Abstract
Background and Aim:
The activity of “gaming” has increased greatly in popularity in recent years, with many gamers using nutritional supplements to aid mood and gaming performance. We evaluated the impact of AmaTea® Max (referred to as AmaTea® throughout; a patented dietary supplement consisting of a blend of caffeine and polyphenol antioxidants), compared to both caffeine and a placebo, on gaming and cognitive performance in active gamers.
Methods:
Subjects reported to the lab on three occasions, separated by approximately 1 week. On each day, they had baseline measurements taken and then played the game Fortnite for four 1-h periods. Measures of cognitive performance, gaming performance, heart rate and blood pressure (BP), and blood cortisol were measured before and at selected times following gameplay.
Results:
Neither caffeine nor AmaTea® impacted gaming or cognitive performance in a statistically significant manner. However, a trend (P=0.075) was noted for the condition effect for kills/match, with values 21% higher for AmaTea® (1.84) compared to placebo (1.51), and 12% higher for AmaTea® compared to caffeine (1.63). Subjective mood was relatively unaffected, although a condition effect was noted for jittery (P=0.05), with values lower for placebo than for caffeine (P=0.02). BP was minimally elevated with both AmaTea® and caffeine, while cortisol followed the normal diurnal variation and was lower for placebo than AmaTea® and caffeine.
Conclusion:
AmaTea® modestly increased kills/match during gameplay. It is possible that a different gaming stimulus, varied time of gameplay, or different dosage of the supplement may have yielded different results.
Relevance for Subjects:
Active gamers who seek to use a dietary supplement for purposes of gaming performance may benefit slightly from ingestion of AmaTea® before gameplay while experiencing greater vigor and lower fatigue as compared to placebo.
Keywords: gaming, caffeine, stimulants, AmaTea®
1. Introduction
Electronic sports (esports) have become increasingly popular globally and, as a result, methods to improve performance are being investigated, since the financial motivation for improving performance is high. In fact, the video gaming industry was estimated to be worth well over 100 billion dollars in 2021, with an audience of nearly 400 million and popularity that parallels or exceeds that of some in-person sporting events [1]. Moreover, many gamers are monetizing their playing time, with elite gamers often earning a full-time salary with this activity alone.
In the USA, esports players have been considered professional athletes for visa purposes since 2013, similar to traditional sports such as soccer, ice hockey, or basketball [2,3]. Given the immense popularity and potential financial gain [4], there has been effort by the beverage and dietary supplement industry to develop products that may improve gaming performance and mood, while not leading to adverse effects which are common with “energy” products (e.g., caffeine-induced irritability) [5].
Gaming success is largely determined by mental functioning and the ability of the individual to think and reason abstractly and solve problems either alone or as a group member (i.e., squad). In the problem-solving mind, inductive skills, spatial awareness, eye-hand coordination, and social competencies are the central competencies of digital games [6]. The competence model has six dimensions including sensorimotor control, cognition, personal competencies, emotion and volition, social competencies, and media literacy [3]. Sensorimotor, cognitive, emotional/volitional, and personal competencies mostly describe physical and psychological abilities that are required to play video games [7].
The video game “Fortnite” has been an overwhelmingly successful and popular online game developed in 2017 by Epic Games. In the debut year, it was claimed to attract more than 125 million players, earning hundreds of millions of dollars in revenue in its first 2 years [8]. In particular, Fortnite Battle Royale is well-known, recognizable, and free to the public, where up to 100 players compete (i.e., fight) to be the last remaining person or team using the aforementioned mental skills and cognitive functioning, coupled with mood and group functioning. However, mental fatigue may impede performance and mood, altering a player’s psychophysiological state during or following sustained periods of cognitive activity. This may result in considerably decreased ability to focus one’s attention and subsequent compromised performance in a variety of cognitive tasks. As a result, it is imperative that players maintain sustained peak cognitive performance, optimal motor behavioral skills, and a natural euphoria for several hours of competition. To that end, many video gamers rely on nootropics, dietary supplements, or chemicals such as caffeine or caffeinated beverages to improve cognition, particularly executive function, memory, creativity, and motivation [9].
Energy drink consumption is pervasive among gamers and such products often contain caffeine, with assertions that mental fatigue will be attenuated and performance improved via increased alertness and wakefulness [10,11]. There is considerable documentation of the nootropic effects of low-dose caffeine [11,12], with over 85% of the US population consuming caffeine daily. Caffeine is a naturally occurring dietary ingredient and is found in coffee beans, cacao beans, kola nuts, guarana berries, and tea leaves including yerba mate. On ingestion, caffeine modulates neurotransmitters in the brain causing both therapeutic psychoactive effects and improvement in mood and overall cognition, but also with potential negative side effects. Reported benefits of caffeine consumption for gamers include reaction time improvement, improved vigilance, greater attention and alertness, more accurate judgment, and reduced perception of effort [11]. This is achieved by obstructing central and peripheral adenosine receptors which initiate and promote sleep, and by triggering the release of dopamine and adrenaline, which both contribute to the ensuing euphoria post-consumption [13]. Consuming traditional forms of caffeine will provoke an adrenergic response with increases in hormonal epinephrine, which controls visceral functions and the fight-or-flight response, but with potential side effects such as migraine headaches, nervousness, irritability, or restlessness particularly in caffeine-naive individuals [13]. As a result, these effects would circumvent the primary reason for consumption.
Caffeine can originate from both synthetic and plant-based sources [14,15]. Interestingly, it appears that not all caffeine sources exert the same effects on physiology and/or mood, even when consumed in similar amounts. AmaTea® is a caffeinated dietary supplement produced from guayusa, a species of naturally caffeinated holly tree native to the Amazon rainforest [16,17]. Guayusa differs from other teas in that it lacks bitter, astringent tannins (high molecular weight polyphenols) but contains a different polyphenolic antioxidant profile with chlorogenic acids (similar to coffee plants) and rutin (flavonoid polyphenol in fruits and vegetables), as well as a distinctive sweet tea-like flavor [17]. Moreover, anecdotally, guayusa has been used for its purported ability to heighten awareness and provide energy without the accompanying restlessness [18]. In a 2016 study, Krieger and coworkers showed that guayusa regulated, in part, epinephrine levels upon caffeine ingestion [19]. In a double-blind, crossover clinical trial with 200 mg of caffeine provided from either a green coffee extract, a guayusa leaf extract, or a synthetic control, it was shown that the guayusa leaf extracts significantly blunted the robust increases in epinephrine observed with the control and green coffee extract.
Preliminary studies with the AmaTea® in rodent models also show that administration of AmaTea®, but not placebo, induced significant elevations of several neurotransmitters, which modulate executive function tasks such as learning, categorization, inhibitory control, cognitive flexibility, working memory, and behavioral flexibility [18]. For example, extracellular concentrations of norepinephrine, 5-hydroxytryptamine, histamine, acetylcholine, and dopamine were significantly increased, but not excessively, compared to the placebo. This may be due to modulation of neurotransmitters in humans, although further research is needed [16]. Collectively, however, data suggest the elaboration of the desirable nootropic effects by AmaTea® for enhanced performance and mood in video gaming, without the negative adrenergic effects often noted with synthetic caffeine consumption.
In the present study, we examined markers of cognitive function and mental and physiological fatigue, as well as gaming performance and blood cortisol concentrations, in active gamers using Fortnite Battle Royale as a representative game. The gamers ingested either caffeine anhydrous (equivalent to 2 strong cups of coffee [270 mg]), placebo, or AmaTea®. We hypothesized that both AmaTea® and caffeine would improve outcome measures more than placebo, with greater improvement noted for the AmaTea® condition due to the addition of the chlorogenic acid and use of guayusa. Active video gamers were recruited to play Fortnite Battle Royale for 4 h-long blocks, with hourly testing of cognition, mood, and gaming performance.
2. Materials and Methods
A total of 49 subjects completed all visits for the 3 test conditions. Of the 49 subjects, 47 were male and two were female. An additional ten subjects started the trial but only completed 1 or 2 of the sessions; hence, data for these subjects are not included here. Another 30 subjects completed all screening procedures and provided informed consent but failed to follow-up for actual testing. Therefore, a total of 89 subjects were recruited and screened into the study. Since the study was completed primarily during the COVID-19 pandemic, we believe that many subjects did not feel comfortable being contained within a lab for approximately 6 h on 3 separate occasions.
All procedures were approved by the University of Memphis Institutional Review Board for Human Subjects Research (protocol FY2020-65) and the study was registered through clinicaltrials.gov (NCT04234529). Subjects were required to be between 18 and 40-years-old, non-tobacco users, not morbidly obese (body mass index under 40 kg/m2), not diabetic and without a history of cardiovascular or neurological disease, and willing to refrain from alcohol and caffeine within 48 h of each test day. It was not a requirement that subjects be a regular consumer of caffeine, with the range of daily intake between approximately 0 and 400 mg. Female subjects were not pregnant. All subjects were active gamers, playing at least 4 days/week, for at least 4 h/day, for the past 12 months. They were also required to have experience playing the game Fortnite, as this was the game played during each trial. Subjects were compensated $300 for their full participation. Subject descriptive characteristics are presented in Table 1.
Table 1. Characteristics of 49 subjects.
| Variable | Value |
|---|---|
| Age (yr) | 22±3 |
| Height (cm) | 178±8 |
| Weight (kg) | 82±19 |
| BMI (kg/m2) | 26±5 |
| Waist Circumference (cm) | 86±12 |
| Hip Circumference (cm) | 105±12 |
| Waist: Hip | 0.82±0.05 |
| Resting HR (bpm) | 76±12 |
| Resting SBP (mm Hg) | 125±10 |
| Resting DBP (mm Hg) | 75±11 |
| Daily Caffeine (mg) | 128±124 |
Values are Mean±SD. HR: Heart rate, SBP: Systolic blood pressure, DBP: Diastolic blood pressure
2.1. Screening visit
During the initial visit to the laboratory, subjects completed the informed consent form, health history, medication, and dietary supplement usage, and physical activity questionnaires. Subjects’ heart rate (HR), blood pressure (BP), height, weight, waist circumference, and hip circumference were measured. To confirm non-pregnancy, females were provided with a urine pregnancy test kit (Clinical Guard®, Atlanta, Georgia, USA), escorted to a private restroom (within the lab), and asked to perform the test, which was then confidentially confirmed by the investigators. Eligible subjects were scheduled for weekly testing visits after screening was completed.
2.2. Independent variable
Three conditions were used in this study. The dosage for all conditions (AmaTea®, caffeine, placebo) was three capsules. The AmaTea® used in this study is a patented dietary supplement consisting of a unique blend of caffeine and polyphenol antioxidants; standardized at 20% caffeine and 30% chlorogenic acids, referred to as AmaTea® Max. The product is also Generally Recognized as Safe (GRAS) and USDA organic. Three capsules of AmaTea® contained a total of 270 mg of caffeine. Caffeine anhydrous was the other condition, and 3 capsules contained a matched total of 270 mg of caffeine. A placebo (cellulose) was also provided in capsule form. The three conditions were provided in random order using a double-blind, cross-over design. A contract manufacturer produced capsules in accordance with Good Manufacturing Practices. Capsules were of similar appearance and provided in blinded packets labeled A, B, or C. Subjects ingested the capsules in the presence of an investigator on each test visit day before beginning gameplay.
2.3. Test visit procedures
Subjects reported to the laboratory a total of 3 times. For each test visit, subjects were instructed to arrive in a 3-h fasted state. Testing took place approximately between 4:00 pm and 10:00 pm, and attempts were made to group the same subjects together for each visit, ideally in teams of 4.
BP, HR, Digit Symbol Substitution Test, Brunel Mood Scale, Subjective Feelings, Reaction Time (Go/No-Go), and a 30-min mental performance test (AX-Continuous Performance Test [CPT]) were conducted and recorded at baseline. E-PRIME 3 software (Psychology Software Tools, Inc., Pittsburgh, PA) was used for Digit Symbol Substitution Test, Brunel Mood Scale, Reaction Time Test, and AX-CPT. A blood sample was also collected before the assigned condition was ingested. Details are provided below.
For each visit, one of the three conditions was consumed 5 min before the initiation of gameplay. This time was chosen to coincide with peak caffeine concentration being approximately 1 h into the 4-h gaming session. The total testing session time was approximately 6 h in duration when considering the additional time needed for obtaining outcome measures. If having subjects wait longer than 5 min following the intake of the condition before commencing gameplay, we would have extended the total time of subject involvement and were concerned that fatigue would be increased and enthusiasm for participation would wane. Water was allowed ad libitum during the entire session. A Clif Builder bar (Clif Builder, Emeryville, CA) was provided after data collection, following 2 h of game play. No additional food or calorie-containing beverages were allowed throughout the study period. Caloric intake was limited to help ensure that absorption of the supplement was similar amongst subjects.
After 1, 2, 3, and 4 h of game play, BP and HR measurements, Digit Symbol Substitution Test, Brunel Mood Scale, Subjective Feelings, and Reaction Time Test were performed. Blood samples were also collected following 2 and 4 h of gameplay. AX-CPT was performed following the 4th h of gameplay. Subjects remained in the lab during the entire time and were supervised throughout, except when using the restroom as needed. Approximately 1 week separated each condition trial. A familiarization trial of the gaming session was provided before the first session. Subjects were encouraged to obtain at least 7 h of sleep the night before each test day.
2.4. Resting BP and HR
Resting BP and HR were obtained using an automated unit (OMRON HEM 907XL, OMRON Healthcare, Tokyo, Japan), following a 5-min seated rest period, with the average of duplicate measures recorded at each time.
2.5. Subjective feelings
Subjective feelings were obtained using a visual analog scale, with 0 representing the lowest rating (none at all; feeling at the absolute lowest value on this scale for the select variable) and 100 representing the highest rating (extreme; feeling at the absolute highest value on this scale for the select variable). Ratings were recorded for the following: attentive, energetic, motivated, irritable, focused, jittery, and moody.
2.6. Cognitive and mood tests
2.6.1. Brunel mood scale test
The Brunel mood scale test measures 6 identifiable affective states through a 24-item self-report inventory, with respondents rating a list of adjectives on a 5-point Likert scale ranging from 0 (not at all) to 4 (extremely), based on subjective feelings. The questions contain simple mood descriptors such as angry, nervous, unhappy, and energetic. The Brunel Mood Scale Test has six subscales, with each of the subscales containing 4 mood descriptors. The subscales are anger, confusion, depression, fatigue, tension, and vigor.
2.6.2 Go/No-Go test
In general, Go/No-Go testing refers to a pass/fail test (or check) principle using 2 boundaries. Go/No-Go tests are used to measure a participant’s capacity for sustained attention and response control. For example, a Go/No-Go test that requires a participant to perform an action given certain stimuli (e.g., press a button – Go) and inhibit that action under a different set of stimuli (e.g., not press that same button – No-Go). Go/No-Go had 100 trials that were random in order, with 80 Go and 20 No-Go trials.
2.6.3 Digit symbol substitution test
The digit symbol substitution test is an evaluation tool used to assess cognitive functioning. It involves a key consisting of the numbers 1-9, each paired with a unique symbol such as a “V,” “+” or “>.” Below the key is a series of the numbers 1-9 in random order, which are repeated several times. The subject was allowed 90 s to enter the corresponding symbol for each number requiring the subject to visually scan the response key (at the top of the test) with entry of the correct symbol to the corresponding number.
2.6.4. AX-CPT
An important concept in the study of cognitive control is the ability to manage cognitive processing, execute current task demands, strategically plan, retain short-term memory, and other similar tasks. The AX-CPT task is simple to perform and used frequently in clinical and developmental populations as a cognitive control task to examine context processing and goal maintenance. During the AX-CPT, the subject views a series of cues and probe sequences (one at a time) and presses different keys (z or / ) on a keyboard to indicate if the stimulus is the target or non-target. The typical 4 cue-probe combinations are AX, AY, BX, and BY, with AX being the only correct target response. We also included A# and B# combinations for which the correct response was pressing no key. There were 325 combinations per session broken down as follows: AX 130 tests, AY 32 tests, YY 97 tests, A# 17 tests, Y# 17 tests, and YX 32 tests in random order. The AX-CPT was determined before and after the conclusion of the 4-h gaming session.
2.7. Video gaming performance
For each hour of gameplay, subjects were asked to view gaming statistics briefly at the end of each match and inform investigators of the number of “kills” and overall placement (ranking) of the squad, which was then totaled for each hour. The number of matches per hour was also noted. Video gamers were observed continuously throughout the gaming and testing sessions to ensure compliance.
2.8. Blood cortisol
In addition to the above variables, blood samples were collected to measure cortisol. Specifically, single venipunctures were used to collect blood samples from subjects before condition ingestion and after 2 and 4 h of gameplay. Blood was collected and processed, and the serum was stored at −80° Celsius until analysis for cortisol using an ELISA kit, according to the instructions of the manufacturer (Calbiotech, El Cajon, CA: item #CO103S).
2.9. Gaming stimulus
Subjects played the game Fortnite in small group format (e.g., 3-4 players) using the team approach. Each “match” was completed in approximately 15-20 min in duration and included various aspects of cognitive demand, stress, anxiety, and competition. The gameplay allowed for the evaluation of cognitive, physiological, and subjective feeling measures between the 3 conditions. All gaming occurred in an isolated and controlled setting, with PlayStation 4 consoles and 27-inch flat-panel television screens/monitors. Subjects logged into Fortnite accounts created specifically for the study to play the matches. After 2, 1- h blocks of match play (roughly 8 matches), subsequent outcome measures were collected, and subjects were provided with a short break to use the restroom and eat a Clif Builder bar (Clif Builder, Emeryville, CA). They then resumed play for an additional 2 h of gaming. After each hour of play, outcome measures were collected.
2.10. Dietary intake and physical activity
Subjects followed their usual activity patterns over the course of the study period but refrained from strenuous activity for the 48 h preceding each lab test day. Dietary intake was to remain similar over the entire study period. Subjects were instructed to consume the same breakfast/lunch meal on each of the test days. Subjects recorded all food and drink consumed during the approximate 60-h period before each test day and were asked to mimic the intake before the first test day during the 2 days leading up to test days 2 and 3.
2.11. Data analysis
The data are presented as mean ± SD. Most data were analyzed using a 3 (condition) × 5 (time) analysis of variance (ANOVA), while the AX-CPT and cortisol were analyzed using a 3 (condition) × 2 or 3 (time) ANOVA. Tukey post hoc testing and contrast analysis was used as appropriate. Analyses were performed using JMP software (SAS, Cary, NC) and statistical significance was set at P≤0.05.
3. Results
3.1. Overview
A total of 49 subjects completed visits for all three conditions. Three minor adverse events were noted, including a complaint of pain following venipuncture that was believed by a medical provider to be due to inflammation. The pain subsided after approximately 1 week. Two reports of syncope were noted following blood sample collection, with both subjects regaining consciousness within a few seconds after each incident. All events were reported to the IRB and the study sponsor.
3.2. HR and BP
For HR, a time effect was noted (P<0.0001), with h 0 higher than h 1 and 2 (P<0.05) and h 3 and 4 higher than h 1 (P<0.05). No condition (P=0.58) or interaction (P=0.98) effects were noted.
For systolic BP (SBP), a condition effect was noted (P=0.002), with placebo lower than caffeine and AmaTea® (P<0.05). A time effect was also noted (P=0.0001), with h 0 lower than all other times (P<0.05). No interaction effect was noted (P=0.71).
For diastolic BP (DBP), a time effect was noted (P<0.0001), with h 0 lower than all other times (P<0.05) and h 1 lower than h 4 (P<0.05). No condition (P=0.14) or interaction (p=0.93) effects were noted.
For rate pressure product (RPP), a condition effect was noted (P=0.04), with placebo lower than caffeine (P<0.05). A time effect was also noted (P=0.005), with h 1 lower than h 3 and 4 (P<0.05). No interaction effect was noted (P=0.97). Data for HR, BP, and RPP are presented in Table 2.
Table 2. HR, BP, and RPP data of men and women before, during, and after 4 h of gaming.
| Variable | Caffeine | AmaTea® | Placebo |
|---|---|---|---|
| HR (bpm) | |||
| Hour 0* | 80±16 | 79±13 | 79±13 |
| Hour 1 | 73±15 | 69±11 | 72±12 |
| Hour 2 | 73±14 | 73±11 | 72±12 |
| Hour 3* | 77±14 | 77±12 | 76±13 |
| Hour 4* | 78±15 | 77±14 | 75±16 |
| SBP (mm Hg) | |||
| Hour 0* | 123±13 | 122±11 | 122±14* |
| Hour 1 | 129±11 | 128±13 | 125±13* |
| Hour 2 | 131±13 | 127±13 | 125±12* |
| Hour 3 | 131±13 | 131±13 | 125±12* |
| Hour 4 | 128±14 | 129±16 | 125±13* |
| DBP (mm Hg) | |||
| Hour 0* | 74±11 | 74±9 | 73±10 |
| Hour 1* | 78±9 | 79±10 | 76±10 |
| Hour 2 | 80±11 | 81±11 | 78±9 |
| Hour 3 | 81±11 | 80±8 | 78±11 |
| Hour 4 | 83±12 | 80±11 | 81±11 |
| RPP | |||
| Hour 0 | 9875±2419 | 9684±1943 | 9582±1907* |
| Hour 1* | 9407±2346 | 8874±1582 | 9053±1878* |
| Hour 2 | 9581±2340 | 9370±1852 | 9013±1747* |
| Hour 3 | 10095±2026 | 10052±1915 | 9513±1759* |
| Hour 4 | 9978±2221 | 9933±2395 | 9444±2318* |
Values are mean±SD. HR: Heart rate, BP: Blood pressure, SBP: Systolic blood pressure, DBP: Diastolic blood pressure, RPP: Rate pressure product. RPP = HR×SBP. HR:
Time effect (P<0.0001); h 0 higher than h 1 and 2 (P<0.05) and h 3 and 4 higher than h 1 (P<0.05). SBP: *Condition effect (P = 0.002); Placebo lower than Caffeine and AmaTea® (P<0.05), Time effect (P=0.0001); h 0 lower than all other times (P<0.05). DBP: *Time effect (P<0.0001); h 0 lower than all other times (P<0.05) and h 1 lower than h 4 (P<0.05). RPP: *Condition effect (P=0.04); Placebo lower than Caffeine (P<0.05), Time effect (P=0.005); h 1 lower than h 3 and 4 (P<0.05). No other differences of statistical significance were noted (P>0.05).
3.3. Subjective feelings
With regards to subjective feelings, a condition effect was noted for jittery (P=0.05), with values lower for placebo than for caffeine (P=0.02) but not statistically different between caffeine and AmaTea®. A trend for a time effect was noted for focused (P=0.07), with values generally increasing initially (in particular for caffeine and AmaTea® and then decreasing (although values remained elevated for caffeine through h 4). No other differences of statistical significance were noted for any variable (P>0.05). Data are presented in Table 3 and in Figure 1 for feelings of jittery.
Table 3. Subjective feelings data of men and women before, during, and after 4 h of gaming.
| Variable | Caffeine | AmaTea® | Placebo |
|---|---|---|---|
| Attentive | |||
| Hour 0 | 62±20 | 60±24 | 62±21 |
| Hour 1 | 65±19 | 63±22 | 62±23 |
| Hour 2 | 64±22 | 62±23 | 60±22 |
| Hour 3 | 63±23 | 58±23 | 60±22 |
| Hour 4 | 66±21 | 56±23 | 57±23 |
| Energetic | |||
| Hour 0 | 57±21 | 61±25 | 59±24 |
| Hour 1 | 65±20 | 61±24 | 63±24 |
| Hour 2 | 60±24 | 62±22 | 58±25 |
| Hour 3 | 63±21 | 55±24 | 56±25 |
| Hour 4 | 60±23 | 54±24 | 54±24 |
| Motivated | |||
| Hour 0 | 62±21 | 62±22 | 61±24 |
| Hour 1 | 64±21 | 61±25 | 64±23 |
| Hour 2 | 63±20 | 62±21 | 59±22 |
| Hour 3 | 59±23 | 56±22 | 59±22 |
| Hour 4 | 61±21 | 53±23 | 55±24 |
| Irritable | |||
| Hour 0 | 19±18 | 22±22 | 20±21 |
| Hour 1 | 21±20 | 17±17 | 18±16 |
| Hour 2 | 18±18 | 18±23 | 21±17 |
| Hour 3 | 18±20 | 18±19 | 20±18 |
| Hour 4 | 19±16 | 24±21 | 18±18 |
| Focused | |||
| Hour 0 | 61±22 | 62±23 | 64±23 |
| Hour 1 | 66±23 | 64±24 | 66±24 |
| Hour 2 | 65±21 | 65±22 | 64±25 |
| Hour 3 | 63±25 | 56±25 | 58±24 |
| Hour 4 | 63±23 | 55±21 | 58±23 |
| Jittery | |||
| Hour 0 | 26±27 | 30±29 | 23±25* |
| Hour 1 | 35±28 | 30±30 | 24±28* |
| Hour 2 | 27±25 | 29±28 | 25±25* |
| Hour 3 | 30±28 | 23±23 | 25±25* |
| Hour 4 | 28±25 | 26±24 | 20±23* |
| Moody | |||
| Hour 0 | 13±14 | 18±20 | 16±20 |
| Hour 1 | 16±16 | 19±20 | 13±15 |
| Hour 2 | 15±20 | 15±18 | 15±17 |
| Hour 3 | 15±16 | 16±17 | 16±18 |
| Hour 4 | 20±21 | 20±22 | 19±21 |
Values are mean±SD.
Condition effect (P=0.05); Values lower for Placebo than for Caffeine (P=0.02). No other differences of statistical significance were noted (P>0.05).
Figure 1. Percentage change from baseline to 1 h post ingestion for feelings of jittery. Paired contrast between caffeine and AmaTea® (P=0.28).
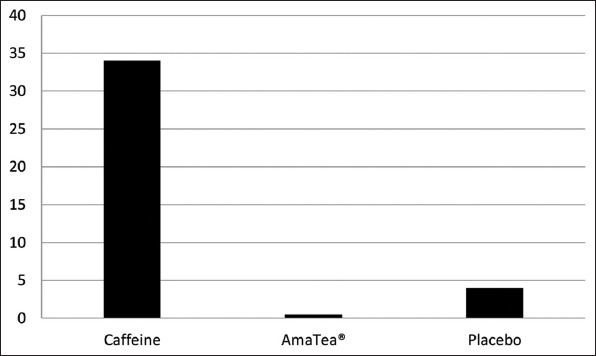
3.4. Brunel mood scale
A time effect was noted for Anger (P=0.04), with values higher at h 3 than h 0 and 1 (P=0.02) and higher at h 4 than h 0 and 1 (P=0.008). No condition (P=0.46) or interaction (P=0.54) effects were noted.
A time effect was noted for Depression (P=0.02), with values higher at h 4 than h 1 (P<0.05). No condition (P=0.74) or interaction (P=0.61) effects were noted.
A time effect was noted for Vigor (P<0.0001), with values higher at h 1 and 2 than h 0 (P<0.05), higher at h 1 than h 3 and 4 (P<0.05), and higher at h 2 than h 4 (P<0.05). A condition effect was also noted for Vigor (P=0.05), with values lower for placebo than for AmaTea® (P=0.02). No interaction effect was noted (P=0.51).
A time effect was noted for Fatigue (P<0.0001), with values higher at h 4 than all other times (P<0.05), and higher at h 3 than h 1 (P<0.05). A condition effect was also noted for Fatigue (P=0.01), with values higher for placebo than for AmaTea® (P<0.05). No interaction effect was noted (P=0.99).
A time effect was noted for Happy (P=0.005), with values lower at h 4 than h 0 and 1 (P<0.05). No condition (P=0.17) or interaction (P=0.86) effects were noted.
A time effect was noted for Calmness (P=0.0002), with values at h 0 higher than all other times (P<0.05). No condition (P=0.77) or interaction (P=0.63) effects were noted.
No other differences of statistical significance were noted for any variable (P>0.05). Data are presented in Table 4.
Table 4. Brunel Mood Scale data of men and women before, during, and after 4 h of gaming.
| Variable | Caffeine | AmaTea® | Placebo |
|---|---|---|---|
| Anger* | |||
| Hour 0 | 4.8±2.1 | 4.3±1.0 | 4.6±1.3 |
| Hour 1 | 4.8±1.6 | 4.7±1.3 | 4.8±1.9 |
| Hour 2 | 4.7±1.5 | 4.9±1.7 | 4.8±1.4 |
| Hour 3 | 5.0±1.8 | 5.3±2.2 | 4.9±1.6 |
| Hour 4 | 5.6±2.7 | 4.9±1.6 | 4.9±2.1 |
| Tension | |||
| Hour 0 | 5.4±2.1 | 5.3±1.8 | 5.3±2.6 |
| Hour 1 | 5.5±2.7 | 5.2±1.7 | 5.2±1.8 |
| Hour 2 | 5.2±2.3 | 5.3±1.6 | 4.8±1.6 |
| Hour 3 | 4.9±1.8 | 5.1±1.6 | 4.7±1.4 |
| Hour 4 | 5.1±1.9 | 4.9±1.6 | 4.8±1.7 |
| Depression* | |||
| Hour 0 | 4.6±1.4 | 4.3±0.9 | 4.4±1.0 |
| Hour 1 | 4.2±0.5 | 4.2±0.6 | 4.2±0.6 |
| Hour 2 | 4.3±0.8 | 4.2±0.7 | 4.5±1.0 |
| Hour 3 | 4.4±0.9 | 4.6±1.1 | 4.4±0.8 |
| Hour 4 | 4.7±1.8 | 4.6±1.2 | 4.4±0.9 |
| Vigor* | |||
| Hour 0 | 11.4±3.3 | 12.9±3.0 | 12.1±3.8* |
| Hour 1 | 14.0±3.3 | 14.3±3.2 | 13.5±3.6* |
| Hour 2 | 13.8±3.3 | 13.8±3.3 | 12.6±3.4* |
| Hour 3 | 13.1±3.1 | 12.5±3.7 | 12.3±3.6* |
| Hour 4 | 11.9±3.5 | 11.6±3.7 | 11.0±3.3* |
| Fatigue* | |||
| Hour 0 | 6.8±3.2 | 6.3±2.5 | 7.1±3.6* |
| Hour 1 | 5.9±2.5 | 5.4±2.0 | 6.3±2.5* |
| Hour 2 | 6.4±3.4 | 5.9±2.2 | 7.0±3.1* |
| Hour 3 | 7.0±3.3 | 6.8±2.8 | 7.8±3.5* |
| Hour 4 | 8.1±3.8 | 8.3±3.4 | 8.6±3.7* |
| Confusion | |||
| Hour 0 | 4.7±1.4 | 5.0±1.7 | 4.9±1.8 |
| Hour 1 | 4.6±1.2 | 4.6±1.1 | 4.8±1.7 |
| Hour 2 | 4.7±1.3 | 4.8±1.6 | 4.5±1.1 |
| Hour 3 | 4.7±1.4 | 4.6±1.3 | 4.4±1.3 |
| Hour 4 | 4.9±2.0 | 4.6±1.2 | 4.6±1.4 |
| Happy* | |||
| Hour 0 | 12.4±2.9 | 13.3±2.4 | 12.8±3.1 |
| Hour 1 | 12.6±3.0 | 13.3±2.8 | 12.6±3.3 |
| Hour 2 | 12.7±2.6 | 12.8±3.0 | 11.9±2.7 |
| Hour 3 | 12.1±2.7 | 12.4±3.4 | 12.0±3.0 |
| Hour 4 | 11.5±3.2 | 11.7±3.3 | 11.9±2.7 |
| Calmness* | |||
| Hour 0 | 12.6±2.8 | 13.9±2.8 | 12.9±3.2 |
| Hour 1 | 11.0±2.9 | 11.9±2.8 | 12.0±3.3 |
| Hour 2 | 12.0±2.9 | 11.7±2.9 | 12.1±2.9 |
| Hour 3 | 11.7±3.1 | 12.1±2.9 | 12.3±3.4 |
| Hour 4 | 11.6±3.3 | 11.8±3.1 | 12.2±2.7 |
Values are mean±SD.
Time effect for Anger (P=0.04); Values higher at h 3 than h 0 and 1 (P=0.02) and higher at h 4 than h 0 and 1 (P=0.008). *Time effect for Depression (P=0.02); Values higher at h 4 than h 1 (P<0.05). *Time effect for Vigor (P<0.0001); Values higher at h 1 and 2 than h 0 (P<0.05), higher at h 1 than h 3 and 4 (P<0.05), and higher at h 2 than h 4 (P<0.05); Condition effect for Vigor (P=0.05) with values lower for Placebo than for AmaTea® (P=0.02). *Time effect for Fatigue (P<0.0001); Values higher at h 4 than all other times (P<0.05), higher at h 3 than h 1 (P<0.05); Condition effect for Fatigue (P=0.01) with values higher for Placebo than for AmaTea® (P<0.05). *Time effect for Happy (P=0.005); Values lower at h 4 than h 0 and 1 (P<0.05). *Time effect for Calmness (P=0.0002); Values at h 0 higher than all other times (P<0.05). No other differences of statistical significance were noted (P>0.05).
3.5. Go/No-Go reaction time
A time effect was noted for accuracy (P=0.04), with values higher at h 1 than h 3 and 4 (P=0.17) and h 2 (P=0.02). A trend for time effect was noted for response time (P=0.06), with the response becoming faster over time. No other differences of statistical significance were noted for either variable (P>0.05). Data are presented in Table 5.
Table 5. Go-No Go data of men and women before, during, and after 4 h of gaming.
| Variable | Caffeine | AmaTea® | Placebo |
|---|---|---|---|
| Go-No Go (ms) | |||
| Hour 0 | 277±36 | 279±47 | 274±37 |
| Hour 1 | 271±29 | 271±44 | 271±41 |
| Hour 2 | 273±32 | 265±33 | 275±35 |
| Hour 3 | 265±30 | 268±44 | 262±32 |
| Hour 4 | 266±35 | 267±34 | 267±36 |
| Accuracy (%)* | |||
| Hour 0 | 96±3 | 96±3 | 96±3 |
| Hour 1 | 97±2 | 96±3 | 96±3 |
| Hour 2 | 96±3 | 95±4 | 95±3 |
| Hour 3 | 96±4 | 96±3 | 95±3 |
| Hour 4 | 95±4 | 96±3 | 95±4 |
Values are mean±SD.
Time effect (P=0.04); Values higher at h 1 than h 3 and 4 (P=0.17) and h 2 (P=0.02). No other differences of statistical significance were noted (P>0.05).
3.6. DSST
A time effect was noted for the DSST (P<0.0001), with values higher at h 2, 3, and 4 than h 0 (P<0.05). No condition (P=0.81) or interaction (P=0.99) effects were noted. Data are presented in Table 6.
Table 6. Digit Symbol Substitution Test data of men and women before, during, and after 4 h of gaming.
| Variable | Caffeine | AmaTea® | Placebo |
|---|---|---|---|
| Correct responses | |||
| Hour 0* | 65±9 | 65±11 | 66±10 |
| Hour 1 | 68±8 | 68±9 | 68±10 |
| Hour 2 | 69±9 | 69±9 | 68±10 |
| Hour 3 | 71±9 | 70±10 | 70±9 |
| Hour 4 | 71±9 | 71±10 | 69±10 |
Values are mean±SD.
Time effect (P<0.0001); Values higher at h 2, 3, and 4 than h 0 (P<0.05). No other differences of statistical significance were noted (P>0.05).
3.7. AX-CPT
No condition (P=0.62), time (P=0.43), or interaction (P=0.40) effects were noted for AX-CPT performance with regards to the percentage of correct responses. While not of statistical significance, a 6% improvement was noted from h 0 to h 4 for AmaTea®, without such improvement noted for caffeine or placebo. Moreover, when investigating only the final one-third of responses at h 4 of the AX-CPT, there was a 5% quicker reaction time for AmaTea® as compared to caffeine and placebo; however, these differences were not of statistical significance (P=0.29 and P=0.27, respectively).
No condition (P=0.37), time (P=0.11), or interaction (P=0.63) effects were noted for AX-CPT performance with regards to reaction time. Data are presented in Table 7.
Table 7. AX-Continuous Performance Task data of men and women before and after 4 h of gaming.
| Variable | Caffeine | AmaTea® | Placebo |
|---|---|---|---|
| % Correct | |||
| Hour 0 | 88±13 | 83±18 | 85±18 |
| Hour 4 | 87±15 | 88±14 | 85±14 |
| Reaction Time (ms) | |||
| Hour 0 | 463±108 | 424±123 | 441±124 |
| Hour 4 | 425±119 | 418±101 | 420±103 |
Values are mean±SD. No differences of statistical significance were noted (P>0.05).
3.8. Gaming performance
When analyzing data by individual hour, overall gaming performance was highest during h 1 and gradually declined with time, with regards to wins (P=0.0004), kills/match (P=0.008), and top 3 performances (P=0.0003). A trend (P=0.075) was noted for the condition effect, with kills/match being 21% higher for AmaTea® (1.84) compared to placebo (1.51), and 12% higher for AmaTea® compared to caffeine (1.63). No interaction effects were noted (P>0.05).
When pooling all 4 h of gaming play together, no condition, time, or interaction effects were noted for any variable related to gaming performance (P>0.05). However, the kills/match were approximately 13% higher for AmaTea® as compared to caffeine and placebo; values that were not of statistical significance. Data are presented in Table 8 and Figure 2.
Table 8. Game data of men and women after 4 h of gaming.
| Variable | Caffeine | AmaTea® | Placebo |
|---|---|---|---|
| Wins | 1.5±1.5 | 1.9±1.8 | 2.2±1.8 |
| Matches | 17.7±3.8 | 16.1±2.8 | 16.8±3.7 |
| Kills | 26.0±15.5 | 26.4±14.8 | 24.3±13.7 |
| Kills/Match | 1.5±1.0 | 1.7±1.1 | 1.5±0.8 |
| Top 3 Finishes | 3.1±1.6 | 3.7±1.9 | 3.7±1.9 |
| Top 5 Finishes | 2.2±1.5 | 1.8±1.5 | 1.9±1.6 |
| Top 10 Finishes | 2.6±1.5 | 2.8±1.4 | 2.7±1.4 |
Values are mean±SD. No differences of statistical significance were noted (P>0.05).
Figure 2. Kills/match of men and women after 4 h of gaming.
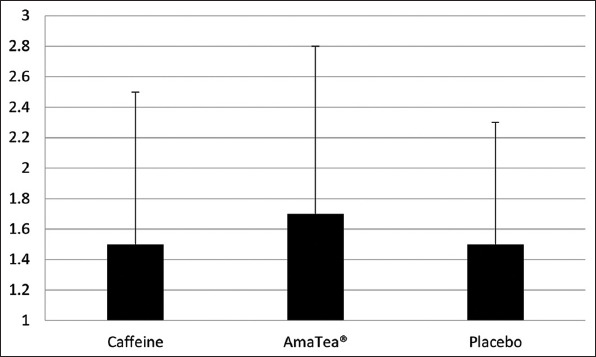
3.8. Cortisol
A time effect was noted for cortisol (P<0.0001), with values higher at h 0 than h 2 and 4 (P<0.05), and higher at h 2 than h 4 (P<0.05). A condition effect was also noted (P=0.005), with values lower for placebo than for caffeine and AmaTea® (P<0.05). No interaction effect was noted (P=0.78). It should be noted that cortisol data are only available for 47 of the 49 subjects. Of the 423 samples (47 subjects × 3 visits × 3 samples per visit), 18 samples were not included in the analyses due to extremely high or low values. Data are presented in Table 9 and Figure 3.
Table 9. Blood cortisol data of men and women before, during, and after 4 h of gaming.
| Time* | Caffeine | AmaTea® | Placebo* |
|---|---|---|---|
| Hour 0 | 67.2±31.6 | 77.2±65.5 | 61.5±26.3 |
| Hour 2 | 61.0±39.6 | 59.7±30.0 | 43.8±24.7 |
| Hour 4 | 46.3±30.5 | 50.0±35.7 | 35.1±33.0 |
Values are mean±SD (ng/mL).
Time effect (P<0.0001); Values higher at h 0 than h 2 and 4 (P<0.05), higher at h 2 than h 4 (P<0.05); Condition effect (P=0.005) with values lower for Placebo than for Caffeine and AmaTea® (P<0.05). No other differences of statistical significance were noted (P>0.05).
Figure 3. Blood cortisol data of men and women before, during, and after 4 h of gaming.
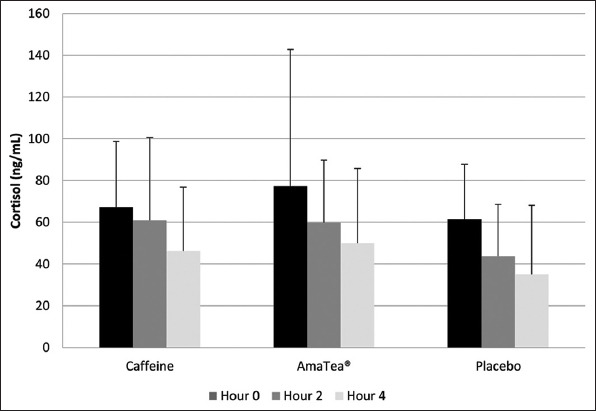
4. Discussion
The present study compared the impact of AmaTea® on gaming performance and related measures, as compared to caffeine and placebo conditions. Actual gaming performance was not impacted in a statistically significant manner for any condition, while a trend was noted for a condition effect for kills/match (P=0.075), with AmaTea® ~12% higher than caffeine and 21% higher than placebo.
It was surprising to us that neither caffeine nor AmaTea® (containing a relative high quantity of caffeine) improved gaming performance or measures of cognition—in particular within subjects with varied caffeine intake (range between 0 and 400 mg daily). Caffeine is a well-known stimulant capable of causing physical sensations similar to that caused by adrenaline, followed by a sudden and tapering lethargy [20]. While not of statistical significance, the data indicate that the form of caffeine (or perhaps the combination of ingredients) in AmaTea® appears to somewhat differently impact subjective feelings, as a condition effect was noted for perceived fatigue (P=0.01), with values higher for placebo than for AmaTea® but not caffeine. Caffeine competes for the adenosine receptor, an inhibitory neurotransmitter promoting sleepiness, in the brain although adenosine production continues. Once caffeine is metabolized, adenosine binds receptors with ensuing fatigue. A time effect was noted for fatigue, with values higher at h 4 than all other times and higher at h 3 than h 1, which seems reasonable given the demands and length of the testing sessions.
An additional finding was that AmaTea® consumption increased perceived feelings of vigor, with values lower for placebo than for AmaTea® (P=0.02). A time effect was noted, with values higher at h 1 and 2 than h 0, higher at h 1 than h 3 and 4, and higher at h 2 than h 4. Thus, it seems that the lengthy gaming session (4 h of gameplay; 6-7 h total visit time) contributed to the loss of vigor or tiredness. Observations by the investigators over the time-period corroborate this notion. In addition, the AX-CPT test completed at the initial visit and at the end of the visit required considerable focus, with multiple cognitive test queries and 30-45 min of sustained attention.
The conditions had no impact on HR but an elevation in SBP was noted for both caffeine and AmaTea® supplemented groups, as expected. It has been reported and is fairly well-recognized that caffeine can cause a transitory, but potentially intense increase in BP even in healthy individuals without hypertension. In a review of 34 studies, consumption of 200-300 mg caffeine as 1.5-2.0 cups of coffee caused an average increase of 8 mm Hg and 6 mm Hg in SBP and DBP, respectively [21]. Moreover, this effect was noted up to 3 h post-consumption in both healthy individuals and those with diagnosed hypertension. A key factor for consideration with participants is the overall consumption patterns since caffeine tolerance can impact BP rises, as well other potential side effects. In our study, we noted that gaming alone increased SBP by 3 mm Hg and caffeine contributed an additional 3-5 mm Hg. This effect is less robust than reported previously, but parameters associated with the experimental design and subject pool could have contributed to these findings.
Overall, mental and gaming performance was not impacted in a statistically significant manner following any of the three conditions. While there were no statistically significant differences with the percentage of correct responses on AX-CPT, there was a 6% improvement from h 0 to h 4 for AmaTea® without the same effect for caffeine or placebo. In addition, when investigating only the final one-third of responses at h 4 of the AX-CPT, there was a 5% quicker reaction time for AmaTea® as compared to caffeine and placebo.
Gaming performance as measured by kills/match was 13% higher for those consuming AmaTea® compared to the caffeine and placebo groups. Although the results suggest a mild and non-statistically significant effect, they should be considered in future studies of AmaTea®, as well as studies involving gaming performance.
While studies of dietary supplements in active gamers are sparse, one recent investigation used a sample of nine elite League of Legends (LoL) gamers (21±2 y, BMI 25.6±3.4 kg/m2), subjects consumed an energy drink (Reload™) or placebo (Placebo) in a randomized, double-blind, placebo-controlled crossover trial with completion of a test battery (attention [Erikson Flanker Test], reaction time [Go/No-Go test] and working memory [n-back test]) before treatment consumption and after playing each of 3 competitive LoL games [5]. The authors reported no significant time, group, or group-by-time interactions for any measured performance index except for the working memory test, where the Reload™ group demonstrated a significant within-group improvement. However, no between-group differences were noted, suggesting that elite gamers did not demonstrate a mental or physical improvement in performance. While we were surprised that the caffeine and AmaTea® conditions did not impact gaming and cognitive performance significantly, our data seem consistent with many aspects of the above findings using a cohort with similar BMI, age, and video gaming experience. Other studies report both positive and null effects of caffeine ingestion on tasks of cognitive performance [11].
We analyzed plasma cortisol levels to determine any effect on overall stress experienced by the gamers. Both caffeine and stress can increase plasma cortisol, leading to potential negative side effects. Gameplay did not appear to affect plasma cortisol, and values were slightly lower overall for placebo as compared to the other conditions, which may be a function of the lower baseline cortisol values for placebo (Table 9; Figure 3). We observed that plasma cortisol, a steroid hormone, basically followed its natural circadian rhythm, with healthy individuals displaying cortisol levels that peak in the morning and slowly decline throughout the day to very low levels around midnight, with subsequent increases throughout the night and a peak in the morning hours. We collected blood for cortisol analysis at approximately 4:30 pm, 7:30 pm, and again close to 10:00 pm, suggesting that levels may have been too low, although detectable, to note any substantive differences between groups.
There are potential confounding factors in the experimental design. For example, to improve consistency amongst subjects and maximize sensitivity, subjects reported to the gaming suite at 4:00 pm after a 3-h fast, which may have impacted response to the treatments. This is particularly relevant since subjects played Fortnite until around 10:00 pm, with a ~300-kcal snack (protein bar) at the midpoint and water throughout the session. The authors assume, however, that any contributing aspect of this design (e.g., hunger or ingestion of calories) to the results would be uniform among all participants and obviate statistical confounding. In addition, as subjects play against various individuals during each match, the number of kills and wins is somewhat dependent on the quality of the opponents. We make the assumption that the average quality of the competition is similar from day to day and across time, regardless of condition ingestion.
5. Conclusions
Neither caffeine nor AmaTea® favorably impacted gaming and cognitive performance in a statistically significant manner. However, AmaTea® did appear to be associated with greater vigor and lower fatigue as compared to placebo. Gaming performance, as indicated by kills/match, in addition to the score on the AX-CPT, was slightly improved with AmaTea® albeit in a non-statistically significant manner. It is possible that a different gaming stimulus, varied time of game play, or different dosage of the supplement may have yielded even more favorable results.
Acknowledgments
Funding for this work was provided in part by Applied Food Sciences, Inc. and the University of Memphis. We thank Tim Harmon and Joe Harmon for their technical/gaming expertise and consultation in developing the gaming suite, as well as in the selection of gaming materials. We thank Brandon L. Pigg, Roddy Morris, Jr., and Lyla Saudi for assistance with data collection. We thank Dr. Brandt Pence for writing R code to extract the data from the computer testing files, calculate the desired outputs, and aggregate the findings. Finally, we thank Matthew Butawan MB for assistance with coding for computerized testing.
Conflict of Interest
No author declares a conflict of interest related to this work. The sponsor had no role in the execution of the study, or in the interpretation of the data.
References
- [1].Yin K, Zi Y, Zhuang W, Gao Y, Tong Y, Song L, et al. Linking Esports to Health Risks and Benefits:Current Knowledge and Future Research Needs. J Sport Health Sci. 2020;9:485–8. doi: 10.1016/j.jshs.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Fiore R, Zampaglione D, Murazzi E, Bucchieri F, Cappello F, Fucarino A. The eSports Conundrum:Is the Sports Sciences Community Ready to Face Them?A Perspective. J Sports Med Phys Fitness. 2020;60:1591–602. doi: 10.23736/S0022-4707.20.10892-2. [DOI] [PubMed] [Google Scholar]
- [3].Nagorsky E, Wiemeyer J. The Structure of Performance and Training in Esports. PLoS One. 2020;15:1–10. doi: 10.1371/journal.pone.0237584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Bányai F, Zsila Á, Griffiths MD, Demetrovics Z, Király O. Career as a Professional Gamer:Gaming Motives as Predictors of Career Plans to Become a Professional Esport Player. Front Psychol. 2020;11:1866. doi: 10.3389/fpsyg.2020.01866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Thomas CJ, Rothschild J, Earnest CP, Blaisdell A. The Effects of Energy Drink Consumption on Cognitive and Physical Performance in Elite League of Legends Players. Sports. 2019;7:196. doi: 10.3390/sports7090196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Emara AK, Ng MK, Cruickshank JA, Kampert MW, Piuzzi NS, Schaffer JL, et al. Gamer's Health Guide:Optimizing Performance, Recognizing Hazards, and Promoting Wellness in Esports. Curr Sports Med Rep. 2020;19:537–45. doi: 10.1249/JSR.0000000000000787. [DOI] [PubMed] [Google Scholar]
- [7].Campbell MJ, Toth AJ, Moran AP, Kowal M, Exton C. eSports:A New Window on Neurocognitive Expertise? Prog Brain Res. 2018;240:161–74. doi: 10.1016/bs.pbr.2018.09.006. [DOI] [PubMed] [Google Scholar]
- [8].Duggan J. How Fortnite Became the Biggest Game in the World, IGN. 2018:1. [Google Scholar]
- [9].Brühl AB, Sahakian BJ. Drugs, Games, and Devices for Enhancing Cognition:Implications for Work and Society. Ann N Y Acad Sci. 2016;1369:195–217. doi: 10.1111/nyas.13040. [DOI] [PubMed] [Google Scholar]
- [10].Onaolapo AY, Obelawo AY, Onaolapo OJ. Brain Ageing, Cognition and Diet:A Review of the Emerging Roles of Food-Based Nootropics in Mitigating Age-related Memory Decline. Curr Aging Sci. 2019;12:2–14. doi: 10.2174/1874609812666190311160754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].McLellan TM, Caldwell JA, Lieberman HR. A Review of Caffeine's Effects on Cognitive, Physical and Occupational Performance. Neurosci Biobehav Rev. 2016;71:294–312. doi: 10.1016/j.neubiorev.2016.09.001. [DOI] [PubMed] [Google Scholar]
- [12].Kamimori GH, McLellan TM, Tate CM, Voss DM, Niro P, Lieberman HR. Caffeine Improves Reaction Time, Vigilance and Logical Reasoning During Extended Periods with Restricted Opportunities for Sleep. Psychopharmacology (Berl) 2015;232:2031–42. doi: 10.1007/s00213-014-3834-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Temple JL, Bernard C, Lipshultz SE, Czachor JD, Westphal JA, Mestre MA. The Safety of Ingested Caffeine:A Comprehensive Review. Front Psychiatry. 2017;8:80. doi: 10.3389/fpsyt.2017.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Verster JC, Koenig J. Caffeine Intake and Its Sources:A Review of National Representative Studies. Crit Rev Food Sci Nutr. 2018;58:1250–9. doi: 10.1080/10408398.2016.1247252. [DOI] [PubMed] [Google Scholar]
- [15].Mahoney CR, Giles GE, Marriott BP, Judelson DA, Glickman EL, Geiselman PJ, et al. Intake of Caffeine From All Sources and Reasons for Use by College Students. Clin Nutr. 2019;38:668–75. doi: 10.1016/j.clnu.2018.04.004. [DOI] [PubMed] [Google Scholar]
- [16].Wise G, Negrin A. A Critical Review of the Composition and History of Safe Use of Guayusa:A Stimulant and Antioxidant Novel Food. Crit Rev Food Sci Nutr. 2020;60:2393–404. doi: 10.1080/10408398.2019.1643286. [DOI] [PubMed] [Google Scholar]
- [17].García-Ruiz A, Baenas N, Benítez-González AM, Stinco CM, Meléndez-Martínez AJ, Moreno DA, et al. Guayusa (Ilex guayusa L.) New Tea:Phenolic and Carotenoid Composition and Antioxidant Capacity. J Sci Food Agric. 2017;97:3929–36. doi: 10.1002/jsfa.8255. [DOI] [PubMed] [Google Scholar]
- [18].Gan RY, Zhang D, Wang M, Corke H. Health Benefits of Bioactive Compounds From the Genus Ilex, a Source of Traditional Caffeinated Beverages. Nutrients. 2018;10:1682. doi: 10.3390/nu10111682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Krieger DR, Kalman DS, Feldman S, Arnillas L, Goldberg D, Gisbert O, et al. The Safety, Pharmacokinetics, and Nervous System Effects of Two Natural Sources of Caffeine in Healthy Adult Males. Clin Transl Sci. 2016;9:246–51. doi: 10.1111/cts.12403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Hussain A, Jiji AK, Barke P, Biswas S, Tabrez SS. Cardiovascular Pathologies Associated with Excessive Energy Drink Consumption:A Review. Crit Rev Eukaryot Gene Expr. 2018;28:107–13. doi: 10.1615/CritRevEukaryotGeneExpr.2018021703. [DOI] [PubMed] [Google Scholar]
- [21].Chrysant SG. The Impact of Coffee Consumption on Blood Pressure, Cardiovascular Disease and Diabetes Mellitus. Expert Rev Cardiovasc Ther. 2017;15:151–6. doi: 10.1080/14779072.2017.1287563. [DOI] [PubMed] [Google Scholar]


