Abstract
Objective:
Elucidate how physicians formulate a neurological prognosis after cardiac arrest and compare differences between experts and general providers.
Methods:
We performed semi-structured interviews with experts in post-arrest care and general physicians. We created an initial model and interview guide based on professional society guidelines. Two authors independently coded interviews based on this initial model, then identified new topics not included in it. To describe individual physicians’ cognitive approach to prognostication, we created a graphical representation. We summarized these individual “mental models” into a single overall model, as well as two models stratified by expertise.
Results:
We performed 36 interviews (17 experts and 19 generalists), most of whom practice in Europe (23) or North America (12). Participants described their approach to prognosis formulation as complex and iterative, with sequential and repeated data acquisition, interpretation, and prognosis formulation. Eventually, this cycle results in a final prognosis and treatment recommendation. Commonly mentioned factors were diagnostic test performance, time from arrest, patient characteristics. Participants also discussed factors rarely discussed in prognostication research including physician and hospital characteristics. We found no substantial differences between experts and general physicians.
Conclusion:
Physicians’ cognitive approach to neurologic prognostication is complex and influenced by many factors, including some rarely considered in current research. Understanding these processes better could inform interventions designed to aid physicians in prognostication.
Keywords: cardiac arrest, prognostication, decision-making, hypoxic-ischemic injury
Introduction
Neurological prognostication can dramatically influence outcomes of severe acute brain injury, including hypoxic-ischemic brain injury after cardiac arrest. Most patients are comatose after resuscitation from cardiac arrest.1 Differentiating those who can eventually awaken from those who have irrecoverable brain injury is challenging. Physicians must formulate a neurological prognosis before engaging families in shared decision-making about withdrawal, continuation, or escalation of life-sustaining therapies.2 In high-income Western nations, nearly half of comatose patients hospitalized after cardiac arrest will die after life-sustaining therapies are withdrawn for perceived poor neurological prognosis.3,4 Despite the stakes, physicians exhibit significant variability in both practice and performance, often making inaccurate predictions and deviating from consensus guidelines.5,6
These problems persist despite decades of research and efforts of professional societies including the European Resuscitation Council (ERC), European Society of Intensive Care Medicine (ESICM) and American Heart Association (AHA) to develop and promote evidence-based practice guidelines. We offer an approach to enhance these efforts by elucidating how physicians gather, integrate, and interpret information related to prognostication.7,8 Drawing from decision science, we seek to understand physicians’ cognitive processes, in terms that can guide interventions that complement their natural ways of thinking, building on their strengths while addressing weaknesses. We used open-ended “mental models” interviews, structured around the medical and institutional realities of clinical practice, to elicit physicians’ perception of their prognostication approach and its constraints.
Methods
Overview
We performed a qualitative study to characterize physicians’ perceptions of neurological prognosis formulation after cardiac arrest. We used semi-structured interviews and recruited both experts in post-arrest care and general physicians in high-income Western nations. We designed our recruitment strategy to allow triangulation of data interpretation.9 The University of Pittsburgh Institutional Review Board approved the study.
Sampling and Recruitment
We performed purposive sampling of both expert and general physicians whose clinical duties include prognostication after cardiac arrest. We defined “experts” as meeting all of the following criteria:1) cared for more than 150 post-arrest patients over their career; 2) published >3 peer-reviewed publications related to post-arrest care or prognostication within the past three years; and, 3) played a leadership role in a multicenter trial enrolling patients after resuscitation from cardiac arrest. We identified experts through international reputation and review of PubMed-indexed literature published since 2010. General physicians did not meet any of these criteria. We identified general physicians by asking each expert to recommend two clinicians in their region. Our goal was to interview at least 16 in each group in order to achieve theoretical saturation, which is typically reached with 12 to 16 interviews in relatively homogeneous populations.10,11 We collected information on the characteristics of participants and the hospital where they practice. All participants provided verbal consent before the interviews.
Interview Design
We created an initial model circumscribing potentially relevant factors based on ERC/ESICM and AHA guidelines and consensus statements.12,13 Based on this initial model, we developed an interview guide with open-ended questions. Questions began with very general ones about how physicians formulated prognoses, then move to more specific questions about the factors in the model, while being open to issues missing from it. We conducted all interviews in English.
We conducted pilot interviews with five University of Pittsburgh physicians, drawn from a consultation service that evaluates post-cardiac arrest patients, including advising on neurological prognosis.14–16 Collectively, their team cares for over 350 post-arrest patients annually. Based on these interviews, we altered our initial model (Supplemental Figure 1) and interview guide. We did not include data from these pilot interviews in our final analysis. Informed by these pilot interviews, the revised model treated prognosis formulation as a dynamic, iterative one, whereas the initial model depicted a static process wherein prognoses reflected the summary influence of varied factors.
We revised our interview guide based on this model, following the same format as the pretests, going from general to specific open-ended questions (Supplemental Table 1). We asked participants to clarify responses and probed for additional details. One investigator (AS) conducted all interviews by phone, in-person, or using an online video platform, depending on participant availability. Interviews lasted between 30 and 60 minutes and were recorded. A professional service transcribed all interviews into text files for analysis. We deidentified each interview prior to analysis.
Analysis
Recognizing that these interviews, like the pilot ones, might reveal new issues, we used a combination of deductive and inductive coding17,18 The deductive component used a pre-defined codebook, with codes based on the revised model. The inductive component supplemented the codebook and model with novel factors emerging in the interviews. We used content analysis, breaking the interview text into units, then coding each unit into topics.
Two authors (AS and EG) coded the interviews using NVivo version 12 (QRS International, Doncaster, Australia). AS and EG coded each interview individually, then met to review their codes, discuss disagreements, and reach consensus. Throughout the coding process, two other authors (BF and JE) reviewed the analysis for internal consistency and external (medical) validity.19 JE is a physician-scientist and expert on post-arrest prognostication and BF is an expert on decision science. We applied any changes in the coding to all previously coded interviews. We double-coded interviews until we reached inter-rater reliability of Cohen’s κ > 0.8, separately for identification of nodes and edges in new interviews. We achieved that criterion after double-coding 70% of interviews, after which a single author coded each interview. We reached theoretical saturation (i.e., no new topics) after coding about 75% of the interviews.
Creating the Mental Model
The study of mental models has a long tradition in psychology for capturing complex cognitive processes, addressing topics as diverse as syllogistic reasoning, intuitive geography, and understanding of physiology.20,21 The present version was developed to study open systems, where it is unclear which elements individuals can or do consider. It involves open-ended interviews, structured around a qualitative formal model (in this case, Figure 1) meant to capture both the elements (nodes) and integrative processes (edges) in individuals’ thinking.22,23 Confirmatory methods include structured surveys, assessing, the population prevalence of processes revealed in the interviews; experimental tests, varying model elements; problem-solving tasks, assessing consistency of inferences with model elements; and interventions, based on needs identified in descriptive research.24–28
Figure 1:
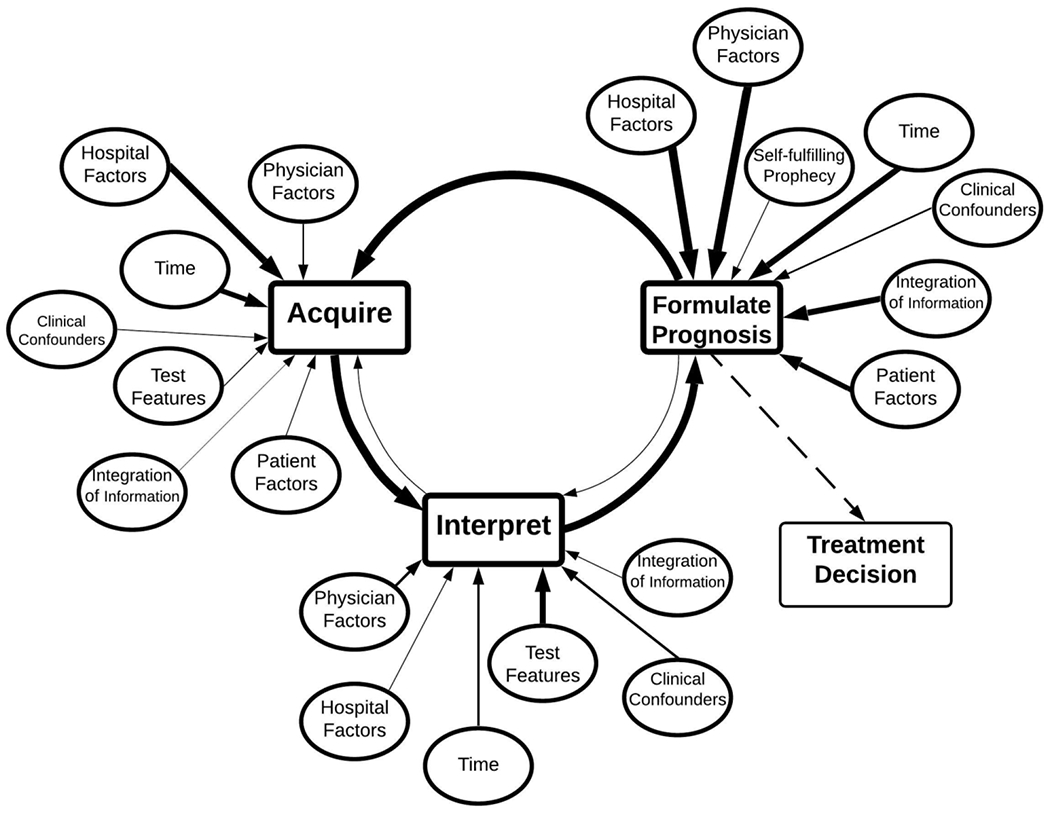
Complex mental model. It contains both content factor or nodes and links or edges from all interviews. It is organized around a three-step diagnostic cycle, eventually exiting to a treatment decision. Weight of arrows corresponds to the cumulative frequency that the edge/connection was mentioned. Dotted lines represents a part of the model but was not the main focus of coding.
We used content analysis to identify important concepts and describe relationships between these concepts as described by interview participants. Consistent with past mental models research, we use the term “node” to describe discrete, well-circumscribed concepts. To qualify as a node, these concept had easily understandable (to the coders) real-world correlates relevant to neurologic prognostication after cardiac arrest and were identified by multiple participants. An example of a node might be “interpretation of prognostic test results.” Coders determined when to aggregate more granular concepts into a single node based on the frequency and reported importance of the concept across interviews. For example, if one respondent described the effect on physician specialty on prognostication, this concept might be classified as “physician factors.” If many respondents indicated their belief that physician specialty is a critical factor, it would be classified as its own node. When participants described a thought process that connected two nodes in their mind, we used the term “edge” to define this connection and the directionality of the relationship. For example, “the lack of resources affects my ability to acquire specific tests,” would be coded as an edge from hospital factors to acquiring data. As new nodes or new edges appeared in interviews, we defined these nodes and edges in a codebook. We defined each possible node and edge in a codebook.
To create an aggregate mental model depicting general trends in the interviews, we counted how often each node and edge was mentioned and by how many physicians. Then, we revised our final complex model based on these empirical observations (Figure 1). We also developed a simplified model including only nodes and edges mentioned by least 70% of physicians (Figure 2). A priori, we planned to compare the models of experts and non-expert providers. During the coding process, we identified themes that differed between North America and Europe, so we made a post-hoc decision to identify differences and similarities.29
Figure 2:
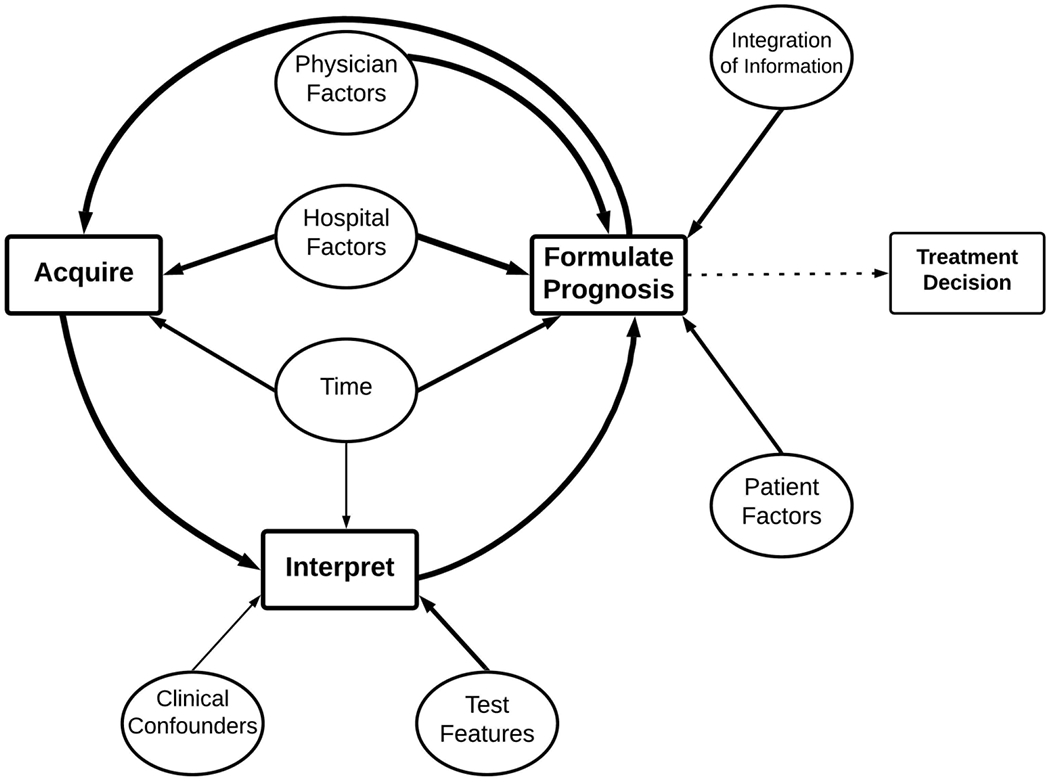
Simplified mental model. Only the most commonly mentioned nodes and edges are included in the model from all interviews. Weight of arrows corresponds to the cumulative frequency that the edge/connection was mentioned. Dotted lines represents a part of the model but was not the main focus of coding.
Results
Demographics
We conducted 36 interviews, 17 (47%) with experts and 19 (53%) with general physicians. Participants had mean 18 (SD 8) years of clinical experience. Twenty-three (64%) practice in Europe, 12 (33%) in North America, and 1 (3%) in Australia (Table 2).
Table 2:
Demographic Information of Participants
| Characteristic | N (%) (36 Total) |
|---|---|
| Participant Type | |
| Expert | 17 (47) |
| General Provider | 19 (53) |
| Location of Practice | |
| Europe | 23 (64) |
| United Kingdom | 6 (17) |
| Sweden | 4 (11) |
| Switzerland | 4 (11) |
| Belgium | 3 (8) |
| Italy | 3 (8) |
| Norway | 2 (6) |
| Finland | 1 (3) |
| North America (United States) | 12 (33) |
| Australia | 1 (3) |
| Clinical specialty* | |
| Critical Care | 24 (67) |
| Neurology | 12 (33) |
| Emergency Medicine | 1 (3) |
| Cardiology | 1 (3) |
| Female Gender | 9 (25) |
| Years in Practice | 18±8 |
| Average Post Arrest Patients Cared for in 2019 | 25 (18 – 50) |
| Hospital size, beds | |
| 100-250 | 2 (6) |
| 250-500 | 8 (22) |
| >500 | 24 (67) |
| ICU size, beds | |
| <20 | 10 (27) |
| 20-50 | 15 (42) |
| 50-100 | 3 (8) |
| >100 | 6 (17) |
| Teaching Hospital | 35 (97) |
| ICU Specialty Type** | |
| General/mixed ICU | 25 (69) |
| Medical | 9 (25) |
| Surgical | 11 (31) |
| Neurologic | 11 (31) |
| Cardiac | 10 (28) |
| Other | 3 (8) |
| Prognostication Protocols available at Hospital | 26 (72) |
Data are presented a raw number with corresponding percentage.
N sums to more than the number of clinical specialty because physician may have more than one specialty (i.e. neurology and critical care)
N sums to more than the number of hospitals since hospitals may have multiple ICUs, percentages are expressed for the total number of ICUs
Physicians’ Mental Model of the Prognostication Process
Physicians’ approach to prognostication after cardiac arrest is complex (Figure 1, Table 3 and Table 4). As one participant noted: “There are so many factors that affect prognostication … that we don’t put in the multimodal strict systems we use.” (Expert, Europe) Figure 2 shows a simplified version of the process, omitting less frequent connections in Figure 1. Both figures include a summary of all interviews.
Table 3.
Representative quotes for core cycle components
| Edges | Representative quotes | Expert N=17 (%) | General N=19 (%) |
|---|---|---|---|
| Acquire to Interpret | “We are defining (EEG) after ACNS (American Clinical Neurophysiology Society) of highly malignant patterns and benign patterns. So that’s how we scored EEG. We scored the evoke potentials bilaterally absent versus present, we don’t use the amplitude. We score the clinical examination using the FOUR score after the Mayo Clinic. We don’t have a clear threshold, but of course if we repeat the examination, we look at the brainstem reflexes and the evolution of the FOUR score. We have a safe threshold for the NSE in our lab at about 70 microgram per milliliter.” (Expert, Europe) “[I aim] to get an idea if there are particularly ominous signs on the brain MRI like damage to the bilateral thalami or extensive laminar necrosis or other findings that are consistent with severe anoxic brain injury” (General, North America) |
17 (100) | 19 (100) |
| Interpret to Formulate Prognosis | “I am looking for cerebral edema that is below where we have seen survivability. For me, that’s if the gray-white landmarks are gone or if the cortex is really tight with sulcal effacement or the cisterns are obliterated. Then, I feel …this person has a very poor prognosis…”(Expert, North America) “If the patient has recovered, for example, continuous background activity, which may be one of the best ways of assessing a potential good outcome. On the contrary where the patient has developed myoclonus seizures or even myoclonus status, which is a very bad sign in combination with a certain cEEG pattern.” (Expert, Europe) “If EEG is burst-identical burst, or NPI is 2 and we don’t have motor response … we are really pessimistic, and we just don’t feel that patient would be able to be awake.” (General, Europe) “Poor prognosis, which I usually think of as like absent corneal responses, absent pupillary responses.” (General, North America) |
17 (100) | 18 (95) |
| Formulate Prognosis to Acquire | “I described that I am constantly revising my estimated chance of recovery for the patient in my own head. I would constantly seek greater certainty, so if I feel that the test would increase my certainty, I would get it”(Expert, North America) “So in other words, if up to 48 to 72 hours, we are still in the gray zone, the patients don’t awaken and on the other side doesn’t have very poor prognosticators. We try to get to more evidence”(Expert, Europe) “So when in doubt, we prolong the observation period. If you don’t have strong predictors suggesting that prognosis will be very poor, or if you don’t have a series of less robust predictors, all consistently indicating and signaling that the prognosis will be poor, generally we wait” (Expert, Europe) |
17 (100) | 18 (95) |
Table 4.
Main factors that affect core cycle elements
| Edges (factors) | Representative quotes | Expert N=17 (%) | General N=19 (%) |
|---|---|---|---|
| Acquire | |||
| Hospital Factors | “Inevitably there’s quite wide variation in access to investigations, and we’re not in a general hospital by UK standards. I think our inability to have access to continuous EEG monitoring is a major issue, and I think that would change the way we go about things quite significantly”(Expert, Europe) “We have tried to do evoked potentials, but I don’t think that the technicians locally have the expertise.”(General, North America) |
17 (100) | 19 (100) |
| Time | “Usually, the first day after the event…we get an EEG. What we do is get a blood exam for neuron specific enolase. So this is the first day. Then we have a follow-up at 48 hours, or 72 hours, with another EEG, a clinical evaluation. We repeat second blood examination for neuron specific enolase and then somatosensory evoked potentials. This is done from 48 to 72 hours.” (General, Europe) | 16 (94) | 14 (74) |
| Physician Factors | “It [SSEPs] would probably be in about 50% of the patients, probably half would get it. It’s probably clinician dependent. Practice varies.” (Expert, North America) “I think there is a range of practice. Some people are more aggressive in their investigations. Some people are less aggressive in their investigations.” (General, Europe) “I’ve seen a wide variety of what I think of as worthless tests my partners are looking to guide management, but I don’t find them useful at all. Maybe I’m an under utilizer of those tests”(General, North America) “No. No, I’ve never ordered a serum enolase or whatever that thing is. I mean I don’t even know how to order that. I don’t even know what the timing on that is. I find brain MRIs to be worthless and disruptive. And, unfortunately, what happens with these tests, again this is one person’s experience”(General, North America) “So yeah, I think that a lot of doctors these days, I don’t want to be like, the kids these days, but yeah they look for technology. They look to technologic diagnostic solutions and biomarkers. And maybe that’s a function of training … there’s mission creep or imaging creep into all of neurology”(General, North America) |
9 (53) | 12 (63) |
| Interpret | |||
| Test Features | “When somebody presents a case to me and says: “The patient didn’t have a corneal, so I think they’re going to do poorly.” my next question is always: “How did you test the corneals?” And they tell me “a saline squirt.” Then, I put on my white coat, get on my Q-tip and say let’s go to the bedside and make sure. Technique is everything.”(Expert, North America) “So, we have an MRI in a gray zone patient. We have an MRI showing … diffusion restriction in the hippocampi, basal ganglia, and somehow widespread over parietal-occipital cortical area. That’s not a good outcome in a functional viewpoint, and we try to integrate that in that way. But it’s more a qualitative approach regarding imaging as opposed to the other tools.” (Expert, Europe) |
15 (88) | 18 (95) |
| Physician Factors | “You always get the raw data. It’s always a little bit subjective. I would have to say that you bring your own personality to interpreting it…”(Expert, North America) “The raw interpretation of an EEG by a clinical neurophysiologist I’ve found to be very variable…In our hospital, we have somebody who is internationally renowned, and even he openly admits that he will present the same EEG to himself and the different colleague. The variance is in the way that it’s reported, and the language that is used, can really bias you.” (General, Europe) “Yeah. It’s of course based on the experience and knowledge you have, and what is published. You never do it alone” (Expert, Europe) “Years of experience, attention to detail, coming in with an open mind and absolute candor with the family”(Expert, Europe) |
11 (65) | 11 (58) |
| Clinical Confounders | “Medications, obviously, as I alluded to. Multi organ dysfunction, or end organ dysfunction, so someone whose liver is not working well, their kidneys are not working well. We evaluate the diagnostic tests like EEG findings and clinical exam in the context of those things.”(Expert, North America) “In the first 24 hours, we don’t put a lot of stock in EEGs since they’re usually cold and usually on a decent amount of sedation”(Expert, North America) |
13 (76) | 14 (74) |
| Time and Trajectory | “So the EEG and seeing its progression over time is as good as watching the clinical exam improve over time. I feel that is a necessary step for recovery and would watch it for at least the first 24-36 hours to get a feeling for the trajectory. Seeing it recover more quickly than that is even more encouraging. But I would not give up hope unless I watched for 36-48 hours and had seen no improvement.”(Expert, North America) “I do find that in between days 3 to 5 you can have quite a significant change in the patient, either good or bad.” (General, Europe) “Any day that goes by with no improvement, I adjust my assessment accordingly, it gets worse with any day that goes by without any improvement, but I don’t have a hard cutoff.” (General, North America) |
14 (82) | 15 (79) |
| Formulate Prognosis | |||
| Hospital Factors | “We have a very strict protocol. If you don’t follow the protocol, I would be upset and I would tell the person that would have consequences…You have to have a very good explanation if you don’t follow a protocol that we have all agreed on”(Expert, Europe) “First of all, I think one issue I would like to stress, going back to the prognostication is that it’s a multidisciplinary approach. We’ll discuss all these cases together, intensivists,neurologists and colleagues from the neuro rehabilitation department.”(Expert, Europe) “One thing that shouldn’t affect, but definitely now and then does, is how full is your ICU? How many beds do you have? What else is going on? Unfortunately, that might influence at times.”(Expert, Europe) |
17 (100) | 19 (100) |
| Physician Factors | “Probably the experience, probably if you talk to all of the physicians, they are more pessimistic about this patient because they are used to how they treat with the outcome of this patient in the last, in the previous years maybe before 2000s. I don’t know. If you talk with a younger physician, they are more optimistic about the outcome of this patient.” (General, Europe) “Several of my colleagues will openly admit that they are much more optimistic, perhaps not the correct way perhaps, but they have a more positive outlook on most patients compared to others… I don’t know what their own personal background values are, but they openly admit that personal values are that, when they are in that situation, they might want more things to be done and tested than otherwise.” (General, Europe) “Well, there is always the bias of the last patient seen, right? And then you swear by the last patient seen, so they’ll keep doing that. So if the last patient that they saw, they predicted a poor outcome, they withdrew, and the patient died. That’s the vicious cycle.” (Expert, North America) “My approach is cautiousness.” (Expert, Europe) |
17 (100) | 19 (100) |
| Integration of Information | “You have to try a multimodal approach, combining several different aspects of the neurologic prognostication, I guess. Using a combination of the radiology, clinical, neurologic examination, lab values, the neurophysiology”(General, Europe) “I try not to interpret any of the prognostic data in a vacuum, so I try to interpret it within the whole context of how the patient is doing clinically. So, for instance, if someone is extremely ill, has may comorbid diagnoses at baseline, but has a reassuring head CT and a reassuring SSEP, but are doing poorly, have refractory ARDS, I’m not so optimistic about their prognosis because they’re ill from a separate, another organ standpoint”(General, North America) |
17 (100) | 16 (84) |
| Time | “We would personally never try to prognosticate before the 72 hours after cardiac arrest” (Expert, Europe) “I think that you’re always formulating their prognosis, right? Like I try and formulate the prognosis right away. Like I wouldn’t put a definitive prognosis right away, but sometimes you have a definitive prognosis right away.”(General, North America) |
17 (100) | 17 (89) |
| Patient Factors | “Well, we touched upon this already and patient wishes and families’ expression of patients’ wishes plays a very big part on how I predict the future, manage a patient.” (General, Europe) “I think younger, healthier bodies are more resilient and somewhat more likely to recover”(General, North America) |
17 (100) | 19 (100) |
For the Edge column: the sub-header node flows into the header node. For instance, under acquire there is hospital factors, so in the model, there is an arrow going from hospital factors to acquire.
An iterative process
The model centers around a cyclical core (Figure 2, Table 1, Table 3), as captured in, “I often don’t form only a single prognosis. I’m prognosticating in a continuous manner from the point that the patient comes into intensive care.” (Expert, Europe) The core cycle includes sequential and repeated data acquisition, interpretation, and prognosis formulation. When coding, we treated references to outcome assessments and perceived uncertainty of assessments as “prognosis formulation” (Table 1). All participants said that, after initial prognosis formulation, they generally acquire more data with the hope of reducing their uncertainty. This data acquisition phase includes continued observation for signs of clinical improvement, additional test acquisition, and consultations with specialists or experts. One physician explained, “When in doubt, we prolong the observation period. If you don’t have strong predictors suggesting that prognosis will be very poor, or if you don’t have a series of less robust predictors all consistently indicating and signaling that the prognosis will be poor, generally we wait.” (Expert, Europe) When physicians become more confident in their prediction, the cycle stops with a final prognosis, leading to a recommended treatment decision. Participants described looking for concordance of multimodality testing and brain-body interactions. However, they also acknowledged times when the cycle stops despite their residual uncertainty, as when a patient’s preference for avoiding prolonged supportive care makes the physician’s treatment recommendation irrelevant.
Table 1.
Definitions of the core prognostic cycle
| Content | Definition |
|---|---|
| Acquire | Collect information on a patient with potential prognostic value. Either, can be objective (lab, physical exam, EEG, imaging) or subjective (patient’s previous quality of life) |
| Interpret | Make a tentative inference about the meaning of the information |
| Prognosis Formulation | Synthesize the data to assess the probability of patient outcomes (awaken, good functional outcome) and confidence in that assessment |
| Treatment Decision | Create an action plan for next steps (withdrawal of life-sustaining therapy vs tracheostomy/PEG) |
Factors affecting the core cycle
Many factors affect each step of the core prognostication cycle (Figure 2, Table 4).
Physician factors
Most participants identified physician factors affecting each step, often phrased in terms such as, “We are human, we are affected by many things. We try to be objective and try to be strong, but of course there are extraneous factors that affect our prediction.” (General, North America) Many described the influence of physicians’ personalities: “I think several of my colleagues will openly admit that they are much more optimistic, perhaps not the correct way perhaps, but they have a more positive outlook on most patients compared to others.” (General, Europe) Many stated: “I always preach patience.” (Expert, North America)
Participants viewed background specialities as influential. “A neurologist always insists on an MRI. The question is whether that is a very good prognostic tool in the hands of the people that look at MRI at my hospital, who are really not neuroradiologists in the same respect [as] in a university hospital.” (General, Europe) Participants typically mentioned how physicians’ experience and knowledge affected test interpretation. “It’s of course based on the experience and knowledge you have and what is published, and you never do it alone.” (Expert, Europe)
Hospital Factors
Participants mentioned many hospital factors as important, with the most common being resource availability, protocols, and participation in trials/research. “We’re a community hospital. We don’t have any fancy stuff. We don’t measure markers or do perfusion studies or anything like that … We can’t even do an MRI on an intubated patient in our hospital.” (General, North America) Other commonly mentioned environmental factors included societal views on withdrawal of life sustaining therapy, bed capacity, and use of multidisciplinary care teams.
Patient and Medical Characteristics
Many physicians described their interpretation of test information as contingent on such clinical confounders as metabolic disarray, sedatives, seizures, and hypothermia. They commonly cited several patient factors as influencing their prognosis formulation, namely, age, frailty, comorbidities, and wishes. “Of course, you use the physical status of the patient, so the frailty of the patient, the age, and these factors that are generally included in the general evaluation of the patient. So, independent life before cardiac arrest and so on.” (Expert, Europe)
Group Differences
When analyzing interviews at the level of the simplified model, we observed no substantive differences between cardiac arrest experts and general physicians, or between North American and European physicians. At the level of the complex model, Europeans were more likely than North Americans to mention reliance on formal protocols. “We’ve got a protocol that’s based on the European Resuscitation guidelines. We follow that very strictly. What then happens, without being too anecdotal, we reach a point where either the outcome is relatively clear on the basis of the algorithm.” (General, Europe)
Discussion
In semi-structured open-ended interviews, physicians describe a complex, iterative process used to formulate a neurological prognosis after severe acute brain injury. They discuss evaluating and refining their initial hypothesis, until they gain sufficient certainty to make a treatment recommendation. Many of the factors that they cited figure prominently in research and guidelines: diagnostic test performance, time since arrest, and patient factors.12,13 Other factors, though, have received less attention, especially those related to characteristics of the physician (e.g., inherent optimism) and their work environment (e.g., protocols, resources). We found little difference between expert and general physicians. We found a difference between physicians in North America and Europe, with the latter describing much greater reliance on institutional protocols.30,31
Our goal is to inform interventions that improve prognostic performance and patient outcomes, and which are sensitive to both physicians and their work environment.8,32 Physicians do not follow evidence based guidelines for post-arrest prognostication, but the reasons for this are uncertain. Stakeholder engagement and identification of barriers are necessary prerequisites to inform effective interventions that promote delivery of guideline-concordant care. By engaging physician stakeholders, we sought to identify novel barriers that can be targeted by future implementation science research. Respondents reported both environmental and physician factors are important. Professional society guidelines and expert consensus statements do not currently address these factors. Insofar as a availability of resources (or lack thereof) drives prognosis formulation, urging providers to acquire guideline-recommended tests is likely to be ineffective. Conversely, regionalization of post-arrest care at specialty centers at which multiple modalities of neurodiagnostics are more easily acquired may be quite effective. Participants also acknowledged that physician factors (experience, clinical background) can result in internal biases that alters predicted outcomes of cardiac arrest patients. The use of multidisciplinary teams may mitigate these biases since physicians would be from a variety of backgrounds. Overall, the cognitive focus of our research complements studies of how physician, hospital, and patient characteristics affect medical decision-making,33,34 especially with respect to cognitive biases that can produce poorly-calibrated decisions and diagnostic errors.35,36
We believe this to be the first such study exploring factors that affect neurologic prognostication after cardiac arrest. We sought to develop a rich picture of the strategies used by a diverse set of physicians. Our next steps are confirmatory research using methods including structured surveys that are well-suited to larger samples needed to assess validity and generalizability of our results. The major focus will be on both hospital and physician factors and how they influence physicians’ predictions of post-arrest outcomes.
Our study has several potential limitations. Even though our interviews asked open-ended questions, we did probe aspects of our initial conceptual model, which could have constrained participants’ accounts. We asked participants to reflect on their own practices, in order to elicit specific accounts of prognostication processes, rather than general summaries or theories.20 Although their reports seemed candid and often self-critical, they may have reflected some self-presentation bias. Our coding procedure divided the interviews into conceptual units, corresponding to elements in the conceptual model that structured the interviews. That procedure allows reliable coding, given the clarity of the conceptual model. However, it limits the ability to detect higher-order patterns. That limitation can be overcome with coding themes instead of only broader concepts, as we plan to do in the next stage of the research. Our sample size was chosen with the goal of theoretical saturation: hearing at least once, any belief held with any frequency in the population. It limited our ability to correlate physician characteristics and prognostic strategy. We also sampled only one or two physicians per institution, limiting our ability to detect institution differences, in the interest of observing a diversity of views. Sampling two physicians per institute could be one reason that we did not find substantial differences between experts and general physicians. Such qualitative research allows for hearing the richness of individuals thinking and discovering new issues. However, it requires confirmatory research, as described above. Those studies would prespecify (and preregister) their analysis, rather than require post hoc exploratory analyses, as completed here. Our sample is also limited by our recruitment procedure, relying on personal connections. Although our experts may roughly represent their peers in academic medical centers, our generalists were less diverse than those in community hospitals in general (e.g., with respect to race, ethnicity, and gender).37,38 Only 25% of our participants were female. The relative homogeneity of the samples may have reduced that of the mental models that we observed. Another limitation to our study is the definition that we used to define expert. This could have led to not finding major differences between expert and general physicians. Since our definition of expert focused on their research efforts, it could be that a general physician has extensive clinical experience and may be involved in local internal protocol creations. There were few physicians interviewed from smaller/community hospitals. One reason for this is that Europe lacks a clear definition of “community” hospital compared to North America. We may have found differences in cognitive processes of prognostication if more community physicians were interviewed.
Many patients globally undergo neurologic prognostication after severe acute brain injury. When asked to describe their approach to this often-challenging task, physicians described a complex, dynamic process, often influenced by factors rarely considered in current prognostication research and guidelines. We hope that a more encompassing approach, building on these observations, can inform interventions that improve physician performance.
Supplementary Material
Conflict of Interest:
Dr. Elmer’s research time is supported by the NIH through grant 5K23NS097629. Dr. White is supported by NIH grant K24 HL148314.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Geocadin RG, Callaway CW, Fink EL, et al. Standards for studies of neurological prognostication in comatose survivors of cardiac arrest: A scientific statement from the american heart association. Circulation 2019;140(9):e517–e542. [DOI] [PubMed] [Google Scholar]
- 2.Scheunemann LP, Ernecoff NC, Buddadhumaruk P, et al. Clinician-Family Communication About Patients’ Values and Preferences in Intensive Care Units. JAMA Intern. Med. 2019;179(5):676–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Laver S, Farrow C, Turner D, Nolan J. Mode of death after admission to an intensive care unit following cardiac arrest. Intensive Care Med. 2004;30(11):2126–2128. [DOI] [PubMed] [Google Scholar]
- 4.Elmer J, Torres C, Aufderheide TP, et al. Association of early withdrawal of life-sustaining therapy for perceived neurological prognosis with mortality after cardiac arrest. Resuscitation 2016;102:127–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steinberg A, Callaway C, Dezfulian C, Elmer J. Are providers overconfident in predicting outcome after cardiac arrest? Resuscitation 2020; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maciel CB, Barden MM, Youn TS, et al. Neuroprognostication practices in postcardiac arrest patients: an international survey of critical care providers. Crit. Care Med. 2020;48(2):e107–e114. [DOI] [PubMed] [Google Scholar]
- 7.Weiss CH. Why do we fail to deliver evidence-based practice in critical care medicine? Curr. Opin. Crit. Care 2017;23(5):400–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rothman AJ, Sheeran P. The operating conditions framework: Integrating mechanisms and moderators in health behavior interventions. Health Psychol. 2020; [DOI] [PubMed] [Google Scholar]
- 9.Carter N, Bryant-Lukosius D, DiCenso A, et al. The use of triangulation in qualitative research. Oncol Nurs Forum 2014;41(5):545–547. [DOI] [PubMed] [Google Scholar]
- 10.Crabtree BF, Miller WL, editors. Doing Qualitative Research. 2nd ed. SAGE Publications; 1999. [Google Scholar]
- 11.Namey E, Guest G, McKenna K, Chen M. Evaluating bang for the buck: A cost-effectiveness comparison between individual interviews and focus groups based on thematic saturation levels. American Journal of Evaluation 2016;37(3):425–440. [Google Scholar]
- 12.Callaway CW, Donnino MW, Fink EL, et al. Part 8: Post-Cardiac Arrest Care: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2015;132(18 Suppl 2):S465–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nolan JP, Soar J, Cariou A, et al. European Resuscitation Council and European Society of Intensive Care Medicine 2015 guidelines for post-resuscitation care. Intensive Care Med. 2015;41(12):2039–2056. [DOI] [PubMed] [Google Scholar]
- 14.Elmer J, Rittenberger JC, Coppler PJ, et al. Long-term survival benefit from treatment at a specialty center after cardiac arrest. Resuscitation 2016;108:48–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rittenberger JC, Guyette FX, Tisherman SA, et al. Outcomes of a hospital-wide plan to improve care of comatose survivors of cardiac arrest. Resuscitation 2008;79(2): 198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elmer J, Callaway CW, Chang C-CH, et al. Long-Term Outcomes of Out-of-Hospital Cardiac Arrest Care at Regionalized Centers. Ann. Emerg. Med. 2019;73(1):29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giacomini MK, Cook DJ. Users’ guides to the medical literature: XXIII. Qualitative research in health care A. Are the results of the study valid? Evidence-Based Medicine Working Group. JAMA 2000;284(3):357–362. [DOI] [PubMed] [Google Scholar]
- 18.Elo S, Kyngäs H. The qualitative content analysis process. J. Adv. Nurs. 2008;62(1):107–115. [DOI] [PubMed] [Google Scholar]
- 19.Patton MQ. Enhancing the quality and credibility of qualitative analysis. Health Serv. Res. 1999;34(5 Pt 2):1189–1208. [PMC free article] [PubMed] [Google Scholar]
- 20.Ericsson A, Simon HA. Protocol Analysis. Cambridge, MA: MIT Press; 1993. [Google Scholar]
- 21.Johnson-Laird P How We Reason. Oxford University Press; 2008. [Google Scholar]
- 22.Morgan MG, Fischhoff B, Bostrom A, Atman CJ. Risk Communication: A Mental Models Approach. New York: Cambridge University Press; 2002. [Google Scholar]
- 23.Bruine de Bruin W, Bostrom A. Assessing what to address in science communication. Proc. Natl. Acad. Sci. USA 2013;110 Suppl 3:14062–14068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Bruin WB, Güvenç U, Fischhoff B, et al. Communicating about xenotransplantation: models and scenarios. Risk Anal 2009;29(8):1105–1115. [DOI] [PubMed] [Google Scholar]
- 25.Downs JS, Murray PJ, Bruine de Bruin W, et al. Interactive video behavioral intervention to reduce adolescent females’ STD risk: a randomized controlled trial. Soc. Sci. Med. 2004;59(8):1561–1572. [DOI] [PubMed] [Google Scholar]
- 26.Haward MF, Janvier A, Lorenz JM, Fischhoff B. Counseling parents at risk of delivery of an extremely premature infant: Differing strategies. AJOB Empir. Bioeth. 2017;8(4):243–252. [DOI] [PubMed] [Google Scholar]
- 27.Scherr CL, Ross Arguedas AA, Getachew-Smith H, et al. A Modern Dilemma: How Experts Grapple with Ambiguous Genetic Test Results. Med Decis Making 2020;40(5):655–668. [DOI] [PubMed] [Google Scholar]
- 28.Wood MD, Thorne S, Kovacs D, et al. Mental Modeling Approach. New York, NY: Springer New York; 2017. [Google Scholar]
- 29.Glaser BG. The Constant Comparative Method of Qualitative Analysis. Soc Probl 1965;12(4):436–445. [Google Scholar]
- 30.Ridic G, Gleason S, Ridic O. Comparisons of health care systems in the United States, Germany and Canada. Mater. Sociomed. 2012;24(2):112–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Papanicolas I, Woskie LR, Jha AK. Health Care Spending in the United States and Other High-Income Countries. JAMA 2018;319(10):1024–1039. [DOI] [PubMed] [Google Scholar]
- 32.Baron RM, Kenny DA. The moderator–mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. J. Pers. Soc. Psychol. 1986;51(6):1173–1182. [DOI] [PubMed] [Google Scholar]
- 33.Croskerry P Adaptive expertise in medical decision making. Med. Teach. 2018;40(8):803–808. [DOI] [PubMed] [Google Scholar]
- 34.Kitson A, Harvey G, McCormack B. Enabling the implementation of evidence based practice: a conceptual framework. Qual. Health Care 1998;7(3):149–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Graber ML, Franklin N, Gordon R. Diagnostic error in internal medicine. Arch. Intern. Med. 2005;165(13):1493–1499. [DOI] [PubMed] [Google Scholar]
- 36.Singh H, Schiff GD, Graber ML, et al. The global burden of diagnostic errors in primary care. BMJ Qual. Saf. 2017;26(6):484–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Casey SD, Mumma BE. Sex, race, and insurance status differences in hospital treatment and outcomes following out-of-hospital cardiac arrest. Resuscitation 2018;126:125–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dehon E, Weiss N, Jones J, et al. A systematic review of the impact of physician implicit racial bias on clinical decision making. Acad. Emerg. Med. 2017;24(8):895–904. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


