Abstract
Cystic echinococcosis (CE) is a chronic zoonotic parasitic disease caused by infection with the larvae of the Echinococcus granulosus sensu lato (s.l.) cluster. Currently, new drugs are urgently required due to the poor therapeutic effect of the existing drugs albendazole and mebendazole. Capparis spinosa, a traditional medicinal plant, has potential therapeutic effects on various diseases based on extracts from its fruit and other parts. The results of this study demonstrated that the water-soluble and ethanolic extracts of C. spinosa fruit had in vitro killing effects on the larvae of E. granulosus sensu stricto (s.s.) and disrupted the ultrastructure of protoscoleces and metacestodes. In vitro cytotoxicity assays showed that the water-soluble and ethanolic extracts of C. spinosa fruit were not significantly toxic to primary mouse hepatocytes at an effective dose to CE. In conclusion, water-soluble and ethanolic extracts of C. spinosa fruit have great potential for the development of new drugs for the treatment of CE.
Keywords: Capparis spinosa, cystic echinococcosis, drug development, Echinococcus granulosus sensu stricto
Cystic echinococcosis (CE) is a chronic zoonotic parasitic disease caused by infection with the larvae of the Echinococcus granulosus sensu lato (s.l.) cluster [21]. CE is distributed worldwide, with areas of high endemicity in western China, South America, Central Asia, Mediterranean countries, and East Africa [33]. The World Health Organization (WHO) reports that the annual costs associated with CE are estimated at $3 billion, thus causing enormous damage to human health and livestock development [2].
Currently, the main treatments for human CE include surgery, percutaneous treatments, and antiparasitic treatment with benzimidazoles; however, antiparasitic medication remains essential for patients who are inoperable or to prevent recurrence after surgery [3]. The main drugs recommended by the WHO for the treatment of CE are albendazole (ABZ) and mebendazole; however, 20–40% of patients do not respond well [23]. In addition, patients usually need to take these drugs for a long time, which may lead to the development of adverse effects such as hepatotoxicity, severe leukopenia, thrombocytopenia, and alopecia [12]. Therefore, the development of new drugs to treat CE is urgently required.
Medicinal plants provide abundant resources for the development of new drugs to treat human diseases [36]. Due to their wide sources and simple extraction process, plant extracts have gradually become a research hotspot in recent years. To date, approximately 35,000–70,000 plant species have been studied for their therapeutic effects [25]. In recent years, many natural extracts, such as Allium sativum extract [27], Foeniculum vulgare mill extract [14], Pistacia vera oil extract [20], and others have shown protoscolicidal effects on the larvae of E. granulosus s.l. This suggests that natural extracts have great potential for the treatment of CE.
Capparis spinosa is an aromatic plant grown in the tropics and subtropics [36]. As a traditional herbal medicine, the root bark, leaves, and the fruit of C. spinosa have definite medicinal value and are used to treat rheumatoid arthritis and gout [24]. As research progresses, an increasing number of studies have shown that C. spinosa has various pharmacological effects, including anti-inflammatory, analgesic, liver protective, hypoglycemic, antioxidant and anticancer effects [5, 22, 30, 31, 35]. In addition, C. spinosa has some antiparasitic effects. Caboni et al. [4] found that methanolic extracts from the leaves, stems and buds (especially the stems) of C. spinosa have a killing effect on Meloidogyne incognita. Mahmoudvand et al. [19] demonstrated that methanolic extract of C. spinosa fruit can be used as a protoscolicidal agent, which can be used during surgery to avoid protoscoleces spillage. However, it is not known whether the water-soluble and ethanolic extracts of C. spinosa fruit have a killing effect on E. granulosus s.l.
This study aims to investigate the in vitro effects of extracts of C. spinosa fruit on protoscoleces (PSCs) and metacestodes (MTCs) of E. granulosus sensu stricto (s.s.) to provide a foundation for the development of antiparasitic drugs.
MATERIALS AND METHODS
Ethics statement
Animal procedures and management were carried out under the protocol (IACUC-20180213-07) approved by the Ethical Committee of the Animal Management Center of Xinjiang Medical University. Throughout the experiment, the animals were placed in a temperature-controlled and light-controlled environment, and euthanasia was performed to prevent the suffering of the animals to the greatest extent.
Preparation of plant extracts
The ripe fruit of C. spinosa was purchased from Jinfurong Traditional Chinese Medicine Decoction Piece Co., Ltd., Anhui Province, China (Approval Number: 170401), and authenticated by an expert herbalist at Xinjiang Medical University. At room temperature, the fruit of C. spinosa was left to dry in a shaded and aerated place until the weight was stable.
The fruits were processed based on the method described by Hamuti et al. [11] with some minor modifications. The dried fruit of C. spinosa (5,000.0 g) was crushed and extracted twice by refluxing 8 volumes of 85% ethanol at reflux for 2 hr each time, and the extracts were pooled. The ethanolic extract was concentrated by desiccation under reduced pressure (50°C) to 800.0 g in dry weight. The ethanolic extract was dissolved in 1,000 ml of distilled water, extracted three times with equal volumes of petroleum ether, chloroform and ethyl acetate, and the remaining aqueous solution was partially concentrated under reduced pressure and dried to obtain 50.3 g of water-soluble extract.
Establishment of a mouse model of E. granulosus s.s. PSC infection
Healthy C57BL/6J female mice (18 ± 1 g, aged 8 weeks) were selected to establish the E. granulosus s.s. PSC-infected mice model. Before the experiment, the mice were allowed to grow for one week in a laboratory environment with a temperature of 20°C and 12 hr of light/12 hr of darkness. The mice were inoculated with 2,000 PSCs (PSCs were derived from hydatid cysts of infected sheep slaughtered in an abattoir located in Urumuqi) in 0.2 ml normal saline by intraperitoneal injection. After 6 months of infection, euthanasia and necropsy were performed on the infected mice to collect MTCs developed from the PSCs.
Chemicals
Water-soluble and ethanolic extracts were dissolved in dimethyl sulfoxide (DMSO) (Merck Millipore, Billerica, MA, USA) at concentrations of 100, 200, and 400 mg/ml. Albendazole sulfoxide (ABZSO, the metabolite of ABZ after liver biotransformation, the anti-parasite activity of ABZ mainly comes from ABZSO, which was used as a positive control group in this study) was dissolved in DMSO at a concentration of 1.5 mM [16]. These were used as stock solutions and stored at −20°C prior to the experiment.
In vitro collection and culture of PSCs and MTCs
Under aseptic conditions, E. granulosus s.s. PSCs were derived from hydatid cysts of infected sheep slaughtered at a local abattoir (Urumqi, Xinjiang, China) and cultured in RPMI-1640 complete medium (Gibco, Grand Island, NY, USA) [10% fetal bovine serum (Gibco), 2% of 100 U/ml penicillin/100 μg/ml streptomycin (Gibco)]. In addition, MTCs were obtained under aseptic conditions from the peritoneal cavity of C57BL/6J mice infected with E. granulosus s.s. The MTC culture method was performed according to the method described by Loos et al. [17] with some minor modifications. MTCs were cultured in RPMI-1640 complete medium (25% fetal bovine serum, 0.45% yeast extract, 0.42% glucose and 1% 100 U/ml penicillin/100 μg/ml streptomycin). PSCs and MTCs were cultured in culture flasks (25-cm2 growth area) at 37°C and 5% CO2 and the same complete medium was changed every two days.
In vitro drug intervention of PSCs
Before the experiment, the viability of PSCs was evaluated by 1% eosin staining, and the viability of PSCs was >90% by three parallel tests. All of the experiments were carried out until the viability of the control was lower than 90% or all treated parasites were dead [18]. PSCs with complete morphology were spread on a 96-well plate (n=200/0.32-cm2 growth area per well). Two microliters of stock solutions of water-soluble and ethanolic extracts of C. spinosa and ABZSO were added to the wells to final concentrations of 1, 2, 4 mg/ml and 15 µM respectively (total volume of 200 µl) [22]. PSCs incubated in culture medium containing 1% DMSO were used as controls. The negative control (NC) group did not undergo any pharmacological intervention. During the drug intervention, the morphology of PSCs was observed every 24 hr under an inverted light microscope, and the viability of PSCs was evaluated every 4 days by a 1% eosin exclusion assay. After 24 days of drug intervention, PSCs were collected and observed by scanning electron microscopy (SEM) for ultrastructural characteristics. The dead PSCs were counted in triplicate for each group and the mean values of the viability were derived from three experiments. During the experiment, the complete culture medium was changed every 2 days, with continual drug supplementation.
In vitro drug intervention of MTCs
MTCs of morphologically healthy and equal size (approximately 2–3 mm in diameter) were randomly assigned to 6-well plates (n=10–20/9.6-cm2 growth area per well). Water-soluble and ethanolic extracts of C. spinosa and ABZSO were added to the MTC culture medium at final concentrations of 1, 4 mg/ml and 15 µM (total volume of 2 ml). The viability of MTCs was observed daily until the viability of the control group was lower than 90% or all treated parasites were dead. The viability of MTCs was assessed by the loss of turgidity and the collapse and ultrastructure of the germinal layer, as described by Elissondo et al. [6]. After 11 days of drug intervention, MTCs were collected and observed by SEM for ultrastructural characteristics. The dead MTCs were counted in triplicate for each group and the mean values of viability were derived from three experiments. During the experiment, the same complete culture medium was changed every 2 days, with continual drug supplementation.
Ultrastructural observation of PSCs and MTCs
After drug intervention, the PSCs and MTCs were collected, placed in 2.5% glutaraldehyde solution (pH 7.2), and stored at 4°C for 24 hr. For SEM, samples of PSCs and MTCs in vitro were dehydrated followed by coating with gold as described by Xin et al. [34]. PSC and MTC samples were washed in PBS (pH 7.2) and then samples were fixed in 1% osmium tetroxide for 2 hr at room temperature. The samples were washed again and then dehydrated through a gradient of tert-butanol (50, 70, 80, 90, and 100%), followed by immersion in 100% tert-butanol, drying to a critical point, and sputtering with gold plating. The samples were processed for SEM observation using an LEO-1430VP (Carl Zeiss AG, Jena, Germany) microscope.
Primary mouse hepatocyte isolation and culture
Primary hepatocytes were isolated from 10- to 12-week-old female C57BL/6 mice as previously described [15]. Isolated primary mouse hepatocytes were seeded at a density of 1 × 104 cells/0.32-cm2 growth area per well in rat tail collagen type I-coated 96-well plates. Then, 200 µl of growth medium [William’s E (Gibco), 10% fetal bovine serum, 0.01% dexamethasone, 0.25% insulin, and 1% of 100 U/ml penicillin/100 μg/ml streptomycin] was added to each well. The hepatocytes were grown at 37°C, 5% CO2 for 4 hr in preparation for the next step.
In vitro toxicity assessment of water-soluble and ethanolic extracts of C. spinosa on primary mouse hepatocytes
A Cell Counting Kit-8 (CCK-8) assay was used to analyze the viability of primary mouse hepatocytes. In short, primary mouse hepatocytes were cultured at 37°C for 4 hr at 5% CO2 and then replaced with growth medium (without 10% fetal bovine serum). Then, water-soluble and ethanolic extracts of C. spinosa were added to the cultures at concentrations of 4 mg/ml and the hepatocytes continued to be cultured at 37°C, and 5% CO2. As controls, hepatocytes were cultured in culture medium containing 1% DMSO. Forty-eight hours later, 10 μl of CCK-8 solution (Bioss, Beijing, China) was added to each well and mixed evenly, and after 2 hr of continuous incubation, zeroing was completed using blank control wells, and the optical density of each well was measured at 450 nm using a microplate reader (Thermo Fisher Scientific, Waltham, MA, USA).
Statistics
The significance of the data in the experiment was determined by the χ2 test and one-way ANOVA, and P<0.05 was considered statistically significant. All data were represented by the arithmetic mean ± SEM.
Linguistic statement
This publication follows the terminology proposed by the World Association of Echinococcosis [32].
RESULTS
In vitro effectiveness of the water-soluble and ethanolic extracts of C. spinosa against PSCs
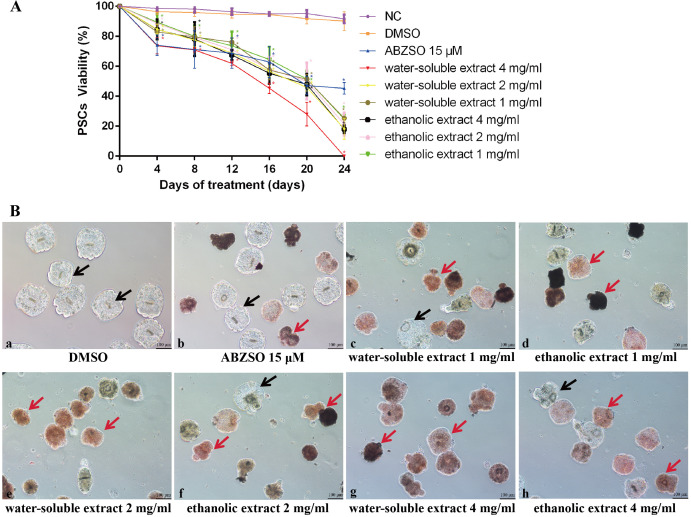
The viability of PSCs after exposure to different concentrations of water-soluble and ethanolic extracts of C. spinosa is shown in Fig. 1A. Compared to the solvent group, the viability of PSCs was significantly reduced (P<0.05) after treatment with water-soluble and ethanolic extracts of C. spinosa for 24 days. Notably, 4 mg/ml of water-soluble and ethanolic extracts of C. spinosa fruit killed 100 and 82% of the PSCs after 24 days of treatment, respectively. Throughout the experimental period, PSCs in the solvent group had an intact morphology and clear calcareous corpuscles. The morphology of some PSCs in the ABZSO group was disrupted, and hooks were lost. After 24 days of intervention with water-soluble and ethanolic extracts of C. spinosa, the morphology of PSCs was altered with the collapse of suckers, disappearance of the hooks, and reduction of calcareous corpuscles. All PSCs died and more severe morphological changes were observed after 24 days of intervention with 4 mg/ml water-soluble extract of C. spinosa (Fig. 1B).
Fig. 1.
In vitro efficacy of the drugs against protoscoleces (PSCs). (A) Viability of PSCs during 24 days of in vitro treatment with albendazole sulfoxide (ABZSO) and water-soluble and ethanolic extracts of Capparis spinosa fruit. Data are the mean ± standard deviation (SD) of three independent experiments. PSCs incubated in complete culture medium containing 1% DMSO (dimethyl sulfoxide) served as a solvent group. PSCs incubated in complete medium (no added drugs) served as a negative control (NC). * Statistically significant difference (P<0.05) compared with solvent group. (B) Light microscopy of PSCs incubated in vitro for 24 days with DMSO, ABZSO and water-soluble and ethanolic extracts of C. spinosa fruit. PSCs incubated in complete culture medium containing 1% DMSO served as a solvent group. Red arrows indicate representative dead PSCs, black arrows indicate representative surviving PSCs.
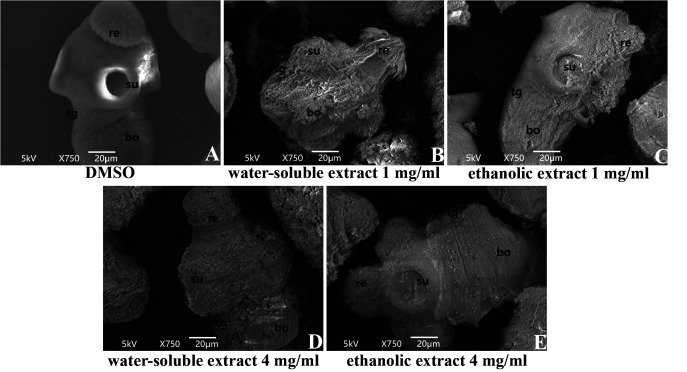
The anti-PSC effect of the extracts of C. spinosa was further assessed at the ultrastructural level using SEM. As shown in Fig. 2A, PSCs in the solvent group did not show ultrastructural changes. Compared with the solvent group, the ultrastructure of PSCs was damaged by the extract of C. spinosa (Fig. 2B–E). After 24 days of intervention with C. spinosa extracts (1 and 4 mg/ml), the tegument of PSCs had a large number of blebs, the rostellar structure was destroyed, the hooks were lost, and the microtriches were shed.
Fig. 2.
Representative scanning electron microscopy (SEM) images of protoscoleces (PSCs) after water-soluble and ethanolic extracts of Capparis spinosa fruit incubation for 24 days. (A) PSCs incubated in complete culture medium containing 1% dimethyl sulfoxide (DMSO) served as a solvent group; (B) PSCs incubated with 1 mg/ml water-soluble extracts of C. spinosa fruit; (C) PSCs incubated with 1 mg/ml ethanolic extracts of C. spinosa fruit; (D) PSCs incubated with 4 mg/ml water-soluble extracts of C. spinosa fruit; (E) PSCs incubated with 4 mg/ml ethanolic extracts of C. spinosa fruit. re, rostellum; su, suckers; bo, body; tg, tegument.
In vitro effectiveness of the water-soluble and ethanolic extracts of C. spinosa against MTCs
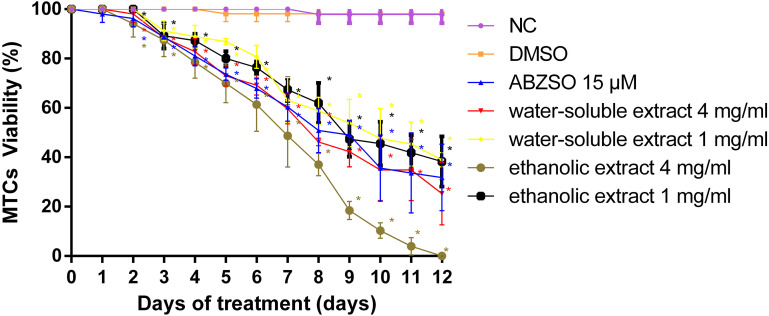
As shown in Fig. 3, after 12 days of in vitro treatment, the viability of MTCs treated with 1 and 4 mg/ml of water-soluble extract of C. spinosa was 38.96 ± 2.35 and 25.27 ± 10.3%, respectively. The viability of MTCs after treatment with 1 and 4 mg/ml ethanolic extracts of C. spinosa was 38.30 ± 8.30 and 0%, and 4 mg/ml water-soluble and ethanolic extracts of C. spinosa were superior to 15 μM ABZSO (the viability of MTCs was 31.81 ± 10.99% when treated with 15 μM ABZSO).
Fig. 3.
Viability of metacestodes (MTCs) during 12 days of in vitro treatment with albendazole sulfoxide (ABZSO) and water-soluble and ethanolic extracts of Capparis spinosa fruit. Data are the mean ± standard deviation (SD) of three independent experiments. MTCs incubated in complete culture medium containing 1% dimethyl sulfoxide (DMSO) served as a solvent group. MTCs incubated in complete medium (no added drugs) served as a negative control (NC). * Statistically significant difference (P<0.05) compared with solvent group.
Next, the SEM results showed that the MTCs in the solvent group exhibited intact typical structures: the morphology of the germinal layer was intact and the germinal layer cells were neatly arranged. The water-soluble and ethanolic extracts of C. spinosa treated MTCs revealed loss or destruction of germinal layer cells in the germinal layer (Fig. 4).
Fig. 4.
Representative scanning electron microscopy (SEM) images of metacestodes (MTCs) after water-soluble and ethanolic extracts of Capparis spinosa fruit incubation for 11 days. (A) MTCs incubated in complete culture medium containing 1% dimethyl sulfoxide (DMSO) served as a solvent group; (B) MTCs incubated with 1 mg/ml water-soluble extracts of C. spinosa fruit; (C) MTCs incubated with 1 mg/ml ethanolic extracts of C. spinosa fruit; (D) MTCs incubated with 4 mg/ml water-soluble extracts of C. spinosa fruit; (E) MTCs incubated with 4 mg/ml ethanolic extracts of C. spinosa fruit. GL, germinal layer.
Cytotoxicity of water-soluble and ethanolic extracts of C. spinosa fruit on primary mouse hepatocytes
To assess the potential cytotoxicity of water-soluble and ethanolic extracts of C. spinosa fruit, cell viability was evaluated by a CCK-8 assay. As shown in Fig. 5, compared with the solvent group, the cell viability of primary mouse hepatocytes treated with 4 mg/ml of water-soluble and ethanolic extracts of C. spinosa for 48 hr was 94.20 ± 12.82 and 98.37 ± 4.86%, respectively.
Fig. 5.
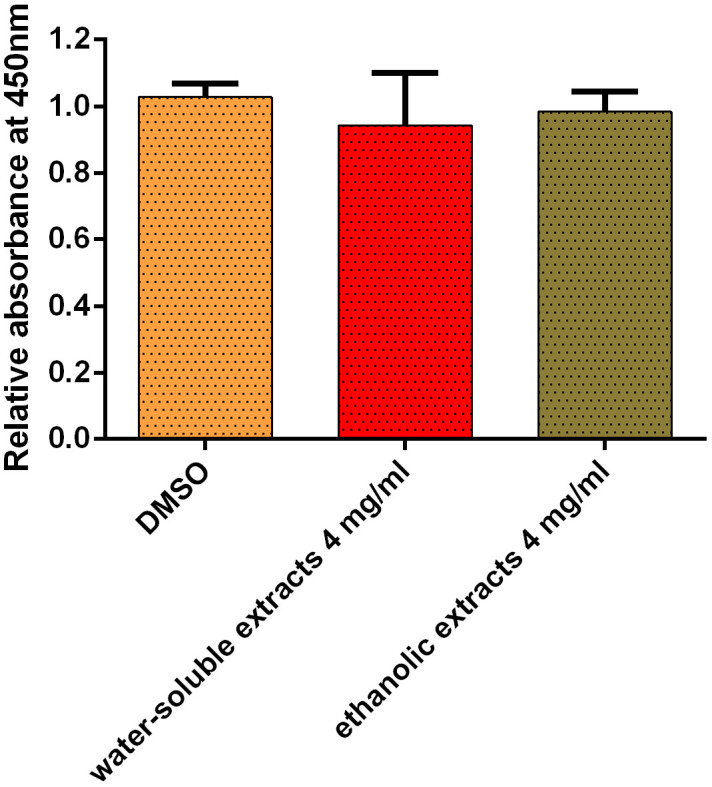
Cytotoxicity of water-soluble and ethanolic extracts of Capparis spinosa fruit on primary mouse hepatocytes. The cells were incubated with 4 mg/ml of water-soluble and ethanolic extracts for 48 hr, respectively, as assessed by the Cell Counting Kit-8 (CCK-8) method. The bars indicate arithmetic mean ± standard error of mean (SEM).
DISCUSSION
At present, a variety of natural extracts can kill PSCs in a short time at very high concentrations and can be used as protoscolicidal agents to reduce the risk of PSC spillage through hydatid cyst surgery [1, 7, 13, 27, 28]. The results of this study indicated that the water-soluble and ethanolic extracts of C. spinosa fruits had significant in vitro killing effects on the larvae of E. granulosus s.s. in a short period of time. SEM results showed that the extracts of C. spinosa fruit caused morphological and ultrastructural damage to PSCs and MTCs, especially to the germinal layer of MCTs. A large number of germinal cells are located in the germinal layer and are mainly responsible for the development of the parasite [29]. The C. spinosa fruit led to the death of these germinal cells, which ultimately manifests as the collapse of the germinal layer and a reduction in the viability of MTCs.
The in vitro data obtained indicated that the ethanolic extract at 4 mg/ml was superior to the water-soluble extract in killing MTCs. The ethanolic extract of C. spinosa fruit contains mainly polyphenols and flavonoids. Several studies have reported that polyphenols and flavonoids in natural extracts have significant inhibitory effects on parasitic infection [8, 26]. In addition, this phenomenon might also be due to the different active chemical compositions and concentrations of water-soluble and ethanolic extracts, and the choice of solvent can affect the chemical composition and content of each extract. The difference in solvent polarities may explain the differences in the antiparasitic effect of C. spinosa extracts. Interestingly, the 4 mg/ml water-soluble extract of C. spinosa fruit showed a more significant killing effect on PSCs. This result suggests that the water-soluble and ethanolic extracts of C. spinosa fruit have different inhibitory abilities on different growth stages of E. granulosus s.s. Although the precise antiparasitic action mechanisms of C. spinosa are not yet agreed upon, it is possible that C. spinosa eliminates the products of oxidative reactions and assists in the immune-mediated destruction of E. granulosus s.l. [5, 24]. We will further isolate the active ingredients in C. spinosa fruit extract to improve the therapeutic effect and investigate the mechanism of C. spinosa for CE.
The results of the cytotoxicity experiments showed that neither extract caused significant damage to mouse hepatocytes. Gadgoli et al. [9] found that compounds contained in C. spinosa, such as p-methoxy benzoic acid, have antihepatotoxic activity [12]. Notably, C. spinosa has been shown to be an effective hepatoprotective agent, and its extract is the main active ingredient in Liv 52, a marketed hepatoprotective drug in India [10]. In CE, clinical symptoms are associated with injury or dysfunction of the target organ, especially the liver (70%), which can still cause severe liver damage to the patient’s liver even after surgery [33]. The safety profile is shown by C. spinosa on hepatocytes and, its satisfactory hepatoprotective effect may repair liver damage and reduce the occurrence of adverse effects during drug treatment in CE patients, which needs to be confirmed in subsequent studies.
In conclusion, the results of this study indicate that water-soluble and ethanolic extracts of C. spinosa fruit have significant killing activity against the larval stage of E. granulosus s.s., thus laying the foundation for the development of new drugs for the treatment of CE. However, further studies are required on the active ingredients of C. spinosa fruit for the treatment of CE and to validate the in vivo efficacy.
POTENTIAL CONFLICTS OF INTEREST
The authors declare no conflict of interest.
Acknowledgments
The authors thank Associate Professor Xiaotao Zhou (School of Basic Medicine, Xinjiang Medical University) for her cooperation. The authors thank the American Journal Experts (AJE) for their helpful critical reading of this manuscript and have received an AJE ‘Editorial Certificate’ for language editing. This research was supported by the National Natural Science Foundation of China (NSFC) (82060373, 81760369 and 81360251); State Key Laboratory of Pathogenesis, Prevention and Treatment of Central Asian High Incidence Diseases Fund (SKL-HIDCA-2020-BC3 and SKL-HIDCA-2021- YG1); Tianshan Talent Project of the Xinjiang Uygur Autonomous Region (No. 2021-37-216).
REFERENCES
- 1.Abdel-Baki A. A., Almalki E., Mansour L., Al-Quarishy S.2016. In Vitro Scolicidal Effects of Salvadora persica root extract against protoscolices of Echinococcus granulosus. Korean J. Parasitol. 54: 61–66. doi: 10.3347/kjp.2016.54.1.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borhani M., Fathi S., Lahmar S., Ahmed H., Abdulhameed M. F., Fasihi Harandi M.2020. Cystic echinococcosis in the Eastern Mediterranean region: Neglected and prevailing! PLoS Negl. Trop. Dis. 14: e0008114. doi: 10.1371/journal.pntd.0008114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brunetti E., Kern P., Vuitton D. A., Writing Panel for the WHO-IWGE.2010. Expert consensus for the diagnosis and treatment of cystic and alveolar echinococcosis in humans. Acta Trop. 114: 1–16. doi: 10.1016/j.actatropica.2009.11.001 [DOI] [PubMed] [Google Scholar]
- 4.Caboni P., Sarais G., Aissani N., Tocco G., Sasanelli N., Liori B., Carta A., Angioni A.2012. Nematicidal activity of 2-thiophenecarboxaldehyde and methylisothiocyanate from caper (Capparis spinosa) against Meloidogyne incognita. J. Agric. Food Chem. 60: 7345–7351. doi: 10.1021/jf302075w [DOI] [PubMed] [Google Scholar]
- 5.El Azhary K., Tahiri Jouti N., El Khachibi M., Moutia M., Tabyaoui I., El Hou A., Achtak H., Nadifi S., Habti N., Badou A.2017. Anti-inflammatory potential of Capparis spinosa L. in vivo in mice through inhibition of cell infiltration and cytokine gene expression. BMC Complement. Altern. Med. 17: 81. doi: 10.1186/s12906-017-1569-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elissondo M., Ceballos L., Dopchiz M., Andresiuk V., Alvarez L., Bruni S. S., Lanusse C., Denegri G.2007. In vitro and in vivo effects of flubendazole on Echinococcus granulosus metacestodes. Parasitol. Res. 100: 1003–1009. doi: 10.1007/s00436-006-0381-y [DOI] [PubMed] [Google Scholar]
- 7.Fabbri J., Maggiore M. A., Pensel P. E., Denegri G. M., Elissondo M. C.2020. In vitro efficacy study of Cinnamomum zeylanicum essential oil and cinnamaldehyde against the larval stage of Echinococcus granulosus. Exp. Parasitol. 214: 107904. doi: 10.1016/j.exppara.2020.107904 [DOI] [PubMed] [Google Scholar]
- 8.Fracasso M., Dutra da Silva A., Bottari N. B., Monteiro S. G., Garzon L. R., Farias de Souza L. A., Schetinger M. R. C., Da Silva A. S.2021. Resveratrol impacts in oxidative stress in liver during Trypanosoma cruzi infection. Microb. Pathog. 153: 104800. doi: 10.1016/j.micpath.2021.104800 [DOI] [PubMed] [Google Scholar]
- 9.Gadgoli C., Mishra S. H.1999. Antihepatotoxic activity of p-methoxy benzoic acid from Capparis spinosa. J. Ethnopharmacol. 66: 187–192. doi: 10.1016/S0378-8741(98)00229-3 [DOI] [PubMed] [Google Scholar]
- 10.Ghosh N., Ghosh R., Mandal V., Mandal S. C.2011. Recent advances in herbal medicine for treatment of liver diseases. Pharm. Biol. 49: 970–988. doi: 10.3109/13880209.2011.558515 [DOI] [PubMed] [Google Scholar]
- 11.Hamuti A., Li J., Zhou F., Aipire A., Ma J., Yang J., Li J.2017. Capparis spinosa fruit ethanol extracts exert different effects on the maturation of dendritic cells. Molecules 22: 97. doi: 10.3390/molecules22010097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Horton J.2003. Albendazole for the treatment of echinococcosis. Fundam. Clin. Pharmacol. 17: 205–212. doi: 10.1046/j.1472-8206.2003.00171.x [DOI] [PubMed] [Google Scholar]
- 13.Jahanbakhsh S., Azadpour M., Tavakoli Kareshk A., Keyhani A., Mahmoudvand H.2016. Zataria multiflora Bioss: lethal effects of methanolic extract against protoscoleces of Echinococcus granulosus. J. Parasit. Dis. 40: 1289–1292. doi: 10.1007/s12639-015-0670-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lashkarizadeh M. R., Asgaripour K., Saedi Dezaki E., Fasihi Harandi M.2015. Comparison of scolicidal effects of amphotricin B, silver nanoparticles,_and Foeniculum vulgare mill on hydatid cysts protoscoleces. Iran. J. Parasitol. 10: 206–212. [PMC free article] [PubMed] [Google Scholar]
- 15.Liu J., Nie C., Xue L., Yan Y., Liu S., Sun J., Fan M., Qian H., Ying H., Wang L., Li Y.2021. Growth hormone receptor disrupts glucose homeostasis via promoting and stabilizing retinol binding protein 4. Theranostics 11: 8283–8300. doi: 10.7150/thno.61192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Loos J. A., Cumino A. C.2015. In Vitro anti-echinococcal and metabolic effects of metformin involve activation of AMP-activated protein kinase in larval stages of Echinococcus granulosus. PLoS One 10: e0126009. doi: 10.1371/journal.pone.0126009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loos J. A., Caparros P. A., Nicolao M. C., Denegri G. M., Cumino A. C.2014. Identification and pharmacological induction of autophagy in the larval stages of Echinococcus granulosus: an active catabolic process in calcareous corpuscles. Int. J. Parasitol. 44: 415–427. doi: 10.1016/j.ijpara.2014.02.007 [DOI] [PubMed] [Google Scholar]
- 18.Loos J. A., Dávila V. A., Rodrígues C. R., Petrigh R., Zoppi J. A., Crocenzi F. A., Cumino A. C.2017. Metformin exhibits preventive and therapeutic efficacy against experimental cystic echinococcosis. PLoS Negl. Trop. Dis. 11: e0005370. doi: 10.1371/journal.pntd.0005370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mahmoudvand H., Khalaf A. K., Beyranvand M.2021. In vitro and ex vivo evaluation of Capparis Spinosa extract to inactivate protoscoleces during hydatid cyst surgery. Curr. Drug Discov. Technol. 18: e18082020185049. doi: 10.2174/1570163817999200819091336 [DOI] [PubMed] [Google Scholar]
- 20.Mahmoudvand H., Kheirandish F., Dezaki E. S., Shamsaddini S., Harandi M. F.2016. Chemical composition, efficacy and safety of Pistacia vera (var. Fandoghi) to inactivate protoscoleces during hydatid cyst surgery. Biomed. Pharmacother. 82: 393–398. doi: 10.1016/j.biopha.2016.05.012 [DOI] [PubMed] [Google Scholar]
- 21.McManus D. P., Gray D. J., Zhang W., Yang Y.2012. Diagnosis, treatment, and management of echinococcosis. BMJ 344: e3866. doi: 10.1136/bmj.e3866 [DOI] [PubMed] [Google Scholar]
- 22.Moghadamnia Y., Mousavi Kani S. N., Ghasemi-Kasman M., Kazemi Kani M. T., Kazemi S.2019. The anti-cancer effects of Capparis spinosa hydroalcoholic extract. Avicenna J. Med. Biotechnol. 11: 43–47. [PMC free article] [PubMed] [Google Scholar]
- 23.Moro P., Schantz P. M.2009. Echinococcosis: a review. Int. J. Infect. Dis. 13: 125–133. doi: 10.1016/j.ijid.2008.03.037 [DOI] [PubMed] [Google Scholar]
- 24.Nabavi S. F., Maggi F., Daglia M., Habtemariam S., Rastrelli L., Nabavi S. M.2016. Pharmacological effects of Capparis spinosa L. Phytother. Res. 30: 1733–1744. doi: 10.1002/ptr.5684 [DOI] [PubMed] [Google Scholar]
- 25.Nguyen-Vo T. H., Nguyen L., Do N., Nguyen T. N., Trinh K., Cao H., Le L.2020. Plant metabolite databases: from herbal medicines to modern drug discovery. J. Chem. Inf. Model. 60: 1101–1110. doi: 10.1021/acs.jcim.9b00826 [DOI] [PubMed] [Google Scholar]
- 26.Penna-Coutinho J., Aguiar A. C., Krettli A. U.2018. Commercial drugs containing flavonoids are active in mice with malaria and in vitro against chloroquine-resistant Plasmodium falciparum. Mem. Inst. Oswaldo Cruz 113: e180279. doi: 10.1590/0074-02760180279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rahimi-Esboei B., Ebrahimzadeh M. A., Fathi H., Rezaei Anzahaei F.2016. Scolicidal effect of Allium sativum flowers on hydatid cyst protoscolices. Eur. Rev. Med. Pharmacol. Sci. 20: 129–132. [PubMed] [Google Scholar]
- 28.Rouhani S., Salehi N., Kamalinejad M., Zayeri F.2013. Efficacy of Berberis vulgaris aqueous extract on viability of Echinococcus granulosus protoscolices. J. Invest. Surg. 26: 347–351. doi: 10.3109/08941939.2013.818746 [DOI] [PubMed] [Google Scholar]
- 29.Thompson R. C.2017. Biology and Systematics of Echinococcus. Adv. Parasitol. 95: 65–109. doi: 10.1016/bs.apar.2016.07.001 [DOI] [PubMed] [Google Scholar]
- 30.Tlili N., Feriani A., Saadoui E., Nasri N., Khaldi A.2017. Capparis spinosa leaves extract: Source of bioantioxidants with nephroprotective and hepatoprotective effects. Biomed. Pharmacother. 87: 171–179. doi: 10.1016/j.biopha.2016.12.052 [DOI] [PubMed] [Google Scholar]
- 31.Vahid H., Rakhshandeh H., Ghorbani A.2017. Antidiabetic properties of Capparis spinosa L. and its components. Biomed. Pharmacother. 92: 293–302. doi: 10.1016/j.biopha.2017.05.082 [DOI] [PubMed] [Google Scholar]
- 32.Vuitton D. A., McManus D. P., Rogan M. T., Romig T., Gottstein B., Naidich A., Tuxun T., Wen H., Menezes da Silva A., World Association of Echinococcosis.2020. International consensus on terminology to be used in the field of echinococcoses. Parasite 27: 41. doi: 10.1051/parasite/2020024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wen H., Vuitton L., Tuxun T., Li J., Vuitton D. A., Zhang W., McManus D. P.2019. Echinococcosis: Advances in the 21st Century. Clin. Microbiol. Rev. 32: e00075–e18. doi: 10.1128/CMR.00075-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xin Q., Yuan M., Li H., Song X., Lu J., Jing T.2019. In vitro and in vivo effects of 3-bromopyruvate against Echinococcus metacestodes. Vet. Res. (Faisalabad) 50: 96. doi: 10.1186/s13567-019-0710-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu L., Yang J., Wang X., Jiang B., Sun Y., Ji Y.2017. Antioxidant and antitumor activities of Capparis spinosa L. and the related mechanisms. Oncol. Rep. 37: 357–367. doi: 10.3892/or.2016.5249 [DOI] [PubMed] [Google Scholar]
- 36.Zhang R., Li C., Wang J., Yang Y., Yan Y.2018. Microbial production of small medicinal molecules and biologics: From nature to synthetic pathways. Biotechnol. Adv. 36: 2219–2231. doi: 10.1016/j.biotechadv.2018.10.009 [DOI] [PubMed] [Google Scholar]