Abstract
Mdm2 is the principal E3-ubiquitin ligase for p53 and contains a C2H2C4 type RING domain wherein the last cysteine residue is followed by an evolutionarily conserved 13 amino acid C-terminal tail. Previous studies have indicated that integrity of the C-terminal tail is critical for Mdm2 function. Recently, a mutation extending the MDM2 length by five amino acids was identified and associated with enhanced p53 response in fibroblasts and premature aging in a human patient. To investigate the importance of the conserved Mdm2 C-terminus length on p53 regulatory function in vivo, we engineered three novel mouse alleles using CRISPR-Cas9 technology. Genetic studies with these murine models showed that curtailing Mdm2 C-terminus length by even a single amino acid leads to p53-dependent embryonic lethality. Extension of the Mdm2 C-terminus length by five amino acids (QLTCL) yielded viable mice that are smaller in size, exhibit fertility problems, and have a shortened life span. Analysis of early passage mouse embryonic fibroblasts indicated impaired Mdm2 function correlates with enhanced p53 activity under stress conditions. Furthermore, analysis in mice showed tissue-specific alterations in p53 target gene expression and enhanced radiosensitivity. These results confirm the physiological importance of the evolutionarily conserved Mdm2 C-terminus in regulating p53 functions.
INTRODUCTION
Murine double minute 2 (Mdm2), an evolutionarily conserved protein (489 and 491 amino acids in mice and humans, respectively) and its homologous protein Mdm4 are bona fide inhibitors of p53 function (1–3). Genetic ablation of Mdm2 in mice results in p53-dependent lethality and underscores its importance in p53 regulation (4–7). Mdm2 directly binds to the N-terminal domain of p53 and squelches its transcriptional activity (8). In addition, Mdm2 promotes proteasomal degradation of the p53 protein by virtue of its inherent E3-ubiquitin ligase activity (9,10). The C-terminus of Mdm2 contains a C2H2C4 RING domain that mediates its interaction with a ubiquitin-conjugating enzyme E2 nucleating the complex that targets p53 for degradation (11). The RING domain also enables Mdm2 to form a heterodimer with Mdm4 which potentiates its ubiquitin ligase activity (12,13). To complicate matters, Mdm2 is also a transcriptional target of p53 and stress- induced p53 promotes Mdm2 transcription to ultimately tame its own activity and stability (14).
Mice with Mdm2 deletion are embryo lethal in a p53-dependent manner (4,7). Other alleles expressing low Mdm2 levels exhibit hyperpigmentation of extremities, fertility problems and radiosensitivity due to enhanced p53 activity (15–17). In recent years, studies from numerous labs have shown that mutation of the RING domain (residues 437–479) disrupts Mdm2 structure, affects its interaction with Mdm4 and impairs its E3 ligase activity (18,19). In addition, mutations at amino acids tyrosine 487 or phenylalanine 490 also impair Mdm2 E3 ligase activity without affecting binding and inhibition of p53 activity (20,21).
Comparative sequence analyses reveal that Mdm2 C-terminal length is conserved throughout evolution and the last cysteine residue of the RING domain is followed by exactly 13 amino acids. Alterations to the Mdm2 protein length compromise its function in cell culture studies (20,22). A recent report of an anti-terminating mutation in Mdm2 in a human patient with a progeria-like phenotype also highlighted the role of C-terminal length in regulating p53 functions (23). Fibroblast cells from the patient exhibit enhanced p53 response and increased p53 stability under stress. However, the single patient and other in vitro studies could not be extrapolated to fully understand the physiological relevance of the Mdm2 C-terminus in p53 regulation.
To examine the physiological importance of the Mdm2 C-terminal tail, we used CRISPR-cas9 technology and engineered mice in which the Mdm2 length was either curtailed, or extended by 5 amino acids. Our results showed that shortening the length is not compatible with life, while extension of 5 amino acids (QLTCL), identical to the human patient, impaired Mdm2 function. Impaired Mdm2 resulted in increased p53 activity and stability that manifested in the form of phenotypic aberrations including small size, hyperpigmentation, fertility issues, and a shortened life span. Furthermore, unregulated p53 activity due to compromised Mdm2 function sensitized mice to DNA damage. Thus, our results provide direct evidence in support of the important role of evolutionary conserved Mdm2 length in its function.
MATERIALS AND METHODS:
Mouse maintenance and genotyping:
Mice were generated by Genetically Engineered Mouse (GEM) facility at MDACC. Sequences for donor and guide RNAs used for CRISPR/Cas9 knock-in were as follows. Donor1– 5’-GTGCAAAGAAGCTAAAAAAAAGAAACAAGCCCTGCCCAGTGTGCAGACAGCCAATCCAAATGATCGTGTTGACTTATTTTAACCAATTAACCTGCCTATAAAAATAGAATTTTATATTTCTAACTATATGACCCCCAAATTAGACAACATGGGTATTATTTTTATACATTAAAGCCAGAAAA-3’. sgRNA1– 5’-GAAATTAATACGACTCACTATAGAGTAAGTTAGCACAATCATTGTTTTAGAGCTAGAAATAGCAAGTTAAAATAAGGCTAGTCCGTTATCAACTTGAAAAAGTGGCACCGAGTCGGTGCTTTT-3’; sgRNA2–5’-AAAAGCACCGACTCGGTGCCACTTTTTCAAGTTGATAACGGACTAGCCTTATTTTAACTTGCTATTTCTAGCTCTAAAACAATGATTGTGCTAACTTACTCTATAGTGAGTCGTATTAATTTC-3’. CRISPR/Cas9 generated chimeric mice were backcrossed to C57BL/6J for 3–4 generations prior to commencing this study. All studies were conducted as per an institutional animal care and use committee approved protocol. Mice were genotyped using PCR with For-5’-GCCAAGAAAGCGTGAAAGAG-3’ and Rev- 5’-GAGGAAGGTCCACCATCATC-3’ primers followed by Hinc II restriction digestion and resolution on 2% agarose gel.
MEF generation:
Timed matings were set up and pregnant dams were sacrificed at 13.5 dpc and embryos used for making MEFs. Early passage MEFs (#2–4) were used in the experiments.
RNA isolation and RT–qPCR:
Total RNA was isolated from MEFs and tissues using TRIzol reagent (Invitrogen,Carlsbad, CA, USA). One microgram of RNA was reverse transcribed using iScript cDNA synthesis kit (Bio-Rad, CA, USA). The reaction was diluted 15-fold and 2 μl of the diluted sample was used in qPCR for p53 targets as described in (24).
IP and Western blotting:
Tissues were homogenized in NP-40 lysis buffer (50 nM Tris-HCl pH 8.0, 100 mM Nacl, 0.5% NP-40) supplemented with 1X complete protease inhibitor (Roche, Indianapolis, IN, USA). 100 μl of protein lysate was resolved on 8% sodium dodecyl sulfate–polyacrylamide gel electrophoresis. IP was performed as described in (25). After transfer on nitrocellulose membranes, blots were probed with p53 (CM5, 1:1000, Novacastra, IL, USA), Mdm2 (2A10, 1:500, Calbiochem, San Diego, CA, USA), Mdm4 (1:500; MX82, Sigma), Actin (A5441, 1:5000, Sigma, St Louis, MO, USA), Cleaved PARP-1 (1051–1, 1:1000, Epitomics/Abcam, Cambridge, UK), p21 (556431, 1:1000, BD Pharmingen, USA) and Vinculin (V9131, 1:5000, Sigma, St Louis, MO, USA) antibodies. Data were analyzed using ImageJ software (National Institutes of Health, Bethesda).
Radiation:
Early passage MEFs or 5 to 6-week old mice were irradiated with 6 Gy in a Cesium-137 irradiator and tissues harvested at different time points. Some irradiated mice were monitored for survival.
Immunohistochemistry:
IHC with cleaved caspase-3 antibody (9661, Cell Signaling, MA, USA) was carried out on formalin fixed testis tissue sections.
Statistical analyses:
GraphPad Prism software (Version 8, Graphpad Software Inc, La Jolla, CA, USA) was used for statistical calculations and data visualization. Student’s two tailed t-test was used to confirm statistical significance and a P-value ≤ 0.05 was considered significant. Data are shown as mean ± s.e.m. Log-rank (Mantel–Cox) test was used to determine statistical significance in survival studies.
RESULTS
Generation and characterization of mice with Mdm2 C-terminal alterations
Mdm2 is an evolutionarily conserved protein entrusted with inhibiting p53 function (2,26). Both N- and C-terminal domains which interact with p53 are conserved across species. To investigate the contribution of C-terminal length in Mdm2 functionality, we generated novel mutant alleles using CRISPR/Cas9 technology. Briefly, the naturally occurring stop codon in Mdm2 was mutated to Glutamine (Q) and four novel amino acids (Lysine (L), Tyrosine (T), Cysteine (C), and Lysine (L)) were added before terminating at a new stop codon. Silent mutations were introduced to prevent re-targeting (Fig 1A). These changes facilitated the extension of the murine Mdm2 coding sequence by 5 amino acids (Mdm25AA) analogous to the anti-terminating MDM2 mutation in a human patient. Chimeric Mdm25AA mice were identified by polymerase chain reaction (PCR) amplification of tail DNA encompassing the mutation site followed by restriction digestion with Hinc II enzyme and resolution on agarose gel. Positive clones were further confirmed by DNA sequencing of the PCR amplified product. Sanger sequencing of PCR amplified DNA at potential off-target sites was performed and ruled out mutations at these sites. Subsequently, we reverse transcribed and sequenced the full length Mdm2 cDNA and ruled out inadvertent mutations in the coding sequence. Mice were backcrossed to C57BL/6J mice for four generations prior to initiating this study.
Figure 1: Generation and characterization of Mdm2 C-terminal mutant alleles.
A). A schematic of three mouse alleles generated. Green letters denote nucleotide changes; amino acids in red are novel. B). Left: Graph shows comparative body weights of Mdm2+/+ (n=7), Mdm25AA/ +(n=17) and Mdm25AA/5AA (n=24) mice. Right: Graph shows comparative body weights of male and female Mdm2+/+ (n=4 and 3 respectively), Mdm25AA/ +(n=12 and 5 respectively) and Mdm25AA/5AA (n=13 and 11 respectively) mice. C). Graph shows comparative organ weights of Mdm2+/+ (n=7), Mdm25AA/+ (n=3) and Mdm25AA/5AA (n=7) mice. D). Graph shows comparative testis weights of Mdm25AA/+ (n=4) and Mdm25AA/5AA (n=4) mice. E). Graph depicts white blood cell counts (WBC) of Mdm25AA/+ (n=3) and Mdm25AA/5AA (n=3) mice. *, p<0.05; **, p<0.005; ***, p<0.001.
Next, we intercrossed heterozygous Mdm25AA/+ mice to obtain homozygous Mdm25AA/5AA mice. Notably, Mdm25AA/5AA mice were viable and born at the normal Mendelian ratio (24/87, expected frequency 25%). Phenotypically, both male and female Mdm25AA/5AA mice were noticeably smaller than their heterozygous or wildtype counterparts (Fig 1B). Major organs weighed less; however, organ weight to body weight ratio remained comparable between Mdm2+/+, Mdm25AA/+ and Mdm25AA/5AA mice (Fig 1C). Mdm25AA/5AA mice also exhibited darker paws and tails than Mdm2+/+ mice. Male Mdm25AA/5AA mice had significantly smaller testes (Fig 1D), and females exhibited fertility problems with small litter size (4–6 pups instead of 8–10) and limited pregnancies (1–3 instead of 5–6) during reproductive period. Complete blood count analysis of 6 week old mice also showed leucopenia in Mdm25AA/5AA mice (Fig 1E).
In the course of generating the Mdm25AA mutant, we serendipitously generated two additional C-terminal mutant Mdm2 alleles. In one allele, a single in frame methionine amino acid was deleted (Mdm2Δ1) while the other encoded Mdm2 protein length shortened by 5 amino acids (Mdm2Δ5) with addition of 2 novel amino acids (Threonine and Cysteine) prior to termination (Fig 1A). To analyze the impact of a shortened Mdm2 tail on its function, we intercrossed heterozygous Mdm2Δ1 or Mdm2Δ5 mutant mice to generate homozygous mice bearing each of these mutations. No homozygous mice for either allele (0/31 for Mdm2Δ1 and 0/28 for Mdm2Δ5) were recovered suggesting an embryo lethal phenotype. Next, to test if this embryonic lethality was p53-dependent, we crossed Mdm2Δ1 or Mdm2Δ5 mice to p53-null mice. Concomitant deletion of p53 from the background yielded live pups for both these alleles. This p53-dependent rescue emphasizes the physiological importance of Mdm2 C-terminal length in p53 regulation. Due to similarities with the Mdm2-null mouse phenotype, we did not perform additional embryo experiments with these mice.
Mdm2–5AA retains the ability to interact with Mdm4 and p53
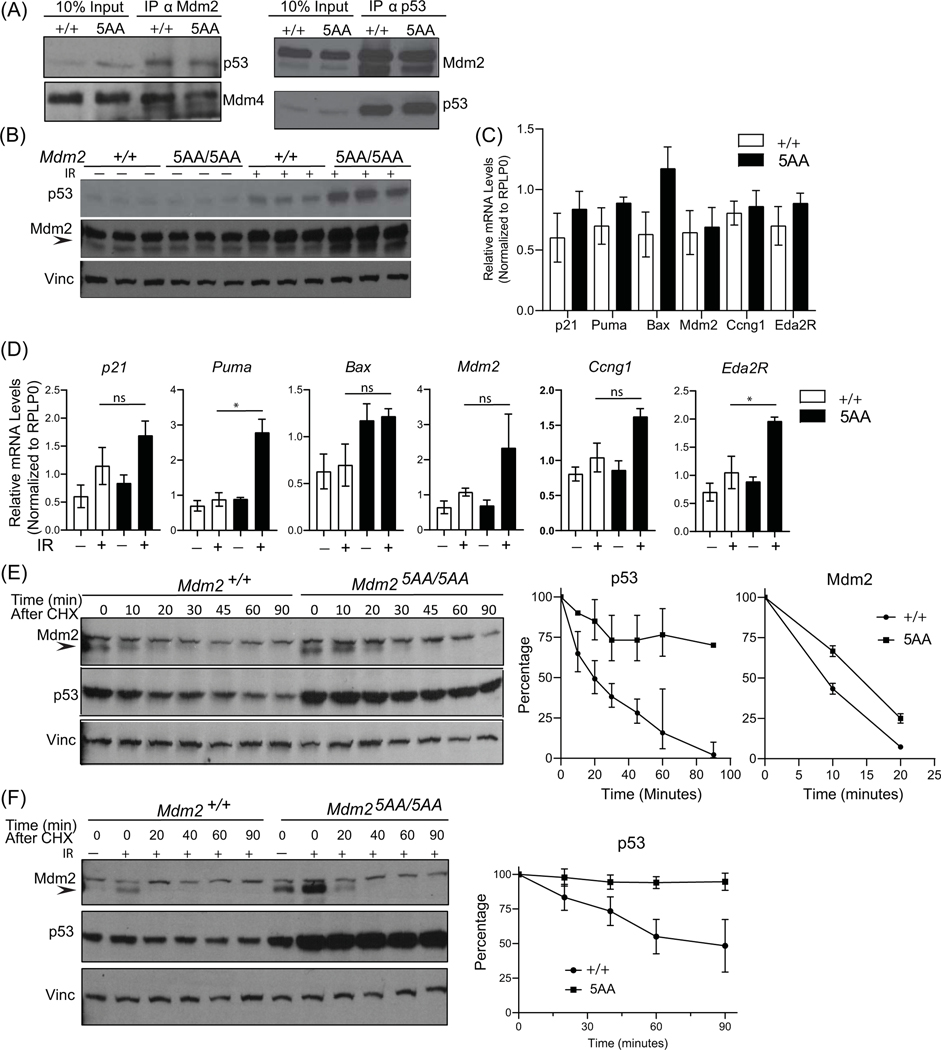
Mdm2 heterodimerizes with Mdm4 through its RING domain and forms a more effective ubiquitin ligase for restraining p53 (13,25,27). Therefore, we first tested whether Mdm2–5AA retains the ability to heterodimerize with Mdm4. We generated mouse embryonic fibroblasts (MEFs) from 13.5 dpc Mdm25AA/5AA embryos and performed immunoprecipitation (IP) assays with an Mdm2 antibody. Western blot (WB) analysis using an antibody against Mdm4 showed that similar to the wild type Mdm2, Mdm2–5AA also interacted with Mdm4 (Fig 2A). Similarly, another IP-WB assay with a p53 antibody revealed that Mdm2–5AA retains the ability to physically interact with p53. Thus, these results indicated that increase in C-terminal length does not interfere with the ability of Mdm2–5AA to physically interact with Mdm4 and p53.
Figure 2: Mdm2–5AA regulates transcriptional activity and stability of p53 in MEFs.
A). Immunoprecipitation (IP) experiments of Mdm2 (αΜdm2) and p53 (αp53) proteins followed by western blot analysis to examine interactions. B). Western blot for p53 and Mdm2 levels in Mdm2+/+ and Mdm25AA/5AA MEFs (n=3 each) 4 hours after 6 Gy IR. C). Real time PCR assays for basal levels of six p53 target genes in Mdm2+/+ and Mdm25AA/5AA MEFs. D). Real time PCR assays for a panel of six p53 target genes in Mdm2+/+ and Mdm25AA/5AA MEFs with or without DNA damage. *, p<0.05; ns, not significant; IR, 6 Gray γ radiation. E). Representative western blots for p53 and Mdm2 in Mdm2+/+ and Mdm25AA/5AA MEF lysates after treatment with cycloheximide (CHX). Min, minutes. Vinculin (Vinc) is used as loading control. Graphs depict the quantification results for p53 and Mdm2 from 3 independent experiments. F). Representative western blots for p53 and Mdm2 in irradiated Mdm2+/+ and Mdm25AA/5AA MEFs following treatment with cycloheximide (CHX). Vinculin (Vinc) is used as loading control. Graph depicts the quantification results for p53 from 3 independent experiments. +/+, Mdm2+/+; 5AA, Mdm25AA/5AA.
Mdm2–5AA regulates transcriptional activity and stability of p53 in MEFs
Mdm2 regulates p53 transcriptional activity by binding and masking its N-terminal transactivation domain. We therefore tested whether Mdm2–5AA binding to p53 could similarly inhibit p53 activity. First, we irradiated Mdm2+/+ and Mdm25AA/5AA MEFs and assessed p53 and Mdm2 protein levels in cell lysates (Fig 2B). After radiation, both Mdm25AA/5AA and Mdm2+/+ MEFs showed increased p53 and Mdm2 levels with Mdm25AA/5AA exhibiting higher levels of p53. Subsequently, we isolated RNA from these irradiated MEFs and performed real time PCR assays on a panel of p53 downstream targets. In the absence of stress, expression of p53 downstream targets was similar in both MEF genotypes. No noticeable change in basal mRNA levels of canonical p53 targets was identified by RT-qPCR assay (Fig 2C). We next analyzed the effect of DNA damage on p53 activity, as DNA damage phosphorylates p53 at the N-terminus and prevents Mdm2 binding (28). After exposure to 6 Gy IR, slightly higher mRNA levels of six p53 downstream targets was observed in Mdm25AA/5AA MEFs, and two targets (Puma and Eda2R) were significantly elevated in Mdm25AA/5AA as compared to Mdm2+/+ MEFs (Fig 2D). These results are quite akin to the reported data from the patient fibroblasts treated with daunorubicin (23) and suggest that Mdm2–5AA can bind and inhibit p53 transcriptional activity although not to the level of wild type Mdm2.
Mdm2 moderates protein levels of p53 and itself owing to its RING domain-associated ubiquitin ligase activity. Next, to examine whether the stability of p53 and Mdm2 proteins is affected in Mdm2–5AA cells, we carried out cycloheximide treatment of Mdm2+/+ and Mdm25AA/5AA MEFs and performed western blot analyses on the cell lysates. Western blot analyses showed that Mdm2–5AA was more stable with an increased half-life of ~15 minutes as compared to 10 minutes for wild type Mdm2 (Fig 2E). Additionally, p53 was also overtly stabilized in the presence of Mdm2–5AA. While p53 half-life remained close to the previously reported 20–30 minute interval in wild type MEFs (25), p53 levels were 2–3 fold higher to begin with in Mdm25AA/5AA and remained high throughout the 90 minutes duration of the experiment. A half-life could not be calculated.
p53 is a stress response protein that is stabilized upon exposure to DNA damage, Mdm2 is its major E3-ligase that returns p53 protein levels to a basal state (29,30). Therefore, in a subsequent assay, we used radiation, a prototypical DNA damaging agent to investigate the stability of stress-induced p53 protein in Mdm2–5AA MEFs. We irradiated Mdm2+/+ and Mdm25AA/5AA MEFs and examined the stability of p53 in cell lysates (Fig 2F). p53 was highly stabilized in irradiated Mdm25AA/5AA MEFs compared to Mdm2+/+. Moreover, while p53 levels in Mdm2+/+ MEFs eventually returned to base line, in mutant Mdm2–5AA MEFs, they remained higher throughout the course of the experiment. These assays indicate an inherent deficiency in the ability of Mdm2–5AA to regulate p53 levels.
Mdm2–5AA function is slightly compromised in vivo
Mdm2-mediated inhibition of p53 activity is critical for survival during development and stress conditions (4–7,18,24). To investigate the function of Mdm2–5AA in moderating p53 in vivo, we compared p53 activity in tissues of Mdm2+/+ and Mdm25AA/5AA mice using RT-qPCR assays for a panel of six p53 downstream targets in thymi, a well-known p53-responsive tissue (Fig 3A). Notably, basal mRNA levels of the p53 target genes remained similar in both Mdm2+/+ and Mdm25AA/5AA thymi suggesting no major changes in p53 activity. Moreover, after DNA damage p53-induced mRNA levels of these genes were comparable in both genotypes suggesting a similar functionality of Mdm2 and Mdm2–5AA in the respective mouse genotypes. Of note, we observed lower induction of Puma and Bax in irradiated Mdm25AA/5AA thymi as compared to Mdm2+/+ thymi for unknown reasons. To rule out the possibility that minor changes in p53 activity might be missed by RT-qPCR, we proceeded to capture functional outcomes of baseline p53 activity by immunohistochemistry for cleaved caspase 3 (CC3) on thymi and testis samples (Fig 3B). CC3 is a marker for apoptosis and reflects cellular death due to increased p53 activity. Both thymi and testis from Mdm25AA/5AA mice showed higher levels of CC3 staining in comparison to Mdm2+/+ organs. Although CC3 staining in the thymus was not statistically different between the two genotypes, quantification of CC3 staining in Mdm25AA/5AA testis depicted significantly more apoptosis correlating with their small size. This suggests that baseline p53 activity is higher in Mdm25AA/5AA testes due to subtle impairment of Mdm2 function.
Figure 3: Mdm2–5AA function is slightly compromised in vivo.
A). Real time PCR assays for a panel of p53 target genes in thymi of Mdm2+/+ (+/+) and Mdm25AA/5AA (5AA) mice (n=4 each). **, p<0.005; ns, not significant; IR, 6 Gray γ radiation. B). Representative image of cleaved caspase-3 staining on mouse testis samples of Mdm2+/+ and Mdm25AA/5AA mice (40X). Graphs depict the quantification of CC3 positive cells from testis and thymus. Eighteen to twenty two 40X view fields per genotype (n=2–4) were counted. C). Western blots for p53 in Mdm2+/+ and Mdm25AA/5AA thymi tissue lysates. Vinculin (Vinc) is used as loading control. #; empty lane, -C (Mdm2−/−p53−/−) and +C (p53515A/515A) irradiated mouse tissue lysates used as negative and positive controls, respectively. D). Western blots for p53, Mdm2, p21 and cleaved PARP-1 in irradiated Mdm2+/+ and Mdm25AA/5AA spleen tissue lysates. Actin is used as loading control. E). Western blot for p53R172H in p53515A/515A and Mdm25AA/5AAp53515A/515A thymi lysates. Vinculin (Vinc) is used as loading control. All mice were 5–6 weeks old.
Next, we investigated whether Mdm2–5AA could regulate p53 protein levels in mouse thymi and spleens. Notably, basal levels of p53 in Mdm2+/+ and Mdm25AA/5AA thymi remained comparable with no overt stabilization of p53 in the absence of DNA damage (Fig 3C). Upon 6 Gy radiation exposure, p53 levels further stabilized in both sets. Moreover, the level of stable p53 between Mdm2+/+ and Mdm25AA/5AA remained indistinguishable in both genotypes. Western blot analysis of spleen tissue lysate also yielded similar results (Fig 3D). p53 was stabilized 4 hours after IR to similar levels in both genotypes and returned to baseline by 8 hours. However, unlike MEFs, stabilization of Mdm2 was not observed in the Mdm25AA/5AA lysates. In addition, no difference in cleaved PARP-1 levels, a marker of apoptotic activity was discernable between the two genotypes. Similar levels of cleaved PARP-1 fragment (24 kDa) was observed in the irradiated tissue samples at 4 hour and 8 hour time points. Nonetheless, p21 levels were increased in irradiated Mdm25AA/5AA spleen lysates compared to Mdm2+/+. While p21 levels reverted to baseline by 8 hours after IR in Mdm2+/+ spleens, they remained higher in corresponding Mdm25AA/5AA spleens. These results suggest that subtle increases in basal wild type p53 levels observed in Mdm25AA/5AA MEFs were not appreciable in thymi and spleens physiologically.
Next, we crossed Mdm25AA/5AA mice with p53515A/515A mice (encoding mutant p53R172H) (31) and compared p53 levels in protein lysates from thymi of p53515A/515A and Mdm25AA/5AA p53515A/515A mice. Previous studies from our lab show that Mdm2 also regulates the protein levels of p53R172H and does not allow its accumulation in normal tissues of young mice (32). In agreement, we noted baseline p53R172H levels in p53515A/515A (Fig 3E). However, p53R172H levels were noticeably higher in Mdm25AA/5AA p53515A/515A thymus lysates. This implies a slight impairment of E3-ligase activity in Mdm2–5AA thymi. Overall, these results indicate a subtle decrease in Mdm2–5AA functional activity towards p53 in some tissues especially when measuring long term effects.
Mdm25AA/5AA mice are hypomorphic
As described earlier, Mdm25AA/5AA mice are smaller in size, have fertility problems and exhibit hyperpigmentation in the extremities. Similar characteristics have been reported in Mdm2PND and Mdm2puro/Δ7–12 mice associated with enhanced p53 activity due to insufficient Mdm2 levels (17,33). These mice, in addition to Mdm2+/− and Mdm2P2P2 mice, are also radiosensitive and succumb to bone marrow aplasia after exposure to 6 Gy IR (16,24). Therefore, to test in vivo whether Mdm2–5AA is also functionally compromised after DNA damage, we irradiated Mdm2+/+ and Mdm25AA/5AA mice (6 Gy IR) and monitored them for 30 days. As expected, all Mdm2+/+ mice (7/7) survived radiation exposure, however, Mdm25AA/5AA mice (8/8) died within 2 weeks post-IR (Fig 4A). These data clearly highlight insufficiency of Mdm2 levels to ground p53 activity.
Figure 4: Mdm25AA/5AA mice are hypomorphic and have a shorter life span.
A). Kaplan-Meier survival curve for Mdm25AA/+ (n=7) and Mdm25AA/5AA (n=8) mice after treatment with 6 Gray (Gy) γ radiation (IR). ***; p<0.0001. B). Kaplan-Meier survival curves for Mdm25AA/+ (n=34) and Mdm25AA/5AA (n=33) mice. **; p<0.005.
Studies have shown that a single functional Mdm2 allele can sufficiently mitigate p53 activity for survival (15,24). To genetically assess the effectiveness of a Mdm25AA allele in dampening p53 activity for survival, we intercrossed Mdm25AA/5AA and Mdm2+/− mice. Surprisingly, the cross did not yield viable Mdm25AA/− mice at weaning age (0/20; expected frequency 50%). Absence of live Mdm25AA/− mice clearly emphasizes impaired activity of the Mdm2–5AA protein in p53 regulation. These experiments underscore the hypomorphic nature of Mdm2 C-terminal mutation in vivo.
Mdm2–5AA mutant mice have a shorter life span
Enhanced p53 activity has been controversially linked with aging (34). In a recent report, segmental progeroid syndrome in a human patient with a homozygous anti-terminating MDM2 mutation, identical to the one studied here, was attributed to increased p53 activity (23). Accelerated aging was also reported in p53+/m and p53TSD mice (35,36). However, no aging phenotype was observed in Super p53, Mdm2+/−, Mdm2P2/P2, Mdm2PND and Mdm2puro/Δ7–12 mice which have slight elevation of p53 levels (15–17,24,37). To directly assess the involvement of Mdm2–5AA mutation in aging, we generated a cohort of homozygous Mdm25AA/5AA and heterozygous Mdm25AA/+ mice and monitored their survival for up to 2 years of age (Fig 4B). We did not observe anything amiss in mutation bearing Mdm25AA/5AA mice. Complete pathological assessment of 12–18 month old Mdm25AA/5AA mouse tissues did not reveal any standout problems. No aging associated phenotypes such as kyphosis, reduced fat mass or hair graying was observed in Mdm25AA/5AA mice. However, Mdm25AA/5AA mice (median survival 473 days) did not live as long as the Mdm25AA/+ mice (median survival undefined) in the cohort and cause of death remains undetermined. Some aged Mdm25AA/+ and Mdm25AA/5AA mice (1/35 and 7/40; respectively) developed tumors, mostly thymic lymphomas, by 18 month of age for unknown reasons and had to be euthanized. These mice were excluded from the survival curves.
DISCUSSION
The Mdm2 C-terminus contains a C2H2C4 type RING domain which is followed by 13 amino acids (22). Evolutionary sequence analysis and cell culture-based studies have highlighted the importance of Mdm2 C-terminal length in p53 regulation (20,22). While disease associated mutations in Mdm2 are very rare, a recent report implicated a homozygous anti-terminating mutation in Mdm2 to increased p53 activity and early aging phenotype in a human patient (23). To further investigate the effect of this mutation and the overall role of C-terminal length in Mdm2 function, we generated multiple mutant mice and carried out biochemical and genetic studies. Our results clearly show that curtailing of Mdm2 length even by a single amino acid negatively impacts its function. Truncation of Mdm2 length in Mdm2Δ1 and Mdm2Δ5 mice resulted in embryonic lethality that could be rescued by eliminating p53. These data suggest lack of Mdm2 function of these alleles. We hypothesize that deletion of terminal amino acids impair the Mdm2 RING domain structure which is needed for heterodimerization with Mdm4 and p53 down modulation during embryogenesis (25,27). Absence of an Mdm2-Mdm4 heterodimer likely led to early embryonic demise of these mice.
A mutation that increased the Mdm2 C-terminal length by 5 novel amino acids (QLTCL) resulted in viable mice, albeit with some defects, indicating enough functional Mdm2 for survival. Several studies support functionality of the Mdm2–5AA protein. Although we did not pursue biophysical assays to evaluate changes to the relative strength of the Mdm2–5AA binding with Mdm4 and p53, immunoprecipitation assays suggest that increase in the C-terminal tail length did not impair their interaction. Furthermore, basal p53 activity as measured by RT-qPCR was comparable between wild type and Mdm25AA/5AA MEFs. Similarly, western blot analysis of thymus and spleen tissue lysates did not show increased p53 stability nor increased transactivation of p53 targets. These data suggest that Mdm2–5AA retained sufficient function in inhibition of p53 activity.
Other studies indicate some deficiency in overall Mdm2–5AA function in MEFs and mice. p53 activity was enhanced in Mdm25AA/5AA MEFs after DNA damage as compared to Mdm2+/+ MEFs. Similarly, p53 stability and half-life was also up modulated in Mdm25AA/5AA MEFs after radiation. Thus, Mdm2–5AA could not effectively degrade p53 via its E3-ubiquitin ligase activity. Similarly, the small size/weight of Mdm25AA/5AA mice, fertility problems, hyperpigmentation of extremities and radiosensitivity to a sub-lethal dose of radiation further attest to Mdm2 insufficiency in Mdm25AA/5AA mice. Additionally, a single allele of Mdm25AA was non-viable in contrast to Mdm2+/− mice. The differences observed in Mdm2–5AA-mediated p53 regulation may be due to tissue/cell specificity. Also, changes in Mdm2–5AA post translational modification could affect its function towards p53. Moreover, the extension of the MDM2 C-terminus by QLTCL amino acids might alter its ability to regulate the transcriptional activity of p53 on a subset of p53 downstream targets especially the pro-apoptotic genes. Thus, the combination of functional and genetic data revealed a dysfunctional Mdm2–5AA protein in various tissues and circumstances.
We did not observe aging associated phenotypes in Mdm25AA/5AA mice although they had a shorter life span than their wild type/heterozygote counterparts. These results are different from the analogous mutation in the human patient. However, as the reported patient was a result of consanguineous relationship, he was genetically homozygous across a wide spectrum of the genome. It is possible that homozygosity of other genes contributed towards the segmental progeria syndrome. Another possibility suggests that enhanced p53 activity alone is insufficient to promote aging in laboratory mice that are not exposed to natural stresses or environmental stimuli during their lifetime. Previous studies with p53-Mdm2 pathway mutant mice also indicate that increased p53 activity in the presence of some functional Mdm2 fails to promote aging phenotypes even though the animal lifespan may be impacted (15,17,37). The shorter life span of Mdm2–5AA mice is reminiscent of the short life span reported in another Mdm2 hypomorphic mouse model from our lab (17). The aging process is an indicator of diminishing ability of stem cells to produce and replace differentiated cells in tissues. A slightly elevated p53 activity in Mdm25AA/5AA mice is insufficient to cause stem cell exhaustion and promote aging. Overall, these in vivo results convey the importance of conserved length of the Mdm2 C-terminus in evolution.
Statement of Significance:
This in vivo study highlights that alterations to the C-terminus of Mdm2 perturb its regulation of the tumor suppressor p53.
ACKNOWLEDGEMENTS
We thank Dr. Carol Prives, Columbia University, for her insights and encouragement. VP is partially supported by R50-CA251703 grant. This study was funded by NCI grant CA47296 to GL.
Footnotes
“The authors declare no potential conflicts of interest.”
REFERENCES
- 1.Moyer SM, Larsson CA, Lozano G. Mdm proteins: critical regulators of embry ogenesis and homeostasis. J Mol Cell Biol 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lane DP, Verma C. Mdm2 in evolution. Genes Cancer 2012;3:320–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eischen CM, Lozano G. The Mdm Network and Its Regulation of p53 Activities: A Rheostat of Cancer Risk. Hum Mutat 2014;35:728–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Montes de Oca Luna R, Wagner DS, Lozano G. Rescue of early embryonic lethality in mdm2-deficient mice by deletion of p53. Nature 1995;378:203–6 [DOI] [PubMed] [Google Scholar]
- 5.Zhang Y, Xiong S, Li Q, Hu S, Tashakori M, Van Pelt C, et al. Tissue-specific and age-dependent effects of global Mdm2 loss. J Pathol 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ringshausen I, O’Shea CC, Finch AJ, Swigart LB, Evan GI. Mdm2 is critically and continuously required to suppress lethal p53 activity in vivo. Cancer Cell 2006;10:501–14 [DOI] [PubMed] [Google Scholar]
- 7.Jones SN, Roe AE, Donehower LA, Bradley A. Rescue of embryonic lethality in Mdm2-deficient mice by absence of p53. Nature 1995;378:206–8 [DOI] [PubMed] [Google Scholar]
- 8.Momand J, Zambetti GP, Olson DC, George D, Levine AJ. The mdm-2 oncogene product forms a complex with the p53 protein and inhibits p53-mediated transactivation. Cell 1992;69:1237–45 [DOI] [PubMed] [Google Scholar]
- 9.Honda R, Tanaka H, Yasuda H. Oncoprotein MDM2 is a ubiquitin ligase E3 for tumor suppressor p53. FEBS Lett 1997;420:25–7 [DOI] [PubMed] [Google Scholar]
- 10.Kubbutat MH, Jones SN, Vousden KH. Regulation of p53 stability by Mdm2. Nature 1997;387:299–303 [DOI] [PubMed] [Google Scholar]
- 11.Kostic M, Matt T, Martinez-Yamout MA, Dyson HJ, Wright PE. Solution structure of the Hdm2 C2H2C4 RING, a domain critical for ubiquitination of p53. J Mol Biol 2006;363:433–50 [DOI] [PubMed] [Google Scholar]
- 12.Tanimura S, Ohtsuka S, Mitsui K, Shirouzu K, Yoshimura A, Ohtsubo M. MDM2 interacts with MDMX through their RING finger domains. FEBS Lett 1999;447:5–9 [DOI] [PubMed] [Google Scholar]
- 13.Kawai H, Lopez-Pajares V, Kim MM, Wiederschain D, Yuan ZM. RING domain-mediated interaction is a requirement for MDM2’s E3 ligase activity. Cancer Res 2007;67:6026–30 [DOI] [PubMed] [Google Scholar]
- 14.Barak Y, Juven T, Haffner R, Oren M. mdm2 expression is induced by wild type p53 activity. EMBO J 1993;12:461–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mendrysa SM, O’Leary KA, McElwee MK, Michalowski J, Eisenman RN, Powell DA, et al. Tumor suppression and normal aging in mice with constitutively high p53 activity. Genes Dev 2006;20:16–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Terzian T, Wang Y, Van Pelt CS, Box NF, Travis EL, Lozano G. Haploinsufficiency of Mdm2 and Mdm4 in tumorigenesis and development. Mol Cell Biol 2007;27:5479–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pant V, Xiong S, Chau G, Tsai K, Shetty G, Lozano G. Distinct downstream targets manifest p53-dependent pathologies in mice. Oncogene 2016;35:5713–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Itahana K, Mao H, Jin A, Itahana Y, Clegg HV, Lindstrom MS, et al. Targeted inactivation of Mdm2 RING finger E3 ubiquitin ligase activity in the mouse reveals mechanistic insights into p53 regulation. Cancer Cell 2007;12:355–66 [DOI] [PubMed] [Google Scholar]
- 19.Nomura K, Klejnot M, Kowalczyk D, Hock AK, Sibbet GJ, Vousden KH, et al. Structural analysis of MDM2 RING separates degradation from regulation of p53 transcription activity. Nature Structural & Molecular Biology 2017;24:578–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poyurovsky MV, Priest C, Kentsis A, Borden KL, Pan ZQ, Pavletich N, et al. The Mdm2 RING domain C-terminus is required for supramolecular assembly and ubiquitin ligase activity. EMBO J 2007;26:90–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tollini LA, Jin A, Park J, Zhang Y. Regulation of p53 by Mdm2 E3 ligase function is dispensable in embryogenesis and development, but essential in response to DNA damage. Cancer Cell 2014;26:235–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dolezelova P, Cetkovska K, Vousden KH, Uldrijan S. Mutational analysis of Mdm2 C-terminal tail suggests an evolutionarily conserved role of its length in Mdm2 activity toward p53 and indicates structural differences between Mdm2 homodimers and Mdm2/MdmX heterodimers. Cell Cycle 2012;11:953–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lessel D, Wu D, Trujillo C, Ramezani T, Lessel I, Alwasiyah MK, et al. Dysfunction of the MDM2/p53 axis is linked to premature aging. J Clin Invest 2017;127:3598–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pant V, Xiong S, Jackson JG, Post SM, Abbas HA, Quintas-Cardama A, et al. The p53-Mdm2 feedback loop protects against DNA damage by inhibiting p53 activity but is dispensable for p53 stability, development, and longevity. Genes Dev 2013;27:1857–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pant V, Xiong S, Iwakuma T, Quintas-Cardama A, Lozano G. Heterodimerization of Mdm2 and Mdm4 is critical for regulating p53 activity during embryogenesis but dispensable for p53 and Mdm2 stability. Proc Natl Acad Sci U S A 2011;108:11995–2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Momand J, Wu HH, Dasgupta G. MDM2--master regulator of the p53 tumor suppressor protein. Gene 2000;242:15–29 [DOI] [PubMed] [Google Scholar]
- 27.Huang L, Yan Z, Liao X, Li Y, Yang J, Wang ZG, et al. The p53 inhibitors MDM2/MDMX complex is required for control of p53 activity in vivo. Proc Natl Acad Sci U S A 2011;108:12001–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shieh SY, Ikeda M, Taya Y, Prives C. DNA damage-induced phosphorylation of p53 alleviates inhibition by MDM2. Cell 1997;91:325–34 [DOI] [PubMed] [Google Scholar]
- 29.Haupt Y, Maya R, Kazaz A, Oren M. Mdm2 promotes the rapid degradation of p53. Nature 1997;387:296–9 [DOI] [PubMed] [Google Scholar]
- 30.Mendrysa SM, Perry ME. The p53 tumor suppressor protein does not regulate expression of its own inhibitor, MDM2, except under conditions of stress. Mol Cell Biol 2000;20:2023–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lang GA, Iwakuma T, Suh YA, Liu G, Rao VA, Parant JM, et al. Gain of function of a p53 hot spot mutation in a mouse model of Li-Fraumeni syndrome. Cell 2004;119:861–72 [DOI] [PubMed] [Google Scholar]
- 32.Terzian T, Suh YA, Iwakuma T, Post SM, Neumann M, Lang GA, et al. The inherent instability of mutant p53 is alleviated by Mdm2 or p16INK4a loss. Genes Dev 2008;22:1337–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mendrysa SM, McElwee MK, Michalowski J, O’Leary KA, Young KM, Perry ME. mdm2 Is critical for inhibition of p53 during lymphopoiesis and the response to ionizing irradiation. Mol Cell Biol 2003;23:462–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu D, Prives C. Relevance of the p53-MDM2 axis to aging. Cell Death Differ 2018;25:169–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu D, Ou L, Clemenson GD Jr., Chao C, Lutske ME, Zambetti GP, et al. Puma is required for p53-induced depletion of adult stem cells. Nat Cell Biol 2010;12:993–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tyner SD, Venkatachalam S, Choi J, Jones S, Ghebranious N, Igelmann H, et al. p53 mutant mice that display early ageing-associated phenotypes. Nature 2002;415:45–53 [DOI] [PubMed] [Google Scholar]
- 37.Garcia-Cao I, Garcia-Cao M, Martin-Caballero J, Criado LM, Klatt P, Flores JM, et al. “Super p53” mice exhibit enhanced DNA damage response, are tumor resistant and age normally. EMBO J 2002;21:6225–35 [DOI] [PMC free article] [PubMed] [Google Scholar]