Abstract
Background
Black women experience worse survival with high grade endometrial cancer. Differences in adjuvant treatment have been proposed to be major contributors to this disparity. Little is known about the differences in type or timing of adjuvant treatment as it relates to race/ethnicity in the Medicare population.
Objectives
To examine patterns of adjuvant therapy and survival for non-Hispanic Black women compared to non-Hispanic White women and Hispanic women who have undergone surgery for high grade endometrial cancer in the Medicare population.
Study Design
We utilized the Surveillance, Epidemiology, and End Results-Medicare Linked database to identify women who underwent surgery as a primary treatment for uterine grade 3 endometrioid adenocarcinoma, carcinosarcoma, clear cell carcinoma, or serous carcinoma between the years 2000 and 2015. Women who did not identify as White or Black race or Hispanic ethnicity were excluded. Multinomial logistic regression was used to estimate odds ratios (OR) and 95% confidence intervals (95% CI) for receiving a treatment delay or not receiving adjuvant treatment (compared to those who received adjuvant treatment within 12 weeks) adjusted for clinical and demographic characteristics. Overall survival was stratified by race/ethnicity, route of surgery, operative complications, and type and timing of adjuvant therapy were analyzed using the Kaplan-Meier method. Cox proportional hazards regression was used to estimate hazard of death by race/ethnicity adjusted for known predictors, as well as surgical outcomes and adjuvant therapy patterns.
Results
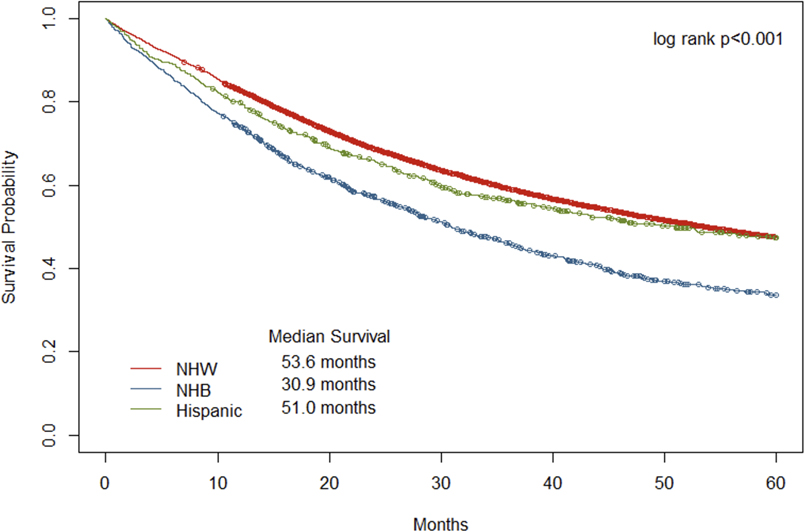
12,201 women met study inclusion criteria. Non-Hispanic Black patients had a significantly worse five-year overall survival than Hispanic and non-Hispanic White patients (30.9 months vs 51.0 months vs 53.6 months, respectively). Approximately 8.6% of patients who received adjuvant treatment experienced a treatment delay (632/7,282). Delay in treatment of greater than or equal to 12 weeks was significantly different by race/ethnicity (p=0.034), with 12% of Hispanic, 9% of non-Hispanic Black, and 8% of non-Hispanic White women experiencing a delay. After adjustment for number of complications, age, histology (endometrioid vs non-endometroid), FIGO stage, marital status, comorbidity count, surgical approach, lymph node dissection, and urban-rural code, Hispanic women had a 71% increased risk of treatment delay (OR 1.71, CI 1.23–2.38) for all stages of disease. In the same model, non-Hispanic Black race was independently predictive of decreased use of adjuvant treatment for FIGO stage II and higher (OR 1.32, CI 1.04–1.68). Non-Hispanic Black race, number of perioperative complications, and non-endometrioid histology were predictive of worse survival in univariate models. Treatment delay was not independently predictive of worse 1- or 5-year survival at any stage.
Conclusions
Non-Hispanic Black race is predictive of worse 5-year survival across all stages and is also associated with omission of adjuvant treatment in ≥FIGO Stage II high grade endometrial cancer. In unadjusted analyses, patients who experience treatment omission or delay experienced poorer overall survival, but these factors were not independently associated in multivariate analyses. This study suggests race/ethnicity is independently associated with type and timing of adjuvant treatment in patients with high grade endometrial cancer. Further efforts to identify specific causes of barriers to care and timely treatment are imperative.
Introduction
Uterine cancer is the most common gynecologic malignancy in the United States with an estimated 65,620 new cases in 2020, accounting for 14% of all new cancers in women.1 The majority of endometrial cancers (EC) are early stage, low grade, and endometrioid histology with a 5-year survival rate of greater than 85%.2,3 Despite this overall favorable prognosis of EC, patients with higher grade, higher stage disease, those who are older at diagnosis, and those with non-endometrioid histology independently have worse 5-year survival.4 High grade endometrial cancers (HGEC) comprise only 7% of newly diagnosed EC but account for 14% of EC attributed deaths.5,6 Non-Hispanic Black (NHB) women appear to be disproportionately affected by higher stage and higher-grade endometrial cancers than their Hispanic (HS) and Non-Hispanic White (NHW) counterparts.7
When other variables are accounted for, contemporary cohorts of NHB women with EC have a 29% increased risk of all-cause mortality compared to NHW women, and are twice as likely to not be recommended definitive surgery.7 NHB women who do undergo surgery for EC generally incur more postoperative complications compared to white women, and receive laparotomy more frequently for all stages of disease. These complications can be mitigated when a minimally invasive (MIS) route is chosen.8,9 Contemporary standards of care dictate that nearly all high-risk cancers be treated with chemotherapy (CT), radiotherapy (RT) or a combination of both (CRT) after definitive surgery.10,11 Increased perioperative complications have been associated with adjuvant treatment delays, which negatively impact survival in ovarian, colorectal, pancreatic cancers.12–14
The majority of treatment disparity research in EC includes only NHW and NHB populations, and there is a limited exploration of the HS population, especially as it pertains to surgical and adjuvant therapy treatment patterns and outcomes.15, 16 We hypothesized that racial differences between NHW, NHB and HS patients in the Medicare population resulted from the effect of surgery for HGEC on type and frequency of adjuvant therapy, and these differences may extend to survival outcomes.
Methods
Data from Medicare-linked Surveillance Epidemiology and End Results (SEER) database was used to conduct this retrospective case study. The SEER program is sponsored by the National Cancer Institute (NCI) and collects incidence and survival data from 18 population-based cancer registries across the United States, representing approximately 35% of the U.S. population17. Medicare is the primary insurance for older Americans (age 65 or older) and the Center for Medicare and Medicaid Services manages the claims for all covered health care services. Taken together, these data sources provide researchers detailed information on cancer diagnosis, outcome, and treatment information. This study was determined to be non-human participant research by Wayne State University’s Institutional Review Board (Review number: HPR #2016–002).
Cohort Selection
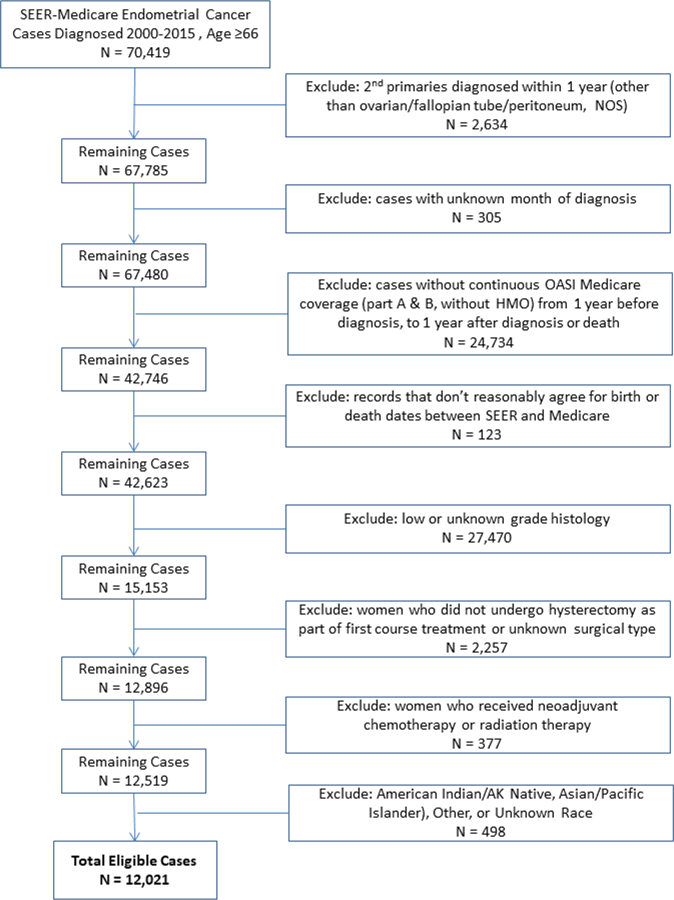
The patient records included in this study were those of women diagnosed with high-grade endometrial cancer between January 1, 2000 and December 31, 2015. High grade cancer was defined by International Federation of Gynecology and Obstetrics (FIGO) grade 3, and the following International Classification of Disease (ICD) – Oncology histology codes: endometrioid (8140, 8143, 8210–8211, 8262–8263, 8380–8384, 8560, 8570), serous (8441, 8460–8461), clear cell (8310,8313), carcinosarcoma (8950–8951,8980–8981), and mixed (8255, 8323). In order to adequately capture pre-existing comorbid conditions at diagnosis from Medicare claims data, only patients age 66 years of age or older were included. In addition, patients had to be a Part A and Part B Medicare enrollee for one year prior to and one year after diagnosis (or until death if survival was less than 1 year), and have received a hysterectomy within 9 months of diagnosis. Women who received neoadjuvant chemotherapy or radiation therapy were excluded, as well as race other than White or Black (due to the small sample size). The study’s inclusion and exclusion criteria are detailed in Figure 1.
Figure 1.
Study inclusion and exclusion criteria
Study Variables
Demographic variables (race, ethnicity, age group, marital status, and urban-rural indicator) were taken from SEER records. Race and ethnicity were combined to create three groups: non-Hispanic White (NHW), non-Hispanic Black (NHB), and Hispanic (HS). Histology subtypes were grouped as described in the cohort selection (endometrioid, serous, clear cell, carcinosarcoma, and mixed) and as a dichotomous variable (endometrioid and non- endometrioid). A comorbidity count was calculated for each patient at the time of surgery using the list of conditions standardly included in the Charlson comorbidity score, but excluding cancer. FIGO stage was based on the SEER extension codes. Surgery type was classified as either open or minimally invasive (MIS) using either HCPCS codes or ICD-9 procedure codes. From SEER records, the receipt of lymphadenectomy and the number of lymph nodes examined were also examined.
Adjuvant treatment, identified from Medicare claims, was defined as the receipt of either chemotherapy or radiation therapy within 6 months of surgery. Adjuvant treatment was then classified as receiving both chemotherapy and radiation therapy, chemotherapy only, radiation therapy only, or neither. Time from surgery to the start of adjuvant treatment was classified as either no delay (within 12 weeks), delayed (started over 12 weeks from surgery), or no adjuvant treatment.
Peri-operative complications were classified using methodology described by Wright et al, and were categorized as intra-operative, post-operative surgical, and post-operative medical complications. Intraoperative complications included bladder injury, ureteral injury, intestinal injury, vascular injury, nephrostomy, or other operative injury.12 Post-operative surgical complications included hemorrhage, abscess, bowel obstruction, ileus, or wound complication within 30 days of surgery. Post-operative medical complications included respiratory failure, VTE, pneumonia, renal failure, shock, stroke, cardiopulmonary arrest, bacteremia/sepsis, or myocardial infraction within 30 days from surgery. Complications from any group were also summed and assessed as a continuous variable and a dichotomous variable (none versus any).
Statistical Analysis
Statistical analyses were performed using SAS software (version 9.4; Cary, NC) and Kaplan-Meier graphs were drawn using the R package “survival”.18 The distributions of demographic and clinical characteristics were summarized by race/ethnicity. The subset of women with surgical complications was examined by race/ethnicity and pair-wise comparisons were made using chi-square tests. Similarly, the type of adjuvant treatment (overall and stratified by FIGO stage) and adjuvant treatment delay were compared using chi-square. Multivariate multinomial logistic regression was used to estimate the association of clinical and demographic variables for either adjuvant treatment delay or not receiving adjuvant treatment (with an outcome reference category of receiving adjuvant treatment without delay). This analysis was limited to those women who survived at least 3 months from surgery to minimize the potential for immortal time bias. To take into consideration the multiple comparisons, p-values <0.005 were considered statistically significant, and all tests were two-sided.
Overall survival was calculated from the date of surgery to death or last follow-up based on Medicare claims. Median survival by race/ethnicity was calculated using the Kaplan Meier method, was compared using log-rank tests, and graphs were drawn to illustrate the 5-year survival. This analysis was further stratified by adjuvant treatment delay or omission and FIGO stage for women who survived at least 3 months. Cox proportional hazards regression was used to estimate the overall hazard of death stratified by FIGO stage (FIGO stage I-II and Stage III-IV). Due to the fact that surgical complications had a large effect size on survival within the first year and a much smaller effect size in the subsequent years, hazard ratios were estimated for two time periods; 1-year survival from surgery and greater than 1 year to 5 years from surgery. The survival analysis was also limited to those who survived at least 3 months. The proportionality assumption was assessed by including an interaction term between the log of survival time and each variable in the final model, and the interaction terms were not significant.
In addition, the cohort of women who received each type of adjuvant treatment was examined by race/ethnicity. This was further examined by time period (2000–2004, 2005–2009, and 2010–2015) and the number of surgical complications. Other variables examined by time period included surgical approach and the number of surgical complications. Stage, surgical complications, and adjuvant treatment were examined by surgical approach for all women, and were then stratified by race.
Results
The data set included 12,021 women diagnosed with high-grade endometrial cancer from 2000 to 2015 (9,891 NHW, 1,474 NHB, and 656 HS, Figure 1). Table 1 summarizes the demographic and clinical characteristics of the study population. NHB and HS women were diagnosed at a younger age than NHW (73.9 vs 74.4 vs 75.8). NHB and HS women were more likely to be diagnosed with non-endometrioid histology, more advanced stage, and to have a higher comorbidity count at the time of surgery than NHW women. NHB patients had a significantly worse five-year overall survival (OS) than HS and NHW patients (30.9 months vs 51.0 months vs 53.6 months, respectively, p<0.001).
Table 1:
Select demographic and clinical characteristics of elderly women diagnosed with high-grade endometrial cancer by race/ethnicity
| Non-Hispanic White | Non-Hispanic Black | Hispanic | ||||
|---|---|---|---|---|---|---|
| Characteristic | N | % | N | % | N | % |
|
| ||||||
| Total | 9,891 | 82% | 1,474 | 12% | 656 | 6% |
|
| ||||||
| Age Group | ||||||
|
| ||||||
| 65–69 | 2,116 | 21% | 417 | 28% | 183 | 28% |
|
| ||||||
| 70–74 | 2,625 | 27% | 470 | 32% | 188 | 29% |
|
| ||||||
| 75–79 | 2,258 | 23% | 325 | 22% | 145 | 22% |
|
| ||||||
| 80–84 | 1,699 | 17% | 165 | 11% | 85 | 13% |
|
| ||||||
| 85+ | 1,193 | 12% | 97 | 7% | 55 | 8% |
|
| ||||||
| mean (std) | 75.8 (6.7) | 73.9 (6.1) | 74.4 (6.5) | |||
|
| ||||||
| Urban Rural Indicator | ||||||
|
| ||||||
| All urban | 6,069 | 61% | 1,101 | 75% | 515 | 79% |
|
| ||||||
| Mostly urban | 2,181 | 22% | 231 | 16% | 100 | 15% |
|
| ||||||
| Mostly rural | 717 | 7% | 61 | 4% | 22 | 3% |
|
| ||||||
| All rural | 919 | 9% | 79 | 5% | 19 | 3% |
|
| ||||||
| Histology Group | ||||||
|
| ||||||
| Clear Cell | 496 | 5% | 85 | 6% | 41 | 6% |
|
| ||||||
| High Grade Endometrioid | 3,744 | 38% | 369 | 25% | 214 | 33% |
|
| ||||||
| Mixed | 1,550 | 16% | 139 | 9% | 92 | 14% |
|
| ||||||
| MMMT | 1,834 | 19% | 437 | 30% | 120 | 18% |
|
| ||||||
| Serous | 2,267 | 23% | 444 | 30% | 189 | 29% |
|
| ||||||
|
| ||||||
| High Grade Endometrioid | 3,744 | 38% | 369 | 25% | 214 | 33% |
|
| ||||||
| Non- Endometrioid High Grade | 6,147 | 62% | 1105 | 75% | 442 | 67% |
|
| ||||||
| FIGO Stage (a) | ||||||
|
| ||||||
| I | 6,084 | 62% | 758 | 52% | 368 | 56% |
|
| ||||||
| II | 1,021 | 10% | 212 | 15% | 77 | 12% |
|
| ||||||
| III | 2,026 | 21% | 362 | 25% | 167 | 26% |
|
| ||||||
| IV | 644 | 7% | 118 | 8% | 44(c) | 6% |
|
| ||||||
| Comorbidity Count at Surgery (b) | ||||||
|
| ||||||
| 0 | 3,569 | 36% | 360 | 24% | 172 | 26% |
|
| ||||||
| 1 | 3,148 | 32% | 468 | 32% | 195 | 30% |
|
| ||||||
| 2 | 1,737 | 18% | 293 | 20% | 147 | 22% |
|
| ||||||
| 3+ | 1,437 | 15% | 353 | 24% | 142 | 22% |
|
| ||||||
| Marital Status | ||||||
|
| ||||||
| Married, or equivalent | 4,467 | 45% | 387 | 26% | 256 | 39% |
|
| ||||||
| Single, separated, divorced or widowed | 5,034 | 51% | 1026 | 70% | 378 | 58% |
|
| ||||||
| Unknown | 390 | 4% | 61 | 4% | 22 | 3% |
|
| ||||||
| Surgery Type | ||||||
|
| ||||||
| Open | 7,153 | 72% | 1186 | 80% | 471 | 72% |
|
| ||||||
| MIS | 2,738 | 28% | 288 | 20% | 185 | 28% |
|
| ||||||
| Lymphadenectomy | ||||||
|
| ||||||
| No | 2,633 | 27% | 462 | 32% | 205 | 31% |
|
| ||||||
| Yes | 7,189 | 73% | 1,000 | 68% | 450 | 69% |
|
| ||||||
| Number of Nodes Examined (b) | ||||||
|
| ||||||
| None | 2,633 | 28% | 462 | 33% | 205 | 32% |
|
| ||||||
| 1–10 nodes | 2,693 | 28% | 457 | 32% | 186 | 29% |
|
| ||||||
| >10 nodes | 4,234 | 44% | 500 | 35% | 251 | 39% |
Unknown values excluded from the table.
Comorbidity count based on claims a year prior to surgery.
Condition list: diabetes, chronic pulmonary disease, congestive heart failure, cerebrovascular disease, paralysis, peripheral vascular disease, myocardial infarction, moderate/severe renal disease, ulcer disease, dementia, rheumatologic disease, and liver disease.
Unknown and Stage IV values combined to maintain anonymity
Non-Hispanic Black patients were significantly more likely to have one or more perioperative complications when separately compared to NHW and HS (Table 2). Approximately 39% of the identified cohort did not receive adjuvant treatment, and among those who did have adjuvant treatment 8.6% (632/7,282) experienced a treatment delay of at least 12 weeks. The proportion of HS and NHB women who experienced a delay in treatment of at least 12 weeks was slightly higher than it was in NHW patients (12% vs 9% vs 8%, p=0.034, Table 3). Predictors of adjuvant treatment delay included race/ethnicity, age, medical comorbidities, and surgical complications.
Table 2:
Intra-operative and post-operative complications for elderly women diagnosed with high-grade endometrial cancer by race/ethnicity
| Non-Hispanic White | Non-Hispanic Black | Hispanic | p-value* | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | NHW:NHB | NHW:H | NHB:H | |
|
| |||||||||
| Intra-Operative Complications | 0.150 | 0.693 | 0.219 | ||||||
|
| |||||||||
| No | 8,957 | 91% | 1,365 | 92% | 591 | 90% | |||
|
| |||||||||
| Yes | 934 | 9% | 122 | 8% | 65 | 10% | |||
|
| |||||||||
| Post-Operative Surgical Complications | 0.001 | 0.166 | 0.470 | ||||||
|
| |||||||||
| No | 7,621 | 77% | 1,079 | 73% | 490 | 75% | |||
|
| |||||||||
| Yes | 2,270 | 23% | 395 | 27% | 166 | 25% | |||
|
| |||||||||
| Post-Operative Medical Complications | <.001 | 0.098 | 0.257 | ||||||
|
| |||||||||
| No | 7,418 | 75% | 1,027 | 70% | 473 | 72% | |||
|
| |||||||||
| Yes | 2,473 | 25% | 447 | 30% | 183 | 28% | |||
|
| |||||||||
| Complications Sum | <.001 | 0.018 | 0.193 | ||||||
|
| |||||||||
| None | 5,534 | 56% | 736 | 50% | 359 | 55% | |||
|
| |||||||||
| 1 | 2,150 | 22% | 326 | 22% | 128 | 20% | |||
|
| |||||||||
| 2 | 1,054 | 11% | 170 | 12% | 63 | 10% | |||
|
| |||||||||
| 3 | 525 | 55 | 103 | 7% | 47 | 7% | |||
|
| |||||||||
| 4 | 294 | 3% | 64 | 4% | 33 | 5% | |||
|
| |||||||||
| 5+ | 334 | 3% | 75 | 5% | 26 | 4% | |||
|
| |||||||||
| <.001 | 0.541 | 0.041 | |||||||
|
| |||||||||
| None | 5,534 | 56% | 736 | 50% | 359 | 55% | |||
|
| |||||||||
| 1 or more | 4,357 | 44% | 738 | 50% | 297 | 45% | |||
Pair-wise comparisons were made using chi-square tests
NHW: Non-Hispanic White; NHB: Non-Hispanic Black
Table 3:
The receipt of adjuvant treatment by race/ethnicity for elderly women diagnosed with high-grade endometrial cancer
| Non-Hispanic White | Non-Hispanic Black | Hispanic | p-value* | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Treatment Variable | N | % | N | % | N | % | NHW:NHB | NHW:H | NHB:H |
|
| |||||||||
| Adjuvant therapy (a) | <0.001 | 0.080 | 0.642 | ||||||
|
| |||||||||
| Chemotherapy and radiation | 1,311 | 13% | 219 | 15% | 87 | 13% | |||
|
| |||||||||
| Chemotherapy only | 2,594 | 26% | 459 | 31% | 200 | 30% | |||
|
| |||||||||
| Radiation therapy only | 2,028 | 21% | 267 | 18% | 117 | 18% | |||
|
| |||||||||
| Neither | 3,958 | 40% | 529 | 36% | 252 | 38% | |||
|
| |||||||||
| FIGO Stage I | |||||||||
|
| |||||||||
| Adjuvant therapy received | 3,258 | 54% | 454 | 60% | 204 | 55% | 0.001 | 0.481 | 0.154 |
|
| |||||||||
| FIGO Stage II | |||||||||
|
| |||||||||
| Adjuvant therapy received | 715 | 70% | 153 | 72% | 59 | 77% | 0.534 | 0.221 | 0.449 |
|
| |||||||||
| FIGO Stage III | |||||||||
|
| |||||||||
| Adjuvant therapy received | 1,465 | 72% | 250 | 69% | 116 | 69% | 0.206 | 0.430 | 0.926 |
|
| |||||||||
| FIGO Stage IV | |||||||||
|
| |||||||||
| Adjuvant therapy received | 440 | 68% | 77 | 65% | 22 | 54% | 0.512 | 0.052 | 0.187 |
|
| |||||||||
| Adjuvant therapy delay (b) | 0.538 | 0.010 | 0.078 | ||||||
|
| |||||||||
| Adjuvant received within 12 wks | 5,435 | 92% | 860 | 91% | 355 | 88% | |||
|
| |||||||||
| Adjuvant delay (>12 wks) | 498 | 8% | 85 | 9% | 49 | 12% | |||
first course treatment defined as adjuvant treatment initiated within 6 months of surgery
among women who received adjuvant therapy
Pair-wise comparisons were made using chi-square tests
NHW: Non-Hispanic White; NHB: Non-Hispanic Black
NHB patients were 36% more likely (OR 1.34; 95% CI 1.07–1.72) and HS patients 43% more likely (OR 1.43, CI 1.01 – 2.03) to not receive adjuvant treatment compared to NHW patients when diagnosed at FIGO stage II-IV, adjusting for differences in surgical complications, age, histology, FIGO stage, marital status, comorbidity score, rural-urban continuum codes, and surgical type. Women with an increasing number of surgical complications were more likely to experience treatment delay, for all stages (p- trend < 0.001) as well as for women diagnosed with FIGO stage II-IV (p-trend <0.001, Table 4).
Table 4:
Predictors of adjuvant treatment delay and omission of adjuvant treatment among patients who survived at least 3 months
| All Stages | FIGO Stage II+ | |||||||
|---|---|---|---|---|---|---|---|---|
| Treatment Delay | No Adjuvant Treatment | Treatment Delay | No Adjuvant Treatment | |||||
| Predictor Variable | OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI |
|
| ||||||||
| Race/Ethnicity | ||||||||
|
| ||||||||
| NHW | Ref | Ref | Ref | Ref | ||||
|
| ||||||||
| NHB | 1.02 | 0.78–1.33 | 0.96 | 0.84–1.11 | 1.17 | 0.80–1.72 | 1.36 | 1.07–1.72 |
|
| ||||||||
| Hispanic | 1.63 | 1.18–2.25 | 1.16 | 0.95–1.41 | 1.98 | 1.23–3.17 | 1.43 | 1.01–2.03 |
|
| ||||||||
| Surgical Complications | ||||||||
|
| ||||||||
| None | Ref | Ref | Ref | Ref | ||||
|
| ||||||||
| 1 | 1.06 | 0.85–1.33 | 1.04 | 0.93–1.16 | 1.08 | 0.76–1.52 | 1.02 | 0.83–1.26 |
|
| ||||||||
| 2 | 1.15 | 0.85–1.55 | 1.09 | 0.94–1.27 | 1.09 | 0.70–1.69 | 1.00 | 0.77–1.29 |
|
| ||||||||
| 3+ | 2.58 | 2.01–3.30 | 1.48 | 1.27–1.73 | 2.69 | 1.87–3.87 | 1.78 | 1.38–2.28 |
|
| ||||||||
| p-trend | <0.001 | 0.001 | <0.001 | 0.004 | ||||
|
| ||||||||
| Age Group | ||||||||
|
| ||||||||
| Ordinal (5 year groups) | 1.01 | 0.99–1.02 | 1.06 | 1.06–1.07 | 1.00 | 0.98–1.03 | 1.11 | 1.09–1.12 |
|
| ||||||||
| Histology | ||||||||
|
| ||||||||
| Endometrioid | Ref | Ref | Ref | Ref | ||||
|
| ||||||||
| Non-Endometrioid | 0.88 | 0.74–1.06 | 0.68 | 0.62–0.74 | 0.71 | 0.54–0.95 | 0.62 | 0.52–0.74 |
|
| ||||||||
| FIGO Stage | ||||||||
|
| ||||||||
| I | Ref | Ref | ||||||
|
| ||||||||
| II | 1.00 | 0.78–1.28 | 0.40 | 0.34–0.46 | Ref | |||
|
| ||||||||
| III-IV | 0.60 | 0.49–0.74 | 0.25 | 0.22–0.28 | 0.64 | 0.48–0.84 | 0.63 | 0.53–0.75 |
|
| ||||||||
| Marital Status | ||||||||
|
| ||||||||
| Married or equivalent | Ref | Ref | Ref | Ref | ||||
|
| ||||||||
| S/D/W | 1.06 | 0.89–1.27 | 1.14 | 1.04–1.25 | 1.04 | 0.79–1.37 | 1.42 | 1.19–1.70 |
|
| ||||||||
| Comorbidity Score | ||||||||
|
| ||||||||
| None | Ref | Ref | Ref | Ref | ||||
|
| ||||||||
| 1 | 1.22 | 0.98–1.52 | 1.08 | 0.97–1.20 | 1.11 | 0.78–1.58 | 1.25 | 1.01–1.55 |
|
| ||||||||
| 2 | 1.33 | 1.04–1.71 | 1.10 | 0.96–1.25 | 1.46 | 1.00–2.14 | 1.35 | 1.06–1.71 |
|
| ||||||||
| 3+ | 1.69 | 1.30–2.20 | 1.46 | 1.27–1.68 | 2.00 | 1.36–2.94 | 1.92 | 1.51–2.45 |
|
| ||||||||
| Surgery Type | ||||||||
|
| ||||||||
| Open | Ref | Ref | Ref | Ref | ||||
|
| ||||||||
| MIS | 1.05 | 0.83–1.33 | 1.18 | 1.05–1.32 | 1.21 | 0.81–1.82 | 1.03 | 0.80–1.31 |
|
| ||||||||
| Lymphadenectomy | ||||||||
|
| ||||||||
| Not Done | Ref | Ref | Ref | Ref | ||||
|
| ||||||||
| 1–10 nodes examined | 0.93 | 0.74–1.16 | 0.68 | 0.60–0.76 | 1.12 | 0.80–1.56 | 0.89 | 0.72–1.09 |
|
| ||||||||
| >10 nodes examined | 0.73 | 0.59–0.91 | 0.55 | 0.49–0.61 | 0.89 | 0.63–1.24 | 0.74 | 0.60–0.90 |
|
| ||||||||
| Urban Rural Indicator | ||||||||
|
| ||||||||
| All urban | Ref | Ref | Ref | Ref | ||||
|
| ||||||||
| Mostly urban | 1.17 | 0.94–1.44 | 1.23 | 1.10–1.37 | 1.44 | 1.04–2.00 | 1.24 | 1.01–1.53 |
|
| ||||||||
| Mostly rural | 0.82 | 0.56–1.22 | 1.25 | 1.05–1.49 | 0.69 | 0.35–1.34 | 1.24 | 0.88–1.74 |
|
| ||||||||
| All rural | 1.19 | 0.87–1.63 | 1.48 | 1.27–1.74 | 1.83 | 1.19–2.82 | 1.26 | 0.93–1.71 |
|
| ||||||||
| Diagnosis Year (continuous) | 0.95 | 0.93–0.97 | 0.93 | 0.92–0.94 | 0.93 | 0.90–0.96 | 0.94 | 0.92–0.96 |
(Multivariate multinomial logistic regression with receiving adjuvant treatment without delay as referent)
NHW: Non-Hispanic White; NHB: Non-Hispanic Black
In multivariate analyses, NHB women had poorer survival from years 1–5 following surgery compared to NHW women across all stages. This persists even when stratified by early stage (HR 1.34, 1.18–1.52) and advanced stage (HR 1.27, 1.07–1.50) as noted in Table 5. Compared to adjuvant treatment without delay, omission of any treatment was significantly associated with worse 1-year survival in advanced stage (FIGO III-IV) high-grade endometrial cancer (HR 2.15, CI 1.83 – 2.54).
Table 5:
Predictors of 1-year and >1 to 5 year survival for endometrial cancer patients with high grade disease who survival at least 3 month stratified by FIGO stage
| FIGO Stage I - II | FIGO Stage III- IV | |||||||
|---|---|---|---|---|---|---|---|---|
| 1 year survival | >1 – 5 year survival | 1 year survival | >1 – 5 year survival | |||||
| Predictor Variable | HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI |
|
| ||||||||
| Race/Ethnicity | ||||||||
|
| ||||||||
| NHW | Ref | Ref | Ref | Ref | ||||
|
| ||||||||
| NHB | 1.42 | 1.14–1.76 | 1.34 | 1.18–1.52 | 1.19 | 0.98–1.44 | 1.27 | 1.07–1.50 |
|
| ||||||||
| Hispanic | 0.70 | 0.46–1.07 | 0.88 | 0.72–1.07 | 1.24 | 0.94–1.64 | 1.10 | 0.87–1.41 |
|
| ||||||||
| Surgical Complications | ||||||||
|
| ||||||||
| None | Ref | Ref | Ref | Ref | ||||
|
| ||||||||
| 1 | 1.31 | 1.08–1.60 | 1.20 | 1.09–1.33 | 1.36 | 1.14–1.63 | 1.23 | 1.07–1.42 |
|
| ||||||||
| 2 | 1.45 | 1.13–1.87 | 1.29 | 1.13–1.49 | 1.36 | 1.10–1.68 | 1.32 | 1.12–1.56 |
|
| ||||||||
| 3+ | 2.21 | 1.76–2.76 | 1.30 | 1.13–1.50 | 1.73 | 1.42–2.11 | 1.33 | 1.12–1.58 |
|
| ||||||||
| p-trend | <0.001 | <0.001 | <0.001 | <0.001 | ||||
|
| ||||||||
| Adjuvant Treatment | ||||||||
|
| ||||||||
| Adjuvant treatment without delay | Ref | Ref | Ref | Ref | ||||
|
| ||||||||
| Treatment Delay | 1.24 | 0.91–1.68 | 1.15 | 0.97–1.36 | 1.31 | 0.97–1.76 | 0.96 | 0.74–1.23 |
|
| ||||||||
| No Adjuvant Treatment | 1.02 | 0.86–1.21 | 0.83 | 0.76–0.91 | 2.17 | 1.84–2.56 | 0.92 | 0.78–1.08 |
|
| ||||||||
| Age Group | ||||||||
|
| ||||||||
| Ordinal (5 year groups) | 1.05 | 1.04–1.06 | 1.04 | 1.04–1.05 | 1.02 | 1.00–1.03 | 1.02 | 1.01–1.03 |
|
| ||||||||
| Histology | ||||||||
|
| ||||||||
| Endometrioid | Ref | Ref | Ref | Ref | ||||
|
| ||||||||
| Non-Endometrioid | 1.71 | 1.44–2.03 | 1.28 | 1.17–1.39 | 1.23 | 1.05–1.45 | 1.26 | 1.11–1.44 |
|
| ||||||||
| Marital Status | ||||||||
|
| ||||||||
| Married or equivalent | Ref | Ref | Ref | Ref | ||||
|
| ||||||||
| S/D/W | 1.27 | 1.07–1.51 | 1.17 | 1.07–1.27 | 1.12 | 0.96–1.31 | 1.11 | 0.98–1.25 |
|
| ||||||||
| Comorbidity Score | ||||||||
|
| ||||||||
| None | Ref | Ref | Ref | Ref | ||||
|
| ||||||||
| 1 | 1.03 | 0.84–1.28 | 1.08 | 0.98–1.20 | 1.08 | 0.90–1.30 | 1.14 | 0.99–1.31 |
|
| ||||||||
| 2 | 1.37 | 1.09–1.71 | 1.31 | 1.17–1.48 | 1.05 | 0.85–1.29 | 1.21 | 1.03–1.43 |
|
| ||||||||
| 3+ | 1.61 | 1.28–2.02 | 1.33 | 1.17–1.51 | 1.28 | 1.04–1.58 | 1.25 | 1.05–1.49 |
|
| ||||||||
| Surgery Type | ||||||||
|
| ||||||||
| Open | Ref | Ref | Ref | Ref | ||||
|
| ||||||||
| MIS | 0.91 | 0.74–1.12 | 1.02 | 0.91–1.13 | 0.83 | 0.67–1.04 | 0.89 | 0.75–1.05 |
|
| ||||||||
| Lymphadenectomy | ||||||||
|
| ||||||||
| Not Done | Ref | Ref | Ref | Ref | ||||
|
| ||||||||
| 1–10 nodes examined | 0.74 | 0.61–0.91 | 0.95 | 0.85–1.05 | 0.80 | 0.67–0.94 | 0.77 | 0.67–0.89 |
|
| ||||||||
| >10 nodes examined | 0.71 | 0.58–0.85 | 0.73 | 0.66–0.81 | 0.58 | 0.49–0.70 | 0.66 | 0.57–0.75 |
|
| ||||||||
| Urban Rural Indicator | ||||||||
|
| ||||||||
| All urban | Ref | Ref | Ref | Ref | ||||
|
| ||||||||
| Mostly urban | 0.98 | 0.80–1.20 | 1.07 | 0.97–1.19 | 1.00 | 0.84–1.20 | 1.15 | 1.00–1.33 |
|
| ||||||||
| Mostly rural | 1.37 | 1.03–1.83 | 0.90 | 0.75–1.08 | 1.10 | 0.82–1.48 | 1.27 | 1.01–1.61 |
|
| ||||||||
| All rural | 1.02 | 0.76–1.36 | 1.03 | 0.89–1.20 | 1.07 | 0.82–1.40 | 1.06 | 0.85–1.32 |
|
| ||||||||
| Diagnosis Year (continuous) | 0.98 | 0.96–0.99 | 1.00 | 0.99–1.01 | 1.00 | 0.98–1.01 | 1.00 | 0.99–1.02 |
NHW: Non-Hispanic White; NHB: Non-Hispanic Black
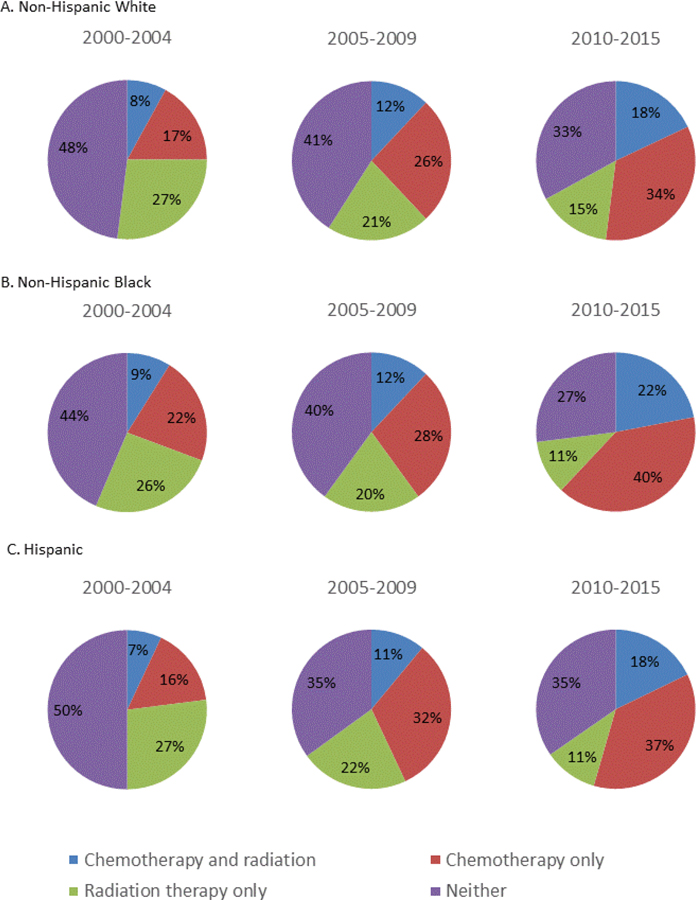
Practice patterns and treatment type for all races changed over the 16 years of the study (Figure 3). Adjuvant therapy differed when stratified by era as well as by race/ethnicity (all p<0.001). The proportion of women who received CRT increased from 8% in Era 1 to 19% in Era 2, while RT and no adjuvant treatment decreased. From Era 1 to Era 3 CRT increased over 200% across all races (p<0.001), and CT increased 17% (p<0.001) while RT and no adjuvant treatment decreased 13% and 14%, respectively (p<0.001).
Figure 3.
Type of adjuvant treatment by year group stratified by race/ethnicity
Comment
a. Principal Findings
Our study reports that, to a degree, Hispanic ethnicity as well as those classified as black race are independent predictors for both treatment delay and omission of adjuvant treatment, especially in 2009 FIGO Stage II or greater, when adjuvant therapy is uniformly recommended.19 NHB race was found to be independently predictive of a 36% higher risk of not receiving any adjuvant treatment for Stage II or higher disease (OR 1.36, CI 1.07 – 1.72). This is of concern given that we also found omission of adjuvant treatment was independently associated with worse overall survival in patients with Stage III or IV disease (HR 2.17). Unfortunately, when a delay in adjuvant therapy or omission of therapy was accounted for, NHB race continued to be an independent predictor of worse survival (Table 5, Supplemental Figure 1B). We also found HS race, across all stages, to be a predictor of treatment delay (OR 1.63, CI 1.18–2.25). Interestingly, this did not extrapolate to worse OS as treatment delay itself was not found to be predictive of worse survival at either 1-year or 5-years after surgery.
b. Results in the Context of What is Known
Our study’s primary focus was the timing and omission of adjuvant therapy and its causes, as this has not been studied before in regards to HGEC but has shown an effect on outcomes in other cancer types.12–14 Our findings are largely consistent with two of the largest population-based studies evaluating racial differences in treatment for HGEC pertaining to the survival differences between racial groups in the US.15,20 A study by Bregar et al. found that on multivariate analysis, NHB women had decreased OS when compared to NHW women, while HS women had improved OS compared to NHW women, but only in early stage (I/II) disease.20 Rauh-Hain et al found that racial disparities for disease specific mortality were eliminated when accounting for socioeconomic and geographical factors, but OS remained worse for African American patients with HGEC in the multivariate model.15 Neither of these studies specifically evaluated the effect of timing of adjuvant treatment on those who undergo primary surgical management. We believe our study contributes to the body of evidence evaluating racial disparities because the impact of surgery on complication rates and adjuvant treatment as it pertains to overall survival has not been thoroughly explored, but is an essential consideration when interrogating these disparities and their effects on survival.
Adjuvant treatment type over the course of our study varied over time and between races. The aforementioned study by Bregar et al. found no significant difference observed in the administration of adjuvant treatment between racial and ethnic groups after controlling for various factors, a finding not supported in this analysis. These discordant findings may be accounted for by differences in the study populations. Our study included only Medicare patients who underwent primary surgery while Bregar et al. included all patients with HGEC and did not stratify adjuvant treatment based on receipt of surgery. Over the course of our study we found use of “no adjuvant treatment” and “radiation-only” decreased substantially among all races, while “chemotherapy-only” and “combined chemoradiation” increased in all races (Figure 3). It is reassuring that the general trend of treatment change is similar among all races across the study period, suggesting that no one group is adopting changes in treatment faster or slower than the others.
c. Clinical Implications
The occurrence of perioperative complications has a profound effect on survival across most major surgical procedures, and this finding was also observed in our study.13,21 We found that the presence of a single surgical complication was independently associated with a significantly worse 1- and 5-year survival across all stages. For the first time in HGEC, the present study found surgical complications also caused a delay or omission of treatment of adjuvant treatment, especially when the patient experienced three or more complications (OR 2.31 and 1.35, respectively, p<0.001). Our findings support the recommendations to individualize surgical plans, and that the extent of the surgery should be adapted to the medical condition of the patient. This is especially true of NHB patients with HGEC, given that NHB patients in our cohort had significantly higher rates of post-operative medical and surgical complications, and were more likely to have one more or perioperative complications compared to NHW patients (44% vs 50%, p<0.001), a finding supported in other population-based studies.15,20
d. Research Implications
When correcting for a history of hysterectomy, black women develop HGEC at two to four times the incidence of NHW patients and have lower cancer-specific and all-cause mortality compared to white women.15, 22 The causes of this are likely multifactorial and significant effort must be made across all aspects of patient care to close the racial gap in survival of patients with HGEC. Although our current study did not find association between delay or omission of treatment and survival in multivariate analysis, the results did show a clear association between race and delay or omission of adjuvant treatment. We have recently shown that access to guideline concordant treatment may reduce these differences.23 Current guidelines are clear that treatment should begin within eight weeks of surgery, and no later than 12 weeks.24 Therefore, it is imperative that the causes of inequitable timing of adjuvant treatment across racial groups identified in the current study must be addressed and mitigated.
e. Limitations
Before considering the results of the study, several limitations must be mentioned. Several types of bias are inherent given the retrospective nature of the study. The length of the study there may lend to disruptions in observed rates related to the coding transition, and coding errors could contribute to limitations of the study31. Using data not created or collected to answer a specific research question, including possible unmeasured confounding, misclassification bias, missing data, and changing participant eligibility over time can affect conclusions. Data are lacking on why certain patients received laparotomy versus minimally invasive surgery, pre-operative imaging reports, the number of chemotherapy cycles delivered, specific regimens of adjuvant therapy, BMI, presence of fibroids and size of uterus, details with respect to types of radiotherapy, insurance, and income status. The clinical reason for the omission of, or delay in, adjuvant therapy is also not available and can only be speculated, particularly in early stage disease when the decision to treat following surgery is more variable. Our study population includes fee-for-service (FFS) Medicare recipients and the findings may not be applicable to younger populations of patients with endometrial cancer, or patients not enrolled in FFS Medicare (e.g. Medicare HMO plans). Lastly, our study includes only patients who had primary surgical management. Our findings can only be applied to patients who underwent surgical management for initial treatment of HGEC. The significant strengths of this study are that it is a large, population-based sample of women with validated claims data and detailed treatment and complication information.
f. Conclusions
In summary, our study is one of only a few to analyze and identify factors involved in adjuvant treatment delays or omission in HGEC; and is the only study that factors in comorbidity score, stage, histologic type, histologic grade, route of surgery and timing of adjuvant therapy stratified by race when examining factors that influence overall survival. Although non-white race and Hispanic ethnicity was predictive of delay and omission of adjuvant therapy, it did not entirely account for the worse survival outcomes seen in NHB women diagnosed with HGEC. It has become apparent that disparities in survival are multifactorial and must be addressed with a holistic approach, including addressing socioeconomic barriers, community views towards cancer treatment, and potential impact of implicit bias in providers. Our research highlights a significant difference in adjuvant treatment that was independently associated with race/ethnicity and provides a well-defined area to address as a means to close the racial and ethnic survival gap in Medicare-aged patients with HGEC.
Supplementary Material
Figure 2.
Overall survival by race/ethnicity
NHW – non-Hispanic White; NHB – non-Hispanic Black
AJOG at a glance:
a. Why was this study conducted?
To identify differences in timing of adjuvant treatment in US women with high grade endometrial cancer stratified by race/ethnicity and subsequent effects on survival.
b. What are the key findings?
This study suggests race/ethnicity is independently associated with timing of adjuvant treatment in patients with high-grade endometrial cancer.
c. What does this study add to what is already known?
This study is the first to identify that delay or omission of adjuvant treatment in high-grade endometrial cancer is independently associated with race/ethnicity, and could be a modifiable factor contributing to the known disparate survival between racial and ethnic groups.
Acknowledgments
This work was supported, in part, by the Epidemiology Research Core (Health and Human Services contract HHS N261201300011I), and NIH Center Grant P30 CA022453 awarded to the Karmanos Cancer Institute at Wayne State University.
Footnotes
Disclosure Statement: The authors report no conflict of interest.
Non clinical trial.
Non systematic review or meta analysis
Never Presented
N/A
Condensation: Non-white race and Hispanic ethnicity are predictors for both treatment delay and omission of adjuvant treatment in US women with high-grade endometrial cancer.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.“Key Statistics for Endometrial Cancer.” n.d. Accessed April 7, 2021. https://www.cancer.org/cancer/endometrial-cancer/about/key-statistics.html.
- 2.Creutzberg Carien L., van Putten Wim L. J., Koper Peter C. M., Lybeert Marnix L. M., Jobsen Jan J., Wárlám-Rodenhuis Carla C., De Winter Karin A. J., et al. 2000. “Surgery and Postoperative Radiotherapy versus Surgery Alone for Patients with Stage-1 Endometrial Carcinoma: Multicentre Randomised Trial.” The Lancet 355 (9213): 1404–11. [DOI] [PubMed] [Google Scholar]
- 3.Endometrial cancer. Practice Bulletin No. 149. American College of Obstetricians and Gynecologists. Obstet Gynecol 2015;125:1006–26. [DOI] [PubMed] [Google Scholar]
- 4.Berek Jonathan S., and Hacker Neville F. 2010. Berek and Hacker’s Gynecologic Oncology. Lippincott Williams & Wilkins. [Google Scholar]
- 5.Long B, Liu FW, and Bristow RE 2013. “Disparities in Uterine Cancer Epidemiology, Treatment, and Survival among African Americans in the United States.” Gynecologic Oncology 130 (3): 652–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bregar Amy J., Rauh-Hain J. Alejandro, Spencer Ryan, Clemmer Joel T., Schorge John O., Rice Laurel W., and Del Carmen Marcela G. 2017. “Disparities in Receipt of Care for High-Grade Endometrial Cancer: A National Cancer Data Base Analysis.” Gynecologic Oncology 145 (1): 114–21. [DOI] [PubMed] [Google Scholar]
- 7.Sud Shivani, Holmes Jordan, Eblan Michael, Chen Ronald, and Jones Ellen. 2018. “Clinical Characteristics Associated with Racial Disparities in Endometrial Cancer Outcomes: A Surveillance, Epidemiology and End Results Analysis.” Gynecologic Oncology 148 (2): 349–56. [DOI] [PubMed] [Google Scholar]
- 8.Lee Jessica, Gerber Deanna, Aphinyanaphongs Yindalon, Curtin John P., and Boyd Leslie R. 2018. “Laparoscopy Decreases the Disparity in Postoperative Complications between Black and White Women after Hysterectomy for Endometrial Cancer.” Gynecologic Oncology 149 (1): 22–27. [DOI] [PubMed] [Google Scholar]
- 9.Fader Amanda Nickles, Seamon Leigh G., Escobar Pedro F., Frasure Heidi E., Havrilesky Laura A., Zanotti Kristine M., Secord Angeles Alvarez, et al. 2012. “Minimally Invasive Surgery versus Laparotomy in Women with High Grade Endometrial Cancer: A Multi-Site Study Performed at High Volume Cancer Centers.” Gynecologic Oncology 126 (2): 180–85. [DOI] [PubMed] [Google Scholar]
- 10.Boer Stephanie M. de, Powell Melanie E., Mileshkin Linda R., Katsaros Dionyssios, Bessette Paul, Haie-Meder Christine, Ottevanger Petronella B., et al. 2017. “Final Results of the International Randomized PORTEC-3 Trial of Adjuvant Chemotherapy and Radiation Therapy (RT) versus RT Alone for Women with High-Risk Endometrial Cancer.” Journal of Clinical Orthodontics: JCO 35 (15_suppl): 5502–5502. [Google Scholar]
- 11.Neoplasms Uterine. n.d. “NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) 2019.” Endo, 4–10. [Google Scholar]
- 12.Wright Jason D., Herzog Thomas J., Neugut Alfred I., Burke William M., Lu Yu-Shiang, Lewin Sharyn N., and Hershman Dawn L. 2012. “Effect of Radical Cytoreductive Surgery on Omission and Delay of Chemotherapy for Advanced-Stage Ovarian Cancer.” Obstetrics and Gynecology 120 (4): 871–81. [DOI] [PubMed] [Google Scholar]
- 13.Gao Peng, Huang Xuan-Zhang, Song Yong-Xi, Sun Jing-Xu, Chen Xiao-Wan, Sun Yu, Jiang Yu-Meng, and Wang Zhen-Ning. 2018. “Impact of Timing of Adjuvant Chemotherapy on Survival in Stage III Colon Cancer: A Population-Based Study.” BMC Cancer 18 (1): 234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Merkow Ryan P., Bilimoria Karl Y., Tomlinson James S., Paruch Jennifer L., Fleming Jason B., Talamonti Mark S., Ko Clifford Y., and Bentrem David J. 2014. “Postoperative Complications Reduce Adjuvant Chemotherapy Use in Resectable Pancreatic Cancer.” Annals of Surgery 260 (2): 372–77. [DOI] [PubMed] [Google Scholar]
- 15.Rauh-Hain J. Alejandro, Buskwofie Ama, Clemmer Joel, Boruta David M., Schorge John O., and del Carmen Marcela G. 2015. “Racial Disparities in Treatment of High-Grade Endometrial Cancer in the Medicare Population.” Obstetrics and Gynecology 125 (4): 843–51. [DOI] [PubMed] [Google Scholar]
- 16.Ghanem Ahmed I., Modh Ankit, Burmeister Charlotte, Mahmoud Omar, and Elshaikh Mohamed A. 2020. “Does the Interval Between Hysterectomy and Start of Adjuvant Radiation Treatment Influence Survival in Women With Endometrial Carcinoma?: A National Cancer Database Analysis.” American Journal of Clinical Oncology 43 (8): 602–6. [DOI] [PubMed] [Google Scholar]
- 17.National Cancer Institute. Overview of the SEER Program. https://seer.cancer.gov/about/overview.html. Accessed March 2021.
- 18.Therneau T A Package for Survival Analysis in S. version 2.38, URL:https://CRAN.R-project.org/package=survival. 2015.
- 19.Koh Wui-Jin, Abu-Rustum Nadeem R., Bean Sarah, Bradley Kristin, Campos Susana M., Cho Kathleen R., Chon Hye Sook, et al. 2018. “Uterine Neoplasms, Version 1.2018, NCCN Clinical Practice Guidelines in Oncology.” Journal of the National Comprehensive Cancer Network: JNCCN 16 (2): 170–99. [DOI] [PubMed] [Google Scholar]
- 20.Bregar Amy J., Rauh-Hain J. Alejandro, Spencer Ryan, Clemmer Joel T., Schorge John O., Rice Laurel W., and Del Carmen Marcela G. 2017. “Disparities in Receipt of Care for High-Grade Endometrial Cancer: A National Cancer Data Base Analysis.” Gynecologic Oncology 145 (1): 114–21. [DOI] [PubMed] [Google Scholar]
- 21.Khuri Shukri F., Henderson William G., DePalma Ralph G., Mosca Cecilia, Healey Nancy A., Kumbhani Dharam J., and Others. 2005. “Determinants of Long-Term Survival after Major Surgery and the Adverse Effect of Postoperative Complications.” Annals of Surgery 242 (3): 326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abel Mary Kathryn, Liao Cheng-I, Chan Chloe, Lee Danny, Rohatgi Atharva, Darcy Kathleen M., Tian Chunqiao, et al. 2021. “Racial Disparities in High-Risk Uterine Cancer Histologic Subtypes: A United States Cancer Statistics Study.” Gynecologic Oncology 161 (2): 470–76. [DOI] [PubMed] [Google Scholar]
- 23.Fucinari Juliana, Elshaikh Mohamed A., Ruterbusch Julie J., Khalil Remonda, Dyson Gregory, Shultz Daniel, Ali-Fehmi Rouba, and Cote Michele L. 2021. “The Impact of Race, Comorbid Conditions and Obesity on Survival Endpoints in Women with High Grade Endometrial Carcinoma.” Gynecologic Oncology, May. 10.1016/j.ygyno.2021.04.036. [DOI] [PubMed] [Google Scholar]
- 24.Colombo N, Creutzberg C, Amant F, Bosse T, González-Martín A, Ledermann J, Marth C, et al. 2016. “ESMO-ESGO-ESTRO Consensus Conference on Endometrial Cancer: Diagnosis, Treatment and Follow-Up†.” Annals of Oncology: Official Journal of the European Society for Medical Oncology / ESMO 27 (1): 16–41. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.