Summary
Myocardial infarction is lethal to patients because of insufficient blood perfusion to vital organs. Several attempts have been made to improve its prognosis, among which nanomaterial research offers an opportunity to address this problem at the molecular level and has the potential to improve disease prevention, diagnosis, and treatment significantly. Up to now, nanomaterial-based technology has played a crucial role in broad novel diagnostic and therapeutic strategies for cardiac repair. This review summarizes various nanomaterial applications in myocardial infarction from multiple aspects, including high precision detection, pro-angiogenesis, regulating immune homeostasis, and miRNA and stem cell delivery vehicles. We also propose promising research hotspots that have not been reported much yet, such as conjugating pro-angiogenetic elements with nanoparticles to construct drug carriers, developing nanodrugs targeting other immune cells except for macrophages in the infarcted myocardium or the remote region. Though most of those strategies are preclinical and lack clinical trials, there is tremendous potential for their further applications in the future.
Keywords: Ischaemic heart disease, Myocardial infarction, Nanomaterials, Cardiac biomarker, Nano-delivery system, New diagnostic and therapeutic strategies
Introduction
Cardiovascular disease (CVD), a collective jargon for diseases damaging the heart and blood vessels, including coronary heart disease, atherosclerosis, rhythm disorders, peripheral arterial disease, myocardial infarction, heart failure, and valvular disease, is a rapid-onset disease with high mortality.1 According to the WHO (World Health Organization),2 it was reported that nearly 18 million people died from cardiovascular-related diseases in 2019, which represented 32% of overall global deaths as the leading cause of death in all diseases. Among CVD, myocardial infarction (MI) due to coronary heart disease (CHD) which accounted for approximately 75% of sudden cardiac death (SCD), had become a major international health problem.3 MI occurs when the blood flow of coronary arteries reduces due to various reasons, causing oxygen deficiency of the cardiomyocytes and subsequent ischemic necrosis in the supply area.4 Even after anticoagulation, antiplatelet, thrombolysis, and reperfusion therapies, some patients still progress into heart failure.5 Hence, an effective treatment is urgently needed to inhibit cardiomyocyte apoptosis and promote local angiogenesis to stop the expansion of irreversible myocardial injury.
With those demands, nanomaterials provided a broad prospect for diagnosing and treating MI. According to ISO/TS 80004,6 nanomedicine refers to a highly selective diagnostic or therapeutic strategy against diseases at the molecular scale by utilizing nano-objects with any external dimension at the nanoscale (from 1 to 100 nm) or nanostructured materials with internal or surface structure in the nanoscale. Since nanomaterials can control or manipulate particles at the atomic level, they exhibit properties different from conventional materials. For example, many of them have an element-associated catalytic activity characterized as nanozymes.7 Furthermore, the ultrahigh area to volume ratio,8 intrinsic stability,9 biocompatibility,10 and low cytotoxicity11 enable nanomaterials to be used as drug delivery systems, which reduce the amount and frequency of medication to decrease side effects and improve efficacy.
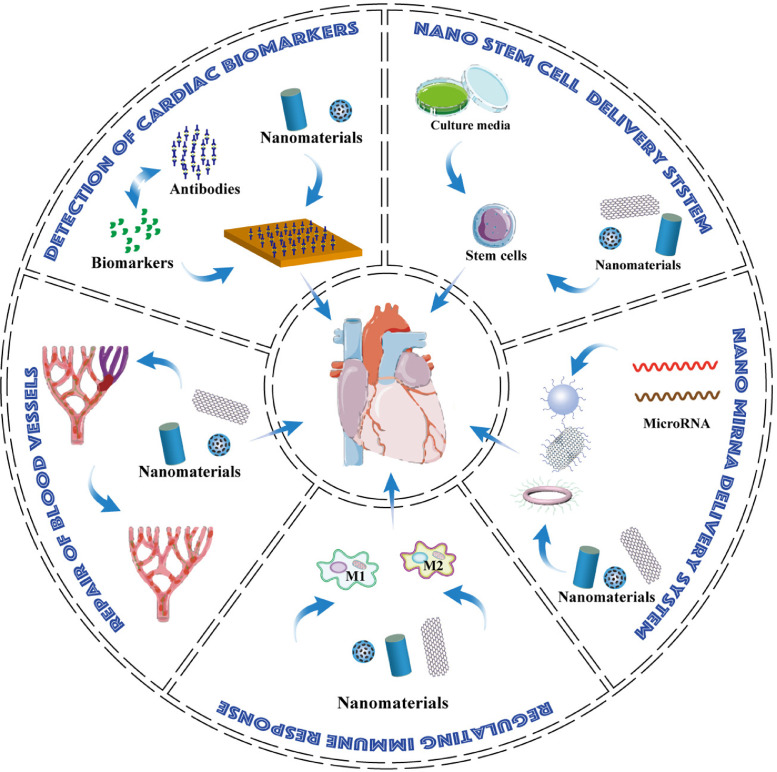
In this review, applications of nanomaterials in MI followed as diagnostic and therapeutic strategies are reviewed. Among therapeutic strategies, we introduce how injectable hydrogels and organic nanofibers can facilitate angiogenesis. After that, owing to the relatively few reports about the neovascularization application of inorganic nano-objects in MI animal models, we focus on their pro-angiogenetic mechanism on endothelial cells and proposed that conjugating pro-angiogenic elements on nano-drug deliver may exhibit extra neovascularization effect when treating MI. We next reported the recent progress using nanomaterials targeting different immune cells in infarcted myocardium or the remote region, mainly from 2019. Macrophage reprogramming in vivo or targeting multiple immune cells may be a bright future. Finally, we review the novel biomolecule-delivery-system role of nanomaterials for MI treatment, where the transportation of miRNA and even an intact cell are discussed, respectively. We also listed suggestions on designing basic science experiments to promote associated clinical trials treating MI (Figure 1).
Figure 1.
The schematic diagram shows the application of nanomaterials in MI. Nanomaterials help lower the detective limit of cardiac biomarkers, thus enabling a timely recognition of potential patients. In basic research, Nanomaterials have been explored to promote angiogenesis and regulate immune responses after MI. They also played a promising role in delivering novel biomolecules like miRNA and stem cells.
Nanomaterials for detection of cardiac biomarkers
Currently, the clinical diagnosis of MI still depends on the presence of myocardial injury symptoms, ECG ischemia changes, and abnormal blood levels of cardiac biomarkers, among which cardiac troponin I (cTnI) is the optimal biomarker for evaluating myocardial injury.12 Generally, the concentration of cTnI is lower than 0.4 ng/ml in a healthy human body, while people would have the potential risk for CVD once higher than 2.0 ng/mL.13 The past several decades have observed the development of many instant analysis assays of cTnI, including enzyme-linked immunosorbent assay (ELISA),14 chemiluminescent immunoassays,15 emerging photoelectrical, and electrical chemiluminescent immunosensors.16 The precision of cTnI measurement depends on two aspects. One is the number of cTnI that combines with capturers. Another is the appreciable amount of this combination outcome, for instance, the staining intensity or the current value. Nanomaterials improve both due to their physicochemical properties, thus providing a sensitive and selective method to monitor MI.
Nanomaterials used for enhancing the captured number of cTnI
Compared to the conventional materials loading primary antibodies, nanomaterials immobilize the location and orientation of antibodies, exposing the fragment antigen-binding (Fab) part better to avoid mutual masking of binding sites with cTnI induced by antibody floating. In addition, the high area-to-volume ratio of nanostructure also increases the density of primary antibodies loading on the surface, which enhances the percentage of captured cTnI in the blood sample. Qian et al.17 fabricated the TB-2HP5@Au-Pd/MnO2 nanocomposites and applied them to the electrochemical immunosensor. Not only did MnO2 nanosheets increase the excellent electrochemical properties of nanocomposites, but they also improved the loading amount of palladium nanoparticles (PdNPs) and gold nanoparticles (AuNPs), which could accelerate electron transfer and broaden the detection range as well.
Apart from antibodies, nanomaterials were used to improve the combinative ability of cTnI and other captures. Aptamers are the oligonucleotides or peptide molecules that have been artificially selected and modified to bind the target molecule.18 Lopa et al.19 deposited AuNPs conjugated with oligonucleotide aptamers onto titanium foil. After the combination between aptamers and cTnI, the negatively charged aptamers folded, inducing the charge perturbation and redox reaction of potassium ferro/ferri-cyanide (K4[Fe(CN)6]/K3[Fe(CN)6]) in the solution. As a result, the potential change negatively correlated with cTnI concentration was produced. This was an improvement of conventional electroanalytical methods.
Nanomaterials used for the signal amplification
In addition to enhancing the captured cTnI in the blood sample, nanomaterials have also been utilized to amplify observable signals induced by the binding of cTnI. As metal nano-objects, AuNPs can act as a wire connecting the redox motif of enzymes to the electrode surface based on its eminent electron transferability. Tan et al.16 designed an immunosensor with photoresponsive materials consisting of dodecahedral AuNPs and N-acetyl-L-cysteine capped CdAgTe quantum dots (NAC-CdAgTe QDs). The redox couple probes were inhibited from being transferred onto the surface of electrodes, which generated current values negatively correlated with cTnI concentration. Here AuNPs recuperated the conductivity of quantum dots which possessed good optical properties and the ability to create photocurrents, thus increasing the detection limit of cTnI to reach 1.756 pg/ml.
Also, the enzyme-like nanomaterials can catalyze the enzymatic reaction, thus accelerating and increasing the color value produced within a specific time. Gao et al.20 firstly reported that Fe3O4 magnetic nanoparticles (MNPs)-modified antibodies that allowed magnetic separation were also characterized by a peroxidase-like activity better than conventional horseradish peroxidase (HRP), which catalyzed the color reaction of substrates conjugated to the cTnI capturers. However, few studies have continued to confirm the detection range of MNP-based ELISA due to development costs. Besides, Fe3O4 MNPs only acted as catalyzers rather than luminescent materials, which limited the maximum value of output signals and further expansion in research, whereas carbon nitride nanosheet (CNNS) with low toxicity, low cost, and high stability21 directly acted as luminophores per se. Jiang et al.22 modified CNNS with metal-organic frameworks conjugated with functional amino-acid (NH2-MIL(Fe)) to produce a molecular probe of secondary antibodies (CNNS@NH2-MIL(Fe)), where NH2-MIL(Fe) accelerated the reduction of S2O82- to produce more abundant reactive intermediate SO4•–, thus enhancing the ECL luminescent reaction and amplifying the cTnI detection-value through the conversion from chemical to electrical energy. Furthermore, Jiang also used Ti3C2 nanosheet as the matrix, which expanded the loading area of capturing antibodies and facilitated the electron transfer to achieve multiple signal amplification.
The studies mentioned above illustrated that by being applied in the synthesis of matrix embedded primary antibodies, primary or secondary antibodies, catalyzers, or luminophores, nanomaterials have the potential to increase the detective accuracy of various cardiac biomarkers significantly Table 1. summarizes the detection limit and linear detection range of cardiac biomarkers via assays mentioned above. Along with developing novel nanomaterials, similar ideas like Jiang et al.22 can be adopted to utilize different nanomaterials in producing different parts of biosensors to increase detection accuracy.
Table 1.
Different materials for MI biomarker detection.
| biomaterials | biomarkers | methods | Detection limit | Linear detection range |
|---|---|---|---|---|
| TiO2 nanotube23 | cTnl | fluorescence immunoassay | 0.1 pg/ml | 0.1 pg/ml-100 ng/ml |
| 2HP5@Au-Pd/MnO217 | cTnI | electrochemical immunosensor | 2 pg/ml | 0.005–20 ng/ml |
| AuNPs/Ti19 | cTnI | electrochemical aptasensor | 0.18 pM | 1–1100 pM |
| NAC-CdAgTe- QDs/AuNPs/GCE16 | cTnI | photoelectric immunosensor | 1.756 pg/ml | 5.0 pg/mL–20.0 ng/ml |
| CNNS@NH2-MIL(Fe)22 | cTnI | electrochemiluminescence | 0.38 fg/ml | 1 fg/ml–10 ng/ml |
| Polyaniline Nanowire24 | Myo | Biosensors | 100 pg mL−1 | - |
| Polyaniline Nanowire24 | cTnl | Biosensors | 250 fg mL−1 | - |
| Polyaniline Nanowire24 Polyaniline Nanowire24 |
CK-MB BNP |
Biosensors Biosensors |
150 fg mL−1 50 fg mL−1 |
- - |
Nanomaterials for the pro-angiogenesis
Angiogenesis plays a crucial role in cardiovascular-related diseases such as atherosclerosis and cardiac ischemic disease. Therapeutic angiogenesis aims to create new blood vessels in ischemic areas to replenish deficient energy and oxygen resources caused by the blocked vessels, thus supporting the contractility and autorhythmicity of residual cardiomyocytes,25 promoting infarction healing and inhibiting cardiac remodeling. Several lines of evidence suggested that angiogenesis after MI mainly relied on the sprouting of endothelial cells from pre-existing vessels rather than the differentiation de novo from endothelial precursor cells to primary vascular plexus, emphasizing the critical role of endothelial cells in cardiac tissue repair.26,27 In this part, we review various nanomaterials about regulating endothelial behavior to promote angiogenesis.
Injectable hydrogels
Hydrogels are insoluble three-dimension (3D) polymeric networks where hydrophilic molecules are immobilized. Characterized by high biocompatibility, nontoxicity, and biodegradation with high absorption capacity, not only can hydrogels repair blood vessels by the carried biologically active components, but they also can be directly used as reparative materials for tissue formation, thus exhibiting a wide range of applications in drug delivery, tissue engineering, and wound healing.28
Hydrogels protect the loaded bioactive components from degradation
Recently, several cytokines/growth factors (GFs), including vascular endothelial growth factor A (VEGF-A),29 and basic fibroblast growth factor (bFGF), 30 have been used to form collateral blood vessels in the ischemic tissues associated with fibrosis and thrombosis. However, in highly vascularized myocardial tissues, the rapid blood flow makes it unlikely for degradation-susceptible GFs to retain bioactivity prolongedly, which partially explains why the direct injection of VEGF towards CHD patients failed to improve their prognosis in clinical trials.31 Moreover, a single injection of high-dose VEGF had been proved to induce edema and vascular leakage.32 Compared to other nanomaterials, the high porosity and the mesh-like structure formed by cross-linkers of hydrogels enhance their contact surface area with cells and protect GFs from proteasome degradation, thus maintaining their release for an extended period of time. O'Dwyer et al.33 combined VEGF with polyglutamic acid (PGA) polypeptides to form nanoparticles, increasing the VEGF-releasing time to 4 weeks in vitro. Subsequently, he encapsulated nanoparticles into a hyaluronic acid hydrogel, which increased the release of VEGF for another week. Similarly, Li et al.34 utilized heparin which possessed a growth factor-like domain to bind VEGF as a stabilizer, tyramine as a linker to form the hydrogel structure, and H2O2 with HRP as a regulator for controlling hydrogel degradation to synthesize subcutaneous injectable hydrogel and proved its pro-angiogenetic ability within soft tissues. Nevertheless, he did not achieve the same result in MI. Furthermore, Rufaihah et al.35 embedded VEGF and angiopoietin-1(ANG-1) that downregulated the endothelial barrier permeability and prompted endothelial survival into polyethylene glycol-fibrinogen hydrogels, injecting them into the peri-infarct area of rats under left anterior descending artery (LAD) ligation subsequently. The result showed the dropped infarction area and the improved cardiac function due to enhanced angiogenesis and mature vascular density.
To control the degradation process of injectable hydrogels, the smart hydrogel that releases active components after reaching cardiac tissues was also fabricated. It has been previously reported that matrix metalloproteinases (MMPs) induced by post-infarction inflammation played a crucial role in the degradation of extracellular matrix (ECM).36 A recent study by Fan et al.37 successfully achieved target delivery of bFGF to MI area by conjugating MMP-2/9-cleavable peptide, a specific substrate of MMP, with hydrogels to form collagen hydrogel composites. Not only did they selectively release bFGF by the degradation of hydrogels via binding of MMP and its substrate peptide, but also this peptide competitively inhibited the degradation of cardiac ECM by MMPs, protecting the heart from ventricular rupture post-MI. In addition to VEGF and FGF,38 insulin-like growth factor 1 (IGF-1),39 Myeloid-Derived Growth Factor (Mydgf)40 has also been applied to increase new blood vessel density in the infarction area. Meanwhile, the limited blood flow caused by fibrin-rich thrombosis also exacerbates myocardial and endothelial cell necrosis. To solve this, a core-shell colloidal architecture hydrogel dual-delivery system was innovatively designed by Mihalko et al.41. The conditional solubility of nanoparticles gave the loaded drugs the possibility of being released under different stimuli, such as temperature, pH,42 etc. Y-27632 is a fibrosis-associated Rhoa/ROCK signaling pathway inhibitor that mitigates fibrosis after IR injury, whereas tissue plasminogen activator (tPA) helps dissolve the clot to restore the coronary artery perfusion. By utilizing those mentioned above, Mihalko designed a mixture of two nanogels comprising a tight crosslinked core containing tPA as well as a loosely crosslinked shell containing Y-27632, successfully limiting the scar formation size in mice after MI.
Hydrogels promote angiogenesis via intrinsic characteristics
Hydrogels consisting of bioactive materials have the potential to promote angiogenesis without loaded nanoparticles or drugs. Previous studies had suggested that nitric oxide (NO) could regulate endothelial cells' survival, migration, and proliferation, but the overexpressed ROS induced by local inflammation converted NO into nitrite, which exacerbated oxidative stress to inhibit mature angiogenesis.43 Vong et al.44 designed a new injection hydrogel based on A-B-A triblock copolymer (PARg-PEg-PARg) and polyacrylic acid (PAAc). The PIC micelles obtained consisted of PEG chains as the ring structure of shells, scavenging overexpressed ROS through the electrostatic crosslinking to maintain the local concentration of pro-angiogenic NO. Moreover, under physiological conditions, the increased temperature could convert the injectable hydrogel into gel to upregulate the retained time of NO for more than ten days, which also prolonged the angiogenetic repair.
Besides, myocardium-derived ECM provides an adequate microenvironment for endothelial cells and cardiomyocytes to survive. Hydrogels made from decellularized matrix naturally contain elastin, collagen, and fibronectin, which promote the adhesion and vascularization of endothelial cells and the differentiation of cardiac progenitor cells with fewer risks of side effects induced by immunogenicity.45 Studies over the past decades have suggested that myocardial matrix-derived hydrogels reduced the scar size and increased angiogenesis in MI rodents or vitro.46, 47, 48 More remarkably, similar results have also been observed in larger mammals like pigs,48 suggesting a broad prospect for natural material-originated hydrogels used in infarction repair.
Considering the two directions of hydrogel application mentioned above, ECM mimetic hydrogels loaded with pro-angiogenic molecules may provide a dual effect of pro-angiogenesis. However, few studies have reported the successful application of this method in rodent or pig infarct models. Besides, the delivery of hydrogels to cardiac tissues mainly depended on infection to the MI area or cardiac tissue patch,49 which increased the risk of arrhythmias due to surgical injury or post-implantation response. Those aspects deserve to be further improved in the future.
Organic nanofibers
Nanofibers refer to fibers with two external dimensions in nano-size, including hollow nanotubes and solid nanorods.50 Compared to other polymeric materials, the average diameter and surface area of organic nanofibers are more similar to the collagen fiber network of ECM, providing an adequate environment for angiogenesis. Chaudhuri et al.51 reported that carbon nano-vectors (CNTs) exerted a pro-angiogenic effect in vitro and vivo. They also assessed the positive impact of doxorubicin conjugated single-walled carbon nanotubes (CNT-Dox) on angiogenesis.
Additionally, carbon-nanofibers also exhibit an advantage of contribution to synchronous ventricular contraction. Along with preserving cardiac function, it also promotes angiogenesis via the downregulation of irregulated contractility between infarction areas and no infarction areas. Tashakori-Miyanroudi et al.52 synthesized a collagen scaffold with carbon-nanofiber, promoting the formation of a vascular network in MI areas of rats. Recently, several other organic materials have also been applied to produce angiogenesis-related nanofibers. Rufaihah and Seliktar53 modified glycosaminoglycans (GAG) mimetic peptide into heparan sulfate glycosaminoglycans analogs to synthesize nanofiber scaffold, by which the endogenous VEGF and FGF were enriched to facilitate angiogenesis. Moreover, organic nanofibers including poly-lactic/glycolic acid (PLGA),54 gelatin shell nanofibers,54 polycaprolactone55 have also been utilized to produce artificial blood vessels, providing a new way of thinking for therapeutic angiogenesis.
Novel inorganic nanoparticles
Inorganic nanoparticles were prepared predominantly by inorganic salt crystallization thermal decomposition and hydrothermal process. Various inorganic nanoparticles (such as copper, europium, zinc, gold, silver, iron oxide, and cerium oxide) have been successfully prepared and applied to multiple preclinical and clinical studies.56 The general mechanism behind the pro-angiogenic role of inorganic nanoparticles locates on the inorganic elements that directly regulate endothelial cell proliferation and migration by stimulating the overexpression of growth factors. However, compared to the field of drug delivery, the research about nanomaterials’ intrinsic characteristics in pro-angiogenesis has remained in the stage of endothelial culture experiments rather than MI animal model experiments.
Copper (Cu)
Several theories have concluded that the lack of copper ions negatively affected neovascularization. The classic view attributed this phenomenon to copper's role in promoting the translocation of structurally stable HIF-1α to the nucleus. Under normoxia, the prolyl hydroxylase domain-containing proteins (PHDs) recognize and hydroxylase the prolyl residues of HIF-1α, making it much easier to recognize E3 ubiquitin protein ligase-like von Hippel–Lindau protein (pVHL) that ubiquitinates HIF-1α and transports it to the proteasome degradation system. In this process, copper facilitates the accumulation of active HIF-1α via the inhibition of PHDs.57 Regarding the decreased copper concentration observed in the ischemic heart,95 researchers used tetraethylenepentamine (TEPA), a copper chelator, to remove copper when culturing HUVEC in vitro, observing the inhibited combination between HIF-1α and hypoxic response elements (HRE) sites of target genes, suggesting the key component of copper in the biofunction of HIF-1α transcriptional complex.58 Meanwhile, it was reported that the high affinity between copper and S100A13, a protein regulating the nonclassical secretion of signal proteins, contributed to the secretion of FGF-1.59 Antioxidant-1 (Atox1), a copper transport protein, also promoted neovascularization in the ischemic limb of mice via the activation of copper enzyme lysyl oxidase to upregulate intracellular ROS to stimulate inflammation-related angiogenesis.60,61
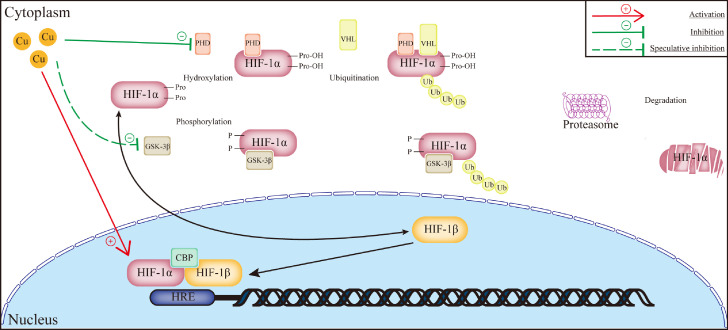
Based on the research, nanomaterials containing copper were utilized in blood vessel regeneration, especially damaged bone repair. Elrayah et al.62 used copper ion-containing solutions to produce a hydroxyapatite scaffold with the nanostructured surface, observing its neovascularization after being implanted into rabbits subcutaneously. However, it is rare to see the direct use of copper nanomaterials to facilitate pro-angiogenesis in MI. Sharma et al.63 treated reperfused MI mice with oral copper nanoparticles for four weeks, observing the decreased MI areas, with a more pronounced effect in the presence of swimming training for 90 days before the ligation surgery. He attributed this phenomenon to the increased phosphorylation (i.e., the inhibition) of glycogen synthase kinase-3β (GSK-3β) that had been proved to induce HIF-1α degradation in human microvascular endothelial cells.64 However, the phosphorylation of GSK-3β had also been reported to maintain mitochondrial permeability, which inhibited cardiomyocyte apoptosis, indicating its sophisticated role in MI development.65 Whether the activated behavior of endothelial cells or the inhibited apoptosis of myocytes was the leading cause of decreased MI areas remains to be discussed (Figure 2).
Figure 2.
Schematic illustration of how copper promotes angiogenesis. The pro-angiogenetic signaling pathway affected by copper is the regulation of HIF-1α degradation, where copper inhibits the hydroxylation of prolyl residues to maintain the intracellular concentration of active HIF-1α. Copper also influences the combination between HIF-1 transcriptional complex and HRE sites. It was also speculated that copper also directly actives HIF-1α via downregulating the expression of GSK-3β. Abbreviations: HIF: hypoxia-inducible facto; PHD: prolyl hydroxylase domain-containing protein; VHF: von Hippel–Lindau protein; GSK-3β: glycogen synthase kinase-3β; CBP: CREB-binding protein; the red straight line with arrow means activation; the green straight line with straight-arrow means inhibition; the green truncated line with straight-arrow means inhibition under speculation.
Cerium (Ce)
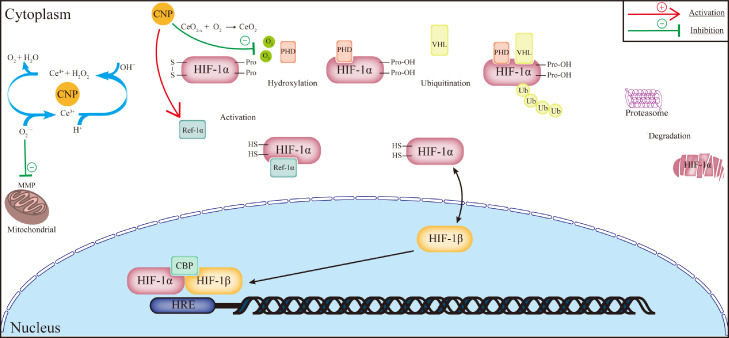
As an element of the rare earth family of metals, cerium has almost no biological activity. However, several studies suggest that cerium oxide nanoparticles (CNP) can regulate endothelial cell behavior, among which the role of a free radical scavenger has been reported most. The anti-ROS ability of CNP depends on the oxidation state of cerium. Due to redox cycling between cerium (IV) and cerium (III), both Ce4+ and Ce3+ exist on the surface of CNP.66 Previous research has proved that Ce3+ can react with O2˙− to produce H2O2, and as a catalase-mimics, Ce4+ subsequently decomposes H2O2 to oxygen and water, whereby CNP downregulates the intracellular concentration of ROS, restoring the mitochondrial membrane potentials to inhibit apoptosis and maintain cell viability of HUVECs in vitro.67,68 Another explanation of CNP pro-angiogenic effect was reported that after being absorbed into the cytoplasm, CNP downregulated the intracellular concentration of oxygen, which considerably upregulated both the expression and intranuclear translocation of HIF-1α.69 Therefore, the induced VEGF promoted the tube formation of endothelial cells.
Besides, another pro-angiogenetic signaling pathway induced by CNP was also reported in limb ischemia rodent models.69 Reduction-oxidation factor 1-apurinic (Ref-1) is a kind of endonuclease that recognizes and removes the apurinic/apyrimidinic (AP) sites in damaged DNA molecules to achieve DNA base excision repair.69 In the studies of retinal diseases,70 researchers observed that Ref-1 could reduce the cysteine residues in specific translational factors, including HIF-1α and NF-κB, which activated the expression of VEGF. Das et al.69 injected CNP into the ligated femoral artery of mice, protecting muscle cells from ischemic necrosis after seven days. He then cultured HUVEC with H2O2 (i.e., ROS-excessive conditions) and CNP, successfully restoring the cell viability and tubular formation compared to only H2O2 treated group. This process was inhibited by adding APX3330 (i.e., a Ref-1 inhibitor), proving that CNP reestablished blood vessels in the ischemic limbs via the Ref-1 signaling pathway.
However, none of those mechanisms has been successfully used to directly facilitate vascularization in MI areas, resulting from the different effects of CNP concentration on endothelial cells or cardiomyocytes. It was suggested that CNP with high concentration had detrimental influences on HUVEC proliferation.71 Also, the inhalation of CNP activated mast cells to produce inflammatory cytokines that increased the infarction area of ischemic/injury models.72 However, low concentration-injection of CNP may be insufficient for its pro-angiogenetic bioactivity in cardiac tissues. Therefore, a more precious concentration and more appropriate CNP administration method are needed (Figure 3).
Figure 3.
Schematic illustration of cerium nanoparticles in pro-angiogenesis. The redox cycling on the surface of cerium nanoparticles helps remove intracellular ROS to maintain the mitochondrial membrane potential and inhibit apoptosis of endothelial cells. It was also assumed that cerium nanoparticles could absorb intracellular oxygen, thus the induced hypoxia environment protected HIF-1α from degradation. Another pro-angiogenetic mechanism proposed was that cerium nanoparticles also upregulated Ref-1α that reduced oxidized cysteine residues in HIF-1α to enhance the expression of VEGF. Abbreviations: MMP: mitochondrial membrane potential; HIF: hypoxia-inducible facto; PHD: prolyl hydroxylase domain-containing protein; VHF: von Hippel–Lindau protein; Ref-1α: reduction-oxidation factor 1; CBP: CREB-binding protein; the red straight line with an arrow means activation; the green straight line with a straight arrow means inhibition.
Europium (Eu)
Over recent years, the pro-angiogenic property of europium has been gradually revealed. In preclinical studies, europium trihydroxide (Eu(OH)3) nanorods were firstly synthesized successfully by a microwave technique by Patra et al.,73 who further explored the produced nanorods and demonstrated its pro-angiogenic property that along with promoting vascular sprouting in vivo, the nanorods could also facilitate endothelial cell proliferation in vitro. The mechanism for angiogenesis focused on the generation of endogenous ROS and the activation of the mitogen-activated protein kinase (MAPK) signaling pathway, which regulated the proliferation and migration of endothelial cells. Additionally, the endothelial cell sprouting was also observed in zebrafish embryos.74 From the perspective of drug-use safety, even in the dose six times higher than the plausible restorative dosage, europium still showed no cytogenic toxicity against mice, which partially supported its application safety in the human body.75
Recently, Eu(OH)3 has exhibited another pro-angiogenetic mechanism. Roy et al.76 reported that when co-cultured with Eu(OH)3 nanorods, cytotoxic heavy metal element cadmium (Cd)-treated endothelial cells showed elevated expression of endothelial nitric oxide synthase (eNOS) and B-cell lymphoma-extra-large protein (Bcl-xL), whereby the improved activities of endothelial cells subsequently attenuate cardiovascular toxicity caused by Cd, protecting chick embryo from developmental abnormalities. However, cardiac toxicity associated with Cd was totally different from the common pathological initiator of MI to induce cardiomyocyte necrosis, which limited its expansion from subtypes to universality.
Nanomaterials for the regulation of immune response
In a healthy heart, the resident leukocytes form the majority of cardiac immune cells, among which the heterogeneous macrophages have the most extensive number, compared to other small populations such as dendritic cells in the aortic valves, mast cells in the myocardium, and lymphocytes in the pericardiac adipose tissues.77 The cardiac ischemic injury surges the release of granules from mast cells and inflammatory cytokines (e.g., IL-1, IL-6, TNF-a) from cardiomyocytes and resident macrophages,78 which triggers robust recruitment of neutrophils, monocytes/macrophages, and lymphocytes from circulation, thus altering the cardiac immune homeostasis and MI progression. A consensus has been gradually reached that targeting those immune cells to reestablish cardiac homeostasis plays a vital role in inhibiting the myocardium injury and acceleration of cardiac repair,79 therefore, restoring the cardiac function. Herein, we summarize the recent progress using nanomaterials to target immune cells, especially macrophages, to treat MI.
Nanomaterials targeting macrophages
The flow cytometry and single-cell RNA sequencing have partitioned the mice cardiac macrophages into three populations: CCR2+ MHC IIhigh, CCR2− MHC IIhigh, and CCR2− MHC IIlow cells, which was partially confirmed in the human cardiac tissues obtained from patients with dilated cardiomyopathy (DCM) and ischemic cardiomyopathy (ICM) that human CCR2− macrophages are predominately HLA-DRhigh.79 With a capacity that quickly expresses high levels of IL-1β, IL-6, and TNF-α in response to inflammatory stimulations, the minor population, CCR2+ macrophages are replenished and originates from monocytes in circulation, which has been further confirmed in a study examining macrophages isolated from the sex-mismatched transplanted heart.79 In contrast, the numerically dominant CCR2− macrophages renew in situ consistently and originate from the yolk sac (CCR2− MHC IIlow) and fetal monocytes (CCR2− MHC IIhigh).80 In addition to inducing cardiac regeneration and neovascularization, they also regulate the maturation and development of coronary arteries,80 probably via expressed IGF and enhanced matrix formation in neonatal mice.
The role of macrophages post-MI hinges on the CCR2 surface marker. Although CCR2− macrophages, the predominant resident cell subtypes, show relatively low pro-inflammatory potentials, they can activate the proliferation of hematopoietic stem and progenitor cells (HSPCs) in the bone marrow, and induce mesenchymal stem cells (MSCs) and peripheral B lymphocytes to secrete CCL2 and CCL7 that leads to leukocytosis after being stimulated by the released granules from mast cells and damaged-associated molecular pattern (DAMP) from apoptotic cardiomyocytes.81,82 A small fraction of HSPCs also transfers to the spleen,83 making it a secondary source for monocytes and neutrophils after MI. Subsequently, those monocytes infiltrate in the infarcted myocardium, which differentiates into CCR2+ macrophages and upregulates TLR4 and TLR9 signaling pathways through the recognition of HMGB1, nDNA (nuclear DNA), and mtDNA (mitochondrial DNA) within seven days,77 respectively. These pro-inflammatory macrophages enhance their phagocytosis to remove dead/dying cells via upregulated scavenge receptors and Mer tyrosine kinase (MerTK),84 which promotes wound healing and scar formation to retard the progression of long-term cardiac dysfunction. However, the sterile inflammation culminates in matrix remodeling via the increased MMP9 and pro-inflammatory cytokine burst,85 recruiting the circulatory neutrophils, especially in the first three days, whereas the accumulation of neutrophils after MI is predominantly considered as an adverse prognostic factor. Another study also reported that the increased ratio of CCR2+ to CCR2− macrophages correspond with persistent left ventricular systolic dysfunction in patients after left ventricular assist device (LVAD) unloading.79
Unlike the relatively high-abundance CCR2+ macrophages in the infarcted myocardium, the number of CCR2− macrophages rise steadily in the remote regions.86, 87, 88 RNA sequencing and qPCR analysis showed that they expressed high levels of IGF-1, IL-10, TGF-β, and VEGF, in correspondence with growth promotion, inflammation inhibition, collagen deposition, and neovascularization, suggesting the reparative subtype of these macrophages. The result of tissue localization immunostaining exhibited that compared to TUNEL positive cardiomyocytes,79 they were considerably adjacent to regenerated coronary endothelial cells, which indicated their pro-repair function rather than pro-inflammation. In summary, the early excessive accumulation of pro-inflammatory macrophages and the decreased transformation of pro-inflammatory macrophages to reparative macrophages aggravate cardiac dysfunction, where nanomaterials can be used to reestablish immune homeostasis after MI.
Nanomaterials blunt the monocyte-derivate macrophages accumulation in the early phase of MI
The ischemia shock induces apoptotic cardiomyocytes to release DAMPs which activate the innate immune system via TLR489 and TLR990 to recruit monocytes from circulation, deteriorating the survival of cardiomyocytes. Although the TLR4 knockout mice developed smaller infarcted myocardium size after I/R injury,91 only a few TLR4 inhibitors have been proved to decrease the damage. In addition, despite that TLR4 competitive inhibitor, Eritoran, alleviated mice infarcted size after I/R injury,92 the ligation of LAD was preceded 10 min by agent intravenous injection, which limited its further application in clinics since many heart attacks occurred without preconditioning. In contrast, simultaneous dosing with reperfusion after ischemia may show higher clinical feasibility, which requires timely enrichment of drugs in the infarcted myocardium. In 2019, Fujiwara et al.93 synthesized TAK-242, a chemical inhibitor of TLR4 intracellular domain containing PLGA nanoparticles. As the bio-polymerization of glycolide and lactide, PLGA can be metabolized entirely through the tricarboxylic acid cycle (TCA)94 in vivo, which is appropriate for damaged fragile cardiomyocytes. The immunostaining visualized the enhanced delivery efficacy of nanodrugs to monocytes/macrophages in circulation, the spleen, and the heart, especially the border region of infarcted cardiac tissues with swollen mitochondrial. 24 h after I/R injury, the flow cytometry showed that nanodrugs considerably reduced the Ly-6Chigh monocytes, a pro-inflammatory monocyte population, and neutrophils to express IL-1β, IL-6, and MCP-1 in the ischemia area, which was consistent with the smaller infarcted size compared to the vector-treated mice. Also, this experiment discovered that CCR2+ monocytes from the spleen of TLR4 ablation mice decreased significantly compared to wild-type mice, which were also associated with improved cardiac function. In support of this notion, Ikeda et al.95 synthesized PLGA nanoparticles containing the cyclosporine A or pitavastatin, with the former one inhibiting the mitochondrial permeability by blocking cyclophilin D to decrease the cell-lethal signals, and the latter one decreased the recruited macrophages from circulation by inhibiting the CCR2 receptor competitively. Irrespective of cooperated or single administration, both nanoparticles successfully downregulated the Ly-6Chigh monocytes and ameliorated infarcted size after 24 h. A similar study from Tokutome et al.96 used aDA-approved classical drug pioglitazone, a peroxisome proliferator-activated receptor-gamma (PPAR-γ) activator, for the treatment of rat I/R models. Previous studies had indicated that oral pioglitazone started three days before ischemic shock reduced infarcted size, but few effects were observed when dosing with reperfusion.97 The author administered pioglitazone loaded PLGA nanoparticles(NPs) to IR mice as soon as reperfusion initiated, successfully blunting the recruitment of Ly-6Chigh monocytes and cardiac inflammation, whereas the administration of pioglitazone alone or using CCR2 ablation mice failed to reach the same result, suggesting the delivery efficacy of NPs targeting monocytes.96 Furthermore, they applied pioglitazone-NPs on MI mini-pigs and received the same interventive effect, which promoted the translation to clinical practice.
In addition to TLR4, mtDNA and nDNA released by local necrotic or apoptotic cardiomyocytes activates cytosolic DNA receptor cGAS(GMP-AMP synthase)-STING (stimulator of interferon genes) pathway and extracellular DNA sensor TLR9/MyD88/CXCL5 signaling pathway respectively,98,99 which also exacerbates the accumulation and transformation of pro-inflammatory macrophages in the initial phase of MI. TLR2100 or TLR3101 ablation mice also exhibited reduced infarcted size after MI. Nevertheless, few studies intervened with them to downregulate the scathing innate immune system response, which was partially attributed to the ambiguous ligands and downstream mediators binding to these receptors on cardiac macrophages. More pharmacologic antagonists deserved to be developed with PLGA NPs or liposomes to regulate pro-inflammatory macrophage functions.
Nanomaterials enhanced macrophage polarization toward M2 phenotype
The different polarization of macrophage towards M1 or M2 phenotype represents the classic classification of macrophage populations. In vitro experiments delineated that lipopolysaccharide (LPS) and IFN-γ induced the bone marrow-derived macrophage (BMDM) to differentiate to M1 macrophage, whereas IL-4 and IL-13 serve as another differentiation factor to switch BMDM to M2 macrophages.102,103 M1 macrophage showed increased production of ROS, culminating in the enrichment of cytotoxic malondialdehyde and unsaturated aldehydes via lipid peroxidation as well as the destruction of nucleobases and the deoxyribose backbone via separating the hydrogen atoms.104 Additionally, ROS catalyzed the oxidative modification of specific amino acids, such as cysteine and methionine, facilitating proteolysis and cellular signaling protein dysfunction.105 So far, ROS has been proved to induce cardiomyocyte death via the access: the activation of ASK-1/JNK-dependent pathways, the downregulation of SIRT3, and the downregulation of apoptosis repressor.106 From the perspective of macrophage metabolism, the production of ROS requires the incomplete electron transfer to oxygen and abnormal oxidative phosphorylation (OXPHOS) in mitochondrial,107 in which case macrophages are prone to synthesize ATP in a glycolysis-dependent model, whereas M1 macrophages exhibit enhanced glycolysis and lactate production.108 The contribution of ROS to macrophage phenotype transformation inspired the development of nanodrugs for regulating cardiac ROS synthesis.
Pro-inflammatory macrophages undergo complex metabolic remodeling. The reversal of succinate dehydrogenase (SDH) induced by the blunt TCA cycle during ischemia culminated in the accumulation of succinate in mitochondrial, which was also the substrate of mitochondrial complex II.109 The excessive oxidation of succinate via SDH induced the excessive reduction of the CoQ pool, leading to the reverse electron transportation (RET) with the help of proton motive force (Δp). The subsequent electron leak aggravated ROS production, which was hypothesized as a key factor for ROS overproduction during myocardial ischemia.107,110 Meanwhile, several studies had reported that the stimulation from TLR4 upregulated the expression of mitochondrial-associated enzyme immunoresponsive gene-1 (IRG-1), thus catalyzing the production of itaconate from cis aconitic acid.111 As an anti-inflammatory bypass metabolite of the TCA cycle, itaconate inhibits SDH to decrease succinate oxidation, thus reducing the production of ROS.110 Dimethyl itaconate, a cell-permeable itaconate derivative, had been reported to downregulate IL-6 release of macrophage to relieve skin inflammation. Borrowed from this, Nakkala et al.112 electrospuned FDA-approved biodegradable poly-ε-caprolactone (PCL) and DMI into nanofibers, which increased the entrapped rate of DMI compared to PLGA. The subcutaneous implantation of nanofibers resulted in the recruited CD86+ M1 macrophages three days after surgery and CD206+ M2 macrophages seven days after surgery compared to the sham-group, suggesting that nanofibers only accelerated the M2 polarization in the late inflammation without altering the M1 macrophage functions in the early phase. He also received similar results in the rat MI model as downregulated local MCP-1, IFNα, IFN-β, IL-23 and IL17A occurred with ameliorated cardiac remodeling. The groundbreaking advance of this research was the subcutaneous administration of cardiac-protective drugs, which showed apparent fewer risks than in situ injections, making it more feasible to be used as a protective vaccine for the prevention of MI.
Some specific components of nanomaterials may show ROS removal without loaded drugs. Zhou et al.108 found that melanin nanoparticles obtained from cuttlefish ink and alginate hydrogels synthesized from ocean algae could react with ROS to produce oxygen, which drives the macrophage polarization from M1 towards M2 phenotype under hypoxia. Moreover, the nanocomplex upregulates PI3K/Akt1/mTOR signaling pathway, which had been regarded as a key factor inducing M2 polarization and NF-κB downregulation.113 The subsequent intervention indicated that the in-situ injection of nanocomplex to infarcted myocardium of rats promoted the ratio of M2/M1 phenotypes and inhibited cardiomyocyte apoptosis. Decreased fibrosis and improved ejection fraction were also observed in 3 days and 28 days after surgery, respectively, suggesting the cardiac protective role of these natural nanomaterials.
Apart from ROS, there were other ways regulating M2 polarization. The inflammation resolution of ischemic or infarcted regions initiated after the uptake of apoptotic neutrophils by macrophages that secreted more anti-inflammatory cytokines.114 Inspired by this phenomenon, Bao et al.115 fused the natural membrane of neutrophil apoptotic bodies with hexyl 5-aminolevulinate hydrochloride (HAL) preloaded mesoporous silica to synthesize nanoparticles (MSN-HAL) and proved that macrophages could uptake them, degrading the mesoporous silica to release the inner HAL that produced bilirubin, an anti-inflammatory metabolite intracellularly. After LAD ligation followed by three-day tail vein injection of nanoparticles, given to the CD11b, CD44, and αLβ2-integrin combined on the surface of apoptotic bodies, the nanoparticles had the capability to target cardiac inflammation actively, thus promoting the M2 polarization and improving cardiac functions. Another interesting result came from Galili et al.,116 who used α-Gal nanoparticles that recruited macrophages via the activation of the complement system, whereas the accumulated macrophages showed an M2 phenotype and expressed high levels of VEGF, IL-1α, FGF, and PDGF, all reparative cytokines, thus decreasing the poor fibrosis and improved cardiac functions.
The studies mentioned in this chapter indicated the two predominant strategies using nanomaterials to regulate macrophage-mediated homeostasis: inhibiting the excessive accumulation of pro-inflammatory macrophages or promoting the macrophage differentiation to reparative phenotype. From the perspective of pharmacokinetics, nanomaterials enhanced the permeability of vessels around the infarcted region, from which the drug maintenance and uptake efficacy benefited. Phagocytosis, pinocytosis, and receptor-mediated endocytosis make it easy for macrophages to uptake biomaterials. Several strategies have been proposed to optimize this process, including modifying nanoparticles with cationic ligands or surfactants which destructed the liposome membrane to release the uptake drugs or regulating nanoparticle size (100–200 nm better) to facilitate endocytosis without quick biodegradation in lysosomes simultaneously.117,118 Adding specific ligands that bind to macrophage receptors also increased the targeting ability of nano-complexes. In the aspect of material type, the FDA-approved PLGA was most widely utilized as a macrophage-targeting carrier, whereas liposomes or biofilm-made materials also played a promising role in this area. However, the restoration of cardiac function depended on the sophisticated collaboration between biomaterials and loaded drugs, which increased the difficulty in affinity matching. In addition, those carriers still existed a potential threat to security resulting from biodegradation and biocompatibility. Therefore, exploring the intrinsic therapeutic characteristic of natural materials like what Zhou et al.108 did is worth trying compared to merely treating them as drug carriers.
Meanwhile, the mechanism in which nanomaterials regulate macrophage behavior can be explored further. Most studies classified the targeted cardiac macrophages into M1 and M2 phenotypes, yet this differentiation resulted from stimulation in vitro, which completely induced extreme cellular functions: pro-inflammatory or anti-inflammatory. In fact, it may be insufficient to describe macrophage molecular features using this classification in vivo since macrophages react with multiple factors dynamically rather than form and stay in a specific population. Moreover, DAMP, not PAMP (pathogen-associated molecular pattern), activates macrophage differentiation in MI, which is not entirely consistent with in vitro stimulator (LPS), thus having the possibility of inducing a different downstream mediator. Besides, M1/M2 differentiation occurs after macrophage maturation, whereas macrophage behavior altered within the whole expanded macrophage family, including monocytes. Therefore, a more detailed classification strategy is required to explore the complex profiles of cardiac macrophages. As an emerging surface marker, CCR2 had been proved to discriminate macrophage origination, mediate macrophage recruitment, and determine cell functions in infarcted myocardium. Except for transformation between M1 and M2 phenotype, researchers can also assess the alteration of CCR2 expression ratio to evaluate the interventive effect of targeted nano-drugs. Also, the removal of apoptotic cells via M1 macrophages plays a vital role in subsequent scar formation, while the excessive activation of M2 macrophage induces poor fibrosis that increases cardiac motion stiffness and precludes improved cardiac functions. Therefore, it is insufficient to assess the ratio of M1 to M2 phenotype alteration alone and regard it as a key factor indicating beneficial effects in MI. Researchers can evaluate M1/M2 ratio with timepoint sequentially to demonstrate the dynamic protective effect of macrophages.
There are other promising research focuses on the therapeutic effect of nanomaterials targeting macrophages. Previous studies had demonstrated that macrophages in the remote region also participated in the development of cardiac remodeling.77 Although resident macrophages died of ischemia in the infarcted area, with the chronic hemodynamic stimulation after MI, for example, the increased intraventricular pressure, macrophages survived and accumulated in the remote region, gradually rising even ten days after MI. The replenishment from monocytes in circulation also aggravated this imbalance that possibly affected matrix remodeling. With RNA sequencing of those macrophages, it is expected to select a new therapeutic target using Nano-drugs.
In early 2022, a study reported a revolutionary strategy using liposome nanoparticles encapsulating mRNA translating chimeric antigen receptor (CAR) to realize T cell reprogramming in vivo via CD5 ligand.119 The synthesized CAR-T cells were able to inhibit myofibroblast proliferation after heart failure but maintained in vivo for less than three days, which induced a transient therapeutic effect after intravenous injection of nanoparticles, thus decreasing the side effect. Such an idea can be expanded on macrophage reprogramming in vivo, which also have the potential to become research hotspots in the future.
Nanomaterials targeting other immune cells
Apart from macrophages, many other immune cells function in the development of MI, including mast cells, neutrophils, dendritic cells (DCs), and T-cells.77 However, only a few studies regulated them to achieve cardiac homeostasis after MI. In 2017, Choo et al.120 reported that the injection of extracted DCs primed by infarcted lysate to mice undergoing MI induced the activation of systematic regulatory T lymphocytes (Treg cells) that ameliorated infarcted size, cardiac remodeling and promoted reparative macrophages to increase mice survival. However, given that the production of tolerant DCs in vitro was too costly and time-consuming to be expanded in clinics, they tried to find another way to produce tolerant DCs in vivo. In 2021, another research team121 developed liposome nanoparticles containing infracted lysates and rapamycin, an inhibitor of mTOR signaling to induce DCs to tolerate, suggesting that merely subcutaneous injection activated Treg cells in infarcted cardiac tissues, which inhibited adverse cardiac remodeling. Similarly, Zhang et al.122 synthesized alginate hydrogels loaded DCs-derived exosomes and injected them around ithe nfarcted myocardium, observing the differentiation of Treg cells and subsequent reparative macrophages, through which cardiac protective effects were also achieved. Even though the authors in the two studies used nanomaterials targeting DCs and Treg cells, the final therapeutic effect was still obtained by regulating macrophage function. The neutrophil burst and mast cell degranulation also played a vital role in cardiac inflammation,77 but there were few nano-drugs being developed to target them. The limitation of targeted immune cells may be attributed to less retention time, like neutrophils which considerably decreased three days after MI, and ambiguous biological effect of cell function or interventive targets like mast cells, which is expected to be explored in the future.
Nanomaterials for micro-RNA and stem cell delivery
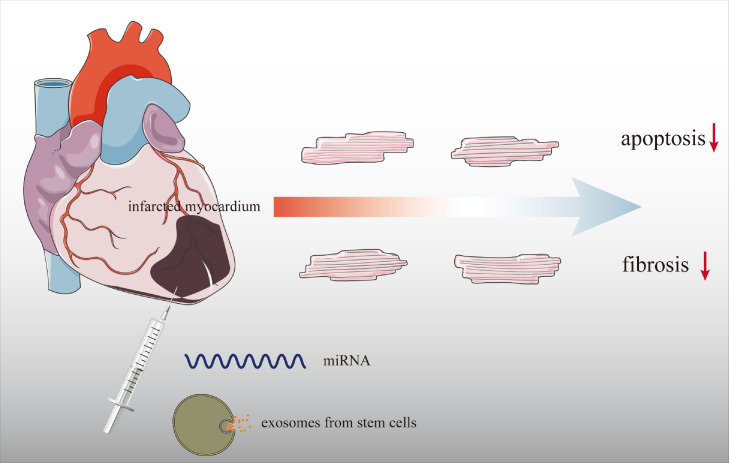
For the past decades, nanomaterial-based drug delivery system has received much more attention in the field of targeted therapy against cancer in advanced stages. However, it was merely the tip of the clinical application iceberg. The nanoparticle drug delivery system (Nano-DDS), including polymeric micelles,123 liposomes,124 polymeric nanoparticles,125 and crystalline metals,126 has been characterized as nearly nontoxic and stable when carrying biomolecules within tissues and has become a central strategy in contemporary cardiovascular medicine.127 Different dosing methods also affected the delivery efficiency of Nano-DDS, among which the biodegradable molecules still depended on the in situ injection (Figure 4).
Figure 4.
Regarding the biodegradation-susceptible of miRNAs and stem cells compared to other biomolecules in vivo, the in-situ injection is applied to enhance drug delivery efficacy. However, this approach decreases the possibility of clinical translation since the in-situ injection induces a second injury to the damaged fragile myocardium.
Nano microRNAs delivery system
MiRNAs are endogenous, non-coding small RNAs composed of about 22 nucleotides that play critical regulatory roles in many biological processes and are associated with various human diseases.128 By binding to the 3’ region of targeted mRNA, miRNAs induce the degradation or silence of that and inhibit the expression of targeted genes.129 In MI, miRNAs play an essential role in anti-apoptosis or regeneration for cardiomyocytes and inhibition of inflammation for immune cells. Despite significant advances in the use of miRNAs for the regulation of disease pathogenesis, several vital challenges remain to be addressed, ranging from ineffective delivery systems to the continuous host immune response or the potential thrombosis130 caused by the off-target effects. In addition, traditional miRNA delivery methods are hard to maintain the expression for miRNAs are prone to degradation in serum.131 Therefore, effective miRNA carriers are urgently required. In this day and age, novel miRNA carriers can be classified roughly into two main categories: one comprises non-viral vectors and the other comprises viral vectors.132 Hoverer, viral vectors have severe drawbacks such as toxicity and limited potential for up-scale expression because of the anti-virus immune response due to its immunogenicity.133 Several non-viral nanomaterial vector systems have been engineered successfully to generate a safe and efficient gene-delivery system, including cationic organic carriers, inorganic nanoparticles and hybrid vectors.134 Herein, we reported nano microRNAs targeting cardiomyocytes.
Nano microRNAs inhibit myocardial apoptosis
Myocardial apoptosis is associated with the altered expression of different miRNAs, among which miRNA-499 acts as an important MI protector.135 According to this, Nie et al.135 designed an unlockable core-shell nanocomplex (Hep@PGEA) which benefited the self-accelerating release of miRNA-499. The basic structure of Hep@PGEA comprised poly glycidyl methacrylate (PGEA) shell and heparin nanoparticles core that impeded intravascular coagulation. The injection of Hep@PGEA conduced to the attenuation of myocardial infarction in the early stage. Another miRNA related to cardiomyocyte apoptosis is miRNA-1, which inhibits the expression of anti-apoptosis proteins like B-Cell Leukemia/Lymphoma 2 (Bcl-2). Hong et al.136 designed the poly-l-lysine (DGL)-loaded miRNA-1 inhibitor, namely antisense oligonucleotide (AMO-1), which inhibited cardiomyocyte apoptosis and improved ventricular remodeling after MI. In addition, a more perfectly target mRNA binding sequence, i.e., small interfering RNA (siRNA), has also been used to alleviate myocardial injury post-MI.137 It is anticipated that there will be more miRNA-regulation therapeutic strategies in CVD treatment.
Nano microRNAs induce myocardial regeneration
Recent studies have revealed that fibroblasts have the potential to be directly converted into induced cardiomyocytes (iCMs),138 demonstrating the potential development for myocardial regeneration in the future. By combining nanoparticles and miRNA, researchers have gigantically improved their survival and retention. miRNA encapsulated by PLGA-polyethyleneimine (PEI) nanocarriers had been successfully designed by Muniyandi et al.139 It was the first time that nanoparticles were directly utilized to regulate myocardial reprogramming. Similarly, in 2021, Yang et al.140 used branched polyethyleneimine coated nitrogen-enriched carbon dots (BP-NCDs) to carry the complex of miRNA, including miRNA-1, miRNA-133, miRNA-208, and miRNA-499. The result showed that targeted fibroblasts were partially converted to cardiomyocytes-like cells. Moreover, the injection of these nanoparticles significantly reduced the areas of fibrosis as well as the pathological thickness of the ventricular wall after MI. In the cellular experiment, Yang et al.141 also observed the proliferation of human embryonic stem cell-derived endothelial cells and cardiomyocytes by injecting their proliferator miRNA-199a-3p nanoparticles, proving the expanded application of nanomaterials in cardiac remodeling.
Given to the compatibility of lipid and cell membrane, Liposomes are prone to be absorbed by cardiomyocytes and released to cytoplasm in the low pH environment inside the endosome,118 which dominate the nucleic acid delivery system. However, miRNA connects to the liposome membrane via relatively weak electrostatic interactions, resulting in its degradation and off-target effects. Compared to liposomes, polyelectrolyte complex, such as poly-lysine, showed increased nucleic acid-retention time through hydrophobic forces, electrostatic, and hydrogen bonding.142 The ratio of DNA/poly-lysine mass concentration between two to three has been proved to carry the highest DNA.143 Yet, there were few studies focusing on the optimal concentration ratio to carry miRNA. Unlike immune cells, cardiomyocytes have not been reported to show specific cell surface markers clearly. Nano-miRNAs always target the infarcted area passively via cardiac injection in situ, limiting its further potential in clinical translation. Researchers can utilize single-cell RNA sequencing to select cardiomyocyte surface receptors with high-affinity ligands and connect them to the outer membrane of nanoparticles to enhance their targeting specificity. Some other reports also pointed out that the smart hydrogel could release its cargos under various stimulation like pH, temperature, and specific metabolites, making it another promising material loading miRNA in the future.
Nano Stem cells delivery system
As a type of adult stem cells, mesenchymal stem cells (MSCs) isolated from the bone marrow, peripheral blood and adipose tissues have great potential in treating many vascular diseases and cardiac disorders.144 MSCs can produce many cell types, such as bone cells, chondrocytes, adipocytes, blood cells, and nerve cells, as well as heart and skeletal muscle cells,145 which are immunologically inert when being transplanted into the host. Likewise, as the major stem cells for cardiac disorder therapy, MSCs have lots of ability followed as differentiation into cardiovascular cells, inflammation and fibrosis inhibition, angiogenesis, and apoptosis resistance.146 However, the effectiveness of stem cell therapy in animal and clinical studies is hampered by fundamental issues, such as poor survival and the low engraftment rate of the transplanted cells in the damaged tissues, hindering the therapeutic efficacy.147 In this case, nano-biomaterials would be a promising approach as a means to increase the survival and engraftment rate of MSCs.
To improve the therapeutic potential of MSCs, Teo et al.148 designed nanoparticles that contained PLGA-block-hyaluronic acid (PLGA-b-HA) by encapsulating naturally antioxidant epigallocatechin gallate (EGCG) with manganese oxide (MnO2) nano-catalysts. The results showed that it maintained an oxidative environment, thus prolonging cellular survival and maintaining its secretion activities. Moreover, this study researched a series of microgel as a delivery vehicle for cardiac stem cell therapy and indicated that a hydrophilic with the negatively charged microenvironment maintained high viability and long-term viability of cardiac stem cells. Injecting the microgel can promote angiogenesis, inhibit cell apoptosis, and promote cardiac repair of myocardial infarction.149
Conclusions and perspectives
As the most severe stage of coronary artery disease, MI causes cardiac arrhythmia, heart failure, cardiac rupture, and sudden death. A considerable part of patients (about a quarter) was currently not capable of receiving timely reperfusion in the presence of the occlusive artery,5 indicating the insufficiency of MI diagnosis. Cardiac markers released under ischemic conditions are an important indicator for subsequent MI, among which cTnI has been regarded as the gold standard. The clinical laboratory often uses ELISA to monitor cTnI and reach a lower limit of detection for 10 pg/ml. However, the high consumption of human resources, time, and money limited its expansion in community clinics.150 Besides, the risky concentration of cTnI is lower than the normal detection limit. Based on that, the high area to volume ratio makes nanomaterials conjugate with more capture antibodies, thus enhancing the combined cTnI in the blood sample. The electrical characteristics of metal nanomaterials also help amplify detective signals of cTnI, strengthening the detection specificity of ELISA, photoelectric immunoassay, and electrochemical immunoassay, making it possible to detect cTnI as low as 0.1 pg/ml. However, the deposable synthesized nanomaterials require repeated production, which costs a lot without mass output technology. Also, such a low detection limit indicated the interference-prone characteristic of nanomaterial-based measurement, which may decrease the specificity in reverse. More stable nanomaterials needed to be explored and applied in clinal trials to verify their efficacy.
For those diagnosed with MI, the timely and indication-compliant antithrombotic drugs, vasodilators, percutaneous coronary intervention (PCI), or coronary artery bypass graft (CABG) significantly reduce patients’ mortality.5 However, those interventions only blunt cardiac remodeling rather than precluding it regarding the long-term effect. The damaged myocardium undergoes acute ischemic shock-reperfusion injury-latter remodeling pathologically caused by disruption of immune homeostasis and irregular as well as decreased neovascularization. To restore them, the Nano drug delivery system has become a promising carrier that protects their cargo from biodegradation and even can reach specific organelles. Liposomes and PLGA nanomaterials are the star carriers targeting cardiac inflammation, which benefits from high affinity with the cell membrane and pro-endocytosis effect considering their size and surface charges. Research hotspots are located on restoring the CCR2− reparative macrophages in the infarcted myocardium. Regulating other immune cells like DCs and Tregs or using liposomes to realize macrophage reprogramming in vivo are expected to be a promising direction.
Hydrogels, carbon nanotubes, organic nanofibers have been used to deliver pro-angiogenic drugs, especially those smart hydrogels which merely open their structure to release loaded drugs after connecting to the stimulators. Also, the intrinsic characteristics of nanomaterials, for example, the enzyme-like activity, make themselves more than merely a carrier. Hence, we discuss the pro-angiogenic effect of some inorganic elements which had been explored in the proliferation of endothelial cells in vitro and other diseases like skin wound healing of diabetes or lower extremity ischemia, but not yet MI. We aim at providing a new idea to design nanocarriers by conjugating them with pro-angiogenic elements to facilitate excess pro-angiogenic effect except for the loaded drugs, thus improving long-term adverse cardiac remodeling.
Currently, miRNAs and stem cells have been reported to treat MI via inhibiting apoptosis or promoting reprogramming of cardiomyocytes. However, both are degradation-prone, especially in circulation, which can be avoided via nanoparticles encapsulation. Also, we noticed no studies reporting nanomaterials carrying organelles like mitochondrial, which may also have a bright future.
The biggest challenge to blunt nanomaterials application in clinics is that few clinical trials have been approved or conducted, especially for the treatment of MI. The administration accesses of nano-drugs consist of intravenous injection, intracardiac injection, and subcutaneous implantation. However, except for liposomes and a specific type of nanoparticles, most nanomaterials still relied on the intracardiac injection to target cardiac tissues passively, which significantly limited their application because of puncture injury. Another reason lies in the selected animal models, as mice or rats occupied a high proportion compared to pigs. Also, toxicity and side effects should be more of our concern. For example, nanomaterials caused macrophage destructions while accumulating in the liver, spleen, lung, and other internal organs.151 To solve those problems above and facilitate the expansion of clinical trials, several strategies can be taken, including selecting nanomaterials that are able to be administered through intravenous injection or subcutaneous implantation, experimenting with the efficacy of nanomaterials on pig MI models, and developing antidotes against toxicity. It is inappropriate to deny the expanded application of nanomaterials just for its potential safety problems. By combining with other biomolecules, including drugs, miRNA, and even an intact cell, the advanced nanomaterials with less toxicity will play an essential role in developing precision medicine in MI or other fields of medicine in the future.
Search and selection criteria
This review is based on a systematic search in PubMed using the term (nanomaterial OR Nano-delivery system) AND (coronary artery disease OR atherosclerosis OR cardiovascular OR myocardial infarction OR cardiac biomarker) . We limited our search to articles written in English and published over the last twenty years.
Contributions
H.Shi, Z.Huang, T.Xu, A.Sun and J.Ge performed the literature search, prepared the figures, and wrote the manuscript. All authors read and approved the final manuscript.
Declaration of interests
The authors declare that they have no conflicts of interest.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81800235); Shanghai Clinical Research Center for Interventional Medicine (19MC1910300).
Contributor Information
Ai-jun Sun, Email: sun.aijun@zs-hospital.sh.cn.
Jun-bo Ge, Email: jbge@zs-hospital.sh.cn.
References
- 1.Dariush Mozaffarian E.J.B., Go A.S., Arnett D.K., et al. Heart disease and stroke statistics—2015 update a report from the American heart association. Circulation. 2015;131(4):e29–e322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 2.Organization W.H. Cardiovascular diseases (CVDs). June 11 2021. https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds). Accessed 24 July 2021.
- 3.Hayashi M., Shimizu W., Albert C.M. The spectrum of epidemiology underlying sudden cardiac death. Circ Res. 2015;116(12):1887–1906. doi: 10.1161/CIRCRESAHA.116.304521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zasada W., Bobrowska B., Plens K., et al. Acute myocardial infarction in young patients. Kardiol Pol. 2021 doi: 10.33963/KP.a2021.0099. [DOI] [PubMed] [Google Scholar]
- 5.Virani S.S., Alonso A., Benjamin E.J., et al. Heart disease and stroke statistics-2020 update: a report from the American heart association. Circulation. 2020;141(9):e139–e596. doi: 10.1161/CIR.0000000000000757. [DOI] [PubMed] [Google Scholar]
- 6.“ISO/TS 80004 - 1:2015 - Nanotechnologies - Vocabulary - Part 1: Core terms”. https://www.iso.org/standard/68058.html?browse=tc.pdf. Accessed 24 July 2021.
- 7.Singh S., Dosani T., Karakoti A.S., Kumar A., Seal S., Self WT. A phosphate-dependent shift in redox state of cerium oxide nanoparticles and its effects on catalytic properties. Biomaterials. 2011;32(28):6745–6753. doi: 10.1016/j.biomaterials.2011.05.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng L., Wang X., Gong F., Liu T., Liu Z. 2D nanomaterials for cancer theranostic applications. Adv Mater. 2020;32(13) doi: 10.1002/adma.201902333. [DOI] [PubMed] [Google Scholar]
- 9.Mofazzal Jahromi M.A., Sahandi Zangabad P., Moosavi Basri S.M., et al. Nanomedicine and advanced technologies for burns: preventing infection and facilitating wound healing. Adv Drug Deliv Rev. 2018;123:33–64. doi: 10.1016/j.addr.2017.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sahle F.F., Kim S., Niloy K.K., et al. Nanotechnology in regenerative ophthalmology. Adv Drug Deliv Rev. 2019;148:290–307. doi: 10.1016/j.addr.2019.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seaberg J., Montazerian H., Hossen M.N., Bhattacharya R., Khademhosseini A., Mukherjee P. Hybrid nanosystems for biomedical applications. ACS Nano. 2021;15(2):2099–2142. doi: 10.1021/acsnano.0c09382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thygesen K., Mair J., Giannitsis E., et al. How to use high-sensitivity cardiac troponins in acute cardiac care. Eur Heart J. 2012;33(18):2252–2257. doi: 10.1093/eurheartj/ehs154. [DOI] [PubMed] [Google Scholar]
- 13.Boriani G., Biffi M., Cervi V., et al. Evaluation of myocardial injury following repeated internal atrial shocks by monitoring serum cardiac troponin I levels. Chest. 2000;118(2):342–347. doi: 10.1378/chest.118.2.342. [DOI] [PubMed] [Google Scholar]
- 14.Rezaee M.A., Rasaee M.J., Mohammadnejad J. Selection of specific inhibitor peptides in enzyme-linked immunosorbent assay (ELISA) of cardiac troponin I using immuno-dominant epitopes as competitor. J Immunoassay Immunochem. 2017;38(1):72–81. doi: 10.1080/15321819.2016.1216444. [DOI] [PubMed] [Google Scholar]
- 15.Lou D., Fan L., Cui Y., Zhu Y., Gu N., Zhang Y. Fluorescent nanoprobes with oriented modified antibodies to improve lateral flow immunoassay of cardiac troponin I. Anal Chem. 2018;90(11):6502–6508. doi: 10.1021/acs.analchem.7b05410. [DOI] [PubMed] [Google Scholar]
- 16.Tan Y., Wang Y.Y., Li M.S., Ye X.X., Wu T.H., Li CY. Enhanced photoelectrochemical immunosensing of cardiac troponin I based on energy transfer between N-acetyl-L-cysteine capped CdAgTe quantum dots and dodecahedral Au nanoparticles. Biosens Bioelectron. 2017;91:741–746. doi: 10.1016/j.bios.2017.01.040. [DOI] [PubMed] [Google Scholar]
- 17.Qian X., Zhou X., Ran X., et al. Facile and clean synthesis of dihydroxylatopillar[5]arene-stabilized gold nanoparticles integrated Pd/MnO2 nanocomposites for robust and ultrasensitive detection of cardiac troponin I. Biosens Bioelectron. 2019;130:214–224. doi: 10.1016/j.bios.2019.01.041. [DOI] [PubMed] [Google Scholar]
- 18.Park K.S. Nucleic acid aptamer-based methods for diagnosis of infections. Biosens Bioelectron. 2018;102:179–188. doi: 10.1016/j.bios.2017.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lopa N.S., Rahman M.M., Ahmed F., et al. Simple, low-cost, sensitive and label-free aptasensor for the detection of cardiac troponin I based on a gold nanoparticles modified titanium foil. Biosens Bioelectron. 2019;126:381–388. doi: 10.1016/j.bios.2018.11.012. [DOI] [PubMed] [Google Scholar]
- 20.Gao L., Zhuang J., Nie L., et al. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat Nanotechnol. 2007;2(9):577–583. doi: 10.1038/nnano.2007.260. [DOI] [PubMed] [Google Scholar]
- 21.Lv Y., Chen S., Shen Y., et al. Competitive multiple-mechanism-driven electrochemiluminescent detection of 8-hydroxy-2′-deoxyguanosine. J Am Chem Soc. 2018;140(8):2801–2804. doi: 10.1021/jacs.8b00515. [DOI] [PubMed] [Google Scholar]
- 22.Jiang X., Wang H., Chai Y., Shi W., Yuan R. High-Efficiency CNNS@NH2-MIL(Fe) electrochemiluminescence emitters coupled with Ti3C2 nanosheets as a matrix for a highly sensitive cardiac troponin I assay. Anal Chem. 2020;92(13):8992–9000. doi: 10.1021/acs.analchem.0c01075. [DOI] [PubMed] [Google Scholar]
- 23.Kar P., Pandey A., Greer J.J., Shankar K. Ultrahigh sensitivity assays for human cardiac troponin I using TiO2 nanotube arrays. Lab Chip. 2012;12(4):821–828. doi: 10.1039/c2lc20892j. [DOI] [PubMed] [Google Scholar]
- 24.Lee I., Luo X., Huang J., Cui X.T., Yun M. Detection of cardiac biomarkers using single polyaniline nanowire-based conductometric biosensors. Biosensors. 2012;2(2):205–220. doi: 10.3390/bios2020205. (Basel) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carmeliet P., Jain R.K. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473(7347):298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oka T., Akazawa H., Naito A.T., Komuro I. Angiogenesis and cardiac hypertrophy: maintenance of cardiac function and causative roles in heart failure. Circ Res. 2014;114(3):565–571. doi: 10.1161/CIRCRESAHA.114.300507. [DOI] [PubMed] [Google Scholar]
- 27.Wu X., Reboll M.R., Korf-Klingebiel M., Wollert K.C. Angiogenesis after acute myocardial infarction. Cardiovasc Res. 2020;117(5):1257–1273. doi: 10.1093/cvr/cvaa287. [DOI] [PubMed] [Google Scholar]
- 28.Liao X., Yang X., Deng H., et al. Injectable hydrogel-based nanocomposites for cardiovascular diseases. Front Bioeng Biotechnol. 2020;8:251. doi: 10.3389/fbioe.2020.00251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Basu S., Nagy J.A., Pal S., et al. The neurotransmitter dopamine inhibits angiogenesis induced by vascular permeability factor/vascular endothelial growth factor. Nat Med. 2001;7(5):569–574. doi: 10.1038/87895. [DOI] [PubMed] [Google Scholar]
- 30.Coultas L., Chawengsaksophak K., Rossant J. Endothelial cells and VEGF in vascular development. Nature. 2005;438(7070):937–945. doi: 10.1038/nature04479. [DOI] [PubMed] [Google Scholar]
- 31.Henry T.D., Annex B.H., McKendall G.R., et al. The VIVA trial: vascular endothelial growth factor in ischemia for vascular angiogenesis. Circulation. 2003;107(10):1359–1365. doi: 10.1161/01.cir.0000061911.47710.8a. [DOI] [PubMed] [Google Scholar]
- 32.Kim J.H., Kim M.H., Jo D.H., Yu Y.S., Lee T.G., Kim J.H. The inhibition of retinal neovascularization by gold nanoparticles via suppression of VEGFR-2 activation. Biomaterials. 2011;32(7):1865–1871. doi: 10.1016/j.biomaterials.2010.11.030. [DOI] [PubMed] [Google Scholar]
- 33.O'Dwyer J., Murphy R., Dolan E.B., et al. Development of a nanomedicine-loaded hydrogel for sustained delivery of an angiogenic growth factor to the ischaemic myocardium. Drug Delivery Transl Res. 2020;10(2):440–454. doi: 10.1007/s13346-019-00684-5. [DOI] [PubMed] [Google Scholar]
- 34.Li Z., Qu T., Ding C., et al. Injectable gelatin derivative hydrogels with sustained vascular endothelial growth factor release for induced angiogenesis. Acta Biomater. 2015;13:88–100. doi: 10.1016/j.actbio.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rufaihah A.J., Johari N.A., Vaibavi S.R., et al. Dual delivery of VEGF and ANG-1 in ischemic hearts using an injectable hydrogel. Acta Biomater. 2017;48:58–67. doi: 10.1016/j.actbio.2016.10.013. [DOI] [PubMed] [Google Scholar]
- 36.Alonso-Herranz L., Sahún-Español Á., Paredes A., et al. Macrophages promote endothelial-to-mesenchymal transition via MT1-MMP/TGFβ1 after myocardial infarction. Elife. 2020;9 doi: 10.7554/eLife.57920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fan C., Shi J., Zhuang Y., et al. Myocardial-infarction-responsive smart hydrogels targeting matrix metalloproteinase for on-demand growth factor delivery. Adv Mater. 2019;31(40) doi: 10.1002/adma.201902900. [DOI] [PubMed] [Google Scholar]
- 38.Fan Z., Xu Z., Niu H., et al. Spatiotemporal delivery of basic fibroblast growth factor to directly and simultaneously attenuate cardiac fibrosis and promote cardiac tissue vascularization following myocardial infarction. J Controlled Release. 2019;311-312:233–244. doi: 10.1016/j.jconrel.2019.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fang R., Qiao S., Liu Y., et al. Sustained co-delivery of BIO and IGF-1 by a novel hybrid hydrogel system to stimulate endogenous cardiac repair in myocardial infarcted rat hearts. Int J Nanomed. 2015;10:4691–4703. doi: 10.2147/IJN.S81451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yuan Z., Tsou Y.H., Zhang X.Q., et al. Injectable citrate-based hydrogel as an angiogenic biomaterial improves cardiac repair after myocardial infarction. ACS Appl Mater Interfaces. 2019;11(42):38429–38439. doi: 10.1021/acsami.9b12043. [DOI] [PubMed] [Google Scholar]
- 41.Mihalko E., Huang K., Sproul E., Cheng K., Brown AC. Targeted treatment of ischemic and fibrotic complications of myocardial infarction using a dual-delivery microgel therapeutic. ACS Nano. 2018;12(8):7826–7837. doi: 10.1021/acsnano.8b01977. [DOI] [PubMed] [Google Scholar]
- 42.Kotsuchibashi Y., Narain R. Dual-temperature and pH responsive (ethylene glycol)-based nanogels via structural design. Polym Chem. 2014;5(8):3061–3070. [Google Scholar]
- 43.Vaziri N.D., Rodríguez-Iturbe B. Mechanisms of disease: oxidative stress and inflammation in the pathogenesis of hypertension. Nat Clin Pract Nephrol. 2006;2(10):582–593. doi: 10.1038/ncpneph0283. [DOI] [PubMed] [Google Scholar]
- 44.Vong L.B., Bui T.Q., Tomita T., Sakamoto H., Hiramatsu Y., Nagasaki Y. Novel angiogenesis therapeutics by redox injectable hydrogel - Regulation of local nitric oxide generation for effective cardiovascular therapy. Biomaterials. 2018;167:143–152. doi: 10.1016/j.biomaterials.2018.03.023. [DOI] [PubMed] [Google Scholar]
- 45.Wang R.M., Christman K.L. Decellularized myocardial matrix hydrogels: In basic research and preclinical studies. Adv Drug Deliv Rev. 2016;96:77–82. doi: 10.1016/j.addr.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Efraim Y., Sarig H., Cohen Anavy N., et al. Biohybrid cardiac ECM-based hydrogels improve long term cardiac function post myocardial infarction. Acta Biomater. 2017;50:220–233. doi: 10.1016/j.actbio.2016.12.015. [DOI] [PubMed] [Google Scholar]
- 47.Singelyn J.M., DeQuach J.A., Seif-Naraghi S.B., Littlefield R.B., Schup-Magoffin P.J., Christman K.L. Naturally derived myocardial matrix as an injectable scaffold for cardiac tissue engineering. Biomaterials. 2009;30(29):5409–5416. doi: 10.1016/j.biomaterials.2009.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Singelyn J.M., Sundaramurthy P., Johnson T.D., et al. Catheter-deliverable hydrogel derived from decellularized ventricular extracellular matrix increases endogenous cardiomyocytes and preserves cardiac function post-myocardial infarction. J Am Coll Cardiol. 2012;59(8):751–763. doi: 10.1016/j.jacc.2011.10.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jackman C.P., Ganapathi A.M., Asfour H., et al. Engineered cardiac tissue patch maintains structural and electrical properties after epicardial implantation. Biomaterials. 2018;159:48–58. doi: 10.1016/j.biomaterials.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aida T., Meijer E.W., Stupp S.I. Functional supramolecular polymers. Science. 2012;335(6070):813–817. doi: 10.1126/science.1205962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chaudhuri P., Harfouche R., Soni S., Hentschel D.M., Sengupta S. Shape effect of carbon nanovectors on angiogenesis. ACS Nano. 2010;4(1):574–582. doi: 10.1021/nn901465h. [DOI] [PubMed] [Google Scholar]
- 52.Tashakori-Miyanroudi M., Rakhshan K., Ramez M., et al. Conductive carbon nanofibers incorporated into collagen bio-scaffold assists myocardial injury repair. Int J Biol Macromol. 2020;163:1136–1146. doi: 10.1016/j.ijbiomac.2020.06.259. [DOI] [PubMed] [Google Scholar]
- 53.Rufaihah A.J., Seliktar D. Hydrogels for therapeutic cardiovascular angiogenesis. Adv Drug Deliv Rev. 2016;96:31–39. doi: 10.1016/j.addr.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 54.Meng Z.X., Wang Y.S., Ma C., Zheng W., Li L., Zheng Y.F. Electrospinning of PLGA/gelatin randomly-oriented and aligned nanofibers as potential scaffold in tissue engineering. Mater Sci Eng C. 2010;30(8):1204–1210. [Google Scholar]
- 55.Mrówczyński W., Mugnai D., de Valence S., et al. Porcine carotid artery replacement with biodegradable electrospun poly-e-caprolactone vascular prosthesis. J Vasc Surg. 2014;59(1):210–219. doi: 10.1016/j.jvs.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 56.Kargozar S., Baino F., Hamzehlou S., Hamblin M.R., Mozafari M. Nanotechnology for angiogenesis: opportunities and challenges. Chem Soc Rev. 2020;49(14):5008–5057. doi: 10.1039/c8cs01021h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Urso E., Maffia M. Behind the link between copper and angiogenesis: established mechanisms and an overview on the role of vascular copper transport systems. J Vasc Res. 2015;52(3):172–196. doi: 10.1159/000438485. [DOI] [PubMed] [Google Scholar]
- 58.Feng W., Ye F., Xue W., Zhou Z., Kang Y.J. Copper regulation of hypoxia-inducible factor-1 activity. Mol Pharmacol. 2009;75(1):174–182. doi: 10.1124/mol.108.051516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sivaraja V., Kumar T.K.S., Rajalingam D., Graziani I., Prudovsky I., Yu C. Copper binding affinity of S100A13, a key component of the FGF-1 nonclassical copper-dependent release complex. Biophys J. 2006;91(5):1832–1843. doi: 10.1529/biophysj.105.079988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fukai T., Ushio-Fukai M., Kaplan J.H. Copper transporters and copper chaperones: roles in cardiovascular physiology and disease. Am J Physiol Cell Physiol. 2018;315(2):C186–C201. doi: 10.1152/ajpcell.00132.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen G.F., Sudhahar V., Youn S.W., et al. Copper transport protein antioxidant-1 promotes inflammatory neovascularization via chaperone and transcription factor function. Sci Rep. 2015;5:14780. doi: 10.1038/srep14780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Elrayah A., Zhi W., Feng S., Al-Ezzi S., Lei H., Weng J. Preparation of micro/nano-structure copper-substituted hydroxyapatite scaffolds with improved angiogenesis capacity for bone regeneration. Materials. 2018;11(9) doi: 10.3390/ma11091516. (Basel) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sharma A.K., Kumar A., Sahu M., Sharma G., Datusalia A.K., Rajput S.K. Exercise preconditioning and low dose copper nanoparticles exhibits cardioprotection through targeting GSK-3β phosphorylation in ischemia/reperfusion induced myocardial infarction. Microvasc Res. 2018;120:59–66. doi: 10.1016/j.mvr.2018.06.003. [DOI] [PubMed] [Google Scholar]
- 64.Flügel D., Görlach A., Kietzmann T. GSK-3β regulates cell growth, migration, and angiogenesis via Fbw7 and USP28-dependent degradation of HIF-1α. Blood. 2012;119(5):1292–1301. doi: 10.1182/blood-2011-08-375014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lal H., Ahmad F., Woodgett J., Force T. The GSK-3 family as therapeutic target for myocardial diseases. Circ Res. 2015;116(1):138–149. doi: 10.1161/CIRCRESAHA.116.303613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nelson B.C., Johnson M.E., Walker M.L., Riley K.R., Sims C.M. Antioxidant cerium oxide nanoparticles in biology and medicine. Antioxidants. 2016;5(2) doi: 10.3390/antiox5020015. (Basel) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhao T., Wu W., Sui L., et al. Reactive oxygen species-based nanomaterials for the treatment of myocardial ischemia reperfusion injuries. Bioact Mater. 2021;7:47–72. doi: 10.1016/j.bioactmat.2021.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen S., Hou Y., Cheng G., Zhang C., Wang S., Zhang J. Cerium oxide nanoparticles protect endothelial cells from apoptosis induced by oxidative stress. Biol Trace Elem Res. 2013;154(1):156–166. doi: 10.1007/s12011-013-9678-8. [DOI] [PubMed] [Google Scholar]
- 69.Das S., Singh S., Dowding J.M., et al. The induction of angiogenesis by cerium oxide nanoparticles through the modulation of oxygen in intracellular environments. Biomaterials. 2012;33(31):7746–7755. doi: 10.1016/j.biomaterials.2012.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Heisel C., Yousif J., Mijiti M., et al. APE1/Ref-1 as a novel target for retinal diseases. J Cell Signal. 2021;2(2):133–138. doi: 10.33696/Signaling.2.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Saghiri M.A., Orangi J., Asatourian A., Sorenson C.M., Sheibani N. Functional role of inorganic trace elements in angiogenesis part III: (Ti, Li, Ce, As, Hg, Va, Nb and Pb) Crit Rev Oncol Hematol. 2016;98:290–301. doi: 10.1016/j.critrevonc.2015.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wingard C.J., Walters D.M., Cathey B.L., et al. Mast cells contribute to altered vascular reactivity and ischemia-reperfusion injury following cerium oxide nanoparticle instillation. Nanotoxicology. 2011;5(4):531–545. doi: 10.3109/17435390.2010.530004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Patra C.R., Bhattacharya R., Patra S., et al. Pro-angiogenic properties of europium(III) hydroxide nanorods. Adv Mater. 2008;20(4):753–756. [Google Scholar]
- 74.Patra C.R., Kim J.H., Pramanik K., et al. Reactive oxygen species driven angiogenesis by inorganic nanorods. Nano Lett. 2011;11(11):4932–4938. doi: 10.1021/nl2028766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bollu V.S., Nethi S.K., Dasari R.K., Rao S.S.N., Misra S., Patra C.R. Evaluation of in vivo cytogenetic toxicity of europium hydroxide nanorods (EHNs) in male and female Swiss albino mice. Nanotoxicology. 2016;10(4):413–425. doi: 10.3109/17435390.2015.1073398. [DOI] [PubMed] [Google Scholar]
- 76.Roy A., Nethi S.K., Suganya N., Raval M., Chatterjee S., Patra C.R. Attenuation of cadmium-induced vascular toxicity by pro-angiogenic nanorods. Mater Sci Eng C. 2020;115 doi: 10.1016/j.msec.2020.111108. [DOI] [PubMed] [Google Scholar]
- 77.Swirski F.K., Nahrendorf M. Cardioimmunology: the immune system in cardiac homeostasis and disease. Nat Rev Immunol. 2018;18(12):733–744. doi: 10.1038/s41577-018-0065-8. [DOI] [PubMed] [Google Scholar]
- 78.Scialo F., Fernandez-Ayala D.J., Sanz A. Role of mitochondrial reverse electron transport in ROS signaling: potential roles in health and disease. Front Physiol. 2017;8:428. doi: 10.3389/fphys.2017.00428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bajpai G., Schneider C., Wong N., et al. The human heart contains distinct macrophage subsets with divergent origins and functions. Nat Med. 2018;24(8):1234–1245. doi: 10.1038/s41591-018-0059-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Epelman S., Lavine K.J., Beaudin A.E., et al. Embryonic and adult-derived resident cardiac macrophages are maintained through distinct mechanisms at steady state and during inflammation. Immunity. 2014;40(1):91–104. doi: 10.1016/j.immuni.2013.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shi C., Jia T., Mendez-Ferrer S., et al. Bone marrow mesenchymal stem and progenitor cells induce monocyte emigration in response to circulating toll-like receptor ligands. Immunity. 2011;34(4):590–601. doi: 10.1016/j.immuni.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dewald O., Zymek P., Winkelmann K., et al. CCL2/monocyte chemoattractant protein-1 regulates inflammatory responses critical to healing myocardial infarcts. Circ Res. 2005;96(8):881–889. doi: 10.1161/01.RES.0000163017.13772.3a. [DOI] [PubMed] [Google Scholar]
- 83.Swirski F.K., Nahrendorf M., Etzrodt M., et al. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science. 2009;325(5940):612–616. doi: 10.1126/science.1175202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wan E., Yeap X.Y., Dehn S., et al. Enhanced efferocytosis of apoptotic cardiomyocytes through myeloid-epithelial-reproductive tyrosine kinase links acute inflammation resolution to cardiac repair after infarction. Circ Res. 2013;113(8):1004–1012. doi: 10.1161/CIRCRESAHA.113.301198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hilgendorf I., Gerhardt L.M.S., Tan T.C., et al. Ly-6Chigh monocytes depend on Nr4a1 to balance both inflammatory and reparative phases in the infarcted myocardium. Circ Res. 2014;114(10):1611–1622. doi: 10.1161/CIRCRESAHA.114.303204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liao X., Chang E., et al. Cardiac macrophages regulate isoproterenol-induced Takotsubo-like cardiomyopathy. JCI Insight. 2022;7(3) doi: 10.1172/jci.insight.156236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sager H.B., Hulsmans M., Lavine K.J., et al. Proliferation and recruitment contribute to myocardial macrophage expansion in chronic heart failure. Circ Res. 2016;119(7):853–864. doi: 10.1161/CIRCRESAHA.116.309001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ismahil M.A., Hamid T., Bansal S.S., Patel B., Kingery J.R., Prabhu S.D. Remodeling of the mononuclear phagocyte network underlies chronic inflammation and disease progression in heart failure: critical importance of the cardiosplenic axis. Circ Res. 2014;114(2):266–282. doi: 10.1161/CIRCRESAHA.113.301720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Karuppagounder V., Giridharan V.V., Arumugam S., et al. Modulation of macrophage polarization and HMGB1-TLR2/TLR4 cascade plays a crucial role for cardiac remodeling in senescence-accelerated prone mice. PLoS One. 2016;11(4) doi: 10.1371/journal.pone.0152922. e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rodríguez-Nuevo A., Díaz-Ramos A., Noguera E., et al. Mitochondrial DNA and TLR9 drive muscle inflammation upon Opa1 deficiency. EMBO J. 2018;37(10) doi: 10.15252/embj.201796553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Oyama J., Blais C., Liu X., et al. Reduced myocardial ischemia-reperfusion injury in toll-like receptor 4-deficient mice. Circulation. 2004;109(6):784–789. doi: 10.1161/01.CIR.0000112575.66565.84. [DOI] [PubMed] [Google Scholar]
- 92.Shimamoto A., Chong A.J., Yada M., et al. Inhibition of Toll-like receptor 4 with eritoran attenuates myocardial ischemia-reperfusion injury. Circulation. 2006;114:I270–I274. doi: 10.1161/CIRCULATIONAHA.105.000901. 1 Suppl. [DOI] [PubMed] [Google Scholar]
- 93.Fujiwara M., Matoba T., Koga J.I., et al. Nanoparticle incorporating toll-like receptor 4 inhibitor attenuates myocardial ischaemia-reperfusion injury by inhibiting monocyte-mediated inflammation in mice. Cardiovasc Res. 2019;115(7):1244–1255. doi: 10.1093/cvr/cvz066. [DOI] [PubMed] [Google Scholar]
- 94.Danhier F., Ansorena E., Silva J.M., Coco R., Le Breton A., Préat V. PLGA-based nanoparticles: an overview of biomedical applications. J Controlled Release. 2012;161(2):505–522. doi: 10.1016/j.jconrel.2012.01.043. [DOI] [PubMed] [Google Scholar]
- 95.Ikeda G., Matoba T., Ishikita A., et al. Nanoparticle-mediated simultaneous targeting of mitochondrial injury and inflammation attenuates myocardial ischemia-reperfusion injury. J Am Heart Assoc. 2021;10(12) doi: 10.1161/JAHA.120.019521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tokutome M., Matoba T., Nakano Y., et al. Peroxisome proliferator-activated receptor-gamma targeting nanomedicine promotes cardiac healing after acute myocardial infarction by skewing monocyte/macrophage polarization in preclinical animal models. Cardiovasc Res. 2019;115(2):419–431. doi: 10.1093/cvr/cvy200. [DOI] [PubMed] [Google Scholar]
- 97.Ye Y., Lin Y., Manickavasagam S., Perez-Polo J.R., Tieu B.C., Birnbaum Y. Pioglitazone protects the myocardium against ischemia-reperfusion injury in eNOS and iNOS knockout mice. Am J Physiol Heart Circ Physiol. 2008;295(6):H2436–H2446. doi: 10.1152/ajpheart.00690.2008. [DOI] [PubMed] [Google Scholar]
- 98.Cao D.J. Macrophages in cardiovascular homeostasis and disease. Circulation. 2018;138(22):2452–2455. doi: 10.1161/CIRCULATIONAHA.118.035736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cao D.J., Schiattarella G.G., Villalobos E., et al. Cytosolic DNA sensing promotes macrophage transformation and governs myocardial ischemic injury. Circulation. 2018;137(24):2613–2634. doi: 10.1161/CIRCULATIONAHA.117.031046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Arslan F., Smeets M.B., O'Neill L.A.J., et al. Myocardial ischemia/reperfusion injury is mediated by leukocytic Toll-Like receptor-2 and reduced by systemic administration of a novel anti-toll-like receptor-2 antibody. Circulation. 2010;121(1):80–90. doi: 10.1161/CIRCULATIONAHA.109.880187. [DOI] [PubMed] [Google Scholar]
- 101.McCartney S.A., Vermi W., Lonardi S., et al. RNA sensor-induced type I IFN prevents diabetes caused by a beta cell-tropic virus in mice. J Clin Invest. 2011;121(4):1497–1507. doi: 10.1172/JCI44005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Murray P.J., Wynn T.A. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 2011;11(11):723–737. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Natoli G., Monticelli S. Macrophage activation: glancing into diversity. Immunity. 2014;40(2):175–177. doi: 10.1016/j.immuni.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 104.Zhao T., Wu W., Sui L., et al. Reactive oxygen species-based nanomaterials for the treatment of myocardial ischemia reperfusion injuries. Bioact Mater. 2022;7:47–72. doi: 10.1016/j.bioactmat.2021.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lugrin J., Rosenblatt-Velin N., Parapanov R., Liaudet L. The role of oxidative stress during inflammatory processes. Biol Chem. 2014;395(2):203–230. doi: 10.1515/hsz-2013-0241. [DOI] [PubMed] [Google Scholar]
- 106.Hafstad A.D., Nabeebaccus A.A., Shah A.M. Novel aspects of ROS signalling in heart failure. Basic Res Cardiol. 2013;108(4):359. doi: 10.1007/s00395-013-0359-8. [DOI] [PubMed] [Google Scholar]
- 107.Murphy M.P. How mitochondria produce reactive oxygen species. Biochem J. 2009;417(1):1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhang D., Tang Z., Huang H., et al. Metabolic regulation of gene expression by histone lactylation. Nature. 2019;574(7779):575–580. doi: 10.1038/s41586-019-1678-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tretter L., Patocs A., Chinopoulos C. Succinate, an intermediate in metabolism, signal transduction, ROS, hypoxia, and tumorigenesis. Biochim. Biophys. Acta (BBA) Bioenerg. 2016;1857(8):1086–1101. doi: 10.1016/j.bbabio.2016.03.012. [DOI] [PubMed] [Google Scholar]
- 110.Chouchani E.T., Pell V.R., Gaude E., et al. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature. 2014;515(7527):431–435. doi: 10.1038/nature13909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lampropoulou V., Sergushichev A., Bambouskova M., et al. Itaconate links inhibition of succinate dehydrogenase with macrophage metabolic remodeling and regulation of inflammation. Cell Metab. 2016;24(1):158–166. doi: 10.1016/j.cmet.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nakkala J.R., Yao Y., Zhai Z., et al. Dimethyl itaconate-loaded nanofibers rewrite macrophage polarization, reduce inflammation, and enhance repair of myocardic infarction. Small. 2021;17(17) doi: 10.1002/smll.202006992. [DOI] [PubMed] [Google Scholar]
- 113.Vergadi E., Ieronymaki E., Lyroni K., Vaporidi K., Tsatsanis C. Akt signaling pathway in macrophage activation and M1/M2 polarization. J Immunol. 2017;198(3):1006–1014. doi: 10.4049/jimmunol.1601515. (Baltimore, Md : 1950) [DOI] [PubMed] [Google Scholar]
- 114.Greenlee-Wacker M.C. Clearance of apoptotic neutrophils and resolution of inflammation. Immunol Rev. 2016;273(1):357–370. doi: 10.1111/imr.12453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bao L., Dou G., Tian R., et al. Engineered neutrophil apoptotic bodies ameliorate myocardial infarction by promoting macrophage efferocytosis and inflammation resolution. Bioact Mater. 2022;9:183–197. doi: 10.1016/j.bioactmat.2021.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Galili U., Zhu Z., Chen J., Goldufsky J.W., Schaer GL. Near complete repair after myocardial infarction in adult mice by altering the inflammatory response with intramyocardial injection of alpha-gal nanoparticles. Front Cardiovasc Med. 2021;8 doi: 10.3389/fcvm.2021.719160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Medrano-Bosch M., Moreno-Lanceta A., Melgar-Lesmes P. Nanoparticles to target and treat macrophages: the Ockham's concept? Pharmaceutics. 2021;13(9):1340. doi: 10.3390/pharmaceutics13091340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sullivan H.L., Gianneschi N.C., Christman K.L. Targeted nanoscale therapeutics for myocardial infarction. Biomater Sci. 2021;9(4):1204–1216. doi: 10.1039/d0bm01677b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rurik J.G., Tombácz I., Yadegari A., et al. CAR T cells produced in vivo to treat cardiac injury. Science. 2022;375(6576):91–96. doi: 10.1126/science.abm0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Choo E.H., Lee J.H., Park E.H., et al. Infarcted myocardium-primed dendritic cells improve remodeling and cardiac function after myocardial infarction by modulating the regulatory T cell and macrophage polarization. Circulation. 2017;135(15):1444–1457. doi: 10.1161/CIRCULATIONAHA.116.023106. [DOI] [PubMed] [Google Scholar]
- 121.Kwon S.P., Hwang B.H., Park E.H., et al. Nanoparticle-mediated blocking of excessive inflammation for prevention of heart failure following myocardial infarction. Small. 2021;17(32) doi: 10.1002/smll.202101207. [DOI] [PubMed] [Google Scholar]
- 122.Zhang Y.M., Cai Z.C., Shen Y.L., et al. Hydrogel-load exosomes derived from dendritic cells improve cardiac function via Treg cells and the polarization of macrophages following myocardial infarction. J Nanobiotechnol. 2021;19(1) doi: 10.1186/s12951-021-01016-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Pu X., Zhao L., Li J., et al. A polymeric micelle with an endosomal pH-sensitivity for intracellular delivery and enhanced antitumor efficacy of hydroxycamptothecin. Acta Biomater. 2019;88:357–369. doi: 10.1016/j.actbio.2019.02.039. [DOI] [PubMed] [Google Scholar]
- 124.Wang Z., Zhao Y., Jiang Y., et al. Enhanced anti-ischemic stroke of ZL006 by T7-conjugated PEGylated liposomes drug delivery system. Sci Rep. 2015;5:12651. doi: 10.1038/srep12651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.de Oliveira E.R., Santos L.C.R., Salomao M.A., et al. Nose-to-brain drug delivery mediated by polymeric nanoparticles: influence of PEG surface coating. Drug Deliv Transl Res. 2020;10(6):1688–1699. doi: 10.1007/s13346-020-00816-2. [DOI] [PubMed] [Google Scholar]
- 126.Miao Y., Zhao X., Qiu Y., Liu Z., Yang W., Jia X. Metal-organic framework-assisted nanoplatform with hydrogen peroxide/glutathione dual-sensitive on-demand drug release for targeting tumors and their microenvironment. ACS Appl Bio Mater. 2019;2(2):895–905. doi: 10.1021/acsabm.8b00741. [DOI] [PubMed] [Google Scholar]
- 127.Sheng Q., Qiao X., Zhou M., Zheng J. Recent progress in electrochemical sensing of cardiac troponin by using nanomaterial-induced signal amplification. Microchim Acta. 2017;184(6):1573–1585. [Google Scholar]
- 128.van Rooij E., Olson E.N. MicroRNA therapeutics for cardiovascular disease: opportunities and obstacles. Nat Rev Drug Discov. 2012;11(11):860–872. doi: 10.1038/nrd3864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wang W., Zheng H. Myocardial infarction: the protective role of miRNAs in myocardium pathology. Front Cardiovasc Med. 2021;8 doi: 10.3389/fcvm.2021.631817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Morelli V.M., Brækkan S.K., Hansen J.B. Role of microRNAs in venous thromboembolism. Int J Mol Sci. 2020;21(7) doi: 10.3390/ijms21072602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Winbanks C.E., Murphy K.T., Bernardo B.C., et al. Smad7 gene delivery prevents muscle wasting associated with cancer cachexia in mice. Sci Transl Med. 2016;8(348) doi: 10.1126/scitranslmed.aac4976. [DOI] [PubMed] [Google Scholar]
- 132.Kataoka K. Gene delivery systems: viral vs. non-viral vectors. Adv Drug Deliv Rev. 2001;52:151. [Google Scholar]
- 133.Mintzer M.A., Simanek E.E. Non-viral vectors for gene delivery. Chem Rev. 2009;109:259–320. doi: 10.1021/cr800409e. [DOI] [PubMed] [Google Scholar]
- 134.Gigante A., Li M., Junghanel S., Hirschhauser C., Knauer S., Schmuck C. Non-viral transfection vectors: are hybrid materials the way forward? MedChemComm. 2019;10(10):1692–1718. doi: 10.1039/c9md00275h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Nie J.J., Qiao B., Duan S., et al. Unlockable nanocomplexes with self-accelerating nucleic acid release for effective staged gene therapy of cardiovascular diseases. Adv Mater. 2018;30(31) doi: 10.1002/adma.201801570. [DOI] [PubMed] [Google Scholar]
- 136.Hong T., Wei Y., Xue X., et al. A novel anti-coagulative nanocomplex in delivering miRNA-1 inhibitor against microvascular obstruction of myocardial infarction. Adv Healthc Mater. 2020;9(11) doi: 10.1002/adhm.201901783. [DOI] [PubMed] [Google Scholar]
- 137.Li Y., Yu H., Zhao L., et al. Effects of carbon nanotube-mediated caspase3 gene silencing on cardiomyocyte apoptosis and cardiac function during early acute myocardial infarction. Nanoscale. 2020;12(42):21599–21604. doi: 10.1039/d0nr05032f. [DOI] [PubMed] [Google Scholar]
- 138.López-Muneta L., Miranda-Arrubla J., Carvajal-Vergara X. The future of direct cardiac reprogramming: any GMT cocktail variety? Int J Mol Sci. 2020;21(21):7950. doi: 10.3390/ijms21217950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Muniyandi P., Palaninathan V., Mizuki T., Maekawa T., Hanajiri T., Mohamed M.S. Poly(lactic-co-glycolic acid)/polyethylenimine nanocarriers for direct genetic reprogramming of microRNA targeting cardiac fibroblasts. ACS Appl Nano Mater. 2020;3(3):2491–2505. [Google Scholar]
- 140.Yang L., Xue S., Du M., Lian F. Highly efficient microRNA delivery using functionalized carbon dots for enhanced conversion of fibroblasts to cardiomyocytes. Int J Nanomed. 2021;16:3741–3754. doi: 10.2147/IJN.S304873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Yang H., Qin X., Wang H., et al. An in vivo miRNA delivery system for restoring infarcted myocardium. ACS Nano. 2019;13(9):9880–9894. doi: 10.1021/acsnano.9b03343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Machtakova M., Thérien-Aubin H., Landfester K. Polymer Nano-systems for the encapsulation and delivery of active biomacromolecular therapeutic agents. Chem Soc Rev. 2022;51(1):128–152. doi: 10.1039/d1cs00686j. [DOI] [PubMed] [Google Scholar]
- 143.Lai E., van Zanten J.H. Monitoring DNA/poly-L-lysine polyplex formation with time-resolved multiangle laser light scattering. Biophys J. 2001;80(2):864–873. doi: 10.1016/S0006-3495(01)76065-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lin H., Sohn J., Shen H., Langhans M.T., Tuan RS. Bone marrow mesenchymal stem cells: aging and tissue engineering applications to enhance bone healing. Biomaterials. 2019;203:96–110. doi: 10.1016/j.biomaterials.2018.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sadhukha T., O'Brien T.D., Prabha S. Nano-engineered mesenchymal stem cells as targeted therapeutic carriers. J Control Release. 2014;196:243–251. doi: 10.1016/j.jconrel.2014.10.015. [DOI] [PubMed] [Google Scholar]
- 146.Tang J., Shen D., Caranasos T.G., et al. Therapeutic microparticles functionalized with biomimetic cardiac stem cell membranes and secretome. Nat Commun. 2017;8:13724. doi: 10.1038/ncomms13724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Qi S., Zhang P., Ma M., et al. Cellular internalization-induced aggregation of porous silicon nanoparticles for ultrasound imaging and protein-mediated protection of stem cells. Small. 2019;15(1) doi: 10.1002/smll.201804332. [DOI] [PubMed] [Google Scholar]
- 148.Teo J.Y., Seo Y., Ko E., et al. Surface tethering of stem cells with H2O2-responsive anti-oxidizing colloidal particles for protection against oxidation-induced death. Biomaterials. 2019;201:1–15. doi: 10.1016/j.biomaterials.2019.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Cui X., Tang J., Hartanto Y., et al. NIPAM-based microgel microenvironment regulates the therapeutic function of cardiac stromal cells. ACS Appl Mater Interfaces. 2018;10(44):37783–37796. doi: 10.1021/acsami.8b09757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Fathil M.F.M., Md Arshad M.K., Ruslinda A.R., et al. Progression in sensing cardiac troponin biomarker charge transductions on semiconducting nanomaterials. Anal Chim Acta. 2016;935:30–43. doi: 10.1016/j.aca.2016.06.012. [DOI] [PubMed] [Google Scholar]
- 151.Allen C. The question of toxicity of nanomaterials and nanoparticles. J Control Release. 2019;304:288. doi: 10.1016/j.jconrel.2019.06.008. [DOI] [PubMed] [Google Scholar]