Abstract
Introduction
Lung cancer is the leading cause of cancer-related death and the second most often diagnosed malignancy worldwide. Males have higher incidence of lung cancer and higher mortality. It is hypothesized that the sex differences in survival are primarily driven by a better response of females to treatment. The primary objective of this work is to analyze and describe outcome differences between males and females diagnosed with having lung cancer.
Methods
Data were obtained from a large hybrid academic-community practice institution and validated with Surveillance, Epidemiology, and End Results (SEER). The initial cohort included patients aged more than or equal to 18 years diagnosed with having primary malignant lung cancer. Patients were excluded from the analysis if they had an unknown diagnosis date, were missing sex, or had prior history of cancer. Chi-square, t test, and Kruskal-Wallis tests were used to compare characteristics of males and females. Risks were estimated by logistic and Cox regressions.
Results
A total of 8909 patients from our institution and 725,018 in SEER were analyzed. Male-to-female ratio was 1.0. Females were more likely to undergo surgery and less likely to be treated with immunotherapy. Females had higher rates of documented psychological affections, depression, anxiety, urinary tract infection, hypothyroidism, and hyperthyroidism, while displaying lower rates of acute kidney injury, myocardial infarction, and myocarditis. Paired multivariable models revealed a lower risk of death for females in SEER (hazard ratio for females = 0.84, confidence interval: 0.69–1.02, p = 0.08) and equal risks in our institution (hazard ratio for females = 0.84, confidence interval: 0.69–1.02, p = 0.08).
Conclusions
Female sex was associated with higher surgical rates, lower immunotherapy use rates, higher rates of endocrinologic complications after immunotherapy use, and higher rates of psychological disorders.
Keywords: Lung cancer, Sex differences, Treatment, Outcomes, SEER
Introduction
Lung cancer is the leading cause of cancer-related death and the second most often diagnosed malignancy worldwide, estimated to have caused 1.8 million deaths and 2.2 million new cases in 2020.1 In the United States, lung cancer represents 12.4% of all new cancer cases, with a median age at diagnosis of 71 years old and a 5-year relative survival of 21.7%.2 Besides the wide-ranging clinicopathologic features, it is classified broadly as non-small cell lung cancer (NSCLC), representing 85% of the cases, and small cell lung cancer (SCLC), with 15% of the cases.3,4
Approximately 80% of the cases are attributable to a history of smoking, with other validated risk factors including radon exposure, air pollution, and occupational workplace exposure.5,6 Despite the availability of low-dose screening computed tomography scans for patients with important tobacco use, patients usually present for diagnostic evaluation after becoming symptomatic. The treatment strategy is tailored to the TNM (primary tumor, regional lymph nodes, and distal metastasis) stage and typically includes surgery, chemotherapy, radiotherapy, and immunotherapy either alone or combined.6
Sex differences in cancer incidence and outcomes are well documented. Epidemiologic data indicate higher incidence rates (>20%) and mortality rates (>40%) in males, with a better prognosis for females when looking at all cancers combined.7,8 Males have a higher incidence of lung cancer, with sex ratios varying from 1.5 to 20, and females tend to be diagnosed younger and at earlier stages.9, 10, 11, 12 A delayed onset of the lung cancer epidemic in females is attributed to changes in smoking patterns, with developed countries demonstrating an increased incidence among females and decreased incidence in males.13 Worldwide lung cancer mortality is approximately threefold higher in males with a current downward trend for males and upward trend for females.9,14, 15, 16 Although lung adenocarcinomas are the most common histologic subtype in both sexes, females have proportionally more adenocarcinoma and less squamous cell carcinoma when compared with males,9 possibly owing to differing smoking patterns in females compared with males.9,14, 15, 16
It is hypothesized that the sex differences in lung cancer survival are driven, in addition to other factors, by a better response of females to treatment. To our knowledge, there are no studies with large cohorts describing sex differences on treatment patterns and treatment effects.9,17 Therefore, the primary objective of this work is to analyze and elucidate potential outcome differences between males and females diagnosed with having lung cancer at a single hybrid academic-community practice institution.
Materials and Methods
This cohort is composed of patients with lung cancer from a single large, hybrid academic-community practice institution (herein referred to as University Hospitals [UH]) in the United States, composed of an academic campus and more than 18 community practice satellites. Its research data repository is based on CAISIS, an open-source, web-based cancer data management system that integrates research with patient care from at least eight disparate sources. The data sources encompass clinical chart notes, coding and reimbursement claims, laboratory results, scheduling, tumor registry, clinical pathway systems, and clinical trial database. Patient records from 2005 to 2020 were deidentified, and the study was approved by the institutional review board. All the analysis were performed in accordance with relevant guidelines and regulations, respecting the Declaration of Helsinki and a waive of the informed consent was consented.
The initial cohort included patients aged more than or equal to 18 years diagnosed with having primary malignant lung cancer (determined using tumor registry or electronic medical record International Classification of Diseases [ICD] codes C34.XX and 162.X, with X standing for any integer).18,19 Patients were excluded from the analysis if they had an unknown diagnosis date, missing sex, or a prior history of cancer.
The data elements retrieved for each patient included the following: demographics, tobacco use, comorbidity index, tumor characteristics (histology -NSCLC or SCLC or other- and stage), treatment modalities, complications of treatment, and survival outcomes. The demographic characteristics included age at diagnosis (categorized as either <65 y or ≥65 y), race (white, black, Asian), ethnicity (Hispanic, non-Hispanic), and medical insurance (commercial insurance, Medicare, Medicaid, not insured).2 Additional demographic features were extracted on the basis of the patient’s zip code using the Social Determinants of Health Database from the Agency for Healthcare Research and Quality20: median income, percentage of population with only high school diploma, percent population with less than high school diploma, and percentage of employed population.
Smoking status (categorized as yes or no or former according to the patient self-report during the anamnesis) and Charlson comorbidity score (extracted from the comorbidities list on the basis of the ICD codes)21 were obtained. Tumor characteristics included cancer diagnosis date, histologic type (NSCLC, SCLC), and clinical staging group (stages 0–II and III–IV, according to the International Association for the Study of Lung Cancer TNM system of the diagnosis year). Stages 0 to II were considered earlier stages and stages III to IV advanced stages.
Treatment patterns included the use of single or multiple treatment modalities: chemotherapy, radiotherapy, immunotherapy, targeted therapy, and surgery. Medications included in each category are described in Supplementary Table 1. Time to and on treatment variables were extracted on the basis of first day of diagnosis, first day of therapy, and last day of therapy and categorized according to median values. Compliance to treatment was calculated based on cancelled appointments divided by the total number of appointments for each patient.
Treatment complications were extracted on the basis of ICD, Ninth Revision, and ICD, Tenth Revision, codes from the comorbidities list, where only diagnoses occurring after the date of first treatment were considered. Complications from any treatment included cognitive decline or dementia (yes, no), psychological disorders (yes, no) and their subtypes, and oxygen dependence after treatment (yes, no). Surgical complications included those most often reported in the literature and 30-day and 90-day mortality after surgery.22, 23, 24, 25 Chemotherapy complications included those most frequently reported in the literature.26,27 Complications from checkpoint inhibitor therapy were described as immune-related adverse events (irAEs) (yes, no) and subtypes of irAEs.28, 29, 30 The ICD codes and categorizations are summarized on Supplementary Table 1. Outcomes included vital status (yes, no) and median survival (mo). Patients who had a reported date of death were considered dead, whereas those with no reported date of death were considered alive.
The Surveillance, Epidemiology, and End Results (SEER) program data set was used to compare and validate our findings with the general U.S. population. Data were obtained from SEER∗stat software on the basis of SEER research plus database for lung cancer diagnosis between 2005 and 2018.31 The variables analyzed were categorized after the methodology applied to the UH database and included age at diagnosis, race, ethnicity, histology, clinical staging, chemotherapy, surgery, vital status, and median survival.
Statistical Analysis
Pearson’s chi-square tests were used to compare categorical variables by sex. To compare continuous variables by sex, the normality was checked using normal plots and the Kolmogorov-Smirnov test, proceeding with t test for normally distributed data, and the nonparametric Kruskal-Wallis for non-normally distributed data. The influence of sex on time to treatment, time on active treatment, treatment complications, surgery complications, and chemotherapy complications variables was assessed by OR with 95% confidence intervals (CIs) using univariable and multivariable logistic regression. Correlation was analyzed by means of correlation plots, and correlated variables were not included simultaneously in the final multivariable models. Overall survival by sex was first assessed using Kaplan-Meier analysis generating median survival by sex with 95% CI and log-rank tests. Cox proportional hazards regressions models were used to assess univariable and multivariable models of overall survival by sex after checking assumptions. The variables selected for the multivariable models were those with p value less than 0.15 on the univariable model and those with clinical importance (and not correlated). A p value less than 0.05 was considered significant, and all analyses were performed using RStudio 1.4.1717 software.32
Results
Institutional Data
A total of 8909 eligible patients with lung cancer were identified from 2005 to 2020, of whom 51% were female, reflecting an overall equitable sex distribution of this population (Table 1). No differences were noted between sexes in the age of diagnosis (p = 0.94). Significant differences were observed in racial and sex distribution, with female black patients accounting for 20.3% of the female cohort, whereas black male patients accounted for 17.6% (p = 0.008). Female patients lived predominantly in zip codes with higher median income (p = 0.01) and a higher education level (p < 0.001).
Table 1.
Demographics, Risk Factors, and Cancer Characteristics by Sex for Patients With Lung Cancer From Our Institution (UH) (2005–2020) and SEER (2005–2018) Databases
| Feature | UH |
SEER |
||||
|---|---|---|---|---|---|---|
| Male 4366 (49%) |
Female 4543 (51%) |
p Value | Male 378,452 (52.2%) |
Female 346,566 (47.8%) |
p Value | |
| Age at diagnosis, n (%)a | ||||||
| <65 y | 1543 (35.5) | 1604 (35.4) | 0.94 | 118,047 (31.2) | 103,886 (30) | <0.001 |
| ≥65 y | 2804 (64.5) | 2926 (64.5) | 260,405 (68.8) | 242,680 (70) | ||
| Unknown | 19 | 13 | — | — | ||
| Race, n (%)a | ||||||
| White | 2798 (81.8) | 2913 (79.4) | 0.008 | 307,077 (81.7) | 289,058 (84) | <0.001 |
| Black | 603 (17.6) | 745 (20.3) | 44,419 (11.8) | 35,630 (10.4) | ||
| Asian | 18 (0.5) | 12 (0.3) | 24,255 (6.5) | 19,289 (5.6) | ||
| Other or unknown | 947 | 873 | 2701 | 2589 | ||
| Ethnicity, n (%)a | ||||||
| Hispanic | 22 (0.5) | 26 (0.6) | 0.77 | 22,018 (5.8) | 19,741 (5.7) | 0.02 |
| Non-Hispanic | 4023 (99.5) | 4203 (99.4) | 356,434 (94.2) | 326,825 (94.3) | ||
| Unknown | 321 | 314 | — | — | — | |
| Primary payer, n (%)a | ||||||
| Insured | 864 (28.5) | 941 (28.9) | 0.52 | — | — | — |
| Medicare | 1789 (59) | 1889 (58) | — | — | — | |
| Medicaid | 262 (8.6) | 311 (9.5) | — | — | — | |
| Not insured | 119 (3.9) | 117 (3.6) | — | — | — | |
| Other or unknown | 1332 | 1285 | — | — | — | |
| Median income, n (%)a | ||||||
| ≤$50,648 | 1888 (51.6) | 1855 (48.6) | 0.01 | |||
| >$50,648 | 1770 (48.4) | 1960 (51.4) | — | — | — | |
| Unknown | 708 | 728 | ||||
| Educational level | ||||||
| % Population with only high school diploma, mean, SDb | 32.3, 9 | 31.3, 8.9 | <0.001 | — | — | — |
| % Population with less than high school diploma, mean, SDb | 10.2, 6.3 | 10, 6.3 | 0.02 | — | — | — |
| Employment | ||||||
| % Population unemployed, mean, SDb | 4.3, 2.8 | 4.3, 2.8 | 0.80 | — | — | — |
| Histology, n (%)a | ||||||
| NSCLC | 2732 (88.2) | 2805 (86.7) | 0.07 | 231,893 (83.8) | 203,630 (80.4) | <0.001 |
| SCLC | 364 (11.8) | 429 (13.3) | 44,753 (16.2) | 49,532 (19.6) | ||
| Other or unknown | 1270 | 1309 | 101,806 | 93,404 | ||
| Histology subtype, n (%)a | ||||||
| Adenocarcinoma | 1150 (62.4) | 1399 (74.2) | <0.001 | — | — | — |
| Squamous cell carcinoma | 692 (37.6) | 486 (25.8) | — | — | ||
| Clinical staging, n (%)a | ||||||
| 0–II | 646 (26.1) | 736 (28.9) | 0.02 | 61,712 (20.3) | 69,596 (25.4) | <0.001 |
| III–IV | 1832 (73.9) | 1807 (71.1) | 242,304 (79.7) | 203,956 (74.6) | ||
| Unknown | 1888 | 2000 | 74,436 | 73,014 | ||
| Smoker, n (%)a | ||||||
| Yes | 543 (26.8) | 492 (23.1) | <0.001 | — | — | — |
| No | 180 (8.9) | 326 (15.3) | — | — | ||
| Former | 1300 (64.3) | 1309 (61.5) | — | — | ||
| Unknown | 2343 | 2416 | ||||
| Charlson score, n (%)a | ||||||
| 1–2 | 2486 (56.9) | 2686 (59.1) | 0.001 | — | — | — |
| 3–4 | 1147 (26.3) | 1221 (26.9) | — | — | ||
| ≥5 | 733 (16.8) | 636 (14) | — | — | ||
SEER, Surveillance, Epidemiology, and End Results; UH, University Hospital.
Compared with Pearson’s chi-square test.
Compared with Kruskal-Wallis test.
When we analyzed risk factors and tumor characteristics (Table 1), female patients were diagnosed at earlier stages (0–II) (28.9% versus 26.1%, p = 0.02), were more likely never smokers (15.3% versus 8.9%, p < 0.001), and had fewer comorbidities (p = 0.001). Although there were higher rates of SCLC among females (13.8% versus 11.8%), this did not reach statistical significance (p = 0.07), whereas for histologic subtype, females were more likely to have adenocarcinoma (74.2% versus 62.4 % for males, p < 0.001).
An analysis of treatment patterns (Table 2) revealed that females were more likely to undergo surgery (26.4% versus 23%, p < 0.001) and less likely to be treated with immunotherapy (p = 0.03). No significant differences were observed between chemotherapy and targeted therapy use rates, time to chemotherapy, time to immunotherapy, time to surgery, or compliance to treatment. The analysis was stratified by stage (Table 3) and reveals that females with earlier stages (0–II) are more likely to undergo surgery (57.6% versus 49.8%, p = 0.004) and less likely to receive chemotherapy (27.9% versus 35.3%, p = 0.003), but they tend to be longer on chemotherapy overall (p = 0.009), whereas females with advanced stages (III–IV) are more likely to receive chemotherapy (69% versus 65.8%, p = 0.04) and take longer to receive this treatment after diagnosis (p = 0.02).
Table 2.
Treatment Patterns by Sex for Patients With Lung Cancer From UH (2005–2020) and SEER (2005–2018) Databases
| Feature | UH |
SEER |
||||
|---|---|---|---|---|---|---|
| Male |
Female |
p Value | Male |
Female |
p Value | |
| 4366 (49%) | 4543 (51%) | 378,452 (52.2%) | 346,566 (47.8%) | |||
| Chemo, n (%)a | ||||||
| Yes | 1941 (44.5) | 1985 (43.7) | 0.48 | 153,915 (40.7) | 132,425 (38.2) | <0.001 |
| Surgery, n (%)a | ||||||
| Yes | 1005 (23) | 1200 (26.4) | <0.001 | 72,937 (19.3) | 80,765 (23.3) | <0.001 |
| Immunotherapy or PD-L1 inhibitors, n (%)a | ||||||
| Yes | 1074 (24.6) | 1028 (22.6) | 0.03 | — | — | — |
| Targeted therapy, n (%)a | ||||||
| Yes | 136 (3.1) | 167 (3.7) | 0.16 | — | — | — |
| Time to chemo, n (%)a | ||||||
| ≤30 d | 723 (48.2) | 691 (45.2) | 0.10 | — | — | — |
| >30 d | 778 (51.8) | 839 (54.8) | — | — | ||
| Time to immunotherapy, n (%)a | ||||||
| ≤50 d | 517 (50.6) | 468 (48.2) | 0.30 | — | — | — |
| >50 d | 505 (49.4) | 503 (51.8) | — | — | ||
| Time to surgery, n (%)a | ||||||
| ≤30 d | 286 (51.6) | 331 (52.8) | 0.73 | — | — | — |
| >30 d | 268 (48.4) | 296 (47.2) | — | — | ||
| Time of chemo, n (%)a | ||||||
| ≤60 d | 421 (41.6) | 345 (35.6) | 0.007 | — | — | — |
| >60 d | 592 (58.4) | 623 (64.4) | — | — | ||
| Compliance to treatmentb | ||||||
| Appointments cancelled (mean, %) | 22.2 | 21.4 | 0.86 | |||
Chemo, chemotherapy; PD-L1, programmed death-ligand 1; SEER, Surveillance, Epidemiology, and End Results; UH, University Hospital.
Compared with Pearson’s chi-square test.
Compared with Kruskal-Wallis test.
Table 3.
Treatment Patterns by Sex and by Stage for Patients With Lung Cancer From UH (2005–2020) Database
| Feature | Stages 0–II |
Stages III–IV |
||||
|---|---|---|---|---|---|---|
| Male 646 (46.7%) |
Female 736 (53.3%) |
pValue | Male 1832 (50.3%) |
Female 1807 (49.7%) |
p Value | |
| Chemo, n (%)a | ||||||
| Yes | 228 (35.3) | 205 (27.9) | 0.003 | 1205 (65.8) | 1246 (69) | 0.04 |
| Surgery, n (%)a | ||||||
| Ye | 322 (49.8) | 424 (57.6) | 0.004 | 225 (12.3) | 231 (12.8) | 0.68 |
| Immunotherapy or PD-L1 inhibitors, n (%)a | ||||||
| Yes | 123 (19) | 111 (15.1) | 0.06 | 549 (30) | 565 (31.3) | 0.41 |
| Targeted therapy, n (%)a | ||||||
| Yes | 9 (1.4) | 7 (1) | 0.60 | 81 (4.4) | 99 (5.5) | 0.16 |
| Time to chemo, n (%)a | ||||||
| ≤30 d | 51 (26.3) | 51 (28) | 0.79 | 540 (57) | 507 (51.8) | 0.02 |
| >30 d | 143 (73.7) | 131 (72) | 407 (43) | 471 (48.2) | ||
| Time to immunotherapy, n (%)a | ||||||
| ≤50 d | 29 (23.8) | 22 (20) | 0.59 | 317 (57.8) | 299 (53.3) | 0.14 |
| >50 d | 93 (76.2) | 88 (80) | 231 (42.2) | 262 (46.7) | ||
| Time to surgery, n (%)a | ||||||
| ≤30 d | 102 (52.6) | 134 (53.6) | 0.90 | 61 (46.6) | 54 (42.2) | 0.55 |
| >30 d | 92 (47.4) | 116 (46.4) | 70 (53.4) | 74 (57.8) | ||
| Time of chemo, n (%)a | ||||||
| ≤60 d | 50 (42.7) | 26 (25.2) | 0.009 | 208 (39.5) | 179 (33.5) | 0.05 |
| >60 d | 67 (57.3) | 77 (74.8) | 318 (60.5) | 355 (66.5) | ||
| Compliance to treatmentb | ||||||
| Appointments cancelled (mean, %) | 17.5 | 16.6 | 0.87 | 19.9 | 19.9 | 0.29 |
Chemo, chemotherapy; PD-L1, programmed death-ligand 1; SEER, Surveillance, Epidemiology, and End Results; UH, University Hospital.
Compared with Pearson’s chi-square test.
Compared with Kruskal-Wallis test.
Complications after treatment (Table 4) were accounted only for patients with associated treatment codes, consisting of 2716 males (48.5%) and 2887 females (51.5%). Females had higher rates of documented psychological disorders (p < 0.001), depression (p = 0.001), and anxiety (p < 0.001). Chemotherapy was prescribed to 2034 males (50.8%) and 1971 females (49.2%), and no sex differences were observed for complications after this treatment in our population. Overall, 1005 males (45.6%) and 1200 females (54.4%) underwent surgery and sex differences for complications after surgery were noticeable for higher rates of urinary tract infection (UTI) in females (3.8% versus 1.1%, p < 0.001) and lower 90-day mortality after surgery (6% versus 8.4%, p = 0.03). Immunotherapy was prescribed for 1074 males (51.1%) and 1028 females (48.9%). In irAEs (Table 5), female patients had higher rates of hypothyroidism (p < 0.001) and hyperthyroidism (p < 0.001), whereas they displayed lower rates of acute kidney injury (p < 0.01) and a lower tendency of myocardial infarction (1.2% versus 2.3% for males) and myocarditis (3.4% versus 5.1% for males).
Table 4.
Complications After Any Treatment, Chemotherapy, and Surgery by Sex for Patients With Lung Cancer From UH (2005–2020) Database
| Feature General treatment |
UH |
||
|---|---|---|---|
| Male 2716 (48.5%) |
Female 2887 (51.5%) |
p Value | |
| Cognitive decline or dementia, n (%)a | |||
| Yes | 24 (0.9) | 29 (1) | 0.74 |
| Psychological disorders, n (%)a | |||
| Yes | 104 (3.8) | 219 (7.6) | <0.001 |
| Subtypes of psychological disorders, n (%)a | |||
| Depression | 73 (2.7) | 123 (4.3) | 0.001 |
| Anxiety | 55 (2) | 143 (5) | <0.001 |
| Bipolar disorder | 8 (0.3) | 11 (0.4) | 0.74 |
| O2 dependence after treatment, n (%)a | |||
| Yes | 30 (1.1) | 44 (1.5) | 0.20 |
| Chemotherapy | Male 2038 (50.8%) |
Female 1971 (49.2%) |
p Value |
|---|---|---|---|
| Complications after chemo, n (%)a | |||
| Yes | 31 (1.5) | 27 (1.4) | 0.78 |
| Types of complications, n (%)a | |||
| Cardiomyopathy | 3 (0.1) | 1 (0.1) | 0.64 |
| Neuropathy | 4 (0.2) | 1 (0.1) | 0.39 |
| Diarrhea or enteritis | 7 (0.3) | 5 (0.3) | 0.81 |
| Fatigue or weakness | 4 (0.2) | 4 (0.2) | 1 |
| Nausea or vomiting | 5 (0.2) | 7 (0.4) | 0.72 |
| Steatohepatitis | 0 | 0 | — |
| Anemia | 4 (0.2) | 7 (0.4) | 0.50 |
| Agranulocytosis | 9 (0.4) | 10 (0.5) | 0.94 |
| Thrombocytopenia | 2 (0.1) | 3 (0.2) | 0.97 |
| Lung disease | 10 (0.5) | 12 (0.6) | 0.77 |
| Related pain | 6 (0.3) | 4 (0.2) | 0.79 |
| Mouth sore | 1 | 1 (0.1) | 1 |
| Hypovolemia | 12 (0.6) | 11 (0.6) | 1 |
| Renal failure | 1 | 1 (0.1) | 1 |
| Drug induced rash | 0 | 1 (0.1) | 0.98 |
| Infusion reaction | 0 | 2 (0.1) | 0.46 |
| Other adverse reaction | 7 (0.3) | 7 (0.4) | 1 |
| Surgery | Male 1005 (45.6%) |
Female 1200 (54.4%) |
p Value |
|---|---|---|---|
| Complications after surgery, n (%)a | |||
| Yes | 99 (9.9) | 136 (11.3) | 0.29 |
| Types of complications, n (%)a | |||
| Atrial fibrillation | 43 (4.3) | 50 (4.2) | 0.98 |
| Arrhythmia | 24 (2.4) | 42 (3.5) | 0.16 |
| Myocardial infarction | 10 (1) | 13 (1.1) | 1 |
| ARDS | 5 (0.5) | 1 (0.1) | 0.14 |
| Pulmonary edema | 6 (0.6) | 9 (0.7) | 0.86 |
| Pulmonary embolism | 9 (0.9) | 18 (1.5) | 0.27 |
| Respiratory failure | 22 (2.2) | 26 (2.2) | 1 |
| Postprocedural complication of respiratory system | 4 (0.4) | 2 (0.2) | 0.52 |
| Subcutaneous emphysema | 3 (0.3) | 3 (0.2) | 1 |
| Gastrointestinal bleeding | 0 | 0 | — |
| Infection after a procedure | 3 (0.3) | 5 (0.4) | 0.91 |
| Cerebrovascular accident | 0 | 0 | — |
| Delirium or disorientation | 16 (1.6) | 24 (2) | 0.57 |
| Deep vein thrombosis | 5 (0.5) | 12 (1) | 0.27 |
| Postoperative bleeding | 1 (0.1) | 4 (0.3) | 0.48 |
| Renal failure | 34 (3.4) | 37 (3.1) | 0.78 |
| Urinary tract infection | 11 (1.1) | 45 (3.8) | <0.001 |
| Conversion to open surgery | 2 (0.2) | 4 (0.3) | 0.84 |
| Other complications | 5 (0.5) | 6 (0.5) | 1 |
| Death 30 d after surgery, n (%)a | |||
| Yes | 41 (4.1) | 35 (2.9) | 0.16 |
| Death 90 d after surgery, n (%)a | |||
| Yes | 84 (8.4) | 72 (6) | 0.03 |
ARDS, Acute Respiratory Distress Syndrome; Chemo, chemotherapy; O2, oxygen; UH, University Hospital.
Compared with Pearson’s chi-square test.
Table 5.
irAEs by Sex for Patients With Lung Cancer From UH (2005–2020) Database
| Feature | UH |
||
|---|---|---|---|
| Male 1074 (51.1%) |
Female 1028 (48.9%) |
p Value | |
| irAEs, n (%)a | |||
| Yes | 472 (43.9) | 448 (43.6) | 0.89 |
| Subtypes of irAEs, n (%)a | |||
| Rash or eczema | 17 (1.6) | 16 (1.6) | 1 |
| Diarrhea | 28 (2.6) | 45 (4.4) | 0.03 |
| Adrenal insufficiency | 1 (0.1) | 2 (0.2) | 0.96 |
| Hyperlipidemia | 306 (28.5) | 244 (23.7) | 0.01 |
| Nephritis | 0 | 0 | — |
| Leukopenia | 15 (1.4) | 15 (1.5) | 1 |
| Hypothyroidism | 53 (4.9) | 101 (9.8) | <0.001 |
| Hyperthyroidism | 0 | 13 (1.3%) | <0.001 |
| Hypophysitis | 25 (2.3) | 27 (2.6) | 0.76 |
| Parathyroidism | 3 (0.3) | 7 (0.7) | 0.30 |
| Acute kidney injury | 68 (6.3) | 39 (3.8) | 0.01 |
| Neuritis | 21 (2) | 23 (2.2) | 0.76 |
| Hepatitis | 11 (1) | 5 (0.5) | 0.24 |
| Colitis | 11 (1) | 9 (0.9) | 0.89 |
| Pancreatitis | 5 (0.5) | 8 (0.8) | 0.52 |
| Mucositis | 7 (0.7) | 4 (0.4) | 0.59 |
| Arrhythmia | 184 (17.1) | 112 (10.9) | <0.001 |
| Myocardial infarction | 25 (2.3) | 12 (1.2) | 0.06 |
| Myocarditis | 55 (5.1) | 35 (3.4) | 0.06 |
| Pericarditis | 20 (1.9) | 13 (1.3) | 0.35 |
| Cardiomyopathy | 33 (3.1) | 12 (1.2) | 0.004 |
| Pneumonitis | 116 (10.8) | 100 (9.7) | 0.46 |
| Meningitis | 0 | 0 | — |
| Encephalopathy | 4 (0.4) | 2 (0.2) | 0.72 |
| Vitiligo | 1 (0.1) | 1 (0.1) | 1 |
| Thrombocytopenia | 22 (2) | 18 (1.8) | 0.73 |
| DM1 | 4 (0.4) | 7 (0.7) | 0.49 |
DM1, diabetes mellitus type 1; irAE, immune-related adverse event; UH, University Hospital.
Compared with Pearson’s chi-square test.
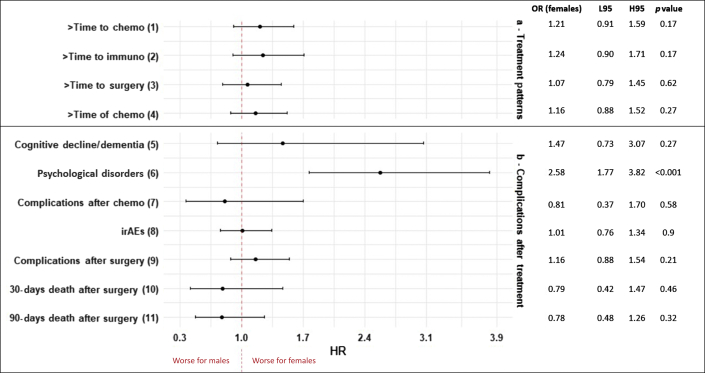
Figure 1 reveals significant associations between sex and treatment patterns, including treatment complications, on multivariable analysis. Females had higher ORs of psychological disorders (OR = 2.58, 95% CI: 1.77–3.82, p < 0.001). All the regression analysis, including univariable and multivariable models, are summarized in Supplementary Table 2.
Figure 1.
Multivariable model forest plot of sex differences in time to treatment, time of treatment, and complications after treatment for lung cancer, UH database, 2005 to 2020. The ORs are calculated for female patients. (1) Adjusted for age at diagnosis, smoking status, histology, Charlson score, stage, and surgery. (2) Adjusted for race, smoking status, histology, surgery, Charlson score, stage, and chemotherapy. (3) Adjusted for age at diagnosis, stage, chemotherapy, and immunotherapy. (4) Adjusted for age at diagnosis, subhistology. (5) Adjusted for age at diagnosis, Charlson score, stage, chemotherapy, and immunotherapy. (6) Adjusted for smoking status, Charlson score, stage, chemotherapy, immunotherapy, and surgery. (7) Adjusted for age at diagnosis, histology, surgery, stage, and immunotherapy. (8) Adjusted for age at diagnosis, smoking status, histology, Charlson score, stage, chemotherapy, and surgery. (9) Adjusted for age at diagnosis, Charlson score, and chemotherapy. (10) Adjusted for histology, stage, chemotherapy, and immunotherapy. (11) Adjusted for ethnicity, histology, stage, chemotherapy, and immunotherapy. H95, higher 95% confidence interval; HR, hazard ratio; irAE, immune-related adverse event; L95, lower 95% confidence interval; UH, University Hospital.
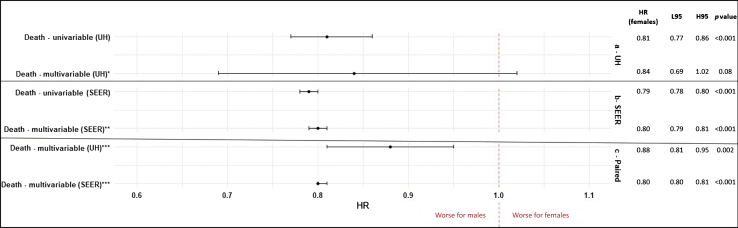
In this patient population overall, the median survival was 33 months (95% CI: 30–37) for females and 21 months (95% CI: 19–22) for males, with a p value less than 0.001 for the log-rank test. When looking by stage, the medial survival for patients with earlier stages (0–II) was 129 months (95% CI: 101–not applicable) for females and 62 months (95% CI: 50–76) for males, with p less than 0.001, whereas for patients with advanced stages (III–IV), 12 months (95% CI: 11–13) for females versus 10 months (95% CI: 9–11) for males, with p equals to 0.08.The univariable Cox model revealed lower risk of death for females (hazard ratio [HR] = 0.81, 95% CI: 0.77–0.86, p < 0.001). Nevertheless, a multivariable analysis accounting for covariates such as age at diagnosis, race, smoking, histology, chemotherapy, surgery, comorbidity index, stage, and time in treatment revealed equal risks for males and females (HR for females = 0.84, 95% CI: 0.69–1.02, p = 0.08). The multivariable models are summarized on Figure 2.
Figure 2.
Multivariable model forest plot of sex differences in survival for lung cancer, UH (2005–2020) and SEER database (2018–2020). The HRs are calculated for female patients. ∗Adjusted for age at diagnosis, race, smoking status, histology, Charlson score, stage, chemotherapy, surgery, time to chemotherapy, and time of chemotherapy. ∗∗Adjusted for age at diagnosis, race, ethnicity, histology, stage, chemotherapy, radiotherapy, and surgery. ∗∗∗Adjusted for age at diagnosis, race, ethnicity, histology, stage, chemotherapy, and surgery. H95, higher 95% confidence interval; HR, hazard ratio; L95, lower 95% confidence interval; SEER, Surveillance, Epidemiology and End Results; UH, University Hospital.
Surveillance, Epidemiology, and End Results Data
A total of 725,018 eligible patients with lung cancer were identified in SEER from 2005 to 2018, of whom 47.8% were female (Table 1). In SEER, female patients were diagnosed at an older age (p < 0.001), with a higher predominance of white (p < 0.001 for race) and Hispanic (p = 0.02 for ethnicity) patients when compared with our large institutional cohort. Analysis of risk factors and tumor characteristics revealed that females had a higher proportion of SCLC histology (p < 0.001) and lower rates of advanced-stage disease (III–IV) on diagnosis (p < 0.001). Analysis of treatment patterns (Table 2) revealed that females had lower rates of chemotherapy (p < 0.001) and higher rates of surgery (p < 0.001) when compared with males.
Survival analysis revealed sex differences with regard to vital status (p < 0.001) with 47.8% (263,374) of females being dead, compared with 82.7% (313,119) of males. The median survival was 8 months (95% CI: 8–9) for males and 12 months (95% CI: 12–12) for females, with a p value less than 0.001 for the log-rank test. A lower risk of death for females was observed on univariable (HR = 0.79, 95% CI: 0.78–0.80, p < 0.001) and multivariable (HR = 0.80, 95% CI: 0.79–0.81, p < 0.001) Cox regression models. Paired multivariable models were performed for SEER and our institution patient populations, adjusting for variables available on both data sets (age at diagnosis, race, ethnicity, histology, stage, chemotherapy, and surgery), and revealed a lower risk of death for females (our institution HR = 0.88, 95% CI: 0.81–0.95, p = 0.002; SEER HR = 0.80, 95% CI: 0.80–0.81, p < 0.001). The models are summarized on Figure 2.
Discussion
This study evaluated sex differences among patients with lung cancer regarding treatment modalities, time to treatment, its adverse events, and survival. Although this work focused on a lung cancer population diagnosed and treated at a single institution, its hybrid academic-community practice setting provided real-world assessment of such variables. By using a comprehensive, open-source web-based data integrating system, we assessed quality and detailed clinical annotations that allowed for an in-depth analysis of patients’ longitudinal trajectories.
The clinical characteristics of our population mirror a contemporaneous SEER database. Here, equitable rates of lung cancer were found among sexes, also reflecting current global epidemiologic data.33 Nevertheless, several studies have revealed that female sex is associated with higher risk for lung cancer after accounting for smoking status.33, 34, 35, 36 This includes higher odds for histologic subtypes highly related with cigarette smoking, such as squamous cell carcinoma and SCLC.37 Hence, it is hypothesized that current equitable lung cancer incidence rates are partially driven by delayed onset of smoking among females and higher incidence rates of lung cancer in females who never smoked.9,34, 35, 36 Notably, despite significantly lower smoking rates, females at UH institution had higher rates of SCLC, as found in the SEER database and described in prior studies.38 In addition to the lower smoking rates, analysis of our population revealed that females are healthier and diagnosed at earlier stages, corroborating the literature.9,39 Importantly, 20% of the female patients at our institution were black, compared with 17% of the males. Those rates are remarkably higher when compared with the SEER population and reflect the racial distribution of the institution’s catchment area. Although this study did not focus on racial disparities, the substantial number of females of black race in this database allows for invaluable representation.
Analysis of sex differences in treatment modalities and adverse events revealed that in this institution, females with lung cancer were more likely to undergo surgical resection than males. We hypothesize that this finding may reflect the fact that females were more likely to be diagnosed at earlier stages (p = 0.02) and had lower Charlson comorbidity scores (p = 0.001); however, causality may not be inferred as our study does not capture the decision-making process taking place between surgeons and patients, including the percentage of patients to which surgery was offered and potentially refused. Female sex has been identified as a negative predictor of undergoing surgery for lung cancer in previous studies.40,41 Nevertheless, our findings corroborate those of a contemporaneous SEER data, where female sex is also associated with higher surgical rates. This female cohort had lower 90-day mortality rates and higher rates of UTI. Although UTI rates are usually higher in women, when we analyzed the entire cohort, we noticed an incidence of 14.9% in females versus 8.2% in males; analysis in postsurgical patients also revealed a higher sex difference between rates (3.8% in females versus 1.1% in males). A previous analysis of the Society of Thoracic Surgeons General Thoracic Surgery Database encompassing more than 34,000 patients between 2002 and 2010 revealed remarkably lower OR for in-hospital and 30-day mortality in female patients.42 It is certainly possible that a more conservative approach when deciding to offer surgical resection for male patients with comorbidities at this institution could account for lack of differences in postoperative complications between sexes.
Chemotherapy treatment rates were not different between sexes. Female patients, however, in a univariable analysis, were more likely to stay on treatment longer. The reasons for this finding are unclear. Sex hormonal and pharmacogenomic differences that could perhaps influence chemotherapy tolerance have been described.43,44 Nevertheless, there were no sex differences in chemotherapy complications in this cohort, which makes tolerability less likely to be a determining factor. Use of maintenance chemotherapy with pemetrexed is the standard of care in treatment of metastatic adenocarcinoma. It is possible that higher rates of adenocarcinoma in females in this cohort could account for this, as adjusted multivariable models revealed loss of significance. We also speculate that this may reflect better clinical outcomes for female patients such as longer progression-free survival and overall survival. Immunotherapy use was slightly higher in males, possibly owing to greater rates of advanced disease and predictors of response to immunotherapy that are associated with smoking.45, 46, 47 We did not however assess for biomarker testing and results in this analysis, and such inferences remain speculative in nature. When it comes to irAEs, females were more likely to have endocrinologic complications, such as hyperthyroidism or hypothyroidism, but less likely to have cardiac-related events, corroborating previous populational reports.48,49 Higher rates of acute kidney injury in females were also noted. The reasons for that are unclear. Interestingly, no sex differences in the use of targeted therapy use were noted, possibly owing to very small number of patients in this category.
Female sex in this database was associated with higher rates of psychological disorders (i.e., anxiety and depression) after treatment. Whereas previous reports describe higher association between emotional problems and female sex at the time of lung cancer diagnosis, provider bias in diagnosing such conditions in female patients is not excluded.50, 51, 52
Finally, although median survival was longer for female patients (33 mo versus 21 mo, p < 0.001), sex was not an independent factor associated with survival after accounting for multiple variables of interest. This differed from SEER data, where female sex is independently associated with lower median survival, likely reflecting the long time analyzed combined with a large population and a higher number of diagnoses in advanced stages. Nevertheless, paired multivariable models adjusting for similar variables in SEER and our institution’s population generate similar associations, underscoring the level of granularity attained with our institution’s cohort data set that cannot be reproduced in less comprehensive databases.
This study has several limitations. Many patients are referred to our institution for second opinions at the academic campus, after undergoing diagnosis and initial treatment elsewhere. Hence, some of the information in the electronic medical record may be incomplete or reflect recall bias owing to the self-reporting nature of some risk factors (such as smoking status). In addition, owing to the retrospective nature of this study some very important treatment variables (radiotherapy appointments and treatments) were not available “and” or “or” associated with a high number of “unknown or not applicable” codes which could lead to a loss of statistical power for this analysis and were excluded. In addition, median income, education, and employment were estimated using a patient’s zip code, which may not accurately reflect the true socioeconomic characteristics of individual patients. Additional studies with other databases, larger cohorts, and with prospective design are needed to corroborate and investigate the findings reported here.
In summary, in this contemporaneous cohort of patients with lung cancer treated at this large hybrid academic-community practice institution, female sex was associated with earlier stage at diagnosis, lower smoking rates, and fewer comorbidities and with a trend toward higher rates of SCLC. Female sex was also associated with living at zip codes with higher income and educational levels. A large proportion of patients in this database are black, reflecting the composition of this institution’s catchment area and providing a heftier representation than SEER. Female sex was associated with higher surgical rates, lower immunotherapy use rates, and higher rates of endocrinologic complications after immunotherapy use. Higher rates of psychological diagnosis were also associated with female sex. Sex was not an independent factor associated with survival at this institution.
CRediT Authorship Contribution Statement
Nickolas Stabellini: Data curation, Formal analysis, Investigation, Methodology, Project administration, Validation, Visualization, Writing - original draft, Writing - review & editing.
Debora S. Bruno: Methodology, Project administration, Supervision, Validation, Writing - original draft, Writing - review & editing.
Mantas Dmukauskas: Data curation, Writing - review & editing.
Amie J. Barda: Data curation, Software, Writing - review & editing.
Lifen Cao: Writing - review & editing.
John Shanahan: Data curation, Resources, Software.
Kristin Waite: Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing - review & editing.
Alberto J. Montero: Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing - review & editing.
Jill Barnholtz-Sloan: Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing - review & editing.
Acknowledgments
Nickolas Stabellini is supported through funding from the Sociedade Beneficente Israelita Brasileira Albert Einstein on the program “Marcos Lottenberg & Marcos Wolosker International Fellowship for Physicians Scientist—Case Western.”
Footnotes
Disclosure: The authors declare no conflict of interest.
Cite this article as: Stabellini N, Bruno DS, Dmukauskas M, et al. Sex differences in lung cancer treatment and outcomes at a large hybrid academic-community practice. JTO Clin Res Rep. 2022;3:100307.
Note: To access the supplementary material accompanying this article, visit the online version of the JTO Clinical and Research Reports at www.jtocrr.org and at https://doi.org/10.1016/j.jtocrr.2022.100307
Supplementary Data
References
- 1.Sung H., Ferlay J., Siegel R.L., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.SEER. Cancer stat facts: lung and bronchus cancer. https://seer.cancer.gov/statfacts/html/lungb.html
- 3.Travis W.D., Brambilla E., Burke A.P., Marx A., Nicholson A.G. Introduction to the 2015 World Health Organization classification of tumors of the lung, pleura, thymus, and heart. J Thorac Oncol. 2015;10:1240–1242. doi: 10.1097/JTO.0000000000000663. [DOI] [PubMed] [Google Scholar]
- 4.Thai A.A., Solomon B.J., Sequist L.V., Gainor J.F., Heist R.S. Lung cancer. Lancet. 2021;398:535–554. doi: 10.1016/S0140-6736(21)00312-3. [DOI] [PubMed] [Google Scholar]
- 5.Malhotra J., Malvezzi M., Negri E., Vecchia C.L., Boffetta P. Risk factors for lung cancer worldwide. Eur Respir J. 2016;48:889–902. doi: 10.1183/13993003.00359-2016. [DOI] [PubMed] [Google Scholar]
- 6.Neal R.D., Sun F., Emery J.D., Callister M.E. Lung cancer. BMJ. 2019;365:l1725. doi: 10.1136/bmj.l1725. [DOI] [PubMed] [Google Scholar]
- 7.Micheli A., Ciampichini R., Oberaigner W., et al. The advantage of women in cancer survival: an analysis of EUROCARE-4 data. Eur J Cancer. 2009;45:1017–1027. doi: 10.1016/j.ejca.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 8.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 9.Kiyohara C., Ohno Y. Sex differences in lung cancer susceptibility: a review. Gend Med. 2010;7:381–401. doi: 10.1016/j.genm.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 10.Pope C.A., III, Burnett R.T., Thun M.J., et al. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. JAMA. 2002;287:1132–1141. doi: 10.1001/jama.287.9.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bilello K.S., Murin S., Matthay R.A. Epidemiology, etiology, and prevention of lung cancer. Clin Chest Med. 2002;23:1–25. doi: 10.1016/s0272-5231(03)00057-1. [DOI] [PubMed] [Google Scholar]
- 12.Sagerup C.M.T., Småstuen M., Johannesen T.B., Helland Å., Brustugun O.T. Sex-specific trends in lung cancer incidence and survival: a population study of 40 118 cases. Thorax. 2011;66:301–307. doi: 10.1136/thx.2010.151621. [DOI] [PubMed] [Google Scholar]
- 13.North C.M., Christiani D.C. Women and lung cancer: what’s new? Semin Thorac Cardiovasc Surg. 2013;25:87–94. doi: 10.1053/j.semtcvs.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boyle P., Ferlay J. Cancer incidence and mortality in Europe, 2004. Ann Oncol. 2005;16:481–488. doi: 10.1093/annonc/mdi098. [DOI] [PubMed] [Google Scholar]
- 15.Payne S. Smoke like a man, die like a man?: a review of the relationship between gender, sex and lung cancer. Soc Sci Med. 2001;53:1067–1080. doi: 10.1016/s0277-9536(00)00402-0. [DOI] [PubMed] [Google Scholar]
- 16.Parkin D.M., Bray F., Ferlay J., Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 17.Wisnivesky J.P., Halm E.A. Sex differences in lung cancer survival: do tumors behave differently in elderly women? J Clin Oncol. 2007;25:1705–1712. doi: 10.1200/JCO.2006.08.1455. [DOI] [PubMed] [Google Scholar]
- 18.International Classification of Diseases (ICD) ICD-10 version. https://icd.who.int/browse10/2019/en
- 19.International Classification of Diseases (ICD) ICD-9 version. https://www.cdc.gov/nchs/icd/icd9.htm
- 20.Agency for Healthcare Research and Quality Social determinants of health database (beta version) http://www.ahrq.gov/sdoh/data-analytics/sdoh-data.html
- 21.Charlson M.E., Pompei P., Ales K.L., MacKenzie C.R. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 22.Imperatori A., Rotolo N., Gatti M., et al. Peri-operative complications of video-assisted thoracoscopic surgery (VATS) Int J Surg Lond Engl. 2008;6(suppl 1):S78–S81. doi: 10.1016/j.ijsu.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 23.Ziarnik E., Grogan E.L. Post-lobectomy early complications. Thorac Surg Clin. 2015;25:355–364. doi: 10.1016/j.thorsurg.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shiono S., Abiko M., Sato T. Postoperative complications in elderly patients after lung cancer surgery. Interact Cardiovasc Thorac Surg. 2013;16:819–823. doi: 10.1093/icvts/ivt034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seely A.J.E., Ivanovic J., Threader J., et al. Systematic classification of morbidity and mortality after thoracic surgery. Ann Thorac Surg. 2010;90:936–942. doi: 10.1016/j.athoracsur.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 26.Muthu V., Mylliemngap B., Prasad K.T., Behera D., Singh N. Adverse effects observed in lung cancer patients undergoing first-line chemotherapy and effectiveness of supportive care drugs in a resource-limited setting. Lung India. 2019;36:32–37. doi: 10.4103/lungindia.lungindia_321_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spiro S.G., Douse J., Read C., Janes S. Complications of lung cancer treatment. Semin Respir Crit Care Med. 2008;29:302–317. doi: 10.1055/s-2008-1076750. [DOI] [PubMed] [Google Scholar]
- 28.Haratani K., Hayashi H., Chiba Y., et al. Association of immune-related adverse events with nivolumab efficacy in non–small-cell lung cancer. JAMA Oncol. 2018;4:374–378. doi: 10.1001/jamaoncol.2017.2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shankar B., Zhang J., Naqash A.R., et al. Multisystem immune-related adverse events associated with immune checkpoint inhibitors for treatment of non–small cell lung cancer. JAMA Oncol. 2020;6:1952–1956. doi: 10.1001/jamaoncol.2020.5012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cathcart-Rake E.J., Sangaralingham L.R., Henk H.J., Shah N.D., Riaz I.B., Mansfield A.S. A population-based study of immunotherapy-related toxicities in lung cancer. Clin Lung Cancer. 2020;21:421–427.e2. doi: 10.1016/j.cllc.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.SEER SEER incidence database—SEER data & software. https://seer.cancer.gov/data/index.html
- 32.RStudio Open source & professional software for data science teams. https://rstudio.com/
- 33.Zang E.A., Wynder E.L. Differences in lung cancer risk between men and women: examination of the evidence. J Natl Cancer Inst. 1996;88:183–192. doi: 10.1093/jnci/88.3-4.183. [DOI] [PubMed] [Google Scholar]
- 34.Pisani P., Srivatanakul P., Randerson-Moor J., et al. GSTM1 and CYP1A1 polymorphisms, tobacco, air pollution, and lung cancer: a study in rural Thailand. Cancer Epidemiol Biomark Prev. 2006;15:667–674. doi: 10.1158/1055-9965.EPI-05-0667. [DOI] [PubMed] [Google Scholar]
- 35.Fidler-Benaoudia M.M., Torre L.A., Bray F., Ferlay J., Jemal A. Lung cancer incidence in young women vs. young men: a systematic analysis in 40 countries. Int J Cancer. 2020;147:811–819. doi: 10.1002/ijc.32809. [DOI] [PubMed] [Google Scholar]
- 36.Jemal A., Miller K.D., Ma J., et al. Higher lung cancer incidence in young women than young men in the United States. N Engl J Med. 2018;378:1999–2009. doi: 10.1056/NEJMoa1715907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khuder S.A. Effect of cigarette smoking on major histological types of lung cancer: a meta-analysis. Lung Cancer Amst, Neth. 2001;31:139–148. doi: 10.1016/s0169-5002(00)00181-1. [DOI] [PubMed] [Google Scholar]
- 38.Ouellette D., Desbiens G., Emond C., Beauchamp G. Lung cancer in women compared with men: stage, treatment, and survival. Ann Thorac Surg. 1998;66:1140–1144. doi: 10.1016/s0003-4975(98)00557-8. [DOI] [PubMed] [Google Scholar]
- 39.Olak J., Colson Y. Gender differences in lung cancer: have we really come a long way, baby? J Thorac Cardiovasc Surg. 2004;128:346–351. doi: 10.1016/j.jtcvs.2004.05.025. [DOI] [PubMed] [Google Scholar]
- 40.Lathan C.S., Neville B.A., Earle C.C. The effect of race on invasive staging and surgery in non-small-cell lung cancer. J Clin Oncol. 2006;24:413–418. doi: 10.1200/JCO.2005.02.1758. [DOI] [PubMed] [Google Scholar]
- 41.Shugarman L.R., Mack K., Sorbero M.E.S., et al. Race and sex differences in the receipt of timely and appropriate lung cancer treatment. Med Care. 2009;47:774–781. doi: 10.1097/MLR.0b013e3181a393fe. [DOI] [PubMed] [Google Scholar]
- 42.Tong B.C., Kosinski A.S., Burfeind W.R., et al. Sex differences in early outcomes after lung cancer resection: analysis of the Society of Thoracic Surgeons General Thoracic Database. J Thorac Cardiovasc Surg. 2014;148:13–18. doi: 10.1016/j.jtcvs.2014.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harichand-Herdt S., Ramalingam S.S. Gender-associated differences in lung cancer: clinical characteristics and treatment outcomes in women. Semin Oncol. 2009;36:572–580. doi: 10.1053/j.seminoncol.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 44.Mederos N., Friedlaender A., Peters S., Addeo A. Gender-specific aspects of epidemiology, molecular genetics and outcome: lung cancer. ESMO Open. 2020;5(suppl 4) doi: 10.1136/esmoopen-2020-000796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Willis C., Fiander M., Tran D., et al. Tumor mutational burden in lung cancer: a systematic literature review. Oncotarget. 2019;10:6604–6622. doi: 10.18632/oncotarget.27287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rizvi N.A., Hellmann M.D., Snyder A., et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348:124–128. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sholl L.M., Hirsch F.R., Hwang D., et al. The promises and challenges of tumor mutation burden as an immunotherapy biomarker: a perspective from the International Association for the Study of Lung Cancer Pathology Committee. J Thorac Oncol. 2020;15:1409–1424. doi: 10.1016/j.jtho.2020.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Flynn R.W.V., MacDonald T.M., Morris A.D., Jung R.T., Leese G.P. The thyroid epidemiology, audit, and research study: thyroid dysfunction in the general population. J Clin Endocrinol Metab. 2004;89:3879–3884. doi: 10.1210/jc.2003-032089. [DOI] [PubMed] [Google Scholar]
- 49.Weidner G. Why do men get more heart disease than women? An international perspective. J Am Coll Health. 2000;48:291–294. doi: 10.1080/07448480009596270. [DOI] [PubMed] [Google Scholar]
- 50.Morrison E.J., Novotny P.J., Sloan J.A., et al. Emotional problems, quality of life, and symptom burden in patients with lung cancer. Clin Lung Cancer. 2017;18:497–503. doi: 10.1016/j.cllc.2017.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Samulowitz A., Gremyr I., Eriksson E., Hensing G. “Brave men” and “emotional women”: a theory-guided literature review on gender bias in health care and gendered norms towards patients with chronic pain. Pain Res Manag. 2018;2018 doi: 10.1155/2018/6358624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Christov-Moore L., Simpson E.A., Coudé G., Grigaityte K., Iacoboni M., Ferrari P.F. Empathy: gender effects in brain and behavior. Neurosci Biobehav Rev. 2014;46:604–627. doi: 10.1016/j.neubiorev.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.