Abstract
This study investigated, for the first time, the role of cerium oxide nanoparticles (CeO2 NPs) on dairy effluent nitrate and phosphate bioremediation using different inoculum sources. Two inoculum sources (wastewater and sludge) were obtained from the dairy wastewater treatment plant unit. A culture was prepared to be tested in the treatment of nitrate and phosphate effluent, and the role of CeO2 NPs was checked to be completely efficient after 5 days of incubation. The reduction efficiency of nitrate using sludge as inoculum source was improved up to 89.01% and 68.12% for phosphate compared to control. In the case of using wastewater as an inoculum source, the nitrate reduction was improved up to 83.30% and 87.75% for phosphate compared to control. The bacterial richness showed a significant variance (higher richness) between control and other samples. The optimal concentration of CeO2 NPs for inoculum richness and nitrate and phosphate reduction was (sludge: 1 × 10−10 ppm) and (wastewater: 1 × 10−12 ppm). The results revealed that CeO2 NPs could enhance the microbial growth of different inoculum sources that have a key role in dairy effluent nitrate and phosphate bioremediation.
Supplementary information
The online version contains supplementary material available at 10.1007/s10661-022-10003-0.
Keywords: Cerium oxide nanoparticles, Nitrate, Phosphate, Activated sludge, Bacterial wastewater treatment, Bioremediation
Introduction
To date, there is a great challenge in the drivers for water change such as population growth, economic development, social, and technological change. These drivers have adversely negative impacts on water resources and climate change (Bassem, 2020). The unsustainable industrial development causes negative pressures on the environment. Industrial wastewater with high concentrations of pollutants (e.g., nitrate and phosphate) adversely affects the environment (Amoatey & Baawain, 2019). Declining the water quality in the countries with a scarcity of water resources reduces the country’s opportunity for sustainable industrial development and threatens public health with spreading infectious diseases (PAHO, 2013).
Nanoparticles (NPs) were investigated for various processes of wastewater treatment. The NP properties (e.g., high surface-to-volume ratios and reduced size) enable them to be highly reactive with distinct characteristics (Singh et al., 2019). Das et al. (2020) reported that NPs are highly effective in wastewater pollutants removal and are considered a promising method for wastewater treatment. Nano-bioremediation achievements flourished the technologies of wastewater treatment (Khan et al., 2019; Singh et al., 2020a, b).
Antioxidant nanomaterials such as cerium oxide (CeO2) NPs had recently obtained good attention for their massive potential in biotechnology (Casals et al., 2020). CeO2 NPs were used as catalysts, physicochemical burnish mediators, coverings, and fuel additives (Hu et al., 2018). CeO2 NPs also obtained a lot of attention in solving many problems through displaying redox action, and biofilm restraint, etc. (Nyoka et al., 2020; Singh et al., 2020a, b).
The formation of CeO2 nanoparticles was characterized using transmission electron microscopy (TEM) which used to assess the size and detailed morphology of the CeO2 NPs, X-ray diffraction (XRD), and zeta potential which applied to recognize the surface charge of CeO2 NPs and to study the stability of nanoparticles. Ultraviolet-visible (UV-vis) spectroscopy was used for the visual observation of the NPs formation, and Fourier transform infrared spectroscopy (FT-IR) was applied to determine the existence of specific surface functional groups in the investigated NPs. The crystalline phase analysis using X-ray diffraction exposed the amorphous nature of CeO2 NPs (Al-Ananzeh, 2021; García et al., 2012; Prabhakar et al., 2017).
CeO2 NPs were effective in the removal of different pollutants from the wastewater (Contreras et al., 2015). CeO2 NPs could improve the growth of some bacterial species that are shared in the bioremediation process. High concentrations of CeO2 NPs could have negative effects on the bioremediation process of phosphate removal using an activated sludge (Kamika & Tekere, 2017).
Different industrial wastewater pollutants, particularly with high nitrate and phosphate concentrations, were successfully removed using the biological treatment processes. The wastewater treatment processes were highly developed to achieve this task with low input of energy (Ahammad et al., 2013; Iloms et al., 2020). The efficiency of these processes can be referred to as the presence of key microorganisms in wastewater and activated sludge (Achmadulina et al., 2017).
High concentrations of nitrate perform adversely in oxygen transport procedures, leading to the hypoxia process and many human health problems (Cheng & Chen, 2001, 2002; Dutra et al., 2020; Luo et al., 2020). In intensive systems, aquatic organisms could be exposed to high nitrate concentrations that could change the water quality and negatively affect the organism’s metabolism (de Farias Lima et al., 2020; Romano & Zeng, 2007, 2009, 2013). The nitrate bioaccumulation in aquatic organism’s tissue can cause undesirable problems in humans after consumption (Wolfe & Patz, 2002). In addition, the ingestion of highly accumulated nitrates led to emerging of carcinogens in the digestive system (de Farias Lima et al., 2020; Song et al., 2015).
Phosphates help in the blood oxidation in the biota and are involved in numerous biochemical procedures (Choi et al., 2020; Naushad et al., 2017; Wiemer, 2020). Despite phosphate is not poisonous, it is responsible for surface water eutrophication; therefore, remediation methods have been constantly investigated to eliminate it in aqueous environments (Luengo et al., 2017; McPherson et al., 2004). Chronic influences of phosphates such as expansion inhibition, reduced fertility, and gene expression were detected in aquatic organisms (Yuan et al., 2018).
This study aims to investigate the role of CeO2 NPs in the activation of microorganisms for the dairy effluent nitrate and phosphate bioremediation process. Specifically, the parameters such as the concentration of CeO2 NPs and their impact on bacterial growth and nitrate and phosphate reduction were investigated and discussed in this study.
Materials and methods
Inoculum sample collection
Fresh inoculum samples of dairy wastewater and activated sludge were collected from a dairy wastewater treatment plant at Jumasa, Egypt. The samples were stored at 4 °C to maintain their inoculum properties.
Cerium oxide nanoparticles
A powder sample (5 mg) of NP CeO2 (within a size: ≤ 25 nm) was obtained from Sigma-Aldrich® chemical company, Ontario, Canada, and used in this study.
Experimental setup
The different inoculum source solutions (100 mL) were inoculated separately in a reactor including 300 mL of culture media (d-glucose anhydrate, 2.5 g/L; MgSO4·7H2O, 0.5 g/L and KNO3, 0.18 g/L; prepared in distilled water) (Kamika & Tekere, 2017). All the chemicals used in the experimental work were obtained from Sigma-Aldrich® chemical company, Ontario, Canada. The inoculum sources were separately treated with different concentrations of CeO2 NPs to investigate their impact on the microbial species in wastewater treatment plants.
The concentrations of CeO2 NPs in the samples were adjusted to be 1 × 10−8, 1 × 10−9, 1 × 10−10, 1 × 10−11, 1 × 10−12, 1 × 10−13, 1 × 10−14, and 1 × 10−15 ppm, and the non-treated was used as a control. The concentrations’ adjustment was done after a pilot study aimed to obtain the bacterial growth enhancement CeO2 NPs start concentration using 1 × 10−1, 1 × 10−2, 1 × 10−3, 1 × 10−4, 1 × 10−5, 1 × 10–6, 1 × 10–7, and 1 × 10−8 ppm. Incubation condition for maximizing the bacterial growth was performed at 35 °C and pH 7.
X-ray diffraction analysis (XRD) and transmission electron microscope (TEM) were performed to determine the mineral composition and shape of the studied CeO2 NPs. Zeta potential analysis was performed to identify the surface charge of CeO2 NPs. These analyses and nano-specifications were carried out in Nano Science and Technology Institute at Kafrelsheikh University, Egypt. The aliquot samples were used to determine nitrate and phosphate concentrations (ppm) using ion chromatography (Thermo Scientific, Dionex ICS-1100) (Yi et al., 2020).
The microbial growth was measured at a wavelength of 450 nm (Domínguez et al., 2001; Mauerhofer et al., 2018), using Jenway Model 6800 Spectrophotometer. Triplicate tests were carried out, and the mean and change percentages to control were recorded.
X-ray diffraction analysis
The XRD analysis was used to show the nano-size and peaks of the CeO2 NPs to approve their crystalline structure and pattern (Arockia et al., 2019; Pillai et al., 2020; Almessiere et al., 2020; Aref & Salem, 2020). The XRD analysis was carried out in Nano Science and Technology Institute at Kafrelsheikh University, Egypt.
Transmission electron microscope
Transmission electron microscopy (TEM) was employed to show the size and morphological investigations of the NPs (Arockia et al., 2019; Aref & Salem, 2020; Pillai et al., 2020). The TEM analysis was carried out in Nano Science and Technology Institute at Kafrelsheikh University, Egypt.
Zeta potential analysis
Zeta potential analysis was used to identify the surface charge of CeO2 NPs and their physical stability in the aqueous solutions (Ding et al., 2018; Gaikwad et al., 2019; Jiang et al., 2009; Joseph & Singhvi, 2019; Selvamani, 2019). The particle sizes, ζ-potential, and polydispersity index (PDI) of nanoparticles had been determined through a Nano-ZS Zetasizer analyzer (Meng et al., 2020). The zeta analysis was carried out in Nano Science and Technology Institute at Kafrelsheikh University, Egypt.
Ultraviolet–visible spectroscopy
Ultraviolet–visible spectroscopy was used for the visual observation of the NP formation by monitoring the alterations in the solution color through incubation time (Arockia et al., 2019). The UV–Vis analysis using was carried out in Nano Science and Technology Institute at Kafrelsheikh University, Egypt, using JASCO NIR Spectrophotometer/ model: V-770.
Fourier transform infrared spectroscopy
FT-IR spectrophotometer was employed in this study to determine the existence of specific surface functional groups of the studied samples (Madubuonu et al., 2020; Pillai et al., 2020; Varadavenkatesan et al., 2020). The FT-IR analysis was carried out in Nano Science and Technology Institute at Kafrelsheikh University, Egypt, using Infrared Spectrum Origin Jasco: model, FT-IR 6800typeA.
Results and discussion
CeO2 nanoparticle characterization
X-ray diffraction analysis
Figure 1 shows the XRD pattern of CeO2 NPs where well-defined peaks were obtained at 28.14°, 32.64°, 47.16°, 56.00°, 58.84°, 68.96°, 76.24°, and 78.90° corresponding to [346], [112], [218], [166], [38], [50], [76], and [50] planes of cubic CeO2 lattice. This diffraction pattern indicated that the NPs have very sharp peaks with ultrafine nature and high crystalline cubic spinel structure that confirm the purity and good formation of the metal-oxide NPs (Romer et al., 2019).
Fig. 1.
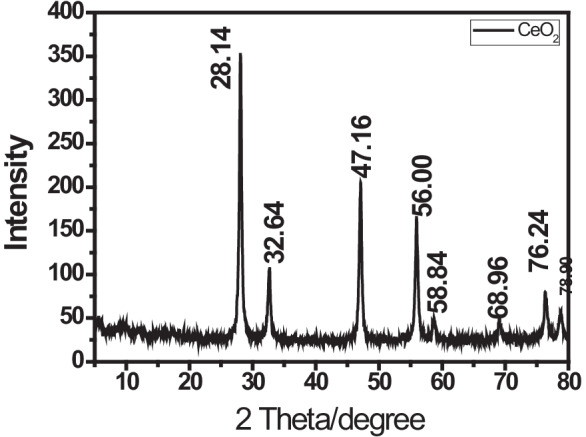
XRD pattern of CeO2 NPs
Transmission electron microscope
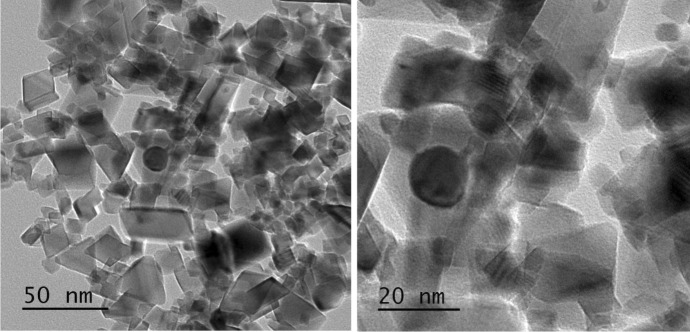
TEM photomicrograph of the prepared CeO2 NPs is shown in Fig. 2 and indicates that the particles have an isotropic shape (Forest et al., 2017), within a range of 20–40 nm in size.
Fig. 2.
TEM photomicrograph of CeO2 NPs
Zeta potential analysis
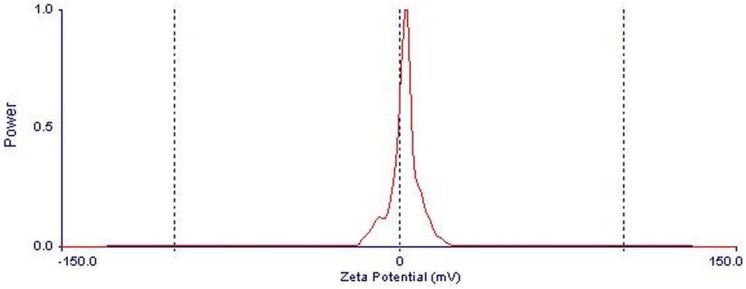
The zeta potential analysis of CeO2 NPs is shown in Fig. 3 indicating that the zeta potential value of CeO2 NPs is 1.5 mV and confirming its negative surface charge. Nanoparticles with zeta potential greater than + 25 mV or less than −25 mV have more colloidal stability with repulsive forces to avoid the agglomeration of NPs (Thakkar et al., 2016). Furthermore, the obtained results of CeO2 NPs indicated that the nanoparticles have a suitable dispersion capability in an aqueous medium.
Fig. 3.
Zeta potential of CeO2 NPs
Ultraviolet–visible spectroscopy
Ultraviolet–visible analysis of CeO2 NPs is shown in Fig. 4 where the UV–Vis spectra at wavelengths of 200–800 nm were used to notice a powerful absorption peak, which related to superficial Plasmon excitation (Aref & Salem, 2020). The sharp peak assumed by the UV–Vis spectrum at the absorption wavelength is 340 nm (Fig. 4).
Fig. 4.
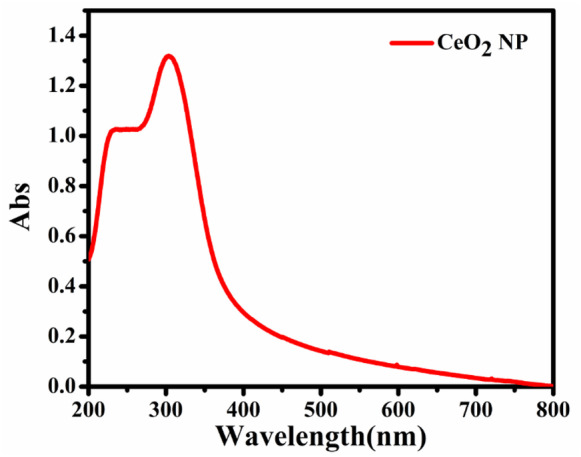
Ultraviolet–visible analysis of CeO2 NPs
Fourier transform infrared spectroscopy
Fourier transform infrared (FT-IR) analysis of CeO2 NPs in terms of wavenumber vs transmittance (%) is shown in Fig. 5. The evaluation was performed by using FT-IR spectrometer; the spectra were scanned in the wavelength range of 400–4000 Cm−1 at a resolution of 2 Cm−1 in KBr pellets (Aref & Salem, 2020; Sobhani-Nasab et al., 2020), where the maximum transmittance is 38.75% and the minimum transmittance is 7.31%.
Fig. 5.
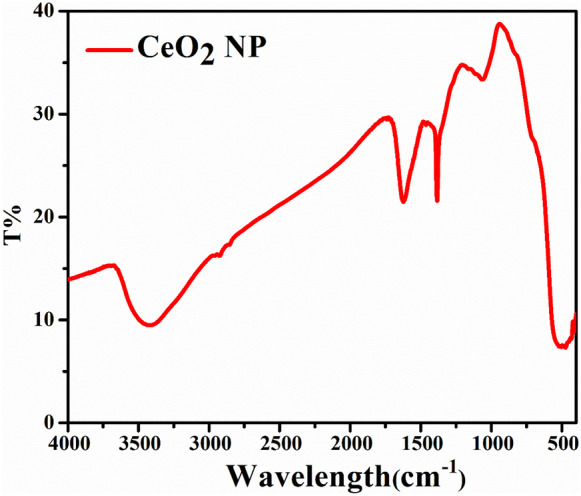
Fourier transform infrared (FT-IR) analysis of CeO2 NPs
Bacteriological wastewater treatment
Microorganisms have a key role in pollutant degradation and biological systems maintenance and stabilization. Applying the technologies of biological wastewater treatment compared to other treatment actions has many advantages such as low cost, low or without secondary excretion of pollutants, and the most significant low adverse effects on the environment (Dadrasnia et al., 2017). Physico-chemical methods that were used for treating nitrate in wastewater (e.g., reverse osmosis (RO), electrodialysis, and ion exchange) generate secondary wastes which made these processes less desirable (Yun et al., 2016). In contrast, biological methods are more reliable and stable in wastewater treatment (McCarty, 2018). The presence of nitrate in polluted wastewater allows bacteria to obtain many metabolic capabilities enabling its adaptation to simulate nitrification-and denitrification processes (Rajta et al., 2020; Sharma & Dwivedi, 2017).
Numerous and diverse chemical, physico-chemical, and biological methods were used to remove phosphorus from wastewater. Chemical methods are less desirable due to their high cost and generate secondary pollution, while physico-chemical methods involve a high expenditure of the processes with a complex use. Furthermore, the biological methods of phosphorus removal are widely used worldwide (Ruzhitskaya & Gogina, 2017). There are series of technologies used for the biological removal of phosphorus such as phostrip, anaerobic/anoxic/oxic, activated sludge, and other technologies (Barnard, 2006).
The study results clearly explained the effectiveness of using microbial consortia (wastewater inoculum and sludge inoculum) in biological nitrogen and phosphorous remediation, which agree with the results of Wu et al. (2019), Zhang et al. (2019), Al Ali et al. (2020), Salama et al. (2022), Liu et al. (2017, 2018), Shomar et al. (2020), and Guemmaz et al. (2019) indicating the high efficiency of microbial consortia in simultaneous removal of nitrogen and phosphorous and particularly are more effective in bioremediation than using other pure microbial species.
Effect of CeO2 nanoparticle concentrations on the bacterial growth
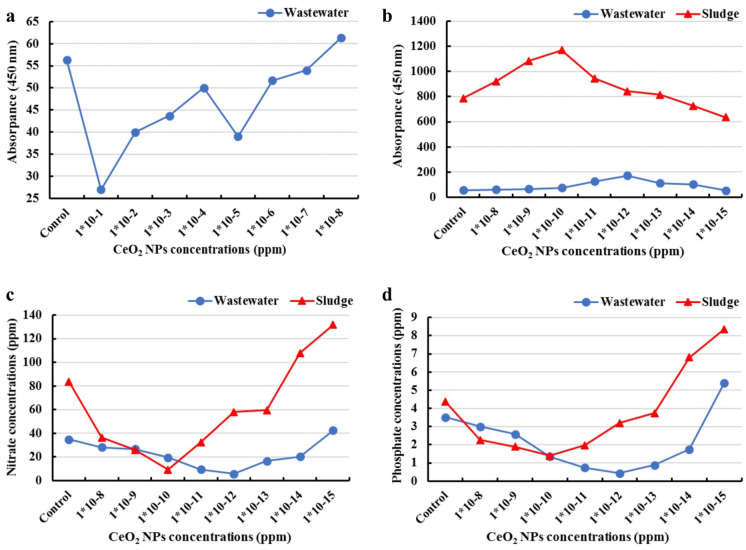
The growth properties of wastewater inoculum were investigated using a spectrophotometer with CeO2 NP concentrations (from 1 × 10−1 to 1 × 10−8 ppm) that were used as a pilot study and were shown in Fig. 6a. While the growth properties of wastewater and sludge inoculum were investigated in presence of concentrations from (1 × 10−8 to 1 × 10−15 ppm) as shown in Fig. 6b.
Fig. 6.
Absorbance pattern of microbial growth media at (450 nm) with wastewater as inoculum source (pilot study) a and using wastewater and sludge as inoculum source b. Reduction patterns of nitrate c and phosphate d concentrations (ppm) using different concentrations (ppm) of CeO2 NPs
Figure 6a, b show bi-phase dose–response relationships. A positive effect of CeO2 NPs on microbial growth was observed with a maximum of 1 × 10−12 ppm for wastewater inoculum and 1 × 10−10 ppm for sludge inoculum. However, a high-dose inhibition in biofilm formation was observed with CeO2 NPs higher than 1 × 10−8 ppm as shown in the pilot study’s results, which indicated the antimicrobial effects of CeO2 NPs. The statistical analysis of the studied data showed significant variations (P < 0.01) in the absorbance pattern of microbial growth media at 450 nm among different experimental factors (Table 1). These findings agree with those of Xu et al. (2019) indicating that the impacts of CeO2 NPs on microbial growth showed a typical effect, which was defined as a bi-phase dose–response relationship with low-dose stimulation and high-dose inhibition (Popov et al., 2017; Qiu et al., 2016; Salama et al., 2021; Xu et al., 2019).
Table 1.
General linear model test for variation in absorbance pattern of bacterial growth media at 450 nm, using different concentrations (ppm) of CeO2 NPs
| Growth media | Source | Degree of freedom | Sequential sums of squares | Adjusted sums of squares | Adjusted mean squares | F-value | P-value |
|---|---|---|---|---|---|---|---|
| Bacterial growth media + different concentrations of CeO2 NPs | Inoculum source | 1 | 8,402,456 | 8,402,456 | 8,402,456 | 996.60 | 0.000 |
| Conc. of NPs (ppm) | 8 | 354,214 | 354,214 | 44,277 | 5.25 | 0.000 | |
| Error | 44 | 370,971 | 370,971 | 8431 | - | - | |
| Total | 53 | 9,127,640 | - | - | - | - |
The low concentrations of CeO2 NPs improved the surface hydrophobicity, the aggregating ability in addition to the protein (PRO), and the polysaccharide (PS) microbial production during the initial attachment and differentiation process. The increased reactive oxygen species (ROS), which are produced by CeO2 NPs, promoted the production of the quorum sensing (QS) molecules by microbial organisms that resulting in the accelerated activation of QS systems (Xu et al., 2018). The QS among bacteria promotes the formation of biofilms, improves the strains’ resistance, promotes bacterial growth, and enhances the metabolic effects (Yang et al., 2020).
Effect of CeO2 nanoparticle concentrations on dairy effluent nitrate and phosphate bioremediation
The bioremediation properties of dairy effluent were evaluated in terms of nitrate and phosphate examination. The role of different microbial inoculum sources on nitrate and phosphate decrease (ppm) was investigated using different concentrations of CeO2 NPs (from 1 × 10−8 to 1 × 10−14 ppm) for wastewater inoculum, and (from 1 × 10−8 to 1 × 10−13 ppm) for sludge inoculum (Fig. 6c, d).
By comparing the results obtained from the microbial growth with nitrate and phosphate reductions, it is noticed that the best microbial growth was at absorbance: 172.67 for wastewater inoculum, and 1170.33 for sludge inoculum, which coincides with the highest nitrate reduction (5.81 and 9.19 ppm) and highest phosphate reduction (0.43 and 1.39 ppm) that were achieved using (1 × 10−12 and 1 × 10−10 ppm) of CeO2 NPs, respectively, in the nutrient media (wastewater and sludge inoculum separately) that were compared to the control sample after 5 days of incubation at temperature 35 °C. Figure 6c, d show that nitrate and phosphate concentrations (ppm) linearly decrease with the increase of CeO2 NPs concentrations from 1 × 10−8 to 1 × 10−14 ppm for wastewater inoculum and from 1 × 10−8 to 1 × 10−13 ppm for sludge inoculum. A higher concentration of CeO2 NPs showed a lower bioremediation efficiency.
Statistical analysis of the data showed significant variations at P < 0.01 in nitrate and phosphate concentrations (ppm) between different experimental factors (microbial inoculum sources with different CeO2 NPs) (Tables 2 and 3).
Table 2.
General linear model test for variation in nitrate (ppm) pattern, using different concentrations (ppm) of CeO2 NPs
| Growth media | Source | Degree of freedom | Sequential sums of squares | Adjusted sums of squares | Adjusted mean squares | F-value | P-value |
|---|---|---|---|---|---|---|---|
| Bacterial growth media + different concentrations of CeO2 NPs | Inoculum source | 1 | 19,399.5 | 19,399.5 | 19,399.5 | 54.04 | 0.000 |
| Conc. of NPs (ppm) | 8 | 26,903.4 | 26,903.4 | 3362.9 | 9.37 | 0.000 | |
| Error | 44 | 15,794.9 | 15,794.9 | 359.0 | - | - | |
| Total | 53 | 62,097.7 | - | - | - | - |
Table 3.
General linear model test for variation in phosphate (ppm) pattern, using different concentrations (ppm) of CeO2 NPs
| Growth media | Source | Degree of freedom | Sequential sums of squares | Adjusted sums of squares | Adjusted mean squares | F-value | P-value |
|---|---|---|---|---|---|---|---|
| Bacterial growth media + different concentrations of CeO2 NPs | Inoculum source | 1 | 34.10 | 34.10 | 34.10 | 31.86 | 0.000 |
| Conc. of NPs (ppm) | 8 | 153.15 | 153.15 | 19.144 | 17.89 | 0.000 | |
| Error | 44 | 47.09 | 47.09 | 1.070 | - | - | |
| Total | 53 | 234.34 | - | - | - | - |
CeO2 NPs have delivered promising approaches in the bioremediation process. The physico-chemical properties of CeO2 NPs (e.g., size and surface charge) play key roles in the ultimate interactions of the nanoparticles with target cells (Charbgoo et al., 2017). Based on this, CeO2 NPs could enhance the metabolic activity of some microbial species while inhibiting those of others (Kamika & Tekere, 2017; Pelletier et al., 2010), depending on the enzymes that play a key role in the bacterial bioremediation (Jaiswal & Shukla, 2020).
Nitrification and denitrification are two major processes for biological nitrogen removal that organize the global nitrogen cycle. Four key enzymes carried out the denitrification process: nitrate reductase, nitrite reductase, nitric oxide reductase, and nitrous oxide reductase (Rajta et al., 2020). Furthermore, bacterial growth, which stimulates nitrate and phosphate removal enzymes, is a result of maximizing the bacterial count (Deng et al., 2020; Wang et al., 2020). According to Farias et al. (2018), the effect of CeO2 NPs on microbial count and activity is concentration dependent. The bacterial count and metabolic activity of some strains were enhanced by sub-lethal concentrations of CeO2 NPs exposure (Martínez et al., 2019; Xu et al., 2019).
The obtained results agree with those of Feng et al. (2019) and summarizing that exposure to higher concentrations of CeO2 NPs caused a sharp decrease in nitrogen and phosphorus removal efficiencies that were consistent with the tendencies of key enzymes (Feng et al., 2019). Specifically, CeO2 NPs at concentrations of 0.1, 1, and 10 ppm decreased the secretion of tightly bound extracellular polymeric substances to 0.13%, 3.14%, and 28.60%, respectively in comparison to the control. According to Wang et al. (2016), the removal rates of nitrate and phosphate show similar variation trends to the microbial enzymatic activities. Additionally, the variations of ROS and lactate dehydrogenase (LDH) indicated that a high concentration of CeO2 NPs could result in biotoxicity to the activated sludge (Wang et al., 2016). Overall, the high concentrations of CeO2 NPs could cause adverse effects on microbial richness and diversity of the activated sludge.
Conclusions
Enhancing the reduction of nitrate and phosphate using bioremediation nanotechnologies is a major challenge. CeO2 NPs with sub-lethal concentrations have attracted interest due to their ability to produce higher bacterial growth, metabolic activity, and accordingly accelerate the nitrate and phosphate reduction. The bacterial growth together with nitrate and phosphate reduction were linearly correlated with the increase of CeO2 NP concentration. Nitrate and phosphate reduction’s efficiency, using sludge as an inoculum source, was improved up to 89.01% (for nitrate) and 68.12% (for phosphate) compared to control. In the case of using wastewater as an inoculum source, the nitrate and phosphate reduction was improved up to 83.30% and 87.75%, respectively, compared to control. The study findings concluded that using various inoculum sources together with the CeO2 NP concentrations is an efficient method for nitrate and phosphate reduction from dairy effluent.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contribution
All authors have equal contributions in this paper.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Availability of data and material
Fresh dairy wastewater and activated sludge inoculum samples were collected from a dairy wastewater treatment plant at Jumasa, Egypt. The dairy factory agreed to give different inoculum samples for this study. The permission’s letter was given without any obligation and commitment on our part.
Code availability
Not applicable.
Declarations
Ethical approval
Not applicable.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Achmadulina FY, Zakirov RK, Balymova ES, Denisova V, Brovdyová T, Trögl J, Neruda M. Comparison of bioindicator eukaryotes of activated sludge biocenoses on two water-treatment plants: A case study. Nova Biotechnologica Et Chimica. 2017;16(1):54–60. doi: 10.1515/nbec-2017-0008. [DOI] [Google Scholar]
- Ahammad SZ, Graham DW, Dolfing GJ. Wastewater treatment: Biological. In: Jorgenson SE, editor. Encyclopedia of Environmental Management. Taylor & Francis; 2013. pp. 2645–2656. [Google Scholar]
- Al Ali, A. A., Naddeo, V., Hasan, S. W., & Yousef, A. F. (2020). Correlation between bacterial community structure and performance efficiency of a full-scale wastewater treatment plant. Journal of Water Process Engineering, 37, 101472.
- Al-Ananzeh NM. Treatment of wastewater from a dairy plant by adsorption using synthesized copper oxide nanoparticles: Kinetics and isotherms modeling optimization. Water Science and Technology. 2021;83(7):1591–1604. doi: 10.2166/wst.2021.089. [DOI] [PubMed] [Google Scholar]
- Almessiere MA, Slimani Y, Baykal A. Synthesis and characterization of Co1–2xNixMnxCeyFe2–yO4 nanoparticles. Journal of Rare Earths. 2020;38(2):188–194. doi: 10.1016/j.jre.2019.07.005. [DOI] [Google Scholar]
- Amoatey P, Baawain MS. Effects of pollution on freshwater aquatic organisms. Water Environment Research. 2019;91:1272–1287. doi: 10.1002/wer.1221. [DOI] [PubMed] [Google Scholar]
- Aref MS, Salem SS. Bio-callus synthesis of silver nanoparticles, characterization, and antibacterial activities via Cinnamomum camphora callus culture. Biocatalysis and Agricultural Biotechnology. 2020 doi: 10.1016/j.bcab.2020.101689. [DOI] [Google Scholar]
- Arockia JD, Sathyavathi K, Umadevi M, Parimaladevi R. Enhanced bioactivity of Fe3O4-Au nanocomposites — A comparative antibacterial study. Materials Letters. 2019;258:126795. doi: 10.1016/j.matlet.2019.126795. [DOI] [Google Scholar]
- Barnard J. Biological nutrient removal: Where we have been, where we are going? Proceedings of the Water Environment Federation. 2006;13:1–25. doi: 10.2175/193864706783710578. [DOI] [Google Scholar]
- Bassem SM. Water pollution and aquatic biodiversity. Biodiversity International Journal. 2020;4(1):10–16. [Google Scholar]
- Casals, E., Zeng, M., Parra-Robert, M., Fernández-Varo, G., Morales‐Ruiz, M., Jiménez, W., Puntes, V., & Casals, G. (2020). Cerium oxide nanoparticles: Advances in biodistribution, toxicity, and preclinical exploration. Small, 16(20), e1907322. [DOI] [PubMed]
- Charbgoo F, Ahmad MB, Darroudi M. Cerium oxide nanoparticles: Green synthesis and biological applications. International Journal of Nanomedicine. 2017;12:1401–1413. doi: 10.2147/IJN.S124855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng SY, Chen JC. Joint action of elevated ambient nitrite and nitrate on hemolymph nitrogenous compounds and nitrogen excretion of tiger shrimp Penaeus monodon. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology. 2002;131(3):303–314. doi: 10.1016/s1532-0456(02)00004-2. [DOI] [PubMed] [Google Scholar]
- Cheng SY, Chen JC. The time-course change of nitrogenous excretion in the Kuruma shrimp Penaeus japonicus following nitrite exposure. Aquatic Toxicology. 2001;51(4):443–454. doi: 10.1016/S0166-445X(00)00122-3. [DOI] [PubMed] [Google Scholar]
- Choi, Y., Jeon, J., Choi, Y., & Kim, S. D. (2020). Characterizing biotransformation products and pathways of the flame retardant triphenyl phosphate in Daphnia magna using non-target screening. Science of The Total Environment, 708, 135106. [DOI] [PubMed]
- Contreras AR, Casals E, Puntes V, Komilis D, Sánchez A, Font X. Use of cerium oxide (CeO2) nanoparticles for the adsorption of dissolved cadmium (II), lead (II) and chromium (VI) at two different pHs in single and multi-component systems. Global Nest Journal. 2015;17(3):536–543. doi: 10.30955/gnj.001687. [DOI] [Google Scholar]
- Dadrasnia, A., Usman, M. M., Lim, K. T., Velappan, R. D., Shahsavari, N., Vejan, P., Mahmud, A. F., & Ismail, S. (2017). Microbial aspects in wastewater treatment – A technical review. Environmental Pollution and Protection. Isaac Scientific Publishing, 2(2), 57–84. www.isaacpub.org/images/PaperPDF/EPP_100034_2017062616455301922.pdf. Accessed 27 February 2022.
- Das, C., Sen, S., Singh, T., Ghosh, T., Paul, S. S., Kim, T. W., Jeon, S., Maiti, D. K., & Im, J. (2020). Biswas G. Green synthesis, characterization and application of natural product coated magnetite nanoparticles for wastewater treatment. Nanomaterials, 10(8), 1615. [DOI] [PMC free article] [PubMed]
- de Farias Lima J, Lobo ET, Bastos AM, Duarte SS. Nitrate level safety to Amazon River shrimp juveniles. Environmental Science and Pollution Research. 2020;27(4):4546–4550. doi: 10.1007/s11356-019-07033-6. [DOI] [PubMed] [Google Scholar]
- Deng M, Dai Z, Senbati Y, Li L, Song K, He X. Aerobic denitrification microbial community and function in zero-discharge recirculating aquaculture system using a single biofloc-based suspended growth reactor: Influence of the carbon-to-nitrogen ratio. Frontiers in Microbiology. 2020;11:1760. doi: 10.3389/fmicb.2020.01760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding, Z., Jiang, Y., & Liu, X. (2018). Nanoemulsions-based drug delivery for brain tumors. In Nanotechnology-based targeted drug delivery systems for brain tumors (pp. 327–358). Academic Press.
- Dominguez MC, Rosa M, Borobio MV. Application of a spectrophotometric method for the determination of post-antibiotic effect and comparison with viable counts in agar. The Journal of Antimicrobial Chemotherapy. 2001;47:391–398. doi: 10.1093/jac/47.4.391. [DOI] [PubMed] [Google Scholar]
- Dutra, F. M., Alab, J. H. C., Gomes, M. K. C., Furtado, P. S., Valenti, W. C., & Ballester, E. L. C. (2020). Nitrate acute toxicity to post larvae and juveniles of Macrobrachium amazonicum (Heller, 1862). Chemosphere, 242, 125229. [DOI] [PubMed]
- Farias IAP, Santos CCL, Sampaio FC. Antimicrobial activity of cerium oxide nanoparticles on opportunistic microorganisms: A systematic review. Bio Med Research International. 2018 doi: 10.1155/2018/1923606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Q, Sun Y, Wu Y, Xue Z, Luo J, Fang F, Li C, Cao J. Physicochemical and biological effects on activated sludge performance and activity recovery of damaged sludge by exposure to CeO2 nanoparticles in sequencing batch reactors. International Journal of Environmental Research and Public Health. 2019;16(20):4029. doi: 10.3390/ijerph16204029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forest V, Leclerca L, Hochepiedd JF, Trouvéd A, Sarry G, Pourchez J. Impact of cerium oxide nanoparticles shape on their in vitro cellular toxicity. Toxicology in Vitro. 2017;38:136–141. doi: 10.1016/j.tiv.2016.09.022. [DOI] [PubMed] [Google Scholar]
- Gaikwad, V. L., Choudhari, P. B., Bhatia, N. M., & Bhatia, M. S. (2019). Characterization of pharmaceutical nanocarriers: In vitro and in vivo studies. In Nanomaterials for Drug Delivery and Therapy (pp. 33–58). William Andrew Publishing.
- García A, Delgado L, Torà JA, Casals E, González E, Puntes V, Sánchez A. Effect of cerium dioxide, titanium dioxide, silver, and gold nanoparticles on the activity of microbial communities intended in wastewater treatment. Journal of Hazardous Materials. 2012;199:64–72. doi: 10.1016/j.jhazmat.2011.10.057. [DOI] [PubMed] [Google Scholar]
- Guemmaz, F., Neffar, S., & Chenchouni, H. (2019). Physicochemical and bacteriological quality of surface water resources receiving common wastewater effluents in drylands of Algeria. In Water Resources in Algeria-Part II (pp. 117–148). Springer, Cham.
- Hu X, Liu X, Yang X, Guo F, Su X, Chen Y. Acute and chronic responses of macrophyte and microorganisms in constructed wetlands to cerium dioxide nanoparticles: Implications for wastewater treatment. Chemical Engineering Journal. 2018;348:35–45. doi: 10.1016/j.cej.2018.04.189. [DOI] [Google Scholar]
- Iloms E, Ololade OO, Ogola HJO, Selvarajan R. Investigating industrial effluent impact on municipal wastewater treatment plant in Vaal, South Africa. International Journal of Environmental Research and Public Health. 2020;17:1096. doi: 10.3390/ijerph17031096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal S, Shukla P. Alternative strategies for microbial remediation of pollutants via synthetic biology. Frontiers in Microbiology. 2020;11:808. doi: 10.3389/fmicb.2020.00808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J, Oberdörster G, Biswas P. Characterization of size, surface charge, and agglomeration state of nanoparticle dispersions for toxicological studies. Journal of Nanoparticle Research. 2009;11(1):77–89. doi: 10.1007/s11051-008-9446-4. [DOI] [Google Scholar]
- Joseph, E., & Singhvi, G. (2019). Multifunctional nanocrystals for cancer therapy: A potential nanocarrier. Nanomaterials for Drug Delivery and Therapy, 91–116.
- Kamika I, Tekere M. Impacts of cerium oxide nanoparticles on bacterial community in activated sludge. AMB Express. 2017;7(63):1–11. doi: 10.1186/s13568-017-0365-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan I, Saeed K, Khan I. Nanoparticles: Properties, applications and toxicities. Arabian Journal of Chemistry. 2019;12(7):908–931. doi: 10.1016/j.arabjc.2017.05.011. [DOI] [Google Scholar]
- Liu, J., Wu, Y., Wu, C., Muylaert, K., Vyverman, W., Yu, H., Munoz, R., & Rittmann, B. (2017). Advanced nutrient removal from surface water by a consortium of attached microalgae and bacteria: A review. Bioresource Technology, 241. [DOI] [PubMed]
- Liu Y, Li X, Zhao J, Wang D, Yang Q, Zeng G. The feasibility of enhanced biological phosphorus removal in the novel oxic/extended idle process using fermentation liquid from sludge fermentation. RSC Advances. 2018;8(6):3321–3327. doi: 10.1039/C7RA12886J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luengo CV, Volpe MA, Avena MJ. High sorption of phosphate on Mg-Al layered double hydroxides: Kinetics and equilibrium. Journal of Environmental Chemical Engineering. 2017;5(5):4656–4662. doi: 10.1016/j.jece.2017.08.051. [DOI] [Google Scholar]
- Luo, G., Xu, J., & Meng, H. (2020). Nitrate accumulation in biofloc aquaculture systems. Aquaculture, 520, 734675.
- Madubuonu N, Aisida SO, Ahmad I, Botha S, Zhao TK, Maaza M, Ezema FI. Bio-inspired iron oxide nanoparticles using Psidium guajava aqueous extract for antibacterial activity. Applied Physics A. 2020;126(1):1–8. doi: 10.1007/s00339-019-3249-6. [DOI] [Google Scholar]
- Martínez NN, Orozco MFS, Castañón GAM, Méndez FT, Ruiz F. Molecular mechanisms of bacterial resistance to metal and metal oxide nanoparticles. International Journal of Molecular Sciences. 2019;20(11):2808. doi: 10.3390/ijms20112808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauerhofer L, Pappenreiter P, Paulik C, Seifert AH, Bernacchi S, Rittmann SKMR. Methods for quantification of growth and productivity in anaerobic microbiology and biotechnology. Folia Microbiologica (praha) 2018;64:321–360. doi: 10.1007/s12223-018-0658-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarty PL. What is the best biological process for nitrogen removal: When and why? Environmental Science & Technology. 2018;52:3835–3841. doi: 10.1021/acs.est.7b05832. [DOI] [PubMed] [Google Scholar]
- McPherson, A., Thorpe, B., & Blake, A. (2004). Brominated flame retardants in dust on computers: The case for safer chemicals and better computer design. Clean Protect, 43.
- Meng R, Wu Z, Xie QT, Cheng JS, Zhang B. Preparation and characterization of zein/carboxymethyl dextrin nanoparticles to encapsulate curcumin: Physicochemical stability, antioxidant activity and controlled release properties. Food Chemistry. 2021;340:127893. doi: 10.1016/j.foodchem.2020.127893. [DOI] [PubMed] [Google Scholar]
- Naushad, M., Sharma, G., Kumar, A., Sharma, S., Ghfar, A. A., Bhatnagar, A., Stadler, F. J., & Khan, M. R. (2017). Efficient removal of toxic phosphate anions from aqueous environment using pectin based quaternary amino anion exchanger. International Journal of Biological Macromolecules, 106, 1–10. 10.1016/j.ijbiomac.2017.07.169 [DOI] [PubMed]
- Nyoka M, Choonara YE, Kumar P, Kondiah PP, Pillay V. Synthesis of cerium oxide nanoparticles using various methods: Implications for biomedical applications. Nanomaterials. 2020;10(2):242. doi: 10.3390/nano10020242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAHO. (2013). Health, Environment and Sustainable Development: Towards the future we want. Pan American Health Organization (PAHO). Washington, DC. https://www.paho.org/hq/dmdocuments/2013/seminario-rio-20-eng.pdf
- Pelletier DA, Suresh AK, Holton GA, McKeown CK, Wang W, Gu B. Effects of engineered cerium oxide nanoparticles on bacterial growth and viability. Applied and Environmental Microbiology. 2010;76(24):7981–7989. doi: 10.1128/AEM.00650-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai AM, Sivasankarapillai VS, Rahdar A, Joseph J, Sadeghfar F, Anuf AR, Rajesh K, Kyzas GZ. Green synthesis and characterization of zinc oxide nanoparticles with antibacterial and antifungal activity. Journal of Molecular Structure. 2020;1211:128107. doi: 10.1016/j.molstruc.2020.128107. [DOI] [Google Scholar]
- Popov, A. L., Shcherbakov, A. B., Zholobak, N. M., Baranchikov, A. Y., & Ivanov, V. K. (2017). Cerium dioxide nanoparticles as third-generation enzymes (nanozymes). Haнocиcтeмы: физикa, xимия, мaтeмaтикa, 8(6).
- Prabhakar R, Samadder SR, Jyotsana. Aquatic and terrestrial weed mediated synthesis of iron nanoparticles for possible application in wastewater remediation. Journal of Cleaner Production. 2017;168:1201–1210. doi: 10.1016/j.jclepro.2017.09.063. [DOI] [Google Scholar]
- Qiu G, Neo SY, Ting YP. Effects of CeO2 nanoparticles on system performance and bacterial community dynamics in a sequencing batch reactor. Water Science and Technology. 2016;73(1):95–101. doi: 10.2166/wst.2015.462. [DOI] [PubMed] [Google Scholar]
- Rajta A, Bhatia R, Setia H, Pathani P. Role of heterotrophic aerobic denitrifying bacteria in nitrate removal from wastewater. Journal of Applied Microbiology. 2020;128(5):1261–1278. doi: 10.1111/jam.14476. [DOI] [PubMed] [Google Scholar]
- Romano N, Zeng C. Effects of potassium on nitrate mediated alterations of osmoregulation in marine crabs. Aquatic Toxicology. 2007;85(3):202–208. doi: 10.1016/j.aquatox.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Romano N, Zeng C. Evaluating the newly proposed protocol of incorporated potassium in nitrate toxicity experiments at different salinities: A case study with the tiger prawn, Penaeus monodon, juveniles. Aquaculture. 2009;289(3–4):304–309. doi: 10.1016/j.aquaculture.2009.01.035. [DOI] [Google Scholar]
- Romano N, Zeng C. Toxic effects of ammonia, nitrite, and nitrate to decapod crustaceans: A review on factors influencing their toxicity, physiological consequences, and coping mechanisms. Reviews in Fisheries Science. 2013;21(1):1–21. doi: 10.1080/10641262.2012.753404. [DOI] [Google Scholar]
- Romer I, Briffa SM, Dasilva YAR, Hapiuk D, Trouillet V, Palmer RE, Jones EV. Impact of particle size, oxidation state and capping agent of different cerium dioxide nanoparticles on the phosphate-induced transformations at different pH and concentration. PLoS ONE. 2019 doi: 10.1371/journal.pone.0217483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruzhitskaya O, Gogina E. Methods for removing of phosphates from wastewater. MATEC Web of Conferences. 2017;106:07006. doi: 10.1051/matecconf/201710607006. [DOI] [Google Scholar]
- Salama, A. M., Abedin, R. M. A., & Elwakeel, K. Z. (2021). Influences of greenly synthesized iron oxide nanoparticles on the bioremediation of dairy effluent using selected microbial isolates. International Journal of Environmental Science and Technology, 1–12.
- Salama A, Beheary MS, Abd Elaal AE, Abdelaal A. Potential of magnetite nanoparticles on dairy effluent nitrate and phosphate bioremediation. Alfarama Journal of Basic & Applied Sciences. 2022;3(1):182–199. [Google Scholar]
- Selvamani, V. (2019). Stability studies on nanomaterials used in drugs. In Characterization and Biology of Nanomaterials for Drug Delivery (pp. 425–444). Elsevier.
- Sharma N, Dwivedi A. Bioremediation of dairy waste water for nitrate reduction. World Journal of Pharmaceutical and Life Sciences. 2017;3(1):375–384. [Google Scholar]
- Shomar, B., Al-Darwish, K., & Vincent, A. (2020). Optimization of wastewater treatment processes using molecular bacteriology. Journal of Water Process Engineering, 33, 101030.
- Singh KR, Nayak V, Sarkar T, Singh RP. Cerium oxide nanoparticles: Properties, biosynthesis and biomedical application. RSC Advances. 2020;10(45):27194–27214. doi: 10.1039/D0RA04736H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R, Behera M, Kumar S. Nano-bioremediation: An innovative remediation technology for treatment and management of contaminated sites. In: Bharagava RN, Saxena G, editors. Bioremediation of industrial waste for environmental safety. Springer Nature; 2020. pp. 165–182. [Google Scholar]
- Singh S, Kumar V, Romero R, Sharma K, Singh J, et al. Applications of nanoparticles in wastewater treatment. In: Prasad R, et al., editors. Nanobiotechnology in bioformulations, nanotechnology in the life sciences. Springer Nature; 2019. pp. 395–418. [Google Scholar]
- Sobhani-Nasab A, Eghbali-Arani M, Hosseinpour-Mashkani SM, Ahmadi F, Rahimi-Nasrabadi M, Ameri V. Eco-friendly preparation and characterization of CuMn2O4 nanoparticles with the green capping agent and their photocatalytic and photovoltaic applications. Iranian Journal of Catalysis. 2020;10(2):91–99. [Google Scholar]
- Song P, Wu L, Guan W. Dietary nitrates, nitrites, and nitrosamines intake and the risk of gastric cancer: A meta-analysis. Nutrients. 2015;7(12):9872–9895. doi: 10.3390/nu7125505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakkar S, Wanjale S, Panzade PD. Green synthesis of gold nanoparticles using Colchicum autumnale and its characterization. International Journal of Advanced Research. 2016;4(4):596–607. doi: 10.21474/IJAR01/257. [DOI] [Google Scholar]
- Varadavenkatesan T, Vinayagam R, Selvaraj R. Green synthesis and structural characterization of silver nanoparticles synthesized using the pod extract of Clitoria ternatea and its application towards dye degradation. Materials Today: Proceedings. 2020;23:27–29. [Google Scholar]
- Wang J, Li R, Zhang H, Wei G, Li Z. Beneficial bacteria activate nutrients and promote wheat growth under conditions of reduced fertilizer application. BMC Microbiology. 2020;20:38. doi: 10.1186/s12866-020-1708-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Gao M, Li Z, She Z, Wu J, Zheng D, Guo L, Zhao Y, Gao F, Wang X. Performance evaluation, microbial enzymatic activity and microbial community of a sequencing batch reactor under long-term exposure to cerium dioxide nanoparticles. Bioresource Technology. 2016;220:262–270. doi: 10.1016/j.biortech.2016.08.086. [DOI] [PubMed] [Google Scholar]
- Wiemer AJ. Metabolic efficacy of phosphate prodrugs and the remdesivir paradigm. ACS Pharmacology & Translational Science. 2020;3(4):613–626. doi: 10.1021/acsptsci.0c00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe, A. H., & Patz, J. A. (2002). Reactive nitrogen and human health: Acute and long-term implications. Ambio: A Journal of the Human Environment, 31(2), 120–125. [DOI] [PubMed]
- Wu L, Ning D, Zhang B, Li Y, Zhang P, Shan X, Zhou J. Global diversity and biogeography of bacterial communities in wastewater treatment plants. Nature Microbiology. 2019;4(7):1183–1195. doi: 10.1038/s41564-019-0426-5. [DOI] [PubMed] [Google Scholar]
- Xu Y, Wang C, Hou J, Wang P, You G, Miao L. Effects of cerium oxide nanoparticles on bacterial growth and behaviors: Induction of biofilm formation and stress response. Environmental Science and Pollution Research. 2019;26:9293–9304. doi: 10.1007/s11356-019-04340-w. [DOI] [PubMed] [Google Scholar]
- Xu Y, Wang C, Hou J, Wang P, You G, Miao L. Mechanistic understanding of cerium oxide nanoparticle-mediated biofilm formation in Pseudomonas aeruginosa. Environmental Science and Pollution Research. 2018;25:34765–34776. doi: 10.1007/s11356-018-3418-8. [DOI] [PubMed] [Google Scholar]
- Yang M, Meng F, Gu W, Li F, Tao Y, Zhang Z, Zhang F, Yang X, Li J, Yu J. Effects of natural products on bacterial communication and network-quorum sensing. BioMed Research International. 2020 doi: 10.1155/2020/8638103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi R, Song P, Liu X, Maruo M, Ba S. Differences in dissolved phosphate in shallow-lake waters as determined by spectrophotometry and ion chromatography. Limnology. 2020;21:329–339. doi: 10.1007/s10201-019-00574-2. [DOI] [Google Scholar]
- Yuan S, Li H, Dang Y, Liu C. Effects of triphenyl phosphate on growth, reproduction and transcription of genes of Daphnia magna. Aquatic Toxicology. 2018;195:58–66. doi: 10.1016/j.aquatox.2017.12.009. [DOI] [PubMed] [Google Scholar]
- Yun Y, Li Z, Chen Y, Saino M, Cheng S, Zheng L. Reduction of nitrate in secondary effluent of wastewater treatment plants by Fe0 reductant and Pd–Cu/graphene catalyst. Water Air and Soil Pollution. 2016;227:111. doi: 10.1007/s11270-016-2792-4. [DOI] [PubMed] [Google Scholar]
- Zhang L, Shen Z, Fang W, Gao G. Composition of bacterial communities in municipal wastewater treatment plant. Science of the Total Environment. 2019;689:1181–1191. doi: 10.1016/j.scitotenv.2019.06.432. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Fresh dairy wastewater and activated sludge inoculum samples were collected from a dairy wastewater treatment plant at Jumasa, Egypt. The dairy factory agreed to give different inoculum samples for this study. The permission’s letter was given without any obligation and commitment on our part.
Not applicable.