Abstract
Objectives
Few studies reported COVID-19 cases in schools during the 2020/21 academic year in a setting of uninterrupted in-person schooling. The main objective was to determine the SARS-CoV-2 seroprevalence among school staff in Vancouver public schools.
Design
Cumulative incident COVID-19 cases among all students and school staff based on public health data, with an embedded cross-sectional serosurvey among a school staff sample that was compared to period, age, sex and geographical location-weighted data from blood donors.
Setting
Vancouver School District (British Columbia, Canada) from kindergarten to grade 12.
Participants
Active school staff enrolled from 3 February to 23 April 2021 with serology testing from 10 February to 15 May 2021.
Main outcome measures
SARS-CoV-2 seroprevalence among school staff, based on spike (S)-based (unvaccinated staff) or N-based serology testing (vaccinated staff).
Results
Public health data showed the cumulative incidence of COVID-19 among students attending in-person was 9.8 per 1000 students (n=47 280), and 13 per 1000 among school staff (n=7071). In a representative sample of 1689 school staff, 78.2% had classroom responsibilities, and spent a median of 17.6 hours in class per week (IQR: 5.0–25 hours). Although 21.5% (363/1686) of surveyed staff self-reported close contact with a COVID-19 case outside of their household (16.5% contacts were school-based), 5 cases likely acquired the infection at school based on viral testing. Sensitivity/Specificity-adjusted seroprevalence in 1556/1689 staff (92.1%) was 2.3% (95% CI: 1.6% to 3.2%), comparable to a sex, age, date and residency area-weighted seroprevalence of 2.6% (95% CI: 2.2% to 3.1%) among 5417 blood donors.
Conclusion
Seroprevalence among staff was comparable to a reference group of blood donors from the same community. These data show that in-person schooling could be safely maintained during the 2020/21 school year with mitigation measures, in a large school district in Vancouver, Canada.
Keywords: COVID-19, public health, paediatrics, epidemiology
Strengths and limitations of this study.
Largest Canadian study in one of the few, if not only, jurisdiction in North America that maintained in-person schooling during the entire 2020–2021 school year.
Reference data from entire school population, and robust reporting of seroprevalence based on accurate serology testing on a representative staff sample.
Non-random participant selection, implying that a selection bias cannot be entirely excluded, although it is unlikely based on comparison of school staff sample with entire population.
Limited power to detect small increase in seroprevalence over representative community reference group of blood donors.
Study predates the emergence of most variants of concerns in Canada, including the delta variant.
Introduction
SARS-CoV-2 forced over a billion students out-of-school globally in the Spring of 2020. Decisions to close schools, motivated by high case mortality in populations, had serious implications for children’s emotional, social, physical and educational outcomes.1 The risk of secondary SARS-CoV-2 transmission within schools has been heavily debated. On the one hand, data support low rates of in-school SARS-CoV-2 transmission,2–12 with little increased transmission when schools re-opened.13–18 On the other hand, few studies have accounted for asymptomatic transmission using antibody testing, and most have reported data early in the pandemic, or in the setting of partial school closure.14 19–21
In the spring of 2020, British Columbia (BC) health authorities ordered a cessation of in-person schooling provincially, with a transition to remote learning from home. Like most of the world, the province went under a nearly complete lockdown between March and early June 2020 when most sectors of the economy were paused. In the global context, BC and Canada observed relatively low incident COVID-19 cases compared with other areas of the world.22 While BC reported relatively low community transmission, roughly >50 times more cases were reported between 1 July 2020 and 15 May 2021 (136 291 cases in a population of 5 017 000), compared with the first pandemic wave from February to May 2020.23 Despite increasing cases in late summer, BC was unique within Canada in that it maintained in-person schooling for the entire duration of the 2020/21 school year starting 8 September 2020, except for regular winter (18 December 2020 to 4 January 2021) and spring (12 March to 29 March 2021) breaks.
The main goal of this study was to determine the SARS-CoV-2 seroprevalence in school staff in Vancouver public schools during the 2020/21 school year. The secondary objective was to compare the seroprevalence in school staff with a reference population of matched Canadian blood donors.
Materials and methods
Study design
This study used baseline, cross-sectional data from a prospective study collected by questionnaire among active school staff of the Vancouver School District (the District) between 10 February and 15 May 2021, with blood samples for serology testing collected from the same school staff between 10 February to 15 May 2021, and serology data obtained between 1 January and 31 May 2021 from Canadian blood donors, weighted for age, sex and geographical area of residency.
Participants
School staff self-enrolled from 3 February to 23 April 2021 after receiving an introduction email from school principals from the District in early February 2021, inviting them to register online at: https://www.bcchr.ca/COVIDatschools, for both a questionnaire and to provide blood for serology testing. A flyer was posted on the District website, and reminder emails were also sent. Interested participants completed a screener to identify whether they met eligibility criteria. Staff were included if they were a current, full or part-time staff member (confirmed by District email address). Staff who reported being temporary staff, on-leave or on-call with no classroom time, or working exclusively in an adult education setting were ineligible.
Study setting
The District is a large, urban school district with 89 elementary schools and 18 secondary schools (47 280 students and 7071 school staff) located in the city of Vancouver (BC, Canada ~600 000 population in the city of Vancouver with a population of ~2.6 million in urban area). Following a complete closure in March 2020, schools opened in a limited fashion, except for students who use English as a second language and those with complex learning needs who were able to attend in-person 5 days/week until 30 June 2020. On 8 September 2020, schools reopened for the 2020/21 school year, except for a regularly scheduled winter break from 18 December 2020 to 4 January 2021, and spring break from 12 to 29 March 2021. COVID-19 mitigations measures implemented in District schools as well as indications for viral testing are detailed in online supplemental appendix 1.
bmjopen-2021-057846supp001.pdf (1.1MB, pdf)
Data collection
To estimate the degree of exposure to known COVID-19 cases, we obtained data from Vancouver Coastal Health (VCH)’s Case and Contact Management Interface. To this end, the District provided student and staff lists attending the District as of 17 May 2021, to VCH, which linked the data to determine the cumulative incidence of COVID-19 cases among all students and staff in District schools (excluding the adult education staff). Staff and students affiliated with Vancouver Alternate Secondary School programmes were counted as attending a single school for the purposes of incidence calculations.
To compare the COVID-19 case data from VCH to the data from the prospective school staff sample obtained via questionnaires, we selected the median date of questionnaire completion (ie, 4 March 2021) as the end date for VCH COVID-19 cases data extraction. We extracted all lab-confirmed, probable and epidemiologically linked COVID-19 cases reported to VCH. To assess the cumulative incidence of known infection among staff over the course of the pandemic, we calculated the incidence of reported staff cases from 15 January 2020 (corresponding to the first case reported to VCH) to 4 March 2021. Similarly, exposure to student cases during the school year was assessed using the incidence of confirmed, probable or epidemiologically linked COVID-19 cases from the beginning of the school year (8 September 2020) to 4 March 2021. Data from (smaller size) school annexes were combined to their corresponding attachment schools, as long as the school staff was shared between the two, for a total of 77 elementary and 18 secondary schools in the analysis.
Data were collected from the school staff sample using a questionnaire that asked, among others, about risk factors for COVID-19, household structure, physical distancing behaviour, close contact with COVID-19 cases (defined by asking: “someone diagnosed with COVID-19 with whom you’d been within two meters of for greater than two min”), history of viral testing (including dates and symptoms) and vaccination, etc.24 Analyses on risk factors for COVID-19 and data from a second questionnaire about mental health and vaccine perception are not reported in this paper. For blood donors, we only had access to age, sex, first 3 digits of postal code of residence and COVID-19 vaccination status at the time of blood donation using questionnaires administered by Canadian Blood Services as part of the routine donation process.
Serology testing
Blood samples were collected at clinics set-up in multiple participating Vancouver schools geographically dispersed across the District, at the BC Children’s Hospital or outpatient clinical laboratories in the Vancouver area. The presence of antibodies against SARS-CoV-2 was used as a marker of prior COVID-19 infection, using dual S-based and N-based serology testing, where S-based serology was used in unvaccinated participants and N-based serology testing was used with vaccinated participants, or for blood donors in whom we did lack reliable data on vaccination status (online supplemental figure 1). Vaccines used in Canada elicit a spike (S) antibody response, whereas natural infection elicits both an S and a nucleocapsid (N) response. Thus, N responses can be used to determine if a participant has had prior infection regardless of vaccination status.
Antibodies directed against the spike (S1) protein were detected using the Ortho T VITROS Anti-SARS-CoV-2 Total antibody assay (Ortho Clinical Diagnostics, Rochester, New York, USA), a Health Canada and FDA-licensed qualitative assay which detects IgA, IgG and IgM antibodies. S-based serology testing was done on a Vitros 5600 analyser at the BC Children’s & Women’s Hospital Laboratory, which is accredited for clinical testing. Literature and in-house validation demonstrated this assay can identify both symptomatic and asymptomatic infected individuals >7 days postillness onset with a sensitivity between 90.7% and 97.7%, and specificities between 99.4% and 100%.25 26 Specimens were considered reactive at a cut-off index ≥1.00. All S-tested negative samples with S-antibody indexes >99th centile were also confirmed to be negative on the Roche assay. Testing for antinucleocapsid (N) protein SARS-CoV-2 antibodies was performed using the Roche Elecsys Anti-SARS-CoV-2 (Roche Diagnostics Canada, Laval, Quebec, Canada). This qualitative total antibody assay is Health Canada-licensed and Food and Drug Administration-licensed with reported sensitivity of 88.5%–100% at least 14 days post-COVID-19 onset and specificity of 99.8%–100%.27–29 Testing was performed on a Cobas e601 analyzer at St. Paul’s Hospital Laboratory.
Blood donors were screened prior to donation, to ensure they were in good health. People were ineligible to donate blood if they had a recent COVID-19 infection 2 weeks after symptoms resolved, or were hospitalised within 3 weeks before. Blood donors were tested for N antibodies using the Roche Elecsys Anti-SARS-CoV-2 assay on a Cobas e801 analyzer (Roche Diagnostics Canada, Laval, Quebec, Canada). Sensitivity of 98.8% and specificity of 99.6% were used for the nucleocapsid-based Roche Elecsys Anti-SARS-CoV-2 assay. N antibodies have been shown to persist in blood after infection with assay sensitivity maintained until at least a year postinfection.30
Bias minimisation strategies
A number of measures were taken to facilitate/encourage participation: (i) strong buy-in from schools (see ‘Patient and public involvement’ section); (ii) easy participation: blood collection sites were set-up in schools over lunch and after work, in four geographically dispersed, centrally located area within the Vancouver School District, to ensure that the blood collection was readily accessible to participants. Other blood collection sites also included partnerships with hundreds of private community clinics in Vancouver (open on weekends), the St. Paul’s Hospital (high sampling volume, located in downtown Vancouver) and the British Columbia Children’s Hospital (located west of the District); (iii) facilitation on the ground: we hired a full-time study coordinator to maintain contact and answer emails 7 days per week, and ensure a smooth study flow, facilitate bookings at blood collection sites with flexible hours, etc (including driving around the city to meet the few participants who were unable to attend the multiple blood clinics); (iv) participant incentives: participants were offered a CAD$20 incentive and serology results were returned to them.
Patient and public involvement
Right from the study design stage, District leaders, teacher and student support worker and parent associations were engaged to obtain support and seek feedback on study feasibility. Weekly meetings occurred from study launching until publication of findings with a District leadership representative (Collette O'Reilly) and a District liaison (Kathy O’Sullivan) to adjust study advertisement and procedures to maximise recruitment. At the end, results were shared immediately, initially with study participants, followed by BC and Canadian Public Health and government authorities, and health providers and experts from other Canadian provinces.
Statistical analyses
In absence of data available at the time on COVID-19 transmission in schools, our initial sample size was set based on an anticipated increase in seroprevalence compared with earlier phases of the pandemic. We estimated that 2410 school staff would achieve 80% power to detect a 2.2-fold increase in prevalence estimates available from April to June 2020.31 The Rogan-Gladen estimator was used to calculate the true prevalence adjusting for test specificity and sensitivity, with 95% CIs estimated using Blaker’s method.32 For the school staff, sensitivity of 95.3% and specificity of 100% were used for the S-based assay,26 33 ignoring the small proportion of N-based assays used for outcome classification. For the blood donors, data were weighted by collection month, postal code, sex and age (online supplemental data). Uncertainty of the serology tests was approached incorporating the uncertainty in test parameters using a Bayesian approach with no meaningful changes to 95% CIs (not shown). All analyses were done on complete cases.
Data statement
De-identified data will be made available upon written request through the COVID-19 Immunity Task Force.
Results
District-wide COVID-19 exposure from students
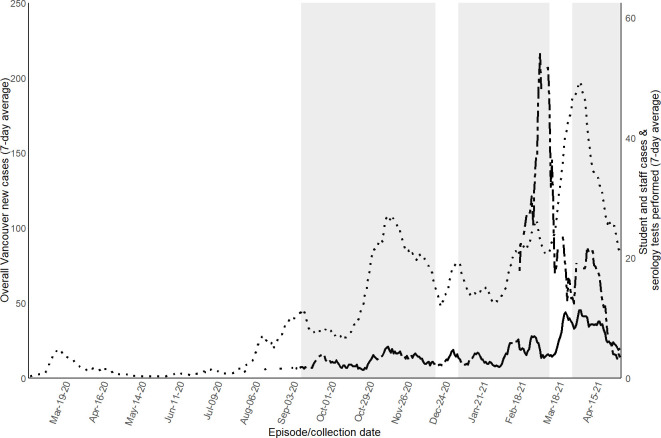
During the 2020/21 school year (September 2020 to June 2021), 46 879 students attended District schools in-person and 401 students attended an alternate District school (total 47 280 students). As shown in figure 1, overall weekly rates of reported COVID-19 cases among staff and students during the pandemic followed a trend similar to the weekly rates among Vancouver residents. Population-level cumulative incidence of COVID-19 cases among students (total of 47 280 students) during the 2020/21 school year was 9.8 cases per 1000 students (median 8.2; range 0–63 cases per 1000 between schools). Each school had between 0 and 36 cases. Twelve schools had zero student cases.
Figure 1.
Weekly reported COVID-19 cases among all school staff and students of the Vancouver School District, compared with all Vancouver residents. Seven-day average of new SARS-CoV-2 cases among Vancouver residents and dates of serology collection. Median date of completion of questionnaire was 4 March. Dotted line shows total weekly Vancouver resident COVID-19 cases. Plain line shows total weekly cases among all students and staff from the Vancouver School District. Dashed line shows cumulative weekly serology tests performed among school staff sample. Grey background denotes when public school was in session.
District-wide COVID-19 cumulative incidence among school staff
The cumulative incidence of COVID-19 cases from 15 January 2020 to 4 March 2021 among the 5091 classroom school staff was 13 cases per 1000, and was 14 cases per 1000 among 1980 other and non-classroom staff (online supplemental table 1). When looking at COVID-19 cases since the beginning of the pandemic, 54 of 95 schools had no staff COVID-19 cases, with a maximum of 3 staff cases per school. The cumulative incidence of COVID-19 cases among 4.5% staff members assigned to more than one school was 21 per 1000 staff.
Characteristics of school staff sample
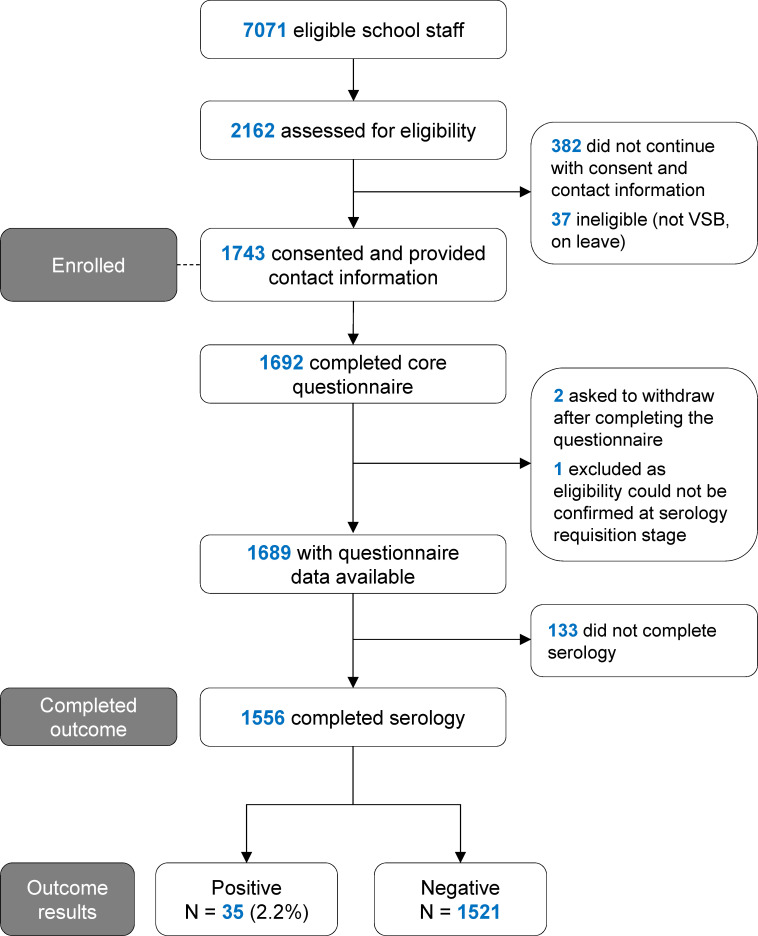
In total, 2162 school staff accessed the initial study screening website, of which 1743 staff provided contact information and consented for serology testing (figure 2). The characteristics of 1689 staff who completed the questionnaire are shown in table 1. This corresponds to 23.9% of all eligible staff. The age and sex of school staff in the sample were representative of the District population (mean age±SD: 47.4±11.2 years; 69.5% female (76.2% female among classroom staff); n=6751 with data available). The proportion of school staff sampled for serology was evenly distributed among low vs high COVID-19 incidence schools (online supplemental figure 2). The residency distribution of the school staff sample was also geographically similar to the District population (online supplemental data).
Figure 2.
Flow diagram for enrollment of school staff study sample. VSB: Vancouver School Board.
Table 1.
Baseline characteristics of school staff sample
| Variable | N* | Completed questionnaire (n=1689) | N* | Completed serology testing (n=1556) |
| Age (mean±SD) | 1684 | 45.4±10.4 | 1556 | 45.7±10.3 |
| Sex, % female, n (%) | 1681 | 1355 (80.6%) | 1550 | 1257 (81.1%) |
| Canadians of indigenous origin, n (%) | 1688 | 31 (1.8%) | 1555 | 31 (2.0%) |
| Ethnicity, n (%) | 1689 | 1556 | ||
| White, Caucasian | 1175 (69.6%) | 1084 (69.7%) | ||
| South Asian | 65 (3.9%) | 57 (3.7%) | ||
| Chinese | 277 (16.4%) | 257 (16.5%) | ||
| Black | 12 (0.7%) | 12 (0.8%) | ||
| Filipino | 35 (2.1%) | 33 (2.1%) | ||
| Latin American | 26 (1.5%) | 26 (1.7%) | ||
| Arab | 4 (0.2%) | 3 (0.2%) | ||
| Southeast Asian | 32 (1.9%) | 27 (1.7%) | ||
| West Asian | 1 (0.1%) | 1 (0.1%) | ||
| Korean | 11 (0.7%) | 9 (0.6%) | ||
| Japanese | 39 (2.3%) | 36 (2.3%) | ||
| Other/No answer | 62 (3.7%) | 57 (3.7%) | ||
| Classroom workers†, n (%) | 1688 | 1320 (78.2%) | 1555 | 1212 (77.9%) |
| Contact time with students (hours/week), median (IQR) | 1684 | 17.6 (5.0–25.0) | 1552 | 17.5 (4.6–25.0) |
| School level, n (%) | 1689 | 1556 | ||
| Elementary | 1076 (63.7%) | 992 (63.8%) | ||
| Secondary | 474 (28.1%) | 436 (28.0%) | ||
| Work at multiple levels | 55 (3.3%) | 48 (3.1%) | ||
| School district office only | 84 (5.0%) | 80 (5.1%) | ||
| No. of people living in household, median (IQR) | 1685 | 3 (2–4) | 1552 | 3 (2–4) |
| Living with an essential worker in household, n (%) | 1671 | 619 (37.0%) | 1541 | 565 (36.7%) |
| At least one comorbidity‡, n (%) | 1689 | 409 (24.2%) | 1556 | 379 (24.4%) |
| Smoker, n (%) | 1686 | 46 (2.7%) | 1553 | 41 (2.6%) |
| Travelled outside BC since 1 January 2020, n (%) | 1687 | 278 (16.5%) | 1554 | 252 (16.2%) |
*N with data available.
†Those who reported being a teacher, teacher librarian, resource teacher, student support worker or family and youth worker in response to the question: “What is your job title? (teacher, teacher librarian, resource teacher, student support worker, family and youth worker, administrator (principal, vice principal), administrative assistant, maintenance staff, school board office staff, other)”.
‡Any the following: hypertension, diabetes, asthma, chronic lung disease, chronic heart disease, chronic kidney disease, liver disease, cancer, chronic blood disorder, immunosuppressed, chronic neurological disorder.
BC, British Columbia.
A total of 78.2% (n=1320) of our sample were classroom staff who spent a median of 17.6 hours of contact time with students per week (table 1). In comparison, the District estimated that 71.9% (n=5091) of the 7071 eligible staff, had classroom responsibilities. Notably, the distribution of the school staff sample between elementary and secondary schools (table 1), as well as the distribution of occupations of the school staff sample also reflected all staff in the District (online supplemental table 2).
About one-third (37%) lived with an essential worker, predominantly in the social services, education/research/healthcare, construction, maintenance and skilled trades and food sectors (table 2). In total, 363 (21.5%) school staff reported close contact with a COVID-19 case at or outside school, including 51 who reported close contact with a COVID-19 case in their household (table 2).
Table 2.
Reported COVID-19 exposures and PCR outcomes among school staff
| Variable | N* | Completed questionnaire (n=1689) |
| COVID-19-like symptoms†, n (%) | 1688 | 664 (39.3%) |
| Number tested for COVID-19 (PCR), n (%) | 1688 | 760 (45.0%) |
| At least one positive COVID-19 viral test | 24 (1.4%) | |
| More than one positive COVID-19 viral test | 1 (0.01%) | |
| All negative COVID-19 viral test | 715 (42.4%) | |
| Did not know/Could not remember test result | 21 (1.2%) | |
| Hospitalised for COVID-19, n (%) | 1683 | 3 (0.2%) |
| Type of occupation for essential worker living in household, n (%) | 1671 | 619 (37.0%) |
| Agriculture and food production | 7 (0.4%) | |
| Community services (sewage and water treatment, waste disposal) | 10 (0.6%) | |
| Construction, maintenance, skilled trades | 77 (4.6%) | |
| Consumer products (hardware, safety, vehicle, sales, garden centres) | 9 (0.5%) | |
| Financial services (banking, real estate, insurance) | 19 (1.1%) | |
| Food (grocery, convenience, liquor, restaurant) | 67 (4.0%) | |
| Healthcare | 99 (5.9%) | |
| Social services, education, research | 244 (14.6%) | |
| Manufacturing, resources, energy, utilities | 21 (1.3%) | |
| Services (pharmacy, gas station, delivery, funeral, vet, etc) | 13 (0.8%) | |
| Sports (professional) | 0 | |
| Supply chain and transportation | 19 (1.1%) | |
| Telecommunications and IT (including the media) | 16 (1.0%) | |
| Other | 84 (5.0%) | |
| COVID-19 case among other household members‡, % yes, n (%) | 1688 | 51 (3.0%) |
| Reported close contact with a COVID-19 case outside household (within 2 m and for >2 min), n (%) | 1686 | 363 (21.5%) |
| Another school staff member/work colleague | 133 (7.9%) | |
| Student in classroom setting | 145 (8.6%) | |
| Family (non-household member) | 46 (2.7%) | |
| Friend | 84 (5.0%) | |
| Unknown | 26 (1.5%) | |
| Wear a mask in public places§, % always or often, n (%) | 1685 | 1677 (98.5%) |
| Co-workers wear masks§, % always or usually, n (%) | 1682 | 1635 (97.2%) |
| Students wear masks§, % always or usually, n (%) | ||
| Elementary | 1058 | 359 (33.9%) |
| Secondary | 465 | 431 (92.7%) |
| Completed serology testing, n (%) | 1689 | 1556 (92.1%) |
*N with data available.
†Any of the following: headache, cough, fever, sore throat, shortness of breath, sore muscles, diarrhoea, decrease sense of smell (specify period). “Did you have any of the following symptoms between January 2020 and present?”
‡"Has anyone in your household (not counting yourself) ever tested positive for COVID-19? ((yes), (not applicable, I live alone), (no one has been tested), (no, they tested negative), (not sure, waiting for the result))”.
§Questions about masking were as follows: “How often have you worn a mask in public places in the past 3 months? (never, rarely, occasionally, often, always)”; “To the best of your knowledge, how often do your co-workers wear a mask in your presence? (never, occasionally, usually, always)”; “To the best of your knowledge, how often do students in your school wear a mask in your presence? (never, occasionally, usually, always)”.
SARS-CoV-2 prevalence in school staff sample by viral testing
Only 24 self-reported having had COVID-19 based on nucleic acid amplification tests, for a cumulative incidence of 1.4% of school staff (table 2). Of the 24 school staff who reported a positive viral test, 4 (16.7%) tested positive prior to the beginning of classes in September 2020. Five (21%) reported that the most likely source of infection was a close contact with a student or co-worker case, including one who required hospitalisation during the 2020/21 school year. Seven (29%) reported close contact with a friend or family member with COVID-19, and one reported close contact with both a co-worker and family member with COVID-19. Eleven had no known source of exposure and were not aware of any close contact with a COVID-19 case.
SARS-CoV-2 seroprevalence in school staff sample by serology
Of 1689 school staff who completed the prospective questionnaire, 1556 completed serology testing (median blood collection date: 11 March 2021). In total, 35 tested positive for SARS-CoV-2 by serology. Therefore, this corresponded to 46% more infections diagnosed by serology compared with infections diagnosed by viral testing.
Thirty-five staff (2.2%) of the 1556 school staff who completed serology were vaccinated at the time of blood testing. Individual serology results are shown in online supplemental tables 3 and 4 for vaccinated and SARS-CoV-2-infected staff, respectively. Accounting for vaccination status, 35 school staff had a serology profile indicative of a previous COVID-19 infection (online supplemental figure 1). Of the 35 school staff who had a positive serology indicative of infection, 29 worked in a classroom setting and one did not work in a classroom setting, but reported >20 hours of contact time with students per week. The proportion of staff who tested positive for SARS-CoV-2 by serology between secondary and elementary schools (table 3) was similar to the proportion of staff in each school level (table 1).
Table 3.
Seropositive cases according to school education level where school staff teaches/assists
| School | Frequency | Cases (%) |
| Elementary | 19 | 54.3 |
| Secondary | 9 | 25.7 |
| Multiple/Mixed | 3 | 8.6 |
| School board office | 4 | 11.4 |
| Total | 35 | 100 |
Among the school staff sample, the unadjusted prevalence was 2.2% (95% CI: 1.6% to 3.1%), and the seroprevalence adjusted for the sensitivity and specificity of the test was 2.3% (95% CI: 1.6% to 3.2%). In comparison, the adjusted seroprevalence among 5417 blood donors was 2.6% (95% CI: 2.2% to 3.1%). Importantly, the postal code area distribution of the school staff sample closely matched the age, sex, period and residency location-weighted blood donor data (online supplemental figure 3).
Poststratification seroprevalence analyses
The proportion of females overall was slightly higher in the school sample compared with the District population. However, the seroprevalence in the school sample was similar, 2.6% (95% CI: 1.9% to 3.6%), after poststratifying for sex. Additionally, if we had sampled equally among schools, the poststratification seroprevalence would be 2.5% (95% CI: 1.8% to 3.5%) and not statistically different than the original estimate in the school staff sample presented above of 2.2% (95% CI: 1.6% to 3.1%).
Discussion
This study found that the seroprevalence among staff in Vancouver public schools was relatively low after a period of widespread community transmission predating the emergence of variants of concerns. Results were consistent with COVID-19 cases reported by VCH. Findings are in keeping with modelling studies34 35 and data from the UK where low seroprevalence was also measured in teachers, but this was earlier in the pandemic.14 To the best of our knowledge, this study is the largest Canadian study, and one of the largest overall, to report seroprevalence estimates in the context of continuously maintained in-person schooling and widespread viral transmission late in the 2020/21 academic year. Despite that the seroprevalence in this study was approximately threefold higher relative to previous estimates of 0.55%–0.6% obtained from Vancouver residents in spring 2020,31 36 37 it remained comparable to the community, as determined from blood donors of the same age, sex and living in the same community.
Another study reported SARS-CoV-2 seroprevalence in the school setting in North America.38 A major advantage of the current study is that it was conducted in BC, one of the few jurisdictions in North America that maintained in-person schooling during the 2020/21 school year. About one-quarter of the ~132 444 COVID-19 cases reported in BC during this period were located in the regional health authority where the city of Vancouver and its District are located. Study results are drawn from a large sample of staff, including a majority of those exposed to COVID-19 in the classroom. The study used sensitive serology testing to identify cumulative SARS-CoV-2 cases that may have not come to clinical attention, but could still contribute to the transmission chain.39 The use of S-based serology assays identified COVID-19 cases up to a year before. Conversely, the N-based serology test allowed us to assess for infections in vaccinated staff towards the end of recruitment.
The high proportion (60%) of cases diagnosed by viral testing who also tested positive via serology contrasts with a recent review finding that on average the ratio of antibody to viral detection of cases was up to over 18.40 Our findings would suggest good access to viral testing in this specific setting, during the study period.
Among our study participants, 21.5% (363) of school staff reported a close contact with a COVID-19 case, and the majority (76.6%, 278/363) identified contact with a COVID-19 case at school. These data alone could reinforce the perception that schools are a risky environment. However, despite the high frequency of school staff who reported close contacts, and symptoms (table 2), 90.1% (598/664) had no serological evidence of infection using a sensitive testing strategy. Thus, we were able to provide a more accurate depiction of actual viral infections. In light of these data we could not find evidence to substantiate the perception that a large number of asymptomatic infections have been missed through contact tracing.
Mitigation strategies employed in BC schools have been shown elsewhere to minimise risk in educators to a level comparable to the risk in the community.41 42 Although non-medical masks were encouraged, but were not required for students in schools in BC until February 2021 (grades 8–12) and end of March 2021 (grades 4–12), we did not observe any difference in seroprevalence between elementary and secondary school staff. Of note all school staff from the District were required to mask indoors (which is reflected in our survey results) and this intervention has been associated with lower risk of infection.41
This study has limitations. First, non-random participant selection among the school staff population implies a potential volunteer bias. However, the similar demographic characteristics, and the similar incidence of COVID-19 cases among the school staff sample (1.4%) compared with the entire District (1.3%) suggests that we did not undersample those at risk. Second, blood donors are healthier and therefore, may not be a reliable estimate of community seroprevalence, though there are likely representative of school staff compared with other socioeconomic-deprived populations at higher risk of COVID-19.43 44 Effectively, underestimation of the seroprevalence in blood donors would only reinforce our conclusion. Third, this study was conducted before the more transmissible delta or omicron variants. Based on contact tracing data, we recently showed that secondary transmission in District schools remained infrequent even in the delta era.45 Further serology testing is planned in three main school district in Vancouver (including the one surveyed in the current study) in the spring of 2022, which should determine if these conclusions will hold true during the omicron era.
In conclusion, this study shows no detectable increase in SARS-CoV-2 infections in school staff working in Vancouver public schools following a period of widespread community transmission (October 2020 to May 2021), compared with a reference group of blood donors from the same age, sex and community area. Vaccination of school staff and older student age groups, together with the introduction of more transmissible variants requires ongoing evaluation of COVID-19 infections within the school community.
Supplementary Material
Acknowledgments
We would like to thank the school staff who participated in the study and have been working tirelessly during this pandemic, and the District leadership, particularly Suzanne Hoffman and David Nelson for providing full support during this study; Kathy O’Sullivan for ongoing work reviewing District communications, documents and liaising with school partners throughout the study; Esther Alonso-Prieto for help with the human resource and financial management of this study; Brandon Bates, John Bhullar and Chemistry Laboratory staff at Children’s and Women’s Hospitals and the BC Children’s Hospital Biobank staff for help with sample collection and processing; the District and BC Children’s Hospital communication teams, Kim Schmidt and Lea Separovic for help with advertising; Laura Burns and Janet Simons, from the St-Paul’s Hospital Laboratory, for coordinating the Roche N-based serology testing; VCH’s Office of the Chief Medical Health Officer, the BC Centre for Disease Control, Dr Sarka Lisonkova and KS Joseph for ongoing discussions that guided this study; Steven Drews and Qi-Long Yi for consultation and assistance with matched donor data from Canadian Blood Services, and LifeLabs and Dynacare Laboratories for partnering with us commercially for the collection of blood samples.
Footnotes
Twitter: @DaveMGoldfarb, @Pascal_M_Lavoie
Contributors: LCM and PML obtained funding for this study; DMG, AWW, SMH, MAI, DC, PML and LCM designed the original study concept; SMH further contributed to data interpretation; FR reviewed the literature; ESB constructed and managed the data collection database; LM set-up and coordinated the recruitment of participants; SS and HRR processed blood samples, under the supervision of VEB; ND analysed the population-level data from students and school staff, under the supervision of AC; MAI performed statistical analyses; SFO'B provided and analysed matched data from Canadian blood donors; AWW performed all other data analyses; CO'R facilitated communications within the District during the study; RYX helped with data analysis; MS contributed to the design of the study; DMG and PML drafted the first manuscript with specific sections written by AWW, SMH, VEB, MAI, AC, CO'R and LCM. All authors revised the manuscript and approved its final version. LM and PML accept full responsibility for the work and/or the conduct of the study, AW, LM and PML had access to the data, and DMG, LM and PML controlled the decision to publish.
Funding: The study was funded by the Government of Canada via its COVID-19 Immunity Task Force (to PML and LCM as co-principal applicant; award # AWD-016994). PML and LCM receive a salary from the British Columbia Children’s Hospital (BCCH) Foundation through the Investigator Grant Award Program (award number is not applicable). MS is supported via salary awards from the BC Children’s Hospital Foundation, the Canadian Child Health Clinician Scientist Program and the Michael Smith Foundation for Health Research (award number is not applicable). The BC Children’s Hospital Healthy Starts Theme provided some seed funding at the beginning of the study (award number is not applicable).
Map disclaimer: The inclusion of any map (including the depiction of any boundaries therein), or of any geographic or locational reference, does not imply the expression of any opinion whatsoever on the part of BMJ concerning the legal status of any country, territory, jurisdiction or area or of its authorities. Any such expression remains solely that of the relevant source and is not endorsed by BMJ. Maps are provided without any warranty of any kind, either express or implied.
Competing interests: CO'R is an employee of the Vancouver School District, but the District was not involved in the design, analysis, interpretation of the data or the drafting of this manuscript; MS has been an investigator on projects funded by GlaxoSmithKline, Merck, Pfizer, Sanofi-Pasteur, Seqirus, Symvivo and VBI Vaccines. All funds have been paid to his institute, and he has not received any personal payments; the authors declare no relevant conflicts of interest. LifeLabs and Dynacare played no role in the study other than providing a service for the collection of blood samples.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the 'Materials and methods' section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data may be obtained from a third party and are not publicly available. Data may be obtained by making a written request to the COVID-19 Immunity Task Force.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study was approved by the University of British Columbia Children’s and Women’s Research Ethics Board Reference Number: H20-03593. Participants gave informed consent to participate in the study before taking part.
References
- 1.Levinson M, Cevik M, Lipsitch M. Reopening primary schools during the pandemic. N Engl J Med 2020;383:981–5. 10.1056/NEJMms2024920 [DOI] [PubMed] [Google Scholar]
- 2.Macartney K, Quinn HE, Pillsbury AJ, et al. Transmission of SARS-CoV-2 in Australian educational settings: a prospective cohort study. Lancet Child Adolesc Health 2020;4:807–16. 10.1016/S2352-4642(20)30251-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heavey L, Casey G, Kelly C, et al. No evidence of secondary transmission of COVID-19 from children attending school in Ireland, 2020. Euro Surveill 2020;25. 10.2807/1560-7917.ES.2020.25.21.2000903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yung CF, Kam K-Q, Nadua KD, et al. Novel coronavirus 2019 transmission risk in educational settings. Clin Infect Dis 2021;72:1055–8. 10.1093/cid/ciaa794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Falk A, Benda A, Falk P, et al. COVID-19 cases and transmission in 17 K-12 schools - wood county, wisconsin, August 31-November 29, 2020. MMWR Morb Mortal Wkly Rep 2021;70:136–40. 10.15585/mmwr.mm7004e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brandal LT, Ofitserova TS, Meijerink H, et al. Minimal transmission of SARS-CoV-2 from paediatric COVID-19 cases in primary schools, Norway, August to November 2020. Euro Surveill 2021;26. 10.2807/1560-7917.ES.2020.26.1.2002011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ismail SA, Saliba V, Lopez Bernal J, et al. SARS-CoV-2 infection and transmission in educational settings: a prospective, cross-sectional analysis of infection clusters and outbreaks in England. Lancet Infect Dis 2021;21:344–53. 10.1016/S1473-3099(20)30882-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hershow RB, Wu K, Lewis NM, et al. Low SARS-CoV-2 transmission in elementary schools - Salt Lake County, Utah, December 3, 2020-January 31, 2021. MMWR Morb Mortal Wkly Rep 2021;70:442–8. 10.15585/mmwr.mm7012e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zimmerman KO, Akinboyo IC, Brookhart MA, et al. Incidence and secondary transmission of SARS-CoV-2 infections in schools. Pediatrics 2021;147. 10.1542/peds.2020-048090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Varma JK, Thamkittikasem J, Whittemore K, et al. COVID-19 infections among students and staff in New York City public schools. Pediatrics 2021;147:050605. 10.1542/peds.2021-050605 [DOI] [PubMed] [Google Scholar]
- 11.Bark D, Dhillon N, St-Jean M, et al. SARS-CoV-2 transmission in kindergarten to grade 12 schools in the Vancouver coastal health region: a descriptive epidemiologic study. CMAJ Open 2021;9:E810–7. 10.9778/cmajo.20210106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bullard J, Funk D, Dust K, et al. Infectivity of severe acute respiratory syndrome coronavirus 2 in children compared with adults. CMAJ 2021;193:E601–6. 10.1503/cmaj.210263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Powell AA, Amin-Chowdhury Z, Mensah A, et al. Severe acute respiratory syndrome coronavirus 2 infections in primary school age children after partial reopening of schools in England. Pediatr Infect Dis J 2021;40:e243–5. 10.1097/INF.0000000000003120 [DOI] [PubMed] [Google Scholar]
- 14.Ladhani SN, Baawuah F, Beckmann J, et al. SARS-CoV-2 infection and transmission in primary schools in England in June-December, 2020 (sKIDs): an active, prospective surveillance study. Lancet Child Adolesc Health 2021;5:417–27. 10.1016/S2352-4642(21)00061-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buonsenso D, De Rose C, Moroni R, et al. SARS-CoV-2 infections in Italian schools: preliminary findings after 1 month of school opening during the second wave of the pandemic. Front Pediatr 2020;8:615894. 10.3389/fped.2020.615894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gras-Le Guen C, Cohen R, Rozenberg J, et al. Reopening schools in the context of increasing COVID-19 community transmission: the French experience. Arch Pediatr 2021;28:178–85. 10.1016/j.arcped.2021.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Doyle T, Kendrick K, Troelstrup T, et al. COVID-19 in primary and secondary school settings during the first semester of school reopening - Florida, August-December 2020. MMWR Morb Mortal Wkly Rep 2021;70:437–41. 10.15585/mmwr.mm7012e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haapanen M, Renko M, Artama M, et al. The impact of the lockdown and the re-opening of schools and day cares on the epidemiology of SARS-CoV-2 and other respiratory infections in children - A nationwide register study in Finland. EClinicalMedicine 2021;34:100807. 10.1016/j.eclinm.2021.100807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Armann JP, Kirsten C, Galow L, et al. SARS-CoV-2 transmissions in students and teachers: seroprevalence follow-up study in a German secondary school in November and December 2020. BMJ Paediatr Open 2021;5:e001036. 10.1136/bmjpo-2021-001036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Szépfalusi Z, Schmidthaler K, Sieber J, et al. Lessons from low seroprevalence of SARS-CoV-2 antibodies in schoolchildren: a cross-sectional study. Pediatr Allergy Immunol 2021;32:762–70. 10.1111/pai.13459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Torres JP, Piñera C, De La Maza V, et al. Severe acute respiratory syndrome coronavirus 2 antibody prevalence in blood in a large school community subject to a coronavirus disease 2019 outbreak: a cross-sectional study. Clin Infect Dis 2021;73:e458–65. 10.1093/cid/ciaa955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rostami A, Sepidarkish M, Leeflang MMG, et al. SARS-CoV-2 seroprevalence worldwide: a systematic review and meta-analysis. Clin Microbiol Infect 2021;27:331–40. 10.1016/j.cmi.2020.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.BC centre for disease control, 2021. Available: http://www.bccdc.ca/health-info/diseases-conditions/covid-19/data [Accessed 1 Sep 2021].
- 24.Field studies working groups core data elements (individual). Available: https://www.covid19immunitytaskforce.ca/wp-content/uploads/2020/07/Core-Elements_English-New.pdf [Accessed 10 May 2021].
- 25.Garnett E, Jung J, Tam E, et al. Clinical validation and performance evaluation of the automated vitros total anti-SARS-CoV-2 antibodies assay for screening of serostatus in COVID-19. Am J Clin Pathol 2020;154:742–7. 10.1093/ajcp/aqaa157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harritshøj LH, Gybel-Brask M, Afzal S, et al. Comparison of 16 serological SARS-CoV-2 immunoassays in 16 clinical laboratories. J Clin Microbiol 2021;59:20. 10.1128/JCM.02596-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bailey D, Konforte D, Barakauskas VE, et al. Canadian society of clinical chemists (CSCC) interim consensus guidance for testing and reporting of SARS-CoV-2 serology. Clin Biochem 2020;86:1–7. 10.1016/j.clinbiochem.2020.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.National SARS-CoV-2 Serology Assay Evaluation Group . Performance characteristics of five immunoassays for SARS-CoV-2: a head-to-head benchmark comparison. Lancet Infect Dis 2020;20:1390–400. 10.1016/S1473-3099(20)30634-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Favresse J, Eucher C, Elsen M, et al. Clinical performance of the Elecsys Electrochemiluminescent immunoassay for the detection of SARS-CoV-2 total antibodies. Clin Chem 2020;66:1104–6. 10.1093/clinchem/hvaa131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Favresse J, Douxfils J. Evaluations of SARS-CoV-2 serological assay performance need inclusion of long-term samples. J Clin Microbiol 2021;59:21. 10.1128/JCM.00487-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Majdoubi A, Michalski C, O'Connell SE, et al. A majority of uninfected adults show preexisting antibody reactivity against SARS-CoV-2. JCI Insight 2021;6:146316. 10.1172/jci.insight.146316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reiczigel J, Földi J, Ozsvári L. Exact confidence limits for prevalence of a disease with an imperfect diagnostic test. Epidemiol Infect 2010;138:1674–8. 10.1017/S0950268810000385 [DOI] [PubMed] [Google Scholar]
- 33.Elecsys anti-SARS-CoV-2 S. Available: https://www.fda.gov/media/144037/download [Accessed 17 May 2021].
- 34.Naimark D, Mishra S, Barrett K, et al. Simulation-based estimation of SARS-CoV-2 infections associated with school closures and community-based nonpharmaceutical interventions in Ontario, Canada. JAMA Netw Open 2021;4:e213793. 10.1001/jamanetworkopen.2021.3793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Viner RM, Russell SJ, Croker H, et al. School closure and management practices during coronavirus outbreaks including COVID-19: a rapid systematic review. Lancet Child Adolesc Health 2020;4:397–404. 10.1016/S2352-4642(20)30095-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saeed S, Drews SJ, Pambrun C, et al. SARS-CoV-2 seroprevalence among blood donors after the first COVID-19 wave in Canada. Transfusion 2021;61:862–72. 10.1111/trf.16296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Skowronski DM, Sekirov I, Sabaiduc S. Low SARS-CoV-2 sero-prevalence based on anonymized residual sero-survey before and after first wave measures in British Columbia, Canada, March-May 2020. Medrxiv [pre-print] 2020. [Google Scholar]
- 38.Lopez L, Nguyen T, Weber G, et al. Seroprevalence of anti-SARS-CoV-2 IgG antibodies in the staff of a public school system in the midwestern United States. PLoS One 2021;16:e0243676. 10.1371/journal.pone.0243676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pollock AM, Lancaster J. Asymptomatic transmission of covid-19. BMJ 2020;371:m4851. 10.1136/bmj.m4851 [DOI] [Google Scholar]
- 40.Bobrovitz N, Arora RK, Cao C, et al. Global seroprevalence of SARS-CoV-2 antibodies: a systematic review and meta-analysis. PLoS One 2021;16:e0252617. 10.1371/journal.pone.0252617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lessler J, Grabowski MK, Grantz KH, et al. Household COVID-19 risk and in-person schooling. Science 2021;372:1092–7. 10.1126/science.abh2939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dawson P, Worrell MC, Malone S, et al. Pilot investigation of SARS-CoV-2 secondary transmission in kindergarten through grade 12 schools implementing mitigation strategies - St. Louis County and City of Springfield, Missouri, December 2020. MMWR Morb Mortal Wkly Rep 2021;70:449–55. 10.15585/mmwr.mm7012e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Castro Dopico X, Muschiol S, Christian M, et al. Seropositivity in blood donors and pregnant women during the first year of SARS-CoV-2 transmission in Stockholm, Sweden. J Intern Med 2021;290:666–76. 10.1111/joim.13304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grant R, Dub T, Andrianou X, et al. SARS-CoV-2 population-based seroprevalence studies in Europe: a scoping review. BMJ Open 2021;11:e045425. 10.1136/bmjopen-2020-045425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Choi A, Mâsse LC, Bardwell S. Symptomatic and asymptomatic transmission of SARS-CoV-2 in K-12 schools, British Columbia, April to June 2021. Medrxiv 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2021-057846supp001.pdf (1.1MB, pdf)
Data Availability Statement
Data may be obtained from a third party and are not publicly available. Data may be obtained by making a written request to the COVID-19 Immunity Task Force.