Abstract
Broiler breeder chickens are commercially feed restricted to slow their growth and improve their health and production, however, there is research demonstrating that this leads to chronic hunger resulting in poor welfare. A challenge in these studies is to account for possible daily rhythms or the effects of time since last meal on measures relating hunger. To address this, we used 3 feed treatments: AL (ad libitum fed), Ram (restricted, fed in the morning), and Rpm (restricted, fed in the afternoon) to control for diurnal effects. We then conducted foraging motivation tests and collected home pen behavior and physiological samples at 4 times relative to feeding throughout a 24-h period. The feed treatment had the largest influence on the data, with AL birds weighing more, having lower concentrations of plasma NEFA, and mRNA expression of AGRP and NPY alongside higher expression of POMC in the basal hypothalamus than Ram or Rpm birds (P < 0.001). R birds were more successful at and had a shorter latency to complete the motivation test, and did more walking and less feeding than AL birds in the home pen (P < 0.01). There was little effect of time since last meal on many measures (P > 0.05) but AGRP expression was highest in the basal hypothalamus shortly after a meal (P < 0.05), blood plasma NEFA was higher in R birds just before feeding (P < 0.001) and glucose was higher in Ram birds just after feeding (P < 0.001), and the latency to complete the motivation test was shortest before the next meal (P < 0.05). Time of day effects were mainly found in the difference in activity levels in the home pen when during lights on and lights off periods. In conclusion, many behavioral and physiological hunger measures were not significantly influenced by time of day or time since the last meal. For the measures that do change, future studies should be designed so that sampling is balanced in such a way as to minimize bias due to these effects.
Key words: broiler breeder, hunger, behavior, physiology, welfare
INTRODUCTION
Many animals used in commercial food production are regularly feed restricted to decrease growth rates and maintain good physical and reproductive health (review by D'Eath et al., 2009). This restriction is especially severe in the growing phase of broiler breeders, the parent stock of broilers (meat chickens). Broiler breeders share the same fast growth potential as their offspring and if fed ad libitum, these birds would have high mortality, lameness, metabolic issues, and poor reproduction (Renema and Robinson, 2004). To combat this, broiler breeders are feed restricted up to about 32 to 33% of what they would choose to eat given free access (De Jong et al 2001) and although broiler breeder genetics will have changed since this publication, increased growth selection for broilers (e.g., Havenstein et al., 2003) will lead to even more severe restriction needed in the parent stock. This chronic feed restriction leads to the welfare concern that they are chronically hungry (reviewed by Mench, 2002; D'Eath et al., 2009). Feed restricted broiler breeders show increased activity and foraging behavior and perform abnormal or stereotypic behaviors such as pacing, spot pecking and polydipsia as well as a high motivation to access feed when available (Savory and Maros, 1993; Hocking et al., 2001; Sandilands et al., 2005; Dixon et al., 2014). Finding methods to increase satiety while maintaining slow growth could improve the welfare of millions of broiler breeders in the UK alone (Sandilands et al., 2006).
In previous research we found that feed restricted broiler breeder hens were more motivated to access an area to forage for food (appetitive feeding behavior) (Dixon et al., 2014) and they had higher levels of agouti-related protein (AGRP) mRNA in the basal hypothalamus (thought to be representative of current hunger and metabolic state; Dunn et al., 2013b) than birds of the same age fed larger portions or ad libitum, adding to the evidence that these birds are chronically hungry. However, a criticism of this work is that the data were collected after restricted birds had run out of food, those on larger portions may or may not have had food left, and that ad libitum fed birds had access to food until they underwent behavioral testing or were killed for physiological sampling. This may have resulted in behavioral and physiological differences in our measures depending on the time of the day data were collected, and the associated time since the last meal.
Daily oscillations in physiological and behavioral measures are known to occur (e.g., Machado et al., 2015). For example, hens are motivated to access nestboxes prior to oviposition and will display nest seeking and inspection behaviors that are not present at other times of the day (Duncan, 1989; Appleby et al., 2004). Circulating glucocorticoids are higher during the active period of animals, including broiler breeder chickens (de Jong et al., 2001) and tend to show a peak at the beginning of the activity period (Chung et al., 2011). From a feeding behavior point of view, most animals establish daily feeding rhythms when given ad libitum access to food. Free-fed domestic fowl tend to eat more at the beginning or end of the light period but less in the middle of the day (Savory, 1980). However, food-restricted animals consume food immediately after being provided access to it, while in ad libitum animals, feeding is related to time since last meal. For example, broiler breeders on a commercial level of feed restriction (from 25 to 51% of what they would choose to eat ad libitum) and those fed twice this amount were more motivated to work for feed by pecking a disc for a food reward than birds fed ad libitum on the same diet. Additionally, when restricted birds were compared to ad libitum birds who had feed withdrawn for 3 to 72 h, the restricted birds did not significantly vary their number of responses throughout the day while ad libitum birds increased their responses as time since last meal increased (Savory et al., 1993). Therefore, time of day and/or time since last meal may have affected the responses of ad libitum birds in our previous motivation tests (Dixon et al., 2014) but effects on the restricted-fed birds may be minimal.
Prior research on daily rhythms of AGRP gene expression is conflicting: there was no effect of time of day on hypothalamic AGRP mRNA levels in Siberian hamsters (Ellis et al., 2008) but there was a diurnal rhythm of AGRP mRNA found in rats, with a peak 4 h after lights off and a trough at 4 h after lights on which was thought to be consistent with a day-night food intake rhythm of this nocturnal animal (Lu et al., 2002). Free feeding mice also had an increase in AGRP neuron electrical activity related to nocturnal feeding behavior, with less activity around dawn than later in the photoperiod when it was some time since they last fed. While in food-restricted mice AGRP neuron activity dropped as food became available but still stayed at higher levels than in freely-fed mice (Mandelblat-Cerf et al., 2015). In birds, Japanese quail fasted for 24 h had higher AGRP mRNA compared to ad libitum-fed individuals (Philips-Singh et al., 2003), and AGRP mRNA decreased in broiler breeder hens released from a period of feed restriction and ad libitum fed for 2.5 d, suggesting expression can change relatively quickly (Dunn et al., 2013b; Caughey et al., 2018). This indicates that the time of day or the time since the last meal, especially with food restriction, could affect AGRP mRNA levels and may influence results depending on when the samples were collected.
Other gene products in the arcuate nucleus of the hypothalamus are also thought to be important in regulating energy balance through feeding stimulation or inhibition. Neuropeptide Y (NPY) is co localized and acts similarly to AGRP by stimulating feeding behavior and by its gene expression being increased in response to food restriction. Broiler breeder males reared on a commercial restriction program had significantly higher NPY gene expression than similarly aged birds fed ad libitum (Boswell et al., 1999) and feed intake can be stimulated in broilers when NPY is injected into the brain (Kuenzel et al., 1987). Pro-opiomelanocortin (POMC) neurons are anorexigenic, having a catabolic effect on energy balance, and would, when activated, be expected to decrease feeding behavior in an opposite, inhibitory manner compared to AGRP. However, food deprivation studies in birds do not always follow this pattern. During short-term food deprivation (24–48 h) and chronic food restriction (7 d) broiler chicks and layer chicks had decreased POMC expression compared to when they were fully fed (Hen et al., 2006; Higgins et al., 2010; Lei and Lixian, 2012; Fang et al., 2014) but there was no change in POMC mRNA levels in Japanese quail and broiler chicks after short term food deprivation and no change in broiler breeder hens after chronic food restriction (6 wk) (Philips-Singh et al., 2003; Song et al., 2012). There is not much currently known about the diurnal rhythms of POMC in birds but in proestrous female rats, levels of POMC mRNA increased in the morning with a peak between 0300 and 1000 and then decreased by 2300 (Wise et al., 1990) and male ad libitum fed rats had a peak around midnight which decreased from 0600 to 1900 (Chen et al., 2004). In mammals, cocaine and amphetamine regulated transcript (CART) is also anorexigenic and involved in regulating food intake and body mass. Less is known about CART and its co-expression with POMC in birds. However decreased expression of CART mRNA and reduced immunoreactive CART fibers have been observed after fasting or food restriction in broiler and layer chickens and in zebra finches, consistent with an anorectic action of these neurons in birds (Cai et al., 2015; Singh et al., 2016; Caughey et al., 2018).
Aside from the abovementioned neurons, there are peripheral peptides which may also impact on hunger/satiety. In a complementary paper where we quantified gene expression of peptide YY (PYY) and pancreatic polypeptide Y (PPY) utilizing the same samples featured in this study, we observed significant effects of time since feeding only for PYY mRNA in the pancreas. However, there were clear treatment effects with gene expression of PYY and PPY both being higher in the pancreas of ad libitum-fed birds (Reid et al., 2017). NPY neurons are also present in the gut and inhibit electrolyte and water secretions and the motility of the gastrointestinal tract (Cox, 2007). There is currently no evidence that NPY in the gut is influenced by hunger or time since feeding but as PYY and PPY did change in the Reid et al. (2017) paper, it is possible that NPY, which is part of the same family, may as well. In chickens, circulating insulin levels are correlated with food intake levels (Simon, 1989) and direct injection of insulin can increase food intake (Honda et al., 2007); however insulin levels did not differ between selected lines of lean and fat birds when both were food restricted (Simon, 1989). Insulin injections also increased gene expression of POMC in chickens but did not inhibit AGRP mRNA and did not consistently inhibit NPY mRNA as it did in similar lab rat studies (Porte, Jr et al., 2002; Honda et al., 2007; Shiraishi et al., 2008). Exogenous cholecystokinin (CCK) inhibits food intake (Dunn et al., 2013a) but CCK receptor type A (CCKAR) is less abundant in chickens bred for fast growth, like modern broilers and broiler breeders, leading to a decreased sensitivity to its satiating effects (Honda, 2016). Several different mRNA transcripts are transcribed from the chicken glucagon gene that undergo tissue-specific processing to produce glucagon (GCG) in the pancreas and glucagon-like peptides-1 and -2 (GLP-1 and GLP-2) in the intestine and brain (Honda, 2016). Both GCG itself and GLP-1 inhibit food intake when injected into the brain (van der Wal et al., 1999). Levels of non-esterified fatty acids (NEFA) and glucose in the blood plasma can indicate metabolic rate and the storage or use of energy substrates (Scheurink et al., 1996). NEFA levels were increased in broilers subjected to short term food restriction (de Jong et al., 2003) but were decreased in broiler breeders subject to high levels of chronic food restriction (similar to commercial restriction levels) compared to birds who were still chronically restricted but at a less severe level and ad libitum fed breeders, while glucose levels were not affected by the different restriction levels (from ad libitum up to a restriction of 25% of the ad libitum food intake; Renema and Robinson, 2004).
Clearly, there are still gaps in our understanding of how these peptides interact to regulate feeding in chickens with even fewer studies exploring the diurnal rhythms of these peptides. In future studies, we plan to feed broiler breeders restricted diets of different compositions that may decrease hunger and improve satiety which may lead to the birds showing more similarities to ad libitum fed birds. Therefore, we need to determine the daily rhythms and influences of feeding times for our key measures to ensure future results are not influenced by these outside factors. This study was specifically set out to ensure feeding-driven changes were discernible from any photoperiod- or circadian-driven cycles. Additionally, these results from a well-powered study may help to improve our understanding of the regulation of energy balance in chickens and what potential changes occur in relation to time of day and hunger status. Therefore, this study aimed to determine how behavior, appetitive feeding motivation, AGRP mRNA in the basal hypothalamus and other neurobiological and physiological measures vary with time after feeding, whilst controlling for effects relating to time of day for restricted and ad libitum-fed broiler breeders. We hypothesized that restricted-fed birds would show the lowest behavioral and physiological measures relating to hunger shortly after a meal and the highest shortly before a meal, with other time points giving intermediate results, and that restricted-fed birds would always show behavioral and physiological signs of increased hunger compared to ad libitum-fed birds.
MATERIALS AND METHODS
Ethical Considerations
Food restriction is likely to result in hunger, but welfare issues which are typical in commercial farming need to be replicated in the laboratory so they can be studied for potential solutions. The levels of food restriction we imposed were similar to those used routinely in the poultry industry, while 1 feed treatment was ad libitum access to feed. Ad libitum feeding of broiler breeders from hatch can cause welfare concerns (Renema and Robinson, 2004); therefore our birds did not begin the ad libitum feeding treatment until they reached 7 wk of age and the experiment was ended when birds were 12 wk old, at which age they were still active and healthy. All procedures in this experiment were carried out under Home Office Licence and with the SRUC Animal Experiment Committee's approval; birds were inspected a minimum of 3 times per day.
Animals and Housing
A total of 216 non–beak-trimmed Ross 308 broiler breeder female chickens (Aviagen, Stratford, UK) were raised from 1-day-old chicks in 2 separate batches, 6 wk apart (108 chicks per batch). Each batch was housed in 2 rooms, with 12 floor pens with wood shavings (1.0 × 1.5 m) in groups of 9 birds per pen. The lighting schedule for the first day was 23.5L:0.5D hours light:dark after which the photoperiod was gradually reduced to 8L:16D over 10 d. Temperature followed commercial recommendations, decreasing from around 30°C at bird level at 1-day-old to around 20°C by 4 wk of age. Chicks were given ad libitum water from bell drinkers and were fed chick starter crumbs for the first 3 wk, chick starter pellets for the following 3 wk and then grower pellets from the beginning of 6 wk of age to the end of the trial (all ABN, Cupar Mills, Fife, UK). The feed formulations were developed in consultation with a broiler breeder producer and feed manufacturer to be in line with commercial broiler breeder standards and are proprietary, however, all diets met the National Research Council requirements. Food was provided ad libitum for the first 7 d and then in restricted amounts given at 9:00 h each day that were gradually increased from 26 to 44 g per bird per day by the beginning of the 6th wk, as per the Ross 308 parent stock guidelines (Aviagen, 2013). At 2 wk of age, all birds were weighed and wing tagged (10 mm × 10 mm padlock-style tags, Roxan Developments Ltd., UK).
At 6 weeks of age, all birds were weighed and regrouped into pens of 9 birds according to matched body weight. The photoperiod was also increased from 8L:16D to 10L:14D hours at this point to allow sufficient hours of light to complete all the necessary training and testing. All birds were weighed about weekly from 2 wk of age to the end of the trial (12 wk of age).
Experimental Design
Pens were in 4 spatial blocks across both rooms in each batch with 3 pens of similar average weight making up each block. In order to optimize balance of feed treatments with average pen weight, the 3 different feed treatments (Ram, Rpm and AL) were allocated at the pen level within each block using 2 3 × 3 latin squares, 1 per batch, plus the addition of a random allocation to the remaining 3 pens in 1 block in batch 1, which was reversed for the remaining block in batch 2. This resulted in 8 pens and 72 birds in each feed treatment over both batches (Figure 1). Birds within pens were allocated to be culled for postmortem at 4 times relative to feeding (see below), randomly allocating the 4 lightest and the 4 heaviest in each pen to the 4 times, and then randomly allocating the remaining 4 birds per treatment in each batch to the 4 times. Birds within pens were allocated to 1 of 3 scheduling groups for which motivation tests were staggered by 1 wk, in such a way that each scheduling group contained equal numbers of birds per batch in each feed treatment by postmortem time relative to feeding. Allocation of the 12 birds of each diet in each scheduling group to 1 of 3 sets of apparatus (see below) was achieved by using 2 3 × 3 latin squares, 1 for each batch. This ensured that scheduling group by apparatus was balanced with feed treatment by postmortem time relative to feeding. Similar approaches were used to ensure balance between each feed treatment by postmortem time relative to feeding whilst also optimizing balance with bird weight for the 3 postmortem teams and 2 d on which postmortems were carried out per batch, the 3 laboratory processing days per batch, the 2 testers carrying out the foraging tests and order of sampling for all the various measurements.
Figure 1.
The allocation of birds to pens, treatments and rooms for both batches of the experiment.
Treatments and Times of Measurements
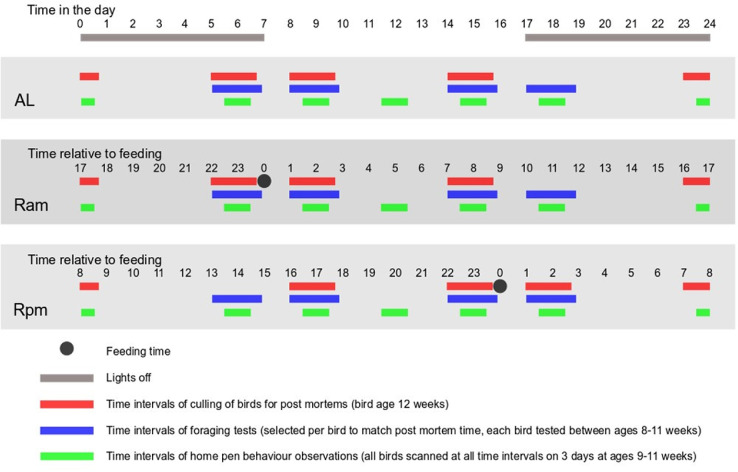
Two treatment groups of 72 birds (8 pens) each were fed the standard commercial restricted diet (R) which was provided to the birds either first thing after lights came on in the morning at 07:00 h (Ram) or at 16:00 h (Rpm) which was 1 h ± 15 min before lights went off in the evening (17:00 h). A third treatment group of birds were fed the commercial diet ad libitum (AL). Behavioral and physiological measures (see below) were collected throughout various 24-h periods, once after the birds had eaten (minimum time since being fed), once before the next feeding (maximum time since being fed) and at various other time points between the minimum and maximum (see Figure 2). Birds had been allocated to be culled for post mortem during ∼2-h intervals starting at 1, 7, 16, and 22 h relative to the feeding time. These specific times were chosen in order that the circadian time of sampling was as similar as possible between Ram and Rpm birds and in order that there were equal sampling points during lights on and lights off. AL birds were fed and sampled at the same time as Ram birds. Home pen scan sessions were chosen to also coincide with the time in the day birds were culled for postmortem, plus the addition of 1 session in the middle of the day, but all birds were observed at all 6 sessions during the day regardless of the time when they were to be culled for postmortem. Foraging tests took place over intervals of 2 h while home pen scan sessions were 1-h long (see Figure 2).
Figure 2.
The treatment structure for the experiment, showing time relative to feeding and actual time of day when measurements took place for the 3 feed treatments. Birds were culled for PMs during ∼2-h intervals starting at 1, 7, 16, and 22 h relative to feeding. Observation times for AL were chosen to match those for Ram. These time intervals were chosen in order to have 1 soon after feeding, 1 just before feeding, and 2 intermediate, and so that 3 of 4 intervals also coincided at the same times in the day. Foraging tests took place for each bird at the same time in the day that the bird was to be culled for post mortem, apart from those culled around midnight for which foraging tests were instead at 17:00–19:00 (Ram) or 5:00–7:00 (Rpm). (Foraging motivation tests were not carried out at midnight as the birds would have been asleep for a few hours and previous experience suggests they would not perform in the motivation test). Home pen scan sessions were chosen to also coincide with the time in the day birds were culled for post mortem, plus the addition of 1 session in the middle of the day. Each 1-h session contained 10 scans during lights on and 5 scans during lights off. Foraging tests took place over 3 wk per batch with different birds being tested each week, and then home pen observations took place for all birds over one 24-h period at the end of each of these weeks, when birds were undisturbed, apart from for feeding.
Behavior Tests
Foraging Motivation Test
Apparatus – Set Up, Habituation, and Training
The foraging motivation apparatus and habituation and training procedures have been described previously (Dixon et al., 2014), but in brief the apparatus consisted of a wooden start platform which had a ramp into a runway which could be filled with varying depths of water and led to a moveable wooden platform where wood shavings were placed during testing (wood shavings platform). The apparatus was covered by a lid that prevented the birds from flying across the runway to avoid water during training and testing.
Before training began, birds were habituated in groups to the apparatus with no water or wood shavings for three 15-min sessions. Birds then received 2 individual habituation sessions in the apparatus as training and testing were done on an individual basis.
Training began at 6 wk of age, coinciding with when the diet treatments began, and took 1 wk. There were 3 training stages. First the birds were placed in the apparatus with the 2 wooden platforms directly next to each other (no ramps), wood shavings were present on the wood shavings platform and birds were given 10 min to move from the start to the wood shavings platform. Next the wood shavings platform was moved 1 m from the start platform and the ramps were added back in. No water was in the runway and again birds were given 10 min to reach the wood shavings platform. Finally, this step was repeated but with enough water in the runway was to just cover the birds’ feet (about 20 mm). Birds did not progress to the next training stage until they had successfully completed the previous one.
Testing
Each batch of birds was divided into 3 groups with each group being tested for 1 wk. Birds were each tested 4 times, once per day for 4 consecutive days, with the 12 birds from each of the 3 diet treatments tested on 1 of the 3 apparatuses (see above). The test time interval for each bird was selected to match the time relative to feeding when they were to be culled for postmortem apart from those culled around midnight for which foraging tests were instead at 17:00–19:00 h (Ram) or 05:00–07:00 h (Rpm). From previous experience, birds' disturbed mid-way through the dark period would not perform well in a test environment and would merely rest, thus not giving accurate data for this test. This arrangement resulted in all birds being tested either 1–3, 7–18 and 22–24 h since last feed and all tests conducted during, or within 2 hours either side of, the period when lights were on (see Figure 2). Testing began with the first group of birds when they were 8 to 9 wk of age, the second group when they were 9 to 10 wk of age and the third group when they were 11 wk of age. For birds in groups 2 and 3, a re-fresher training session (similar to the third training session) was conducted to ensure they were still familiar with the apparatus. For the first test, the wood shavings platform was moved 1.5 m from the start platform, with 0.8 m between the bottom of the ramps and water was added to the runway. Because birds on the different feed treatments grew at different rates over the test, the water depth was proportional to mean leg length of the 12 birds to be tested on each apparatus in each test week. To do this, the length of the birds’ legs was measured from the ground to the top of the hock before their test week.
Over subsequent tests, the ‘cost’ of accessing the wood shavings platform, in terms of water depth and length was increased in a stepwise manner: water depth was increased in increments relative to the average length of the birds’ legs for each feed treatment (water depth: test 1 = 2/6 leg length, test 2=4/6 leg length, test 3 = 6/6 leg length, test 4 = 8/6 leg length). This resulted in water depth levels that ranged from 18mm at the first test to 73 to 94 mm at the 4th test. As the water depth increased with each test, the length of the runway between the bottom of the 2 ramps was also increased from 0.8 m at the first test by 0.8 m each time up to a length of 3.2 m at the 4th test.
Each test lasted about 20 min. At the beginning of a test, a bird was placed on the start platform and could spend the test time in whatever areas of the apparatus she chose to. After the 20 min were up, the bird was removed from the apparatus. Due to the number of birds being tested, 3 identical apparatuses were used and 2 people took shifts placing the birds on the start platform at the beginning of each test.
Measurements
Measurements were made from videos of the foraging tests by 1 observer using The Observer XT (Version 11, Noldus, Wageningen, the Netherlands). For all tests, time spent in the different parts of the apparatus was recorded and from this whether the bird reached the wood shavings platform (defined by the bird having both feet on it) and latency to reach the wood shavings platform were derived. Behavior on the wood shavings platform was also recorded using the Observer XT giving total durations that the birds spent in the foraging area foraging, sitting, standing, walking or preening using the same behavior definitions as in the Home Pen observations (below). For tests 1 and 4 of each week, start platform behavior was also recorded to determine how the birds were using the start platform and to increase the amount of data available on the AL birds that spent most of their time on the start platform.
All birds were tested with all platform distances and water depths, even if they gave up crossing the water to reach the wood shavings in earlier tests. This allowed statistical analyses of a full complement of longitudinal data resulting in more power than would be the case for analyses of summary measures such as the maximum cost paid (distance/depth overcome) to get to the wood shavings platform.
Home Pen Observations
All pens were video recorded for 24 h periods once a week for 3 wk during days when foraging motivation testing was not occurring when birds were aged 9 to 11 wk. Each bird in a pen was individually identified by a pattern made with black livestock marker. Scan sampling was carried out by 1 observer during 6 1-h sessions throughout the 24-h period, chosen to coincide with the time of day birds were to be culled for postmortem, plus the addition of 1 session in the middle of the day (see Figure 2). The behavior of each bird in each pen was recorded for 10 scans, 6 min apart, for the 3 sessions during lights on and 5 scans, 15 min apart, for the 3 sessions during lights off. The behaviors recorded were inactive (standing/sitting/sleeping), walking (including running), foraging (pecking and scratching at litter), feeding (pecking at feed), drinking (pecking at and swallowing water), object pecking (pecking at feeder, drinker, pen walls), preening (while sitting or standing), dustbathing, aggressive pecking (peck directed to the head of another bird, delivered in a sharp, downwards manner), nonaggressive pecking (gentle and vigorous feather pecking, pecking at another bird's beak), and other (wing flap, shake, stretch, bill wipe). Walking and foraging were also combined for statistical analysis to form the category ‘active behavior’.
Physiological Measures
At 12 wk of age, blood, brain, and gut tissue samples were collected from all birds. Due to the number of birds, sampling was done for each batch over 2 nonconsecutive 24-h periods and 3 teams of 3 people each were involved in the sampling during all 4 periods. The sampling times for these collections were relative to feeding times (see Figure 2). At the beginning of a sampling time, a bird was removed from their home pen, weighed, and had 2 mL blood drawn from the brachial wing vein. This was split equally into 2 1.5 mL microfuge tubes (Sarstedt, Leicester, UK), 1 containing 100 µL 0.6 M NaF/ 0.18M K Oxalate solution (for glucose measurements) and the other 50 µL Heparin (1,000 IU/mL) (for NEFA measurements). These tubes were mixed and then stored on ice for up to 1 h before being centrifuged at 8,000 g for 10 min at 4°C and the plasma removed and stored at −20°C until analysis. The bird was then euthanized with an overdose of IV pentobarbital. Once death had been confirmed, digestive organs, and contents were weighed. Tissue samples (40–100 mg) were taken from the gut and immediately stored in liquid nitrogen until transfer to −80°C freezer: proventriculus (ProV), gizzard, pancreas, liver, and gallbladder. Basal hypothalamus was dissected as described previously (Dunn et al., 2013b). Contents from the crop was weighed and scored on appearance: 1: Empty - no liquid or solid food evident, 2: Wet mush - mainly liquid with some soft solid food., 3: Solid mush - soft solid food, 4: Mix of dry pellets/solid mush - mainly soft solid food with few dry whole food pellets, 5: Dry pellets - whole dry food pellets, very little or no soft solid food.
RNA extraction and reverse transcription and measurement of anorectic (POMC, CART) and orexigenic peptide (AGRP, NPY) genes in the basal hypothalamus and genes related to metabolism in the pancreas (cholecystokinin A receptor [CCKAR], NPY) were carried out by RTPCR as reported previously (Dunn et al., 2013b; a) and PPY was measured as reported (Reid et al., 2017). Glucagon (GCG) and Insulin (INS) were measured in the same way as the other RTPCR assays using the following primers; GCG: Forward – 5’-TGATAGTTCAAGGCAGCTGG; Reverse – 5’-AAAATCCTGAGCTCGTCTGC; Insulin: Forward – 5’-TCCTTGTCTTTTCTGGCCCT; Reverse – 5’-GCTCAACAATCCCTCGCTTG.
Glucose and NEFA were measured at the Easter Bush pathology lab (R(D)SVS, Easter Bush, UK) on an Instrumentation Laboratory 650 analyzer (Werfen, Warrington, UK) using Instrumentation Laboratory and Randox Laboratories (Crumlin, N Ireland) analysis kits respectively.
Statistical Analysis
Foraging Motivation Test
Linear mixed models (LMM) were fitted to latency to reach the wood shavings platform, and durations on the start platform and wood shavings platform, calculated as a proportion of total test time (all angular transformed). LMM were fitted to durations for different behaviors exhibited on the wood shavings platform for successful birds and on the start platform for all birds at test numbers 1 and 4 only calculated as a proportion of time spent there (all angular transformed).
Generalized linear mixed models (GLMM) were fitted to the binary variable whether a bird successfully reached the wood shavings platform or not, with logit link function, binomially distributed errors and offset by total test time (log transformed).
Random effects were included for batch, for individual pens of birds and individual birds, and for LMM only blocks within batches and test numbers within pens, but they were all fairly small apart from the variability between birds and between test numbers within birds (i.e., the residual for LMMs).
Fixed effects were included for the 3 apparatuses, the 2 testers (main effects only) and the 4 test numbers, bird age (fitted as a 3 level factor), dietfeedtime (AL, Ram, Rpm) and the time interval relative to feeding category (1.2–2.6, 7.2–17.5, 22.2–23.6 h) at which birds were tested and all interactions. These models were fitted to 4 different subsets of the data (depending on the measurement, on availability of data, and on what was of interest): the whole data set, R birds only, R birds that successfully reached the wood shavings platform only or test numbers 1 and 4 only. In some cases, due to sparse and/or missing data, it was necessary to obtain results from simpler fixed effects models with fewer interaction terms than 4 way. For the GLMM for whether a bird successfully reached the wood shavings platform, for all data only main effects were included whereas for R birds only interactions up to 3 way were included. For LMMs applied to behaviors on the wood shavings platform for successful birds, only interactions up to 3 way were included.
Home Pen Behavior
Classifications from the original ethogram of behaviors statistically analyzed were feeding (pecking at feed), foraging (pecking and scratching at litter), drinking (pecking at and swallowing water), object pecking (pecking at feeder, drinker, pen walls), preening, walking (including running), inactive (standing/sitting/sleeping), as well as active (walking, running, or foraging classes combined). Behaviors dustbathing, aggressive pecking, nonaggressive pecking, and other occurred too rarely to be statistically analyzed. For each of these classifications, the data was summarized up (over the 10 scans for lights on sessions and the 5 scans for light off sessions) into tables of counts by the classes for each bird in each session, prior to subsequent statistical analyses. So that is 18 tables per bird (3 wk by 6 sessions per 24-h period). These tables of counts were constructed both including the not visible class and excluding it. Initial data exploration for the 8 resulting classifications suggested that exclusion of not visible birds had no impact on the results and so results presented here exclude these scans. Initial data exploration showed that whether lights were on or off dominated behaviors, with many behavior counts very low at night, so it was necessary to analyze data separately for lights on and lights off.
In order to analyze the proportions of scans in each different behavior class GLMMs were fitted to the binomial count for that behavior class for each bird in each session with binomial total the number of scans for which the bird was visible in that session, logit link function and binomially distributed errors.
Random effects were included for batch, for individual pens of birds and individual birds, and for different weeks within pens and within birds, and for different sessions within pens and weeks (flocking behavior), and dispersion was fixed at 1. All the variance components were fairly small apart from the variability between birds and for flocking behavior for some behavior classes.
Fixed effects were included for the week of observation (a proxy for bird age), the time during lights on (8:00–10:30, 10:30–13:30, 13:30–16:00 h) or lights off (16:30–20:00, 22:30–01:45, 4:30–7:45 h) and dietfeedtime (AL, Ram, Rpm), all fitted as 3 level factors, and all interactions. Where the data was sparse it was necessary to obtain results from simpler fixed effects models with fewer interaction terms than 3 way. Only main effects were included for feeding, drinking, foraging, and object pecking when lights were off and only interactions up to 2 way were included for active (locomotion or foraging) and locomotion when lights were off and feeding when lights were on.
Physiological Measures
LMMs were fitted to bird and organ weights (log transformed), crop content weight (log plus 1 transformed), an ordinal variable for the crop content score (1: Empty, 2: Wet mush, 3: Solid mush, 4: Dry pellets/solid mush, 5: Dry pellets), blood plasma NEFA, and glucose concentrations (both log transformed) and expression measures (log transformed). Expression measures were standardized by dividing by values for the housekeeping gene before calculating logs.
Random effects were included for batch, the 4 different days on which PMs were done (identical to the lab day for expression measures), each pen of birds and for LMMs only blocks of these pens within each batch, the 4 different days on which PMs were done within pens and individual birds (the residual). Fixed effects were included for the 3 PM teams (main effect only) and for bird age (fitted as a 2 level factor), dietfeedtime (AL, Ram, Rpm) and the time interval relative to feeding category (1.2–3.2, 6.9–8.7, 15.9–18.3, 22.0–23.8 h) at which birds were tested and all interactions.
For LMMs models were fitted to all data and also to data omitting outliers (as defined by the linear mixed model residuals) to confirm that results for all data reported here are not just attributable to the outliers.
Pearson's correlation coefficient (ρ) was calculated between continuous measures.
All Statistical Analyses
Fixed effects were tested sequentially in the order given above, so, for example, effects of dietfeedtime and time relative to feeding or time in the day are tested after adjusting for effects of apparatus, tester, postmortem team, and so on. Although the experimental design ensured balance with these factors, where only a subset of data was analyzed (such as behavior on the foraging platform) confounding is likely to occur so test order is important. Alternative parameterizations of the above models were fitted including fixed effects of both diet (AL,R) and of dietfeedtime (AL,Ram,Rpm), because testing dietfeedtime after diet provides an explicit test of whether there is an effect of feeding time for the R birds (i.e., tests explicitly for a difference between Ram and Rpm). This also provides explicit tests of whether there is evidence that an effect of time relative to feeding, or time in the day, differs for Ram and Rpm birds or whether significant interactions between dietfeedtime and times are just due to differences in trends between AL and R birds.
P values are based on approximate F tests when available but otherwise are based on Wald tests. Model estimates (±SE) were obtained from the model with dietfeedtime (not diet) in the fixed effects back transformed onto the original scale to aid interpretation. Post hoc tests were carried out by using Fisher's least significant difference test for which residual degrees of freedom were the same as those used in the approximate F tests.
All data was compiled in MS Excel. Genstat 18 was used for the study design, data processing, and all statistical analyses.
RESULTS
Ad Libitum vs. Restricted Diets
The feed treatment had the largest effect on all measures compared to other factors. As expected, the birds fed AL were heavier than both R treatment birds when weighed before culling at 12 wk of age (P < 0.001; Table 1). Consistent with their greater body weight, AL birds also had heavier gall bladders (empty), gizzards, livers, pancreas, and proventriculus (all P ≤ 0.001). Correlations were highest between bird weight, and weights of liver, pancreas, and proventriculus (all Pearson's ρ > 0.88). Averaged over sampling times, AL birds had slightly higher crop content scores (indicating more recent feeding; P = 0.033) and lower plasma NEFA concentrations (P < 0.001) than Ram and Rpm birds. Additionally, AGRP and NPY mRNA levels in the basal hypothalamus were lower in AL than both R treatment birds (P < 0.001) while POMC and PPY mRNA were higher in AL birds (P < 0.001 and P = 0.002, respectively). PPY results were previously reported in (Reid et al., 2017). AGRP and NPY mRNA levels in the basal hypothalamus were highly correlated (Pearson's ρ = 0.83), while CCKAR, insulin and PPY mRNA levels in the pancreas were also correlated (Pearson's ρ > 0.64). Correlation between CART and POMC mRNA levels in the basal hypothalamus was more marginal (Pearson's ρ = 0.45) as was correlation between NPY and GCG in the pancreas (Pearson's ρ = 0.45). There was no statistically significant effect of feed quantity treatment on any of the other physiological measures (P > 0.05).
Table 1.
Effects of the feed treatments on physiological measurements.
| Feed treatment |
Statistics |
|||||
|---|---|---|---|---|---|---|
| Physiological measures | AL | Ram | Rpm | SEM | F or Wald† | P |
| Weight at PM (g) | 8.012a (3,016) | 7.083b (1,191) | 7.098b (1,210) | 0.015 | 1,234.32 | <0.001 |
| Plasma NEFA | −2.45a (0.086) | −2.09b (0.124) | −1.88b (0.152) | 0.14 | 16.31 | <0.001 |
| Plasma glucose | 2.409 (11.1) | 2.410 (11.1) | 2.377 (10.8) | 0.043 | 3.05 | ns |
| AGRP (bh) | −6.71a | −3.67b | −3.61b | 0.35 | 252.59 | <0.001 |
| NPY (bh) | −4.00a | −3.09b | −2.97b | 0.10 | 84.82 | <0.001 |
| POMC (bh) | −4.29b | −5.11a | −5.19a | 0.28 | 26.17 | <0.001 |
| CART (bh) | −4.01 | −4.09 | −3.98 | 0.27 | 1.55 | ns |
| CCKAR (pan) | −0.67 | −0.42 | −0.46 | 0.12 | 1.57 | ns |
| GCG (pan) | −2.31 | −1.67 | −2.48 | 0.35 | 2.31 | ns |
| insulin (pan) | 2.46 | 2.29 | 2.13 | 0.14 | 1.32 | ns |
| NPY (pan) | −5.60 | −5.43 | −5.38 | 0.10 | 2.25 | ns |
| PPY (pan) | 3.82a | 3.08b | 2.98b | 0.19 | 10.74 | 0.002 |
| Gall bladder (empty) (g) | −0.848c (0.428) | −1.586a (0.205) | −1.462b (0.232) | 0.044 | 80.94 | <0.001 |
| Gizzard (g) | 4.173a (64.9) | 3.970b (53.0) | 3.988b (54.0) | 0.048 | 12.81 | 0.001 |
| Liver (g) | 4.18a (65.3) | 3.031b (20.7) | 3.11b (22.5) | 0.042 | 399.62 | <0.001 |
| Pancreas (g) | 1.812a (6.12) | 1.056b (2.88) | 1.040b (2.83) | 0.023 | 440.99 | <0.001 |
| Proventriculus (g) | 2.371c (10.7) | 1.599b (4.95) | 1.542a (4.67) | 0.021 | 618.84 | <0.001 |
| Crop content weight (g) | 2.87 (16.7) | 2.43 (10.4) | 2.58 (12.2) | 0.16 | 2.23 | ns |
| Crop content Score (1–5) | 2.84a | 2.52b | 2.56b | 0.21 | 6.83 | 0.033 |
Values are means and SEMs estimated from LMMs. If the data were analysed on transformed scale these values are shown, with back-transformed values shown in brackets where biologically meaningful.
bh, measured from the basal hypothalamus; pan, measured from the pancreas; ns, nonsignificant (P > 0.05); SEM, highest standard error of the mean for each factor.
abcSuperscripted letters indicate where differences lie.
Treatments sharing a letter do not differ significantly from each other.
ndf = 2, ddf = 13-181, italic text indicates Wald tests used.
In the foraging motivation test, R birds spent less time on the start platform, were more successful at completing the test (reaching the wood shavings platform), had a shorter latency to reach the wood shavings platform and spent longer on it than AL birds (all P < 0.001; see Table 2). While on the start platform, AL birds, when compared to R, stand/sit or preen more, and forage or walk less (all P < 0.001), with Rpm birds performing more walking than Ram birds (P = 0.043). Both R treatment birds spent similar amounts of time foraging, walking or standing on the wood shavings platform (P > 0.05) but Ram birds spent slightly more time preening (P = 0.044).
Table 2.
Effects of the feed treatments on the foraging motivation test measurements.
| Feed treatment |
Statistics |
||||||
|---|---|---|---|---|---|---|---|
| Foraging motivation test measurements | AL | Ram | Rpm | SEM | F or Wald† | P | |
| Foraging test success (proportion of birds) (GLMM)§ | 5.46a (0.004) | 0.51b (0.624) | 0.27b (0.567) | 0.84 | 42.35 | <0.001 | |
| R birds: Foraging test success (proportion of birds) (GLMM) | NA | 0.59 (0.644) | 0.29 (0.572) | 0.43 | 0.22 | ns | |
| Latency to wood shavings platform (s) | 89.1a (1,200 s) | 55.2b (809 s) | 53.8b (781 s) | 3.0 | 97.12 | <0.001 | |
| R birds: Latency to wood shavings platform (s) | NA | 55.2 (809 s) | 53.8 (781 s) | 3.5 | 0.03 | ns | |
| Proportion of test spent on start platform | 78.6a (0.961) | 44.6b (0.492) | 44.9b (0.498) | 2.8 | 126.26 | <0.001 | |
| Proportion of test spent on wood shavings platform | 0.5b (0.000) | 19.3a (0.109) | 18.6a (0.102) | 1.9 | 74.22 | <0.001 | |
| R birds: Proportion of test spent on wood shavings platform | NA | 19.3 (0.109) | 18.6 (0.102) | 2.2 | 0.41 | ns | |
| Proportion of time on the start platform spent standing/sitting (test numbers 1 and 4) | 71.9a (0.904) | 49.4b (0.576) | 51.0b (0.604) | 1.7 | 73.94 | <0.001 | |
| Proportion of time on the start platform spent standing (test numbers 1 and 4) | 44.8b (0.497) | 49.4ab (0.577) | 51.0a (0.603) | 1.8 | 6.18 | 0.009 | |
| Proportion of time on the start platform spent preening (test numbers 1 and 4) | 5.1c (0.0078) | 13.3a (0.0528) | 8.8b (0.0235) | 2.3 | 11.72 | <0.001 | |
| Proportion of time on the start platform spent foraging (test numbers 1 and 4) | 14.2b (0.061) | 26.9a (0.204) | 26.6a (0.200) | 1.6 | 24.75 | <0.001 | |
| Proportion of time on the start platform spent walking (test numbers 1 and 4) | 3.54c (0.0038) | 11.15b (0.0374) | 13.66a (0.0558) | 0.91 | 37.93 | <0.001 | |
| Successful R birds: Proportion of time on the wood shavings platform spent standing | NA | 16.1 (0.0767) | 16.2 (0.078) | 2.0 | 0.29 | ns | |
| Successful R birds: Proportion of time on the wood shavings platform spent preeningǂ | NA | 9.5a (0.0270) | 4.8b (0.0071) | 1.4 | 4.06 | 0.044 | |
| Successful R birds: Proportion of time on the wood shavings platform spent foraging | NA | 60.7 (0.760) | 65.7 (0.831) | 2.6 | 0.14 | ns | |
| Successful R birds: Proportion of time on the wood shavings platform spent walking | NA | 9.4 (0.0265) | 10.1 (0.0308) | 1.7 | 2.32 | ns | |
Values are means and SEMs estimated from LMMs or GLMMs. If the data were analyzed on transformed scale these values are shown, with back-transformed values shown in brackets where biologically meaningful.
ns, nonsignificant (P > 0.05); SEM, highest standard error of the mean for each factor.
abcSuperscripted letters indicate where differences lie.
Treatments sharing a letter do not differ significantly from each other.
ndf = 2 or 1 for R birds only, ddf = 18-183, italic text indicates Wald tests used.
Only main fixed effects included.
Only 2 way interaction and main fixed effects included.
During lights on in their home pens, averaging over time in the day effects, AL birds spent more time feeding than R birds, and Ram birds drank more and did more object pecking and spent less time being inactive than Rpm and AL birds (all P < 0.001; see Table 3); although there were also significant interactions between feed treatment and time of day. AL birds also preened more and walked less than R birds (P < 0.001), whilst Rpm birds preened more than Ram birds (P = 0.029); however all birds performed similar amounts of foraging (P > 0.05). In the lights off period, averaging over time in the night effects, Rpm birds foraged more, drank more, did more object pecking and were more active overall than the Ram and AL birds (P ≤ 0.006). They also walked more than the Ram birds with AL birds walking the least (P = 0.001). Ram birds spent less time feeding than AL and Rpm birds (P < 0.001) and Rpm birds spent less time being inactive during lights off (P < 0.001). However, there were significant interactions between feed treatment and time of night.
Table 3.
Effects of the feed treatments on the home pen behavior measurements.
| Feed treatment |
Statistics |
|||||
|---|---|---|---|---|---|---|
| Lights ON | AL | Ram | Rpm | SEM | F or Wald† | P |
| Proportion of time spent feedingǂ | −2.32a (0.0891) | −3.15b (0.0411) | −3.53b (0.0285) | 0.19 | 11.69 | <0.001 |
| Proportion of time spent foraging | −1.94 (0.125) | −2.02 (0.118) | −1.76 (0.147) | 0.36 | 1.01 | ns |
| Proportion of time spent drinking | −2.33b (0.089) | −1.16a (0.239) | −2.08b (0.111) | 0.15 | 17.46 | <0.001 |
| Proportion of time spent object pecking | −3.59b (0.0268) | −1.97a (0.1224) | −3.24b (0.0376) | 0.20 | 18.44 | <0.001 |
| Proportion of time spent preening | −1.89a (0.1315) | −2.92c (0.0513) | −2.54b (0.0729) | 0.12 | 31.93 | <0.001 |
| Proportion of time spent walking | −2.65b (0.0657) | −2.10a (0.1091) | −2.09a (0.1103) | 0.14 | 21.14 | <0.001 |
| Proportion of time spent being active (walking + foraging) | −1.39b (0.200) | −1.14ab (0.242) | −0.90a (0.289) | 0.32 | 4.70 | 0.021 |
| Proportion of time spent being inactive (standing, sitting, sleeping) | −0.63a (0.347) | −2.17b (0.103) | −0.71a (0.330) | 0.22 | 40.51 | <0.001 |
| Lights OFF | AL | Ram | Rpm | SEM | F or WaldŦ | P |
|---|---|---|---|---|---|---|
| Proportion of time spent feeding§ | −4.34a (0.01289) | −6.41b (0.00164) | −4.62a (0.00972) | 0.42 | 20.47 | <0.001 |
| Proportion of time spent foraging§ | −6.59b (0.00137) | −7.69b (0.00046) | −5.00a (0.00671) | 0.80 | 5.97 | 0.006 |
| Proportion of time spent drinking§ | −4.46b (0.0114) | −5.41c (0.0044) | −3.18a (0.0398) | 0.30 | 24.04 | <0.001 |
| Proportion of time spent object pecking§ | −6.92b (0.000986) | −8.06b (0.000316) | −5.79a (0.003042) | 0.76 | 12.33 | 0.002 |
| Proportion of time spent preening | −3.20 (0.0392) | −3.31 (0.0352) | −3.53 (0.0285) | 0.13 | 2.62 | ns |
| Proportion of time spent walkingǂ | −4.41b (0.0121) | −4.00b (0.0180) | −3.34a (0.0341) | 0.20 | 9.30 | 0.001 |
| Proportion of time spent being active (walking + foraging)ǂ | −4.20b (0.0147) | −3.91b (0.0196) | −3.03a (0.0461) | 0.19 | 12.47 | <0.001 |
| Proportion of time spent being inactive (standing, sitting, sleeping) | 2.35b (0.913) | 2.73a (0.939) | 1.78c (0.855) | 0.11 | 19.67 | <0.001 |
Values are means and SEMs estimated from GLMMs. If the data were analysed on transformed scale these values are shown, with back-transformed values shown in brackets where biologically meaningful.
ns, non-significant (P > 0.05); SEM, highest standard error of the mean for each factor.
abcSuperscripted letters indicate where differences lie.
Treatments sharing a letter do not differ significantly from each other.
ndf = 2, ddf = 19-290.
ndf = 2, ddf = 22-129. Italic text indicates Wald tests used.
Only 2 way interaction and main fixed effects included.
Only main fixed effects included.
Time Relative to Last Meal
Bird weight at culling was lighter at 1-3 h and slightly heavier from 7 to 18 h after feeding then decreased again before the next feeding time (P = 0.047; Table 4). Averaged over feed treatments, crop content was heaviest right after being fed (1–3 h) and decreased over time, being lightest right before their next feed (22–24 h; P < 0.001) and crop content scores decreased as time after feeding increased (P < 0.001); although there were some significant interactions between feed treatment and time since feeding for these measures. Averaged over feed treatments, plasma NEFA concentrations decreased at 7 to 9 h since the last feed then increased to their highest before being fed the next meal (P < 0.001) while plasma glucose concentrations were highest 1 to 3 h since the last feed then decreased with time maintaining the same level from 16 h since the last feed (P < 0.001); although again there were some significant interactions of feed treatment and time since feeding for these measures. Of all the brain and pancreas gene expression measures, only AGRP mRNA expression in the basal hypothalamus changed with time since feeding. This was highest right after feeding, then decreased and stayed fairly constant from 7 h after feeding (P = 0.028). Empty gallbladder weights were heaviest at 22 to 24 h since the last feed (P = 0.009) while gizzard weight decreased from 16 to 18 h post-feeding (P = 0.012). Averaged over feed treatments, liver weights were lowest at 1 to 3 h, and then increased at 7 to 18 h before decreasing at the time before the next feed (P < 0.001); although there were some marginally significant interactions between feed treatment and time since feeding. There was no effect of time relative to last meal on any other physiological measures (P > 0.05; Table 4).
Table 4.
Effects of time since last feed on physiological measurements.
| Time since last feed |
Statistics |
||||||
|---|---|---|---|---|---|---|---|
| Physiological measures | 1–3 | 7–9 | 16–18 | 22–24 | SEM | F or WaldŦ | P |
| Weight at PM (g) | 7.375b (1,595) | 7.431a (1,688) | 7.413ab (1,658) | 7.371b (1,590) | 0.018 | 2.70 | 0.047 |
| Plasma NEFA | −2.32b (0.098) | −2.75a (0.064) | −2.04c (0.130) | −1.45d (0.234) | 0.15 | 41.21 | <0.001 |
| Plasma glucose | 2.465a (11.8) | 2.398b (11.0) | 2.354c (10.5) | 2.377bc (10.8) | 0.043 | 13.10 | <0.001 |
| AGRP (bh) | −4.23b | −4.86a | −4.82a | −4.74a | 0.36 | 9.10 | 0.028 |
| NPY (bh) | −3.24 | −3.38 | −3.44 | −3.35 | 0.11 | 1.95 | ns |
| POMC (bh) | −4.79 | −4.86 | −4.93 | −4.87 | 0.28 | 0.29 | ns |
| CART (bh) | −4.07 | −3.97 | −4.04 | −4.03 | 0.28 | 0.49 | ns |
| CCKAR (pan) | −0.45 | −0.52 | −0.47 | −0.64 | 0.14 | 0.37 | ns |
| GCG (pan) | −2.09 | −1.94 | −2.69 | −1.89 | 0.39 | 1.15 | ns |
| insulin (pan) | 2.44 | 2.40 | 2.26 | 2.08 | 0.16 | 1.04 | ns |
| NPY (pan) | −5.49 | −5.50 | −5.49 | −5.39 | 0.11 | 0.30 | ns |
| PPY (pan) | 3.42 | 3.37 | 3.21 | 3.17 | 0.20 | 0.70 | ns |
| Gall bladder (empty) (g) | −1.330a (0.264) | −1.377a (0.252) | −1.327a (0.265) | −1.159b (0.314) | 0.050 | 3.97 | 0.009 |
| Gizzard (g) | 4.080ab (59.1) | 4.096a (60.1) | 4.016bc (55.5) | 3.983c (53.7) | 0.046 | 3.77 | 0.012 |
| Liver (g) | 3.359c (28.8) | 3.528a (34.1) | 3.476ac (32.3) | 3.401bc (30.0) | 0.041 | 7.07 | <0.001 |
| Pancreas (g) | 1.287 (3.62) | 1.325 (3.76) | 1.293 (3.65) | 1.307 (3.70) | 0.026 | 0.45 | ns |
| Proventriculus (g) | 1.838 (6.29) | 1.849 (6.35) | 1.815 (6.14) | 1.846 (6.33) | 0.024 | 0.45 | ns |
| Crop content weight (g) | 3.79a (43.2) | 3.46a (30.9) | 2.36b (9.5) | 0.90c (1.5) | 0.14 | 128.79 | <0.001 |
| Crop content Score (1–5) | 3.50a | 2.80b | 2.44b | 1.81c | 0.22 | 98.33 | <0.001 |
Values are means and SEMs estimated from LMMs. If the data were analyzed on transformed scale these values are shown, with back-transformed values shown in brackets where biologically meaningful.
bh, measured from the basal hypothalamus; pan, measured from the pancreas; ns, nonsignificant (P > 0.05); SEM, highest standard error of the mean for each factor
abcSuperscripted letters indicate where differences lie.
Treatments sharing a letter do not differ significantly from each other.
ndf = 3, ddf = 147-181, italic text indicates Wald tests used.
For the foraging motivation test, averaging over feed treatments, there was no effect of time since last feeding on test success (reaching the wood shavings platform) or time spent on the start platform (P > 0.05; Table 5); although there were some significant interactions of feed treatment and time since last feed. However, latency to reach the wood shavings platform decreased at 22 to 24 h after the last feed (P = 0.028) but time since the last feed did not affect the proportion of time birds spent on the wood shavings platform (P > 0.05). On the wood shavings platform (Ram and Rpm birds only in analysis) the amount of standing and walking birds performed 7 to 18 h since last feeding was less than just before their next feed (P = 0.010 and P = 0.012, respectively); however the amount of time spent standing and walking at 1 to 3 h after their last feed was not significantly different from either of these times since last feeding (P > 0.05). These birds also had a corresponding peak in foraging behavior at 7 to 18 h since their last feed which decreased at 22 to 24 h (P = 0.020). For behavior on the start platform, birds were found to preen and walk more (P = 0.012, 0.013, respectively) and forage less (P < 0.001) at 22 to 24 h since their last feed and stand and sit more 7 to 18 h since their last feed (P = 0.027) compared to 1 to 3 h since their last feed; however, standing and sitting at 22 to 24 h was not significantly different from either of those times since last feed (P > 0.05).
Table 5.
Effects of the time since last feed on the foraging motivation test measurements.
| Time since last feed |
Statistics |
|||||
|---|---|---|---|---|---|---|
| Foraging motivation test measurements | 1–3 | 7–18 | 22–24 | SEM | F or WaldŦ | P |
| Foraging test success (proportion of birds) (GLMM)§ | −1.91 (0.129) | −1.96 (0.124) | −0.82 (0.305) | 0.50 | 4.72 | ns |
| R birds: Foraging test success (proportion of birds) (GLMM) | −0.14 (0.465) | −0.01 (0.499) | 1.47 (0.813) | 0.58 | 4.58 | ns |
| Latency to wood shavings platform (s) | 69.1a (1048 s) | 69.4a (1051 s) | 59.6b (893 s) | 3.3 | 7.13 | 0.028 |
| R birds: Latency to wood shavings platform (s) | 59.8a (896 s) | 59.3a (887 s) | 44.4b (587 s) | 4.7 | 7.69 | 0.021 |
| Proportion of test spent on start platform | 55.8 (0.684) | 58.3 (0.724) | 54.0 (0.654) | 3.1 | 1.56 | ns |
| Proportion of test spent on wood shavings platform | 10.9 (0.036) | 12.3 (0.046) | 15.2 (0.068) | 2.1 | 2.57 | ns |
| R birds: Proportion of test spent on wood shavings platform | 15.6 (0.072) | 18.5 (0.101) | 22.8 (0.15) | 3.0 | 2.94 | ns |
| Proportion of time on the start platform spent standing/sitting (test numbers 1 and 4) | 54.2b (0.657) | 59.5a (0.743) | 58.6ab (0.729) | 1.8 | 3.67 | 0.027 |
| Proportion of time on the start platform spent standing (test numbers 1 and 4) | 48.6 (0.562) | 48.0 (0.551) | 48.7 (0.564) | 2.0 | 0.05 | ns |
| Proportion of time on the start platform spent preening (test numbers 1 and 4) | 8.2b (0.0201) | 7.1b (0.0152) | 11.9a (0.0428) | 2.4 | 4.54 | 0.012 |
| Proportion of time on the start platform spent foraging (test numbers 1 and 4) | 27.1a (0.207) | 22.9a (0.151) | 17.7b (0.093) | 1.8 | 7.27 | <0.001 |
| Proportion of time on the start platform spent walking (test numbers 1 and 4) | 8.82b (0.0235) | 8.00b (0.0194) | 11.53a (0.0399) | 0.98 | 4.45 | 0.013 |
| Successful R birds: Proportion of time on the wood shavings platform spent standing | 16.5ab (0.0809) | 12.5b (0.0471) | 19.4a (0.1102) | 2.8 | 9.12 | 0.010 |
| Successful R birds: Proportion of time on the wood shavings platform spent preeningǂ | 6.1 (0.0113) | 7.6 (0.0173) | 7.8 (0.0183) | 2.0 | 0.46 | ns |
| Successful R birds: Proportion of time on the wood shavings platform spent foraging | 61.8ab (0.777) | 68.2a (0.862) | 59.6b (0.744) | 3.8 | 7.80 | 0.020 |
| Successful R birds: Proportion of time on the wood shavings platform spent walking | 10.0ab (0.0302) | 6.6b (0.0133) | 12.6a (0.0474) | 2.3 | 8.82 | 0.012 |
Values are means and SEMs estimated from LMMs or GLMMs. If the data were analyzed on transformed scale these values are shown, with back-transformed values shown in brackets where biologically meaningful.
ns, nonsignificant (P > 0.05); SEM, highest standard error of the mean for each factor.
abcSuperscripted letters indicate where differences lie.
Treatments sharing a letter do not differ significantly from each other.
ndf = 2, ddf = 167-183, italic text indicates Wald tests used.
Only main fixed effects included.
Only 2 way interaction and main fixed effects included.
In the home pen during the lights on period, averaging over feed treatments, birds decreased their drinking and object pecking (P < 0.001; see Table 6) and to a lesser extent foraging (P = 0.042), and increased walking and being inactive (P < 0.001), with time in the day; although there were some significant interactions between feed treatment and time in the day. Preening had a peak around the mid-light period (P < 0.001). During the dark period, averaging over feed treatments, the amounts of drinking had a dip in the middle of the night (P = 0.003) when inactivity peaked (P < 0.001), object pecking was highest just after lights off (P = 0.014), and walking, preening and overall activity increased shortly before the lights came back on (P < 0.001); although there were significant interactions between feed treatment and time in the day.
Table 6.
Effects of the time in the day on the home pen behaviour measurements.
| Time in the day/night |
Statistics |
|||||
|---|---|---|---|---|---|---|
| Lights ON | 8:00–10:30 | 10:30–13:30 | 13:30–16:00 | SEM | F or Wald‡ | P |
| Proportion of time spent feedingǂ | −2.78 (0.0582) | −3.20 (0.0391) | −3.02 (0.0467) | 0.16 | 1.49 | ns |
| Proportion of time spent foraging | −1.79a (0.143) | −1.87ab (0.134) | −2.06b (0.113) | 0.35 | 3.25 | 0.042 |
| Proportion of time spent drinking | −1.49a (0.184) | −1.89b (0.131) | −2.19c (0.101) | 0.10 | 38.90 | <0.001 |
| Proportion of time spent object pecking | −2.61a (0.0686) | −3.05b (0.0451) | −3.14b (0.0414) | 0.14 | 8.17 | <0.001 |
| Proportion of time spent preening | −2.54b (0.0732) | −2.23a (0.0975) | −2.58b (0.0703) | 0.10 | 9.03 | <0.001 |
| Proportion of time spent walking | −2.38b (0.0845) | −2.44b (0.0799) | −2.02a (0.1175) | 0.14 | 27.94 | <0.001 |
| Proportion of time spent being active (walking + foraging) | −1.12 (0.246) | −1.23 (0.227) | −1.09 (0.252) | 0.31 | 1.56 | ns |
| Proportion of time spent being inactive (standing, sitting, sleeping) | −1.76b (0.147) | −0.93a (0.282) | −0.82a (0.306) | 0.20 | 62.19 | <0.001 |
| Lights OFF | 16:30–20:00 | 22:30–01:45 | 4:30–07:45 | SEM | F or Wald¥ | P |
|---|---|---|---|---|---|---|
| Proportion of time spent feeding§ | −5.00 (0.00671) | −5.49 (0.00410) | −4.89 (0.00750) | 0.26 | 5.08 | ns |
| Proportion of time spent foraging§ | −5.88 (0.00278) | −7.10 (0.00083) | −6.29 (0.00185) | 0.52 | 2.87 | ns |
| Proportion of time spent drinking§ | −4.26a (0.0140) | −4.82b (0.0080) | −3.98a (0.0184) | 0.21 | 6.13 | 0.003 |
| Proportion of time spent object pecking§ | −6.16a (0.002106) | −7.40b (0.000612) | −7.21b (0.000737) | 0.55 | 8.48 | 0.014 |
| Proportion of time spent preening | −3.57b (0.0275) | −3.84b (0.0210) | −2.64a (0.0668) | 0.14 | 33.33 | <0.001 |
| Proportion of time spent walkingǂ | −4.08b (0.0167) | −4.28b (0.0136) | −3.39a (0.0327) | 0.19 | 10.31 | <0.001 |
| Proportion of time spent being active (walking + foraging)ǂ | −3.79b (0.0222) | −4.12b (0.0160) | −3.24a (0.0376) | 0.17 | 11.07 | <0.001 |
| Proportion of time spent being inactive (standing, sitting, sleeping) | 2.42b (0.918) | 2.76a (0.941) | 1.68c (0.842) | 0.10 | 29.94 | <0.001 |
Values are means and SEMs estimated from GLMMs. If the data were analyzed on transformed scale these values are shown, with back-transformed values shown in brackets where biologically meaningful.
ns, nonsignificant (P > 0.05); SEM, highest standard error of the mean for each factor.
abcSuperscripted letters indicate where differences lie.
Treatments sharing a letter do not differ significantly from each other.
ndf = 2, ddf = 104-156.
ndf = 2, ddf = 83-209; italic text indicates Wald tests used.
Only 2 way interaction and main fixed effects included.
Only main fixed effects includeFigure Titles.
Feed Treatment by Time Relative to Last Meal Interactions
Crop content weight was fairly consistent for AL birds across the day, with a small peak at 7 to 9 h post-feed top up, while crop content was heaviest at the start for both Ram and Rpm birds then decreased as time since last feed increased (P < 0.001; Figure 3A). Birds fed AL had a fairly constant crop content score over time with a slight increase after 7 to 9 h post feed (ranging from a score of 2.5–3) but Ram and Rpm crop content scores were higher than for AL birds just after feeding and decreased as time since last feeding increased (ranging from scores of 4 down to 1, P < 0.001; Figure 3B), indicating a shift from fuller, drier crop contents to emptier/wetter. Plasma concentrations of NEFA also stayed fairly consistent for AL birds throughout the day but NEFA increased for Ram and Rpm birds by 22 to 24 h since being fed (P < 0.001; Figure 3C). Rpm and AL birds had consistent plasma glucose concentrations while glucose levels in Ram birds were higher just after being fed (1–3 h) and then decreased to a level similar to AL and Rpm by 7 to 9 h since being fed (P < 0.001; Figure 3D). Both R treatment birds had constant liver weights throughout the day (averaging Ram = 20.8 g, Rpm = 22.6 g, back-transformed values) but AL birds had an increase in liver weight after 7 h from the last feed (ranging from 55.9 to 73.0, back transformed values) (F6, 163 = 2.34, P = 0.034). For crop content weight, NEFA and liver weight the interaction between time in the day of feeding for R birds and the time since last feeding is not significant after adjusting for the interaction between AL vs. R birds and the time since last feeding, which confirms that the highly significant interactions are due only to differences in time since last feeding between AL and R birds and are unaffected by the time in the day of feeding for R birds. In contrast for glucose, the interaction between time of feeding for Ram and Rpm birds and the time since last feed is highly significant (P < 0.001) after adjusting for the interaction between AL vs. R birds and the time since last feed. There were no statistically significant interactions between treatment and time relative to last meal for any of the other physiological measures (P > 0.05).
Figure 3.
Back-transformed crop content weight (A), crop content score (B), plasma NEFA levels (C) and plasma glucose levels (D) for each feed treatment at the 4 sampling times relative to last feed (hours). Data are back-transformed means ± SEMs estimated from LMMs. * indicates a significant difference between AL and R treatments. ** indicates a significant difference between Ram and Rpm treatments. ‡ indicates a significant difference between AL/Rpm and Ram treatments. Ω indicates a significant difference between AL and Rpm treatments. ¥ indicates a significant difference between AL/Ram and Rpm
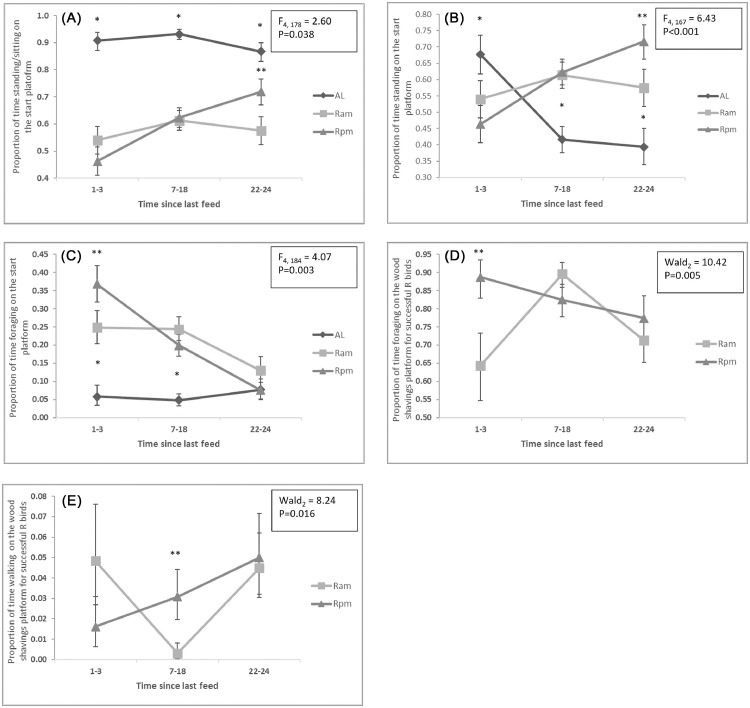
For the Foraging Test, as time since last feeding increased, AL birds maintained high levels of standing/sitting on the Start Platform, while Ram and Rpm birds increased their standing/sitting with time relative to feeding (P = 0.038; Figure 4A). AL birds decreased time standing on the Start Platform whilst R birds increased time standing on the start platform with time relative to feeding (P < 0.001; Figure 4B). AL birds spent little time foraging on the start platform whilst Ram and Rpm birds spent less time foraging with increased time relative to feeding (P = 0.003; Figure 4C). For all these behaviors the significant differences were between the AL and R feed treatments not between the differences in feed time of Ram and Rpm birds (P > 0.05). For the successful birds (i.e., they reached the wood shavings platform), Rpm birds showed a slight decrease with time since last feed in the amount of foraging and a slight increase in walking in relation to time since last feed, while Ram birds had a peak in foraging and a decrease in walking at 7 to 18 h since last feeding (foraging: P = 0.005; Figure 4D, walking: P = 0.016; Figure 4E). There were no significant interaction effects for any of the other motivation test measures (P > 0.05).
Figure 4.
Back-transformed means and SEM for the proportion of the test time spent standing/sitting (A), standing only (B) and foraging (C) on the start platform and for the proportion of the test time spent foraging (D) and walking (E) for the successful R birds on the wood shavings platform at the 3 sampling times relative to last feed (hours). Data are back-transformed means ± SEMs estimated from LMMs. * indicates a significant difference between AL and R treatments. ** indicates a significant difference between Ram and Rpm treatments.
In the home pen, as the daylight period progressed, AL birds increased their feeding, and Ram and Rpm birds decreased their feeding/pecking at the feeder by 10:30 h (P = 0.018; Figure 5A). AL and Ram birds maintained constant levels of foraging and walking throughout the day while Rpm birds decreased foraging and increased walking toward the end of the light period (P < 0.001 for both; Figure 5B, E). AL and Ram birds also drank more consistently throughout the light period, with Ram birds drinking more than AL birds and more so at the start, while Rpm birds starting off drinking more than AL birds, then decreased their drinking to lower levels than AL birds by the end of the light period (P < 0.001; Figure 5C). AL birds decreased their preening behavior after 13:30 h, while R birds maintained broadly constant lower levels of preening throughout the day (P < 0.001; Figure 5D). While Ram birds are less inactive throughout the day (Figure 5F) inactivity increased with time in the day more for R birds than AL birds (P < 0.001) but the trend was slightly different for Ram and Rpm birds (P = 0.026). In the dark period, AL and Ram birds increased preening and walking behavior in the period before lights on, whereas Rpm birds decreased preening and walking mid-dark period, with preening increasing again before lights on and walking being the highest just after lights off (P = 0.002, <0.001 respectively; Figures 6A and 6B). In general, AL and Ram birds were most active just before lights on while Rpm birds were most active just after lights off; although their activity levels were similar to those of the AL birds before lights on (P < 0.001; Figure 6C). Conversely, AL and Rpm birds were least inactive just before lights on whilst Rpm birds were least inactive just after lights off (P < 0.001; Figure 6D).
Figure 5.
Back-transformed means of the time spent feeding (A), foraging (B), drinking (C), preening (D), walking (E), and inactive (F) during the lights on period in the home pen. Data are back-transformed means±SEMs estimated from GLMMs. * indicates a significant difference between AL and R treatments. ** indicates a significant difference between Ram and Rpm treatments. ‡ indicates a significant difference between AL/Rpm and Ram treatments.
Figure 6.
Back-transformed means of the time spent preening (A), walking (B), active (C), and inactive (D) during the lights off period in the home pen. Data are back-transformed means ± SEMs estimated from GLMMs. * indicates a significant difference between AL and R treatments. ** indicates a significant difference between Ram and Rpm treatments. Ω indicates a significant difference between AL and Rpm treatments. ‡ indicates a significant difference between AL/Rpm and Ram treatments.
Foraging Test Increase in Cost
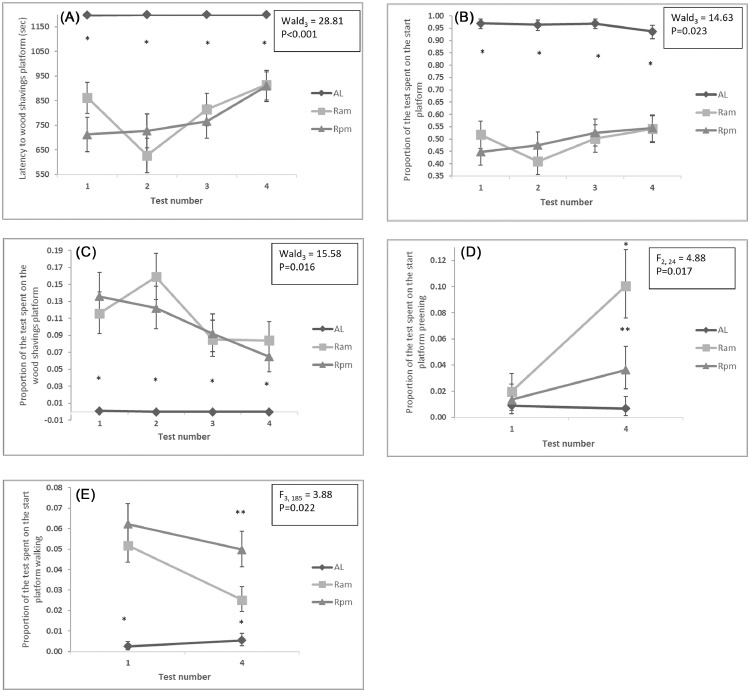
The proportion of R birds successfully reaching the wood shavings platform decreased with tests 3 and 4 (range mean ± SEM estimated from GLMM: test 1 (63%,79%), test 2 (66%,82%), test 3 (45%,65%), test 4 (30%,48%), Wald3 = 14.48, P = 0.002). AL birds maintained a high latency to reach the wood shavings platform throughout the 4 tests (P < 0.001) while the latency for Rpm increased in test 4 and Ram had a decreased latency in test 2 which increased again in tests 3 and 4 (P = 0.001; Figure 7A). AL birds consistently spent the majority of all tests on the start platform and little time on the wood shavings platform whilst R birds only spent about 50% of test time on the start platform (Figure 7B) and around 10% of test time on the wood shavings platform (Figure 7C). More variation between test numbers was seen for R than AL birds on the start platform (P = 0.023; Figure 7B) and on the wood shaving platform (P = 0.016; Figure 7C), with R birds generally spending less time on the wood shavings platform with increased test number. Although the trend with test number of time spent on the start and wood shaving platforms differed for Ram and Rpm this was not statistically significant (P > 0.05). The amount of preening and walking behavior on the start platform remained consistent for tests 1 and 4 for AL birds, whilst preening behavior increased in test 4 compared to test 1 for R birds (P = 0.017; Figure 7D) and walking decreased (P = 0.022; Figure 7E). These effects were more apparent for Ram birds although tests indicated no significant difference in behavior on the start platform between Ram and Rpm birds (P > 0.05). There were no significant interactions between feed treatment and test number for any other foraging motivation test measures (P > 0.05).
Figure 7.
Back-transformed means of the latency to reach the wood shaving platform (A), the proportion of the test time spent on the start platform (B) and the proportion of the test time spent on the wood shavings platform (C) over the 4 tests and the proportion of the test time spent preening (D) and walking (E) on the start platform over tests 1 and 4. Data are back-transformed means ± SEMs from LMMs. * indicates a significant difference between AL and R treatments. ** indicates a significant difference between Ram and Rpm treatments.
Other Factors Influencing Results
There were other factors in the design of the experiment and processing of samples that influenced the results. For example, the amount of time spent feeding in the home pens during lights on decreased in wk 3 (bird age 82 d) compared to the other test weeks (1, bird age 63–68 d and 2, bird age 69–75 d; P < 0.001; Supplementary Tables 1a and b). From the 3 teams collecting data during post mortem sampling, higher plasma glucose levels were recorded from samples collected by Team C than by Team B (P = 0.009; Supplementary Tables 2a and b) with Team A intermediate. Higher AGRP and POMC values were measured in tissues dissected by Team A than those for the other teams (P ≤ 0.001). Birds had a shorter latency to reach the wood shavings platform when tested in apparatus 3 compared to identically designed apparatuses 1 and 2 (back-transformed means––apparatus 1: 1,093 s, apparatus 2: 1,040 s, apparatus 3: 842 s, Wald2 = 7.17, P = 0.028) and for 1 of the testers (back-transformed means – tester LB: 950 s, tester LD: 1,049 s, Wald1 = 4.42, P = 0.036). Birds also spent a smaller proportion of the test time on the start platform standing in apparatus 2 compared to 1 and 3 (back-transformed means – apparatus 1: 0.67, apparatus 2: 0.41, apparatus 3: 0.59, F2, 174 = 3.68, P = 0.027) and a larger proportion of the test time walking on the start platform in apparatus 2 compared to 1 (back-transformed means: apparatus 1: 0.020, apparatus 2: 0.034, apparatus 3: 0.027, F2, 180 = 4.45, P = 0.013). While these results are interesting and important in relation to experimental design and balancing, these factors were not the main objectives of this experiment, so the full details of these results have been included as online Supplementary Materials.
DISCUSSION
Time Relative to Last Meal
The aim of this study was to determine what effects time since last feeding had on behavioral and physiological measures relating to feed intake and hunger while accounting for time of day in restricted and ad libitum fed broiler breeders. For the many of measures there was no evidence of effects related to the time since last feed from this study, for example, NPY, POMC, and CART gene expression, pancreas weight, foraging test success, proportions of time spent on the start and wood shavings platforms. Additionally home pen behavior was highly influenced by light/dark status, not time relative to last meal, leading to these measures being analyzed separately for the lights on and lights off periods.
However, some measures did show changes: AGRP mRNA expression was highest after being fed then decreased and maintained a consistent level from 7 to 9 h post-feed. At first sight, this is an unexpected finding, since in previous work, higher levels of AGRP are associated with feed restriction over the longer term. The high levels may suggest a lag between the activity of the AGRP neurones and the expression of AGRP as well as the need for the nutrient signals to be translated into satiety signals which can be read by the orexigenic second order neurones in the brain. It may also reflect the fact that AGRP seems to be involved with regulation of energy intake in the medium and long term in the chicken, rather than on a shorter term meal to meal basis (Boswell and Dunn, 2017). Latency to reach the wood shavings platform in the motivation test decreased just before being fed indicating an increase in motivation at that point. It has previously been found that motivation increases as time since last feeding increases (e.g., Savory and Lariviere, 2000) but these tests involve the birds working for a food reward whereas our motivation test only allowed appetitive feeding behavior (foraging) and may account for the lack of change in motivation until shortly before the next feeding (see D'Eath et al., 2009 for criticisms of feeding motivation tests).
Ad Libitum vs. Restricted Diets
Feed treatment (AL vs. Ram and Rpm) had a more significant impact on our measures than time since last feeding: AL birds were heavier (grew faster) and had some larger digestive organs (gall bladder, gizzard, liver, pancreas, and proventriculus) compared to R treatment birds. Additionally, AL birds had lower levels of physiological indicators of hunger, such as gene expression of the orexigenic neuropeptides AGRP and NPY, higher levels of factors related to satiety, such as expression of the anorectic gene POMC in the basal hypothalamus and PYY and PPY in the pancreas (Reid et al., 2017). However, previously we did not detect any changes in POMC mRNA expression in the AL vs. R fed birds but this may be due to a smaller sampler size or greater variation in the previous study (Dunn et al., 2013b). It may be that in an even larger powered study, differences in POMC expression over the 24 h would also be observed since it was numerically highest 1 to 3 h from lights on and then decreased with time since feeding.
Plasma NEFA concentrations were also lower in AL birds just prior to feeding, than in restricted-fed which indicates that AL birds were able to store more energy and R treatment birds had to use more energy reserves. CCK has previously been found to inhibit food intake (Savory, 1980), and its receptors are less abundant in chickens bred for fast growth (Dunn et al., 2013a). The types of broiler breeders used in this study are the parent stock to one of the fastest growing broiler strains commercially available (Ross 308: Aviagen, 2013). However, the results suggest that although CCKAR expression may underlie growth differences, the expression of this receptor is not responsive to diet-induced changes in growth and feed intake. Additionally, similar to the results found by de Jong et al (2003), although there were no differences found in plasma glucose concentrations between ad libitum and restricted fed birds at most time points sampled after feeding the levels in the Ram group immediately after feeding were higher.
We also found no changes in CART gene expression in response to food restriction. This may reflect that we used females in our study because previous observations of decreased CART mRNA in chickens in response to food deprivation or restriction have only been observed in males (Cai et al., 2015; Caughey et al., 2018). Additionally, circulating insulin and glucagon peptide levels are positively correlated, respectively, with feed intake and fasting in chickens (Simon, 1989; Richards and McMurtry, 2008) but we did not see any differences in their gene expressions between AL birds and R birds, despite a 3- to 4-fold difference in feed intake. This suggests that changes in circulating insulin and glucagon are produced by post-translational effects or changes in secretion rather than by altered gene expression as indicated for glucagon by Richards and McMurtry (2008).
From the behavioral data, AL birds spent more time feeding and less time walking during the lights on period in the home pen than the restricted fed birds. AL birds also spent less time walking during the dark period than Rpm birds; although the majority of the lights off period were spent inactive for all feed treatments as birds naturally sleep during darkness periods (Blokhuis, 1984). Additionally, AL birds were also less motivated (less successful, higher latency) to access an area with a foraging substrate than similarly aged Ram and Rpm birds.
These results are similar to our previous experiments (e.g., (Boswell et al., 1999, 2002; Dunn et al., 2013a,b; Dixon et al., 2014; Reid et al., 2017) and others who have compared ad libitum or larger portion fed broiler breeders with those that were restricted in food quantity (e.g., Hocking et al., 1993; de Jong et al., 2003; Bokkers and Koene, 2004; Lees et al., 2017; Arrazola et al., 2020).
Feed Treatment by Time Relative to Last Meal Interactions
The combination of time since last feeding and feed treatment corresponded with changes in several measures. As AL birds could feed throughout the day, they had similar crop weight scores with a significant peak at 7 to 9 h after feeding which then decreased over time, crop content scores which had a slight increase over time and NEFA concentrations which had a peak at 16 to 18 h after feeding. In contrast Ram and Rpm birds had very high crop weight and content scores just after feeding, while the crop essentially becomes empty 22 to 24 h after feeding. Our finding that both R treatment birds showed high plasma NEFA concentrations just before feeding is consistent with other research that found a peak in plasma NEFA at 20 to 24 h postfeeding in restricted birds (de Beer et al., 2008), and is consistent with a mobilization of body energy reserves.
Ram birds had a peak in plasma glucose right after being fed which decreased as time since feeding increased, while AL and Rpm birds had consistent glucose levels. This may be due to the slower digestion times in Rpm birds which were fed shortly before lights off and have less demand for glucose due to reduced activity in the dark period.
In a complementary paper (Reid et al., 2017) which sought to correct mistakes in the chicken genome regarding the PP fold family of peptides, we measured both PPY and PYY expression. This paper adds the expression in the pancreas of the third member of the family, NPY but the primary surprise was that PYY is expressed highly in the pancreas of chickens, something which is not an obvious feature of mammalian physiology. In the pancreas, PYY is known for its roles in maintaining glucose while PPY is related to satiety, principally thought to be secreted from the small intestine (Boey et al., 2007). In the Reid et al. (2017) study, we found that PPY was clearly different between feeding treatments and was numerically but not significantly lower in the AL group during the night.
This finding for PPY was replicated in this paper on a larger set of the same samples. PYY expression was higher in the pancreas of chickens than in other gut tissues sampled, and both PPY and PYY were higher in the pancreas of AL fed birds. PYY did change with time of sampling relative to feeding: PYY expression was higher 7 h after feeding in Ram and Rpm birds and lower in AL fed birds at night, reaching expression levels similar to those seen in both R treatment birds. In contrast in the present study NPY showed no effect of time of day or treatment consistent with its role in the gut as a neurotransmitter in peripheral nerves rather than as a secreted peptide. Therefore, PYY may also act as a short term satiety factor in birds (Reid et al., 2017) and may show good correlation with behavioral effects on feeding motivation which we aim to test further in the future.
In the foraging motivation test, the behavior of the Rpm birds on the platforms changed as the time post feeding increased; they increased standing and the standing/sitting combined measure on the start platform and walking on the wood shavings platform. Both Ram and Rpm birds decreased their foraging on the start platform by 22 to 24 h since last feed while Ram birds had a decrease in walking and increase in foraging at 7 to 18 h since last feed on the wood shavings platform but these reversed at 22 to 24 h with walking increasing and foraging decreasing. Broiler breeders have been shown to increase locomotor (walking) behavior leading up to feeding time especially when they are food restricted (Kostal et al., 1992; Savory and Maros, 1993). Ram birds did not show a similar increase in walking on the start platform but the dark period (when birds are generally less active) was just before their meal time, while Rpm birds were fed towards the end of the light period which may account for this difference (Savory, 1980; Dixon et al., 2016).
For home pen behavior, the time of day had a larger effect than time since last feeding. During the light period, AL birds increased their feeding throughout the light period but Ram and Rpm birds decreased their feeding and pecking at the feeder, most likely because the feeders got emptied quickly. AL and Ram birds also foraged and walked regularly throughout the light period while Rpm birds decreased foraging and increased walking as it got closer to their feeding time, showing the pre-feeding increase in locomotor behavior mentioned above and found in other studies (reviews in Mason and Mendl 1997; D'Eath et al., 2009). AL and Ram birds drank uniformly throughout the light period while the Rpm birds decreased their drinking. Restricted broiler breeders often display polydipsia as an attempt to gut fill and commercial breeders are often water restricted to prevent this (Savory et al., 1992). It is possible the Rpm birds drank enough to achieve gut fill earlier in the day and therefore did not need to continue at high drinking levels, or they may have reduced drinking to ‘leave room’ for their expected afternoon meal.
During the dark period, AL and Ram birds were most active before lights on with mainly walking and preening behavior, possibly in anticipation of their upcoming feeding (Mistlberger and Rusak, 1987; Wichman et al., 2012) whereas Rpm birds were most active after lights off (shortly after they were fed), again mainly with walking and preening behavior, but their activity levels were still similar to AL birds before lights on.
Foraging Test Success
A typical design of motivation tests is to increase the cost of accessing the resource over subsequent tests, which was done here as an increase in the length and depth of the water runway over 4 tests. Animals who are highly motivated to access a resource should continue to work for it, while those not motivated should stop responding (Dawkins, 1990). The proportion of birds reaching the wood shavings platform (successful birds) did decrease and the latency to the wood shavings platform did increase in tests 3 and 4. However, the success rate was only 25% at its highest and decreased to 9% at its lowest. These numbers are low because of the inclusion of AL birds in the analysis, who were rarely successful in completing the motivation test. Only 0.4% of AL birds were successful over the 4 tests combined while 62% Ram birds and 57% Rpm birds succeeded in reaching the wood shavings platform. AL birds always had access to feed so would not be expected to be motivated to reach an area where they can search for more food, especially given the increase in cost to reach that area over the 4 tests and this is similar to previous results (Dixon et al., 2014).
When examining the R treatments only, test success ranged from 46 to 69% for Ram birds and 44 to 59% for Rpm birds. These values are a little lower than those found in previous work, where we found a success rate of over 90% in R birds for the tests with the easier costs, reducing to over 60% success in the hardest test (Dixon et al., 2014). The main difference between the current study and Dixon et al (Dixon et al., 2014) was the training and testing of birds. Previously birds were given 10 min to reach the wood shavings platform and if they were successful, they were then allowed 5 more minutes to spend on the wood shavings platform (although birds could leave the wood shavings platform before the 5 min were up if they chose to). In this experiment, the test was ∼20 min in total and the birds could spend this time in any area of the apparatus that they chose. This means the birds had more time to visit the wood shavings platform and this may have led to more rapid learning that there is no food in the foraging area, which would de-value the reward (Apps et al., 2015). Successfully reaching the wood shavings platform was never rewarded with feed so it may be expected that the responses might extinguish (Bouton, 2004). However, a large proportion of the Ram and Rpm birds continued to work for access to the wood shavings platform even as the cost increased indicating that they were still motivated to search for food (Stephens and Krebs, 1986).
In conclusion, there were changes to several behavioral and physiological measures throughout the 24-h period. However, there are time windows where future data can be collected where changes due to time of day and/or time since last feeding will not have a major influence on findings. Additionally, this experiment provides further evidence that feed restricted birds show behavioral and physiological signs of hunger and that the amount of feed provided has the largest effect on most of these measures compared to any other feeding driven or diurnal rhythms produced by feeding time. In terms of hunger/satiety regulation, it appears that AGRP, NPY (basal hypothalamus), POMC, and plasma NEFA are most sensitive to feeding history in fast growing chickens than other potential physiological indicators. From an animal welfare perspective, restricted feeding of broiler breeders is still a concern that needs to be addressed. In subsequent studies we have used these measures to investigate the feeding of broiler breeders with adjusted diets to try and improve satiety and therefore welfare. For example, increased dietary fiber and/or lower energy and protein diets has been investigated. If a feeding solution to feed restriction in broiler breeders can be found, it has the potential to improve the welfare of millions of birds in the UK and worldwide.
Acknowledgments
ACKNOWLEDGMENTS
The work described in the present study was funded by BBSRC grant BB/L000288/1 ‘Investigating how the type and quantity of food affect foraging behavior and the neural circuits controlling feeding in broiler breeder chickens’. SRUC and BioSS receive funding from the Scottish Government's Environment, Agriculture and Food Strategic Research Programme and the Roslin Institute is funded by the BBSRC through Institute Strategic Grant funding BB/J004316/1. The authors would like to thank the staff at the Monogastric Science Research Centre for providing excellent animal care and the manuscript reviewers for their helpful feedback.
DISCLOSURES
The authors have no conflicts of interest to report.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.psj.2022.101838.
Appendix. Supplementary materials
REFERENCES
- Appleby M.C., Mench J.A., Hughes B.O. CABI Publishing; Wallingford, UK: 2004. Poultry Behaviour and Welfare. [Google Scholar]
- Apps M.A.J., Grima L.L., Manohar S., Husain M. The role of cognitive effort in subjective reward devaluation and risky decision-making. Sci. Rep. 2015;5:1–11. doi: 10.1038/srep16880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrazola A., Mosco E., Widowski T.M., Guerin M.T., Kiarie E.G., Torrey S. The effect of alternative feeding strategies during rearing on the behaviour of broiler breeder pullets. Appl. Anim. Behav. Sci. 2020;224 [Google Scholar]
- Aviagen. 2013. Ross 308 Parent stock management handbook. Accessed Feb. 2014. http://en.aviagen.com/brands/ross/products/ross-308.
- Blokhuis H.J. Rest in poultry. Appl. Anim. Behav. Sci. 1984;12:289–303. [Google Scholar]
- Boey D., Sainsbury A., Herzog H. The role of peptide YY in regulating glucose homeostasis. Peptides. 2007;28:390–395. doi: 10.1016/j.peptides.2006.07.031. [DOI] [PubMed] [Google Scholar]
- Bokkers E.A.M., Koene P. Motivation and ability to walk for a food reward in fast- and slow-growing broilers to 12 weeks of age. Behav. Process. 2004;67:121–130. doi: 10.1016/j.beproc.2004.03.015. [DOI] [PubMed] [Google Scholar]
- Boswell T., Dunn I.C. Regulation of agouti-related protein and pro-opiomelanocortin gene expression in the avian arcuate nucleus. Front. Endocrinol. 2017;8:1–11. doi: 10.3389/fendo.2017.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boswell T., Dunn I.C., Corr S.A. Hypothalamic neuropeptide Y mRNA is increased after feed restriction in growing broilers animals and husbandry. Poult. Sci. 1999;78:1203–1207. doi: 10.1093/ps/78.8.1203. [DOI] [PubMed] [Google Scholar]
- Boswell T., Li Q., Takeuchi S. Neurons expressing neuropeptide Y mRNA in the infundibular hypothalamus of Japanese quail are activated by fasting and co-express agouti-related protein mRNA. Mol. Brain Res. 2002;100:31–42. doi: 10.1016/s0169-328x(02)00145-6. [DOI] [PubMed] [Google Scholar]
- Bouton M.E. Context and behavioral processes in extinction. Learn. Mem. 2004;11:485–494. doi: 10.1101/lm.78804. [DOI] [PubMed] [Google Scholar]
- Cai G., Mo C., Huang L., Li J., Wang Y. Characterization of the two CART genes (CART1 and CART2) in chickens (Gallus gallus) PLoS One. 2015;10 doi: 10.1371/journal.pone.0127107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caughey S.D., Wilson P.W., Mukhtar N., Brocklehurst S., Reid A., D'Eath R.B., Boswell T., Dunn I.C. Sex differences in basal hypothalamic anorectic and orexigenic gene expression and the effect of quantitative and qualitative food restriction. Biol. Sex Differ. 2018;9:1–12. doi: 10.1186/s13293-018-0178-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C.P., Kuhn P., Advis J.P., Sarkar D.K. Chronic ethanol consumption impairs the circadian rhythm of pro-opiomelanocortin and period genes mRNA expression in the hypothalamus of the male rat. J. Neurochem. 2004;88:1547–1554. doi: 10.1046/j.1471-4159.2003.02300.x. [DOI] [PubMed] [Google Scholar]
- Chung S., Son G.H., Kim K. Circadian rhythm of adrenal glucocorticoid : its regulation and clinical implications. Biochim. Biophys. Acta Mol. Basis Dis. 2011;1812:581–591. doi: 10.1016/j.bbadis.2011.02.003. [DOI] [PubMed] [Google Scholar]
- Cox H.M. Neuropeptide Y receptors; antisecretory control of intestinal epithelial function. Auton. Neurosci. 2007;133:76–85. doi: 10.1016/j.autneu.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Dawkins M.S. From an animal's point of view: motivation, fitness and animal welfare. Behav. Brain Sci. 1990;13:1–61. [Google Scholar]
- de Beer M., McMurtry J.P., Brocht D.M., Coon C.N. An examination of the role of feeding regimens in regulating metabolism during the broiler breeder grower period. 2. Plasma hormones and metabolites. Poult. Sci. 2008;87:264–275. doi: 10.3382/ps.2007-00196. [DOI] [PubMed] [Google Scholar]
- de Jong I.C., van Voorst A.S., Blokhuis H.J. Parameters for quantification of hunger in broiler breeders. Physiol. Behav. 2003;78:773–783. doi: 10.1016/s0031-9384(03)00058-1. [DOI] [PubMed] [Google Scholar]
- de Jong I.C., van Voorst A.S., Erkens J.H.F., Ehlhardt D.A., Blokhuis H.J. Determination of the circadian rhythm in plasma corticosterone and catecholamine concentrations in growing broiler breeders using intravenous cannulation. Physiol. Behav. 2001;74:299–304. doi: 10.1016/s0031-9384(01)00562-5. [DOI] [PubMed] [Google Scholar]
- D'Eath R.B., Tolkamp B.J., Kyriazakis I., Lawrence A.B. Freedom from hunger” and preventing obesity: the animal welfare implications of reducing food quantity or quality. Anim. Behav. 2009;77:275–288. [Google Scholar]
- Dixon L.M., Brocklehurst S., Sandilands V., Bateson M., Tolkamp B.J., D'Eath R.B. Measuring motivation for appetitive behaviour: food-restricted broiler breeder chickens cross a water barrier to forage in an area of wood shavings without food. PLoS One. 2014;9 doi: 10.1371/journal.pone.0102322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon L.M., Sandilands V., D'Eath R.B. A preliminary investigation into resting behavior in feed-restricted and ad lib fed female broiler breeders. Br. Poult. Abstracts. 2016;12:19. [Google Scholar]
- Duncan I.J.H. Nest site selection and nest-building behaviour in domestic fowl. Anim. Behav. 1989;37:215–231. [Google Scholar]
- Dunn I.C., Meddle S.L., Wilson P.W., Wardle C.A., Law A.S., Bishop V.R., Hindar C., Robertson G.W., Burt D.W., Ellison S.J.H., Morrice D.M., Hocking P.M. Decreased expression of the satiety signal receptor CCKAR is responsible for increased growth and body weight during the domestication of chickens. Am. J. Physiol. Endocrinol. Metabolism. 2013;304:909–921. doi: 10.1152/ajpendo.00580.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn I.C., Wilson P.W., v. Smulders T., Sandilands V., D'Eath R.B., Boswell T. Hypothalamic agouti-related protein expression is affected by both acute and chronic experience of food restriction and re-feeding in chickens. J. Neuroendocrinol. 2013;25:920–928. doi: 10.1111/jne.12088. [DOI] [PubMed] [Google Scholar]
- Ellis C., Moar K.M., Logie T.J., Ross A.W., Morgan P.J., Mercer J.G., Ellis C., Km M., Tj L., Aw R., Pj M., Jg M. Diurnal profiles of hypothalamic energy balance gene expression with photoperiod manipulation in the Siberian hamster, Phodopus sungorus. Am. J. Physiol. Regulat. Integ. Compar. Physiol. 2008;294:1148–1153. doi: 10.1152/ajpregu.00825.2007. [DOI] [PubMed] [Google Scholar]
- Fang X.L., Zhu X.T., Chen S.F., Zhang Z.Q., Zeng Q.J., Deng L., Peng J.L., Yu J.J., Wang L.N., Wang S.B., Gao P., Jiang Q.Y., Shu G. Differential gene expression pattern in hypothalamus of chickens during fasting-induced metabolic reprogramming: functions of glucose and lipid metabolism in the feed intake of chickens. Poult. Sci. 2014;93:2841–2854. doi: 10.3382/ps.2014-04047. [DOI] [PubMed] [Google Scholar]
- Havenstein G.B., Ferket P.R., Qureshi M.A. Growth, liveability, and feed conversion of 1957 versus 2001 broilers when fed representative 1957 and 2001 broiler diets. Poult. Sci. 2003;82:1500–1508. doi: 10.1093/ps/82.10.1500. [DOI] [PubMed] [Google Scholar]
- Hen G., Yosefi G., Simchaev V., Shinder D., Hruby V.J., Friedman-Einat M. The melanocortin circuit in obese and lean strains of chicks. J. Endocrinol. 2006;190:527–535. doi: 10.1677/joe.1.06783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins S.E., Ellestad L.E., Trakooljul N., Mccarthy F., Saliba J., Cogburn L.A., Porter T.E. Transcriptional and pathway analysis in the hypothalamus of newly hatched chicks during fasting and delayed feeding. BMC Genomics. 2010;11:1–17. doi: 10.1186/1471-2164-11-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hocking P.M., Maxwell M.H., Mitchell M.A. Welfare of broiler breeder and layer females subjected to food and water control during rearing. Br. Poult. Sci. 1993;34:443–458. [Google Scholar]
- Hocking P.M., Maxwell M.H., Robertson G.W., Mitchell M.A. Welfare assessment of modified rearing programmes for broiler breeders. Br. Poult. Sci. 2001;42:424–432. doi: 10.1080/00071660120070677. [DOI] [PubMed] [Google Scholar]
- Honda K. Glucagon-related peptides and the regulation of food intake in chickens. Anim. Sci. J. 2016;87:1090–1098. doi: 10.1111/asj.12619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda K., Kamisoyama H., Saneyasu T., Sugahara S., Hasegawa S. Central administration of insulin suppresses food intake in chicks. Neurosci. Lett. 2007;423:153–157. doi: 10.1016/j.neulet.2007.07.004. [DOI] [PubMed] [Google Scholar]
- Kostal L., Savory C.J., Hughes B.O. Diurnal and individual variation in behaviour of restricted-fed broiler breeders. Appl. Anim. Behav. Sci. 1992;32:361–374. [Google Scholar]
- Kuenzel W.J., Douglass L.W., Davison B.A. Robust feeding following central administration of neuropeptide Y or peptide YY in chicks, Gallus domesticus. Peptides. 1987;8:823–828. doi: 10.1016/0196-9781(87)90066-0. [DOI] [PubMed] [Google Scholar]
- Lees J.J., Lindholm C., Batakis P., Busscher M., Altimiras J. The physiological and neuroendocrine correlates of hunger in the Red Junglefowl The physiological and neuroendocrine correlates of hunger in the Red Junglefowl. Sci. Rep. 2017;7:17984–17995. doi: 10.1038/s41598-017-17922-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei L., Lixian Z. Effect of 24 h fasting on gene expression of AMPK, appetite regulation peptides and lipometabolism related factors in the hypothalamus of broiler chicks. Asian-Australas. J. Anim. Sci. 2012;25:1300–1308. doi: 10.5713/ajas.2012.12153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X., Shieh K., Kabbaj M., Barsh G.S., Akil H., Watson S.J., Health M. Diurnal rhythm of agouti-related protein and its relation to corticosterone and food intake. Endocrinology. 2002;143:3905–3915. doi: 10.1210/en.2002-220150. [DOI] [PubMed] [Google Scholar]
- Machado F.S.M., Rodovalho G.V., Coimbra C.C. Physiology & Behavior - The time of day differently influences fatigue and locomotor activity : is body temperature a key factor? Physiol. Behav. 2015;140:8–14. doi: 10.1016/j.physbeh.2014.11.069. [DOI] [PubMed] [Google Scholar]
- Mandelblat-Cerf Y., Ramesh R.N., Burgess C.R., Patella P., Yang Z., Lowell B.B., Andermann M.L. Arcuate hypothalamic AgRP and putative POMC neurons show opposite changes in spiking across multiple timescales. eLife. 2015;4:e07122. doi: 10.7554/eLife.07122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason G., Mendl M. Do the stereotypies of pigs, chickens and mink reflective adaptive species differences in the control of foraging? Appl. Anim. Behav. Sci. 1997;53:45–58. [Google Scholar]
- Mench J.A. Broiler breeders: feed restriction and welfare. Worlds Poult. Sci. J. 2002;58:23–29. [Google Scholar]
- Mistlberger R., Rusak B. Palatable daily meals entrain anticipatory activity rhythms in free-feedings rats: dependence on meal size and nutrient content. Physiol. Behav. 1987;41:219–226. doi: 10.1016/0031-9384(87)90356-8. [DOI] [PubMed] [Google Scholar]
- Philips-Singh D., Li Q., Takeuchi S., Ohkubo T., Sharp P.J., Boswell T. Fasting differentially regulates expression of agouti-related peptide, pro-opiomelanocortin, prepro-orexin, and vasoactive intestinal polypeptide mRNAs in the hypothalamus of Japanese quail. Cell Tissue Res. 2003;313:217–225. doi: 10.1007/s00441-003-0755-8. [DOI] [PubMed] [Google Scholar]
- Porte D., Jr., Baskin D.G., Schwartz M.W. Leptin and insulin action in the central nervous system. Nutr. Rev. 2002;60:S20–S29. doi: 10.1301/002966402320634797. [DOI] [PubMed] [Google Scholar]
- Reid A.M.A., Wilson P.W., Caughey S.D., Dixon L.M., D'Eath R.B., Sandilands V., Boswell T., Dunn I.C. Pancreatic PYY but not PPY expression is responsive to short-term nutritional state and the pancreas constitutes the major site of PYY mRNA expression in chickens. Gen. Comp. Endocrinol. 2017;252:226–235. doi: 10.1016/j.ygcen.2017.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renema R.A., Robinson F.E. Defining normal: comparison of feed restriction and full feeding of female broiler breeders. Worlds Poult. Sci. J. 2004;60:508–522. [Google Scholar]
- Richards M.F., McMurtry J.P. Expression of proglucagon and proglucagon-derived peptide hormone receptor genes in the chicken. Gen. Comp. Endocrinol. 2008;1:323–328. doi: 10.1016/j.ygcen.2008.01.014. [DOI] [PubMed] [Google Scholar]
- Sandilands V., Tolkamp B.J., Kyriazakis I. Behaviour of food restricted broilers during rearing and lay - effects of an alternative feeding method. Physiol. Behav. 2005;85:115–123. doi: 10.1016/j.physbeh.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Sandilands V., Tolkamp B.J., Savory C.J., Kyriazakis I. Behaviour and welfare of broiler breeders fed qualitatively restricted diets during rearing: are there viable alternatives to quantitative restriction? Appl. Anim. Behav. Sci. 2006;96:53–67. [Google Scholar]
- Savory C.J. Diurnal feeding patterns in domestic fowls: a review. Appl. Anim. Ethol. 1980;6:71–82. [Google Scholar]
- Savory C.J., Lariviere J.M. Effects of qualitative and quantitative food restriction treatments on feeding motivational state and general activity level of growing broiler breeders. Appl. Anim. Behav. Sci. 2000;69:135–147. doi: 10.1016/s0168-1591(00)00123-4. [DOI] [PubMed] [Google Scholar]
- Savory C.J., Maros K. Influence of degree of food restriction, age and time of day on behaviour of broiler breeder chickens. Behav. Process. 1993;29:179–189. doi: 10.1016/0376-6357(93)90122-8. [DOI] [PubMed] [Google Scholar]
- Savory C.J., Seawright E., Watson A. Stereotyped behaviour in broiler breeders in relation to husbandry and opioid receptor blockade. Appl. Anim. Behav. Sci. 1992;32:349–360. [Google Scholar]
- Savory C., Maros K., Rutter S. Assessment of hunger in growing broiler breeders in relation to a commercial restricted feeding programme. Anim. Welfare. 1993;2:131–152. [Google Scholar]
- Scheurink A.W., Balkan B., Strubbe J.H., van Dijk G.J., Steffens A.B. Overfeeding, autonomic regulation and metabolic consequences. Cardiovasc. Drugs Ther. 1996;10:263–274. doi: 10.1007/BF00120496. [DOI] [PubMed] [Google Scholar]
- Shiraishi J., Yanagita K., Fujita M., Bungo T. Central insulin suppresses feeding behavior via melanocortins in chicks. Domest. Anim. Endocrinol. 2008;34:223–228. doi: 10.1016/j.domaniend.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Simon J. Chicken as a useful species for the comprehension of insulin action. Crit. Rev. Poult. Biol. 1989;2:121–248. [Google Scholar]
- Singh O., Kumar S., Singh U., Kumar V., Lechan R.M., Singru P.S. Cocaine- and amphetamine-regulated transcript peptide (CART) in the brain of zebra finch, Taeniopygia guttata: organization, interaction with neuropeptide Y, and response to changes in energy status. J. Comp. Neurol. 2016;524:3014–3041. doi: 10.1002/cne.24004. [DOI] [PubMed] [Google Scholar]
- Song Z., Liu L., Yue Y., Jiao H., Lin H., Sheikhahmadi A., Everaert N., Decuypere E., Buyse J. Fasting alters protein expression of AMP-activated protein kinase in the hypothalamus of broiler chicks (Gallus gallus domesticus) Gen. Comp. Endocrinol. 2012;178:546–555. doi: 10.1016/j.ygcen.2012.06.026. [DOI] [PubMed] [Google Scholar]
- Stephens D.W., Krebs J.R. Princeton University Press; New Jersey, USA: 1986. Foraging Theory. [Google Scholar]
- van der Wal P.G., Reimert H.G., Goedhart H.A., Uijttenboogaart T.G. The effect of feed withdrawal on broiler blood glucose and nonesterified fatty acid levels, postmortem liver pH values, and carcass yield. Poult. Sci. 1999;78:569–573. doi: 10.1093/ps/78.4.569. [DOI] [PubMed] [Google Scholar]
- Wichman A., Keeling L.J., Forkman B. Cognitive bias and anticipatory behaviour of laying hens housed in basic and enriched pens. Appl. Anim. Behav. Sci. 2012;140:62–69. [Google Scholar]
- Wise P.M., Scarbrought K., Weiland N.G., Larsont G.H. Diurnal pattern of proopiomelanocortin gene expression in the arcuate nucleus of proestrous, ovariectomized, and steroid-treated rats : a possible role in cyclic luteinizing hormone secretion. Mol. Endocrinol. 1990;4:886–892. doi: 10.1210/mend-4-6-886. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.