Graphical abstract
Keywords: Gut microbiota, Dietary nutrients, Cardiovascular disease, Microbial metabolites, Lipid metabolism, Therapeutic strategies
Abstract
Cardiovascular diseases (CVD) are a group of disorders of the heart and blood vessels and remain the leading cause of morbidity and mortality worldwide. Over the past decades, accumulating studies indicated that the gut microbiota, an indispensable “invisible organ”, plays a vital role in human metabolism and disease states including CVD. Among many endogenous and exogenous factors that can impact gut microbial communities, the dietary nutrients emerge as an essential component of host-microbiota relationships that can be involved in CVD susceptibility. In this review, we summarize the major concepts of dietary modulation of the gut microbiota and the chief principles of the involvement of this microbiota in CVD development. We also discuss the mechanisms of diet-microbiota crosstalk that regulate CVD progression, including endotoxemia, inflammation, gut barrier dysfunction and lipid metabolism dysfunction. In addition, we describe how metabolites produced by the microbiota, including trimethylamine-N-oxide (TMAO), secondary bile acids (BAs), short chain fatty acids (SCFAs) as well as aromatic amino acids (AAAs) derived metabolites play a role in CVD pathogenesis. Finally, we present the potential dietary interventions which interacted with gut microbiota as novel preventive and therapeutic strategies for CVD management.
1. Introduction
The gut microbiota, the most abundant microbial community living in the human body, has recently been considered an indispensable “invisible organ” that regulates essential functions of host physiology, including nutrients transformation and metabolism, maintenance of intestinal barrier integrity and host immunity homeostasis [1], [2], [3]. Numerous studies indicated that dietary nutrient intake, as a key environmental factor impacting gut microbiota composition, can induce “dysbiosis” which is defined as an altered microbial community including richness, diversity and composition. The dysbiosis can accelerate the homeostatic imbalance which contributes to multiple disease states [4], [5], [6], [7], [8], [9], including obesity, diabetes, liver diseases, cancer, brain disorders, as well as cardiovascular disease (CVD) which remains the leading cause of morbidity and mortality worldwide [10], [11].
In human gut, more than 1,000 bacterial species have been identified with high-throughput metagenomics sequencing techniques (e.g. 16S, Shotgun) and about 160 species are largely shared among individuals [12]. Two main phyla of bacteria, Firmicutes and Bacteroidetes, are predominant with more than 90% of total bacteria, and their ratio is usually considered a relative signature for health state although it is still controversial [13]. Besides, the phyla Actinobacteria, Proteobacteria and Verrucomicrobia are also members of the gut microbiota but to a much lesser abundance [14]. At the genus level, Eubacterium, Ruminococcus, Clostridium, Lactobacillus, and also Bacteroides are dominant in healthy adult humans [12], [13], [14]. Particularly, the concept of “enterotype” firstly emerged in 2011 by comparing large scales of human subjects to demonstrate the stratification of gut microbiota which were divided into three robust clusters including Bacteroides, Prevotella, and Ruminococcus as predominant genera in the different enterotypes. Subsequently, a new addition to enterotypes separates the Bacteroides into two sub-enterotypes B1 and B2 in which B2 enterotype is highly associated with systemic inflammation [15], [16]. In general, the compositional and functional features of the gut microbiota include α-diversity (richness of community in one sample) and β-diversity (community differences across samples), as well as microbial metabolites production [17].
CVD is a general term for diseases affecting heart and blood vessels encompassing multiple disorders like coronary artery disease (CAD), heart failure, stroke and other conditions [18]. As a chronic progressive health burden, the development of CVD relates to various risk factors including hypertension (HT), dyslipidemia as well as insulin resistance and inflammation, most of which could damage vascular structure and eventually lead to more direct processes like atherosclerosis and thrombosis susceptibility [19]. Besides these physiological factors, dietary nutrients have been highlighted as one of the key modifiable contributors that could interact with the gut microbiota implicating a diet-microbiota-dependent mechanism for the development of CVD [20].
Specifically, the large and diverse intestinal microbial community serves as a “metabolic filter” in the gastrointestinal (GI) tract to what we eat, our predominant environmental exposures [21]. Trillions of microbial cells residing in the intestine contribute to the nutrient digestion and metabolism, in which the bacteria are the most abundant community belonging to thousands of species, but yeasts, fungi, and archaea are also involved [22]. With the variation of pH, the redox potential and adhesion sites, the microbial density gradually increases along the GI tract until reaching the highest level in the distal intestine where the metabolites are produced in response to dietary stimuli, some of which being correlated with the development of CVD [23]. “You are what you eat” – indeed, a growing body of studies have provided strong evidences to support the diet-gut microbiota crosstalk on CVD in both positive and negative ways [24], [25]. On one side, unhealthy dietary nutrient intake like high consumption of saturated fat, refined carbohydrates, and dietary choline, has been refined to increase the abundance of pathogenic bacteria or metabolites which trigger systemic inflammation, damage intestinal barrier to promote CVD susceptibility [26]. Contrarily, healthy dietary patterns with high intake of diet rich in fibers such as fruits, vegetables, and whole grain stimulate the growth of beneficial bacteria which might exert potential preventive effects on CVD [26].
Herein, large body of data is reviewed in supporting our understanding of the diet-gut microbiota interactions in CVD. Thus, we precisely discuss the role of gut microbiota in the pathogenic development of CVD, the diet-microbial metabolites interactions in the development of CVD, as well as the potential dietary interventions through gut microbiota axis as novel preventive and therapeutic strategies for CVD management.
2. Dietary modulation of gut microbiota
Numerous studies have suggested that diet is an essential modulator of the compositional and functional features of the gut microbiota in both humans and animals. The impacts of dietary changes on microbial communities in the gut can be summarized in three major aspects: (1) Rapid/short-term effect: this theme is supported by the research on human subjects who switched between plant-based (high-fiber) and animal-based (high-fat) diet. The microbiota composition of all subjects shifted within 1–2 days with increased abundance of Firmicutes which metabolized dietary fiber with plant-based diet and increased bile-tolerant microorganisms Alistipes and Bilophila with animal-based diet [27]. However, short-term diet changes had no impact on enterotypes even after 10 days intervention [28]. (2) Long-term effect: despite of rapid modulations of microbial community, long-term dietary interventions are not only associated with the compositional alterations, but also related to physiological changes. For instance, feeding rats with high fat diet (HFD) for 8 or 12 weeks induced increased abundance of Enterobacteriales (Proteobacteria phylum) which was coupled with the elevation of systemic inflammation, intestinal permeability and obese phenotype [29]. On the contrary, human cohorts intervened with 3 months low-carbohydrate or low-fat healthy diet resulted in 14 or 12 taxonomic changes correlating with weight loss suggesting that long-term interventions are necessary [30]. Besides, enterotypes are mostly related to long-term dietary effects instead of short-term impacts [28]. (3) Specific microbial changes in response to particular diet: For example, dietary fiber intake promoted the increase of gut microbiota richness or diversity [31] as well as phylum of Firmicutes [27]. Bacterial species Ruminococcus bromii bloomed under the intervention of resistant-starch diet [32]. Interestingly, not only the microbial composition but specific microbial metabolism is correlated with specific diet and disease patterns. For instance, subjects with red meat enriched diet had more trimethylamine-N-oxide (TMAO) (intestinal microbial metabolite of choline present in red meat) in plasma than vegetarians. The elevation of TMAO levels has been identified in human subjects with the enriched proportions of enterotype Prevotella and is associated with increased risk of CVD [33].
3. Microbial alterations in CVD
Extensive investigations have identified that the gut microbiota plays an essential role in CVD. Indeed, early studies suggested that the depletion of gut microbiota by antibiotic treatment raised blood pressure in rats [34]. These discoveries have been further confirmed in germ-free (GF) rats implicating the crucial role of gut microbiota in the regulation of blood pressure [35]. Also, the absence of microbiota in ApoE−/− mice model in standard diet accelerated the formation of atherosclerotic plaques in the aorta and the development of heart diseases when compared with conventional ones [36]. Interestingly, the opposite effect of the microbiota was observed with mice infused with Angiotensin II in which the absence of gut microbiota attenuated arterial HT and vascular dysfunction [37]. In addition, the imbalance or maladaptation of microbial community, defined as “dysbiosis”, has been discovered to be associated with the incidence of CVD risk and to affect the progression of CVD. For instance, a significant dysbiosis has been identified in hypertensive animals and was characterized by decreased microbial richness and diversity, and increased Firmicutes/Bacteroidetes (F/B) ratio [38]. These alterations in microbiota were also discovered in Ldlr−/− mice (a hypercholesterolemic atherosclerosis model) with an acceleration of arterial thrombosis [39].
In human cohorts, large number of studies have observed a gut microbial dysbiosis in patients with HT, atherosclerosis, CAD, heart failure and stroke (Table1). For instance, alterations of the gut microbiota in patients with HT include a lower α-diversity and a significant shift of β-diversity with a higher abundance of potential pathogenic bacteria including Parabacteroides, Desulfovibrio, Prevotella and Oscillibacter which are gram-negative bacteria that may produce endotoxins (e.g. lipopolysaccharides, LPS) associated with inflammatory status. Some gram-positive bacteria such as Clostridium have also been found in higher abundance in patients with HT [40], [41], [42]. Concurrently, lower abundance of beneficial bacteria including SCFAs producing bacteria like Lachnospiraceae, Lactobacillus, Faecalibacterium, Ruminococcus was detected in patients with HT [40], [41], [42]. Similar findings (Table1) were also discovered in most of the human studies related to atherosclerosis, CAD, heart failure and stroke [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54]. Additionally, alterations of some different bacterial groups were also found in CVD cohorts such as a decreased proportion of Bacteroides and Roseburia [43], [44], [45], [46], [47], [48], [52], [54]. Besides, a gut microbial dysbiosis was also correlated with cardiac risk parameters in plasma. For instance, the alterations of microbiota were associated to the lipid metabolism dysfunctions like lower levels of High-density lipoprotein (HDL) in plasma in CVD patients [48]. Moreover, increased abundance of Escherichia/Shigella was positively correlated to the elevated plasmatic levels of TMAO, a gut microbial metabolite contributing to CVD pathogenesis [51]. However, some CAD and stroke patients have also been identified with higher presence of potential beneficial bacteria such as Lactobacillales or Akkermansia which was associated with the depletion of other SCFAs producers [48], [53], [54]. This discrepancy in human studies might be explained by the following aspects: (1) The methodology of gut microbiota analysis: multiple factors during the gut microbiota analysis could affect the taxonomic resolution including different protocols for fecal samples collection, storage, DNA extraction as well as different sequencing platforms or different sequencing methods (e.g. 16S or shotgun) [55], [56], [57]. (2) Population level related parameters in clinical studies: numerous covariates of the subjects recruited in the clinical studies could interfere with the fecal microbiota variation such as blood parameters, dietary habits, lifestyle, anthropometrics as well as stool consistency [58]. (3) Medication: this is another essential factor which might result in the variation of gut microbiota. It has been reported that individual medication or multimedication as well as the drug dosage exhibited a strong relation to the alteration of the gut microbiota [58], [59]. Therefore, clinical studies in human subjects should consider the above aspects to design properly new investigations on gut microbiota related CVD pathogenesis.
Table1.
The alteration of gut microbiota associated with CVD conditions in human cohorts.
| CVD Condition | Cohorts | Sequencing Method | Diversity | ↑Increased Abundance | ↓Decreased Abundance | Ref. |
|---|---|---|---|---|---|---|
| Hypertension | 99 HT 56 Pre-HT 41 Controls |
Shotgun | ↓α-diversity *β-diversity |
Klebsiella Prevotella Desulfovibrio |
Faecalibacterium Oscillibacter Roseburia Bifidobacterium Coprococcus Butyrivibrio |
[40] |
| 67 HT 62 Controls |
16S | NAα-diversity *β-diversity |
Acetobacteroides Alistipes Bacteroides Barnesiella Clostridium Desulfovibrio Megasphaera Microvirgula Parabacteroides |
Lactobacillus Olsenella Paraprevotella Prevotella Romboutsia Ruminococcus |
[41] | |
| 183 HT 346 Controls |
16S | ↓α-diversity *β-diversity |
Catabacter Veillonella Clostridium Oscillibacter Robinsoniella |
Akkermansia Ruminococcus Anaerovorax Sporobacter Asaccharobacter |
[42] | |
| Atherosclerosis | 13 Patients 12 Controls |
Shotgun | NA |
Collinsella Clostridiales Clostridium |
Bacteroides Roseburia Eubacterium Faecalibacterium |
[43] |
| 218 Patients 187 Controls |
Shotgun | NA |
Escherichia coli Klebsiella spp. Enterobacter aerogenes |
Bacteroides spp. Prevotella copri Alistipes shahii |
[44] | |
| 223 Patients 181 Controls |
16S | ↓α-diversity *β-diversity |
Streptococcus anginosus Atopobium parvulum Actinomyces graevenitzii Streptococcus mitis/oralis/pneumonia |
Bacteroides xylanisolvens Odoribacter splanchnicus Eubacterium eligens Roseburia inulinivorans Roseburia intestinalis |
[45] | |
| CAD | 39 Patients 30 High-risks 50 Controls |
16S | *β-diversity | F/B ratio Lactobacillales |
Bacteroides Prevotella |
[46] |
| 70 Patients 98 Controls |
16S | ↓α-diversity *β-diversity |
Escherichia-Shigella Enterococcus |
Faecalibacterium Subdoligranulum Roseburia Eubacterium rectale |
[47] | |
| 161 Patients 40 Controls |
16S | NA |
Enterobacteriaceae Enterococcus Streptococcus |
Faecalibacterium Roseburia Ruminococcus Lachnospiraceae |
[48] | |
| Heart Failure | 60 Patients 20 Controls |
Culture |
NA |
Candida Campylobacter Shigella Yersinia |
NA | [49] |
| 84 Patients 266 Controls |
16S | ↓α-diversity *β-diversity |
Succiniclasticum Prevotella |
Lachnospiraceae Eubacterium hallii |
[50] | |
| 22 Patients 11 Controls |
16S | NA α-diversity *β-diversity |
Actinobacteria Bifidobacterium Escherichia/Shigella |
Megamonas | [51] | |
| Stroke | 141 Patients 94 Controls |
16S | ↑α-diversity *β-diversity |
Enterobacter Megasphaera Oscillibacter Desulfovibrio |
Bacteroides Prevotella Faecalibacterium |
[52] |
| 30Patients 30Controls |
16S | NA α-diversity NA β-diversity |
Odoribacter Akkermansia Christensenellaceae Ruminococcaceae Enterobacter |
Anaerostipes Ruminiclostridium |
[53] | |
| 140Patients 92Controls |
16S | *β-diversity | F/B ratio Lactobacillaceae Akkermansia Enterobacteriaceae Porphyromonadaceae |
Roseburia Bacteroides Lachnospiraceae Faecalibacterium Blautia, Anaerostipes |
[54] |
4. The signature of diet-gut microbiota crosstalk in CVD pathogenesis
4.1. Intestinal barrier dysfunctions and inflammation
In healthy state, the appropriate intestinal barrier provides a crucial first line of defense against pathogens that is supported by several physiological components including mucus layer, epithelial cells connected by tight junction proteins and immune cells [60]. However, CVD patients in heart failure or HT were often observed with dysfunctional intestinal barrier accompanied with increase of the systemic microbial component LPS and inflammation [61], [62], [63]. What are the risk factors to trigger the gut leakiness and inflammation in the process of CVD? One of the hypotheses is that long-term consumption of western diet or HFD could induce dysbiosis and impair the gut barrier which enhanced LPS translocation and systemic inflammation resulting in increase of CVD risk [64], [65], [66].
In view of this, higher intake of diet rich in saturated fat or trans fatty acids is highly associated with the increase of CVD risk while a lower intake of dietary saturated fats reduced CVD by about 30% [67]. In large cohortstudies, long-term (6-months) consumption of HFD induced a microbial dysbiosis with increased proportion of Gram-negative bacteria such as Alistipes and Bacteroides accompanied with higher levels of genes involved in LPS biosynthesis [64]. Meanwhile, dietary fats have been identified to impair intestinal barrier through activating the secretion of pro-inflammatory cytokines (e.g. TNF-α, IFNγ and IL-1β) [68], [69]. The upregulation of the pro-inflammatory cytokines further activated MLCK (Myosin light-chain kinase) signaling pathway which reorganizes the tight junction proteins including occludin, ZO-1 (Zonula occludens-1) and results in the leaky gut [70], [71], [72].
When the intestinal barrier is disrupted, LPS or pathogens could translocate into the circulation causing endotoxemia that stimulates the release of systemic pro-inflammatory cytokines [73]. Once translocated in the bloodstream, endotoxin can trigger the damage of endothelial cells through interaction with TLR-4 (Toll-like receptor 4) on cellular surface and enhance the generation of ROS (Reactive oxygen species) to reduce endothelial NO (Nitric oxide) bioavailability resulting in the formation of plaque and atherosclerosis lesion [73], [74]. This hypothesis has been confirmed in animal models, in which ApoE−/− mice under western diet displayed an aggravation of atherosclerotic lesions with a significant increase of Proteobacteria (Gram-negative pro-inflammatory bacteria) and systemic LPS levels [75]. Moreover, western diet promotes the upregulation of inflammatory cytokines (e.g. TNF-α and IL-1β) and increases intestinal permeability coupled with modification of tight junction proteins (e.g. occludin) in ApoE−/− mice as well [75], [76]. However, there is still a lack of data in human cohorts to decipher at what point the impaired intestinal barrier and associated increased endotoxemia due to western diet can induce CVD pathogenesis.
4.2. Lipid metabolism disorders
In addition to diet-microbiota interactions on inflammation and gut barrier functions, intestinal microbes also influence CVD via the host lipid metabolism. Indeed, a growing body of animal and human studies have suggested that the gut microbiota is implicated in lipid metabolism disorders such as dyslipidemia or hyperlipidemia, which are major risk factors in the development of CVD [77], [78], [79], [80]. For example, GF mice have an altered cholesterol metabolism [81] and the depletion of gut microbiota in ApoE−/− mice caused a marked elevation of plasma cholesterol accompanied with larger aortic lesions compared to conventional ApoE−/− mice [77], [78]. Moreover, microbiota transplantation from high plasma cholesterol humans to mice instigated the phenotype of upregulated circulating cholesterol coupled with the reduction of hepatic cholesterol synthesis [78]. This could be due to cholesterol-to-coprostanol conversion by the intestinal microbiota which could facilitate the elimination of cholesterol from the body and lower cholesterolemia [82]. This has been confirmed using a mathematical model of cholesterol metabolism, and recently revealed that both bile salt metabolism and cholesterol-to-coprostanol conversion by the gut microbiota can influence blood cholesterol level [83]. In addition, an interesting recent study on human cohorts also confirmed this point and identified that individuals harboring coprostanol-forming microbes such as Eubacterium coprostanoligenes which contain cholesterol metabolizing enzymes ismA have significantly lower fecal cholesterol levels and lower serum total cholesterol [84]. However, controversial results were also discovered in GF ApoE−/− mice with smaller plaque area despite of the upregulation of plasmatic total cholesterol (TC) which might be due to the absence of endotoxemia linked to the GF status [79].
Interestingly, the absence of gut microbiota seems to attenuate atherogenic effects of long-term dietary lipids consumption. Specifically, HFD-fed GF Ldlr−/− mice were identified with a significant reduction in thrombus size compared with conventional counterparts [39]. Although there is no difference in plasmatic TC levels in both GF and conventional Ldlr−/−mice fed with HFD, lipids enriched diet still induced about twice the level of TC in GF mice (TC≈1.6 mg/dlx103) versus GF mice fed with chow diet (TC≈0.8 mg/dlx103) [39]. In comparison, HFD induced about eight fold increase in plasmatic TC in conventional mice (≈1.6 mg/dlx103) versus mice raised with chow diet (TC≈0.2 mg/dlx103) [39]. Similar discoveries for VLDL were also found in this study [39]. Lipids enriched diet also enhanced the microbiota dysbiosis in Ldlr−/− mice with an increased abundance of Clostridiaceae, Staphylococcaceae, Bacillales and decreased abundance of Lactobacillaceae [39]. However, recent study suggested that no significant difference was discovered between GF Ldlr−/− vs conventional mice on late aortic atherosclerosis [85]. Taken together, the different studies show different impacts of the gut microbiota on blood lipid metabolism. Whether this impact has a protective or aggravating effect on CVD development is still not clear. This discrepancy might depend on the animal model, the age of animals, the type of diet, the feeding period as well as the housing conditions. Future studies could take these factors into their consideration for a better investigation.
5. Gut microbiota metabolites in CVD pathogenesis
5.1. TMAO, a dietary induced microbial biomarker for the risk of CVD
Diet-intestinal microbiota derived metabolite TMAO - TMAO (trimethylamine-N-oxide), a gut microbial co-metabolite derived from dietary nutrients, was first discovered and reported to predict the risk for CVD a decade ago [86]. The dietary precursors phosphatidylcholine [86], choline [87], and L-carnitine [33], which are commonly present in cheese, red meat, seafood, egg yolks and other western style nutrients [88], are primarily metabolized by specific gut microbial enzymes to produce high levels of trimethylamine (TMA) [63], [89], [90], [91]. Specifically, TMA lyase containing functional microbial CutC/D genes is responsible for choline related TMA transformation [63], [66]. TMA is further absorbed into blood stream and oxidized in the liver by flavin monooxygenases (FMOs, mainly FMO3) to generate TMAO [63], [92]. Seven different bacterial strains expressing TMA lyase CutC/D were identified in human gut including Anaerococcus hydrogenalis, Clostridium asparagiforme, Clostridium hathewayi, Clostridium sporogenes, Escherichia fergusonii, Proteus penneri, and Providencia rettgeri [87]. Additionally, TMA can be synthesized from L-carnitine via microbial Rieske-type l-carnitine oxygenase CntA/B [90]. Although CntA/B encoding genes were identified in Proteobacteria, the L-carnitine dependent TMA formation by commensal gut microbiota has not been demonstrated [93]. Whereas, a very recent study discovered a novel combination of two bacterial strains Emergencia timonensis and Ihubacter massiliensis as potential important players in carnitine converted TMA accumulation [94]. Interestingly, the bacteria E. timonensis has lately been identified to promote TMAO production via l-carnitine → γBB (gamma-butyrobetaine, precursors of carnitine) → TMA → TMAO pathway [95]. However, the specific commensal microbiota associated with carnitine-TMA transformation pathways still need to be further discovered.
Diet-microbiota derived TMAO in the modulation of CVD pathogenesis – In order to directly discover the diet-microbiota related TMAO pathway in CVD progression, initial studies suggested that mice raised with high choline or carnitine diet showed an elevation in circulating TMAO levels, an increase of macrophage foam cell formation and enhancement of aortic atherosclerotic plaque development (Fig. 1) [33], [86]. On the contrary, the capacity to TMAO production, and choline or carnitine diet related atherosclerotic plaque burden were respectively eliminated or suppressed in GF or antibiotic treated ApoE−/− mice (C57BL/6 strain) [33], [86]. Interestingly, ApoE−/− mice were discovered to develop higher choline diet dependent aggregation of aortic lesions when receiving cecal microbiota transplantation from high TMA/TMAO producing donor C57BL/6 mice than from low TMA/TMAO producing donor NZW/LacJ mice [96]. Similarly, transplant of high TMA producing microbes in GF mice induced platelet hyperresponsiveness and enhanced thrombosis associated with high plasmatic TMAO levels [97]. Therefore, microbiota is necessary for TMAO production which is involved in atherosclerosis progression through several mechanisms: (1) foam cell formation: Microbiota derived TMAO can activate the expression of the stress-induced heat-shock proteins (HSP) HSP70 or HSP60, which may trigger the activation of Scavenger receptors (e.g. SR-A1) and CD36 in macrophages to stimulate the uptake of oxidized low-density lipoprotein (ox-LDL) and foam cell formation [33], [86], [98]. (2) Inflammation: TMAO induced the proatherogenic inflammatory markers expression including IL-6, cyclooxygenase 2 (COX-2), and intracellular adhesion molecule 1 via the activation of mitogen-activated protein kinase (MAPK) and NF-κB signaling pathway in Ldlr−/− mice fed a choline-rich diet. [98], [99]. In addition, the increase in circulating TMAO was associated with the elevation of pro-inflammatory cytokines TNF-α and IL-1β, and reduction of the anti-inflammatory cytokine IL-10 [98], [100]. (3) Lipid Metabolism: TMAO could suppress the reverse cholesterol transport (RCT) which results in the arterial cholesterol deposition to accelerate atherosclerotic lesions [98]. (4) Platelet hyperreactivity and thrombosis: Diet induced high levels of microbial TMAO could stimulate platelet to activate sub-maximal stimulus including thrombin, adenosine diphosphate (ADP) and collagen as well as to induce the release of intracellular calcium resulting in platelet hyperresponsiveness [97]. However, some studies have shown opposite results illustrating that dietary TMAO, choline or carnitine did not induce the atherosclerosis in ApoE−/− or Ldlr−/− mice model [77], [101]. This discrepancy might be due to the housing conditions and mice models, but precise reasons still need to be further discovered. Interestingly, Jaworska et al recently illustrated that TMA, but not TMAO reduced cardiomyocytes and vascular smooth muscle cells viability [102], [103]. They also found that intravenous infusions of TMA instead of TMAO in rats showed a significant increase of the mean arterial blood pressure indicating the deleterious effect of TMA on CVD [104]. More in vivo and in vitro studies still need to be done to further confirm the role of TMA on CVD pathogenesis and validate the related mechanism.
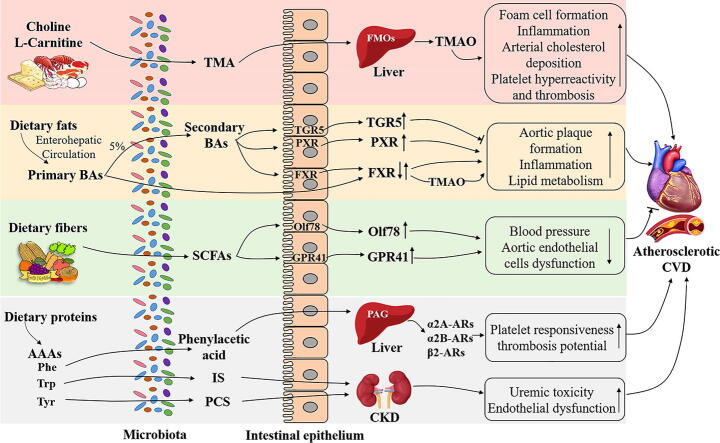
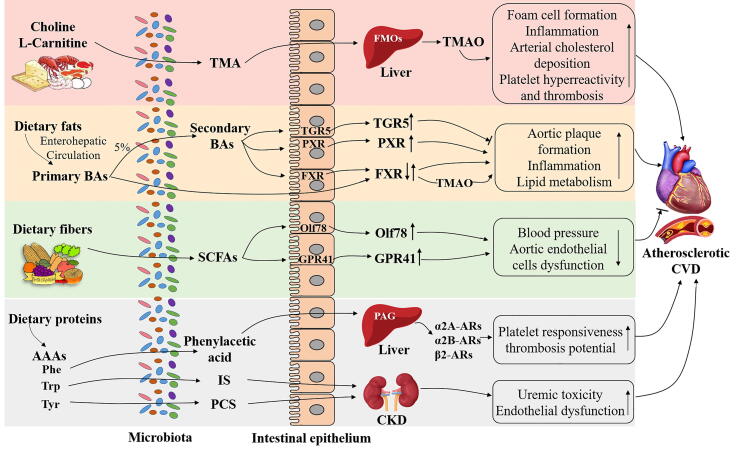
Fig. 1.
Potential mechanisms of dietary metabolites produced by gut microbiota in CVD pathogenesis. Dietary choline or L-carnitine could be metabolized by specific gut microbiota to TMA which is further oxidized in the liver by FMOs to produce TMAO. TMAO has been identified as an essential biomarker to stimulate foam cell formation, induce inflammation, suppress RCT, as well as accelerate platelet hyperreactivity and thrombosis. Primary BAs are synthesized from dietary fats or cholesterol via enterohepatic circulation. Only about 5% of primary BAs are non-reabsorbed and deconjugated by gut microbiota to produce secondary BAs. BAs can interact with receptors including FXR, PXR or TGR5 (↓) to stimulate vascular lesion formation, increase inflammation and increased severity of lipid metabolism defects. Dietary proteins rich in AAAs such as Phe could be converted into phenylacetic acid via the gut microbiota and then transferred into PAG in the liver. PAG further responded to G-protein coupled receptors including α2A, α2B and β2-ARs to facilitate platelet responsiveness, thrombosis potential to promote atherosclerotic CVD. The other gut microbial derived metabolites IS from Trp and PCS from Tyr could also predict CVD events in CKD patients which might be associated with uremic toxicity and endothelial dysfunction. On the contrary, dietary fibers could be fermented by microbiota to produce SCFAs which activate the receptors Olf78 and GPR41 involving in the decrease of blood pressure and aortic endothelial cells dysfunction to improve CVD conditions. TMA: trimethylamine; FMOs: flavin monooxygenases; TMAO: trimethylamine-N-oxide; RCT: reverse cholesterol transport; BAs: bile acids; FXR: Farnesoid X-activated receptor; PXR: Pregnane X receptor; TGR5: Takeda G protein-coupled receptor 5; Phe:phenylalanine; PAG: phenylacetylglutamine; ARs:adrenergic receptors; IS: indoxyl sulfate; Trp: tryptophan; PCS: p-cresol sulfate; Tyr: tyrosine; CKD: chronic kidney disease; SCFAs: short chain fatty acids; Olf78: olfactory receptor 78; GPR41: G-protein receptor 41.
Human studies on circulating TMAO in CVD prediction and prognosis - Numerous human studies have proved the role of gut microbial-derived TMAO in prediction of CVD risk. The original study investigated a human cohort with more than 1800 subjects and found that elevated plasmatic TMAO were related with the occurrence of multiple CVD subtypes including peripheral artery disease (PAD), CAD, and history of myocardial infarction (MI) [86]. In clinical outcome studies, large cohorts of participants indicated that circulating TMAO was positively associated with increased risks of major adverse cardiovascular events, incident mortality and artery infarction [105], [106]. The cutoff value of plasmatic TMAO in many studies is over 6 μM to predict the risk of all-cause mortality [21], [63] and a recent meta-analysis of over 10,000 subjects proposed 5.1 μM for CVD prognosis [107]. Additionally, high levels of TMAO have been found associated with the increase of pro-inflammatory monocytes and cardiovascular risk in human cohorts [108]. Similarly, a systematic review and dose–response meta-analysis recruited more than 13,000 participants and discovered a non-linear association between the elevation of plasmatic TMAO levels and the increase of inflammatory marker C-reactive protein (CRP) [109]. However, not all the human studies found similar data. For instance, there were no obvious change in gut microbiota and blood TMAO levels in human subjects with asymptomatic atherosclerosis. Whereas, patients with stroke and transient ischemic attack exhibited a significant dysbiosis of the gut microbiota but with a decrease of plasma TMAO levels [52]. Comparatively, there was no significant relation between TMAO concentrations and atherosclerosis progression in a cohort of participants (n = 817) of ages 35–55 over a 10-year follow-up [110]. Interestingly, a recent study uncovered that TMA but not TMAO was related to high blood pressure load and CVD risk factors, and with decreased abundance of genera Akkermansia, Faecalibacterium, Ruminococcus, and Subdoligranulum in human subjects with early-stage chronic kidney disease (CKD) [111]. However, further studies in human cohorts still need to be conducted to investigate whether the TMAO precursor TMA is a forgotten toxin or predictor in the modulation of early-stage CVD pathogenesis.
5.2. Bile acids
Bile acids (BAs) are hydroxylated and saturated steroids, and facilitate the emulsification and intestinal absorption of dietary fat and fat-soluble molecules [112], [113]. In human hepatocytes, primary Bas (cholic acid (CA) and chenodeoxycholic acid (CDCA)) are synthesized from cholesterol via catalytic enzymes such as cholesterol 7a-hydroxylase (CYP7A1), sterol-27-hydroxylase (CYP27A1), oxysterol 7a-hydroxylase (CYP7B1) which expressions are regulated by the gut microbiota [114]. Then, primary BAs are conjugated to glycine or taurine, and over 95% primary BAs are reabsorbed and recirculated back to the liver [115]. The non-reabsorbed BAs can be deconjugated by catalyzed enzyme bile salt hydrolases (BSHs), which are expressed by several commensal gut bacteria, including the Gram-positive Bifidobacterium, Clostridium, Enterococcus, Lactobacillus and the Gram-negative Bacteroides [116], [117]. Besides deconjugation, gut microbes such as Clostridium and Eubacterium are a source of 7-dehydoxylase to generate secondary BAs including lithocholic acid (LCA) from CDCA and deoxycholic acid (DCA) from CA [118]. In addition, the oxidation and epimerization of BAs are catalyzed via hydroxysteroid dehydrogenases (HSDHs), which have been discovered in various bacteria including Actinobacteria, Proteobacteria, Clostridium and others [119], [120].
Once the microbial metabolized BAs enter into the circulating blood, BA receptors can mediate the signaling pathways to regulate host metabolism which has been discovered to contribute to CVD development (Fig. 1). One of the most essential BA receptors is Farnesoid X-activated receptor (FXR) that is the main sensor with both primary BAs in the liver and secondary BAs in the intestine [121]. FXR has been identified in the modulation of lipid and glucose metabolism [121]. Interestingly, the activation of FXR in atherosclerosis prone mice showed protective effects in the formation of atherosclerotic lesions [122]. Correspondingly, the deletion of FXR in ApoE−/− caused an increased severity of lipid metabolism defects with enhanced aortic plaque formation [123]. In contrast, other studies in FXR/ApoE or FXR/Ldlr double deficiency mice showed reductions of aortic lesions and plasma LDL cholesterol [124], [125]. Of interest, FXR has also been discovered to modulate TMAO pathway via regulating FMO3 activity [126]. Another important BAs receptor is Takeda G protein-coupled receptor 5 (TGR5), whose activation by secondary BAs has been identified to attenuate vascular lesion formation via decreased intraplaque inflammation, plaque macrophage content and lipid loading [127]. Pregnane X receptor (PXR) is another nuclear receptor which is associated with BAs metabolism and is activated by secondary BAs (e.g. LCA) [128]. In contrast to other receptors, the activation of PXR elevated the level of lipoproteins VLDL, LDL and CD36 expression to aggregate the atherosclerotic formation in ApoE−/− mice [128], while the inhibition of PXR in ApoE−/− mice alleviated the aortic lesions area with the decrease of lipid uptake in macrophages and CD36 expression [129].
Although most studies on the mechanism of BAs in CVD pathogenesis are on mice model, circulating levels of BAs have been discovered in association of CVD phenotypes in clinical cohorts. For instance, decreased primary and secondary BAs levels in human subjects have been found with reduced overall survival in chronic heart failure patients [130]. In addition, lower fasting plasmatic total BAs were significantly associated with the severity of CAD, MI and the presence of coronary lesions [131]. Collectively, gut microbiota derived BAs regulate the development of CVD via multiple types of BA receptors, and plasmatic BAs might be another essential predictor for CVD occurrence which still need to be further investigated.
5.3. Short chain fatty acids
Short chain fatty acids (SCFAs) are the major microbial products of dietary fibers (mainly polysaccharides) fermentation, and consist mainly of acetate, butyrate and propionate [132]. Specific members of the gut microbiota participate in particular fermenting pathways for SCFAs synthesis [133], [134].
Interestingly, the intestinal microbiota has been identified to modulate the protective association between the diet rich in fibers and CVD risk. Specifically, numerous studies have illustrated the functional role of dietary fibers or SCFAs in alleviating HT or other CVD subtypes (Fig. 1). One of these studies has found that both high fiber diet and acetate supplementation could reduce systolic and diastolic blood pressures, cardiac fibrosis, and left ventricular hypertrophy, which was associated with improved gut dysbiosis and increased abundance of Bacteroides acidifaciens [135]. Similarly, propionate treatment protected the mice from hypertensive cardiovascular damage, while butyrate producing bacteria (e.g.Roseburia intestinalis) decreased the aortic atherosclerotic lesion area [136], [137]. The studies discovered that the G-protein-coupled SCFAs olfactory receptor 78 (Olfr78) and G-protein receptor 41 (GPR41) participated in the regulation of host blood pressure and endothelial function [138], [139]. In specific, propionate induced an acute hypotensive response in wild-type (WT) mice by modulating the disruption of Olfr78 and GPR41 expression [138]. Whereas, antibiotic treated Olfr78−/− mice but not wild-type mice showed an elevation in blood pressure, and GPR41−/− mice also had systolic HT compared with WT mice [138], [139]. Moreover, a very recent study suggested that both acetate and butyrate improved rat aortic endothelial dysfunction by increasing the bioavailability of NO, through GPR41/43 activation for butyrate only [140]. Further studies still need to be conducted to uncover the mechanistic role of SCFAs in regulation of CVD pathogenesis.
In humans, most the studies of CVD risk are about SCFAs related blood pressure modulation. Early clinical intervention study found that increase intake of dietary fibers was associated with the reduction of blood pressure in patients with HT [141]. Similar protective effects of viscous soluble fibers on blood pressure were also discovered in a meta-analysis study [142]. By contrast, a recent intervention study reported that high fiber high protein diet might increase the risk of CVD by upregulating circulating SCFAs level [143]. Specifically, high protein high fiber diet induced higher propionate level which was associated with upregulation of LDL cholesterol and blood pressure; and higher butyrate level which was correlated with upregulation of glucose and downregulation of HDL cholesterol [143]. However, it is still limited on the direct demonstration of the SCFAs effect on human CVD risk or protection which need to be further clarified.
5.4. Other gut microbial metabolites
Aromatic amino acids (AAAs) are aromatic ring containing amino acids including phenylalanine (Phe), tryptophan (Trp) and tyrosine (Tyr) [144]. The major sources of AAAs are dietary proteins such as beef, pork, chicken or fish [145]. Interestingly, researchers discovered a pathway from the gut microbiota Clostridium sporogenes that generates AAAs metabolites [144]. Recently, several striking studies uncovered a robust relation between the Phe-derived microbial metabolite phenylacetylglutamine (PAG) and major adverse cardiac events (e.g. myocardial infarction/MI, acute ischemic stroke or coronary artery disease) (Fig. 1) [146], [147], [148]. In specific, dietary Phe was converted into phenylacetic acid via the gut microbiota enriched in porA gene and subsequent converted into PAG in the liver. PAG further activated G-protein coupled receptors including α2A, α2B and β2-adrenergic receptors to facilitate platelet responsiveness, thrombosis potential in animal models of arterial injury [146]. Similarly, gut microbial derived metabolite indoxyl sulfate (IS) from Trp and p-cresol sulfate (PCS) from Tyr have also been identified as valuable markers to predict CVD events in patients with CKD [149], [150]. This might be due to the deleterious effects of IS and PCS through induction of uremic toxicity and endothelial dysfunction [150], [151], [152]. However, some studies discovered that IS, PCS, or PAG were not associated with CVD outcomes [153], [154]. The discrepancies might be due to the threshold effect from different studies. Further investigations still need to be conducted to confirm the role of these gut microbial metabolites on CVD progression..
6. Dietary interventions in CVD prevention through gut microbiota
6.1. Dietary patterns
Healthy dietary patterns have been suggested to prevent the CVD progression (Fig. 2) including Mediterranean Diet (Med-diet), Dietary Approaches to Stop Hypertension (DASH) [10] and feeding patterns such as intermittent fasting (IF) [155].
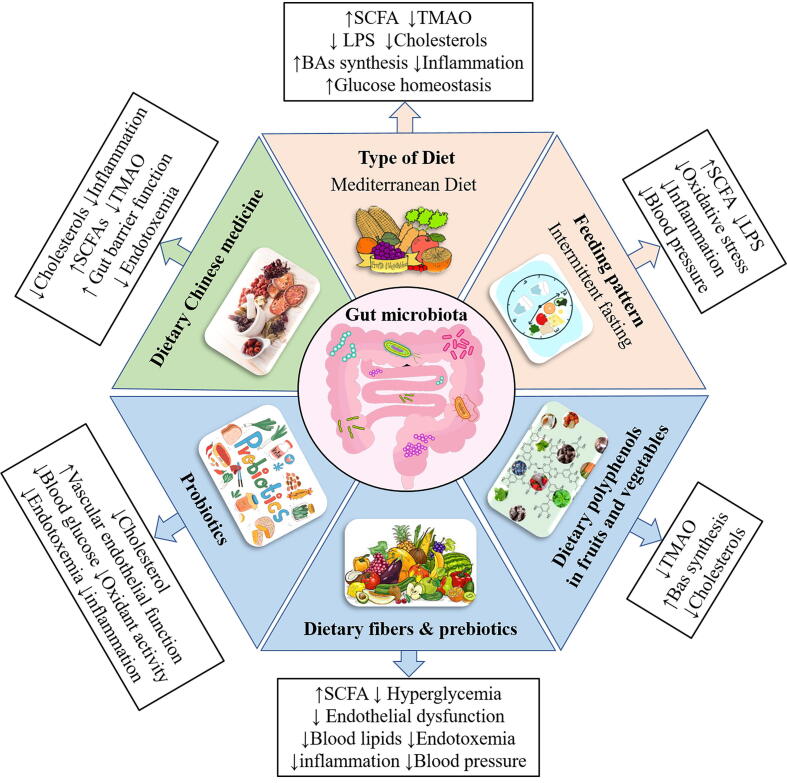
Fig. 2.
Dietary interventions that target the gut microbiota for potential therapeutics in CVD prevention. Dietary patterns such as healthy Mediterranean diet and feeding pattern like intermittent fasting showed strong protective effects on CVD risk factors including inflammation, endotoxemia, hypertension and oxidative stress. These effects may be due to changes in microbiota composition and microbial metabolites production. In addition, multiple dietary components have been identified to exert therapeutic potentials on CVD through microbiota modulation. For instance, the consumption of dietary polyphenols from fruits and vegetables could enrich the “beneficial bacteria” and increase production of SCFAs, decrease TMA production as well as improve lipid metabolism to protect CVD. Dietary consumption of food rich in fibers, prebiotics and probiotics could indirectly or directly interact with gut microbiota to increase the SCFAs production and attenuate blood lipids, endotoxemia, inflammation, hyperglycemia, blood pressure and vascular endothelia dysfunction to improve CVD conditions. Similarly, dietary Chinese medicine also showed the potential properties for CVD prevention via the interactions with the microbiota by improving lipid metabolism, alleviate inflammation and TMAOs as well as improve the gut barrier functions. CVD: Cardiovascular disease; SCFAs: short chain fatty acids; TMAOs: Trimethylamine N-oxide.
Types of diets – Multiple clinical trials have confirmed the protective effect of Med-diet on major vascular events, coronary events, stroke and heart failure [156], [157]. This effect is associated with the increase of microbiota diversity and microbial metabolite SCFAs [158] as well as lower levels of gut microbiota derived metabolite TMAO [159] and plasmatic LPS [160]. However, no direct finding has been investigated until very recently that the long-term intervention of Med-diet could protect CVD through gut microbiota modulation [161]. Specifically, long-term intervention of Med-diet could significantly alter the overall gut microbiome profiles with the enrichment of dietary fiber metabolizers such as Faecalibacterium prausnitzii and Bacteroides cellulosilyticus. In particular, Med-diet showed a strong protective effect on CVD risk factors including lipid metabolism, inflammation and glucose homeostasis in the absence of Prevotella copri [161]. Although multiple data have illustrated that DASH diet could improve cardiac risk factors with decrease of HT and dyslipidemia [162], there is still lack of data about the direct linkage between DASH diet and microbiota modifications in CVD preventions.
Feeding patterns – Intermittent fasting (IF), one of the important dietary feeding patterns, is a practice of periodic energy restriction which has been discovered to reduce the CVD risk via the alteration of gut microbiota [155], [163]. Specifically, spontaneously hypertensive stroke-prone rats showed a striking shift of gut microbiota in β-diversity after 50 days of IF intervention which was associated with the reduction of HT via modulation of the bile acids metabolism [155]. Those findings have been confirmed using fecal transplantation to GF rats [155]. Moreover, clinical interventions in human cohorts with IF during 8 weeks significantly improved vasodilatory parameters and attenuated oxidative stress, inflammation associated with increased SCFAs production by the microbiota and decreased plasmatic LPS [163]. Interestingly, short-term 5 days fasting also reduced the blood pressure and body weight with the modulation of the microbiota including Desulfovibrionaceae, Akkermansia, and Ruminococcaceae [164].
6.2. Dietary components
Dietary polyphenols in fruits and vegetables – Polyphenols are a large family of organic compounds commonly found in plant products, particularly fruits and vegetables. More than 90% of total polyphenols is non-absorbable in the small intestine and further metabolized by the gut microbiota in the large intestine [165]. A growing body of studies supported the effect of dietary polyphenols on the modification of gut microbiota and CVD protection [166], [167], [168]. For instance, resveratrol (found in fruits such as grapes, apples and berries) has been identified to attenuate atherosclerosis in ApoE−/− mice by downregulating TMAO levels and upregulating BAs synthesis which was associated with increased abundance of beneficial bacteria Bacteroides, Lactobacillus, Bifidobacterium, and Akkermansia [166]. Oral administration of quercetin (found in vegetables such as onions, broccoli and tomatoes) has been discovered to suppress body weight gains and ameliorate the extent of atherosclerotic lesions with diminished levels of cholesterol, atherogenic lysophosphatidylcholine and reduced abundance of gram-negative bacteria Verrucomicrobia together with the increase of microbiome diversity [167]. In human subjects, interventions with diet rich in polyphenols found that dietary polyphenols could significantly increase microbial diversity and Ruminococcaceae related with the improvement of cardiometabolic risk factors such as plasmatic triglycerides and cholesterol in large VLDL [168]. Collectively, polyphenols in fruits and vegetables might be potential therapeutic interventions for CVD, and a part of their protective effect could be mediated through gut microbiota modifications.
Dietary fibers and prebiotics – Dietary fibers are non-digestible carbohydrates including water-soluble or insoluble forms which are commonly presented in fruits, vegetables, whole grains, nuts and legumes etc. [169]. Dietary fibers could not be absorbed in the small intestine and “feed” the healthy gut microbiota leading to increased diversity and production of SCFAs [169]. As mentioned previously, SCFAs activate specific receptors leading to improved HT and aortic endothelial cells dysfunction [138], [139], [140]. Indeed, a very recent study discovered that chickpea dietary fiber increased the microbial diversity and the relative abundance of Bacteroides and Lactobacillus and upregulated levels of propionic acid [170]. Additionally, chickpea dietary fiber could improve hyperglycemia via similar modification of gut microbiota [170]. Whole grain oat also decreased plasma cholesterol levels and improve insulin sensitivity which correlated with increased beneficial Lactobacillaceae in the microbiota [171], [172]. Similarly, whole grain products consumption in human cohorts also showed lower levels of total and LDL cholesterol and higher abundance of Bifidobacterium [173].
Prebiotics are plant derived or non-digestible food ingredients which stimulate the growth of “friendly” microorganisms in the GI tract [174]. Most prebiotics are dietary fibers, while not all dietary fibers can be categorized as prebiotics [175]. Common prebiotics include oligosaccharides and polysaccharides such as inulin, oligofructose, β-glucans which can ordinarily induce specific modifications of the gut microbiota [174]. Many studies have interestingly investigated the beneficial effects of prebiotics on host metabolism to improve CVD conditions (Fig. 2) through three main aspects: (1) Reduce blood lipids: Parnell et al. discovered that supplementation of prebiotic fibers (e.g. inulin) could lower the plasmatic cholesterol levels as well as reduce the TAG accumulation in the liver [176], [177], [178]; (2) Diminish endotoxemia and inflammation: Cani et al. found that prebiotic oligofructose could increase the population of Bifidobacteria with negative relation to endotoxemia and inflammation in plasma and adipose tissues [179]; (3) Decrease blood pressure: Kaye et al. uncovered very recently that the supplementation of diet rich in prebiotic fibers could diminish both systolic and diastolic blood pressure through GPR43 signaling pathway [180].
Probiotics - Probiotics are defined as “live microorganisms that, when administered in adequate amounts, confer a health benefit on the host.” [174]. Large scales of fermented products in human diets such as yogurt, sauerkraut, kefir, Kimchi contain probiotic strains [174]. As prebiotics, probiotic strains have also been identified to protect against CVD progresses (Fig. 2) in more aspects: (1) Ameliorate vascular endothelial function: administration of Lactobacillus plantarum 299v showed the improvement of endothelium-dependent vasodilation in resistance arteries from patients with CAD [181]. Similarly, Lactobacillus fermentum CECT5716 treatment reduced vascular oxidative stress and improvemed endothelial function in rats [182]. (2) Decrease blood glucose and oxidant activity: intervention with probiotic yogurt significantly lowered the blood glucose and increase total antioxidant status [183]. (3) Reduce cholesterol: supplementation with Bifidobacterium longum BB536 has significant effects on the reduction of total cholesterol, liver lipid deposition and adipocyte size [184]. (4) Attenuate endotoxemia and inflammation: oral introduction of Akkermansia muciniphila has been shown to reduce atherosclerotic lesions by improving systemic endotoxemia-induced inflammation through restoration of the gut barrier function [64]. In addition, supplementation with Lactobacillus reuteri V3401 reduced the levels of inflammatory markers such as TNF-α, IL-6, IL-8 which was associated with the reduction of CVD risk [185].
6.3. Dietary Chinese medicine
Some natural ingredients from Chinese medicine have also been used as potential CVD therapeutics via the modulation of gut microbiota (Fig. 2). Particularly, berberine (BBR), a bioactive isoquinoline alkaloid which is widely presented and extracted from various Chinese medicinal plants has been proved to exert many beneficial effects. Wu et al. recently discovered that high dose of BBR not only improved lipid metabolism via attenuating reduced total cholesterol and VLDL cholesterol levels, but also downregulated pro-inflammatory cytokines TNF-α, Il-1β, IL-6 and upregulated anti-inflammatory IL-10 levels which were correlated with the increased abundance of Alistipes and Roseburia involved in SCFA production [186]. Additionally, BBR could reduce choline-induced atherosclerosis by suppressing TMAO production through remodeling gut microbiota compositions [187]. Similarly, red yeast rice (RYR), another Chinese folk medicine, could alleviate the plaque formation with the reduction of total cholesterol and LDL levels which are associated with decreased ratio of Firmicutes/ Bacteroidetes as well as reduced abundance of Alistipes and Flavonifractor [76], [188]. RYR intervention also improved gut barrier function, and attenuated inflammation via TLR signaling pathway [76]. Furthermore, Ganoderma lucidum (also known as Lingzhi), a type of medicinal mushroom, has been discovered to reduce obesity, endotoxemia, chronic inflammation as well as restore intestinal barrier function by decreasing endotoxin bearing Proteobacteria levels and increasing beneficial bacteria including Clostridium and Eubacterium [189].
7. Summary and outlook
Over the past decades, accumulating studies demonstrated an essential and complex association between gut microbiota and cardiovascular disease. As one of the important modulators in gut microbiota, dietary components have been identified to modify the microbial compositions which were linked with the systemic endotoxemia, inflammation, gut barrier dysfunction, as well as lipid metabolism dysfunction to increase the CVD risk. While, more research data suggested the principal effect of intestinal microbiota on dietary metabolism in modulating CVD pathogenesis including: (1) metabolizing dietary choline or L-carnitine to induce the release of TMAO which promote the atherosclerotic progression; (2) regulating BAs metabolism which is likely to regulate the atherosclerotic formation via multiple receptors pathway; (3) generating AAAs metabolites PAG, IS, IPA or PCS which can accelerate the atherosclerosis formation; (4) fermenting dietary fibers to generate SCFAs which mostly exert some beneficial effects on CVD progression. These findings provide some excellent opportunities for developing novel potential prevention and therapeutic methods for CVD such as healthy diets and feeding patterns, dietary interventions with healthy components including dietary polyphenols from fruits and vegetables, dietary fibers and prebiotics, probiotics as well as the dietary Chinese medicine interventions.
Although numerous intestinal bacteria have been identified in association with CVD risk, much more works remain to discover the specific commensal microbes and the precise mechanisms or pathways behind those complex relationship between the dietary induced gut microbiota modification and CVD pathogenesis. However, there are still some limitations in current research: (1) Regarding gut microbiota analysis, different studies follow different protocols or methodologies for fecal samples collection, storage and DNA extraction, or choose different sequencing platform or methods. These dissimilarities might impact the consistency and result in the variations of gut microbiota. (2) In the clinical human cohort studies, numerous parameters of the recruited human subjects might affect the consistency of microbiota discoveries, such as dietary habits, lifestyle, and medications. (3) In animal studies, different age, housing condition, or different diet, feeding period could also impact the gut microbiota. Therefore, it is crucial to design the study properly to reach the reliable discoveries for future investigations.
CRediT authorship contribution statement
Xufei Zhang: Conceptualization, Writing – original draft. Philippe Gérard: Conceptualization, Writing – review & editing, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Funding: This study is supported by the ERA-Net Cofund “Interrelation of the INtesTInal MICrobiome, Diet and Health” (HDHL-INTIMIC) (grant ANR-17-HDIM-0007-04).
References
- 1.Li X., Liu L., Cao Z., Li W., Li H., et al. Gut microbiota as an “invisible organ” that modulates the function of drugs. Biomed Pharmacother. 2020;121 doi: 10.1016/j.biopha.2019.109653. [DOI] [PubMed] [Google Scholar]
- 2.Baquero F., Nombela C. Wiley Online Library; 2012. The microbiome as a human organ. [DOI] [PubMed] [Google Scholar]
- 3.Bapteste E., Gerard P., Larose C., Blouin M., Not F., et al. The epistemic revolution induced by microbiome studies: an interdisciplinary view. Biology. 2021;10:651. doi: 10.3390/biology10070651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gérard P. Gut microbiome and obesity. How to prove causality? Ann Am Thorac Soc. 2017;14:S354–S356. doi: 10.1513/AnnalsATS.201702-117AW. [DOI] [PubMed] [Google Scholar]
- 5.Gérard P. Gut microbiota and obesity. Cell Mol Life Sci. 2016;73:147–162. doi: 10.1007/s00018-015-2061-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Safari Z., Gérard P. The links between the gut microbiome and non-alcoholic fatty liver disease (NAFLD) Cell Mol Life Sci. 2019;76:1541–1558. doi: 10.1007/s00018-019-03011-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duca F., Gérard P., Covasa M., Lepage P. How gut and brain control metabolism. 2014. Metabolic interplay between gut bacteria and their host; pp. 73–82. [DOI] [PubMed] [Google Scholar]
- 8.Sofi F., Dinu M., Pagliai G., Pierre F., Gueraud F., et al. Fecal microbiome as determinant of the effect of diet on colorectal cancer risk: comparison of meat-based versus pesco-vegetarian diets (the MeaTIc study) Trials. 2019;20:1–9. doi: 10.1186/s13063-019-3801-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singer-Englar T., Barlow G., Mathur R. Obesity, diabetes, and the gut microbiome: an updated review. Expert Rev Gastroenterol Hepatol. 2019;13:3–15. doi: 10.1080/17474124.2019.1543023. [DOI] [PubMed] [Google Scholar]
- 10.Tang W.W., Li D.Y., Hazen S.L. Dietary metabolism, the gut microbiome, and heart failure. Nat Rev Cardiol. 2019;16:137–154. doi: 10.1038/s41569-018-0108-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jonsson A.L., Bäckhed F. Role of gut microbiota in atherosclerosis. Nat Rev Cardiol. 2017;14:79–87. doi: 10.1038/nrcardio.2016.183. [DOI] [PubMed] [Google Scholar]
- 12.Qin J., Li R., Raes J., Arumugam M., Burgdorf K.S., et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eckburg P.B., Bik E.M., Bernstein C.N., Purdom E., Dethlefsen L., et al. Diversity of the human intestinal microbial flora. Science. 2005;308:1635–1638. doi: 10.1126/science.1110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gill S.R., Pop M., DeBoy R.T., Eckburg P.B., Turnbaugh P.J., et al. Metagenomic analysis of the human distal gut microbiome. Science. 2006;312:1355–1359. doi: 10.1126/science.1124234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vandeputte D., Kathagen G., D'Hoe K., Vieira-Silva S., Valles-Colomer M., et al. Quantitative microbiome profiling links gut community variation to microbial load. Nature. 2017;551:507–511. doi: 10.1038/nature24460. [DOI] [PubMed] [Google Scholar]
- 16.Vieira-Silva S., Falony G., Belda E., Nielsen T., Aron-Wisnewsky J., et al. Statin therapy is associated with lower prevalence of gut microbiota dysbiosis. Nature. 2020;581:310–315. doi: 10.1038/s41586-020-2269-x. [DOI] [PubMed] [Google Scholar]
- 17.Martinez K.B., Leone V., Chang E.B. Western diets, gut dysbiosis, and metabolic diseases: Are they linked? Gut microbes. 2017;8:130–142. doi: 10.1080/19490976.2016.1270811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mozaffarian D., Benjamin E.J., Go A.S., Arnett D.K., Blaha M.J., et al. Executive summary: heart disease and stroke statistics—2016 update: a report from the American Heart Association. Circulation. 2016;133:447–454. doi: 10.1161/CIR.0000000000000366. [DOI] [PubMed] [Google Scholar]
- 19.Zhou W., Cheng Y., Zhu P., Nasser M., Zhang X., et al. (2020) Implication of gut microbiota in cardiovascular diseases. Oxid Med Cell Longevity. 2020 doi: 10.1155/2020/5394096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown J.M., Hazen S.L. Microbial modulation of cardiovascular disease. Nat Rev Microbiol. 2018;16:171–181. doi: 10.1038/nrmicro.2017.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tang W.W., Bäckhed F., Landmesser U., Hazen S.L. Intestinal microbiota in cardiovascular health and disease: JACC state-of-the-art review. J Am Coll Cardiol. 2019;73:2089–2105. doi: 10.1016/j.jacc.2019.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sekirov I., Russell S.L., Antunes L.C.M., Finlay B.B. Gut microbiota in health and disease. Physiol Rev. 2010 doi: 10.1152/physrev.00045.2009. [DOI] [PubMed] [Google Scholar]
- 23.O'Hara A.M., Shanahan F. The gut flora as a forgotten organ. EMBO Rep. 2006;7:688–693. doi: 10.1038/sj.embor.7400731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moszak M., Szulińska M., Bogdański P. You are what you eat—the relationship between diet, microbiota, and metabolic disorders—A review. Nutrients. 2020;12:1096. doi: 10.3390/nu12041096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zmora N., Suez J., Elinav E. You are what you eat: diet, health and the gut microbiota. Nat Rev Gastroenterol Hepatol. 2019;16:35–56. doi: 10.1038/s41575-018-0061-2. [DOI] [PubMed] [Google Scholar]
- 26.Sonnenburg J.L., Bäckhed F. Diet–microbiota interactions as moderators of human metabolism. Nature. 2016;535:56–64. doi: 10.1038/nature18846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.David L.A., Maurice C.F., Carmody R.N., Gootenberg D.B., Button J.E., et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505:559–563. doi: 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu G.D., Chen J., Hoffmann C., Bittinger K., Chen Y.-Y., et al. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334:105–108. doi: 10.1126/science.1208344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de La Serre C.B., Ellis C.L., Lee J., Hartman A.L., Rutledge J.C., et al. Propensity to high-fat diet-induced obesity in rats is associated with changes in the gut microbiota and gut inflammation. Am J Physiol-Gastrointestinal Liver Physiol. 2010;299:G440–G448. doi: 10.1152/ajpgi.00098.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fragiadakis G.K., Wastyk H.C., Robinson J.L., Sonnenburg E.D., Sonnenburg J.L., et al. Long-term dietary intervention reveals resilience of the gut microbiota despite changes in diet and weight. Am J Clin Nutr. 2020;111:1127–1136. doi: 10.1093/ajcn/nqaa046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cotillard A., Kennedy S.P., Kong L.C., Prifti E., Pons N., et al. Dietary intervention impact on gut microbial gene richness. Nature. 2013;500:585–588. doi: 10.1038/nature12480. [DOI] [PubMed] [Google Scholar]
- 32.Walker A.W., Ince J., Duncan S.H., Webster L.M., Holtrop G., et al. Dominant and diet-responsive groups of bacteria within the human colonic microbiota. ISME J. 2011;5:220–230. doi: 10.1038/ismej.2010.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koeth R.A., Wang Z., Levison B.S., Buffa J.A., Org E., et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19:576–585. doi: 10.1038/nm.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Honour J. The possible involvement of intestinal bacteria in steroidal hypertension. Endocrinology. 1982;110:285–287. doi: 10.1210/endo-110-1-285. [DOI] [PubMed] [Google Scholar]
- 35.Joe B., McCarthy C.G., Edwards J.M., Cheng X., Chakraborty S., et al. Microbiota introduced to germ-free rats restores vascular contractility and blood pressure. Hypertension. 2020;76:1847–1855. doi: 10.1161/HYPERTENSIONAHA.120.15939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stepankova R., Tonar Z., Bartova J., Nedorost L., Rossman P., et al. Absence of microbiota (germ-free conditions) accelerates the atherosclerosis in ApoE-deficient mice fed standard low cholesterol diet. J Atherosclerosis Thrombosis. 2010;17:796–804. doi: 10.5551/jat.3285. [DOI] [PubMed] [Google Scholar]
- 37.Karbach S.H., Schonfelder T., Brandao I., Wilms E., Hormann N., et al. Gut microbiota promote angiotensin II-induced arterial hypertension and vascular dysfunction. J Am Heart Assoc. 2016:5. doi: 10.1161/JAHA.116.003698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang T., Santisteban M.M., Rodriguez V., Li E., Ahmari N., et al. Gut dysbiosis is linked to hypertension. Hypertension. 2015;65:1331–1340. doi: 10.1161/HYPERTENSIONAHA.115.05315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kiouptsi K., Jäckel S., Pontarollo G., Grill A., Kuijpers M.J., et al. The microbiota promotes arterial thrombosis in low-density lipoprotein receptor-deficient mice. MBio. 2019;10:e02298–02219. doi: 10.1128/mBio.02298-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li J., Zhao F., Wang Y., Chen J., Tao J., et al. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome. 2017;5:1–19. doi: 10.1186/s40168-016-0222-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dan X., Mushi Z., Baili W., Han L., Enqi W., et al. Differential analysis of hypertension-associated intestinal microbiota. Int J Med Sci. 2019;16:872. doi: 10.7150/ijms.29322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sun S., Lulla A., Sioda M., Winglee K., Wu M.C., et al. Gut microbiota composition and blood pressure: the CARDIA study. Hypertension. 2019;73:998–1006. doi: 10.1161/HYPERTENSIONAHA.118.12109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Karlsson F.H., Fåk F., Nookaew I., Tremaroli V., Fagerberg B., et al. Symptomatic atherosclerosis is associated with an altered gut metagenome. Nat Commun. 2012;3:1–8. doi: 10.1038/ncomms2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jie Z., Xia H., Zhong S.-L., Feng Q., Li S., et al. The gut microbiome in atherosclerotic cardiovascular disease. Nat Commun. 2017;8:1–12. doi: 10.1038/s41467-017-00900-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu S., Zhao W., Liu X., Cheng L. Metagenomic analysis of the gut microbiome in atherosclerosis patients identify cross-cohort microbial signatures and potential therapeutic target. FASEB J. 2020;34:14166–14181. doi: 10.1096/fj.202000622R. [DOI] [PubMed] [Google Scholar]
- 46.Emoto T., Yamashita T., Sasaki N., Hirota Y., Hayashi T., et al. Analysis of gut microbiota in coronary artery disease patients: a possible link between gut microbiota and coronary artery disease. J Atherosclerosis Thrombosis. 2016;32672 doi: 10.5551/jat.32672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhu Q., Gao R., Zhang Y., Pan D., Zhu Y., et al. Dysbiosis signatures of gut microbiota in coronary artery disease. Physiol Genomics. 2018;50:893–903. doi: 10.1152/physiolgenomics.00070.2018. [DOI] [PubMed] [Google Scholar]
- 48.Liu H., Chen X., Hu X., Niu H., Tian R., et al. Alterations in the gut microbiome and metabolism with coronary artery disease severity. Microbiome. 2019;7:1–14. doi: 10.1186/s40168-019-0683-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pasini E., Aquilani R., Testa C., Baiardi P., Angioletti S., et al. Pathogenic gut flora in patients with chronic heart failure. JACC Heart Failure. 2016;4:220–227. doi: 10.1016/j.jchf.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 50.Kummen M., Mayerhofer C.C., Vestad B., Broch K., Awoyemi A., et al. Gut microbiota signature in heart failure defined from profiling of 2 independent cohorts. J Am Coll Cardiol. 2018;71:1184–1186. doi: 10.1016/j.jacc.2017.12.057. [DOI] [PubMed] [Google Scholar]
- 51.Hayashi T., Yamashita T., Watanabe H., Kami K., Yoshida N., et al. Gut microbiome and plasma microbiome-related metabolites in patients with decompensated and compensated heart failure. Circ J. 2018;83:182–192. doi: 10.1253/circj.CJ-18-0468. [DOI] [PubMed] [Google Scholar]
- 52.Yin J., Liao S.X., He Y., Wang S., Xia G.H., et al. Dysbiosis of gut microbiota with reduced trimethylamine-N-oxide level in patients with large-artery atherosclerotic stroke or transient ischemic attack. J Am Heart Assoc. 2015;4 doi: 10.1161/JAHA.115.002699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li N., Wang X., Sun C., Wu X., Lu M., et al. Change of intestinal microbiota in cerebral ischemic stroke patients. BMC Microbiol. 2019;19:1–8. doi: 10.1186/s12866-019-1552-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tan C., Wu Q., Wang H., Gao X., Xu R., et al. Dysbiosis of gut microbiota and short-chain fatty acids in acute ischemic stroke and the subsequent risk for poor functional outcomes. J Parenteral Enteral Nutr. 2021;45:518–529. doi: 10.1002/jpen.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Panek M., Cipcic Paljetak H., Baresic A., Peric M., Matijasic M., et al. Methodology challenges in studying human gut microbiota - effects of collection, storage, DNA extraction and next generation sequencing technologies. Sci Rep. 2018;8:5143. doi: 10.1038/s41598-018-23296-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Whon T.W., Chung W.H., Lim M.Y., Song E.J., Kim P.S., et al. The effects of sequencing platforms on phylogenetic resolution in 16 S rRNA gene profiling of human feces. Sci Data. 2018;5 doi: 10.1038/sdata.2018.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Durazzi F., Sala C., Castellani G., Manfreda G., Remondini D., et al. Comparison between 16S rRNA and shotgun sequencing data for the taxonomic characterization of the gut microbiota. Sci Rep. 2021;11:3030. doi: 10.1038/s41598-021-82726-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Falony G., Joossens M., Vieira-Silva S., Wang J., Darzi Y., et al. Population-level analysis of gut microbiome variation. Science. 2016;352:560–564. doi: 10.1126/science.aad3503. [DOI] [PubMed] [Google Scholar]
- 59.Forslund S, Chakaroun R, Zimmermann-Kogadeeva M, Markó L, Aron-Wisnewsky J, et al. (2021) Combinatorial, additive and dose-dependent drug–microbiome associations. Nature 600. [DOI] [PubMed]
- 60.König J., Wells J., Cani P.D., García-Ródenas C.L., MacDonald T., et al. Human intestinal barrier function in health and disease. Clin Transl Gastroenterol. 2016;7 doi: 10.1038/ctg.2016.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sandek A., Bauditz J., Swidsinski A., Buhner S., Weber-Eibel J., et al. Altered intestinal function in patients with chronic heart failure. J Am Coll Cardiol. 2007;50:1561–1569. doi: 10.1016/j.jacc.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 62.Kim S., Goel R., Kumar A., Qi Y., Lobaton G., et al. Imbalance of gut microbiome and intestinal epithelial barrier dysfunction in patients with high blood pressure. Clin Sci. 2018;132:701–718. doi: 10.1042/CS20180087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Witkowski M., Weeks T.L., Hazen S.L. Gut microbiota and cardiovascular disease. Circ Res. 2020;127:553–570. doi: 10.1161/CIRCRESAHA.120.316242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wan Y., Wang F., Yuan J., Li J., Jiang D., et al. Effects of dietary fat on gut microbiota and faecal metabolites, and their relationship with cardiometabolic risk factors: a 6-month randomised controlled-feeding trial. Gut. 2019;68:1417–1429. doi: 10.1136/gutjnl-2018-317609. [DOI] [PubMed] [Google Scholar]
- 65.Leech B., McIntyre E., Steel A., Sibbritt D. Risk factors associated with intestinal permeability in an adult population: a systematic review. Int J Clin Pract. 2019;73 doi: 10.1111/ijcp.13385. [DOI] [PubMed] [Google Scholar]
- 66.Gérard P. The crosstalk between the gut microbiota and lipids. OCL. 2020;27:70. [Google Scholar]
- 67.Sacks F.M., Lichtenstein A.H., Wu J.H., Appel L.J., Creager M.A., et al. Dietary fats and cardiovascular disease: a presidential advisory from the American Heart Association. Circulation. 2017;136:e1–e23. doi: 10.1161/CIR.0000000000000510. [DOI] [PubMed] [Google Scholar]
- 68.Yang Y., Zhong Z., Wang B., Xia X., Yao W., et al. Early-life high-fat diet-induced obesity programs hippocampal development and cognitive functions via regulation of gut commensal Akkermansia muciniphila. Neuropsychopharmacology. 2019;44:2054–2064. doi: 10.1038/s41386-019-0437-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rohr M.W., Narasimhulu C.A., Rudeski-Rohr T.A., Parthasarathy S. Negative effects of a high-fat diet on intestinal permeability: a review. Adv Nutr. 2020;11:77–91. doi: 10.1093/advances/nmz061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang F., Graham W.V., Wang Y., Witkowski E.D., Schwarz B.T., et al. Interferon-γ and tumor necrosis factor-α synergize to induce intestinal epithelial barrier dysfunction by up-regulating myosin light chain kinase expression. Am J Pathol. 2005;166:409–419. doi: 10.1016/s0002-9440(10)62264-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Al-Sadi R., Ye D., Dokladny K., Ma T.Y. Mechanism of IL-1β-induced increase in intestinal epithelial tight junction permeability. J Immunol. 2008;180:5653–5661. doi: 10.4049/jimmunol.180.8.5653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cunningham K.E., Turner J.R. Myosin light chain kinase: pulling the strings of epithelial tight junction function. Ann N Y Acad Sci. 2012;1258:34. doi: 10.1111/j.1749-6632.2012.06526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Manco M., Putignani L., Bottazzo G.F. Gut microbiota, lipopolysaccharides, and innate immunity in the pathogenesis of obesity and cardiovascular risk. Endocr Rev. 2010;31:817–844. doi: 10.1210/er.2009-0030. [DOI] [PubMed] [Google Scholar]
- 74.Incalza M.A., D'Oria R., Natalicchio A., Perrini S., Laviola L., et al. Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. Vascul Pharmacol. 2018;100:1–19. doi: 10.1016/j.vph.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 75.Li J., Lin S., Vanhoutte P.M., Woo C.W., Xu A. Akkermansia muciniphila protects against atherosclerosis by preventing metabolic endotoxemia-induced inflammation in Apoe−/− mice. Circulation. 2016;133:2434–2446. doi: 10.1161/CIRCULATIONAHA.115.019645. [DOI] [PubMed] [Google Scholar]
- 76.Dong Y., Cheng H., Liu Y., Xue M., Liang H. Red yeast rice ameliorates high-fat diet-induced atherosclerosis in Apoe−/− mice in association with improved inflammation and altered gut microbiota composition. Food Funct. 2019;10:3880–3889. doi: 10.1039/c9fo00583h. [DOI] [PubMed] [Google Scholar]
- 77.Lindskog Jonsson A., Caesar R., Akrami R., Reinhardt C., Fåk Hållenius F., et al. Impact of gut microbiota and diet on the development of atherosclerosis in Apoe−/− mice. Arterioscler Thromb Vasc Biol. 2018;38:2318–2326. doi: 10.1161/ATVBAHA.118.311233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Le Roy T., Lécuyer E., Chassaing B., Rhimi M., Lhomme M., et al. The intestinal microbiota regulates host cholesterol homeostasis. BMC Biol. 2019;17:1–18. doi: 10.1186/s12915-019-0715-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kasahara K., Tanoue T., Yamashita T., Yodoi K., Matsumoto T., et al. Commensal bacteria at the crossroad between cholesterol homeostasis and chronic inflammation in atherosclerosis. J Lipid Res. 2017;58:519-528. doi: 10.1194/jlr.M072165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Koren O., Spor A., Felin J., Fåk F., Stombaugh J., et al. Human oral, gut, and plaque microbiota in patients with atherosclerosis. Proc Natl Acad Sci. 2011;108:4592–4598. doi: 10.1073/pnas.1011383107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rabot S., Membrez M., Bruneau A., Gérard P., Harach T., et al. Germ-free C57BL/6J mice are resistant to high-fat-diet-induced insulin resistance and have altered cholesterol metabolism. FASEB J. 2010;24:4948–4959. doi: 10.1096/fj.10-164921. [DOI] [PubMed] [Google Scholar]
- 82.Juste C., Gérard P. Cholesterol-to-coprostanol conversion by the gut microbiota: what we know, suspect, and ignore. Microorganisms. 2021;9:1881. doi: 10.3390/microorganisms9091881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bourgin M., Labarthe S., Kriaa A., Lhomme M., Gérard P., et al. Exploring the bacterial impact on cholesterol cycle: a numerical study. Front Microbiol. 2020;11:1121. doi: 10.3389/fmicb.2020.01121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kenny D.J., Plichta D.R., Shungin D., Koppel N., Hall A.B., et al. Cholesterol metabolism by uncultured human gut bacteria influences host cholesterol level. Cell Host Microbe. 2020;28 doi: 10.1016/j.chom.2020.05.013. 245-257 e246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kiouptsi K., Pontarollo G., Todorov H., Braun J., Jackel S., et al. Germ-free housing conditions do not affect aortic root and aortic arch lesion size of late atherosclerotic low-density lipoprotein receptor-deficient mice. Gut Microbes. 2020;11:1809–1823. doi: 10.1080/19490976.2020.1767463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang Z., Klipfell E., Bennett B.J., Koeth R., Levison B.S., et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Romano K.A., Vivas E.I., Amador-Noguez D., Rey F.E. Intestinal microbiota composition modulates choline bioavailability from diet and accumulation of the proatherogenic metabolite trimethylamine-N-oxide. MBio. 2015;6:e02481–02414. doi: 10.1128/mBio.02481-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang Z., Bergeron N., Levison B.S., Li X.S., Chiu S., et al. Impact of chronic dietary red meat, white meat, or non-meat protein on trimethylamine N-oxide metabolism and renal excretion in healthy men and women. Eur Heart J. 2019;40:583–594. doi: 10.1093/eurheartj/ehy799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Craciun S., Balskus E.P. Microbial conversion of choline to trimethylamine requires a glycyl radical enzyme. Proc Natl Acad Sci. 2012;109:21307–21312. doi: 10.1073/pnas.1215689109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhu Y., Jameson E., Crosatti M., Schäfer H., Rajakumar K., et al. Carnitine metabolism to trimethylamine by an unusual Rieske-type oxygenase from human microbiota. Proc Natl Acad Sci. 2014;111:4268–4273. doi: 10.1073/pnas.1316569111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Papandreou C, More M, Bellamine A (2020) Trimethylamine N-oxide in relation to cardiometabolic health-cause or effect? Nutrients 12. [DOI] [PMC free article] [PubMed]
- 92.Shih D.M., Wang Z., Lee R., Meng Y., Che N., et al. Flavin containing monooxygenase 3 exerts broad effects on glucose and lipid metabolism and atherosclerosis [S] J Lipid Res. 2015;56:22–37. doi: 10.1194/jlr.M051680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rath S., Heidrich B., Pieper D.H., Vital M. Uncovering the trimethylamine-producing bacteria of the human gut microbiota. Microbiome. 2017;5:1–14. doi: 10.1186/s40168-017-0271-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wu W.-K., Panyod S., Liu P.-Y., Chen C.-C., Kao H.-L., et al. Characterization of TMAO productivity from carnitine challenge facilitates personalized nutrition and microbiome signatures discovery. Microbiome. 2020;8:1–17. doi: 10.1186/s40168-020-00912-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Koeth R.A., Lam-Galvez B.R., Kirsop J., Wang Z., Levison B.S., et al. l-Carnitine in omnivorous diets induces an atherogenic gut microbial pathway in humans. J Clin Investig. 2019;129:373–387. doi: 10.1172/JCI94601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gregory J.C., Buffa J.A., Org E., Wang Z., Levison B.S., et al. Transmission of atherosclerosis susceptibility with gut microbial transplantation. J Biol Chem. 2015;290:5647–5660. doi: 10.1074/jbc.M114.618249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhu W., Gregory J.C., Org E., Buffa J.A., Gupta N., et al. Gut microbial metabolite TMAO enhances platelet hyperreactivity and thrombosis risk. Cell. 2016;165:111–124. doi: 10.1016/j.cell.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yang S., Li X., Yang F., Zhao R., Pan X., et al. Gut microbiota-dependent marker TMAO in promoting cardiovascular disease: inflammation mechanism, clinical prognostic, and potential as a therapeutic target. Front Pharmacol. 2019;10:1360. doi: 10.3389/fphar.2019.01360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Seldin M.M., Meng Y., Qi H., Zhu W., Wang Z., et al. Trimethylamine N-oxide promotes vascular inflammation through signaling of mitogen-activated protein kinase and nuclear factor-κB. J Am Heart Assoc. 2016;5 doi: 10.1161/JAHA.115.002767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chen K., Zheng X., Feng M., Li D., Zhang H. Gut microbiota-dependent metabolite trimethylamine N-oxide contributes to cardiac dysfunction in western diet-induced obese mice. Front Physiol. 2017;8:139. doi: 10.3389/fphys.2017.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Aldana-Hernández P., Leonard K.-A., Zhao Y.-Y., Curtis J.M., Field C.J., et al. Dietary choline or trimethylamine N-oxide supplementation does not influence atherosclerosis development in Ldlr−/− and Apoe−/− male mice. J Nutr. 2020;150:249–255. doi: 10.1093/jn/nxz214. [DOI] [PubMed] [Google Scholar]
- 102.Jaworska KA-OX, Hering DA-O, Mosieniak G, Bielak-Zmijewska A, Pilz MA-O, et al. (2019) TMA, A Forgotten Uremic Toxin, but Not TMAO, Is Involved in Cardiovascular Pathology. LID - 10.3390/toxins11090490 [doi] LID - 490. Toxins 11: 490. [DOI] [PMC free article] [PubMed]
- 103.Jaworska K., Konop M., Hutsch T., Perlejewski K., Radkowski M., et al. Trimethylamine but not trimethylamine oxide increases with age in rat plasma and affects smooth muscle cells viability. J Gerontol A Biol Sci Med Sci. 2020;75:1276–1283. doi: 10.1093/gerona/glz181. [DOI] [PubMed] [Google Scholar]
- 104.Jaworska K., Bielinska K., Gawrys-Kopczynska M., Ufnal M. TMA (trimethylamine), but not its oxide TMAO (trimethylamine-oxide), exerts haemodynamic effects: implications for interpretation of cardiovascular actions of gut microbiome. Cardiovasc Res. 2019;115:1948–1949. doi: 10.1093/cvr/cvz231. [DOI] [PubMed] [Google Scholar]
- 105.Tang W.W., Wang Z., Levison B.S., Koeth R.A., Britt E.B., et al. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med. 2013;368:1575–1584. doi: 10.1056/NEJMoa1109400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Senthong V., Wang Z., Li X.S., Fan Y., Wu Y., et al. Intestinal microbiota-generated metabolite trimethylamine-N-oxide and 5-year mortality risk in stable coronary artery disease: the contributory role of intestinal microbiota in a COURAGE-like patient cohort. J Am Heart Assoc. 2016;5 doi: 10.1161/JAHA.115.002816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yao M.-E., Liao P.-D., Zhao X.-J., Wang L. Trimethylamine-N-oxide has prognostic value in coronary heart disease: a meta-analysis and dose-response analysis. BMC Cardiovasc Disord. 2020;20:1–9. doi: 10.1186/s12872-019-01310-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Haghikia A., Li X.S., Liman T.G., Bledau N., Schmidt D., et al. Gut microbiota–dependent trimethylamine N-oxide predicts risk of cardiovascular events in patients with stroke and is related to proinflammatory monocytes. Arterioscler Thromb Vasc Biol. 2018;38:2225–2235. doi: 10.1161/ATVBAHA.118.311023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Farhangi M.A., Vajdi M. Novel findings of the association between gut microbiota–derived metabolite trimethylamine N-oxide and inflammation: results from a systematic review and dose-response meta-analysis. Crit Rev Food Sci Nutr. 2020;60:2801–2823. doi: 10.1080/10408398.2020.1770199. [DOI] [PubMed] [Google Scholar]
- 110.Meyer K.A., Benton T.Z., Bennett B.J., Jacobs D.R., Jr, Lloyd-Jones D.M., et al. Microbiota-dependent metabolite trimethylamine N-oxide and coronary artery calcium in the coronary artery risk development in young adults study (CARDIA) J Am Heart Assoc. 2016;5 doi: 10.1161/JAHA.116.003970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hsu C.N., Chang-Chien G.P., Lin S., Hou C.Y., Lu P.C., et al. Association of trimethylamine, trimethylamine n-oxide, and dimethylamine with cardiovascular risk in children with chronic kidney disease. J Clin Med. 2020;9 doi: 10.3390/jcm9020336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gérard P. Metabolism of cholesterol and bile acids by the gut microbiota. Pathogens. 2014;3:14–24. doi: 10.3390/pathogens3010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Heaton K. The importance of keeping bile salts in their place. Gut. 1969;10:857. doi: 10.1136/gut.10.10.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sayin S.I., Wahlström A., Felin J., Jäntti S., Marschall H.-U., et al. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab. 2013;17:225–235. doi: 10.1016/j.cmet.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 115.Dawson P.A., Lan T., Rao A. Bile acid transporters. J Lipid Res. 2009;50:2340–2357. doi: 10.1194/jlr.R900012-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yao L., Seaton S.C., Ndousse-Fetter S., Adhikari A.A., DiBenedetto N., et al. A selective gut bacterial bile salt hydrolase alters host metabolism. Elife. 2018;7 doi: 10.7554/eLife.37182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gérard P. Health consequences of microbial interactions with hydrocarbons oils, and lipids. 2020. Gastrointestinal tract: microbial metabolism of steroids. pp. 389–399. [Google Scholar]
- 118.Ridlon J.M., Kang D.-J., Hylemon P.B. Bile salt biotransformations by human intestinal bacteria. J Lipid Res. 2006;47:241–259. doi: 10.1194/jlr.R500013-JLR200. [DOI] [PubMed] [Google Scholar]
- 119.Kisiela M., Skarka A., Ebert B., Maser E. Hydroxysteroid dehydrogenases (HSDs) in bacteria–a bioinformatic perspective. J Steroid Biochem Mol Biol. 2012;129:31–46. doi: 10.1016/j.jsbmb.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 120.Lepercq P., Gérard P., Béguet F., Raibaud P., Grill J.-P., et al. Epimerization of chenodeoxycholic acid to ursodeoxycholic acid by Clostridium baratii isolated from human feces. FEMS Microbiol Lett. 2004;235:65–72. doi: 10.1016/j.femsle.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 121.Wahlström A., Sayin S.I., Marschall H.-U., Bäckhed F. Intestinal crosstalk between bile acids and microbiota and its impact on host metabolism. Cell Metab. 2016;24:41–50. doi: 10.1016/j.cmet.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 122.Hartman H.B., Gardell S.J., Petucci C.J., Wang S., Krueger J.A., et al. Activation of farnesoid X receptor prevents atherosclerotic lesion formation in LDLR−/− and apoE−/− mice. J Lipid Res. 2009;50:1090–1100. doi: 10.1194/jlr.M800619-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hanniman E.A., Lambert G., McCarthy T.C., Sinal C.J. Loss of functional farnesoid X receptor increases atherosclerotic lesions in apolipoprotein E-deficient mice. J Lipid Res. 2005;46:2595–2604. doi: 10.1194/jlr.M500390-JLR200. [DOI] [PubMed] [Google Scholar]
- 124.Guo G.L., Santamarina-Fojo S., Akiyama T.E., Amar M.J., Paigen B.J., et al. Vol. 1761. 2006. Effects of FXR in foam-cell formation and atherosclerosis development; pp. 1401–1409. (Biochim Biophys Acta (BBA)-Mol Cell Biol Lipids). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhang Y., Wang X., Vales C., Lee F.Y., Lee H., et al. FXR deficiency causes reduced atherosclerosis in Ldlr−/− mice. Arterioscler Thromb Vasc Biol. 2006;26:2316–2321. doi: 10.1161/01.ATV.0000235697.35431.05. [DOI] [PubMed] [Google Scholar]
- 126.Bennett B.J., de Aguiar Vallim T.Q., Wang Z., Shih D.M., Meng Y., et al. Trimethylamine-N-oxide, a metabolite associated with atherosclerosis, exhibits complex genetic and dietary regulation. Cell Metab. 2013;17:49–60. doi: 10.1016/j.cmet.2012.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Pols T.W., Nomura M., Harach T., Sasso G.L., Oosterveer M.H., et al. TGR5 activation inhibits atherosclerosis by reducing macrophage inflammation and lipid loading. Cell Metab. 2011;14:747–757. doi: 10.1016/j.cmet.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhou C., King N., Chen K.Y., Breslow J.L. Activation of PXR induces hypercholesterolemia in wild-type and accelerates atherosclerosis in apoE deficient mice [S] J Lipid Res. 2009;50:2004–2013. doi: 10.1194/jlr.M800608-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sui Y., Xu J., Rios-Pilier J., Zhou C. Deficiency of PXR decreases atherosclerosis in apoE-deficient mice. J Lipid Res. 2011;52:1652–1659. doi: 10.1194/jlr.M017376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mayerhofer C.C., Ueland T., Broch K., Vincent R.P., Cross G.F., et al. Increased secondary/primary bile acid ratio in chronic heart failure. J Cardiac Fail. 2017;23:666–671. doi: 10.1016/j.cardfail.2017.06.007. [DOI] [PubMed] [Google Scholar]
- 131.Li W., Shu S., Cheng L., Hao X., Wang L., et al. Fasting serum total bile acid level is associated with coronary artery disease, myocardial infarction and severity of coronary lesions. Atherosclerosis. 2020;292:193–200. doi: 10.1016/j.atherosclerosis.2019.11.026. [DOI] [PubMed] [Google Scholar]
- 132.Canfora E.E., Jocken J.W., Blaak E.E. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat Rev Endocrinol. 2015;11:577–591. doi: 10.1038/nrendo.2015.128. [DOI] [PubMed] [Google Scholar]
- 133.Koh A., De Vadder F., Kovatcheva-Datchary P., Bäckhed F. From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell. 2016;165:1332–1345. doi: 10.1016/j.cell.2016.05.041. [DOI] [PubMed] [Google Scholar]
- 134.Nogal A., Valdes A.M., Menni C. The role of short-chain fatty acids in the interplay between gut microbiota and diet in cardio-metabolic health. Gut Microbes. 2021;13:1–24. doi: 10.1080/19490976.2021.1897212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Marques F.Z., Nelson E., Chu P.-Y., Horlock D., Fiedler A., et al. High-fiber diet and acetate supplementation change the gut microbiota and prevent the development of hypertension and heart failure in hypertensive mice. Circulation. 2017;135:964–977. doi: 10.1161/CIRCULATIONAHA.116.024545. [DOI] [PubMed] [Google Scholar]
- 136.Bartolomaeus H., Balogh A., Yakoub M., Homann S., Markó L., et al. Short-chain fatty acid propionate protects from hypertensive cardiovascular damage. Circulation. 2019;139:1407–1421. doi: 10.1161/CIRCULATIONAHA.118.036652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kasahara K., Krautkramer K.A., Org E., Romano K.A., Kerby R.L., et al. Interactions between Roseburia intestinalis and diet modulate atherogenesis in a murine model. Nat Microbiol. 2018;3:1461–1471. doi: 10.1038/s41564-018-0272-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Pluznick J.L., Protzko R.J., Gevorgyan H., Peterlin Z., Sipos A., et al. Olfactory receptor responding to gut microbiota-derived signals plays a role in renin secretion and blood pressure regulation. Proc Natl Acad Sci. 2013;110:4410–4415. doi: 10.1073/pnas.1215927110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Natarajan N., Hori D., Flavahan S., Steppan J., Flavahan N.A., et al. Microbial short chain fatty acid metabolites lower blood pressure via endothelial G protein-coupled receptor 41. Physiol Genomics. 2016;48:826–834. doi: 10.1152/physiolgenomics.00089.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Robles-Vera I., Toral M., de la Visitación N., Aguilera-Sánchez N., Redondo J.M., et al. Protective effects of short-chain fatty acids on endothelial dysfunction induced by angiotensin II. Front Physiol. 2020;11:277. doi: 10.3389/fphys.2020.00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Whelton S.P., Hyre A.D., Pedersen B., Yi Y., Whelton P.K., et al. Effect of dietary fiber intake on blood pressure: a meta-analysis of randomized, controlled clinical trials. LWW. 2005 doi: 10.1097/01.hjh.0000160199.51158.cf. [DOI] [PubMed] [Google Scholar]
- 142.Khan K., Jovanovski E., Ho H., Marques A., Zurbau A., et al. The effect of viscous soluble fiber on blood pressure: A systematic review and meta-analysis of randomized controlled trials. Nutr Metabol Cardiovas Dis. 2018;28:3–13. doi: 10.1016/j.numecd.2017.09.007. [DOI] [PubMed] [Google Scholar]
- 143.Mueller N.T., Zhang M., Juraschek S.P., Miller E.R., Appel L.J. Effects of high-fiber diets enriched with carbohydrate, protein, or unsaturated fat on circulating short chain fatty acids: results from the OmniHeart randomized trial. Am J Clin Nutr. 2020;111:545–554. doi: 10.1093/ajcn/nqz322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Dodd D., Spitzer M.H., Van Treuren W., Merrill B.D., Hryckowian A.J., et al. A gut bacterial pathway metabolizes aromatic amino acids into nine circulating metabolites. Nature. 2017;551:648–652. doi: 10.1038/nature24661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Tessari P., Lante A., Mosca G. Essential amino acids: master regulators of nutrition and environmental footprint? Sci Rep. 2016;6:1–13. doi: 10.1038/srep26074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Nemet I., Saha P.P., Gupta N., Zhu W., Romano K.A., et al. A cardiovascular disease-linked gut microbial metabolite acts via adrenergic receptors. Cell. 2020;180 doi: 10.1016/j.cell.2020.02.016. 862-877. e822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Yu F., Feng X., Li X., Luo Y., Wei M., et al. Gut-derived metabolite phenylacetylglutamine and white matter hyperintensities in patients with acute ischemic stroke. Front Aging Neurosci. 2021;13 doi: 10.3389/fnagi.2021.675158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ottosson F., Brunkwall L., Smith E., Orho-Melander M., Nilsson P.M., et al. The gut microbiota-related metabolite phenylacetylglutamine associates with increased risk of incident coronary artery disease. J Hypertens. 2020;38:2427–2434. doi: 10.1097/HJH.0000000000002569. [DOI] [PubMed] [Google Scholar]
- 149.Lin C.J., Liu H.L., Pan C.F., Chuang C.K., Jayakumar T., et al. Indoxyl sulfate predicts cardiovascular disease and renal function deterioration in advanced chronic kidney disease. Arch Med Res. 2012;43:451–456. doi: 10.1016/j.arcmed.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 150.Glorieux G., Vanholder R., Van Biesen W., Pletinck A., Schepers E., et al. Free p-cresyl sulfate shows the highest association with cardiovascular outcome in chronic kidney disease. Nephrol Dial Transplant. 2021;36:998–1005. doi: 10.1093/ndt/gfab004. [DOI] [PubMed] [Google Scholar]
- 151.Nguyen C., Edgley A.J., Kelly D.J., Kompa A.R. Aryl hydrocarbon receptor inhibition restores indoxyl sulfate-mediated endothelial dysfunction in rat aortic rings. Toxins (Basel) 2022;14 doi: 10.3390/toxins14020100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Vanholder R., Schepers E., Pletinck A., Nagler E.V., Glorieux G. The uremic toxicity of indoxyl sulfate and p-cresyl sulfate: a systematic review. J Am Soc Nephrol. 2014;25:1897–1907. doi: 10.1681/ASN.2013101062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Cason C.A., Dolan K.T., Sharma G., Tao M., Kulkarni R., et al. Plasma microbiome-modulated indole-and phenyl-derived metabolites associate with advanced atherosclerosis and postoperative outcomes. J Vasc Surg. 2018;68 doi: 10.1016/j.jvs.2017.09.029. 1552-1562. e1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Shafi T., Sirich T.L., Meyer T.W., Hostetter T.H., Plummer N.S., et al. Results of the HEMO Study suggest that p-cresol sulfate and indoxyl sulfate are not associated with cardiovascular outcomes. Kidney Int. 2017;92:1484–1492. doi: 10.1016/j.kint.2017.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Shi H., Zhang B., Abo-Hamzy T., Nelson J.W., Ambati C.S.R., et al. Restructuring the gut microbiota by intermittent fasting lowers blood pressure. Circ Res. 2021;128:1240–1254. doi: 10.1161/CIRCRESAHA.120.318155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Liyanage T., Ninomiya T., Wang A., Neal B., Jun M., et al. Effects of the Mediterranean diet on cardiovascular outcomes—a systematic review and meta-analysis. PLoS ONE. 2016;11 doi: 10.1371/journal.pone.0159252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Estruch R., Ros E., Salas-Salvado J., Covas M.I., Corella D., et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med. 2013;368:1279–1290. doi: 10.1056/NEJMoa1200303. [DOI] [PubMed] [Google Scholar]
- 158.Nagpal R., Shively C.A., Register T.C., Craft S., Yadav H. Gut microbiome-Mediterranean diet interactions in improving host health. F1000Research. 2019;8 doi: 10.12688/f1000research.18992.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Vázquez-Fresno R., Llorach R., Urpi-Sarda M., Lupianez-Barbero A., Estruch R., et al. Metabolomic pattern analysis after mediterranean diet intervention in a nondiabetic population: a 1-and 3-year follow-up in the PREDIMED study. J Proteome Res. 2015;14:531–540. doi: 10.1021/pr5007894. [DOI] [PubMed] [Google Scholar]
- 160.Pastori D., Carnevale R., Nocella C., Novo M., Santulli M., et al. Gut-derived serum lipopolysaccharide is associated with enhanced risk of major adverse cardiovascular events in atrial fibrillation: Effect of adherence to mediterranean diet. J Am Heart Assoc. 2017;6 doi: 10.1161/JAHA.117.005784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wang D.D., Nguyen L.H., Li Y., Yan Y., Ma W., et al. The gut microbiome modulates the protective association between a Mediterranean diet and cardiometabolic disease risk. Nat Med. 2021;27:333–343. doi: 10.1038/s41591-020-01223-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Siervo M., Lara J., Chowdhury S., Ashor A., Oggioni C., et al. Effects of the Dietary Approach to Stop Hypertension (DASH) diet on cardiovascular risk factors: a systematic review and meta-analysis. Br J Nutr. 2015;113:1–15. doi: 10.1017/S0007114514003341. [DOI] [PubMed] [Google Scholar]
- 163.Guo Y., Luo S., Ye Y., Yin S., Fan J., et al. Intermittent fasting improves cardiometabolic risk factors and alters gut microbiota in metabolic syndrome patients. J Clin Endocrinol Metab. 2021;106:64–79. doi: 10.1210/clinem/dgaa644. [DOI] [PubMed] [Google Scholar]
- 164.Maifeld A., Bartolomaeus H., Lober U., Avery E.G., Steckhan N., et al. Fasting alters the gut microbiome reducing blood pressure and body weight in metabolic syndrome patients. Nat Commun. 2021;12:1970. doi: 10.1038/s41467-021-22097-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Man A.W., Xia N., Daiber A., Li H. The roles of gut microbiota and circadian rhythm in the cardiovascular protective effects of polyphenols. Br J Pharmacol. 2020;177:1278–1293. doi: 10.1111/bph.14850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Chen M-l, Yi L., Zhang Y., Zhou X., Ran L., et al. Resveratrol attenuates trimethylamine-N-oxide (TMAO)-induced atherosclerosis by regulating TMAO synthesis and bile acid metabolism via remodeling of the gut microbiota. MBio. 2016;7:e02210–02215. doi: 10.1128/mBio.02210-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Nie J., Zhang L., Zhao G., Du X. Quercetin reduces atherosclerotic lesions by altering the gut microbiota and reducing atherogenic lipid metabolites. J Appl Microbiol. 2019;127:1824–1834. doi: 10.1111/jam.14441. [DOI] [PubMed] [Google Scholar]
- 168.Vetrani C., Maukonen J., Bozzetto L., Della Pepa G., Vitale M., et al. Diets naturally rich in polyphenols and/or long-chain n-3 polyunsaturated fatty acids differently affect microbiota composition in high-cardiometabolic-risk individuals. Acta Diabetol. 2020;57:853–860. doi: 10.1007/s00592-020-01494-9. [DOI] [PubMed] [Google Scholar]
- 169.Makki K., Deehan E.C., Walter J., Bäckhed F. The impact of dietary fiber on gut microbiota in host health and disease. Cell Host Microbe. 2018;23:705–715. doi: 10.1016/j.chom.2018.05.012. [DOI] [PubMed] [Google Scholar]
- 170.Han J., Zhang R., Muheyati D., Lv M.X., Aikebaier W., et al. The effect of chickpea dietary fiber on lipid metabolism and gut microbiota in high-fat diet-induced hyperlipidemia in rats. J Med Food. 2021;24:124–134. doi: 10.1089/jmf.2020.4800. [DOI] [PubMed] [Google Scholar]
- 171.Zhou A.L., Hergert N., Rompato G., Lefevre M. Whole grain oats improve insulin sensitivity and plasma cholesterol profile and modify gut microbiota composition in C57BL/6J mice. J Nutr. 2015;145:222–230. doi: 10.3945/jn.114.199778. [DOI] [PubMed] [Google Scholar]
- 172.Tosh S.M., Bordenave N. Emerging science on benefits of whole grain oat and barley and their soluble dietary fibers for heart health, glycemic response, and gut microbiota. Nutr Rev. 2020;78:13–20. doi: 10.1093/nutrit/nuz085. [DOI] [PubMed] [Google Scholar]
- 173.Eriksen A.K., Brunius C., Mazidi M., Hellström P.M., Risérus U., et al. Effects of whole-grain wheat, rye, and lignan supplementation on cardiometabolic risk factors in men with metabolic syndrome: a randomized crossover trial. Am J Clin Nutr. 2020;111:864–876. doi: 10.1093/ajcn/nqaa026. [DOI] [PubMed] [Google Scholar]
- 174.Oniszczuk A., Oniszczuk T., Gancarz M., Szymańska J. Role of gut microbiota, probiotics and prebiotics in the cardiovascular diseases. Molecules. 2021;26:1172. doi: 10.3390/molecules26041172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Holscher H.D. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes. 2017;8:172–184. doi: 10.1080/19490976.2017.1290756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Parnell J.A., Reimer R.A. Effect of prebiotic fibre supplementation on hepatic gene expression and serum lipids: a dose–response study in JCR: LA-cp rats. Br J Nutr. 2010;103:1577–1584. doi: 10.1017/S0007114509993539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Rault-Nania M.-H., Gueux E., Demougeot C., Demigné C., Rock E., et al. Inulin attenuates atherosclerosis in apolipoprotein E-deficient mice. Br J Nutr. 2006;96:840–844. doi: 10.1017/bjn20061913. [DOI] [PubMed] [Google Scholar]
- 178.Tiwari U., Cummins E. Meta-analysis of the effect of β-glucan intake on blood cholesterol and glucose levels. Nutrition. 2011;27:1008–1016. doi: 10.1016/j.nut.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 179.Cani P.D., Neyrinck A.M., Fava F., Knauf C., Burcelin R.G., et al. Selective increases of bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetologia. 2007;50:2374–2383. doi: 10.1007/s00125-007-0791-0. [DOI] [PubMed] [Google Scholar]
- 180.Kaye D.M., Shihata W.A., Jama H.A., Tsyganov K., Ziemann M., et al. Deficiency of prebiotic fiber and insufficient signaling through gut metabolite-sensing receptors leads to cardiovascular disease. Circulation. 2020;141:1393–1403. doi: 10.1161/CIRCULATIONAHA.119.043081. [DOI] [PubMed] [Google Scholar]
- 181.Malik M., Suboc T.M., Tyagi S., Salzman N., Wang J., et al. Lactobacillus plantarum 299v supplementation improves vascular endothelial function and reduces inflammatory biomarkers in men with stable coronary artery disease. Circ Res. 2018;123:1091–1102. doi: 10.1161/CIRCRESAHA.118.313565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Robles-Vera I., Toral M., de la Visitacion N., Sanchez M., Romero M., et al. The probiotic Lactobacillus fermentum prevents dysbiosis and vascular oxidative stress in rats with hypertension induced by chronic nitric oxide blockade. Mol Nutr Food Res. 2018;62 doi: 10.1002/mnfr.201800298. [DOI] [PubMed] [Google Scholar]
- 183.Ejtahed H.S., Mohtadi-Nia J., Homayouni-Rad A., Niafar M., Asghari-Jafarabadi M., et al. Probiotic yogurt improves antioxidant status in type 2 diabetic patients. Nutrition. 2012;28:539–543. doi: 10.1016/j.nut.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 184.Al-Sheraji S., Amin I., Azlan A., Manap M., Hassan F. Effects of Bifidobacterium longum BB536 on lipid profile and histopathological changes in hypercholesterolaemic rats. Beneficial Microbes. 2015;6:661–668. doi: 10.3920/BM2014.0032. [DOI] [PubMed] [Google Scholar]
- 185.Tenorio-Jiménez C., Martínez-Ramírez M.J., Tercero-Lozano M., Arraiza-Irigoyen C., Del Castillo-Codes I., et al. Evaluation of the effect of Lactobacillus reuteri V3401 on biomarkers of inflammation, cardiovascular risk and liver steatosis in obese adults with metabolic syndrome: a randomized clinical trial (PROSIR) BMC Complementary Alternative Med. 2018;18:1–8. doi: 10.1186/s12906-018-2371-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Wu M., Yang S., Wang S., Cao Y., Zhao R., et al. Effect of berberine on atherosclerosis and gut microbiota modulation and their correlation in high-fat diet-fed ApoE−/− mice. Front Pharmacol. 2020;11:223. doi: 10.3389/fphar.2020.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Li X., Su C., Jiang Z., Yang Y., Zhang Y., et al. Berberine attenuates choline-induced atherosclerosis by inhibiting trimethylamine and trimethylamine-N-oxide production via manipulating the gut microbiome. NPJ Biofilms Microbiomes. 2021;7:1–14. doi: 10.1038/s41522-021-00205-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Yang H, Pan R, Wang J, Zheng L, Li Z, et al. (2020) Modulation of the gut microbiota and liver transcriptome by red yeast rice and monascus pigment fermented by purple monascus SHM1105 in rats fed with a high-fat diet. Front Pharmacol 11. [DOI] [PMC free article] [PubMed]
- 189.Chang C.-J., Lin C.-S., Lu C.-C., Martel J., Ko Y.-F., et al. Ganoderma lucidum reduces obesity in mice by modulating the composition of the gut microbiota. Nat Commun. 2015;6:1–19. doi: 10.1038/ncomms8489. [DOI] [PMC free article] [PubMed] [Google Scholar]