Highlights
-
•
Moderate high intensity ultrasound (HIU) could affect the structure of proteins positively.
-
•
Potential mechanisms of HIU on functionality of proteins are elucidated clearly.
-
•
HIU combined with other methods can achieve synergistic modification effects.
-
•
Multi-mode HIU devices can overcome the shortcomings of traditional HIU devices.
Keywords: High intensity ultrasound, Acoustic cavitation, Proteins, Modification, Functionality
Abstract
High intensity ultrasound (HIU) is an efficient and green technology that has recently received enormous research attention for modification of food proteins. However, there are still several knowledge gaps in the possible mechanisms, synergistic effects of HIU with other strategies and improvement of HIU equipment that contribute to its application in the food industry. This review focuses on the recent research progress on the effects and potential mechanisms of HIU on the structure (including secondary and tertiary structure) and functionality (including solubility, emulsibility, foamability, and gelability) of proteins. Furthermore, the combination methods and innovations of HIU equipment for proteins modification in recent years are also detailed. Meanwhile, the possible future trends of food proteins modification by HIU are also considered and proposed.
1. Introduction
Proteins are a crucial component of the human diet and essential for improving the immunity of the human body, maintaining normal metabolism, transporting various functional substances in the body, and providing energy for life activities [1], [2], [3]. At present, food proteins in the human diet are mainly derived from plants and animals. Apart from the directly consumed product forms such as protein powder, peptide powder, and muscle building powder, most proteins are indirectly present in meat, eggs, milk, and agricultural products, and these protein-based products often rely on functional properties such as emulsibility, foamability, and gelability of proteins [4], [5]. However, the poor solubility of most natural plant-derived and animal-derived proteins, as well as their extreme sensitivity to environmental stimulation such as pH, ionic strength, and temperature in food processing and preservation, significantly limit their application in the food industry. Therefore, there is an urgent need to modify the structure of food proteins to make their original rigid structure more flexible for enhancing their functional properties [4], [5], [6].
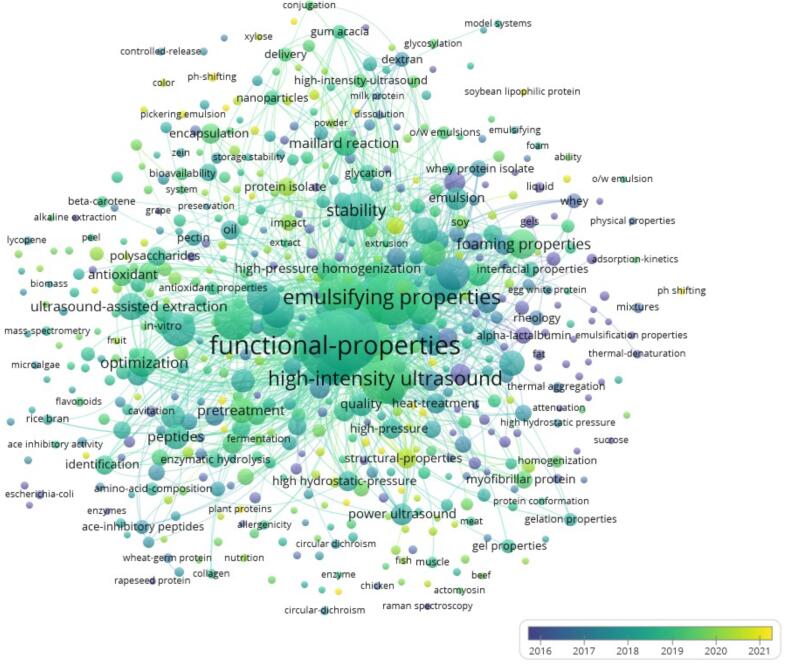
Currently, the main methods of protein modification include physical technologies such as microwave [7], high isostatic pressure [8],ultrasonics [9], cold plasma [10], pulsed electric field [11]; chemical technologies such as pH-shifting [12], glycosylation [13], phosphorylation [14], deamidation [15] and biological technologies such as enzyme hydrolysis [16], enzyme crosslinking [17]. Among them, high intensity ultrasound (HIU), a physical processing technology with the superiority of efficient, economical, and environmentally friendly, is the core ultrasonic method used in food science and industrialization because of its high output energy with the appropriate frequency range from 20 kHz to 100 kHz and strong cavitation effects on food materials [18], [19]. Thus, the HIU has been widely used in the modification of food proteins in recent years (Fig. 1).
Fig. 1.
Visualization of trends in modification of food proteins by HIU for enhancing functional properties.
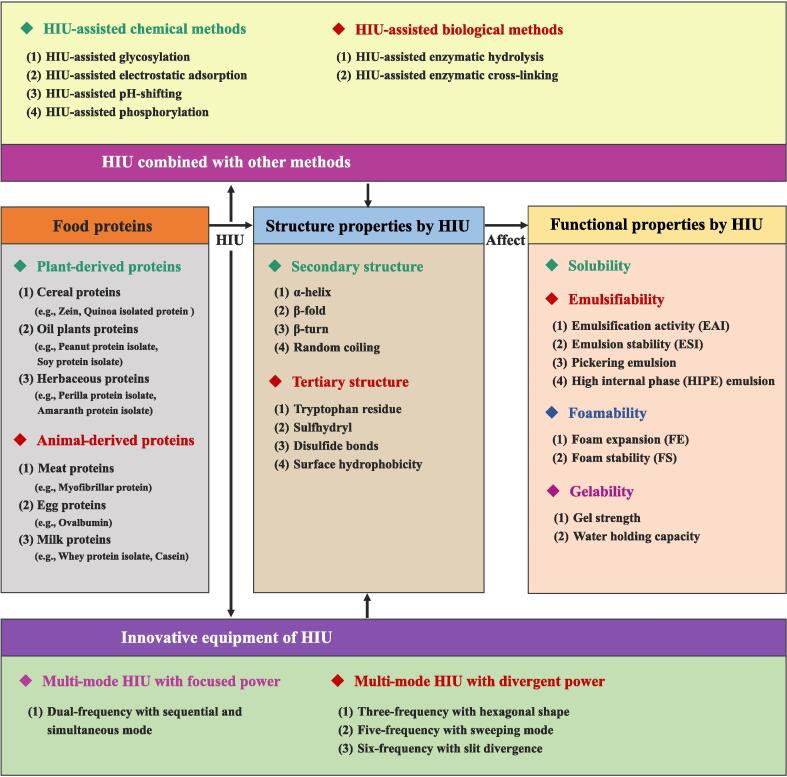
There are several recent review articles for modification of plant and animal-derived proteins [[20], [21], [22]]. However, the current review is centered around the insight into the latest research progress of the effects and the potential mechanisms of HIU on the structure and functionality of food proteins. At the same time, several vital technologies combined with HIU on the functionality of food proteins were also summarized and discussed comprehensively. Moreover, the innovations of HIU equipment for proteins modification in recent years were introduced, and some suggestions for the further development of HIU in modification of proteins were considered. Fig. 2 presented an overview of this review. Ultimately, we hope to provide the theoretical basis and advance the application of HIU on functionally modified proteins in the food industry.
Fig. 2.
An overview diagram of food proteins modified by HIU.
2. Acoustic cavitation effects of HIU
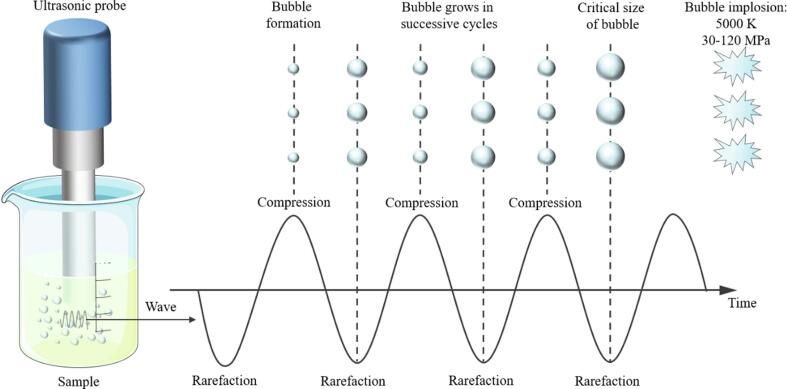
Ultrasound can produce the famous acoustic cavitation effects (Fig. 3), whose essence is the cycle of growth and contraction of preexisting microbubbles and their collapse in the liquid medium under the ultrasonic field [23]. According to whether the cavitation bubble ruptures or not, the cavitation status can be divided into stable cavitation and transient cavitation [24]. The role of food proteins modification was mainly attributed to the transient cavitation of HIU due to the cavitation bubble will expand rapidly at high acoustic pressure and collapse when it reaches the critical point of resonance, which can generate instantaneous high temperature of up to 5000 K and high pressure of up to 30 MPa in the local area, and then induce a series of physical, chemical, and thermal effects [22], [25], [26], [27].
Fig. 3.
The acoustic cavitation effect produced by HIU. Adapted from Rahman & Lamsal [22].
The physical effects caused by acoustic cavitation mainly include strong mechanical shear, high pressure, shock waves, microjets, turbulence, and acoustic flow [23]. These physical perturbations intermingle during sonication and can increase the mass transfer effect while causing folding and unfolding of protein molecules, with significant effects on protein-water interactions and protein–protein interactions [28].
If the medium of ultrasonic treatment is water, then acoustic cavitation will cause the covalent bonds of water molecules to break evenly, forming H∙ and ∙OH radicals and even producing H2O2 [23]. Thus, the free sulfhydryl groups in the protein molecules can be oxidized to disulfide bonds. Ashokkumar et al. [29] has also pointed out that choosing relatively low ultrasonic frequency for food processing minimizes the harmful reactions between the free radicals generated by ultrasound and the food components. This opinion further established the rationality of HIU in the field of food proteins modification.
3. Changes of the structure of proteins by HIU
The acoustic cavitation effects of HIU mainly affect the secondary and tertiary structure of proteins. Table.S1 in Supplementary presented the representative research on structure modification of proteins by HIU. Here, the main changes and possible reasons of HIU on these structures are reviewed in this section.
3.1. Secondary structure
The secondary structure of proteins is mainly maintained by the behaviors of α-helix, β-fold, β-turn, and random coiling of peptide chains. Under the acoustic cavitation effects of HIU, these behaviors are transformed to some extent by each other. For example, the percentage of α-helix of perilla isolated protein enhanced with the increase of ultrasonic power, but the percentage of β-fold, β-turn gradually decreased [30]. This may be attributed to the increased chance of collision between protein molecules under the effects of mechanical vibrations and turbulence generated by the collapse of cavitation bubbles, and the increase in the percentage of α-helix indicates that the protein structure becomes denser under the ultrasonic field. However, a contrary phenomenon was studied by Zou et al. [31]. They reported a decrease in the percentage of α-helix, β-turn, and random coiling, and an increase in the percentage of β-fold after 0–20 min of HIU treatment of mussel sarcoplasmic proteins. Meanwhile, excessive sonication (30 min) had a counterproductive effect on the secondary structure. This is because the aggregation and dissociation of proteins occur simultaneously under the action of HIU, and excessive ultrasonication causes protein molecules that were already dispersed to regroup together. In addition, HIU does not absolutely change the secondary structure of the proteins. Another study showed that HIU treatment had little effect on the secondary structure of ovalbumin [32]. This suggested that the effects of HIU on proteins secondary structure depends on many factors such as protein type, the original aggregation state of the proteins, the degree of proteins denaturation and ultrasound parameters.
3.2. Tertiary structure
The tertiary structure of proteins has a more direct impact on its functional properties than the secondary structure. Tryptophan residue changes, sulfhydryl content, disulfide bonds, and surface hydrophobicity are four essential indicators of the tertiary structure. The changes in the tertiary structure of chicken myogenic fibronectin (MP) by different times of HIU treatment were investigated by Li et al. [33]. Through intrinsic fluorescence spectroscopy, they found that the maximum emission wavelength of MP increased from 331.0 nm to 335.8 nm with a red-shifted peak and a significant increase in fluorescence intensity as the sonication time increased from 0 min to 6 min, indicating that HIU induced the tertiary structure of the MP molecule to unfold and more tryptophan residues were exposed to the polar environment. Meanwhile, the free sulfhydryl content in MP also increased significantly with the addition of sonication time, indicating that the sulfhydryl groups that were encapsulated inside the protein molecules were exposed to the surface under the unfolding of the tertiary structure. Besides, part of the disulfide bonds might also be broken under the effect of acoustic cavitation, leading to the increase of sulfhydryl content. Similar phenomena have also been reported by Zou et al. [34] and Saleem & Ahmad [35]. However, excessive sonication also causes the increased sulfhydryl groups to further form disulfide bonds under the oxidation of free radicals such as H∙ and ∙OH generated by the cavitation effects, making the already loosened structure of proteins rigid again [12].
Hydrophobic interactions are the main driver of protein folding and play the primary role in maintaining the tertiary structure of proteins, which are mainly influenced by the surface hydrophobicity of protein molecules. Meng et al. [36] found that the surface hydrophobicity of whey isolates increased significantly after moderate sonication, which can be attributed to the unfolding of proteins due to the tertiary structural changes of whey isolates caused by sonication, changing the local environment of hydrophobic groups. In contrast, excessive sonication resulted in a decrease in surface hydrophobicity due to protein reaggregation to re-embed the hydrophobic region into the interior of the protein molecule [36]. Not only animal-derived proteins but similar phenomena were also seen in the effects of HIU on the structure of amaranth isolates and chickpea isolates studied by Tomé Constantino & Garcia-Rojas [37] and Xu et al. [38], respectively. Further, a recent study found that the surface hydrophobicity of soybean 7S proteins with higher ionic strength was significantly higher than that of soybean 7S proteins with lower ionic strength after HIU treatment at pH = 7.0 [39]. However, they also found that increasing the ionic strength did not significantly improve the hydrophobicity of the protein surface under the HIU field at pH = 3.0. This may be attributed to the fact that proteins are highly protonated in high-acid environments, which increases the electrostatic repulsion between molecules. At this time, increasing ionic strength weakens this electrostatic repulsion and protects protein molecules from the cavitation effects induced by HIU.
4. Effects and mechanisms of HIU on functionality of proteins
HIU can effectively regulate the polymerization and depolymerization (folding and unfolding) of protein molecules, which in turn affects the solubility, emulsification, foaming, and gelling properties of proteins. Thus, it is now widely used in the improvement of functional properties of plant and animal-derived proteins to produce diverse delicious foods or food ingredients such as sausages, dairy products, butter, sauces and ice cream with the convenience and safety of operation and the low cost of production.
4.1. Solubility
The solubility of proteins lays the foundation and plays a crucial role in other functional properties [20], which depends mainly on the pH and ionic strength of the system. A recent study used HIU to treat isolated lupin protein with initial pH = 5 and 9, respectively, and then adjusted the pH of protein with two different initial pH values to 2, 4, 6, 8, and 10 after the treatment [40]. It was found that HIU not only significantly enhanced the solubility of isolated lupin protein with initial pH = 9 near the isoelectric point (pH = 4.6), but also boosted the solubility of the protein in other pH ranges across the board. This was attributed to the distinction in particle size of the two isolated lupin proteins with various initial pH, leading to differences in their solubility after the same sonication treatment. Moreover, HIU breaks the protein intermolecular bonds through acoustic cavitation and weakens protein–protein interaction, while reducing the particle size and increasing the specific surface area of protein particles, which promotes the interaction between protein molecules and water, thus increasing the solubility of proteins [41], [42].
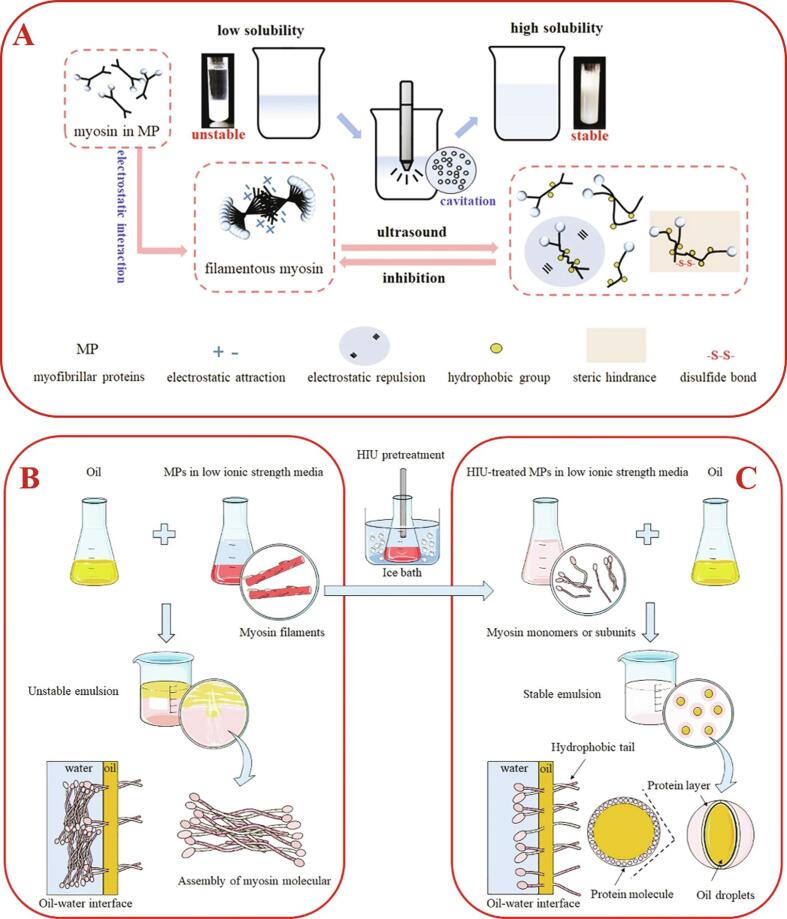
In addition, the level of ionic strength can also cause salt solubility or salt precipitation due to the charge shielding effect and ionic effect, which affects the solubility of proteins. Compared with most plant-derived proteins, myofibrillar proteins (MP) need high salt conditions (typically 0.47–0.68 M NaCl) to realize complete solubilization due to their highly ordered self-assembled myofilament structure [41], [43]. However, high salt levels are detrimental to human dietary health, so how to improve the solubility of MP under low salt conditions has become an important issue for researchers in recent years. Liu et al. [41] found that under low salt conditions of less than 1.3 mM NaCl, the solubility of MP gradually increased from 1.49% to 61.28% with the improvement of HIU power until 600 W. Meanwhile, the HIU did not cause significant changes in the pH and ionic strength of the system. They hypothesized that apart from reducing the particle size of MP through the physical effects of acoustic cavitation to increase the specific surface area of the protein particles, the highly reactive radicals generated by HIU could also oxidize and bond the exposed sulfhydryl groups of the protein molecules to form disulfide bonds, thus forming soluble myosin oligomers to inhibit the assembly of filamentous myosin for improving the solubility and dispersion stability of MP in water (Fig. 4A).
Fig. 4.
Potential mechanisms of HIU to enhance solubility (A) and emulsifiability ((B) before HIU pretreatment; (C) after HIU pretreatment) of myofibrillar proteins under low salt conditions. Adapted from Liu et al. [41], [50].
4.2. Emulsifiability
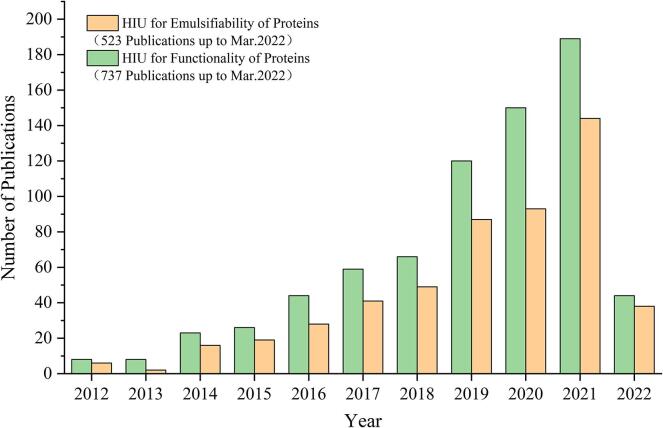
Because of the amphiphilic properties, proteins have become a suitable alternative to replace the traditional surfactant for stabilizing emulsions in recent years [44]. However, proteins in their natural state possess poor flexibility, surface hydrophobicity, and surface hydrophilicity, and are sensitive to environmental conditions such as temperature, pH, and ionic strength. Thus, they cannot rapidly unfold and reorient at the water–oil interface to form a strong viscoelastic protein film to stabilize emulsion, resulting in destabilization phenomena such as aggregation, flocculation, settling, and Ostwald ripening of emulsion [45]. In recent years, except directly used in homogeneous emulsification [25], [46], HIU has also been heavily applied to the structure modification of proteins to improve their emulsification properties such as emulsification activity (EAI) and emulsion stability (ESI). Besides, it was also found that emulsifiability is one of the most studied functional properties by HIU from Fig. 5.
Fig. 5.
The number of publications of HIU for emulsifiability and functionality of food proteins obtained from a search of the Web of Science database with the year from 2012 to 2022 (up to Mar.2022).
Soybean proteins and myofibrillar proteins are two proteins that have been widely modified using HIU to improve their emulsification properties due to their many characteristics such as vast sources, high yields, and high consumption. Huang et al. [47] found that structure modification of soybean protein isolate (SPI) induced by moderate sonication successfully improved its EAI and ESI. On this basis, Yan et al. [48] investigated the relationship between the molecular flexibility as well as surface hydrophobicity and the emulsifying properties of the SPI treated with different ultrasonic power. They found that both molecular flexibility and surface hydrophobicity of SPI were positively correlated with EAI and ESI, but the protein flexibility had a more significant effect on emulsification performance compared to surface hydrophobicity due to the unfolding of the primitive globular SPI and the exposure of the hypervariable region of globulin and the extended region of α-conglycinin under the ultrasonic field.
Amiri et al. [49] and Li et al. [33] successively found that moderate HIU treatment led to more myofibrillar proteins involved in the formation of interfacial protein layer, resulting in a significant increase in emulsification efficiency. However, the possible mechanism was still unknown at that time. Later, Liu et al. [50] creatively discovered that HIU could disrupt and inhibit the formation of filamentous polymers by unravelling the double chain helix of myosin. The resulting myosin monomer or subunit can be uniformly dispersed in the continuous phase so that its hydrophobic end is oriented toward the oil and its hydrophilic end is oriented toward the water. Thus, the emulsion can be stabilized over time by electrostatic repulsion and spatial site resistance (Fig. 4 B-C).
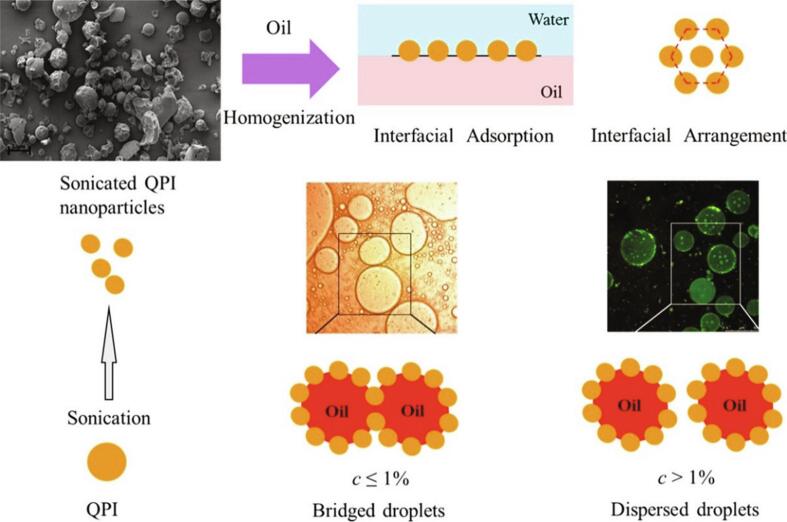
On top of that, HIU has also been creatively used to induce the formation of nanoparticles from modified proteins to stabilize Pickering emulsions. Gao et al. [51] were the first to use HIU to promote the formation of complex colloidal particles of zein and stearate to stabilize Pickering emulsions, although got more research interest in recent years. Later, Qin et al. [52] investigated the interfacial adsorption and alignment properties of quinoa isolated protein (QPI) nanoparticles stabilizing Pickering emulsion before and after HIU treatment. They found that the three-phase contact angle used to evaluate the wettability of QPI nanoparticles at the water–oil interface increased from 58.0° to 68.5° after sonication, and the sonicated QPI nanoparticles could be adsorbed at the water–oil interface sufficiently and form a thicker interfacial protein layer, exhibiting superior emulsification efficiency and higher anti-agglomeration and anti-coalescence ability (Fig. 6). On this basis, a recent study showed that the morphology of QPI nanoparticles gradually changed from irregular to spherical, and the Pickering high internal phase emulsions (HIPEs) stabilized by HIU treated QPI nanoparticles showed high viscoelasticity and excellent freeze–thaw stability with the increase of ultrasonic power [53]. A similar phenomenon has also been found by Zhang et al. [54] recently. Therefore, Pickering emulsions (including HIPEs) stabilized by HIU-induced protein nanoparticles not only provide an important reference for antifreeze in frozen foods, but also become a vital choice for high-fat alternative foods.
Fig. 6.
Potential mechanisms for stabilization of Pickering emulsions by QPI nanoparticles formed through HIU [52].
4.3. Foamability
The foamability of proteins, mainly used in foods such as ice cream, bread, and butter, belongs to the property of the interface between the continuous aqueous phase and the dispersed gas phase, and depends mainly on the movement, unfolding, and rearrangement of protein molecules at the gas–water interface. Foam expansion (FE) and foam stability (FS) are commonly used to measure the foaming properties of proteins [36]. Xiong et al. [55] observed that HIU induced partial unfolding of the pea protein isolate and rapid adsorption at the air–water interface to form a relatively viscoelastic interfacial film, thereby increasing the FS from 58% to 73.3% (Fig. S1). Later, Zhao et al. [30] found that the FE and FS of perilla protein isolate enhanced with increasing ultrasound power, reaching a maximum of 50% and 30.5% at 750 W, respectively. These studies may provide significant inspiration for the development of HIU induced plant-based ice cream. A similar phenomenon was also shown in animal-derived proteins such as whey isolates [36] and egg white proteins [56] after HIU treatment. This is attributed to the cavitation effect of HIU which improves the surface hydrophobicity and molecular flexibility of protein molecules, enhances the solubility of protein molecules, reduces the particle size of protein particles, and thus improves the absorption ability of proteins in the interface. However, it should be noted that excessive sonication can also reduce foam stability due to protein reaggregation resulting in its desorption from the gas–water interface [30].
4.4. Gelability
The gelatinization of proteins is widely found in the daily diet of human beings such as eggs, tofu, meat, and yogurts, which depends mainly on protein–protein interactions as well as on pH, divalent ions, and temperature. However, HIU mainly affects the gel network formed between proteins molecules by promoting the folding and unfolding of proteins. For example, Wang et al. [57] found that HIU was a more effective technique for preparing soy proteins with better gelling ability than heat treatment, and that the higher the concentration used in the ultrasonic process, the greater the gelling ability of the soy proteins. Later, another study showed that moderate HIU power at 300 W improved the gel strength and water holding capacity of the immersion thawing myofibrillar proteins of chicken and formed a dense gel network [58]. It was attributed that HIU shortened the thawing time of the fish, leading to a lower degree of denaturation of the proteins, reducing the number and size of voids in the gel network, and avoiding excessive destruction of the gel microstructure (Fig. S2). Further, the formation of an excellent gel network of proteins under HIU field can also improve the performance of 3D printing. For instance, Chen et al. [59] found that the surimi treated by HIU-assisted immersion thawing at ultrasonic frequency of 100 kHz had the best 3D printing performance due to the least damage to the structure of myofibrillar proteins. Food 3D printing technology represents one of the frontier directions of food science. However, the application of HIU in modified protein gel-based 3D printed food is still limited, which needs further research in the future.
5. HIU combined with other technologies
Apart from the modification of proteins by HIU alone, some studies on the synergistic action of HIU in combination with chemical means including glycosylation, electrostatic adsorption, pH-shifting, phosphorylation, and biological means including enzymatic hydrolysis and enzymatic cross-linking have started to emerge and generally achieved better modification results in recent years. Table 1 presented representative research on the synergistic effect of HIU with other modification strategies on function changes of proteins.
Table.1.
Summary of representative research on function changes of modified proteins through HIU treatment combined with other technologies Table 1. Summary of representative research on function changes of modified proteins through HIU treatment combined with other technologies.
| Proteins | Method of combination | HIU conditions | Solubility | Emulsibility | Foamability | Gelability | References |
|---|---|---|---|---|---|---|---|
| Ovalbumin | HIU + Glycosylation | Frequency (kHz):20;Power (W) :400,600;Time (min) :15 |
— | ↑ | ↑ | — | [63] |
| Whey protein isolate |
HIU + Glycosylation | Frequency (kHz):20;Power (W) :300;Time (min) :60 |
— | ↑ | ↑ | — | [61] |
| Zein | HIU + Glycosylation | Frequency (kHz):20;Power (W) :400;Time (min) :25 |
— | ↑ | — | — | [69] |
| Soy protein isolate | HIU + Electrostatic adsorption | Frequency (kHz):22;Power (W) :360,450, 540,630,720;Time (min) :5,10,15,20 |
— | ↑ | — | — | [65] |
| Whey protein concentrate | HIU + Electrostatic adsorption | Frequency (kHz):20;Power (W) :320;Time (min) :5 |
— | ↑ | — | — | [71] |
| Whey protein isolate |
HIU + Electrostatic adsorption | Frequency (kHz):24;Power (W) :200,400;Time (min) :2,4 |
↓ | ↑ | ↑ | ↓ | [70] |
| Faba bean protein | HIU+pH-shifting | Frequency (kHz):20;Power (W) :360;Time (min) :10, 20;Pulse on/off (s) :5/5 |
↑ | ↑ | ↑ | — | [12] |
|---|---|---|---|---|---|---|---|
| Protein isolated from rainbow trout filleting by-products | HIU+pH-shifting | Frequency (kHz):20;Power (W) :100,400;Time (min) :5,10,20;Pulse on/off (s) :2/3 |
↑ | ↑ | ↑ | — | [74] |
| Rapeseed protein | HIU+pH-shifting | Frequency (kHz):20,40,60, 20±2,40±2,60±2;Power (W/L) :50;Time (min) :10;Pulse on/off (s) :3/3 |
↑ | — | — | — | [73] |
| Goose liver protein | HIU+Phosphorylation | Frequency (kHz):20;Power (W) :300;Time (min) :25 |
↑ | ↑ | — | — | [86] |
| Myofibrillar protein | HIU+Enzyme hydrolysis | Frequency (kHz):20;Power (W) :200;Time (min) :15,30 |
↑ | ↑ | ↑ | — | [16] |
| Sunflower protein | HIU+Enzyme hydrolysis | Frequency (kHz):20/40;Power (W) :220;Time (min) :15Pulse on/off (s) :2/5 |
↑ | ↑ | ↑ | — | [87] |
| α-lactalbumin | HIU+Enzyme (laccase) crosslinking | Frequency (kHz):20;Power (W) : 400;Time (min) : 20,40,60,80,100;Pulse on/off (s) :2/5 |
— | — | — | ↑ | [91] |
|---|---|---|---|---|---|---|---|
| Whey protein isolate |
HIU+Heat+Enzyme (transglutaminase) crosslinking | Frequency (kHz):20;Power (W) :600;Time (min) :20,40,60 |
— | ↓ | ↑ | — | [92] |
Note: “—” represents the corresponding functionality was not mentioned in the paper; “↑” represents the corresponding functionality was improved after HIU, while “↓” represents the opposite.
5.1. HIU-assisted chemical methods
5.1.1. HIU-assisted glycosylation
Glycosylation is a covalent reaction between the amino group of proteins and the carbonyl group of polysaccharides, with the advantage that the modification process is free of contamination by chemical reagents [60], [61]. It was first initiated by Kato et al. [62] to improve the functional properties of ovalbumin (OVA). However, the conventional dry heating and wet heating Maillard reaction had several disadvantages, including long time consuming and low glycation grafting rate, and are prone to produce harmful late-stage Maillard reactants. In recent years, HIU has been used as an auxiliary method to improve the efficiency of glycosylation [20]. For example, Yang et al. [63] found by high-resolution mass spectrometry that HIU promoted the glycosylation of OVA by increasing the number of exposed glycosylation sites, significantly improving the emulsification and foaming properties of OVA. A recent study further showed that HIU-assisted OVA glycosylation promoted the formation of more disulfide bonds in OVA, while enhancing the interaction between protein and water, forming a regular and homogeneous OVA gel network and improving the gel strength of OVA gels [64]. In addition, HIU-assisted glycosylation likewise improved the functional properties of plant proteins such as soybean protein isolate [65], [66], peanut isolate [67], and mung bean isolate [68]. However, it should be noted that Ma et al. [69] proposed a method for HIU-assisted zein glycosylation and its effect. Different from water-soluble proteins, the solvent of zein in the Maillard reaction is NaOH with high alkaline. They found that the Maillard reaction between zein and gum Arabic could be promoted by HIU treatment for only 25 min with the power of 400 W, resulting in a zein-gum Arabic conjugate that was more excellent in solubility and emulsifiability than Zein, and was able to stabilize the oil phase up to 80% of the high internal phase emulsion. This is the first study to propose HIU-assisted glycosylation of alcohol-soluble proteins, which is of great significance for its functional modification and high-value utilization.
5.1.2. HIU-assisted electrostatic adsorption
Apart from the covalent binding of proteins and polysaccharides through the Maillard reaction, they can also form complexes by electrostatic adsorption to improve the functionality of proteins [70]. However, the traditional protein-polysaccharide complexes are unstable due to the large particle size and the interference of intermolecular interactions. Recently, Albano & Nicoletti [71] used HIU to adsorb pectin as anionic polysaccharide onto the outer layer of positively charged whey protein isolate. They found that HIU can disrupt the intermolecular interactions between protein molecules, promote the conversion of a large amount of precipitate to colloidal suspension, reduce the viscosity of the suspension and the particle size of the complex, and thus improve the emulsifying properties of the complex. Later, Ma et al. [72]systematically investigated the changes in the emulsification properties of soybean protein isolate-pectin electrostatic complexes at different sonication powers and sonication times. They found that the EAI and ESI of electrostatic complexes were significantly enhanced after 10 min under the HIU field at 630 W. Meanwhile, HIU increased the absolute value of zeta potential of the complexes and reduced the particle size to obtain more stable colloidal dispersions, further validating the findings of Albano & Nicoletti [71].
5.1.3. HIU-assisted pH-shifting
pH shifting is an emerging chemical modification method to improve the functional properties of proteins by adjusting the microenvironment to extreme pH conditions and maintaining it for a while to partially unfold the protein molecules and then adjusting it back to neutral to refold the protein molecules, thus forming a flexible structure called “molten spherical state”[73]. However, it has also been found that pH-shifting of proteins under HIU fields can further improve the functionality of proteins in recent years. For instance, Pezeshk et al. [74] found that HIU with pH-shifting treatment not only improved the yield of proteins extracted from rainbow trout filleting by-products, but also effectively enhanced the emulsification and foaming properties of proteins, which was attributed to the fact that unfolded proteins are more susceptible to HIU under extreme pH conditions. Similarly, two recent studies [75], [76] also found that HIU-assisted pH-shifting facilitated the formation of more viscous interfacial membranes of proteins at the air–water interface and thus enhancing the foaming properties of proteins as the unfolding of protein molecules increased the exposure of their hydrophobic regions. However, compared to glycosylation, the use of green acids/alkalis for pH-shifting needs to be considered in the future to achieve a non-polluting modification process.
5.1.4. HIU-assisted phosphorylation
Phosphorylation refers to the addition of phosphoric acid groups to protein molecules for changing structure and electrification of proteins, thus modifying their functionality to a certain degree. As early as 1983 [77]and 1989 [78], there were studies on chemical phosphorylation and enzymatic phosphorylation of soybean protein isolate to improve their functional properties, respectively. However, enzymatic phosphorylation can only bring few phosphate groups to proteins with a high cost of enzyme, thus limiting its large-scale use in food industry [79]. Since then, most studies have used sodium tripolyphosphate (STP) [14], [80] or sodium trimetaphosphate (STMP) [81], [82], [83] for chemical phosphorylation. Recently, thermal treatment [84], microwave [85], and other methods have been gradually involved in the assisted phosphorylation of proteins, while the first study on HIU-assisted phosphorylation did not appear until 2022 [86]. Hu et al. [86] found that HIU can promote the combination of phosphate groups with goose liver protein, improve the degree of goose liver protein phosphorylation, and thus increase solubility and emulsifiability of proteins. This was attributed to the fact that HIU promoted the unfolding of the proteins to expose more phosphorylation sites, which is a key aspect of facilitating phosphorylation. However, there is no relevant research on HIU-assisted phosphorylation of plant-derived proteins and their effects on foaming and gelling properties, which still need to be strengthened in the future.
5.2. HIU-assisted biological methods
5.2.1. HIU-assisted enzymatic hydrolysis
Enzymatic hydrolysis leads to the exposure of the hydrophobic groups hidden in the protein and the reduction of molecular weight, paving the way for conformational change and improved functional properties of the protein. Based on promoting unfolding of proteins, HIU can increase the contact site and contact probability between enzyme and protein, further facilitate the enzymatic hydrolysis process and enhance the functionality of protease hydrolysates [87]. For example, Dabbour et al. [88] found that HIU treatment improved the solubility, emulsification, and foaming ability of sunflower protein isolates with different degrees of hydrolysis. Similarly, a recent study by Amiri et al. [16] showed that HIU treatment combined with papain can maximize the functional properties of myofibrillary proteases under the condition of power of 200 W and time of 15 min. However, excessive ultrasonication can also lead to reaggregation of proteins due to the re-enhancement of protein–protein interactions, thus reducing enzymatic hydrolysis efficiency and solubility, emulsification, and foamability of the hydrolysates [16]. Therefore, the optimization of HIU conditions and the economic cost of enzymes should be considered in the future to further improve the possibility of HIU-assisted enzymatic hydrolysis in the production of modified protein foods.
5.2.2. HIU-assisted enzymatic cross-linking
Transglutaminase (TGase) and laccase are two important enzymes used for protein cross-linking to improve the functional properties of proteins, especially gelability. TGase crosslinks protein residues by forming γ-carboxyl (glutamine)-ε-amino (lysine) isopeptide bond [89], while laccase produces crosslinked proteins by oxidizing phenolic groups in proteins with the addition or modification of phenolic compounds as electron transfer agents [90]. However, some proteins are not favorable substrates for enzyme cross-linking due to their rigid structure, while the cross-linkability of proteins could be significantly enhanced under the action of the ultrasonic field. For instance, whey protein cannot be directly crosslinked by enzymes due to its special rigid spherical structure. Yuan et al. [91] used HIU to assist laccase crosslinking in the presence of ferulic acid. They found that HIU induced exposure of hydrophobic residues and formation of disulfide bonds during laccase-catalyzed crosslinking, thus may contribute to the generation of protein–protein aggregates, resulting in gel networks with better gel strength. However, HIU-assisted enzyme cross-linking does not absolutely improve the functional properties of proteins. In a later study, the emulsifiability and foamability were inferior to that of whey protein isolate (WPI) without cross-linking after HIU combined with thermal pretreatment assisted TGase, although the cross-linking degree was improved [92]. This may be attributed to the increase of viscosity of WPI caused by enzyme crosslinking, which leads to protein particles cannot being fully stabilized at the water–oil and water–gas interface [92].
6. Innovations of HIU equipment for modifying proteins
At present, the HIU equipment used for protein modification mainly concentrates on the single frequency with focused energy probe. However, such ultrasonic equipment generally has many shortcomings, such as single working mode, uneven distribution of ultrasonic field, excessive power density and serious cavitation damage (Fig. S3A) [93]. In addition, single-frequency ultrasound is also prone to form standing waves (Fig. S3B), resulting in insufficient cavitation effects and low energy efficiency [94], which is far from playing the role of HIU in the field of proteins modification.
Based on the above defects, the development of multi-mode HIU equipment for modifying proteins began to rise in recent years. For example, our research team developed 12 kinds of HIU working mode, including sweeping frequency and fixed frequency, multi-frequency and single frequency, multi-frequency with sequential and simultaneous mode, pulse and continuous, contact and non-contact, reflux circulation and optional flow [24]. The four main multi-mode HIU devices for proteins modification were presented in Fig. 7. These innovative ultrasonic devices greatly make up for the disadvantages of traditional HIU equipment and are expected to lead to large-scale industrialization of food proteins modification by HIU. The following sections are divided into multi-mode HIU with focused power and multi-mode HIU with divergent power to concentrate on the recent research progress of multi-mode HIU equipment for modifying proteins.
Fig. 7.
Four main types of multi-mode HIU devices for modifying proteins. (A) Dual-frequency with focused power; (B) Three frequency with hexagonal shape; (C) Five frequency with sweeping mode; (D) Six frequency with slit divergence. Adapted from Xu et al. [24].
6.1. Multi-mode HIU with focused power
Compared with conventional single-frequency HIU with focused power (usually fixed at 20 kHz), a frequency spectrum with a more complex waveform and a wider range to avoid the formation of standing waves can be realized by dual-frequency HIU with focused power. Moreover, the combined effect of the simultaneous radiation of dual-frequency ultrasound was significantly greater than the effect of the single-frequency ultrasound, which could produce stronger and more uniform proteins modification effects. Recently, the film-forming ability of the myofibrillar proteins at the water–oil interface and the emulsification performance of the protein under mild ultrasound (≤6 min) was improved greatly by dual-frequency HIU with focused power [95]. Later, another study showed that the solubility, emulsification, and foaming ability of soybean protein are completely different with various angles of two transducers of HIU under the action of 20/40 kHz dual-frequency ultrasonic field (Fig. 7A) [96]. When the angle of the two transducers was from 0° to 30° and then to 45°, the solubility of soybean protein increased first and then decreased, reaching the maximum of 87.09% at 30°. However, the emulsion stability (ESI) and foam expansion rate (FE) of soybean protein reached the highest at 0°, which was consistent with the experiment of Chen et al. [95], indicating that the cavitation effects could be stronger when the angle of the two transducers was 0° under the action of dual-frequency ultrasound. On top of that, Chen et al. [97] also found that dual-frequency HIU with focused power induced the binding of myosin with hydrophilic and non-hydrophilic polyphenols to produce a quorum sensing effect, which improved the crosslinking ability of myosin and promoted a stronger gelation process.
6.2. Multi-mode HIU with divergent power
The multi-mode HIU with focused power will inevitably pollute the protein samples due to the cavitation damage of the ultrasonic probe. In contrast, multi-mode HIU with divergent power avoids direct contact between sample and transducer, and can achieve a more uniform distribution of sonication field. Cheng et al. [98] used a three-frequency ultrasonic device with hexagonal shape (Fig. 7B) to conduct sequential HIU pretreatment of whey protein isolate. They found that this ultrasonic working mode could enhance the gel properties and adjust in vitro digestion behavior of the emulsion gel formed by heated whey protein isolate, especially under the condition of the frequency of 20/28 kHz and treatment time of 10 min. To strengthen the resonance probability of proteins in the ultrasonic field, Li et al. [73] creatively adopted a sweeping frequency ultrasonic device (Fig. 7C) that could fluctuate up and down around the central frequency with a certain sweep period to assist pH-shifting for modifying rapeseed protein. They found that the solubility of proteins could be facilitated by a maximum of 7.5 times under an ultrasound field of 20 ± 2 kHz compared to the control group. For further increasing the contact area between ultrasonic field and proteins to improve the mass transfer effects and overcoming the problem of uneven ultrasonic radiation, Wen et al. [99] employed a six-frequency ultrasound device with slit divergence (Fig. 7D) to assist the wet-heating Maillard reaction for glycosylation of soybean protein isolates. They found that this type of ultrasonic device can expose more hidden amino groups in protein molecules and reduce particle size of proteins, thus improving graft degree and surface hydrophobicity compared to the traditional glycosylation method. Moreover, the emulsion stabilized by the soybean protein isolates-lentinan conjugation formed by this HIU equipment can resist environmental stress including pH, temperature, and ionic strength, which has broad application prospects in food industry.
7. Conclusions and future trends
As a physical processing technology, HIU has many advantages in modifying proteins to improve their functional properties, which has been recognized by researchers in recent years. As discussed in this review, structural change of proteins after treatment of HIU is a major determinant of the functionality of proteins, and in turn the quality of finished foods formed by modified proteins. Meanwhile, HIU has excellent auxiliary effects on conventional chemical or biological modification methods. Moreover, the innovative multi-mode HIU devices can also overcome the shortcomings of traditional HIU equipment for modifying food proteins. However, there are still the following deficiencies in the future development of HIU for enhancing the functionality of proteins, which need further improvement:
-
(1)
The structure of proteins is essentially a dynamic change process under the HIU field. It is necessary to establish real-time online in situ monitoring models and develop corresponding software systems to intelligently monitor and precisely control the spatial structure of proteins that are conducive to the improvement of functional properties of proteins.
-
(2)
Emerging techniques such as foodomics and molecular dynamic simulation need to be introduced to deeply reveal the mechanism of modifying structure by HIU and its effects on digestion and nutrition.
-
(3)
A great deal of attention has been focused on modifying bulk plant-derived and animal-derived proteins. However, little information is available on the proteins derived from low-value utilized material such as oil cake and insects, which need to be utilized by HIU modification in the future.
-
(4)
It is necessary to further develop multi-mode HIU equipment that can be used for the large-scale industrialization. At the same time, the noise generated by HIU needs to be reduced to the acceptable range of human hearing for ensuring the auditory health of workers.
-
(5)
At present, HIU only stays at the level of improving the functional properties of proteins, and few studies continue to explore the impact of enhanced functionality on their downstream products. For example, the influence of HIU on the physicochemical properties of 3D printed food formed by modified proteins and on the properties of electrospun nanofiber films or edible coating films fabricated by modified proteins could be explored in the future.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the National 863 Program of China: Research and development of key technologies for high bioavailability proteins preparation (2013AA102203).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ultsonch.2022.105993.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Day L., Cakebread J.A., Loveday S.M. Food proteins from animals and plants: Differences in the nutritional and functional properties. Trends Food Sci. Technol. 2022;119:428–442. doi: 10.1016/j.tifs.2021.12.020. [DOI] [Google Scholar]
- 2.Ashfaq A., Jahan K., Islam R.U., Younis K. Protein-based functional colloids and their potential applications in food: A review. LWT. 2022;154 doi: 10.1016/j.lwt.2021.112667. [DOI] [Google Scholar]
- 3.Daroit D.J., Brandelli A. In vivo bioactivities of food protein-derived peptides – a current review. Curr. Opin. Food Sci. 2021;39:120–129. doi: 10.1016/j.cofs.2021.01.002. [DOI] [Google Scholar]
- 4.Kang D., Zhang W., Lorenzo J.M., Chen X. Structural and functional modification of food proteins by high power ultrasound and its application in meat processing. Crit. Rev. Food Sci. Nutr. 2021;61:1914–1933. doi: 10.1080/10408398.2020.1767538. [DOI] [PubMed] [Google Scholar]
- 5.Sagis L.M., Yang J. Protein-stabilized interfaces in multiphase food: comparing structure-function relations of plant-based and animal-based proteins. Curr. Opin. Food Sci. 2022;43:53–60. doi: 10.1016/j.cofs.2021.11.003. [DOI] [Google Scholar]
- 6.Akharume F.U., Aluko R.E., Adedeji A.A. Modification of plant proteins for improved functionality: A review. Compr. Rev. Food Sci. Food Saf. 2021;20:198–224. doi: 10.1111/1541-4337.12688. [DOI] [PubMed] [Google Scholar]
- 7.Chang C., Su Y., Gu L., Li J., Yang Y. Microwave induced glycosylation of egg white protein:study on physicochemical properties and baking performance. Food Hydrocoll. 2021;118 doi: 10.1016/j.foodhyd.2020.106569. [DOI] [Google Scholar]
- 8.Baier A.K., Knorr D. Influence of high isostatic pressure on structural and functional characteristics of potato protein. Food Res. Int. 2015;77:753–761. doi: 10.1016/j.foodres.2015.05.053. [DOI] [Google Scholar]
- 9.Wang T., Chen X., Wang W., Wang L., Jiang L., Yu D., Xie F. Effect of ultrasound on the properties of rice bran protein and its chlorogenic acid complex. Ultrason. Sonochem. 2021;79 doi: 10.1016/j.ultsonch.2021.105758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu J., Ji H., Chen Y., Zhang Y., Zheng X., Li S., Chen Y. Analysis of the glycosylation products of peanut protein and lactose by cold plasma treatment: Solubility and structural characteristics. Int. J. Biol. Macromol. 2020;158:1194–1203. doi: 10.1016/j.ijbiomac.2020.04.255. [DOI] [PubMed] [Google Scholar]
- 11.Xu F.-Y., Wen Q.-H., Wang R., Li J., Chen B.-R., Zeng X.-A. Enhanced synthesis of succinylated whey protein isolate by pulsed electric field pretreatment. Food Chem. 2021;363 doi: 10.1016/j.foodchem.2021.129892. [DOI] [PubMed] [Google Scholar]
- 12.Alavi F., Chen L., Emam-Djomeh Z. Effect of ultrasound-assisted alkaline treatment on functional property modifications of faba bean protein. Food Chem. 2021;354 doi: 10.1016/j.foodchem.2021.129494. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Z., Chen W., Zhou X., Deng Q., Dong X., Yang C., Huang F. Astaxanthin-loaded emulsion gels stabilized by Maillard reaction products of whey protein and flaxseed gum: Physicochemical characterization and in vitro digestibility. Food Res. Int. 2021;144 doi: 10.1016/j.foodres.2021.110321. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y.-R., Zhang B., Fan J.-L., Yang Q., Chen H.-Q. Effects of sodium tripolyphosphate modification on the structural, functional, and rheological properties of rice glutelin. Food Chem. 2019;281:18–27. doi: 10.1016/j.foodchem.2018.12.085. [DOI] [PubMed] [Google Scholar]
- 15.Jiang Y., Wang Z., He Z., Zeng M., Qin F., Chen J. Effect of heat-induced aggregation of soy protein isolate on protein-glutaminase deamidation and the emulsifying properties of deamidated products. LWT. 2022;154 doi: 10.1016/j.lwt.2021.112328. [DOI] [Google Scholar]
- 16.Amiri A., Sharifian P., Morakabati N., Mousakhani-Ganjeh A., Mirtaheri M., Nilghaz A., Guo Y., Pratap-Singh A. Modification of functional, rheological and structural characteristics of myofibrillar proteins by high-intensity ultrasonic and papain treatment. Innov. Food Sci. Emerg. Technol. 2021;72 doi: 10.1016/j.ifset.2021.102748. [DOI] [Google Scholar]
- 17.Qayum A., Li M., Shi R., Bilawal A., Gantumur M.-A., Hussain M., Ishfaq M., Waqas Ali Shah S., Jiang Z., Hou J. Laccase cross-linking of sonicated α-Lactalbumin improves physical and oxidative stability of CLA oil in water emulsion. Ultrason. Sonochem. 2021;71 doi: 10.1016/j.ultsonch.2020.105365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muñoz-Almagro N., Morales-Soriano E., Villamiel M., Condezo-Hoyos L. Hybrid high-intensity ultrasound and microwave treatment: A review on its effect on quality and bioactivity of foods. Ultrason. Sonochem. 2021;80 doi: 10.1016/j.ultsonch.2021.105835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nguyen Huu C., Rai R., Yang X., Tikekar R.V., Nitin N. Synergistic inactivation of bacteria based on a combination of low frequency, low-intensity ultrasound and a food grade antioxidant. Ultrason. Sonochem. 2021;74 doi: 10.1016/j.ultsonch.2021.105567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin D., Zhang Q., Xiao L., Huang Y., Yang Z., Wu Z., Tu Z., Qin W., Chen H., Wu D., Zhang Q., Li S. Effects of ultrasound on functional properties, structure and glycation properties of proteins: a review. Crit. Rev. Food Sci. Nutr. 2021;61:2471–2481. doi: 10.1080/10408398.2020.1778632. [DOI] [PubMed] [Google Scholar]
- 21.O’Sullivan J.J., Park M., Beevers J., Greenwood R.W., Norton I.T. Applications of ultrasound for the functional modification of proteins and nanoemulsion formation: A review. Food Hydrocoll. 2017;71:299–310. doi: 10.1016/j.foodhyd.2016.12.037. [DOI] [Google Scholar]
- 22.Rahman M.M., Lamsal B.P. Ultrasound-assisted extraction and modification of plant-based proteins: Impact on physicochemical, functional, and nutritional properties. Compr. Rev. Food Sci. Food Saf. 2021;20:1457–1480. doi: 10.1111/1541-4337.12709. [DOI] [PubMed] [Google Scholar]
- 23.Ashokkumar M. The characterization of acoustic cavitation bubbles – An overview. Ultrason. Sonochem. 2011;18:864–872. doi: 10.1016/j.ultsonch.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 24.Xu B., Azam S.M.R., Feng M., Wu B., Yan W., Zhou C., Ma H. Application of multi-frequency power ultrasound in selected food processing using large-scale reactors: A review. Ultrason. Sonochem. 2021;81 doi: 10.1016/j.ultsonch.2021.105855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou L., Zhang J., Xing L., Zhang W. Applications and effects of ultrasound assisted emulsification in the production of food emulsions: A review. Trends Food Sci. Technol. 2021;110:493–512. doi: 10.1016/j.tifs.2021.02.008. [DOI] [Google Scholar]
- 26.Zhang X., Chen X., Gong Y., Li Z., Guo Y., Yu D., Pan M. Emulsion gels stabilized by soybean protein isolate and pectin: Effects of high intensity ultrasound on the gel properties, stability and β-carotene digestive characteristics. Ultrason. Sonochem. 2021;79 doi: 10.1016/j.ultsonch.2021.105756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Flores E.M.M., Cravotto G., Bizzi C.A., Santos D., Iop G.D. Ultrasound-assisted biomass valorization to industrial interesting products: state-of-the-art, perspectives and challenges. Ultrason. Sonochem. 2021;72 doi: 10.1016/j.ultsonch.2020.105455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ashokkumar M. Applications of ultrasound in food and bioprocessing. Ultrason. Sonochem. 2015;25:17–23. doi: 10.1016/j.ultsonch.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 29.Ashokkumar M., Sunartio D., Kentish S., Mawson R., Simons L., Vilkhu K., C. (Kees) Versteeg, Modification of food ingredients by ultrasound to improve functionality: A preliminary study on a model system. Innov. Food Sci. Emerg. Technol. 2008;9:155–160. doi: 10.1016/j.ifset.2007.05.005. [DOI] [Google Scholar]
- 30.Zhao Q., Xie T., Hong X., Zhou Y., Fan L., Liu Y., Li J. Modification of functional properties of perilla protein isolate by high-intensity ultrasonic treatment and the stability of o/w emulsion. Food Chem. 2022;368 doi: 10.1016/j.foodchem.2021.130848. [DOI] [PubMed] [Google Scholar]
- 31.Zou H., Zhao N., Sun S., Dong X., Yu C. High-intensity ultrasonication treatment improved physicochemical and functional properties of mussel sarcoplasmic proteins and enhanced the stability of oil-in-water emulsion. Colloids Surf. Physicochem. Eng. Asp. 2020;589 doi: 10.1016/j.colsurfa.2020.124463. [DOI] [Google Scholar]
- 32.Xiong W., Wang Y., Zhang C., Wan J., Shah B.R., Pei Y., Zhou B., Li J., Li B. High intensity ultrasound modified ovalbumin: Structure, interface and gelation properties. Ultrason. Sonochem. 2016;31:302–309. doi: 10.1016/j.ultsonch.2016.01.014. [DOI] [PubMed] [Google Scholar]
- 33.Li K., Fu L., Zhao Y.-Y., Xue S.-W., Wang P., Xu X.-L., Bai Y.-H. Use of high-intensity ultrasound to improve emulsifying properties of chicken myofibrillar protein and enhance the rheological properties and stability of the emulsion. Food Hydrocoll. 2020;98 doi: 10.1016/j.foodhyd.2019.105275. [DOI] [Google Scholar]
- 34.Zou Y., Xu P., Wu H., Zhang M., Sun Z., Sun C., Wang D., Cao J., Xu W. Effects of different ultrasound power on physicochemical property and functional performance of chicken actomyosin. Int. J. Biol. Macromol. 2018;113:640–647. doi: 10.1016/j.ijbiomac.2018.02.039. [DOI] [PubMed] [Google Scholar]
- 35.Saleem R., Ahmad R. Effect of low frequency ultrasonication on biochemical and structural properties of chicken actomyosin. Food Chem. 2016;205:43–51. doi: 10.1016/j.foodchem.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 36.Meng Y., Liang Z., Zhang C., Hao S., Han H., Du P., Li A., Shao H., Li C., Liu L. Ultrasonic modification of whey protein isolate: Implications for the structural and functional properties. LWT. 2021:112272. doi: 10.1016/j.lwt.2021.112272. [DOI] [Google Scholar]
- 37.Tomé Constantino A.B., Garcia-Rojas E.E. Modifications of physicochemical and functional properties of amaranth protein isolate (Amaranthus cruentus BRS Alegria) treated with high-intensity ultrasound. J. Cereal Sci. 2020;95 doi: 10.1016/j.jcs.2020.103076. [DOI] [Google Scholar]
- 38.Xu L., Yan W., Zhang M., Hong X., Liu Y., Li J. Application of ultrasound in stabilizing of Antarctic krill oil by modified chickpea protein isolate and ginseng saponin. LWT. 2021;149 doi: 10.1016/j.lwt.2021.111803. [DOI] [Google Scholar]
- 39.Geng M., Liu J., Hu H., Qin L., Taha A., Zhang Z. A comprehensive study on structures and characterizations of 7S protein treated by high intensity ultrasound at different pH and ionic strengths. Food Chem. 2022;373 doi: 10.1016/j.foodchem.2021.131378. [DOI] [PubMed] [Google Scholar]
- 40.Lo B., Kasapis S., Farahnaky A. Effect of low frequency ultrasound on the functional characteristics of isolated lupin protein. Food Hydrocoll. 2022;124 doi: 10.1016/j.foodhyd.2021.107345. [DOI] [Google Scholar]
- 41.Liu H., Zhang H., Liu Q., Chen Q., Kong B. Solubilization and stable dispersion of myofibrillar proteins in water through the destruction and inhibition of the assembly of filaments using high-intensity ultrasound. Ultrason. Sonochem. 2020;67 doi: 10.1016/j.ultsonch.2020.105160. [DOI] [PubMed] [Google Scholar]
- 42.Ren X., Li C., Yang F., Huang Y., Huang C., Zhang K., Yan L. Comparison of hydrodynamic and ultrasonic cavitation effects on soy protein isolate functionality. J. Food Eng. 2020;265 doi: 10.1016/j.jfoodeng.2019.109697. [DOI] [Google Scholar]
- 43.Chen X., Xiong Y.L., Xu X. High-pressure homogenization combined with sulfhydryl blockage by hydrogen peroxide enhance the thermal stability of chicken breast myofibrillar protein aqueous solution. Food Chem. 2019;285:31–38. doi: 10.1016/j.foodchem.2019.01.131. [DOI] [PubMed] [Google Scholar]
- 44.Yan X., Ma C., Cui F., McClements D.J., Liu X., Liu F. Protein-stabilized Pickering emulsions: Formation, stability, properties, and applications in foods. Trends Food Sci. Technol. 2020;103:293–303. doi: 10.1016/j.tifs.2020.07.005. [DOI] [Google Scholar]
- 45.Tang C.-H. Emulsifying properties of soy proteins: A critical review with emphasis on the role of conformational flexibility. Crit. Rev. Food Sci. Nutr. 2017;57:2636–2679. doi: 10.1080/10408398.2015.1067594. [DOI] [PubMed] [Google Scholar]
- 46.Taha A., Ahmed E., Ismaiel A., Ashokkumar M., Xu X., Pan S., Hu H. Ultrasonic emulsification: An overview on the preparation of different emulsifiers-stabilized emulsions. Trends Food Sci. Technol. 2020;105:363–377. doi: 10.1016/j.tifs.2020.09.024. [DOI] [Google Scholar]
- 47.Huang L., Zhang W., Ding X., Wu Z., Li Y. Effects of dual-frequency ultrasound with different energy irradiation modes on the structural and emulsifying properties of soy protein isolate. Food Bioprod. Process. 2020;123:419–426. doi: 10.1016/j.fbp.2020.07.021. [DOI] [Google Scholar]
- 48.Yan S., Xu J., Zhang S., Li Y. Effects of flexibility and surface hydrophobicity on emulsifying properties: Ultrasound-treated soybean protein isolate. LWT. 2021;142 doi: 10.1016/j.lwt.2021.110881. [DOI] [Google Scholar]
- 49.Amiri A., Sharifian P., Soltanizadeh N. Application of ultrasound treatment for improving the physicochemical, functional and rheological properties of myofibrillar proteins. Int. J. Biol. Macromol. 2018;111:139–147. doi: 10.1016/j.ijbiomac.2017.12.167. [DOI] [PubMed] [Google Scholar]
- 50.Liu H., Zhang J., Wang H., Chen Q., Kong B. High-intensity ultrasound improves the physical stability of myofibrillar protein emulsion at low ionic strength by destroying and suppressing myosin molecular assembly. Ultrason. Sonochem. 2021;74 doi: 10.1016/j.ultsonch.2021.105554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gao Z.-M., Yang X.-Q., Wu N.-N., Wang L.-J., Wang J.-M., Guo J., Yin S.-W. Protein-based pickering emulsion and oil gel prepared by complexes of zein colloidal particles and stearate. J. Agric. Food Chem. 2014;62:2672–2678. doi: 10.1021/jf500005y. [DOI] [PubMed] [Google Scholar]
- 52.Qin X.-S., Luo Z.-G., Peng X.-C. Fabrication and characterization of quinoa protein nanoparticle-stabilized food-grade pickering emulsions with ultrasound treatment: interfacial adsorption/arrangement properties. J. Agric. Food Chem. 2018;66:4449–4457. doi: 10.1021/acs.jafc.8b00225. [DOI] [PubMed] [Google Scholar]
- 53.Cen K., Yu X., Gao C., Feng X., Tang X. Effects of different vegetable oils and ultrasonicated quinoa protein nanoparticles on the rheological properties of Pickering emulsion and freeze-thaw stability of emulsion gels. J. Cereal Sci. 2021;102 doi: 10.1016/j.jcs.2021.103350. [DOI] [Google Scholar]
- 54.Zhang X., Zuo Z., Ma W., Yu P., Li T., Wang L. Assemble behavior of ultrasound-induced quinoa protein nanoparticles and their roles on rheological properties and stability of high internal phase emulsions. Food Hydrocoll. 2021;117 doi: 10.1016/j.foodhyd.2021.106748. [DOI] [Google Scholar]
- 55.Xiong T., Xiong W., Ge M., Xia J., Li B., Chen Y. Effect of high intensity ultrasound on structure and foaming properties of pea protein isolate. Food Res. Int. 2018;109:260–267. doi: 10.1016/j.foodres.2018.04.044. [DOI] [PubMed] [Google Scholar]
- 56.Sheng L., Wang Y., Chen J., Zou J., Wang Q., Ma M. Influence of high-intensity ultrasound on foaming and structural properties of egg white. Food Res. Int. 2018;108:604–610. doi: 10.1016/j.foodres.2018.04.007. [DOI] [PubMed] [Google Scholar]
- 57.Wang J., Na X., Navicha W.B., Wen C., Ma W., Xu X., Wu C., Du M. Concentration-dependent improvement of gelling ability of soy proteins by preheating or ultrasound treatment. LWT. 2020;134 doi: 10.1016/j.lwt.2020.110170. [DOI] [Google Scholar]
- 58.Zhang C., Liu H., Xia X., Sun F., Kong B. Effect of ultrasound-assisted immersion thawing on emulsifying and gelling properties of chicken myofibrillar protein. LWT. 2021;142 doi: 10.1016/j.lwt.2021.111016. [DOI] [Google Scholar]
- 59.Chen H., Zhang M., Rao Z. Effect of ultrasound-assisted thawing on gelling and 3D printing properties of silver carp surimi. Food Res. Int. 2021;145 doi: 10.1016/j.foodres.2021.110405. [DOI] [PubMed] [Google Scholar]
- 60.Zhang Q., Li L., Lan Q., Li M., Wu D., Chen H., Liu Y., Lin D., Qin W., Zhang Z., Liu J., Yang W. Protein glycosylation: a promising way to modify the functional properties and extend the application in food system. Crit. Rev. Food Sci Nutr. 2018 doi: 10.1080/10408398.2018.1507995. https://www.tandfonline.com/doi/abs/10.1080/10408398.2018.1507995 (accessed January 6, 2022. [DOI] [PubMed] [Google Scholar]
- 61.Dev M.J., Pandit A.B., Singhal R.S. Ultrasound assisted vis-à-vis classical heating for the conjugation of whey protein isolate-gellan gum: Process optimization, structural characterization and physico-functional evaluation. Innov. Food Sci. Emerg. Technol. 2021;72 doi: 10.1016/j.ifset.2021.102724. [DOI] [Google Scholar]
- 62.Kato A., Sasaki Y., Furuta R., Kobayashi K. Functional protein-polysaccharide conjugate prepared by controlled dry-heating of ovalbumin-dextran mixtures. Agric. Biol. Chem. 1990;54:107–112. doi: 10.1271/bbb1961.54.107. [DOI] [PubMed] [Google Scholar]
- 63.Yang W., Tu Z., Li Q., Kaltashov I.A., McClements D.J. Utilization of sonication-glycation to improve the functional properties of ovalbumin: A high-resolution mass spectrometry study. Food Hydrocoll. 2021;119 doi: 10.1016/j.foodhyd.2021.106822. [DOI] [Google Scholar]
- 64.Sheng L., Liu Q., Dong W., Cai Z. Effect of high intensity ultrasound assisted glycosylation on the gel properties of ovalbumin: Texture, rheology, water state and microstructure. Food Chem. 2022;372 doi: 10.1016/j.foodchem.2021.131215. [DOI] [PubMed] [Google Scholar]
- 65.Ma X., Hou F., Zhao H., Wang D., Chen W., Miao S., Liu D. Conjugation of soy protein isolate (SPI) with pectin by ultrasound treatment. Food Hydrocoll. 2020;108 doi: 10.1016/j.foodhyd.2020.106056. [DOI] [Google Scholar]
- 66.Cui Q., Zhang A., Li R., Wang X., Sun L., Jiang L. Ultrasonic treatment affects emulsifying properties and molecular flexibility of soybean protein isolate-glucose conjugates. Food Biosci. 2020;38 doi: 10.1016/j.fbio.2020.100747. [DOI] [Google Scholar]
- 67.Chen L., Chen J., Wu K., Yu L. Improved Low pH Emulsification Properties of Glycated Peanut Protein Isolate by Ultrasound Maillard Reaction. J. Agric. Food Chem. 2016;64:5531–5538. doi: 10.1021/acs.jafc.6b00989. [DOI] [PubMed] [Google Scholar]
- 68.Wang Z., Han F., Sui X., Qi B., Yang Y., Zhang H., Wang R., Li Y., Jiang L. Effect of ultrasound treatment on the wet heating Maillard reaction between mung bean [Vigna radiate (L.)] protein isolates and glucose and on structural and physico-chemical properties of conjugates. J. Sci. Food Agric. 2016;96:1532–1540. doi: 10.1002/jsfa.7255. [DOI] [PubMed] [Google Scholar]
- 69.Ma C., Jiang W., Chen G., Wang Q., McClements D.J., Liu X., Liu F., Ngai T. Sonochemical effects on formation and emulsifying properties of zein-gum Arabic complexes. Food Hydrocoll. 2021;114 doi: 10.1016/j.foodhyd.2020.106557. [DOI] [Google Scholar]
- 70.Vargas S.A., Delgado-Macuil R.J., Ruiz-Espinosa H., Rojas-López M., Amador-Espejo G.G. High-intensity ultrasound pretreatment influence on whey protein isolate and its use on complex coacervation with kappa carrageenan: Evaluation of selected functional properties. Ultrason. Sonochem. 2021;70 doi: 10.1016/j.ultsonch.2020.105340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Albano K.M., Nicoletti V.R. Ultrasound impact on whey protein concentrate-pectin complexes and in the O/W emulsions with low oil soybean content stabilization. Ultrason. Sonochem. 2018;41:562–571. doi: 10.1016/j.ultsonch.2017.10.018. [DOI] [PubMed] [Google Scholar]
- 72.Ma X., Yan T., Hou F., Chen W., Miao S., Liu D. Formation of soy protein isolate (SPI)-citrus pectin (CP) electrostatic complexes under a high-intensity ultrasonic field: Linking the enhanced emulsifying properties to physicochemical and structural properties. Ultrason. Sonochem. 2019;59 doi: 10.1016/j.ultsonch.2019.104748. [DOI] [PubMed] [Google Scholar]
- 73.Li Y., Cheng Y., Zhang Z., Wang Y., Mintah B.K., Dabbour M., Jiang H., He R., Ma H. Modification of rapeseed protein by ultrasound-assisted pH shift treatment: Ultrasonic mode and frequency screening, changes in protein solubility and structural characteristics. Ultrason. Sonochem. 2020;69 doi: 10.1016/j.ultsonch.2020.105240. [DOI] [PubMed] [Google Scholar]
- 74.Pezeshk S., Rezaei M., Hosseini H., Abdollahi M. Impact of pH-shift processing combined with ultrasonication on structural and functional properties of proteins isolated from rainbow trout by-products. Food Hydrocoll. 2021;118 doi: 10.1016/j.foodhyd.2021.106768. [DOI] [Google Scholar]
- 75.Figueroa-González J.J., Lobato-Calleros C., Vernon-Carter E.J., Aguirre-Mandujano E., Alvarez-Ramirez J., Martínez-Velasco A. Modifying the structure, physicochemical properties, and foaming ability of amaranth protein by dual pH-shifting and ultrasound treatments. LWT. 2022;153 doi: 10.1016/j.lwt.2021.112561. [DOI] [Google Scholar]
- 76.Wang Y., Wang S., Li R., Wang Y., Xiang Q., Li K., Bai Y. Effects of combined treatment with ultrasound and pH shifting on foaming properties of chickpea protein isolate. Food Hydrocoll. 2022;124 doi: 10.1016/j.foodhyd.2021.107351. [DOI] [Google Scholar]
- 77.Sung H.-Y., Chen H.-J., Liu T.-Y., Su J.-C. Improvement of the Functionalities of Soy Protein Isolate through Chemical Phosphorylation. J. Food Sci. 1983;48:716–721. doi: 10.1111/j.1365-2621.1983.tb14882.x. [DOI] [Google Scholar]
- 78.Ross L.F., Bhatnagar D. Enzymatic phosphorylation of soybean proteins. J. Agric. Food Chem. 1989;37:841–844. doi: 10.1021/jf00088a001. [DOI] [Google Scholar]
- 79.Li C.-P., Enomoto H., Hayashi Y., Zhao H., Aoki T. Recent advances in phosphorylation of food proteins: A review. LWT - Food Sci. Technol. 2010;43:1295–1300. doi: 10.1016/j.lwt.2010.03.016. [DOI] [Google Scholar]
- 80.Liu Y., Wang D., Wang J., Yang Y., Zhang L., Li J., Wang S. Functional properties and structural characteristics of phosphorylated pea protein isolate. Int. J. Food Sci. Technol. 2020;55:2002–2010. doi: 10.1111/ijfs.14391. [DOI] [Google Scholar]
- 81.Sánchez-Reséndiz A., Rodríguez-Barrientos S., Rodríguez-Rodríguez J., Barba-Dávila B., Serna-Saldívar S.O., Chuck-Hernández C. Phosphoesterification of soybean and peanut proteins with sodium trimetaphosphate (STMP): Changes in structure to improve functionality for food applications. Food Chem. 2018;260:299–305. doi: 10.1016/j.foodchem.2018.04.009. [DOI] [PubMed] [Google Scholar]
- 82.Huang T., Tu Z., Shangguan X., Wang H., Sha X., Bansal N. Rheological behavior, emulsifying properties and structural characterization of phosphorylated fish gelatin. Food Chem. 2018;246:428–436. doi: 10.1016/j.foodchem.2017.12.023. [DOI] [PubMed] [Google Scholar]
- 83.Yan C., Zhou Z. Solubility and emulsifying properties of phosphorylated walnut protein isolate extracted by sodium trimetaphosphate. LWT. 2021;143 doi: 10.1016/j.lwt.2021.111117. [DOI] [Google Scholar]
- 84.Wang Y.-R., Yang Q., Fan J.-L., Zhang B., Chen H.-Q. The effects of phosphorylation modification on the structure, interactions and rheological properties of rice glutelin during heat treatment. Food Chem. 2019;297 doi: 10.1016/j.foodchem.2019.124978. [DOI] [PubMed] [Google Scholar]
- 85.Hadidi M., Jafarzadeh S., Ibarz A. Modified mung bean protein: Optimization of microwave-assisted phosphorylation and its functional and structural characterizations. LWT. 2021;151 doi: 10.1016/j.lwt.2021.112119. [DOI] [Google Scholar]
- 86.Hu Y., Wu Z., Sun Y., Cao J., He J., Dang Y., Pan D., Zhou C. Insight into ultrasound-assisted phosphorylation on the structural and emulsifying properties of goose liver protein. Food Chem. 2022;373 doi: 10.1016/j.foodchem.2021.131598. [DOI] [PubMed] [Google Scholar]
- 87.Dabbour M., Xiang J., Mintah B., He R., Jiang H., Ma H. Localized enzymolysis and sonochemically modified sunflower protein: Physical, functional and structure attributes. Ultrason. Sonochem. 2020;63 doi: 10.1016/j.ultsonch.2019.104957. [DOI] [PubMed] [Google Scholar]
- 88.Dabbour M., He R., Mintah B., Xiang J., Ma H. Changes in functionalities, conformational characteristics and antioxidative capacities of sunflower protein by controlled enzymolysis and ultrasonication action. Ultrason. Sonochem. 2019;58 doi: 10.1016/j.ultsonch.2019.104625. [DOI] [PubMed] [Google Scholar]
- 89.Zeeb B., Gibis M., Fischer L., Weiss J. Crosslinking of interfacial layers in multilayered oil-in-water emulsions using laccase: Characterization and pH-stability. Food Hydrocoll. 2012;27:126–136. doi: 10.1016/j.foodhyd.2011.08.005. [DOI] [Google Scholar]
- 90.Ma H., Forssell P., Partanen R., Buchert J., Boer H. Improving Laccase Catalyzed Cross-Linking of Whey Protein Isolate and Their Application as Emulsifiers. J. Agric. Food Chem. 2011;59:1406–1414. doi: 10.1021/jf103591p. [DOI] [PubMed] [Google Scholar]
- 91.Yuan X., Li X., Zhang X., Mu Z., Gao Z., Jiang L., Jiang Z. Effect of ultrasound on structure and functional properties of laccase-catalyzed α-lactalbumin. J. Food Eng. 2018;223:116–123. doi: 10.1016/j.jfoodeng.2017.12.008. [DOI] [Google Scholar]
- 92.Jiang Z., Wang C., Li T., Sun D., Gao H., Gao Z., Mu Z. Effect of ultrasound on the structure and functional properties of transglutaminase-crosslinked whey protein isolate exposed to prior heat treatment. Int. Dairy J. 2019;88:79–88. doi: 10.1016/j.idairyj.2018.08.007. [DOI] [Google Scholar]
- 93.Wang Y., Zhang Z., He R., Liu D., Kumah Mintah B., Dabbour M., Ma H. Improvement in enzymolysis efficiency and changes in conformational attributes of corn gluten meal by dual-frequency slit ultrasonication action. Ultrason. Sonochem. 2020;64 doi: 10.1016/j.ultsonch.2020.105038. [DOI] [PubMed] [Google Scholar]
- 94.Ding Y., Ma H., Wang K., Azam S.M.R., Wang Y., Zhou J., Qu W. Ultrasound frequency effect on soybean protein: Acoustic field simulation, extraction rate and structure. LWT. 2021;145 doi: 10.1016/j.lwt.2021.111320. [DOI] [Google Scholar]
- 95.Chen J., Xu Y., Pius B.A., Wang P., Xu X. Changes of myofibrillar protein structure improved the stability and distribution of baicalein in emulsion. LWT. 2021;137 doi: 10.1016/j.lwt.2020.110404. [DOI] [Google Scholar]
- 96.Zhang L., Wang X., Hu Y., Abiola Fakayode O., Ma H., Zhou C., Hu Z., Xia A., Li Q. Dual-frequency multi-angle ultrasonic processing technology and its real-time monitoring on physicochemical properties of raw soymilk and soybean protein. Ultrason. Sonochem. 2021;80 doi: 10.1016/j.ultsonch.2021.105803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chen J., Zhang X., Chen Y., Zhao X., Anthony B., Xu X. Effects of different ultrasound frequencies on the structure, rheological and functional properties of myosin: Significance of quorum sensing. Ultrason. Sonochem. 2020;69 doi: 10.1016/j.ultsonch.2020.105268. [DOI] [PubMed] [Google Scholar]
- 98.Cheng Y., Ofori Donkor P., Yeboah G.B., Ayim I., Wu J., Ma H. Modulating the in vitro digestion of heat-set whey protein emulsion gels via gelling properties modification with sequential ultrasound pretreatment. LWT. 2021;149 doi: 10.1016/j.lwt.2021.111856. [DOI] [Google Scholar]
- 99.Wen C., Zhang J., Qin W., Gu J., Zhang H., Duan Y., Ma H. Structure and functional properties of soy protein isolate-lentinan conjugates obtained in Maillard reaction by slit divergent ultrasonic assisted wet heating and the stability of oil-in-water emulsions. Food Chem. 2020;331 doi: 10.1016/j.foodchem.2020.127374. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.