Abstract
Fetal, perinatal, and neonatal asphyxia are vital health issues for the most vulnerable groups in human beings, including fetuses, newborns, and infants. Severe reduction in oxygen and blood supply to the fetal brain can cause hypoxic-ischemic encephalopathy, leading to long-term neurological disorders, including mental impairment and cerebral palsy. Such neurological disorders are major healthcare concerns. Therefore, there has been a continuous effort to develop clinically useful diagnostic tools for accurately and quantitatively measuring and monitoring blood and oxygen supply to the fetal and neonatal brain to avoid severe consequences of asphyxia Hypoxic-Ischemic Encephalopathy (HIE) and Neonatal Encephalopathy (NE). Major diagnostic technologies used for this purpose include fetal heart rate monitoring (FHRM), fetus scalp blood sampling (FBS), ultrasound (US) imaging, magnetic resonance imaging (MRI), x-ray computed tomography (CT), and nuclear medicine. In addition, given the limitations and shortcomings of traditional diagnostic methods, emerging technologies such as near-infrared spectroscopy (NIRS) and photoacoustic (PA) imaging have also been introduced as stand-alone or complementary solutions to address this critical gap in fetal and neonatal care. This review provides a thorough overview of the traditional and emerging technologies for monitoring fetal and neonatal brain oxygenation status and describes their clinical utility, performance, advantages, and disadvantages.
Keywords: Fetal and Neonatal, Blood flow, Oxygenation, Hypoxic-Ischemic, Neonatal Encephalopathy, Diagnostic Imaging, Ultrasound, Photoacoustic, MRI, X-Ray CT
Introduction
Perinatal asphyxia, or oxygen deprivation, is believed to cause approximately 23% of newborn deaths globally [1–4]. Furthermore, a severe reduction in oxygen and blood supply to the fetal brain can cause hypoxic-ischemic encephalopathy (HIE), leading to long-term neurological disorders, including mental impairment and cerebral palsy [5–12]. Such neurological disorders are major healthcare concerns [13–15]. Pregnancy complications, such as prolonged, arrested, and obstructed labor and inadequate use of oxytocin, are some of the major causes of perinatal asphyxia and HIE [1–18]. Neonatal encephalopathy (NE) is a clinical syndrome characterized by disturbed neurologic function in the earliest days of life in an infant born at or beyond 35 weeks of gestation. It manifests as a reduced level of consciousness or seizures, often accompanied by difficulty initiating and maintaining respiration and by depression of tone and reflexes [1]. HIE, or birth asphyxia, is one of the leading causes of NE. To date, there is not a gold-standard test for diagnosis of HIE, and it remains challenging to determine whether acute hypoxic-ischemic events contributed to NE. The incidence of NE varies between 2 and 9 per 1000 term births [2, 3]. In a 2010 comprehensive review, the estimated incidence of NE was 3.0 per 1000 live births (95% CI 2.7–3.3), while the estimated incidence of HIE (a subset of NE) was between 1.3 and 1.7 per live births (95% CI 1.3–1.7) [4]. It is estimated that 30% of NE cases in developed populations and 60% in developing populations have some evidence of intrapartum hypoxia-ischemia [4]. Newborns presenting with signs of NE warrant immediate clinical assessment to determine eligibility for therapeutic hypothermia, which is typically started within six hours of birth.
During development, the fetus has a significant margin of safety for oxygenation. Physiological adaptation mechanisms include increased affinity of fetal hemoglobin to oxygen, increased basal blood flow to most tissues, and release of oxygen to the fetal tissues at a lower oxygen tension [5–7]. Acute hypoxia in late gestation fetuses leads to decreased fetal breathing movements and decreased heart rate (bradycardia), leading to less oxygen consumption, as one of the fetal defense mechanisms [8]. Bradycardia allows cardiac output and perfusion pressure maintenance by prolonging cardiac end-diastolic filling time, increasing end-diastolic volume [9]. Another defense mechanism, known as the fetal brain sparing effect, is explained by the redistribution of blood flow away from peripheral vessels to vital organs, including the brain, heart, adrenal glands, and kidneys. Carotid chemoreflex activation leads to bradycardia through the vagal nerve stimulation and increased peripheral vasoconstriction [10] (Figure Legends Fig. 1). Hormones and local vascular mediators further maintain peripheral vasoconstriction. However, prolonged, and inadequate fetal defense mechanisms trigger a cascade of cellular processes, leading to inflammation, cell death, and subsequent HIE.
Fig. 1.
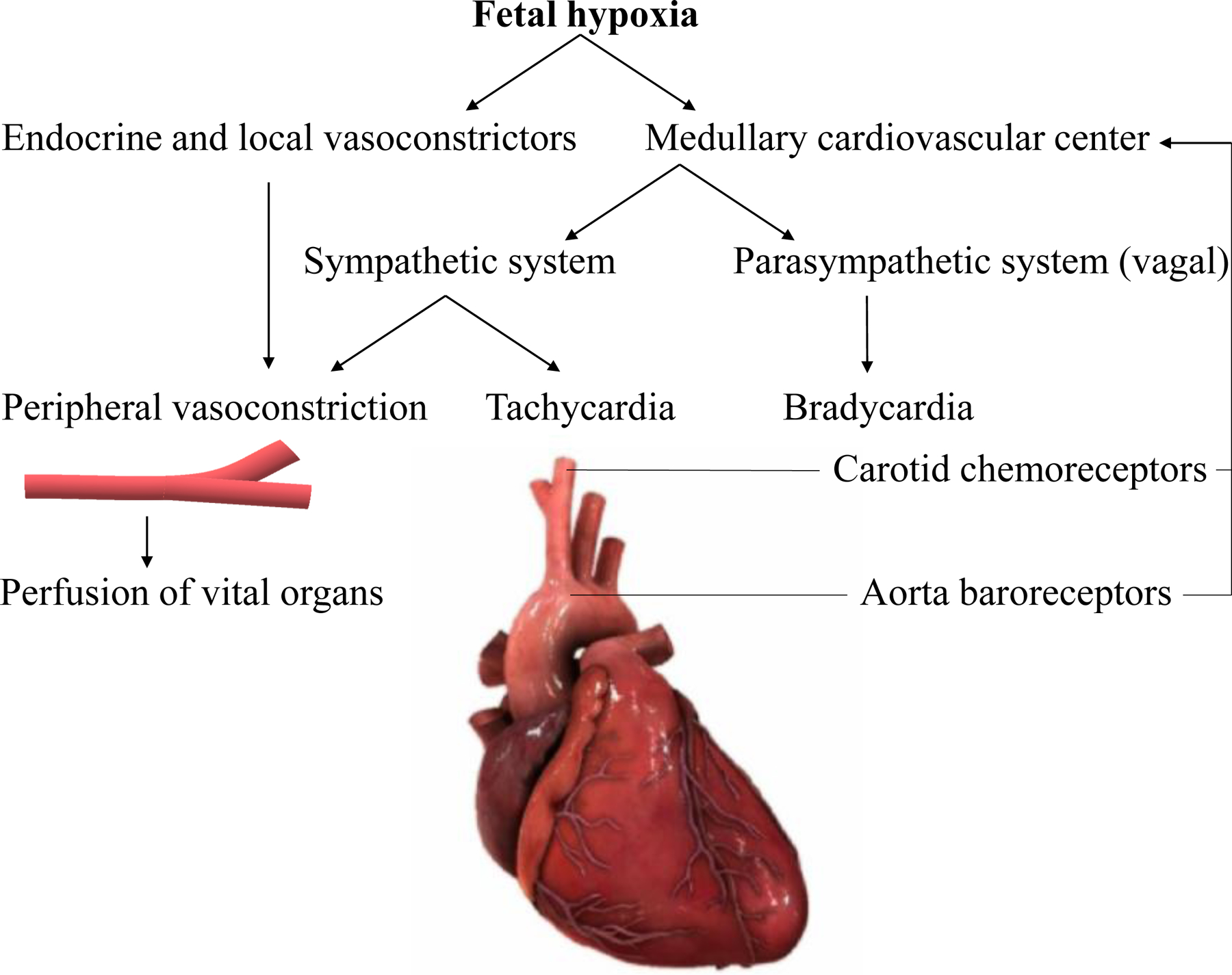
Physiology of fetal brain sparing during hypoxia. Carotid chemoreflex activation leads to bradycardia through the vagal nerve stimulation and increase in peripheral vasoconstriction. Hormones and local vascular mediators further maintain peripheral vasoconstriction.
Based on a report by the American College of Obstetricians and Gynecologists (ACOG) guidelines for HIE, all of the following conditions should be present for the infant to be considered as having HIE: Apgar score of <5 at 5 minutes and 10 minutes, fetal umbilical artery pH <7.0, or base deficit ≥12 mmol/L, or both, acute brain injury seen on brain MRI or magnetic resonance spectroscopy (MRS) consistent with hypoxia-ischemia, presence of multisystem organ failure consistent with HIE (Fig. 2). Other factors that can introduce a sentinel hypoxic or ischemic event occurring immediately before or during labor and delivery include the ruptured uterus or severe abruptio placentae. Fetal heart rate monitor patterns are consistent with an acute peripartum or intrapartum event, such as a category III pattern. Additionally, the neuroimaging evidence shows a pattern of brain injury typical of hypoxic-ischemic injury in the newborn, including deep nuclear gray matter or watershed zone injury, and no evidence of other factors contributing to the encephalopathy [1]. The National Institute of Child Health and Human Development (NICHD) category III FHR tracing is defined as having either sinusoidal pattern or absent baseline variability plus recurrent late decelerations, recurrent variable decelerations, or bradycardia [19]. Whether neuroimaging or other methods for fetal hypoxia monitoring can sooner or later be used as a gold-standard for diagnosing prenatal hypoxia and hypoxic-ischemic brain injury remains to be established.
Fig. 2.
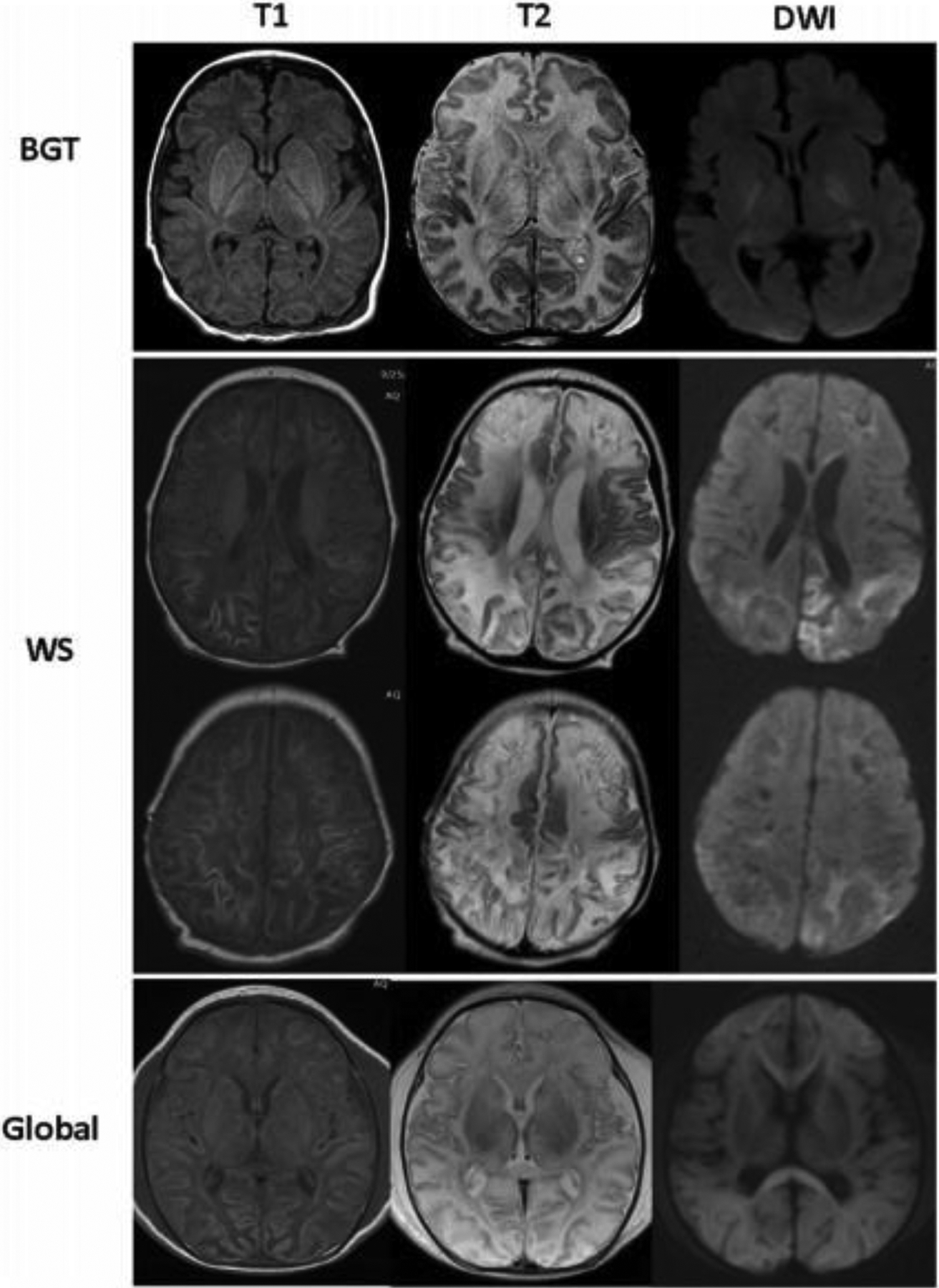
Patterns of brain injury in neonates with HIE on T1-weighted, and diffusion-weighted (DWI) images. BGT, basal ganglia/thalamic predominant injury; WS, watershed injury with cortical laminar necrosis and subcortical white matter injury, and global pattern with total cerebral injury (Adopted from [11] Figure 1)
Currently, available indicators such as fetal heart rate monitoring (FHRM) and fetal scalp blood sampling (FBS) have certain diagnostic/prognosis limitations [12]. As the gold-standard for diagnosing fetal intolerance of labor, FHRM is most widely used to monitor fetal well-being in obstetrics despite its poor sensitivity. This has led to an increase in operative deliveries [13]. On the other hand, FBS was proposed as a complementary test to increase FHRM specificity to reduce unnecessary interventions. However, FBS was also reported as having a limited contribution to FHRM and for being an invasive procedure only applicable in late stages of labor after rupture of membranes. Currently, its use in high-risk pregnancies is very low (<1.5 to 2%) [14].
For the past decade, the well-accepted and conventional neuroimaging modalities, including cranial ultrasonography (CUS), computed tomography (CT), magnetic resonance imaging (MRI), and nuclear medicine methods (PET, SPECT), have been the standard of care for evaluating infants with neurological pathology [14–17, 20, 21]. While CUS is widely used for fetal and neonatal brain evaluation, fetal MRI is studied to confirm and characterize brain abnormalities detected by routine CUS. Fetal MRI is not a common tool in practice and is considered when high-quality neurosonography is unavailable or cannot provide specific diagnostic information [22, 23]. Among various neuroimaging modalities for neonatal brain evaluation, the CUS is the preferred and most widely used modality. It can be used to image the evolution of lesions and brain maturation due to its rapid, safe, and easy technology. The CUS can detect cerebral edema within 24 hours after the hypoxic-ischemic insult and provide findings related to intrapartum hypoxia, i.e., increased echogenicity [16, 17]. The vascular abnormalities (Fig. 3) [24] can be detected by color Doppler sonography (CDS). The CT imaging modality has been rarely used for neonatal brain evaluation due to radiation exposure and reported low sensitivity to detect early, small or infra-tentorial infarcts [20]. Neonatal MRI is a relatively new technique that has rapidly become the study of choice for the evaluation in newborns. It is considered the most sensitive technique to detect early fetal hypoxia [21]. Still, there are some limitations, such as its non-portability and high cost. Nuclear medicine offers non-invasive imaging methods using positron emission tomography (PET) and single-photon emission computed tomography (SPECT) to study cerebral perfusion and metabolism. Some significant limitations restrict nuclear medicine use in neonates, including ionizing radiation and high operating cost. Additionally, PET is not accessible as a point of care testing for observation/investigation in patients for a routine checkup or during emergencies. The purpose of this review article is to provide an objective overview of the utility and limitations of existing methods for fetal and neonatal brain hypoxia assessment and to introduce new emerging technologies such as photoacoustic (PA) imaging as a potential technique for monitoring to overcome existing limitations in current practice.
Fig. 3.
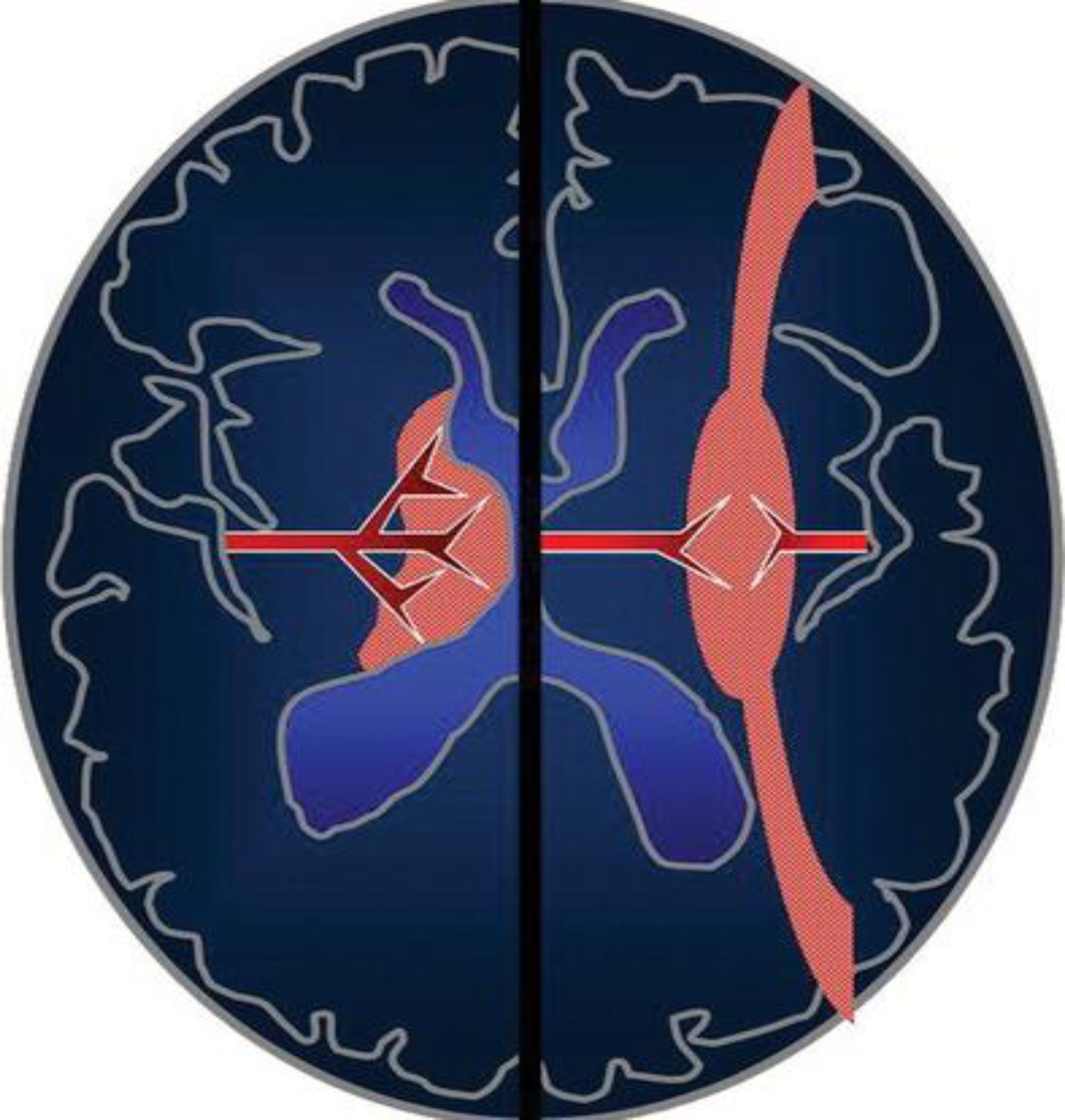
Patterns of brain injury in HIE. Schematic shows the premature neonatal brain (left side) and term infant brain (right side). It illustrates how the vascular supply changes with maturation and affects the pattern of brain injury in HIE. The premature neonatal brain (left side) has a ventriculopetal vascular pattern, and hypoperfusion results in a periventricular border zone (red shaded area) of white matter injury. In the term infant (right side), a ventriculofugal vascular pattern develops as the brain matures, and the border zone during hypoperfusion is more peripheral (red shaded area) with subcortical white matter and parasagittal cortical injury. (Adopted from [24] Figure 2)
Diagnostic methods for monitoring fetal brain blood flow and oxygenation
I. Fetal Heart Rate Monitor (FHRM)
Fetal Heart Rate Monitor (FHRM) or Cardiotocography (CTG) is an electronic technology for recording the fetal heart rate referenced to the uterine contractions during pregnancy and labor [25]. The technique was introduced as a screening test in the 1960s to improve the detection of fetal hypoxemia and reduce cerebral palsy and perinatal mortality, particularly in high-risk pregnancies [26, 27]. By detecting the pattern changes in the CTG signals, the FHRM system was anticipated to identify signs of fetal hypoxia and suggest cesarean section or instrumental vaginal birth [28, 29]. A value below 110 bpm is considered as bradycardia and above 160 bpm tachycardia. For example, NICHD category III tracing indicates current fetal hypoxia. It is defined as having either sinusoidal pattern or absent baseline variability plus recurrent late decelerations, recurrent variable decelerations, or bradycardia [30].
Several antepartum fetal surveillance techniques based on FHRM are in clinical use. These include the contraction stress test (CST), a nonstress test (NST), biophysical profile (BPP), and modified BPP. The clinical studies suggest that initiating antepartum fetal testing at 32 0/7 weeks of gestation or later is appropriate for most at-risk patients [31–34]. However, in pregnancies with high-risk conditions, testing might begin at an earlier gestational age when delivery would be considered for the perinatal benefit [35–40].
The CST is based on the response of the FHR to uterine contractions. It relies on the hypothesis that uterine contractions will transiently worsen fetal oxygenation. Worsening in oxygenation status will lead to the FHR pattern of late decelerations (Fig. 4c). The NST is based on the hypothesis that the heart rate of a fetus that is not acidotic or neurologically depressed will temporarily accelerate with fetal movement. Heart rate reactivity is thought to be a good indicator of normal fetal autonomic function. Loss of reactivity is usually associated with a fetal sleep cycle but may result from any cause of central nervous system depression, including fetal acidemia. The BPP consists of an NST combined with four observations made by real-time ultrasonography (fetal breathing movements, body or limb movements, fetal tone, and amniotic fluid volume) [41]. The modified BPP combines the NST, an indicator of fetal acid-base status, with an amniotic fluid volume assessment, as an indicator of long-term placental function [42]. Because antepartum fetal surveillance tests have high false-positive rates and low positive predictive values, abnormal test results are usually followed by another test or delivery based on consideration of test results, maternal and fetal condition, and gestational age [43, 44].
Fig. 4.
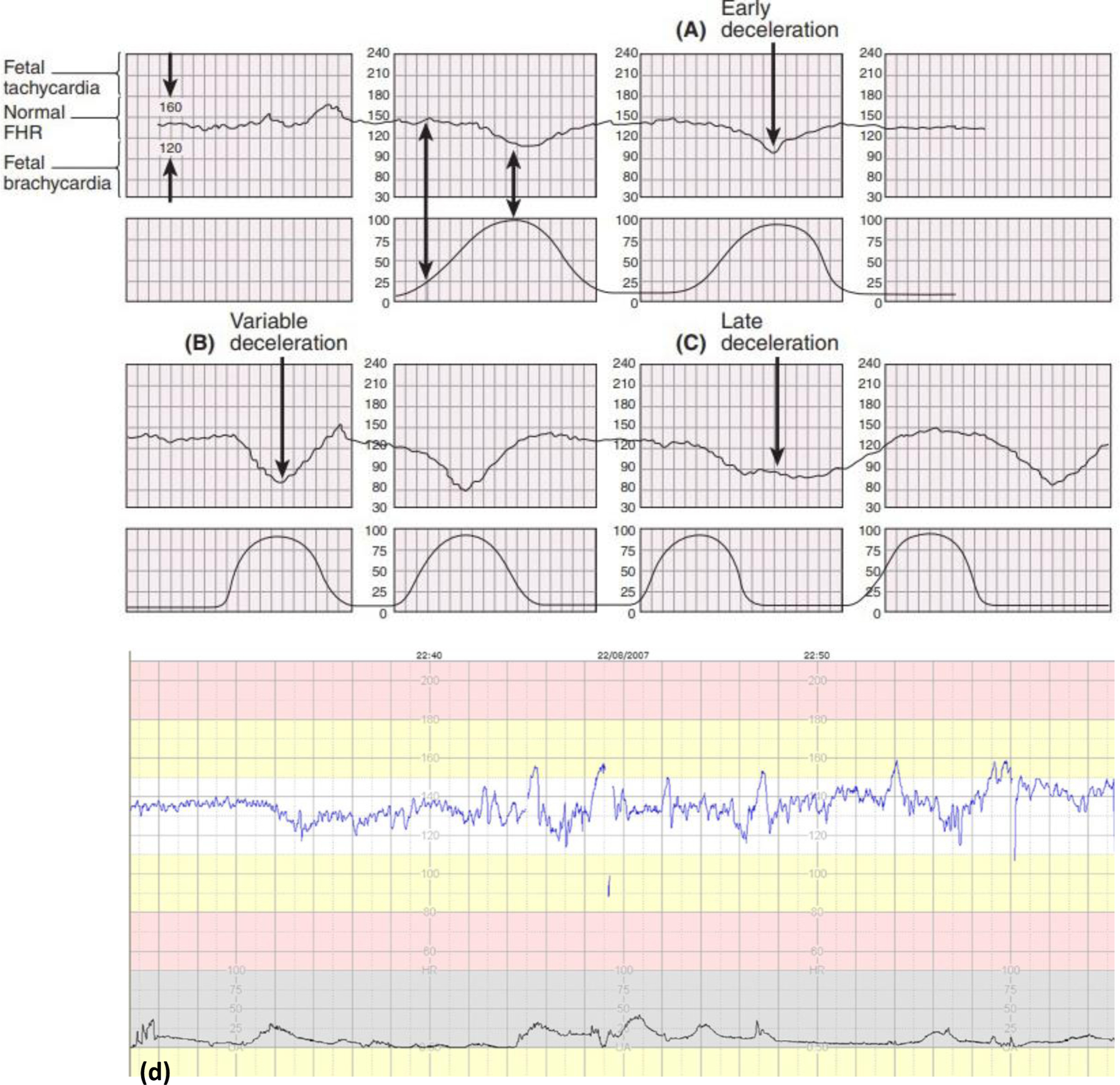
Fetal Heart Rate patterns (a) Early deceleration. (b) Variable deceleration. These decelerations may start before, during, or after a uterine contraction starts. (c) Late deceleration. The onset, nadir, and recovery of the deceleration occur, respectively, after the beginning, peak, and end of the contraction (Adopted from [45] Fig. 9.11). (d) An example of normal CTG showing stable baseline fetal HR of about 130 bpm. It shows no decelerations, normal baseline variability, accelerations, and fetal cycling activity (Adopted from [47]).
The specificity of FHRM for the prediction of cerebral palsy is low, with a reported false positive rate as high as 99.8% [25], even in the presence of multiple late decelerations or decreased variability (Fig. 4a–c) [45]. Another significant limitation is its inability to assess fetal oxygen status directly [46]. Approximately 30% of fetuses will have a non-reassuring FHR pattern at some time during labor [47]. However, it has been estimated that even the most ominous fetal heart rate patterns are associated with at most a 50% to 65% incidence of neonatal asphyxia or neonatal acidemia [48, 49]. Although some evidence suggests that intrapartum fetal monitoring is associated with a reduction in intrapartum death [50], a reduction in long-term neurologic impairment has not been proven. All available data are derived from trials comparing continuous electronic monitoring with intermittent auscultation. No randomized trials are comparing intrapartum fetal monitoring versus no intrapartum fetal monitoring.
II. Fetal Scalp Blood Sampling (FBS)
Fetal scalp blood sampling (FBS) was introduced for obstetric care in the late 1960s. In the FBS procedure (Fig. 5a), a small blood sample from the fetal scalp is drawn for examination and tested for two constituents: pH and lactate, indicators of acid-base homeostasis [51]. As an ancillary method, FBS is now considered a less useful test for intrapartum fetal evaluation. An amnioscope with a light source is used to expose the fetal scalp, and after puncturing the scalp with 2-mm blade, the blood is collected into capillary tubes. The technical difficulty in obtaining fetal blood samples, especially in morbid obesity patients, is challenging. The test requires that the cervix be dilated at least 2 to 3 cm, and is often uncomfortable for the mother. Rare complications include infection, hemorrhage, and leakage of cerebrospinal fluid [52–56]. Intrapartum pH and lactate measurements have not been clearly proven to reduce emergency cesarean deliveries or operative vaginal births or improve long-term perinatal outcomes [57–59]. Schiermeier et al. (Fig. 5b) [14] evaluated the sensitivity and specificity of intrapartum computerized FIGO criteria for CTG and fetal scalp pH during labor. The study concluded that computerized CTG analysis is highly sensitive for fetal acidosis and can be used as an objective adjunctive criterion during delivery. More recently, the study group performed a multicenter observational study to evaluate the value of FBS as an adjunct test to CTG to predict adverse neonatal outcomes [59]. The primary outcome was the prediction of neonatal acidaemia diagnosed as umbilical cord arterial pH < 7.05. The secondary outcomes were the prediction of Apgar scores < 7 at 1st and 5th minutes and admission to the neonatal intensive care unit (NICU). As an adjuncts test to CTG, FBS offered limited value to predict neonatal acidaemia, low Apgar scores, and admission to NICU. For this reason and many others, including difficulties with quality control, cost, patient discomfort, sample failure rates up to 10 percent, and unavailability of sampling kits, FBS is performed rarely in the United States and worldwide.
Fig. 5.
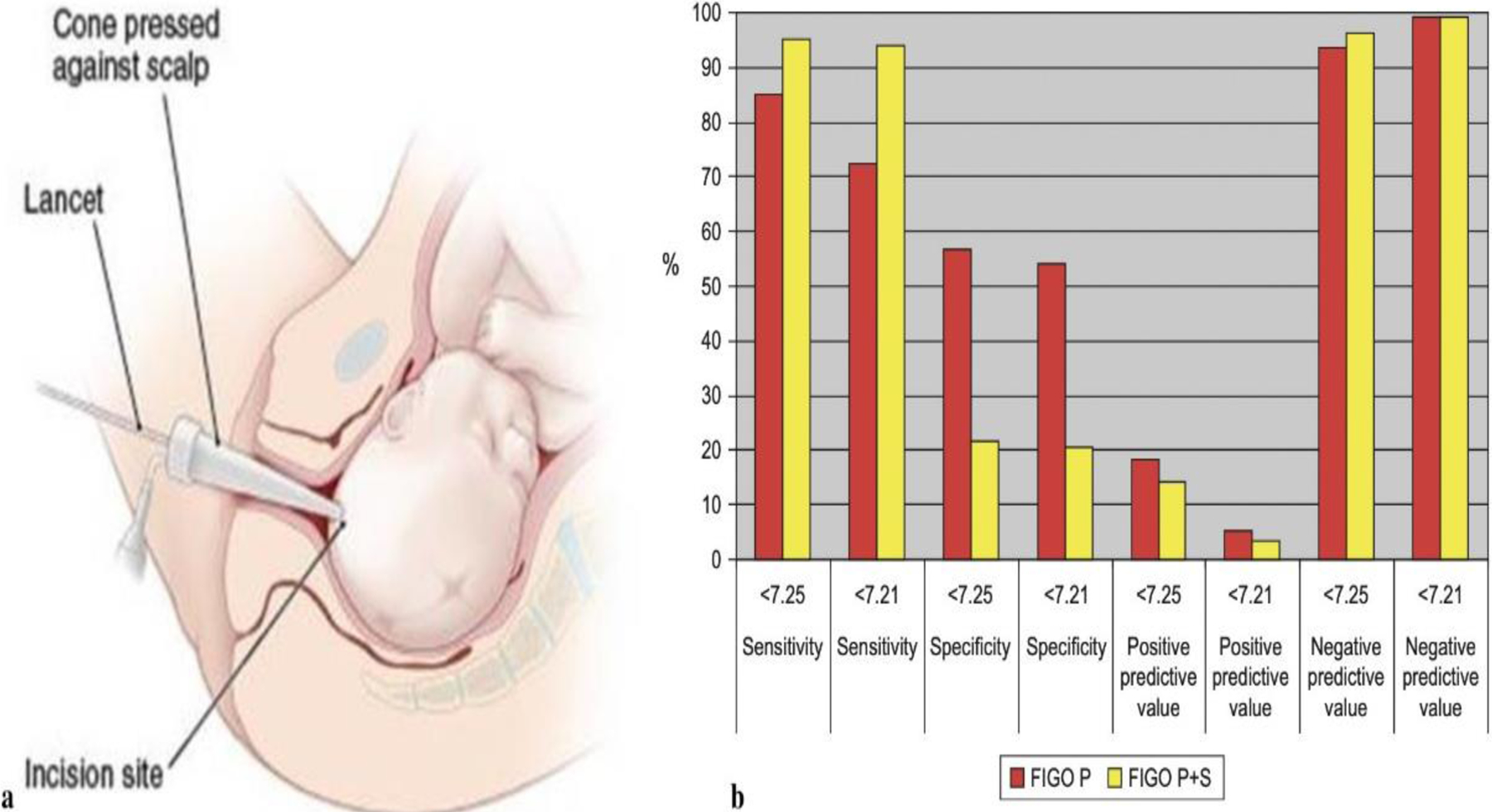
(a) Fetal scalp Blood Sampling. The Figure shows the incision site of the lancet and placement of the cone against the scalp. (Adopted from [45] Fig 9.12). (b) Shown the sensitivity and specificity for fetal scalp PH values as well as active and negative predictive values for pathological (P), and pathological and suspect (P + S) FIGO criteria. (Adopted from [14]).
Diagnostic methods for monitoring neonatal brain blood flow and oxygenation
I. Computed Tomography (CT)
Computed Tomography (CT) scan of the head was an imaging technique that uses x-rays and effectively detects hemorrhage with the added advantage of limited sedation need. CT was sometimes used for neuroimaging in neonates with NE despite potential harm from radiation exposure [60, 61]. However, due to the noticeable risk of depositing harmful radiation exposures, CT scans have been abandoned in the past decade [62, 63]. It has also been reported to suffer from low sensitivity and specificity towards a diagnosis of HIE [15, 64, 65]. Given the excessive radiation exposure, fetal CT is not recommended for brain imaging, and in rare cases, the prenatal low-dose CT is used to detect congenital skeletal abnormalities [66]. Multidetector CT can be used for screening intracranial hemorrhage in very sick neonates without the need for sedation or when an MRI is not accessible [67].
Clinical approaches for monitoring both fetal and neonatal brain blood flow and oxygenation
I. Near-Infrared Spectroscopy (NIRS)
Cerebral Near-Infrared Spectroscopy (NIRS) monitoring uses light in the near-infrared spectrum region, which is absorbed by oxygenated and deoxygenated hemoglobin and cytochrome oxidase (CytOx). Total hemoglobin is an index of cerebral blood volume, and cytochrome oxidase is the terminal complex of the mitochondrial respiratory chain and adenosine triphosphate (ATP) [68]. Cerebral oximetry assessed via NIRS has been proposed as a “standard of care”, and this technique is widely used now (Fig. 6) [69, 70]. A major advantage of this technique is that it allows for non-invasive continuous monitoring of cerebral oxygenation.
Fig. 6.
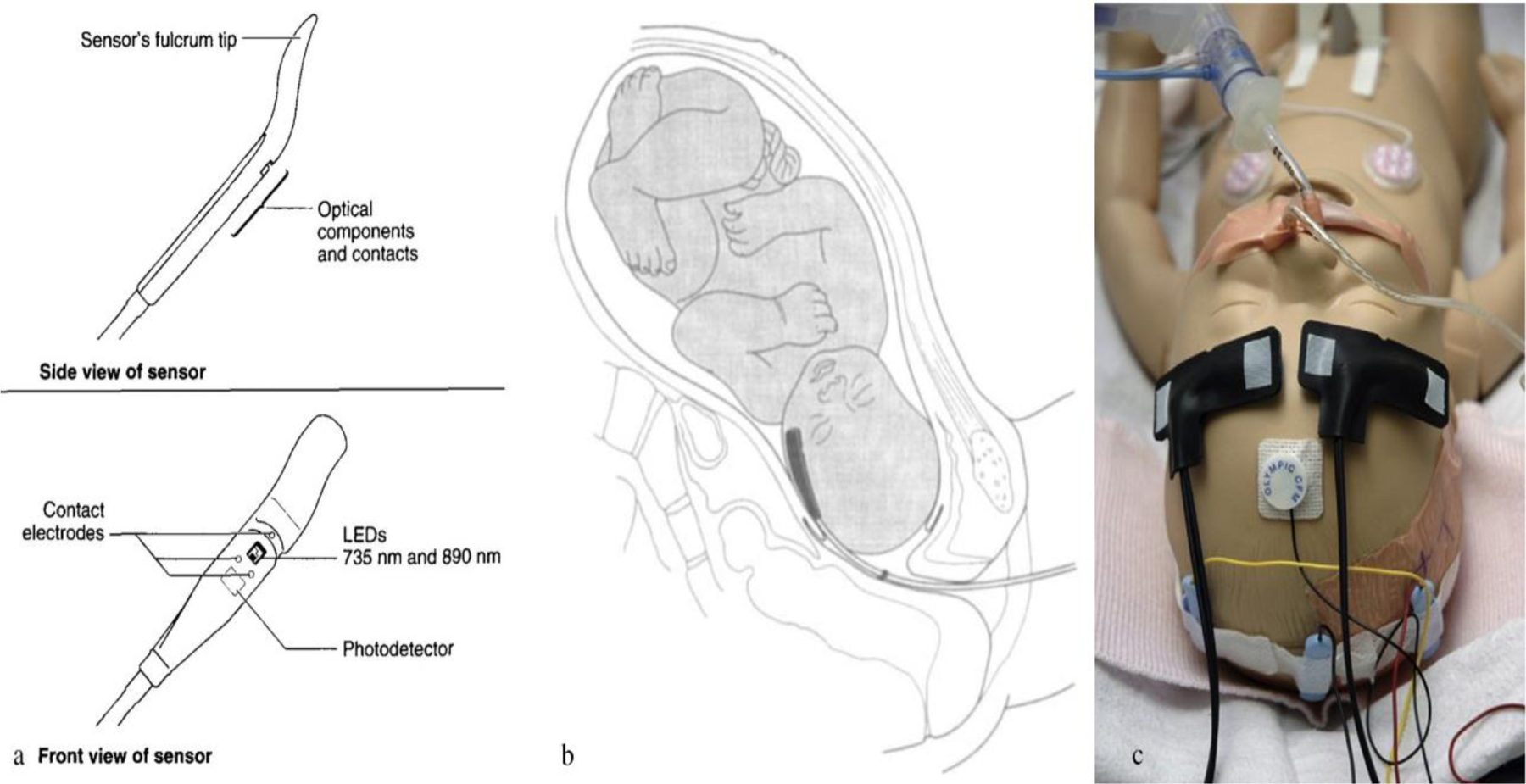
The FSpO2 monitoring system: a) and b) show a single use, sterile, disposable sensor that is inserted through the cervix into the uterus which rests against the fetal temple, cheek or forehead (Adopted from [70] Figure 1 and 2), c) shows position of the NIRS sensors for the purpose of monitoring continuously mixed venous saturation, two neonatal NIRS sensors are placed respectively on each side of the newborn’ s forehead, over the area of frontal lobes. (Adopted from [69] Figure 1).
NIRS can provide data about several important variables of cerebral oxygenation and hemodynamics (Fig. 7) [71]. Since 1985 it has been used as a research tool for investigating the newborn infant’s brain [72]. More recently, the technique has been adapted to monitor the human fetal brain during labor [73]. Commercial continuous-wave NIRS systems have been used in HIE to monitor relative cerebral hemoglobin oxygen saturation (SO2). OxiFirst® fetal oxygen saturation monitoring system is a new type of fetal monitor that measures oxygen saturation in the fetuses’ blood as a sign of fetal health during labor and delivery [74]. The sensor is inserted into the mother’s uterus and placed against the temple or cheek of the fetus. The monitor displays fetal oxygen saturation as the percentage of oxygen in the blood of the fetus. In a randomized, controlled clinical trial of more than 1,000 births conducted at nine sites throughout the United States, fetal oxygen monitoring proved to be safe and efficient for measuring the oxygenation of a fetus during periods of non-reassuring fetal heart rate patterns [75, 76].
Fig. 7.
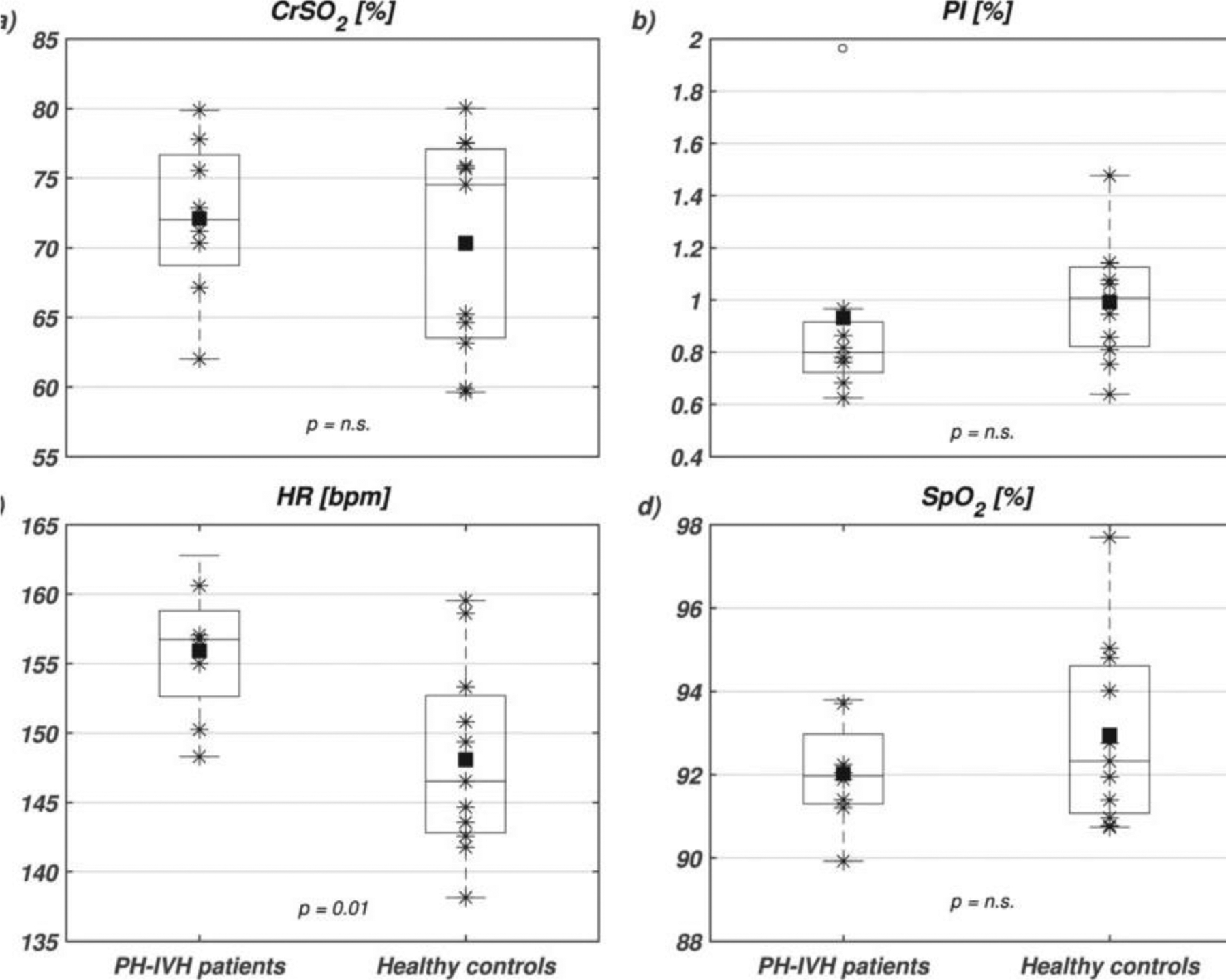
Boxplots of (a) near infrared spectroscopy (NIRS) cerebral regional haemoglobin oxygen saturation (CrSO2), (b) peripheral perfusion index (PI), (c) heart rate (HR) and (d) capillary oxygen saturation (SpO2) in PH-IVH patients (left boxplots) and healthy age-matched controls (right boxplots). On each box, the central mark is the median, the square is the mean, the stars are the individual data, the edges of the box are the 25th and 75th percentiles, and the whiskers show the 95%onfidence interval. Empty circles denote outliers and statistical comparisons are indicated with corresponding p-values (n.s., non-significant). (Adopted from [71] Figure 1).
NIRS is an old technique dating back to 1977 used initially as a non-invasive neuroimaging tool that can measure local hemodynamic changes in the brain. NIRS can continuously and simultaneously monitor cerebral, intestinal, and renal perfusion and oxygenation in different organ systems at the bedside without interrupting routine care [77] (Fig. 8). Thus, near-infrared spectroscopy can augment current physiologic monitoring to increase awareness of abnormal perfusion status in the preterm population and potentially reduce risks associated with several diseases that may lead to ischemic injury.
Fig. 8.
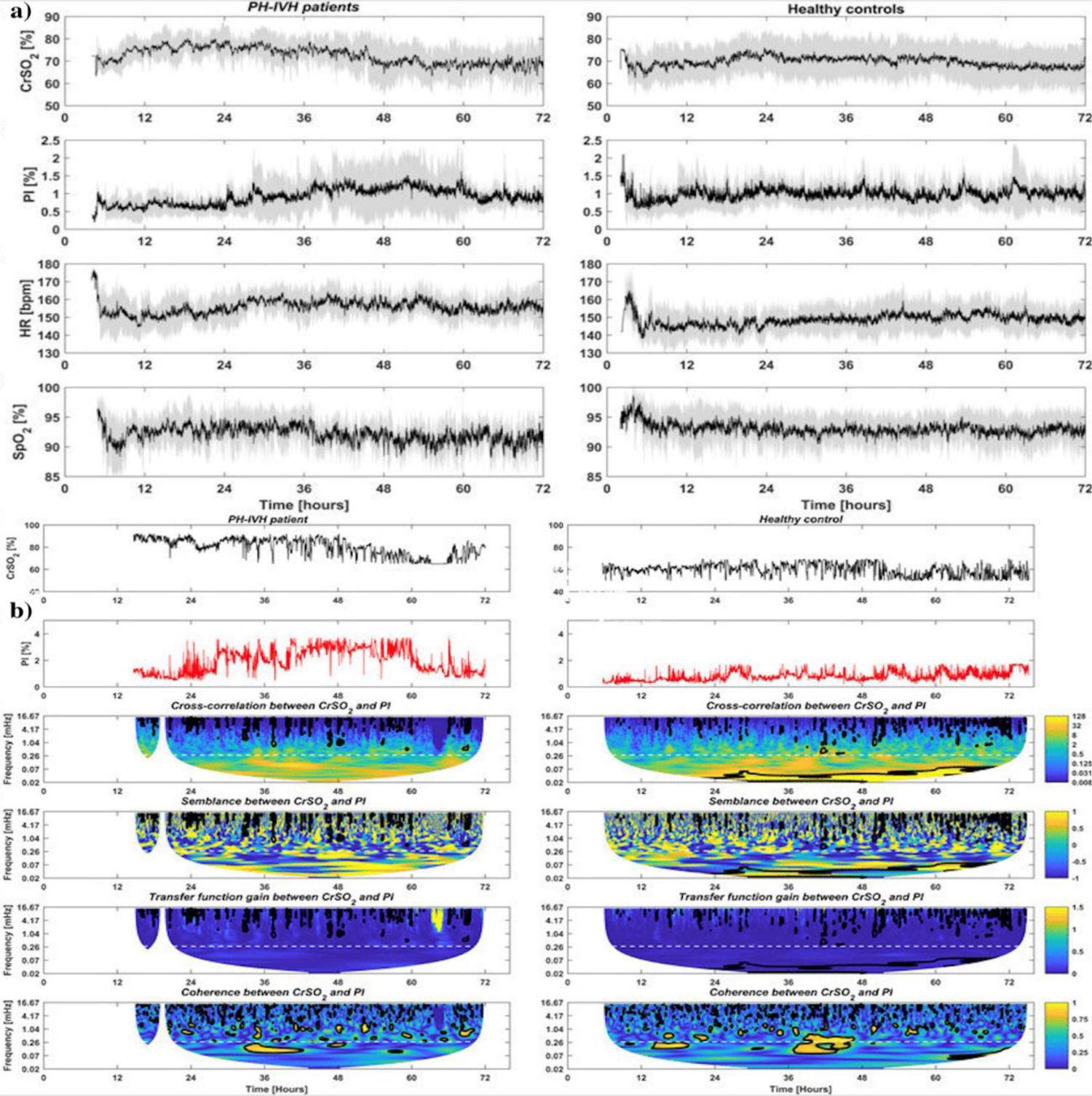
(a) Temporal distributions of (first line) near infrared spectroscopy (NIRS) cerebral regional hemoglobin oxygen saturation (CrSO2), (second line) peripheral perfusion index (PI), (third line) heart rate (HR) and (fourth line) capillary oxygen saturation (SpO2) in PH-IVH patients (left column) and healthy age-matched controls (right column) in the first 72 hr of life. On each plot, the black curve is the mean, and the grey shaded region represents one standard deviation of the group. (b) Example of the complete analytical workow in a healthy control (left column) and in an infant with pulmonary (PH) and/or intraventricular haemorrhage (IVH, right column): (first line) and (second line) depict temporal distributions of near infrared spectroscopy (NIRS) cerebral regional hemoglobin oxygen saturation (CrSO2) and peripheral perfusion index (PI) in the first 72 hr of life, respectively; (third-sixth line) display the amplitude of the cross-correlation, the semblance (anti-phase and in-phase), the amplitude of the gain (transfer function) and the coherence between CrSO2 and PI in the time-frequency space. Regions that are statistically significant are comprised in a black bold contour. A dashed white line indicates the selected ultra-frequency band of slow and prolonged periods of >1 h (<0.28 mHz) used for statistical analysis. (Adopted from [71] Figure 2 and 3).
NIRS is a promising technique for fetal hypoxia. Still, its use as a sole diagnostic measure of the balance between the brain oxygen requirement and the brain oxygen supply is still premature. A major problem with NIRS monitoring is that the percentage of arteries, capillaries, and veins mixing in the brain circulation is not constant. In cerebral circulation, arteries are estimated to represent 10–20% of the total vessel volume, capillaries 5%, and veins the remaining 75–85%. Thus, SO2 predominantly represents the venous saturation in the brain [78]. Another limitation is the influence of hemoglobin values on NIRS monitoring and the effect of pCO2. In addition, hemodilution, transfusion, hypocapnia, and hypercapnia lead to significant variations in cerebral oximetry [79]. Nevertheless, NIRS has been increasingly used in clinical application, and further studies may widen the fields of application in fetal hypoxia monitoring.
II. Cranial Ultrasound (CUS)
Cranial ultrasound (CUS) is a technique for scanning the brain, and it is used almost exclusively in fetus and neonates because its fontanelle provides an “acoustic window.” The CUS is the preferred modality to image the fetal and neonatal brain for the evolution of lesions and brain maturation due to its rapid and easy technology and safety [80] (Fig. 9). The advantages of the CUS are numerous: it is an effective, non-invasive, and portable method of investigation. Several studies have reported its capability to differentiate hypoxic-ischemic injury from other causes of neonatal encephalopathies, including metabolic diseases [81, 82]. Moreover, color Doppler sonography (CDS) enables the assessment of brain perfusion and its pathology. Thus, the CDS options are essential prerequisites for the modern US – not only for assessing anatomy and patency of major vessels but also for the assessment of flow dynamics and peripheral vasculature, which in several conditions can be the clue to the diagnosis, e.g., asphyxia, brain death, high-pressure hydrocephalus, venous thrombosis.
Fig. 9.
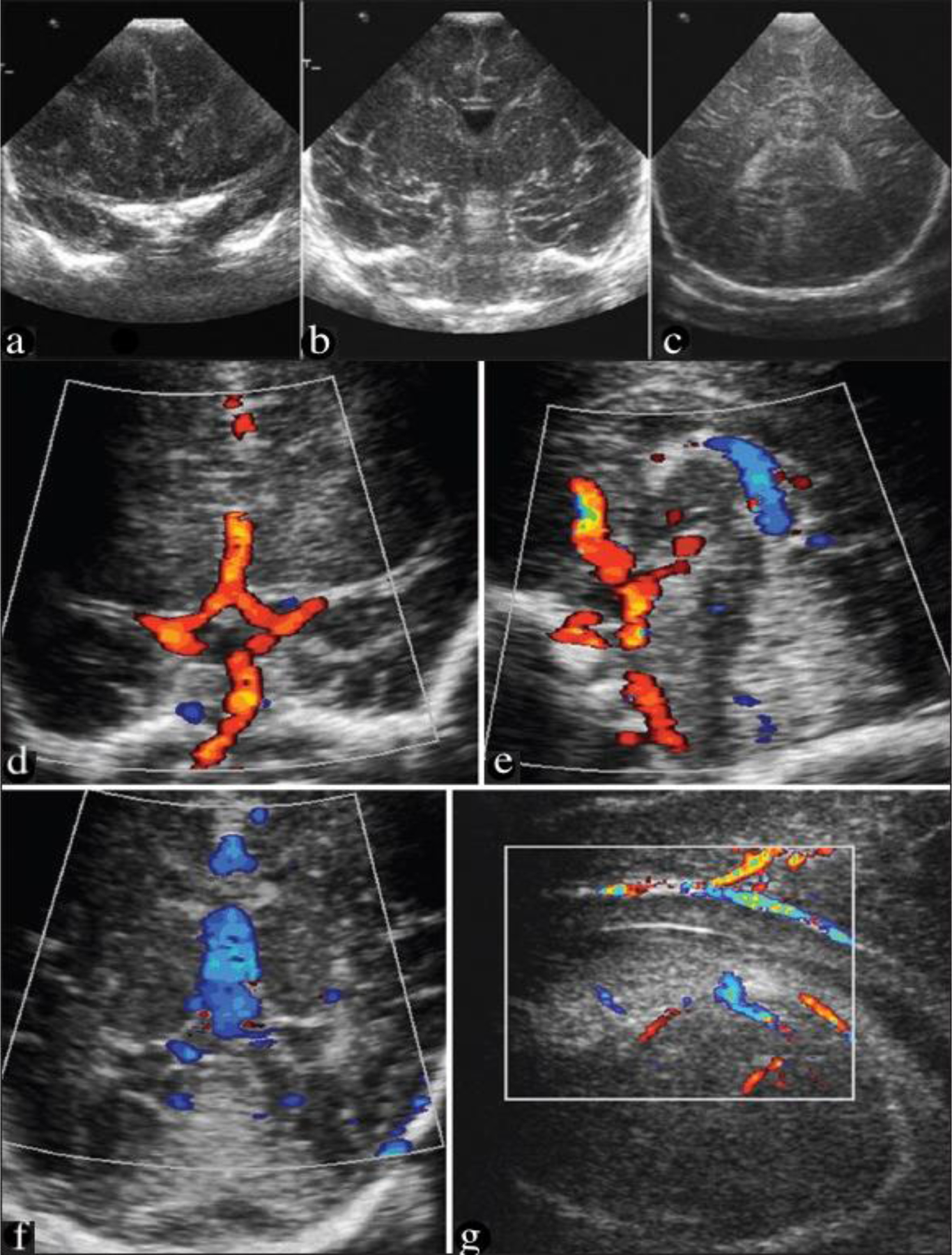
Coronal USG at the level of frontal lobes (a), foramina of Monro (b), and trigone (c), demonstrating the interhemispheric fissure, lateral ventricles, and periventricular parenchyma. Doppler images (d & e) demonstrating the circle of Willis. Coronal USG is demonstrating the vein of Galen, f) a parasagittal study showing the small periventricular veins in the region of the caudothalamic groove (g). (Adopted from [80] Figure 9 A-D and Figure 6 A-C).
Several limitations of CUS have been described in the literature, including a small field of view, limited soft-tissue acoustic contrast, beam attenuation by adipose tissue, poor image quality in oligohydramnios, and limited visualization of the posterior fossa in some cases due to variations in the size of the acoustic window, such as at the 33 weeks’ gestation [83–86]. Thus, sonographic findings are occasionally inconclusive or insufficient to guide treatment choices. In addition, the quality of images depends on the skills and experience of the sonographer, as some areas of the brain are challenging to visualize, and several abnormalities (i.e., coagulation necrosis and colliquative necrosis) remain beyond the scope of its abilities.
Although fetal malformations of the posterior fossa encompass a heterogeneous group of abnormalities, they can have similar characteristics on ultrasound. Therefore, multiplanar ultrasound with high-frequency probes and a transvaginal approach for fetuses in vertex presentation can be beneficial in such circumstances. In addition, when sonographic findings are inconclusive or insufficient to guide treatment choices, fetal MRI can represent a clinically valuable supplement to neurosonography [15, 87, 88]. However, neonatal CUS is not routinely used to predict outcomes in HIE, and it is not as sensitive as fetal magnetic resonance imaging (MRI) in diagnosing brain injury and may take several days to become apparent [22, 23]. Therefore, fetal MRI has become an addition to ultrasound in prenatal diagnosis.
III. Magnetic Resonance Imaging (MRI)
Magnetic Resonance Imaging (MRI) is an imaging technique based on nuclear magnetic resonance (NMR) science. When placed in an external magnetic field, certain atomic nuclei can absorb and emit radio-frequency energy. In clinical and research MRI, hydrogen atoms are most often used to generate a detectable radio-frequency signal received by antennas near the examined anatomy [89]. Fetal MRI initially emerged as a clinically useful supplement to sonography in the 1980s but was limited due to slow sequences requiring fetal and maternal sedation [90].
While ultrasound has been the primary imaging modality for fetal and neonatal evaluation, MRI has offered multiple advantages over ultrasound in this field [91]. The advantages of using fetal MRI include improved soft-tissue contrast and characterization, a larger field of view, and access to functional data. Multiple modalities of MRI have become available, including diffusion-weighted imaging (DWI), perfusion-weighted imaging (PWI), MR spectroscopy (MRS), and blood oxygenation level-dependent (BOLD) MRI. The latter is highly sensitive to changes in blood oxygenation levels and has been used in the quantitative assessment of cerebral metabolic rate in stroke and multiple sclerosis patients [92, 93]. Conventional MRI images (T1w and T2w), usually performed after day four from delivery, provide information on myelination status and preexisting brain developmental defects. DWI is highly sensitive for areas of edema and allows earlier identification of injury patterns in the first 24–48 hours. Both conventional MRI sequences (T1w and T2w) and DWI have a good specificity (>90%) and positive predictive value (>85%) in predicting death or major disability at age two years [94].
MR imaging of the fetus can be done at both 1.5T and 3.0T field strengths. A major advantage of 3.0T MRI compared to 1.5T is its increased signal-to-noise ratio (basically linearly proportional to the imaging field strength) [91]. The benefits of this increase in signal-t-noise include: (1) higher imaging resolution, (2) improved tissue biochemical profiling through MRS, (3) shorter imaging times and (4) improved functional data. However, to keep susceptibility related artifacts similar to those seen at 1.5T, shorter echo times are required. However, the susceptibility effects can be particularly advantageous when using susceptibility weighted imaging (SWI). Furthermore, unless the sequences are appropriately redesigned, the operating frequency of 128 MHz of 3.0T MRI will lead to higher radio-frequency energy deposition as measured by specific absorption rate (SAR). Recently, it has been shown that SAR can be kept well within FDA guidelines using modified pulse sequences [95] Some studies using 3T scanners have been reported around the world. Therefore, fetal brain imaging at 3.0T can provide higher resolution and better imaging quality while simultaneously reducing the SAR and can serve as a viable means to image the fetal brain, body and vasculature [91, 96].
BOLD-based signal changes in MR susceptibility-weighted imaging (SWI) have been previously used to evaluate and study stroke in adults and pediatric and neonatal populations [97] (Fig. 10). SWI has also been used to study traumatic brain injury in the pediatric population due to its sensitivity to hemorrhagic lesions. SWI is a high resolution, fully flow compensated MRI technique, which is more sensitive than the typical T2* based BOLD acquisition because of its unique combination of the T2* weighted magnitude data with the phase data [97]. Phase image from gradient-echo sequences like SWI provides distinctive contrast, unlike the conventional T1, T2, and T2* contrast, emphasizing small differences in tissue magnetic susceptibility property. Hence, SWI is particularly useful when differentiating arteries from veins. It is also considered highly sensitive to micro-bleeds or changes in cerebral venous oxygen saturation [98]. During a stroke, an increased oxygen extraction in the tissue with reduced perfusion leads to an increase in deoxyhemoglobin content in the veins, leading to their prominent appearance on SWI [99–101]. In addition, the unusually prominent deep medullary veins are seen in SWI during a hypoxic-ischemic injury in neonates [100].
Fig. 10.
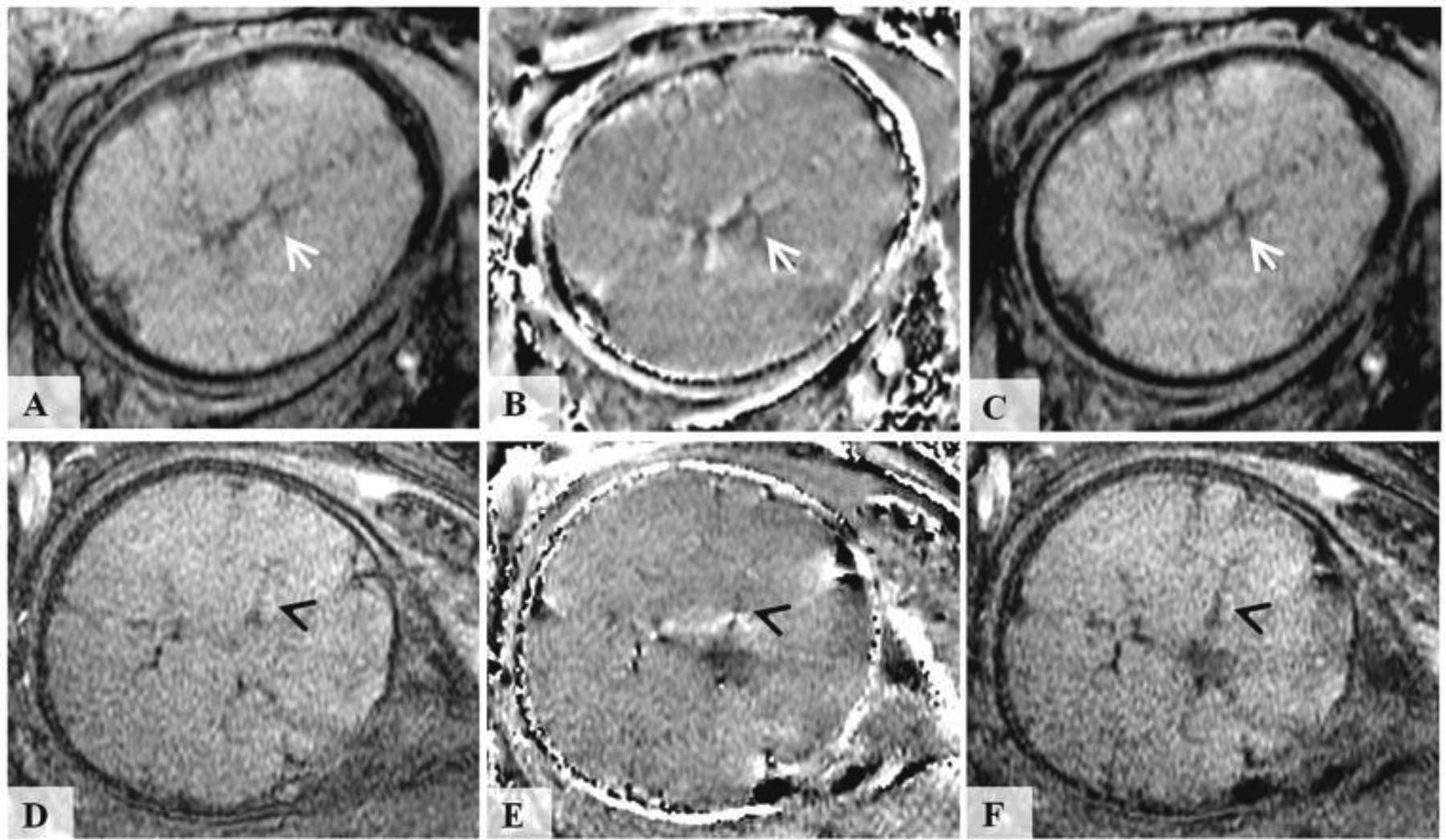
Susceptibility weighted images of the fetal brain. The top row images were acquired in the axial orientation relative to the fetus using 3D SWI sequence at 3.0T field strength from a fetus at 36 weeks of gestation. The bottom row images were acquired in coronal orientation relative to the fetus using a 2D SWI sequence at 1.5T field strength from a fetus at 35 weeks of gestation. Fig (a, d) show the original magnitude images. Fig (b, e) corresponds to filtered phase images. Fig (c, f) corresponds to susceptibility weighted magnitude image (SWI). (Adopted from [97] Figure 1).
Visibility of the veins in SWI depends on the phase signature of the venous vasculature, which is a function of blood oxygenation level, blood hematocrit, and the magnetic susceptibility of fetal blood [97]. Physiological variations in hematocrit, cerebral blood flow, and magnetic susceptibility can affect the venous phase and visibility of veins in SWI. Other SWI sequence parameters such as echo time (TE) and image resolution can also influence the visibility of veins: the longer the TE, the better the phase definition within the veins, and the smaller the voxel size, the better the visibility of smaller veins. Moreover, the fetal head orientation relative to the main magnetic field also influences the venous phase in SWI. This problem was overcome by appropriately modulating the phase mask generation step to account for arbitrary head orientation. A linear phase mask with an appropriate sign for enhancing venous vasculature was chosen depending on the sign of the venous phase [97].
Chronic hypoxia is known to be a predictor of abnormal neurodevelopment and cognitive disabilities. Therefore, the fetal cerebral blood oxygenation status could be an important physiological parameter in identifying fetuses at risk of brain injury. Recent works on quantitative MRI have either used the transverse relaxation rate property, T2, of blood or the paramagnetic nature of deoxyhemoglobin (dHb) with MR susceptometry to measure blood oxygenation status in the fetus [102, 103]. The former method was successfully applied in the major vessels of the heart of fetuses of third-trimester, but it was susceptible to radio-frequency field inhomogeneities and was also associated with a high SAR, especially when imaging at 3.0T [103]. The MR susceptometry measures the magnetic susceptibility of blood which is related to the amount of deoxyhemoglobin present.
Quantitative susceptibility mapping (QSM) is a recently developed MRI technique for quantifying the spatial distribution of magnetic susceptibility within biological tissues [104]. QSM uses both the intravascular and extravascular phase to estimate the magnetic susceptibility on a pixel-by-pixel basis. QSM is independent of the orientation and the shape/size of the structure of interest. It has been used to measure venous oxygen saturation in the cerebral veins and detect hemorrhages or micro-bleeds [103]. A study reported that the measured venous oxygen saturation values in the second-trimester groups were higher than in the third-trimester group. This finding supports previous studies where blood oxygenation level (both pO2 and SO2) in umbilical venous blood samples was found to decrease with advancing gestational age. In addition, it is known that the cerebral blood flow rate to the human fetal brain increases from the second trimester to the third trimester. Altogether, the flow and oxygen saturation changes may occur to accommodate the increased metabolic demand usually seen during the third trimester. While QSM holds tremendous potential to measure the blood venous oxygen saturation non-invasively, more studies are needed to evaluate the feasibility of performing QSM in the human fetus. The following limitations have been outlined by the research group: (1) the mask generation using manual intervention was time-consuming; (2) a longer TE was used to achieve a good phase signal-to-noise ratio (SNR) in SWI. A shorter TE can prove to be more useful in QSM processing due to less T2* signal loss and phase aliasing inside and at the boundaries of the veins. Additionally, it could also help reduce the imaging time without sacrificing the phase SNR [103].
At seven days after birth, brain MRI is the standard of care for all infants at risk of hypoxic-ischemic cerebral injuries in full-term newborns undergoing hypothermia therapy [105, 106]. MRI of the brain in newborns is indicated for early evaluation of infarction, hemorrhage, injuries of prematurity, myelination, white and gray matter differentiation [107–109] (Fig. 11). Thayyil et al. included thirty-two studies (860 infants with NE) in the meta-analysis and compared conventional MRI vs. proton MR spectroscopy performed during the neonatal period with neurodevelopmental outcome at ≥one year [110]. Conventional MRI during the neonatal period (days 1–30) showed a pooled sensitivity of 91% (95% confidence interval [CI]: 87%–94%) and specificity of 51% (95% CI: 45%–58%). Late MRI (days 8–30) had higher sensitivity but lower specificity than early MRI (days 1–7). Proton MR spectroscopy allows identification of cerebral lactate, which persists for weeks following a significant hypoxic-ischemic injury. Proton MR spectroscopy deep gray matter lactate/N-acetyl aspartate (Lac/NAA) peak-area ratio (days 1–30) had 82% overall pooled sensitivity (95% CI: 74%–89%) and 95% specificity (95% CI: 88%–99%). On common study analysis, Lac/NAA had better diagnostic accuracy than conventional MRI performed at any time during neonatal period.
Fig. 11.
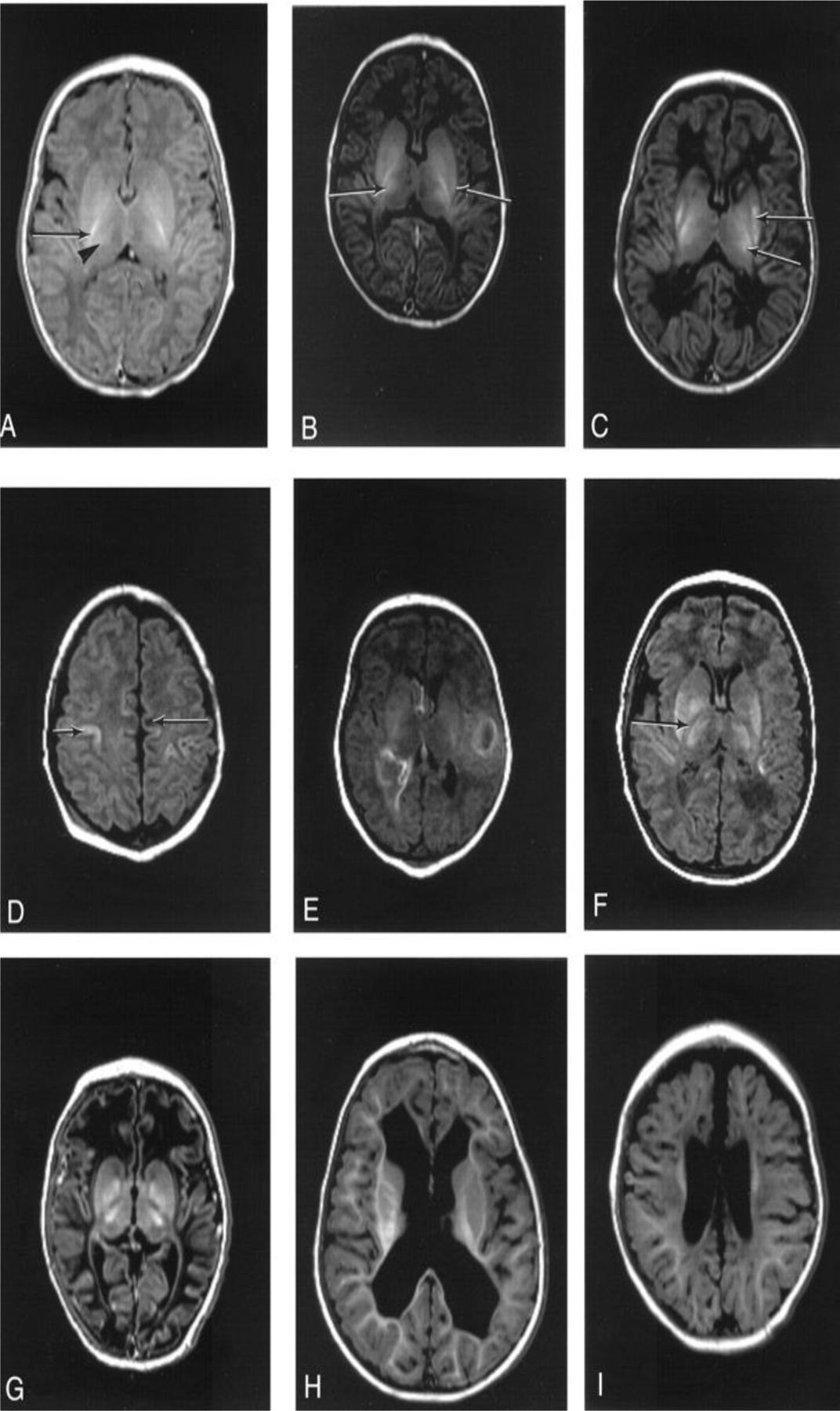
Patterns of injury identified on neonatal MRI. A, Normal appearances control term infant 2 days old). There are: normal high signal from myelin in the posterior half of the PLIC (arrow), more diffuse high signal in the lateral thalamus secondary to myelination in the nuclei (arrowhead), normal gray/white matter differentiation in the cerebral hemispheres. B, Mild basal ganglia: infant 4 days old with stage 2 HIE. There are small focal high-signal areas in the inferior thalamus and lentiform. C, Moderate basal ganglia: infant 20 days old with stage 2 HIE. There are: focal high-signal intensity regions in the lentiform and thalami (arrows), equivocal signal intensity within the PLIC (arrowhead). D, Moderate white matter: infant 20 days old with stage 2 HIE. There are: areas of focal low-signal intensity in the subcortical white matter (arrow), abnormal high-signal intensity in the cortex around the central sulcus (arrowhead). E, Severe white matter with hemorrhage: infant 5 days old with stage 2 HIE. There are: areas of high-signal intensity consistent with hemorrhage and areas of loss of gray/white matter differentiation. F, Severe basal ganglia lesions: infant 8 days old with stage 2 HIE. There are: abnormal areas of high-signal intensity in the lentiform and thalami, abnormal low-signal intensity within the PLIC. G, Severe basal ganglia, and severe white matter lesions: infant 11 days old with stage 2 HIE. There are: abnormal signal intensity in the basal ganglia and thalami, loss of the normal high-signal intensity from myelin within the posterior limb, abnormal low-signal intensity in the white matter with loss of the normal gray/white matter differentiation, particularly in the frontal lobes. H, Follow-up imaging/ventricular dilatation: infant 1 year 5 months of age with stage 2 HIE (same case as E). There is marked irregular dilatation of the ventricles. The extracerebral space is normal. I, Follow-up imaging/ventricular dilatation with widened extracerebral space: infant 1 year old with stage 2 HIE (same case as G). There is moderate ventricular dilatation and widening of the frontal extracerebral space and interhemispheric fissure. (Adopted from [109] Figure 1.)
Despite numerous advantages, there are material, technical and interpretative challenges, including elevated costs, limited accessibility, and limited time to acquire fast sequences, and limited training opportunities and expertise in the field of fetal MRI. In addition, MRI is more expensive to perform than in the US, with less equipment availability. Furthermore, it is impractical as an early screening tool or as a tool to optimize the therapy of HIE due to its non-portability and high cost.
IV. Nuclear medicine (PET, SPECT)
Nuclear medicine offers non-invasive imaging methods using positron emission tomography (PET) and single-photon emission computed tomography (SPECT) to study cerebral perfusion and metabolism [111]. The PET technique measures radiopharmaceuticals labeled with positron emitters using a PET scanner [112]. The unstable nucleus of radio-ligand emits positrons, combining with neighboring electrons to produce gamma-rays in the opposite direction at 180 degrees. These gamma rays are detected by the ring of detectors placed within the donut-shaped body of the scanner. The energy and location of these gamma rays are recorded and used by a computer program to reconstruct three-dimensional (3D) images of tracer concentration within the body.
Glucose metabolism provides the major energy supply required for brain function. Therefore, it is highly correlated with neuronal activity. In the newborn, the highest glucose metabolism was observed in the primary sensorimotor cortex, thalamus, brain stem, cingulate cortex, cerebellar vermis, and hippocampal region [113]. A study evaluating the correlation between glucose metabolism rate and gestational age was conducted on 36 preterm and term infants without brain injury and 24 infants with HIE. Cerebral glucose metabolic rate was increased with gestational age, especially at ≥ 37 weeks, and 18F-fluorodeoxyglucose uptake was lower in infants with HIE than in healthy term infants, especially in the subcortical white matter, thalamus, and basal ganglia areas, and correlated with the degree of severity of HIE, except for the basal ganglia [114]. In addition, a high correlation between cerebral glucose metabolism and the severity of HIE was detected in a study conducted on 20 term infants with HIE after perinatal asphyxia [115] (Fig. 12).
Fig. 12.
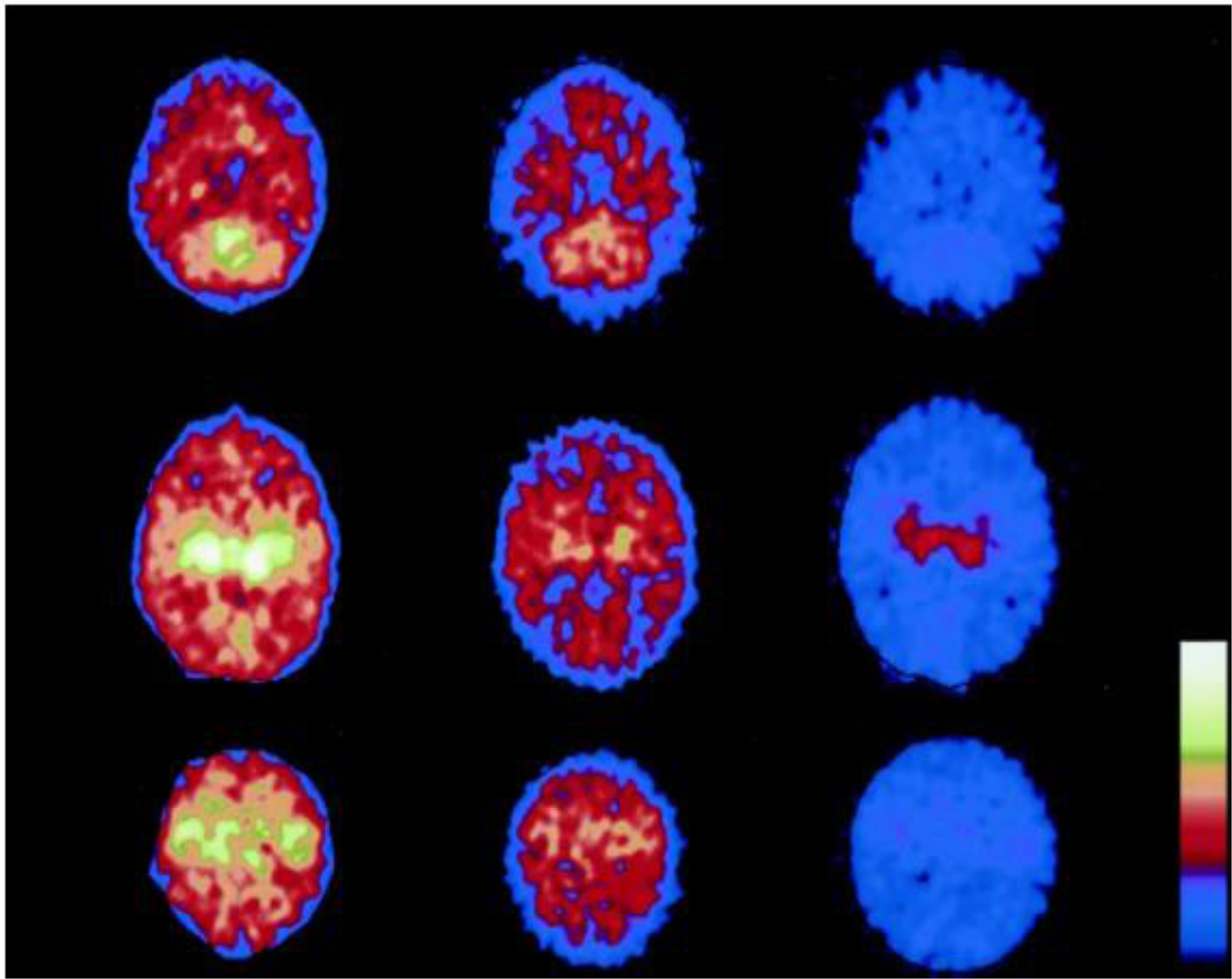
Total and regional cerebral glucose metabolism (CMRgl) measured in the subacute period (10–11 d) after birth asphyxia in three infants with different degrees of HIE. PET scan shows that the most metabolically active brain areas were the deep subcortical parts, thalamus, basal ganglia, and sensorimotor cortex, whereas the cortical areas (frontal, parietal, and occipital regions) were less metabolically active. Regional CMRglc in cerebellum (top), thalamus (middle), and sensorimotor cortex (bottom). The infant to the right (HIE-3) has developed CP (tetraplegia) with complicated seizures. The infant to the left (HIE-1) is healthy (2-y follow-up). The scale of the different images is identical. (Adopted from [115] Figure 2.)
Quantitative measurement of cerebral blood flow can be obtained using injected H2O or inhaled CO2 labeled with the isotope oxygen 15 [116]. In 1983, Volpe et al. [117], for the first time, demonstrated PET for determining regional CBF in neonates with major intraventricular hemorrhage and hemorrhagic intracerebral involvement. Later, the same group of investigators studied regional CBF by PET in 17 asphyxiated term infants during the acute period of illness. A study showed a relative decrease in CBF to parasagittal regions, generally symmetrical and more marked posteriorly than anteriorly. In addition, the single patient studied at postmortem examination exhibited parasagittal ischemic cerebral injury that correlated well with the PET abnormality of regional CBF [118].
SPECT is another type of nuclear medicine technique used to acquire the three-dimensional (3D) distribution of a radiopharmaceutical using a gamma camera [119]. Technetium-99m (Tc-99m) labeled compounds such as ethyl cysteine dimer (ECD), and hexamethyl propylene amine oxime (HMPAO) can be used to measure cerebral perfusion. These agents are unstable and are able to cross the blood-brain barrier in their lipophilic form and are retained within the brain by their conversion into hydrophilic form, which is unable to pass the blood-brain barrier and is trapped in the brain. Their extraction from plasma to the brain occurs rapidly within 1–2 minutes. This initial tracer uptake remains almost unchanged for several hours, allowing the acquisition of a constant image of regional cerebral blood flow at the time of tracer injection [120]. A prospective cohort study of CBF using SPECT was conducted in tertiary level ICU [121]. Shah et al. evaluated CBF on SPECT scan in neonates with HIE, CBF correlation with immediate neurological status and neurodevelopmental outcome, as well as comparison of SPECT to ultrasonography. Results showed that the most common pattern of defect noted was parasagittal hypoperfusion. Neonates with severe perfusion defects had a higher incidence of difficulty to control seizures and longer duration of altered sensorium. The study concluded that SPECT scan is superior to ultrasonography in predicting neurodevelopmental outcomes in neonates with HIE.
Several limitations restrict the use of nuclear medicine in neonates. PET uses ionizing radiation, and the technique is not widely available (there is a need for proximity to a cyclotron) because the tracer has an extremely short half-life [122]. Moreover, PET is not available at the bedside or for emergencies. Another disadvantage of PET scanners is their operating cost [123]. SPECT is a suitable bedside method that is cheaper and more widely available than PET imaging. The greatest disadvantage of using the SPECT technique in children is the ionizing radiation. The technique also yields poor resolution and requires a long examination time (20–25 minutes). In addition, data processing to obtain maps takes about 5 minutes.
Emerging diagnostic technologies for monitoring fetal and neonatal brain status
I. Photoacoustic (PA) Imaging
In recent years, a novel US-based modality called photoacoustic (PA) imaging has shown tremendous potential by providing complementary functional and cellular, and molecular information [124–129]. The key advantage that makes PA imaging a suitable diagnostic modality for clinical applications is the ability to provide functional images (e.g., tissue vascularity and SO2 level) at clinically relevant depths (> 30 mm, depending on tissue type) with high resolution (typically < 200 μm, depending on the acoustic resolution for deep-tissue imaging) and in real-time. Moreover, PA imaging can be easily combined with US imaging because both modalities use similar acquisition hardware components and signal detection regimens. Therefore, combining the US and PA imaging makes it possible to simultaneously obtain anatomic and functional information [124, 125, 130]. In PA imaging, short (typically in the nano-seconds range) laser pulses are utilized to excite the tissue chromophores such as oxy- and de-oxyhemoglobin, and consequent acoustic signals (PA signals) [124, 128] is acquired by an acoustic (or ultrasonic) receiver to form PA images that are representing the tissue’s optical absorption map. In addition, similar to NIRS, the contrast between oxy- and deoxy hemoglobin provides PA with a unique capability to measure the blood oxygen saturation (SO2= ratio of oxy- to total hemoglobin). PA imaging has been previously applied for breast cancer, melanoma, osteoarthritis imaging, cerebral blood oxygen saturation assessment, and neuro-vasculature evaluation with and without functional hemodynamics [131–134]. Like US imaging, a major challenge in PA imaging of the brain is that the skull limits the penetration depth and causes image distortions due to acoustic aberrations [135, 136]. However, fetuses and neonates are more suitable for PA imaging due to their relatively thin skull and open fontanelles [137–140]. These unique anatomical properties create minimal optical, ultrasonic attenuation and distortion as compared to adults.
The use of a single-element US transducer for non-invasive trans-fontanelle PA imaging of neonatal brains is reported in the literature (Fig. 13) [139, 141]. Two laser excitation wavelengths, 752 and 797 nm, were selected for spectroscopic PA (sPA) and are suggested to be suitable for spectral unmixing and differentiation between oxy- and deoxy- hemoglobin. The results revealed that the PA imaging could detect the blood SO2 in a vessel through the infant skull at 5 mm depth (Fig. 13 a and b). sPA imaging results indicated a high correlation between the measurements to the gold standard pulse-oximeter (Fig. 13 c). Another group proposed an integrated US, US Doppler, and PA imaging system around a clinical transvaginal US probe [133] (Fig. 14). Their proposed multi-parametric imaging system aims to visualize blood flow and estimate the blood SO2 in venous and arterial vessels, such as superior sagittal sinus (SSS) and cortical branches of the anterior cerebral artery (ACA). Estimating the SO2 in selected vessels and combining it with quantitative arterial and venous blood flow (measured by fractional moving blood volume (FMBV)) allows the calculation for metabolic rate of oxygen consumption (CMRO2) in the fetal brain. This proposed multi-parametric imaging probe have clinically acceptable size for an intrapartum imaging (total diameter 29 mm, Fig 14. a–c), high accuracy in monitoring blood volume (Fig 14. d), and blood oxygenation level (Fig 14. e). Therefore, it may pave the way for developing effective diagnostic modalities and favorable fetal and maternal outcomes.
Fig. 13.
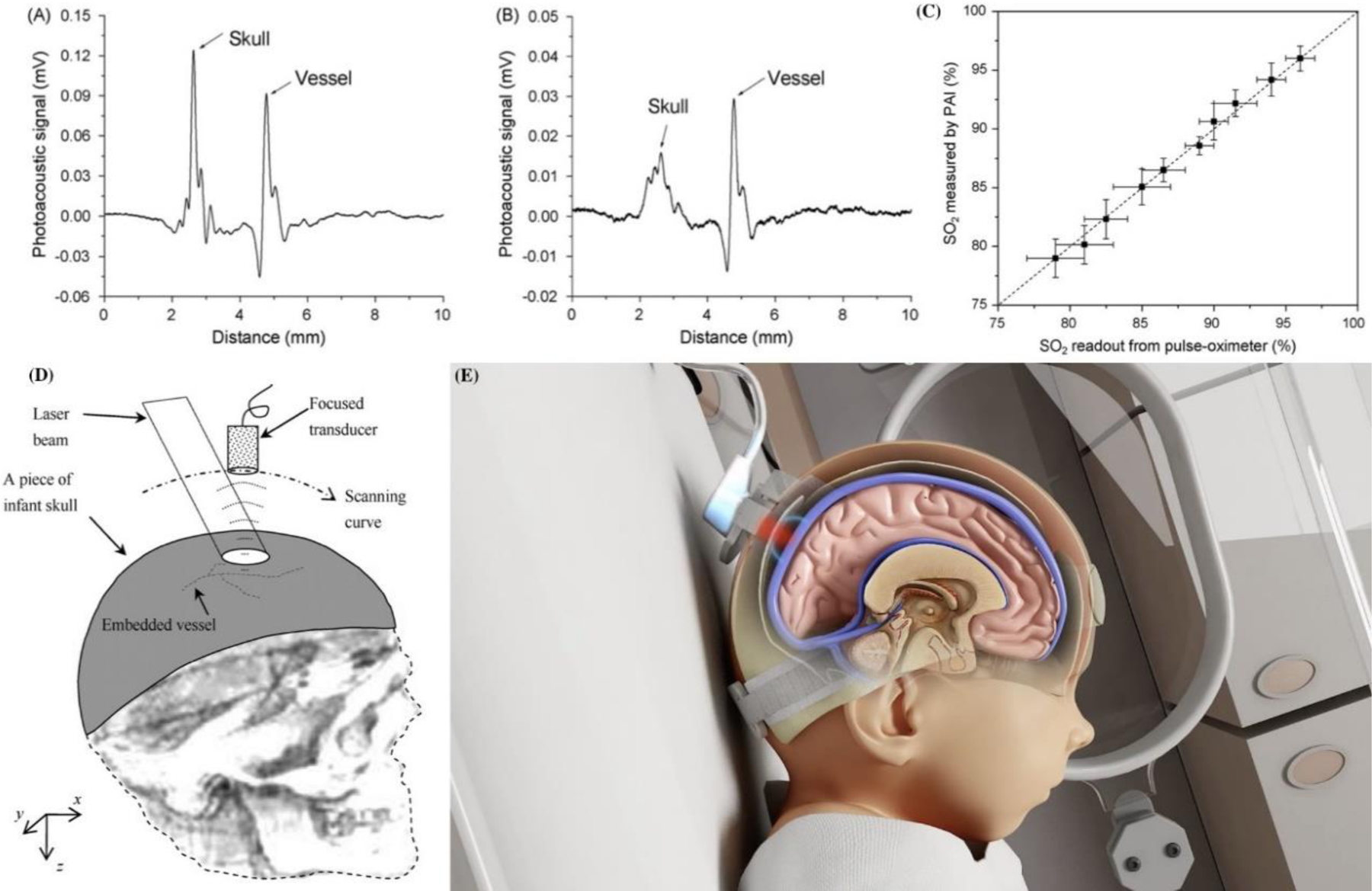
Single element focused USPA system for imaging the neonatal brain. Fig (A) & (B) show photoacoustic signals from a vessel embedded beneath the infant skull, where (A) is induced by solid-beam illumination and (B) is induced by dark-field illumination. Fig (C) shows photoacoustic measurements of the blood oxygenation levels through the infant skull in comparison with the readouts from a pulse-oximeter. Fig (D) represents the geometry of reflection mode transcranial photoacoustic imaging of brain. Adopted from [139]). Fig (E) Noninvasix® Photoacoustic oxymetry System. Noninvasix’s system utilizes optoacoustic monitoring of cerebral venous oxygenation to accurately measure the amount of oxygen a neonate is receiving in real time.
Fig. 14.
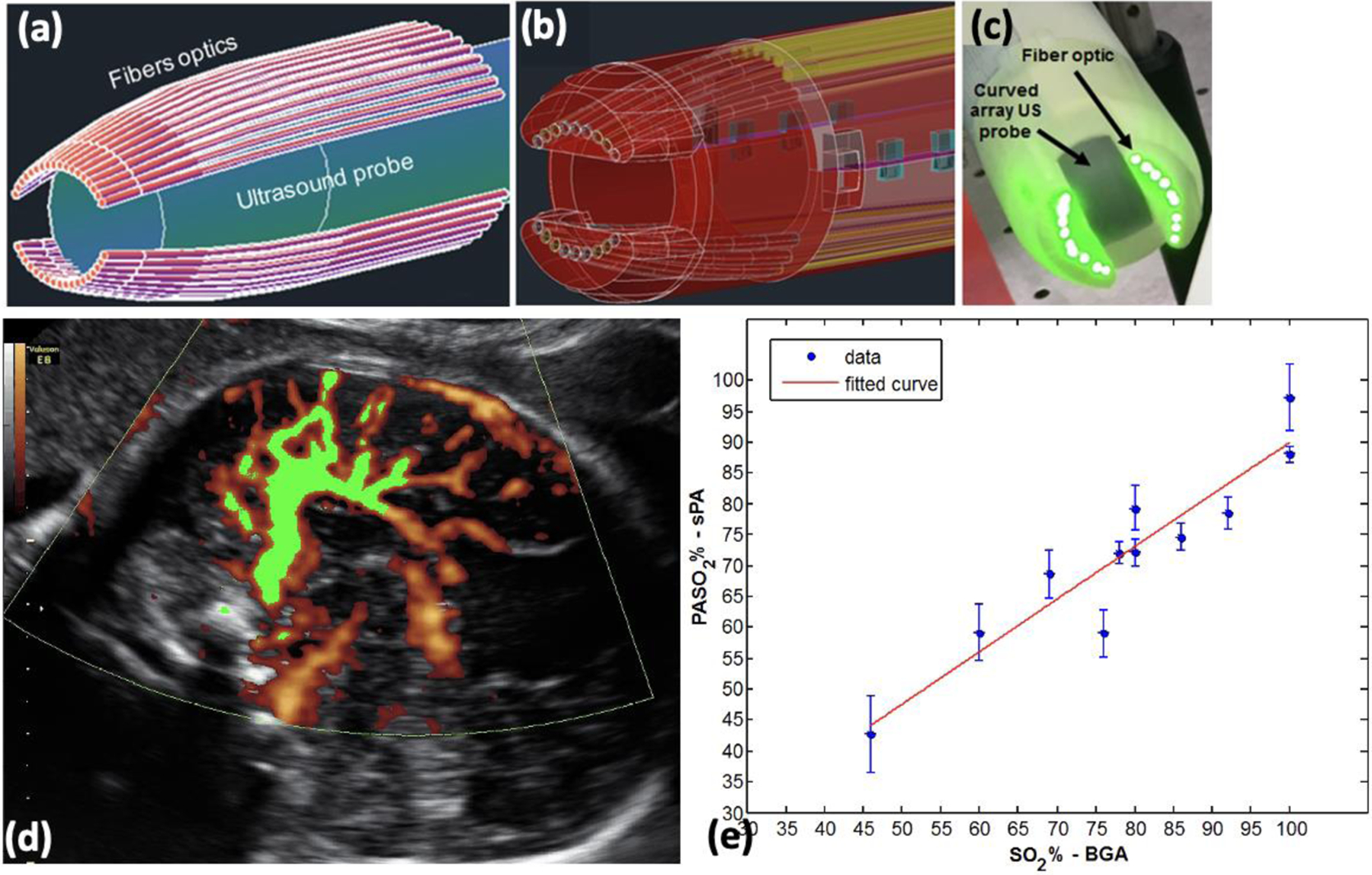
(a) Schematic of a TVUS and fiber optics light delivery. (b) CAD design of fiber holding sheath. (c) Photograph of the prototype an Endocavity US and PA probe, which illuminates the target tissue with a focused (line-like) illumination pattern at 25 mm away from probe surface. The system has a diameter of 29 mm (under clinical requirements for transvaginal imaging). It can provide functional and molecular images at high resolution (200 μm @ 25 mm depth), and high penetration depth (> 20 mm) in brain. (d) Normalized FMBV image (green mask) overlaid on top of acquired Doppler image, indicating the blood volume is 31%. (e) Blood SO2 measure by sPA vs. gold standard blood gas analysis. A high correlation (R2=0.87) was found between sPA measurements of blood SO2 and the gold standard. These results suggest high accuracy and reliability of sPA imaging to evaluate blood oxygenation. The 46% oxygenation, as the lowest SO2 level is relatively close to the fetal SO2 threshold during labor (%35 to %40). (Adopted from [133]).
In summary, the integrated US and PA imaging technology have the following advantages in imaging fetal and neonatal brains. First, the PA signal directly measures cerebral venous oxygenation with high spatial resolution, accurate blood oxygenation measurements, and continuous real-time monitoring. Second, the SO2 levels in the ACA and SSS estimated by sPA in combination with blood flow detected by US Doppler give the accessibility for calculating CMRO2, which may help to potentially lower the risk of hypoxic events during delivery and labor. Additionally, summarizes the performance of existing clinical diagnostic tools and emerging technologies in detecting HIE and NE.
Conclusion
HIE is a significant cause of morbidity and mortality in the neonatal period, and cerebral palsy is a late neurologic sequela in the postnatal period. The Cranial Ultrasound (CUS) provides a convenient, non-invasive, relatively low-cost screening examination of the hemodynamically unstable neonate at the bedside. CT has the least sensitive modality for the evaluation of HIE for poor parenchymal contrast resolution. The most sensitive and specific imaging technique for examining infants with suspected HIE is MR imaging. CUS, CT, MRI, nuclear medicine techniques, each with its advantages and disadvantages, show characteristic patterns of brain injury that correlate well with the degree of hypotension and the level of brain maturity at the time of the insult, thus excluding other causes of encephalopathy. The development of an ideal fetal and neonatal neuroimaging modality is an ongoing challenge within the world of intrapartum care and neonatal intensive care medicine. Innovations including low radiation, rapid CT, and diffusion-weighted MRI have changed the approach to which infants with neurologic dysfunction are evaluated and managed. However, despite these advances, we continue to lack the ability to non-invasively detect and follow structural and functional variations in real-time at the bedside of a critically ill neonate. In recent years, a novel ongoing imaging modality, photoacoustic (PA) imaging, has shown the potential to develop a point-of-care, non-invasive, real-time method to monitor cerebral hemodynamic, assess the cortical injury and assist in developing neuroprotective strategies within the walls of the neonatal intensive care and intrapartum units. It may pave the way for developing practical solutions and good fetal and neonatal care and potentially lower the risk of hypoxic events in fetuses and newborns.
Table 1.
Performance of existing clinical diagnostic tools and emerging technologies in detecting HIE and NE.
| Method [References] | Advantages | Limitations |
|---|---|---|
| External fetal heart rate monitoring (FHRM) References: [25–29, 45–49, 142–146] |
|
|
| Internal fetal heart rate monitoring (FHRM) References: [25–29, 45–49, 142–146] |
|
|
| Fetal scalp blood sampling (FBS) References: [14, 51–54, 147] |
|
|
| Cranial ultrasound (CUS) References: [22, 80–85] |
|
|
| Magnetic resonance imaging (MRI) References: [89–93, 97, 98, 100, 103–110] |
|
|
| Computed tomography (CT) References: [60–67] |
|
|
| Nuclear imaging (PET, SPECT) References: [111–123] |
|
|
| Near-infrared spectroscopy (NIRS) References: [68, 69, 71–79] |
|
|
| Photoacoustic (PA) imaging References: [124–140] |
|
|
Acknowledgments
We would like to thank Dr. E. Mark Haacke for his valuable and insightful comments for the 3.0T MRI imaging in fetus, and Mr. David Bustamante from Wayne State University for proofreading this manuscript.
Funding Sources
This research was supported by the National Institute of Biomedical Imaging and Bioengineering (NIBIB/NIH) under grant No. 1R01EB030058.
Footnotes
Conflict of Interest Statement
Dr. Hernandez is an Editorial Board Member of Fetal Diagnosis and Therapy. The other authors have no conflicts of interest to declare.
References
- [1].Encephalopathy N, and Neurologic Outcome, “Report of the American College of Obstetricians and Gynecologists’ Task Force on neonatal encephalopathy,” Obstet Gynecol, vol. 123, no. 4, pp. 896–901, 2014. [DOI] [PubMed] [Google Scholar]
- [2].Graham EM, Ruis KA, Hartman AL, Northington FJ, and Fox HE, “A systematic review of the role of intrapartum hypoxia-ischemia in the causation of neonatal encephalopathy,” American journal of obstetrics and gynecology, vol. 199, no. 6, pp. 587–595, 2008. [DOI] [PubMed] [Google Scholar]
- [3].Thornberg E, Thiringer K, Odeback A, and Milsom I, “Birth asphyxia: incidence, clinical course and outcome in a Swedish population,” Acta paediatrica, vol. 84, no. 8, pp. 927–932, 1995. [DOI] [PubMed] [Google Scholar]
- [4].Kurinczuk JJ, White-Koning M, and Badawi N, “Epidemiology of neonatal encephalopathy and hypoxic–ischaemic encephalopathy,” Early human development, vol. 86, no. 6, pp. 329–338, 2010. [DOI] [PubMed] [Google Scholar]
- [5].Barcroft J, “The Croonian lecture-Foetal respiration,” Proceedings of the Royal Society of London. Series B-Biological Sciences, vol. 118, no. 808, pp. 242–263, 1935. [Google Scholar]
- [6].Rudolph AM and Heymann MA, “The fetal circulation,” Annual review of medicine, vol. 19, no. 1, pp. 195–206, 1968. [DOI] [PubMed] [Google Scholar]
- [7].Maurer HS, Behrman RE, and Honig GR, “Dependence of the oxygen affinity of blood on the presence of foetal or adult haemoglobin,” Nature, vol. 227, no. 5256, pp. 388–390, 1970. [DOI] [PubMed] [Google Scholar]
- [8].Boddy K, Dawes G, Fisher R, Pinter S, and Robinson J, “Foetal respiratory movements, electrocortical and cardiovascular responses to hypoxaemia and hypercapnia in sheep,” The Journal of physiology, vol. 243, no. 3, pp. 599–618, 1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Anderson P, Glick KL, Killam AP, and Mainwaring R, “The effect of heart rate on in utero left ventricular output in the fetal sheep,” The Journal of physiology, vol. 372, no. 1, pp. 557573, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Giussani DA, “The fetal brain sparing response to hypoxia: physiological mechanisms,” The Journal of physiology, vol. 594, no. 5, pp. 1215–1230, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Massaro AN, “MRI for neurodevelopmental prognostication in the high-risk term infant,” in Seminars in perinatology, 2015, vol. 39, no. 2, pp. 159–167: Elsevier. [DOI] [PubMed] [Google Scholar]
- [12].Azzopardi D, “Clinical applications of cerebral function monitoring in neonates,” in Seminars in Fetal and Neonatal Medicine, 2015, vol. 20, no. 3, pp. 154–163: Elsevier. [DOI] [PubMed] [Google Scholar]
- [13].Dash S, Quirk JG, and Djurić PM, “Fetal heart rate classification using generative models,” IEEE Transactions on Biomedical Engineering, vol. 61, no. 11, pp. 2796–2805, 2014. [DOI] [PubMed] [Google Scholar]
- [14].Schiermeier S et al. , “Sensitivity and specificity of intrapartum computerised FIGO criteria for cardiotocography and fetal scalp pH during labour: multicentre, observational study,” BJOG: An International Journal of Obstetrics & Gynaecology, vol. 115, no. 12, pp. 1557–1563, 2008. [DOI] [PubMed] [Google Scholar]
- [15].Barnette AR et al. , “Neuroimaging in the evaluation of neonatal encephalopathy,” Pediatrics, vol. 133, no. 6, pp. e1508–e1517, 2014. [DOI] [PubMed] [Google Scholar]
- [16].Arthurs G and Nicholls B, Ultrasound in Anesthesia, Critical Care and Pain Management with Online Resource. Cambridge University Press, 2017. [Google Scholar]
- [17].Kudrevičienė A et al. , “The value of ultrasonography and Doppler sonography in prognosticating long-term outcomes among full-term newborns with perinatal asphyxia,” Medicina, vol. 50, no. 2, pp. 100–110, 2014. [DOI] [PubMed] [Google Scholar]
- [18].Creasy JL, “The General Appearance of Edema and Hemorrhage on CT, MR and US (Including a General Introduction to CT, MR and US Scanning),” in Dating Neurological Injury:: Springer, 2011, pp. 43–58. [Google Scholar]
- [19].Macones GA, Hankins GD, Spong CY, Hauth J, and Moore T, “The 2008 National Institute of Child Health and Human Development workshop report on electronic fetal monitoring: update on definitions, interpretation, and research guidelines,” Journal of Obstetric, Gynecologic, & Neonatal Nursing, vol. 37, no. 5, pp. 510–515, 2008. [DOI] [PubMed] [Google Scholar]
- [20].Accardo J, Kammann H, and Hoon AH Jr, “Neuroimaging in cerebral palsy,” The Journal of pediatrics, vol. 145, no. 2, pp. S19–S27, 2004. [DOI] [PubMed] [Google Scholar]
- [21].Lequin M, Dudink J, Tong KA, and Obenaus A, “Magnetic resonance imaging in neonatal stroke,” in Seminars in Fetal and Neonatal Medicine, 2009, vol. 14, no. 5, pp. 299–310: Elsevier. [DOI] [PubMed] [Google Scholar]
- [22].Merrill JD, Piecuch RE, Fell SC, Barkovich AJ, and Goldstein RB, “A new pattern of cerebellar hemorrhages in preterm infants,” Pediatrics, vol. 102, no. 6, pp. e62–e62, 1998. [DOI] [PubMed] [Google Scholar]
- [23].Rutherford MA, Pennock JM, and Dubowitz LM, “Cranial ultrasound and magnetic resonance imaging in hypoxic-ischaemic encephalopathy: a comparison with outcome,” Developmental Medicine & Child Neurology, vol. 36, no. 9, pp. 813–825, 1994. [DOI] [PubMed] [Google Scholar]
- [24].Chao CP, Zaleski CG, and Patton AC, “Neonatal hypoxic-ischemic encephalopathy: multimodality imaging findings,” Radiographics, vol. 26, no. suppl_1, pp. S159–S172, 2006. [DOI] [PubMed] [Google Scholar]
- [25].Alfirevic Z, Gyte GM, Cuthbert A, and Devane D, “Continuous cardiotocography (CTG) as a form of electronic fetal monitoring (EFM) for fetal assessment during labour,” Cochrane database of systematic reviews, no. 2, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].HOM E, “An atlas fetal heart rate pattern,” Hew Heaven Conn, 1968. [Google Scholar]
- [27].Parer JT and King T, “Fetal heart rate monitoring: is it salvageable?,” American journal of obstetrics and gynecology, vol. 182, no. 4, pp. 982–987, 2000. [DOI] [PubMed] [Google Scholar]
- [28].Sharbaf FR, Amjadi N, Alavi A, Akbari S, and Forghani F, “Normal and indeterminate pattern of fetal cardiotocography in admission test and pregnancy outcome,” Journal of Obstetrics and Gynaecology Research, vol. 40, no. 3, pp. 694–699, 2014. [DOI] [PubMed] [Google Scholar]
- [29].Sholapurkar S, “The unresolved role of cardiotocography (CTG), fetal ECG (STAN) and intrapartum fetal pulse oximetry (IFPO) as diagnostic methods for fetal hypoxia,” Journal of Obstetrics and Gynaecology, vol. 34, no. 8, pp. 757–757, 2014. [DOI] [PubMed] [Google Scholar]
- [30].Denney J, Holmgren C, Henry E, Rose N, Jackson M, and Esplin S, “561: Category III fetal heart rate tracings: a rare occurrence strongly associated with adverse neonatal outcome,” American Journal of Obstetrics & Gynecology, vol. 201, no. 6, pp. S208–S209, 2009. [Google Scholar]
- [31].Rouse DJ, Owen J, Goldenberg RL, and Cliver SP, “Determinants of the optimal time in gestation to initiate antenatal fetal testing: a decision-analytic approach,” American journal of obstetrics and gynecology, vol. 173, no. 5, pp. 1357–1363, 1995. [DOI] [PubMed] [Google Scholar]
- [32].Lagrew DC, Pircon RA, Towers CV, Dorchester W, and Freeman RK, “Antepartum fetal surveillance in patients with diabetes: when to start?,” American journal of obstetrics and gynecology, vol. 168, no. 6, pp. 1820–1826, 1993. [DOI] [PubMed] [Google Scholar]
- [33].Pircon RA, Lagrew DC, Towers CV, Dorchester WL, Gocke SE, and Freeman RK, “Antepartum testing in the hypertensive patient: when to begin,” American journal of obstetrics and gynecology, vol. 164, no. 6, pp. 1563–1570, 1991. [DOI] [PubMed] [Google Scholar]
- [34].A. C. o. Obstetricians and Gynecologists, “ACOG Committee opinion no. 828: Indications for outpatient antenatal fetal surveillance,” Obstetrics and gynecology, vol. 137, pp. e178–197, 2021. [DOI] [PubMed] [Google Scholar]
- [35].Powers WF and Kiely JL, “The risks confronting twins: a national perspective,” American journal of obstetrics and gynecology, vol. 170, no. 2, pp. 456–461, 1994. [DOI] [PubMed] [Google Scholar]
- [36].Houlton M, Marivate M, and Philpott R, “The prediction of fetal growth retardation in twin pregnancy,” BJOG: An International Journal of Obstetrics & Gynaecology, vol. 88, no. 3, pp. 264–273, 1981. [DOI] [PubMed] [Google Scholar]
- [37].Sassoon DA, Castro LC, Davis JL, and Hobel CJ, “Perinatal outcome in triplet versus twin gestations,” Obstetrics and Gynecology, vol. 75, no. 5, pp. 817–820, 1990. [PubMed] [Google Scholar]
- [38].Alexander JM, Hammond KR, and Steinkampf MP, “Multifetal reduction of high-order multiple pregnancy: comparison of obstetrical outcome with nonreduced twin gestations,” Fertility and sterility, vol. 64, no. 6, pp. 1201–1203, 1995. [DOI] [PubMed] [Google Scholar]
- [39].Silver RK, Helfand BT, Russell TL, Ragin A, Sholl JS, and MacGregor SN, “Multifetal reduction increases the risk of preterm delivery and fetal growth restriction in twins: a casecontrol study,” Fertility and sterility, vol. 67, no. 1, pp. 30–33, 1997. [DOI] [PubMed] [Google Scholar]
- [40].Machin G, “Velamentous cord insertion in monochorionic twin gestation. An added risk factor,” The Journal of reproductive medicine, vol. 42, no. 12, pp. 785–789, 1997. [PubMed] [Google Scholar]
- [41].Manning F, Morrison I, Lange I, Harman C, and Chamberlain P, “Fetal biophysical profile scoring: selective use of the nonstress test,” American Journal of Obstetrics and Gynecology, vol. 156, no. 3, pp. 709–712, 1987. [DOI] [PubMed] [Google Scholar]
- [42].Clark SL, Sabey P, and Jolley K, “Nonstress testing with acoustic stimulation and amniotic fluid volume assessment: 5973 tests without unexpected fetal death,” American journal of obstetrics and gynecology, vol. 160, no. 3, pp. 694–697, 1989. [DOI] [PubMed] [Google Scholar]
- [43].Evertson LR, Gauthier RJ, Schifrin BS, and Paul RH, “Antepartum fetal heart rate testing: I. Evolution of the nonstress test,” American Journal of Obstetrics and Gynecology, vol. 133, no. 1, pp. 29–33, 1979. [DOI] [PubMed] [Google Scholar]
- [44].Staisch K, Westlake J, and Bashore R, “Blind oxytocin challenge test and perinatal outcome,” American journal of obstetrics and gynecology, vol. 138, no. 4, pp. 399–403, 1980. [DOI] [PubMed] [Google Scholar]
- [45].A. E. T. Areas. (6/1/2021). Abnormal Labor and Intrapartum Fetal Surveillance (Obstetrics and Gynecology 7 Ed. Chapter 9 ed.).
- [46].Westerhuis ME et al. , “Identification of cases with adverse neonatal outcome monitored by cardiotocography versus ST analysis: secondary analysis of a randomized trial,” Acta obstetricia et gynecologica Scandinavica, vol. 91, no. 7, pp. 830–837, 2012. [DOI] [PubMed] [Google Scholar]
- [47].Ugwumadu A, “Understanding cardiotocographic patterns associated with intrapartum fetal hypoxia and neurologic injury,” Best practice & research Clinical obstetrics & gynaecology, vol. 27, no. 4, pp. 509–536, 2013. [DOI] [PubMed] [Google Scholar]
- [48].Martin CB Jr, “Electronic fetal monitoring: a brief summary of its development, problems and prospects,” European Journal of Obstetrics & Gynecology and Reproductive Biology, vol. 78, no. 2, pp. 133–140, 1998. [DOI] [PubMed] [Google Scholar]
- [49].Gilbert ES, Manual of High Risk Pregnancy and Delivery E-Book. Elsevier Health Sciences, 2010. [Google Scholar]
- [50].Vintzileos AM, Nochimson DJ, Guzman ER, Knuppel RA, Lake M, and Schifrin BS, “Intrapartum electronic fetal heart rate monitoring versus intermittent auscultation: a meta-analysis,” Obstetrics & Gynecology, vol. 85, no. 1, pp. 149–155, 1995. [DOI] [PubMed] [Google Scholar]
- [51].Higgins C, “Fetal scalp blood sampling,” 2014.
- [52].Chandraharan E, “Fetal scalp blood sampling during labour: is it a useful diagnostic test or a historical test that no longer has a place in modern clinical obstetrics?,” BJOG: An International Journal of Obstetrics & Gynaecology, vol. 121, no. 9, pp. 1056–1062, 2014. [DOI] [PubMed] [Google Scholar]
- [53].East CE, Kane SC, Davey M-A, Kamlin CO, and Brennecke SP, “Protocol for a randomised controlled trial of fetal scalp blood lactate measurement to reduce caesarean sections during labour: the Flamingo trial [ACTRN12611000172909],” BMC pregnancy and childbirth, vol. 15, no. 1, pp. 1–10, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Jaiyesimi RK and Hickey WN, “Fetal haemorrhage after fetal scalp blood sampling,” The Lancet, vol. 336, no. 8718, pp. 819–820, 1990. [DOI] [PubMed] [Google Scholar]
- [55].Sabir H, Stannigel H, Schwarz A, and Hoehn T, “Perinatal hemorrhagic shock after fetal scalp blood sampling,” Obstetrics & Gynecology, vol. 115, no. 2, pp. 419–420, 2010. [DOI] [PubMed] [Google Scholar]
- [56].Schaap TP et al. , “Cerebrospinal fluid leakage, an uncommon complication of fetal blood sampling: a case report and review of the literature,” Obstetrical & gynecological survey, vol. 66, no. 1, pp. 42–46, 2011. [DOI] [PubMed] [Google Scholar]
- [57].Chandraharan E, “Should national guidelines continue to recommend fetal scalp blood sampling during labor?,” The Journal of Maternal-Fetal & Neonatal Medicine, vol. 29, no. 22, pp. 3682–3685, 2016. [DOI] [PubMed] [Google Scholar]
- [58].Carbonne B, Pons K, and Maisonneuve E, “Foetal scalp blood sampling during labour for pH and lactate measurements,” Best Practice & Research Clinical Obstetrics & Gynaecology, vol. 30, pp. 62–67, 2016. [DOI] [PubMed] [Google Scholar]
- [59].Al Wattar BH et al. , “Evaluating the value of intrapartum fetal scalp blood sampling to predict adverse neonatal outcomes: A UK multicentre observational study,” European Journal of Obstetrics & Gynecology and Reproductive Biology, vol. 240, pp. 62–67, 2019. [DOI] [PubMed] [Google Scholar]
- [60].Brenner DJ and Hall EJ, “Computed tomography—an increasing source of radiation exposure,” New England Journal of Medicine, vol. 357, no. 22, pp. 2277–2284, 2007. [DOI] [PubMed] [Google Scholar]
- [61].Bano S, Chaudhary V, and Garga UC, “Neonatal hypoxic-ischemic encephalopathy: A radiological review,” Journal of pediatric neurosciences, vol. 12, no. 1, p. 1, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Strauss KJ et al. , “Image gently: ten steps you can take to optimize image quality and lower CT dose for pediatric patients,” American Journal of Roentgenology, vol. 194, no. 4, pp. 868–873, 2010. [DOI] [PubMed] [Google Scholar]
- [63].Menoch MJ, Hirsh DA, Khan NS, Simon HK, and Sturm JJ, “Trends in computed tomography utilization in the pediatric emergency department,” Pediatrics, vol. 129, no. 3, pp. e690–e697, 2012. [DOI] [PubMed] [Google Scholar]
- [64].Liu J-B, Zhang T-F, Wu X-Z, Shen D-G, and Lin J, “Role of cerebral computed tomography in the evaluation of brain injury following hypoxia in neonates,” Zhongguo Dang dai er ke za zhi= Chinese Journal of Contemporary Pediatrics, vol. 8, no. 3, pp. 195–197, 2006. [PubMed] [Google Scholar]
- [65].Barkovich AJ, “The encephalopathic neonate: choosing the proper imaging technique,” American journal of neuroradiology, vol. 18, no. 10, pp. 1816–1820, 1997. [PMC free article] [PubMed] [Google Scholar]
- [66].Victoria T et al. , “Low-dose fetal CT in the prenatal evaluation of skeletal dysplasias and other severe skeletal abnormalities,” American Journal of Roentgenology, vol. 200, no. 5, pp. 989–1000, 2013. [DOI] [PubMed] [Google Scholar]
- [67].Sorokan ST, Jefferies AL, and Miller SP, “Imaging the term neonatal brain,” Paediatrics & child health, vol. 23, no. 5, pp. 322–328, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Murkin JM and Arango M, “Near-infrared spectroscopy as an index of brain and tissue oxygenation,” British journal of anaesthesia, vol. 103, no. suppl_1, pp. i3–i13, 2009. [DOI] [PubMed] [Google Scholar]
- [69].Wintermark P, Hansen A, Warfield SK, Dukhovny D, and Soul JS, “Near-infrared spectroscopy versus magnetic resonance imaging to study brain perfusion in newborns with hypoxic–ischemic encephalopathy treated with hypothermia,” Neuroimage, vol. 85, pp. 287–293, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Simpson KR, “Fetal oxygen saturation monitoring during labor: Implications for perinatal risk management,” Journal of Healthcare Risk Management, vol. 21, no. 1, pp. 17–27, 2001. [Google Scholar]
- [71].Beausoleil TP, Janaillac M, Barrington KJ, Lapointe A, and Dehaes M, “Cerebral oxygen saturation and peripheral perfusion in the extremely premature infant with intraventricular and/or pulmonary haemorrhage early in life,” Scientific reports, vol. 8, no. 1, pp. 1–13, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Wyatt J et al. , “Quantitation of cerebral blood volume in human infants by near-infrared spectroscopy,” Journal of Applied Physiology, vol. 68, no. 3, pp. 1086–1091, 1990. [DOI] [PubMed] [Google Scholar]
- [73].Peebles D and Wyatt J, “Near infrared spectroscopy and intrapartum fetal monitoring,” Contemporary Reviews in Obstetrics and Gynaecology, vol. 5, pp. 124–129, 1993. [Google Scholar]
- [74].Bloom SL et al. , “Fetal pulse oximetry and cesarean delivery,” New England Journal of Medicine, vol. 355, no. 21, pp. 2195–2202, 2006. [DOI] [PubMed] [Google Scholar]
- [75].Chez BF, Harvey MG, and Harvey CJ, “Intrapartum fetal monitoring: past, present, and future,” The Journal of perinatal & neonatal nursing, vol. 14, no. 3, pp. 1–18, 2000. [DOI] [PubMed] [Google Scholar]
- [76].Dildy GA, “The future of intrapartum fetal pulse oximetry,” Current Opinion in Obstetrics and Gynecology, vol. 13, no. 2, pp. 133–136, 2001. [DOI] [PubMed] [Google Scholar]
- [77].Marin T and Moore J, “Understanding near-infrared spectroscopy,” Advances in Neonatal Care, vol. 11, no. 6, pp. 382–388, 2011. [DOI] [PubMed] [Google Scholar]
- [78].Van Bel F, Lemmers P, and Naulaers G, “Monitoring neonatal regional cerebral oxygen saturation in clinical practice: value and pitfalls,” Neonatology, vol. 94, no. 4, pp. 237–244, 2008. [DOI] [PubMed] [Google Scholar]
- [79].Jarraya A, Mohamed S, Sofiene L, and Kolsi K, “Near-infrared spectrometry in pregnancy: progress and perspectives, a review of literature,” Pan African Medical Journal, vol. 23, no. 1, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Bhat V and Bhat V, “Neonatal neurosonography: A pictorial essay,” The Indian journal of radiology & imaging, vol. 24, no. 4, p. 389, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Bhide A, Chandraharan E, and Acharya G, “Fetal monitoring in labor: Implications of evidence generated by new systematic review,” Acta obstetricia et gynecologica Scandinavica, vol. 95, no. 1, pp. 5–8, 2016. [DOI] [PubMed] [Google Scholar]
- [82].van Wezel-Meijler G, Steggerda SJ, and Leijser LM, “Cranial ultrasonography in neonates: role and limitations,” in Seminars in perinatology, 2010, vol. 34, no. 1, pp. 28–38: Elsevier. [DOI] [PubMed] [Google Scholar]
- [83].Garel C, Brisse H, Sebag G, Elmaleh M, Oury J-F, and Hassan M, “Magnetic resonance imaging of the fetus,” Pediatric radiology, vol. 28, no. 4, pp. 201–211, 1998. [DOI] [PubMed] [Google Scholar]
- [84].Angtuaco TL, Shah HR, Mattison DR, and Quirk J Jr, “MR imaging in high-risk obstetric patients: a valuable complement to US,” Radiographics, vol. 12, no. 1, pp. 91–109, 1992. [DOI] [PubMed] [Google Scholar]
- [85].Sonigo PC, Rypens FF, Carteret M, Delezoide A-L, and Brunelle FO, “MR imaging of fetal cerebral anomalies,” Pediatric radiology, vol. 28, no. 4, pp. 212–222, 1998. [DOI] [PubMed] [Google Scholar]
- [86].Fumagalli M, Parodi A, Ramenghi L, Limperopoulos C, and Steggerda S, “Ultrasound of acquired posterior fossa abnormalities in the newborn,” Pediatric research, vol. 87, no. 1, pp. 25–36, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].de Vries LS and Groenendaal F, “Patterns of neonatal hypoxic–ischaemic brain injury,” Neuroradiology, vol. 52, no. 6, pp. 555–566, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Groenendaal F and de Vries LS, “Fifty years of brain imaging in neonatal encephalopathy following perinatal asphyxia,” Pediatric research, vol. 81, no. 1, pp. 150–155, 2017. [DOI] [PubMed] [Google Scholar]
- [89].Kudrevičienė A, Lukoševičius S, Laurynaitienė J, Marmienė V, Tamelienė R, and Basevičius A, “Ultrasonography and magnetic resonance imaging of the brain in hypoxic full-term newborns,” Medicina, vol. 49, no. 1, p. 8, 2013. [PubMed] [Google Scholar]
- [90].Yuh EL et al. , “Magnetic resonance imaging improves 3-month outcome prediction in mild traumatic brain injury,” Annals of neurology, vol. 73, no. 2, pp. 224–235, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Krishnamurthy U et al. , “MR imaging of the fetal brain at 1.5 T and 3.0 T field strengths: comparing specific absorption rate (SAR) and image quality,” Journal of perinatal medicine, vol. 43, no. 2, pp. 209–220, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Sørensen A et al. , “Changes in human fetal oxygenation during maternal hyperoxia as estimated by BOLD MRI,” Prenatal diagnosis, vol. 33, no. 2, pp. 141–145, 2013. [DOI] [PubMed] [Google Scholar]
- [93].Ge Y et al. , “Characterizing brain oxygen metabolism in patients with multiple sclerosis with T2-relaxation-under-spin-tagging MRI,” Journal of Cerebral Blood Flow & Metabolism, vol. 32, no. 3, pp. 403–412, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].van Laerhoven H, de Haan TR, Offringa M, Post B, and van der Lee JH, “Prognostic tests in term neonates with hypoxic-ischemic encephalopathy: a systematic review,” Pediatrics, vol. 131, no. 1, pp. 88–98, 2013. [DOI] [PubMed] [Google Scholar]
- [95].Neelavalli J et al. , “Magnetic resonance angiography of fetal vasculature at 3.0 T,” European radiology, vol. 26, no. 12, pp. 4570–4576, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Weisstanner C et al. , “Fetal MRI at 3T—ready for routine use?,” The British journal of radiology, vol. 90, no. 1069, p. 20160362, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Neelavalli J et al. , “MR venography of the fetal brain using susceptibility weighted imaging,” Journal of Magnetic Resonance Imaging, vol. 40, no. 4, pp. 949–957, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Tong KA, Ashwal S, Obenaus A, Nickerson J, Kido D, and Haacke E, “Susceptibility-weighted MR imaging: a review of clinical applications in children,” American Journal of Neuroradiology, vol. 29, no. 1, pp. 9–17, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Darekar A, Schreglmann M, Joy H, Gawne-Cain M, Kirkham F, and Vollmer B, “Susceptibility-Weighted Magnetic Resonance Imaging (SWI) in Newborns with Hypoxic-Ischemic Encephalopathy,” Neuropediatrics, vol. 47, no. S 01, pp. P08–07, 2016. [Google Scholar]
- [100].Kitamura G, Kido D, Wycliffe N, Jacobson JP, Oyoyo U, and Ashwal S, “Hypoxic-ischemic injury: utility of susceptibility-weighted imaging,” Pediatric neurology, vol. 45, no. 4, pp. 220–224, 2011. [DOI] [PubMed] [Google Scholar]
- [101].Yadav BK et al. , “Dual-Imaging Modality Approach to Evaluate Cerebral Hemodynamics in Growth-Restricted Fetuses: Oxygenation and Perfusion,” Fetal diagnosis and therapy, vol. 47, no. 2, pp. 145–155, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Yadav BK et al. , “Imaging putative foetal cerebral blood oxygenation using susceptibility weighted imaging (SWI),” European radiology, vol. 28, no. 5, pp. 1884–1890, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Yadav BK et al. , “Quantitative susceptibility mapping in the human fetus to measure blood oxygenation in the superior sagittal sinus,” European radiology, vol. 29, no. 4, pp. 2017–2026, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Liu C, Wei H, Gong N-J, Cronin M, Dibb R, and Decker K, “Quantitative susceptibility mapping: contrast mechanisms and clinical applications,” Tomography, vol. 1, no. 1, p. 3, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Coakley FV, Glenn OA, Qayyum A, Barkovich AJ, Goldstein R, and Filly RA, “Fetal MRI: a developing technique for the developing patient,” American Journal of Roentgenology, vol. 182, no. 1, pp. 243–252, 2004. [DOI] [PubMed] [Google Scholar]
- [106].Vergales BD et al. , “Depressed heart rate variability is associated with abnormal EEG, MRI, and death in neonates with hypoxic ischemic encephalopathy,” American journal of perinatology, vol. 31, no. 10, pp. 855–862, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Gano D et al. , “Evolution of pattern of injury and quantitative MRI on days 1 and 3 in term newborns with hypoxic–ischemic encephalopathy,” Pediatric research, vol. 74, no. 1, pp. 82–87, 2013. [DOI] [PubMed] [Google Scholar]
- [108].van Doormaal PJ, Meiners LC, Ter Horst HJ, van der Veere CN, and Sijens PE, “The prognostic value of multivoxel magnetic resonance spectroscopy determined metabolite levels in white and grey matter brain tissue for adverse outcome in term newborns following perinatal asphyxia,” European radiology, vol. 22, no. 4, pp. 772–778, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Mercuri E et al. , “Head growth in infants with hypoxic–ischemic encephalopathy: correlation with neonatal magnetic resonance imaging,” Pediatrics, vol. 106, no. 2, pp. 235–243, 2000. [DOI] [PubMed] [Google Scholar]
- [110].Thayyil S et al. , “Cerebral magnetic resonance biomarkers in neonatal encephalopathy: a meta-analysis,” Pediatrics, vol. 125, no. 2, pp. e382–e395, 2010. [DOI] [PubMed] [Google Scholar]
- [111].Rahmim A and Zaidi H, “PET versus SPECT: strengths, limitations and challenges,” Nuclear medicine communications, vol. 29, no. 3, pp. 193–207, 2008. [DOI] [PubMed] [Google Scholar]
- [112].Kapoor M and Kasi A, “PET Scanning,” 2020. [PubMed]
- [113].Chugani HT, “A critical period of brain development: studies of cerebral glucose utilization with PET,” Preventive medicine, vol. 27, no. 2, pp. 184–188, 1998. [DOI] [PubMed] [Google Scholar]
- [114].Shi Y et al. , “Changes of positron emission tomography in newborn infants at different gestational ages, and neonatal hypoxic-ischemic encephalopathy,” Pediatric neurology, vol. 46, no. 2, pp. 116–123, 2012. [DOI] [PubMed] [Google Scholar]
- [115].Thorngren-Jerneck K et al. , “Cerebral glucose metabolism measured by positron emission tomography in term newborn infants with hypoxic ischemic encephalopathy,” Pediatric research, vol. 49, no. 4, pp. 495–501, 2001. [DOI] [PubMed] [Google Scholar]
- [116].Fan AP, Jahanian H, Holdsworth SJ, and Zaharchuk G, “Comparison of cerebral blood flow measurement with [15O]-water positron emission tomography and arterial spin labeling magnetic resonance imaging: a systematic review,” Journal of Cerebral Blood Flow & Metabolism, vol. 36, no. 5, pp. 842–861, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Volpe JJ, Herscovitch P, Perlman JM, and Raichle ME, “Positron emission tomography in the newborn: extensive impairment of regional cerebral blood flow with intraventricular hemorrhage and hemorrhagic intracerebral involvement,” Pediatrics, vol. 72, no. 5, pp. 589–601, 1983. [PubMed] [Google Scholar]
- [118].Volpe JJ, Herscovitch P, Perlman JM, Kreusser KL, and Raichle ME, “Positron emmission tomography in the asphyxiated term newborn: Parasagittal impairment of cerebral blood flow,” Annals of Neurology: Official Journal of the American Neurological Association and the Child Neurology Society, vol. 17, no. 3, pp. 287–296, 1985. [DOI] [PubMed] [Google Scholar]
- [119].Khalil MM, Tremoleda JL, Bayomy TB, and Gsell W, “Molecular SPECT imaging: an overview,” International journal of molecular imaging, vol. 2011, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Akdemir ÜÖ and Kapucu LÖA, “Nuclear medicine imaging in pediatric neurology,” Molecular imaging and radionuclide therapy, vol. 25, no. 1, p. 1, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Shah S, Fernandez A, and Chirla D, “Role of brain SPECT in neonates with hypoxic ischemic encephalopathy and its correlation with neurodevelopmental outcome,” Indian pediatrics, vol. 38, no. 7, pp. 705–713, 2001. [PubMed] [Google Scholar]
- [122].Zanotti-Fregonara P, Laforest R, and Wallis JW, “Fetal radiation dose from 18F-FDG in pregnant patients imaged with PET, PET/CT, and PET/MR,” Journal of Nuclear Medicine, vol. 56, no. 8, pp. 1218–1222, 2015. [DOI] [PubMed] [Google Scholar]
- [123].Vaquero JJ and Kinahan P, “Positron emission tomography: current challenges and opportunities for technological advances in clinical and preclinical imaging systems,” Annual review of biomedical engineering, vol. 17, pp. 385–414, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Wang LV and Hu S, “Photoacoustic tomography: in vivo imaging from organelles to organs,” science, vol. 335, no. 6075, pp. 1458–1462, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Wang X, Pang Y, Ku G, Xie X, Stoica G, and Wang LV, “Noninvasive laser-induced photoacoustic tomography for structural and functional in vivo imaging of the brain,” Nature biotechnology, vol. 21, no. 7, pp. 803–806, 2003. [DOI] [PubMed] [Google Scholar]
- [126].Beard P, “Biomedical photoacoustic imaging,” Interface focus, vol. 1, no. 4, pp. 602–631, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Wang X, Xie X, Ku G, Wang LV, and Stoica G, “Noninvasive imaging of hemoglobin concentration and oxygenation in the rat brain using high-resolution photoacoustic tomography,” Journal of biomedical optics, vol. 11, no. 2, p. 024015, 2006. [DOI] [PubMed] [Google Scholar]
- [128].Mehrmohammadi M, Joon Yoon S, Yeager D, and Y Emelianov S, “Photoacoustic imaging for cancer detection and staging,” Current Molecular Imaging (Discontinued), vol. 2, no. 1, pp. 89–105, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Xu M and Wang LV, “Photoacoustic imaging in biomedicine,” Review of scientific instruments, vol. 77, no. 4, p. 041101, 2006. [Google Scholar]
- [130].Mallidi S, Luke GP, and Emelianov S, “Photoacoustic imaging in cancer detection, diagnosis, and treatment guidance,” Trends in biotechnology, vol. 29, no. 5, pp. 213–221, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Laufer J, Elwell C, Delpy D, and Beard P, “In vitro measurements of absolute blood oxygen saturation using pulsed near-infrared photoacoustic spectroscopy: accuracy and resolution,” Physics in Medicine & Biology, vol. 50, no. 18, p. 4409, 2005. [DOI] [PubMed] [Google Scholar]
- [132].Yan Y et al. , “Ultrasound, elasticity, and photoacoustic imaging of cervix: towards a more accurate prediction of preterm delivery (Conference Presentation),” in Medical Imaging 2018: Ultrasonic Imaging and Tomography, 2018, vol. 10580, p. 105800U: International Society for Optics and Photonics. [Google Scholar]
- [133].Mehrmohammadi M, Hernandez-Andrade E, Gelovani JG, Hassan SS, and Yan Y, “Ultrasound and photoacoustic systems and methods for fetal brain assessment during delivery,” ed: Google Patents, 2021. [Google Scholar]
- [134].Mehrmohammadi M, Yan Y, and Hernandez‐Andrade E, “OP01. 03: Development of an endocavity ultrasound and photoacoustic imaging device for non‐invasive assessment of blood oxygenation and perfusion in fetuses and neonates,” Ultrasound in Obstetrics & Gynecology, vol. 54, pp. 84–85, 2019. [Google Scholar]
- [135].Manwar R, Kratkiewicz K, and Avanaki K, “Investigation of the Effect of the Skull in Transcranial Photoacoustic Imaging: A Preliminary Ex Vivo Study,” Sensors, vol. 20, no. 15, p. 4189, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Liang B, Wang S, Shen F, Liu QH, Gong Y, and Yao J, “Acoustic impact of the human skull on transcranial photoacoustic imaging,” Biomedical optics express, vol. 12, no. 3, pp. 1512–1528, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Tavakolian P, Kosik I, Chamson-Reig A, Lawrence KS, and Carson JJ, “Potential for photoacoustic imaging of the neonatal brain,” in Photons Plus Ultrasound: Imaging and Sensing 2013, 2013, vol. 8581, p. 858147: International Society for Optics and Photonics. [Google Scholar]
- [138].Huang C et al. , “Aberration correction for transcranial photoacoustic tomography of primates employing adjunct image data,” Journal of biomedical optics, vol. 17, no. 6, p. 066016, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Wang X, Chamberland DL, and Xi G, “Noninvasive reflection mode photoacoustic imaging through infant skull toward imaging of neonatal brains,” Journal of neuroscience methods, vol. 168, no. 2, pp. 412–421, 2008. [DOI] [PubMed] [Google Scholar]
- [140].Di Salvo DN, “A new view of the neonatal brain: clinical utility of supplemental neurologic US imaging windows,” Radiographics, vol. 21, no. 4, pp. 943–955, 2001. [DOI] [PubMed] [Google Scholar]
- [141].SCHLETT J. (June). NIR and Optoacoustic Spectroscopy Cerebral Oximeters Aim to Save Preemies. Available: https://www.photonics.com/EDU/Handbook.aspx?AID=60904&PID=4
- [142].U. o. R. M. Center, “External and Internal Heart Rate Monitoring of the Fetus,” in Health Encyclopedia, Bowers NA, Foster S, Akin L, Bowers NA, and Foster S, Eds., ed. https://www.urmc.rochester.edu/encyclopedia/content.aspx?contenttypeid=92&contentid=P07776: Univeristy of Rochester Medical Center, 2020. [Google Scholar]
- [143].Simpson KR and Knox GE, “Risk management and electronic fetal monitoring: Decreasing risk of adverse outcomes and liability exposure,” The Journal of perinatal & neonatal nursing, vol. 14, no. 3, pp. 40–52, 2000. [DOI] [PubMed] [Google Scholar]
- [144].Ayres-de-Campos D, Spong CY, and Chandraharan E, “FIGO consensus guidelines on intrapartum fetal monitoring: Cardiotocography,” International Journal of Gynecology & Obstetrics, vol. 131, no. 1, pp. 13–24, 2015. [DOI] [PubMed] [Google Scholar]
- [145].Graham EM, Petersen SM, Christo DK, and Fox HE, “Intrapartum electronic fetal heart rate monitoring and the prevention of perinatal brain injury,” Obstetrics & Gynecology, vol. 108, no. 3, pp. 656–666, 2006. [DOI] [PubMed] [Google Scholar]
- [146].Holzmann M, Wretler S, Cnattingius S, and Nordström L, “Cardiotocography patterns and risk of intrapartum fetal acidemia,” Journal of perinatal medicine, vol. 43, no. 4, pp. 473–479, 2015. [DOI] [PubMed] [Google Scholar]
- [147].Stener Jørgensen J, “Fetal scalp blood sampling should be abandoned: AGAINST: Fetal scalp blood sampling in conjunction with electronic fetal monitoring reduces the risk of unnecessary operative delivery,” BJOG: An International Journal of Obstetrics & Gynaecology, vol. 123, no. 11, pp. 1771–1771, 2016. [DOI] [PubMed] [Google Scholar]


