Abstract
Bioaugmentation, the addition of cultured microorganisms to enhance the currently existing microbial community, is an option to remediate contaminated areas. Several studies reported the success of the bioaugmentation method in treating heavy metal contaminated soil, but concerns related to the applicability of this method in real-scale application were raised. A comprehensive analysis of the mechanisms of heavy metal treatment by microbes (especially bacteria) and the concerns related to the possible application in the real scale were juxtaposed to show the weakness of the claim. This review proposes the use of bioaugmentation-assisted phytoremediation in treating heavy metal contaminated soil. The performance of bioaugmentation-assisted phytoremediation in treating heavy metal contaminated soil as well as the mechanisms of removal and interactions between plants and microbes are also discussed in detail. Bioaugmentation-assisted phytoremediation shows greater efficiencies and performs complete metal removal from soil compared with only bioaugmentation. Research related to selection of hyperaccumulator species, potential microbial species, analysis of interaction mechanisms, and potential usage of treating plant biomass after treatment are suggested as future research directions to enhance this currently proposed topic.
Keywords: Biosorption, Phytotechnology, Environmental pollution, Real scale, Recovery, Separation
Biosorption, Phytotechnology, Pollution, Real scale, Recovery, Separation.
1. Introduction
Heavy metal is commonly found on Earth and is widely acknowledged as metalloids and metals in the periodic table (Yadav et al., 2019). The property of heavy metal with its high atomic weight and density makes it a good candidate for conducting electricity (Jaishankar et al., 2014; Shadman et al., 2019). Heavy metal processing industries are increasing with the utilization of heavy metals in daily life (C. He et al., 2020). The increment of heavy metal utilization leads to increasing cases of heavy metal pollution that is very dangerous to the environment and human life (Hejna et al., 2018).
Several heavy metals such as chromium in hexavalent form, Cr(VI), lead (Pb), and arsenic, which is generated from anthropogenic activities, are poisonous to living organisms (Oliveira, 2012; Kamaruzzaman et al., 2019; Titah et al., 2018). Mercury (Hg) also poses several hazardous characteristics such as neurotoxicity and immunotoxicity to living organisms, including humans (Bjørklund et al., 2017). Arsenic also causes enzyme reaction inhibition, especially related to phosphate uptake and utilization (Titah et al., 2018). Cadmium (Cd), which is abundantly found in soil, could reduce soil fertility due to its hazardous properties (Li et al., 2019). Specifically, Cd in soil is directly transferred to most plants and animals, inhibiting their actual growth and function (Chibuike and Obiora, 2014; Scaccabarozzi et al., 2020). Humans may also be affected and may possess health risks such as lung cancer and kidney problem because food sources are contaminated with toxic heavy metals (Li et al., 2019). Cd and Cr mining could also cause severe environmental pollution and an ecological disaster as it involves large operating areas. Mining activities, as one of the most reported sources of heavy metal in soil, release heavy metals into the surrounding environment (Ighalo et al., 2022). They cause most organisms to become exposed to hazardous pollutants and lose their natural habitat, hence creating another alarming issue of resistant bacteria in the environment (Oladipo et al., 2018; Schippers et al., 2010). The abundance of toxic waste accumulated in the environment has a remarkable effect on the entire ecosystem (Igwegbe et al., 2022). Therefore, the effect of anthropogenic activities should be reduced to minimize heavy metal pollutants in nature.
Bioaugmentation is a widely known approach to remediate heavy metal from contaminated environment by adding indigenous and exogenous microorganisms that can resist and reduce the toxicity of heavy metals (Hassan et al., 2019; Purwanti et al., 2020). Indigenous microorganisms are isolated from contaminated soil and reinoculated back into the corresponding contaminated soil (Purwanti et al., 2018a, Purwanti et al., 2018b). By contrast, exogenous microorganisms are isolated outside the contaminated areas and introduced into the desired contaminated area (Huang and Ye 2019). Several of the processes involved in remediating heavy metals using microbes are biosorption, bioaccumulation, bio chelation, bio digestion, biomineralization, and biotransformation (Nwaehiri et al., 2020).
Several studies claimed the success of bioaugmentation in treating heavy metal contaminated soil. Mahbub et al. (2017) reported the successful removal of Hg from artificial contaminated soil using Sphingobium SA2 with removal efficiency reaching 50%. Ibarrolaza et al. (2011) also mentioned the removal of hexavalent chromium from artificial contaminated soil by Sphingomonas paucimobilis 20006FA with removal efficiency of 90%. Polti et al. (2014) also reported removal of hexavalent chromium from artificial contaminated soil by using consortium of Streptomyces sp. M7, Streptomyces sp. MC1, Streptomyces sp. A5, and Amycolatopsis tucumanensis with removal efficiency of 86%.
However, most of the treatments were conducted in laboratory scale using artificial contaminated soil under controlled condition. Questions related to the applicability of this method in real-scale contaminated soil, especially in terms of the segregation of deposited metal from soil, to obtain remediated clean medium free from toxic heavy metals were elevated (Agnello et al., 2016; Purwanti et al., 2019a). Even if toxic metals are deposited as stable complexed precipitates in soil, stable heavy metals may turn back into mobilized phase due to uncontrolled climate or weather changes. This review highlights several concerns related to the applicability of bioaugmentation in treating contaminated soil, focusing on the failure of separating soil with metal after treatment. This review article identifies the mechanism of bacteria in detoxifying heavy metal correlated with the removal mechanisms occurring during the bioaugmentation in treating heavy metal contaminated soil. Phytoremediation is suggested as an alternative solution for the concerns, whereas the introduction of plant growth promoting rhizobacteria inside the heavy metal contaminated soil phytoremediation system will alleviate the removal processes. This review is expected to shed a light on the low-real-scale applicability of bioaugmentation to treat heavy metal contaminated soil while also providing the application of phytoremediation as a promising approach to treat heavy metal contaminated soil.
2. Heavy metal contaminated soil and bioaugmentation treatment
2.1. Sources of heavy metals in contaminated soil
Toxic heavy metals contaminate the soil largely due to anthropogenic activities such as mining, electroplating, tanneries, textile dye industry, automotive, electronics, batteries, and others, as shown in Table 1. Most of the areas are contaminated with Cu, As, Cr, Cu, Ni, Zn, Pb, Fe, Mn, and Cd at concentrations that are higher than maximum permissible addition (MPA) values, defined on (Vodyanitskii, 2016).
Table 1.
Sources of heavy metals that contaminate soil and their concentration.
| Source | Locality | Heavy metals∗∗ and concentration (mg/kg)∗ | References |
|---|---|---|---|
| Anthropogenic activities (nonspecific) | Australia | Cr (41.5) | Ghobadi et al. (2021) |
| Cu (185) | |||
| Mn (288.5) | |||
| Ni (12.5) | |||
| Pb (214.5) | |||
| Zn (311) | |||
| Anthropogenic activities (nonspecific) | Canada | Cr (1.67) | Dhiman et al. (2020) |
| Cu (7.11) | |||
| Fe (669) | |||
| Pb (0.04) | |||
| Zn (35) | |||
| Animal waste that has not been treated, metal industry, and fertilizers | Indonesia | As (67) | Sindern et al. (2016) |
| Cr (85) | |||
| Cu (128) | |||
| Ni (29) | |||
| Pb (111) | |||
| Zn (595) | |||
| Electroplating industries | India | Cr (2,195) | Sainger et al. (2011) |
| Cu (374) | |||
| Fe (2,228) | |||
| Ni (335) | |||
| Zn (3,112) | |||
| Landfills | Malaysia | As (163) | Hussein et al. (2021) |
| Cd (1.88) | |||
| Cr (346.82) | |||
| Cu (61.63) | |||
| Fe (58.12) | |||
| Mn (693.13) | |||
| Ni (15.63) | |||
| Pb (171.72) | |||
| Zn (79.06) | |||
| Metal factories | Egypt | Cd (18.6) | El-Saadony et al. (2021) |
| Ni (259) | |||
| Pb (254) | |||
| Metal factories | Fiji | Cd (2.62) | Diarra et al. (2021) |
| Cr (47.45) | |||
| Cu (72.58) | |||
| Fe (22,856) | |||
| Mn (70.26) | |||
| Ni (40.25) | |||
| Pb (110.35) | |||
| Zn (34.8) | |||
| Metal factories | Kazakhstan | Cd (8.9) | Ramazanova et al. (2021) |
| Cr (7.1) | |||
| Cu (137.9) | |||
| Pb (441.9) | |||
| Zn (178) | |||
| Mining area | China | Cd (0.6) | Jin et al., 2019a, Jin et al., 2019b |
| Cr (68.99) | |||
| Cu (138.92) | |||
| Ni (43.15) | |||
| Pb (2,021.71) | |||
| Zn (401.93) | |||
| Mining area | Korea | As (0.77) | Kwon et al. (2017) |
| Cd (0.98) | |||
| Cu (29.6) | |||
| Pb (3.34) | |||
| Zn (38) | |||
| Mining area | Iran | Cd (0.9) | Shojaei et al. (2021) |
| Cr (0.87) | |||
| Mn (0.88) | |||
| Pb (0.94) | |||
| Mining area | India | Cd (5.16) | Pradhan et al., 2020 |
| Co (3,097.01) | |||
| Cr (3,012.45) | |||
| Cu (7.51) | |||
| Fe (5,485.01) | |||
| Mn (5,840.5) | |||
| Ni (2,281.01) | |||
| Zn (226.1) | |||
| Mining area | Zambia | Cd (0.45) | Kaninga et al. (2020) |
| Cu (979) | |||
| Ni (44) | |||
| Pb (10.2) | |||
| Zn (32) | |||
| Mining area | Morocco | Cu (964.1) | Raklami et al. (2021) |
| Pb (110.6) | |||
| Zn (519.6) |
Bold highlighted items are concentrations that exceed the maximum permissible addition value.
Maximum permissible addition in mg/kg: As (4.5), Cd (0.76), Co (24), Cr (3.8), Cu (3.5), Ni (2.6), Pb (55), and Zn (16).
2.2. Bioaugmentation of microorganisms in treating heavy metal contaminated soil
Microorganisms are selected based on two main criteria: ability to degrade targeted pollutants and ability to resist and survive in a wide range of environments. Several microorganisms such as bacteria, fungi, yeast, actinomycetes, and algae can resist and survive in a wide range of environments, including the ability to remove heavy metals from contaminated areas. This capability is mostly originated from microbial cell walls made up of polysaccharides, lipids, and protein that play an essential role in attaching metal ions with carboxylate, hydroxyl, and amino and phosphate groups (Girma, 2015; Purwanti et al., 2018a, Purwanti et al., 2018b) resulting in nontoxic complexed compounds.
Table 2 summarizes several types of heavy metal resistant microorganisms and their removal percentage. Most of the microorganisms are isolated from heavy metal contaminated sites (indigenous) because they can easily adapt to the environment compared with exogenous microorganisms. By contrast, exogenous microorganisms face challenges for colonizing and maintaining immobilized effects in contaminated soils because they have to compete with indigenous microorganisms (Wang et al., 2020). Heavy metal contamination using microorganisms (either indigenous or exogenous) can be treated using either single or consortium cultures (Agnello et al., 2016; Purwanti et al., 2018a, Purwanti et al., 2018b, 2019b). Consortium of bacteria and actinomyces showed a higher reduction of heavy metal in the range 50%–86 % compared with single cultures. However, several single cultures are more effective than consortium cultures in removing heavy metals from contaminated soils. S. paucimobilis 20006FA (Ibarrolaza et al., 2011), Pannonibacter phragmitetus BB (Wang et al., 2015), and Staphylococcus aureus (Giwa and Ibitoye 2017) managed to reduce 90% of Cr(VI), 99% of Cr(VI), and 89.5% of Pb, respectively.
Table 2.
Heavy metal removal from contaminated soil by microorganisms.
| Microorganisms | Source | Type of soil | Exposure period (days) | Heavy metal | Removal (%) | Reference |
|---|---|---|---|---|---|---|
| Bacteria | ||||||
| Consortium of Serratia marcescens, Pseudomonas pyogenes, Erwnia amylovora, and Enterobacter cloacae | Soil and effluent of paper mill | Contaminated paper mill: 1.223 mg/L Pb, 0.093 mg/L Cd, 13.169 mg/L Zn, 0.613 mg/L As, 0.358 mg/L Cr, 1.756 mg/L Cu, and 0.059 mg/L Ni | 180 | Pb | 74.7 | Nwaehiri et al. (2020) |
| Cd | 51.6 | |||||
| Zn | 71.6 | |||||
| As | 72.6 | |||||
| Cr | 78.2 | |||||
| Cu | 55.9 | |||||
| Ni | 78.1 | |||||
| Consortium of Bacillus subtilis and Staphylococcus aureus | Soil and effluent of paper mill | Contaminated paper mill: 1.223 mg/L Pb, 0.093 mg/L Cd, 13.169 mg/L Zn, 0.613 mg/L As, 0.358 mg/L Cr, 1.756 mg/L Cu, and 0.059 mg/L Ni | 180 | Pb | 67.2 | |
| Cd | 53.8 | |||||
| Zn | 66.3 | |||||
| As | 66.1 | |||||
| Cr | 65.8 | |||||
| Cu | 59.4 | |||||
| Ni | 65.3 | |||||
| Brochothrix thermosphacta | Aluminum contaminated site | Artificial: 48 mg/kg of Al | 5 | Al | 4.58 | Purwanti et al. (2019a) |
| Vibrio alginolyticus | 5.48 | |||||
| Sphingobium SA2 | Mercury contaminated soil | Artificial: 100 mg/kg Hg Contaminated field: 200 mg/kg Hg |
28 | Hg | 30–50 | Mahbub et al. (2017) |
| Azotobacter S8 | N/A | Artificial: 19 mg/kg of Cr | 14 | Cr(III) | 22.82 | Purwanti et al., 2017a, Purwanti et al., 2017b |
| Bacillus subtilis | N/A | Artificial: 29 mg/kg of Cr | 14 | Cr(III) | 11 | Purwanti et al., 2017a, Purwanti et al., 2017b |
| Bacillus sp., Lysinibacillus sp. and Rhodococcus sp. | Landfill soil | Landfill and leachate: N/A | 100 | Cu | 86 | Emenike et al. (2016) |
| Zn | 73 | |||||
| Pb | 71 | |||||
| Sphingomonas paucimobilis 20006FA | Uncontaminated soil | Artificial: 100 mg/kg of Cr(VI) | 62 | Cr(VI) | 90 | Ibarrolaza et al. (2011) |
| Pannonibacter phragmitetus BB | Chromium containing slag | Steel alloy factory: 518.84 mg/kg of Cr(VI) | 2 | Cr(VI) | 99 | Wang et al. (2015) |
| Micrococcus sp. | Crude oil contaminated soil | Crude oil contaminated soil: N/A | 60 | Cd | 36 | Giwa and Ibitoye (2017) |
| Staphylococcus aureus | Crude oil contaminated soil: N/A | 60 | Pb | 89.5 | ||
| Actinomycetes | ||||||
| Streptomyces sp. M7 | Pesticides and heavy metals contaminated soil | Artificial: 50 mg/kg of Cr(VI) | 14 | Cr (VI) | 51 | Polti et al. (2014) |
| Streptomyces sp. MC1 | 40 | |||||
| Streptomyces sp. A5 | 5 | |||||
| Amycolatopsis tucumanensis | 5 | |||||
| Consortium of Streptomyces sp. M7, Streptomyces sp. MC1, Streptomyces sp. A5, and Amycolatopsis tucumanensis | 86 | |||||
| Fungi | ||||||
| Consortium of Perenniporia subtephropora, Daldinia starbaeckii, Phanerochaete concrescens, Cerrena aurantiopora, Fusarium equiseti, Polyporales sp., Aspergillus niger, Aspergillus fumigatus, and Trametes versicolor |
Landfill soil | Landfill: N/A | 100 | As | 62 | Hassan et al. (2019) |
| Cr | 42 | |||||
| Cu | 49 | |||||
| Fe | 38 | |||||
| Mn | 59 | |||||
| Consortium of Paecilomyces lilacinus, Antrodia serialis, and Penicillium cataractum | Landfill soil | Landfill: N/A | 100 | As | 48 | |
| Cr | 36 | |||||
| Cu | 41 | |||||
| Fe | 35 | |||||
| Mn | 41 | |||||
| Yeast | ||||||
| Candida tropicalis | Chromium contaminated soil | Artificial: 40 mg/kg of Cr(VI) | 8 | Cr (VI) | 58.7–72.25 | Bahafid et al. (2013) |
∗N/A: not available.
Each microbe has different potential and effectiveness in reducing heavy metals from contaminated soil at various concentrations (Medfu Tarekegn et al., 2020). Microorganisms used in remediating various types of heavy metals are as follows: Cr by Aspergillus niger (Xu et al., 2021) and Bacillus sp. MNU16 (Upadhyay et al., 2017), Hg by Pseudomonas aeruginosa (Imron et al., 2019) and Vibrio parahaemolyticus PG02 (Jafari et al., 2015), nickel (Ni) and Cd by Bacillus megaterium and Rhizopus stolonifera (Njoku et al., 2020), and copper (Cu) and Pb by Micrococcus luteus DE2008 (Puyen et al., 2012).
Recent technological advancement involved the utilization of microalgae (Leong and Chang, 2020) such as Chlorella vulgaris and Scenedesmus almeriensis for treating arsenic, Parachlorella sp. and C. minutissima for Cd, S. quadricauda and C. vulgaris for Cr, and Chaetoceros sp. and Phormidium sp. for Pb. Microalgae are considered microplants with great capability of metal adsorption and can perform complex enzymatic reactions such as bacteria for metal transformation (Lee et al., 2022). However, application of microalgae for treating metal-contaminated medium is limited to only in aqueous medium, due to the natural habitat of the used agents (Fernández et al., 2018). Additionally, engineered gene emerged as one of the options to improve the capability of certain bacterial species (Jaiswal et al., 2019), especially Actinobacteria (Mawang et al., 2021), to obtain faster metal transformation, greater metal adsorption capacity, and modified pathways for metal resistance. Utilization of engineered gene microorganisms requires further concern, especially in terms of cost.
3. Analysis of bioaugmentation applicability in treating heavy metal contaminated soil
3.1. Mechanisms of heavy metal removal by bacteria
Microorganisms, particularly bacteria, that live in harsh or toxic environments can combat contaminants by developing adaptation mechanisms (Kapahi and Sachdeva, 2019). This process occurs due to natural selection, in which the surrounding environment forces bacteria to alter their phenotype and genotype to stay alive in the natural environment (Lenski, 2017). Specifically, bacteria protect themselves by becoming resistant to the environment contaminated by poisonous pollutants, such as heavy metal, to maintain their population. Resistant bacteria can withstand the exposure of hazardous heavy metals and eliminate them biologically by developing mechanisms such as biotransformation, bioreduction/bio-oxidation, biosorption, and bioaccumulation (Ahemad, 2019; Fernández et al., 2018; Juwarkar and Yadav, 2010), as illustrated in Figure 1.
Figure 1.
Heavy metal removal mechanisms.
Biosorption and bioaccumulation mechanisms involve the uptake of toxic chemicals but vary in a few processes and pathways (Timková et al., 2018). Biosorption is the process of metal attachment in the outer cell wall or the transfer of metal from the medium into the cell (Kurniawan et al., 2019). Biosorption and bioaccumulation are sequential processes that result in the concentration of metal inside the cell (Titah et al., 2018). Biosorption mechanisms can be demonstrated extracellularly by animate and inanimate microorganism cells due to their metabolic independence, whereas bioaccumulation takes place intracellularly by animate cells, which primarily determine this process as metabolic based (Jobby et al., 2018; Srinath et al., 2002). Specifically, in the biosorption mechanism for heavy metal–bacteria interaction, heavy metals can be bound to active and inactive bacteria cells without the use of adenosine triphosphate (ATP) because this process does not require energy to activate (Mohapatra et al., 2017; Timková et al., 2018). In comparison, bioaccumulation initiates quickly and proceeds to a moderate stage allowing ion exchange or physical adsorption for metal attachment and transportation, based on metabolic activity involving ATP (Srinath et al., 2002). Metal inside cells are generally accumulated in the form of complex–metal compounds, as the result of their resistance mechanisms through several enzymatic reactions (Imron et al., 2021).
Biotransformation pathways consist of two mechanisms, namely, bio-oxidation and bioreduction. These mechanisms can occur extracellularly or intracellularly. Bio-oxidation mechanisms change the toxic heavy metal oxidation state into a nontoxic or less toxic oxidation state through which usually occurs extracellularly (Ahemad, 2019), such as oxidizing As(III) to a lesser toxic As(V) (Titah et al., 2018). Bioreduction, such as from Cr(VI) to Cr(III), reduces the Cr oxidation state from 6 + to 3 + that commonly happens inside the bacteria cell (Pradhan et al., 2017). Both pathways typically involve enzymes to reduce or transform the toxic heavy metal before releasing the less toxic heavy metal into the ecosystem (Jobby et al., 2018). The bound between functional groups or compounds in the bacterial cell wall may also occur as the resistance mechanisms to heavy metals (Imron et al., 2021). The bounded metals and the functional group form minerals surrounding the cell walls, which also serve as further protection, commonly called as biomineralization (Dhami et al., 2013; Zahoor et al., 2017). In general, bacteria use their adaptation mechanisms to defend their cell from harm while indirectly decontaminating the surrounding environment during heavy metal removal.
3.2. Issues in real-scale application
Several issues related to the real-scale application of bioaugmentation in treating heavy metal contaminated soil are summarized in Figure 2.
Figure 2.
Issues related to bioaugmentation in real-scale application.
3.2.1. Impractical metal separation from soil
Understanding the mechanisms of heavy metal removal by bacteria, several concerns are raised if bioaugmentation is applied to treat heavy metal contaminated soil. Treated heavy metals in the form of nonionic/nonsoluble/nonmobile compounds may be concentrated inside bacteria cells, without going anywhere (Titah et al., 2018, 2019). The bacteria, containing metals, will be still inside the soil matrix. Separation of the bacteria and/or the stable metal after treatment need to be carried out (Purwanti et al., 2019b), but sophisticated laboratory technology is often required (discussed further in Section 3.2.2). Purwanti et al. (2019a) reported that separation of aluminum (Al) from soil had a very low efficiency due to the difficulty in the distinguishing soil matrix, bacteria, and the stable metal. After separation, stable metal still needs to be excavated to obtain the free pollutant treated soil. In real-scale application, creating saturated soil condition for solid/solid separation requires a barrier to localize the contaminated area (Casasso et al., 2019); thus, excavation is considered more applicable because the barrier should be placed horizontally and vertically to prevent the leaching of heavy metals into the surrounding area, especially groundwater (Fouad et al., 2017).
3.2.2. Requirement of sophisticated laboratory equipment
Several studies reported the separation of stable heavy metals (converted from ionic to solid form) by centrifugation (Purwanti et al., 2019b), magnetic separation (Feng et al., 2007), and electro separation (Xu et al., 2019). The utilized sophisticated laboratory technology successfully separated clean soil medium with heavy metal containing bacteria from the stable heavy metal itself. Application of centrifugation requires the soil medium in water saturated condition while conducted in laboratory condition (Purwanti et al., 2019b). Moreover, centrifugation of small mass in laboratory scale is applicable inside the centrifugation tube, whereas centrifugation of real contaminated soil masses requires large equipment (which is not available) or sequential/repeated processing that requires high energy consumption and may takes time, leading to impractical practice for field application for large contaminated areas. Separation efficiency of stable heavy metal and clean soil medium report is currently scarce. Purwanti et al. (2019a) reported that only 5.5% of stable metal can be recovered after bioaugmentation in Al contaminated soil. Scale up of the centrifugation technique does not guarantee a high efficiency due to the limitation in the utilized equipment and the difficulties during real-scale application. Another method of separation that can be used is electro-separation, but this option requires abundant energy consumption if conducted in real scale (Xu et al., 2019), as discussed further in Section 3.2.3.
3.2.3. High energy and chemical consumption
Utilization of electrochemical separation technology to separate stable metals after applying bioaugmentation is also considered feasible in laboratory scale (Xu et al., 2019), whereas in real scale, the separated layers of soil, bacteria, and stable metal need to be analyzed further. Stable metal has a higher density than clean soil medium; thus, stable metal layer forms at the bottom of the treated area after separation using electrochemical separation. Separation using the electrochemical method requires the soil to be soaked with certain solutions (commonly solvent) as the medium to transfer the state of the pollutant from the soil matrix, which is then treated further (Song et al., 2022). Utilization of electric-related technology for large scale is highly not recommended due to the high cost and hardly controlled operation. Song et al. (2022) reported 200 mL of solvents and 19.12 Wh of electricity for treating only 40 g of contaminated soil in 48 h treatment period.
3.2.4. Potential of metal releaching
If metal particles remain in the soil matrix after being stabilized via the bioaugmentation method, the changing environmental conditions, especially in terms of pH and redox condition, may affect the stability of the metals (Bourg and Loch, 1995; Jin and Kirk, 2018) and transform the stable deposits back to mobile phase. The change of pH, especially into more acidic levels, may transform the stable metal compounds back into their ionic form, increasing their toxicity, mobility, and bioavailability (Jin and Kirk, 2018). Chen et al. (2021) reported that the bioavailability of Pb in soil was substantially reduced by up to 76.3% in neutral pH after bioremediation, but the effect of pH changing was not assessed. The change of redox condition may change the structure of stable complex-heavy metal compounds, increasing its solubility that then intensifies its potential toxicity (Bourg and Loch, 1995). Liu et al. (2021) mentioned that leachability test for metal-contaminated soil after bioremediation still showed ∼80% of Pb, and ∼77% of Ni was still detected even in neutral pH (7.4–8.2).
3.2.5. Considered as noncomplete remediation
Remediation is considered successful if the contaminant can be fully broken down, degraded, or separated from the contaminated medium (Purwanti et al., 2017b, 2020). However, heavy metals are nonbiodegradable and can only be reduced, oxidized, transformed, or complexed (Atieh et al., 2017; Ayangbenro and Babalola, 2017); thus, heavy metal treatment via any mean either through physical, biological, and chemical approaches is only transformation from one medium to another. Looking at the above demerits, bioaugmentation in treating heavy metal contaminated soil is not a complete remediation (Purwanti et al., 2019b). As previously stated, contaminants need to be completely separated from the contaminated medium to achieve complete remediation. Different from organic pollutant, heavy metals do not undergo degradation process but transformation processes: from ionic form to solid form (Titah et al., 2018), ionic into stable-complex form (Ismail et al., 2020), or even from mobile to immobile form (Rieuwerts, 2007). Complete remediation of heavy metal contaminated soil can be achieved once the stable metal/concentrated bacteria in cells are separated from the medium.
3.2.6. More suitable for metal-containing wastewater/organic contaminated medium
Bioaugmentation is considered more suitable for application in treating heavy metal containing wastewater because the formed stable metal can be easily separated from the wastewater by depositing them at the bottom of the treatment area, producing complete separation with clear differences between phases (water and metal) (Shahid et al., 2020; Titah et al., 2019; Xia et al., 2020). The treatment of heavy metal contaminated soil may show a reduction in metal toxicity due to the reduction from ionic to nonionic form (Joutey et al., 2015; Wiatrowski et al., 2006), but the particle itself remains inside the soil matrix. Soil matrix makes distinguishing and separating stable metals to obtain clean treated medium more difficult. Purwanti et al. (2019a) reported that recovery of Al from contaminated water was remarkably higher compared with soil (59.7% versus 5.5%). Moreover, bioaugmentation was more suggested to be applied in treating organic-contaminated medium because degradation of pollutant will occur, thus resulting in complete remediation (Rahim et al., 2022; Said et al., 2021). Bioaugmentation in organic contaminated medium achieved >90% complete degradation of pollutants (Dalecka et al., 2021; Imron et al., 2020; Muhamad et al., 2021).
4. Role of rhizobacteria in phytoremediation of heavy metal contaminated soil
Phytoremediation or phytotechnology is one of environmentally friendly approaches that currently capture and attract researchers’ attention to exploit the capability of plants together with their associated microorganisms in rhizosphere to treat, remove, degrade, and detoxify different types of pollutants encompassing heavy metals, dyes, hydrocarbons, nutrients, carbon, mining, and radioactive (Abdullah et al., 2020; Al-Ajalin et al., 2022; AL Falahi et al., 2021). Interaction of plant and microbe is a natural process occurring in the environment and now has been engineered to combat pollution problems. Plant–microbe interaction clearly causes this technology to be more advantageous compared with solely microbe-bioaugmentation approach (Kurniawan et al., 2021).
4.1. Bioaugmentation of bacteria in phytoremediation of heavy metal contaminated soil
Even though certain heavy metals, such as Cu, Mn, Mo, Ni, and Zn (Alloway, 2013), are categorized as essential micronutrient for organisms, their elevated concentrations eventually cause reverse effects, leading to acute toxic contamination (Manoj et al., 2020). Bioaugmented phytoremediation or also known as bacteria/fungi-assisted phytoremediation, a treatment with addition of bacteria (most used) or fungi into existing plant rhizosphere, is one of the approaches widely studied for heavy metal remediation. Microbial inoculum is added into the process on the basis that sole phytoremediation treatment in elevated metal contaminated soil is usually restricted to slow plant growth. Existence of metals leads to change in microbial metabolic process and its cellular function, in addition to inhibiting plant's photosynthesis and respiration activities (Manoj et al., 2020). Several studies reported that phytoremediation plants exposed to heavy metal soil with addition of plant growth promoting bacteria (PGPB) stay healthier physically in a longer duration; in terms of plant condition, height and growth of root networks, compared with non-inoculated plants (Ismail et al., 2020; Wang et al., 2019; Wu et al., 2020) and increased contaminant removal efficiency (Purwanti et al., 2020). Root tissues take up more metal concentration than shoots in bioaugmented and non-bioaugmented treatment (Tirry et al., 2018). Table 3 lists studies highlighting on increment of phytoremediation performance with addition of bacteria/fungi.
Table 3.
Performance of bioaugmentation-assisted phytoremediation in heavy metal remediation.
| Bacteria/fungi species | Contaminated media | Hyperaccumulator plants | Operating condition | Performance | Reference |
|---|---|---|---|---|---|
|
Bacillus cereus strain NII Bacillus subtilis strain NII Brevibacterium sp. strain NII |
Synthetic mining wastewater | Scirpus grossus |
|
Increment:
|
Ismail et al. (2020) |
|
Burkholderia sp. strain S6-1 Pseudomonas sp. strain S2-3 |
Contaminated agricultural soil | Sorghum bicolor L. |
|
Total accumulation:
|
Wu et al. (2019) |
| Cellulosimicrobium sp. NF2 | Artificial contaminated soil | Medicago sativa (Alfalfa) |
|
Increment:
|
Tirry et al. (2018) |
| Pseudomonas libanensis TR1 | Contaminated soil | Brassica oxyrrhina |
|
Increment in metal stress:
|
Ma et al. (2016) |
| Klebsiella pneumonia | Artificial contaminated soil | Scirpus triqueter |
|
Pyrene dissipation rate:
|
Zhang et al. (2020) |
| Bacillus sp. QX8 and QX13 | Contaminated soil | Solanum nigrum |
|
Increment in Cd soil:
|
He et al. (2020) |
| Vibrio alginolyticus | Artificial contaminated soil | Scirpus grossus and Thypa angustifolia |
|
Aluminium removal:
|
Purwanti et al. (2020) |
|
Paenibacillus mucilaginosis Sinorhizobium meliloti |
Contaminated soil | Medicago sativa (Alfalfa) |
|
Increment:
|
Ju et al. (2020) |
| Simplicillium chinense QD10 | Artificial contaminated soil | Phragmites communis |
|
Biosorption capacity:
|
Jin et al., 2019a, Jin et al., 2019b |
|
Pseudomonas libanensis TR1 Claroideoglomus claroideogmolus claroideum BEG210 |
Contaminated saline soil | Helianthus annuus |
|
Increment of Ni accumulation:
|
Ma et al. (2019) |
Table 3 shows that Bacillus sp. and Pseudomonas sp. indicate good increment of heavy metal accumulation by plants or removal from contaminated soil. Not only elevating the heavy metal performance, the addition bacteria or fungi also boost the plants’ growth, indicated by the increment of dry weight, shoot, and root length. The increase of heavy metal removal from contaminated soil may be obtained from the increment of plant biomass and extracting more metals from the soil, and may occur due to the complex interactions between plants and rhizomicrobial species existing in the rhizosphere (Adeyemi et al., 2021).
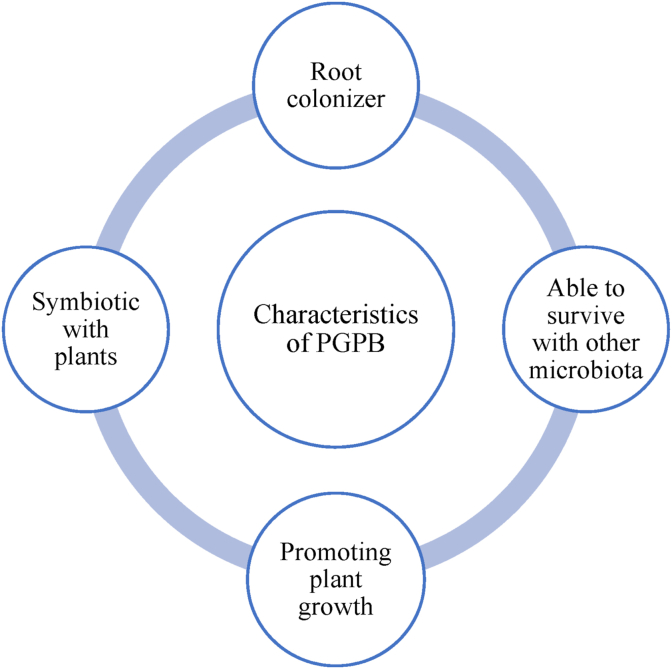
Bacteria added can reduce metal toxicity and promote plant growth via secretion of respected chelating agent, enzymes, acidification, and growth-promoting substance (Manoj et al., 2020); thus, they are widely known as PGPB or specifically known as plant growth promoting rhizobacteria (PGPR). Distinct characteristics of PGPB are as listed in Figure 3. Generally, they assist in improving potential of plant growth and its stress tolerance toward heavy metals (Kong et al., 2019; Mello et al., 2020) through nutrient recycling, providing stable soil structure, increasing metal bioavailability, and reducing their toxicity (X. He et al., 2020; Manoj et al., 2020). These combination actions are a mutual symbiosis, in which plants receive benefits from the ability of bacteria to provide suitable growth condition for plants while bacteria benefit from available nutrients from plant's exudates for its enzymatic reaction and suitable living environment leading it to exhibit phytostimulation mechanism (Jin et al., 2019a, Jin et al., 2019b; Kamaruzzaman et al., 2019; Purwanti et al., 2020).
Figure 3.
General properties of plant growth promoting bacteria (PGPB).
Several factors of metal uptake are pH of soil, solubilization of metal, and community of rooting system secretion (Dhiman et al., 2020; Raklami et al., 2021; Shahid et al., 2020). Owing to pH drop, exposure of heavy metal in soil definitely reduces its microbial community, which then decreases soil fertility that is correlated closely to microbe soil organic transformation activity (Shah and Daverey, 2020). In a research by Ismail et al. (2020), reduction in microbial community was observed in the non-bioaugmented system, whereas the reverse observation was found for bioaugmented system. An increment was observed in bacterial population in the bioaugmented system due to their symbiosis mechanism (Ismail et al., 2020). Performance of PGPB that is reproduced gradually by time in new environment also depends on its resistivity to environmental changes including competition between native microbial community (Kong et al., 2019). Different species of bacteria involve and react differently in plant–microbe symbiosis as not every single species of bacteria can perform simultaneously as toxicity reducer and growth promoter (Kamaruzzaman et al., 2019; Mello et al., 2020).
Even though not as wide as bacteria application, fungi also have the same benefits of mediating phytoremediation, where it often calls as plant growth promoting fungi (PGPF). Jin et al., 2019a, Jin et al., 2019b studied the addition of fungi S. chinense in phytoremediation of Cd and Pb contaminated soil. These findings state that while S. chinense improved the bioavailability of the metals in soil media, remediation of Cd and Pb were due to different mechanisms, namely, formation of Cd-chelate and adsorption by extracellular polymeric substance, respectively. In this case of adding fungi into phytoremediation system, dosage is not as crucial as its colonization, due to the high growth rate of fungi (Jin et al., 2019a, Jin et al., 2019b).
4.2. Mechanisms of bacteria-assisted phytoremediation of heavy metal contaminated soil
As widely discussed in previous reviews, phytoremediation works by several mechanisms, namely, phytoextraction, phytostabilization, rhizodegradation, rhizofiltration, phytovolatilization, phytodegradation, and phytofiltration (Almaamary et al., 2022; Sharma, 2021). Phytoremediation of heavy metal focuses on two divisions; 1) metal immobilization in soil and roots in the rhizosphere (Raklami et al., 2021); 2) mobilization, absorption, and conversion of metals in the aerial part (Adeyemi et al., 2021; Diarra et al., 2021; Purwanti et al., 2020). Here, focus is more on the former division, with the help of PGPB/PGPF together with local microbial community in assisting phytoremediation.
Efficiency of heavy metal phytoremediation depends greatly on the amounts of bioavailable metal for plant uptake (Shah and Daverey, 2020). Here, microorganisms in the rhizosphere, also recognized as rhizospheric microorganisms, play a crucial role in regulating the surrounding into suitable environment for phytoremediation. PGPB/PGPF are involved actively to increase metal bioavailability and reduce it into a less toxic form by various biological mechanism such as transformation, chelating, accumulation, sorption, volatilization, leaching, and degradation to be easily adsorbed/absorbed/extracted by plants (Manoj et al., 2020). As discussed in the earlier section, existence of alleviated heavy metal concentration in soils also leads to plant growth stunt. Thus, addition of these microorganisms also facilitates promoting plant growth for better phytoremediation, and they can be divided according to their functionality as biofertilizer, phytostimulators, rhizomediators, and biopesticide. Purwanti et al. (2020) reported that the addition of bacteria can promote mechanisms such as phytostimulation and rhizostabilization in addition to assisting in reducing metal toxicity and increasing its bioavailability, contributing to higher phytoextraction rate. Availability of heavy metals is associated with biogeochemical process such as precipitation, adsorption, mineralization, and protonation that depend on soil and its rhizosphere characteristics (Shah and Daverey, 2020).
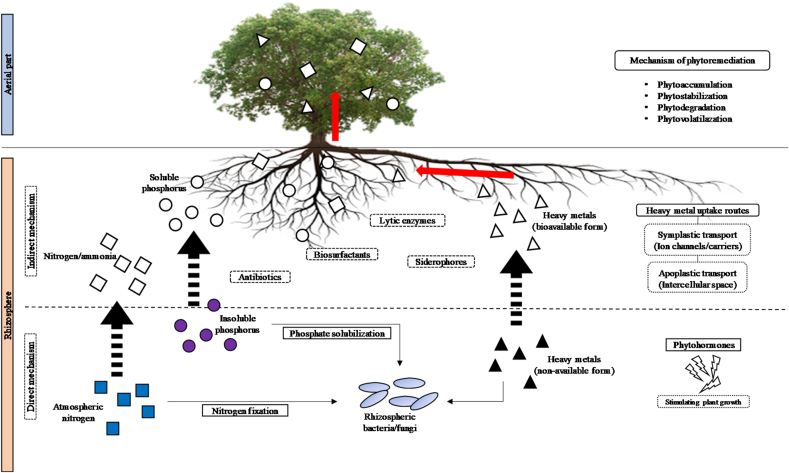
In general, plant promoting mechanisms by additional microorganism can be categorized into two: direct mechanism that is responsible in direct growth stimulation and indirect mechanisms that assist more into reducing plant stress and pathogenic effect through secretion of specific substances, as detailed in Table 4. Tirry et al. (2018) mentioned that production of indole acetic acid (IAA), siderophores, and phosphate solubilization were the indication characteristics in determining the ability of bacteria as PGPB in metal contaminated media. Figure 4 summarizes graphical explanation of respected aspects. Phytohormones, which are involved in direct mechanism, is a group of physiological and metabolic plant growth regulators (Ma et al., 2019; Manoj et al., 2020; Novo et al., 2018). Major hormones associated with plant growth are IAA, 1-aminocyclopropane-1-carboxylate (ACC) deaminase, cytokinin, and gibberellin. IAA is an auxin hormone that is responsible for root development such as cell division, tissue expansion, and nodule formation by providing great access to nutrient and minerals (Ma et al., 2016; Manoj et al., 2020). IAA also increases bacteria reproduction rate in roots by nutrient mobilization (Tirry et al., 2018).
Table 4.
Plant growth-promoting substances and their respective functions.
| Division of mechanism | Plant growth promoting aspects | Components/substance involved | Functions/ability |
|---|---|---|---|
| Direct mechanism | Phytohormones | Indole acetic acid-auxins | Improve root development by enhancing mineral adsorption and nutrient uptake (Bahadur et al., 2017; Manoj et al., 2020; Tirry et al., 2018; Kamaruzzaman et al., 2019) Stimulate germination; cell, plant, and root growth development, resistance to stress, and various metabolites' production (Novo et al., 2018) |
| 1-aminocyclopropane-1-carboxylate deaminase | Regulate excess ethylene content produced in stress condition (Manoj et al., 2020) | ||
| Cytokinin | Stimulate plant growth physiologically (shoot initiation, cell differentiation, nutritional signaling, and chlorophyll production) in normal or unfavorable conditions (Manoj et al., 2020; Novo et al., 2018) | ||
| Gibberellins | Stimulate shoot growth, seed germination, stem elongation, leaf expansion, pollen, and fruit development (Manoj et al., 2020; Novo et al., 2018) | ||
| Phosphate solubilization | Inorganic phosphorus: Low molecular weight organic acids (acetic, lactic, malic, succinic, tartaric, gluconic, oxalic, and citric acids) Organic phosphorus: enzymes (phosphatase, phytase, phosphonoacetate hydrolase, d--glycerophosphatase, and C–P lyase) |
Ability of bacteria to convert insoluble phosphate into soluble form (Novo et al., 2018; Kamaruzzaman et al., 2019) Organic acid: Alter soil pH and increase chelation efficiency (Shah and Daverey, 2020) |
|
| Nitrogen fixation | Nitrogenase | Ability of bacteria to convert atmospheric nitrogen into ammonia and nitrate form (Manoj et al., 2020; Novo et al., 2018; Kamaruzzaman et al., 2019) | |
| Indirect mechanism | Antibiotics | Prevent growth of other bacteria and fungi (Novo et al., 2018) | |
| Lytic enzymes | Hydrolyze components of cell wall to avert proliferation of pathogenic fungi (Novo et al., 2018) | ||
| Siderophores | Supply assimilable iron by converting insoluble iron (Fe3+) into soluble form (Fe2+), thus increasing its bioavailability in soil (Manoj et al., 2020; Novo et al., 2018) Reduce free radical production (Tirry et al., 2018) Hamper propagation of phytopathogen (Ribeiro and Rode, 2019) Form stable complexes with heavy metals, enhancing metal bioavailability in rhizosphere (Ma et al., 2016; Manoj et al., 2020; Tirry et al., 2018) |
||
| Biosurfactant | Desorb metal form soil matrix to be available for plant (Shah and Daverey, 2020) | ||
Figure 4.
Direct and indirect mechanism in bacteria-assisted phytoremediation.
ACC is another type of phytohormone responsible for producing ethylene, an important component for plant growth regulation. However, in biotic and abiotic stress conditions, ACC produces extra ethylene that then causes disruptions in root development and plant metabolism (Reed and Glick, 2005; Saleem et al., 2007). Here, ACC deaminase plays an important task in degrading respective ACC enzyme that then produces carbon and nitrogen byproducts that are useful for plant growth (Manoj et al., 2020; Novo et al., 2018). Cytokinin is known as an important phytohormone after auxins. It involves not only in numerous aspects of plant growth, as listed in Table 4, but also protect plants from pathogen infection. Cytokinin can be produced by the plant itself and microorganism in the surroundings (Akhtar et al., 2020). Gibberellins act as growth hormones that help in stem elongation and seed germination (Novo et al., 2018).
Another aspect in direct mechanism is nitrogen fixation. It is a process of converting atmospheric nitrogen (N2) into plants-available form involving a complex enzyme known as nitrogenase, which can be found in symbiotic and nonsymbiotic N2 fixing bacteria (Novo et al., 2018). Symbiotic bacteria are involved directly in this process by producing root nodules, whereas nonsymbiotic bacteria locate in nearby root area to allow sorption of its excess nitrogen into plants (Novo et al., 2018). Second to nitrogen, phosphorus (P) is also one of the important elements for plant growth. Access of P is also limited due to its existing insoluble form while the plant can only accept two forms of monobasic (H2PO4−) and dibasic (H2PO42−) soluble ions (Novo et al., 2018). Thus, phosphate solubilizing bacteria act as biofertilizer to supply sufficient P to the plant.
Indirect PGPB mechanism is not involved directly in plant growth, but it helps by providing better growing condition and reducing stresses caused by biotic and abiotic factors such as temperature, metal toxicity, and organic pollutants (Manoj et al., 2020; Novo et al., 2018). PGPB produces biocompounds such as antibiotic, lytic enzymes, and siderophores to suppress phytopathogen, thus proving better growth environment (Novo et al., 2018; Kamaruzzaman et al., 2019).
Siderophores, a compound with high affinity toward Fe3+ is naturally secreted by bacteria in stressed conditions to convert iron into soluble form (Fe2+) or to form iron-siderophore complexes, enhancing respected metal bioavailability (Novo et al., 2018). Iron (Fe) is another important element that is responsible for various enzymatic activities for plant growth, but it is not in readily absorbed available form despite being the fourth most abundant element on earth (Novo et al., 2018). Siderophores produced by bacteria demonstrate higher affinity than phyto-siderophores (Novo et al., 2018). Siderophores also protect auxins from degradation; thus, they involve involuntary in plant growth (Tirry et al., 2018). However, Novo et al. (2018) mentioned that in general, PGPB do not really greatly contribute toward metal bioavailability in mine lands, whereas PGPB-aided phytoremediation clearly substantially contributes to plant growth and metal tolerability.
5. Future research direction
Accommodating previous explanation, analysis on the potential specific-type heavy metal hyper accumulator plants is suggested to be explored further. Certain plant species can adsorb and accumulate certain heavy metal type inside their cell, thus resulting in higher removal of heavy metals from the contaminated medium (Li et al., 2020). In the search of the hyper accumulator species, certain criteria need to be concerned, including the species should be nonedible plants (Ekperusi et al., 2019; Shahid et al., 2020), highest concentration that can be tolerated by plants (maximum tolerable concentration) (Purwanti et al., 2018a, Purwanti et al., 2018b), minimum concentration of heavy metals that starts to inhibit the plant's growth (minimum inhibitory concentration) (Imron et al., 2021), and perennial plant type is suggested to minimize harvesting that can increase operation and maintenance cost (Al-Baldawi et al., 2015; Im et al., 2019).
In addition, searching for a specific microbial species that could promote plant growth that also enhances the removal of heavy metals from soil is highly endorsed. Microbial species (whether bacteria or fungi) may have specific mechanisms to certain heavy metals that can result in higher removal of heavy metals from contaminated soil during bioaugmentation-assisted phytoremediation (Ismail et al., 2020; Kamaruzzaman et al., 2020; Li et al., 2020). Analysis of the interaction between specific microorganisms and specific plants species is also recommended to analyze heavy metal elimination from soil during bioaugmentation-assisted phytoremediation (Abdullah et al., 2020; Shahid et al., 2020). The analysis of microbial community interaction with certain plant species can be detailed to understand which species and mechanisms enhance the treatment processes opening to analysis at biomolecular levels (metabolomic and metagenomic analysis). The fate of heavy metals inside soil/plant parts/PGPB can be explored and revealed.
After the selection of the PGPB, addition of the selected species during the real field phytoremediation of heavy metal contaminated soil should be concerned (Wu et al., 2020). Appropriate bacterial concentration (Li et al., 2020), carrying medium (Mubashar et al., 2020), and volume (Wang et al., 2019) need to be determined to obtain the maximum removal efficiencies. Accommodating the parametric analysis on the bacterial addition, optimization of the treatment condition is also recommended to obtain the highest performance. Factors contributing to performance of heavy metal contaminated soil phytoremediation such as operational pH (Varma et al., 2021), bioavailability of metals (Tangahu et al., 2021), existence of chelating agents (Diarra et al., 2021), nutrient availability (Schwammberger et al., 2019), and water content (Mohan and Tippa, 2019) can be optimized to obtain the right environmental condition for highest metal removal.
Post treatment also becomes a topic to be explored further because current research on biomass utilization after treatment of heavy metal contaminated medium is limited. Produced biomass after treatment of heavy metal contaminated area is categorized as hazardous compound because it contains heavy metals and its corresponding intermediate components as the results of the treatment (García et al., 2016; Lee et al., 2019; Tangahu et al., 2011). Harvested phytoremediation plant biomass is commonly not utilized any further and processed further to incineration or secured landfill (Abhilash and Yunus, 2011). The Toxicity Characteristic Leaching Procedure can be carried out on treating plants to determine the toxicity of the plants before they can be used for other purposes. Analysis of heavy metal components inside plants after treatment may enable selecting a suitable action for further processing (Alshekhli et al., 2020). In addition, analysis of heavy metal components inside plants may open new opportunities in phytomining or the recovery of valuable heavy metals from plant biomass after phytoremediation (Ahmad et al., 2016; Dodbiba et al., 2015).
6. Conclusions
Bioaugmentation is not suitable to be applied alone in treating heavy metal contaminated soil in real-scale area due to incomplete separation of heavy metals (and its intermediate compounds) from the treated medium after the treatment. Several research works report successful heavy metal removal from contaminated soil that are mostly conducted at the laboratory scale under a controlled environment. The said research studies also limitedly discuss the separation of heavy metals from the medium to obtain pollutant-free soil after treatment. In addition, separation technology used in laboratory scale is considered applicable for use in real application due to extensive energy consumption and difficult operation. Phytoremediation is suggested for use as heavy metal contaminated soil treatment method, whereas bioaugmentation of PGPB to assist the phytoremediation is proven to be the future promising technology with higher removal efficiencies. Searching for potential hyper accumulator plant species of certain heavy metal type, potential microbial species, and its interaction with plants during bioaugmentation-assisted phytoremediation, fate of heavy metals, and post treatment handling of produced plant biomass are future research directions to be explored further to enrich the knowledge of the treatment of heavy metal contaminated soil by bioaugmentation-assisted phytoremediation.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This work was supported by Ministry of Higher Education, Malaysia (FRGS/1/2019/TK02/UKM/01/1) and the Universitas Airlangga via MANDAT reseach grant (No. 1519/UN3.15/PT/2021).
Data availability statement
Data will be made available on request.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
Image used in Figure 4 was licensed from enterphoto (https://www.istockphoto.com/id/foto/pohon-beringin-gm875854076-244496040) under order number 2078011759.
Contributor Information
Muhammad Fauzul Imron, Email: fauzul.01@gmail.com.
Siti Rozaimah Sheikh Abdullah, Email: rozaimah@ukm.edu.my.
References
- Abdullah S.R.S., Al-Baldawi I.A., Almansoory A.F., Purwanti I.F., Al-Sbani N.H., Sharuddin S.S.N. Plant-assisted remediation of hydrocarbons in water and soil: application, mechanisms, challenges and opportunities. Chemosphere. 2020;247:125932. doi: 10.1016/j.chemosphere.2020.125932. [DOI] [PubMed] [Google Scholar]
- Abhilash P.C., Yunus M. Can we use biomass produced from phytoremediation? Biomass Bioenergy. 2011;35:1371–1372. [Google Scholar]
- Adeyemi N.O., Atayese M.O., Sakariyawo O.S., Azeez J.O., Abayomi Sobowale S.P., Olubode A., Mudathir R., Adebayo R., Adeoye S. Alleviation of heavy metal stress by arbuscular mycorrhizal symbiosis in Glycine max (L.) grown in copper, lead and zinc contaminated soils. Rhizosphere. 2021;18:100325. [Google Scholar]
- Agnello A.C., Bagard M., van Hullebusch E.D., Esposito G., Huguenot D. Comparative bioremediation of heavy metals and petroleum hydrocarbons co-contaminated soil by natural attenuation, phytoremediation, bioaugmentation and bioaugmentation-assisted phytoremediation. Sci. Total Environ. 2016;563–564:693–703. doi: 10.1016/j.scitotenv.2015.10.061. [DOI] [PubMed] [Google Scholar]
- Ahemad M. Remediation of metalliferous soils through the heavy metal resistant plant growth promoting bacteria: paradigms and prospects. Arab. J. Chem. 2019;12:1365–1377. [Google Scholar]
- Ahmad T., Ahmad K., Alam M. Sustainable management of water treatment sludge through 3’R’ concept. J. Clean. Prod. 2016;124:1–13. [Google Scholar]
- Akhtar S.S., Mekureyaw M.F., Pandey C., Roitsch T. Role of cytokinins for interactions of plants with microbial pathogens and pest insects. Front. Plant Sci. 2020 doi: 10.3389/fpls.2019.01777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Ajalin F.A.H., Abdullah S.R.S., Idris M., Kurniawan S.B., Ramli N.N., Imron M.F. Removal of ammonium, phosphate, and COD by bacteria isolated from Lepironia articulata and Scirpus grossus root system. Int. J. Environ. Sci. Technol. 2022 [Google Scholar]
- Al-Baldawi I.A., Abdullah S.R.S., Anuar N., Suja F., Mushrifah I. Phytodegradation of total petroleum hydrocarbon (TPH) in diesel-contaminated water using Scirpus grossus. Ecol. Eng. 2015;74:463–473. [Google Scholar]
- AL Falahi O.A., Abdullah S.R.S., Hasan H.A., Othman A.R., Ewadh H.M., Al-Baldawi I.A., Kurniawan S.B., Imron M.F., Ismail N.I. Simultaneous removal of ibuprofen, organic material, and nutrients from domestic wastewater through a pilot-scale vertical sub-surface flow constructed wetland with aeration system. J. Water Proc. Eng. 2021;43:102214. [Google Scholar]
- Alloway B.J. 2013. Heavy Metals and Metalloids as Micronutrients for Plants and Animals; pp. 195–209. [Google Scholar]
- Almaamary E.A.S., Abdullah S.R.S., Ismail N. ‘Izzati, Idris M., Kurniawan S.B., Imron M.F. Comparative performance of Scirpus grossus for phytotreating mixed dye wastewater in batch and continuous pilot subsurface constructed wetland systems. J. Environ. Manag. 2022;307:114534. doi: 10.1016/j.jenvman.2022.114534. [DOI] [PubMed] [Google Scholar]
- Alshekhli A.F., Hasan H.A., Muhamad M.H., Sheikh Abdullah S.R. Development of adsorbent from phytoremediation plant waste for methylene blue removal. J. Ecol. Eng. 2020;21:207–215. [Google Scholar]
- Atieh M.A., Ji Y., Kochkodan V. Metals in the environment: toxic metals removal. Bioinorgan. Chem. Appl. 2017;2017:1–2. doi: 10.1155/2017/4309198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayangbenro A., Babalola O. A new strategy for heavy metal polluted environments: a review of microbial biosorbents. Int. J. Environ. Res. Publ. Health. 2017;14:94. doi: 10.3390/ijerph14010094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahadur A., Ahmad R., Afzal A., Feng H., Suthar V., Batool A., Khan A., Mahmood-ul-Hassan M. The influences of Cr-tolerant rhizobacteria in phytoremediation and attenuation of Cr (VI) stress in agronomic sunflower (Helianthus annuus L.) Chemosphere. 2017;179:112–119. doi: 10.1016/j.chemosphere.2017.03.102. [DOI] [PubMed] [Google Scholar]
- Bahafid W., Tahri Joutey N., Sayel H., Boularab I., El Ghachtouli N. Bioaugmentation of chromium-polluted soil microcosms with Candida tropicalis diminishes phytoavailable chromium. J. Appl. Microbiol. 2013;115:727–734. doi: 10.1111/jam.12282. [DOI] [PubMed] [Google Scholar]
- Bjørklund G., Dadar M., Mutter J., Aaseth J. The toxicology of mercury: current research and emerging trends. Environ. Res. 2017 doi: 10.1016/j.envres.2017.08.051. [DOI] [PubMed] [Google Scholar]
- Bourg A.C.M., Loch J.P.G. Biogeodynamics of Pollutants in Soils and Sediments. Springer Berlin Heidelberg; Berlin, Heidelberg: 1995. Mobilization of heavy metals as affected by pH and redox conditions; pp. 87–102. [Google Scholar]
- Casasso A., Tosco T., Bianco C., Bucci A., Sethi R. How can we make pump and treat systems more energetically sustainable? Water. 2019;12:67. [Google Scholar]
- Chen M., Li Y., Jiang X., Zhao D., Liu X., Zhou J., He Z., Zheng C., Pan X. Study on soil physical structure after the bioremediation of Pb pollution using microbial-induced carbonate precipitation methodology. J. Hazard Mater. 2021;411 doi: 10.1016/j.jhazmat.2021.125103. [DOI] [PubMed] [Google Scholar]
- Chibuike G.U., Obiora S.C. Heavy metal polluted soils: effect on plants and bioremediation methods. Appl. Environ. Soil Sci. 2014;2014:1–12. [Google Scholar]
- Dalecka B., Strods M., Cacivkins P., Ziverte E., Rajarao G.K., Juhna T. Removal of pharmaceutical compounds from municipal wastewater by bioaugmentation with fungi: an emerging strategy using fluidized bed pelleted bioreactor. Environ. Adv. 2021;5 [Google Scholar]
- Dhami N.K., Reddy M.S., Mukherjee A. Biomineralization of calcium carbonates and their engineered applications: a review. Front. Microbiol. 2013;4:1–7. doi: 10.3389/fmicb.2013.00314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhiman J., Prasher S.O., ElSayed E., Patel R.M., Nzediegwu C., Mawof A. Heavy metal uptake by wastewater irrigated potato plants grown on contaminated soil treated with hydrogel based amendments. Environ. Technol. Innovat. 2020;19:100952. [Google Scholar]
- Diarra I., Kotra K.K., Prasad S. Assessment of biodegradable chelating agents in the phytoextraction of heavy metals from multi–metal contaminated soil. Chemosphere. 2021;273:128483. doi: 10.1016/j.chemosphere.2020.128483. [DOI] [PubMed] [Google Scholar]
- Dodbiba G., Ponou J., Fujita T. Microbiology for Minerals, Metals, Materials and the Environment. CRC Press; 2015. Urban biomining: new challenges for a successful exploitation of WEEE by means of a biotechnological approach; pp. 347–376. [Google Scholar]
- Ekperusi A.O., Sikoki F.D., Nwachukwu E.O. Application of common duckweed (Lemna minor) in phytoremediation of chemicals in the environment: state and future perspective. Chemosphere. 2019;223:285–309. doi: 10.1016/j.chemosphere.2019.02.025. [DOI] [PubMed] [Google Scholar]
- El-Saadony M.T., Desoky E.S.M., Saad A.M., Eid R.S.M., Selem E., Elrys A.S. Biological silicon nanoparticles improve Phaseolus vulgaris L. yield and minimize its contaminant contents on a heavy metals-contaminated saline soil. J. Environ. Sci. (China) 2021;106:1–14. doi: 10.1016/j.jes.2021.01.012. [DOI] [PubMed] [Google Scholar]
- Emenike C.U., Agamuthu P., Fauziah S.H. Blending Bacillus sp., lysinibacillus sp. and rhodococcus sp. for optimal reduction of heavy metals in leachate contaminated soil. Environ. Earth Sci. 2016;75:26. [Google Scholar]
- Feng N., Bitton G., Yeager P., Bonzongo J.-C.C., Boularbah A. Heavy metal removal from soils using magnetic separation: 1. Laboratory experiments. Clean – Soil, Air, Water. 2007;35:362–369. [Google Scholar]
- Fernández P.M., Viñarta S.C., Bernal A.R., Cruz E.L., Figueroa L.I.C. Bioremediation strategies for chromium removal: current research, scale-up approach and future perspectives. Chemosphere. 2018;208:139–148. doi: 10.1016/j.chemosphere.2018.05.166. [DOI] [PubMed] [Google Scholar]
- Fouad M.M., El-Gendy A.S., Razek T.M.A. Evaluation of leached metals in recovered aluminum coagulants from water treatment slurry. Water Sci. Technol. 2017;75:998–1006. doi: 10.2166/wst.2016.582. [DOI] [PubMed] [Google Scholar]
- García R., Campos J., Cruz J.A., Calderón M.E., Raynal M.E., Buitrón G. Biosorption of Cd, Cr, Mn, and Pb from aqueous solutions by Bacillus sp strains isolated from industrial waste activate sludge. TIP. 2016;19:5–14. [Google Scholar]
- Ghobadi R., Altaee A., Zhou J.L., Karbassiyazdi E., Ganbat N. Effective remediation of heavy metals in contaminated soil by electrokinetic technology incorporating reactive filter media. Sci. Total Environ. 2021;794:148668. doi: 10.1016/j.scitotenv.2021.148668. [DOI] [PubMed] [Google Scholar]
- Girma G. Microbial bioremediation of some heavy metals in soils: an updated review. Egypt. Acad. J. Biol. Sci. G. Microbiol. 2015;7:29–45. [Google Scholar]
- Giwa O., Ibitoye F. Bioremediation of heavy metal in crude oil contaminated soil using isolated Indigenous microorganism cultured with E coli DE3 BL21. Int. J. Eng. Appl. Sci. 2017;4:257436. [Google Scholar]
- Hassan A., Pariatamby A., Ahmed A., Auta H.S., Hamid F.S. Enhanced bioremediation of heavy metal contaminated landfill soil using filamentous fungi consortia: a demonstration of bioaugmentation potential. Water Air Soil Pollut. 2019;230 [Google Scholar]
- He C., Gu L., Xu Z., He H., Fu G., Han F., Huang B., Pan X. Cleaning chromium pollution in aquatic environments by bioremediation, photocatalytic remediation, electrochemical remediation and coupled remediation systems. Environ. Chem. Lett. 2020;18:561–576. [Google Scholar]
- He X., Xu M., Wei Q., Tang M., Guan L., Lou L., Xu X., Hu Z., Chen Y., Shen Z., Xia Y. Promotion of growth and phytoextraction of cadmium and lead in Solanum nigrum L. mediated by plant-growth-promoting rhizobacteria. Ecotoxicol. Environ. Saf. 2020;205:111333. doi: 10.1016/j.ecoenv.2020.111333. [DOI] [PubMed] [Google Scholar]
- Hejna M., Gottardo D., Baldi A., Dell’Orto V., Cheli F., Zaninelli M., Rossi L. Review: nutritional ecology of heavy metals. Animal. 2018;12:2156–2170. doi: 10.1017/S175173111700355X. [DOI] [PubMed] [Google Scholar]
- Huang H., Ye L. Elsevier Inc; 2019. Biological Technologies for cHRPs and Risk Control, High-Risk Pollutants in Wastewater. [Google Scholar]
- Hussein M., Yoneda K., Mohd-Zaki Z., Amir A., Othman N. Heavy metals in leachate, impacted soils and natural soils of different landfills in Malaysia: an alarming threat. Chemosphere. 2021;267:128874. doi: 10.1016/j.chemosphere.2020.128874. [DOI] [PubMed] [Google Scholar]
- Ibarrolaza A., Coppotelli B.M., Del Panno M.T., Donati E.R., Morelli I.S. Application of the knowledge-based approach to strain selection for a bioaugmentation process of phenanthrene- and Cr(VI)-contaminated soil. J. Appl. Microbiol. 2011;111:26–35. doi: 10.1111/j.1365-2672.2011.05036.x. [DOI] [PubMed] [Google Scholar]
- Ighalo J.O., Kurniawan S.B., Iwuozor K.O., Aniagor C.O., Ajala O.J., Oba S.N., Iwuchukwu F.U., Ahmadi S.S., Igwegbe C.A. A review of treatment technologies for the mitigation of the toxic environmental effects of acid mine drainage (AMD) Process Saf. Environ. Protect. 2022;157:37–58. [Google Scholar]
- Igwegbe C.A., Obiora-Okafo I.A., Iwuozor K.O., Ghosh S., Kurniawan S.B., Rangabhashiyam S., Kanaoujiya R., Ighalo J.O. Treatment technologies for bakers’ yeast production wastewater. Environ. Sci. Pollut. Res. 2022;29:11004–11026. doi: 10.1007/s11356-021-17992-4. [DOI] [PubMed] [Google Scholar]
- Im S., Lee B.E., Lee H.G., Bae Y.J., Kim J.G. Perennial emergent macrophytes as the main determinant of Hydrochara affinis inhabitation. J. Asia Pac. Entomol. 2019;22:1070–1081. [Google Scholar]
- Imron M.F., Kurniawan S.B., Abdullah S.R.S. Resistance of bacteria isolated from leachate to heavy metals and the removal of Hg by Pseudomonas aeruginosa strain FZ-2 at different salinity levels in a batch biosorption system. Sustain. Environ. Res. 2021;31:14. [Google Scholar]
- Imron M.F., Kurniawan S.B., Ismail N. ‘Izzati, Abdullah S.R.S. Future challenges in diesel biodegradation by bacteria isolates: a review. J. Clean. Prod. 2020;251:119716. [Google Scholar]
- Imron M.F., Kurniawan S.B., Soegianto A. Characterization of mercury-reducing potential bacteria isolated from Keputih non-active sanitary landfill leachate, Surabaya, Indonesia under different saline conditions. J. Environ. Manag. 2019;241:113–122. doi: 10.1016/j.jenvman.2019.04.017. [DOI] [PubMed] [Google Scholar]
- Ismail N.I., Abdullah S.R.S., Idris M., Kurniawan S.B., Effendi Halmi M.I., AL Sbani N.H., Jehawi O.H., Hasan H.A. Applying rhizobacteria consortium for the enhancement of Scirpus grossus growth and phytoaccumulation of Fe and Al in pilot constructed wetlands. J. Environ. Manag. 2020;267:110643. doi: 10.1016/j.jenvman.2020.110643. [DOI] [PubMed] [Google Scholar]
- Jafari S.A., Cheraghi S., Mirbakhsh M., Mirza R., Maryamabadi A. Employing response surface methodology for optimization of mercury bioremediation by Vibrio parahaemolyticus PG02 in coastal sediments of bushehr, Iran. Clean - Soil, Air, Water. 2015;43:118–126. [Google Scholar]
- Jaishankar M., Tseten T., Anbalagan N., Mathew B.B., Beeregowda K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscipl. Toxicol. 2014;7:60–72. doi: 10.2478/intox-2014-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal S., Singh D.K., Shukla P. Gene editing and systems biology tools for pesticide bioremediation: a review. Front. Microbiol. 2019;10:1. doi: 10.3389/fmicb.2019.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin G., Fang W., Shafi M., Wu D., Li Y., Zhong B., Ma J., Liu D. Source apportionment of heavy metals in farmland soil with application of APCS-MLR model: a pilot study for restoration of farmland in Shaoxing City Zhejiang, China. Ecotoxicol. Environ. Saf. 2019;184:109495. doi: 10.1016/j.ecoenv.2019.109495. [DOI] [PubMed] [Google Scholar]
- Jin Q., Kirk M.F. pH as a primary control in environmental microbiology: 1. Thermodynamic perspective. Front. Environ. Sci. 2018;6:1–7. [Google Scholar]
- Jin Z., Deng S., Wen Y., Jin Y., Pan L., Zhang Y., Black T., Jones K.C., Zhang H., Zhang D. Application of Simplicillium chinense for Cd and Pb biosorption and enhancing heavy metal phytoremediation of soils. Sci. Total Environ. 2019;697:134148. doi: 10.1016/j.scitotenv.2019.134148. [DOI] [PubMed] [Google Scholar]
- Jobby R., Jha P., Yadav A.K., Desai N. Biosorption and biotransformation of hexavalent chromium [Cr(VI)]: a comprehensive review. Chemosphere. 2018;207:255–266. doi: 10.1016/j.chemosphere.2018.05.050. [DOI] [PubMed] [Google Scholar]
- Joutey N.T., Sayel H., Bahafid W., Ghachtouli N. El. Mechanisms of hexavalent chromium resistance and removal by microorganisms. Rev. Environ. Contam. Toxicol. 2015;233:45–69. doi: 10.1007/978-3-319-10479-9_2. [DOI] [PubMed] [Google Scholar]
- Ju W., Liu L., Jin X., Duan C., Cui Y., Wang J., Ma D., Zhao W., Wang Y., Fang L. Co-inoculation effect of plant-growth-promoting rhizobacteria and rhizobium on EDDS assisted phytoremediation of Cu contaminated soils. Chemosphere. 2020;254:126724. doi: 10.1016/j.chemosphere.2020.126724. [DOI] [PubMed] [Google Scholar]
- Juwarkar A.A., Yadav S.K. Springer Netherlands; Dordrecht: 2010. Bioaccumulation and Biotransformation of Heavy Metals, Bioremediation Technology. [Google Scholar]
- Kamaruzzaman M.A., Abdullah S.R.S., Hasan H.A., Hassan M., Idris M., Ismail N. Potential of hexavalent chromium-resistant rhizosphere bacteria in promoting plant growth and hexavalent chromium reduction. J. Environ. Biol. 2019;40:427–433. [Google Scholar]
- Kamaruzzaman M.A., Abdullah S.R.S., Hasan H.A., Hassan M., Othman A.R., Idris M. Characterisation of Pb-resistant plant growth-promoting rhizobacteria (PGPR) from Scirpus grossus. Biocatal. Agric. Biotechnol. 2020;23:101456. [Google Scholar]
- Kaninga B., Chishala B.H., Maseka K.K., Sakala G.M., Young S.D., Lark R.M., Tye A., Hamilton E.M., Gardner A., Watts M.J. Do soil amendments used to improve agricultural productivity have consequences for soils contaminated with heavy metals? Heliyon. 2020;6 doi: 10.1016/j.heliyon.2020.e05502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapahi M., Sachdeva S. Bioremediation options for heavy metal pollution. J. Heal. Pollut. 2019;9:191203. doi: 10.5696/2156-9614-9.24.191203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong Z., Wu Z., Glick B.R., He S., Huang C., Wu L. Co-occurrence patterns of microbial communities affected by inoculants of plant growth-promoting bacteria during phytoremediation of heavy metal-contaminated soils. Ecotoxicol. Environ. Saf. 2019;183:109504. doi: 10.1016/j.ecoenv.2019.109504. [DOI] [PubMed] [Google Scholar]
- Kurniawan S.B., Ahmad A., Said N.S.M., Imron M.F., Abdullah S.R.S., Othman A.R., Purwanti I.F., Hasan H.A. Macrophytes as wastewater treatment agents: nutrient uptake and potential of produced biomass utilization toward circular economy initiatives. Sci. Total Environ. 2021;790:148219. doi: 10.1016/j.scitotenv.2021.148219. [DOI] [PubMed] [Google Scholar]
- Kurniawan S.B., Imron M.F., Purwanti I.F. Biosorption of chromium by living cells of Azotobacter s8, Bacillus subtilis and Pseudomonas aeruginosa using batch system reactor. J. Ecol. Eng. 2019;20:184–189. [Google Scholar]
- Kwon J.C., Nejad Z.D., Jung M.C. Arsenic and heavy metals in paddy soil and polished rice contaminated by mining activities in Korea. Catena. 2017;148:92–100. [Google Scholar]
- Lee W.J., Bao Y., Hu X., Lim T.-T. Hybrid catalytic ozonation-membrane filtration process with CeOx and MnOx impregnated catalytic ceramic membranes for micropollutants degradation. Chem. Eng. J. 2019;378:121670. [Google Scholar]
- Lee X.J., Ong H.C., Ooi J., Yu K.L., Tham T.C., Chen W.H., Ok Y.S. Engineered macroalgal and microalgal adsorbents: synthesis routes and adsorptive performance on hazardous water contaminants. J. Hazard Mater. 2022;423:126921. doi: 10.1016/j.jhazmat.2021.126921. [DOI] [PubMed] [Google Scholar]
- Lenski R.E. What is adaptation by natural selection? Perspectives of an experimental microbiologist. PLoS Genet. 2017;13 doi: 10.1371/journal.pgen.1006668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong Y.K., Chang J.-S. Bioremediation of heavy metals using microalgae: recent advances and mechanisms. Bioresour. Technol. 2020;303:122886. doi: 10.1016/j.biortech.2020.122886. [DOI] [PubMed] [Google Scholar]
- Li C., Zhou K., Qin W., Tian C., Qi M. Soil and sediment contamination : an international A review on heavy metals contamination in soil : effects , sources , and remediation techniques A review on heavy metals contamination in soil : E ff ects , sources , and remediation techniques. Soil Sediment Contam. An Int. J. 2019:1–15. 0. [Google Scholar]
- Li Y., Lin J., Huang Y., Yao Y., Wang X., Liu C., Liang Y., Liu K., Yu F. Bioaugmentation-assisted phytoremediation of manganese and cadmium co-contaminated soil by Polygonaceae plants (Polygonum hydropiper L. and Polygonum lapathifolium L.) and Enterobacter sp. FM-1. Plant Soil. 2020;448:439–453. [Google Scholar]
- Liu P., Zhang Y., Tang Q., Shi S. Bioremediation of metal-contaminated soils by microbially-induced carbonate precipitation and its effects on ecotoxicity and long-term stability. Biochem. Eng. J. 2021;166 [Google Scholar]
- Ma Y., Rajkumar M., Oliveira R.S., Zhang C., Freitas H. Potential of plant beneficial bacteria and arbuscular mycorrhizal fungi in phytoremediation of metal-contaminated saline soils. J. Hazard Mater. 2019;379:120813. doi: 10.1016/j.jhazmat.2019.120813. [DOI] [PubMed] [Google Scholar]
- Ma Y., Rajkumar M., Zhang C., Freitas H. Inoculation of Brassica oxyrrhina with plant growth promoting bacteria for the improvement of heavy metal phytoremediation under drought conditions. J. Hazard Mater. 2016;320:36–44. doi: 10.1016/j.jhazmat.2016.08.009. [DOI] [PubMed] [Google Scholar]
- Mahbub K.R., Krishnan K., Andrews S., Venter H., Naidu R., Megharaj M. Bio-augmentation and nutrient amendment decrease concentration of mercury in contaminated soil. Sci. Total Environ. 2017;576:303–309. doi: 10.1016/j.scitotenv.2016.10.083. [DOI] [PubMed] [Google Scholar]
- Manoj S.R., Karthik C., Kadirvelu K., Arulselvi P.I., Shanmugasundaram T., Bruno B., Rajkumar M. Understanding the molecular mechanisms for the enhanced phytoremediation of heavy metals through plant growth promoting rhizobacteria: a review. J. Environ. Manag. 2020;254:109779. doi: 10.1016/j.jenvman.2019.109779. [DOI] [PubMed] [Google Scholar]
- Mawang C.-I., Azman A.-S., Fuad A.-S.M., Ahamad M. Actinobacteria: an eco-friendly and promising technology for the bioaugmentation of contaminants. Biotechnol. Rep. 2021;32 doi: 10.1016/j.btre.2021.e00679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medfu Tarekegn M., Zewdu Salilih F., Ishetu A.I. Microbes used as a tool for bioremediation of heavy metal from the environment. Cogent Food Agric. 2020;6:1783174. [Google Scholar]
- Mello I.S., Targanski S., Pietro-Souza W., Frutuoso Stachack F.F., Terezo A.J., Soares M.A. Endophytic bacteria stimulate mercury phytoremediation by modulating its bioaccumulation and volatilization. Ecotoxicol. Environ. Saf. 2020;202:110818. doi: 10.1016/j.ecoenv.2020.110818. [DOI] [PubMed] [Google Scholar]
- Mohan S., Tippa A. World Environ. Water Resour. Congr. 2019 Groundwater, Sustain. Hydro-Climate/Climate Chang. Environ. Eng. - Sel. Pap. from World Environ. Water Resour. Congr. 2019. 2019. Role of wetland soil bacteria in enhancing the phytoremediation process through bioavailability phenomenon; pp. 1–10. [Google Scholar]
- Mohapatra R.K., Parhi P.K., Patra J.K., Panda C.R., Thatoi H.N. Microbial Biotechnology. Springer Singapore; Singapore: 2017. Biodetoxification of toxic heavy metals by marine metal resistant bacteria- a novel approach for bioremediation of the polluted saline environment; pp. 343–376. [Google Scholar]
- Mubashar M., Naveed M., Mustafa A., Ashraf S., Baig K.S., Alamri S., Siddiqui M.H., Zabochnicka-światek M., Szota M., Kalaji H.M. Experimental investigation of chlorella vulgaris and enterobacter sp. Mn17 for decolorization and removal of heavy metals from textile wastewater. Water (Switzerland) 2020;12 [Google Scholar]
- Muhamad M.H., Abdullah S.R.S., Hasan H.A., Bakar S.N.H.A., Kurniawan S.B., Ismail N.I. A hybrid treatment system for water contaminated with pentachlorophenol: removal performance and bacterial community composition. J. Water Proc. Eng. 2021;43 [Google Scholar]
- Njoku K.L., Akinyede O.R., Obidi O.F. Microbial remediation of heavy metals contaminated media by Bacillus megaterium and Rhizopus stolonifer. Sci. African. 2020;10 [Google Scholar]
- Novo L.A.B., Castro P.M.L., Alvarenga P., da Silva E.F. Bio-Geotechnologies for Mine Site Rehabilitation. Elsevier; 2018. Plant growth–promoting rhizobacteria-assisted phytoremediation of mine soils; pp. 281–295. [Google Scholar]
- Nwaehiri U.L., Akwukwaegbu P.I., Nwoke B.E.B. Bacterial remediation of heavy metal polluted soil and effluent from paper mill industry. Environ. Health Toxicol. 2020;35:1–10. doi: 10.5620/eaht.e2020009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oladipo O.G., Ezeokoli O.T., Maboeta M.S., Bezuidenhout J.J., Tiedt L.R., Jordaan A., Bezuidenhout C.C. Tolerance and growth kinetics of bacteria isolated from gold and gemstone mining sites in response to heavy metal concentrations. J. Environ. Manag. 2018;212:357–366. doi: 10.1016/j.jenvman.2018.01.038. [DOI] [PubMed] [Google Scholar]
- Oliveira H. Chromium as an environmental pollutant: insights on induced plant toxicity. J. Bot., Le. 2012;2012:1–8. [Google Scholar]
- Polti M.A., Aparicio J.D., Benimeli C.S., Amoroso M.J. Simultaneous bioremediation of Cr(VI) and lindane in soil by actinobacteria. Int. Biodeterior. Biodegrad. 2014;88:48–55. [Google Scholar]
- Pradhan D., Sukla L.B., Sawyer M., Rahman P.K.S.M. Recent bioreduction of hexavalent chromium in wastewater treatment: a review. J. Ind. Eng. Chem. 2017 [Google Scholar]
- Pradhan S.K., Singh N.R., Kumar U., Mishra S.R., Perumal R.C., Benny J., Thatoi H. Illumina MiSeq based assessment of bacterial community structure and diversity along the heavy metal concentration gradient in Sukinda chromite mine area soils, India. Ecol. Genet. Genom. 2020;15:100054. [Google Scholar]
- Purwanti I.F., Kurniawan S.B., Ismail N. ‘Izzati, Imron M.F., Abdullah S.R.S. Aluminium removal and recovery from wastewater and soil using isolated indigenous bacteria. J. Environ. Manag. 2019;249:109412. doi: 10.1016/j.jenvman.2019.109412. [DOI] [PubMed] [Google Scholar]
- Purwanti I.F., Kurniawan S.B., Simanjuntak D.Y. Removal of aluminium in contaminated soil using locally isolated vibrio alginolyticus. J. Ecol. Eng. 2019;20:135–140. [Google Scholar]
- Purwanti I.F., Kurniawan S.B., Tangahu B.V., Rahayu N.M. Bioremediation of trivalent chromium in soil using bacteria. Int. J. Appl. Eng. Res. 2017;12:9346–9350. [Google Scholar]
- Purwanti Ipung Fitri, Kurniawan S.B., Titah H.S., Tangahu B.V. Identification of acid and aluminium resistant bacteria isolated from aluminium recycling area. Int. J. Civ. Eng. Technol. 2018;9:945–954. [Google Scholar]
- Purwanti I.F., Obenu A., Tangahu B.V., Kurniawan S.B., Imron M.F., Abdullah S.R.S. Bioaugmentation of Vibrio alginolyticus in phytoremediation of aluminium-contaminated soil using Scirpus grossus and Thypa angustifolia. Heliyon. 2020;6 doi: 10.1016/j.heliyon.2020.e05004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purwanti I.F., Putri T.P., Kurniawan S.B. AIP Conference Proceedings. 2017. Treatment of chromium contaminated soil using bioremediation; p. 40008. [Google Scholar]
- Purwanti I.F., Simanjuntak D.Y., Kurniawan S.B. AIP Conference Proceedings. 2018. Toxicity test of aluminium to Vibrio alginolyticus as a preliminary test of contaminated soil remediation. [Google Scholar]
- Puyen Z.M., Villagrasa E., Maldonado J., Diestra E., Esteve I., Solé A. Biosorption of lead and copper by heavy-metal tolerant Micrococcus luteus DE2008. Bioresour. Technol. 2012;126:233–237. doi: 10.1016/j.biortech.2012.09.036. [DOI] [PubMed] [Google Scholar]
- Rahim F., Abdullah S.R.S., Hasan H.A., Kurniawan S.B., Mamat A., Yusof K.A., Ambak K.I. A feasibility study for the treatment of 1,2-dichloroethane-contaminated groundwater using reedbed system and assessment of its natural attenuation. Sci. Total Environ. 2022;814:152799. doi: 10.1016/j.scitotenv.2021.152799. [DOI] [PubMed] [Google Scholar]
- Raklami A., Tahiri A. ilah, Bechtaoui N., Abdelhay E.G., Pajuelo E., Baslam M., Meddich A., Oufdou K. Restoring the plant productivity of heavy metal-contaminated soil using phosphate sludge, marble waste, and beneficial microorganisms. J. Environ. Sci. (China) 2021;99:210–221. doi: 10.1016/j.jes.2020.06.032. [DOI] [PubMed] [Google Scholar]
- Ramazanova E., Lee S.H., Lee W. Stochastic risk assessment of urban soils contaminated by heavy metals in Kazakhstan. Sci. Total Environ. 2021;750:141535. doi: 10.1016/j.scitotenv.2020.141535. [DOI] [PubMed] [Google Scholar]
- Reed M.L.E., Glick B.R. Growth of canola (Brassica napus) in the presence of plant growth-promoting bacteria and either copper or polycyclic aromatic hydrocarbons. Can. J. Microbiol. 2005;51:1061–1069. doi: 10.1139/w05-094. [DOI] [PubMed] [Google Scholar]
- Ribeiro A.P., Rode M. Residual biomass energy potential: perspectives in a peripheral region in Brazil. Clean Technol. Environ. Policy. 2019;21:733–744. [Google Scholar]
- Rieuwerts J.S. The mobility and bioavailability of trace metals in tropical soils: a review. Chem. Speciat. Bioavailab. 2007;19:75–85. [Google Scholar]
- Said N.S.M., Kurniawan S.B., Abdullah S.R.S., Hasan H.A., Othman A.R., Ismail N.I. Competence of Lepironia articulata in eradicating chemical oxygen demand and ammoniacal nitrogen in coffee processing mill effluent and its potential as green straw. Sci. Total Environ. 2021;799:149315. doi: 10.1016/j.scitotenv.2021.149315. [DOI] [PubMed] [Google Scholar]
- Sainger P.A., Dhankhar R., Sainger M., Kaushik A., Singh R.P. Ecotoxicology and Environmental Safety Assessment of heavy metal tolerance in native plant species from soils contaminated with electroplating effluent. Ecotoxicol. Environ. Saf. 2011;74:2284–2291. doi: 10.1016/j.ecoenv.2011.07.028. [DOI] [PubMed] [Google Scholar]
- Saleem M., Arshad M., Hussain S., Bhatti A.S. Perspective of plant growth promoting rhizobacteria (PGPR) containing ACC deaminase in stress agriculture. J. Ind. Microbiol. Biotechnol. 2007;34:635–648. doi: 10.1007/s10295-007-0240-6. [DOI] [PubMed] [Google Scholar]
- Scaccabarozzi D., Castillo L., Aromatisi A., Milne L., Búllon Castillo A., Muñoz-Rojas M. Soil, site, and management factors affecting cadmium concentrations in cacao-growing soils. Agronomy. 2020;10:806. [Google Scholar]
- Schippers A., Breuker A., Blazejak A., Bosecker K., Kock D., Wright T.L. The biogeochemistry and microbiology of sulfidic mine waste and bioleaching dumps and heaps, and novel Fe(II)-oxidizing bacteria. Hydrometallurgy. 2010;104:342–350. [Google Scholar]
- Schwammberger P.F., Lucke T., Walker C., Trueman S.J. Nutrient uptake by constructed floating wetland plants during the construction phase of an urban residential development. Sci. Total Environ. 2019;677:390–403. doi: 10.1016/j.scitotenv.2019.04.341. [DOI] [PubMed] [Google Scholar]
- Shadman S.M., Daneshi M., Shafiei F., Azimimehr M., Khorasgani M.R., Sadeghian M., Motaghi H., Mehrgardi M.A. Electrochemical Biosensors. Elsevier; 2019. Aptamer-based electrochemical biosensors; pp. 213–251. [Google Scholar]
- Shah V., Daverey A. Phytoremediation: a multidisciplinary approach to clean up heavy metal contaminated soil. Environ. Technol. Innovat. 2020;18:100774. [Google Scholar]
- Shahid M.J., AL-surhanee A.A., Kouadri F., Ali S., Nawaz N., Afzal M., Rizwan M., Ali B., Soliman M.H. Role of microorganisms in the remediation of wastewater in floating treatment wetlands: a review. Sustainability. 2020;12:5559. [Google Scholar]
- Sharma P. Efficiency of bacteria and bacterial assisted phytoremediation of heavy metals: an update. Bioresour. Technol. 2021;328:124835. doi: 10.1016/j.biortech.2021.124835. [DOI] [PubMed] [Google Scholar]
- Shojaei Saeed, Jafarpour A., Shojaei Siroos, Gyasi-Agyei Y., Rodrigo-Comino J. Heavy metal uptake by plants from wastewater of different pulp concentrations and contaminated soils. J. Clean. Prod. 2021;296:126345. [Google Scholar]
- Sindern S., Tremöhlen M., Dsikowitzky L., Gronen L., Schwarzbauer J., Siregar T.H., Ariyani F., Irianto H.E. Heavy metals in river and coast sediments of the Jakarta Bay region (Indonesia) — geogenic versus anthropogenic sources. Mar. Pollut. Bull. 2016;110:624–633. doi: 10.1016/j.marpolbul.2016.06.003. [DOI] [PubMed] [Google Scholar]
- Song Y., Gao S., Yuan X., Sun R., Wang R. Two-compartment membrane electrochemical remediation of heavy metals from an aged electroplating-contaminated soil: a comparative study of anodic and cathodic processes. J. Hazard Mater. 2022;423 doi: 10.1016/j.jhazmat.2021.127235. [DOI] [PubMed] [Google Scholar]
- Srinath T., Verma T., Ramteke P.W., Garg S.K. Chromium (VI) biosorption and bioaccumulation by chromate resistant bacteria. Chemosphere. 2002;48:427–435. doi: 10.1016/s0045-6535(02)00089-9. [DOI] [PubMed] [Google Scholar]
- Tangahu B.V., Sheikh Abdullah S.R., Basri H., Idris M., Anuar N., Mukhlisin M. Lead (Pb) removal from contaminated water using constructed wetland planted with Scirpus grossus: optimization using response surface methodology (RSM) and assessment of rhizobacterial addition. Chemosphere. 2021:132952. doi: 10.1016/j.chemosphere.2021.132952. [DOI] [PubMed] [Google Scholar]
- Tangahu B.V., Sheikh Abdullah S.R., Basri H., Idris M., Anuar N., Mukhlisin M. A review on heavy metals (As, Pb, and Hg) uptake by plants through phytoremediation. Int. J. Chem. Eng. 2011;2011:1–31. [Google Scholar]
- Timková I., Sedláková-Kaduková J., Pristaš P. Biosorption and bioaccumulation abilities of actinomycetes/streptomycetes isolated from metal contaminated sites. Separations. 2018;5:54. [Google Scholar]
- Tirry N., Tahri Joutey N., Sayel H., Kouchou A., Bahafid W., Asri M., El Ghachtouli N. Screening of plant growth promoting traits in heavy metals resistant bacteria: prospects in phytoremediation. J. Genet. Eng. Biotechnol. 2018;16:613–619. doi: 10.1016/j.jgeb.2018.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titah H.S., Purwanti I.F., Tangahu B.V., Kurniawan S.B., Imron M.F., Abdullah S.R.S., Ismail N.I. Kinetics of aluminium removal by locally isolated Brochothrix thermosphacta and Vibrio alginolyticus. J. Environ. Manag. 2019;238:194–200. doi: 10.1016/j.jenvman.2019.03.011. [DOI] [PubMed] [Google Scholar]
- Titah H.S., Rozaimah S., Abdullah S.R.S., Idris M., Anuar N., Basri H., Mukhlisin M., Tangahu B.V., Purwanti I.F., Kurniawan S.B. Arsenic resistance and biosorption by isolated Rhizobacteria from the roots of Ludwigia octovalvis. Internet J. Microbiol. 2018;2018:1–10. doi: 10.1155/2018/3101498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhyay N., Vishwakarma K., Singh J., Mishra M., Kumar V., Rani R., Mishra R.K., Chauhan D.K., Tripathi D.K., Sharma S. Tolerance and reduction of chromium(VI) by Bacillus sp. MNU16 isolated from contaminated coal mining soil. Front. Plant Sci. 2017;8:1–5. doi: 10.3389/fpls.2017.00778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varma M., Gupta A.K., Ghosal P.S., Majumder A. A review on performance of constructed wetlands in tropical and cold climate: insights of mechanism, role of influencing factors, and system modification in low temperature. Sci. Total Environ. 2021;755 doi: 10.1016/j.scitotenv.2020.142540. [DOI] [PubMed] [Google Scholar]
- Vodyanitskii Y.N. Standards for the contents of heavy metals in soils of some states. Ann. Agrar. Sci. 2016;14:257–263. [Google Scholar]
- Wang Q., Ma L., Zhou Q., Chen B., Zhang X., Wu Y., Pan F., Huang L., Yang X., Feng Y. Inoculation of plant growth promoting bacteria from hyperaccumulator facilitated non-host root development and provided promising agents for elevated phytoremediation efficiency. Chemosphere. 2019;234:769–776. doi: 10.1016/j.chemosphere.2019.06.132. [DOI] [PubMed] [Google Scholar]
- Wang Y., Luo Y., Zeng G., Wu X., Wu B., Li X., Xu H. Characteristics and in situ remediation effects of heavy metal immobilizing bacteria on cadmium and nickel co-contaminated soil. Ecotoxicol. Environ. Saf. 2020;192:110294. doi: 10.1016/j.ecoenv.2020.110294. [DOI] [PubMed] [Google Scholar]
- Wang Y., Peng B., Yang Z., Chai L., Liao Q., Zhang Z., Li C. Bacterial community dynamics during bioremediation of Cr(VI)-contaminated soil. Appl. Soil Ecol. 2015;85:50–55. [Google Scholar]
- Wiatrowski H.A., Ward P.M., Barkay T. Novel reduction of mercury(II) by mercury-sensitive dissimilatory metal reducing bacteria. Environ. Sci. Technol. 2006;40:6690–6696. doi: 10.1021/es061046g. [DOI] [PubMed] [Google Scholar]
- Wu Y., Ma L., Liu Q., Sikder M.M., Vestergård M., Zhou K., Wang Q., Yang X., Feng Y. Pseudomonas fluorescens promote photosynthesis, carbon fixation and cadmium phytoremediation of hyperaccumulator Sedum alfredii. Sci. Total Environ. 2020;726 doi: 10.1016/j.scitotenv.2020.138554. [DOI] [PubMed] [Google Scholar]
- Wu Z., Kong Z., Lu S., Huang C., Huang S., He Y., Wu L. Isolation, characterization and the effect of indigenous heavy metal-resistant plant growth-promoting bacteria on sorghum grown in acid mine drainage polluted soils. J. Gen. Appl. Microbiol. 2019;65:254–264. doi: 10.2323/jgam.2018.11.004. [DOI] [PubMed] [Google Scholar]
- Xia L., Tan J., Wu P., He Q., Song S., Li Y. Biopolymers extracted from Klebsiella sp. and Bacillus sp. in wastewater sludge as superb adsorbents for aqueous Hg(II) removal from water. Chem. Phys. Lett. 2020;754:137689. [Google Scholar]
- Xu H., Hao R., Xu X., Ding Y., Lu A., Li Y. Removal of hexavalent chromium by Aspergillus Niger through reduction and accumulation. Geomicrobiol. J. 2021;38:20–28. [Google Scholar]
- Xu J., Liu C., Hsu P.-C., Zhao J., Wu T., Tang J., Liu K., Cui Y. Remediation of heavy metal contaminated soil by asymmetrical alternating current electrochemistry. Nat. Commun. 2019;10:2440. doi: 10.1038/s41467-019-10472-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav M., Gupta R., Sharma R.K. Advances in Water Purification Techniques. Elsevier; 2019. Green and sustainable pathways for wastewater purification; pp. 355–383. [Google Scholar]
- Zahoor M., Irshad M., Rahman H., Qasim M., Afridi S.G., Qadir M., Hussain A. Alleviation of heavy metal toxicity and phytostimulation of Brassica campestris L. by endophytic Mucor sp. MHR-7. Ecotoxicol. Environ. Saf. 2017;142:139–149. doi: 10.1016/j.ecoenv.2017.04.005. [DOI] [PubMed] [Google Scholar]
- Zhang X., Su C., Liu X., Liu Z., Liang X., Zhang Y., Feng Y. Effect of plant-growth-promoting rhizobacteria on phytoremediation efficiency of Scirpus triqueter in pyrene-Ni co-contaminated soils. Chemosphere. 2020;241:125027. doi: 10.1016/j.chemosphere.2019.125027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.