Abstract
Background
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection is associated with significant cognitive impairment and increased risk for mental health comorbidities. This study aimed to identify specific associations between cognitive impairment, self-reported disruptions in memory, and psychiatric symptoms including depression, anxiety, posttraumatic stress disorder (PTSD), and perceived sleep concerns.
Methods
Data collected from all consecutive patients with Post-Acute Sequelae of SARS-CoV-2 infection (PASC) who presented to a dedicated Post-COVID Clinic were used to evaluate whether certain psychiatric symptoms were more strongly associated with cognitive impairment and self-reported memory disturbances.
Results
Univariate and multivariable analyses revealed that depression symptom severity was significantly associated with the severity of cognitive impairment among patients with PASC. This association was driven primarily by lower performance on verbal fluency, attention, and delayed recall tasks among patients with higher depression symptoms severity. Perceived sleep concerns were an important predictor of self-reported memory disturbances. Conversely, neither PTSD symptom severity nor anxiety symptom severity were significant predictors of cognitive impairment or self-reported memory disturbances.
Conclusions
These findings have important clinical implications for justifying the need for screening patients with PASC for both depression and cognitive impairment.
Keywords: COVID-19, Cognitive impairment, Depression, Sleep, Anxiety, PTSD, Memory
Highlights
-
•
Depression was positively associated with cognitive impairment post-COVID-19 infection.
-
•
Depression was associated with lower performance on verbal fluency, attention, and delayed recall.
-
•
Perceived sleep concerns predicted self-reported memory disturbances.
-
•
Neither PTSD nor anxiety predicted cognitive impairment memory.
1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection is associated with significant cognitive impairment in some individuals. Up to 36% of patients in the acute stages of viral illness and up to 84% of patients in intensive care units for SARS-CoV-2 infection have at least mild cognitive impairment (Alonso-Lana et al., 2020; Helms et al., 2020; Jaywant et al., 2021; Mao et al., 2020; Parker et al., 2021; Patel et al., 2021; Tomasoni et al., 2021). In a systematic review, concentration difficulties were one of the most common symptoms of COVID-19, affecting up to 26% of patients, with memory impairment affecting 15% of non-hospitalized and 34% of hospitalized patients (Michelen et al., 2021). Tasks that rely on frontoparietal regions of the brain appear most impacted by SARS-CoV-2 (Hosp et al., 2021). While some studies suggest recovery of cognitive impairment during rehabilitation (Patel et al., 2021), others found that cognitive impairment can last at least 3–6 months after infection among some patients (Blazhenets et al., 2021; van den Borst et al., 2020).
SARS-CoV-2 infection is also associated with increased risk for mental health comorbidities. Among individuals previously hospitalized with SARS-CoV-2 infection, up to 40% screened positive for psychiatric symptoms during the acute phase (Ma et al., 2020; Michelen et al., 2021; Parker et al., 2021) with 20–30% during the post-acute period (Huang et al., 2021; Lorenzo et al., 2020; Méndez et al., 2021; Michelen et al., 2021; Tomasoni et al., 2021)/Psychiatric comorbidity in individuals with PASC remained at around 36% up to three-months after discharge (Mazza et al., 2021). Whereas anxiety, PTSD symptoms, and insomnia may decrease in the period following discharge from the hospital, depression may persist (Mazza et al., 2021). Even among patients with COVID-19 who are not hospitalized, psychiatric comorbidity is notable, with up to 52% meeting criteria for depression or anxiety (Graham et al., 2021; Michelen et al., 2021).
Some studies suggest that psychiatric comorbidities are collectively associated with higher prevalence of neurocognitive impairment among individuals infected with SARS-CoV-2 in both acute hospitalization and in the post-acute period (Huang et al., 2021; Lorenzo et al., 2020; Ma et al., 2020; Méndez et al., 2021; Miskowiak et al., 2021; Tomasoni et al., 2021). On the other hand, at least one study did not find significant associations between cognitive impairment and psychiatric comorbidity in patients recovering from prolonged COVID-19 hospitalization who required acute inpatient rehabilitation prior to discharge (Jaywant et al., 2021). To further complicate matters, prior studies on the association between mental health and cognitive impairment did not evaluate the independent associations between anxiety, depression, or PTSD. The intersection of cognitive impairment and mental health symptoms are cause for concern among individuals infected with SARS-CoV-2, as both are associated with increased risk of mortality among hospitalized patients (Bayrak and Çadirci, 2021). Given that the presence of psychiatric symptoms (in general) and cognitive impairment can significantly increase risk for deleterious health consequences among individuals with SARS-CoV-2 infection, it is essential to better understand which types of psychiatric symptoms are most strongly associated with cognitive impairment in patients with PASC. Associations between psychiatric symptoms and cognitive impairment may vary drastically between acutely hospitalized patients and PASC patients, further necessitating the need for more research on this topic.
In this study, we used data collected from COVID-19 patients with PASC who presented to a dedicated Post-COVID-19 clinic to evaluate associations between objectively measured cognitive impairment, self-reported disruptions in memory, and psychiatric symptoms including depression, anxiety, PTSD, and perceived sleep concerns. This sample is unique compared to the existing literature given that some of our participants were hospitalized whereas others were not, which enhances generalizability of the study to the larger population of patients with PASC. We hypothesized that all four psychiatric symptom types would be significantly associated with both cognitive impairment and self-reported memory concerns. We also hypothesized that these associations would remain after controlling for demographic variables and severity of the initial infection, as proxied by hospitalization. Given the possible impact of SARS-CoV-2 infection on frontoparietal regions of the brain, we hypothesized that psychiatric symptoms (an indicator of disruption emotional reactivity, regulation or both) would be most strongly associated with poor performance on tasks involving attention, memory, and speech and language.
2. Materials and methods
2.1. Participants
Participants (N = 297) were primarily white (n = 203, 68.4%) women (n = 206, 70.1%) who were middle-aged (M = 46.9, SD = 14.1, range 19–91). In terms of the severity of their illness, 22.2% (n = 66) had been hospitalized for COVID-19 and 6.7% (n = 20) had been in the intensive care unit. Data from all participants on the Post-COVID-19 Rehabilitation Unit were de-identified and included in the study; only participants missing data on the cognitive assessment were excluded from analyses. This observational cohort study includes all consecutive, unique patients receiving treatment at the study health system Post-COVID clinic from June 2020 to April 2021 to address their PASC symptoms.
2.2. Measures
HADS - The Hospital Anxiety and Depression Scale (HADS) is a 14-item scale consisting of two, seven-item subscales for anxiety and depression. Scoring for each item ranges from zero to three. An anxiety subscale score >8 denotes severe anxiety, and a depression subscale score >8 denotes severe depression. The HADS has demonstrated satisfactory psychometric properties when used to assess symptom severity and presence of anxiety disorders and depression within primary care and general populations (Bjelland et al., 2002; Cameron et al., 2008). The measure has strong internal consistency (α = 0.83; Bjelland et al., 2002).
IES - The Impact of Event Scale – Revised (IES-6) is a 6-item self-report measure assessing posttraumatic stress reactions following a specific traumatic event. Respondents rated how distressing each item was experienced in the past week on a 4-point scale ranging from not at all (item score 0) to extremely (item score 4). An abbreviated version of the widely used 22-item IES-R, the 6-item IES-6 used in this study captures three symptom subscales: intrusion (2 items; α = 0.78), avoidance (2 items; α = 0.69), and hyperarousal (2 items, α = 0.78; Giorgi et al., 2015). An IES-6 score >1.75 indicates clinically significant PTSD symptoms. The IES-6 has been shown to correlate strongly with longer versions of the instrument and has demonstrated reliability and statistical validity as a measure of posttraumatic stress reactions in clinical and research settings (Giorgi et al., 2015; Hosey et al., 2019). The IES-6 was found to have good internal consistency (6 items; α = 0.88) and split-half reliability (Giorgi et al., 2015).
Perceived Sleep Concerns - The following open-ended question was included to assess participants’ perceived sleep concerns: “Do you have any concerns regarding your sleep?” This item was rated on a binary (0 = no, 1 = yes) scale.
MoCA-Blind – The Montreal Cognitive Assessment (MoCA) is a brief screening instrument measuring cognitive dysfunction, and the Blind adaptation removes visual items. The assessment examines nine subscales across multiple cognitive domains: attention and concentration, memory, language, conceptual thinking, calculations, and orientation. The MoCA requires approximately 10 min to administer. A total score of >18 indicates the absence of cognitive impairment. The MoCA has demonstrated satisfactory psychometric properties as an overall measure of cognitive performance, showing discriminant and generalized validity of MoCA scores (Freitas et al., 2014; Sala, 2020). The MoCA-Blind (referred to throughout as MoCA, for simplicity) has excellent specificity and adequate sensitivity (Wittich et al., 2010).
Self-reported memory disturbance – The following open-ended question was used to assess memory disturbances: “Do you have any concerns regarding your memory?” This item was rated on a binary (0 = no, 1 = yes) scale.
Procedures. Upon clinical evaluation, individuals completed a cognitive evaluation with Physical Medicine and Rehabilitation (PM&R) care team members who were closely trained on the administration of the MoCA, followed by self-report questionnaires. De-identified archival data were analyzed following approval from the Institutional Review Board at the University of Pennsylvania. Data are available upon request.
Data analysis. Stata v. 15.1 was used for all analyses. First, univariate regression analyses examined associations between each of the psychiatric variables (anxiety, depression, PTSD and perceived sleep disturbance) and MoCA total score. Then, a multivariable linear regression model included all psychiatric variables to examine their association with MoCA total score. These analyses were repeated after controlling for key demographic variables and indicators of COVID-19 severity. Given that depression severity was the psychiatric variable that was most strongly associated with MoCA total score, this variable was then examined in relation to the 9 MoCA subscales to determine which subscale was most strongly associated with depression severity. To accomplish this, depression univariate linear regressions were conducted with the 9 MoCA subscales as the independent variables and depression as the dependent variable. Then, a multivariable linear regression was conducted with all 9 MoCA subscales entered as simultaneous predictors of depression severity. Finally, self-reported memory problems were evaluated in relation to each of the psychiatric variables, first using univariate logistic regression (with the binary self-reported memory problem as the dependent variable and each of the psychiatric variables as independent variables), and then in a multivariable logistic regression (wherein all psychiatric variables were examined simultaneously). This analysis was repeated after controlling for several critical demographic variables and indicators of COVID-19 severity.
3. Results
3.1. Prevalence of positive screens for psychiatric comorbidities
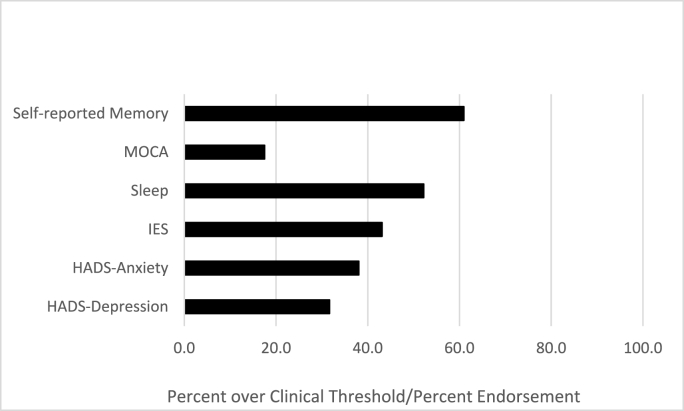
The prevalence of scoring in the clinical range or of endorsing disruption in a given domain is presented in Fig. 1. Psychiatric variables were all significantly associated, but the largest correlation was between anxiety and PTSD (r = 0.61), though the correlation was not large enough to suggest strong multicollinearity in multivariable models (correlation range for all other associations: r = 0.13, for self-report sleep disturbance and PTSD to r = 0.58 for anxiety and depression). Associations between psychiatric variables and weeks since COVID-19 symptom onset were small (range: r = 0.03 - 0.07), whereas associations were moderate for psychiatric variables and number of cardiopulmonary symptoms (range: r = 0.33 - 0.40).
Fig. 1.
Prevalence of psychiatric and cognitive concerns.
3.2. MoCA total score results
In univariate analyses, depression symptom severity was significantly associated with MoCA total score (β = −0.207, t = −3.62, p < .001) whereas anxiety symptom severity was only marginally associated with MoCA total score (p = .051). Both PTSD symptom severity (p = .096) and self-reported sleep disturbance (p = .599) were not significantly associated with MoCA total score. When depression symptom severity, anxiety symptom severity, PTSD symptom severity and self-reported sleep disturbance were entered simultaneously in a multivariable model, only depression symptom severity was significantly associated with MoCA total score (p < .01, see Table 1). These results held after controlling for age (which was also significant, p < .001), sex, ethnicity, and hospitalization status, time since COVID-19 symptom onset, and number of cardiopulmonary function symptoms, except that with the inclusion of these covariates, anxiety was significantly associated with MoCA total score (β = −0.150, t = −2.42, p < .01) in the univariate analysis (but not the multivariable analysis).
Table 1.
Demographic characteristics.
| M (SD) | MoCA ≥ 18 (Absence of cognitive impairment, N = 245) | MoCA <18 (Possible cognitive impairment, N = 52) | Test Statistic | |
|---|---|---|---|---|
| Age | 46.9 (14.1); Range: 19 - 91 | 45.7 (13.5); Range: 19-78 | 52.6 (15.2); Range: 19–91 | F = 10.47, p < .01 |
| Weeks since COVID-19 symptoms began | 22.4 (12.8); Range: 1 - 51 | 22.1 (12.8); Range: 1-51 | 23.6 (12.8); Range: 3 - 50 | F = 0.51, p = .47 |
| Number of cardiopulmonary symptoms | 2.7 (1.6); Range: 0 - 7 | 2.7 (1.6); Range: 0 -6 | 2.8 (1.7); Range: 0–7 | F = 0.36, p = .55 |
| Sex | χ2 = 0.58, p = .45 | |||
| Women | 206 (69.4%) | 168 (68.6%) | 38 (73.1%) | |
| Men | 88 (29.6%) | 75 (30.6%) | 13 (25%) | |
| Missing | 3 (1.0%) | 2 (0.8%) | 1 (1.9%) | |
| Race | Fisher's exact: p < .01 | |||
| White | 218 (73.4%) | 187 (76.3%) | 31 (59.6%) | |
| Native American | 1 (0.3%) | 0 (0.0%) | 1 (1.9%) | |
| Asian | 5 (1.7%) | 5 (2.0%) | 0 (0.0%) | |
| Black | 49 (16.5%) | 32 (13.1%) | 17 (32.7%) | |
| Other | 9 (3.0%) | 5 (2.0%) | 1 (1.9%) | |
| Missing | 18 (6.0%) | 16 (6.5%) | 2 (3.8%) | |
| Hospitalization | χ2 = 10.3, p < .01 | |||
| None | 227 (76.4%) | 196 (80.8%) | 31 (59.6%) | |
| Yes, ICU | 20 (6.7%) | 33 (13.5%) | 13 (25%) | |
| Yes, General Hospitalization | 46 (15.5%) | 13 (5.3%) | 7 (13.5%) | |
| Missing | 4 (1.3%) | 3 (1.2%) | 1 (1.9%) | |
| Difficulties with ADLs | χ2 = 4.7, p < .05 | |||
| No | 171 (86.8%) | 145 (59.2%) | 26 (50.0%) | |
| Yes | 96 (32.3%) | 71 (29.0%) | 25 (48.1%) | |
| Missing | 30 (10.1%) | 29 (11.8%) | 1 (1.9%) | |
| Comfort with community activities | Fisher's exact p < .001 | |||
| 0 | 46 (15.5%) | 26 (10.6%) | 20 (38.5%) | |
| 1 | 7 (2.4%) | 4 (1.6%) | 3 (5.8%) | |
| 2 | 5 (1.7%) | 4 (1.6%) | 1 (1.9%) | |
| 3 | 10 (3.4%) | 8 (3.3%) | 2 (3.8%) | |
| 4 | 196 (66.0%) | 172 (70.0%) | 24 (46.2%) | |
| Missing | 33 (11.1%) | 31 (12.7%) | 2 (3.8%) | |
| Education | Fisher's exact p = .087 | |||
| Less than 12 years | 33 (11.1%) | 31 (12.7%) | 2 (3.9%) | |
| More than 12 years | 264 (88.9%) | 214 (87.4%) | 50 (96.2%) | |
| No | 202 (68.2%) | 172 (70.5%) | 30 (57.7%) | |
| Yes | 94 (31.8%) | 72 (29.5%) | 22 (42.3%) | |
| Clinically significant PTSD | χ2 = 4.46, p < .05 | |||
| No | 166 (56.5%) | 144 (59.3%) | 22 (43.1%) | |
| Yes | 128 (43.5%) | 99 (40.7%) | 29 (56.9%) | |
| Perceived sleep difficulties | χ2 = 0.51, p = .474 | |||
| No | 126 (44.8%) | 105 (45.9%) | 21 (40.4%) | |
| Yes | 155 (55.2%) | 124 (54.2%) | 31 (59.6%) | |
Note: ADLs = Activities of Daily Living; ICU = Intensive Care Unit; Community activities was measured as a count variable of comfort with shopping, using the restroom, transportation and driving.
3.3. MoCA subscale results
In light of the significant association between depression symptom severity and MoCA Total Score, we extended the analysis to determine which specific dimension captured by each of the 9 MoCA subscales was most strongly associated with depression symptom severity. Our findings indicated that MoCA Digit Span-Backward, the MoCA Fluency and the MoCA Delayed recall were all significantly associated with depression. Specifically, a greater likelihood of errors on Digit Span Backward, less than 11 words generated on Verbal Fluency, and fewer words on Delayed Recall were associated with higher depression symptom severity (see Table 2).
Table 2.
The relationship of Depression, Anxiety, PTSD and Sleep Disturbance to Cognitive Impairment.
| β | Coeff. | SE | [95% CI] | t | p | ||
|---|---|---|---|---|---|---|---|
| MoCA Total Score | |||||||
| Depression | −0.236 | −0.126 | 0.041 | −0.208 | −0.045 | −3.05 | .003 |
| Anxiety | −0.016 | −0.009 | 0.045 | −0.097 | 0.080 | −0.19 | .848 |
| PTSD | 0.034 | 0.085 | 0.204 | −0.316 | 0.486 | 0.42 | .676 |
| Sleep | 0.005 | 0.025 | 0.300 | −0.566 | 0.616 | 0.08 | .934 |
| Constant | 20.114 | 0.315 | 19.494 | 20.735 | 63.80 | <.001 | |
| MoCA Total Score | |||||||
| Depression | −0.166 | −0.089 | 0.041 | −0.170 | −0.008 | −2.17 | .031 |
| Anxiety | −0.088 | −0.048 | 0.044 | −0.136 | 0.039 | −1.09 | .277 |
| PTSD | 0.072 | 0.185 | 0.200 | −0.209 | 0.580 | 0.93 | .355 |
| Sleep | 0.005 | 0.022 | 0.291 | −0.551 | 0.595 | 0.08 | .939 |
| Hospitalized (0 = No, 1 = Yes) | −0.112 | −0.627 | 0.350 | −1.315 | 0.061 | −1.79 | .074 |
| Sex (0 = Female; 1 = male) | 0.023 | 0.123 | 0.300 | −0.469 | 0.714 | 0.41 | .684 |
| Age | −0.273 | −0.046 | 0.010 | −0.065 | −0.026 | −4.59 | <.001 |
| Ethnicity (0 = White, 1 = Non-White | −0.015 | −0.074 | 0.302 | −0.669 | 0.521 | −0.25 | .806 |
| Constant | 22.293 | 0.556 | 21.198 | 23.388 | 40.08 | <.001 | |
Cognitive impairment is captured by the MoCA total score. Hospitalized refers to a history of hospitalization for COVID-19. MoCA = Montreal Cognitive Assessment. PTSD = posttraumatic stress disorder.
As a sensitivity analysis, the association between MoCA subscales and depression symptom severity was evaluated with hospitalization status entered as a moderator. The associations between depression symptom severity and each of the subscales was not moderated by hospitalization status (MoCA Digit Span-Forward (p = .137), MoCA Digit Span-Backward (p = .724), MoCA Letter List (p = .086), MoCA Serial Sevens (p = .08), MoCA Sentence Repetition (p = .421), MoCA Fluency (p = .272), MoCA Delayed Recall (p = .109), MoCA Abstraction (p = .617), MoCA Orientation moderation could not be tested because of small cell sizes).
3.4. Self-reported memory problems
In univariate analyses with self-reported memory problems as the dependent variable, depression symptom severity, OR = 1.23, 95% CI [1.15, 1.33], SE 0.044, z = 5.83, p < .001, anxiety symptom severity, OR = 1.16, 95% CI [1.09, 1.23], SE = 0.04, z = 4.52, p < .001, PTSD OR = 1.99, 95% CI [1.49, 2.65], SE = 0.29, z = 4.69, p < .001, and self-reported sleep disturbance, OR = 3.35, 95% CI [2.00, 5.59], z = 4.62, p < .001, were all significantly associated with self-reported memory problems. When depression symptom severity, anxiety symptom severity, PTSD symptom severity and self-reported sleep disturbance were entered simultaneously in a multivariable model, only depression symptom severity (p < .01) and sleep disturbance (p < .001) were significantly associated with a greater likelihood of self-reported memory problems (Table 3). These results held after controlling for age, sex, ethnicity, hospitalization status, time since COVID-19 symptom onset, and number of cardiopulmonary function symptoms. In a sensitivity analysis, the association between depression and self-reported memory problems was not significant (p = .328), nor was the association between depression and self-reported sleep disturbance (p = .238) (see Table 4).
Table 3.
The relationship of Depression Symptom Severity and Cognitive Impairment Subscales in Univariate Analyses.
| Depression | β |
Coeff. |
SE |
[95% CI] |
t |
p |
|
|---|---|---|---|---|---|---|---|
| Binary predictors | |||||||
| MoCA Digit Span-Backward | −0.120 | −1.917 | 0.926 | −3.739 | −0.095 | −2.07 | .039 |
| Constant | 8.417 | 0.888 | 6.670 | 10.163 | 9.48 | .000 | |
| MoCA Digit Span-Forward | −0.098 | −1.884 | 1.120 | −4.089 | 0.321 | −1.68 | .094 |
| Constant | 8.438 | 1.090 | 6.293 | 10.582 | 7.74 | <.001 | |
| MoCA Verbal Fluency | −0.129 | −1.404 | 0.632 | −2.648 | −0.161 | −2.22 | .027 |
| Constant | 7.780 | 0.565 | 6.667 | 8.892 | 13.76 | <.001 | |
| MoCA Letter List | −0.090 | −1.606 | 1.034 | −3.641 | 0.430 | −1.55 | 0.122 |
| Constant | 8.158 | 1.001 | 6.189 | 10.127 | 8.15 | 0.000 | |
| Categorical predictors | |||||||
| MoCA Delayed recall (Reference = 0) |
Omnibus test |
5 (290) |
2.30 |
.045 |
|||
| β |
Coeff. |
SE |
[95% CI] |
t |
p |
||
| 1 | 0.030 | 0.638 | 1.819 | −2.942 | 4.219 | 0.35 | .726 |
| 2 | 0.143 | 2.006 | 1.567 | −1.077 | 5.090 | 1.28 | .201 |
| 3 | −0.056 | −0.666 | 1.506 | −3.630 | 2.298 | −0.44 | .659 |
| 4 | −0.088 | −0.9 | 1.461 | −3.775 | 1.975 | −0.62 | .538 |
| 5 | −0.046 | −0.404 | 1.422 | −3.203 | 2.395 | −0.28 | .777 |
| Constant | 6.9 | 1.368 | 4.208 | 9.592 | 5.05 | .000 | |
| MoCA Serial Sevens (Reference = 0) |
Omnibus test |
3 (292) |
2.41 |
.068 |
|||
| β |
Coeff. |
SE |
[95% CI] |
t |
p |
||
| 1 | 0.013 | 0.201 | 1.679 | −3.103 | 3.505 | 0.12 | .905 |
| 2 | −0.114 | −1.277 | 1.561 | −4.349 | 1.795 | −0.82 | .414 |
| 3 | −0.204 | −1.936 | 1.478 | −4.845 | 0.974 | −1.31 | .191 |
| Constant | 8.222 | 1.447 | 5.374 | 11.070 | 5.68 | <.001 | |
| MoCA sentence repetition (Reference = 0) |
Omnibus test |
2 (293) |
2.62 |
.075 |
|||
| β |
Coeff. |
SE |
[95% CI] |
t |
p |
||
| 1 | −0.137 | −1.453 | 1.368 | −4.145 | 1.239 | −1.06 | .289 |
| 2 | −0.240 | −2.395 | 1.289 | −4.932 | 0.141 | −1.86 | .064 |
| Constant | 8.75 | 1.255 | 6.280 | 11.220 | 6.97 | <.001 | |
| MoCA Abstraction (Reference = 0) |
Omnibus test |
2 (293) |
0.73 |
.483 |
|||
| β |
Coeff. |
SE |
[95% CI] |
t |
p |
||
| 1 | −0.096 | −1.105 | 1.664 | −4.380 | 2.170 | −0.66 | .507 |
| 2 | −0.146 | −1.598 | 1.573 | −4.694 | 1.498 | −1.02 | .311 |
| Constant | 8.125 | 1.547 | 5.080 | 11.170 | 5.25 | <.001 | |
| MoCA Orientation (Reference = 6) |
Omnibus test |
3 (292) |
0.60 |
.617 |
|||
| β |
Coeff. |
SE |
[95% CI] |
t |
p |
||
| 3 | 0.033 | 2.450 | 4.389 | −6.189 | 11.088 | 0.56 | .577 |
| 4 | 0.031 | 1.050 | 1.977 | −2.842 | 4.942 | 0.53 | .596 |
| 5 | 0.066 | 1.116 | 0.993 | −0.837 | 3.070 | 1.12 | .262 |
| Constant | 6.550 | 0.267 | 6.024 | 7.076 | 24.52 | <.001 | |
Coeff. = coefficient; MoCA = Montreal Cognitive Assessment; SE = standard error.
Table 4.
The relationship of Depression, Anxiety, PTSD and Sleep Disturbance to Memory Impairment.
| Self-reported memory difficulties | OR | SE | [95% CI] | z | p | |
|---|---|---|---|---|---|---|
| Depression | 1.158 | 0.053 | 1.058 | 1.266 | 3.19 | .001 |
| Anxiety | 1.007 | 0.046 | 0.921 | 1.101 | 0.15 | .881 |
| IES | 1.434 | 0.300 | 0.951 | 2.160 | 1.72 | .085 |
| Sleep | 2.910 | 0.855 | 1.636 | 5.177 | 3.64 | <.001 |
| Constant |
0.231 |
0.077 |
0.121 |
0.444 |
4.41 |
<.001 |
|
Self-reported memory difficulties |
OR |
SE |
[95% CI] |
z |
p |
|
| Depression | 1.153 | 0.055 | 1.049 | 1.267 | 2.95 | .003 |
| Anxiety | 1.001 | 0.047 | 0.912 | 1.098 | 0.01 | .988 |
| PTSD | 1.512 | 0.326 | 0.990 | 2.308 | 1.91 | .056 |
| Sleep | 3.021 | 0.907 | 1.677 | 5.443 | 3.68 | <.001 |
| Sex (0 = Female; 1 = male) | 0.930 | 0.294 | 0.501 | 1.728 | −0.23 | .819 |
| Age | 1.012 | 0.011 | 0.990 | 1.034 | 1.08 | .282 |
| Ethnicity (0 = White, 1 = Non-White) | 0.598 | 0.195 | 0.315 | 1.134 | −1.57 | .115 |
| Hospitalized | 0.773 | 0.296 | 0.365 | 1.636 | −0.67 | .501 |
| Constant | 0.166 | 0.101 | 0.051 | 0.546 | −2.96 | .003 |
PTSD = posttraumatic stress disorder.
4. Discussion
In this sample of patients with PASC, depression was the only significant psychological predictor of objectively measured mild cognitive dysfunction. These findings held even after controlling for demographic factors, including age, gender, ethnicity, and after controlling for illness severity through a hospitalization status proxy variable. The association between depression and mild cognitive dysfunction was driven by lower performance on measures of attention (Digit Span Backward), verbal fluency, and delayed recall. These associations were not moderated by hospitalization status. In contrast, anxiety was marginally associated with MoCA total score (p = .051) in a univariate analysis but not in the multivariable analysis. Both PTSD symptoms and perceived sleep concerns were not significantly associated with mild cognitive dysfunction in univariate or multivariate analyses. Univariate analyses revealed significant associations between all four psychological variables and self-reported memory impairment. Here again, in a multivariable analysis, only depression and perceived sleep concerns were significantly associated with self-reported memory impairments, and this association was not moderated by hospitalization status. This is the first study to our knowledge to explore the unique psychological predictors of mild cognitive impairment and self-reported memory loss a large and diverse sample of persons with PASC.
As described above, the prevalence rate of cognitive impairment among individuals infected with SARS-CoV-2 is high (Alonso-Lana et al., 2020; Helms et al., 2020; Jaywant et al., 2021; Mao et al., 2020; Miskowiak et al., 2021; Parker et al., 2021; Patel et al., 2021; Tomasoni et al., 2021). In the current study of patients in treatment in a Post-COVID Clinic, over 17% of participants scored in the range indicative of cognitive impairment on the MoCA, and 61% endorsed concerns about their memory. Multiple prior studies have demonstrated that a combination of anxiety and depression are associated with a greater likelihood of cognitive impairment among patients with SARS-CoV-2 infection during the acute phase (Ma et al., 2020) and for PASC patients (Huang et al., 2021; Lorenzo et al., 2020; Méndez et al., 2021; Tomasoni et al., 2021). However, these studies combined anxiety and depression as a composite indicator of psychiatric distress. Our study advances the literature by disentangling that relationship and revealing that depression, but not anxiety, was significantly associated with cognitive impairment in the context of COVID-19. Importantly, our findings also indicated that PTSD symptoms and perceived sleep concerns did not also contribute to the experience of cognitive impairment in this sample.
Prior studies have demonstrated that SARS-CoV-2 is associated with disruption in communication fluency (Mazza et al., 2021; Ramage, 2020) and in attention (Zhou et al., 2020). Going beyond these findings, our study is the first to examine and detect associations between challenges in communication (in the form of verbal fluency), attention (Digit Span Backward) and symptoms of depression among patients in recovery from SARS-CoV-2. One prior study (Mazza et al., 2021) reported on associations between psychopathology broadly speaking and both verbal fluency and attention (finding significant negative associations), but did not evaluate the type of psychopathology underlying this effect. This study revealed that patients recovering from COVID-19 are more likely to have difficulties with verbal fluency, attention, and delayed recall if they also have symptoms of depression.
Our findings are also consistent with some prior research on associations between depression and memory among individuals recovering from SARS-CoV-2. For instance, in a small sample (N = 35) of patients with confirmed SARS-CoV-2 infection, poorer memory was detected among patients presenting with psychological symptoms of anxiety and depression (Almeria et al., 2020). Like in the studies reported above, Almeria et al. (2020) used a combined measure of depression and anxiety, which reduces the ability to understand the relative contribution of each type of affect to memory disturbances. In the current study, perceived sleep concerns were also significantly associated with self-reported memory disturbances. While the COVID-19 pandemic has had a significant impact on symptoms of insomnia (Dzierzewski et al., 2021; Pizzonia et al., 2021; Wang et al., 2020), this is the first study to our knowledge to demonstrate that perceptions of sleep concerns were significantly associated with self-reported memory disturbances.
There are several important limitations of this study that require consideration. First, only 17.5% of the sample scored in the clinically significant range for cognitive impairment on the MoCA, which is lower than in some other samples, especially those in acute rehabilitation (Alonso-Lana et al., 2020; Helms et al., 2020; Jaywant et al., 2021; Mao et al., 2020; Miskowiak et al., 2021; Parker et al., 2021; Patel et al., 2021; Tomasoni et al., 2021). While all patients in this study experienced significant symptoms of COVID-19 as the basis for inclusion in the Post-COVID Clinic, differences in rates of cognitive impairment are likely to be different than patients who are actively admitted to the intensive care unit, for instance. Indeed, rates of cognitive impairment in this sample fall between established rates in the literature in patients who were hospitalized (34%) and not-hospitalized (15%) for COVID-19 (Michelen et al., 2021). Nevertheless, this prevalence (nearly one-fifth of the sample) represents a significant minority of patients with cognitive impairment. That our sample included both hospitalized and non-hospitalized patients likely contributed to lower rates of cognitive impairment, but also significantly enhances the generalizability of our findings for the general population of patients with PASC. Second, while approximately one-third of participants identified as minorities, the majority of participants in the study were white (68.4%) women (70.1%) who were middle-aged (M = 46.9, SD = 14.1). Thus, findings may not generalize to other samples although the true prevalence of long-COVID in different demographics groups has not been clearly established. Third, this study evaluated cross-sectional associations between psychopathology, self-reported memory disturbances, and cognitive impairment. Longitudinal studies are needed to evaluate the associations between depression, sleep, cognitive impairment, and memory over time as well as to determine their directionality. Finally, future studies should include biological or objective indicators of symptoms as all data included in the study (except the MoCA) reflected self-reported symptom distress, and should explore the influence of other psychiatric comorbidities (e.g., psychosis, history of manic episodes, substance use, sleep apnea, etc.).
These findings offer important clinical implications for improving the management and care of patients with COVID-19. Prior research has demonstrated that symptoms of depression remain persistent up to 3 months following hospitalization discharge for patients with SARS-CoV-2 infection, whereas symptoms of anxiety, PTSD, and insomnia each demonstrated relative improvement (Mazza et al., 2021). In light of the relative persistence of depression symptoms and its association with cognitive impairment, it is essential to offer evidence-based treatments to reduce depression symptoms among patients with PASC. Furthermore, in patients with PASC, cognitive impairment may drive symptoms of depression regardless of whether patients were hospitalized for their COVID-19 infection. Prior research suggests the promise of cognitive rehabilitation for improving depression (Jang & Jun 2012). Thus, it may be advantageous to offer both cognitive rehabilitation and evidence-based treatments for depression to patients with PASC. It may also be important to offer evidence-based practices for insomnia, such as cognitive behavioral therapy for insomnia (CBT-I; Taylor and Pruiksma, 2014) for patients recovering from COVID-19, especially among patients who are reporting concerns about memory challenges. The recent widespread availability of telehealth during the COVID-19 pandemic allows for the delivery of evidence-based practices for both depression and insomnia (or other sleep disorders) even among patients who are recovering with significant physical limitations following SARS-COV 2 infection.
5. Conclusions
In summary, this is the first study of its kind to evaluate the type of psychopathology that is most strongly associated with cognitive impairment and self-reported memory disturbances among patients with PASC. Symptoms of depression were significantly associated with severity of cognitive impairment among patients recovering from SARS-CoV-2 infection in both univariate and multivariable analyses. The association between depression and cognitive impairment was driven primarily by lower performance on tasks of verbal fluency, attention, and delayed recall among patients with higher severity of depression symptoms. Perceived sleep concerns were also an important predictor of self-reported memory disturbances. In contrast, neither symptoms of PTSD nor anxiety were significant predictors of cognitive impairment or self-reported memory disturbances in multivariable analyses. These findings have important implications for targeting which patients are most likely to be struggling with symptoms of depression, sleep concerns, cognitive impairment, and self-reported memory concerns as they recover from COVID-19.
Funding
This research was supported in part by a research grant from the National Institute on Aging (NIA) to Drs. Dillingham and Pezzin (5-R01-AG058718).
Declaration of competing interest
None.
References
- Almeria M., Cejudo J.C., Sotoca J., Deus J., Krupinski J. Cognitive profile following COVID-19 infection: clinical predictors leading to neuropsychological impairment. Brain Behav. Immun. Health. 2020;9 doi: 10.1016/j.bbih.2020.100163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso-Lana S., Marquié M., Ruiz A., Boada M. Cognitive and neuropsychiatric manifestations of COVID-19 and effects on elderly individuals with dementia. Front. Aging Neurosci. 2020;12 doi: 10.3389/fnagi.2020.588872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayrak M., Çadirci K. The associations of life quality, depression, and cognitive impairment with mortality in older adults with COVID-19: a prospective, observational study. Acta Clin. Belg. 2021:1–8. doi: 10.1080/17843286.2021.1916687. 0(0) [DOI] [PubMed] [Google Scholar]
- Bjelland I., Dahl A.A., Haug T.T., Neckelmann D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J. Psychosom. Res. 2002;52(2):69–77. doi: 10.1016/s0022-3999(01)00296-3. [DOI] [PubMed] [Google Scholar]
- Blazhenets G., Schroeter N., Bormann T., Thurow J., Wagner D., Frings L., Weiller C., Meyer P.T., Dressing A., Hosp J.A. Slow but evident recovery from neocortical dysfunction and cognitive impairment in a series of chronic COVID-19 patients. J. Nucl. Med.: Off. Publ. Soc. Nucl. Med. 2021;62(7):910–915. doi: 10.2967/jnumed.121.262128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron I.M., Crawford J.R., Lawton K., Reid I.C. Psychometric comparison of PHQ-9 and HADS for measuring depression severity in primary care. Br. J. Gen. Pract.: J. Roy. Coll. Gen. Pract. 2008;58(546):32–36. doi: 10.3399/bjgp08X263794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzierzewski J.M., Dautovich N.D., Ravyts S.G., Perez E., Soto P., Donovan E.K. Insomnia symptoms during the COVID-19 pandemic: an examination of biopsychosocial moderators. Sleep Med. 2021;S1389–9457(21) doi: 10.1016/j.sleep.2021.02.018. 00116-00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitas S., Prieto G., Simões M.R., Santana I. Psychometric properties of the montreal cognitive assessment (MoCA): an analysis using the rasch model. Clin. Neuropsychol. 2014;28(1):65–83. doi: 10.1080/13854046.2013.870231. [DOI] [PubMed] [Google Scholar]
- Giorgi G., Fiz Perez F.S., Castiello D'Antonio A., Mucci N., Ferrero C., Cupelli V., Arcangeli G. Psychometric properties of the Impact of Event Scale-6 in a sample of victims of bank robbery. Psychol. Res. Behav. Manag. 2015;8:99–104. doi: 10.2147/PRBM.S73901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham E.L., Clark J.R., Orban Z.S., Lim P.H., Szymanski A.L., Taylor C., DiBiase R.M., Jia D.T., Balabanov R., Ho S.U., Batra A., Liotta E.M., Koralnik I.J. Persistent neurologic symptoms and cognitive dysfunction in non-hospitalized Covid-19 “long haulers. Ann. Clin. Transl. Neurol. 2021;8(5):1073–1085. doi: 10.1002/acn3.51350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms J., Kremer S., Merdji H., Clere-Jehl R., Schenck M., Kummerlen C., Collange O., Boulay C., Fafi-Kremer S., Ohana M., Anheim M., Meziani F. Neurologic features in severe SARS-CoV-2 infection. N. Engl. J. Med. 2020 doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosey M.M., Leoutsakos J.-M.S., Li X., Dinglas V.D., Bienvenu O.J., Parker A.M., Hopkins R.O., Needham D.M., Neufeld K.J. Screening for posttraumatic stress disorder in ARDS survivors: validation of the impact of event scale-6 (IES-6) Crit. Care. 2019;23(1):276. doi: 10.1186/s13054-019-2553-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosp J.A., Dressing A., Blazhenets G., Bormann T., Rau A., Schwabenland M., Thurow J., Wagner D., Waller C., Niesen W.D., Frings L., Urbach H., Prinz M., Weiller C., Schroeter N., Meyer P.T. Cognitive impairment and altered cerebral glucose metabolism in the subacute stage of COVID-19. Brain: J. Neurol. 2021;144(4):1263–1276. doi: 10.1093/brain/awab009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Huang L., Wang Y., Li X., Ren L., Gu X., Kang L., Guo L., Liu M., Zhou X., Luo J., Huang Z., Tu S., Zhao Y., Chen L., Xu D., Li Y., Li C., Peng L., Cao B. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet (London, England) 2021;397(10270):220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang Y., Jun S. Effect of cognitive rehabilitation program on cognitive function, depression, and activities of daily living among patients with strokes. Kor. J. Adult Nurs. 2012;24(3) https://www.koreascience.or.kr/article/JAKO201227935977208.page [Google Scholar]
- Jaywant A., Vanderlind W.M., Alexopoulos G.S., Fridman C.B., Perlis R.H., Gunning F.M. Frequency and profile of objective cognitive deficits in hospitalized patients recovering from COVID-19. Neuropsychopharmacology. 2021;1–6 doi: 10.1038/s41386-021-00978-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo R.D., Conte C., Lanzani C., Benedetti F., Roveri L., Mazza M.G., Brioni E., Giacalone G., Canti V., Sofia V., D'Amico M., Napoli D.D., Ambrosio A., Scarpellini P., Castagna A., Landoni G., Zangrillo A., Bosi E., Tresoldi M., Rovere-Querini P. Residual clinical damage after COVID-19: a retrospective and prospective observational cohort study. PLoS One. 2020;15(10) doi: 10.1371/journal.pone.0239570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y.-F., Li W., Deng H.-B., Wang L., Wang Y., Wang P.-H., Bo H.-X., Cao J., Wang Y., Zhu L.-Y., Yang Y., Cheung T., Ng C.H., Wu X., Xiang Y.-T. Prevalence of depression and its association with quality of life in clinically stable patients with COVID-19. J. Affect. Disord. 2020;275:145–148. doi: 10.1016/j.jad.2020.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao L., Jin H., Wang M., Hu Y., Chen S., He Q., Chang J., Hong C., Zhou Y., Wang D., Miao X., Li Y., Hu B. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazza M.G., Palladini M., De Lorenzo R., Magnaghi C., Poletti S., Furlan R., Ciceri F., Rovere-Querini P., Benedetti F. Persistent psychopathology and neurocognitive impairment in COVID-19 survivors: effect of inflammatory biomarkers at three-month follow-up. Brain Behav. Immun. 2021;94:138–147. doi: 10.1016/j.bbi.2021.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Méndez R., Balanzá-Martínez V., Luperdi S.C., Estrada I., Latorre A., González-Jiménez P., Feced L., Bouzas L., Yépez K., Ferrando A., Hervás D., Zaldívar E., Reyes S., Berk M., Menéndez R. Short-term neuropsychiatric outcomes and quality of life in COVID-19 survivors. J. Intern. Med. 2021;290(3):621–631. doi: 10.1111/joim.13262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelen M., Cheng V., Manoharan L., Elkheir N., Dagens D., Hastie C., O'Hara M., Suett J.C., Dahmash D.T., Bugaeva P., Rigby I., Munblit D., Harriss E., Burls A., Foote C., Scott J.T., Carson G., Olliaro P., Sigfrid L., Stavropoulou C. Characterising long term Covid-19: a living systematic review. medRxiv. 2021;2020 doi: 10.1101/2020.12.08.20246025. 12.08.20246025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miskowiak K., Johnsen S., Sattler S., Nielsen S., Kunalan K., Rungby J., Lapperre T., Porsberg C. Cognitive impairments four months after COVID-19 hospital discharge: pattern, severity and association with illness variables. Eur. Neuropsychopharmacol. 2021;46:39–48. doi: 10.1016/j.euroneuro.2021.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker C., Shalev D., Hsu I., Shenoy A., Cheung S., Nash S., Wiener I., Fedoronko D., Allen N., Shapiro P.A. Depression, anxiety, and acute stress disorder among patients hospitalized with COVID-19: a prospective cohort study. J. Acad. Consult. Liais. Psychiatr. 2021;62(2):211–219. doi: 10.1016/j.psym.2020.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel R., Savrides I., Cahalan C., Doulatani G., O'Dell M.W., Toglia J., Jaywant A. Cognitive impairment and functional change in COVID-19 patients undergoing inpatient rehabilitation. medRxiv. 2021;2021 doi: 10.1101/2021.03.15.21253637. 03.15.21253637. [DOI] [PubMed] [Google Scholar]
- Pizzonia K.L., Koscinski B., Suhr J.A., Accorso C., Allan D.M., Allan N.P. Insomnia during the COVID-19 pandemic: the role of depression and COVID-19-related risk factors. Cognit. Behav. Ther. 2021;50(3):246–260. doi: 10.1080/16506073.2021.1879241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramage A.E. Potential for cognitive communication impairment in COVID-19 survivors: a call to action for speech-language pathologists. Am. J. Speech Lang. Pathol. 2020;29(4):1821–1832. doi: 10.1044/2020_AJSLP-20-00147. [DOI] [PubMed] [Google Scholar]
- Sala G. The psychometric properties of the Montreal Cognitive Assessment (MoCA): a comprehensive investigation. Swiss J. Psychol. Schweiz. Z. Psychol. Rev. Suisse Psychol. 2020;79(3–4):155. doi: 10.1024/1421-0185/a000242. [DOI] [Google Scholar]
- Taylor D.J., Pruiksma K.E. Cognitive and behavioural therapy for insomnia (CBT-I) in psychiatric populations: a systematic review. Int. Rev. Psychiatr. 2014;26(2):205–213. doi: 10.3109/09540261.2014.902808. [DOI] [PubMed] [Google Scholar]
- Tomasoni D., Bai F., Castoldi R., Barbanotti D., Falcinella C., Mulè G., Mondatore D., Tavelli A., Vegni E., Marchetti G., Monforte A. d'Arminio. Anxiety and depression symptoms after virological clearance of COVID-19: a cross-sectional study in Milan, Italy. J. Med. Virol. 2021;93(2):1175–1179. doi: 10.1002/jmv.26459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Borst B., Peters J.B., Brink M., Schoon Y., Bleeker-Rovers C.P., Schers H., van Hees H.W.H., van Helvoort H., van den Boogaard M., van der Hoeven H., Reijers M.H., Prokop M., Vercoulen J., van den Heuvel M. Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America; 2020. Comprehensive Health Assessment Three Months after Recovery from Acute COVID-19. ciaa1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Zhu L.-Y., Ma Y.-F., Bo H.-X., Deng H.-B., Cao J., Wang Y., Wang X.-J., Xu Y., Lu Q.-D., Wang H., Wu X.-J. Association of insomnia disorder with sociodemographic factors and poor mental health in COVID-19 inpatients in China. Sleep Med. 2020;75:282–286. doi: 10.1016/j.sleep.2020.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittich W., Phillips N., Nasreddine Z.S., Chertkow H. Sensitivity and specificity of the montreal cognitive assessment modified for individuals who are visually impaired. J. Vis. Impair. Blind. (JVIB) 2010;104(6):360–368. doi: 10.1177/0145482X1010400606. [DOI] [Google Scholar]
- Zhou H., Lu S., Chen J., Wei N., Wang D., Lyu H., Shi C., Hu S. The landscape of cognitive function in recovered COVID-19 patients. J. Psychiatr. Res. 2020;129:98–102. doi: 10.1016/j.jpsychires.2020.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]