Abstract
Introduction
The eight TNM classification of lung tumors provides a more precise prediction of prognosis than previous classification systems, especially in T1 tumors, the invasion size of which are less than or equal to 3 cm. T1 is divided into T1a (6–10 mm), T1b (11–20 mm), and T1c (21–30 mm), but the relationship between pathologic T (pT)1 categories and other pathologic factors has not been thoroughly evaluated.
Methods
Surgically resected pulmonary adenocarcinomas (N = 551) were extracted on the basis of computed tomography-based tumor size measurements, including 302 pT1a to c cases (pT1a: n = 98, pT1b: n = 156, and pT1c: n = 48). Pathologic factors, including a minor component of micropapillary or solid subtype, were analyzed by new T categories. Recurrence-free and disease-specific survivals (DSSs) were evaluated using univariable and multivariable analyses and Cox proportional hazards models.
Results
Lymphatic invasion, vascular invasion, and nodal metastasis increased remarkably from pT1a to pT1c, step-wisely. Visceral pleural invasion was elevated from 7% (6–10 mm) to 33% (21–30 mm) along with an increase in invasion size. Recurrence-free survival (RFS) and DSS relevantly deteriorated from the group of pathologic stages 0, IA1, and IA2 to the group IA3 and IB. Multivariable analysis revealed that lymph node metastasis and solid components were independent prognostic factors for both RFS and DSS in pT1a to c cases.
Conclusions
The new TNM classification precisely predicts prognosis. Tumor invasion size is closely associated with lymphatic and vascular invasion, nodal metastasis, and visceral pleural invasion. As a minor component, solid subtype was a potent adverse prognostic factor affecting both RFS and DSS after surgery in T1 categories.
Keywords: Lung, Small adenocarcinoma, TNM classification, T1 stage, Prognostic factors
Introduction
Lung cancer has been a leading cause of cancer-related deaths for decades. Accurate staging is crucial in management of patients. Pathologic staging after surgery is one of the most important predictors of cancer recurrence and survival. The eight edition of the TNM staging system has been effective internationally since January 1, 2017. The latest version of the TNM system is the product of an extensive initiative by the International Association for the Study of Lung Cancer, proposing a subdivision of T1 categories, that is, T1a (6 < invasion size ≤ 10 mm), T1b (10 < invasion size ≤ 20 mm), and T1c (21 < invasion size ≤ 30 mm) for lung cancers. A new classification has been reported to be prognostically more precise.1 Furthermore, several studies have reported that nonanatomical factors, such as lymphatic vessel invasion and a minor component of high-grade subtypes, affect prognosis.2, 3, 4 Nevertheless, the association between the clinicopathologic parameters and prognosis of surgically treated T1 adenocarcinomas has not been fully understood. A better understanding of the relationship between T1 categories and other prognostic factors would be beneficial for postoperative monitoring of patients and can contribute to prognostic prediction for small adenocarcinomas. In this study, we evaluated the relationship between the eight TNM classification system, especially the subclassification of the T1 categories, and other factors, including lymphatic and vascular invasion, visceral pleural invasion, and nodal metastasis. Moreover, we sought to evaluate the prognostic capacity of each factor in T1a, T1b, and T1c categories of lung adenocarcinomas.
Materials and Methods
Case Selection
We reviewed chest computed tomography (CT) images of patients who underwent radical surgery in the Thoracic Surgery Department of The Cancer Institute Hospital between January 2006 and December 2011. Patients with adenocarcinoma were selected on the basis of maximal tumor size being 3 cm or less derived using soft tissue/mediastinal window settings on CT images. Data regarding clinical, pathologic, and treatment information were also obtained from hospital records. Age at the time of surgery, sex, smoking status (nonsmoker, ex-smoker, and current smoker), and types of surgery were also obtained. Cases with incomplete resection or multiple invasive cancers were excluded. All patients provided informed consent for medical research, and the institutional review board of the Japanese Foundation for Cancer Research approved the study plan.
Patient Follow-up
Postoperative workup was performed every 6 months in the first 2 years and annually thereafter. Follow-up studies included blood tumor marker testing, chest CT imaging, brain magnetic resonance imaging, and when necessary, positron emission tomography scanning. Imaging diagnostics, including chest CT, were performed at any time recurrence was clinically suspected.
Histologic Evaluation
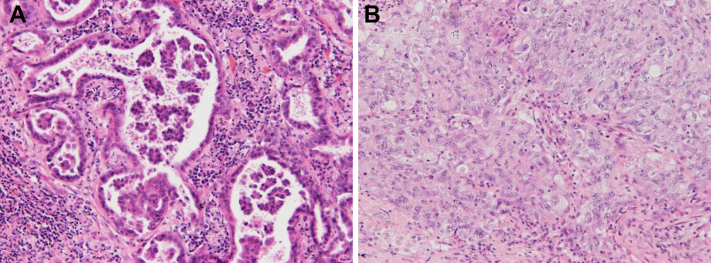
Surgical specimens were fixed with 15% buffered formalin and sectioned to 5 mm. Blocks were made from all tumor tissues embedded in paraffin. Slices, 4-μm thick, were stained with hematoxylin and eosin. Elastica van Gieson staining was also used to highlight elastic fibers, which made it easier to detect vascular invasion and visceral pleural invasion and to recognize pathologically invasive areas and destruction of alveolar structures. According to the revised classification, all tumor histologic sections were evaluated and comprehensive histologic subtyping was performed semiquantitatively with 5% increments. The existence of micropapillary (Fig. 1A) and solid (Fig. 1B) components, representing a high-grade subtype, was defined as 5% or more area of the lesion occupied by either one of them.3,5,6 Two pathologists (HN and YI) reviewed all slides and discussed cases until consensus was achieved.
Figure 1.
High-grade subtypes of pulmonary adenocarcinoma. (A) Micropapillary component. Small clusters composed of low numbers of tumor cells floating in the airspace. (B) Solid component. Polygonal cells proliferating in a solid fashion.
Pathologic Diagnosis
Pathologic diagnosis was made on the basis of the eight edition of the TNM staging system classification. In short, adenocarcinoma in situ was defined as small-sized (≤3 cm) lesions with growth restricted to neoplastic cells along a preexisting alveolar structure, namely with lepidic growth, which lacks vascular, stromal, or pleural invasion. Minimally invasive adenocarcinoma was histopathologically defined as small-sized (≤3 cm) solitary lesions, exhibiting a predominantly lepidic pattern, with invasion areas of less than or equal to 5 mm. Pathologic T (pT)1a, pT1b, and pT1c have invasion sizes of 6 to 10 mm, 11 to 20 mm, and 21 to 30 mm, respectively. Visceral pleural invasion 1 and 2 in stage I (invasion size ≤ 3 cm) was upgraded to pT2a. The PL1/3 status was defined as invasion beyond the elastic layer of the visceral pleura (PL1 or higher) but having unclear parietal pleural invasion7 and categorized as pT3 in this study according to the eighth edition of the General Rule for Clinical and Pathological Record of Lung Cancer.8 Vessel invasion was also found to be positive on staining with Elastica van Gieson. Lymphatic invasion was defined as the presence of tumor emboli within a lymphatic vessel lumen, which was detected by hematoxylin and eosin staining.
Statistical Analysis
All statistical analyses were performed using EZR, a graphical user interface for R. More precisely, it is a modified version of R commander designed to add statistical functions frequently used in biostatistics.9 Recurrence-free survival (RFS) was defined as the period from the date of surgical operation to the date of lung cancer recurrence. Disease-specific survival (DSS) was defined as the period from the date of operation to the date of lung cancer-related death. Loss to follow-up for any other reason or death was considered a censored event. The log-rank test was used to compare differences in RFS and DSS between adjacent groups. The value of statistical significance was set at p value less than or equal to 0.05, and marginal significance was at 0.05 less than p value less than 0.1. The Cox proportional hazards model was used to determine the risk factors for recurrence and cancer-related death. To investigate the prognostic value of clinicopathologic variables in terms of recurrence and cancer-related death, we adopted both univariable and multivariable analyses. chi-square or Fisher’s exact tests were used for categorical variables, as appropriate.
Results
Patient Characteristics and TNM Staging
A total of 551 cases were included in this study (Table 1). Among them, 324 (58.8%) were male and 232 (41.2%) were female patients. The average age was 63.7 years (range 33–86 y). In the female group, 260 cases (80.2%) were nonsmokers and 64 cases (19.8%) were current- and ex- (>30 d after cessation) smokers. Alternatively, in the male group, 182 patients (80.2%) were current-/ex-smokers, whereas 45 (19.8%) were nonsmokers. As for pT stages, 100 cases were in pTis (pathologic stage [pStage] 0, 18.1%) and pStage IA1 cases (including pT1 mi and pT1a) represented the largest proportion (n = 168, 30.5%), followed by pStage IA2 (n = 137, 24.9%). As a result, a total of 302 pT1a to c cases were included in this cohort.
Table 1.
Patient Profiles and Pathologic Stage
| Sex (M/F) Age, y (Mean) |
227/324 63.7 |
Types of Cancer | n | % | |
|---|---|---|---|---|---|
| n | % | ||||
| Smoking status | |||||
| Non | 305 | 55.3 | Adenocarcinoma in situ | 100 | 18.1 |
| Ex | 155 | 28.1 | MIA (mucinous, nonmucinous) | 73 | 13.2 |
| Current | 91 | 16.5 | Invasive adenocarcinoma | 378 | 68.6 |
| Pathologic stage | |||||
| 0 | 100 | 18.1 | Lepidic | 54 | |
| IA1 | 168 | 30.5 | Papillary | 256 | |
| IA2 | 137 | 24.9 | Acinar | 30 | |
| IA3 | 40 | 7.3 | Micropapillary | 5 | |
| IB | 28 | 5.1 | Solid | 19 | |
| IIB | 46 | 8.3 | Enteric | 1 | |
| IIIA | 30 | 5.4 | Invasive mucinous adenocarcinoma | 13 | |
| IIIB | 2 | 0.4 | |||
F, female; M, male; MIA, minimally invasive adenocarcinoma.
Survival Stratified by the Eight TNM Staging System
RFS of subsets of patients stratified by T and N descriptors is found in Supplementary Figure 1A. Each curve reveals a stepwise deterioration according to the upstaging of pStages from 0, IA1, IA2, and IA3 to II. No cancer recurrence occurred among pTis (pStage 0) and pT1mi (a part of pStage IA1) groups. Patients with pStage 0 to IA2 had excellent prognosis even after a 10-year follow-up. Differences between stages IA2 and IA3 (p = 0.03), IA3 and IB (p = 0.03), and IIB and IIIA (p = 0.02) were statistically significant, and the differences between IB and IIB (p = 0.05) were marginally so, on the basis of log-rank test results.
DSS curves are found in Supplementary Figure 1B. Although differences between pStage 0 and IA1 (p = 0.44) and IA1 and IA2 (p = 0.22) were not significant, DSS rates between IA2 and IA3 (p = 0.004), IA3 and IB (p = 0.043), and IIB and IIIA (p = 0.023) differed significantly. Differences in DSS between IB and IIB were not significant (p = 0.66) as patients with IIB had relatively good prognosis.
Correlation Between Clinicopathologic Factors and Recurrence and Cancer Death of pT1a to c Category Tumors
Statistical analysis was performed on clinicopathologic variables to analyze factors affecting cancer recurrence in pT1a, pT1b, and pT1c cases (Table 2). Clinicopathologic factors were as follows: sex, age (<65 y, ≥65 y), lymphatic invasion, vascular invasion, nodal metastasis, and 5% or more micropapillary or solid components. Some studies have revealed that minor components of micropapillary or solid histology are associated with poor prognosis.3,5,6 Lymphatic invasion (p = 0.011), vascular invasion (p = 0.03), nodal metastasis (p < 0.0001), and more than or equal to 5% micropapillary (p < 0.0001) or solid (p < 0.0001) components were significant adverse prognostic factors for recurrence. Moreover, lymphatic invasion (p < 0.0001), vascular invasion (p < 0.001), nodal metastasis (p < 0.00001), and more than or equal to 5% micropapillary (p < 0.001) or solid (p < 0.05) components were negative prognostic factors for DSS.
Table 2.
Correlation Between Clinicopathological Factors and Recurrence and Cancer-Death in pT1 Categories
| Variable | pT1a–c (n = 302) |
||||||
|---|---|---|---|---|---|---|---|
| Recurrence |
Disease-Specific Death |
||||||
| Yes 35 |
No 167 |
p-Value | Yes 21 |
No 181 |
p-Value | ||
| Sex (M/F) | M | 16 | 109 | 0.58 | 12 | 113 | 0.168 |
| F | 19 | 158 | 9 | 168 | |||
| Age (<65, ≥65), y | <65 | 17 | 123 | 0.77 | 12 | 128 | 0.367 |
| ≥65 | 18 | 144 | 9 | 153 | |||
| Lymphatic invasion | Positive | 19 | 31 | 0.011 | 11 | 38 | <0.0001 |
| Negative | 16 | 236 | 10 | 243 | |||
| Vascular invasion | Positive | 23 | 58 | 0.03 | 13 | 68 | <0.001 |
| Negative | 12 | 209 | 8 | 213 | |||
| Nodal metastasis | Positive | 17 | 13 | <0.0001 | 12 | 18 | <0.00001 |
| Negative | 18 | 254 | 9 | 263 | |||
| MP component (<5%, ≥5%) | <5% | 11 | 30 | <0.0001 | 9 | 32 | <0.001 |
| ≥5% | 24 | 237 | 12 | 249 | |||
| Solid component (<5%, ≥5%) | <5% | 14 | 35 | <0.0001 | 8 | 41 | <0.05 |
| ≥5% | 21 | 232 | 13 | 240 | |||
Note: Bold values denote statistical significance at the p < 0.05 level.
F, female; M, male; MP, micropapillary; pT, pathologic T.
Univariable and Multivariable Cox Regression Analyses to Identify Clinicopathologic Factors Affecting RFS and DSS of pT1 Categories (n = 302)
Univariable and multivariable analyses using the Cox proportional hazards model were performed to identify prognostic factors for RFS and DSS in pT1a to c categories (Table 3). Univariable analysis revealed that invasion size, nodal metastasis, lymphatic and vascular invasion, and more than or equal to 5% high-grade component (micropapillary and solid subtypes) were significant prognostic factors affecting both RFS and DSS. Multivariable analysis revealed that nodal metastasis (hazard ratio [HR] = 4.043, 95% confidence interval [CI]: 1.807–9.042, p < 0.001), lymphatic invasion (HR = 3.341, 95% CI: 1.593–7.008, p < 0.05), and more than or equal to 5% solid component (HR = 2.371, 95% CI: 1.098–5.117, p < 0.05) were significant prognostic factors affecting RFS. As for DSS, male sex (HR = 0.366, 95% CI: 0.136–0.988, p < 0.05), nodal metastasis (HR = 5.783, 95% CI: 1.982–16.88, p < 0.05), and more than or equal to 5% solid component (HR = 3.147, 95% CI: 1.078–9.185, p < 0.05) were significant prognostic factors.
Table 3.
Clinicopathologic Factors Affecting Survival of Pathological T1 Cases (n = 302) Examined by Univariable and Multivariable Analyses Using Cox Proportional Hazards Model
| Variable | Recurrence-Free Survival |
Disease-Specific Survival |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariable |
Multivariable |
Univariable |
Multivariable |
|||||||||
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | HR | 95% CI | p-Value | HR | 95% CI | p-Value | |
| Sex (M/F) | 0.825 | 0.424–1.604 | 0.571 | 0.850 | 0.417–1.735 | 0.656 | 0.495 | 0.208–1.177 | 0.111 | 0.366 | 0.136–0.988 | <0.05 |
| Age (<65, ≥65), y | 0.931 | 0.479–1.806 | 0.832 | 0.725 | 0.358–1.468 | 0.372 | 0.710 | 0.299–1.687 | 0.438 | 0.491 | 0.186–1.297 | 0.151 |
| Invasion size | 2.021 | 1.241–3.289 | <0.005 | 1.299 | 0.759–2.224 | 0.339 | 2.659 | 1.377–5.132 | <0.01 | 2.075 | 0.933–4.617 | 0.074 |
| Nodal metastasis | 13.14 | 6.733–25.65 | <0.00001 | 4.043 | 1.807–9.042 | <0.001 | 13.870 | 5.832–32.97 | <0.00001 | 5.783 | 1.982–16.88 | <0.01 |
| Lymphatic invasion | 7.674 | 3.938–14.95 | <0.00001 | 3.341 | 1.593–7.008 | <0.01 | 7.103 | 2.998–16.83 | <0.00001 | 2.670 | 0.983–7.254 | 0.054 |
| Vascular invasion | 6.010 | 2.988–12.09 | <0.00001 | 1.893 | 0.860–4.163 | 0.113 | 4.737 | 1.963–11.43 | <0.001 | 1.093 | 0.400–2.988 | 0.863 |
| MP component (<5%, ≥5%) | 3.185 | 1.560–6.505 | <0.01 | 2.032 | 0.925–4.463 | 0.078 | 4.579 | 1.927–10.88 | <0.001 | 1.937 | 0.703–5.34 | 0.201 |
| Solid component (<5%, ≥5%) | 4.001 | 2.034–7.872 | <0.0001 | 2.371 | 1.098–5.117 | <0.05 | 3.442 | 1.424–8.32 | <0.01 | 3.147 | 1.078–9.185 | <0.05 |
Note: Bold values denote statistical significance at the p < 0.05 level.
CI, confidence interval; F, female; HR, hazard ratio; M, male; MP, micropapillary.
Rate of Lymphatic and Vascular Invasion and Lymph Node Metastasis Stratified by pT Categories
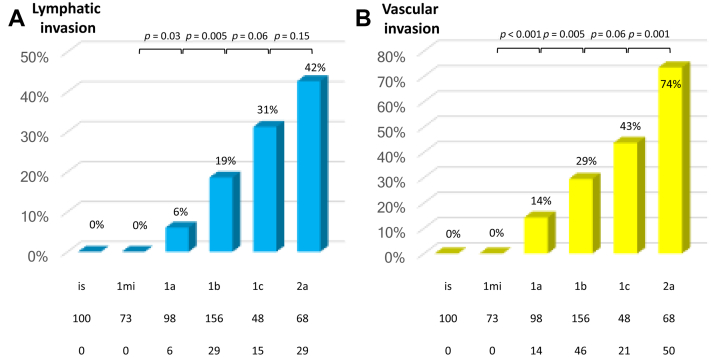
Lymphatic and vascular invasion rates stratified by pT subclassifications are found in Figure 2. First, the lymphatic invasion rate increased as the pT stage elevated from T1a to T1b, T1c, and T2a (Fig. 2A). The differences between pT1mi and pT1a and between pT1a and pT1b were statistically significant (p = 0.03 and p = 0.005, respectively). As for vascular invasion (Fig. 2B), the relationship between positivity rates and pT stage indicated a similar trend and differences between pT1mi and pT1a, pT1a and pT1b, and pT1c and pT2a were statistically significant (p < 0.001, p = 0.005, and p = 0.001, respectively).
Figure 2.
Correlation between (A) lymphatic and (B) vascular invasion rate and pathologic T descriptor.
The relationship between nodal metastatic status and pT stage is found in Figure 3A. Nodal metastatic rates indicated similar stepwise elevation along with the advancement of the pT stage. These differed significantly between pT1a and pT1b (3% and 12%, p = 0.011). The statistical difference was remarkable between pT1c and pT2a (17% and 49%, p < 0.001). The difference did not reach statistical significance between pT1b and pT1c (12% and 17%, p = 0.42).
Figure 3.
Increased rates of (A) lymph node metastasis by pathologic T stages and (B) visceral pleural invasion by invasion size.
Correlation Between Visceral Pleural Invasion and Invasion Size
Visceral pleural invasion is a potent prognostic factor that affects pT categories and can be upgraded to T2 even with a tumor invasion size measuring 3 cm or less. The correlation between visceral pleural invasion and pathologic invasion size of tumors is found in Figure 3B. Each tumor was reclassified solely according to invasion size (regardless of the existence of pleural invasion). The rate of visceral pleural invasion differed significantly between the two adjacent groups sorted by invasion size: 1 to 5 mm (0%), 6 to 10 mm (7%), 11 to 20 mm (16%), and 21 to 30 mm (33%) (p = 0.02, p = 0.02, and p = 0.001, respectively). This graph (Fig. 3B) clearly indicated that the invasion rate of the visceral pleura markedly and significantly increased in accordance with the invasion size.
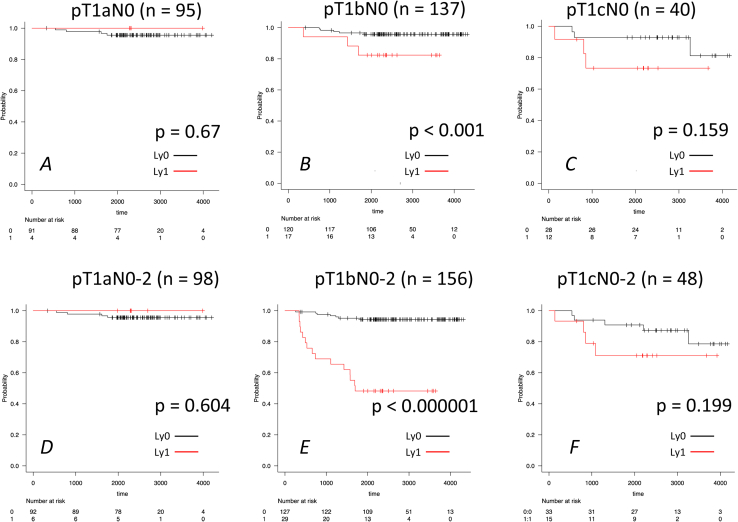
RFS Curves Stratified by Adverse Prognostic Factors in Each pT1 Category
We analyzed the difference of RFS with or without each factor depending on nodal metastasis status to evaluate the effect of each prognostic factor on survival of pT1a to c cases. Survival curves of each pT1 category, including N0 to 2 or only N0, stratified by each prognostic factor, are found in Supplementary Figures 2 to 5. In pT1a category, there was almost no prognostic difference between the two groups with or without adverse prognostic factors (Supplementary Figs. 2–5A and D). Notably, in pT1bN0 to 2, RFS differed significantly concerning any factor: lymphatic invasion (p < 0.000001; Supplementary Fig. 2E), vascular invasion (p < 0.000001; Supplementary Fig. 3E), solid component (≥5%) (p < 0.01; Supplementary Fig. 4E), and micropapillary component (≥5%) (p < 0.01; Supplementary Fig. 5E). In contrast, in pT1bN0 group, RFS had significant difference with or without lymphatic invasion (p < 0.001; Supplementary Fig. 2B), vascular invasion (p < 0.0001; Supplementary Fig. 3B), and solid component (≥5%) (p < 0.05; Supplementary Fig. 4B). Interestingly, the statistical difference of RFS compared with or without adverse prognostic factors changed depending on nodal metastasis presence. In other words, the prognostic impact on RFS is less potent given no nodal metastases. As for pT1c category, adverse prognostic factor-positive groups tended to have unfavorable outcomes (red line), although the statistical difference was not significant between two groups with or without any adverse prognostic factor in both N0 to 2 and N0 cases (lymphatic invasion: p = 0.199/0.159, vascular invasion: p = 0.136 and 0.125, solid component (≥5%): p = 0.075 (marginal) and 0.395, and micropapillary component (≥5%): p = 0.22 and 0.517; Supplementary Figs. 2–5C and F). In summary, as for pT1a category, patients had an excellent prognosis regardless of adverse prognostic factors, such as lymphatic or vascular invasion. In pT1b category, RFS differed significantly compared with or without any adverse prognostic factor in both N0 to 2 and N0 cases. In pT1c category, RFS tended to worsen when the adverse prognostic factors existed, although the difference was not statistically significant.
Discussion
In this study, we sought to investigate potential relationships between invasion size-based T subclassification and other pathologic factors. Furthermore, we intended to clarify pathologic factors affecting prognosis among T1 categories. To accomplish that, we compared the outcome with or without optional adverse prognostic factors. In the eight TNM classification, T1 is divided into T1a, T1b, and T1c, but no detailed analyses have ever been performed concerning the factors that affect prognosis among T1 subcategories. In this study, particular attention was paid to clarifying the clinicopathologic factors that affected RFS and DSS of pT1 categories. Early stage cancers are composed of a relatively homogeneous population and are suitable to evaluate the potential prognostic impact of nonanatomical factors, such as vascular invasion and existence of high-grade subtypes (micropapillary or solid). First, our results revealed that the rate of lymphatic invasion, vascular invasion, and nodal metastasis increased step-wisely along with the elevation of pT1 category. In addition, when confined to pT1a to pT1c cases, multivariable analysis revealed that nodal metastasis, lymphatic invasion, and more than or equal to 5% solid components were independent risk factors for RFS. Furthermore, nodal metastasis and more than or equal to 5% solid subtype components were independent risk factors for DSS.
The cancer staging system is critical for providing an accurate prognosis and stratification for appropriate treatment. Moreover, it is vital to transmit useful information concerning the prognosis and treatment choices for patients. One of the major changes in the eight TNM classification is the determination of pT subdivisions on the basis of the maximum dimension of the invasive component and excludes lepidic components.10 Since implementation of this revision, prognostic validity has been externally evaluated.11, 12, 13, 14 We evaluated the relationship with T subclassification and other pathologic factors, including nodal metastasis and pleural invasion, paying particular attention to T1 and T2a cases. Furthermore, we focused on other nonanatomical factors, that is, lymphatic and vascular invasion, which do not currently affect the T categories. There have been a small number of reports referring to the relationship between invasion size and other factors.15 Our results revealed that lymphatic and vessel invasion and nodal metastasis increased along with the elevation of pT categories, particularly in T1 subcategories. Visceral pleural invasion rate also elevated along with increasing invasion size (Fig. 3B). Such invasion has been found to be significantly associated with higher levels of lymph node metastasis.16 These results revealed that tumor invasion size was positively related to various factors corresponding to tumor invasiveness.2,15,17, 18, 19
When based on the current TNM staging, pTis and pT1mi were classified as stage 0 and stage IA, respectively. Nevertheless, our results also indicated that there were neither lymphatic/vascular invasions nor metastasis to lymph nodes in pTis and pT1mi cases (Figs. 2 and 3A). Moreover, both RFS and DSS were extremely good as found in Supplementary Figure 1. These findings propose that pTis and pT1mi can be regarded as one group, expecting no recurrence after surgery.6
Our results also revealed that minor components of high-grade subtypes, such as micropapillary and solid subtypes, even when representing only a small proportion of the whole tumor (≥5%) were found to have adverse prognostic impact on pT1a to c categories. In the multivariable analyses of pT1a to c categories, the presence of nodal metastasis and more than or equal to 5% solid subtypes affected both RFS and DSS. Some studies have reported that minor components of high-grade subtypes (micropapillary or solid components) could have a significant impact on prognosis.3, 4, 5,20 Recently, a grading system on the basis of high-grade subtypes has been found to be useful in prognostic terms and included in the new WHO classification.21,22 This classification is expected to enable selection of patients who could potentially benefit from additional therapy after surgery. Our result indicated that minor component of solid subtype had more potent prognostic potential than micropapillary subtype on both RFS and DSS. Nevertheless, further research is required to reveal that these factors should be considered in pathologic staging.
There were certain limitations to this study. It was a retrospective study that was conducted at a single institution involving a relatively small sample size, indicating possible selection bias. As for prognostic factor, frequently encountered EGFR mutation status was not included for analysis. Therefore, prospective multi-institutional randomized clinical trials are necessary to validate our results.
In conclusion, detailed classification based on tumor invasion size is closely associated with optional descriptors, such as lymphatic and vascular invasion, including nodal metastasis and the rate of visceral pleural invasion. Even a minor component of high-grade subtypes, micropapillary and solid components had adverse prognostic effects on small adenocarcinomas and solid component had more potent impact both for RFS and DSS. We emphasize that optional descriptors, including lymphatic/vascular invasion and a small proportion of poorly differentiated subtypes, can predict poorer prognosis, especially in pT1b or pT1c categories.
CRediT Authorship Contribution Statement
Hironori Ninomiya: Conceptualization, Methodology, Formal analysis, Investigation.
Kentaro Inamura, Mingyon Mun, Makoto Nishio: Writing - review & editing.
Yuichi Ishikawa: Visualization, Supervision, Validation, Writing - original draft.
Acknowledgments
The authors are grateful to all the other members of Asia-Australasia Pulmonary Pathology Society for useful discussion. The authors also thank Enago (www.enago.jp) for the English language review.
Footnotes
Disclosure: Dr. Inamura reports receiving research grants from Konica Minolta, Inc., and Daiichi Sankyo Co., Ltd. outside of the submitted work. The remaining authors declare no conflict of interest.
Cite this article as: Ninomiya H, Inamura K, Mun M, et al. Relationship between pathologic T1 categories and pathologic factors affecting prognosis in pulmonary adenocarcinoma. JTO Clin Res Rep. 2022;3:100293.
Note: To access the supplementary material accompanying this article, visit the online version of the JTO Clinical and Research Reports at www.jtocrr.org and at https://doi.org/10.1016/j.jtocrr.2022.100293
Supplementary Data
Supplementary Figure 1.
Supplementary Figure 2.
Supplementary Figure 3.
Supplementary Figure 4.
Supplementary Figure 5.
References
- 1.Goldstraw P., Chansky K., Crowley J., et al. The IASLC Lung Cancer Staging Project: proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM Classification for Lung Cancer. J Thorac Oncol. 2016;11:39–51. doi: 10.1016/j.jtho.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 2.Noma D., Inamura K., Matsuura Y., et al. Prognostic effect of lymphovascular invasion on TNM staging in stage I non-small-cell lung cancer. Clin Lung Cancer. 2018;19:e109–e122. doi: 10.1016/j.cllc.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 3.Zhao Y., Wang R., Shen X., et al. Minor components of micropapillary and solid subtypes in lung adenocarcinoma are predictors of lymph node metastasis and poor prognosis. Ann Surg Oncol. 2016;23:2099–2105. doi: 10.1245/s10434-015-5043-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen T., Luo J., Gu H., et al. Impact of solid minor histologic subtype in postsurgical prognosis of stage I lung adenocarcinoma. Ann Thorac Surg. 2018;105:302–308. doi: 10.1016/j.athoracsur.2017.08.018. [DOI] [PubMed] [Google Scholar]
- 5.Miyoshi T., Satoh Y., Okumura S., et al. Early-stage lung adenocarcinomas with a micropapillary pattern, a distinct pathologic marker for a significantly poor prognosis. Am J Surg Pathol. 2003;27:101–109. doi: 10.1097/00000478-200301000-00011. [DOI] [PubMed] [Google Scholar]
- 6.Yambayev I., Sullivan T.B., Suzuki K., et al. Pulmonary adenocarcinomas of low malignant potential: proposed criteria to expand the spectrum beyond adenocarcinoma in situ and minimally invasive adenocarcinoma. Am J Surg Pathol. 2021;45:567–576. doi: 10.1097/PAS.0000000000001618. [DOI] [PubMed] [Google Scholar]
- 7.Mikubo M., Nakashima H., Naito M., et al. Prognostic impact of uncertain parietal pleural invasion at adhesion sites in non-small cell lung cancer patients. Lung Cancer. 2017;108:103–108. doi: 10.1016/j.lungcan.2017.03.002. [DOI] [PubMed] [Google Scholar]
- 8.The Japan Lung Cancer Society . revised version. 8th ed. Kanehara Publishing Co; Tokyo, Japan: 2021. General Rule for Clinical and Pathological Record of Lung Cancer. [Google Scholar]
- 9.Kanda Y. Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transplant. 2013;48:452–458. doi: 10.1038/bmt.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Travis W.D., Asamura H., Bankier A.A., et al. The IASLC lung cancer staging project: proposals for coding T categories for subsolid nodules and assessment of tumor size in part-solid tumors in the forthcoming eighth edition of the TNM classification of lung cancer. J Thorac Oncol. 2016;11:1204–1223. doi: 10.1016/j.jtho.2016.03.025. [DOI] [PubMed] [Google Scholar]
- 11.Wang J., Wu N., Lv C., Yang Y. External validation of the eighth edition of the TNM classification for lung cancer in 3,611 surgically treated patients at a single institution. Ann Transl Med. 2020;8:122. doi: 10.21037/atm.2020.01.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yun J.K., Lee G.D., Kim H.R., et al. Validation of the 8th edition of the TNM staging system in 3,950 patients with surgically resected non-small cell lung cancer. J Thorac Dis. 2019;11:2955–2964. doi: 10.21037/jtd.2019.07.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moon Y., Choi S.Y., Park J.K., Lee K.Y. Prognostic factors in stage IB non-small cell lung cancer according to the 8th edition of the TNM staging system after curative resection. J Thorac Dis. 2019;11:5352–5361. doi: 10.21037/jtd.2019.11.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu W., Zhao Q., Xia C., et al. Validation of stage groupings in the eighth edition of the tumor node metastasis classification for lung adenocarcinoma. Thorac Cancer. 2019;10:483–491. doi: 10.1111/1759-7714.12961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ichinose Y., Yano T., Yokoyama H., Inoue T., Asoh H., Katsuda Y. The correlation between tumor size and lymphatic vessel invasion in resected peripheral stage I non-small-cell lung cancer. A potential risk of limited resection. J Thorac Cardiovasc Surg. 1994;108:684–686. [PubMed] [Google Scholar]
- 16.Shimizu K., Yoshida J., Nagai K., et al. Visceral pleural invasion is an invasive and aggressive indicator of non-small cell lung cancer. J Thorac Cardiovasc Surg. 2005;130:160–165. doi: 10.1016/j.jtcvs.2004.11.021. [DOI] [PubMed] [Google Scholar]
- 17.Moon Y., Park J.K., Lee K.Y., Sung S.W. Lymphatic invasion is a more significant prognostic factor than visceral pleural invasion in non-small cell lung cancer with tumours of 3 cm or less. Respirology. 2017;22:1179–1184. doi: 10.1111/resp.13029. [DOI] [PubMed] [Google Scholar]
- 18.Matsuura N., Go T., Fujiwara A., et al. Lymphatic invasion is a cause of local recurrence after wedge resection of primary lung cancer. Gen Thorac Cardiovasc Surg. 2019;67:861–866. doi: 10.1007/s11748-019-01095-6. [DOI] [PubMed] [Google Scholar]
- 19.Al-Alao B.S., Gately K., Nicholson S., McGovern E., Young V.K., O’Byrne K.J. Prognostic impact of vascular and lymphovascular invasion in early lung cancer. Asian Cardiovasc Thorac Ann. 2014;22:55–64. doi: 10.1177/0218492313478431. [DOI] [PubMed] [Google Scholar]
- 20.Matsuoka Y., Yurugi Y., Takagi Y., et al. Prognostic significance of solid and micropapillary components in invasive lung adenocarcinomas measuring≤ 3 cm. Anticancer Res. 2016;36:4923–4930. doi: 10.21873/anticanres.11058. [DOI] [PubMed] [Google Scholar]
- 21.WHO Classification of Tumours Editorial Board. Thoracic Tumours. 5th ed. International Agency for Research on Cancer; Lyon, France: 2021. [Google Scholar]
- 22.Moreira A.L., Ocampo P.S.S., Xia Y., et al. A grading system for invasive pulmonary adenocarcinoma: a proposal from the International Association for the Study Lung Cancer Pathology Committee. J Thorac Oncol. 2020;15:1599–1610. doi: 10.1016/j.jtho.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]