Abstract
Background
Research on the disease severity of COVID-19 in patients with rheumatic immune-mediated inflammatory diseases (IMIDs) has been inconclusive, and long-term prospective data on the development of SARS-CoV-2 antibodies in these patients are lacking.
Methods
Adult patients with rheumatic IMIDs from the Amsterdam Rheumatology and Immunology Center, Amsterdam were invited to participate. All patients were asked to recruit their own sex-matched and age-matched control subject. Clinical data were collected via online questionnaires (at baseline, and after 1–4 and 5–9 months of follow-up). Serum samples were collected twice and analysed for the presence of SARS-CoV-2-specific antibodies. Subsequently, IgG titres were quantified in samples with a positive test result.
Findings
In total, 3080 consecutive patients and 1102 controls with comparable age and sex distribution were included for analyses. Patients were more frequently hospitalised compared with controls when infected with SARS-CoV-2; 7% vs 0.7% (adjusted OR: 7.33, 95% CI: 0.96 to 55.77). Only treatment with B-cell targeting therapy was independently associated with an increased risk of COVID-19-related hospitalisation (adjusted OR: 14.62, 95% CI: 2.31 to 92.39). IgG antibody titres were higher in hospitalised compared with non-hospitalised patients, and slowly declined with time in similar patterns for patients in all treatment subgroups and controls.
Interpretation
We observed that patients with rheumatic IMIDs, especially those treated with B-cell targeting therapy, were more likely to be hospitalised when infected with SARS-CoV-2. Treatment with conventional synthetic disease-modifying antirheumatic drugs (DMARDs) and biological DMARDs other than B-cell targeting agents is unlikely to have negative effects on the development of long-lasting humoral immunity against SARS-CoV-2.
Keywords: antirheumatic agents, autoimmune diseases, biological therapy, COVID-19, epidemiology
Key messages.
What is already known about this subject?
Patients with rheumatic immune-mediated inflammatory diseases (IMIDs) seem to be at an increased risk of COVID-19-related hospitalisation, but results are inconclusive.
Effects of immunosuppressive drugs other than B-cell targeting agents on the development of humoral immunity after COVID-19 vaccination are minimal, but long-term effects, especially after COVID-19 infection instead of vaccination, are still unknown.
What does this study add?
Patients with rheumatic IMIDs, especially those treated with B-cell targeting therapy, were more frequently hospitalised when infected with SARS-CoV-2 compared with the general population.
Treatment with conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) and biological DMARD (bDMARDs) other than B-cell targeting agents does not seem to impair the development of long-term humoral immunity against SARS-CoV2.
How might this impact on clinical practice or future developments?
Our results regarding effects of csDMARDs and bDMARDs on the maintenance of humoral immunity after primary infection with SARS-CoV-2 over time are reassuring, but future studies are needed to assess whether these findings are similar for long-term humoral immunity after COVID-19 vaccinations.
Introduction
Since the start of the COVID-19 pandemic, concerns have been raised regarding the safety of those who are vulnerable to infections, which includes patients with a rheumatic immune-mediated inflammatory disease (IMID).1 Both the underlying disease and immunosuppressive treatment regimens prescribed to these patients make them susceptible for infections,2 and therefore possibly a worse disease outcome of COVID-19. Moreover, treatment with immunosuppressive drugs may hamper the maintenance of immunological memory against SARS-CoV-2, which might increase the susceptibility of patients with rheumatic diseases to (severe) COVID-19 reinfections as well. Although current data on disease severity of COVID-19 in patients with rheumatic diseases seem reassuring,3 4 definitive conclusions have not yet been drawn due to lack of studies that provide a high quality of evidence. So far, most data have been derived from cross-sectional cohort studies or retrospective registry studies that often suffer from considerable methodological disadvantages, such as selection bias.5 Hence, experts of the EULAR recently agreed that although existing evidence does not point towards an increased risk of a worse disease course of COVID-19 in patients with rheumatic diseases, patients should still be advised to strictly follow infection prevention measures, even after receiving vaccinations.6
In contrast to the increasing amount of data on COVID-19 disease severity in patients with rheumatic diseases, data on the development of SARS-CoV-2 antibodies in this patient group are still scarce. Existing data in patients with non-rheumatic IMIDs, such as multiple sclerosis or inflammatory bowel disease, point towards strong inhibitory effects of B-cell targeting agents,7 8 and considerable attenuating effects of tumour necrosis factor (TNF) inhibitors on the production of SARS-CoV-2 antibodies in the first 2–12 weeks after infection.9 In addition, we recently observed that treatment with methotrexate was associated with lower seroconversion rates after first COVID-19 vaccinations,10 which might suggest that seroconversion after an infection with SARS-CoV-2 are decreased as well. Therefore, since previous studies demonstrated that the presence of SARS-CoV-2 antibodies protect against symptomatic reinfections, it is relevant to evaluate the impact of immunosuppressive therapies on the development and maintenance of SARS-CoV-2 antibodies over time. In this study, we describe the results of a large prospective controlled cohort study that was designed to compare both disease severity of COVID-19, and the development of SARS-CoV-2 antibodies over time between patients with rheumatic IMIDs and healthy controls.
Methods
Study design
Between April 2020 and March 2021, all adult patients with rheumatic IMIDs from the Amsterdam Rheumatology and Immunology Center were invited to participate in this study. All patients were asked, but not obliged, to recruit their own control subject of the same sex, comparable age (age difference of <5 years) and without a rheumatic IMID. Clinical data were collected at baseline and up to two times during follow-up via online questionnaires distributed by email. Serum samples were collected up to two times via regular blood withdrawal at the local research institutes or via a finger prick that could be performed at home, prior to COVID-19 vaccination.
Procedures
Data collection on the incidence and disease severity of COVID-19 ended on 30 April 2021, due to the start of the Dutch national COVID-19 vaccination programme. The baseline questionnaire was sent to participants when they were included in the study. The first and second follow-up questionnaires were sent to participants 1–4 and 5–9 months after completion of the baseline questionnaire. Information on demographic data were only collected at baseline and included age, sex, height, weight, smoking status, ethnicity, educational level, type of IMID and the presence of comorbidities. At baseline and during follow-up, participants reported their disease activity, medication use and COVID-19-related characteristics. Disease activity was evaluated only for patients with rheumatoid arthritis or spondyloarthritis using the multidimensional Health Assessment Questionnaire (HAQ) (Routine Assessment of Patient Index Data-3 (RAPID-3)/HAQ2) or Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), respectively. COVID-19-related characteristics included information on type and severity of COVID-19-related symptoms (cough, dyspnoea, fever, ageusia/agnosia, malaise, fatigue, headache, vomiting/diarrhoea), recent travelling history, (probable) contact with confirmed COVID-19 cases, social distancing measures, COVID-19 test results (PCR tests, loop-mediated isothermal amplification tests, rapid antigen tests, serological antibody test results acquired outside the study, hospitalisation and starting January 2021 (when the vaccination campaign started) COVID-19 vaccination status.
Between May and November 2020, all included patients were invited for their first blood draw at the local research institute of Reade, preferably at the time of completion of the first follow-up survey. Between October and December 2020, a self-administered finger prick test kit was sent to healthy controls and patients who were unable to visit the research institute. Between January and March 2021, all participants were invited for a first or second blood draw at the research institute, again preferably at the time of completing a follow-up survey. A test kit was sent to participants who had indicated their preference for the finger prick method in a previous survey or via direct contact with the researchers. All serum samples were collected prior to COVID-19 vaccination.
All collected serum samples were analysed for the presence of SARS-CoV-2-specific antibodies with a receptor binding domain antibody (RBD-Ab) bridging ELISA (in house) with a 98.1% sensitivity and a 99.5% specificity.11 Subsequently, titres of IgG antibodies against the RBD protein were quantified in samples with a positive test result in the bridging assay.12 Signals were compared with a serially diluted calibrator (arbitrary assigned a value of 100 arbitrary units (AU) per mL (AU/mL)) consisting of pooled convalescent plasma that was included on each plate. Anti-RBD IgG titres were expressed as AU/mL. Neutralisation assays are considered to be more biologically relevant compared with total antibody assays, but we previously demonstrated high correlations between both assays.12
Outcomes
The primary objective of the study was to compare the disease severity of COVID-19 between patients with rheumatic IMIDs and healthy controls. Confirmed COVID-19 cases were defined as participants who reported a positive PCR test to SARS-CoV-2 at any moment during follow-up but before COVID-19 vaccination, and/or participants in whom SARS-CoV-2-specific antibodies were detected using the RBD-Ab bridging ELISA. Severe COVID-19 disease was defined as an (unplanned) hospital admission due to confirmed COVID-19. All participants who indicated in the questionnaires that they were hospitalised as a consequence of COVID-19 disease were contacted to verify the reason and duration of their hospitalisation. To confirm if participants died during the study, an email requesting a response was sent to all participants who did not complete a questionnaire since 1 January 2021 from whom it was known that they did not stop participation in this study. In case of non-response, electronic health records were checked for patients, but this was not possible for healthy controls. Relatives of participants who died during the conduct were contacted to verify the cause of death of the participant.
Secondary objectives of this study were to assess the effects of treatment with conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) (including methotrexate, hydroxychloroquine, sulfasalazine, leflunomide, azathioprine and ciclosporin), biological (b)DMARDs (including TNF, interleukin (IL)-1 and IL-6 inhibitors, abatacept and B-cell targeting agents) and prednisone on disease severity of COVID-19, seropositivity for SARS-CoV-2 antibodies and the maintenance of SARS-CoV-2 IgG antibody titres over time. For a subgroup of patients (patients with rheumatoid arthritis or spondyloarthritis), we aimed to assess whether the impact of the COVID-19 pandemic on patient care had influenced rheumatic disease activity. Proportions of seropositivity for SARS-CoV-2 were compared only between patients and controls with a diagnosis of COVID-19 confirmed by PCR. The infection date of COVID-19 was determined for SARS-CoV-2 seropositive participants. The infection date could be determined when participants had received a positive PCR test result and/or experienced symptoms indicative of COVID-19 (at least one of the following symptoms: cough, dyspnoea or loss of taste/smell, or at least two of the following symptoms: fever, general malaise, excessive tiredness, headache, rhinorrhoea, vomiting/diarrhoea).
Statistical analyses
Participants were included for analyses if they completed at least one survey part that assessed patient characteristics and one survey part that assessed COVID-19-related characteristics. Patient-related and COVID-19-related characteristics are presented as mean±SD, median (IQR) or frequencies and proportions depending on the type and distribution of the data. Additionally, logarithmically transformed IgG antibody titres are presented in scatter plots stratified for treatment groups.
Univariable and multivariable logistic regression analyses were used to compare COVID-19-related hospitalisation rates between patients and controls with a confirmed COVID-19 diagnosis, and subsequently between patients with or without treatment with csDMARDs, bDMARDs or prednisone. A priori, all multivariable models were adjusted for age, sex and the presence of cardiovascular, diabetes or chronic pulmonary diseases, which have previously been demonstrated to be associated with severe COVID-19 disease.13 Analyses that compared the above-described treatment regimens were also adjusted for concomitant treatment with other immunosuppressive drugs.
Univariable and multivariable logistic and linear mixed model analyses were used to compare seropositivity rates for SARS-CoV-2 and SARS-CoV-2 IgG antibody titres between patients and controls, and subsequently between patients with or without treatment with csDMARDs, bDMARDs or prednisone. A random intercept for subject was added to the models to account for repeated measures, and no random slopes were added. IgG titres were transformed logarithmically before analyses. The relationship of logarithmically transformed IgG titres with time was best described with a linear trend. A priori, all multivariable models were adjusted for age, sex, time since COVID-19 disease and severe COVID-19 disease. Again, analyses that compared treatment regimens were also adjusted for concomitant treatment with other immunosuppressive drugs. Univariable linear mixed model analyses were used to investigate the longitudinal development of disease activity over time in patients with rheumatoid arthritis (RAPID-3 scores) or ankylosing spondylitis (BASDAI scores).
Because previous studies demonstrated opposing and varying effects of individual csDMARDs and bDMARDs on COVID-19 disease severity and antibody development,14–17 additional exploratory regression or mixed model analyses were performed to assess effects of methotrexate, TNF inhibitors and B-cell targeting agents on COVID-19 disease severity, seropositivity rates for SARS-CoV-2 and SARS-CoV-2 IgG antibody titres. All multivariable models were adjusted for a predefined set of confounders as described above. For the primary objective, a p value <0.05 was considered significant. For secondary objectives and exploratory analyses, the Benjamini-Hochberg method was used to correct for multiple tests to ensure a family-wise false discovery rate of 5%.18 No effect modification was investigated. All statistical analyses were performed in SPSS V.27.0. Scatter plots of IgG antibody titres were created in GraphPad Prism (V.6.0).
Patient or public involvement
Patients or the public were not involved in the design, or conduct, or reporting, or dissemination plans of our research.
Results
Patient characteristics
In total, 3673 consecutive patients with rheumatic diseases and 1243 healthy controls were included in the study, of which 3080 patients and 1102 controls were included for analyses (figure 1). Serum samples were collected from 2343 (76%) patients and 862 (78%) controls; a single sample from 1253 patients and 549 controls, and multiple samples from 1090 patients and 313 controls. Baseline characteristics of patients and controls are shown in table 1. The mean age of patients and controls was 57 years (SD 14) and 55 years (SD 14), respectively, and the majority were female (65% of patients and 69% of controls) and of Caucasian race (86% of patients and 92% of controls). The prevalence of chronic pulmonary diseases, cardiovascular diseases, diabetes and obesity was slightly higher in patients compared with controls. The study population included a broad range of rheumatic diseases, and as expected, rheumatoid arthritis was the most common diagnosis among patients (56%). Most patients were on immunosuppressive treatment (78%); 52% used csDMARDs and 36% used bDMARDs. Methotrexate was used by 38% of patients, frequently concomitant with TNF inhibitors: 304 (26%) of 1183 patients on methotrexate also used TNF inhibitors. Only a small proportion of patients were on interleukin (IL)-6 inhibitors (tocilizumab) or B cell targeting therapy (rituximab or belimumab): 1% and 2%, respectively. Lastly, 12% of patients were treated with prednisone (median dose: 5 mg/day, range of IQR: 5–8). An overview of treatment alterations including type and reason of change can be found in the online supplemental table S4.
Figure 1.
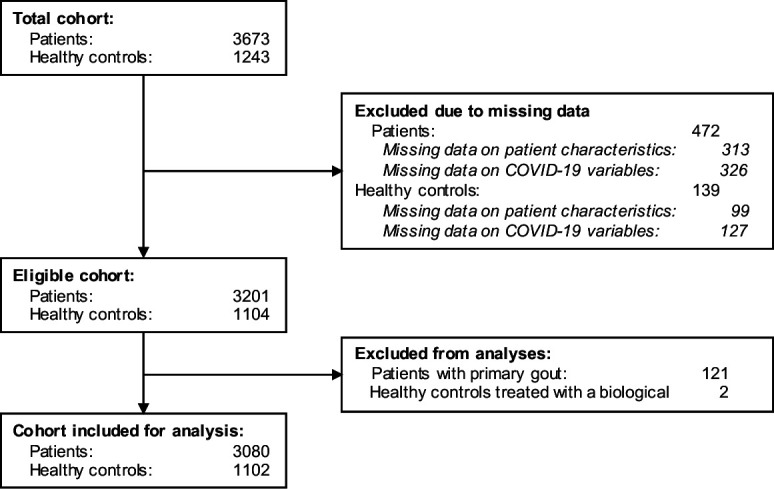
Flow chart of the study population.
Table 1.
Characteristics of patients with rheumatic immune-mediated inflammatory diseases compared with healthy controls
| Patient characteristics | Patients (n=3080) | Healthy controls (n=1102) |
| Age, years—mean (SD) | 57 (14) | 55 (14) |
| Female sex—no. (%) | 1990 (65) | 759 (69) |
| Male sex—no. (%) | 1089 (35) | 343 (31) |
| Body mass index, kg/m2—mean (SD) | 26 (5) | 25 (4) |
| Caucasian race—no. (%) | 2346 (86) | 909 (92) |
| Educational level—no. (%) | ||
| High | 1148 (46) | 534 (58) |
| Middle | 775 (31) | 246 (27) |
| Low | 553 (22) | 139 (15) |
| Current smoking status—no. (%) | 387 (13) | 80 (7) |
| Coexisting conditions—no. (%) | ||
| Cardiovascular disease | 392 (13) | 83 (8) |
| Chronic pulmonary disease | 376 (12) | 65 (6) |
| Diabetes | 183 (6) | 37 (3) |
| Obesity | 497 (16) | 112 (10) |
| Immune-mediated inflammatory disease type—no. (%) | ||
| Rheumatoid arthritis | 1714 (56) | N.A. |
| Psoriatic arthritis | 505 (16) | N.A. |
| Ankylosing spondylitis | 459 (15) | N.A. |
| Axial or peripheral spondyloarthritis | 76 (3) | N.A. |
| Juvenile idiopathic arthritis | 51 (2) | N.A. |
| Systemic lupus erythematosus | 175 (6) | N.A. |
| Vasculitis | 81 (3) | N.A. |
| Polymyalgia rheumatica | 125 (4) | N.A. |
| Sjögren’s disease | 190 (6) | N.A. |
| Systemic sclerosis | 61 (2) | N.A. |
| Mixed connective tissue disease | 27 (0.9) | N.A. |
| Other rheumatic diseases | 131 (4) | N.A. |
| Disease activity—mean (SD) | ||
| RAPID-3 score | 11 (5.6) | N.A. |
| BASDAI | 4 (2.0) | N.A. |
| Immunosuppressive medication—no. (%) | ||
| No immunosuppressive medication | 679 (22) | 1098 (99.6) |
| csDMARDs | 1605 (52) | 3 (0.3) |
| Methotrexate | 1183 (38) | 1 (0.1) |
| Hydroxychloroquine | 362 (12) | 2 (0.2) |
| Sulfasalazine | 176 (6) | N.A. |
| bDMARDs | 1111 (36) | N.A. |
| TNF inhibitor | 885 (29) | N.A. |
| Tocilizumab | 34 (1) | N.A. |
| Abatacept | 55 (2) | N.A. |
| B-cell targeting therapy | 71 (2) | N.A. |
| JAK inhibitor | 32 (1) | N.A. |
| Oral glucocorticoids | 358 (12) | 1 (0.1) |
| Dose—median (IQR) | 5 (5–8) | 5 N.A. |
| COVID-19 tests—no. (%) | ||
| PCR test | 1267 (41) | 451 (41) |
| Solely negative results | 1068 (35) | 385 (35) |
| At least one positive result | 199 (7) | 66 (6) |
| RBD-Ab bridging ELISA | 2343 (76) | 862 (78) |
| Solely negative results | 2052 (67) | 742 (67) |
| At least one positive result | 291 (9) | 120 (11) |
Data are mean (SD), median (IQR) or n (%). Educational levels were based on the International Standard Classification of Education. Other rheumatic diseases included Raynaud’s disease, sarcoidosis, myositis, dermatomyositis, polymyositis, reactive arthritis, relapsing polychondritis, remitting seronegative symmetrical synovitis with pitting oedema, IgG4-mediated diseases, SAPHO syndrome, oeosinophilic fasciitis and diffuse idiopathic skeletal hyperostosis. Other bDMARDs were ustekinumab, secukinumab, anakinra, ixekizumab and sarilumab. Other csDMARDs were leflunomide, azathioprine, ciclosporin and gold injections. One person can be diagnosed with more than one autoimmune disease and receive more than one immunosuppressive drug. RAPID-3 scores were only assessed in patients with RA. BASDAI scores were only assessed in patients with AS, PsA or axial/peripheral SpA. Healthy controls treated with csDMARDs were diagnosed with lichen planopilaris, chronic discoid lupus erythematosus. Only data on educational level and race had missing values, valid percentages are shown.
AS, ankylosing spondylitis; BASDAI, Bath Ankylosing Spondylitis Disease Activity Index; bDMARDs, biological DMARDs; csDMARDs, conventional synthetic disease-modifying antirheumatic drugs; JAK, janus kinase; N.A., not applicable; PsA, psoriatic arthritis; RA, rheumatoid arthritis; RAPID-3, Routine Assessment of Patient Index Data-3; RBD-Ab, receptor binding domain antibody; SAPHO, synovitis, acne, pustulosis, hyperostosis, osteitis; SpA, spondyloarthritis; TNF, tumour necrosis factor.
rmdopen-2021-002035supp001.pdf (91.9KB, pdf)
Prevalence, symptomology and severity of COVID-19
Patient-related and COVID-19-related characteristics of study participants with a confirmed COVID-19 diagnosis (347 patients and 133 controls) are presented in tables 2 and 3. Between January and March 2021, the prevalence of SARS-CoV-2 antibodies was lower in patients compared with controls: 289 (14.7%) of 1971 patients and 119 (16.0%) of 742 controls had detectable antibodies. Symptomology of COVID-19 disease was similar for patients and controls, with the exception of dyspnoea, headaches and excessive tiredness, which were reported more frequently by patients. A minority of COVID-19 cases were hospitalised (23 (7%) of 347 patients and 1 (0.7%) of 133 controls). Three (13%) of 23 patients (one treated with methotrexate monotherapy, one treated with a TNF inhibitor concomitant with methotrexate and one patient not treated with immunosuppressive drugs) were admitted to the intensive care unit (ICU), of whom one patient died (a man aged 71 years with psoriatic arthritis and cardiovascular comorbidity). None of the healthy control subjects was admitted to the ICU and all survived. Unadjusted regression analyses demonstrated that the risk of hospitalisation was 9 times higher for patients compared with controls (OR: 9.37, 95% CI: 1.25 to 70.10, p=0.029; table 4). This ratio slightly decreased after adjusting for known risk factors for severe COVID-19 disease (age, sex and presence of comorbidities), and no longer reached statistical significance (OR: 7.33, 95% CI: 0.96 to 55.77, p=0.055; table 4).
Table 2.
Baseline characteristics of patients with rheumatic immune-mediated inflammatory diseases and healthy controls with a confirmed diagnosis of COVID-19
| Patient characteristics | Patients (n=347) | Healthy controls (n=133) |
| Age, years—mean (SD) | 56 (13) | 54 (13) |
| Female sex—no. (%) | 240 (69) | 102 (77) |
| Male sex—no. (%) | 107 (31) | 31 (23) |
| Body mass index, kg/m2—mean (SD) | 25 (5) | 25 (4) |
| Caucasian race—no. (%) | 282 (83) | 122/131 (93) |
| Educational level—no. (%) | ||
| High | 149 (45) | 68 (53) |
| Middle | 114 (35) | 39 (30) |
| Low | 67 (20) | 21 (16) |
| Current smoking status—no. (%) | 27 (8) | 2 (2) |
| Coexisting conditions—no. (%) | ||
| Cardiovascular disease | 34 (10) | 7 (5) |
| Chronic pulmonary disease | 42 (12) | 9 (7) |
| Diabetes | 14 (4) | 0 (0) |
| Obesity | 65 (19) | 14 (10) |
| COVID-19-related symptoms—no. (%) | ||
| Sore throat | 154 (44) | 54 (41) |
| Cough | 183 (53) | 66 (50) |
| Rhinorrhoea | 206 (59) | 81 (61) |
| Dyspnoea | 124 (36) | 39 (29) |
| Fever | 138 (40) | 58 (44) |
| Loss of taste or smell | 163 (47) | 69 (52) |
| Headache | 192 (55) | 59 (44) |
| Excessive tiredness | 229 (66) | 70 (53) |
| General malaise | 148 (43) | 56 (42) |
| Vomiting and/or diarrhoea | 46 (13) | 11 (8) |
| Diagnosis of COVID-19—no. (%) | ||
| PCR test | 249(72) | 95 (71) |
| Solely negative results | 50 (14) | 29 (22) |
| At least one positive result | 199 (57) | 66 (50) |
| RBD-Ab bridging ELISA | 296 (85) | 125 (94) |
| Solely negative results | 30 (9) | 9 (7) |
| At least one positive result | 291 (84) | 120 (90) |
| Disease severity of COVID-19 | ||
| Asymptomatic—no. (%) | 45 (13) | 10 (8) |
| Hospitalisation—no. (%) | 23 (7) | 1 (0.7) |
| Duration, days—median (IQR) | 10 (2–11) | 15 N.A. |
| ICU admission—no. (%) | 3 (0.9) | 0 (0) |
| Deceased—no. (%) | 1 (0.3) | 0 (0) |
Data are mean (SD), median (IQR) or n (%).
Only data on educational level and race had missing values, valid percentages are shown. Duration of hospitalisation was only assessed in patients who survived COVID-19.
ICU, intensive care unit; N.A., not available; RBD-Ab, receptor binding domain antibody.
Table 3.
Characteristics of patients with rheumatic immune-mediated inflammatory diseases: non-hospitalised versus hospitalised COVID-19 cases
| Patient characteristics | Non-hospitalised patients (n=324) | Hospitalised patients (n=23) |
| Age, years—mean (SD) | 54 (13) | 61 (11) |
| Female sex—no. (%) | 231 (71) | 9 (39) |
| Male sex—no. (%) | 93 (29) | 14 (61) |
| Body mass index, kg/m2—mean (SD) | 25 (5) | 27 (5) |
| Caucasian race—no. (%) | 266 (84) | 16 (76) |
| Educational level—no. (%) | ||
| High | 142 (46) | 7 (35) |
| Middle | 106 (34) | 8 (40) |
| Low | 62 (20) | 5 (25) |
| Current smoking status—no. (%) | 26 (8) | 1 (5) |
| Coexisting conditions—no. (%) | ||
| Cardiovascular disease | 30 (9) | 4 (17) |
| Chronic pulmonary disease | 34 (11) | 8 (35) |
| Diabetes | 10 (3) | 4 (17) |
| Obesity | 60 (19) | 5 (22) |
| Immune-mediated inflammatory disease type—no. (%) | ||
| Rheumatoid arthritis | 188 (58) | 10 (43) |
| Psoriatic arthritis | 54 (17) | 6 (26) |
| Ankylosing spondylitis | 40 (12) | 5 (22) |
| Axial or peripheral spondyloarthritis | 6 (2) | 1 (4) |
| Juvenile idiopathic arthritis | 8 (3) | 0 (0) |
| Systemic lupus erythematosus | 14 (4) | 0 (0) |
| Vasculitis | 16 (5) | 1 (4) |
| Polymyalgia rheumatica | 12 (4) | 1 (4) |
| Sjögren’s disease | 16 (5) | 0 (0) |
| Systemic sclerosis | 7 (2) | 0 (0) |
| Mixed connective tissue disease | 2 (0.6) | 0 (0) |
| Other rheumatic diseases | 17 (5) | 1 (4) |
| Disease activity—mean (SD) | ||
| RAPID-3 score | 11 (7) | 13 (6) |
| BASDAI | 4 (2.1) | 5 (1.9) |
| Immunosuppressive medication*—no. (%) | ||
| No immunosuppressive medication | 68 (21) | 5 (22) |
| csDMARDs | 176 (54) | 11 (48) |
| Methotrexate | 129 (40) | 8 (35) |
| Hydroxychloroquine | 48 (15) | 1 (4) |
| Sulfasalazine | 22 (7) | 1 (4) |
| bDMARDs | 121 (37) | 8 (35) |
| TNF inhibitor | 101 (31) | 4 (17) |
| Tocilizumab | 5 (2) | 0 (0) |
| Abatacept | 7 (2) | 0 (0) |
| B-cell targeting therapy | 4 (1) | 3 (13) |
| JAK inhibitor | 2 (0.6) | 0 (0) |
| Oral glucocorticoids | 38 (12) | 4 (17) |
| Dose—median (IQR) | 6 (5–13) | 5 (5–5) |
Data are mean (SD), median (IQR) or n (%).
*Immunosuppressive treatment at the time of SARS-CoV-2 infection. When the infection date was unknown due to lack of a PCR test result, medication use at baseline was used.
BASDAI, Bath Ankylosing Spondylitis Disease Activity Index; bDMARDs, biological DMARDs; csDMARDs, conventional synthetic disease-modifying antirheumatic drugs; JAK, janus kinase; RAPID-3, Routine Assessment of Patient Index Data-3; TNF, tumour necrosis factor.
Table 4.
Logistic regression comparing hospitalisation rates between patients with rheumatic immune-mediated inflammatory diseases and healthy controls, and different treatment groups
| Hospitalisation rate | Crude model | Adjusted model | ||||||
| No. | % | OR | 95% CI | P value | OR | 95% CI | P value | |
| Healthy controls* | 1/134 | 0.7 | 1.00 | N.A. | 1.00 | N.A. | ||
| Rheumatic patients | 23/347 | 7 | 9.37 | (1.25 to 70.10) | 0.029 | 7.33 | (0.96 to 55.77) | 0.055 |
| No treatment with csDMARDs* | 12/160 | 8 | 1.00 | N.A. | 1.00 | N.A. | ||
| Treatment with any csDMARD | 10/186 | 5 | 0.77 | (0.33 to 1.80) | 0.55 | 0.83 | (0.36 to 2.28) | 0.91 |
| Methotrexate | 8/137 | 6 | – | – | – | 1.44 | (0.39 to 5.31) | 0.59 |
| No treatment with bDMARDs* | 14/217 | 6 | 1.00 | N.A. | 1.00 | N.A. | ||
| Treatment with any bDMARD | 8/129 | 6 | 0.90 | (0.37 to 2.17) | 0.81 | 1.00 | (0.37 to 2.66) | 1.00 |
| TNF inhibitor | 4/105 | 4 | – | – | – | 0.40 | (0.11 to 1.45) | 0.16 |
| B-cell targeting therapy | 3/7 | 4 | – | – | – | 14.62 | (2.31 to 92.39) | 0.004† |
| No treatment with prednisone* | 18/304 | 6 | 1.00 | N.A. | 1.00 | N.A. | ||
| Prednisone | 2/12 | 17 | 2.74 | (0.50 to 15.03) | 0.25 | 1.73 | (0.22 to 13.83) | 0.61 |
Data are ORs with 95% CIs in parentheses, and p values. All adjusted ORs are adjusted for age, sex and presence of cardiovascular or chronic pulmonary diseases. Adjusted ORs for treatment groups are also adjusted for concomitant treatment with other immunosuppressive drugs.
Bold=p<0.050.
*Reference group.
†Below the Benjamini threshold.
bDMARDs, biological DMARDs; csDMARDs, conventional synthetic disease-modifying antirheumatic drugs; N.A., not available; TNF, tumour necrosis factor.
Table 3 compares characteristics of patients with rheumatic disease with a confirmed COVID-19 diagnosis between hospitalised and non-hospitalised patients. Hospitalised patients were older and more frequently male patients compared with non-hospitalised patients. In addition, prevalence of comorbidities, in particular chronic pulmonary disease and diabetes, was higher in hospitalised patients compared with non-hospitalised patients. The proportion of patients treated with any immunosuppressive therapy was similar for non-hospitalised and hospitalised patients, but differences between the type of immunosuppressive therapy were found. Remarkedly, hospitalised patients were more frequently treated with B-cell depleting agents compared with non-hospitalised patients (13% vs 1%), but less frequently treated with TNF inhibitors (17% vs 31%) or hydroxychloroquine (5% vs 15%). However, associations between COVID-19-related hospitalisation and immunosuppressive therapy only reached statistical significance for treatment with B-cell targeting therapy (adjusted OR: 14.62, 95% CI: 2.31 to 92.39, p=0.004; table 4).
Disease activity
Online supplemental table S1 of the appendix shows the development of rheumatic disease activity over time for patients with rheumatoid arthritis and ankylosing spondylitis. Both RAPID-3 scores in patients with rheumatoid arthritis and BASDAI scores in patients with ankylosing spondylitis were slightly higher in January–March 2021 (over a year since the start of the pandemic) compared with April–July 2020 (the first few months of the pandemic) (mean difference RAPID-3: 1.7, 95% CI: 1.4 to 2.0, p<0.0001, and mean difference BASDAI: 0.39, 95% CI: 0.21 to 0.56, p<0.0001).
Humoral immunity against COVID-19
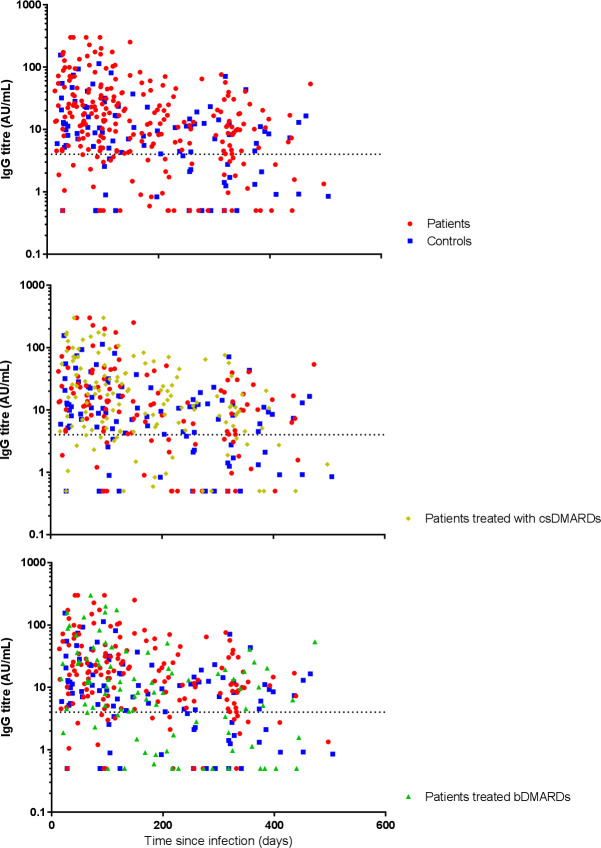
In total, 323 samples of 220 patients and 118 samples of 70 controls with a PCR-confirmed COVID-19 diagnoses were collected. Seropositivity rates for SARS-CoV-2 were comparable between patients and controls (OR: 0.88, 95% CI: 0.42 to 1.83, p=0.73; table 5), and stable over time (table 5 and online supplemental table S3). A trend towards diminished seropositivity was observed for all bDMARDs (OR: 0.38, 95% CI: 0.18 to 0.82, p=0.014), and B-cell targeting agents individually (OR: 0.10, 95% CI: 0.010 to 1.08, p=0.058). The development of IgG antibody titres over time is visualised in figure 2. Antibody titres were comparable for patients and controls (overall median (IQR) IgG titre: 12.6 AU/mL (4.5–33.6) vs 9.1 AU/mL (3.7–19.4), respectively; online supplemental table S3), independent of age, sex, time and COVID-19-related hospitalisation (adjusted ratio: 1.59, 95% CI: 0.88 to 3.02, p=0.12; table 5). However, IgG antibody titres slowly declined with time in a first-order decay (adjusted ratio per month: 0.86, 95% CI: 0.81 to 0.91, p<0.0001, table 5), and titres were higher in patients who were hospitalised compared with patients who were not hospitalised due to COVID-19 (adjusted ratio: 6.58, 95% CI: 1.76 to 24.56, p=0.005; table 5). Antibody titres were comparable for all treatment groups (online supplemental tables S2 and S3).
Table 5.
Multivariable logistic and linear regression comparing proportions of SARS-CoV-2 seropositivity and IgG antibody titres between patients with rheumatic immune-mediated inflammatory diseases and controls
| Seropositivity | IgG antibody titre | ||||||
| OR | 95% CI | P value | Beta | Ratio | 95% CI | P value | |
| Healthy controls* | 1.00 | N.A. | 0.00 | 1.00 | N.A. | ||
| Patients with rheumatic diseases | 0.88 | (0.42 to 1.83) | 0.73 | 0.49 | 1.59 | (0.88 to 3.02) | 0.12 |
| Covariables | |||||||
| Age | 0.97 | (0.95 to 1.0) | 0.040 | −0.0083 | 0.99 | (0.97 to 1.01) | 0.43 |
| Sex | |||||||
| Male* | 1.00 | N.A. | 0.00 | 1.00 | N.A. | ||
| Female | 0.83 | (0.41 to 1.66) | 0.59 | 0.30 | 1.35 | (0.75 to 2.43) | 0.32 |
| COVID-19-related hospitalisation | 0.83 | (0.41 to 1.66) | 0.67 | −1.88 | 6.58 | (1.76 to 24.56) | 0.005† |
| Time since COVID-19 in months | 0.95 | (0.88 to 1.01) | 0.10 | −0.15 | 0.86 | (0.81 to 0.91) | <0.0001† |
Seropositivity data are adjusted ORs with 95% CIs in parentheses, and p values. IgG antibody titre data are adjusted β, back-transformed β (ratio) with corresponding 95% CI in parentheses, and p values. Data on IgG antibody titre were missing for 14 (4%) of 367 samples due to shortage of serum volume.
*Reference group.
†Below the Benjamini threshold.
N.A., not available.
Figure 2.
Development of SARS-CoV-2 IgG antibody titres after primary infection over time. Y-axis: IgG antibody titres in arbitrary units (AU) per mL (AU/mL) on a logarithmically transformed scale. X-axis: time in days since date of positive PCR test result. Dotted line: cut-off value for seropositivity used in this assay (4.0 AU/mL). Red dots: all patients, blue squares: all control subjects, yellow diamonds: all patients treated with any conventional synthetic disease-modifying antirheumatic drug (csDMARD), green triangles: all patients treated with any biological DMARD (bDMARD).
Discussion
We described the results of a large-scale prospective cohort study in patients with rheumatic IMIDs and controls, which provided data with a minimised risk of bias and unique long-term data on the development of humoral immunity in patients with rheumatic IMIDs. We found that patients with rheumatic IMIDs, especially those treated with B-cell depleting therapy, were more frequently hospitalised due to COVID-19 compared with the general population. Rheumatic disease activity slightly deteriorated during the first year of the COVID-19 pandemic, but observed differences were small and below previously established thresholds of minimal clinically important differences.19 20 Lastly, we observed similar proportions of seropositivity and patterns of antibody decay for patients in all treatment subgroups and controls during the first 12 months after infection, which suggests that the development of long-lasting humoral immunity against SARS-CoV-2 is not impaired in patients with rheumatic IMIDs who are treated with csDMARDs and/or bDMARDs other than B-cell targeting agents.
Since the start of the COVID-19 pandemic, numerous studies on the incidence and severity of COVID-19 in patients with rheumatic autoimmune disease have been published. Although varying incidence rates have been observed, most studies reported similar or lower incidences for COVID-19 in patients compared with controls.21–23 These findings correspond with our own results, and are in line with our earlier observation that patients are less likely to be exposed to SARS-CoV-2 compared with the general population due to stricter adherence to social distancing measures.24 Results of studies investigating the risk of patients with rheumatic diseases to develop severe COVID-19 manifestations, such as hospital/ICU admission or death, have been contradictory as well. This can partially be explained by the fact that most data are derived from cross-sectional or retrospective cohort studies with a limited sample size that often lack a control group. These study designs have considerable methodological limitations, such as high risks of selection, misclassification and confounding bias, which generates varying results that only provide a low quality of evidence.5 National registry studies with a previously established capacity to generate high-quality data largely overcome these issues,25 although these studies are still prone to other sources of bias directly related to the COVID-19 pandemic. For example, estimated risk ratios of severe COVID-19 outcomes were often based on the total incidence of COVID-19 in patients and control subjects of a certain population, which was subsequently based on the number of registered positive PCR test results. Basing the incidence of COVID-19 on PCR test results can introduce bias, as the proportion of asymptomatic COVID-19 cases and the extent in which people have social contacts during the pandemic differ between patients and controls.24 26 This results in a different likelihood of being tested (positive) for COVID-19 for patients compared with controls, and consequently in either over- or underestimated incidence rates. Registry studies that have assessed severe COVID-19 outcomes (eg, ICU admission or death) in a population that was hospitalised due to COVID-19 avoided using these (possibly) biased incidence rates, and may therefore provide more accurate risk estimates for severe COVID-19 manifestations. These studies include a Danish and Spanish national registry study that both observed similar COVID-19-related mortality rates for patients with rheumatic diseases and the general population.3 4 This is in line with our own data, as we observed low absolute numbers of patients with rheumatic diseases that were admitted to the ICU or who died (n=3), although we were unable to estimate relative risks due to these low number and absence of controls who required ICU admission. In contrast, we observed that COVID-19-related hospitalisation rates were higher in patients compared with controls (7% vs 1%), especially in patients treated with B-cell targeting therapy (42%). However, this increased risk may partially be explained by a higher prevalence of cardiovascular and chronic pulmonary diseases in patients compared with controls, and an increased awareness of physicians towards possible vulnerable patients, such as patients with rheumatic IMIDs, who develop COVID-19 symptoms as compared with the general population.27 Therefore, our results support the current body of evidence that despite increased COVID-19-related hospitalisation rates, unfavourable COVID-19 disease outcomes such as ICU admission or death in patients with rheumatic IMIDs are rare and comparable to the general population,6 with the exception of patients treated with B-cell targeting therapy for whom risk ratios observed in our own study and previous studies are more pronounced.28 29
Long-term protective immunity against SARS-CoV-2 is generated via multiple components of the immune system, including memory CD4+ and CD8+ T cells, memory B cells and antibodies.30 The presence of SARS-CoV-2 antibodies has independently been associated with protection against reinfection,31–33 which emphasises the relevance of studying the impact of autoimmune diseases and immunosuppressive drugs on antibody development. Especially B-cell targeting agents have well-known pronounced inhibitory effects on antibody production,34 and recent studies indeed confirmed this by demonstrating reduced seroconversion rates for SARS-CoV-2 in patients treated with rituximab or ocrelizumab.7 8 We were unable to demonstrate these effects in the current dataset, but this could be explained by the small number of patients treated with B-cell targeting therapy who were infected with SARS-CoV-2, and bias in the identification process of COVID-19 cases within this patient group, as identification was for an important part based on the detection of SARS-CoV-2 antibodies. Furthermore, in line with our current data, a more severe disease course of COVID-19 has been associated with higher antibody concentrations.35 As three out of seven infected patients on B-cell targeting therapy were hospitalised, this may have further biased our results in patients treated with B-cell targeting therapy towards a seemingly proper humoral immune response. In addition to B-cell targeting agents, a recent British study in patients with inflammatory bowel disease suggested that TNF inhibitors and methotrexate may attenuate SARS-CoV-2 antibody production,9 as they observed significantly lower seroconversion rates for SARS-CoV-2 and lower IgG antibody titres 28 days after infection with SARS-CoV2 in patients treated with infliximab compared with patients treated with vedolizumab (a gut-selective anti-integrin a4b monoclonal antibody), which was even more pronounced in patients concomitantly treated with methotrexate.9 We did not observe significant associations between seropositivity rates or IgG antibody titres and immunosuppressive agents, including methotrexate and TNF inhibitors. Moreover, observed absolute differences in antibody concentrations between patients treated with those drugs and controls were much lower compared with reported differences in the study by Kennedy et al.9 This discrepancy might be explained by differences in the applied serological assay, as Kennedy et al determined the presence of antibodies against the nucleocapsid protein of SARS-CoV-2, while we measured antibodies against the receptor binding domain of the spike protein, since antibodies against the spike protein are considered to be clinically most relevant.36 Therefore, because absolute differences in antibody titres between patients treated with bDMARDs and patients treated with other drugs or healthy controls were marginal, it can be speculated that csDMARDs and bDMARDs other than B-cell targeting agents do not have clinically relevant effects on the risk of developing (severe) symptomatic COVID-19 reinfections in the first 12 months following a primary infection with SARS-CoV-2, assuming that memory T-cell and B-cell function is not impaired by these DMARDs.
Strengths and limitations
Our study has several important strengths. First, our prospective design with a digital platform allowed real-time monitoring of participants and reduced the risk of selection bias, as participants were mostly included prior to infection with SARS-CoV-2. Observational cohort studies with a prospective design are most suitable to investigate causal relationships when the conduction of a randomised controlled trial is considered unethical.37 With our platform, where we combined digital data collection with serological data collection via finger pricks, we demonstrated that conducting a large-scale prospective controlled study is feasible and highly efficient, even during a pandemic.38 Second, we included a control group that was well-matched with regard to age and sex distribution to our patients. Third, because we used a highly sensitive serological assay to screen the vast majority of our study population for the presence of SARS-CoV-2 antibodies, we were able to reliably compare the prevalence of both asymptomatic and symptomatic COVID-19 disease, as well as hospitalisation rates of COVID-19 between patients and controls. Fourth, because we followed our patients and controls since the start of the COVID-19 pandemic, we were able to collect unique long-term data on SARS-CoV-2 antibody development in patients with rheumatic IMIDs, and compare data from these patients between different immunosuppressive treatment regimens and with healthy controls. Fifth, we included a wide range rheumatic IMIDs and immunosuppressive treatment regimens, patients from all adult age groups up to 95 years, and patients with varying social economic status or racial backgrounds. This makes our patient population very heterogeneous and thus our results generalisable to the western rheumatic patient population.
However, our study also has some limitations. First, although the heterogeneity of our patient population increases the generalisability of our results, it reduces the ability to assess independent effects of disease or drug types on COVID-19 disease severity or SARS-CoV-2 antibody development. Second, effective application of infection prevention measures considerably reduced the spread of SARS-CoV-2 among the Dutch population, resulting in a relatively low sample size of infected study participants. Consequently, severe COVID-19 manifestations (hospitalisation, ICU admission and death) were infrequent, thereby limiting our ability to estimate precise effects and draw definitive conclusions. Hence, reported effect estimates for COVID-19 disease severity, especially those stratified for treatment groups, should be interpreted with caution. Third, because we collected data via digital questionnaires, reasons for loss of follow-up, which may have been related to severe COVID-19 disease, were difficult to verify. This may have introduced selection bias, especially since loss of follow-up rates were higher among patients compared with controls, but we tried to minimise this by checking patient records and actively contacting participants for verification of reasons for drop-out. Fourth, study enrolment was voluntary, and not all patients were able to find a control subject to participate in the study. This might have introduced selection bias. Lastly, longitudinal data on SARS-CoV-2 antibody concentrations were sparse, thereby limiting our ability to compare the development of antibody concentrations on the individual level.
Conclusion
We found that patients with rheumatic IMIDs, especially those treated with B-cell depleting therapy, were at increased risk of COVID-19-related hospitalisation compared with the general population, although subsequent ICU admission or death were infrequent. We did not observe associations with other immunosuppressive agents, although our power to detect smaller associations was limited due to a small number of hospitalisations. Furthermore, no clinically relevant deterioration of rheumatic disease activity was observed during the first year of the COVID-19 pandemic, despite profound changes in patient care. Lastly, patterns in decay of SARS-CoV-2 IgG antibody titres during the first 12 months after infection were similar for patients and controls, and proportions seropositivity remained high in both groups, independent of treatment with immunosuppressive agents. Treatment with csDMARDs or bDMARDs other than B-cell targeting agents are therefore unlikely to impair the development of long-term humoral immunity against SARS-CoV-2.
Acknowledgments
We thank Fabio Catarinella of Brightfish for helping us to develop the digital study platform. We would like to acknowledge Sanquin Diagnostic services for their support in logistics of the study design. We would like to thank the Nationaal Screeningslaboratorium Sanquin (NSS) for processing the finger prick envelopes. In addition, we would also like to acknowledge the support of our colleagues in the Dutch Target to B! Consortium on COVID-19 in Autoimmune Diseases. Data provided in the current manuscript have been presented at this year’s edition of the Dutch conference for rheumatology (NVR) and is accepted for a poster presentation for the ACR conference of 2021.
Footnotes
Contributors: LB wrote the manuscript. LB and FH did the statistical analyses. MS, OC, SK, JK and TR did the serological assays to measure SARS-CoV-2 antibody response. FH, EHV, YRB, ML, SA, RFvV, CAW, MG, CK, BD, SB, JJC, AV, IEvdH-B, WL, TWK, SMvH, LW, FE, LYK, PJKvD, EWS, MS, SK, OC, JK, FCL, SWT, MTN, MB, TR and GJW helped revise the manuscript for important intellectual content. GJW supervised the manuscript. LB and FH had access to and verified the underlying data. LB, FH and GJW had full access to all the data in the study and GJW had final responsibility for the decision to submit for publication. GJW is the guarantor for this paper.
Funding: ZonMw and Reade Foundation provided funding for the study.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available on reasonable request. We intend to share de-identified participant-level data on request after we have published all data on our predefined research objectives. Researchers who are interested in doing additional analyses using these data can contact the corresponding author. Data can only be used for scientific research without conflict of interests. After data collection has been completed and data on our predefined research objectives have been published, there will be no end date before which researchers can request access to the data. Additional documents that will be made available on request are the protocol (including all amendments) and informed consent forms.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s)
Ethics approval
The research protocol was approved by the medical ethical committee of the VU University Medical Center (registration number 2020.169). Participants gave informed consent to participate in the study before taking part.
References
- 1.Favalli EG, Ingegnoli F, Cimaz R, et al. What is the true incidence of COVID-19 in patients with rheumatic diseases? Ann Rheum Dis 2021;80:e18. 10.1136/annrheumdis-2020-217615 [DOI] [PubMed] [Google Scholar]
- 2.Listing J, Gerhold K, Zink A. The risk of infections associated with rheumatoid arthritis, with its comorbidity and treatment. Rheumatology 2013;52:53–61. 10.1093/rheumatology/kes305 [DOI] [PubMed] [Google Scholar]
- 3.Ayala Gutiérrez MDM, Rubio-Rivas M, Romero Gómez C, et al. Autoimmune diseases and COVID-19 as risk factors for poor outcomes: data on 13,940 hospitalized patients from the Spanish nationwide SEMI-COVID-19 registry. J Clin Med 2021;10. 10.3390/jcm10091844. [Epub ahead of print: 23 04 2021]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kjeldsen J, Nielsen J, Ellingsen T, et al. Outcome of COVID-19 in hospitalized patients with chronic inflammatory diseases. A population based national register study in Denmark. J Autoimmun 2021;120:102632. 10.1016/j.jaut.2021.102632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sauerland S, Lefering R, Neugebauer EAM. Retrospective clinical studies in surgery: potentials and pitfalls. J Hand Surg Br 2002;27:117–21. 10.1054/JHSB.2001.0703 [DOI] [PubMed] [Google Scholar]
- 6.Eular recommendations for the management of rheumatic and musculoskeletal diseases in the context of SARS-CoV-2: the July 2021 update. Available: https://www.eular.org/myUploadData/files/eular_recommendations_for_the_management_of_rheumatic_and_musculoskeletal_diseases_in_the_context_of_sars_cov_2_(07_21).pdf
- 7.Conte WL. Attenuation of antibody response to SARS-CoV-2 infection in patients with multiple sclerosis on ocrelizumab: a case-control study. Mult Scler Relat Disord 2021;52:103014. 10.1016/j.msard.2021.103014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Kempen ZLE, Strijbis EMM, Al MMCT, Al M, et al. SARS-CoV-2 antibodies in adult patients with multiple sclerosis in the Amsterdam MS cohort. JAMA Neurol 2021;78:880. 10.1001/jamaneurol.2021.1364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kennedy NA, Goodhand JR, Bewshea C, et al. Anti-SARS-CoV-2 antibody responses are attenuated in patients with IBD treated with infliximab. Gut 2021;70:865–75. 10.1136/gutjnl-2021-324388 [DOI] [PubMed] [Google Scholar]
- 10.Boekel L, Steenhuis M, Hooijberg F, et al. Antibody development after COVID-19 vaccination in patients with autoimmune diseases in the Netherlands: a substudy of data from two prospective cohort studies. Lancet Rheumatol 2021;3:e778–88. 10.1016/S2665-9913(21)00222-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vogelzang EH, Loeff FC, Derksen NI. Development of a SARS-CoV-2 total antibody assay and the dynamics of antibody response over time in hospitalized and non-hospitalized patients with COVID-19. medRxiv 2020. 10.1101/2020.06.17.20133793 [DOI] [PubMed] [Google Scholar]
- 12.Steenhuis M, van Mierlo G, Derksen NI, et al. Dynamics of antibodies to SARS-CoV-2 in convalescent plasma donors. Clin Transl Immunology 2021;10:e1285. 10.1002/cti2.1285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Biswas M, Rahaman S, Biswas TK, et al. Association of sex, age, and comorbidities with mortality in COVID-19 patients: a systematic review and meta-analysis. Intervirology 2020:1–12. 10.1159/000512592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fomin I, Caspi D, Levy V, et al. Vaccination against influenza in rheumatoid arthritis: the effect of disease modifying drugs, including TNF alpha blockers. Ann Rheum Dis 2006;65:191–4. 10.1136/ard.2005.036434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kapetanovic MC, Roseman C, Jönsson G, et al. Antibody response is reduced following vaccination with 7-valent conjugate pneumococcal vaccine in adult methotrexate-treated patients with established arthritis, but not those treated with tumor necrosis factor inhibitors. Arthritis Rheum 2011;63:3723–32. 10.1002/art.30580 [DOI] [PubMed] [Google Scholar]
- 16.Kapetanovic MC, Saxne T, Nilsson J-A, et al. Influenza vaccination as model for testing immune modulation induced by anti-TNF and methotrexate therapy in rheumatoid arthritis patients. Rheumatology 2007;46:608–11. 10.1093/rheumatology/kel366 [DOI] [PubMed] [Google Scholar]
- 17.Strangfeld A, Schäfer M, Gianfrancesco MA, et al. Factors associated with COVID-19-related death in people with rheumatic diseases: results from the COVID-19 global rheumatology alliance physician-reported registry. Ann Rheum Dis 2021;80:930–42. 10.1136/annrheumdis-2020-219498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Glickman ME, Rao SR, Schultz MR. False discovery rate control is a recommended alternative to Bonferroni-type adjustments in health studies. J Clin Epidemiol 2014;67:850–7. 10.1016/j.jclinepi.2014.03.012 [DOI] [PubMed] [Google Scholar]
- 19.Ward MM, Castrejon I, Bergman MJ, et al. Minimal clinically important improvement of routine assessment of patient index data 3 in rheumatoid arthritis. J Rheumatol 2019;46:27–30. 10.3899/jrheum.180153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kviatkovsky MJ, Ramiro S, Landewé R, et al. The minimum clinically important improvement and Patient-acceptable symptom state in the BASDAI and BASFI for patients with ankylosing spondylitis. J Rheumatol 2016;43:1680–6. 10.3899/jrheum.151244 [DOI] [PubMed] [Google Scholar]
- 21.Espinosa G, Prieto-González S, Llevadot M, et al. The impact of SARS-CoV-2 coronavirus infection in patients with systemic lupus erythematosus from a single center in Catalonia. Clin Rheumatol 2021;40:2057–63. 10.1007/s10067-021-05675-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Quartuccio L, Valent F, Pasut E, et al. Prevalence of COVID-19 among patients with chronic inflammatory rheumatic diseases treated with biologic agents or small molecules: a population-based study in the first two months of COVID-19 outbreak in Italy. Joint Bone Spine 2020;87:439–43. 10.1016/j.jbspin.2020.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yousefghahari B, Navari S, Sadeghi M, et al. Risk of COVID-19 infection in patients with rheumatic disease taking disease-modifying anti-rheumatic drugs. Clin Rheumatol 2021;40:4309–15. 10.1007/s10067-021-05779-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hooijberg F, Boekel L, Vogelzang EH, et al. Patients with rheumatic diseases adhere to COVID-19 isolation measures more strictly than the general population. Lancet Rheumatol 2020;2:e583–5. 10.1016/S2665-9913(20)30286-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmidt M, Schmidt SAJ, Sandegaard JL, et al. The Danish national patient registry: a review of content, data quality, and research potential. Clin Epidemiol 2015;7:449–90. 10.2147/CLEP.S91125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Druyan A, Lidar M, Brodavka M, et al. The risk for severe COVID 19 in patients with autoimmune and/or inflammatory diseases: first wave lessons. Dermatol Ther 2021;34:e14627. 10.1111/dth.14627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Serling-Boyd N, D'Silva KM, Hsu TY, et al. Coronavirus disease 2019 outcomes among patients with rheumatic diseases 6 months into the pandemic. Ann Rheum Dis 2021;80:660–6. 10.1136/annrheumdis-2020-219279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.FAI2R /SFR/SNFMI/SOFREMIP/CRI/IMIDIATE consortium and contributors . Severity of COVID-19 and survival in patients with rheumatic and inflammatory diseases: data from the French RMD COVID-19 cohort of 694 patients. Ann Rheum Dis 2020;80:527–38. 10.1136/annrheumdis-2020-218310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sormani MP, De Rossi N, Schiavetti I, et al. Disease-Modifying therapies and coronavirus disease 2019 severity in multiple sclerosis. Ann Neurol 2021;89:780–9. 10.1002/ana.26028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dan JM, Mateus J, Kato Y, et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science 2021;371. 10.1126/science.abf4063. [Epub ahead of print: 05 02 2021]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deng W, Bao L, Liu J, et al. Primary exposure to SARS-CoV-2 protects against reinfection in rhesus macaques. Science 2020;369:818–23. 10.1126/science.abc5343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chandrashekar A, Liu J, Martinot AJ, et al. SARS-CoV-2 infection protects against rechallenge in rhesus macaques. Science 2020;369:812–7. 10.1126/science.abc4776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cromer D, Steain M, Reynaldi A, et al. Neutralising antibody titres as predictors of protection against SARS-CoV-2 variants and the impact of boosting: a meta-analysis. Lancet Microbe 2022;3:e52–61. 10.1016/S2666-5247(21)00267-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pescovitz MD, Rituximab PMD. Rituximab, an anti-CD20 monoclonal antibody: history and mechanism of action. Am J Transplant 2006;6:859–66. 10.1111/j.1600-6143.2006.01288.x [DOI] [PubMed] [Google Scholar]
- 35.Yan X, Chen G, Jin Z, et al. Anti-SARS-CoV-2 IgG levels in relation to disease severity of COVID-19. J Med Virol 2022;94:380-383. 10.1002/jmv.27274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dispinseri S, Secchi M, Pirillo MF, et al. Neutralizing antibody responses to SARS-CoV-2 in symptomatic COVID-19 is persistent and critical for survival. Nat Commun 2021;12:2670. 10.1038/s41467-021-22958-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oria MP, Kumanyika S, National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Food and Nutrition Board; Committee on the Development of Guiding Principles for the Inclusion of Chronic Disease Endpoints in Future Dietary Reference Intakes, editors . Guiding principles for developing dietary reference intakes based on chronic disease. Washington (DC): National Academies Press (US), 2017. [PubMed] [Google Scholar]
- 38.Boekel L, Hooijberg F, Vogelzang EH, et al. Spinning straw into gold: description of a disruptive rheumatology research platform inspired by the COVID-19 pandemic. Arthritis Res Ther 2021;23:207. 10.1186/s13075-021-02574-z [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
rmdopen-2021-002035supp001.pdf (91.9KB, pdf)
Data Availability Statement
Data are available on reasonable request. We intend to share de-identified participant-level data on request after we have published all data on our predefined research objectives. Researchers who are interested in doing additional analyses using these data can contact the corresponding author. Data can only be used for scientific research without conflict of interests. After data collection has been completed and data on our predefined research objectives have been published, there will be no end date before which researchers can request access to the data. Additional documents that will be made available on request are the protocol (including all amendments) and informed consent forms.