Abstract
Although Escherichia coli is a commensal bacterium of the bovine vaginal microbiota, it is an important pathogenic bacterium that causes diseases of the reproductive tract and sub-fertility. Recent studies have focused on virulence factors (VFs) of intrauterine E. coli; however, actual endometrial VFs have not been clearly identified. The purpose of this study was to identify the VFs of E. coli associated with clinical metritis and endometritis. Thirty-two strains of E. coli and four mixed Trueperella pyogenes (TP) strains were detected in the uterus of 19 Holstein dairy cows with obvious clinical signs (between 8 and 66 days postpartum). The presence of six E. coli VFs (fimH, fyuA, kpsMTII, hra1, csgA, and astA) was examined by PCR, and clinical signs and reproductive performance (mixed TP, the percentage of polymorphonuclear neutrophils [PMN%], days to uterine involution, etc.) were evaluated. Four VFs (fimH, hra1, csgA, and astA) were detected in all E. coli strains, whereas fyuA and kpsMTII were detected in 94% and 50% of strains, respectively. Cows with E. coli strains harboring kpsMTII exhibited significantly severe clinical scores (vaginal discharge score, PMN%, uterine involution), suggesting that kpsMTII is a key VF for progression of clinical metritis and endometritis. In the present study, we clearly identified six VFs associated with clinical metritis and endometritis. In addition, E. coli strains with kpsMTII probably play a crucial role in the progression of clinical metritis and endometritis.
Keywords: Escherichia coli, kpsMTII, postpartum uterine disease, uterine restoration time, virulence factor
Almost all cows are contaminated or become infected in the uterus immediately after parturition by bacteria ascending from the external environment through the open cervix. Although the uterus harbors a normal flora in approximately 50% of cows by 40 days after parturition and 90% by 60 days after parturition [36], uterine infection can develop in cows in which bacterial clearance does not proceed effectively. Postpartum uterine infections can cause puerperal fever, clinical and subclinical endometritis, and prolong the open period, consequently having a negative impact [6] manifesting as low reproductive performance [16] or increased culling rate [27].
The definition of metritis is described in the report by Sheldon et al. [39]. Puerperal metritis is characterized by an enlarged uterus and reddish-brown, moist, watery intrauterine discharge with signs of systemic disease (decreased milk production, dull pain, toxic symptoms) and fever >39.5°C within 21 days of calving [39]. Cows that are not clearly ill but have an abnormally enlarged uterus and purulent uterine discharge detected in the vagina within 21 days after parturition are classified as having clinical metritis [39]. Clinical endometritis is characterized by the presence of purulent or mucopurulent uterine exudate in the vagina after 21 days postpartum, without systemic symptoms [39]. Treatment for endometritis generally involves antibiotics; however, antibiotic therapies can have negative economic impacts due to a suspension of milk production and animal shipping. Preventing genital disease and shifting to the next prosperous lactation period are thus economically important in postpartum cow management.
Escherichia coli and Trueperella pyogenes (TP) are the most widely known bacteria that cause postpartum endometritis [3, 39, 44]. Colonization of the upper reproductive tract by E. coli is reportedly associated with severe damage to the endometrium and disruption of ovarian cycle activity, followed by infertility [20, 30, 38, 45]. In the early stages of intrauterine infection, E. coli is often the infectious agent, but the source of infection then shifts to bacteria such as TP, with persistent infection leading to chronic uterine disease [12]. Bacterial lipopolysaccharide (LPS) acts on ovarian luteinizing hormone–stimulated theca cells to inhibit steroid production [30]. The presence of E. coli and LPS in the lochia during the early postpartum period favors the development of uterine infections by TP and gram-negative anaerobes during the later postpartum period [12]. It is assumed that preventing infection by E. coli shortly after parturition prevents the adverse effects associated with LPS and contributes to the prevention of other bacterial infections. For these reasons, it is important for commercial dairies to respond early to infections with E. coli.
In uterus, infections can involve diarrheagenic E. coli (DEC) and extra-intestinal pathogenic E. coli (ExPEC), such as uropathogenic E. coli (UPEC), which can infect tissues other than the endometrium. In addition, previously unidentified E. coli strains might also infect the endometrium. Specific adhesion to host tissue cells is an essential virulence factor (VF) of most bacterial pathogens. The fundamental processes that determine the ability of bacteria to attach to host cells are mediated by microbial adhesins [25]. Uterine isolates of E. coli often lack common VF genes associated with DEC or ExPEC strains and are more likely to adhere to and invade endometrial epithelial and stromal cells, causing more severe endometritis. These particular strains are known as endometrial pathogenic E. coli (EnPEC) [40]. Although the evolutionary background of EnPEC is not clear, the ferric yersiniabactin uptake receptor (fyuA) gene was found in pelvic inflammatory disease–associated EnPEC but not E. coli from the uterus of clinically unaffected animals [19].
Phylogenetic analyzes have been used to determine the evolutionary origins of pathogenic E. coli strains. Four major phylogenetic groups of E. coli have been described to date, designated A, B1, B2, and D [21]. Animals with uterine disease are more likely to harbor group A or B1 bacteria [40]. Several E. coli VFs have been suggested as promoting the development of infections with TP, necrotic bacilli, or other organisms [8]. However, although a wide variety of coliforms can infect the uterus, research regarding their VFs is insufficient. A number of recent studies have focused on the pathogenicity of VFs, but these studies have drawn differing conclusions regarding which VFs are decisive factors in determining pathogenesis [8, 9, 17, 24].
Bicalho et al. reported that six VFs common to extraintestinal and enteroaggregative E. coli and associated with puerperal metritis and clinical endometritis have been identified: fimH, hlyA, cdt, kpsMTII, ibeA, and astA, with fimH exhibiting a synergetic relationship with the other five VFs [9]. Another study concluded that only hra1 and kpsMTII are associated with postpartum puerperal metritis [24]. Moreno et al. reported that harboring the fyuA and csgA VF genes may be a risk factor for endometrial disorders [17]. These previous studies suggested an association between E. coli VFs and puerperal metritis and clinical endometritis. However, these studies could not analyze E. coli VFs isolated from cows diagnosed with puerperal metritis or clinical endometritis because most of the E. coli were isolated from the uterus soon after delivery (5–7 days postpartum). Furthermore, no research has focused on the effect of E. coli VFs on clinical metritis. Thus, details regarding the characteristics of EnPEC remain unclear, and there have been no studies examining the course of clinical symptoms and the relationship of these symptoms to VFs. The objective of present study, therefore, was to clarify the impact of VFs on clinical metritis and endometritis and the clinical course of infection. The presence of the fimH, fyuA, kpsMTII, csgA, hra1, and astA VF genes was examined in E. coli isolates obtained from clinical metritis and endometritis samples from animals with obvious clinical symptoms at three Holstein dairy farms.
MATERIALS AND METHODS
Animals
This study was conducted according to the institutional guidelines for animal experiments of Rakuno Gakuen University (approval no. VH17C10). A total of 19 Holstein Friesian cows from three dairy farms were examined in the study. All animals delivered alone in the calving pen, were milked twice daily, and determined not to have other perinatal disorders without uterine diseases.
Clinical examination
Reproductive examinations were started at 7 days after parturition (± 3 days) and performed weekly (± 3 days) until uterine involution was completed. Any signs of systemic illness were recorded. The uterine involution status of each cow was evaluated by reproductive examinations that included transrectal palpation, vaginoscopy, and ultrasonography (HS-101v; Honda Electronics Co., Ltd., Toyohashi, Japan). Cows that were not ill but had an abnormally enlarged uterus and a purulent uterine discharge from the vagina within 21 days after parturition were determined to have clinical metritis [39]. Clinical metritis and endometritis were also confirmed using ultrasonography (Supplementary Fig. 1). Clinical endometritis was diagnosed by the scoring system of Sheldon et al. at ≥22 days postpartum [37] (Table 1). The diameters of the largest part of uterine horn and the cervix were measured by transrectal palpation and ultrasonography and scored on three levels from 0 to 2 as follows: the diameter of the uterine horn was scored 0 for small (<3.5 cm for primiparous cows, <4.0 cm for multiparous cows), 1 for medium (3.5 to 5.5 cm for primiparous cows, 4.0 to 6.0 cm for multiparous cows), and 2 for large (>5.5 cm for primiparous cows, >6.0 cm for multiparous cows). Vaginal discharge was scored based on the amount and characteristics by vaginoscopy. A vaginal discharge score of 0 was given for clear or translucent, 1 for <50 ml with a small amount of thick white flakes, 2 for <50 ml with <50% white or off-white mucosal purulent material, and 3 for exudates of ≥50 ml with ≥50% white or yellow purulent material. A score of 3 was added for foul smell or bloody discharge [37, 44]. Cows with total scores of ≥1 were diagnosed as having clinical endometritis. The day of complete uterine involution was defined as the examination day when the total score was 0, the polymorphonuclear neutrophil percentage (PMN%) was normal, and no bacteria were isolated. In the present study, PMN% values of <18%, <10%, and <5% at 21–33 days, 34–47 days, and 48–60 days postpartum, respectively, were defined as normal [16, 44].
Table 1. Scoring system for the assessment of the severity of endometritis.
| Clinical sign | Score | |||
|---|---|---|---|---|
| Diameter of largest uterine horn | Primipara | Multipara | ||
| Large | >5.5 cm | >6.0 cm | 2 | |
| Medium | 3.5–5.5 cm | 4.0–6.0 cm | 1 | |
| Normal | <3.5 cm | <4.0 cm | 0 | |
| Diameter of cervix | Primipara | Multipara | ||
| Large | >7.5 cm | >7.5 cm | 2 | |
| Midium | 4.5–7.0 cm | 5.0–7.5 cm | 1 | |
| Normal | <4.5 cm | <5.0 cm | 0 | |
| Vaginal discharge | Caracter | Foul smell | 3 | |
| No smell | 0 | |||
| Bloody | 3 | |||
| Volume | ≥50 ml, pus (≥50%) | 3 | ||
| <50 ml, pus (<50%) | 2 | |||
| White clumps | 1 | |||
| Normal | 0 | |||
Uterine smear collection and bacteriology
Intrauterine smears were collected from all cows by endometrial cytology using a sterilized cytobrush instrument (Metricbrush; Fujihira Industry, Tokyo, Japan) [4]. The perineum and vulva were wiped with a dry paper towel and then disinfected with benzalkonium chloride and 70% alcohol. A cytobrush instrument covered with a plastic sheath was introduced into the vagina. The tip of the instrument was guided to the external uterine orifice by rectal palpation. The cytobrush instrument alone was introduced into the uterine body through the cervix after rupturing the tip of the sheath at the external uterine orifice. After inserting the cytobrush into the uterine body, the brush was rotated once to obtain endometrial cells. The instrument was then withdrawn from the uterus and rotated on a sterilized glass slide to prepare a cell smear. All smeared glass slides were stained with modified Giemsa stain, and >400 cells were counted at 200 × magnification using an optical microscope. The ratio of PMNs to all cells on the surface of the endometrium was then calculated (Table 2).
Table 2. Virulence factor genes of Escherichia coli isolated from the postpartum uterus and associated clinical symptoms.
| Cow No. | Parity | Bacterial no. |
Days after parturition |
PMN (%) |
Identified bacteria |
Phylogenetic group |
fimH | fyuA | kpsMT II | hra1 | csgA | astA | Uterine restoration day |
Days to first AI | No. of AI | Days open |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| s2 | 1 | s2-1 | 14 | 6.5 | E. coli | D | + | + | + | + | + | + | 40 | 82 | 3 | 152 |
| s2-2 | 14 | 6.5 | E. coli | B2 | + | + | − | + | + | + | ||||||
| s5 | 2 | s5-1 | 38 | 1.4 | E. coli | D | + | − | − | + | + | + | 45 | 82 | 1 | 82 |
| s6 | 7 | s6-1 | 32 | 6.8 | E. coli | B2 | + | + | − | + | + | + | 39 | (−) | (−) | (−) |
| s7 | 3 | s7-1 | 6 | 74.3 | E. coli | B2 | + | + | + | + | + | + | 97 | 169 | 3 | 225 |
| s7-2 | 13 | 83.9 | E. coli | B2 | + | + | − | + | + | + | ||||||
| s7-3 | 13 | 83.9 | TP | |||||||||||||
| s7-4 | 21 | 53.8 | TP | |||||||||||||
| s7-6 | 28 | 79.4 | TP | |||||||||||||
| s7-7 | 45 | 41.5 | TP | |||||||||||||
| s7-8 | 48 | 49.2 | TP | |||||||||||||
| s9 | 3 | s9-1 | 9 | 83.7 | E. coli | B2 | + | + | − | + | + | + | 40 | 103 | 1 | (−) |
| s9-2 | 9 | 83.7 | E. coli | A | + | + | − | + | + | + | ||||||
| s9-3 | 9 | 83.7 | E. coli | B2 | + | + | + | + | + | + | ||||||
| s9-4 | 9 | 83.7 | TP | |||||||||||||
| s9-5 | 15 | 56.9 | TP | |||||||||||||
| s11 | 1 | s11-1 | 33 | 6.0 | E. coli | B2 | + | + | + | + | + | + | 40 | 62 | 1 | 62 |
| s12 | 5 | s12-1 | 8 | 39.6 | E. coli | B2 | + | + | + | + | + | + | 60 | (−) | (−) | (−) |
| s12-2 | 8 | 39.6 | TP | |||||||||||||
| s12-4 | 15 | 13.1 | TP | |||||||||||||
| s12-5 | 22 | 16.8 | TP | |||||||||||||
| s12-6 | 29 | 41.8 | E. coli | B2 | + | + | + | + | + | + | ||||||
| s12-7 | 29 | 41.8 | TP | |||||||||||||
| s13 | 1 | s13-1 | 16 | – | E. coli | B2 | + | + | + | + | + | + | 57 | 203 | 3 | 304 |
| s13-2 | 16 | – | TP | |||||||||||||
| s13-3 | 22 | 26.2 | TP | |||||||||||||
| s13-4 | 43 | 4.9 | E. coli | B2 | + | + | − | + | + | + | ||||||
| s14 | 1 | s14-1 | 34 | 7.4 | E. coli | B2 | + | + | + | + | + | + | 55 | 90 | 4 | 209 |
| s14-2 | 48 | 0.5 | E. coli | B2 | + | + | − | + | + | + | ||||||
| s15 | 1 | s15-1 | 31 | 0.0 | E. coli | B2 | + | + | + | + | + | + | 80 | 106 | 3 | 175 |
| s15-2 | 45 | 9.1 | E. coli | B2 | + | + | + | + | + | + | ||||||
| s15-3 | 66 | 9.1 | E. coli | B2 | + | + | + | + | + | + | ||||||
| s16 | 3 | s16-1 | 20 | 71.3 | E. coli | B2 | + | + | + | + | + | + | 62 | 98 | 1 | 98 |
| s17 | 6 | s17-1 | 29 | 10.3 | E. coli | B2 | + | + | − | + | + | + | 42 | 78 | 1 | 78 |
| s18 | 4 | s18-1 | 50 | 0.01 | E. coli | B2 | + | + | + | + | + | + | 36 | (−) | (−) | (−) |
| s21 | 1 | s21-1 | 30 | 1.8 | E. coli | B2 | + | + | − | + | + | + | 44 | 60 | 7 | 280 |
| s21-2 | 30 | 1.8 | E. coli | B2 | + | + | − | + | + | + | ||||||
| s23 | 2 | s23-1 | 33 | 0.74 | E. coli | B1 | + | − | − | + | + | + | 33 | 99 | 1 | 99 |
| s24 | 2 | s24-1 | 43 | 4.8 | E. coli | D | + | + | + | + | + | + | 85 | 90 | 3 | (−) |
| s25 | 7 | s25-1 | 38 | 35.2 | E. coli | B2 | + | + | − | + | + | + | 52 | (−) | (−) | (−) |
| s27 | 1 | s27-1 | 34 | 10.8 | E. coli | B2 | + | + | − | + | + | + | 87 | 93 | 2 | 116 |
| s27-2 | 34 | 10.8 | E. coli | B2 | + | + | + | + | + | + | ||||||
| s27-3 | 34 | 10.8 | E. coli | B2 | + | + | + | + | + | + | ||||||
| s27-4 | 48 | 2.8 | E. coli | B2 | + | + | + | + | + | + | ||||||
| s30 | 5 | s30-1 | 29 | 1.6 | E. coli | B1 | + | + | − | + | + | + | 29 | 146 | 4 | (−) |
(−) indicates no implementation. PMN; polymorphonuclear neutrophil, AI; artificial insemination, TP; Trueperella pyogenes.
Immediately after smearing, the cytobrush instrument was suspended in a sterile plastic tube containing 1 ml of sterile saline. After stirring the suspension well, 50 µl of the resulting suspension was cultured on 5% sheep blood agar at 37°C for 24–48 hr. After incubation, bacteria were identified based on colony characteristics, Gram stain, and morphology, with E. coli the primary bacterium recorded in this study. Mixed infection with TP was also recorded. In addition, the presence of E. coli was confirmed by API 20 E biochemical profiling (Sysmex Corp., Kobe, Japan). Finally, 32 samples of E. coli were isolated from 19 cows (Table 2) and stored at –80°C in Luria-Bertani broth containing 25% glycerol.
DNA extraction and PCR
Bacterial DNA samples were purified from overnight cultures in LB broth using a GenEluteTM Bacterial Genomic DNA kit (Sigma-Aldrich/Merck, Darmstadt, Germany). All reactions were performed in a 20-μl volume using 18 μl of premix containing NEbuffer, dNTP mix (0.2 mM each), 0.5 units of Taq DNA polymerase (New England Biolabs, Ipswich, MA, USA), primer mix (0.5 μM each), and finally, 2 μl of DNA extract (50 ng). All thermal cycling protocols were as follows: 94°C for 1 min, 55–63°C for 1 min (according to each primer set, Supplementary Table 1 [2, 17, 24, 28]), 72°C for 1 min, and a final step of 72°C for 2 min. Negative controls consisting of the PCR mixture without DNA were included in all PCR runs. Amplification products were separated by electrophoresis on a 2% (wt/vol) agarose gel.
Random amplified polymorphic DNA PCR
PCR was carried out in 25 μl containing 50 ng of E. coli genomic DNA, 20 pmol of primers, 0.2 mM each dNTP, 1.25 units of Taq DNA polymerase, and standard Taq reaction buffer (New England Biolabs). We used the following primer: 5′-GCGATCCCCA-3′ (primer name: 1283) [1]. The cycling program was 94°C for 1 min, 36°C for 1 min, 72°C for 2 min, and a final step of 72°C for 2 min. Amplification products were separated by electrophoresis on a 2% (wt/vol) agarose gel.
Statistical analysis
The 32 isolated E. coli strains were divided into a clinical metritis group (n=10) and endometritis group (n=22) (Table 3). Cows in the clinical metritis group and endometritis group were within 21 and after 22 days of parturition, respectively. The number of days after parturition, vaginal discharge score, PMN%, and total score were compared between the two groups using the Student’s t-test after normality testing. The parity, number of days after parturition, vaginal discharge score, PMN%, and reproductive parameters were compared between the E. coli kpsMTII-positive group (n=10) and kpsMTII-negative group (n=9) using the Student’s t-test or Welch’s t-test after normality testing and the F test (Table 4). In addition, Fisher’s exact test was performed to compare the ratio of TP mixed infections. Data are presented as the mean ± SD. The significance level was set as <5%.
Table 3. Clinical metritis and endometritis with Escherichia coli diagnosed during and after the puerperium period.
| Group | Number of E. coli strains |
Days after parturition |
Evaluation of uterine repair |
VF prevalence of E. coli strains (%) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Vaginal mucus score | PMN% | Total score | fimH | fyuA | kpsMTII | hra1 | csgA | astA | |||
| Clinical metritis | 10 | 11.8 ± 4.3a | 3.2 ± 2.3a | 59.2 ± 33.0a | 6.9 ± 2.4a | 100 | 100 | 60.0 | 100 | 100 | 100 |
| Endometritis | 22 | 37.8 ± 9.3b | 0.5 ± 0.8b | 8.1 ± 7.7b | 1.7 ± 1.3b | 100 | 90.9 | 50.0 | 100 | 100 | 100 |
Data are shown as mean ± standard deviation. a/b, P<0.05. PMN%; the percentage of polymorphonuclear neutrophils, VF; virulence factor.
Table 4. Comparison of reproductive parameters between cows with kpsMTII-positive and kpsMTII-negative Escherichia coli.
| Group | n | Parity | Days after parturition | Ratio of mixed infection with TP (%) |
Evaluation of uterine repair |
Days to uterine involution |
Days to 1st AI | Days open in pregnant cows |
||
|---|---|---|---|---|---|---|---|---|---|---|
| Vaginal mucus score |
PMN% | Total score | ||||||||
| Detected | 12 | 2.2 ± 1.4a | 30.6 ± 17.3 | 33.3 (4/12) | 1.7 ± 1.7a | 24.5 ± 29.7a | 3.4 ± 2.8 | 61.6 ± 21.1a | 109.6 ± 42.8 | 167.6 ± 78.0 |
| Non-detected | 7 | 4.3 ± 2.6b | 32.7 ± 3.9 | 0 (0/7) | 0.1 ± 0.4b | 8.3 ± 12.4b | 2.9 ± 1.3 | 40.6 ± 7.7b | 93.0 ± 32.7 | 134.8 ± 97.3 |
Data are shown as mean ± standard deviation. a/b, P<0.05. AI; artificial insemination, PMN%; the percentage of polymorphonuclear neutrophils.
RESULTS
E. coli isolated from the postpartum uterus and phylogenetic distribution
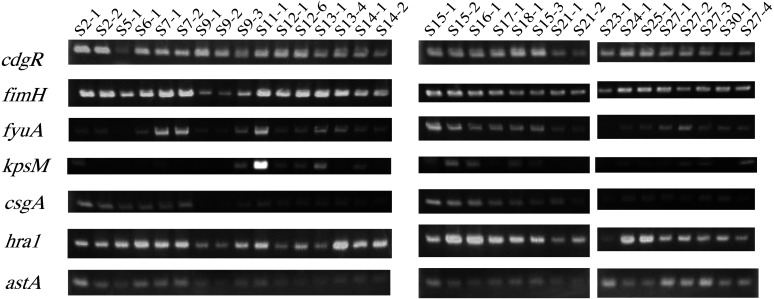
A total of 32 strains of E. coli were isolated from 19 cows, of which 4 had mixed infection with TP. The data of the examinations in which E. coli were detected are summarized in Table 2. All 32 E. coli strains obtained by culturing on blood agar medium were confirmed as E. coli by PCR (Fig. 1). Almost all of the isolates were confirmed as different strains based on RAPD PCR results (Supplementary Fig. 2). To determine the phylogenetic distribution of these strains, the genes chuA, yjaA, and TSPE4C2 were examined by PCR and classified into four phylogenetic groups, A, B1, B2, and D, using the method of Clermont et al. [10, 21] (Table 2 and Fig. 2). Eighty-one percent of the strains were classified as group B2, whereas groups A, B1, and D accounted for 3, 6, and 9%, respectively. Strains lacking both the fyuA and kpsMTII genes were other than B2 and classified as group B1 or D (Table 2).
Fig. 1.
PCR amplification of the chuA and yjaA genes and DNA fragment TSPE4-C2 specific for E. coli phylogenetic groups. PCR fragments of chuA, yjaA, and TSPE4-C2 were confirmed as 279-, 211- and 152-bp fragments, respectively. These amplification patterns allowed phylogenetic group strain determination (Table 2).
Fig. 2.
PCR amplification of six Virulence Factor genes from Escherichia coli strains isolated from endometritis samples. PCR fragments of fimH, fyuA, kpsMTII, csgA, hra1, and astA were confirmed. The data are summarized in Table 2.
VF genes and clinical symptoms in cows
To determine which VFs are associated with which clinical symptoms, six VF genes were investigated by PCR for each E. coli strain isolated (Table 2 and Fig. 1). A total of 10 and 22 E. coli strains were isolated from the clinical metritis and endometritis groups, respectively (Table 3). The evaluation of uterine involution in the clinical metritis group revealed a severe level of disease in these cows (Table 3). Five VF-encoding genes (fimH, fyuA, hra1, csgA, astA) were detected in all 10 E. coli strains isolated from the uterus of clinical metritis cows (Table 3). The prevalence of these five VFs in E. coli strains isolated from the uterus of endometritis cows was as high as 90.9–100% (Table 3). Of the 6 VFs investigated in the study, the gene encoding fyuA was detected in all strains isolated from the endometritis group except strains S5-1 and S23-1 (Table 2). The prevalence of kpsMTII in E. coli isolated from the clinical metritis and endometritis groups was 60 and 50%, respectively (Table 3). In addition, strains lacking fyuA also lacked kpsMTII (Table 2).
VF genes and reproductive performance
Reproductive performance was compared between the kpsMTII-positive and kpsMTII-negative groups (Table 4). Significant differences were found between the two groups in terms of parity, vaginal discharge score, and PMN% (P<0.05) (Table 4). The rates of mixed infection with TP in the kpsMTII-positive and kpsMTII-negative groups were 33.3 and 0% (Table 4), respectively, with all mixed infections with TP occurring in the kpsMTII-positive group (Table 2). Although the number of days of uterine involution in the kpsMTII-positive group was greater than that in the kpsMTII-negative group, there was no significant difference in the number of days to first artificial insemination (AI) and days open (Table 4).
DISCUSSION
In this study, the association between clinical symptoms and VFs of strains isolated from cows with clinical metritis and endometritis was investigated. Although E. coli has been reported as a member of the normal microbiota of the bovine reproductive tract in healthy heifers [35], data indicate it also plays a crucial role in the establishment of metritis and endometritis [15]. These contradictory results may be due to the diversity of E. coli strains, namely, the diversity of specific strains carrying VF genes. To evaluate which VFs are associated with particular clinical symptoms, six VF genes were investigated by PCR for each E. coli strain isolated from cows with obvious clinical metritis or endometritis (Table 2 and Fig. 1).
In our study, there was a difference in the rates of phylogenetic groups compared with previous reports. Duriez et al. reported that ExPEC strains primarily belong to phylogenetic group B2 and harbor several VF genes. In contrast, most commensal E. coli strains belong to groups A and B1 and harbor few VF genes compared with the corresponding pathogenic strains [13]. Clermont et al. also clearly demonstrated that strains harboring chuA, a gene required for heme transport in O157:H7 and other clinical E. coli strains, are distributed in groups B2 and D. However, some reports of phylogenetic classification studies have shown that E. coli isolated from the postpartum uterus (of cows with metritis and endometritis) belong mainly to groups A and B1 [17, 40]. Those results may reflect commensal strains isolated from the uterus of postpartum dairy cows. In this study, most of the strains isolated from animals with severe clinical manifestations of both clinical metritis and endometritis belonged to group B2 (Table 2 and Fig. 2), suggesting that they were ExPEC. These data suggest that ExPEC is involved in the pathogenesis of clinical metritis and endometritis, and we speculate that VFs encoded by genes other than the three that determine phylogenetic group are involved in the pathogenicity of ExPEC.
VF genes were detected at a higher rate in the present study compared with previous reports [9, 17, 24, 40]. Kassé et al. investigated as many as 40 VFs and concluded that statistically, only hra1 and kpsMTII are associated with postpartum puerperal metritis, but they did not find a clearly associated VF [24]. Bicalho et al. reported that six VFs common to extraintestinal and enteroaggregative E. coli, such as fimH, hlyA, cdt, kpsMTII, ibeA, and astA, are associated with puerperal metritis and clinical endometritis, with a synergistic relationship between fimH and the other five VFs [9]. Moreno et al. reported that vaginal E. coli populations harboring the fyuA and csgA VF genes may represent a risk factor for endometrial disorders; specifically, those that also possess kpsMTII may have pathogenic potential to cause repeat breeder syndrome [17]. In all of these reports, the percentage of detected VFs was not as high as in our study, probably because a relatively higher rate of commensal E. coli strains without VFs were isolated in these previous studies. This difference is also consistent with the results of phylogenetic analyzes, in which a low percentage of B2 isolates was reported in previous studies, in contrast to a high percentage of B2 isolates in our study.
The fimH gene encodes one of the most extensively studied adhesins, which binds directly to D-mannose, which in turn is bound to a carrier protein. The fimH protein is understood to be one of the most important VFs because it is uniquely involved in determining receptor specificity and can be a stepping stone to infection [26]. Typically, type I pili including the fimH adhesin are involved in mediating the attachment of E. coli to mammalian cells [23]. The inhibition of E. coli adhesion to uterine endometrial cells by D-mannose as described by Sheldon et al. indicates that fimH is involved in adhesion to uterine endometrial cells. In previous studies, fimH has consistently been the most frequently detected VF in E. coli isolated from the uterus [9, 17, 24]. In addition, fimH is often detected in healthy vagina and also in the healthy uterus after parturition [9, 17, 24]. Therefore, fimH may be necessary for enabling E. coli to remain in the uterus, although further studies are needed to confirm this hypothesis.
The heat-resistant agglutinin 1, hra1, was originally described as an autoaggregating and hemagglutinating protein from an O9:H10:K99 E. coli strain pathogenic in piglets and lambs [29]. In addition, hra1 was first reported by Kassé et al. as a VF associated with postpartum puerperal metritis in dairy cows [24]. In strains of E. coli that are exceptional colonizers, hra1 is an integral outer membrane protein that confers the ability to adhere to human epithelial cells, resulting in bacterial autoaggregation. Although hra1 is also found in non-pathogenic E. coli, its presence enhances colonization by diarrheagenic E. coli such as enteroaggregative E. coli (EAEC) and of invasive E. coli [7, 14, 31]. The bacterial autoaggregation mediated by hra1 can lead to the formation of large bacterial aggregates that are not phagocytosed and may be involved in the formation of biofilms that enable the bacteria to remain in the uterine mucosa [24]. The astA gene encodes EAEC heat-stable toxin 1 (EAST1), which has been implicated in the development of human diarrhea [43]. Bicarho et al. reported that EAST1 may play an important role in the development of postpartum uterine infections and that the VF gene astA is the second most important predictor of puerperal metritis after fimH [9].
Both fyuA and csgA are VFs involved in biofilm formation [19, 41]. The fyuA gene encodes a ferric scavenger receptor with a high prevalence in UPEC, which is among the most prevalent agents of urinary tract infections [18]. It is one of the most upregulated genes, and in biofilm, the ferric yersiniabactin uptake receptor fyuA plays an important role in iron uptake and biofilm formation during urinary tract infections caused by E. coli [19]. The csgA gene encodes a major structural subunit of Curli, the biofilm-forming amyloid. Curli is expressed on the surface of gram-negative enterobacteria and known to interact with a wide range of proteins that contribute to bacterial virulence. Curli mediates host cell adhesion and invasion and is a potent inducer of the host inflammatory response [5, 42]. The onset of CsgA aggregation is delayed by stoichiometric concentrations of fibrinogen, which inhibits the initial events of CsgA assembly [41]. Moreno et al. reported that E. coli of phylogenetic group B1, which possess the fyuA and csgA genes, are highly motile, express Curli fimbriae and cellulose, and have the ability to form biofilms, may be widely involved in the development of postpartum disorders [17].
In our study, surprisingly, four VFs (fimH, hra1, csgA, and astA) were detected in all 32 E. coli strains isolated from the postpartum uterus, even though they were isolated from cows diagnosed with clinical metritis or endometritis lacking systemic symptoms (Table 3). All of these VFs are reportedly associated with uterine infections [3, 13, 16]. Although the relationship between bovine uterine infections and these VFs of E. coli has not been elucidated, the data suggest these six VFs play important roles in uterine infection. Identifying the VFs associated with the development of uterine infections will require the isolation of E. coli from the uterus of cows that have not shown clinical symptoms in a large population, followed by characterization of the differences in E. coli VFs between cows diagnosed with puerperal, clinical metritis, or clinical endometritis and evaluation of the association of E. coli VFs with systemic symptoms. Of the 32 strains isolated in the present study, only two strains lacking fyuA were identified (Table 2). These strains, which also lacked kpsMTII and produced relatively mild clinical symptoms, belonged to phylogenetic groups B1 and D (Table 2). Although the VFs were clearly associated with clinical metritis and endometritis, whether the differences in pathogenicity of the isolates were due to the presence or absence of particular VFs or to differences in phylogenetic group has not been determined.
The mechanism associated with clinical symptoms affecting the postpartum genital tract could involve fimH-mediated adhesion to the endometrium, hra1-promotion of colony formation, fyuA- and csgA-mediated promotion of biofilm formation, and astA functioning as a toxin. The detailed mechanisms by which each VF causes infection and inflammation of the uterus should be the subject of future research.
Interestingly, in our study, kpsMTII was detected at a lower rate than the other VFs examined (Table 4). kpsMTII encodes capsular protein K1 or K5 [24]. These proteins play a role in evading or counteracting host nonspecific immunity by interacting with the bacterial surface and complement system in the early stages of infection [22]. The presence of kpsMTII has been linked to cellulitis in chickens [11] and urinary tract infections in women [33, 34]. Bicalho et al. reported that cows in which the uterus is contaminated with E. coli carrying the kpsMTII gene are 9.2 times less likely to become pregnant than cows with an uncontaminated uterus [9]. Kassé et al. reported that cows harboring E. coli with kpsMTII detected in the uterus at 1–7 days in milk are 6.2 times more likely than cows with no E. coli detected and 3.2 times more likely than cows with E. coli lacking kpsMTII to subsequently develop postpartum puerperal metritis [24]. Moreno et al. reported that the presence of E. coli harboring kpsMTII can cause repeat breeder syndrome [17]. Thus, previous reports consistently indicate that kpsMTII causes postpartum uterine problems, and we hypothesize that kpsMTII is also associated with clinical metritis and endometritis.
In the present study, the presence of kpsMTII was found to significantly worsen vaginal discharge score and PMN% compared to its absence (Table 4). However, the higher vaginal discharge score and PMN% in cows with fewer days postpartum is a physiologic phenomenon, and it is therefore difficult to compare these parameters between cows. Groups must be compared according to the number of days postpartum using a larger number of samples. In addition to the five VFs associated with clinical metritis and endometritis mentioned above, kpsMTII was found to further aggravate the condition. The present study demonstrated that kpsMTII is associated with a delay in postpartum uterine involution in dairy cows. The postpartum uterine involution time for cows in the kpsMTII-positive group was 61.3 days, whereas it was 40.6 days for cows in the kpsMTII-negative group, indicating that uterine involution required a significantly longer amount of time in the kpsMTII-positive group (Table 4). This may be the result of a system in which the K1 or K5 proteins encoded by kpsMTII evade immune recognition by interacting with the bacterial surface and complement system and by employing molecular mimicry [22]. While kpsMTII was found to delay uterine involution, no significant difference was observed in number of days to first AI or days open. This may be due to other factors affecting ovarian function or to environmental factors that collectively preserve sperm activity in the uterus between the time of uterine involution and conception. Furthermore, as reproductive examinations were conducted weekly, the absence of significant differences could have been due to the fact that as soon as all conditions other than uterine involution were achieved, conception could have occurred immediately. During the clinical course, some cows in which kpsMTII was not detected exhibited improvement in symptoms, and some cows in which kpsMTII was detected exhibited stable or worsening of symptoms; more extensive research will be needed to explain this difference.
Although a statistically significant difference was not demonstrated, the rates of mixed infections with TP in the kpsMTII-positive and kpsMTII-negative groups were 33.3 and 0%, respectively (Table 3). From all cases of mixed infection, E. coli with kpsMTII were detected in the early stage (Table 2). As the presence of E. coli and LPS in lochia in the early postpartum period predisposes to uterine infections caused by TP and gram-negative anaerobic bacteria in the late postpartum period [12], the VF kpsMTII may play a role in this transition.
In addition, cases of mixed infection with E. coli and TP are common in young animals (Table 4). Moreover, the animals with E. coli harboring kpsMTII in this study exhibited significantly lower parity (Table 4). To date, there have not been any reports on the relationship between VFs in E. coli and TP. The possibility that TP may have an effect on the delay in uterine involution mediated by kpsMTII cannot be denied. Although it is clear that the presence of kpsMTII delays uterine involution, further large-scale studies are needed to clarify whether TP is involved in this delay. Moore et al. reported detecting the s16 rRNA gene sequence of TP in the uterus of virgin heifers [32]. These data are consistent with the fact that TP infections (umbilical cord inflammation, abscesses, and mastitis, etc.) that we encounter in clinical practice are more common in calves, heifers, and younger cows. These data may provide an opportunity to study the relationship between age and TP infection and between VFs in E. coli and TP infection. Prior to the present study, VFs associated with postpartum uterine infection had not been clearly identified, but we did so by carefully isolating bacteria from animals clearly exhibiting clinical symptoms.
In summary, we demonstrated that at least six VFs (fimH, fyuA, hra1, csgA, astA, and kpsMTII) are strongly associated with the development of clinical metritis and endometritis and that kpsMTII in particular is involved in prolonged uterine involution and worsening of symptoms. In addition, a possible relationship between E. coli VFs and TP infection in the uterus was identified. Further studies are required to clarify the mechanisms through which these factors mediate the pathogenesis of postpartum uterine infections and to examine the relationship between E. coli and TP in mixed infections. This study thus enhances understanding of the pathogenicity of E. coli VFs.
CONFLICT OF INTEREST
The authors declare no conflicts of interest associated with this manuscript.
Supplementary
Acknowledgments
This study was supported by grants for Scientific Research on Innovative Areas and by the International Group of the Ministry of Education, Culture, Sports, Science and Technology, Japan (MEXT)/JSPS KAKENHI (JP17H01506 and JP19K15985).
REFERENCES
- 1.Akopyanz N., Bukanov N. O., Westblom T. U., Kresovich S., Berg D. E.1992. DNA diversity among clinical isolates of Helicobacter pylori detected by PCR-based RAPD fingerprinting. Nucleic Acids Res. 20: 5137–5142. doi: 10.1093/nar/20.19.5137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antikainen J., Tarkka E., Haukka K., Siitonen A., Vaara M., Kirveskari J.2009. New 16-plex PCR method for rapid detection of diarrheagenic Escherichia coli directly from stool samples. Eur. J. Clin. Microbiol. Infect. Dis. 28: 899–908. doi: 10.1007/s10096-009-0720-x [DOI] [PubMed] [Google Scholar]
- 3.Azawi O. I.2008. Postpartum uterine infection in cattle. Anim. Reprod. Sci. 105: 187–208. doi: 10.1016/j.anireprosci.2008.01.010 [DOI] [PubMed] [Google Scholar]
- 4.Barlund C. S., Carruthers T. D., Waldner C. L., Palmer C. W.2008. A comparison of diagnostic techniques for postpartum endometritis in dairy cattle. Theriogenology 69: 714–723. doi: 10.1016/j.theriogenology.2007.12.005 [DOI] [PubMed] [Google Scholar]
- 5.Barnhart M. M., Chapman M. R.2006. Curli biogenesis and function. Annu. Rev. Microbiol. 60: 131–147. doi: 10.1146/annurev.micro.60.080805.142106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bartlett P. C., Kirk J. H., Wilke M. A., Kaneene J. B., Mather E. C.1986. Metritis complex in Michigan Holstein-Friesian cattle: incidence, descriptive epidemiology and estimated economic impact. Prev. Vet. Med. 4: 235–248. doi: 10.1016/0167-5877(86)90026-7 [DOI] [PubMed] [Google Scholar]
- 7.Bhargava S., Johnson B. B., Hwang J., Harris T. A., George A. S., Muir A., Dorff J., Okeke I. N.2009. Heat-resistant agglutinin 1 is an accessory enteroaggregative Escherichia coli colonization factor. J. Bacteriol. 191: 4934–4942. doi: 10.1128/JB.01831-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bicalho M. L. S., Machado V. S., Oikonomou G., Gilbert R. O., Bicalho R. C.2012. Association between virulence factors of Escherichia coli, Fusobacterium necrophorum, and Arcanobacterium pyogenes and uterine diseases of dairy cows. Vet. Microbiol. 157: 125–131. doi: 10.1016/j.vetmic.2011.11.034 [DOI] [PubMed] [Google Scholar]
- 9.Bicalho R. C., Machado V. S., Bicalho M. L. S., Gilbert R. O., Teixeira A. G. V., Caixeta L. S., Pereira R. V. V.2010. Molecular and epidemiological characterization of bovine intrauterine Escherichia coli. J. Dairy Sci. 93: 5818–5830. doi: 10.3168/jds.2010-3550 [DOI] [PubMed] [Google Scholar]
- 10.Clermont O., Bonacorsi S., Bingen E.2000. Rapid and simple determination of the Escherichia coli phylogenetic group. Appl. Environ. Microbiol. 66: 4555–4558. doi: 10.1128/AEM.66.10.4555-4558.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Brito B. G., Gaziri L. C. J., Vidotto M. C.2003. Virulence factors and clonal relationships among Escherichia coli strains isolated from broiler chickens with cellulitis. Infect. Immun. 71: 4175–4177. doi: 10.1128/IAI.71.7.4175-4177.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dohmen M. J. W., Joop K., Sturk A., Bols P. E. J., Lohuis J. A. C. M.2000. Relationship between intra-uterine bacterial contamination, endotoxin levels and the development of endometritis in postpartum cows with dystocia or retained placenta. Theriogenology 54: 1019–1032. doi: 10.1016/S0093-691X(00)00410-6 [DOI] [PubMed] [Google Scholar]
- 13.Duriez P., Clermont O., Bonacorsi S., Bingen E., Chaventré A., Elion J., Picard B., Denamur E.2001. Commensal Escherichia coli isolates are phylogenetically distributed among geographically distinct human populations. Microbiology (Reading) 147: 1671–1676. doi: 10.1099/00221287-147-6-1671 [DOI] [PubMed] [Google Scholar]
- 14.Fagan R. P., Lambert M. A., Smith S. G. J.2008. The hek outer membrane protein of Escherichia coli strain RS218 binds to proteoglycan and utilizes a single extracellular loop for adherence, invasion, and autoaggregation. Infect. Immun. 76: 1135–1142. doi: 10.1128/IAI.01327-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galvão K. N., Bicalho R. C., Jeon S. J.2019. Symposium review: The uterine microbiome associated with the development of uterine disease in dairy cows. J. Dairy Sci. 102: 11786–11797. doi: 10.3168/jds.2019-17106 [DOI] [PubMed] [Google Scholar]
- 16.Gilbert R. O., Shin S. T., Guard C. L., Erb H. N., Frajblat M.2005. Prevalence of endometritis and its effects on reproductive performance of dairy cows. Theriogenology 64: 1879–1888. doi: 10.1016/j.theriogenology.2005.04.022 [DOI] [PubMed] [Google Scholar]
- 17.Gonzalez Moreno C., Torres Luque A., Oliszewski R., Rosa R. J., Otero M. C.2020. Characterization of native Escherichia coli populations from bovine vagina of healthy heifers and cows with postpartum uterine disease. PLoS One 15: e0228294. doi: 10.1371/journal.pone.0228294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Habibi M., Asadi Karam M. R., Bouzari S.2017. Evaluation of prevalence, immunogenicity and efficacy of FyuA iron receptor in uropathogenic Escherichia coli isolates as a vaccine target against urinary tract infection. Microb. Pathog. 110: 477–483. doi: 10.1016/j.micpath.2017.07.037 [DOI] [PubMed] [Google Scholar]
- 19.Hancock V., Ferrières L., Klemm P.2008. The ferric yersiniabactin uptake receptor FyuA is required for efficient biofilm formation by urinary tract infectious Escherichia coli in human urine. Microbiology (Reading) 154: 167–175. doi: 10.1099/mic.0.2007/011981-0 [DOI] [PubMed] [Google Scholar]
- 20.Herath S., Lilly S. T., Santos N. R., Gilbert R. O., Goetze L., Bryant C. E., White J. O., Cronin J., Sheldon I. M.2009. Expression of genes associated with immunity in the endometrium of cattle with disparate postpartum uterine disease and fertility. Reprod. Biol. Endocrinol. 7: 55. doi: 10.1186/1477-7827-7-55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herzer P. J., Inouye S., Inouye M., Whittam T. S.1990. Phylogenetic distribution of branched RNA-linked multicopy single-stranded DNA among natural isolates of Escherichia coli. J. Bacteriol. 172: 6175–6181. doi: 10.1128/jb.172.11.6175-6181.1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jann K., Jann B.1992. Capsules of Escherichia coli, expression and biological significance. Can. J. Microbiol. 38: 705–710. doi: 10.1139/m92-116 [DOI] [PubMed] [Google Scholar]
- 23.Jones C. H., Pinkner J. S., Roth R., Heuser J., Nicholes A. V., Abraham S. N., Hultgren S. J.1995. FimH adhesin of type 1 pili is assembled into a fibrillar tip structure in the Enterobacteriaceae. Proc. Natl. Acad. Sci. USA 92: 2081–2085. doi: 10.1073/pnas.92.6.2081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kassé F. N., Fairbrother J. M., Dubuc J.2016. Relationship between Escherichia coli virulence factors and postpartum metritis in dairy cows. J. Dairy Sci. 99: 4656–4667. doi: 10.3168/jds.2015-10094 [DOI] [PubMed] [Google Scholar]
- 25.Klemm P., Schembri M. A.2000. Bacterial adhesins: function and structure. Int. J. Med. Microbiol. 290: 27–35. doi: 10.1016/S1438-4221(00)80102-2 [DOI] [PubMed] [Google Scholar]
- 26.Krogfelt K. A., Bergmans H., Klemm P.1990. Direct evidence that the FimH protein is the mannose-specific adhesin of Escherichia coli type 1 fimbriae. Infect. Immun. 58: 1995–1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.LeBlanc S. J., Duffield T. F., Leslie K. E., Bateman K. G., Keefe G. P., Walton J. S., Johnson W. H.2002. Defining and diagnosing postpartum clinical endometritis and its impact on reproductive performance in dairy cows. J. Dairy Sci. 85: 2223–2236. doi: 10.3168/jds.S0022-0302(02)74302-6 [DOI] [PubMed] [Google Scholar]
- 28.Lindsey R. L., Garcia-Toledo L., Fasulo D., Gladney L. M., Strockbine N.2017. Multiplex polymerase chain reaction for identification of Escherichia coli, Escherichia albertii and Escherichia fergusonii. J. Microbiol. Methods 140: 1–4. doi: 10.1016/j.mimet.2017.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lutwyche P., Rupps R., Cavanagh J., Warren R. A. J., Brooks D. E.1994. Cloning, sequencing, and viscometric adhesion analysis of heat-resistant agglutinin 1, an integral membrane hemagglutinin from Escherichia coli O9:H10:K99. Infect. Immun. 62: 5020–5026. doi: 10.1128/iai.62.11.5020-5026.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Magata F., Horiuchi M., Miyamoto A., Shimizu T.2014. Lipopolysaccharide (LPS) inhibits steroid production in theca cells of bovine follicles in vitro: distinct effect of LPS on theca cell function in pre- and post-selection follicles. J. Reprod. Dev. 60: 280–287. doi: 10.1262/jrd.2013-124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mancini J., Weckselblatt B., Chung Y. K., Durante J. C., Andelman S., Glaubman J., Dorff J. D., Bhargava S., Lijek R. S., Unger K. P., Okeke I. N.2011. The heat-resistant agglutinin family includes a novel adhesin from enteroaggregative Escherichia coli strain 60A. J. Bacteriol. 193: 4813–4820. doi: 10.1128/JB.05142-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moore S. G., Ericsson A. C., Poock S. E., Melendez P., Lucy M. C.2017. Hot topic: 16S rRNA gene sequencing reveals the microbiome of the virgin and pregnant bovine uterus. J. Dairy Sci. 100: 4953–4960. doi: 10.3168/jds.2017-12592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moreno E., Johnson J. R., Pérez T., Prats G., Kuskowski M. A., Andreu A.2009. Structure and urovirulence characteristics of the fecal Escherichia coli population among healthy women. Microbes Infect. 11: 274–280. doi: 10.1016/j.micinf.2008.12.002 [DOI] [PubMed] [Google Scholar]
- 34.Moreno E., Planells I., Prats G., Planes A. M., Moreno G., Andreu A.2005. Comparative study of Escherichia coli virulence determinants in strains causing urinary tract bacteremia versus strains causing pyelonephritis and other sources of bacteremia. Diagn. Microbiol. Infect. Dis. 53: 93–99. doi: 10.1016/j.diagmicrobio.2005.05.015 [DOI] [PubMed] [Google Scholar]
- 35.Otero C., Saavedra L., Silva de Ruiz C., Wilde O., Holgado A. R., Nader-Macías M. E.2000. Vaginal bacterial microflora modifications during the growth of healthy cows. Lett. Appl. Microbiol. 31: 251–254. doi: 10.1046/j.1365-2672.2000.00809.x [DOI] [PubMed] [Google Scholar]
- 36.Sheldon I. M., Dobson H.2004. Postpartum uterine health in cattle. Anim. Reprod. Sci. 82–83: 295–306. doi: 10.1016/j.anireprosci.2004.04.006 [DOI] [PubMed] [Google Scholar]
- 37.Sheldon I. M., Noakes D. E.1998. Comparison of three treatments for bovine endometritis. Vet. Rec. 142: 575–579. doi: 10.1136/vr.142.21.575 [DOI] [PubMed] [Google Scholar]
- 38.Sheldon I. M., Cronin J., Goetze L., Donofrio G., Schuberth H. J.2009. Defining postpartum uterine disease and the mechanisms of infection and immunity in the female reproductive tract in cattle. Biol. Reprod. 81: 1025–1032. doi: 10.1095/biolreprod.109.077370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sheldon I. M., Lewis G. S., LeBlanc S., Gilbert R. O.2006. Defining postpartum uterine disease in cattle. Theriogenology 65: 1516–1530. doi: 10.1016/j.theriogenology.2005.08.021 [DOI] [PubMed] [Google Scholar]
- 40.Sheldon I. M., Rycroft A. N., Dogan B., Craven M., Bromfield J. J., Chandler A., Roberts M. H., Price S. B., Gilbert R. O., Simpson K. W.2010. Specific strains of Escherichia coli are pathogenic for the endometrium of cattle and cause pelvic inflammatory disease in cattle and mice. PLoS One 5: e9192. doi: 10.1371/journal.pone.0009192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Swasthi H. M., Bhasne K., Mahapatra S., Mukhopadhyay S.2018. Human fibrinogen inhibits amyloid assembly of biofilm-forming CsgA. Biochemistry 57: 6270–6273. doi: 10.1021/acs.biochem.8b00841 [DOI] [PubMed] [Google Scholar]
- 42.Tükel C., Raffatellu M., Humphries A. D., Wilson R. P., Andrews-Polymenis H. L., Gull T., Figueiredo J. F., Wong M. H., Michelsen K. S., Akçelik M., Adams L. G., Bäumler A. J.2005. CsgA is a pathogen-associated molecular pattern of Salmonella enterica serotype Typhimurium that is recognized by Toll-like receptor 2. Mol. Microbiol. 58: 289–304. doi: 10.1111/j.1365-2958.2005.04825.x [DOI] [PubMed] [Google Scholar]
- 43.Veilleux S., Dubreuil J. D.2006. Presence of Escherichia coli carrying the EAST1 toxin gene in farm animals. Vet. Res. 37: 3–13. doi: 10.1051/vetres:2005045 [DOI] [PubMed] [Google Scholar]
- 44.Williams E. J., Fischer D. P., Pfeiffer D. U., England G. C. W., Noakes D. E., Dobson H., Sheldon I. M.2005. Clinical evaluation of postpartum vaginal mucus reflects uterine bacterial infection and the immune response in cattle. Theriogenology 63: 102–117. doi: 10.1016/j.theriogenology.2004.03.017 [DOI] [PubMed] [Google Scholar]
- 45.Williams E. J., Sibley K., Miller A. N., Lane E. A., Fishwick J., Nash D. M., Herath S., England G. C. W., Dobson H., Sheldon I. M.2008. The effect of Escherichia coli lipopolysaccharide and tumour necrosis factor alpha on ovarian function. Am. J. Reprod. Immunol. 60: 462–473. doi: 10.1111/j.1600-0897.2008.00645.x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.