Abstract
Alcohol use disorder (AUD) is frequently comorbid with mood disorders, and these co-occurring neuropsychiatric disorders contribute to the development and maintenance of alcohol dependence and relapse. In preclinical models, mice chronically exposed to alcohol display anxiety-like and depressive-like behaviors during acute withdrawal and protracted abstinence. However, in total, results from studies using voluntary alcohol drinking paradigms show variable behavioral outcomes in assays measuring negative affective behaviors. Thus, the main objective of this review is to summarize the literature on the variability of negative affective behaviors in mice after chronic alcohol exposure. We compare the behavioral phenotypes that emerge during abstinence across different exposure models, including models of alcohol and stress interactions. The complicated outcomes from these studies highlight the difficulties of assessing negative affective behaviors in mouse models designed for the study of AUD. We discuss new behavioral assays, comprehensive platforms, and unbiased machine-learning algorithms as promising approaches to better understand the interaction between alcohol and negative affect in mice. New data-driven approaches in the understanding of mouse behavior hold promise for improving the identification of mechanisms, cell subtypes, and neurocircuits that mediate negative affect. In turn, improving our understanding of the neurobehavioral basis of alcohol-associated negative affect will provide a platform to test hypotheses in mouse models that aim to improve the development of more effective strategies for treating individuals with AUD and co-occurring mood disorders.
Introduction
Alcohol use disorder (AUD) is a leading cause of worldwide disease burden, affecting over 280 million people worldwide, and harmful alcohol consumption is responsible for over 3 million deaths per year (WHO, 2018). AUD is comorbid with a variety of neuropsychiatric disorders, and nearly all cases present with negative mood symptoms, including depression (Brière, Rohde, Seeley, Klein, & Lewinsohn, 2014), bipolar disorder (Farren, Hill, & Weiss, 2012), and anxiety disorders (Grant et al., 2004; Lai, Cleary, Sitharthan, & Hunt, 2015; Schneier et al., 2010). Some studies have reported that up to 40% of AUD patients have a mood disorder, and a third suffer from an anxiety disorder. Multiple diagnoses lead to impaired responses to treatment and poorer disease outcome in individuals with AUD (Prior, Mills, Ross, & Teesson, 2017). The relationship between AUD and neuropsychiatric disorders is likely to be bidirectional, since alcohol itself worsens the course of mood disorders, while negative mood symptoms may promote alcohol consumption (Berglund & Ojehagen, 1998). Withdrawal from chronic alcohol engenders long-lasting negative emotional states (i.e., hyperkatifeia) and deficiencies in reward responses that may promote craving and relapse (Driessen et al., 2001; Gilpin & Koob, 2008; Heilig, Egli, Crabbe, & Becker, 2010; Koob, 2021; Thompson, Maleki, Kelly, Sy, & Oscar-Berman, 2021). The physical symptoms of withdrawal from heavy alcohol consumption are not necessary for the development of negative affective states, as harmful alcohol consumption patterns such as binge drinking, which do not produce significant physical withdrawal, are also associated with depressive symptoms, especially in women in their 20’s and 30’s (Paljärvi et al., 2009; Powers, Duffy, Burns, & Loxton, 2016). Binge drinking is common among those 12 or older (Clark Goings et al., 2019) and is rising in women and adults ≥ 65 years of age (NIAAA, 2021).
Although AUD is more prevalent in adult men (7.3%) than women (4.0%) in the US, the gap between sexes has been diminishing with alcohol use in women increasing sharply (Slade et al., 2016; White et al., 2015). Women with AUD have an increased risk of comorbid anxiety or mood disorder, and exhibit more severe depressive symptoms and craving (Anthenelli, 2010; Bott, Meyer, Rumpf, Hapke, & John, 2015; Goldstein, Dawson, Chou, & Grant, 2012). Additionally, women more commonly report drinking to alleviate negative mood and exhibit longer lasting negative affective states during withdrawal that may contribute to a higher relapse rate and exacerbate the disease course (Erol & Karpyak, 2015). Independent of AUD, women have a two-fold higher life time prevalence of major depressive disorder as well as a higher risk for anxiety disorders compared to men (Rubinow & Schmidt, 2019). Thus, the interaction between mood disorders and alcohol misuse can contribute to the establishment of psychological dependence in spite of harmful health consequences, and these processes may present differently in men compared to women. The components of negative affect that influence persistent craving are driven by adaptations in brain limbic and autonomic systems (Koob, 2021; Sinha et al., 2009) that may differ between men and women. To disentangle the complex relationship between AUD and comorbid neuropsychiatric disorders in males and females, highly controlled studies using animal models are necessary.
The development of valid animal models recapitulating AUD and negative affective behaviors is critical in order to find individualized and sex-specific treatment options (Gururajan, Reif, Cryan, & Slattery, 2019; Kokras & Dalla, 2014; Litten et al., 2015). While previous reviews summarized alcohol and negative affect interactions (Heilig et al., 2010; Holleran & Winder, 2017; Kliethermes, 2005), a number of studies have expanded alcohol exposure models by including repeated stressors and multiple measures of anxiety-like and negative affective behaviors. The outcomes from these studies are complex and prompt re-examination of the literature on the interaction between alcohol and negative affect. This review will focus on current findings in mouse models of alcohol drinking and emergence of negative affective behaviors, emphasizing opportunities for improvement in applying genetic tools combined with the increased use of mice as a model organism for studying anxiety-like behaviors after alcohol exposure and stress interactions. Finally, we comment on future directions using innovative approaches to assess the emergence of negative affect behaviors in alcohol drinking and dependent mice.
Alcohol exposure models to study negative affective behaviors
Studying anxiety-like, avoidance, and other negative affect-related behaviors during acute withdrawal from chronic alcohol exposure or after extended abstinence has been an ongoing focus of the alcohol field. Before discussing recent data, we first provide a brief description of each of the major alcohol exposure models that are used to study negative affective behaviors in mice. For more in-depth discussions of chronic alcohol exposure and voluntary drinking models in rodents, see these thorough reviews (H. C. Becker, 2013; Carnicella, Ron, & Barak, 2014; Griffin, 2014; Thiele & Navarro, 2014).
In early studies on alcohol and negative affective behaviors, alcohol was administered repeatedly to mice by intraperitoneal injections, intragastric gavage, or through the addition of alcohol into a liquid diet given as the sole source of nutrition (Kliethermes, 2005). Inhalation of alcohol vapor is another widely used model that produces rapid dependence, withdrawal-related behaviors, increased operant self-administration of alcohol, and relapse-like enhanced voluntary alcohol drinking in rats and mice (Lopez & Becker, 2014; Vendruscolo & Roberts, 2014). With the exception of the liquid diet, an advantage of these models is the precise control over the duration, pattern, and amount of alcohol exposure, and the parameters can be set to sustain equal blood alcohol levels in male and female mice (Jury, DiBerto, Kash, & Holmes, 2017). For example, exposure to alcohol vapor in inhalation chambers can maintain high blood alcohol concentrations (>174 mg/dL) in a continuous (e.g., 72 hours) or intermittent (e.g., 16 hours plus 8 hours of withdrawal) pattern prior to assessment of behavioral adaptations during withdrawal. One drawback of these intense exposure paradigms is that they can decrease ambulatory activity during acute withdrawal, which could potentially confound interpretation of alcohol-induced anxiety-like behaviors (Kliethermes, 2005). However, these models provide significant construct and predictive validity (Nieto, Grodin, Aguirre, Izquierdo, & Ray, 2021; Spanagel & Holter, 2000) and produce important behavioral phenotypes, such as increased alcohol drinking and withdrawal-associated phenotypes including anxiety-like behaviors (described below).
A number of voluntary alcohol drinking models have been used to study the emergence of negative affective behaviors during abstinence. Perhaps the easiest and least invasive chronic drinking model is the continuous access 2-bottle-choice paradigm. In this model, 10% alcohol is presented with a choice of water continuously for the duration of the study, which is typically 4–6 weeks. Daily alcohol consumption and the corresponding blood alcohol concentrations (BACs) in this model range from ~10–18 g/kg/day and ~30–110 mg/dL, respectively (Holleran et al., 2016; Stevenson et al., 2009), and higher consumption is observed in female compared with male mice (Centanni et al., 2019). This procedure differs from the intermittent-access 2-bottle-choice drinking model originally developed to initiate and maintain consumption of large amounts of alcohol in rats without the use of a secondary procedure, such as sucrose fading (Simms et al., 2008; Wise, 1973). The intermittent-access model was then applied to C57BL/6J mice (Hwa et al., 2011; Melendez, 2011), and produced escalation of alcohol (20%) drinking, a hallmark symptom of human AUD. The intermittent-access model generally produces high BACs (~70–170 mg/dL) and heavy intake (~15–20 g/kg/day) that is more pronounced in female than male mice (Hwa et al., 2011; Joffe, Winder, & Conn, 2020). Finally, in the drinking-in-the-dark (DID) model, mice are typically given access to a single bottle of 20% alcohol for 2 to 4 hours a day for 4–5 days each week (Rhodes, Best, Belknap, Finn, & Crabbe, 2005; Thiele & Navarro, 2014). This model gained popularity because mice consume high amounts of alcohol in a short period (~8 g/kg/4 hr), consistently producing BACs above the NIAAA threshold (i.e., ≥ 80 mg/dL) for defining a binge-like pattern of drinking. Notably, while mice are not food- or fluid-deprived and have an option to abstain from drinking alcohol, during the period of alcohol availability there is no access to water, and it occurs when mice naturally consume higher volumes of fluid during their activity cycle, which may contribute to the high amounts of intake.
Modeling negative affective states
In this review, we use “negative affective states” as an umbrella term encompassing various symptoms that are associated with anxiety, depression, and AUD, including anger, disgust, depressed mood, irritability, anxiety, and fear (Koob, 2021). Behavioral phenotypes of human psychiatric diseases such as depression have been historically difficult to replicate in animal models (Anyan & Amir, 2018; de Kloet & Molendijk, 2016; Yin, Guven, & Dietis, 2016). This is due in part to the heterogeneity of symptoms observed in the human diseases and our limited knowledge of the comorbid relationships between syndromes such as depression and anxiety disorders (Girolamo et al., 2017; Nestler & Hyman, 2010). The limitations of studying mental disorders that show heterogeneity within their diagnoses and comorbidity between diagnoses has been well recognized and was the main motivation behind the development of the Research Domain Criteria (RDoC) research initiative (Insel et al., 2010; Morris & Cuthbert, 2012). This strategy advocates for deconstructing mental health disorder research into component domains and constructs to enhance their understanding and, therefore, reveal how these domains lead to the emergence of and interactions between multiple disorders. Negative valence systems are one of the domains of interest and include anxiety, fear, and frustrative nonreward, which can encompass symptoms of negative affective states during acute withdrawal and prolonged abstinence from heavy alcohol drinking. By using animal models, we will be able to understand the dimensions of negative affect and mechanistic neural processes that contribute to maladaptive heavy alcohol drinking and help to better define and explain the complex relationship between AUD and mood disorders.
Symptoms of negative affect in individuals with AUD include anxiety, dysphoria, irritability, sleep disturbances, enhanced sensitivity to stress, physical and emotional pain, and general malaise. There are a wide range of behavioral assays used by alcohol addiction and stress researchers to model and study negative affective behaviors in mice (Deslauriers, Toth, Der-Avakian, & Risbrough, 2018; Gururajan et al., 2019; Ohl, 2005; Q. Wang, Timberlake, Prall, & Dwivedi, 2017). Since any measure of behavior is a proxy for the internal emotional state of the mouse, there is a focus on conflict-based paradigms to study negative affective behaviors, such as measuring approach-avoidance in dark or enclosed spaces vs light or open spaces. The translational domains of these assays are general anxiety-like and avoidance behaviors, depressive-like behaviors, social interactions, pain perception, fear learning and extinction, aggression, and arousal (Table 1). Conflict-based paradigms are often used to study anxiety-like behaviors in alcohol-exposed mice. Standard assays of conflicting approach and avoidance behavior include assessment of spatio-temporal parameters in the elevated plus maze (EPM), elevated zero maze, open field test (OFT), and light-dark box (LDB). Mice tend to more often avoid the open or brightly lit areas in the tests, which is considered a measure of anxiety-like behavior. Novelty suppressed feeding (NSF) and marble burying assays have also been widely used to measure affective disturbances and changes in natural behaviors, such as digging (de Brouwer, Fick, Harvey, & Wolmarans, 2019), following chronic alcohol. Assays to model depressive-like behaviors and other mood disturbances, such as aggression and anhedonia, include sucrose preference, social interaction or social novelty, and bottle brush tests. The forced swim test, while originally designed to measure depressive-like behaviors, measures adaptive processes underlying active and passive coping strategies during an inescapable stressor (Commons, Cholanians, Babb, & Ehlinger, 2017; Molendijk & de Kloet, 2015), and possibly anxiety-like behaviors (Anyan & Amir, 2018). Intracranial self-stimulation has revealed alcohol withdrawal-induced anhedonia in rats but has primarily been used for acute alcohol studies in mice (Bilbao et al., 2015; Fish, DiBerto, Krouse, Robinson, & Malanga, 2014; Fish et al., 2010; Fish et al., 2012; Kornetsky, Bain, Unterwald, & Lewis, 1988).
Table 1.
Mouse behavioral assays to assess negative affective behaviors during abstinence from chronic alcohol exposure or voluntary alcohol drinking.
| Behavioral Phenotypes | Paradigms | Comments |
|---|---|---|
| Approach, Avoidance, & Anxiety | EPM/EZM, LDB, MB, NSF, OF, AS | Easy to implement; Anxiety is a complex phenotype and requires multiple tests to fully characterize; Procedural differences across labs affect reproducibility; MB is an index of digging activity, and may not be a direct measure of anxiety-like behavior |
| Anhedonia, Despair, & Stress Coping | FS, ICSS, SP | Measures anhedonia and despair; Some concerns related to face, construct, and predictive validity; FS may measure coping strategies under stressful conditions rather than depressive-like behaviors |
| Social Interaction & Irritability | SA, SD, BB | High face, construct, and predictive validity; Testing time can be extensive |
AS, acoustic startle; BB, bottle-brush; EPM/EZM, elevated plus maze/elevated zero maze; FS, forced swim; ICSS, intracranial self-stimulation; LDB, light-dark box; MB, marble burying; NSF, novelty-suppressed feeding; OF, open field; SA, social approach; SD, social defeat; SP, sucrose/saccharin preference.
Negative affective states during abstinence
The remainder of the review focuses on the influence of alcohol exposure on negative affective behaviors during abstinence. The studies included here vary in the length of alcohol exposure from 3 days to 14 weeks and in the time of behavioral testing during abstinence, which ranged from a few hours to >2 months since the last alcohol exposure or drinking session. To visualize the exposure duration of the studies described below and summarized in Figures 1 and 2, we categorized the length of alcohol exposure into short, intermediate, and chronic paradigms that were 7 days or less, 8 days to 3 weeks, and 4 weeks or more, respectively. Second, because the strain or sex of mice can influence alcohol-related behaviors, such as acute functional tolerance (Kirstein et al., 2002), alcohol drinking (Crabbe, 2014), and handling-induced convulsions during alcohol withdrawal (Metten, Sorensen, Cameron, Yu, & Crabbe, 2010), we reported these variables for each study described in Tables 2 and 3.
Figure 1.
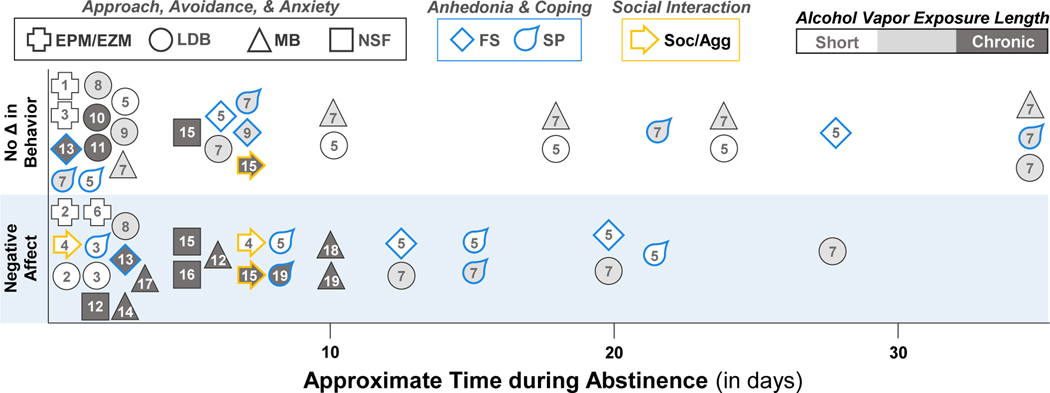
Behavioral disruption during abstinence from alcohol vapor inhalation in mice. The alcohol exposure length (short: 7 d or less, intermediate: 8 d to 3 weeks, or chronic: 4+ weeks) is represented by different shading. See Table 2 for references. EPM/EZM, elevated plus maze/elevated zero maze; FS, forced swim; LDB, light-dark box; MB, marble burying; NSF, novelty-suppressed feeding; Soc/Agg, social interaction/aggression; SP, sucrose/saccharin preference.
Figure 2.
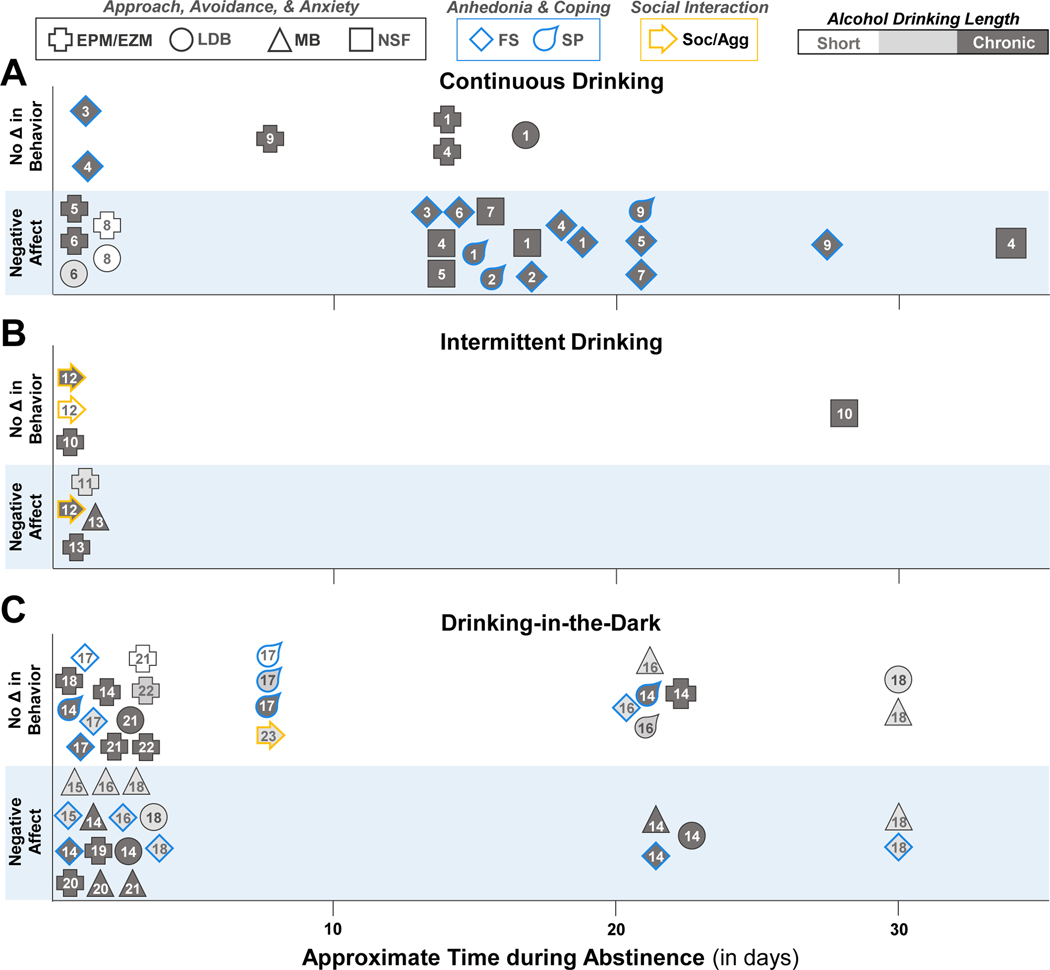
Behavioral changes during early and late abstinence from A) continuous alcohol drinking, B) intermittent access to alcohol, and C) drinking-in-the-dark. The length of the alcohol drinking paradigm (short: 7 d or less, intermediate: 8 d to 3 weeks, or chronic: 4+ weeks) is represented by different shading. See Table 3 for references. EPM/EZM, elevated plus maze/elevated zero maze; FS, forced swim; LDB, light-dark box; MB, marble burying; NSF, novelty-suppressed feeding; 5CSRT, five-choice serial reaction time; Soc/Agg, social interaction/aggression; SP, sucrose/saccharin preference.
Table 2.
Studies measuring negative affective behaviors during abstinence from alcohol vapor inhalation.
| Study | Model (duration) | Strain (sex) | Test (time into abstinence) | |
|---|---|---|---|---|
| Short-term exposure (7 d or less) | ||||
| 1 | Finn et al., 2000 | Continuous (3 d) | C57BL/6J & DBA/2J (M) | EPM (5–10 h) |
| 2 | Kliethermes et al., 2004 | Continuous (3 d) | WSC-2 (F+M) | EZM (1–2 d); LDB (10 h, 1 d, 36 h, 2 d) |
| 3 | Metten et al., 2018 | Continuous (3 d) | HS/Npt (F+M) | LDB (12 h, 1 d); EZM (1 d), SP (2 d) |
| 4 | Lowery-Gionta et al., 2015 | Intermittent (5 d) | DBA2/J (M) | SA (1 d, 7 d) |
| 5 | Hartmann et al., 2020 | Intermittent (1 wk) | C57BL/6J & /6NJ (F+M) | SP, LDB, FS (1 – 27 d) |
| 6 | Kash et al., 2009 | Intermittent (1 wk) | C57BL/6J (M) | EPM (4–6 h) |
| Intermediate exposure (8 d to 3 wk) | ||||
| 7 | Hartmann et al., 2019 | Intermittent (10 d) | WSP-2 & WSR-2 (F+M) | MB, LDB, SP (1 d - 7 wk) |
| 8 | McCool & Chappell, 2015 | Intermittent (2 wk) | C57BL/6J & DBA/2J (M) | LDB (3 d) |
| 9 | Bray et al., 2017 | Intermittent (3 wk) | C57BL/6J & CCL2-tg (F+M) | LDB (3 d); FST (7 d) |
| Chronic exposure (4+ wk) | ||||
| 10 | Daut et al., 2015 | Intermittent (4 wk) | C57BL/6J (F+M) | LDB (2 d) |
| 11 | Holmes et al., 2012 | Intermittent (4 wk) | C57BL/6J (M) | LDB (2 d) |
| 12 | Jury et al., 2017 | Intermittent (4 wk) | C57BL/6J (F+M) | NSF (3 d); MB (6 d) |
| 13 | Maldonado-Devincci et al., 2016 | Intermittent (4 wk) | C57BL/6J (M) | FS (8 h); FS (3 d) |
| 14 | Pleil et al., 2015 | Intermittent (4 wk) | C57BL/6J (M) | MB (2 d) |
| 15 | Sidhu et al., 2018 | Intermittent (4 –6 wk) | C57BL/6J & DBA/2J (M) | NSF (5 d); BB (3–7 d); SA (6–7 d); MB (10 d) |
| 16 | Warden, et al., 2020 | Intermittent (4 wk) | C57BL/6J (M) | NSF (5 d) |
| 17 | Rose et al., 2016 | Intermittent (5 wk) | C57BL/6J (M) | MB (3 d) |
| 18 | Kimbrough et al., 2020 | Intermittent (6 wk) | C57BL/6J (M) | BB (7 d); MB (10 d) |
| 19 | Starski et al. 2020 | Intermittent ’binge” (4 h/d; 6–9 wk) | C57BL/6J (M) | OF (20 d), SP (1–3 wk) |
Bold font indicates an alcohol-induced change in behavior. Study numbers are identified in Figure 1. BB, bottle-brush; EPM/EZM, elevated plus maze/elevated zero maze; FS, forced swim; LDB, light-dark box; MB, marble burying; NO, novel object encounter; NSF, novelty-suppressed feeding; OF, open field; SA, social approach; SP, sucrose/saccharin preference. M, male; F, female.
Table 3.
Negative affective behaviors that emerge during abstinence from voluntary alcohol drinking in mice.
| Study | Model (duration) | Strain (sex) | Test (time into abstinence) | |
|---|---|---|---|---|
| Continuous alcohol drinking | ||||
| 1 | Pang et al., 2013a | 2BC (24 h/d; 6 wk) | C57BL/6J (F) | SP, EPM (14 d); LDB, NSF (17 d); FS (19 d) |
| 2 | Pang et al., 2013b | 2BC (24 h/d; 6 wk) | C57BL/6J (F) | SP (15 d); FS (17 d) |
| 3 | Stevenson et al., 2009 | 2BC (24 h/d; 4 wk) | C57BL/6J (M) | FS, OF (1 d, 14 d) |
| 4 | Holleran et al., 2016 | 2BC (24 h/d; 6 wk) | C57BL/6J (F) | FS (1 d, 18 d); EPM (14 d); NSF (15 d, 35 d) |
| 5 | Vranjkovic et al., 2018 | 2BC (24 h/d; 6 wk) | C57BL/6J (F) | EPM (1 d, 7 d); NSF (14 d); FS (21 d) |
| 6 | Gong et al., 2017 | 2/3BC (24 h/d; 3–6 wk) | C57BL/6J (M) | OF, LDB (12 h); EPM (1 d), FS (13 d), TS (14 d) |
| 7 | Centanni et al., 2019 | 2BC (24 h/d; 6 wk) | C57BL/6J (F+M) | NSF (15 d); FS (21 d) |
| 8 | van Rijn et al., 2010 | 2BC (4 h/d; 5d) | C57BL/6J (M) | EPM, LDB (24 h) |
| 9 | Dao et al., 2020 | 2BC (24 h/d; 6 wk) | C57BL/6J (F+M) | EPM (7 d); OF (14 d); SP (21 d); FS (28 d) |
| Intermittent drinking | ||||
| 10 | Bloch et al., 2020 | 2BC (3 d/wk; 7 wk) | C57BL/6J (F+M) | EZM (1d); NO (19–20 d); NSF (27–28 d) |
| 11 | Wang et al., 2021 | 2BC (3 d/wk; 3 wk) | C57BL/6J (M) | OF (12 h); EPM (12 h) |
| 12 | Hwa et al., 2015 | 2BC (3 d/wk; 1– 8 wk) | Swiss-Webster (M) | Agg, SA (6–8 h) |
| 13 | Quijano Carde et al 2022 | 2BC (3 d/wk; 10 wk) | C57BL/6J (F+M) | EPM, MB, OF (24 h) |
| Drinking-in-the-dark | ||||
| 14 | Lee et al., 2015 | DID (6 wk) | C57BL/6J (M) | SP, LDB, NO, FS, EPM, MB (1–2 d, 21–22 d) |
| 15 | Lee et al., 2016 | DID (14 d) | C57BL/6J (M) | NO, FS (1 d); MB (2 d) |
| 16 | Lee, Coehlo, Solton et al., 2017 | DID (14 d) | C57BL/6J (M) | MB, FS, SP (1 d); MB, FS, SP (28 d) |
| 17 | Olney et al., 2018 | DID (1, 3, 6 wk) | C57BL/6J (M) | FS (1 d); SP (1 wk) |
| 18 | Szumlinski et al., 2019 | DID (14 d) | C57BL/6J (F+M) | ACS, NO, FS, LDB, MB (1–2 d, 30–31 d) |
| 19 | Bloodgood et al., 2020 | DID (4 wk) | OPRK1/Pdyn KO mice (F+M) | EPM (8 h) |
| 20 | Belmer et al., 2018 | DID (12–14 wk) | C57BL/6J (M) | EPM, MB, OF (24 h) |
| 21 | Rath 2020 | DID (8–19 wk) | C57BL/6J (F+M) | EPM, LDB, MB (36 h) |
| 22 | Cox et al., 2013 | DID (1, 3, 6, 10 wk) | C57BL/6J (M) | EPM, OF (1 d) |
| 23 | Flanigan et al., 2022 | DID (3 wk) | C57BL/6J (F+M) | SA, OF, ACS (7 d) |
Bold font indicates an alcohol-induced change in behavior. Study numbers are identified in Figure 2. ACS, acoustic startle; Agg, aggression; BB, bottle-brush; EPM/EZM, elevated plus maze/elevated zero maze; FS, forced swim; L-D, light-dark box; MB, marble burying; NO, novel object encounter; NSF, novelty-suppressed feeding; OF, open field; SA, social approach; SP, sucrose/saccharin preference; TS, tail suspension; M, male; F, female; KO, knockout.
As reviewed previously (Holleran & Winder, 2017; Kliethermes, 2005), repeated injections or gavage of alcohol and liquid diets containing alcohol tend to produce anxiety-like behaviors during acute withdrawal and early abstinence in mice. The EPM and LDB tests were predominantly used in these studies. While increases in anxiety-like or avoidance behaviors were reported in many studies and were often consistent across administration routes (for example, see (Perez & De Biasi, 2015)), they were not always observed in alcohol-exposed mice using these models (Holleran & Winder, 2017; Kliethermes, 2005). Many studies have also examined negative affective behaviors following alcohol exposure in vapor inhalation chambers (Table 2 and Figure 1). The duration of alcohol vapor inhalation ranged from as short as 72 hours to as long as 8 weeks on either continuous or intermittent (e.g., 16 hours on/8 hours off for 4 days/week) schedules. These studies also tested a wide range of negative affective behaviors across 7 weeks of forced abstinence from alcohol vapor inhalation. The short-term alcohol vapor models revealed mixed effects on negative affective behaviors, even in the same task measured during early withdrawal (Finn, Gallaher, & Crabbe, 2000; Hartmann, Haney, Smith, Kumar, & Rosenwasser, 2020; Kash, Baucum, Conrad, Colbran, & Winder, 2009; Kliethermes, Cronise, & Crabbe, 2004; Lowery-Gionta, Marcinkiewcz, & Kash, 2015; Metten et al., 2018). The only persistent behavioral changes during protracted withdrawal (>2 weeks) from short-term alcohol vapor were in the sucrose preference and forced swim tests, which were time- and strain-dependent (Hartmann et al., 2020). Of the three studies that used an intermediate duration of alcohol vapor (Bray, Roberts, & Gruol, 2017; Hartmann, Holbrook, Haney, Crabbe, & Rosenwasser, 2019; McCool & Chappell, 2015), there were mixed effects in the marble burying, LDB, and sucrose preference tests that were also time- and strain-dependent. Many of the behavioral changes induced by alcohol vapor returned to control levels after 15 days of abstinence with the exception of some behaviors in the LDB test (Hartmann et al., 2019). Chronic alcohol vapor exposure did not affect behaviors in the LDB, NSF, and forced swim tests during early abstinence in some studies (Daut et al., 2015; Holmes et al., 2012; Maldonado-Devincci et al., 2016; Sidhu, Kreifeldt, & Contet, 2018). However, prolonged vapor exposure produced negative affective behaviors in the marble burying, social approach, bottle brush, sucrose seeking, and NSF tests up to 10 days into abstinence (Jury et al., 2017; Kimbrough et al., 2020; Maldonado-Devincci et al., 2016; Pleil et al., 2015; Rose et al., 2016; Sidhu et al., 2018; Starski et al., 2020; Warden et al., 2020). Persistent behavioral changes (>2 weeks) in the chronic vapor model have not been tested to our knowledge other than in the open field test. Overall, in models of chronic alcohol exposure with tight regulation of the dose and duration, there are still inconsistencies in alcohol-induced negative affective states in mice, even within individual strains and sex. As illustrated in Figure 1 and Table 2, variables such as sex, strain, type of behavioral test, duration of vapor exposure, and time of testing during abstinence might contribute to the variability in behavioral profiles.
In the continuous access 2-bottle choice paradigm, negative affective behaviors were reported in the EPM and LDB tests, but not the forced swim test, during early abstinence (Gong et al., 2017; Holleran et al., 2016; Stevenson et al., 2009; van Rijn, Brissett, & Whistler, 2010; Vranjkovic, Winkler, & Winder, 2018) (Figure 2A). During protracted abstinence, behaviors in the EPM and LDB box test return to normal within 2 weeks (Holleran et al., 2016; Terence Y. Pang, Thibault Renoir, Xin Du, Andrew J. Lawrence, & Anthony J. Hannan, 2013), whereas negative affective behaviors are present in the alcohol drinking mice across multiple tasks, including forced swim, NSF, and sucrose preference tests that persists for at least 30 days (Centanni et al., 2019; Dao et al., 2020; Gong et al., 2017; Holleran et al., 2016; T. Y. Pang, X. Du, et al., 2013; Terence Y. Pang et al., 2013; Stevenson et al., 2009; Vranjkovic et al., 2018). However, even in the continuous access drinking model, the dichotomy between anxiety-like behaviors during early abstinence and depressive-like behaviors during late abstinence is not as clear as originally proposed. The NSF test is a measure of approach-avoidance when given a choice to consume familiar chow in an unfamiliar environment under food deprivation conditions (Samuels & Hen, 2011). Mice drinking alcohol in the continuous access model display a longer latency to begin feeding in the NSF test during protracted abstinence (14–35 days), suggestive of an impairment in approach-avoidance choice behaviors. How this type of avoidance behavior compares with the choice to stay in the walled arm of the EPM or in the dark arena in the LDB is unclear, but the difference in alcohol-exposure effects during protracted abstinence implies that there may be different neural processes that drive avoidance behavior in the NSF test compared to the other assays, such as engagement of corticotropin-releasing factor signaling during food deprivation (N. A. Chen et al., 2014; Shalev, Finnie, Quinn, Tobin, & Wahi, 2006). Nonetheless, while there are only a handful of studies examining negative affective behaviors during abstinence from continuous access drinking, the behavioral manifestations appear robust and reproducible across multiple tests and different durations of drinking, though they differ between early and protracted abstinence.
A few recent studies have assessed the emergence of negative affective behaviors during acute and prolonged abstinence from intermittent 2-bottle choice drinking. The negative affective behaviors that emerge during abstinence from intermittent drinking do not completely parallel those described for some of the other alcohol exposure models (Figure 2B). Indeed, there are mixed results in negative affective behaviors that were dependent upon task and drinking duration (Bloch, Rinker, Marcus, & Mulholland, 2020; Neira et al., 2022; Quijano Carde & De Biasi, 2022; N. Wang et al., 2021). A decrease in open arm time in the EPM and an increase in marble burying was reported during acute abstinence (Quijano Carde & De Biasi, 2022; N. Wang et al., 2021), whereas another study reported that alcohol drinking increased open zone entries in the elevated zero maze (Bloch et al., 2020). In addition, an increase in social exploration has been reported during early abstinence in the intermittent drinking mice (Hwa et al., 2015). The same study also reported elevated aggressiveness during early abstinence in mice that were drinking in the intermittent model for 8, but not 1 or 4 weeks (Hwa et al., 2015). Latency to feed in the NSF test was similar between water and alcohol drinking mice tested at 27–28 days into abstinence (Bloch et al., 2020), and long-term intermittent alcohol drinking did not affect center time in the open field (Neira et al., 2022; Quijano Carde & De Biasi, 2022). While mice that are drinking in the intermittent model consume large amounts of alcohol and achieve higher BACs compared with other models (Hwa et al., 2011), the negative affective behaviors that appear during abstinence are variable. Additional studies are needed to better understand the complexities and differences across drinking models.
In the DID model, negative affective behaviors in male C57BL/6J mice were reported during early abstinence in intermediate and chronic drinking paradigms across multiple studies (Belmer, Patkar, Lanoue, & Bartlett, 2018; Bloodgood et al., 2020; Flanigan et al., 2022; Lee, Coehlo, McGregor, Waltermire, & Szumlinski, 2015; Lee, Coehlo, Solton, & Szumlinski, 2017; Lee, Coelho, Class, Sern, et al., 2018; Lee, Coelho, Class, & Szumlinski, 2018; Lee et al., 2016; Lee, Coelho, et al., 2017; Lee, Coelho, Sern, & Szumlinski, 2018; Rath et al., 2020) (Figure 2C). Some of the negative affective behaviors persist beyond early abstinence in male mice that consumed alcohol in the DID model for 3 or 6 weeks (Flanigan et al., 2022; Lee et al., 2015), but not 14 days (Lee, Coehlo, et al., 2017). Persistent deficits in negative affective behaviors were also reported in female mice that drank alcohol in the 14-day continuous model (Szumlinski et al., 2019). In contrast, a number of studies using short and chronic DID drinking models did not observe negative affective behaviors during early abstinence in male and female C57BL/6J mice (Bloodgood et al., 2020; Cox et al., 2013; Lee et al., 2015; Lee et al., 2016; Olney, Marshall, & Thiele, 2018; Rath et al., 2020). Moreover, negative affective behaviors were not observed when male mice consumed relatively low amounts of alcohol in the 14-day continuous DID drinking model (Lee, Coelho, Sern, et al., 2018; Szumlinski et al., 2019). In fact, the low drinking male mice exhibited behaviors that were more consistent with a lower anxiety-like phenotype during early abstinence (Szumlinski et al., 2019). In general, the DID model produces anxiety-like behaviors during acute and protracted abstinence that may depend on critical factors, such as drinking duration and amount.
Alcohol-stress interaction models
General life stressors have mixed effects on alcohol drinking (Keyes, Hatzenbuehler, Grant, & Hasin, 2012; Park, Armeli, & Tennen, 2004; Thomas, Randall, & Carrigan, 2003), whereas trauma and more severe forms of stress, especially during early life, increase the risk of developing AUD (Enoch, 2011; Keyes et al., 2012; Ramchandani et al., 2018). Alcohol drinking can serve as a coping strategy for individuals experiencing repeated stressors, and an acute stress experience can trigger relapse (Keyes et al., 2012; Ramchandani et al., 2018). Because of this complex and bidirectional relationship between stressful life experiences and chronic alcohol, preclinical studies have thoroughly examined the interaction of alcohol-stress models on alcohol drinking (H. C. Becker, 2017). More recently, studies modeling alcohol-stress interactions have examined negative affective behaviors during acute and protracted abstinence (Table 4). One alcohol-stress co-exposure model that combines chronic intermittent alcohol exposure with repeated forced swim episodes in adult mice produces an escalation of drinking, morphological and functional neuronal adaptations, cognitive impairments (Anderson, Lopez, & Becker, 2016a, 2016b; Cannady et al., 2021; Lopez, Anderson, & Becker, 2016; Rodberg et al., 2017), and negative affective behaviors in mice (den Hartog et al., 2020; Padula et al., 2020; Rodberg et al., 2017). Results from the marble burying test varied across studies, ranging from no change in the number of buried marbles (Rodberg et al., 2017) to an increase in marbles buried in alcohol dependent, stressed mice compared with stress-only mice (Padula et al., 2020). Another study reported increased marble burying in chronic alcohol vapor exposed mice, regardless of their history of repeated forced swim (den Hartog et al., 2020). A robust interaction between chronic alcohol vapor and forced swim on latency to feed in the NSF test was reported, where the alcohol+stress group had much longer latencies to initiate feeding compared with the other three treatment groups (Padula et al., 2020). Notably, the majority of mice in the combined treatment group did not attempt to feed in the novel environment, but showed normal feeding behavior when returned to their home cages. Studies examining alcohol effects on social interaction in adult stressed mice reported that repeated (10 d) alcohol exposure via gavage and 4 weeks of intermittent access drinking produced a decrease in social interaction 24 h following a modified (subthreshold) social defeat stress when tested during early abstinence (Nelson, Sequeira, & Schank, 2018; Nennig et al., 2020). Although there were large differences in BACs produced by the gavage exposure (~240 mg/dL) and the voluntary drinking paradigm (~40 mg/dL), each model caused a similar decrease in social interaction following social defeat stress. In mice that experienced early life stressors, alcohol-stress interactions were reported in some assays of negative affective behaviors (tail suspension test, EPM), but not others (LDB, digging test) (de Almeida Magalhaes, Correia, de Carvalho, Damasceno, & Brunialti Godard, 2018; Okhuarobo et al., 2020). Together, the studies highlight the importance of stress not only as a trigger for relapse to alcohol drinking, but also as a trigger for maladaptive behavioral responses and impaired coping strategies in mice with a history of alcohol exposure.
Table 4.
Influence of alcohol-stress interactions on affective phenotypes in mice.
| Study | Alcohol-Stress Model | Strain (sex) | Test (time into abstinence) |
|---|---|---|---|
| Rodberg et al., 2017 | CIE + FS | C57BL/6J (M) | MB (3–5 d) |
| den Hartog et al., 2020 | CIE + FS | C57BL/6J (F+M) | MB (4 d) |
| Padula et al., 2020 | CIE + FS | C57BL/6J (M) | NSF, MB (12–13 d) |
| Nelson et al., 2018 | i.g. EtOH + SDS | C57BL/6J (M) | SI (1 d) |
| Nennig et al., 2020 | IA Drinking + SDS | C57BL/6J (M) | SI (1 d) |
| de Almeida Magalhaes et al., 2018 | 2BC + MS | C57BL/6 (F+M) | L-D (<1 d) |
| Okhuarobo et al., 2020 | CIE + LBN | C57BL/6J (F+M) | EPM (10–11 d); Digging (13); Grooming (17 d); TS (19 d) |
Bold font indicates a significant change in negative affective behavior. 2BC, two-bottle choice; IA, intermittent access; CIE, chronic intermittent ethanol; EPM, elevated plus maze; FS, forced swim; LBN, limited bedding and nesting; L-D, light-dark box; MB, marble burying; MS, maternal separation; NSF, novelty-suppressed feeding; SDS, social defeat stress; SI, social interaction; TS, tail suspension; M, male; F, female.
The high co-morbidity between AUD and mood disorders in humans implies that there may be shared mechanisms that drive heavy alcohol drinking and negative affect. Genome-wide association studies have demonstrated multiple genetic variants that influence these co-occurring neuropsychiatric conditions (Stoychev, Dilkov, Naghavi, & Kamburova, 2021). However, current combination pharmacological approaches to treat patients with AUD and mood disorders are largely inconclusive (Agabio & Leggio, 2018; Gimeno et al., 2017; Ipser, Wilson, Akindipe, Sager, & Stein, 2015). Although understudied, preclinical research has attempted to determine shared common mechanisms by examining genes and pharmacological approaches that influence both alcohol drinking and negative affect. For example, Avp, Gsk3b, Kcnn3, and Tacr1 genes have been associated with alcohol consumption, stress susceptibility, and negative affective behaviors in C57BL/6J and genetically-diverse strains of mice (Nelson et al., 2018; Padula et al., 2020; van der Vaart et al., 2018). Pharmacological approaches targeting the α1-adrenergic receptor and KCa2 channels reduced alcohol drinking and anxiety-like behaviors in mice exposed to an alcohol-stress interaction model (den Hartog et al., 2020; Lopez et al., 2020; Padula et al., 2020). Similarly, inhibition of GluK1-containing kainate receptors reduced alcohol consumption and physical signs of withdrawal in mice drinking in the intermittent access model (Quijano Carde, Perez, Feinn, Kranzler, & De Biasi, 2021). Thus, these initial preclinical findings support the suggestion that the mechanisms driving alcohol drinking and negative affect are related, and that pharmacological interventions for selective targets would be effective at reducing heavy drinking and mood disturbances in individuals with co-occurring disorders.
Sex, alcohol, and negative affect
An important consideration for assessment of negative affective states following chronic alcohol intake is biological sex. Historically, in the human population, alcohol intake has been more prevalent in males; however, it is now becoming increasingly apparent that the sex difference gap is continually shrinking (Koob, 2021). Further, women display so-called “telescoping” behavior, which is a faster progression from drug sampling to substance/alcohol use disorder across drug classes (J. B. Becker & Hu, 2008; Hernandez-Avila, Rounsaville, & Kranzler, 2004; McCance-Katz, Carroll, & Rounsaville, 1999; Westermeyer & Boedicker, 2000), which has been recapitulated in animal models. Compounding this issue, women have demonstrated both higher baseline disorders of negative affect, as well as increased withdrawal-induced negative affect across numerous drug classes including cocaine, nicotine, and cannabis (J. B. Becker & Koob, 2016).
However, models of chronic alcohol intake have revealed a surprising resilience in female mice to both intoxicating effects of alcohol as well as withdrawal-induced negative affect. Female mice have been shown to consistently consume alcohol in greater quantities than males (J. B. Becker & Koob, 2016; Bloch et al., 2020; Centanni et al., 2019; Eriksson & Pikkarainen, 1968; Hutchins, Allen, Cole-Harding, & Wilson, 1981; Middaugh, Kelley, Bandy, & McGroarty, 1999; Priddy et al., 2017); however, greater intake in males is occasionally observed (Lopez et al., 2020). Females are resistant to loss of righting reflex (LORR) (Naassila, Ledent, & Daoust, 2002) and handling-induced convulsions (HICs) following withdrawal from acute alcohol administration (Devaud & Chadda, 2001; Kosobud & Crabbe, 1986). Faster recovery from withdrawal has been linked to a tolerance to hypnotic effects of ethanol in females (Walls, Macklin, & Devaud, 2012). Though there has been some argument that female rodents are resistant to the development of alcohol dependence, these studies rely on HICs as the defining criteria of dependence. Because HICs are reduced in females, they may not be a reliable sole indicator of dependence (Devaud & Chadda, 2001). The reduced withdrawal symptomatology in females has been linked in part to circulating hormones, particularly progesterone, which has anxiolytic-like effects (Carroll & Anker, 2010). Female mice have increased activity of progesterone (Tanchuck-Nipper et al., 2015), and following adrenalectomy and gonadectomy, female mice show increased alcohol withdrawal severity (Strong, Kaufman, Crabbe, & Finn, 2009). On the contrary, withdrawal severity in males is not impacted following similar procedures (Strong et al., 2009).
In terms of baseline negative affective behavior, results are mixed, but generally males appear to show greater anxiety-like and depression-like behavior in a number of behavioral tasks (Kokras & Dalla, 2014). In contrast, models of post-traumatic stress disorder (PTSD) have demonstrated greater vulnerability in females (Kokras & Dalla, 2014). However, it is critical to consider that behavioral assays used to assess negative affective behavior have historically been developed using male animals (and, indeed most behavioral models suffer from a similar concern). Additionally, the interpretations of output measures were largely established using male animals. Thus, an assay may be ethologically relevant for male animals, with predictive output data that reflect a reasonable interpretation of a given behavior, but the same assay may not be relevant for female behavior, and could yield data that seem inconsistent or oppositional, when in reality the assay was never well suited to query that behavioral measure in females (Bangasser & Cuarenta, 2021; Kokras & Dalla, 2014). For example, in the widely-used elevated plus maze, behavior in males is strongly correlated to anxiety-like states, but female behavior is thought to be governed more by general activity (Fernandes, Gonzalez, Wilson, & File, 1999). Further, negative affective behavior often differs between males and females. In fear conditioning paradigms, males display freezing behavior, whereas females engage in darting behavior—both behaviors can be bidirectionally modulated by interventions that are thought to increase or decrease negative affect (Kokras & Dalla, 2014; Shansky, 2015).
The combination of chronic alcohol administration and stress exposure increases negative affect-like behavior in both male and female mice (den Hartog et al., 2020). However, female mice have been shown to be resistant to alcohol withdrawal-induced negative affect in some tasks. Of note, alcohol withdrawal has been shown to increase corticosterone levels in male, but not female, rodents (Janis, Devaud, Mitsuyama, & Morrow, 1998), indicating that there may be a reduction in alcohol’s ability to act as a physiological stressor in females. However, the impact of sex on apparent negative affect is largely dependent on the task that has been chosen and experimental conditions employed – for instance, adolescent females appear to show less profound anxiety-like behavior in the marble burying task following DID than males, but adult females and males show equal levels of anxiety-like behavior (Jimenez Chavez et al., 2020). Other tasks have shown inconsistent expression of negative-affect like behavior following chronic alcohol exposure in females, such as the novelty-suppressed feeding task in which alcohol withdrawal induced anxiety-like behaviors in females have been observed in some cases (Centanni et al., 2019; K. M. Holleran et al., 2016; T. Y. Pang, T. Renoir, X. Du, A. J. Lawrence, & A. J. Hannan, 2013; Vranjkovic et al., 2018) but not in others (Bloch et al., 2020). However, most peculiarly, some studies have found opposing effects between male and female animals following chronic alcohol exposure. For instance, adult female C57 mice show decreased latency to enter the light side of a light/dark box following DID, while males display increased latency, which is typically thought of as an anxiogenic-like behavior (Jimenez Chavez et al., 2020). There remains much work to do in order to fully delineate the interaction between stress and chronic alcohol exposure between sexes. However, it is clear that the relationship between these factors is complex, both in terms of neurobiology as well as in our understanding of what constitutes negative affect-like behavior, particularly in female mice.
Summary of alcohol and negative affect behaviors
As described in this review, alcohol exposure and voluntary drinking can produce different profiles when assessing negative affective behaviors during abstinence in mice. In general, the continuous access model of alcohol availability was the most consistent drinking model to elicit negative affective behaviors in mice. Alcohol drinking mice repeatedly showed deficits on the marble burying, forced swim, and NSF assays. Popular tests that are purported to measure anxiety-like behaviors (e.g., EPM, LDB, and OF) produce inconsistent results across labs and alcohol drinking models, and there are even some reports of low anxiety-like phenotypes (e.g., more open zone entries) in mice with a history of alcohol drinking (Bloch et al., 2020; Szumlinski et al., 2019). Inconsistent findings are not just limited to alcohol exposure models. Variability in behavioral profiles of negative affect is also induced by chronic stress paradigms in mice (Ennaceur, 2014; Lezak, Missig, & Carlezon, 2017; Willner, 2017). As an extreme example of this variable response, one study compared behavioral profiles in classical ‘anxiety’ tests in mice exposed to two widely used stress models: unpredictable chronic mild stress (UCMS) and chronic restraint stress (CRS) (Prevot et al., 2019). While UCMS and CRS stressed mice showed the expected weight reductions, behaviors in the OF, EPM, NSF, and novelty-induced hypophagia task varied across the two stress models. Anxiety-like behaviors were reported in the OF assay in mice exposed to UCMS (but not CRS) and the EPM in mice exposed to CRS (but not UCMS). Whereas CRS did not affect behaviors in the NSF or novelty-induced hypophagia assays, bidirectional effects were reported in the latency to feed in the NSF and novelty-induced hypophagia assays in mice treated with UCMS. Potential concerns related to the validity of some of these assays and their translation to humans with mood-related neuropsychiatric disorders have been raised previously (Ennaceur, 2014; Lezak et al., 2017). In addition, tests of negative affective behaviors may be limited by a lack of standardized protocols leading to variations within and across labs. Thus, potential limitations of these tests, as well as differences in the alcohol drinking and chronic stress models themselves, could account for the mixed profiles of negative affective behaviors.
Considerations
Alcohol intake amounts and patterns
Why are there discrepancies across the same behavioral assays in the same alcohol exposure model? Are the varied behavioral responses due to a lack of standardized protocols or other inconspicuous factors? While these are complicated questions to answer, there are a number of possibilities that may explain some of the variability in behavioral outcomes during abstinence. One important factor that could explain differences in behavioral outcomes is the total amount or pattern of alcohol intake. Female C57BL/6J mice that consumed nearly 800 g/kg across 6 weeks of continuous alcohol access showed an anxiety-like phenotype in the EPM and NSF tests (Vranjkovic et al., 2018). In comparison, female C57BL/6J mice consumed a total of ~430 g/kg of alcohol across 7 weeks of intermittent alcohol access, but did not show negative affective behaviors (Bloch et al., 2020). These findings would suggest that the consumption of large amounts of alcohol in a continuous pattern is required to produce negative affective behaviors. Complicating the interpretation that total alcohol exposure amount in a continuous pattern are critical factors, one study reported anxiety-like behaviors in mice that consumed ≤ 25 g/kg of total alcohol in 5 days of limited access (4 hours) drinking (van Rijn et al., 2010). Moreover, anxiety-like behaviors were reported in mice that consumed a total of 120 g/kg of alcohol in the DID model (Lee et al., 2015). While there is not a clear pattern of alcohol availability that consistently produces negative affective behaviors, another factor that may drive these behaviors is the level of intoxication. A set of studies using the DID model reported negative affective behaviors after mice reached high, but not low BACs (Lee et al., 2015; Lee, Coehlo, et al., 2017; Lee, Coelho, Class, & Szumlinski, 2018; Lee et al., 2016; Szumlinski et al., 2019). While the intermittent drinking model has been shown to produce higher amounts of intake during the first 2 h compared with the DID model and 24 h compared with the continuous access model (Hwa et al., 2011), this model does not always produce an anxiety-like phenotype (Figure 2B). Thus, greater alcohol exposure generally increases the probability of negative affective behaviors, however the relationship between amount, duration, and pattern of alcohol exposure and emergence of negative affective behaviors in mice is complex.
Rodent housing and feeding conditions
Additional important considerations for the variability in drinking level and anxiety-like phenotypes across studies are mouse housing conditions and the diet on which the mice are maintained. Often, the light cycle in which testing occurs, type of bedding and cages (ventilated versus unventilated), and specific diet are not reported. Housing conditions are known to affect anxiety-like behaviors in mice (Ahlgren & Voikar, 2019; Pasquarelli, Voehringer, Henke, & Ferger, 2017; Shimizu, Wakita, Tsuchiya, & Nabeshima, 2020). The variation in content of individual diets can vary considerably in nutrient content, including fat content and the presence or not of soy-based products that introduce phytoestrogens (e.g., isoflavones like genistein and daidzein) into the diet. Two studies in mice found that providing mice with higher isoflavone containing diets significantly increased alcohol consumption compared to those that contained lower to little isoflavones (Marshall et al., 2015; Quadir et al., 2020). Additionally, studies have also shown that isoflavones can alter anxiety-like behavior differentially in males and females, and differentially across the estrous cycle, and that these differences are dependent on interaction with estrogen receptors (Patisaul, Blum, Luskin, & Wilson, 2005; Rodriguez-Landa et al., 2017; Sandini et al., 2019). Another key possibility is the time of day, as many studies found differences in performance in multiple assays depending on the phase of the light cycle when testing occurs (Bilu & Kronfeld-Schor, 2013; Richetto, Polesel, & Weber-Stadlbauer, 2019; Tsao, Flint, & Huang, 2022; Verma, Hellemans, Choi, Yu, & Weinberg, 2010). The field would benefit from systematic examination of the influence of housing and testing conditions, as well as diet, on negative affective behaviors during abstinence from prolonged alcohol drinking.
Test batteries and sex
Some of the studies discussed in this review used a battery of behavioral assays where mice were tested on multiple tasks throughout abstinence and male and female mice were tested concurrently. These efforts provide a necessary and comprehensive assessment of alcohol-induced negative affective behaviors across both sexes. However, there are additional points of consideration when designing studies to measure affective behaviors after voluntary chronic alcohol drinking. Mouse behaviors in popular assays are influenced by repeated testing in the same maze or prior testing on a single assay or a battery of tests (Henderson, 1967; McIlwain, Merriweather, Yuva-Paylor, & Paylor, 2001; Rodgers & Shepherd, 1993). While there are reproducible negative affective behaviors in male mice drinking in the DID model, these behavioral changes were not observed in male mice when tested concurrently with females (Jimenez Chavez et al., 2020). The lack of negative affective behaviors in the drinking male mice might have been influenced by the female pheromones that are known to affect behavior in the EPM (Aikey, Nyby, Anmuth, & James, 2002). In addition, estrous cycle and ovarian hormones influence anxiety-like behaviors in mice (Fernandez-Guasti & Picazo, 1992; Gangitano, Salas, Teng, Perez, & De Biasi, 2009). To date, estrous cycle has not been systematically investigated in alcohol models of negative affect. Additional parametric behavioral studies across the different alcohol drinking models will be required to gain a better understanding of the factors driving the variability in behavioral outcomes in male and female mice when tested separately or concurrently. We direct the readers to a number of useful resources that provide strategies for designing and analyzing rigorous experiments that include sex as a biological variable (J. B. Becker & Koob, 2016; Garcia-Sifuentes & Maney, 2021; Shansky & Murphy, 2021; Tannenbaum, Ellis, Eyssel, Zou, & Schiebinger, 2019).
Future approaches
In this review, we identified variability in negative affective behavioral outcomes across a variety of alcohol exposure models and discussed limitations of the traditional behavioral assays and other considerations, some of which relate to experimental design (e.g., using a battery of assays), that may influence negative affect during abstinence. We recognize that some limitations will be difficult to overcome, as combining traditional assays provides researchers with practical approaches while reducing the number of mice required for each study. In this next section, we provide some specific suggestions for new tasks and approaches with the goal of establishing assays with improved validity and higher reproducibility across labs and drinking models to better assess the influence of chronic alcohol on negative affective behaviors. Our suggestions for future approaches include multidimensional analyses by studying translational endpoints and physiological endophenotypes in combination with the application of novel behavioral assays, large-scale and comprehensive behavioral analysis systems, and machine learning to examine the different behavioral phenotypes (approach-avoidance, depression, anhedonia, etc.) of negative affect.
Translational measures
To resolve the species gap, researchers use endpoints that can be measured in mice and humans or explore new approaches to improve validity in assessing mouse behavior. One focus has been to assess additional ethologically relevant behaviors beyond the spatio-temporal parameters on approach-avoidance tasks, such as stretch-attend posture, head dips, digging, grooming, and rearing. However, the study of ethological parameters has produced mixed results, suggesting that additional scrutiny is necessary if they are to complement or replace spatio-temporal measures of anxiety-like behaviors (Ennaceur, 2014). Alternatively, the acoustic startle response is one task that can be tested in mice and humans, is modified by negative affect, and is enhanced in both humans with AUD (Miranda, Meyerson, Buchanan, & Lovallo, 2002; Miranda, Meyerson, Myers, & Lovallo, 2003) and rodents with a history of chronic alcohol exposure (Barrenha & Chester, 2012; Chester & Barrenha, 2007; Ponomarev & Crabbe, 1999). In addition, the acoustic startle response in alcohol-naïve rats predicted an increase in alcohol drinking (Rasmussen & Kincaid, 2015). Thus, the acoustic startle response in mice is an attractive behavioral phenotype to study altered affect during abstinence and test novel treatments that might have high translational value.
Novel behavioral assays
Additional assays to measure innate fearful behaviors may better inform the human condition. One such task is the looming/sweeping disk that assesses behavioral responses to predator-like overhead visual stimuli that can induce freezing or fleeing instinctual behaviors (De Franceschi, Vivattanasarn, Saleem, & Solomon, 2016; Yang et al., 2020; Yilmaz & Meister, 2013). The fleeing response to the looming stimuli is accelerated by repeated stress exposure (Li et al., 2018). Two additional tasks have been developed that expose mice to an environment with ambiguous stimuli (i.e., potential escape routes) and uncertainty of the outcome when choosing between the ambiguous stimuli (Ennaceur, 2014). One of these tasks (3D maze) is a modification of an 8-arm radial maze where the first portion of each arm is inclined that acts as a bridge to the elevated portion of each open arm (Ennaceur, Michalikova, van Rensburg, & Chazot, 2006). The other assay is an elevated platform with steep downward slopes on two opposite sides of the open area (Ennaceur, Michalikova, van Rensburg, & Chazot, 2010; Michalikova, van Rensburg, Chazot, & Ennaceur, 2010). Strains of mice with different trait anxiety (i.e., C57BL/6J, BALB/C) showed the expected behavioral phenotype for exploration of the open arms of the 3D and elevated slopes mazes, respectively. Performance in these tasks is sensitive to pharmacological manipulations with anxiolytics or selective serotonin reuptake inhibitors (Ennaceur, 2011, 2014; Ennaceur, Michalikova, van Rensburg, & Chazot, 2008; Ennaceur et al., 2010; Michalikova et al., 2010). A final assay that leverages innate conflicted choice behavior is a robotic predator (“robopredator”) task that is used to study fear mechanisms (Choi & Kim, 2010; Kim et al., 2018). In this task, an animal is exposed to an arena where it can forage for food at different distances from a nest. After reaching a stable baseline, a programmable LEGO® robotic predator is introduced to the arena that rapidly moves toward the animal as it approaches the food pellet. A recent study used the looming disk and robopredator tasks and reported sex-dependent changes in behavioral response to the different threat stimuli in the alcohol drinking mice (Neira et al., 2022). While these are promising initial results, a more thorough evaluation of these open space assays with modified stimuli (i.e., overhead visual threat, sloped escape routes, or robopredators) will determine their usefulness as models to test novel compounds or study the mechanisms driving negative affect in alcohol drinking mice.
Comprehensive behavioral analysis systems
Large-scale systems for behavioral analysis are additional promising approaches to study the effects of alcohol on negative affect. For example, the proprietary SmartCube® system is an automated and high-throughput phenotyping platform that uses machine learning to evaluate 2000+ mouse behavioral ‘features’ that are combined into ~60 behavioral ‘clusters’ (Alexandrov, Brunner, Hanania, & Leahy, 2015). This platform can successfully differentiate open field behaviors in mice with susceptible or resilient phenotypes induced by chronic social defeat stress (Lorsch et al., 2020). Another comprehensive platform called PsyCoP was designed to assess behavioral endophenotypes (10 assays, 19 behavioral parameters, and 5 RDoC domains combined with dimension reduction analysis) in mice that were established from the RDoC framework, including negative and positive valence systems and cognitive domains (Volkmann, Stephan, Krackow, Jensen, & Rossner, 2020). To avoid experimenter bias and increase throughput, these platforms and assays have been developed to monitor longitudinal behaviors that can differentiate across behavioral domains, such as anxiety and locomotor activity, that can complicate interpretation of traditional assays of approach-avoidance behaviors (Kas, de Mooij-van Malsen, Olivier, Spruijt, & van Ree, 2008; Spruijt & DeVisser, 2006). Recently, analysis of behavioral changes in the home cage has been studied in mouse models of acute and chronic stress, as well as alcohol drinking. For example, a single exposure to a stressor or a history of long-term alcohol drinking shifted the expression of multiple home cage behaviors in mice, such as grooming, rearing, and digging (Fuzesi, Daviu, Wamsteeker Cusulin, Bonin, & Bains, 2016; Neira et al., 2022). When challenged with a bright light above their food for 1 hour during natural feeding times in an assay called the ‘light spot’ test, control mice will avoid the food zone and spend more time in a shelter only during the 1-hour illumination phase (Aarts et al., 2015; Nikolova et al., 2018; Prevot et al., 2019). In chronically stressed mice, the light challenge produced a ‘residual avoidance” of the food zone that persisted for hours after the challenge ended (Maluach et al., 2017; Nikolova et al., 2018; Prevot et al., 2019). Notably, avoidance behavior in the light spot test was observed in mice that were treated with UCMS and CRS protocols that produced varied responses on other traditional assays of negative affect (Prevot et al., 2019). We propose that application of these platforms and novel approaches will improve the validity of the mouse models and the translation for the study of AUD and co-occurring mood disorders.
Machine learning
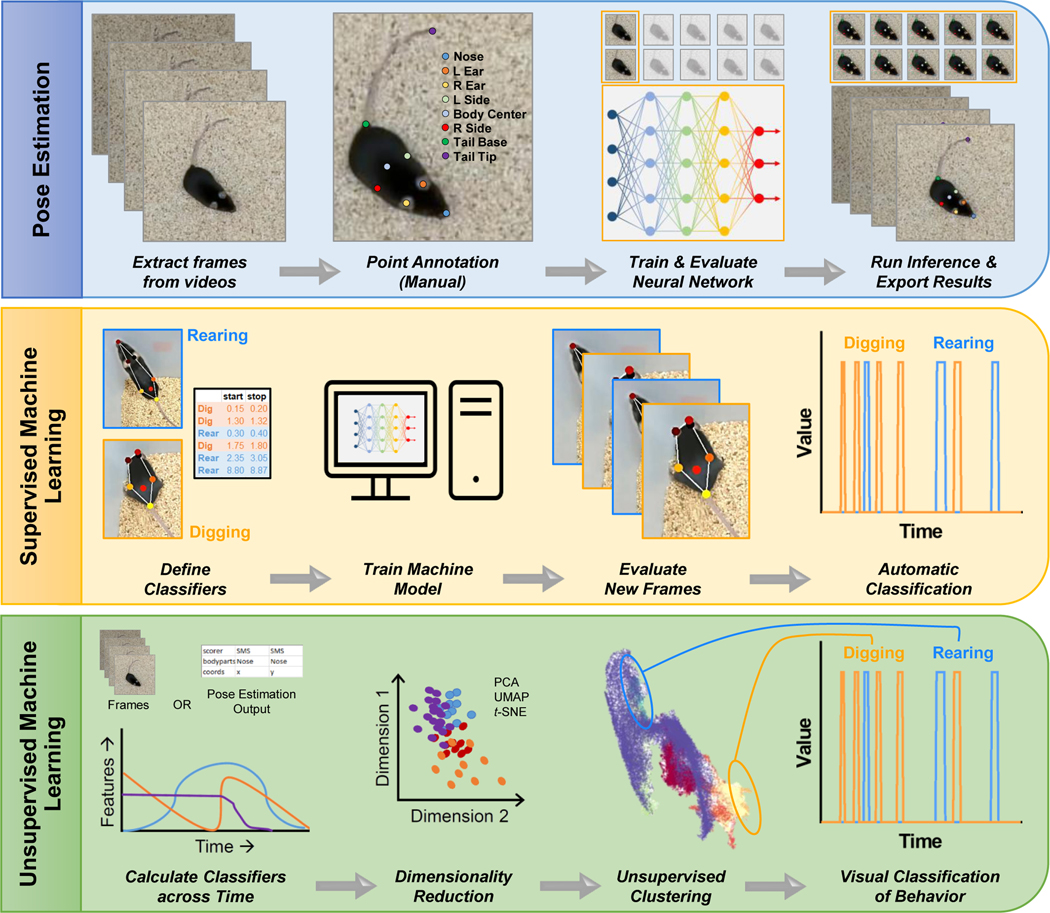
In addition to these large-scale behavioral systems, there are open source and unbiased machine learning pose estimation and behavioral tracking approaches (Graving et al., 2019; Mathis et al., 2018; Pereira et al., 2019; Wiltschko et al., 2015) that would allow for more complex and precise examination of negative affect. These machine-learning algorithms go beyond the simple spatio-temporal location or binary classification of behavior on a maze or in a home cage. For example, DeepLabCut is a deep neural network learning-based algorithm that can detect limb configurations and perform pose estimation (i.e., geometrical configuration of multiple body parts) and behavioral tracking in laboratory animals with high accuracy during performance on complex behavioral tasks (Mathis et al., 2018). Once data are collected with pose estimation algorithms, the output can be combined with secondary algorithms, such as SimBA (Nilsson et al., 2020), AlphaTracker (Z. Chen et al., 2020), VAME (Luxem et al., 2022), and B-SOiD (Hsu & Yttri, 2020), to identify and track ethological behavioral domains (mousebehavior.org). The secondary behavioral algorithms can be trained to track specified behaviors, such as rearing, digging, and grooming, or can identify behaviors using unsupervised learning classifiers (Sturman et al., 2020). An example of the pipeline for assessment of ethological behaviors using DeepLabCut and secondary behavioral classifiers in mice is shown in Figure 3. The combination of deep network learning of pose estimation and behavioral analysis provides a promising and high throughput approach to study altered ethological behaviors (e.g., escape behaviors, such as climbing or defensive behaviors, such as crouching) in mice during alcohol drinking and across acute to protracted withdrawal states, particularly those that occur in the home cage. Indeed, chronic alcohol drinking alters home cage behaviors in male mice that were assessed using machine learning and supervised behavioral classifiers (Neira et al., 2022). Home cage behavioral analysis has the advantage of monitoring and scoring behavior without the added stress of moving mice to a different apparatus. This is especially important when considering alcohol-stress interactions as spontaneous behavior in the home cage is highly sensitive to stress (Neira et al., 2022). In addition, capturing behavior in combination with automatic and synchronized recording of alcohol intake variables (e.g., bottle contacts, temporal distribution of drinking bouts, etc.) could help to determine how negative affective behaviors influence voluntary alcohol intake. Thus, application of new assays in conjunction with deep neural learning will streamline the collection of behavioral data and potentially reveal nuanced differences in home cage behavior that may inform assessments of negative affect in other assays, thereby reducing the time and resource requirements and improving the accuracy of behavioral outcomes in an effort to understand alcohol-induced negative affect in mouse models.
Figure 3.
Machine learning approaches for pose estimation and clustering of behaviors in mice. Top: Pose estimation algorithms, such as DeepLabCut, can track mouse body parts across time. Middle: Supervised machine learning approaches (e.g., SiMBA) can identify specific behaviors when classifiers are predefined. Bottom: Unsupervised machine learning algorithms (e.g., B-SOID, VAME) are used to identify different sets of behaviors in an unbiased manner.
Physiological endophenotypes
Although there is an apparent need to develop improved behavioral models or analytical approaches to better study negative affect in mice, an important bridge outside of the realm of behavior is to assay physiological endophenotypes that are highly translatable to human neuropsychiatric disorders. Physiological markers of autonomic nervous system and neuroendocrine function, such as blood pressure, heart rate variability, and hormone status, are all altered in individuals with depression and anxiety or those who have experienced severe or chronic stressors. Similarly, alterations in stress responses and autonomic dysregulation have been reported during acute withdrawal and protracted abstinence in alcohol dependent individuals (Adinoff, Junghanns, Kiefer, & Krishnan-Sarin, 2005; Bernardy, King, & Lovallo, 2003; Krystal et al., 1996; Sinha et al., 2009). Similar to individuals with AUD that show disturbances in sleep architecture (Koob & Colrain, 2020), studies have also reported altered sleep characteristics and other chronobiological behaviors in mice exposed to repeated alcohol (Huitron-Resendiz et al., 2018; Logan, McCulley, Seggio, & Rosenwasser, 2012; Logan, Seggio, Robinson, Richard, & Rosenwasser, 2010). There are commercially available telemeters (e.g., manufactured by Data Sciences International, Emka Technologies, and TSE Systems) that can acquire these physiological markers in mice and rats across long time scales (>2 months) without major disruption to normal behaviors. Telemetry has been applied to mouse models for the study of anxiety- and depressive-like behaviors (Camp et al., 2012; Gaburro et al., 2011; Wells et al., 2017), but assessment of autonomic endophenotypes using telemetry is underutilized in long-term alcohol exposure models beyond measures of body temperature. Thus, integrating telemetry technology across the development of excessive drinking or alcohol dependence with behavioral measures of negative affect will be a powerful approach to determine which behaviors and endophenotypes are closer to the human condition.
Conclusion
In this review, we present evidence of varied negative affective behavioral outcomes in mice with a history of alcohol exposure, regardless of the type of exposure model. The reasons for the complex findings vary depending on paradigms for assessing anxiety-like behaviors in male and female mice across different strains and the pattern, duration, and amount of alcohol drinking, and potentially diet-based variation in nutrition content. Newer models for assessing negative affective behaviors have been developed and validated using pharmacology and genetic strains of mice with differences in trait anxiety. When combined with high throughput automated analysis of behavior, these new assays and platforms aim to improve assessments of changes in innate behaviors in an unbiased and comprehensive manner. These new assays and computational approaches align with the goals of the RDoC initiative and offer the alcohol field the opportunity to identify reproducible behavioral outcomes in longitudinal studies that measure negative affect. Furthermore, an RDoC strategy can be used to improve use of previous negative affective models by providing a framework by which to interpret the behavior on such models. For example, approach/avoidance tasks may better model complex constructs like ambiguity/risk (reward valuation) under threat rather than negative affective behaviors and thus still provide a valuable understanding of basic processes in the context of AUD. Once improvements in preclinical mouse models or comprehensive platforms to study negative affective behaviors are established, they will provide a valuable approach toward defining the mechanisms and adaptations in cell subtypes and neurocircuits underlying behavioral dysfunction in alcohol drinking mice. In addition, screening of novel pharmacological targets using these innovative tools and platforms in behavioral assessment can accelerate the development of effective therapies for the treatment of co-occurring AUD and mood disorders.
Highlights.
AUD and co-morbid mood disorders contribute to poor treatment outcomes
Alcohol drinking paradigms in mice produce variable affective behaviors
New behavioral assays and analytical approaches are promising future directions
Advancements in negative affect assays will improve treatment strategies for AUD
Funding
This work was support of the NIH-funded INIAstress Consortium (AA013641), the Charleston Alcohol Research Center (AA010761), and NIH grants to PJM (AA020930, AA010761, and AA023288), JAR (AA025110), MFL (AA020929), and EMV (AA025481).
Footnotes
Declarations of interest: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aarts E, Maroteaux G, Loos M, Koopmans B, Kovacevic J, Smit AB, et al. (2015). The light spot test: Measuring anxiety in mice in an automated home-cage environment. Behav Brain Res, 294, 123–130. [DOI] [PubMed] [Google Scholar]
- Adinoff B, Junghanns K, Kiefer F, & Krishnan-Sarin S. (2005). Suppression of the HPA axis stress-response: implications for relapse. Alcohol Clin Exp Res, 29(7), 1351–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agabio R, & Leggio L. (2018). Baclofen in the Treatment of Patients With Alcohol Use Disorder and Other Mental Health Disorders. Front Psychiatry, 9, 464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahlgren J, & Voikar V. (2019). Housing mice in the individually ventilated or open cages-Does it matter for behavioral phenotype? Genes Brain Behav, 18(7), e12564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aikey JL, Nyby JG, Anmuth DM, & James PJ (2002). Testosterone rapidly reduces anxiety in male house mice (Mus musculus). Horm Behav, 42(4), 448–460. [DOI] [PubMed] [Google Scholar]
- Alexandrov V, Brunner D, Hanania T, & Leahy E. (2015). High-throughput analysis of behavior for drug discovery. Eur J Pharmacol, 750, 82–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RI, Lopez MF, & Becker HC (2016a). Forced swim stress increases ethanol consumption in C57BL/6J mice with a history of chronic intermittent ethanol exposure. Psychopharmacology (Berl), 233(11), 2035–2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RI, Lopez MF, & Becker HC (2016b). Stress-Induced Enhancement of Ethanol Intake in C57BL/6J Mice with a History of Chronic Ethanol Exposure: Involvement of Kappa Opioid Receptors. Front Cell Neurosci, 10, 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthenelli RM (2010). Focus On: Comorbid Mental Health Disorders. Alcohol Research & Health, 33(1–2), 109–117. [PMC free article] [PubMed] [Google Scholar]
- Anyan J, & Amir S. (2018). Too Depressed to Swim or Too Afraid to Stop? A Reinterpretation of the Forced Swim Test as a Measure of Anxiety-Like Behavior Neuropsychopharmacology (Vol. 43, pp. 931–933). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser DA, & Cuarenta A. (2021). Sex differences in anxiety and depression: circuits and mechanisms. Nat Rev Neurosci, 22(11), 674–684. [DOI] [PubMed] [Google Scholar]
- Barrenha GD, & Chester JA (2012). Effects of cross-fostering on alcohol preference and correlated responses to selection in high- and low-alcohol-preferring mice. Alcohol Clin Exp Res, 36(12), 2065–2073. [DOI] [PubMed] [Google Scholar]
- Becker HC (2013). Animal models of excessive alcohol consumption in rodents. Curr Top Behav Neurosci, 13, 355–377. [DOI] [PubMed] [Google Scholar]
- Becker HC (2017). Influence of stress associated with chronic alcohol exposure on drinking. Neuropharmacology, 122, 115–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, & Hu M. (2008). Sex differences in drug abuse. Front Neuroendocrinol, 29(1), 36–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, & Koob GF (2016). Sex Differences in Animal Models: Focus on Addiction. Pharmacol Rev, 68(2), 242–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmer A, Patkar OL, Lanoue V, & Bartlett SE (2018). 5-HT1A receptor-dependent modulation of emotional and neurogenic deficits elicited by prolonged consumption of alcohol. Sci Rep, 8(1), 2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berglund M, & Ojehagen A. (1998). The Influence of Alcohol Drinking and Alcohol Use Disorders on Psychiatric Disorders and Suicidal Behavior. Alcoholism, clinical and experimental research, 22(7 Suppl). [DOI] [PubMed] [Google Scholar]
- Bernardy NC, King AC, & Lovallo WR (2003). Cardiovascular responses to physical and psychological stress in female alcoholics with transitory hypertension after early abstinence. Alcohol Clin Exp Res, 27(9), 1489–1498. [DOI] [PubMed] [Google Scholar]
- Bilbao A, Robinson JE, Heilig M, Malanga CJ, Spanagel R, Sommer WH, et al. (2015). A pharmacogenetic determinant of mu-opioid receptor antagonist effects on alcohol reward and consumption: evidence from humanized mice. Biol Psychiatry, 77(10), 850–858. [DOI] [PubMed] [Google Scholar]
- Bilu C, & Kronfeld-Schor N. (2013). Effects of circadian phase and melatonin injection on anxiety-like behavior in nocturnal and diurnal rodents. Chronobiol Int, 30(6), 828–836. [DOI] [PubMed] [Google Scholar]
- Bloch S, Rinker JA, Marcus MM, & Mulholland PJ (2020). Absence of effects of intermittent access to alcohol on negative affective and anxiety-like behaviors in male and female C57BL/6J mice. Alcohol, 88, 91–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloodgood DW, Hardaway JA, Stanhope CM, Pati D, Pina MM, Neira S, et al. (2020). Kappa opioid receptor and dynorphin signaling in the central amygdala regulates alcohol intake. Mol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bott K, Meyer C, Rumpf H-J, Hapke U, & John U. (2015). Psychiatric disorders among at-risk consumers of alcohol in the general population. Journal of Studies on Alcohol. [DOI] [PubMed] [Google Scholar]
- Bray JG, Roberts AJ, & Gruol DL (2017). Transgenic mice with increased astrocyte expression of CCL2 show altered behavioral effects of alcohol. Neuroscience, 354, 88–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brière FN, Rohde P, Seeley JR, Klein D, & Lewinsohn PM (2014). Comorbidity between major depression and alcohol use disorder from adolescence to adulthood. Comprehensive Psychiatry, 55(3), 526–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camp MC, Macpherson KP, Lederle L, Graybeal C, Gaburro S, Debrouse LM, et al. (2012). Genetic strain differences in learned fear inhibition associated with variation in neuroendocrine, autonomic, and amygdala dendritic phenotypes. Neuropsychopharmacology, 37(6), 1534–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannady R, Nguyen T, Padula AE, Rinker JA, Lopez MF, Becker HC, et al. (2021). Interaction of chronic intermittent ethanol and repeated stress on structural and functional plasticity in the mouse medial prefrontal cortex. Neuropharmacology, 182, 108396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnicella S, Ron D, & Barak S. (2014). Intermittent ethanol access schedule in rats as a preclinical model of alcohol abuse. Alcohol, 48(3), 243–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll ME, & Anker JJ (2010). Sex differences and ovarian hormones in animal models of drug dependence. Horm Behav, 58(1), 44–56. [DOI] [PubMed] [Google Scholar]
- Centanni SW, Morris BD, Luchsinger JR, Bedse G, Fetterly TL, Patel S, et al. (2019). Endocannabinoid control of the insular-bed nucleus of the stria terminalis circuit regulates negative affective behavior associated with alcohol abstinence. Neuropsychopharmacology, 44(3), 526–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen NA, Jupp B, Sztainberg Y, Lebow M, Brown RM, Kim JH, et al. (2014). Knockdown of CRF1 receptors in the ventral tegmental area attenuates cue- and acute food deprivation stress-induced cocaine seeking in mice. J Neurosci, 34(35), 11560–11570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Zhang R, Eva Zhang Y, Zhou H, Fang H-S, Rock RR, et al. (2020). AlphaTracker: A Multi-Animal Tracking and Behavioral Analysis Tool. bioRxiv, 2020.2012.2004.405159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chester JA, & Barrenha GD (2007). Acoustic startle at baseline and during acute alcohol withdrawal in replicate mouse lines selectively bred for high or low alcohol preference. Alcohol Clin Exp Res, 31(10), 1633–1644. [DOI] [PubMed] [Google Scholar]
- Choi JS, & Kim JJ (2010). Amygdala regulates risk of predation in rats foraging in a dynamic fear environment. Proc Natl Acad Sci U S A, 107(50), 21773–21777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark Goings T, Salas-Wright CP, Belgrave FZ, Nelson EJ, Harezlak J, & Vaughn MG (2019). Trends in binge drinking and alcohol abstention among adolescents in the US, 2002–2016. Drug Alcohol Depend, 200, 115–123. [DOI] [PubMed] [Google Scholar]
- Commons KG, Cholanians AB, Babb JA, & Ehlinger DG (2017). The Rodent Forced Swim Test Measures Stress-Coping Strategy, Not Depression-like Behavior. ACS Chem Neurosci, 8(5), 955–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox BR, Olney JJ, Lowery-Gionta EG, Sprow GM, Rinker JA, Navarro M, et al. (2013). Repeated cycles of binge-like ethanol (EtOH)-drinking in male C57BL/6J mice augments subsequent voluntary EtOH intake but not other dependence-like phenotypes. Alcohol Clin Exp Res, 37(10), 1688–1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe JC (2014). Rodent models of genetic contributions to motivation to abuse alcohol. Nebr Symp Motiv, 61, 5–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dao NC, Suresh Nair M, Magee SN, Moyer JB, Sendao V, Brockway DF, et al. (2020). Forced Abstinence From Alcohol Induces Sex-Specific Depression-Like Behavioral and Neural Adaptations in Somatostatin Neurons in Cortical and Amygdalar Regions. Front Behav Neurosci, 14, 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daut RA, Busch EF, Ihne J, Fisher D, Mishina M, Grant SG, et al. (2015). Tolerance to ethanol intoxication after chronic ethanol: role of GluN2A and PSD-95. Addict Biol, 20(2), 259–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Almeida Magalhaes T, Correia D, de Carvalho LM, Damasceno S, & Brunialti Godard AL (2018). Maternal separation affects expression of stress response genes and increases vulnerability to ethanol consumption. Brain Behav, 8(1), e00841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Brouwer G, Fick A, Harvey BH, & Wolmarans W. (2019). A critical inquiry into marble-burying as a preclinical screening paradigm of relevance for anxiety and obsessive-compulsive disorder: Mapping the way forward. Cogn Affect Behav Neurosci, 19(1), 1–39. [DOI] [PubMed] [Google Scholar]
- De Franceschi G, Vivattanasarn T, Saleem AB, & Solomon SG (2016). Vision Guides Selection of Freeze or Flight Defense Strategies in Mice. Curr Biol, 26(16), 2150–2154. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, & Molendijk ML (2016). Coping with the Forced Swim Stressor: Towards Understanding an Adaptive Mechanism. Neural Plast, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Hartog CR, Blandino KL, Nash ML, Sjogren ER, Grampetro MA, Moorman DE, et al. (2020). Noradrenergic tone mediates marble burying behavior after chronic stress and ethanol. Psychopharmacology (Berl), 237(10), 3021–3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deslauriers J, Toth M, Der-Avakian A, & Risbrough VB (2018). Current Status of Animal Models of Posttraumatic Stress Disorder: Behavioral and Biological Phenotypes, and Future Challenges in Improving Translation. Biol Psychiatry, 83(10), 895–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaud LL, & Chadda R. (2001). Sex differences in rats in the development of and recovery from ethanol dependence assessed by changes in seizure susceptibility. Alcohol Clin Exp Res, 25(11), 1689–1696. [PubMed] [Google Scholar]
- Driessen M, Meier S, Hill A, Wetterling T, Lange W, & Junghanns K. (2001). The course of anxiety, depression and drinking behaviours after completed detoxification in alcoholics with and without comorbid anxiety and depressive disorders. Alcohol Alcohol, 36(3), 249–255. [DOI] [PubMed] [Google Scholar]
- Ennaceur A. (2011). Omission of the habituation procedure in the acquisition of a working memory task - evidence from Balb/c, C57/BL6J, and CD-1 mice. Behav Brain Res, 223(1), 203–210. [DOI] [PubMed] [Google Scholar]
- Ennaceur A. (2014). Tests of unconditioned anxiety - pitfalls and disappointments. Physiol Behav, 135, 55–71. [DOI] [PubMed] [Google Scholar]
- Ennaceur A, Michalikova S, van Rensburg R, & Chazot PL (2006). Models of anxiety: responses of mice to novelty and open spaces in a 3D maze. Behav Brain Res, 174(1), 9–38. [DOI] [PubMed] [Google Scholar]
- Ennaceur A, Michalikova S, van Rensburg R, & Chazot PL (2008). Are benzodiazepines really anxiolytic? Evidence from a 3D maze spatial navigation task. Behav Brain Res, 188(1), 136–153. [DOI] [PubMed] [Google Scholar]
- Ennaceur A, Michalikova S, van Rensburg R, & Chazot PL (2010). Distinguishing anxiolysis and hyperactivity in an open space behavioral test. Behav Brain Res, 207(1), 84–98. [DOI] [PubMed] [Google Scholar]
- Enoch MA (2011). The role of early life stress as a predictor for alcohol and drug dependence. Psychopharmacology (Berl), 214(1), 17–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson K, & Pikkarainen PH (1968). Differences between the sexes in voluntary alcohol consumption and liver ADH-activity in inbred strains of mice. Metabolism, 17(11), 1037–1042. [DOI] [PubMed] [Google Scholar]
- Erol A, & Karpyak VM (2015). Sex and gender-related differences in alcohol use and its consequences: Contemporary knowledge and future research considerations. Drug and Alcohol Dependence, 156, 1–13. [DOI] [PubMed] [Google Scholar]
- Farren CK, Hill KP, & Weiss RD (2012). Bipolar Disorder and Alcohol Use Disorder: A Review. Current Psychiatry Reports, 14(6), 659–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes C, Gonzalez MI, Wilson CA, & File SE (1999). Factor analysis shows that female rat behaviour is characterized primarily by activity, male rats are driven by sex and anxiety. Pharmacol Biochem Behav, 64(4), 731–738. [DOI] [PubMed] [Google Scholar]
- Fernandez-Guasti A, & Picazo O. (1992). Changes in burying behavior during the estrous cycle: effect of estrogen and progesterone. Psychoneuroendocrinology, 17(6), 681–689. [DOI] [PubMed] [Google Scholar]
- Finn DA, Gallaher EJ, & Crabbe JC (2000). Differential change in neuroactive steroid sensitivity during ethanol withdrawal. J Pharmacol Exp Ther, 292(1), 394–405. [PubMed] [Google Scholar]
- Fish EW, DiBerto JF, Krouse MC, Robinson JE, & Malanga CJ (2014). Different contributions of dopamine D1 and D2 receptor activity to alcohol potentiation of brain stimulation reward in C57BL/6J and DBA/2J mice. J Pharmacol Exp Ther, 350(2), 322–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish EW, Riday TT, McGuigan MM, Faccidomo S, Hodge CW, & Malanga CJ (2010). Alcohol, cocaine, and brain stimulation-reward in C57Bl6/J and DBA2/J mice. Alcohol Clin Exp Res, 34(1), 81–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish EW, Robinson JE, Krouse MC, Hodge CW, Reed C, Phillips TJ, et al. (2012). Intracranial self-stimulation in FAST and SLOW mice: effects of alcohol and cocaine. Psychopharmacology (Berl), 220(4), 719–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanigan ME, Hon OJ, D’Ambrosio S, Boyt KM, Hassanein L, Castle M, et al. (2022). Sex-specific regulation of binge drinking and affective behaviors by subcortical serotonin 5HT2c receptors. bioRxiv, 2022.2001.2028.478036. [Google Scholar]
- Fuzesi T, Daviu N, Wamsteeker Cusulin JI, Bonin RP, & Bains JS (2016). Hypothalamic CRH neurons orchestrate complex behaviours after stress. Nat Commun, 7, 11937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaburro S, Stiedl O, Giusti P, Sartori SB, Landgraf R, & Singewald N. (2011). A mouse model of high trait anxiety shows reduced heart rate variability that can be reversed by anxiolytic drug treatment. Int J Neuropsychopharmacol, 14(10), 1341–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangitano D, Salas R, Teng Y, Perez E, & De Biasi M. (2009). Progesterone modulation of alpha5 nAChR subunits influences anxiety-related behavior during estrus cycle. Genes Brain Behav, 8(4), 398406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Sifuentes Y, & Maney DL (2021). Reporting and misreporting of sex differences in the biological sciences. Elife, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, & Koob GF (2008). Neurobiology of Alcohol Dependence: Focus on Motivational Mechanisms Alcohol Res Health (Vol. 31, pp. 185–195). [PMC free article] [PubMed] [Google Scholar]
- Gimeno C, Dorado ML, Roncero C, Szerman N, Vega P, Balanza-Martinez V, et al. (2017). Treatment of Comorbid Alcohol Dependence and Anxiety Disorder: Review of the Scientific Evidence and Recommendations for Treatment. Front Psychiatry, 8, 173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girolamo G, Gureje O, Haro JM, He Y, Hinkov H, Hu C, et al. (2017). Cross-sectional Comparison of the Epidemiology of DSM-5 Generalized Anxiety Disorder Across the Globe. JAMA psychiatry, 74(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RB, Dawson DA, Chou SP, & Grant BF (2012). Sex Differences in Prevalence and Comorbidity of Alcohol and Drug Use Disorders: Results From Wave 2 of the National Epidemiologic Survey on Alcohol and Related Conditions. Journal of Studies on Alcohol and Drugs, 73(6), 938–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong MF, Wen RT, Xu Y, Pan JC, Fei N, Zhou YM, et al. (2017). Attenuation of ethanol abstinence-induced anxiety- and depressive-like behavior by the phosphodiesterase-4 inhibitor rolipram in rodents. Psychopharmacology (Berl), 234(20), 3143–3151. [DOI] [PubMed] [Google Scholar]
- Grant BF, Stinson FS, Dawson DA, Chou SP, Dufour MC, Compton W, et al. (2004). Prevalence and Co-occurrence of Substance Use Disorders and IndependentMood and Anxiety Disorders: Results From the National Epidemiologic Survey on Alcohol and RelatedConditions. Archives of General Psychiatry, 61(8), 807–816. [DOI] [PubMed] [Google Scholar]
- Graving JM, Chae D, Naik H, Li L, Koger B, Costelloe BR, et al. (2019). DeepPoseKit, a software toolkit for fast and robust animal pose estimation using deep learning. Elife, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin WC 3rd. (2014). Alcohol dependence and free-choice drinking in mice. Alcohol, 48(3), 287–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gururajan A, Reif A, Cryan JF, & Slattery DA (2019). The future of rodent models in depression research. Nat Rev Neurosci, 20(11), 686–701. [DOI] [PubMed] [Google Scholar]
- Hartmann MC, Haney MM, Smith CG, Kumar V, & Rosenwasser AM (2020). Affective Disruption During Forced Ethanol Abstinence in C57BL/6J and C57BL/6NJ Mice. Alcohol Clin Exp Res, 44(10), 2019–2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann MC, Holbrook SE, Haney MM, Crabbe JC, & Rosenwasser AM (2019). Affective Behavior in Withdrawal Seizure-Prone and Withdrawal Seizure-Resistant Mice during Long-Term Alcohol Abstinence. Alcohol Clin Exp Res, 43(7), 1478–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig M, Egli M, Crabbe JC, & Becker HC (2010). REVIEW: Acute withdrawal, protracted abstinence and negative affect in alcoholism: are they linked? Addiction Biology, 15(2), 169–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson ND (1967). Prior treatment effects on open field behaviour of mice--a genetic analysis. Anim Behav, 15(2), 364–376. [DOI] [PubMed] [Google Scholar]
- Hernandez-Avila CA, Rounsaville BJ, & Kranzler HR (2004). Opioid-, cannabis- and alcohol-dependent women show more rapid progression to substance abuse treatment. Drug Alcohol Depend, 74(3), 265–272. [DOI] [PubMed] [Google Scholar]
- Holleran Wilson H.H., Fetterly TL, Bluett RJ, Centanni SW, Gilfarb RA, et al. (2016). Ketamine and MAG Lipase Inhibitor-Dependent Reversal of Evolving Depressive-Like Behavior During Forced Abstinence From Alcohol Drinking. Neuropsychopharmacology, 41(8), 2062–2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holleran KM, Wilson HH, Fetterly TL, Bluett RJ, Centanni SW, Gilfarb RA, et al. (2016). Ketamine and MAG Lipase Inhibitor-Dependent Reversal of Evolving Depressive-Like Behavior During Forced Abstinence From Alcohol Drinking. Neuropsychopharmacology, 41(8), 2062–2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holleran KM, & Winder DG (2017). Preclinical voluntary drinking models for alcohol abstinence-induced affective disturbances in mice. Genes Brain Behav, 16(1), 8–14. [DOI] [PubMed] [Google Scholar]
- Holmes A, Fitzgerald PJ, MacPherson KP, DeBrouse L, Colacicco G, Flynn SM, et al. (2012). Chronic alcohol remodels prefrontal neurons and disrupts NMDAR-mediated fear extinction encoding. Nat Neurosci, 15(10), 1359–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu AI, & Yttri EA (2020). B-SOiD: An Open Source Unsupervised Algorithm for Discovery of Spontaneous Behaviors. bioRxiv, 770271. [Google Scholar]
- Huitron-Resendiz S, Nadav T, Krause S, Cates-Gatto C, Polis I, & Roberts AJ (2018). Effects of Withdrawal from Chronic Intermittent Ethanol Exposure on Sleep Characteristics of Female and Male Mice. Alcohol Clin Exp Res, 42(3), 540–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchins JB, Allen DL, Cole-Harding LS, & Wilson JR (1981). Behavioral and physiological measures for studying ethanol dependence in mice. Pharmacol Biochem Behav, 15(1), 55–59. [DOI] [PubMed] [Google Scholar]
- Hwa LS, Chu A, Levinson SA, Kayyali TM, DeBold JF, & Miczek KA (2011). Persistent escalation of alcohol drinking in C57BL/6J mice with intermittent access to 20% ethanol. Alcohol Clin Exp Res, 35(11), 1938–1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwa LS, Nathanson AJ, Shimamoto A, Tayeh JK, Wilens AR, Holly EN, et al. (2015). Aggression and increased glutamate in the mPFC during withdrawal from intermittent alcohol in outbred mice. Psychopharmacology (Berl), 232(16), 2889–2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, et al. (2010). Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry, 167(7), 748–751. [DOI] [PubMed] [Google Scholar]
- Ipser JC, Wilson D, Akindipe TO, Sager C, & Stein DJ (2015). Pharmacotherapy for anxiety and comorbid alcohol use disorders. Cochrane Database Syst Rev, 1, CD007505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janis GC, Devaud LL, Mitsuyama H, & Morrow AL (1998). Effects of chronic ethanol consumption and withdrawal on the neuroactive steroid 3alpha-hydroxy-5alpha-pregnan-20-one in male and female rats. Alcohol Clin Exp Res, 22(9), 2055–2061. [PubMed] [Google Scholar]
- Jimenez Chavez CL, Coelho MA, Brewin LW, Swauncy I, Tran T, Albanese T, et al. (2020). Incubation of Negative Affect during Protracted Alcohol Withdrawal Is Age-, but Not Sex-Selective. Brain Sci, 10(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joffe ME, Winder DG, & Conn PJ (2020). Contrasting sex-dependent adaptations to synaptic physiology and membrane properties of prefrontal cortex interneuron subtypes in a mouse model of binge drinking. Neuropharmacology, 178, 108126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jury NJ, DiBerto JF, Kash TL, & Holmes A. (2017). Sex differences in the behavioral sequelae of chronic ethanol exposure. Alcohol, 58, 53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kas MJ, de Mooij-van Malsen AJ, Olivier B, Spruijt BM, & van Ree JM (2008). Differential genetic regulation of motor activity and anxiety-related behaviors in mice using an automated home cage task. Behav Neurosci, 122(4), 769–776. [DOI] [PubMed] [Google Scholar]
- Kash TL, Baucum AJ 2nd, Conrad KL, Colbran RJ, & Winder DG (2009). Alcohol exposure alters NMDAR function in the bed nucleus of the stria terminalis. Neuropsychopharmacology, 34(11), 2420–2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyes KM, Hatzenbuehler ML, Grant BF, & Hasin DS (2012). Stress and alcohol: epidemiologic evidence. Alcohol Res, 34(4), 391–400. [PMC free article] [PubMed] [Google Scholar]
- Kim EJ, Kong MS, Park SG, Mizumori SJY, Cho J, & Kim JJ (2018). Dynamic coding of predatory information between the prelimbic cortex and lateral amygdala in foraging rats. Sci Adv, 4(4), eaar7328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimbrough A, Lurie DJ, Collazo A, Kreifeldt M, Sidhu H, Macedo GC, et al. (2020). Brain-wide functional architecture remodeling by alcohol dependence and abstinence. Proceedings of the National Academy of Sciences, 117(4), 2149–2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirstein SL, Davidson KL, Ehringer MA, Sikela JM, Erwin VG, & Tabakoff B. (2002). Quantitative trait loci affecting initial sensitivity and acute functional tolerance to ethanol-induced ataxia and brain cAMP signaling in BXD recombinant inbred mice. J Pharmacol Exp Ther, 302(3), 1238–1245. [DOI] [PubMed] [Google Scholar]
- Kliethermes CL (2005). Anxiety-like behaviors following chronic ethanol exposure. Neurosci Biobehav Rev, 28(8), 837–850. [DOI] [PubMed] [Google Scholar]
- Kliethermes CL, Cronise K, & Crabbe JC (2004). Anxiety-like behavior in mice in two apparatuses during withdrawal from chronic ethanol vapor inhalation. Alcohol Clin Exp Res, 28(7), 1012–1019. [DOI] [PubMed] [Google Scholar]
- Kokras N, & Dalla C. (2014). Sex differences in animal models of psychiatric disorders. British Journal of Pharmacology, 171(20), 4595–4619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF (2021). Drug Addiction: Hyperkatifeia/Negative Reinforcement as a Framework for Medications Development. Pharmacol Rev, 73(1), 163–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, & Colrain IM (2020). Alcohol use disorder and sleep disturbances: a feed-forward allostatic framework. Neuropsychopharmacology, 45(1), 141–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornetsky C, Bain GT, Unterwald EM, & Lewis MJ (1988). Brain stimulation reward: effects of ethanol. Alcohol Clin Exp Res, 12(5), 609–616. [DOI] [PubMed] [Google Scholar]
- Kosobud A, & Crabbe JC (1986). Ethanol withdrawal in mice bred to be genetically prone or resistant to ethanol withdrawal seizures. J Pharmacol Exp Ther, 238(1), 170–177. [PubMed] [Google Scholar]
- Krystal JH, Webb E, Cooney NL, Kranzler HR, Southwick SW, Heninger GR, et al. (1996). Serotonergic and noradrenergic dysregulation in alcoholism: m-chlorophenylpiperazine and yohimbine effects in recently detoxified alcoholics and healthy comparison subjects. Am J Psychiatry, 153(1), 83–92. [DOI] [PubMed] [Google Scholar]
- Lai HMX, Cleary M, Sitharthan T, & Hunt GE (2015). Prevalence of comorbid substance use, anxiety and mood disorders in epidemiological surveys, 1990–2014: A systematic review and meta-analysis. Drug and Alcohol Dependence, 154, 1–13. [DOI] [PubMed] [Google Scholar]
- Lee KM, Coehlo M, McGregor HA, Waltermire RS, & Szumlinski KK (2015). Binge alcohol drinking elicits persistent negative affect in mice. Behav Brain Res, 291, 385–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KM, Coehlo MA, Solton NR, & Szumlinski KK (2017). Negative Affect and Excessive Alcohol Intake Incubate during Protracted Withdrawal from Binge-Drinking in Adolescent, But Not Adult, Mice. Front Psychol, 8, 1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KM, Coelho MA, Class MA, Sern KR, Bocz MD, & Szumlinski KK (2018). mGlu5 Receptor Blockade Within the Nucleus Accumbens Shell Reduces Behavioral Indices of Alcohol Withdrawal-Induced Anxiety in Mice. Front Pharmacol, 9, 1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KM, Coelho MA, Class MA, & Szumlinski KK (2018). mGlu5-dependent modulation of anxiety during early withdrawal from binge-drinking in adult and adolescent male mice. Drug Alcohol Depend, 184, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KM, Coelho MA, McGregor HA, Solton NR, Cohen M, & Szumlinski KK (2016). Adolescent Mice Are Resilient to Alcohol Withdrawal-Induced Anxiety and Changes in Indices of Glutamate Function within the Nucleus Accumbens. Front Cell Neurosci, 10, 265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KM, Coelho MA, Sern KR, Class MA, Bocz MD, & Szumlinski KK (2017). Anxiolytic effects of buspirone and MTEP in the Porsolt Forced Swim Test. Chronic Stress (Thousand Oaks), 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KM, Coelho MA, Sern KR, & Szumlinski KK (2018). Homer2 within the central nucleus of the amygdala modulates withdrawal-induced anxiety in a mouse model of binge-drinking. Neuropharmacology, 128, 448–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lezak KR, Missig G, & Carlezon WA Jr. (2017). Behavioral methods to study anxiety in rodents. Dialogues Clin Neurosci, 19(2), 181–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Feng X, Zhou Z, Zhang H, Shi Q, Lei Z, et al. (2018). Stress Accelerates Defensive Responses to Looming in Mice and Involves a Locus Coeruleus-Superior Colliculus Projection. Curr Biol, 28(6), 859–871 e855. [DOI] [PubMed] [Google Scholar]
- Litten RZ, Ryan ML, Falk DE, Reilly M, Fertig JB, & Koob GF (2015). Heterogeneity of Alcohol Use Disorder: Understanding Mechanisms to Advance Personalized Treatment. Alcoholism, clinical and experimental research, 39(4). [DOI] [PubMed] [Google Scholar]
- Logan RW, McCulley WD 3rd, Seggio JA, & Rosenwasser AM (2012). Effects of withdrawal from chronic intermittent ethanol vapor on the level and circadian periodicity of running-wheel activity in C57BL/6J and C3H/HeJ mice. Alcohol Clin Exp Res, 36(3), 467–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan RW, Seggio JA, Robinson SL, Richard GR, & Rosenwasser AM (2010). Circadian wheel-running activity during withdrawal from chronic intermittent ethanol exposure in mice. Alcohol, 44(3), 239–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez MF, Anderson RI, & Becker HC (2016). Effect of different stressors on voluntary ethanol intake in ethanol-dependent and nondependent C57BL/6J mice. Alcohol, 51, 17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez MF, & Becker HC (2014). Operant ethanol self-administration in ethanol dependent mice. Alcohol, 48(3), 295–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez MF, Reasons SE, Carper BA, Nolen TL, Williams RL, & Becker HC (2020). Evaluation of the effect of doxasozin and zonisamide on voluntary ethanol intake in mice that experienced chronic intermittent ethanol exposure and stress. Alcohol, 89, 37–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorsch ZS, Ambesi-Impiombato A, Zenowich R, Morganstern I, Leahy E, Bansal M, et al. (2020). Computational Analysis of Multidimensional Behavioral Alterations After Chronic Social Defeat Stress. Biol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowery-Gionta EG, Marcinkiewcz CA, & Kash TL (2015). Functional alterations in the dorsal raphe nucleus following acute and chronic ethanol exposure. Neuropsychopharmacology, 40(3), 590–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luxem K, Mocellin P, Fuhrmann F, Kuersch J, Remy S, & Bauer P. (2022). Identifying Behavioral Structure from Deep Variational Embeddings of Animal Motion. BioRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado-Devincci AM, Kampov-Polevoi A, McKinley RE, Morrow DH, O’Buckley TK, & Morrow AL (2016). Chronic Intermittent Ethanol Exposure Alters Stress Effects on (3alpha,5alpha)-3-hydroxy-pregnan-20-one (3alpha,5alpha-THP) Immunolabeling of Amygdala Neurons in C57BL/6J Mice. Front Cell Neurosci, 10, 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maluach AM, Misquitta KA, Prevot TD, Fee C, Sibille E, Banasr M, et al. (2017). Increased Neuronal DNA/RNA Oxidation in the Frontal Cortex of Mice Subjected to Unpredictable Chronic Mild Stress. Chronic Stress (Thousand Oaks), 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall SA, Rinker JA, Harrison LK, Fletcher CA, Herfel TM, & Thiele TE (2015). Assessment of the Effects of 6 Standard Rodent Diets on Binge-Like and Voluntary Ethanol Consumption in Male C57BL/6J Mice. Alcohol Clin Exp Res, 39(8), 1406–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathis A, Mamidanna P, Cury KM, Abe T, Murthy VN, Mathis MW, et al. (2018). DeepLabCut: markerless pose estimation of user-defined body parts with deep learning. Nat Neurosci, 21(9), 1281–1289. [DOI] [PubMed] [Google Scholar]
- McCance-Katz EF, Carroll KM, & Rounsaville BJ (1999). Gender differences in treatment-seeking cocaine abusers--implications for treatment and prognosis. Am J Addict, 8(4), 300–311. [DOI] [PubMed] [Google Scholar]
- McCool BA, & Chappell AM (2015). Chronic intermittent ethanol inhalation increases ethanol self-administration in both C57BL/6J and DBA/2J mice. Alcohol, 49(2), 111–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIlwain KL, Merriweather MY, Yuva-Paylor LA, & Paylor R. (2001). The use of behavioral test batteries: effects of training history. Physiol Behav, 73(5), 705–717. [DOI] [PubMed] [Google Scholar]
- Melendez RI (2011). Intermittent (every-other-day) drinking induces rapid escalation of ethanol intake and preference in adolescent and adult C57BL/6J mice. Alcohol Clin Exp Res, 35(4), 652–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metten P, Schlumbohm JP, Huang LC, Greenberg GD, Hack WR, Spence SE, et al. (2018). An alcohol withdrawal test battery measuring multiple behavioral symptoms in mice. Alcohol, 68, 19–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metten P, Sorensen ML, Cameron AJ, Yu CH, & Crabbe JC (2010). Withdrawal severity after chronic intermittent ethanol in inbred mouse strains. Alcohol Clin Exp Res, 34(9), 1552–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalikova S, van Rensburg R, Chazot PL, & Ennaceur A. (2010). Anxiety responses in Balb/c, c57 and CD-1 mice exposed to a novel open space test. Behav Brain Res, 207(2), 402–417. [DOI] [PubMed] [Google Scholar]
- Middaugh LD, Kelley BM, Bandy AL, & McGroarty KK (1999). Ethanol consumption by C57BL/6 mice: influence of gender and procedural variables. Alcohol, 17(3), 175–183. [DOI] [PubMed] [Google Scholar]
- Miranda R Jr., Meyerson LA, Buchanan TW, & Lovallo WR (2002). Altered emotion-modulated startle in young adults with a family history of alcoholism. Alcohol Clin Exp Res, 26(4), 441–448. [PubMed] [Google Scholar]
- Miranda R Jr., Meyerson LA, Myers RR, & Lovallo WR (2003). Altered affective modulation of the startle reflex in alcoholics with antisocial personality disorder. Alcohol Clin Exp Res, 27(12), 1901–1911. [DOI] [PubMed] [Google Scholar]
- Molendijk ML, & de Kloet ER (2015). Immobility in the forced swim test is adaptive and does not reflect depression. Psychoneuroendocrinology, 62, 389–391. [DOI] [PubMed] [Google Scholar]
- Morris SE, & Cuthbert BN (2012). Research Domain Criteria: cognitive systems, neural circuits, and dimensions of behavior. Dialogues Clin Neurosci, 14(1), 29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naassila M, Ledent C, & Daoust M. (2002). Low ethanol sensitivity and increased ethanol consumption in mice lacking adenosine A2A receptors. J Neurosci, 22(23), 10487–10493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neira S, Hassanein LA, Stanhope CM, Buccini MC, D’Ambrosio SL, Flanigan ME, et al. (2022). Chronic Alcohol Consumption Alters Home-Cage Behaviors and Responses to Ethologically Relevant Predator Tasks in Mice. bioRxiv, 2022.2002.2004.479122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson BS, Sequeira MK, & Schank JR (2018). Bidirectional relationship between alcohol intake and sensitivity to social defeat: association with Tacr1 and Avp expression. Addict Biol, 23(1), 142–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nennig SE, Fulenwider HD, Eskew JE, Whiting KE, Cotton MR, McGinty GE, et al. (2020). Intermittent Ethanol Access Increases Sensitivity to Social Defeat Stress. Alcohol Clin Exp Res, 44(3), 600–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, & Hyman SE, (2010). Animal models of neuropsychiatric disorders. [ReviewPaper]. Nature Neuroscience, 13(10), 1161–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIAAA. (2021). Binge Drinking. [Google Scholar]
- Nieto SJ, Grodin EN, Aguirre CG, Izquierdo A, & Ray LA (2021). Translational opportunities in animal and human models to study alcohol use disorder. Transl Psychiatry, 11(1), 496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolova YS, Misquitta KA, Rocco BR, Prevot TD, Knodt AR, Ellegood J, et al. (2018). Shifting priorities: highly conserved behavioral and brain network adaptations to chronic stress across species. Transl Psychiatry, 8(1), 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson SR, Goodwin NL, Choong JJ, Hwang S, Wright HR, Norville ZC, et al. (2020). Simple Behavioral Analysis (SimBA) – an open source toolkit for computer classification of complex social behaviors in experimental animals. bioRxiv, 2020.2004.2019.049452. [Google Scholar]
- Ohl F. (2005). Animal models of anxiety. Handb Exp Pharmacol(169), 35–69. [DOI] [PubMed] [Google Scholar]
- Okhuarobo A, Bolton JL, Igbe I, Zorrilla EP, Baram TZ, & Contet C. (2020). A novel mouse model for vulnerability to alcohol dependence induced by early-life adversity. Neurobiol Stress, 13, 100269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olney JJ, Marshall SA, & Thiele TE (2018). Assessment of depression-like behavior and anhedonia after repeated cycles of binge-like ethanol drinking in male C57BL/6J mice. Pharmacol Biochem Behav, 168, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padula AE, Rinker JA, Lopez MF, Mulligan MK, Williams RW, Becker HC, et al. (2020). Bioinformatics identification and pharmacological validation of Kcnn3/KCa2 channels as a mediator of negative affective behaviors and excessive alcohol drinking in mice. Transl Psychiatry, 10(1), 414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paljärvi T, Koskenvuo M, Poikolainen K, Kauhanen J, Sillanmäki L, & Mäkelä P. (2009). Binge Drinking and Depressive Symptoms: A 5-year Population-Based Cohort Study. Addiction (Abingdon, England), 104(7). [DOI] [PubMed] [Google Scholar]
- Pang TY, Du X, Catchlove WA, Renoir T, Lawrence AJ, & Hannan AJ (2013). Positive environmental modification of depressive phenotype and abnormal hypothalamic-pituitary-adrenal axis activity in female C57BL/6J mice during abstinence from chronic ethanol consumption. Front Pharmacol, 4, 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang TY, Renoir T, Du X, Lawrence AJ, & Hannan AJ (2013). Depression-related behaviours displayed by female C57BL/6J mice during abstinence from chronic ethanol consumption are rescued by wheel-running. European Journal of Neuroscience, 37(11), 1803–1810. [DOI] [PubMed] [Google Scholar]
- Pang TY, Renoir T, Du X, Lawrence AJ, & Hannan AJ (2013). Depression-related behaviours displayed by female C57BL/6J mice during abstinence from chronic ethanol consumption are rescued by wheel-running. Eur J Neurosci, 37(11), 1803–1810. [DOI] [PubMed] [Google Scholar]
- Park CL, Armeli S, & Tennen H. (2004). The daily stress and coping process and alcohol use among college students. J Stud Alcohol, 65(1), 126–135. [DOI] [PubMed] [Google Scholar]
- Pasquarelli N, Voehringer P, Henke J, & Ferger B. (2017). Effect of a change in housing conditions on body weight, behavior and brain neurotransmitters in male C57BL/6J mice. Behav Brain Res, 333, 35–42. [DOI] [PubMed] [Google Scholar]
- Patisaul HB, Blum A, Luskin JR, & Wilson ME (2005). Dietary soy supplements produce opposite effects on anxiety in intact male and female rats in the elevated plus-maze. Behav Neurosci, 119(2), 587–594. [DOI] [PubMed] [Google Scholar]
- Pereira TD, Aldarondo DE, Willmore L, Kislin M, Wang SS, Murthy M, et al. (2019). Fast animal pose estimation using deep neural networks. Nat Methods, 16(1), 117–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez EE, & De Biasi M. (2015). Assessment of affective and somatic signs of ethanol withdrawal in C57BL/6J mice using a short-term ethanol treatment. Alcohol, 49(3), 237–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleil KE, Lowery-Gionta EG, Crowley NA, Li C, Marcinkiewcz CA, Rose JH, et al. (2015). Effects of chronic ethanol exposure on neuronal function in the prefrontal cortex and extended amygdala. Neuropharmacology, 99, 735–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponomarev I, & Crabbe JC (1999). Genetic association between chronic ethanol withdrawal severity and acoustic startle parameters WSP and WSR mice. Alcohol Clin Exp Res, 23(11), 1730–1735. [PubMed] [Google Scholar]
- Powers J, Duffy L, Burns L, & Loxton D. (2016). Binge drinking and subsequent depressive symptoms in young women in Australia. Drug Alcohol Depend, 161, 86–94. [DOI] [PubMed] [Google Scholar]
- Prevot TD, Misquitta KA, Fee C, Newton DF, Chatterjee D, Nikolova YS, et al. (2019). Residual avoidance: A new, consistent and repeatable readout of chronic stress-induced conflict anxiety reversible by antidepressant treatment. Neuropharmacology, 153, 98–110. [DOI] [PubMed] [Google Scholar]
- Priddy BM, Carmack SA, Thomas LC, Vendruscolo JC, Koob GF, & Vendruscolo LF (2017). Sex, strain, and estrous cycle influences on alcohol drinking in rats. Pharmacol Biochem Behav, 152, 61–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prior K, Mills K, Ross J, & Teesson M. (2017). Substance use disorders comorbid with mood and anxiety disorders in the Australian general population. Drug and Alcohol Review, 36(3), 317–324. [DOI] [PubMed] [Google Scholar]
- Quadir SG, Rohl CD, Zeabi A, Moore CF, Cottone P, & Sabino V. (2020). Effect of different standard rodent diets on ethanol intake and associated allodynia in male mice. Alcohol, 87, 17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quijano Carde NA, & De Biasi M. (2022). Behavioral characterization of withdrawal following chronic voluntary ethanol consumption via intermittent two-bottle choice points to different susceptibility categories. Alcohol Clin Exp Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quijano Carde NA, Perez EE, Feinn R, Kranzler HR, & De Biasi M. (2021). Antagonism of GluK1containing kainate receptors reduces ethanol consumption by modulating ethanol reward and withdrawal. Neuropharmacology, 199, 108783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramchandani VA, Stangl BL, Blaine SK, Plawecki MH, Schwandt ML, Kwako LE, et al. (2018). Stress vulnerability and alcohol use and consequences: From human laboratory studies to clinical outcomes. Alcohol, 72, 75–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen DD, & Kincaid CL (2015). Acoustic startle in alcohol-naive male rats predicts subsequent voluntary alcohol intake and alcohol preference. Alcohol Alcohol, 50(1), 56–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rath M, Guergues J, Pinho JPC, Zhang P, Nguyen TG, MacFadyen KA, et al. (2020). Chronic Voluntary Binge Ethanol Consumption Causes Sex-Specific Differences in Microglial Signaling Pathways and Withdrawal-associated Behaviors in Mice. Alcohol Clin Exp Res, 44(9), 1791–1806. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Best K, Belknap JK, Finn DA, & Crabbe JC (2005). Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiol Behav, 84(1), 53–63. [DOI] [PubMed] [Google Scholar]
- Richetto J, Polesel M, & Weber-Stadlbauer U. (2019). Effects of light and dark phase testing on the investigation of behavioural paradigms in mice: Relevance for behavioural neuroscience. Pharmacol Biochem Behav, 178, 19–29. [DOI] [PubMed] [Google Scholar]
- Rodberg EM, den Hartog CR, Anderson RI, Becker HC, Moorman DE, & Vazey EM (2017). Stress Facilitates the Development of Cognitive Dysfunction After Chronic Ethanol Exposure. Alcohol Clin Exp Res, 41(9), 1574–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers RJ, & Shepherd JK (1993). Influence of prior maze experience on behaviour and response to diazepam in the elevated plus-maze and light/dark tests of anxiety in mice. Psychopharmacology (Berl), 113(2), 237–242. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Landa JF, Cueto-Escobedo J, Puga-Olguin A, Rivadeneyra-Dominguez E, Bernal-Morales B, Herrera-Huerta EV, et al. (2017). The Phytoestrogen Genistein Produces Similar Effects as 17beta-Estradiol on Anxiety-Like Behavior in Rats at 12 Weeks after Ovariectomy. Biomed Res Int, 2017, 9073816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose JH, Karkhanis AN, Chen R, Gioia D, Lopez MF, Becker HC, et al. (2016). Supersensitive Kappa Opioid Receptors Promotes Ethanol Withdrawal-Related Behaviors and Reduce Dopamine Signaling in the Nucleus Accumbens. Int J Neuropsychopharmacol, 19(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinow DR, & Schmidt PJ (2019). Sex differences and the neurobiology of affective disorders. Neuropsychopharmacology, 44(1), 111–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels BA, & Hen R. (2011). Novelty-Suppressed Feeding in the Mouse. In Gould T(Ed.), Mood and Anxiety Related Phenotypes in Mice (Vol. 63): Humana Press. [Google Scholar]
- Sandini TM, Reis-Silva TM, Moreira N, Bernardi MM, Lebrun I, & Spinosa HS (2019). Effects of isoflavones on behavior, estradiol, glutamate, and GABA levels in intact middle-aged female rats. Nutr Neurosci, 22(11), 805–816. [DOI] [PubMed] [Google Scholar]
- Schneier FR, Foose TE, Hasin DS, Heimberg RG, Liu S-M, Grant BF, et al. (2010). Social anxiety disorder and alcohol use disorder co-morbidity in the National Epidemiologic Survey on Alcohol and Related Conditions. Psychological Medicine, 40(6), 977–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev U, Finnie PS, Quinn T, Tobin S, & Wahi P. (2006). A role for corticotropin-releasing factor, but not corticosterone, in acute food-deprivation-induced reinstatement of heroin seeking in rats. Psychopharmacology (Berl), 187(3), 376–384. [DOI] [PubMed] [Google Scholar]
- Shansky RM (2015). Sex differences in PTSD resilience and susceptibility: Challenges for animal models of fear learning. Neurobiol Stress, 1, 60–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shansky RM, & Murphy AZ (2021). Considering sex as a biological variable will require a global shift in science culture. Nat Neurosci, 24(4), 457–464. [DOI] [PubMed] [Google Scholar]
- Shimizu C, Wakita Y, Tsuchiya Y, & Nabeshima T. (2020). Influence of Housing Systems on Physical, Emotional, and Cognitive Functions with Aging in DBA/2CrSlc Mice. Animals (Basel), 10(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidhu H, Kreifeldt M, & Contet C. (2018). Affective Disturbances During Withdrawal from Chronic Intermittent Ethanol Inhalation in C57BL/6J and DBA/2J Male Mice. Alcohol Clin Exp Res, 42(7), 1281–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simms JA, Steensland P, Medina B, Abernathy KE, Chandler LJ, Wise R, et al. (2008). Intermittent access to 20% ethanol induces high ethanol consumption in Long-Evans and Wistar rats. Alcohol Clin Exp Res, 32(10), 1816–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Fox HC, Hong KA, Bergquist K, Bhagwagar Z, & Siedlarz KM (2009). Enhanced negative emotion and alcohol craving, and altered physiological responses following stress and cue exposure in alcohol dependent individuals. Neuropsychopharmacology, 34(5), 1198–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slade T, Chapman C, Swift W, Keyes K, Tonks Z, & Teesson M. (2016). Birth cohort trends in the global epidemiology of alcohol use and alcohol-related harms in men and women: systematic review and meta-regression. BMJ Open, 6(10), e011827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanagel R, & Holter SM (2000). Pharmacological validation of a new animal model of alcoholism. J Neural Transm (Vienna), 107(6), 669–680. [DOI] [PubMed] [Google Scholar]
- Spruijt BM, & DeVisser L. (2006). Advanced behavioural screening: automated home cage ethology. Drug Discov Today Technol, 3(2), 231–237. [DOI] [PubMed] [Google Scholar]
- Starski P, Hong SI, Peyton L, Oliveros A, Wininger K, Hutchison C, et al. (2020). Ethanol induces maladaptive impulse control and decreased seeking behaviors in mice. Addict Biol, 25(3), e12754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson JR, Schroeder JP, Nixon K, Besheer J, Crews FT, & Hodge CW (2009). Abstinence following alcohol drinking produces depression-like behavior and reduced hippocampal neurogenesis in mice. Neuropsychopharmacology, 34(5), 1209–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoychev K, Dilkov D, Naghavi E, & Kamburova Z. (2021). Genetic Basis of Dual Diagnosis: A Review of Genome-Wide Association Studies (GWAS) Focusing on Patients with Mood or Anxiety Disorders and Co-Occurring Alcohol-Use Disorders. Diagnostics (Basel), 11(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong MN, Kaufman KR, Crabbe JC, & Finn DA (2009). Sex differences in acute ethanol withdrawal severity after adrenalectomy and gonadectomy in Withdrawal Seizure-Prone and Withdrawal Seizure-Resistant mice. Alcohol, 43(5), 367–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturman O, von Ziegler L, Schlappi C, Akyol F, Privitera M, Slominski D, et al. (2020). Deep learning-based behavioral analysis reaches human accuracy and is capable of outperforming commercial solutions. Neuropsychopharmacology, 45(11), 1942–1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szumlinski KK, Coelho MA, Lee KM, Tran T, Sern KR, Bernal A, et al. (2019). DID it or DIDn’t it? Exploration of a failure to replicate binge-like alcohol-drinking in C57BL/6J mice. Pharmacol Biochem Behav, 178, 3–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanchuck-Nipper MA, Ford MM, Hertzberg A, Beadles-Bohling A, Cozzoli DK, & Finn DA (2015). Sex Differences in Ethanol’s Anxiolytic Effect and Chronic Ethanol Withdrawal Severity in Mice with a Null Mutation of the 5alpha-Reductase Type 1 Gene. Behav Genet, 45(3), 354–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannenbaum C, Ellis RP, Eyssel F, Zou J, & Schiebinger L. (2019). Sex and gender analysis improves science and engineering. Nature, 575(7781), 137–146. [DOI] [PubMed] [Google Scholar]
- Thiele TE, & Navarro M. (2014). “Drinking in the dark” (DID) procedures: a model of binge-like ethanol drinking in non-dependent mice. Alcohol, 48(3), 235–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas SE, Randall CL, & Carrigan MH (2003). Drinking to cope in socially anxious individuals: a controlled study. Alcohol Clin Exp Res, 27(12), 1937–1943. [DOI] [PubMed] [Google Scholar]
- Thompson BL, Maleki N, Kelly JF, Sy KTL, & Oscar-Berman M. (2021). Brain, behavioral, affective, and sex correlates of recovery from alcohol use disorders. Alcohol Clin Exp Res, 45(8), 1578–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao CH, Flint J, & Huang GJ (2022). Influence of diurnal phase on behavioral tests of sensorimotor performance, anxiety, learning and memory in mice. Sci Rep, 12(1), 432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Vaart A, Meng X, Bowers MS, Batman AM, Aliev F, Farris SP, et al. (2018). Glycogen synthase kinase 3 beta regulates ethanol consumption and is a risk factor for alcohol dependence. Neuropsychopharmacology, 43(13), 2521–2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rijn RM, Brissett DI, & Whistler JL (2010). Dual efficacy of delta opioid receptor-selective ligands for ethanol drinking and anxiety. J Pharmacol Exp Ther, 335(1), 133–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendruscolo LF, & Roberts AJ (2014). Operant alcohol self-administration in dependent rats: focus on the vapor model. Alcohol, 48(3), 277–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma P, Hellemans KG, Choi FY, Yu W, & Weinberg J. (2010). Circadian phase and sex effects on depressive/anxiety-like behaviors and HPA axis responses to acute stress. Physiol Behav, 99(3), 276–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkmann P, Stephan M, Krackow S, Jensen N, & Rossner MJ (2020). PsyCoP - A Platform for Systematic Semi-Automated Behavioral and Cognitive Profiling Reveals Gene and Environment Dependent Impairments of Tcf4 Transgenic Mice Subjected to Social Defeat. Front Behav Neurosci, 14, 618180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vranjkovic O, Winkler G, & Winder DG (2018). Ketamine administration during a critical period after forced ethanol abstinence inhibits the development of time-dependent affective disturbances. Neuropsychopharmacology, 43(9), 1915–1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walls SA, Macklin ZL, & Devaud LL (2012). Ethanol-induced loss-of-righting response during ethanol withdrawal in male and female rats: associations with alterations in Arc labeling. Alcohol Clin Exp Res, 36(2), 234–241. [DOI] [PubMed] [Google Scholar]
- Wang N, Liu X, Li XT, Li XX, Ma W, Xu YM, et al. (2021). 7,8-Dihydroxyflavone Alleviates Anxiety-Like Behavior Induced by Chronic Alcohol Exposure in Mice Involving Tropomyosin-Related Kinase B in the Amygdala. Mol Neurobiol, 58(1), 92–105. [DOI] [PubMed] [Google Scholar]
- Wang Q, Timberlake MA, Prall K, & Dwivedi Y. (2017). The recent progress in animal models of depression. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 77, 99–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warden AS, Wolfe SA, Khom S, Varodayan FP, Patel RR, Steinman MQ, et al. (2020). Microglia Control Escalation of Drinking in Alcohol-Dependent Mice: Genomic and Synaptic Drivers. Biol Psychiatry, 88(12), 910–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells AM, Ridener E, Bourbonais CA, Kim W, Pantazopoulos H, Carroll FI, et al. (2017). Effects of Chronic Social Defeat Stress on Sleep and Circadian Rhythms Are Mitigated by Kappa-Opioid Receptor Antagonism. J Neurosci, 37(32), 7656–7668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westermeyer J, & Boedicker AE (2000). Course, severity, and treatment of substance abuse among women versus men. Am J Drug Alcohol Abuse, 26(4), 523–535. [DOI] [PubMed] [Google Scholar]
- White A, Castle IJ, Chen CM, Shirley M, Roach D, & Hingson R. (2015). Converging Patterns of Alcohol Use and Related Outcomes Among Females and Males in the United States, 2002 to 2012. Alcohol Clin Exp Res, 39(9), 1712–1726. [DOI] [PubMed] [Google Scholar]
- WHO. (2018). WHO | Global status report on alcohol and health 2018. WHO. [Google Scholar]
- Willner P. (2017). Reliability of the chronic mild stress model of depression: A user survey. Neurobiol Stress, 6, 68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiltschko AB, Johnson MJ, Iurilli G, Peterson RE, Katon JM, Pashkovski SL, et al. (2015). Mapping Sub-Second Structure in Mouse Behavior. Neuron, 88(6), 1121–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA (1973). Voluntary ethanol intake in rats following exposure to ethanol on various schedules. Psychopharmacologia, 29(3), 203–210. [DOI] [PubMed] [Google Scholar]
- Yang X, Liu Q, Zhong J, Song R, Zhang L, & Wang L. (2020). A simple threat-detection strategy in mice. BMC Biol, 18(1), 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz M, & Meister M. (2013). Rapid innate defensive responses of mice to looming visual stimuli. Curr Biol, 23(20), 2011–2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin X, Guven N, & Dietis D. (2016). Stress-based Animal Models of Depression: Do We Actually Know What We Are Doing? Brain research, 1652. [DOI] [PubMed] [Google Scholar]