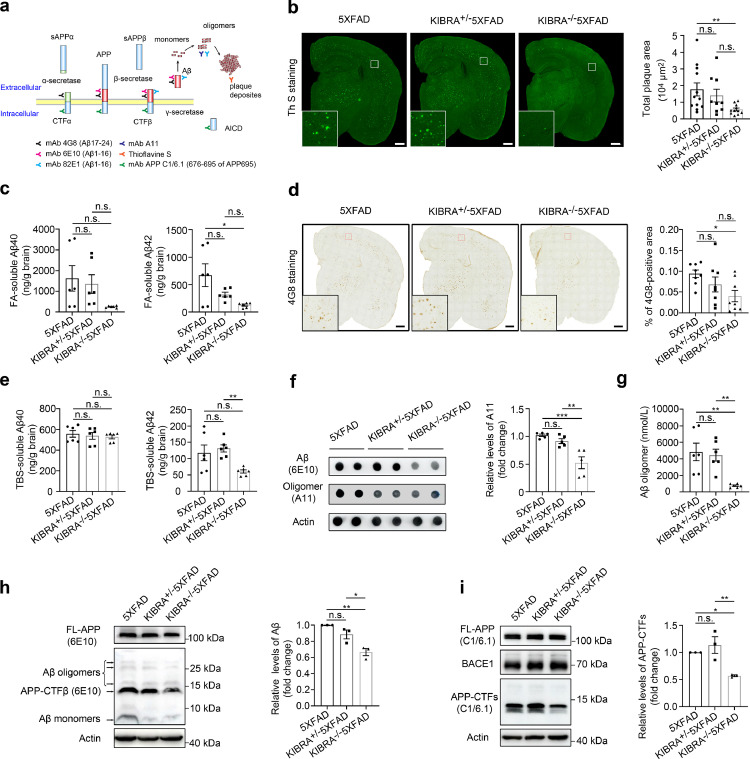
Figure 1.
KIBRA Knockout decreases Aβ from monomers, oligomers to extracellular deposits in 5XFAD mice. (a) Schematic representation of Aβ-specific antibody recognition sites. (b) Representative images and quantification analysis of ThS-stained insoluble Aβ plaques in whole brain of 3–5-month-old mice. Scale bars = 500 µm. 5XFAD (n = 6M/6F), KIBRA+/−5XFAD (n = 4M/5F), KIBRA−/−5XFAD (n = 6M/5F) mice. (c) ELISA analysis of Aβ40 and Aβ42 in formic-acid (FA)-soluble fractions isolated from the whole brain of 3–5-month-old mice. Brain lysates from six mice per group were analyzed. (d) Images and quantification analysis of the whole brains from 3–5-month-old mice of corresponding genotypes, labeled with the anti-APP/Aβ antibody (clone 4G8). Scale bar = 500 µm. Eight mice per group were analyzed. (e) ELISA analysis of Aβ40 and Aβ42 in TBS-soluble fractions isolated from the whole brain of 3–5-month-old mice. Brain lysates from six mice per group were analyzed. (f and g) Analysis of Aβ oligomer in the whole brain of the indicated groups. Dot blot analysis of anti-amyloidogenic protein oligomer A11 and quantification analysis of oligomer A11 (f), and ELISA analysis of 82E1-specific Aβ oligomers (g) from the whole brain lysates of 3–5-month-old mice (n = 6) in the whole brain lysates of the indicated groups. (h) WB analysis of brain lysates from indicated 5XFAD mice for full length APP (FL-APP), oligomer Aβ, CTF-β, and Aβ monomer (anti-Aβ: clone 6E10). (i) WB analysis of brain lysates from the indicated 5XFAD mice for APP/APP-CTFβ (anti-C1/6.1) and BACE1.Data are represented as the mean ± SE. n.s.≥0.05, ⁎⁎⁎P < 0.001, ⁎⁎P < 0.01, and *P < 0.05 as determined by one-way ANOVA followed by Tukey's post hoc comparisons tests.