Graphical abstract
Keywords: PBPs, Protein structure, Bioactive molecules, Biotechnology
Abstract
Phycobiliproteins (PBPs) are fluorescent proteins of various colors, including fuchsia, purple-blue and cyan, that allow the capture of light energy in auxiliary photosynthetic complexes called phycobilisomes (PBS). PBPs have several highly preserved structural and physicochemical characteristics. In the PBS context, PBPs function is capture luminous energy in the 450–650 nm range and delivers it to photosystems allowing photosynthesis take place. Besides the energy harvesting function, PBPs also have shown to have multiple biological activities, including antioxidant, antibacterial and antitumours, making them an interesting focus for different biotechnological applications in areas like biomedicine, bioenergy and scientific research. Nowadays, the main sources of PBPs are cyanobacteria and micro and macro algae from the phylum Rhodophyta. Due to the diverse biological activities of PBPs, they have attracted the attention of different industries, such as food, biomedical and cosmetics. This is why a large number of patents related to the production, extraction, purification of PBPs and their application as cosmetics, biopharmaceuticals or diagnostic applications have been generated, looking less ecological impact in the natural prairies of macroalgae and less culture time or higher productivity in cyanobacteria to satisfy the markets and applications that require high amounts of these molecules. In this review, we summarize the main structural characteristics of PBPs, their biosynthesys and biotechnological applications. We also address current trends and future perspectives of the PBPs market.
1. Introduction
The largest source of energy on our planet comes from the sun in the form of light, in form of indivisible particles called photons. Another way to define light is electromagnetic energy, which can be detected by the human eye at ranges from 400 nm to 700 nm, approximately. This range is used by several organisms to perform photosynthesis. [1]
Photosynthesis is a biological oxide-reduction process, in which chemical energy is obtained from inorganic compounds as a substrate and the electromagnetic energy of light, captured using specialized pigments. The energy captured by these pigments is transformed and stored in different compounds, mainly carbohydrates, as chemical energy [2].
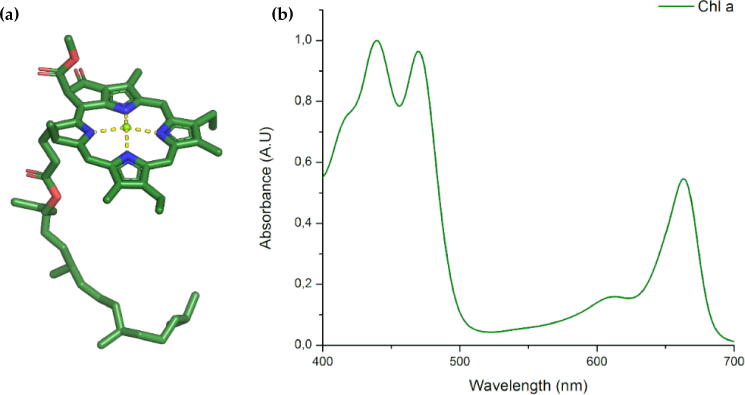
Chlorophyll (Chl), the main photosynthetic pigment, is associated with multiprotein complexes known as reaction centers of photosystems [3], [4]. Chl is made up of a porphyrin ring formed by four pyrrolic rings. It also has a magnesium ion associated with the pyrrolic rings through coordinate covalent bonds and a hydrocarbon chain known as phytol (Fig. 1A) [5], [6]. All photosynthetic organisms contains Chlorophyll a, since they all derived from an common ancestor [7]. To date, six different types of chlorophylls have been described (a, b, c1, c2, d, and f), varying in the radical groups attached to the carbon atoms of their porphyrin ring resulting in different spectrums of absorption between the different chlorophylls. All different Chls can efficiently absorb light energy between 400 and 500 nm and between 650 and 720 nm. Despite the great variety of Chls, they all have poor absorption of wavelength light energy in the range of 500 to 650 nm, a gap zone commonly known as the “Green Gap” (Fig. 1B) [4], [8], [9], [10].
Fig. 1.
(a) Molecular structure of chlorophyll a. (b) Absorption spectrum of Chlorophyll a from Agarophyton chilense. (Chlorphyll a molecule created with pymol from PDB:5B66. Absorption spectrum of Chl a made by the authors, unpublished data).
About 45% of photosynthesis on Earth is carried out in aquatic environments [7], mainly by microalgae. However, in these environments, the irradiance and spectral distribution of light are not uniform due to a series of physical phenomena such as reflection, absorption, and scattering [1]. Organisms such as cyanobacteria and red algae have auxiliary photosynthetic complexes that allow to colonize environments where solar energy is not optimal to carry out photosynthesis. This complexeses make it possible to capture green and yellow light (500 nm-600 nm) and later transfer it to Chl molecules, allowing these organisms to carry out photosynthesis and populate ecological niches that could not be colonized by organisms that only have chlorophyll as their main photosynthetic pigment. Among these light collecting accessory systems are phycobilisomes.
The phycobilisome (PBS) is a multiprotein complex, which efficiently captures light energy in the wavelength range of 450–650 nm, transferring it in a unidirectional way within its structure and delivering it to the Chl molecules present in photosystems [11]. The major component of phycobilisomes are phycobiliproteins (PBPs) (85%) and the remaining 15% corresponds to linker proteins [12]. PBPs are responsible for capturing and transferring light energy and linkers allow the correct assembly of the PBS and modulate energy transfer [13], [14], [15], [16], [17], [18].
Electron microscopy experiments have shown that PBS possess 2 clearly defined substructures: the rods and the core [19], [20], [21]. The rods are composed of two or three PBPs, namely phycoerythrin (PE) and phycoerythrocyanin (PEC), these being the most distal PBPs from the core. The rods are also composed of phycocyanin (PC), which is the phycobiliprotein closest to the core. However, PE and PEC are not always part of the rods of the phycobilisomes. For examplo, there are some species of cyanobacteria, such as Spirulina platensis, whose rods only have PC and protein linkers that remain associated with the core of the phycobilisome [15]. The core is formed by allophycocyanin (APC), together with linker proteins. This core transfers the energy captured by the rods to the chlorophyll present in the thylakoid membrane [22].
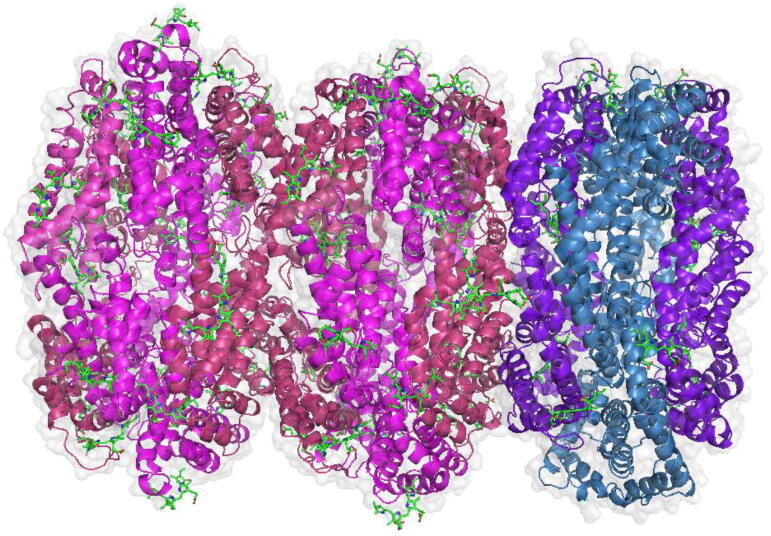
In 2020 and 2021, the structure of the phycobilisomes of Porphyridium purpureum [17], Anabaena sp. PCC 7120 and Synechococcus sp. PCC 7002 [18] were reported. These structures provided new information on energy transfer within this complex. The PBS of Porphyridium purpureum is composed by more than 860 protein molecules (Fig. 2) and more than 2000 chromophores, forming a large complex with dimensions of approximately 680 Å wide, 350 Å height, and 450 Å deep. Its estimated mass has been reported to be close to 16.8 MDa [23], [24].
Fig. 2.
Structure of Porphyridium purpureum phycobilisome. In pink and light pink PE are shown. In Purple and light purple PC are shown. In turqoise and light turqoise APC are shown. (Phycobilisome image created with pymol from PDB: 6xwk). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
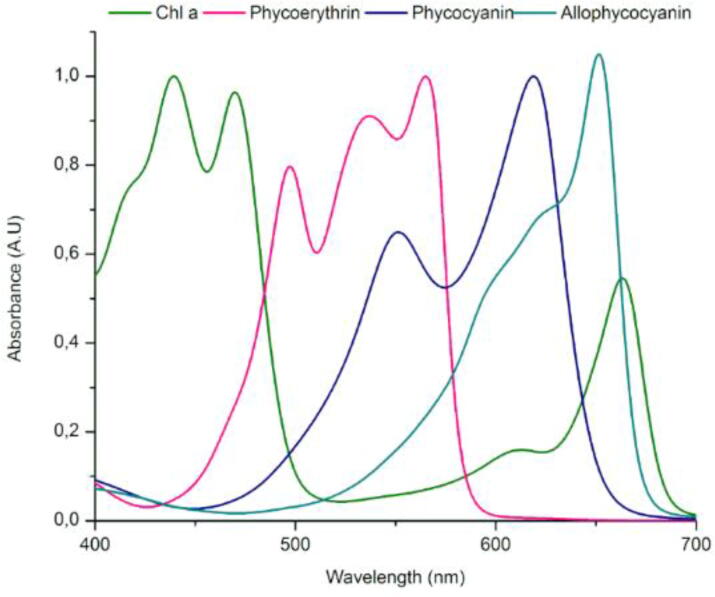
PBPs are fluorescent hydrophilic proteins, characterized by having several chromophores called phycobilins attached to their polypeptide structure. Phycobilins consist of open chain tetrapyrroles derived from the heme group [25]. The phycobilins can have 64 different isomers which differ in configurations (Z/E) and conformations [sys (s)/anti (a)] [26], but the most common isomers in PBPs are ZZZ-ssa and ZZZ-asa isomers [17]. Phycobilins are covalently bonded to specific and highly preserved cysteine residues, which give PBPs their spectroscopic characteristics. There are four phycobilins, each showing different colors: Phycocyanobilin (PCB) is blue; phycoviolobilin (PVB) is violet, phycoerythroblin (PEB) is red and phycourobilin (PUB) is yellow [27]. Each phycobilin possess a system of conjugated double bonds that give it a spectroscopic characteristic. This characteristic depends on the number of conjugated double bonds that each phycobilin possesses, the greater the number of conjugated double bonds, the greater the shift in the absorption maximum towards red. The PCB molecule has nine conjugated double bonds, PVB has eight, PEB has six, and finally, PUB has five conjugated double bonds [28]. The phycobilins present in the PBPs are responsible for the absorption of light energy in the PBS, in the range between 500 and 650 nm. (Fig. 3).
Fig. 3.
Absorption spectrum of PBPs from rhodophytes. PE spectrum ref [29]. PC spectrum ref [30]. APC spectrum ref [31]
The precise relationship in the absorption and emission spectrum of PBPs makes this light-collecting complex one of the most efficient in nature [23], [32]. Recent studies related to PBS structure have helped to explain the high efficiency of energy transfer in this complex [17], [18], these studies demonstrate that it is not only necessary to know the type and number of chromophores present in the PBS, but also the structural organization or the architecture of its components, as they play ab important role. Another important factor in the energy transfer in the PBS is the geometry and spectroscopic properties of chromophores, together with the protein environment.
All PBPs share a series of very similar structural and functional characteristics. Knowing the structure features of PBPs, as the main component of PBS, is of high relevance in order to understand their biological activities and potential biotechnological uses. Several studies have reported noumerous bioactive properties of PBPs, including antioxidant, anticancer, antimicrobial and anti-inflammatory activity. All these properties have contributed to a growing interest in PBPs in the pharmaceutical, food and cosmetic industries. In this context, the aim of this article is to provide an overview of the structural characteristics of PBPs, their biosynthesis and their spectroscopic propierties and summarize PBPs biotechnological applications in the form of patents related to extraction, production, purification. Finally, future perspectives and food industry applications are briefly emphasized.
2. Structural characteristics of PBPs
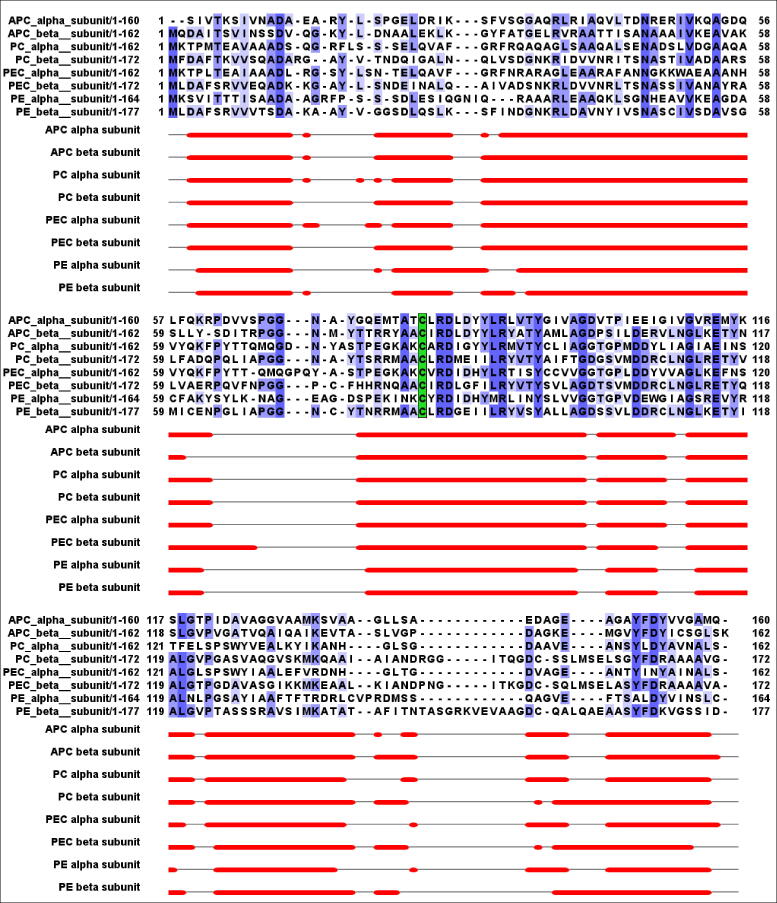
To date, there are sixty-one phycobiliprotein structures deposited in the Protein Data Bank (PDB). Of these, twelve structures correspond to PE; three are from phycoerythrocyanin; thirty-six structures are phycocyanin and ten are allophycocyanin. All these structures have been obtained by means of the x-ray diffraction technique and most of them belong to cyanobacteria and red algae. All this proteins structures shows that PBPs are composed of two subunits, consisting of two polypeptide chains known as α and β. These subunits show similar basic structures, with 6–8 helical zones separated by loops and a similar globulin-like fold [33]. Functionally, each subunit can have one to three phycobilin molecules attached to the polypeptide skeleton in highly preserved cysteine residues (50, 60, 81 and 150), but Cys 81 residue always shows a bonded phycobilin [34]. Fig. 4 shows a structural aligment of APC (PDB: 6YX7), PC (PDB: 6XWK), PEC (PDB: 2C7L) and PE (PDB: 1B8D) and with their respectives secondary structure elements of each PBP. From this alignment, the cysteine residue which is always chromophorylated is highlighted in green. Along with the above, the alpha helices of each of the subunits of the different PBPs are shown as red cylinders. The number of the α-helices of each structure was obtained from the information reported in the PDB by the authors of the respective crystallographic structures. The structural alignment was done with the PDBeFold server and the visualization of the alignment was done with Jalview. The selection of the structures used in this alignment was made based on the criterion of best resolution of the crystallographic structure.
Fig. 4.
Structural aligment of α and β subunits of APC, PC, PEC and PE. Green residue shows the cysteine residue which is always chrmoforilated. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
The analysis of amino acid sequences and the structures of different phycobiliprotein subunits suggests that they come from a common ancestor and that the duplication of the ancestral gene gave rise to the α and β subunits of the different phycobiliproteins [33]. Despite the low similarity of the subunit sequences, there are a series of residues that remained unchanged, as they play a critical role in the structure or function of the phycobiliprotein, such as the chromophore binding site and residues of the interface surface between the α and β subunits [33].
From a physicochemical point of view, the subunits of the different PBPs have molecular weights that range between 16.000 and 18.000 Dalton and with an isoelectric point close to 6. α subunits are composed of 161–167 residues, while β subunits are composed by 161–177 residues, depending on the type of phycobiliprotein (Table 1).
Table 1.
Physicochemical, sequence comparision and biological assembly information of PBPs present in the protein data bank (PDB). CB means Cyanobacteria, RA means Red algae. In bold are show the structure used for the sequence and RMSD analysis.
| Phycobiliprotein | Specie | Subunit | Residues | Molecular Mass (Da) | Isoelectric Point | % Sequence Identity/amino acids similarity | RMSD (Å) | Biological assembly | PDB code |
|---|---|---|---|---|---|---|---|---|---|
| Phyco erythrin | Gloeobacter Violaceus (CB) | α | 164 | 17658.04 | 6.73 | 23,7/52,5 | 2, 18 | (αβ)6 | 2VJH |
| β | 177 | 18427.16 | 7.52 | ||||||
| Phormidium rubidum A09DM (CB) | α | 164 | 17643.87 | 5.84 | 25/44,5 | 2,06 | (αβ)6 | 5NB4, 5NB3, 5AQD, 5FVB | |
| β | 184 | 19336.16 | 6.57 | ||||||
| Agarophyton chilense (RA) | α | 164 | 17751.88 | 5.42 | 27/48,6 | 2, 13 | (αβ)6 | 1EYX | |
| β | 177 | 18604.18 | 5.15 | ||||||
| Griffithsia monilis (RA) | α | 164 | 17668.86 | 5.69 | 24,5/46,8 | 2, 13 | (αβ)6 | 1B8D | |
| β | 177 | 18481.94 | 5.10 | ||||||
| Palmaria palmata (RA) | α | 164 | 17638.79 | 5.40 | 24,6/47,6 | 2, 14 | (αβ)6 | 5B13 | |
| β | 177 | 18408.00 | 5.42 | ||||||
| Polysiphonia urceolata (RA) | α | 164 | 17836.07 | 5.70 | 25/50,5 | 2, 14 | (αβ)6 | 1LIA, 1F99 | |
| β | 177 | 18721.38 | 5.15 | ||||||
| Porphyridium cruentum (RA) | α | 164 | 17805.05 | 5.42 | 27,2/51,1 | 2, 12 | (αβ)3 | 3 V57, 3 V58 | |
| β | 177 | 18554.18 | 5.43 | ||||||
| Phyco Erythro cyanin | Mastigocladus laminosus (CB) | α | 162 | 17563.74 | 6.82 | 27,5/44,4 | 2,00 | (αβ)3 | 2C7L, 2C7K, 2C7J |
| β | 172 | 18486.95 | 6.72 | ||||||
| Phyco cyanin | Acaryochloris marina (CB) | α | 162 | 17375.43 | 5.28 | 26,9/52,6 | 2,01 | (αβ)3 | 5OOK |
| β | 172 | 18022.41 | 4.94 | ||||||
| Cyanidium caldarium (CB) | α | 162 | 17505.66 | 5.81 | 27,6/54 | 1,91 | (αβ)6 | 1PHN | |
| β | 172 | 18252.74 | 4.96 | ||||||
| Gloeobacter Violaceus (CB) | α | 162 | 17662.90 | 7.74 | 23,7/53,2 | 2,03 | (αβ)6 | 2VML, 2VJR | |
| β | 172 | 18459.99 | 5.46 | ||||||
| Leptolyngbya sp (CB) | α | 162 | 17585.87 | 5.83 | 24,6/45 | 1,78 | (αβ)6 | 4L1E, 2UUN | |
| β | 172 | 18066.54 | 4.80 | ||||||
| Microchaete diplosiphon (CB) | α | 162 | 17228.34 | 6.56 | 24,6/52,6 | 1,66 | (αβ)6 | 1CPC | |
| β | 172 | 17920.38 | 5.10 | ||||||
| Phormidium rubidum A09DM (CB) | α | 162 | 17308.34 | 4.64 | 24,7/52,2 | 1,88 | (αβ)6 | 6XWK, 2UUL | |
| β | 172 | 18124.49 | 5.35 | ||||||
| Pseudanabaena sp (CB) | α | 162 | 17222.29 | 5.79 | 22,9/51,4 | 1,82 | (αβ)6 | 5TOU | |
| β | 172 | 17842.27 | 5.40 | ||||||
| Arthrospira platensis (CB) | α | 162 | 17601.87 | 5.83 | 25,7/45,5 | 1,82 | (αβ)6 | 1HA7,1GH0, 2UUM | |
| β | 172 | 18093.57 | 4.96 | ||||||
| Synechococcus elongatus (CB) | α | 162 | 17442.70 | 5.36 | 27/52,3 | 1,77 | (αβ)6 | 3L0F, 4ZIZ, 4Q70, 5MJP, 5MJM, 5O7M, 4Z8K, 5MJQ, 5UVK, 4H0M, 1JBO | |
| β | 172 | 18186.67 | 5.12 | ||||||
| Synechocystis sp. PCC 6803 (CB) | α | 162 | 17586.60 | 5.35 | 26,9/49,7 | 2,07 | (αβ)3 | 4F0T | |
| β | 172 | 18126.47 | 4.98 | ||||||
| Thermo synechococcus vulcanus (CB) | α | 162 | 17456.72 | 5.36 | 26,4/52,3 | 1,74 | (αβ)3 | 3O18, 3O2C, 1KTP, 4N6S, 4GY3, 1I7Y, 1ON7, 4GXE | |
| β | 172 | 18186.67 | 5.12 | ||||||
| Agarophyton chilense (RA) | α | 162 | 17576.64 | 5.15 | 25,4/53,7 | 1,90 | (αβ)6 | 2BV8 | |
| β | 172 | 18162.25 | 4.65 | ||||||
| Galdieria Sulphuraria (RA) | α | 162 | 17506.65 | 6.57 | 27,6/54 | 1,84 | (αβ)6 | 3KVS | |
| β | 172 | 18224.69 | 4.96 | ||||||
| Polysiphonia urceolata (RA) | α | 162 | 17569.78 | 5.81 | 27,5/56,7 | 1,85 | (αβ)6 | 1F99 | |
| β | 172 | 18000.54 | 4.92 | ||||||
| Allophycocyanin | Arthrospira platensis (CB) | α | 161 | 17220.67 | 5.07 | 37/64,2 | 1,48 | (αβ)3 | 1ALL |
| β | 161 | 17243.70 | 7.67 | ||||||
| Phormidium rubidum A09DM (CB) | α | 161 | 17475.92 | 4.85 | 34,6/62,3 | 1,53 | (αβ)3 | 4RMP | |
| β | 161 | 17408.82 | 5.15 | ||||||
| Gloeobacter Violaceus (CB) | α | 161 | 17522.09 | 4.92 | 38,3/66 | 1,41 | (αβ)3 | 2VJT | |
| β | 161 | 17211.69 | 6.24 | ||||||
| Synechocystis sp. PCC 6803 (CB) | αB | 161 | 17923.45 | 5.44 | 33,7/60,7 | 1,53 | (αβ)3 | 4PO5 | |
| β | 161 | 17215.64 | 5.43 | ||||||
| Mastigocladus laminosus (CB) | α | 161 | 17252.63 | 4.90 | 38,3/65,4 | 1,77 | (αβ)3 | 1B33 | |
| β | 161 | 17373.84 | 5.45 | ||||||
| Synechococcus elongatus (CB) | α | 161 | 17421.76 | 4.79 | 38/67,5 | 1,57 | (αβ)3 | 4F0U | |
| β | 161 | 17392.77 | 5.45 | ||||||
| Thermosynechococcus vulcanus (CB) | α | 161 | 17538.96 | 4.85 | 39,5/67,9 | 1,38 | (αβ)3 | 3DBJ, 2V8A | |
| β | 161 | 17358.89 | 5.45 | ||||||
| Agarophyton chilense (RA) | α | 161 | 17519.01 | 4.90 | 38,3/63,6 | 1,42 | (αβ)3 | 5TJF | |
| β | 161 | 17471.82 | 5.10 | ||||||
| Porphyra yezoensis (RA) | α | 161 | 17496.96 | 5.09 | 35,8/62,3 | 1,47 | (αβ)6 | 1KN1 | |
| β | 161 | 17443.89 | 5.45 |
The sequence analysis of α and β subunits shows a identity that ranges between 30% − 40% and data of structural alignments between the main chains of both subunits, showing an RMSD value of less than 2 Å (Table 1). Although the level of sequence identity between the α and β subunits of the PBPs is relatively low, both subunits possess nearly identical structure (Fig. 4). It can be seen that the RMSD value between the α and β subunits of APC is lower when compared to the other PBPs. The sequence identity, similarity and RMSD values between the analyzed α and β subunits were obtained with the Lalign and PDBeFold servers, respectively.
From the analysis of the structures of the PBPs present in the PDB, it can be noted that those PBPs that are part of the PBS rod, PE and PC, have hexameric-type oligomers, this being the biological assembly in charge of the protein function (Table 1). On the other hand, APC, the only PBP present in the core of the PBS, possess a trimeric biological assembly. Table 1 shows data from biological assembly of PE, PEC, PC, and APC from structures deposited in the PDB.
2.1. Subunits and heterodimers (αβ)
The basic component of PBPs are subunits. Both α and β subunits belong to all-alpha class, with a globin-like fold and are recognized as part of PF00502.
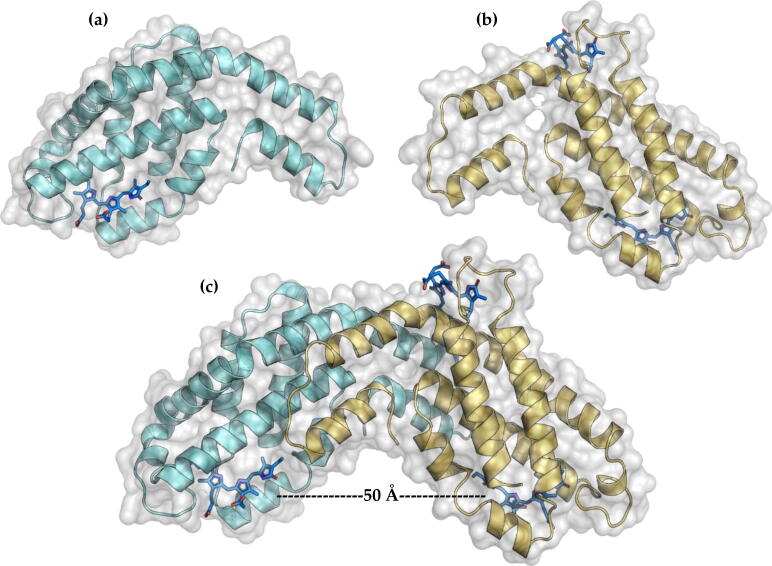
The analysis of different structures obtained by x-ray diffraction of PBPs heterodimers shows that the α (Fig. 5 A) and β subunits (Fig. 5 B) stabilize each other forming an αβ heterodimer (Fig. 5 C). The approximate distance between the chromophores within a heterodimer is approximately 50 Å, a large distance that prevents energy transfer within the heterodimer.
Fig. 5.
Structural level of PBPs. (a) Subunit α, (b) subunit β. (c) heterodimer αβ.
The area of interaction between the α and β subunits is approximately 1400 Å2, formed by 35–40 residues and is stabilized by 12–15 hydrogen bonds. These interactions allow the formation of a very stable surface, which can only be separated under denaturant conditions.
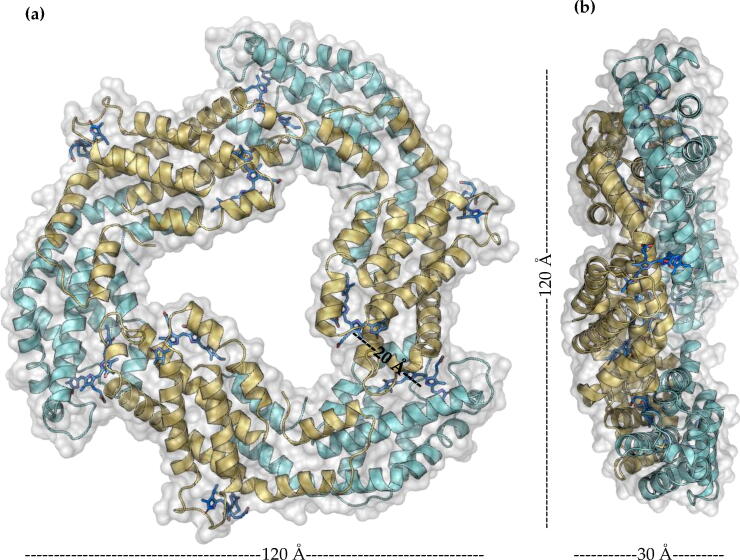
2.2. Trimers(αβ)3
Heterodimers are arranged symmetrically around a central axis (Fig. 6 A), forming (αβ)3 trimers. Trimers show a discoidal structure of 120 Å in diameter, 30 Å in thickness, and a central hole of approximately 10 Å in diameter [13], [14], [25]. Within a trimer, the distance between the chromophores of two adjacent heterodimers is close to 20 Å, allowing the transfer of energy between α and β subunits. This characteristic make trimers the minimum functional unit of PBPs and the biological unit of APC. (αβ)3 trimers have a curvature, where the concave surface is called the face and the convex surface is called back (Fig. 6 B). In the case of APC, the trimer is its functional biological assembly. The trimeric structure of APC is sensitive to temperature and chemical denaturants, which change its absorption and emission spectrum. However, both spectra recover to native state upon removal of the disturber [35], [36], [37]. Together with the above, the spectrum observed under denaturing conditions corresponds to that of the αβ heterodimer, which is not affected under the conditions tested [35]. When analyzing the contact surfaces and interactions that stabilize the trimeric structure of APC, an average area of 500 Å2 with 8 hydrogen bonds and 2 contact salt bridges between each pair of heterodimers can be observed [38].
Fig. 6.
Structural level of PBPs (a) trimer (αβ)3 front view. (b) trimer (αβ)3 side view.
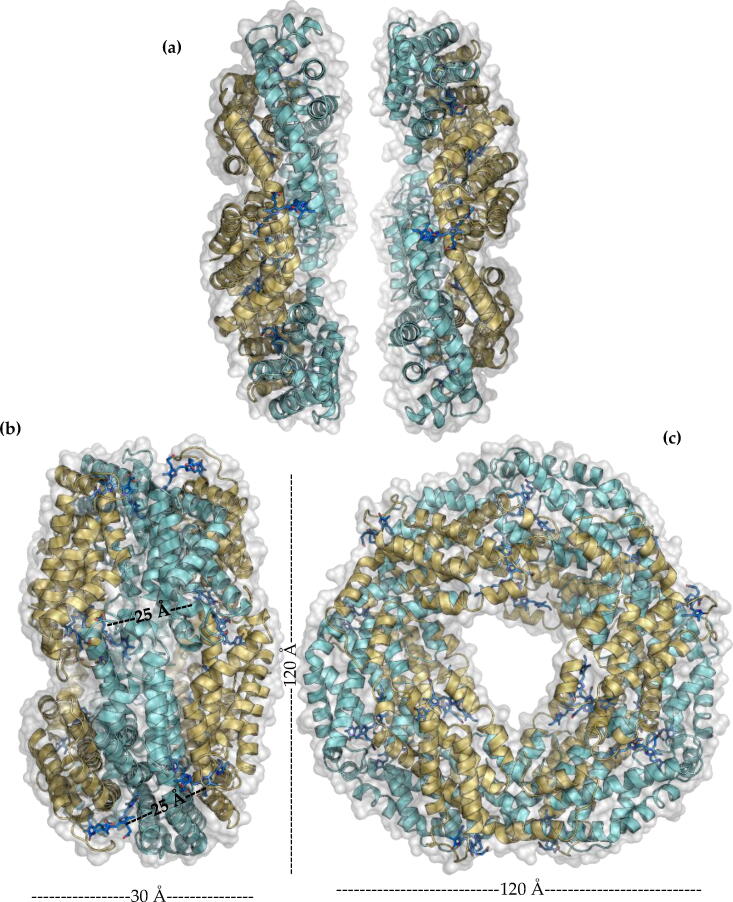
2.3. Hexamers (αβ)6
Trimers (αβ)3 associate face-to-face (Fig. 7 a) to form hexamers (αβ)6 (Fig. 7 b and c), these being the functional biological unit of PBPs, with the exception of APC [39], [40], [41], [42]. In the face-to-face association of the two trimers, one trimer is rotated approximately 30 degrees relative to the other [43]. The contact surface that allows the formation of hexamers is stabilized by interactions between residues of the α subunits of each trimer [43]. The distance of inter-trimeric chromophores in a hexamer is approximately 25 Å.Theoretical studies based on structures [17], [18] and molecular models [21] of PBPs propose energy transfers in a hexamer from one trimer to the other using the chromophores of the α subunits that form the binding surface.
Fig. 7.
Structural level of PBPs. (a) trimers (αβ)3 face-to-face, (b) hexamer(αβ)6 side view. (c) hexamer(αβ)6 front view.
Hexamers and trimers of PBPs associate and form PBS functional structures, such as the rod and core [13], [25], [44].
2.4. Rods and core
PBS rods are cylinder-shaped structures composed of multiple hexamers of PE or To form these cylinders the hexamers interact in a back-to-back using the β subunits to form the interaction surface PC (Fig. 8).
Fig. 9.
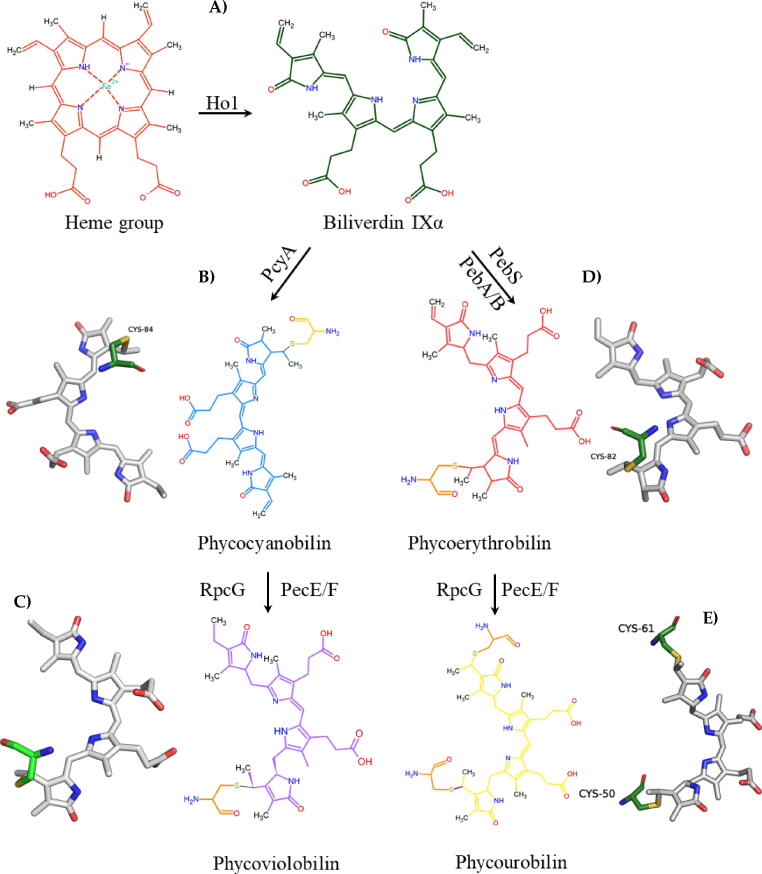
Phycobilins biosynthesis. 2D representantions are based in experimental 3D spatial orientation of phycobilins.
Fig. 8.
PBS rod. In blue α subunits. of PC. In purple β subunits of PC. In magenta α subunits of PE. In fuchsia β subunits of PE. In green PCB and PEB molecules. Figure made with pymol using P. Purpureum PBS structure (PDB id: 6KGX).
The core of the PBS is made up of three cylinders, each one is formed by three APC trimers. The cylinders that are in contact with the membrane have an α subunit replaced by ApcD and a β subunit replaced by ApcF. A second α unit is replaced by the globular domain (PB domain) of the Linker core-membrane (ApcE) [18], [45]. ApcD, ApcF and PB domain are essential to energy tansfer to the photosystems [46], [47], [48], [49]
Both structures, rods and core, are essential for the function of energy transfer to the photosystems, since they allow the chromophores present in each of the subunits that make up these PBS elements to be positioned in space.
3. Phycobiliprotein maturation
To obtain a fully functional PBP, two independent processes are required. The first corresponds to the biosynthesis of bilins and the second stage corresponds to a process of post-translational modifications that allow the chromophores to bind covalently to the polypeptide skeleton of the PBPs.
3.1. Phycobilins biosynthesis
Phycobilins are responsible of the spectroscopic propierties of PBPs and understanding how these molecules are synthetised is important in the context of potential biotechnological applications of PBPs. Four types of bilins are present in red algae and cyanobacterial PBPs; namely phycocyanobilin (PCB), a blue-colored bilin with maximum absorption at 620 nm; phycoviolobilin (PVB), that shows a violet color with a maximum absorption of 561 nm; phycoerythrobilin (PEB), which has a reddish color and has its maximum absorption at 560 nm and phycourobilin (PUB), with a yellowish color and maximum absorption at 495 nm [32], [50]. The main precursor of phycobilins is Biliverdin IXα (BV IXα), which in turn is the resulting product of the action of the enzyme heme oxygenase 1 (HO1) [51] (Fig. 7A). BV IXα acts as substrate for the biosynthesis of PCB and PEB. PCB biosynthesis is mediated by the ferredoxin-dependent enzyme phycocyanobilin: ferredoxin oxidoreductase (PcyA). PcyA catalyzes a BV IXα reduction reaction in two steps, starting with the synthesis of 18, 18-Dihydrobiliverdin (18–18 DHVB) and subsequently 3Z-PCB [51] (Fig. 7 B). On the other hand, PVB is synthesized after the isomerization of PCB mediated by the enzyme [C-phycocyanin α-subunit]: Phycourobilin lyase/isomerase (RpcG) or by the action of the heterodimeric phycoerythrocyanin liase/isomerase (PecE/F) [52], [53] (Fig. 7C). Finally, PEB biosynthesis starts with BV IXα as a precursor, which undergoes a modification by the action of the enzyme 15, 16-dihydrobiliverdin: ferredoxin oxidoreductase (PebA), forming 15, 16-dihydro biliverdin IXa (15, 16-DHBV). Subsequently, 15,16-DHBV is transformed by the action of the enzyme Phycoerythrobilin:ferredoxin oxidoreductase (PebB) to 3Z-PEB [51]. Another route for PEB biosynthesis has been reported by Dammeyer and collaborators [54], which relies on the action of the enzyme phycoerythrobilin synthase (PebS). This enzyme also uses BV IXα as substrate, combining the action of PebA and PebB in one step to form PEB (Fig. 7 D).
PUB is derived from a PEB modification by isomerization mediated by phycoerythrocyanin lyase/isomerase (PecE/F) after binding to the apoprotein [55] (Fig. 7 E). Fig. 7 shows a schematic view of these phycobilins biosynthesis and their spatial orientation as obtained by x-ray diffraction.
3.2. Phycobilins binding to PBPs
Chromophore binding to PBPs consists of post-translational modifications mediated by specific lyase enzymes. To date, four types of bilin lyases have been described including E/F-type, S/U-type, T-type, and Y/Z-type [55], [56]. These different lyases enzymes show specificity for both the chromophore as well as for binding residue in each subunit of the corresponding phycobiliprotein [57]. These enzymes ensure the binding of the correct bilin to the corresponding cysteine residue with the correct stereochemistry. In this way, one or two of the pyrrolic rings at the ends are linked through thioester bonds to cysteine residues [34].
To date, more than 1500 bilin lyases have been described, all belonging to cyanobacteria. Some of them have already been characterized at functional levels. Most cyanobacteria bilin lyases correspond to heterodimeric enzymes composed of two different subunits that, together, possess lyase activity. Each of these subunits, individually, has no activity. Three families of lyase enzymes have been described to date (E/F; CpcS/CpcU and T families) based on phylogenetic, structural, and biochemical studies, based on their respective substrates and their enzymatic activities [58].
The E/F family consists of heterodimeric lyases, with CpcE / F lyase being the first to be described in 1992 [59]. This lyase binds the PCB molecule to cysteine residue 84 of the phycocyanin α subunit. Another interesting member of this family is the PecE/F lyase. This enzyme has two activities, an isomerase activity, capable of transforming PCB into PVB or PEB into PUB, and a second activity consisting of binding these to the α subunit of phycocyanin or phycoerythrocyanin [52]. Kronfel and collaborators reported that a member of E-F family can bind PEB to Cys 48/59 residues of PE β subunit [60]. A recent study show that MpeV lyase-isomerase is responsible of binding double linked PUB molecules to CpeB in Synechococcus. [61].
The CpcS/CpcU family is responsible for the binding of PCBs to cysteine 81 residues of the α, β, αB, and β18 subunits of allophycocyanin, β subunit of phycocyanin, and the binding of PEB in the α and β subunits of PE. Interestingly, the CpcS/U lyase does not bind PCBs to the phycocyanin α subunit [62], [63].
Finally, the T family differs from the others since they are monomeric enzymes. It has been reported that these enzymes are responsible for the binding of PCB and PEB at cysteine residue 153 of the β subunit of phycocyanin and phycoerythrocyanin [52]. Recent studies on the CpeT lyase suggest that this enzyme could be responsible for the binding of PEB at residues 155 of PE [64]. Table 2 shows a summary of lyase enzymes and their activity on PBPs.
Table 2.
Bilin lyases family substrate specificity. PCB = phycocyanobilin. PVB = Phycoviolobilin. PEB = Phycoerythrobilin. PUB = Phycourobilin.
| Lyase family | Bilin | Gene | Binding Subunit | Binding Residue |
|---|---|---|---|---|
| E/F | PCB | rpcA | PC-α | Cys-84 |
| pecA | PEC-α | Cys-84 | ||
| PVB | pecA | PEC-α | Cys-84 | |
| RpcA | PC-α | Cys-84 | ||
| PUB | pecA | PEC-α | Cys-84 | |
| S/U | PCB | apcA | APC-α | Cys-81 |
| apcB | APC-β | Cys-81 | ||
| apcD | APC-αB | Cys-81 | ||
| apcF | APC-β18 | Cys-81 | ||
| cpcB | PC-β | Cys-81 | ||
| rpcB | PC-β | Cys-81 | ||
| pecB | PEC-β | Cys-81 | ||
| PEB | cpeA | PE-α | Cys-81 | |
| cpeB | PE-β | Cys-81 | ||
| T | PCB | cpcB | PC-β | Cys-153 |
| pecB | PEC-β | Cys-153 | ||
| PEB | cpeB | PE-β | Cys-153 | |
| cpcB | PC-β | Cys-153 |
Most of the data regarding bilin lyases are preserved and ordered in a database Cyanolyase [58].
3.3. Other post-translational modifications
The β-chain of PBPs has a methylation at residue asparagine 72, this modification is realized by the enzyme CpcM. The function of this change is increase energy transfer efficiency within the PBS and prevent photoinhibition [65]. Intrestly, CpcM enzyme only modify β subunits and not α subunits, despite their similar sequence and structure.
4. Spectroscopic characteristics of PBPs
PBPs have been classified into large groups based on their color, highlighting four types: phycoerythrin (PE), phycoerythrocyanin (PEC), phycocyanin (PC), and allophycocyanin (APC). These, in turn, have been subclassified according to their source of origin, namely C, R, and B, from cyanobacteria, Rhodophyta algae, or algae of the bangiales order, respectively. The visible energy absorption maxima of the respective fluorescence emission maxima are 576 nm in PE, 641 nm in PC, and 661 nm in APC [13], [25], [44], allowing a very precise spectral coupling [66]. These spectroscopic characteristics of absorption and emission of PBPs allow a very efficient energy transfer in the PBS.
4.1. Phycoerythrin
Phycoerythrins (PE) have an intense pink color and the greatest diversity of chromophores among PBPs. Along with these, PE is characterized by a high quantum yield, higher than 0.9 [67]. PE is located in the distal part of the PBS rods [17], [24], [25]. The α subunit of PE is encoded by the cpeA gene and the β subunit by the cpeB gene [14]. Three types of PE have been reported in cyanobacteria or algae. C-PE is subdivided into C-PE-I and C-PE-II, R-PE and B-PE. They differ according to the number and type of chromophores that they have attached.
Type I (C-PE-I) contains 5 PEB molecules linked at residues α-84, α-140 or 143, β-84 or 82, β-50 or β-61, and β-155 or 159. C-PE-II has 5 PEB molecules located in the same positions as C-PE-I and has a PUB molecule bound to α-75 cysteine. R-PE has 4 PEB molecules attached to residues α-84, α-140, β-84, and β-155, and a molecule of PUB linked to residues β-50 and β-61 respectively. In the case of red algae, B-PE has 5 PEB molecules, 2 in the α subunit and 3 in the β subunit in residues α-84, α-140, β-84, β-155, and β-50 / β-61.
Spectroscopically C-PE, B-PE, and R-PE differ in their number of absorption maxima. C-PE shows only one absorption maximum at 542 nm, while R-PE and B-PE absorbance spectra show two absorption maxima; the highest at 566 nm, followed by another at 545 nm, corresponding to PEB molecules and the third maximum of lower absorbance at 495 nm, corresponding to PUB molecules present exclusively in the β subunit of R-PE. The fluorescence emission in the different types of PE does not present great differences with a maximum emission value at 575 nm [68], [69], [70]. Table 3 shows a summary of spectroscopic characteristics reported for different PEs.
Table 3.
Spectroscopic properties of different phycoerythrins. PEB = Phycoerythrobilin. PUB = Phycourobilin.
| Protein | Binding Residue | λAbs Max (nm) | λEmMax (nm) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Cysα-75* | Cysα-84 | Cysα-143 | Cysβ-[50 61] | Cysβ-84 | Cysβ-155 | ||||
| C-PE | C-PE I | PEB | PEB | PEB | PEB | PEB | 565 | 575 | |
| C-PE II | PUB | PEB | PEB | PUB | PEB | PEB | 565 | 575 | |
| R-PE | PEB | PEB | PUB | PEB | PEB | 495, 545, 566 | 574 | ||
| B-PE | PEB | PEB | PEB | PEB | PEB | 545, 565 | 576 | ||
4.2. Phycoerythrocyanin
Phycoerythrocyanin (PEC) is, to date, the only phycobiliprotein exclusively present in cyanobacteria. It is a purple-bluish color phycobiliprotein, with one PVB molecule at the residue 84 of the α subunit and two PCB molecules at residues 84 and 153 of the β subunit [68]. One of the main characteristics of phycoerythrocyanin is that its abundance is strongly related to the intensity and type of light during its growth, increasing significantly in low light conditions with green light. [71]. Their presence in PBSs is not exclusive to PE, although they can absorb similar areas of the visible light spectrum. [68]. The maximum absorbance of phycoerythrocyanin is at 570 nm with a shoulder at 590 nm. Its fluorescence emission has its maximum at 625 nm [68]. Table 4 shows the spectroscopic characteristics phycoerythocyanin.
Table 4.
Spectroscopic properties of different phycoerythrocyanin. PCB = phycocyanobilin. PVB = Phycoviolobilin.
| Protein | Binding Residue | λAbs Max (nm) | λEmMax (nm) | ||
|---|---|---|---|---|---|
| Cysα-84 | Cysβ-84 | Cysβ-155 | |||
| PEC | PVB | PCB | PCB | 570 | 625 |
4.3. Phycocyanin
Phycocyanin (PC), an intense, blue-colored protein, is found in the proximal part of the rods of PBS. Unlike PE, PC is always present in cyanobacteria and red algae PC. Two types of phycocyanins have been recognized to date; PC present in red algae and cyanobacteria, whose expression is constitutive and come from the products of cpcA and cpcB genes, corresponding to the α and β subunits of phycocyanin, respectively. The second type of phycocyanins are those present only in cyanobacteria and have an inducible character through a chromatic adaptation system. These are the product of cpcA2 and cpcB2 genes [72].
PC is also classified according to their spectroscopic properties [69]. C-PC, present in most cyanobacteria, has PCB molecules attached to cysteine residues at position 84 in the α subunit and cysteines 84 and 155 of the β subunit. Its absorption spectrum shows a single absorption maximum at 620 nm and an emission maximum at 640 nm [25], [50]. R-PC has different chromophores attached, depending on the origin of the phycobiliprotein: R-PC-I is the most abundant, present in red algae and it was the first to be characterized spectroscopically. This protein has a PCB molecule attached to the Cis residues −84 of the α and β subunit and a PEB molecule attached to the Cys-155 residue of the β subunit. The absorption spectrum of this type of PC shows two absorption maxima, the first in less than 555 nm, attributable to a PEB molecule and a second higher maximum, associated with PCB molecules, at 619 nm. This protein has an emission maximum of 640 nm [25], [73].
R-PC-II was the first cyanobacterial PC reported to possess a PEB molecule. This type of phycocyanin has a PEB molecule linked to cysteine 84 in its α subunit, while the β subunit has a PCB molecule linked to cysteine 84 and a PEB molecule to cysteine 155. Its absorption spectrum shows three peaks at 533, 545, and 615 nm. Its fluorescence emission is 646 nm [25], [50].
R-PC-III has two PCB molecules and one PEB, but unlike R-PC-I and R-PC-II, the PCB molecules are both at residues 84 and 153 of the β subunit and the molecule of PEB at residue 84 of the α subunit. It has two absorption maxima, on at 560 nm and the second of less intensity at 603 nm. The emission maximum of this phycocyanin is at 648 nm [50].
R-PC-IV differs from other types by having a PUB molecule attached at residue 84 of the α subunit and two PCB molecules at residues 84 and 155 of the β subunit. Its absorption spectrum shows two maxima, one at 490 nm and the other at 592 nm. The emission maximum of this protein is at 644 nm [25].
In 2009, Blot and collaborators. described a fifth type of phycocyanin, R-PC-V. This protein is characterized by having three different types of PUB chromophores at residue 84 of the α subunit, a PCB molecule at residue 82, and a PEB molecule at residue 153 of the β subunit.
R-PC-V has three absorption maxima, at 495 nm, 540 nm, and 590 nm. Fluorescence emission at 640 nm [52]. Table 5 shows a summary of the spectroscopic properties of the different phycocyanins.
Table 5.
Spectroscopic propierties of different phycocyanins. PCB = phycocyanobilin. PEB = Phycoerythrobilin. PUB = Phycourobilin.
| Protein | Binding Residue | λAbs Max (nm) | λEmMax (nm) | |||
| Cysα-84 | Cysβ-84 | Cysβ-155 | ||||
| C-PC | PCB | PCB | PCB | 620 | 640 | |
| R-PC | R-PC-I | PCB | PCB | PEB | 555, 619 | 640 |
| R-PC-II | PEB | PCB | PEB | 533, 545, 615 | 646 | |
| R-PC-III | PEB | PCB | PCB | 560, 603 | 648 | |
| R-PC-IV | PUB | PCB | PCB | 490, 592 | 644 | |
| R-PC-V | PUB | PCB | PEB | 495, 540, 590 | 640 | |
4.4. Allophycocyanin
Allophycocyanin (APC) has a bright turquoise color and is exclusively found as part of the PBS core. Spectroscopically, it is the simplest phycobiliprotein since it only has one PCB molecule at residue 81 of the α and β subunit. The biological unit of allophycocyanin is trimeric. APC differs from other PBPs as it has 2 codifying genes for the α subunit, called apcA and apcD, whose gene products are known as α subunit and αB subunit, respectively. It also has 2 codifying genes for the β subunit, called apcB and apcF, responsible for the β and β [18] subunits, respectively. Both ApcD and ApcF have lower abundance and replace the α and β subunits in different trimers that form the core of the phycobilisome.
It has been demonstrated from the complete sequencing of the chloroplastidial genome of Gracilaria teniustipiatata var liui [74], Agarophyton chilense [75], and Gloeobacter violaceus [76], that the genes of allophycocyanin are encoded in the apcEABC cluster, however, the apcD and apcF genes are located isolated in different areas of the chloroplastid genome [74].
Trimers composed only by α and β form an (αβ)3 complex, which are the most common trimers and have an absorption maximum of 650 nm and a fluorescence emission maximum of 660 nm. It has been reported an allophycocyanin trimer (αβ)3 that also includes a linker protein know as linker core (Lc) of 8.9 kDa, the presence of this protein produces an spectroscopic change in 2 nm in the absorption maximum and 2 nm in the emission maximum [25].
The replacement of an α subunit by the αB subunit within a trimer (αBα2 β3) shows spectroscopic properties that differ from trimer (αβ)3, with a shift of absorption maxima at 654 nm and emission maxima at 679 nm [22], [25].
The trimers that have the β [18] subunit differ from others since, in addition to replacing a β subunit, there is a change of an α subunit for the PB domain at the core-membrane linker (Lcm) [22] forming a (α2 Lcm β2 β18) trimer. The β [18] subunit is also distinguished from others by its size, since it is made up of 169 residues, unlike the other three allophycocyanin subunits which are made up of 161 residues. This type of trimer has an absorption maximum at 654 nm and an emission maximum at 670 nm [25].
The absence of the αB and β [18] subunits in cyanobacteria has shown to decrease the efficiency of energy transfer from the phycobilisome to photosystem I and photosystem II, respectively [48], [49], [77], [78]. Table 6 shows a summary of the spectroscopic properties of the different types of allophycocyanin trimers.
Table 6.
Spectroscopic propierties of different allophycocyanins. PCB = phycocyanobilin.
| Trimer | Binding Residue | λA max(nm) | λE max (nm) | |
|---|---|---|---|---|
| Cysα-84 | Cysβ-84 | |||
| (αβ)3 | PCB | PCB | 651 | 660 |
| (αβ)3 + Lc | PCB | PCB | 653 | 662 |
| αBα2 β3 | PCB | PCB | 654 | 679 |
| α2β2β18 + Lcm | PCB | PCB | 654 | 670 |
In summary, the information reviewed shows that the different PBPs share a series of general characteristics, such as their structure and function. The presence of these proteins in the context of the PBS has allowed different organisms to be able to populate and survive in adverse environmental conditions, giving an advantage to the species that possess them. However, PBPs have shown more activities than just energy capture. For example, in cases of nitrogen deprivation, PBPs are used as source of nitrogen, allowing the synthesis of other proteins [79]. The truncation of the PBS in Synechocystis sp. PCC 6803 shows alteration in the utilization and regulation of iron and bicarbonate, also has implications in the proteomic profile and cellular membrane associated functions [80], [81]. which suggest that PBS and PBPs have more functions than be only a light harvest complex
The following section reviews multiple industrial applications of PBPs associated with its biotechnological processes.
5. Biotechnological perspectives of PBPs
PBPs has important biological properties for humans and animals that motivate the development of new biotechnological products and processes [82], [83]. Recently, several studies have reported the bioactivities of the PBPs, which can be summarized in anti-oxidant, anti-immflamatory, anti-metabolic diseases, anti-cancer, anti-neurodegenerative, and anti-pathogenic microorganisms. The details of the different biological activities of the PBPs are extensively reviewed in previous works [84], [85], [86], [87], [88]. In this section we will briefly see the biological activities of the PBPs present in publications of the last two years (Table 8).
Table 8.
Biological effect of PBP and its proposed application. Studies reported between 2019 and 2021.
| PBP | Biological activity | Source of PBP / Biological Effect | Proposed Application | Reference |
|---|---|---|---|---|
| PC | Antioxidant | Arthrospira maxima / PCB prevents alterations in oxidative stress markers, antioxidant enzymes, and caspase 9 activities | Nephro-protective action on acute kidney injury caused by mercury | [93] |
| Spirulina sp. / Attenuate radiation-induced oxidative stress damage in liver by activating Nrf2/ HO-1 signaling pathway and reduce DNA damage. | Protective effect on hepatic damage induced by X-ray | [92] | ||
| Spirulina sp. / Dietary supplementation reduces the oxidative stress in liver and kidney induced by a diet enriched with lipid peroxides in Wistar strain rats. | Diminish the risk of pathologies related to oxidative stress due to high oxidize oil consumption. | [115] | ||
| Spirulina sp. / Lengthening delivery of PC, encapsulated in poly(ethylene glycol)-b-(poly(l-glutamic acid)-g-polyethylenimine nano-carrier, through the abdominal subcutaneous injection in rats. | Attenuation of hepatic ischemia/reperfusion-induced pancreatic islet injury. | [116] | ||
| Phormidium versicolor / Prevents cadmium-induced elevation of ALAT, ASAT and bilirubin levels in rats. Enhance the levels of antioxidant enzymes. | Prevention action against hepatotoxicity caused by cadmium. | [117] | ||
| Anti-inflammatory | Spirulina sp. / Inhibits of albumin denaturation, anti-proteinase, hypotonicity-induced haemolysis and anti-lipoxygenase activities. | Potential drug development. | [95] | |
| Spirulina platensis / Reduce the micturition frequency and bladder inflammation in mice with cyclophosphamide-induced cystitis by inhibiting COX-2 and prostaglandin E receptor 4. | Countermeasure for the cyclophosphamide-Induced Cystitis anticancer chemotherapy | [118] | ||
| Spirulina platensis / Inhibits COX-2 expression during the radiation therapy of the colon cancer cell lines and in normal colonic cells. | Countermeasure reduction by C-PC treatment during colonic cancer radiation therapy. | [94] | ||
| Spirulina platensis / Attenuates the pulmonary fibrosis by inhibition of the production of interleukin-1 beta, tumor necrosis factor-α, and lipopolysaccharide. Increases the intestinal bacterial diversity, richness, and reduces pro-inflammatory bacterias. | Idiopathic pulmonary fibrosis treatment | [119] |
| PBP | Biological activity | Source of PBP / Biological Effect | Proposed Application | Reference |
|---|---|---|---|---|
| PC | Anti-metabolic diseases | Arthrospira platensis / APC and C-PC tryptic peptides inhibit enzyme DPP-IV activity in vitro, therefore, acting as hypoglycemic peptides. | Nutraceuticals orfunctional foods for the management of type 2diabetes. | [98] |
| Spirulina platensis / Reduce lipid accumulation in the steatosis L02 cells and in the liver of NASH mice. Improve the antioxidant capacity of liver by activating AMPK pathway of hepatocytes. | Nutraceuticals and therapeutics of non-alcoholic fatty liver disease. | [45] | ||
| Spirulina platensis / Inhibits α-amylase and β-glucosidase enzymes activity. | Potential drug development for diabetes type 2 treatment. | [95] | ||
| Anti-cancer | Limnothrix sp. / Regulates both anti- and pro-apoptotic genes by increasing levels of Bax, Apaf-1 and activates caspase-8, caspase-9, and caspase-3. Decreasing expression of Bcl-2, Mcl-1, and survivin . In combination with Topotecan, increase expression of the death receptor FAS and cleaved PARP. | Prostate cancer cells (LNCaP) treatment with reduction of conventional chemotherapeutic agents such as Topotecan. | [102] | |
| Spirulina platensis / Anti-proliferative effect against HepG-2 cell lines. | Potential drug development for cancer treatment. | [95] | ||
| Limnothrix sp. KNUA002 / The use of PC before cisplatin anti-cancer chemotherapy inhibits apoptosis and protected mitochondrial function by preventing ROS accumulation in cisplatin-treated House Ear Institute-Organ of Corti 1cells. Maintains Bax and Bcl-2 levels close to untreated control. | PC protection of cells against cisplatin-induced cytotoxicity as an anti-cancer therapy countermeasure | [120] | ||
| Spirulina sp. / Ameliorates the radiation-induced intestinal injury and regulates the effect on gut microbiota by TLR4/Myd88/NF-κB pathway | Countermeasure for the radiation-induced gastrointestinal syndrome | [121] | ||
| Spirulina platensis / Chlorophyllin-PC mixture plus diode laser irradiation reduced the ex-vivo cariogenic biofilm of Streptococcus mutans by increasing its ROS generation. | Antimicrobial photodynamic therapy for caries treatment as an ancillary approach | [112] |
| PBP | Biological activity | Source of PBP / Biological Effect | Proposed Application | Reference |
|---|---|---|---|---|
| PC | Anti-cancer | Spirulina sp. / Induce apoptosis, suppressed the growth of NSCLC cells by down regulation of TIRAP/NF-κB activity | Non-small cell lung cancer treatment. | [104] |
| Spirulina platensis / Anti-proliferative and anti-migratory function by the reduction of RIPK1/NF-κB activity in the NSCLC cells. | Non-small cell lung cancer treatment. | [105] | ||
| Spirulina platensis / Anti-proliferation of esophageal squamous cell carcinoma by cell cycle arrest, induction apoptosis and suppression invasion ability. Elevation of Bax, PARP, and cleaved-caspase-3 protein, but reduced cyclin D1, CDK4, Bcl-2, MMP-2, and MMP-9 expression. | Esophageal squamous cell carcinoma treatment | [122] | ||
| Spirulina sp. / The C-PC/CMC-CD59sp nanoparticles up-regulates the expression of p21, and then down-regulates the expressions of Cyclin D1 and CDK4 in BALB/c nude mice. Up-regulates the expression of cleave caspase-3, down-regulates the expression of bcl-2, and inhibit MMP-2 protein expression. | New drug delivery system with antitumor effects containing C-PC as main bioactive molecule. | [123] | ||
| Spirulina platensis LEB 52 / In multiple drug resistance phenotype of erythroleukemic cells, C-PC modulates the expression of COX2 and ABCB1 for the K562-Lucena cells in a ROS-dependent manner and the expression of ALOX5 for the FEPS cells in a ROS-independent manner. | Erythroleukemic cells multiple drug resistance (MDR) phenotype treatment. | [124] | ||
| Galdieria sulphuraria / Aqueous extract containing C-PC has antioxidant activity and exert cytotoxic activity in the human adenocarcinoma A549 cells. | Anticancer candidate for therapeutic treatments | [125] |
| PBP | Biological activity | Source of PBP / Biological Effect | Proposed Application | Reference |
|---|---|---|---|---|
| Anti-neuro-degenerative | Spirulina platensis / PCB reduces brain injury in PC12 neuronal cells after endothelin-1- induced focal cerebral ischaemia in Wistar rats. PCB restored the myelin basic protein and CNPase enzyme expression levels in ischaemic rats. | PCB as a therapeutic pharmacological alternative for ischaemic stroke patients. | [126] | |
| Spirulina sp. / Prevents streptozotocin-induced increase activity of hippocampal cholinesterases, BAX, and the levels of BCL-2 and ChAT. Also, attenuates neuro-inflammation and dysfunctional insulin signaling. | Therapeutic agent in managing Alzheimer's Disease | [110] | ||
| Spirulina sp. / Inhibits A53Tα-synuclein and Aβ40/42 fibril formation, but do not inhibit the reduction-induced amorphous aggregation of ADH and heat-induced aggregation of catalase. | Development of therapeutics to prevent Alzheimer's and/or Parkinson's disease. | [109] | ||
| Anti-micro-organism | Spirulina sp. / PCB inhibits the main protease and papain-like protease activities from coronaviruses (CoV) in vitro. In silico, showed inhibitory activity using other phycobilins such as PUB, PEB, and PVB | PCB as broad-spectrum antiviral drugs against coronaviruses. | [114] | |
| Spirulina sp. / Bacterial abundance and diversity increase in mice intestinal. Decrease intestinal permeability and increases barrier function. | Basis for a positive modulation of intestinal microbes by treatment with PC. | [127] | ||
| Spirulina sp. / Reduce the culture viability of E. faecalis in human premolar teeth by increasing the bacterial internal ROS. | Adjunct therapy in endodontic infection treatment | [128] | ||
| APC | Antioxidant | Phormidium sp. / APC increase anti-aging effect in worm model by improving physiological markers. Moderates the expression of human Aβ1–42. | The use of APC as drug development for age-related disorders. | [129] |
| Anti-inflammatory | Pyropia pseudolinearis / Peptides produced from the water-soluble proteins (riched in PE, PC, APC, and Rubisco) had inhibitory effect on the angiotensin-I-converting enzyme | Prevention of cardiovascular diseases and high blood pressure. | [130] |
| PBP | Biological activity | Source of PBP / Biological Effect | Proposed Application | Reference |
|---|---|---|---|---|
| PE | Antioxidant | Pyropia yezoensis / PE had in vitro antioxidant activity and cytotoxicity against HepG2 cells (hepatocellular carcinoma cells) | Prevention of the hepatocellular carcinoma cells. | [131] |
| Phormidium persicinum / C-PE reduce expression of nephrin and podocin in a mouse model of HgCl2-induced acute kidney injury. Reduced activation of IRE1α pathway and avoids caspase-mediated cell death. | Prevention of HgCl2-induced acute kidney injury. | [132] | ||
| Anti-inflammatory | Colaconema sp. / Enhance prophenoloxidase and phagocytosis activity but inhibits ROS in hemocytes from whiteleg shrimp. In vivo, PE induce immune genes and enhance the innate immune parameters. | Potential immunomodulator in shrimp culture to prevent the Vibrio parahaemolyticus or white spot syndrome virus | [133] | |
| Anti-metabolic diseases | Porphyridium purpureum / Peptide from PE reduce the foam cell formation, intracellular lipid accumulation and the secretion of TNF-α and IL-6 in RAW 264.7 cell line by targeting the scavenging receptors CD36, SRA1, and Map Kinase p38. | Prevention atherogenic foam cell formation. | [134] | |
| Anti-cancer | Inhibits growth and induce apoptosis of SKOV-3 cells. The ROS/JNK/Bcl-2 signaling pathway, upregulation of JNK, GADD45A, RAD23, and downregulation of XBP1 and OS9 are critical in PE -induced apoptosis in this cell. | PE as a potential treatment for human ovarian cancer. | [135] | |
| R-PE has modulated the innate and adaptive immune systems through TLR4/NF-κB mediated CD4+ T cell activation and differentiation in a hydrocortisone immune-suppressive model. | Immuno-modulator during long-term high-dose glucocorticoids therapy for autoimmune diseases and cancer | [96] | ||
| PE | Anti-neurodegenerative | Porphyra haitanensis / C-PE inhibits the precursor protein of BACE1, therefore, reduce accumulation of amyloid-β precursor protein. | Therapeutic drug for complement Alzheimer's disease treatment | [111] |
| Anti-microorganism | Recombinant PE / R-PE increase the abundance of beneficial bacteria in the intestinal flora and reduce the detrimental bacteria from H22-bearing mice. | Gut microbiota modulation for prevention of resistance diseases and nutritional in animals. | [136] |
5.1. Biological activies
5.1.1. Anti-oxidant activity
Cellular oxidative stress can be prevent by PBP, specifically, PC (Table 8). PC can inhibit the oxidative activity of various radicals such as peroxyl, hydroxyl, and superoxide, inhibiting lipid peroxidation [89], [90]. Together with the above, PC can inhibit the action of peroxynitrite radicals, thus reducing DNA damage [91].
Recently, the anti-oxidant activity of C-PC was reported in different application areas as for the reduction of acute liver oxidative damage caused by X-ray (in vivo study) [92] or for prevention of the oxidative stress in acute kidney damage caused by HgCl2 [93].
5.1.2. Anti-inflammatory activity
PBP have been reported to have a series of anti-inflammatory activities, especially phycocyanin (Table 8). In general, the anti-inflammatory activity is related to the inhibition of the COX-2 activity, myeloperoxidase activity, suppression of apoptosis and reduction of autoimmune response [84], [87]. In addition, to reduce patient radiation therapy countermeasure, C-PC was proposed as radiosensitizing due to the inhibition of COX-2 expression during the colon cancer radiation therapy [94]. Recently, anti-inflammatory effects such as in vitro inhibition of albumin denaturation, anti-proteinase, hypotonicity-induced haemolysis and anti-lipoxygenase activities were determined using phycocyanin [95]. On the other hand, immuno-modulatory activity of R-PE in the innate and adaptive immune systems via TLR4/NF-κB-dependent immunocyte differentiation was identify in a hydrocortisone (HC)-induced immunosuppressive model [96].
5.1.3. Anti-metabolic disease activity
PBP, mainly PCB and PC (Table 8), have been reported with anti-diabetes activity due to their inhibition of NADPH oxidase and protective effect against hLEC (human lymphatic endothelial cells) apoptosis [84]. Recently, PC is a potential candidate for anti-diabetic (type 2) natural therapeutic agents due to the in vitro inhibition of carbohydrate-metabolisms (α-amylose and α-glucosidase) and dipeptidyl peptidase IV enzymes (PC and its tryptic peptides) [95], [97], [98]. Anti-obesity activity was identified for PC by inhibition of the pancreatic lipase activity [99]. In addition, C-PC reduce the lipid accumulation in the steatosis L02 cells and liver of non-alcoholic steatohepatitis (NASH) mice, and improve the antioxidant capacity of liver [45].
5.1.4. Anti-cancer activity
PBP anti-cancer activity has been identify in several cancer types and/or tumor cells [84], [87], [100], [101] (Table 8). PC presents the highest activity by altering the growth of various tumor cell lines at different levels of their molecular mechanisms. Its anticancer effect on tumor cell (in vitro and/or in vivo studies) has been reported on breast, cervical, prostate, liver, lung, pancreatic, colon, leukemia and bone marrow cancers, where the cell cycle arrest/reduced proliferation, reduced tumor cell migration/invasion, and apoptosis/necrosis are the principal molecular mechanisms [100], [102]. Recently, MacCarty and collaborators [103] hypothesized the ability of PBP to prevent cancer cachexia by reduction of TLR4 signaling in skeletal muscles and the effect of phycocyanobilin on pancreatic cancer by inhibition of mitochondrial ROS. In addition, C-PC suppresses the in vitro proliferation and migration of non-small-cell lung cancer cells through the reduction of RIPK1/NF-κB and TIRAP/NF-κB activity [104], [105]. In general, PC had almost no or slight proliferative effects on cells from normal tissue, and high level concentration does not affect normal cell viability [106]. Nevertheless, the main mechanisms of action and its cellular targets have not yet been defined [101].
5.1.5. Anti-neurodegenerative activity
Parkinson’s and Alzheimer’s disease (Table 8), which are mediated by the misfolding and aggregation of proteins such as α-synuclein (αS) and amyloid-β (Aβ), respectively [107], where inhibited by C-PC [108], but was ineffective in inhibiting the reduction-induced amorphous aggregation of ADH and heat-induced aggregation of catalase [109]. In addition, intracerebroventricular cognitive decline in the Alzheimer's disease [110]. PE also was proposed as a putative therapeutic drug for the Alzheimer’s disease due to the inhibition of the beta-site amyloid precursor protein cleaving enzyme-1 (BACE1) [111]. Future studies should focus on the understanding of the mechanisms responsible for the formation and inhibition of these protein aggregates and potential therapeutic development.
5.1.6. Anti-microorganism activity
PBP with anti-bacterial, anti-fungal and anti-virus activities were identified, mainly for PC by growth inhibition of selected microorganism. Recently (Table 8), antimicrobial photodynamic therapy using a diode laser (DL) plus chlorophyllin-phycocyanin mixture as photosensitizer reduced dental caries produced by Streptococcus mutans [112]. This indicates the broad range of applications in the field of antibacterials. Anti-fungal activity of C-PC was also identified by the inhibition of cytopathic effects [113]. Anti-virus activity was reported for PCB, PUB, PEB, and PVB, which inhibits the SARS-CoV-2 and others coronavirus (CoV) proteases [114].
5.2. Biotechnological applications
The PBPs are used in several industrial sectors [137], [138]. Among the different commercial applications, they can be found as food coloring additives, and in cosmetic products. Also, they can be found in clinical or research laboratories as fluorophores [139]. PBPs are used as natural food colorants over synthetic dyes which exhibit carcinogenicity. S. platensis and Phorphyridium aerugineum extracted PE is utilized as confectionary colors, gelatin desserts, and cosmetic products. Also, PC is a pharmaceutically active phycopigment due to its antioxidant, neuroprotective, and hepatoprotective properties [139].
5.2.1. Production of PBPs from natural sources
Appropriate optimization of growth conditions is key for the development of PBPs industry and its further processing. Because the production and growth of metabolites in cyanobacteria depend not only on biotic factors but also on abiotic factors (light, temperature, nutrient concentration, pH, salinity and chemical composition of the medium can modify the metabolism of the organism) [84]. The culture conditions to increase the biomass production of different microalgae, such as Spirulina platensis, is one of the tools that are used experimentally and industrially for the accumulation of PBPs. Among the factors that have been used in these studies is the obtaining of biomass rich in PBPs under photo, hetero and mixotrophic conditions [140].The optimization of photobioreactors, medium composition, and culture environments are the major variables researchers use for rapid biomass accumulation and high production [140], [141].
The other main source of obtaining PBPs, apart from cyanobacteria, are red algae. These can be micro or macro algae. For years, various efforts have been made to cultivate them and promote the production of PBPs. In 2021 Rula and collaborators testing three factors (Light intensity, water motion and poblational density) to enhance groth and the PE production of Halymenia durvillei [142]. Another perspective in search of increasing the production of PBP in red algae was used by Souza and collaborators, who used biostimulants, derived of brown algae, to increase the amount of PE, PC and APC of Gracilaria caudata and Laurencia catarinensis with considerable success [143]. Not only the amount of light has been proven to increase the production of PBPs, but also the spectral composition and its effect on increasing the production of PBPs in the algae Halyenia floresii [144]. Similar approaches have been made for the macroalga Colaconema sp and Porphiridium purpureum [145], [146], [147]. Achieving the balance between the increase in biomass and the productivity of PBPs is the current challenge, both for cyanobacteria and for red algae.
5.2.2. Recombinant PBPs
Another alternative for the production of PBPs is the use of bacteria such as E. coli for their biosynthesis.via heterolog expression. This aproximation has been succeeded by genetic engineering techniques [31], [50], [56], [148], [149]. Two processes are involved in the biosynthesis of PBPs: 1) the synthesis of apoproteins and phycobilins and 2) the binding of phycobilins to apoproteins by enzymatic catalysis [150], [151].
After being synthesized in E. coli, phycobilins need to bind to the correct site of the apoprotein. In recombinant PBPs, the linkage between a phycobilin and apoprotein is formed with higher efficiency and correctness when catalyzed by a lyase. Therefore, the use of PBP lyases is the key to efficiently synthesize recombinant PBPs in vitro [86].
A current common problem with recombinantly synthesized PBPs is that the recombinant only binds to one cromophore e molecule, and its Stokes shift is small, usually 10–25 nm. In E. coli genes were introduced as phycobiliprotein lyase genes cpcS and cpcT, the phycocyanin β subunit gene cpcB, the heme oxidase Ho1 gene, and the phycoerythrobilin reductase pebS. This methodology constructs a synthesis pathway of the phycobiliprotein fluorescent protein and obtains the recombinant fluorescent protein with the large stokes shift [152].
By molecular design, several improved functions could be obtained with multiples applications. For example, a fusion protein has been developed between phycocyanin α-subunit CPCA gen and Light-Harvesting Complex LHC-II gen in E. coli. This new product displays a wide-range absorption spectrum, a great feature for photosensitizers, used as a natural dye in solar panels [153].
5.2.3. Extraction of PBPs
Technology approaches are available for the industrial extraction of PBPs from algal sources. However, the selection of methodology depends on factors such as organism composition, stability, and cell wall resistance [139]. In addition to the yield of PBP biosynthesis, the recovery of PBPs from biomass should also be considered. Different grades of purity are required depending on the intended application of the PBP. The purity of PBPs is usually expressed by the ratio Amax/A280 [86], For foods and cosmetics, the reported purity values are between 0.56 and 4.4.
Extraction processes can be used to obtain the released pigments from ruptured biomass (organic solvent extraction, pressurised solvent extraction, ionic liquid extraction, and supercritical carbon dioxide extraction [139]
Several technologies are available for rupturing microalgal cell walls to release intracellular pigments mechanical treatments (high-pressure homogenization (HPH), bead milling, ultrasonication, pulse electric field, freezing-thawing process, osmotic shock, and microwave), enzymatic treatments, and chemical treatments (e.g., phosphate buffer), However, is species-specific and the cell wall structure [154]. Comparing the different treatments, one can find clear advantages and disadvantages for production. Mechanical methods are not selective and consume considerable amounts of energy, however, they are non-toxic, fast, and suitable for large scale production. In contrast, chemical and enzymatic treatments are described as less suitable for scaling-up due to their high cost, low stability, time consumption, and toxicity [141].
5.2.4. Purification of PBPs
It is possible to purify the PBPs by precipitation, sugar gradient centrifugation, and chromatographic techniques [84]. Different amounts of ammonium sulfate precipitation activated carbon and chitosan precipitation are used. Among them are aqueous two-phase extraction with polyethylene glycol, concentration with ultrafiltration or tangential flow ultrafiltration (30–50 kDa). Then, the extract can be purified by a chromatographic column due to differences in the colors and polarity of the pigments or their size (anion exchange chromatography with Q-Sepharose column, gel permeation chromatography with Sephadex G-150 column, and anionic chromatography with diethylamino ethanol cellulose).
Previous studies investigated the recovery of PBP from microalgal and cyanobacterial biomass [155], developed a rapid method for the extraction and quantification of cryptophytic PE from Rhodomonas salina, and the cell disruption and extraction conditions were freezing and phosphate buffer. Cryptophytes do not have a cell wall; they possess a fragile periplast beneath the plasma membrane that can be easily ruptured by lyophilization action. A simple filtration was required to obtain a maximum yield/recovery of 8.04 ρg PE/cell. [155].
Another method is that of Rodrigues and collaborators [156] who reported the application of protic ionic liquids in the microwave-assisted extraction (MAE) of PBPs from Arthrospira platensis. They worked focusing on APC, PC and PE. The extraction was combined with the use of protic ionic liquids, 2-hydroxyethyl ammonium acetate (2-HEAA), and 2-hydroxyethyl ammonium formate (2-HEAF) (1:1) The purity obtained for PC, APC and PE was 1.22, 1.03, and 0.71, respectively [156].
Thus far, it has been reported that effective separation and purification methods to obtain higher purity PBPs are still lacking, which has greatly restricted their applications [84], [86].
The cell disruption, extraction, and purification process have been developed by a combination of the above-mentioned methods. For example, in Spirulin, a method was developed where samples were placed in a freezer at −28 degrees C. Then, they were three times freeze-thawed, the biomass was homogenized for approximately 5 min to break up the cells, and later centrifuged [157].
Chitosan was used for the purification of these extracts at 2% and ion-exchange chromatography [157] showed high purity of PBPs. Another solution to obtain a high purity product is a powder from algae mix Porphyra yezoensis, red algae, cyanobacteria, and Spirulina platensis, which are triturated, precipitated with ammonium sulphate and hidrolyzate with tripsin [158]. This strategy generates phycobiliprotein polypeptides that are easy to digest and absorb, destined for the medical, cosmetic, and food industry.
5.2.5. Encapsulation of phycoliliproteins
The encapsulation of PBP improves its thermal protection and preserves its active properties. In this context, the electrospraying method showed high encapsulation efficiency of phycocyanin [159], with especial emphasis on the food industry. In addition, other techniques have been applied in the last years for the microencapsulation of phycocyanin [160], [161], [162], [163], [164] in order to increase its stability. Nevertheless, a specific delivery of the PBPs should ensure its bioactivity in the target cells or tissues, especially for the treatment of tumor cells or other diseases in humans. In this contex, similar approaches have been reported recently. A nano-drug containing C-PC encapsulated in carboxymethyl-chitosan tagged to CD59sp was developed to treat the cervical cancer in in vivo female BALB/c nude mice and HeLa cells [123], [165], [166]. Peng and collaborators and Yan Wen and collaborators have encapsulated phycocyanin in an electrospun fiber, to prevent colon cancer. Furthermore, the addition of PC to well-established anti-cancer drugs could improve cancer therapy significantly [100], [102].
5.2.6. Biomedical use of PBPs
Inventions aimed at the application in biomedical sciences have been developed, due to their antioxidant and antitumor effects, among others. PBPs showed anti-oxidative properties both in vitro and in vivo. PC could effectively eliminate hydroxyl radicals (•OH) and alkoxy radicals (RO•) and inhibit lipid peroxidation [86], [89]. Moreover, the selenium-containing PCs (Se-PCs) has shown an ability to scavenge superoxide and hydrogen peroxide radicals positively correlated with the Se content [167]. In this context, it has been developed a selenization process in Spirulina, useful for the treatments of inflammatory intestinal diseases [168]. As in vivo and in vitro PBPs have shown anti-tumoral properties, C-PC purified from Oscillatoria tenuis inhibited the growth of HT-29 (colon cancer) and A549 (lung cancer) cells, and induce apoptotic cell death [169]. Skin tumors can be induced by f12-O-tetradecanoyl-phorbol-13-acetate (TPA). In the early stage of tumor progression, oral administration of C-PC extracts from Spirulina, reverse the expression pattern of proteins related to cancer as interlukin-6 and pSTAT3 [170].
On the other hand, the administration of recombinant APC inhibited the size of tumors in H22 hepatoma model mice [171]. Similarly, recombinant PE could inhibit the growth of HeLa cells, concentration dependent [172]. These studies indicate that native PBP and recombinant PBP have potential medical value in anti-tumor applications. Additionally, oral formulations of PBPs polysaccharide extract have been developed for treatment or prevention of pancreatic cancer [173]. Also, PBPs are useful in photodynamic antitumoral therapies in skin cancer [174] also has been designed a methodology to obtain powered of PBPs in photodynamic antitumoral therapies, cosmetic and food industry [158]. Table 9 Summarizes the patents related to production, extraction, purification, and biomedical aplications in different industries.
Table 9.
Patents related to phycocyanins in the fields of production, extraction, purification, and biomedical applications.
| Type of patent | Title | Reference |
|---|---|---|
| Production | Method for inducing the synthesis of PBPs. | [175] |
| A recombinant photosynthetic protein molecule with a wide range of absorption and its construction process | [153] | |
| Recombinant phycobiliprotein fluorescent protein with large stokes shift and preparation method thereof | [152] | |
| Extraction | Methods for extracting and purifying Nostoc sphaeroides Kutzing phycobiliprotein, and purified phycoerythrin | [176] |
| Extracting and purifying method for Nostoc sphaeroids Kutzing phycobiliprotein and purified phycoerythrin | [177] | |
| Extraction method of phycobiliprotein of Nostoc sphaeroides Kutzing | [178] | |
| Phycobiliprotein draws with many fallers corona discharge reaction unit | [179] | |
| Purification | Technology for separating and purifying phycobiliprotein from Gracilaria tenuistipitata deep processing natron solution | [180] |
| Method for extracting and purifying phycobiliprotein and purified phycocyanin | [181] | |
| The process of achieving the highest degree of drug's purity of c-phycocyanin from Spirulina alga | [157] | |
| Diagnostic application | Kit for early detection of liver cancer and preparation method thereof | [182] |
| A kind of utilize recombinant detection reagent bar of fluorescence phycobniliprotein subunit and preparation method thereof | [183] | |
| Pharmaceutical application | Composition containing phycobiliprotein polysaccharide extract and use of a composition containing phycobiliprotein polysaccharide extract | [173] |
| Preparation method of phycobiliprotein polypeptide powder | [158] | |
| Clinicalandcosmetic Application | Antitumor drug carrier and application method thereof | [184] |
| Preparation method of double-network hydrogel loaded with phycobiliprotein | [185] | |
| Anti-enteritis Spirulina selenylation Phycobiliproteinpolypeptide complex and preparation and application thereof | [168] | |
| Compositions for protecting skin comprising DNA repair enzymes and phycobiliprotein | [186] | |
| Heart valve prosthesis, preparation method, and in-vivo heart valve prosthesis sterilization method | [187] | |
| Phycocyanin composition for use in inhibiting bone resorption | [188] | |
| Novel material based on natural diatom shell and phycobiliprotein and application | [174] |
6. Future perspectives
Currently, there is great concern about the use of synthetic colorants in the food industry, due to the negative impact they can have on human health and the environment. In this regard, it has been demonstrated that Allura Red accelerates the formation of tumors in the immune system of mice and causes hyperactivity in children [189]. Indigo carmine has been associated with the development of brain tumors [190] and Sunset yellow may be related to hyperactivity and hypersensitivity in children [191]. In addition to their harmful effects on health, these synthetic colorants are difficult to remove from industrial wastewater, causing environmental damage. In this sense, the colorants obtained from natural sources are an attractive alternative. Thus, the natural colorant market is expected to reach 3.2 billion dollars by 2027. In this scenario, PBPs can provide coloring properties, along with diverse biological activities, providing added value to these compounds. However, the most common natural colorants used as food ingredients are obtained from plants or algae. This involves a large use of land or aquatic environments, long harvesting times and considerable energy consumption. As a result, a promising and economically viable alternative is to obtain these colorants from microbes. There are several successful examples of this strategy. Guerrero-Rubio and collaborators produced betalains (betacyanins (red-violet) and betaxanthins (yellow-orange)) from L-DOPA in a 2L bioreactor of Escherichia coli [192]. Wehrs and collaborators produced a natural blue pigment Indigoidine from Saccharomyces cerevisiae in a 2L bioreactor [193]. Another example was developed by Wang and collaborators, who engineered a tyrosinase from Bacillus megaterium into a bacterium Vibrio natriegens, resulting in a pigment similar to melanin [194]. In order to validate industrial production of these compounds and ensure maintenance of color, it is necessary to integrate omic techniques together with biological synthesis and metabolic engineering. In addition to this, selecting microorganisms considered Generally Recognized As Safe (GRAS) is crucial to guarantee their safety. Finally, after taking into consideration the spectroscopic characteristics of PBPs discussed above, an interesting approach would be to use metabolic engineering to have a single microorganism producing PBPs of different colors, thus contributing to reduce the costs of obtaining natural colorants and enhance productivity.
7. Fundings
Authors would like to acknowledge the support of the Agencia Nacional de Investigación y Desarrollo (ANID). This work was funded by grant FONDECYT Postdoctorado 3180614, Fondo de Fomento al Desarrollo Científico y Tecnológico (FONDEF) under grant number ID19I-10295 and FONDEQUIP EQM180201. Opinions expressed and conclusions arrived at are those of the authors and are not necessarily attributed to the funding agencies.
CRediT authorship contribution statement
Jorge Dagnino-Leone: Conceptualization, Writing - original draft, Visualization, Writing - review & editing, Project administration. Cristina Pinto Figueroa: Writing - original draft. Mónica Latorre Castañeda: Writing - original draft. Andrea Donoso Youlton: Writing - review & editing. Alejandro Vallejos-Almirall: Writing - original draft. Andrés Agurto-Muñoz: Writing - review & editing. Jessy Pavón Pérez: Writing - review & editing. Cristian Agurto-Muñoz: Supervision, Funding acquisition, Resources.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Jorge Dagnino-Leone, Email: jdagnino@udec.cl.
Cristina Pinto Figueroa, Email: cristinapinto@udec.cl.
Mónica Latorre Castañeda, Email: alevallejos@udec.cl.
Andrea Donoso Youlton, Email: molatorre@udec.cl.
Alejandro Vallejos-Almirall, Email: andreadonoso@udec.cl.
Andrés Agurto-Muñoz, Email: andagurto@udec.cl.
Jessy Pavón Pérez, Email: jpavon@udec.cl.
Cristian Agurto-Muñoz, Email: cagurto@udec.cl.
References
- 1.Kirk JT. O. Light and Photosynthesis in Aquatic Ecosystems 3rd Edition. (Cambridge, 2011).
- 2.Sven Beer, Mats Bjork, J. B. Photosynthesis in the Marine Environment. 2014).
- 3.Fischer W.W., Hemp J., Johnson J.E. Evolution of oxygenic photosynthesis. Annu Rev Earth Planet Sci. 2016;44(1):647–683. [Google Scholar]
- 4.Tanaka A, Tanaka R. The biochemistry, physiology, and evolution of the chlorophyll cycle. Advances in Botanical Research 90, (Elsevier Ltd., 2019).
- 5.Morançais M., Mouget J.-L., Dumay J. Microalgae in Health and Disease Prevention. Elsevier; 2018. Proteins and pigments; pp. 145–175. [DOI] [Google Scholar]
- 6.Nobel P.S. Photochemistry of photosynthesis. Physicochem Environ Plant Physiol. 2009;228–275 doi: 10.1016/b978-0-12-374143-1.00005-3. [DOI] [Google Scholar]
- 7.Falkowski PG. Raven J. A. Aquatic Photosynthesis. (Princeton University Press, 2013). 10.1515/9781400849727
- 8.Chen M., Blankenship R.E. Expanding the solar spectrum used by photosynthesis. Trends Plant Sci. 2011;16:427–431. doi: 10.1016/j.tplants.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 9.Larkum A, Douglas S, Raven J. Govindjee. Photosynthesis in algae. (2003). 10.1007/978-94-007-1038-2_13
- 10.Solovchenko A., Yahia E.M., Chen C. Postharvest Physiology and Biochemistry of Fruits and Vegetables. Elsevier; 2019. pp. 225–252. [DOI] [Google Scholar]
- 11.David L., et al. Structural studies show energy transfer within stabilized phycobilisomes independent of the mode of rod-core assembly. Biochim Biophys Acta - Bioenerg. 2014;1837:385–395. doi: 10.1016/j.bbabio.2013.12.014. [DOI] [PubMed] [Google Scholar]
- 12.De Marsac N.T., et al. Phycobiliproteins and phycobilisomes: the early observations. Photosynth Res. 2003;76:193–205. doi: 10.1023/A:1024954911473. [DOI] [PubMed] [Google Scholar]
- 13.MacColl R. Cyanobacterial phycobilisomes. J Struct Biol. 1998;124(2-3):311–334. doi: 10.1006/jsbi.1998.4062. [DOI] [PubMed] [Google Scholar]
- 14.Adir N., Dines M., Klartag M. Assembly and Disassembly of Phycobilisomes. Microbiology Monographs. 2006;2:47–77. [Google Scholar]
- 15.Su H., Xie B., Chen X., Wang J. Efficient separation and purification of allophycocyanin from Spirulina (Arthrospira) platensis. J Appl Phycol. 2010;22:65–70. [Google Scholar]
- 16.Liu L., Chen X., Zhang Y., Zhou B. Characterization, structure and function of linker polypeptides in phycobilisomes of cyanobacteria and red algae: an overview. BBA. 2005;1708:133–142. doi: 10.1016/j.bbabio.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 17.Ma J., You X., Sun S., Wang X., Qin S., Sui S.-F. Structural basis of energy transfer in Porphyridium purpureum phycobilisome. Nature. 2020;579(7797):146–151. doi: 10.1038/s41586-020-2020-7. [DOI] [PubMed] [Google Scholar]
- 18.Zheng L., et al. Structural insight into the mechanism of energy transfer in cyanobacterial phycobilisomes. Nat Commun. 2021;12:1–11. doi: 10.1038/s41467-021-25813-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arteni A., Ajlani G., Boekema E.J. Structural organisation of phycobilisomes from Synechocystis sp. strain PCC6803 and their interaction with the membrane. BBA. 2009;1787:272–279. doi: 10.1016/j.bbabio.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 20.Arteni A.A., et al. Structure and organization of phycobilisomes on membranes of the red alga Porphyridium cruentum. Photosynth Res. 2008;95:169–174. doi: 10.1007/s11120-007-9264-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Figueroa M., et al. In silico model of an antenna of a phycobilisome and energy transfer rates determination by theoretical Förster approach. Protein Sci. 2012;21:1921–1928. doi: 10.1002/pro.2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.MacColl R. Allophycocyanin and energy transfer. BBA. 2004;1657:73–81. doi: 10.1016/j.bbabio.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 23.Adir N., Bar-Zvi S., Harris D. The amazing phycobilisome. Biochim Biophys Acta - Bioenerg. 2020;1861 doi: 10.1016/j.bbabio.2019.07.002. [DOI] [PubMed] [Google Scholar]
- 24.Zhang J., Ma J., Liu D., Qin S., Sun S., Zhao J., et al. Structure of phycobilisome from the red alga Griffithsia pacifica. Nature. 2017;551(7678):57–63. doi: 10.1038/nature24278. [DOI] [PubMed] [Google Scholar]
- 25.Sidler W.A. In: The Molecular Biology of Cyanobacteria. Bryant D.A., editor. Springer Netherlands; Dordrecht: 1994. Phycobilisome and Phycobiliprotein Structures. in The Molecular Biology of Cyanobacteria 139–216; pp. 139–216. [DOI] [Google Scholar]
- 26.Mroginski M.A., et al. Determination of the chromophore structures in the pholoinduced reaction cycle of phytochrome. J Am Chem Soc. 2004;126:16734–16735. doi: 10.1021/ja043959l. [DOI] [PubMed] [Google Scholar]
- 27.Frank HA. Cogdell RJ. Light capture in photosynthesis. Comprehensive Biophysics 8, (Elsevier Ltd., 2012).
- 28.Mimuro M, Kikuchi H. Antenna Systems and Energy Transfer in Cyanophyta and Rhodophyta. in 281–306 (2003). 10.1007/978-94-017-2087-8_9
- 29.Sepúlveda-Ugarte J., Brunet J.E., Matamala A.R., Martínez-Oyanedel J., Bunster M. Spectroscopic parameters of phycoerythrobilin and phycourobilin on phycoerythrin from Gracilaria chilensis. J Photochem Photobiol A Chem. 2011;219:211–216. [Google Scholar]
- 30.Ma Y., Xie J., Zhang R., Hu C., Zhao J. Molecular properties of R-phycocyanin subunits from Polysiphonia urceolata in potassium phosphate buffer. Photochem Photobiol Sci. 2008;7:263–268. doi: 10.1039/b714837b. [DOI] [PubMed] [Google Scholar]
- 31.Dagnino‐Leone J., Figueroa M., Uribe E., Hinrichs M.V., Ortiz‐López D., Martínez‐Oyanedel J., et al. Biosynthesis and characterization of a recombinant eukaryotic allophycocyanin using prokaryotic accessory enzymes. Microbiologyopen. 2020;9(3) doi: 10.1002/mbo3.v9.310.1002/mbo3.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Glazer A.N. N. Light guides. Directional energy transfer in a photosynthetic antenna. J Biol Chem. 1989;264:1–4. [PubMed] [Google Scholar]
- 33.Apt K.E., Collier J.L., Grossman A.R. Evolution of the phycobiliproteins. J Mol Biol. 1995;248(1):79–96. doi: 10.1006/jmbi.1995.0203. [DOI] [PubMed] [Google Scholar]
- 34.Scheer H. MicroReview Biliprotein maturation : the chromophore attachment. Mol Microbiol. 2008;68:263–276. doi: 10.1111/j.1365-2958.2008.06160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.MacColl R., Eisele L.E., Menikh A. Allophycocyanin: Trimers, monomers, subunits, and homodimers. Biopolymers. 2003;72(5):352–365. doi: 10.1002/bip.10437. [DOI] [PubMed] [Google Scholar]
- 36.MacColl, R. Stability of allophycocyanin’s quaternary structure. 223, 24–32 (1983). [DOI] [PubMed]
- 37.MacColl R, Csatorday K, Berns S. The Relationship of the Quaternary Structure Allophycocyanin to Its Spectrum. 208, 42–48 (1981). [DOI] [PubMed]
- 38.Dagnino-Leone J., Figueroa M., Mella C., Vorphal M.A., Kerff F., Vásquez A.J., et al. Structural models of the different trimers present in the core of phycobilisomes from Gracilaria chilensis based on crystal structures and sequences. PLoS ONE. 2017;12(5):e0177540. doi: 10.1371/journal.pone.0177540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brejc K., Ficner R., Huber R., Steinbacher S. Isolation, crystallization, crystal structure analysis and refinement of allophycocyanin from the cyanobacterium Spirulina platensis at 2.3 A resolution. J Mol Biol. 1995;249:424–440. doi: 10.1006/jmbi.1995.0307. [DOI] [PubMed] [Google Scholar]
- 40.Marx A., Adir N. Allophycocyanin and phycocyanin crystal structures reveal facets of phycobilisome assembly. BBA. 2013;1827:311–318. doi: 10.1016/j.bbabio.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 41.McGregor A., Klartag M., David L., Adir N. Allophycocyanin trimer stability and functionality are primarily due to polar enhanced hydrophobicity of the phycocyanobilin binding pocket. J Mol Biol. 2008;384:406–421. doi: 10.1016/j.jmb.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 42.Reuter W., Wiegand G., Huber R., Than M.E. Structural analysis at 2.2 A of orthorhombic crystals presents the asymmetry of the allophycocyanin-linker complex, AP*LC7.8, from phycobilisomes of Mastigocladus laminosus. Proc Natl Acad Sci. 1999;96:1363–1368. doi: 10.1073/pnas.96.4.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.structural implications for thermal stability in phycobilisome assembly Adir, N., Dobrovetsky, Y. & Lerner, N. Structure of c-phycocyanin from the thermophilic cyanobacterium Synechococcus vulcanus at 2.5 A. J Mol Biol. 2001;313:71–81. doi: 10.1006/jmbi.2001.5030. [DOI] [PubMed] [Google Scholar]
- 44.Adir N. Elucidation of the molecular structures of components of the phycobilisome: reconstructing a giant. Photosynth Res. 2005;85(1):15–32. doi: 10.1007/s11120-004-2143-y. [DOI] [PubMed] [Google Scholar]
- 45.Ma P., et al. Potential use of C-phycocyanin in non-alcoholic fatty liver disease. Biochem Biophys Res Commun. 2020;526:906–912. doi: 10.1016/j.bbrc.2020.04.001. [DOI] [PubMed] [Google Scholar]
- 46.Maxson P., Sauer K., Zhou J., Bryant D.A., Glazer A.N. Spectroscopic studies of cyanobacterial phycobilisomes lacking core polypeptides. Biochim Biophys Acta - Bioenerg. 1989;977(1):40–51. doi: 10.1016/s0005-2728(89)80007-6. [DOI] [PubMed] [Google Scholar]
- 47.Kuzminov F.I., Bolychevtseva Y.V., Elanskaya I.V., Karapetyan N.V. Effect of APCD and APCF subunits depletion on phycobilisome fluorescence of the cyanobacterium Synechocystis PCC 6803. J Photochem Photobiol, B. 2014;133:153–160. doi: 10.1016/j.jphotobiol.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 48.Calzadilla P.I., Muzzopappa F., Sétif P., Kirilovsky D. Different roles for ApcD and ApcF in Synechococcus elongatus and Synechocystis sp. PCC 6803 phycobilisomes. Biochim Biophys Acta - Bioenerg. 2019;1860:488–498. doi: 10.1016/j.bbabio.2019.04.004. [DOI] [PubMed] [Google Scholar]
- 49.Ashby M.K., Mullineaux C.W. The role of ApcD and ApcF in energy transfer from phycobilisomes to PS I and PS II in a cyanobacterium. Photosynth Res. 1999;61:169–179. [Google Scholar]
- 50.Alvey R.M., Biswas A., Schluchter W.M., Bryant D.A. Attachment of Noncognate Chromophores to CpcA of Synechocystis sp. PCC 6803 and Synechococcus sp. PCC 7002 by Heterologous Expression in Escherichia coli. Biochemistry. 2011;50(22):4890–4902. doi: 10.1021/bi200307s. [DOI] [PubMed] [Google Scholar]
- 51.Frankenberg-Dinkel N., Terry M.J. In: Tetrapyrroles. Warren M.J., Smith A.G., editors. Springer New York; New York, NY: 2009. Synthesis and Role of Bilins in Photosynthetic Organisms; pp. 208–220. [DOI] [Google Scholar]
- 52.Blot N., et al. Phycourobilinin trichromatic phycocyanin from oceanic cyanobacteria is formed post-translationally by a phycoerythrobilin lyase-isomerase. J Biol Chem. 2009;284:9290–9298. doi: 10.1074/jbc.M809784200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhao K.H., et al. Novel activity of a phycobiliprotein lyase: Both the attachment of phycocyanobilin and the isomerization to phycoviolobilin are catalyzed by the proteins PecE and PecF encoded by the phycoerythrocyanin operon. FEBS Lett. 2000;469:9–13. doi: 10.1016/s0014-5793(00)01245-x. [DOI] [PubMed] [Google Scholar]
- 54.Dammeyer T., Hofmann E., Frankenberg-Dinkel N. Phycoerythrobilin synthase (PebS) of a marine virus. Crystal structures of the biliverdin complex and the substrate-free form. J Biol Chem. 2008;283:27547–27554. doi: 10.1074/jbc.M803765200. [DOI] [PubMed] [Google Scholar]
- 55.Schluchter WM. et al. Recent Advances in Phototrophic Prokaryotes. 675, 211–228 (2010).
- 56.Biswas A., et al. Biosynthesis of Cyanobacterial Phycobiliproteins in Escherichia coli: Chromophorylation Efficiency and Specificity of All Bilin Lyases from Synechococcus sp. Strain PCC 7002. Appl Environ Microbiol. 2010;76:2729–2739. doi: 10.1128/AEM.03100-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Scheer H., Zhao K.-H. Biliprotein maturation: the chromophore attachment. Mol Microbiol. 2008;68:263–276. doi: 10.1111/j.1365-2958.2008.06160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bretaudeau A., et al. CyanoLyase: A database of phycobilin lyase sequences, motifs and functions. Nucleic Acids Res. 2013;41:396–401. doi: 10.1093/nar/gks1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhou J., Gasparich G.E., Stirewalt V.L., De Lorimier R., Bryant D.A. The cpcE and cpcF genes of Synechococcus sp. PCC 7002. Construction and phenotypic characterization of interposon mutants. J Biol Chem. 1992;267:16138–16145. [PubMed] [Google Scholar]
- 60.Kronfel C.M., et al. CpeF is the bilin lyase that ligates the doubly linked phycoerythrobilin on -phycoerythrin in the cyanobacterium Fremyella diplosiphon. J Biol Chem. 2019;294:3987–3999. doi: 10.1074/jbc.RA118.007221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Carrigee L.A., et al. MpeV is a lyase isomerase that ligates a doubly linked phycourobilin on the β-subunit of phycoerythrin I and II in marine Synechococcus. J Biol Chem. 2021;296 doi: 10.1074/jbc.RA120.015289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Saunée N.A., Williams S.R., Bryant D.A., Schluchter W.M. Biogenesis of phycobiliproteins: II. CpcS-I and CpcU comprise the heterodimeric bilin lyase that attaches phycocyanobilin to CYS-82 OF beta-phycocyanin and CYS-81 of allophycocyanin subunits in Synechococcus sp. PCC 7002. J Biol Chem. 2008;283:7513–7522. doi: 10.1074/jbc.M708165200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shen G., Schluchter W.M., Bryant D.A. Biogenesis of phycobiliproteins: I. cpcS-I and cpcU mutants of the cyanobacterium Synechococcus sp. PCC 7002 define a heterodimeric phyococyanobilin lyase specific for beta-phycocyanin and allophycocyanin subunits. J Biol Chem. 2008;283:7503–7512. doi: 10.1074/jbc.M708164200. [DOI] [PubMed] [Google Scholar]
- 64.Nguyen A.A., et al. CpeT is the phycoerythrobilin lyase for Cys-165 on β-phycoerythrin from Fremyella diplosiphon and the chaperone-like protein CpeZ greatly improves its activity. Biochim Biophys Acta - Bioenerg. 2020;1861 doi: 10.1016/j.bbabio.2020.148284. [DOI] [PubMed] [Google Scholar]
- 65.Miller C.A., et al. Biogenesis of phycobiliproteins. III. CpcM is the asparagine methyltransferase for phycobiliprotein beta-subunits in cyanobacteria. J Biol Chem. 2008;283:19293–19300. doi: 10.1074/jbc.M802734200. [DOI] [PubMed] [Google Scholar]
- 66.Palenik B. Chromatic Adaptation in Marine Synechococcus Strains. Society 67, 991–994 (2001). [DOI] [PMC free article] [PubMed]
- 67.Grabowski J., Gantt E. photophysical properties of phycobiliproteins from phycobilisomes: fluorescence lifetimes, quantum yields, and polarization spectra. Photochem Photobiol. 1978;28:39–45. [Google Scholar]
- 68.Bryant D.A. Phycoerythrocyanin and phycoerythrin: properties and occurrence in cyanobacteria. J Gen Microbiol. 1982;128(4):835–844. [Google Scholar]
- 69.Gault P., Marler H. Handbook on cyanobacteria: biochemistry, biotechnology and applications. Bacteriology Research Developments (Nova Science Publishers, Inc, 2009).
- 70.MacColl R. Fluorescence studies on R-phycoerythrin and C-phycoerythrin. J Fluoresc. 1991;1(2):135–140. doi: 10.1007/BF00865209. [DOI] [PubMed] [Google Scholar]
- 71.Hirose Y., et al. Diverse chromatic acclimation processes regulating phycoerythrocyanin and rod-shaped phycobilisome in cyanobacteria. Mol Plant. 2019;12:715–725. doi: 10.1016/j.molp.2019.02.010. [DOI] [PubMed] [Google Scholar]
- 72.Debreczeny M.P., Sauer K., Zhou J., Bryant D.A. Monomeric C-phycocyanin at room temperature and 77 K: resolution of the absorption and fluorescence spectra of the individual chromophores and the energy-transfer rate constants. J Phys Chem. 1993;97:9852–9862. [Google Scholar]
- 73.Contreras-Martel C., et al. The structure at 2 Å resolution of Phycocyanin from Gracilaria chilensis and the energy transfer network in a PC–PC complex. Biophys Chem. 2007;125:388–396. doi: 10.1016/j.bpc.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 74.Hagopian J.C., Reis M., Kitajima J.P., Bhattacharya D., de Oliveira M.C. Comparative analysis of the complete plastid genome sequence of the red alga Gracilaria tenuistipitata var. liui provides insights into the evolution of rhodoplasts and their relationship to other plastids. J Mol Evol. 2004;59:464–477. doi: 10.1007/s00239-004-2638-3. [DOI] [PubMed] [Google Scholar]
- 75.Lee J., et al. Reconstructing the complex evolutionary history of mobile plasmids in red algal genomes. Sci Rep. 2016;6:23744. doi: 10.1038/srep23744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nakamura Y. Complete Genome Structure of Gloeobacter violaceus PCC 7421, a Cyanobacterium that Lacks Thylakoids (Supplement) DNA Res. 2003;10(4):181–201. doi: 10.1093/dnares/10.4.181. [DOI] [PubMed] [Google Scholar]
- 77.Jallet D., Gwizdala M., Kirilovsky D. ApcD, ApcF and ApcE are not required for the Orange Carotenoid Protein related phycobilisome fluorescence quenching in the cyanobacterium Synechocystis PCC 6803. Biochim Biophys Acta - Bioenerg. 2012;1817:1418–1427. doi: 10.1016/j.bbabio.2011.11.020. [DOI] [PubMed] [Google Scholar]
- 78.Zhao K.-H., et al. Chromophore attachment in phycocyanin. Functional amino acids of phycocyanobilin–alpha-phycocyanin lyase and evidence for chromophore binding. FEBS J. 2006;273:1262–1274. doi: 10.1111/j.1742-4658.2006.05149.x. [DOI] [PubMed] [Google Scholar]
- 79.Hsieh-Lo M., Castillo G., Ochoa-Becerra M.A., Mojica L. Phycocyanin and phycoerythrin: Strategies to improve production yield and chemical stability. Algal Res. 2019;42 [Google Scholar]
- 80.Liberton M., Chrisler W.B., Nicora C.D., Moore R.J., Smith R.D., Koppenaal D.W., et al. Phycobilisome truncation causes widespread proteome changes in Synechocystis sp. PCC 6803. PLoS ONE. 2017;12(3):e0173251. doi: 10.1371/journal.pone.0173251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Collins A.M., et al. Photosynthetic pigment localization and thylakoid membrane morphology are altered in synechocystis 6803 phycobilisome mutants. Plant Physiol. 2012;158:1600–1609. doi: 10.1104/pp.111.192849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Venkatraman K.L., Mehta A. Health Benefits and Pharmacological Effects of Porphyra Species. Plant Foods Hum Nutr. 2019;74(1):10–17. doi: 10.1007/s11130-018-0707-9. [DOI] [PubMed] [Google Scholar]
- 83.MU., N., Mehar JG, Mudliar SN, Shekh AY. Recent Advances in Microalgal Bioactives for Food, Feed, and Healthcare Products: Commercial Potential, Market Space, and Sustainability. Compr. Rev. Food Sci. Food Saf. 18, 1882–1897 (2019). [DOI] [PubMed]
- 84.Pagels F., Guedes A.C., Amaro H.M., Kijjoa A., Vasconcelos V. Phycobiliproteins from cyanobacteria: Chemistry and biotechnological applications. Biotechnol Adv. 2019;37:422–443. doi: 10.1016/j.biotechadv.2019.02.010. [DOI] [PubMed] [Google Scholar]
- 85.Piniella-Matamoros B., Marín-Prida J., Pentón-Rol G. Nutraceutical and therapeutic potential of Phycocyanobilin for treating Alzheimer’s disease. J Biosci. 2021;46(2) doi: 10.1007/s12038-021-00161-7. [DOI] [PubMed] [Google Scholar]
- 86.Li W., et al. Phycobiliproteins: Molecular structure, production, applications, and prospects. Biotechnol Adv. 2019;37:340–353. doi: 10.1016/j.biotechadv.2019.01.008. [DOI] [PubMed] [Google Scholar]
- 87.Bannu S.M., Lomada D., Gulla S., Chandrasekhar T., Reddanna P., Reddy M.C. Potential Therapeutic Applications of C-Phycocyanin. Curr Drug Metab. 2020;20(12):967–976. doi: 10.2174/1389200220666191127110857. [DOI] [PubMed] [Google Scholar]
- 88.de Andrade A.F., Porto A.L.F., Bezerra R.P. Photosynthetic microorganisms and their bioactive molecules as new product to healing wounds. Appl Microbiol Biotechnol. 2022;106:497–504. doi: 10.1007/s00253-021-11745-6. [DOI] [PubMed] [Google Scholar]
- 89.Romay C., et al. Antioxidant and anti-inflammatory properties of C-phycocyanin from blue-green algae. Inflamm Res. 1998;47:36–41. doi: 10.1007/s000110050256. [DOI] [PubMed] [Google Scholar]
- 90.Romay C., Gonzalez R., Ledon N., Remirez D., Rimbau V. C-Phycocyanin: A Biliprotein with Antioxidant, Anti-Inflammatory and Neuroprotective Effects. Curr Protein Pept Sci. 2003;4:207–216. doi: 10.2174/1389203033487216. [DOI] [PubMed] [Google Scholar]
- 91.Bhat V.B., Madyastha K.M. Scavenging of peroxynitrite by phycocyanin and phycocyanobilin from Spirulina platensis: Protection against oxidative damage to DNA. Biochem Biophys Res Commun. 2001;285:262–266. doi: 10.1006/bbrc.2001.5195. [DOI] [PubMed] [Google Scholar]
- 92.Liu Q., Li W., Qin S. Therapeutic effect of phycocyanin on acute liver oxidative damage caused by X-ray. Biomed Pharmacother. 2020;130 doi: 10.1016/j.biopha.2020.110553. [DOI] [PubMed] [Google Scholar]
- 93.Garcia-Pliego E., et al. Phycocyanobilin is the molecule responsible for the nephroprotective action of phycocyanin in acute kidney injury caused by mercury. Food Funct. 2021;12:2985–2994. doi: 10.1039/d0fo03294h. [DOI] [PubMed] [Google Scholar]
- 94.Kefayat A., Ghahremani F., Safavi A., Hajiaghababa A., Moshtaghian J. C-phycocyanin: a natural product with radiosensitizing property for enhancement of colon cancer radiation therapy efficacy through inhibition of COX-2 expression. Sci Rep. 2019;9:1–13. doi: 10.1038/s41598-019-55605-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Prabakaran G., Sampathkumar P., Kavisri M., Moovendhan M. Extraction and characterization of phycocyanin from Spirulina platensis and evaluation of its anticancer, antidiabetic and antiinflammatory effect. Int J Biol Macromol. 2020;153:256–263. doi: 10.1016/j.ijbiomac.2020.03.009. [DOI] [PubMed] [Google Scholar]
- 96.Wang C., et al. Immunomodulatory activity of R-phycoerythrin from: Porphyra haitanensis via TLR4/NF-κB-dependent immunocyte differentiation. Food Funct. 2020;11:2173–2185. doi: 10.1039/c9fo02444a. [DOI] [PubMed] [Google Scholar]
- 97.Machmudah S., Diono W., Kanda H., Goto M. Supercritical Fluids Extraction of Valuable Compounds from Algae: Future Perspectives and Challenges. Eng. J. 2018;22:13–30. [Google Scholar]
- 98.Li Y., Aiello G., Bollati C., Bartolomei M., Arnoldi A., Lammi C. Phycobiliproteins from Arthrospira Platensis (Spirulina): A New Source of Peptides with Dipeptidyl Peptidase-IV Inhibitory Activity. Nutrients. 2020;12(3):794. doi: 10.3390/nu12030794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chakdar H., Pabbi S. Algal Green Chemistry. Elsevier; 2017. Algal Pigments for Human Health and Cosmeceuticals; pp. 171–188. [DOI] [Google Scholar]
- 100.Braune S., Krüger-Genge A., Kammerer S., Jung F., Küpper J.H. Phycocyanin from arthrospira platensis as potential anti-cancer drug: Review of in vitro and in vivo studies. Life. 2021;11:1–14. doi: 10.3390/life11020091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fernandes e Silva E., Figueira F.d.S., Lettnin A.P., Carrett-Dias M., Filgueira D.D.M.V.B., Kalil S., et al. C-Phycocyanin: Cellular targets, mechanisms of action and multi drug resistance in cancer. Pharmacol Rep. 2018;70(1):75–80. doi: 10.1016/j.pharep.2017.07.018. [DOI] [PubMed] [Google Scholar]
- 102.Kaur P., Dhandayuthapani S., Venkatesan T., Gantor M., Rathinavelu A. Molecular mechanism of C-phycocyanin induced apoptosis in LNCaP cells. Bioorganic Med Chem. 2020;28(3):115272. doi: 10.1016/j.bmc.2019.115272. [DOI] [PubMed] [Google Scholar]
- 103.McCarty M.F., Iloki-Assanga S., Lujany L.M.L. Nutraceutical targeting of TLR4 signaling has potential for prevention of cancer cachexia. Med Hypotheses. 2019;132:109326. doi: 10.1016/j.mehy.2019.109326. [DOI] [PubMed] [Google Scholar]
- 104.Hao S., Li S., Wang J., Yan Y., Ai X., Zhang J., et al. Phycocyanin Exerts Anti-Proliferative Effects through Down-Regulating TIRAP/NF-κB Activity in Human Non-Small Cell Lung Cancer Cells. Cells. 2019;8(6):588. doi: 10.3390/cells8060588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hao S., Li S., Wang J., Zhao L., Yan Y., Wu T., et al. C-phycocyanin suppresses the in vitro proliferation and migration of non-small-cell lung cancer cells through reduction of RIPK1/NF-κB activity. Mar Drugs. 2019;17(6):362. doi: 10.3390/md17060362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Liu Q., Huang Y., Zhang R., Cai T., Cai Y. Medical application of spirulina platensis derived c-phycocyanin. Evidence-based complement. Altern Med. 2016;2016:1–14. doi: 10.1155/2016/7803846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chiti F., Dobson C.M. Protein misfolding, functional amyloid, and human disease. Annu Rev Biochem. 2006;75(1):333–366. doi: 10.1146/annurev.biochem.75.101304.123901. [DOI] [PubMed] [Google Scholar]
- 108.Barbalace M.C., Malaguti M., Giusti L., Lucacchini A., Hrelia S., Angeloni C. Anti-Inflammatory Activities of Marine Algae in Neurodegenerative Diseases. Int J Mol Sci. 2019;20(12):3061. doi: 10.3390/ijms20123061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Liu Y., Jovcevski B., Pukala T.L. C-Phycocyanin from Spirulina Inhibits α-Synuclein and Amyloid-β Fibril Formation but Not Amorphous Aggregation. J Nat Prod. 2019;82:66–73. doi: 10.1021/acs.jnatprod.8b00610. [DOI] [PubMed] [Google Scholar]
- 110.Agrawal M., Perumal Y., Bansal S., Arora S., Chopra K. Phycocyanin alleviates ICV-STZ induced cognitive and molecular deficits via PI3-Kinase dependent pathway. Food Chem Toxicol. 2020;145 doi: 10.1016/j.fct.2020.111684. [DOI] [PubMed] [Google Scholar]
- 111.Chaubey M.G., et al. Therapeutic potential of cyanobacterial pigment protein phycoerythrin: in silico and in vitro study of BACE1 interaction and in vivo Aβ reduction. Int J Biol Macromol. 2019;134:368–378. doi: 10.1016/j.ijbiomac.2019.05.006. [DOI] [PubMed] [Google Scholar]
- 112.Afrasiabi S., Pourhajibagher M., Chiniforush N., Aminian M., Bahador A. Anti-biofilm and anti-metabolic effects of antimicrobial photodynamic therapy using chlorophyllin-phycocyanin mixture against Streptococcus mutans in experimental biofilm caries model on enamel slabs. Photodiagnosis Photodyn. Ther. 2020;29 doi: 10.1016/j.pdpdt.2019.101620. [DOI] [PubMed] [Google Scholar]
- 113.Murugan T., Radhamadhavan T. Screening for Antifungal and Antiviral activity of C-phycocyanin from Spirulina platensis. J Pharm Res. 2011;4:4161–4163. [Google Scholar]
- 114.Pendyala B., Patras A., Dash C. Phycobilins as Potent Food Bioactive Broad-Spectrum Inhibitors Against Proteases of SARS-CoV-2 and Other Coronaviruses: A Preliminary Study. Front Microbiol. 2021;12 doi: 10.3389/fmicb.2021.645713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ould Amara-Leffad L., Ramdane H., Nekhoul K., Ouznadji A., Koceir E.A. Spirulina effect on modulation of toxins provided by food, impact on hepatic and renal functions. Arch Physiol Biochem. 2019;125:184–194. doi: 10.1080/13813455.2018.1444059. [DOI] [PubMed] [Google Scholar]
- 116.Tong F., Tang X., Liu D. Phycocyanin/PEG-b-(PG-g-PEI) attenuated hepatic ischemia/reperfusion-induced pancreatic islet injury and enlarged islet functionality. Int J Nanomedicine. 2019;14:339–351. doi: 10.2147/IJN.S190938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gammoudi S., et al. Optimization, isolation, characterization and hepatoprotective effect of a novel pigment-protein complex (phycocyanin) producing microalga: Phormidium versicolorNCC-466 using response surface methodology. Int J Biol Macromol. 2019;137:647–656. doi: 10.1016/j.ijbiomac.2019.06.237. [DOI] [PubMed] [Google Scholar]
- 118.Bao X.-Q., Huang Y.-C., Chen F. C-Phycocyanin Alleviates Bladder Inflammation and Dysfunction in Cyclophosphamide-Induced Cystitis in a Mouse Model by Inhibiting COX-2 and EP4. Evid Based Complement Alternat Med. 2019;2019:8424872. doi: 10.1155/2019/8424872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Xie Y., et al. The effects of phycocyanin on bleomycin-induced pulmonary fibrosis and the intestinal microbiota in C57BL/6 mice. Appl Microbiol Biotechnol. 2019;103:8559–8569. doi: 10.1007/s00253-019-10018-7. [DOI] [PubMed] [Google Scholar]
- 120.Kim Y.-R., Do J.-M., Kim K.H., Stoica A.R., Jo S.-W., Kim U.-K., et al. C-phycocyanin from Limnothrix Species KNUA002 Alleviates Cisplatin-Induced Ototoxicity by Blocking the Mitochondrial Apoptotic Pathway in Auditory Cells. Mar Drugs. 2019;17(4):235. doi: 10.3390/md17040235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lu L., et al. Phycocyanin Ameliorates Radiation-Induced Acute Intestinal Toxicity by Regulating the Effect of the Gut Microbiota on the TLR4/Myd88/NF-κB Pathway. JPEN J Parenter Enteral Nutr. 2020;44:1308–1317. doi: 10.1002/jpen.1744. [DOI] [PubMed] [Google Scholar]
- 122.Zhang X., et al. C-Phycocyanin elicited antitumor efficacy via cell-cycle arrest, apoptosis induction, and invasion inhibition in esophageal squamous cell carcinoma. J Recept Signal Transduct Res. 2019;39:114–121. doi: 10.1080/10799893.2019.1638400. [DOI] [PubMed] [Google Scholar]
- 123.Jiang L., Wang Y., Zhu F., Liu G., Liu H., Ji H., et al. Molecular mechanism of anti-cancer activity of the nano-drug C-PC/CMC-CD59sp NPs in cervical cancer. J Cancer. 2019;10(1):92–104. doi: 10.7150/jca.27462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Fernandes E., Silva E., et al. Modulation of reactive oxygen levels and gene expression in sensitive and resistant tumoral cells by C-phyocyanin. Mol Biol Rep. 2019;46:1349–1356. doi: 10.1007/s11033-018-4569-x. [DOI] [PubMed] [Google Scholar]
- 125.Bottone C., et al. Antioxidant and anti-proliferative properties of extracts from heterotrophic cultures of Galdieria sulphuraria. Nat Prod Res. 2019;33:1659–1663. doi: 10.1080/14786419.2018.1425853. [DOI] [PubMed] [Google Scholar]
- 126.Pavón-Fuentes N., et al. Phycocyanobilin reduces brain injury after endothelin-1- induced focal cerebral ischaemia. Clin Exp Pharmacol Physiol. 2020;47:383–392. doi: 10.1111/1440-1681.13214. [DOI] [PubMed] [Google Scholar]
- 127.Xie Y., Li W., Zhu L., Zhai S., Qin S., Du Z. Effects of phycocyanin in modulating the intestinal microbiota of mice. Microbiologyopen. 2019;8(9) doi: 10.1002/mbo3.v8.910.1002/mbo3.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Pourhajibagher M., Chiniforush N., Bahador A. Antimicrobial action of photoactivated C-Phycocyanin against Enterococcus faecalis biofilms: Attenuation of quorum-sensing system. Photodiagnosis Photodyn. Ther. 2019;28:286–291. doi: 10.1016/j.pdpdt.2019.10.013. [DOI] [PubMed] [Google Scholar]
- 129.Chaubey M.G., Patel S.N., Rastogi R.P., Madamwar D., Singh N.K. Cyanobacterial pigment protein allophycocyanin exhibits longevity and reduces Aβ-mediated paralysis in C. elegans: complicity of FOXO and NRF2 ortholog DAF-16 and SKN-1. 3. Biotech. 2020;10:332. doi: 10.1007/s13205-020-02314-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kumagai Y., Toji K., Katsukura S., Morikawa R., Uji T., Yasui H., et al. Characterization of ACE Inhibitory Peptides Prepared from Pyropia pseudolinearis Protein. Mar Drugs. 2021;19(4):200. doi: 10.3390/md19040200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ulagesan S., Nam T.-J., Choi Y.-H. Extraction and Purification of R-Phycoerythrin Alpha Subunit from the Marine Red Algae Pyropia Yezoensis and Its Biological Activities. Molecules. 2021;26(21):6479. doi: 10.3390/molecules26216479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Blas-Valdivia V., Rojas-Franco P., Serrano-Contreras J.I., Sfriso A.A., Garcia-Hernandez C., Franco-Colín M., et al. C-phycoerythrin from Phormidium persicinum Prevents Acute Kidney Injury by Attenuating Oxidative and Endoplasmic Reticulum Stress. Mar Drugs. 2021;19(11):589. doi: 10.3390/md19110589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lee P.-T., Huang J., Huang C.-Y., Liu Z.-X., Yeh H.-Y., Huang H.-T., et al. Phycoerythrin from Colaconema sp. Has Immunostimulatory Effects on the Whiteleg Shrimp Litopenaeus vannamei and Increases Resistance to Vibrio parahaemolyticus and White Spot Syndrome Virus. Anim. an open access J. from MDPI. 2021;11(8):2371. doi: 10.3390/ani11082371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kavitha M.D., et al. Atheroprotective effect of novel peptides from Porphyridium purpureum in RAW 264.7 macrophage cell line and its molecular docking study. Biotechnol Lett. 2019;41:91–106. doi: 10.1007/s10529-018-2621-5. [DOI] [PubMed] [Google Scholar]
- 135.Ying J., et al. Transcriptionomic Study on Apoptosis of SKOV-3 Cells Induced by Phycoerythrin from Gracilaria lemaneiformis. Anticancer Agents Med Chem. 2021;21:1240–1249. doi: 10.2174/1871520620666200908102621. [DOI] [PubMed] [Google Scholar]
- 136.Qi H., Liu Y., Qi X., Liang H., Chen H., Jiang P., et al. Dietary Recombinant Phycoerythrin Modulates the Gut Microbiota of H22 Tumor-Bearing Mice. Mar Drugs. 2019;17(12):665. doi: 10.3390/md17120665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sekar S., Chandramohan M. Phycobiliproteins as a commodity: trends in applied research, patents and commercialization. J Appl Phycol. 2007;20:113–136. [Google Scholar]
- 138.Sonani R.R. Recent advances in production, purification and applications of phycobiliproteins. World J Biol Chem. 2016;7:100. doi: 10.4331/wjbc.v7.i1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Vinothkanna A, Sekar S. Diagnostic Applications of Phycobiliproteins. In: Pigments from Microalgae Handbook 585–610 (Springer International Publishing, 2020). 10.1007/978-3-030-50971-2_24
- 140.Kannaujiya VK, Kumar D, Pathak J, Sinha RP. Phycobiliproteins and Their Commercial Significance. in Cyanobacteria 207–216 (Elsevier, 2019). 10.1016/B978-0-12-814667-5.00010-6
- 141.Corrêa P.S., Morais Júnior W.G., Martins A.A., Caetano N.S., Mata T.M. Microalgae biomolecules: Extraction, separation and purification methods. Processes. 2021;9:1–40. [Google Scholar]
- 142.Rula N.A.M., Ganzon-Fortes E.T., Pante M.J.R., Trono G.C. Influence of light, water motion, and stocking density on the growth and pigment content of Halymenia durvillei (Rhodophyceae) under laboratory conditions. J Appl Phycol. 2021;33:2367–2377. [Google Scholar]
- 143.Souza J.M.C., Castro J.Z., Critchley A.T., Yokoya N.S. Physiological responses of the red algae Gracilaria caudata (Gracilariales) and Laurencia catarinensis (Ceramiales) following treatment with a commercial extract of the brown alga Ascophyllum nodosum (AMPEP) J Appl Phycol. 2019;31:1883–1888. [Google Scholar]
- 144.Godínez-Ortega J.L., Snoeijs P., Robledo D., Freile-Pelegrín Y., Pedersén M. Growth and pigment composition in the red alga Halymenia floresii cultured under different light qualities. J Appl Phycol. 2008;20:253–260. [Google Scholar]
- 145.Lee M.C., et al. Enhancing growth, phycoerythrin production, and pigment composition in the red alga Colaconema sp. Through optimal environmental conditions in an indoor system. Bioresour Technol. 2021;333 doi: 10.1016/j.biortech.2021.125199. [DOI] [PubMed] [Google Scholar]
- 146.Xu Y., et al. Induced cultivation pattern enhanced the phycoerythrin production in red alga Porphyridium purpureum. Bioprocess Biosyst Eng. 2020;43:347–355. doi: 10.1007/s00449-019-02230-6. [DOI] [PubMed] [Google Scholar]
- 147.Sosa-Hernández J.E., et al. Light intensity and nitrogen concentration impact on the biomass and phycoerythrin production by porphyridium purpureum. Mar Drugs. 2019;17:1–12. doi: 10.3390/md17080460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Bryant D.A., et al. Molecular cloning and nucleotide sequence of the α and β subunits of allophycocyanin from the cyanelle genome of Cyanophora paradoxa. Proc Natl Acad Sci USA. 1985;82:3242–3246. doi: 10.1073/pnas.82.10.3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Liu S., et al. Biosynthesis of fluorescent cyanobacterial allophycocyanin trimer in Escherichia coli. Photosynth Res. 2010;105:135–142. doi: 10.1007/s11120-010-9574-4. [DOI] [PubMed] [Google Scholar]
- 150.Zhao K.-H., et al. Reconstitution of phycobilisome core-membrane linker, LCM, by autocatalytic chromophore binding to ApcE. BBA. 2005;1706:81–87. doi: 10.1016/j.bbabio.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 151.Gambetta G.A., Lagarias J.C. Genetic engineering of phytochrome biosynthesis in bacteria. Proc Natl Acad Sci USA. 2001;98(19):10566–10571. doi: 10.1073/pnas.191375198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Huaxin C, Peng J. Recombinant phycobiliprotein fluorescent protein with large stokes shift and preparation method thereof. Patent N° CN108841846 B, China (2018).
- 153.Ge B, Hou Q, Yu Q. A recombinant photosynthetic protein molecule with a broad absorption spectrum and its construction method. Patent N° DE 102018125376 A1, China (2020).
- 154.Nemer G., Louka N., Vorobiev E., Salameh D., Nicaud J.-M., Maroun R.G., et al. Mechanical cell disruption technologies for the extraction of dyes and pigments from microorganisms: A review. Fermentation. 2021;7(1):36. doi: 10.3390/fermentation7010036. [DOI] [Google Scholar]
- 155.Thoisen C., Hansen B.W., Nielsen S.L. A simple and fast method for extraction and quantification of cryptophyte phycoerythrin. MethodsX. 2017;4:209–213. doi: 10.1016/j.mex.2017.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Rodrigues R.D.P., et al. Application of protic ionic liquids in the microwave-assisted extraction of phycobiliproteins from Arthrospira platensis with antioxidant activity. Sep Purif Technol. 2020;252 [Google Scholar]
- 157.Hoseini F., Hoseini S. The process of achieving the highest degree of drug’s purity of c-phycocyanin from Spirulina alga. Patent Application N° WO. 2019;2019/193614 A2:Iran. [Google Scholar]
- 158.Wenming J. et al. Preparation method of phycobiliprotein polypeptide powder. Patent Application N° CN 109536557 A, China (2019).
- 159.Schmatz D.A., da Silveira Mastrantonio D.J., Vieira Costa J.A., de Morais M.G. Encapsulation of phycocyanin by electrospraying: A promising approach for the protection of sensitive compounds. Food Bioprod Process. 2020;119:206–215. [Google Scholar]
- 160.Pradeep H.N., Nayak C.A. Enhanced stability of C-phycocyanin colorant by extrusion encapsulation. J Food Sci Technol. 2019;56:4526–4534. doi: 10.1007/s13197-019-03955-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Pan-utai W., Iamtham S. Enhanced microencapsulation of C-phycocyanin from Arthrospira by freeze-drying with different wall materials. Food Technol. Biotechnol. 2020;58:423–432. doi: 10.17113/ftb.58.04.20.6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Hashimoto T., et al. Encapsulation of biomacromolecules by soaking and co-crystallization into porous protein crystals of hemocyanin. Biochem Biophys Res Commun. 2019;509:577–584. doi: 10.1016/j.bbrc.2018.12.096. [DOI] [PubMed] [Google Scholar]
- 163.Chandralekha A., Prashanth H.S., Tavanandi H., Raghavarao K.S.M.S. A novel method for double encapsulation of C-phycocyanin using aqueous two phase systems for extension of shelf life. J Food Sci Technol. 2021;58:1750–1763. doi: 10.1007/s13197-020-04684-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Chen D., et al. Development of MOF “Armor-Plated” phycocyanin and synergistic inhibition of cellular respiration for hypoxic photodynamic therapy in patient-derived xenograft models. Adv Healthc Mater. 2021;10:2001577. doi: 10.1002/adhm.202001577. [DOI] [PubMed] [Google Scholar]
- 165.Wen P., et al. Targeted delivery of phycocyanin for the prevention of colon cancer using electrospun fibers. Food Funct. 2019;10:1816–1825. doi: 10.1039/c8fo02447b. [DOI] [PubMed] [Google Scholar]
- 166.Wen Y., et al. Encapsulation of phycocyanin by prebiotics and polysaccharides-based electrospun fibers and improved colon cancer prevention effects. Int J Biol Macromol. 2020;149:672–681. doi: 10.1016/j.ijbiomac.2020.01.189. [DOI] [PubMed] [Google Scholar]
- 167.Huang Z., Guo B., Wong R., Jiang Y. Characterization and antioxidant activity of selenium-containing phycocyanin isolated from Spirulina platensis. Food Chem. 2007;100:1137–1143. [Google Scholar]
- 168.Zhi, H., Qinjie, L., Chenghui, Z. H. . & Zhihui, C. A. . Anti-enteritis spirulina selenylation phycobiliprotein polypeptide complex and preparation and application thereof. Patent Application N° CN 106491659 A, China (2017).
- 169.Thangam R., et al. C-Phycocyanin from Oscillatoria tenuis exhibited an antioxidant and in vitro antiproliferative activity through induction of apoptosis and G 0/G1 cell cycle arrest. Food Chem. 2013;140:262–272. doi: 10.1016/j.foodchem.2013.02.060. [DOI] [PubMed] [Google Scholar]
- 170.Gupta N.K., Gupta K.P. Effects of C-Phycocyanin on the representative genes of tumor development in mouse skin exposed to 12-O-tetradecanoyl-phorbol-13-acetate. Environ Toxicol Pharmacol. 2012;34:941–948. doi: 10.1016/j.etap.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 171.Ge B., et al. Pilot-scale fermentation and purification of the recombinant allophycocyanin over-expressed in Escherichia coli. Biotechnol Lett. 2005;27:783–787. doi: 10.1007/s10529-005-5794-7. [DOI] [PubMed] [Google Scholar]
- 172.Wen R., Sui Z., Zhang X., Zhang S., Qin S. Expression of the phycoerythrin gene of Gracilaria lemaneiformis (Rhodophyta) in E. coli and evaluation of the bioactivity of recombinant PE. J Ocean Univ Chin. 2007;6(4):373–377. [Google Scholar]
- 173.Qing, Q. . Composition containing phycobiliprotein polysaccharide extract and use of composition containing phycobiliprotein polysaccharide extract. Granted Patent N° CN 108472323 B, China (2019).
- 174.Zhao, H., Pu, Y., Wei, M., Li, W. & Qin, S. Novel material based on natural diatom shell and phycobiliprotein and application. Granted Patent N° CN 110292635 B, China (2021).
- 175.Ouada, H. Ben & Ammar, J. Methods for inducing the synthesis of phycobiliproteins. (2017).
- 176.Jia, Y. et al. Methods for extracting and purifying Nostoc Sphaeroides kutzing phycobiliprotein, and purified phycoerythrin. Granted Patent N°CN 107200774 B, China (2020).
- 177.Jia, Y. et al. Extracting and purifying method for Nostoc sphaeroids kutzing phycobiliprotein and purified phycoerythrin. Granted Patent N°CN 107200774 B, China (2020).
- 178.Hongying L. et al. Extraction method of phycobiliprotein of Nostoc sphaeroides kutzing. Patent Application N° CN 106432480 A, China (2017).
- 179.Li Z, Yiliang P, Wenjun S. Phycobiliprotein draws with many fallers corona discharge reaction unit. Limited Patent N° CN 206219594 U, China (2017).
- 180.Heshan X, Zhenxiang C. Technology for separating and purifying phycobiliprotein from gracilaria tenuistipitata deep processing natron solution. Patent Application N°CN 106220728 A, China (2016).
- 181.Yu, J. et al. Method for extracting and purifying phycobiliprotein and purified phycocyanin. Granted Patent N° CN 107011433 B, China (2020).
- 182.Baosheng G, Xiangfa W, Fang H, Haixia CH. Kit for early detection of liver cancer and preparation method thereof. Patent Application N° CN 109085362 A, China (2018).
- 183.Xudong J, Wenjun L. Song QI. Detection reagent strip utilizing recombinant fluorescent phycobiliprotein subunit, and preparation method thereof. Patent Application N° CN 107064490 A,China (2017).
- 184.Tianfeng C, Wenjie Z, Yanyu H. Antitumor drug carrier and application method thereof. Granted Patent N° CN 104667289 B, China (2018).
- 185.Wenjun L. et al. Preparation method of double-network hydrogel loaded with phycobiliprotein. Granted Patent N° CN 109535448 B, China (2021).
- 186.Ruiz Canovas E. et al. Compositions For Protecting Skin Comprising Dna Repair Enzymes And Phycobiliprotein. Granted Patent N° US 11071707 B2, Spain (2021).
- 187.Yang J, Zheng Y. Heart valve prosthesis, preparation method and in-vivo heart valve prosthesis sterilization method. Granted Patent N° CN 109999219 B, China (2021).
- 188.Coxam R, Davicco M-J, Leotoing L, Wauquier F. Phycocyanin Composition For Use In Inhibiting Bone Resorption. Granted Patent N° FR 3066919 B1, France (2020).
- 189.Noorafshan A., Hashemi M., Karbalay-Doust S., Karimi F. High dose Allura Red, rather than the ADI dose, induces structural and behavioral changes in the medial prefrontal cortex of rats and taurine can protect it. Acta Histochem. 2018;120:586–594. doi: 10.1016/j.acthis.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 190.Altunay N. An optimization approach for fast, simple and accurate determination of indigo-carmine in food samples. Spectrochim Acta Part A Mol Biomol Spectrosc. 2021;257 doi: 10.1016/j.saa.2021.119791. [DOI] [PubMed] [Google Scholar]
- 191.Coros M., et al. Thermally reduced graphene oxide as green and easily available adsorbent for Sunset yellow decontamination. Environ Res. 2020;182 doi: 10.1016/j.envres.2019.109047. [DOI] [PubMed] [Google Scholar]
- 192.Guerrero-Rubio M.A., López-Llorca R., Henarejos-Escudero P., García-Carmona F., Gandía-Herrero F. Scaled-up biotechnological production of individual betalains in a microbial system. Microb Biotechnol. 2019;12:993–1002. doi: 10.1111/1751-7915.13452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Wehrs M., et al. Production efficiency of the bacterial non-ribosomal peptide indigoidine relies on the respiratory metabolic state in S. cerevisiae. Microb. Cell Factories. 2018;171(17):1–11. doi: 10.1186/s12934-018-1045-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Wang Z., Tschirhart T., Schultzhaus Z., Kelly E.E., Chen A., Oh E., et al. Melanin produced by the fast-growing marine bacterium vibrio natriegens through heterologous biosynthesis: Characterization and application. Appl Environ Microbiol. 2020;86(5) doi: 10.1128/AEM.02749-19. [DOI] [PMC free article] [PubMed] [Google Scholar]