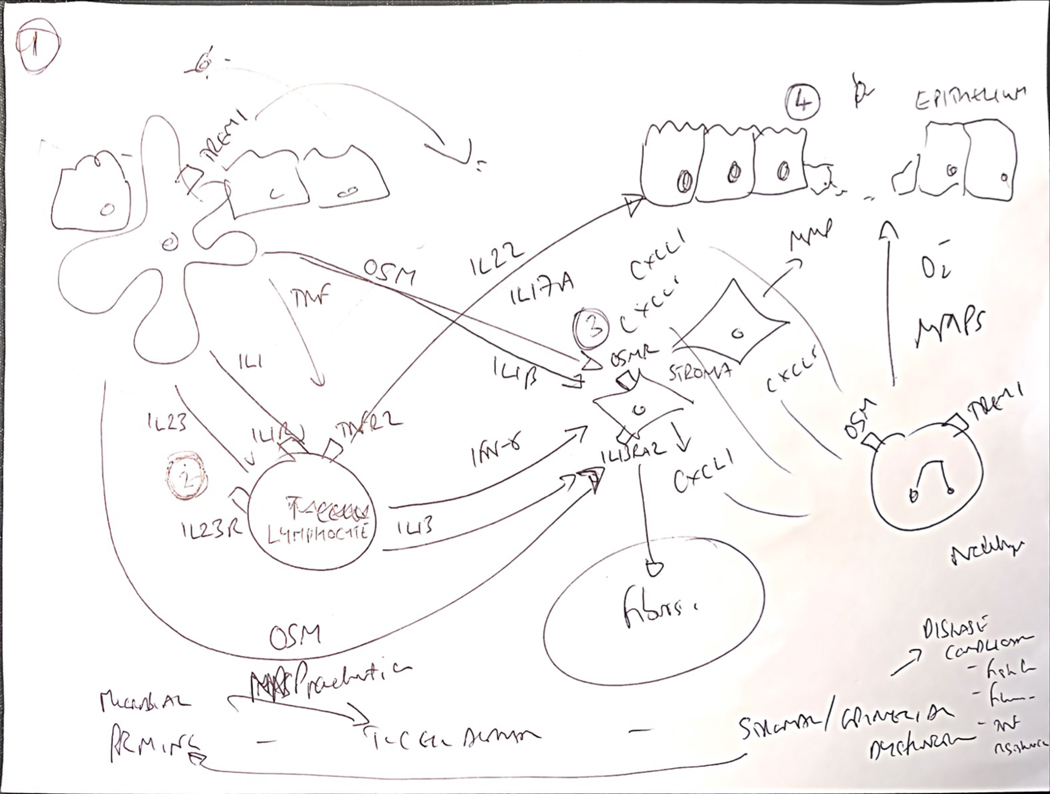
Figure 1:
Towards a new conceptual framework of immunological mechanisms of treatment resistance integrating cross-talk between immune, stromal and epithelial interactions.
The immune mechanisms of treatment resistance are complex and likely multifactorial. Key observations from different independent experiments, including high resolution single cell transcriptomics and cytometry experiments, indicate suggests common immunological themes in treatment resistance, typically involving communication circuits between mucosal immune cells and non-immune compartments, including stromal cells and epithelial cells. It is possible to integrate these paradigms into a potentially unifying hypothetic framework to identify at least one of the biological pathways associated with poor patient outcomes, tissue injury and treatment refractoriness. 1. Key proximal steps responsible for initiating severe inflammation are likely to involve mononuclear phagocytes (MPs), and especially CD14+ CX3CR1+ subsets, which are enriched in diseased tissue of IBD patients and play an important role sampling and responding to luminal antigens, including dysbiotic bacterial communities and other components of the exposome. 2. These MPs are a key source of cytokines implicated in treatment resistance, such as IL-1β, IL-23, oncostatin M and TNF, and other immune molecules, such as TREM1. 3. Some of these cytokines act directly on key non-immune cells, including the stromal and epithelial compartments. The transcriptional response of different intestinal stromal cells to oncostatin M, and IL-1β in particular, is linked to resistance to steroids, anti-TNF and vedolizumab. Analysis of the biological pathway driven by these cytokines indicate activation of neutrophil attracting chemokines (CXCL1, CXCL2, CXCL3, CXCL5, CXCL6, CXCL8), extracellular matrix disruption (including expression of metalloproteinases MMP1, MMP3, MMP13) and augmented expression of hallmark fibroblast genes, such as THY1, PDPN, IL1l, IL13RA2. 4. In agreement with increased expression of neutrophil-selective CXC family chemokines, treatment resistant patients with significant tissue injury, including ulcers have increased neutrophil accumulation in inflamed tissue. IL-1 and IL-23 are important drivers of lymphocyte activation, including conventional CD4+ T-cells, MAIT cells and innate lymphoid cells (ILCs), triggering induction of multiple effector cytokines, such as IFNγ and IL-22. Indeed, IL-22 is also a potent inducer of neutrophil-active cytokines, particularly in intestinal epithelial cells. Notably, IL-22-responsive genes are also enriched in IBD tissue, including ulcers. The mechanisms that underpin the association of enhanced neutrophil recruitment, tissue injury and poor patient outcomes are not known, but neutrophils themselves have potent inflammatory activity as producers of reactive oxygen species, tissue damaging metalloproteinases, extracellular traps and can produce cytokines.