Abstract
BACKGROUND:
Hysterectomy is one of the most frequent gynecologic surgeries in the United States. Women undergoing hysterectomy commonly are offered bilateral oophorectomy for ovarian and breast cancer prevention. Although bilateral oophorectomy may dramatically reduce the risk of gynecologic cancers, some studies suggested that bilateral oophorectomy may be associated with an increased risk of other types of cancer, such as lung cancer and colorectal cancer. However, the results are conflicted.
OBJECTIVE:
To study the association between bilateral oophorectomy and the risk of subsequent cancer of any type.
STUDY DESIGN:
This population-based cohort study included all premenopausal women who underwent bilateral oophorectomy for a nonmalignant indication before the age of 50 years between January 1, 1988 and December 31, 2007 in Olmsted County, Minnesota, and a random sample of age-matched (±1 year) referent women who did not undergo bilateral oophorectomy. Women with cancer before oophorectomy (or index date) or within 6 months after the index date were excluded. Time-to-event analyses were performed to assess the risk of de novo cancer. Cancer diagnosis and type were confirmed using medical record review.
RESULTS:
Over a median follow-up of 18 years, the risk of any cancer did not significantly differ between 1562 women who underwent bilateral oophorectomy before natural menopause and 1610 referent women (adjusted hazard ratio (HR), 0.82, 95% CI, 0.66–1.03). However, women who underwent bilateral oophorectomy had a decreased risk of gynecologic cancers (HR, 0.15; 95% CI, 0.06–0.34) but not of non-gynecologic cancers (HR, 0.99; 95% CI, 0.78–1.26). In particular, the risk of breast cancer, gastrointestinal cancer, and lung cancer did not differ between these two cohorts. Use of estrogen therapy through the age of 50 years in women who underwent bilateral oophorectomy did not modify the results.
CONCLUSIONS:
Women who underwent bilateral oophorectomy before menopause have a reduced risk of gynecologic cancer but not of other types of cancer including breast cancer. Women at average risk of ovarian cancer should not consider bilateral oophorectomy for the prevention of breast cancer or other non-gynecologic cancers.
Keywords: cancer, gynecologic cancer, breast cancer, bilateral oophorectomy, menopause, incidence, estrogen therapy
Introduction
Hysterectomy is one of the most frequent gynecologic surgeries in the United States.1 Women undergoing hysterectomy commonly are offered bilateral oophorectomy for ovarian and breast cancer prevention.1, 2 In addition, prophylactic bilateral oophorectomy is usually recommended for women with an inherited high risk variant in the BRCA1 or BRCA2 genes. As a result, it is estimated that one in eight US women have their ovaries removed before reaching natural menopause.1 Indeed, premenopausal hysterectomy with bilateral oophorectomy may dramatically reduce the risk of gynecologic cancers, such as uterine, fallopian, and ovarian cancers.3
On the other hand, the effect of bilateral oophorectomy on the risk of breast cancer remains controversial. Previous studies have reported conflicting results, especially among women with BRCA1 and BRCA2 variants.4–11 For example, findings from a large prospective study indicated that premenopausal bilateral oophorectomy was only associated with a reduced risk of breast cancer before age 50 years in BRCA2 mutation carriers.12 By contrast, a systematic review of the literature concluded that premenopausal bilateral oophorectomy was associated with a reduced risk of breast cancer in women with BRCA1 mutations but not with BRCA2 mutations.13 Finally, a few observational studies suggested that bilateral oophorectomy may reduce the risk of breast cancer in the general population (in which most women do not carry the BRCA1 or BRCA2 pathogenic variants), only when performed at younger age.14–17
Some studies suggested that bilateral oophorectomy may be associated with an increased risk of other types of cancer. For example, one study reported that premenopausal bilateral oophorectomy was associated with an increased risk of lung cancer.14 Studies examining the risk of colorectal cancer after bilateral oophorectomy have been inconsistent.15, 18 In addition, increased attention has been directed at determining the risk-to-benefit ratio of prophylactic bilateral oophorectomy because of the increased risk of long-term non-cancer morbidity and mortality.19–22 In this study, we investigated the association between premenopausal bilateral oophorectomy and the risk of subsequent cancer overall and by specific cancer type, using an established population-based cohort.
Materials and Methods
Data source and study population
The study design and clinical characteristics for women included in the Mayo Clinic Cohort Study of Oophorectomy and Aging-2 (MOA-2) have been previously described.19, 20, 23, 24 Briefly, we included all premenopausal women who underwent bilateral oophorectomy between January 1, 1988 and December 31, 2007 in Olmsted County, Minnesota. We excluded women who underwent oophorectomy to treat ovarian cancer (primary or metastatic), to treat another estrogen-sensitive malignant disorder (usually breast cancer), or because they were considered at high risk of ovarian cancer (strong family history as judged by the gynecologist or carriers of BRCA1 or BRCA2 pathogenic variants). Bilateral oophorectomy was defined as the removal of both ovaries or as the removal of the remaining ovary for women who underwent two separate procedures. The date of the surgical procedure was considered the index date. Each woman who underwent bilateral oophorectomy was randomly matched to a referent woman of same age (±1 year) who had not undergone bilateral oophorectomy before the index date from the same Olmsted County population. Prior hysterectomy or unilateral oophorectomy were not exclusion criteria for referent women. Data were collected by abstracting medical records from the Rochester Epidemiology Project (REP) medical records-linkage system. Extensive details about the REP were published elsewhere.25–28 All research activities were approved by the Mayo Clinic and Olmsted Medical Center Institutional Review Boards.
Ascertainment of cancer
For all women, we extracted from the electronic indexes of the REP the International Classification of Diseases (ICD; eighth revision, ninth revision, or tenth revision) codes assigned for cancer at any time through December 31, 2018. ICD codes listed in any position on the death certificates were also obtained for deaths through December 31, 2018. We included all of the ICD codes for cancer recommended by the US Department of Health and Human Services (DHHS).29 However, we removed the codes for secondary cancer or metastasis, recurrence of cancer, nonmelanoma skin cancer, male-specific cancer (eg, prostate cancer), carcinoma in situ, benign neoplasms, and for abnormal results of Papanicolaou smears.
The medical records for all women with at least one diagnostic code for cancer were manually reviewed by a physician (N.H.) to confirm the presence of a primary cancer, the date of diagnosis, and the type of primary cancer. Cancers were categorized as gynecologic (ie, ovaries, fallopian tubes, uterus, cervix, vagina, and vulva), breast, gastrointestinal (ie, esophagus, stomach, colon, rectum and anus, liver and intrahepatic bile duct, pancreas, etc.), lung (including bronchus and intrathoracic), head and neck, bone and connective tissue, melanomas of skin, urinary (ie, bladder, kidney and renal pelvis, etc.), brain and nervous system, thyroid, hematologic (ie, Hodgkin’s disease, non-Hodgkin’s lymphoma, leukemia, and multiple myeloma), and other primary cancer. If a woman had two or more primary cancers, they were considered separately in analyses by type.
Other variables
Demographic and clinical characteristics at the index date were manually abstracted for all women from the medical records, and included age, education, race, ethnicity, household income, body mass index (BMI), smoking status, reproductive characteristics, and systemic estrogen therapy after the index date. The indication and the pathology results for each bilateral oophorectomy were defined by the gynecologist and pathologist at the time of surgery.23, 24 In addition, we considered 16 of the 20 chronic conditions used by the DHHS to define multi-morbidity plus anxiety that were present at the index date (total of 17 conditions): depression, anxiety, substance abuse disorders, dementia, schizophrenia or psychosis, hyperlipidemia, hypertension, diabetes, cardiac arrhythmias, coronary artery disease, stroke, congestive heart failure, arthritis, asthma, chronic obstructive pulmonary disease, osteoporosis, and chronic kidney disease.29 From the DHHS list we excluded: cancer (study outcome), human immunodeficiency virus infection (HIV), autism spectrum disorder, and hepatitis. All chronic conditions were assessed by extracting ICD diagnostic codes from the REP diagnostic indexes at any time before the index date. Women needed to have at least two diagnostic codes in a given category separated by >30 days to reduce false positive diagnoses.23, 30
Statistical analyses
All women who were diagnosed with any type of cancer before the index date or within 6 months after the index date were excluded from the primary analyses. Each woman was followed from 6 months after the index date to the first cancer diagnosis or was censored at the earliest occurring of three end-points: date of death, last visit with a REP provider, or the end of the study (December 31, 2018). Inverse probability weights (IPW) derived from a logistic regression model were used to adjust for age at index date (continuous), calendar year (continuous), race (white versus nonwhite), BMI (<30 versus ≥30 kg/m2), years of education (≤12, 13–16, or >16), quartiles of household income (<$42,000, $42,000–56,999, $57,000–71,999, or ≥$72,000), smoking status (current or former vs never), and 17 chronic conditions at baseline. These adjustments were done overall and separately in each stratum to maximize the balance at the index date. After the IPW adjustment, the standardized differences for all of the conditions or characteristics considered were below the recommended threshold of 0.10 (ie, negligible imbalance between the two cohorts).
Cox proportional hazards regression models using age as the time scale and IPW adjustment were used to calculate the hazard ratios (HR) and 95% confidence intervals. The proportional hazards assumptions were checked using time-dependent covariates and with graphical methods; the assumptions were satisfied.31 Differences between the two cohorts were also measured using the absolute risk increase (ARI) or absolute risk reduction (ARR) obtained by subtracting the two absolute risks at 25 years of follow-up. The analyses were conducted in the overall sample and stratified by age at index date (≤45 vs 46–49 years), ovarian indication (benign vs none), and estrogen therapy within each age stratum (estrogen therapy continued to the 50th birth date vs otherwise). We also conducted a further stratification of the age group ≤45 years at index date into <40 and 40–45 years; however, the numbers were small for some specific types of cancer. Finally, we conducted stratified analyses by decade of the index date (1988–1997 vs 1998–2007). The analyses for gastrointestinal and lung cancer were only stratified by age at index date because of the small number of outcomes.
We performed four sets of sensitivity analysis. First, we considered each type of cancer separately, and we excluded only the women who had that type of cancer before the index date or within the 6 months after the index date. Second, we censored at the date of surgery those referent women who underwent bilateral oophorectomy after the index date and before age 50 years. Third, we excluded from analyses all women who had any of the 17 DHHS chronic conditions at the index date. Fourth, we repeated the primary analyses using the traditional multivariable adjustment method rather than the inverse probability weighting method. In the last three sets of sensitivity analyses, women with any type of cancer before the index date or within 6 months of follow-up were excluded. All analyses were conducted using SAS version 9.4 (SAS Institute, Inc., Cary, NC), and tests of statistical significance were conducted at the two-tailed α-level of 0.05.
Results
Characteristics at the index date
Figure 1 shows detailed flowcharts for the two cohorts. Women who underwent bilateral oophorectomy were more likely to have diagnoses of gynecologic cancer before the index date or within 6 months after the index date (Supplemental Figure 1). After excluding women with any type of cancer before the index date or within 6 months after the index date, there were 1562 women who underwent bilateral oophorectomy and 1610 age-matched referent women (Table 1). Most women in both cohorts were white. At the index date, women who underwent bilateral oophorectomy had a greater number of chronic conditions, were more likely to be overweight or obese, and were more likely to be former or current smokers compared with referent women (Table 1).
FIGURE 1.
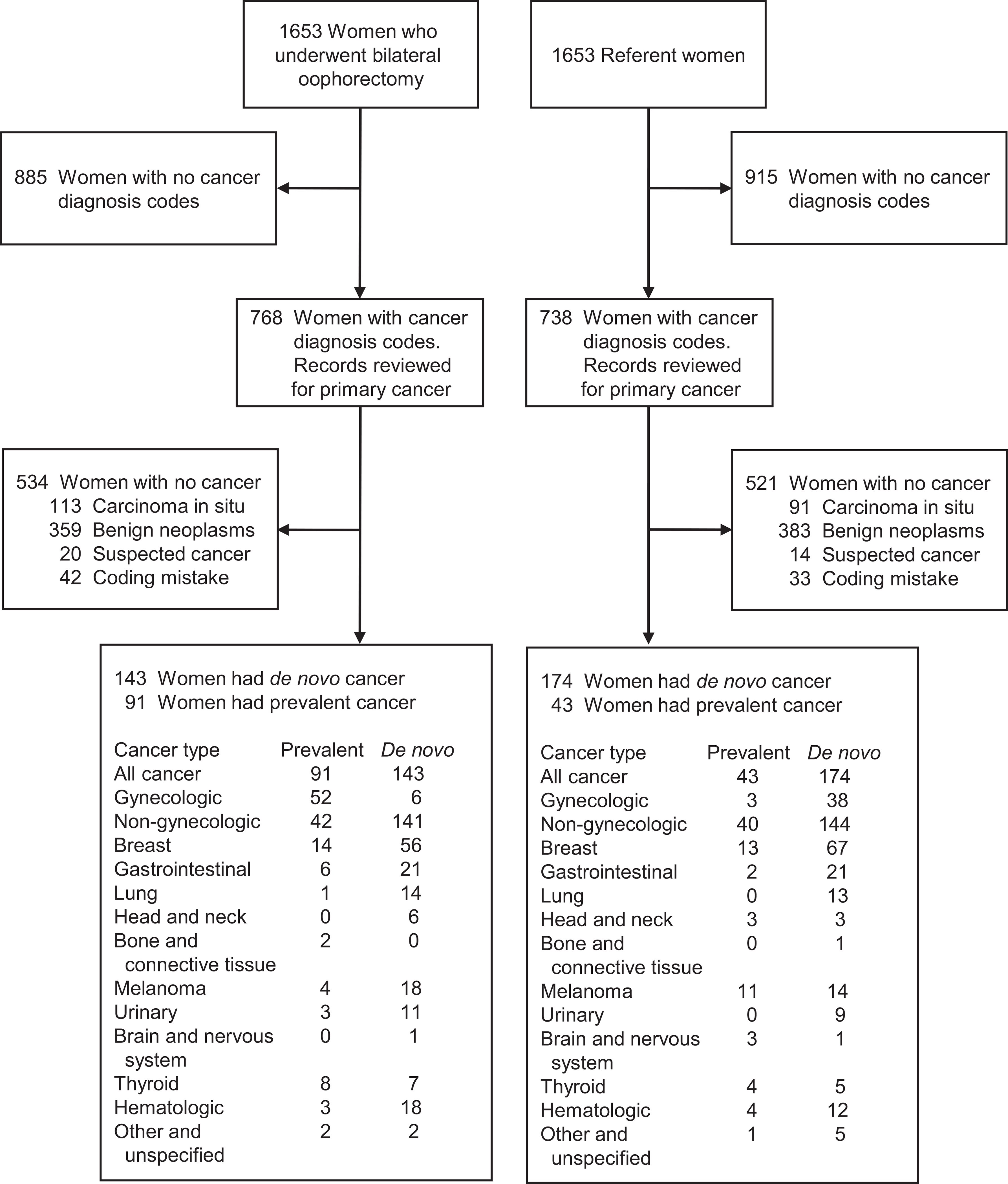
Diagnostic codes for cancer were obtained electronically from the diagnostic indexes of the Rochester Epidemiology Project for all women in the bilateral oophorectomy and the referent cohorts. Medical record review was used to confirm cancer status, diagnosis date, and type of cancer for all women who received at least one diagnostic code for cancer. Women with cancer diagnosed before the index date (date of oophorectomy) or within 6 months after the index date were considered to have prevalent cancer and were excluded. Women with cancer diagnosed more than 6 months after the index date were considered to have de novo cancer. Some women (18 who underwent bilateral oophorectomy, 15 referent women) had two or more types of primary cancer. Gynecologic cancer includes cancer of the ovaries, fallopian tubes, uterus, cervix, vagina, and vulva. Non-gynecologic cancer includes all remaining types of cancer.
TABLE 1.
Baseline sociodemographic and clinical characteristics of women who underwent bilateral oophorectomy and referent women, excluding women with cancer of any type before the index date or within 6 months of follow-up
| Characteristics | Bilateral oophorectomy (n=1562) |
Referent women (n=1610) |
|||
|---|---|---|---|---|---|
| N | % | N | % | P valuea | |
|
| |||||
| Age at index date (years) | .88 | ||||
| ≤45 | 985 | 63.1 | 1011 | 62.8 | |
| 46–49 | 577 | 36.9 | 599 | 37.2 | |
| Index year | .72 | ||||
| 1988–1997 | 679 | 43.5 | 710 | 44.1 | |
| 1998–2007 | 883 | 56.5 | 900 | 55.9 | |
| Race | <.001 | ||||
| White | 1523 | 97.5 | 1528 | 94.9 | |
| Black | 17 | 1.1 | 29 | 1.8 | |
| Asian | 16 | 1.0 | 48 | 3.0 | |
| Other | 6 | 0.4 | 5 | 0.3 | |
| Hispanic ethnicity | 17 | 1.1 | 23 | 1.4 | .39 |
| Years of education | .01 | ||||
| ≤12 | 499 | 32.0 | 461 | 29.3 | |
| 13–16 | 846 | 54.3 | 840 | 53.3 | |
| >16 | 214 | 13.7 | 275 | 17.4 | |
| Missingb | 3 | -- | 34 | -- | |
| Income quartiles | .36 | ||||
| <$42,000 | 394 | 25.3 | 397 | 24.7 | |
| $42,000–56,999 | 417 | 26.8 | 405 | 25.2 | |
| $57,000–71,999 | 393 | 25.3 | 400 | 24.9 | |
| ≥$72,000 | 352 | 22.6 | 406 | 25.2 | |
| Missingb | 6 | -- | 2 | -- | |
| Body mass index (kg/m2) | <.001 | ||||
| <25.0 | 564 | 36.1 | 679 | 42.8 | |
| 25.0–29.9 | 460 | 29.4 | 479 | 30.2 | |
| ≥30.0 | 538 | 34.4 | 430 | 27.1 | |
| Missingb | 0 | -- | 22 | -- | |
| Smoking | .13 | ||||
| Never | 848 | 54.3 | 927 | 57.6 | |
| Former | 371 | 23.8 | 369 | 22.9 | |
| Current | 343 | 22.0 | 314 | 19.5 | |
| Number of chronic conditionsc | <.001 | ||||
| 0 | 650 | 41.6 | 908 | 56.4 | |
| 1 | 402 | 25.7 | 374 | 23.2 | |
| 2 | 245 | 15.7 | 171 | 10.6 | |
| ≥3 | 265 | 17.0 | 157 | 9.8 | |
| Hysterectomy status | <.001 | ||||
| None | 21 | 1.3 | 1453 | 90.2 | |
| Before | 149 | 9.5 | 157 | 9.8 | |
| Concurrent | 1392 | 89.1 | 0 | 0.0 | |
| Prior unilateral oophorectomy | 139 | 8.9 | 51 | 3.2 | <.001 |
| Indication for oophorectomyd | -- | ||||
| Benign ovarian condition | 635 | 40.7 | -- | -- | |
| No ovarian condition | 927 | 59.3 | -- | -- | |
The P values were calculated using chi-squared tests.
In the logistic regression models used to derive the inverse probability weights, women with unknown education were assigned to the ≤12 years group, women with unknown household income were assigned to the $42,000–56,999 quartile, and women with unknown body mass index were assigned to the <30 kg/m2 group.
A total of 17 chronic conditions defined by the US Department of Health and Human Services (DHHS) were considered, including depression, anxiety, substance abuse disorders, dementia, schizophrenia or psychosis, hyperlipidemia, hypertension, diabetes mellitus, cardiac arrhythmias, coronary artery disease, stroke, congestive heart failure, arthritis, asthma, chronic obstructive pulmonary disease, osteoporosis, and chronic kidney disease. Cancer was excluded from the DHHS list because it was an exclusion criterion for our study.
The indication was listed by the gynecologist in the medical record at the time of oophorectomy.
Risk of cancer
Starting at 6 months after the index date, the median follow-up was 18.0 years (interquartile interval 13.6–22.5) for the 1562 women who underwent bilateral oophorectomy. A total of 143 women had a de novo diagnosis of cancer (6 women had 2 types of cancer). The median follow-up was 17.8 years (interquartile interval 13.5–22.6) for the 1610 referent women. A total of 174 women had a de novo diagnosis of cancer (10 women had 2 types of cancer).
After adjustment using IPW, the overall risk of cancer was not significantly different between the two cohorts (HR, 0.82; 95% CI, 0.66–1.03, ARR 3.6%, Table 2, Figure 2). The risk of cancer was significantly lower in women who underwent bilateral oophorectomy before age 46 years (HR, 0.69; 95% CI, 0.51–0.94; ARR, 3.9%), but not in women who underwent bilateral oophorectomy at 46–49 years (HR, 1.03; 95% CI, 0.74–1.45; ARR, 2.6%). However, the HRs did not differ significantly across the two age strata, or in strata by estrogen therapy or by ovarian indication (Table 2, footnote b).
TABLE 2.
De novo cancer outcomes after bilateral oophorectomy, overall and in strata by age at oophorectomy, estrogen therapy, and surgical indication; excluding women with cancer of any type before the index date or within 6 months of follow-up
| Cancer type and strata | Bilateral oophorectomy |
Referent women |
Unweighted modelsa |
Weighted modelsb |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N at risk | Person-years | N of events | Absolute riskc (95% CI) | N at risk | Person-years | N of events | Absolute riskc (95% CI) | Hazard ratio (95% CI) | P value | Hazard ratio (95% CI) | P value | |
|
| ||||||||||||
| All cancer | 1562 | 26,786 | 143 | 13.2% (10.9–16.0) | 1610 | 26,928 | 174 | 16.8% (14.3–19.8) | 0.84 (0.67–1.04) | .11 | 0.82 (0.66–1.03) | .09 |
| Age ≤45 y | 985 | 17,173 | 75 | 11.4% (8.7–14.8) | 1011 | 16,758 | 102 | 15.3% (12.3–18.9) | 0.73 (0.54–0.97) | .03 | 0.69 (0.51–0.94) | .02 |
| Age <40 y | 332 | 5961 | 18 | 5.6% (2.9–10.5) | 340 | 5544 | 27 | 11.5% (7.2–18.2) | 0.62 (0.35–1.12) | .11 | 0.57 (0.31–1.06) | .07 |
| Age 40–45 y | 653 | 11,212 | 57 | 14.2% (10.4–19.2) | 671 | 11,214 | 75 | 17.2% (13.3–22.0) | 0.76 (0.54–1.07) | .12 | 0.74 (0.52–1.04) | .08 |
| ET >49d | 507 | 6246 | 46 | 22.4% (15.2–32.4) | 446 | 5617 | 45 | 18.0% (13.1–24.5) | 0.92 (0.61–1.39) | .71 | 0.87 (0.57–1.32) | .52 |
| No ET or ≤49 | 322 | 3057 | 16 | 25.5% (8.9–60.4) | 281 | 2820 | 17 | 26.2% (11.9–51.7) | 0.86 (0.45–1.66) | .65 | 0.78 (0.39–1.55) | .47 |
| Age 46–49 y | 577 | 9614 | 68 | 17.1% (13.0–22.4) | 599 | 10,170 | 72 | 19.7% (15.3–25.0) | 1.00 (0.72–1.38) | .98 | 1.03 (0.74–1.45) | .84 |
| ET >49d | 393 | 6222 | 47 | 21.4% (15.0–30.1) | 373 | 6087 | 49 | 20.1% (14.7–27.2) | 0.94 (0.63–1.40) | .75 | 0.96 (0.64–1.45) | .86 |
| No ET or ≤49 | 107 | 1396 | 6 | 5.3% (2.1–13.0) | 127 | 1729 | 12 | 14.3% (7.6–25.9) | 0.61 (0.23–1.61) | .32 | 0.59 (0.22–1.60) | .30 |
| Benign indicatione | 635 | 10,987 | 53 | 12.1% (8.9–16.3) | 662 | 11,040 | 72 | 15.5% (12.1–19.8) | 0.76 (0.54–1.08) | .13 | 0.71 (0.49–1.01) | .06 |
| No indicationf | 927 | 15,800 | 90 | 14.2% (11.0–18.2) | 948 | 15,888 | 102 | 18.4% (14.8–22.7) | 0.89 (0.67–1.18) | .42 | 0.89 (0.67–1.19) | .43 |
| Gynecologic cancerg | 1562 | 27,718 | 6 | 0.5% (0.2–1.3) | 1610 | 27,865 | 37 | 4.1% (2.9–5.8) | 0.17 (0.07–0.38) | <.001 | 0.15 (0.06–0.34) | <.001 |
| Age ≤45 y | 985 | 17,604 | 4 | 0.5% (0.1–2.0) | 1011 | 17,309 | 25 | 4.5% (2.9–6.8) | 0.16 (0.06–0.45) | <.001 | 0.13 (0.05–0.36) | <.001 |
| Age <40 y | 332 | 6060 | 2 | 0.8% (0.1–5.6) | 340 | 5685 | 7 | 5.4% (2.5–11.8) | 0.28 (0.07–1.14) | .08 | 0.16 (0.05–0.54) | .003 |
| Age 40–45 y | 653 | 11,544 | 2 | 0.1% (0.0–1.3) | 671 | 11,624 | 18 | 3.9% (2.4–6.4) | 0.11 (0.03–0.49) | .003 | 0.10 (0.02–0.42) | .002 |
| ET >49d | 512 | 6540 | 2 | 2.4% (0.3–16.8) | 462 | 5963 | 14 | 8.6% (2.8–24.8) | 0.13 (0.03–0.58) | .008 | 0.11 (0.03–0.50) | .004 |
| No ET or ≤49 | 327 | 3159 | 1 | 0.3% (0.0–3.4) | 285 | 2905 | 5 | 3.4% (1.3–8.5) | 0.19 (0.02–1.60) | .13 | 0.10 (0.01–0.92) | .04 |
| Age 46–49 y | 577 | 10,114 | 2 | 0.4% (0.1–1.6) | 599 | 10,556 | 12 | 3.4% (1.9–6.1) | 0.17 (0.04–0.78) | .02 | 0.17 (0.04–0.76) | .02 |
| ET >49d | 394 | 6562 | 1 | 0.3% (0.0–2.1) | 373 | 6374 | 8 | 3.6% (1.7–7.5) | 0.12 (0.02–0.98) | .047 | 0.11 (0.01–0.91) | .04 |
| No ET or ≤49 | 114 | 1510 | 1 | 0.8% (0.1–7.5) | 127 | 1775 | 2 | 1.9% (0.5–7.3) | 0.55 (0.05–6.09) | .63 | 0.43 (0.04–4.80) | .49 |
| Benign indicatione | 635 | 11,279 | 3 | 0.7% (0.2–3.0) | 662 | 11,396 | 18 | 4.3% (2.6–7.1) | 0.17 (0.05–0.56) | .003 | 0.15 (0.05–0.48) | .002 |
| No indicationf | 927 | 16,439 | 3 | 0.2% (0.1–1.0) | 948 | 16,469 | 19 | 3.9% (2.4–6.3) | 0.16 (0.05–0.54) | .003 | 0.14 (0.04–0.48) | .002 |
| Non-gynecologic cancerh | 1562 | 26,815 | 137 | 12.8% (10.5–15.5) | 1610 | 27,184 | 143 | 13.4% (11.1–16.1) | 0.99 (0.78–1.24) | .92 | 0.99 (0.78–1.26) | .95 |
| Age ≤45 y | 985 | 17,189 | 71 | 10.9% (8.3–14.3) | 1011 | 16,918 | 82 | 11.6% (8.9–14.9) | 0.87 (0.63–1.19) | .38 | 0.85 (0.61–1.17) | .32 |
| Age <40 y | 332 | 5974 | 16 | 4.8% (2.4–9.3) | 340 | 5584 | 21 | 6.2% (3.5–10.8) | 0.71 (0.37–1.36) | .30 | 0.72 (0.37–1.43) | .35 |
| Age 40–45 y | 653 | 11,215 | 55 | 14.1% (10.3–19.1) | 671 | 11,334 | 61 | 14.3% (10.6–19.1) | 0.92 (0.64–1.32) | .65 | 0.90 (0.62–1.30) | .58 |
| ET >49d | 507 | 6249 | 44 | 20.3% (13.9–29.0) | 447 | 5678 | 34 | 14.4% (9.8–20.8) | 1.19 (0.76–1.85) | .45 | 1.15 (0.73–1.82) | .55 |
| No ET or ≤49 | 323 | 3067 | 15 | 25.3% (8.8–60.4) | 283 | 2875 | 14 | 37.4% (15.8–72.1) | 1.02 (0.51–2.05) | .96 | 0.87 (0.41–1.83) | .71 |
| Age 46–49 y | 577 | 9626 | 66 | 16.8% (12.6–22.1) | 599 | 10,266 | 61 | 16.9% (12.8–22.1) | 1.15 (0.82–1.63) | .42 | 1.22 (0.86–1.73) | .27 |
| ET >49d | 393 | 6222 | 46 | 21.2% (14.8–29.9) | 373 | 6149 | 41 | 16.7% (11.7–23.5) | 1.11 (0.73–1.69) | .63 | 1.16 (0.75–1.78) | .51 |
| No ET or ≤49 | 107 | 1409 | 5 | 4.5% (1.6–12.1) | 127 | 1746 | 11 | 15.5% (8.0–28.9) | 0.57 (0.20–1.62) | .29 | 0.58 (0.20–1.70) | .32 |
| Benign indicatione | 635 | 11,013 | 50 | 11.4% (8.3–15.5) | 662 | 11,171 | 58 | 12.1% (9.0–16.2) | 0.91 (0.63–1.32) | .62 | 0.85 (0.58–1.25) | .42 |
| No indicationf | 927 | 15,802 | 87 | 14.0% (10.8–18.0) | 948 | 16,012 | 85 | 14.9% (11.6–18.9) | 1.04 (0.78–1.41) | .78 | 1.07 (0.79–1.45) | .66 |
| Breast cancer | 1562 | 27,282 | 54 | 5.0% (3.6–6.8) | 1610 | 27,673 | 67 | 6.9% (5.2–9.1) | 0.83 (0.58–1.18) | .30 | 0.87 (0.61–1.24) | .44 |
| Age ≤45 y | 985 | 17,368 | 32 | 5.3% (3.5–7.8) | 1011 | 17,217 | 37 | 5.3% (3.7–7.7) | 0.87 (0.55–1.39) | .57 | 0.86 (0.54–1.39) | .54 |
| Age <40 y | 332 | 6015 | 6 | 2.7% (1.0–7.2) | 340 | 5679 | 10 | 4.0% (1.9–8.4) | 0.58 (0.21–1.58) | .29 | 0.65 (0.23–1.84) | .42 |
| Age 40–45 y | 653 | 11,353 | 26 | 6.5% (4.1–10.2) | 671 | 11,538 | 27 | 6.0% (3.8–9.3) | 0.99 (0.58–1.70) | .96 | 0.95 (0.55–1.63) | .84 |
| ET >49d | 512 | 6399 | 20 | 10.3% (5.5–19.0) | 454 | 5865 | 13 | 5.0% (2.5–10.0) | 1.43 (0.71–2.88) | .32 | 1.48 (0.71–3.05) | .29 |
| No ET or ≤49 | 324 | 3082 | 7 | 6.1% (2.7–13.4) | 286 | 2919 | 8 | 16.5% (6.4–38.6) | 0.84 (0.33–2.16) | .72 | 0.71 (0.26–1.96) | .51 |
| Age 46–49 y | 577 | 9914 | 22 | 4.3% (2.7–7.0) | 599 | 10,455 | 30 | 10.2% (6.8–15.2) | 0.77 (0.45–1.33) | .35 | 0.83 (0.47–1.45) | .50 |
| ET >49d | 394 | 6450 | 15 | 9.1% (4.5–18.0) | 373 | 6302 | 19 | 9.6% (5.6–16.0) | 0.77 (0.39–1.50) | .44 | 0.79 (0.40–1.57) | .51 |
| No ET or ≤49 | 108 | 1432 | 0 | 0.0% (0.0–0.0) | 127 | 1752 | 8 | 12.0% (5.2–26.4) | -- | -- | -- | -- |
| Benign indicatione | 635 | 11,141 | 22 | 6.0% (3.8–9.6) | 662 | 11,345 | 31 | 6.8% (4.6–10.0) | 0.75 (0.44–1.29) | .30 | 0.76 (0.44–1.31) | .32 |
| No indicationf | 927 | 16,141 | 32 | 4.3% (2.8–6.6) | 948 | 16,327 | 36 | 7.4% (5.0–10.8) | 0.90 (0.56–1.45) | .68 | 0.95 (0.59–1.55) | .85 |
| Gastrointestinal canceri | 1562 | 27,667 | 19 | 2.4% (1.4–4.0) | 1610 | 28,031 | 20 | 1.7% (1.0–2.9) | 0.98 (0.52–1.83) | .94 | 1.07 (0.56–2.03) | .83 |
| Age ≤45 y | 985 | 17,590 | 11 | 2.3% (1.2–4.5) | 1011 | 17,416 | 15 | 2.1% (1.1–4.0) | 0.74 (0.34–1.61) | .44 | 0.78 (0.35–1.72) | .53 |
| Age <40 y | 332 | 6073 | 1 | 0.0% (0.0–0.0) | 340 | 5723 | 3 | 0.3% (0.0–3.2) | 0.30 (0.03–2.54) | .27 | 0.35 (0.04–3.21) | .35 |
| Age 40–45 y | 653 | 11,517 | 10 | 3.6% (1.8–7.1) | 671 | 11,693 | 12 | 3.0% (1.5–5.9) | 0.85 (0.37–1.97) | .71 | 0.87 (0.37–2.03) | .75 |
| Age 46–49 y | 577 | 10,077 | 8 | 2.5% (1.1–5.5) | 599 | 10,615 | 5 | 1.0% (0.4–2.6) | 1.67 (0.55–5.10) | .37 | 2.07 (0.65–6.55) | .22 |
| Lung cancer | 1562 | 27,705 | 12 | 1.0% (0.5–1.9) | 1610 | 28,105 | 13 | 1.1% (0.6–1.9) | 0.96 (0.45–2.03) | .91 | 0.84 (0.39–1.82) | .65 |
| Age ≤45 y | 985 | 17,608 | 4 | 0.3% (0.1–1.0) | 1011 | 17,463 | 5 | 0.7% (0.3–1.8) | 0.82 (0.22–3.06) | .76 | 0.57 (0.15–2.21) | .42 |
| Age <40 y | 332 | 6072 | 1 | 0.0% (0.0–0.0) | 340 | 5726 | 1 | 0.0% (0.0–0.0) | 0.91 (0.06–13.9) | .95 | 0.92 (0.06–14.2) | .95 |
| Age 40–45 y | 653 | 11,535 | 3 | 0.4% (0.1–1.5) | 671 | 11,738 | 4 | 0.8% (0.3–2.3) | 0.77 (0.17–3.47) | .74 | 0.60 (0.13–2.73) | .51 |
| Age 46–49 y | 577 | 10,097 | 8 | 2.4% (1.1–5.1) | 599 | 10,642 | 8 | 1.6% (0.8–3.5) | 1.05 (0.42–2.63) | .91 | 1.14 (0.45–2.89) | .78 |
CI, confidence interval; ET, estrogen therapy.
Hazard ratios were calculated using Cox proportional hazards models with age as the time scale. Follow-up for these analyses was started at 6 months after index date.
Hazard ratios were calculated using Cox proportional hazards models with age as the time scale and adjusted using inverse probability weights derived from a logistic regression model including 17 chronic conditions present at baseline, years of education (≤12, 13–16, >16), quartiles of household income (<$42,000, $42,000–56,999, $57,000–71,999, ≥$72,000), race (white vs non-white), body mass index (<30 vs ≥30 kg/m2), cigarette smoking (current or former vs never), age at index date (continuous), and calendar year at index date (continuous). These adjustments were done separately in each stratum to maximize the balance at index date. Follow-up for these analyses was started at 6 months after index date. No significant interactions by age (≤45 y vs 46–49 y), estrogen therapy, or surgical indication were found.
Absolute cumulative risk at 25 years after bilateral oophorectomy (or index) calculated using the Kaplan-Meier method. The estimates were adjusted using inverse probability weights (see details in footnote b). These adjustments were done separately in each stratum to maximize the balance at index date.
Women who were taking systemic ET (only oral or transdermal) on their 50th birth date, after bilateral oophorectomy. Women who died or were lost to follow-up prior to their 50th birth date, had reached age 50 years within 6 months after index date, or had not reached age 50 years as of December 31, 2018 were not included in this analysis. Follow-up for these analyses was started at age 50 years.
The benign condition (eg, benign tumors, cysts, endometriomas) was listed by the gynecologist as the surgical indication in the medical record at the time of oophorectomy, but may not have been the sole indication for the surgery.
Women without a benign ovarian condition. Historically, the terms “prophylactic”, “elective”, or “incidental” oophorectomy we re used; however, we prefer to avoid these terms.
Includes cancer of the ovaries, fallopian tubes, uterus, cervix, vagina, and vulva. Six women had gynecologic cancer after bilateral oophorectomy (2 ovaries, 1 uterus, 1 vagina, and 2 vulva), and 37 referent women had gynecologic cancer after index date (7 ovaries, 26 uterus, 2 cervix, 1 vulva, and 1 unspecified uterine adnexa).
Includes all types of cancer other than cancer of the ovaries, fallopian tubes, uterus, cervix, vagina, and vulva.
Includes cancer of the esophagus, stomach, colon, rectum, liver, pancreas, peritoneum, duodenum, etc. There were 19 women who developed gastrointestinal cancer after bilateral oophorectomy (1 esophagus, 4 stomach, 3 colon, 1 rectum, 4 liver, 3 pancreas, 1 peritoneum, 1 gallbladder, 1 bile duct) and 20 referent women who developed gastrointestinal cancer after index date (10 colon, 4 rectum, 1 liver, 3 pancreas, 1 peritoneum, 1 bile duct).
FIGURE 2.
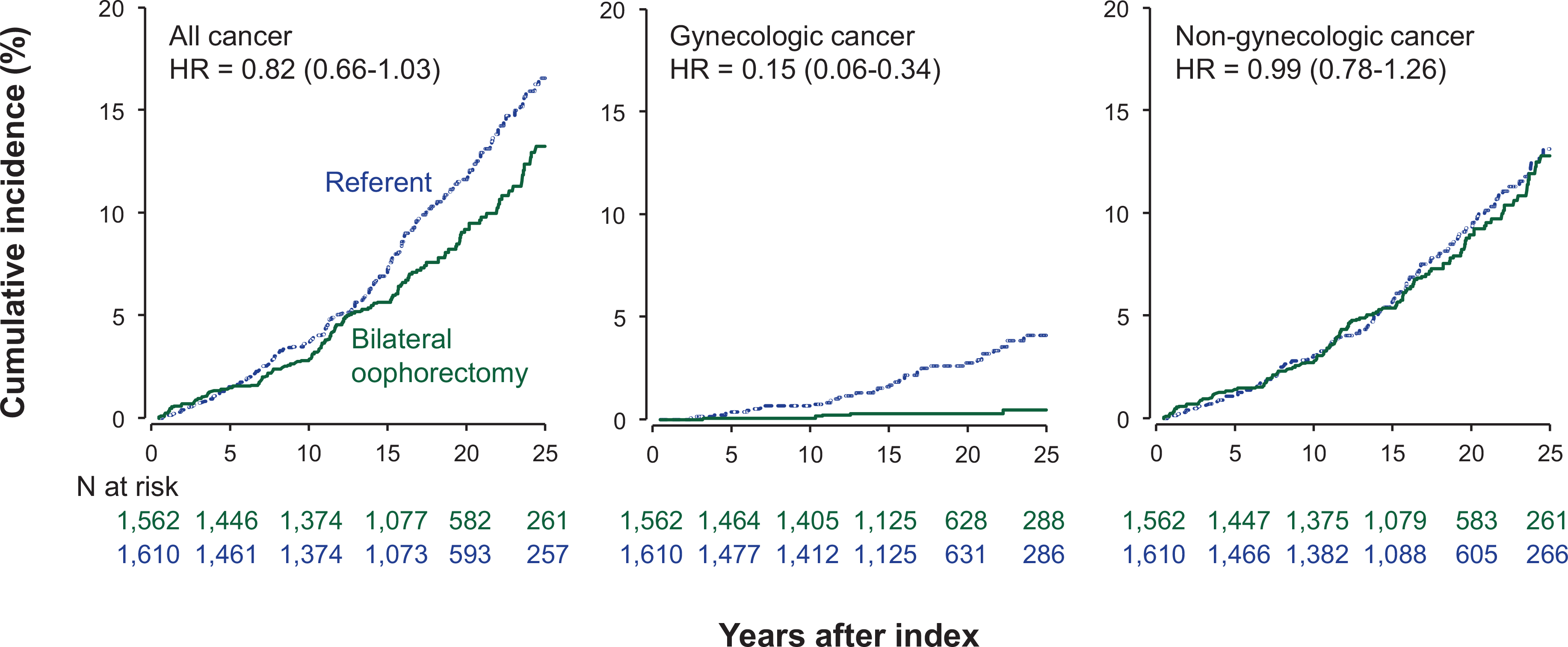
Cumulative incidence curves for cancer overall, gynecologic cancer, and non-gynecologic cancer in women who underwent bilateral oophorectomy compared with referent women. The curves were adjusted using inverse probability weights derived from a logistic regression model including 17 chronic conditions present at baseline (list provided in text), years of education, quartiles of household income, race, body mass index, cigarette smoking, and age and calendar year at the index date.
Women who underwent premenopausal bilateral oophorectomy had a reduced risk of gynecologic cancer compared to referent women overall (HR, 0.15; 95% CI, 0.06–0.34; ARR, 3.6%, Table 2, Figure 3), and in strata by age and ovarian indication. However, there was no significant interaction by estrogen therapy. Details about the gynecologic cancers experienced by the two cohorts are provided in Table 2 (footnote g). In particular, ovarian cancer developed in 2 women in the bilateral oophorectomy cohort vs 7 women in the referent cohort.
FIGURE 3.
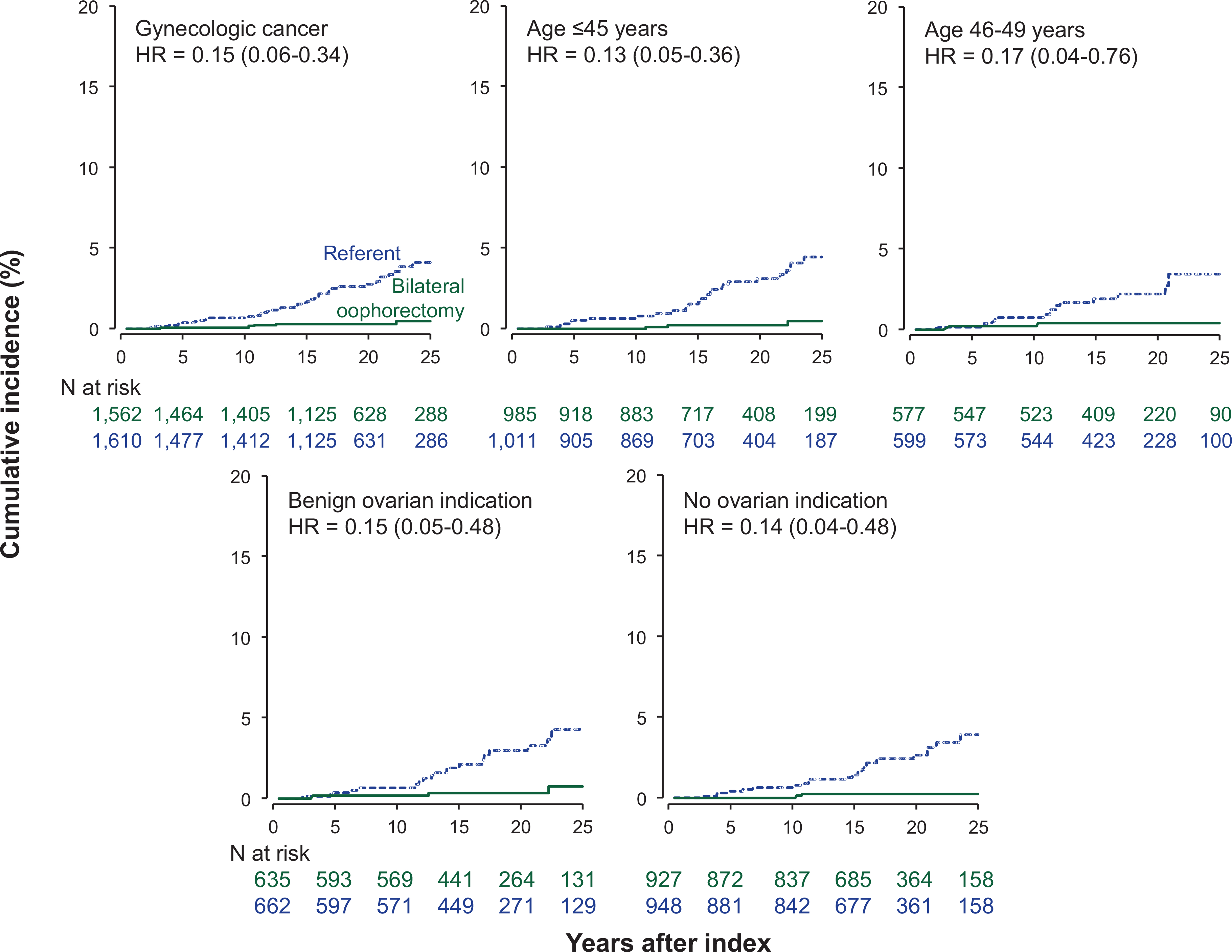
Cumulative incidence curves for gynecologic cancer in women who underwent bilateral oophorectomy compared with referent women, overall and in strata by age at the index date and indication for the oophorectomy. The curves were adjusted using inverse probability weights derived from a logistic regression model including 17 chronic conditions present at baseline (list provided in text), years of education, quartiles of household income, race, body mass index, cigarette smoking, and age and calendar year at the index date.
By contrast, there was no association between bilateral oophorectomy and non-gynecologic cancers overall (HR, 0.99; 95% CI, 0.78–1.26, ARR, 0.6%, Table 2), or in strata by age and ovarian indication. There was no significant interaction by estrogen therapy. In particular, women with or without bilateral oophorectomy before natural menopause had a similar risk of breast cancer (HR, 0.87; 95% CI, 0.61–1.24; ARR, 1.9%), gastrointestinal cancer (HR, 1.07; 95% CI, 0.56–2.03; ARI, 0.7%), and lung cancer (HR, 0.84; 95% CI, 0.39–1.82; ARR, 0.1%) (Table 2).
Sensitivity analyses
In our four sets of sensitivity analyses, the results were similar to the primary analyses. The results for the first set of sensitivity analyses are reported in Supplemental Table 1. The results of the remaining three sets of sensitivity analyses are not shown.
Comment
Principal findings
In this population-based cohort study, women who underwent bilateral oophorectomy before spontaneous menopause had a significantly reduced risk of gynecologic cancer compared to age-matched referent women. However, there were no significant differences for all types of cancer, all non-gynecological cancers, or specifically for breast cancer, gastrointestinal cancer, or lung cancer. The results remained similar when the analyses were stratified by age, use of estrogen therapy, or by ovarian indication for bilateral oophorectomy.
Results and comparison with other studies
Our finding of a lower risk of all types of gynecological cancers, including ovarian cancer, among women who underwent bilateral oophorectomy is consistent with previous studies.3, 14, 15, 32 Ovarian cancer is the fifth most common cause of cancer death, and the most common cause of gynecologic cancer death in women, with an estimated 22,240 new diagnoses and 14,070 deaths each year in the United States.33 Unfortunately, our numbers were small to consider each one of the gynecological cancers separately. Because almost all women underwent concurrent or prior hysterectomy (98.7%), the difference was driven by the dramatic reduction in ovarian and uterine cancer.
By contrast, the risk of breast cancer was not significantly different in our study. Accumulating evidence suggests that sex hormones may play an important role in the development of breast cancer, and that bilateral oophorectomy before natural menopause may reduce the risk, especially among BRCA2 mutation carriers. For example, Kauff and colleagues reported a 72% decrease in breast cancer risk among BRCA2 mutation carriers after bilateral oophorectomy.7 Another study from the Hereditary Breast Cancer Clinical Study Group reported a significantly reduced risk of breast cancer diagnosed before age 50 years among BRCA2 mutation carriers, but not among BRCA1 mutation carriers.12 However, a 2018 systematic review of the literature concluded that the association is more certain for BRCA1 mutation carriers than for BRCA2 mutation carriers.13
The association between premenopausal bilateral oophorectomy and breast cancer risk among women in the general population (ie, in women at average risk of ovarian cancer) is less clear and may vary by age at the time of oophorectomy.14–17 For example, a study using the Cancer Prevention Study-II Nutrition Cohort showed a 20% reduction in breast cancer risk in women who underwent bilateral oophorectomy with hysterectomy at any age compared with no surgery.15 However, the Nurses’ Health Study showed a significant reduction in the risk of breast cancer only for women who underwent bilateral oophorectomy with hysterectomy at ages younger than 45 years compared with women who underwent hysterectomy alone. The risk was not decreased for women who underwent oophorectomy at ages 45–54 or 55 years or older.14
Similarly, a recent study from Australia reported a significant difference in the risk of breast cancer only for women who underwent bilateral oophorectomy with hysterectomy at ages younger than 45 years, but not at ages 45–54 or 55+ years compared to women with no surgery.17 A large case-control study showed reduced odds of breast cancer with hysterectomy and bilateral oophorectomy performed at age ≤40 years but not at age older than 40 years.16 We did not find a significant reduction in risk both at ages ≤45 years or at ages 46–49 years. When we further stratified the age group ≤45 years, there was a trend toward more reduced risk in women with age <40 years compared to age 40–45 years; however, our numbers were too small and we did not have adequate power to test the association restricted to women age <40 years. Therefore, we did not observe a reduced risk of breast cancer; however, we cannot exclude a reduction in risk restricted to women with very early oophorectomy.
We did not observe an increased risk of gastrointestinal cancer after bilateral oophorectomy. Similarly, a recent study from Australia did not report an increased risk of colorectal cancer in women who underwent hysterectomy with bilateral oophorectomy vs no surgery.17 These results contrast with findings from a previous study by Segelman and colleagues.34 Possible reasons for the discrepancy are the small number of events or the frequent use of estrogen therapy among women who underwent bilateral oophorectomy in our study. The use of oral contraceptives or menopausal hormone therapy has been associated with a lower risk of developing colorectal cancer,35, 36 which is the second most common type of cancer and the third leading cause of cancer deaths in women.37 Estrogen receptors are present in the human colorectal tissues, and physiological levels of estrogen stimulate humoral and cell-mediated immune response.38 These observations suggest that estrogen may reduce the risk of colorectal cancer risk in women; however, the studies are inconsistent.39
We did not observe an increased risk of lung cancer. Previous studies have examined multiple sex-specific risk factors for lung cancer (eg, age at menarche, age at first pregnancy, age at first live birth, parity, and lactation), with mixed results.40–43 A study showed that early menopause or oophorectomy were associated with an increased risk of lung cancer.43 However, the investigators did not control for smoking, one of the strongest risk factors for lung cancer. Higher risk after bilateral oophorectomy was also reported from the Nurses’ Health Study.14
In our study, the use of estrogen therapy after bilateral oophorectomy did not significantly modify the risk of any cancer types, including gynecological cancers and breast cancer. Several previous studies suggested that estrogen therapy may increase the risk of breast cancer.44, 45 However, the impact of estrogen therapy on breast cancer risk after bilateral oophorectomy remains unclear.46–48 In addition, it remains unknown whether the increased risk of breast cancer is associated with an increased duration of estrogen use, time since menopause, and with estrogen receptor-positive disease.49, 50 For example, a meta-analysis, showed a progressively greater risk with longer use, and a greater risk for estrogen receptor-positive cancers.50
Implications
In this population-based study, we did not observe an association between premenopausal bilateral oophorectomy and risk of non-gynecological cancers, including breast cancer, among women at average risk of ovarian cancer. Thus, for the general population of women at average risk of ovarian cancer, these results suggest that the ovaries should not be removed prior to spontaneous menopause to reduce the risk of non-gynecological cancers including breast cancer. Considering the additional increased risk for long-term morbidity and mortality not related to cancer after premenopausal bilateral oophorectomy, the benefits of undergoing the surgery may not outweigh possible risk for women in whom the absolute risk for developing ovarian cancer or breast cancer is low.19–22
Strengths and limitations
This study has several strengths. First, the bilateral oophorectomy cohort and the referent cohort were representative of a well-defined population with up to 30 years of follow-up.23 Second, details about the bilateral oophorectomy, baseline characteristics, estrogen therapy, and cancer were confirmed through abstraction of medical records from a medical records-linkage system, thus limiting recall bias.23
However, limitations also warrant consideration. First, participants were predominantly white, and all women resided in Olmsted County, Minnesota. Thus, results may not be generalizable to other populations with different racial, ethnic, or socioeconomic characteristics.25 Second, the observational nature of our study limits causal inference. For example, women with multiple clinically recognized chronic conditions may be more likely to undergo a bilateral oophorectomy. However, we performed a sensitivity analysis that excluded all women with a documented history of chronic conditions before the index date, and the results did not change noticeably. Third, the majority of women in our study were not tested for BRCA1 or BRCA2 variants. In the study time frame (1988–2007), genetic testing was seldom performed even in women judged by the gynecologist to be at high genetic risk (strong family history). Fourth, the study had limited power to study specific types of cancer such as gastrointestinal and lung cancer. On the other hand, the sample size was dictated by the geographically-defined population and by the study time frame rather than by a power calculation conducted during the design of the study. Fifth, the median length of follow-up was 18.0 years in women who underwent bilateral oophorectomy, and 17.8 years in the referent cohort. Therefore, women in our study were still relatively young at the end of follow-up (median age of 62 years). It is possible that we would observe additional significant associations if the women were followed for a longer time. Therefore, we remain cautious in our interpretations until these findings can be replicated elsewhere. We also plan to continue to follow our cohorts. Sixth, the two cohorts were balanced for several possible confounders present at the index date using inverse probability weights. In particular, balancing for 17 chronic conditions present at baseline should have balanced indirectly also for several other possible variables that were not directly measured. The results did not vary using traditional multivariable adjustments. However, some residual confounding is possible. Finally, surgical and medical practices may have changed over the 20-year study time frame. However, analyses stratified in two decades did not show significant differences (data not shown).
Conclusion
This large cohort study showed that the risk of non-gynecologic cancers, including breast cancer, was similar for women with premenopausal bilateral oophorectomy and referent women. Thus, bilateral oophorectomy should not be considered for the prevention of non-gynecological cancers, including breast cancer, in the general population. These findings, in conjunction with the results of other studies showing the increased risk of multiple chronic conditions after premenopausal bilateral oophorectomy, may help women to better evaluate the risk-to-benefit ratio of undergoing bilateral oophorectomy before natural menopause for the prevention of ovarian and other cancers.
Supplementary Material
AJOG at a Glance.
A. Why was this study conducted?
Some studies suggest that premenopausal bilateral oophorectomy may be associated with an increased risk of lung or colon cancer and a decreased risk of breast cancer. However, evidence in the general population is lacking.
B. What are the key findings
In this population-based cohort study, women who underwent premenopausal bilateral oophorectomy had a reduced risk of gynecologic cancers, but not of other types of cancer including breast cancer.
C. What does this study add to what is already known?
Premenopausal bilateral oophorectomy among women at average risk of ovarian cancer (ie, without a strong family history or a high risk genetic variant) does not reduce the risk of non-gynecological cancers including breast cancer and should not be utilized for the prevention of these cancers.
Condensation.
Premenopausal bilateral oophorectomy in the general population does not reduce the risk of non-gynecologic cancers including breast cancer and should not be utilized for the prevention of these cancers.
Acknowledgments
Grant Support: The Mayo Clinic Cohort Study of Oophorectomy and Aging (MOA-2) is partly supported by the National Institute on Aging (NIA grant U54 AG044170, RF1 AG55151) and uses the resources of the Rochester Epidemiology Project (REP) medical records-linkage system. The REP is supported by the National Institute on Aging (NIA grant AG 058738), by the Mayo Clinic Research Committee, and by fees paid annually by REP users. However, the content of this article is solely the responsibility of the authors and does not represent the official views of the National Institute on Aging (NIA), or of the Mayo Clinic. Dr Rocca was partly funded by the Ralph S and Beverley E Caulkins Professorship of Neurodegenerative Diseases Research of the Mayo Clinic.
Potential Competing Interests: Dr Mielke receives funding from the National Institutes of Health and consults for Biogen and Brain Protection Company. Dr Rocca receives funding from the National Institutes of Health. All other authors report no conflict of interest.
Footnotes
SUPPLEMENTAL ONLINE MATERIAL
Supplementary material is available.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Nan HUO, Division of Epidemiology, Department of Quantitative Health Sciences, Mayo Clinic, Rochester, MN.
Carin Y. SMITH, Division of Clinical Trials and Biostatistics, Department of Quantitative Health Sciences, Mayo Clinic, Rochester, MN.
Liliana GAZZUOLA ROCCA, Division of Epidemiology, Department of Quantitative Health Sciences, Mayo Clinic, Rochester, MN.
Walter A. ROCCA, Division of Epidemiology, Department of Quantitative Health Sciences, Mayo Clinic, Rochester, MN; Department of Neurology, Mayo Clinic, Rochester, Minnesota; Mayo Clinic Specialized Research Center of Excellence (SCORE) on Sex Differences, Mayo Clinic, Rochester, Minnesota.
Michelle M. MIELKE, Division of Epidemiology, Department of Quantitative Health Sciences, Mayo Clinic, Rochester, MN; Department of Neurology, Mayo Clinic, Rochester, Minnesota; Mayo Clinic Specialized Research Center of Excellence (SCORE) on Sex Differences, Mayo Clinic, Rochester, Minnesota.
Role of the Funding Source:
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study, and all authors had final responsibility for the decision to submit for publication.
References
- 1.ASANTE A, WHITEMAN MK, KULKARNI A, COX S, MARCHBANKS PA, JAMIESON DJ. Elective oophorectomy in the United States: trends and in-hospital complications, 1998–2006. Obstet Gynecol 2010;116:1088–95. [DOI] [PubMed] [Google Scholar]
- 2.FINCH AP, LUBINSKI J, MOLLER P, et al. Impact of oophorectomy on cancer incidence and mortality in women with a BRCA1 or BRCA2 mutation. J Clin Oncol 2014;32:1547–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.AVERETTE HE, NGUYEN HN. The role of prophylactic oophorectomy in cancer prevention. Gynecol Oncol 1994;55:S38–41. [DOI] [PubMed] [Google Scholar]
- 4.HEEMSKERK-GERRITSEN BA, SEYNAEVE C, VAN ASPEREN CJ, et al. Breast cancer risk after salpingo-oophorectomy in healthy BRCA1/2 mutation carriers: revisiting the evidence for risk reduction. J Natl Cancer Inst 2015;107. [DOI] [PubMed] [Google Scholar]
- 5.FAKKERT IE, MOURITS MJ, JANSEN L, et al. Breast Cancer Incidence After Risk-Reducing Salpingo-Oophorectomy in BRCA1 and BRCA2 Mutation Carriers. Cancer Prev Res (Phila) 2012;5:1291–7. [DOI] [PubMed] [Google Scholar]
- 6.DOMCHEK SM, FRIEBEL TM, SINGER CF, et al. Association of risk-reducing surgery in BRCA1 or BRCA2 mutation carriers with cancer risk and mortality. JAMA 2010;304:967–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.KAUFF ND, DOMCHEK SM, FRIEBEL TM, et al. Risk-reducing salpingo-oophorectomy for the prevention of BRCA1- and BRCA2-associated breast and gynecologic cancer: a multicenter, prospective study. J Clin Oncol 2008;26:1331–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.EISEN A, LUBINSKI J, KLIJN J, et al. Breast cancer risk following bilateral oophorectomy in BRCA1 and BRCA2 mutation carriers: an international case-control study. J Clin Oncol 2005;23:7491–6. [DOI] [PubMed] [Google Scholar]
- 9.KLAREN HM, VAN’T VEER LJ, VAN LEEUWEN FE, ROOKUS MA. Potential for bias in studies on efficacy of prophylactic surgery for BRCA1 and BRCA2 mutation. J Natl Cancer Inst 2003;95:941–7. [DOI] [PubMed] [Google Scholar]
- 10.WACHOLDER S. Bias in intervention studies that enroll patients from high-risk clinics. J Natl Cancer Inst 2004;96:1204–7. [DOI] [PubMed] [Google Scholar]
- 11.CHOI YH, TERRY MB, DALY MB, et al. Association of Risk-Reducing Salpingo-Oophorectomy With Breast Cancer Risk in Women With BRCA1 and BRCA2 Pathogenic Variants. JAMA Oncol 2021;7:585–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.KOTSOPOULOS J, HUZARSKI T, GRONWALD J, et al. Bilateral Oophorectomy and Breast Cancer Risk in BRCA1 and BRCA2 Mutation Carriers. J Natl Cancer Inst 2017;109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.ELEJE GU, EKE AC, EZEBIALU IU, IKECHEBELU JI, UGWU EO, OKONKWO OO. Risk-reducing bilateral salpingo-oophorectomy in women with BRCA1 or BRCA2 mutations. Cochrane Database Syst Rev 2018;8:CD012464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.PARKER WH, BRODER MS, CHANG E, et al. Ovarian conservation at the time of hysterectomy and long-term health outcomes in the nurses’ health study. Obstet Gynecol 2009;113:1027–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.GAUDET MM, GAPSTUR SM, SUN J, TERAS LR, CAMPBELL PT, PATEL AV. Oophorectomy and hysterectomy and cancer incidence in the Cancer Prevention Study-II Nutrition Cohort. Obstet Gynecol 2014;123:1247–55. [DOI] [PubMed] [Google Scholar]
- 16.NICHOLS HB, VISVANATHAN K, NEWCOMB PA, et al. Bilateral oophorectomy in relation to risk of postmenopausal breast cancer: confounding by nonmalignant indications for surgery? American journal of epidemiology 2011;173:1111–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.WILSON LF, TUESLEY KM, WEBB PM, DIXON-SUEN SC, STEWART LM, JORDAN SJ. Hysterectomy and Risk of Breast, Colorectal, Thyroid, and Kidney Cancer–an Australian Data Linkage Study. Cancer Epidemiology and Prevention Biomarkers 2021;30:904–11. [DOI] [PubMed] [Google Scholar]
- 18.LUO G, ZHANG Y, WANG L, et al. Risk of colorectal cancer with hysterectomy and oophorectomy: A systematic review and meta-analysis. Int J Surg 2016;34:88–95. [DOI] [PubMed] [Google Scholar]
- 19.ROCCA WA, GAZZUOLA-ROCCA L, SMITH CY, et al. Accelerated accumulation of multimorbidity after bilateral oophorectomy: a population-based cohort study. Mayo Clin Proc 2016;91:1577–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.ROCCA WA, GAZZUOLA ROCCA L, SMITH CY, et al. Bilateral oophorectomy and accelerated aging: cause or effect? J Gerontol A Biol Sci Med Sci 2017;72:1213–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.HUO N, SMITH CY, GAZZUOLA ROCCA L, ROCCA WA, MIELKE MM. Association of Premenopausal Bilateral Oophorectomy With Restless Legs Syndrome. JAMA Netw Open 2021;4:e2036058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.ROCCA WA, GAZZUOLA ROCCA L, SMITH CY, et al. Loss of Ovarian Hormones and Accelerated Somatic and Mental Aging. Physiology (Bethesda) 2018;33:374–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.ROCCA WA, GAZZUOLA ROCCA L, SMITH CY, et al. Cohort profile: the Mayo Clinic Cohort Study of Oophorectomy and Aging-2 (MOA-2) in Olmsted County, Minnesota (USA). BMJ Open 2017;7:e018861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.ROCCA WA, GAZZUOLA ROCCA L, SMITH CY, et al. Personal, reproductive, and familial characteristics associated with bilateral oophorectomy in premenopausal women: A population-based case-control study. Maturitas 2018;117:64–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.ST. SAUVER JL, GROSSARDT BR, LEIBSON CL, YAWN BP, MELTON LJ 3RD, ROCCA WA. Generalizability of epidemiological findings and public health decisions: an illustration from the Rochester Epidemiology Project. Mayo Clin Proc 2012;87:151–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.ST. SAUVER JL, GROSSARDT BR, YAWN BP, et al. Data resource profile: the Rochester Epidemiology Project (REP) medical records-linkage system. Int J Epidemiol 2012;41:1614–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.ST. SAUVER JL, GROSSARDT BR, YAWN BP, MELTON LJ 3RD, ROCCA WA. Use of a medical records linkage system to enumerate a dynamic population over time: the Rochester epidemiology project. Am J Epidemiol 2011;173:1059–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.ROCCA WA, YAWN BP, ST. SAUVER JL, GROSSARDT BR, MELTON LJ 3RD History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin Proc 2012;87:1202–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.GOODMAN RA, POSNER SF, HUANG ES, PAREKH AK, KOH HK. Defining and measuring chronic conditions: imperatives for research, policy, program, and practice. Prev Chronic Dis 2013;10:E66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.ST SAUVER JL, CHAMBERLAIN AM, BOBO WV, et al. Implementing the US Department of Health and Human Services definition of multimorbidity: a comparison between billing codes and medical record review in a population-based sample of persons 40–84 years old. BMJ open 2021;11:e042870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.THERNEAU TM, GRAMBSCH PM. The Cox model. In: Modeling Survival Data: Extending the Cox Model. New York, NY: Springer-Verlag; Number of pages. [Google Scholar]
- 32.JACOBY VL, GRADY D, WACTAWSKI-WENDE J, et al. Oophorectomy vs ovarian conservation with hysterectomy: cardiovascular disease, hip fracture, and cancer in the Women’s Health Initiative Observational Study. Arch Intern Med 2011;171:760–8. [DOI] [PubMed] [Google Scholar]
- 33.TORRE LA, TRABERT B, DESANTIS CE, et al. Ovarian cancer statistics, 2018. CA Cancer J Clin 2018;68:284–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.SEGELMAN J, LINDSTROM L, FRISELL J, LU Y. Population-based analysis of colorectal cancer risk after oophorectomy. Br J Surg 2016;103:908–15. [DOI] [PubMed] [Google Scholar]
- 35.CHLEBOWSKI RT, WACTAWSKI-WENDE J, RITENBAUGH C, et al. Estrogen plus progestin and colorectal cancer in postmenopausal women. N Engl J Med 2004;350:991–1004. [DOI] [PubMed] [Google Scholar]
- 36.NEWCOMB PA, STORER BE. Postmenopausal hormone use and risk of large-bowel cancer. J Natl Cancer Inst 1995;87:1067–71. [DOI] [PubMed] [Google Scholar]
- 37.BRAY F, FERLAY J, SOERJOMATARAM I, SIEGEL RL, TORRE LA, JEMAL A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 38.CAIAZZA F, RYAN EJ, DOHERTY G, WINTER DC, SHEAHAN K. Estrogen receptors and their implications in colorectal carcinogenesis. Front Oncol 2015;5:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.KHOSROW-KHAVAR F, YIN H, BARKUN A, BOUGANIM N, AZOULAY L. Aromatase inhibitors and the risk of colorectal cancer in postmenopausal women with breast cancer. Ann Oncol 2018;29:744–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.ELLIOTT AM, HANNAFORD PC. Use of exogenous hormones by women and lung cancer: evidence from the Royal College of General Practitioners’ Oral Contraception Study. Contraception 2006;73:331–5. [DOI] [PubMed] [Google Scholar]
- 41.KABAT GC, MILLER AB, ROHAN TE. Reproductive and hormonal factors and risk of lung cancer in women: a prospective cohort study. Int J Cancer 2007;120:2214–20. [DOI] [PubMed] [Google Scholar]
- 42.KREUZER M, GERKEN M, HEINRICH J, KREIENBROCK L, WICHMANN HE. Hormonal factors and risk of lung cancer among women? Int J Epidemiol 2003;32:263–71. [DOI] [PubMed] [Google Scholar]
- 43.KOUSHIK A, PARENT ME, SIEMIATYCKI J. Characteristics of menstruation and pregnancy and the risk of lung cancer in women. Int J Cancer 2009;125:2428–33. [DOI] [PubMed] [Google Scholar]
- 44.ROSSOUW JE, ANDERSON GL, PRENTICE RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA 2002;288:321–33. [DOI] [PubMed] [Google Scholar]
- 45.CHLEBOWSKI RT, ROHAN TE, MANSON JE, et al. Breast Cancer After Use of Estrogen Plus Progestin and Estrogen Alone: Analyses of Data From 2 Women’s Health Initiative Randomized Clinical Trials. JAMA Oncol 2015;1:296–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.EISEN A, LUBINSKI J, GRONWALD J, et al. Hormone therapy and the risk of breast cancer in BRCA1 mutation carriers. J Natl Cancer Inst 2008;100:1361–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.KOTSOPOULOS J, HUZARSKI T, GRONWALD J, et al. Hormone replacement therapy after menopause and risk of breast cancer in BRCA1 mutation carriers: a case-control study. Breast Cancer Res Treat 2016;155:365–73. [DOI] [PubMed] [Google Scholar]
- 48.KOTSOPOULOS J, GRONWALD J, KARLAN BY, et al. Hormone Replacement Therapy After Oophorectomy and Breast Cancer Risk Among BRCA1 Mutation Carriers. JAMA Oncol 2018;4:1059–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.COLLABORATIVE GROUP ON HORMONAL FACTORS IN BREAST CANCER. Breast cancer and hormone replacement therapy: collaborative reanalysis of data from 51 epidemiological studies of 52,705 women with breast cancer and 108,411 women without breast cancer. Lancet 1997;350:1047–59 [PubMed] [Google Scholar]
- 50.COLLABORATIVE GROUP ON HORMONAL FACTORS IN BREAST CANCER. Type and timing of menopausal hormone therapy and breast cancer risk: individual participant meta-analysis of the worldwide epidemiological evidence. Lancet 2019;394:1159–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


