Abstract
The public health impact of genomic screening can be enhanced by cascade testing. However, cascade testing depends on communication of results to family members. While the barriers and facilitators of family communication have been researched following clinical genetic testing, the factors impacting the dissemination of genomic screening results are unknown. Using the pragmatic Electronic Medical Records and Genomics Network-3 (eMERGE-3) study, we explored the reported sharing practices of participants who underwent genomic screening across the United States. Six eMERGE-3 sites returned genomic screening results for mostly dominant medically actionable disorders and surveyed adult participants regarding communication of results with first-degree relatives. Across the sites, 279 participants completed a 1-month and/or 6-month post-results survey. By 6 months, only 34% of the 156 re-spondents shared their results with all first-degree relatives and 4% did not share with any. Over a third (39%) first-degree relatives were not notified of the results. Half (53%) of participants who received their results from a genetics provider shared them with all first-degree relatives compared with 11% of participants who received their results from a non-genetics provider. The most frequent reasons for sharing were a feeling of obligation (72%) and that the information could help family members make medical decisions (72%). The most common reasons indicated for not sharing were that the family members were too young (38%), or they were not in contact (25%) or not close to them (25%). These data indicate that the professional returning the results may impact sharing patterns, suggesting that there is a need to continue to educate healthcare providers regarding approaches to facilitate sharing of genetic results within families. Finally, these data suggest that interventions to increase sharing may be universally effective regardless of the origin of the genetic result.
Keywords: cascade testing, communication, family, population screening, predictive genetic testing
1 ∣. INTRODUCTION
Realizing the full potential of genomic medicine depends on cascade testing of at-risk family members, which can dramatically amplify the impact of screening individuals for actionable genetic variants, especially those associated with dominant disorders (Cornel & van El, 2017). Typically, cascade testing is initiated when individuals share their genetic results with family members. Most research on how families communicate genetic risk has studied individuals affected with a disease or families with a history of the disease (Conley et al., 2020; Elrick et al., 2017; Lerman et al., 2002; Koehly et al., 2009). Similarly, prior research has examined results returned by genetics providers, who are trained to care for their patients’ families and are highly aware of the importance of cascade testing (Korf et al., 2008; Young et al., 2019). Less is known about the sharing practices of people who are found to have genetic risk through population genomic screening and may not learn about their results from genetics professionals.
Studies on clinical genetic testing have shown that while most individuals share their genetic results with at least one at-risk family member, many do not share them with all relatives (Cheung et al., 2010; Finlay et al., 2008; Tab er et al., 2015). Across studies, as many as a third of at-risk family members are not notified and therefore do not pursue testing (Baroutsou et al., 2021; Finlay et al., 2008; Patenaude et al., 2006). A number of factors have been reported to influence individuals’ tendency to share their genetic information with at-risk family members, including gender, familial relationship, culture, education level, genetic knowledge, disease severity, and treatment options (Cheung et al., 2010; Daly et al., 2016; Finlay et al., 2008; Koehly et al., 2009; Lerman et al., 2002; Lieberman et al., 2018; Makhnoon et al., 2020; Patenaude et al., 2006; Peipins et al., 2018; Shah & Daack-Hirsch, 2018; Smit et al., 2021; Stoffel et al., 2008; Young et al., 2019). How genetic results are returned, the informational content and resources provided have also been reported to impact communication with family members (Allison, 2015; Baroutsou et al., 2021; Cornel & van El, 2017; Mikat-Stevens et al., 2015; Smit et al., 2021).
Before adapting existing methods or developing novel tools aimed at enhancing cascade testing following genomic screening, it is first important to estimate the frequency of sharing following screening. It is also crucial to understand whether the factors impacting sharing practices of genomic screening results are similar to those identified in the frame of clinical genetic testing. Here, we explore the reported sharing practices of research participants who received medically actionable genetic findings identified through genomic screening. We describe motivations to share or not share results. We also explore how attributes of the participant, content of the results, and the return of results process may be associated with sharing practices.
2 ∣. METHODS
2.1 ∣. eMERGE-3 study
The Electronic Medical Records and Genomics Network (eMERGE study), funded by the National Institutes of Health, focuses on integrating genomics with electronic health records for genomic discovery and genomic medicine implementation research (Zouk et al., 2019). The third phase of eMERGE (eMERGE-3, September 2015–May 2019) implemented population genomic screening, including 58 of the 59 American College of Medical Genetics and Genomics incidental genes (Green et al., 2013), and each site chose a specific reportable list of genes and single nucleotide variants (SNVs) (Zouk et al., 2019). Approximately 25,000 participants were enrolled and underwent sequencing across ten clinical sites in the United States. There was no central institutional review board (IRB) for the eMERGE-3 study, so each site had its own IRB that reviewed and approved the study. Written consent was obtained from all participants in the study as required by all the IRBs.
2.2 ∣. Return of results in eMERGE-3
Sites offered return of actionable pathogenic or likely pathogenic (P/LP) variants to eligible participants. Some sites also returned risk variants, pharmacogenetic findings, and/or carrier status. The genes and SNVs for which results were returned varied by site, study population, and research interest (Zouk et al., 2019). Participant eligibility to receive results depended on site's study protocol. Some sites offered participants a choice about which results they wanted to receive (Hoell et al., 2020); some returned results that were already known to the participants. Results disclosure to participants was conducted by the study team, which included genetics providers (genetic counselors and medical geneticists) and non-genetics providers (primary care physicians, nurses, cardiologists, nephrologists, and trained research coordinators). In a small number of cases, this study team member was the participant's clinical provider. The information regarding who returned the result was logged in a common RedCap database developed by the eMERGE Outcomes working group. The procedure for returning results varied by site and included return by phone, secure email, patient portal, US postal mail, or face-to-face (Wiesner et al., 2020). Site-specific materials were developed to explain results, including patient letters, family letters, and other resources (Lynch et al., 2020). All participants with a positive result received a summary letter providing recommendations based on their results. These letters were site-specific, and some but not all letters discussed the importance of sharing the results with first-degree family members (Lynch et al., 2020).
2.3 ∣. Participants
Participants included in this analysis received results of P/LP variants or risk variants and completed at least one study survey. Excluded participants were those who received mosaic results or did not complete the questions of interest for this analysis. Even though 10 eMERGE-3 sites administered participant surveys, this study included six sites: Columbia University (CU), Geisinger (GE), Kaiser Permanente of Washington/University of Washington (KPW/UW), Northwestern University (NU), Mass General Brigham (MGB; formerly Partners Healthcare), and Vanderbilt University Medical Center (VUMC). Two sites were excluded as their surveys did not capture sharing results with family members (Mayo Clinic and Meharry Medical College), and two pediatric sites were excluded (Cincinnati Children's Hospital Medical Center and Children's Hospital of Philadelphia) because the survey asked the parents about their experience sharing their child's results.
2.4 ∣. Data sources
2.4.1 ∣. Participant surveys
Sites conducted surveys at approximately 1 month and/or 6 months after the return of results. All but three sites administered the survey at both time points: KPW/UW and GE did not administer a 1-month survey, and VUMC did not administer a 6-month survey (Figure S1). Surveys were conducted on paper or electronically, by mail, in person, or by telephone call, and in English or Spanish. A ‘Participant Survey subgroup’ was formed to coordinate cross-site data collection and analysis. To address the network's shared research questions, the subgroup identified and adapted existing instrument measures. These measures were incorporated into the surveys at each site independently. Some sites modified the questions to address their specific study protocols and asked additional questions specific to their research questions. The survey responses from each site were collected, and responses for network-shared questions were consolidated into a single dataset.
The shared measures included demographic information (i.e., age, race, ethnicity, educational attainment, and number of living first-degree relatives), and result sharing patterns. At 1 month after return of results, participants were asked about intention to share and actual sharing in a single question, ‘Do you plan to or have you shared your results with…?’ (i.e., mother, father, sister/s, brother/s, children). At 6 months, surveys asked about actual sharing with the question stem, ‘Have you shared your results with…?’ Participants indicated the categories and number of first-degree relatives with whom they shared their results. At 6 months, they were also asked to endorse reasons for sharing or not sharing the genetic results with their relatives.
2.4.2 ∣. Outcome forms
The eMERGE Outcomes working group identified data to be collected via manual chart review on participants with P/LP and/or risk variants (Williams et al., 2018). This information was extracted and submitted by each site. We used the information regarding the process of return of results (provider type, how the result was returned) and characteristics of the participant, including whether they were previously aware of the genetic finding and the number of living first-degree relatives if not reported in the participant survey.
2.4.3 ∣. Sequencing center data
Variant classification results were collected from the two eMERGE-3 sequencing centers (Zouk et al., 2019). The Participant Survey subgroup categorized the genes according to their associations with three main groups of diseases: cancer, cardiovascular, and other.
2.5 ∣. Statistical analysis
Descriptive statistics are presented in means, ranges, and frequencies. Participants who reported sharing with all first-degree relatives were classified as ‘ALL’. Those who shared with some but not all were classified as ‘SOME’. Those who did not share with any first-degree relatives were classified as ‘NONE’. Participants who shared with at least some first-degree relatives, but missing data did not allow for the determination of how many, were classified as ‘UNKNOWN’. Those who did not have a first-degree relative were excluded from the analysis. To identify factors potentially influencing sharing, we conducted an exploratory analysis of responses to the 6-month survey of whether and how sharing patterns reported on the 6-month survey varied across three major categories covered by the survey: attributes of the participant, the content of the results, and the return of results process between those who shared with ALL and those who did not (NONE, SOME, and UNKNOWN). However, due to the heterogeneity of the dataset and its limited size, the statistical significance of the results was not reported. Analysis was repeated excluding participants who were aware of their results prior to the study. It was also repeated for the more granular groups of ALL, NONE, SOME, and UNKNOWN, and results are in the supplemental data. Analysis was completed in SAS (SAS Institute Inc. SAS 9.4 [computer program], 2014) and R (RStudio Team, 2016).
3 ∣. RESULTS
3.1 ∣. Completion rates
Across the six sites, 766 participants received P/LP and/or risk variants and were invited to complete at least one survey post-receipt of their result. Site protocols varied in whether they administered 1-month, 6-month post-results, or both surveys. Overall, 499 participants were invited to complete a 1-month post-results survey, and 534 participants were invited to complete a 6-month post-results survey (Table S1). Of those, 267 were invited to complete both (Figure S1). The final response rate was 36% (279/766) with one or both surveys for a 42% (211/499) response rate for the 1-month survey and a 30% (158/534) rate for the 6-month survey. One-month surveys were completed between April 2018 and September 2019, and 6-month surveys were completed between September 2018 and March 2020.
3.2 ∣. Participant characteristics
Participants from NU comprised the largest proportion of the sample (33%) with other sites representing 3%–24% of the total sample (Table 1). The majority of the participants were women (66%), white non-Latinx (88%), older than 45 years of age (55%), and had a bachelor's degree or higher (59%). All but eight participants reported having at least one living first-degree relative.
TABLE 1.
Characteristics of the 279 participants who completed a 1-month or 6-month survey
| n | Proportion | |||
|---|---|---|---|---|
| Total | 279 | |||
| Demographic information | Gender | Male | 94 | 34% |
| Female | 183 | 66% | ||
| Missing | 2 | 1% | ||
| Age | Mean (range), years | 54 | (22–93) | |
| Under 45 yrs | 126 | 45% | ||
| 45 yrs and older | 153 | 55% | ||
| Race and ethnicity | White, not Latinx | 245 | 88% | |
| Other, not Latinx | 17 | 6% | ||
| Other, Latinx | 14 | 5% | ||
| Missing | 3 | 1% | ||
| Education | High school or less | 26 | 9% | |
| Some college | 44 | 16% | ||
| Bachelors | 73 | 26% | ||
| Graduate or postgraduate | 92 | 33% | ||
| Missing | 44 | 16% | ||
| Reported living relative(s) | Father | 179 | 64% | |
| Mother | 185 | 66% | ||
| Sister | 174 | 62% | ||
| Brother | 173 | 62% | ||
| Children | 194 | 70% | ||
| At Least one relative | 271 | 97% | ||
| Knew of results prior to study | Yes | 68 | 24% | |
| No | 191 | 68% | ||
| Unknown | 20 | 7% | ||
| Return of results format | Enrollment site | GE | 56 | 20% |
| CU | 68 | 24% | ||
| NU | 91 | 33% | ||
| MGB | 15 | 5% | ||
| KPW/UW | 8 | 3% | ||
| VUMC | 41 | 15% | ||
| Return of results method | Letter/portal/email | 32 | 11% | |
| Phone | 135 | 48% | ||
| Face-to-face | 70 | 25% | ||
| Missing | 37 | 13% | ||
| Who returned the result | Genetics provider | 152 | 54% | |
| Non-genetics provider | 104 | 37% | ||
| 23 | 8% | |||
| Information about family risk in summary letters | Yes | 256 | 92% | |
| No | 23 | 8% | ||
| Result type | Result category | Cancer | 158 | 57% |
| Cardiovascular | 88 | 32% | ||
| Other | 33 | 12% | ||
| Variant type (most deleterious) | Pathogenic/likely pathogenic | 229 | 82% | |
| Risk factor | 50 | 18% | ||
| Survey | Time of response | One-month survey only | 121 | 43% |
| One-month and 6-month survey | 90 | 32% | ||
| Six-month survey only | 68 | 24% |
A total of 162 unique variants (158 P/LP and 4 risk variants) in 46 genes were returned to the 279 participants included in these analyses (Table S2). P/LP variants in the BRCA2, CHEK2, and LDLR genes, and the I1307K risk variant in the APC gene each represented approximately 10% of the variants returned. Twelve participants had two variants returned. The majority of participants received results related to cancer risk (56%), followed by cardiovascular risk (32%), and the remaining received risk information for other types of disorders (12%) such as MC4R obesity risk or homozygous hemochromatosis. A quarter of participants were aware of their genetic results prior to participation in the study (24%, Table 1). Half of the participants received their results by phone (48%) and 25% had a face-to-face appointment. A third (37%) received their results from non-genetics providers. Most participants received a letter that discussed family member risk (92%).
3.3 ∣. Sharing patterns on the 1-month survey
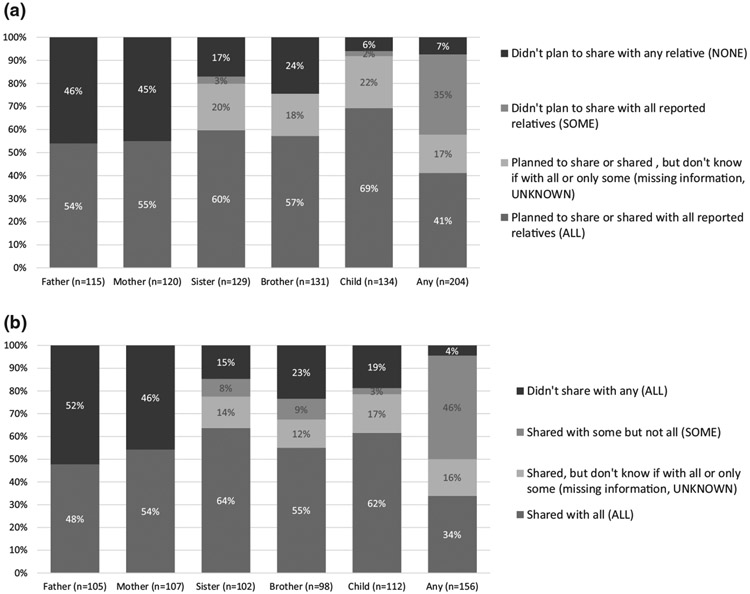
Among the 211 participants who completed the 1-month survey, 204 reported having on average 4 relatives (max 17) and sharing with an average of 3 relatives (max 12). Of the total 939 first-degree relatives, participants reported planning to share or have shared with 569 (61%) relatives and not sharing with 370 (39%). Of those who reported having at least one living first-degree relative, a small majority reported planning to share, or having shared, the results with all their living sisters and brothers (60% of 129 with sisters and 57% of 131 with brothers) and all their living children (69% of 231 with children; Figure 1a). About half of participants with a living parent reported planning to share or having shared the results with one or both parents (55% of 107 with their mother and 54% of 105 with their father), and 28% reported they had not shared or were not planning to share with any parent. Overall, 41% of the participants planned to share, or had shared, their results with ALL first-degree relatives and 35% with SOME (Table S3). Only 7% participants reported that they had not shared or did not plan to share with any first-degree relative and 17% that they shared, or were planning to, but it is UNKNOWN with how many. After excluding the 54 participants who were aware of their genetic results prior to participation, a smaller proportion shared or planned to share with ALL (35% versus 41%) and a larger proportion shared or planned to share with SOME (40% versus 35%).
FIGURE 1.
(a) Reported planned and actual sharing patterns with first-degree relatives by relative type on the 1-month survey for the 204 who reported a first-degree relative. (b) Reported sharing patterns with first-degree relatives by relative type on the 6-month survey for the 154 who reported a first-degree relative
3.4 ∣. Sharing patterns on the 6-month survey
Among the 158 participants who completed the 6-month survey, 156 reported having on average 5 first-degree relatives (max 15) and sharing with an average of 3 relatives (max 10). Of the total 811 first-degree relatives, participants reported sharing with 488 (60%) relatives and not sharing with 323 (40%). A small majority of them reported sharing the results with all their living sisters and brothers (64% of the 102 with sisters and 55% of the 98 with brothers) and their living children (62% of the 112 with children; Figure 1b). About half of participants with a living parent reported sharing results with the parent (54% of the 107 with their mother and 48% of the 105 with their father), and 36% reported they had not shared with any parent. Overall, a third (34%) of the participants shared their results with ALL first-degree relatives, almost half shared with SOME but not all first-degree relatives (45%), and 16% were UNKNOWN (Table S3). A small minority, 4% (n = 7), reported not sharing their genetic results with any first-degree relative. No commonalities were observed among these seven participants. They included three men and four women, ages 45–85 years. They were from multiple sites and received different types of results in different ways from both genetics and non-genetics providers. After excluding the 43 participants who were aware of their genetic results prior to participation, a smaller proportion of participants shared with ALL (24% versus 34%) and a larger proportion shared with SOME (51% versus 45%).
3.5 ∣. Concordant reports at 1 month and 6 months after the return of results
A total of 90 participants completed both a 1-month survey and a 6-month survey (Table 1, Figure S1). The reported sharing on the 6-month survey was concordant with the planned and reported sharing on the 1-month survey (71% answered the same on both surveys; Table S4). Twelve participants responded that they planned to share with all first-degree relatives on the 1-month survey but responded that they had not shared with all on the 6-month survey (13%), and seven had not planned or not yet shared with all at 1 month but had shared with all at the 6-month survey (8%). The other seven participants had missing data on one of the two surveys so the responses could not be compared (8%).
3.6 ∣. Reasons for sharing or not sharing
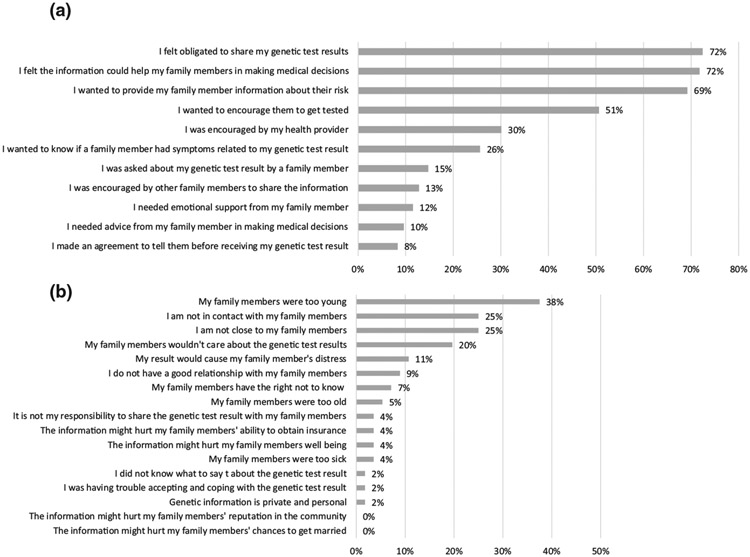
On the 6-month survey, participants were asked to identify one or more reasons for sharing. Of the 149 participants who responded to these questions, the two most frequent reasons for sharing were feeling ‘obligated to share’ (72%) and that ‘the information could help family members make medical decisions’ (72%; Figure 2a). A similar number of participants expressed a desire to provide their ‘family members with information about their risk’ (69%). Half of participants reported sharing the results ‘to encourage family members to have testing’ (51%). Only a third reported ‘encouraged by their health care provider’ as a reason to share their results (30%).
FIGURE 2.
(a) Participants who provided one or more responses to the question, ‘Thinking about the family members you DID share your genetic test result with, which of the following were important to your decision to share?’ (n = 149 who provided at least one response). (b) Participants who provided one or more responses to the question, ‘If you did not disclose your genetic test results to SOME or ALL of your family members, which of the following were reasons for NOT sharing?’ (n = 56 who provided at least one response)
Participants were also asked about reasons for not sharing. Of the 56 participants who responded, the most common responses were ‘my family members were too young’ (38%), ‘I am not in contact with my family members’ (25%), and ‘I am not close to my family members’ (25%, Figure 2b).
3.7 ∣. Sharing patterns by participants’ characteristics, type of results received, and mode of return of results
Because the study protocols varied across sites and therefore potential sources of confounding were variable, we did not include p-values for the following comparisons. We observed no difference in the frequency of sharing between the groups by gender or race/ethnicity (Table 2). Participants younger than 45 years and participants with a college degree or greater more frequently reported sharing with ALL than those who were older or had lower education levels (48% versus 28% and 43% versus 16%, respectively). Participants who had been aware of their results prior to receiving them from the study also more frequently reported having shared with ALL compared with those who first learned about the results during the study (60% versus 24%). We observed no difference between the groups when examined by disease category of result or type of variant (Table 3).
TABLE 2.
Reported sharing on the 6-month survey by demographics of the participant
| Total |
Did not share with all (SOME/ UNKNOWN/ NONE, n = 103) |
Shared with ALL (n = 53) |
|||
|---|---|---|---|---|---|
| n | n | % (row) | n | % (row) |
|
| Male | 56 | 38 | 68% | 18 | 32% |
| Female | 99 | 64 | 65% | 35 | 35% |
| Missing data | 1 | 1 | 0 | ||
| Less than 45 | 46 | 24 | 52% | 22 | 48% |
| 45 or older | 110 | 79 | 72% | 31 | 28% |
| White, non-Latinx | 142 | 95 | 67% | 47 | 33% |
| Not white, non-Latinx | 7 | 7 | 100% | 0 | 0% |
| Missing data | 7 | 1 | 6 | ||
| Less than college degree | 49 | 41 | 84% | 8 | 16% |
| College degree or more | 105 | 60 | 57% | 45 | 43% |
| Missing data | 2 | 0 | 2 | ||
| Did not know prior to study | 110 | 84 | 76% | 26 | 24% |
| Knew prior to study | 43 | 17 | 40% | 26 | 60% |
| Missing data | 3 | 2 | 1 | ||
Note: ALL: Shared with all first-degree relatives, SOME: shared with some but not all, UNKNOWN: shared but do not know number, NONE: did not share.
TABLE 3.
Reported sharing on the 6-month survey by characteristics of the results
| Total |
Did NOT share with all (SOME/ UNKNOWN/ NONE, n = 103) |
Shared with ALL (n = 53) |
|||
|---|---|---|---|---|---|
| n | n | % (row) | n | % (row) |
|
| Pathogenic/likely pathogenic | 138 | 91 | 66% | 47 | 34% |
| Risk factor | 18 | 12 | 67% | 6 | 33% |
| Cancer | 90 | 54 | 60% | 36 | 40% |
| Cardiac | 48 | 36 | 75% | 12 | 25% |
| Other | 18 | 13 | 72% | 5 | 28% |
Note: ALL: shared with all first-degree relatives, SOME: shared with some but not all, UNKNOWN: shared but do not know number, NONE: did not share.
Participants who received their results from a genetics provider more frequently reported sharing with ALL than those who received their results from other providers (52% versus 11%; Table 4). Most sites included discussion of family risk in the summary letters, and sharing with ALL was higher among participants who received letters with this information than those who did not (39% versus 5%). Only one of the 21 participants who did not receive a letter discussing family risk reported sharing with ALL. We repeated all analyses after excluding the 43 participants who knew their results prior and observed the same patterns (data not shown Table S5a-c). We also repeated the analysis with those who did not share with all broken into the more granular categories of did not share with any, shared with some but not all, shared but do not know number compared with those who share with all. The patterns were similar to those observed in the main analysis (Table S6a-c)
TABLE 4.
Reported sharing on the 6-month survey by how the results were returned
| Total |
Did NOT share with all (SOME/ UNKNOWN/ NONE, n = 103) |
Shared with ALL (n = 53) |
|||
|---|---|---|---|---|---|
| n | n | % (row) |
n | % (row) |
|
| By genetics provider | 87 | 42 | 48% | 45 | 52% |
| By non-genetics provider | 62 | 55 | 89% | 7 | 11% |
| Missing data | 7 | 6 | 1 | ||
| Letter/portal/email | 2 | 0 | 0% | 2 | 100% |
| Face-to-face | 60 | 49 | 82% | 11 | 18% |
| Phone | 79 | 39 | 49% | 40 | 51% |
| Missing data | 15 | 13 | 2 | ||
| Family risk in | 135 | 83 | 61% | 52 | 39% |
| summary letter | |||||
| Family risk not discussed in summary letter | 21 | 20 | 95% | 1 | 5% |
Note: ALL: shared with all first-degree relatives, SOME: shared with some but not all, UNKNOWN: shared but do not know number, NONE: did not share.
4 ∣. DISCUSSION
This study examines family communication practices following receipt of genomic screening results in a non-phenotype-driven research setting, as opposed to individuals undergoing genetic testing because of a personal or familial indication. Most participants reported sharing or planning to share their results with at least one first-degree relative, an important first step in facilitating cascade testing. However, sharing varied across relative types and over a third of the first-degree relatives were not notified of the results. We observed concordance between participants’ responses on the 1-month and 6-month surveys, suggesting that the decision regarding sharing was made shortly after learning about the results. Interestingly, participants who received their results from a genetics provider more frequently shared an association not previously studied.
Over half of the participants did not share results with all first-degree relatives. When asked reasons for not sharing, two of the most common responses were that they were not in contact or not close with their first-degree relative(s), which could indicate family estrangement, a known barrier to sharing (Finlay et al., 2008; Lieberman et al., 2018; Patenaude et al., 2006). In agreement with studies on genetic testing, planned and reported sharing were not homogeneous for all types of family members (Patenaude et al., 2006). Participants shared more frequently with their siblings, regardless of gender, and their children than with their living parents. While the genetic result may have limited medical relevance for aging parents, not informing parents could significantly reduce the impact of broader cascade testing, as parental testing can be critical for informing risk of more genetically and socially distant relatives. Interestingly, we did not observe variation in sharing practices based on gender or type of test results, though prior literature has suggested that these factors can influence sharing (Finlay et al., 2008; Hamilton et al., 2016; Lieberman et al., 2018; Patenaude et al., 2006). This may reflect differences between sharing screening results and clinical genetic test results.
As we consider scaling genomic medicine while maintaining quality of care, we need to better understand the impact of the method of returning results on sharing practices. It is common practice for genetics providers to recommend that patients share their own result letters with family members, or to provide patients with a family letter written specifically for at-risk family members (van den Nieuwenhoff et al., 2006). Such letters communicate the risk of the family members and available resources for cascade testing. Provision of these types of materials enables family-mediated communication while ensuring that the information that is shared is medically accurate. The overwhelming majority of the participants in this study received a letter that recommended sharing results with family members. Those who received such a letter more frequently shared with all first-degree relatives than those who did not—pointing to the importance of conveying this information in a written format. Interestingly, only one participant who did not receive such a letter shared the information with all his first-degree relatives. On the contrary, the reduced sharing observed among participants with less than a college degree may be associated with the complexity of the letters. The letters distributed across the network had an average 10th-grade reading level and length of 660 words (Lynch et al., 2020). Studies have also found that lengthy letters can result in anxiety and confusion, overwhelm individuals, and be a barrier to family communication (Brown et al., 2016; Dheensa et al., 2018; Hodgson et al., 2016; Montgomery et al., 2013; Roggenbuck et al., 2015). Future research accounting for patients’ literacy and the complexity of the summary letter or other resources will provide greater clarity on how to effectively facilitate family communication and cascade testing.
The healthcare provider returning the result can choose to highlight the importance of sharing result information with family members, though not all providers have received specific training to do so. Participants who received their results from a genetics provider more frequently shared their results with all first-degree relatives than those who received their results from a non-genetics provider. Genetics providers are more familiar, comfortable, and likely more consistent in discussing cascade family testing. Beyond recommending sharing of the results, genetics providers are trained to provide guidance and resources about how to share with family members and to facilitate cascade testing (Korf et al., 2008). These practices are less familiar to non-genetic providers (Cornel & van El, 2017; Mikat-Stevens et al., 2015). These results suggest that the importance of encouraging cascade testing should be one of the foci when teaching providers how to disclose genetic results.
Disclosure of results via phone is frequently used in clinical practice, and other studies suggested that it is an effective mode of results communication (Beri et al., 2019; Bradbury et al., 2018; Christensen et al., 2018). We observed even greater sharing when results were disclosed by phone compared with in-person sessions. The small sample prevented a multivariable analysis on the interaction between phone and type of provider returning the result or prior knowledge of the genetic result. Other studies have found no significant differences in recall, and satisfaction between those receiving the results in person or by phone (Beri et al., 2019; Bradbury et al., 2018; Christensen et al., 2018). In one study, when given a choice between in person, telephone, or letter for results disclosure, a minority of individuals preferred an in-person disclosure (O’Shea et al., 2016). The impact of the disclosure modality on family sharing has not previously been studied. However, these studies and our observations suggest that phone counseling may not be a barrier in communicating the importance of family sharing.
4.1 ∣. Limitations
Family communication is only the first step in cascade testing, and the eMERGE-3 study did not have access to actual genetic testing of family members. The data are based on self-reported survey responses, which can be affected by recall bias, completion bias, or misunderstanding of survey questions; however, it enabled collection of information across healthcare systems and did not depend on access to the family members. Survey questions were not validated, and the format of the surveys varied across sites as each site administered survey to their participants. Survey questions were limited to who they shared with and why they did or did not shared. The survey did not capture other factors that may have impacted sharing such as family dynamics. The modest response rate to the surveys might be due to changes in contact information, and some participants may not have received the invitation to complete the survey and those who did not respond to the surveys may have had different sharing practices. Even though the analysis was also limited as the 1-month survey lack of distinction between ‘having shared’ and ‘planning to share’, we observed very similar trends at 1 and 6 months, suggesting that the decision on sharing was taken shortly after the reception of the genetic result. The consortium enabled to collect information on a relatively large number of participants from different areas in the United States and using different healthcare systems. However, as each site returned the results differently, provided site-specific written materials, and some returned other results’ types, including carrier and pharmacogenetics, we elected to restrict the study to descriptive analysis of the data. Prior knowledge of the results, or a known family history of the condition, probably also confounded the results as we observed greater sharing with ALL in those who knew previously. Though when we repeated the analysis excluding participants who knew their results prior to this study, we observed the same patterns in sharing based on participant characteristics, result type, and how the result was disclosed.
4.2 ∣. Future research
More research is needed to identify the methods of return of genetic results most effective in prompting cascade testing. Randomized controlled trials comparing different approaches and analyzing testing uptake by family members are therefore desirable (Baroutsou et al., 2021). Inclusion of a diverse study sample and limited-resource facilities is needed to ensure greater generalizability of findings. In addition, a better understanding of the barriers preventing individuals from sharing their results with their living parents will be central to the expansion of cascade testing to second-degree family members. Finally, research into individuals’ expectations from the healthcare system to support family sharing could help developing policies and potentially amending the laws currently limiting the healthcare system role in cascade testing.
4.3 ∣. Practice implications
There is a need for scalable strategies to assist individuals in sharing genomic results with family members. Healthcare providers, both genetics and non-genetics providers, need to be educated on the importance of cascade testing including engaging patients in a conversation about how to communicate the results to their relatives (Burns et al., 2018) Additional resources, such as template materials discussing the importance of familial sharing or video education modules, may also aid them in this discussion with their patients. With a goal of familial sharing with all first-degree relatives, providers should discuss potential barriers to sharing with their patients and provide resources to overcome them, including strategies in cases of familial estrangement.
5 ∣. CONCLUSIONS
By and large, participants receiving genomic screening results shared them with at least one of their relatives, but over a third of at-risk first-degree relatives were not made aware of their risk. Sharing patterns, proportion of relatives with whom results were shared, and motivations to share or not share were consistent with prior literature on sharing practices after clinical genetic testing. Those similarities suggest that interventions to increase sharing may be universally effective regardless of the origin of the genetic result.
Supplementary Material
What is known about this topic?
The full potential of genomic screening relies on sharing of results among family members. While the factors impacting sharing after clinical genetic testing have been studied, there are little data on sharing following genomic screening.
What this paper adds?
Individuals share their genomic screening research results with some but not all at-risk relatives, as was reported regarding sharing of clinical genetic test results. Return of these results by genetics providers may be associated with increased sharing of genomic screening results compared with return by non-genetics providers.
ACKNOWLEDGMENTS
We would like to thank the participants in the eMERGE Network. This phase of the eMERGE Network was initiated and funded by the NHGRI through the following grants: U01HG008657 (Group Health Cooperative/University of Washington); U01HG008685 (Brigham and Women's Hospital); U01HG008672 (Vanderbilt University Medical Center); U01HG008666 (Cincinnati Children's Hospital Medical Center); U01HG006379 (Mayo Clinic); U01HG008679 (Geisinger Clinic); U01HG008680 (Columbia University Irving Medical Center); U01HG008684 (Children's Hospital of Philadelphia); U01HG008673 (Northwestern University); U01HG008701 (Vanderbilt University Medical Center serving as the Coordinating Center); U01HG008676 (Partners Healthcare/Broad Institute); U54MD007593-10 (Meharry Medical College); and U01HG008664 (Baylor College of Medicine).
Footnotes
CONFLICT OF INTEREST
Julia Wynn, Hila Milo Rasouly, Tania Vasquez-Loarte, Robyn Weiss, Robyn Weiss, Sonja I. Ziniel, Ellen Wright Clayton, Kurt D. Christensen, David Fasel, Robert C. Green, Heather S. Hain, Margaret Harr, Christin Hoell, Iftikhar J Kullo, Kathleen A. Leppig, Melanie F. Myers, Joel E. Pacyna, Emma F. Perez, Cynthia A. Prows, Alanna Kulchak Rahm, Gemme Campbell-Salome, Richard R. Sharp, Maureen E Smith, Georgia L. Wiesner, Janet L. Williams, Carrie L. Blout Zawatsky, Ali G. Gharavi, Wendy K. Chung, Ingrid A. Holm, Akilan M. Saami, and Paul S. Appelbaum declare that they have no conflict of interest.
HUMAN STUDIES AND INFORMED CONSENT
Approval to conduct this human subjects research was obtained by the Columbia University (CU), Geisinger (GE), Kaiser Permanente of Washington/University of Washington (KPW/UW), Northwestern University (NU), Mass General Brigham (MGB; formerly Partners Healthcare), and Vanderbilt University Medical Center (VUMC) individual institutional review boards. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000. Informed consent was obtained from all participants for being included in the study.
ANIMAL STUDIES
No non-human animal studies were carried out by the authors for this article.
SUPPORTING INFORMATION
Additional supporting information may be found in the online version of the article at the publisher’s website.
DATA AVAILABILITY
Raw de-identified data are available on request to Julia Wynn jw2500@cumc.columbia.edu.
REFERENCES
- Allison M (2015). Communicating risk with relatives in a familial hypercholesterolemia cascade screening program: A summary of the evidence. Journal of Cardiovascular Nursing, 30(4), E1–E12. 10.1097/JCN.0000000000000153 [DOI] [PubMed] [Google Scholar]
- Baroutsou V, Underhill-Blazey ML, Appenzeller-Herzog C, & Katapodi MC (2021). Interventions facilitating family communication of genetic testing results and cascade screening in hereditary breast/ovarian cancer or lynch syndrome: A systematic review and meta-analysis. Cancers, 13(4), 925. 10.3390/cancers13040925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beri N, Patrick-Miller LJ, Egleston BL, Hall MJ, Domchek SM, Daly MB, Ganschow P, Grana G, Olopade OI, Fetzer D, Brandt A, Chambers R, Clark DF, Forman A, Gaber R, Gulden C, Horte J, Long J, Lucas T, … Bradbury AR (2019). Preferences for in-person disclosure: Patients declining telephone disclosure characteristics and outcomes in the multicenter Communication Of GENetic Test Results by Telephone study. Clinical Genetics, 95(2), 293–301. 10.1111/cge.13474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury AR, Patrick-Miller LJ, Egleston BL, Hall MJ, Domchek SM, Daly MB, Ganschow P, Grana G, Olopade OI, Fetzer D, Brandt A, Chambers R, Clark DF, Forman A, Gaber R, Gulden C, Horte J, Long JM, Lucas T, … Yao X(S (2018). Randomized noninferiority trial of telephone vs in-person disclosure of germline cancer genetic test results. Journal of the National Cancer Institute, 110(9), 985–993. 10.1093/jnci/djy015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown E, Skinner M, Ashley S, Reed K, & Dixon SDL (2016). Assessment of the readability of genetic counseling patient letters. Journal of Genetic Counseling, 25(3), 454–460. 10.1007/s10897-015-9890-0 [DOI] [PubMed] [Google Scholar]
- Burns C, James CA, & Ingles J (2018). Communication of genetic information to families with inherited rhythm disorders. Heart Rhythm, 15(5), 780–786. 10.1016/J.HRTHM.2017.11.024 [DOI] [PubMed] [Google Scholar]
- Cheung EL, Olson AD, Yu TM, Han PZ, & Beattie MS (2010). Communication of BRCA results and family testing in 1,103 high-risk women. Cancer Epidemiology Biomarkers and Prevention, 19(9), 2211–2219. 10.1158/1055-9965.EPI-10-0325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen KD, Uhlmann WR, Roberts JS, Linnenbringer E, Whitehouse PJ, Royal CDM, Obisesan TO, Cupples LA, Butson MB, Fasaye G-A, Hiraki S, Chen CA, Siebert U, Cook-Deegan R, & Green RC (2018). A randomized controlled trial of disclosing genetic risk information for Alzheimer disease via telephone. Genetics in Medicine, 20(1), 132–141. 10.1038/gim.2017.103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley CC, Ketcher D, Reblin M, Kasting ML, Cragun D, & Kim J et al. (2020). The big reveal: Family disclosure patterns of BRCA genetic test results among young Black women with invasive breast cancer. Journal of Genetic Counseling. John Wiley and Sons Inc. 10.1002/jgc4.1196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornel MC, & van El CG (2017). Barriers and facilitating factors for implementation of genetic services: A public health perspective. Frontiers in Public Health, 5, 195. 10.3389/fpubh.2017.00195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly MB, Montgomery S, Bingler R,& Ruth K (2016).Communicating genetic test results within the family: Is it lost in translation? A survey of relatives in the randomized six-step study. Familial Cancer, 15(4), 697–706. 10.1007/s10689-016-9889-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dheensa S, Lucassen A, & Fenwick A (2018). Limitations and pitfalls of using family letters to communicate genetic risk: A qualitative study with patients and healthcare professionals. Journal of Genetic Counseling, 27(3), 689–701. 10.1007/s10897-017-0164-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elrick A, Ashida S, Ivanovich J, Lyons S, Biesecker BB, Goodman MS, & Kaphingst KA (2017). Psychosocial and clinical factors associated with family communication of cancer genetic test results among women diagnosed with breast cancer at a young age. Journal of Genetic Counseling, 26(1), 173–181. 10.1007/S10897-016-9995-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay E, Stopfer JE, Burlingame E, Evans KG, Nathanson KL, Weber BL et al. (2008). Factors determining dissemination of results and uptake of genetic testing in families with known BRCA1/2 mutations. Genetic Testing, 12(1), 81–91. 10.1089/gte.2007.0037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green RC, Berg JS, Grody WW, Kalia SS, Korf BR, Martin CL, McGuire AL, Nussbaum RL, O’Daniel JM, Ormond KE, Rehm HL, Watson MS, Williams MS, & Biesecker LG (2013). ACMG recommendations for reporting of incidental findings in clinical exome and genome sequencing. Genetics in Medicine, 15(7), 565–574. 10.1038/gim.2013.73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton JG, Shuk E, Arniella G, González CJ, Gold GS, Gany F, Robson ME, & Hay JL (2016). Genetic testing awareness and attitudes among latinos: exploring shared perceptions and gender-based differences. Public Health Genomics, 19(1), 34–46. 10.1159/000441552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgson J, Metcalfe S, Gaff C, Donath S, Delatycki MB, Winship I, Skene L, Aitken MA, & Halliday J (2016). Outcomes of a randomised controlled trial of a complex genetic counselling intervention to improve family communication. European Journal of Human Genetics, 24(3), 356–360. 10.1038/ejhg.2015.122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoell C, Wynn J, Rasmussen LV, Marsolo K, Aufox SA, Chung WK, Connolly JJ, Freimuth RR, Kochan D, Hakonarson H, Harr M, Holm IA, Kullo IJ, Lammers PE, Leppig KA, Leslie ND, Myers MF, Sharp RR, Smith ME, & Prows CA (2020). Participant choices for return of genomic results in the eMERGE Network. Genetics in Medicine, 22(11), 1821–1829. 10.1038/s41436-020-0905-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes C, Lerman C, Schwartz M, Peshkin BN, Wenzel L, Narod S, Corio C, Tercyak KP, Hanna D, Isaacs C, & Main D (2002). All in the family: Evaluation of the process and content of sisters’ communication about BRCA1 and BRCA2 genetic test results. American Journal of Medical Genetics, 107(2), 143–150. 10.1002/ajmg.10110 [DOI] [PubMed] [Google Scholar]
- Koehly LM, Peters JA, Kenen R, Hoskins LM, Ersig AL, Kuhn NR, Loud JT, & Greene MH (2009). Characteristics of health information gatherers, disseminators, and blockers within families at risk of hereditary cancer: Implications for family health communication interventions. American Journal of Public Health, 99(12), 2203–2209. 10.2105/AJPH.2008.154096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korf BR, Ledbetter D, & Murray MF (2008). Report of the Banbury Summit Meeting on the evolving role of the medical geneticist, February 12–14 (2006). Genetics in Medicine. Nature Publishing Group, 10.1097/GIM.0b013e31817701fe [DOI] [PubMed] [Google Scholar]
- Lieberman S, Lahad A, Tomer A, Koka S, BenUziyahu M, Raz A, & Levy-Lahad E (2018). Familial communication and cascade testing among relatives of BRCA population screening participants. Genetics in Medicine, 20(11), 1446–1454. 10.1038/gim.2018.26 [DOI] [PubMed] [Google Scholar]
- Lynch J, Sharp R, Aufox S, Bland S, Blout C, Bowen D, Buchanan A, Halverson C, Harr M, Hebbring S, Henrikson N, Hoell C, Holm I, Jarvik G, Kullo I, Kochan D, Larson E, Lazzeri A, Leppig K, … Williams J (2020). Understanding the return of genomic sequencing results process: Content review of participant summary letters in the eMERGE research network. Journal of. Personalized Medicine, 10(2), 38. 10.3390/jpm10020038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makhnoon S, Bowen DJ, Shirts BH, Fullerton SM, Meischke HW, Larson EB, Ralston JD, Leppig K, Crosslin DR, Veenstra D, & Jarvik GP (2021). Relationship between genetic knowledge and familial communication of CRC risk and intent to communicate CRCP genetic information: Insights from FamilyTalk eMERGE III. Translational Behavioral Medicine, 11(2), 563–572. 10.1093/tbm/ibaa054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikat-Stevens NA, Larson IA, & Tarini BA (2015). Primary-care providers’ perceived barriers to integration of genetics services: A systematic review of the literature. Genetics in Medicine, 17(3), 169–176. 10.1038/gim.2014.101 [DOI] [PubMed] [Google Scholar]
- Montgomery SV, Barsevick AM, Egleston BL, Bingler R, Ruth K, Miller SM, Malick J, Cescon TP, & Daly MB (2013). Preparing individuals to communicate genetic test results to their relatives: Report of a randomized control trial. Familial Cancer, 12(3), 537–546. 10.1007/s10689-013-9609-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Shea R, Meany M, Carroll C, Cody N, Healy D, Green A, & Lynch SA (2016). Predictive genetic testing and alternatives to face to face results disclosure: A retrospective review of patients preference for alternative modes of BRCA 1 and 2 results disclosure in the Republic of Ireland. Journal of Genetic Counseling, 25(3), 422–431. 10.1007/s10897-015-9887-8 [DOI] [PubMed] [Google Scholar]
- Patenaude AF, Dorval M, DiGianni LS, Schneider KA, Chittenden A, & Garber JE (2006). Sharing BRCA1/2 test results with first-degree relatives: Factors predicting who women tell. Journal of Clinical Oncology, 24(4), 700–706. 10.1200/JCO.2005.01.7541 [DOI] [PubMed] [Google Scholar]
- Peipins LA, Rodriguez JL, Hawkins NA, Soman A, White MC, Hodgson ME, DeRoo LA, & Sandler DP (2018). Communicating with daughters about familial risk of breast cancer: Individual, family, and provider influences on women’s knowledge of cancer risk. Journal of Women’s Health, 27(5), 630–639. 10.1089/jwh.2017.6528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roggenbuck J, Temme R, Pond D, Baker J, Jarvis K, Liu M, Dugan S, & Mendelsohn NJ (2015). The long and short of genetic counseling summary letters: A case–control study. Journal of Genetic Counseling, 24(4), 645–653. 10.1007/s10897-014-9792-6 [DOI] [PubMed] [Google Scholar]
- RStudio Team (2016). RStudio: Integrated Development for R. http://www.rstudio.com/ [Google Scholar]
- SAS Institute Inc (2014). 9.4 [computer program]. SAS Institute Inc. [Google Scholar]
- Shah LL, & Daack-Hirsch S (2018). Family Communication About Genetic Risk of Hereditary Cardiomyopathies and Arrhythmias: An Integrative Review. Journal of Genetic Counseling, 27(5), 1022. 10.1007/S10897-018-0225-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit AK, Bartley N, Best M, Napier C, Butow P, Newson A, Tucker K, Ballinger M, Thomas D, Jacobs C, Meiser B, Goldstein D, Savard J, & Juraskova I (2021). Family communication about genomic sequencing: A qualitative study with cancer patients and relatives. Patient Education and Counseling, 104(5), 944–952. 10.1016/J.PEC.2020.10.022 [DOI] [PubMed] [Google Scholar]
- Stoffel EM, Ford B, Mercado RC, Punglia D, Kohlmann W, Conrad P, Blanco A, Shannon KM, Powell M, Gruber SB, Terdiman J, Chung DC, & Syngal S (2008). Sharing genetic test results in lynch syndrome: Communication with close and distant relatives. Clinical Gastroenterology and Hepatology, 6(3), 333–338. 10.1016/j.cgh.2007.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taber JM, Chang CQ, Lam TK, Gillanders EM, Hamilton JG, & Schully SD (2015). Prevalence and correlates of receiving and sharing high-penetrance cancer genetic test results: Findings from the health information national trends survey. Public Health Genomics, 18(2), 67–77. 10.1159/000368745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Nieuwenhoff HWP, Mesters I, Nellissen JJTM, Stalenhoef AF, & de Vries NK (2006). The importance of written information packages in support of case-finding within families at risk for inherited high cholesterol. Journal of Genetic Counseling, 15(1), 29–40. 10.1007/s10897-005-9001-8 [DOI] [PubMed] [Google Scholar]
- Wiesner GL, Kulchak Rahm A, Appelbaum P, Aufox S, Bland ST, Blout CL, Christensen KD, Chung WK, Clayton EW, Green RC, Harr MH, Henrikson N, Hoell C, Holm IA, Jarvik GP, Kullo IJ, Lammers PE, Larson EB, Lindor NM, … Leppig KA (2020). Returning results in the genomic Era: Initial experiences of the eMERGE network. Journal of Personalized Medicine, 10(2), 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J, Chung W, Fedotov A, Kiryluk K, Weng C, Connolly J, Harr M, Hakonarson H, Leppig K, Larson E, Jarvik G, Veenstra D, Hoell C, Smith M, Holm I, Peterson J, & Williams M (2018). Harmonizing outcomes for genomic medicine: Comparison of eMERGE outcomes to clingen outcome/intervention pairs. Healthcare, 6(3), 83. 10.3390/healthcare6030083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young AL, Butow PN, Rhodes P, Tucker KM, Williams R, Healey E, & Wakefield CE (2019). Talking across generations: Family communication about BRCA1 and BRCA2 genetic cancer risk. Journal of Genetic Counseling, 28(3), 516–532. 10.1002/JGC4.1055 [DOI] [PubMed] [Google Scholar]
- Zouk H, Venner E, Lennon NJNJ, Muzny DMDM, Abrams D, Adunyah S et al. (2019). Harmonizing Clinical Sequencing and Interpretation for the eMERGE III. Network, 105(3), 588–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw de-identified data are available on request to Julia Wynn jw2500@cumc.columbia.edu.