Pharmacol . 2017;9:59–66. doi: 10.2147/JEP.S130491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Merlin L., Willcox G. B. Traditional herbal medicines for malaria. Br Med J . 2004;329:1156–1159. doi: 10.1136/bmj.329.7475.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tomas A. M., Van Der Wel A. M., Thomas A. W., Janse C. J., Waters A. P. Transfection systems for animal models of malaria. Parasitology Today . 1998;14(6):245–249. doi: 10.1016/S0169-4758(98)01248-4. [DOI] [PubMed] [Google Scholar]
- 36.Waako P. J., Gumede B., Smith P., Folb P. I. The in vitro and in vivo antimalarial activity of Cardiospermum halicacabum and Momordica foetida. Journal of Ethnopharmacology . 2005;99(1):137–143. doi: 10.1016/j.jep.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 37.Melese E., Asres K., Asad M., Engidawork E. Evaluation of the antipeptic ulcer activity of the leaf extract of Plantago lanceolata L. in rodents. Phyther Res . 2011;25(8):1174–1180. doi: 10.1002/ptr.3411. [DOI] [PubMed] [Google Scholar]
- 38.Peter W., Portus H., Robinson L. The four-day suppressive in vivo antimalarial test. Annals of Tropical Medicine and Parasitology . 1995;69:155–171. [Google Scholar]
- 39.Ryley J. F., Peters W. The antimalarial activity of some quinoline esters. Annals of Tropical Medicine and Parasitology . 1995;84:209–222. doi: 10.1080/00034983.1970.11686683. [DOI] [PubMed] [Google Scholar]
- 40.Baah M. K., Mensah A. Y., Asante-Kwatia E., et al. Antiplasmodial activity of different solvent extracts of Myrianthus libericus stem bark and its constituents in Plasmodium berghei-infected mice. Evidence-based Complementary and Alternative Medicine . 2020;2020:1–10. doi: 10.1155/2020/8703197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fentahun S., Makonnen E., Awas T., Giday M. In vivo antimalarial activity of crude extracts and solvent fractions of leaves of Strychnos mitis in Plasmodium berghei infected mice. BMC Complement Altern Med . 2017;17(1):1–12. doi: 10.1186/s12906-016-1529-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jani N. A., Azizi N. A. A., Aminudin N. I. Phytochemical screening and anti-oxidant activity of Psidium guajava. Malaysian J Anal Sci . 2020;24(2):173–178. [Google Scholar]
- 43.Gupta K., Kumar Gupta P., Vinayak Lokur A., Praveen Kumar Gupta C., Rithu B. Phytochemical screening and qualitative analysis of Cymbopogon citratus. Journal of Pharmacognosy and Phytochemistry . 2019;8(4):3338–3343. [Google Scholar]
- 44.Shaikh J. R., Patil M. Qualitative tests for preliminary phytochemical screening: an overview. Int J Chem Stud . 2020;8(2) doi: 10.22271/chemi.2020.v8.i2i.8834. [DOI] [Google Scholar]
- 45.Anwar F., Przybylski R. Effect of solvents extraction on total phenolics and antioxidant activity of extracts from flaxseed (Linum usitatissimum L.) Acta Sci Pol Technol Aliment . 2012;11(3):293–301. [PubMed] [Google Scholar]
- 46.Teklu T., Engidawork E., Nedi T., Teklehaymanot T., Gebremeskel L. Evaluation of the antimalarial activity of the hydroalcoholic extract of leaf of Leonotis ocymifolia (Burm. f.) Iwarsson (lamiaceae) against Plasmodium berghei in mice. Evidence-based Complement Altern Med. . 2020;2020:1–8. doi: 10.1155/2020/5384804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nik Mat Zin N. N. I., Mohamad M. N., Mohamad M. N., et al. In vitro antimalarial and toxicological activities of Quercus infectoria (Olivier) gall extracts. Malaysian J Med Sci . 2020;27(4) doi: 10.21315/mjms2020.27.4.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Biruk H., Sentayehu B., Alebachew Y., Tamiru W., Ejigu A., Assefa S. Antimalarial activity of 80% methanol and aqueous bark extracts of Terminalia brownii Fresen. (Combretaceae) against Plasmodium berghei in mice. Biochem Res Int . 2020;2020:2–7. doi: 10.1155/2020/9749410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Günthardt B. F., Hollender J., Hungerbühler K., Scheringer M., Bucheli T. D. Comprehensive toxic plants-phytotoxins database and its application in assessing aquatic micropollution potential. Journal of Agricultural and Food Chemistry . 2018;66(29):7577–7588. doi: 10.1021/acs.jafc.8b01639. [DOI] [PubMed] [Google Scholar]
- 50.Sisay W., Andargie Y., Molla M., Norahun A. Hydromethanolic crude extract of the leaf of Urtica simensis Hochst . ex . A. Rich. (Urticaceae) acquires appreciable antiulcer effect: validation for in vivo antiulcer activity. Evidence-Based Complement Altern Med An. . 2021;2021:1–12. doi: 10.1155/2021/6591070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Langhorne J., Quin S. J., Sanni L. A. Mouse models of blood-stage malaria infections: immune responses and cytokines involved in protection and pathology. Chemical Immunology . 2002;80:204–228. doi: 10.1159/000058845. [DOI] [PubMed] [Google Scholar]
- 52.Tchatat Tali M. B., Jiatsa Mbouna C. D., Yamthe Tchokouaha L. R., et al. In vivo antiplasmodial activity of Terminalia mantaly stem bark aqueous extract in mice infected by Plasmodium berghei. J Parasitol Res . 2020;2020:1–9. doi: 10.1155/2020/4580526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nyandwaro K., Oyweri J., Kimani F., Mbugua A. Evaluating antiplasmodial and antimalarial activities of soybean (Glycine max) seed extracts on P. falciparum parasite cultures and P. berghei-infected mice. J Pathog . 2020;2020:1–8. doi: 10.1155/2020/7605730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ayisi F., Mensah C. N., Borquaye L. S. In vivo antiplasmodial activity and toxicological analyses of the ethanolic leaf and twig extract of Faurea speciosa Welw. (Proteaceae) J Parasitol Res . 2021;2021 doi: 10.1155/2021/7347532.7347532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bitew H., Mammo W., Hymete A., Yeshak M. Y. Antimalarial activity of acetylenic thiophenes from Echinops hoehnelii Schweinf. Molecules . 2017;22(11):1–10. doi: 10.3390/molecules22111965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mekuria A. B., Geta M., Birru E. M., Gelayee D. A. Antimalarial activity of seed extracts of Schinus molle against Plasmodium berghei in mice. J Evidence-Based Integr Med. . 2021;2021:p. 26. doi: 10.1177/2515690X20984287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ezenyi I. C., Verma V., Singh S., Okhale S. E., Adzu B. Ethnopharmacology-aided antiplasmodial evaluation of six selected plants used for malaria treatment in Nigeria. Journal of Ethnopharmacology . 2020;254(112694):1–7. doi: 10.1016/j.jep.2020.112694. [DOI] [PubMed] [Google Scholar]
- 58.Nadia N. A. C., Cédric Y., Christian N. O., et al. In vivo antiplasmodial activity of Entandrophragma cylindricum (Sprague) sprague ethyl acetate extract in Plasmodium berghei -infected mice. J Parasitol Res . 2020;2020:1–7. doi: 10.1155/2020/8846067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ounjaijean S., Romyasamit C., Somsak V. Evaluation of antimalarial potential of aqueous crude Gymnema inodorum leaf extract against Plasmodium berghei infection in mice. Evidence-based Complement Altern Med. . 2021;2021:1–7. doi: 10.1155/2021/9932891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Udu R., Oyweri J., Gathirwa J. Antimalarial activity of Nigella sativa L. Seed extracts and selection of resistance in Plasmodium berghei ANKA in a mouse model. J Pathog . 2021;2021:1–10. doi: 10.1155/2021/6165950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zewdu Wondafrash D., Bhoumik D., Masresha Altaye B., Bitew Tareke H., Teklebrhan Assefa B. Antimalarial activity of Cordia africana (lam.) (Boraginaceae) leaf extracts and solvent fractions in Plasmodium berghei-infected mice. Evidence-Based Complement Altern Med . 2019;2019:1–14. doi: 10.1155/2019/8324596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Aragaw T. J., Getahun K. A. Antimalarial activity of hydromethanolic crude extract and chloroform fraction of Brucea antidysenterica leaves in Plasmodium berghei-infected mice. Evidence-Based Complement Altern Med . 2021;2021:1–14. doi: 10.1155/2021/2089114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Atsbha G. H., Balasubramanian R., Gebre A. K. Antimalarial effect of the root of Silene macrosolen A. Rich (Caryophyllaceae) on Plasmodium berghei-infected mice. Evidence-based Complement Altern Med. . 2021;2021:1–11. doi: 10.1155/2021/8833865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Umar M. B., Ogbadoyi E. O., Ilumi J. Y., Salawu Ayt O. A., Hassan I. M. Antiplasmodial efficacy of methanolic root and leaf extracts of Morinda lucida. Journal of Natural Sciences Research . 2013;3:112–122. doi: 10.3906/biy-1203-62. [DOI] [Google Scholar]
- 65.Nadia N. A. C., Cédric Y., Raoul S. N. S., et al. Antimalarial activity of ethyl acetate extract and fraction of Bidens pilosa against Plasmodium berghei (ANKA) J Parasitol Res . 2020;2020:1–8. doi: 10.1155/2020/8832724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hagazy K., Sibhat G. G., Karim A., Tekulu G. H., Periasamy G., Hiben M. G. Antimalarial activity of Meriandra dianthera leaf extracts in Plasmodium berghei-infected mice. Evidence-based Complementary and Alternative Medicine . 2020;2020 doi: 10.1155/2020/8980212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Assen Y., Woldearegay M., Haile A. An ethnobotanical study of medicinal plants in Kelala district, South wollo zone of Amhara region, Northeastern Ethiopia. Evidence-Based Complementary and Alternative Medicine . 2021;2021:p. 10. doi: 10.1155/2021/6651922.6651922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Megersa M. O. A., Assefa B., Megersa M. O. A., Jima T. T. Ethnobotanical study of medicinal plants used to treat human diseases in Gura Damole District, Bale Zone, Southeast Ethiopia. Asian J Ethnobiol . 2021;4(1):42–52. doi: 10.13057/asianjethnobiol/y040105. [DOI] [Google Scholar]
- 69.Toma A., Deyno S., Eyado S., Fikru A. In vivo antimalarial activity of solvent fractions of Echinops kebericho roots against Plasmodium berghei infected mice. EC Microbiol . 2017;12(5):204–212. [Google Scholar]
- 70.Seifu T., Mehariffi B., Atlabachew M., Chandravanshi B. Polyphenolic content and antioxidant activity of leaves of Urtica simensis grown in Ethiopia. Latin American Applied Research . 2017;47(1):35–40. doi: 10.52292/j.laar.2017.295. [DOI] [Google Scholar]
- 71.Aragaw T. J., Afework D. T., Getahun K. A. Antimalarial activities of hydromethanolic crude extract and chloroform fraction of Gardenia ternifolia leaves in plasmodium berghei-infected mice. Evidence-based Complementary and Alternative Medicine . 2020;2020:1–11. doi: 10.1155/2020/6674002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tekulu G. H., D’Alessandro S., Parapini S., Basilico N., Karim A. In vitro antimalarial activity of selected medicinal plants native to Tigray region of Ethiopia. Res Sq . 2019;8(42):1–13. doi: 10.21203/rs.2.19327/v1. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.


