Abstract
Early life stress (ELS) is a well-established risk factor for many psychiatric and medical disorders, including substance use disorders (SUDs). The relationship between ELS and SUDs is complex and there are likely multiple pathways from ELS to adverse substance use outcomes. The association between ELS and substance use emerges in adolescence. Adolescence is a critical period in development during which substance exposure markedly increases risk for SUDs. Therefore, this review focuses on the literature supporting the hypothesis that ELS increases risk for the development of SUDs through its influence on adolescent substance use. We discuss studies substantiating the role of ELS in adolescent substance use and explore how internalizing and externalizing psychopathology may be antecedents of substance use in adolescence. We examine clinical work suggesting ELS sculpts the Hypothalamic-Pituitary-Adrenal (HPA) Axis and developing brain—particularly subcortical brain regions that underlie stress response, mesocorticolimbic brain systems associated with reward sensitivity, and prefrontal regions that underlie executive control—in a way that increases risk for adolescent substance use and SUDs. We further explore how substance use during adolescence alters structure and function of these same systems, and how brain changes following ELS and adolescent substance use may independently, additively, or interactively contribute to risk for addiction. We conclude by discussing how the current literature can inform interventions aimed at reducing risk for SUDs in individuals with a history of ELS.
Keywords: Substance-related disorders, adverse childhood experiences, maltreatment, adolescence, psychological stress, neuroimaging
1. Introduction
Early life stress (ELS)—defined as childhood maltreatment (sexual, physical, and emotional abuse and physical and emotional neglect) and household dysfunction—is highly prevalent. Fifty-three percent of adults have experienced ELS prior to the age of 18 (Green, McLaughlin et al. 2010). ELS is a major public health concern and increases risk for a wide range of psychiatric and medical illnesses (Nemeroff 2016, Lippard and Nemeroff 2020), including substance use disorders (SUDs) (Kirsch, Nemeroff et al. 2020). The landmark Adverse Childhood Experiences (ACE) study (N=17,337) found number of ACEs ‘dose-dependently’ related to greater risk for alcoholism and drug abuse. ACEs were associated with 65% of population attributable risk for alcoholism, 50% for drug abuse, and 78% for intravenous drug use (Dube, Felitti et al. 2003). Anda and colleagues (2006) also demonstrated a graded relationship between ELS and risk for alcohol use disorders; individuals having experienced four or more categories of ELS possessed a 7.2-fold increase in risk for developing an alcohol use disorder (Anda, Felitti et al. 2006). This relationship has since been replicated by a large number of retrospective cross-sectional studies (Enoch 2011). Prospective, longitudinal studies have also emerged, and have begun to establish the temporality of this association (Shin, Edwards et al. 2009, Skeer, McCormick et al. 2009, Kisely, Mills et al. 2020, Kisely, Strathearn et al. 2020). Decades of preclinical work also supports an association between ELS and SUDs, thereby strengthening inferences about the causality of this relationship (Higley, Hasert et al. 1991, Kosten, Miserendino et al. 2000, Huot, Thrivikraman et al. 2001, Kosten, Sanchez et al. 2004, Kosten, Zhang et al. 2006, Vazquez, Penit-Soria et al. 2006, Moffett, Vicentic et al. 2007).
ELS impacts each stage of the addiction cycle, from compulsive drug seeking and use, to loss of control over limiting intake, and to the emergence of negative emotional states (Kirsch, Nemeroff et al. 2020). ELS has been associated with an earlier onset of addiction and a more pernicious illness course marked by increased risk of relapse and poor treatment response. Patients with a SUD and a history of ELS remain abstinent for shorter periods of time (Greenfield, Kolodziej et al. 2002), relapse more often (Heffner, Blom et al. 2011, Van Dam, Rando et al. 2014), and are less adherent to treatment (Jaycox, Ebener et al. 2004), compared to patients with a SUD who do not have a history of ELS. The association between ELS and increased risk for SUDs is observed across a broad range of substances of abuse, including alcohol, cannabis, nicotine, opioids, and cocaine (Le Moal and Koob 2007, Sinha 2008, Pilowsky, Keyes et al. 2009, Enoch 2011, Garami, Valikhani et al. 2019). Despite this significant public health concern, the exact mechanisms by which ELS is translated into risk for SUDs is not entirely understood. A wealth of literature supports the hypothesis that ELS shapes neurobiological development in a way that increases risk for addiction (Andersen and Teicher 2009). Indeed, ELS-related neurobiological differences are strikingly similar to those observed in individuals with SUDs. The pathway from ELS to SUDs, however, is complex and likely mediated and moderated by a plethora of internal and external factors.
The current review focuses on evidence supporting the hypothesis that ELS increases risk for the development of SUDs through its influence on adolescent substance use. The association between ELS and substance use emerges in early adolescence (Tonmyr, Thornton et al. 2010, Brajović, Bellis et al. 2019)—a vulnerable period during which exposure to substances of abuse is more likely to increase risk for SUDs (Orlando, Tucker et al. 2004, King and Chassin 2007). As a high proportion of individuals with ELS are exposed to substances of abuse during this vulnerable window, adolescent substance use may be a critical intermediate step in the pathway from ELS to SUDs. We discuss the literature substantiating the relationship between ELS and adolescent substance use and explore psychological and neurobiological pathways by which ELS may confer risk for adolescent substance use. We further examine how substance use during the adolescent period may uniquely increase risk for the development of SUDs. We conclude by discussing future areas of research and opportunities for intervention aimed at reducing risk for the development of SUDs following ELS through intervention during adolescence.
2. ELS and Adolescent Substance Use
2.1. Age of Substance Use Initiation
ELS is associated with a younger age of substance use and abuse initiation compared to what is observed in typical development (Andersen and Teicher 2009). Earlier age of first substance use is robustly associated with progression towards heavy substance use and increased risk for SUDs (Grant, Stinson et al. 2001, Englund, Egeland et al. 2008, Richmond-Rakerd, Slutske et al. 2017). For example, alcohol use before the age of 15 increases risk for alcohol use disorders by 40% (SAMHSA, 1999). Longitudinal research supports a causal role of early substance use initiation and subsequent development of SUDs (Irons, Iacono et al. 2015). Large scale studies have documented associations between ELS and early age of substance use initiation (Harrison, Fulkerson et al. 1997, Dube, Felitti et al. 2003, Ompad, Ikeda et al. 2005, Mills, Alati et al. 2014, Sartor, Grant et al. 2018), with these findings observed across sociocultural contexts (Ramos-Olazagasti, Bird et al. 2017). Bensley and colleagues (1999) found past physical and sexual abuse was associated with more than a 12-fold increase in the odds that regular drinking or marijuana use occurred by the age of 10 (Bensley, Spieker et al. 1999). Another study found childhood neglect was associated with early onset of smoking and alcohol use in a large (N=5158) prospective birth cohort (Mills, Alati et al. 2014). In a nationally representative sample of 3,592 young adults, Rothman and colleagues (2008) showed ACEs predict early onset drinking (initiation prior to the age of 15) (Rothman, Edwards et al. 2008). This association remained significant when controlling for parental and peer alcohol use and parental attitudes toward drinking. The study also found drinking initiation occurred after the age of 20 in young adults without a history of ACEs. There appears to be an inverse dose-response relationship between number of ACEs and age of substance use initiation. For example, Dube and colleagues (2006) found that for every ACE, the likelihood of having initiated alcohol and illicit drugs by the age of 14 increased 2- to 3-fold (Dube, Miller et al. 2006). Harsh parenting, parental substance abuse, and poor parental verbal reasoning have also been prospectively linked with early substance use initiation (Kaplow, Curran et al. 2002, Dodge, Malone et al. 2009).
2.2. Heavy Use
ELS has also been associated with heavy adolescent substance use (i.e., binge drinking). Using data from the US National Longitudinal Study of Adolescent Health (n=12,478 adolescents), Shin and colleagues found that childhood maltreatment prior to the age of 11 increased risk for adolescent (ages 12-18 years) binge drinking (Shin, Edwards et al. 2009). In a separate study of 8503 adolescents from the National Longitudinal Study of Adolescent Health, Shin and colleagues (2013) also showed childhood neglect and physical abuse was associated with faster increases in heavy episodic drinking and persistently elevated heavy episodic drinking across adolescence and young adulthood (Shin, Miller et al. 2013). Hamburger and colleagues (2008) similarly observed a link between childhood sexual abuse and heavy episodic drinking in males (Hamburger, Leeb et al. 2008). Bensely and colleagues (1999) found childhood physical and sexual abuse was associated with an 8-fold increase in risk for heavy drinking in 8th grade (Bensley, Spieker et al. 1999). Studies have also examined the impact of ELS on cigarette smoking, marijuana use, and polysubstance use. Mills and colleagues’ (2014) aforementioned prospective birth cohort study also found childhood neglect was associated with heavy smoking (and alcohol use) in adolescence (Mills, Alati et al. 2014). In a subset of youth (n=903) from the Longitudinal Studies of Child Abuse and Neglect, Yoon and colleagues (2020) found ELS predicts longitudinal trajectories of marijuana and alcohol use (Yoon, Shi et al. 2020). Specifically, emotional abuse during early childhood and physical abuse during adolescence was associated with marijuana and alcohol use trajectories with increasing use observed across ages 12, 16, and 18. Shin and colleagues (2010) found adolescent girls with a history of childhood sexual abuse were five times more likely to be heavy polysubstance users (including alcohol, cannabis, amphetamine, and hallucinogens) compared to those without a history of childhood sexual abuse (Shin, Hong et al. 2010).
2.3. Implications for SUDs
While adolescent substance use appears to increase risk for development of SUDs across the lifespan (DeWit, Adlaf et al. 2000, Englund, Egeland et al. 2008, Tonmyr, Thornton et al. 2010, Brajović, Bellis et al. 2019), it may be uniquely associated with early-onset SUDs. Early onset of SUDs is associated with a more severe illness course characterized by more severe dependence (Hingson et al., 2006), more severe and frequent withdrawal complications (Le Strat et al., 2008), and higher rates of co-occurring psychopathology, including psychotic and mood disorders (Subodh et al., 2019). ELS is associated with a faster transition from substance use to SUDs (Larance, Gisev et al. 2018) and is more prevalent in individuals with early-onset SUDs (before the age of 25), compared to individuals with late-onset SUDs (Dom, De Wilde et al. 2007). DuMont and colleagues (2007) found 56% of young adults who experienced court-documented cases of physical abuse or neglect or sexual abuse between ages 0 and 11 met criteria for alcohol or drug abuse or dependence by young adulthood (DuMont, Widom et al. 2007). In a large-scale (N=1421) longitudinal study, Skeer and colleagues (2009) showed individuals having experienced five or more adverse childhood experiences were seven- to ten-fold more likely to exhibit illicit drug use problems and addiction in late adolescence and emerging adulthood (Skeer, McCormick et al. 2009). Overall, childhood maltreatment has been associated with up to an 8.3-fold increase in risk for youth alcohol and cannabis use disorders (Kilpatrick, Acierno et al. 2000, Scott, Smith et al. 2010, Copeland, Angold et al. 2012).
The studies discussed above indicate exposure to ELS shifts initiation of substance use to younger ages and increases heavy adolescent substance use. Adolescence is a critical period of development during which drug exposure is more likely to lead to dependence (Orlando, Tucker et al. 2004, King and Chassin 2007). Taken together, this work may suggest early and heavy adolescent substance use acts as an intermediate step between ELS and SUDs. While few studies have directly tested this pathway, Skinner and colleagues’ (2012) longitudinal study followed 332 participants from childhood (18 months to 6 years old) to adulthood (31-41 years old) and found the influence of childhood sexual abuse on adult substance use problems was fully mediated by adolescent alcohol use and depression (Skinner, Hong et al. 2016). Widom and colleagues (2007) found women with documented cases of childhood physical abuse, sexual abuse, and/or neglect prior to age 11 were at greater risk for alcohol abuse or dependence in young adulthood (age 29), compared to non-maltreated women, and that alcohol abuse or dependence in young adulthood mediated the relation between childhood abuse and/or neglect with excessive drinking in middle adulthood (age 40) (Widom et al., 2007). Another prospective longitudinal study of 585 families showed a direct effect of physical abuse (before the age of 5) in females on substance use at age 12, with substance use at age 12 mediating the relationship between physical abuse and substance use at ages 16 and 24 (Lansford, Dodge et al. 2010). Additional longitudinal studies directly testing the mediating role for adolescent substance use on the link between ELS and SUDs are needed, as results could have serious implications for informing prevention and early intervention strategies aimed at delaying onset and quantity of substance use in maltreated youth.
3. Pathways from ELS to Adolescent Substance Use: Internalizing and Externalizing Psychopathology
The psychological antecedents of adolescent substance use in maltreated youth marks an area of research that has received considerable attention. Studies aiming to identify potential pathways from ELS to adolescent substance use and SUDs have focused on the mediating role of internalizing and externalizing symptoms. Indeed, ELS is a well-established risk factor for internalizing (i.e., major depression, bipolar disorder, and anxiety disorders) and externalizing (i.e., disruptive behavioral disorders and conduct problems) psychopathology, and for their comorbidity with SUDs in adolescence (Weinberg, Rahdert et al. 1998, Cicchetti and Rogosch 1999, Kessler, McLaughlin et al. 2010, Lippard and Nemeroff 2020). Rates of ELS are especially high in individuals with SUDs and comorbid internalizing/externalizing psychopathology. Wu and colleagues (2010) found that 95% of individuals with comorbid SUDs and mental health problems had experienced a traumatic event during childhood (Wu, Schairer et al. 2010). While findings are mixed—and there is more than one pathway to this comorbidity—prospective data has suggested psychopathology can precede substance use and SUDs in adolescents (Costello 2007, Wittchen, Fröhlich et al. 2007). The subsequent sections will discuss literature examining internalizing and externalizing pathways to adolescent substance use and SUDs following ELS. It is important to keep in mind that these pathways are complex in that they are not mutually exclusive and may even bidirectionally interact to contribute to substance use outcomes.
3.1. Internalizing Psychopathology
Studies have provided empirical support for an internalizing pathway from ELS to adolescent substance use. For example, Shin and colleagues (2020) found depression symptoms mediated the association between childhood maltreatment and problem drinking in young adulthood (Shin, Ksinan Jiskrova et al. 2020). Another study showed childhood maltreatment prospectively predicted cigarette use at age 16 via internalizing symptoms at age 14 (Lewis, Kotch et al. 2011). ELS is also associated with high rates of post-traumatic stress disorder (PTSD) symptoms in adolescence (De Bellis and Zisk 2014), and PTSD symptoms have been consistently found to mediate the relationship between childhood maltreatment and problematic adolescent/young adult substance use (Klanecky, McChargue et al. 2016). The antecedents of adolescent substance use in individuals experiencing internalizing, including PTSD, symptoms following ELS is an active area of investigation; a greater understanding of these precursors is essential for informing interventions.
Substance use coping motives mark one commonly observed antecedent of adolescent substance use in individuals with internalizing symptoms and history of ELS. Large-scale studies have found individuals with a history of ELS are more likely to use substances to cope with problems than individuals without a history of ELS (Harrison, Fulkerson et al. 1997, Grayson and Nolen-Hoeksema 2005, Rothman, Edwards et al. 2008). Coping motives are associated with problematic substance use in adolescence/young adulthood, and with risk for development of SUDs (Merrill et al., 2014). A recent study by Shin and colleagues (2020) found childhood maltreatment was indirectly associated with alcohol-related problems in young adults (n=208) via coping motives (Shin, Jiskrova et al. 2020). Another study of 314 young adults found childhood emotional abuse was associated with higher levels of negative emotionality, which in turn predicted coping drinking motives and problematic drinking (Mezquita, Ibáñez et al. 2014). PTSD symptoms following ELS are also associated with substance use coping motives. For example, Park and colleagues (2019) found childhood maltreatment was associated with posttraumatic stress symptoms, post-traumatic stress symptoms predicted higher coping motives, and coping motives predicted higher levels of alcohol misuse in adolescence (n=564) (Park, Thompson et al. 2019). Similarly, Hannan and colleagues (2015) found childhood sexual abuse was associated with PTSD, PTSD predicted drinking to regulate emotional experiences, which was then associated with alcohol-related problems in females during college (n=579) (Hannan, Orcutt et al. 2015). As substance use coping motives is a well-established risk factor for problematic substance use, interventions aimed at promoting more effective methods for managing negative affect may be especially beneficial in reducing problematic alcohol use in individuals with a history of ELS.
Mechanisms extending beyond coping motives may also contribute to co-occurring internalizing psychopathology and substance use following ELS. Stress sensitization, for example, is well documented in mood disorders (Dienes et al., 2006) and may be another mechanism by which ELS increases risk for substance use in adolescents exhibiting internalizing symptoms. Young-Wolff and colleagues (2012) observed a relationship between childhood maltreatment and greater stress-related drinking even after controlling for mood-related symptoms (Young-Wolff, Kendler et al. 2012). Additionally, stress and reward systems have been found to interact (Koob 2008), and differences in stress sensitivity may alter the rewarding properties of drugs of abuse. Indeed, studies have found individuals with mood and anxiety disorders report drinking to enhance euphoric mood (Norberg et al., 2010, McDonald and Meyer, 2011). There also appears to be shared risk factors (i.e., genetic variation) for mood and SUDs. The internalizing pathway from ELS to SUDs is undoubtedly complex, and recent work has shown it is moderated by various internal and external factors, not limited to, but including genetics, biological sex, parental and peer context, intergenerational effects, and race and ethnicity (Box 1). More work is needed to better delineate the mechanisms by which internalizing symptoms may increase risk for adolescent substance use following ELS, as results could have important implications for prevention and early intervention strategies.
Box 1. Pathways from ELS to SUDs are complex and appear to be moderated by a multitude of factors. Not all individuals with a history of ELS develop SUDs; a greater understanding of how these factors moderate the relationship between ELS and SUDs could help us understand who is at greatest risk for adverse outcomes.
Genetic Variation:
It has become clear that genes interact with environmental stress to confer risk or resiliency to psychiatric outcomes (Moffitt 2005). Genetic studies have largely focused on how specific candidate genes interact with ELS to increase risk for SUDs. This literature suggests possessing certain genetic variants confers risk for psychopathology only in individuals who have experienced ELS. Conversely, lacking certain genetic variants may confer resilience to the effects of ELS. Variation within the corticotropin-releasing hormone receptor 1 (CRHR1) gene, for example, has been shown to interact with childhood and adolescent stressful events to predict age of drinking onset, progression to heavy alcohol consumption, and lifetime alcohol use (Blomeyer, Treutlein et al. 2008, Nelson, Agrawal et al. 2010, Schmid, Blomeyer et al. 2010). Variation in Solute Carrier Family 6 Member 4 (SLC6A4), the serotonin transporter promoter gene, is also reported to interact with ELS to increase risk for adverse alcohol and drugs use behaviors. Specifically, the short allele interacts with childhood maltreatment to predict early initiation of alcohol use (Kaufman, Yang et al. 2007) and use of illicit drugs (Gerra, Zaimovic et al. 2010). Additional work investigating the moderating role of genetic factors is critically needed to determine how genetic variation may put certain individuals at greater risk for adverse outcomes following ELS. To gain a more comprehensive understanding of these complex gene by environment interactions, studies should extend beyond investigating genetic polymorphisms of candidate genes and also focus on epigenetic changes and polygenetic risk.
Biological Sex:
Sex differences in pathways to adolescent substance use following ELS have been commonly observed (Hyman, Paliwal et al. 2008). Hudson and colleagues (2017) found childhood sexual abuse and adolescent problem drinking was mediated by anxiety and anger symptoms in females but only by anger symptoms in males (Hudson, Wekerle et al. 2017). Another study found coping-depression motives mediated the relationship between childhood abuse and alcohol consequences in women, but enhancement motives mediated the relationship in men (Goldstein, Flett et al. 2010). One possible explanation is that internalizing symptoms are more prevalent in females while externalizing symptoms, like anger and sensation seeking, are more prevalent in males. Indeed, sex-differences in the development of the corticolimbic circuitry that underlies emotional regulation has been observed in adolescents with a history of ELS (Colich et al., 2017). However, studies have also supported an externalizing pathway from ELS to adolescent substance use specifically in females (Bailey and McCloskey 2005). Understanding sex differences in these pathways can help inform more targeted intervention in youth with a history of ELS.
Parental and Peer Context:
Parental and peer context influences adolescent substance use outcomes following ELS. Adolescence is characterized by increased time spent with peers and decreased time spent with parents (Spear 2000). This is also a time of greater autonomy and exposure to illicit substances. Adolescent peer relationships may result in exposure and high pressure to engage in illicit substance use, potentially through social motives or pressures (Tarter 2002, Brown, McGue et al. 2008). Indeed, affiliation with substance using peers is a predictor of adolescent substance use (Chuang, Ennett et al. 2005, Dubowitz et al., 2016). Despite this decrease in time spent with family, parents and parenting styles do appear to critically influence adolescent substance use behavior (Wood, Read et al. 2004). For example, maladaptive parenting styles may interact with childhood temperament and behavior (externalizing symptoms) to enhance risk for substance use (King, Iacono et al. 2004, Costello 2007, Zucker, Heitzeg et al. 2011, Chassin, Sher et al. 2013). A recent study observed a sequential association between childhood maltreatment, low levels of parental attachment, higher levels of involvement with pro-marijuana peers, and ultimately adolescent and adult marijuana use (Alex Mason et al., 2017). As discussed in Summary and Future Directions, supportive relationships with family and peers can act as a strong protective factor, as such relationships can teach healthy stress coping strategies and foster healthy stress responses.
Intergenerational Effects of ELS:
The adverse effects of ELS are multigenerational; they persist beyond an individual’s lifespan and are transmitted to future generations (Cowan et al., 2016). Human studies have observed a greater risk of psychiatric illness in offspring of parents previously exposed to trauma. For example, grown children of Holocaust survivors—who were born after the war, or after their parents escaped to safety—show higher rates depression, anxiety disorders, and PTSD compared to Jewish adults without a Holocaust surviving parent (Yehuda et al., 2005). Preclinical studies provide experimental support for the hypothesis that behavioral and neurobiological consequences of ELS exposure are transmitted across generations (Fairbanks, 1996, Dias and Ressler, 2014). Parental behavior and epigenetic programming mark two potential mechanisms thought to underlie the intergenerational transmission of ELS (Cowan et al., 2016). As discussed, parenting style can have a profound influence on adolescent substance use as well as internalizing/externalizing behaviors. Maladaptive parenting, including emotional abuse and neglect (i.e., emotional disengagement), physical punishment and violence, and poor parental adjustment have been observed in parents with histories of ELS (Cowan et al., 2016). Such maladaptive parenting styles are a form of ELS and may increase risk for adverse substance use outcomes in their offspring. Epigenetic programming is one mechanism that may contribute to maladaptive parenting through experience-driven changes (Kundakovic and Champagne, 2015). For example, epigenetic changes in the neural circuitry thought to regulate parenting behavior (e.g., hypermethylation of the been associated with abusive and inattentive caregiving. Emerging evidence also suggests epigenetic changes can be passed on to offspring via germline inheritance. While more research on the exact mechanisms of transmission is needed, future work should also aim to identify targets underlying intergenerational transmission to interrupt this cycle of transmission to future generations.
Race and Ethnicity:
Recently, studies have begun to investigate the role of ELS across different racial and ethnic groups. This work has suggested the vulnerability to the adverse effects of ELS may vary across race and ethnicity (Martin-Gutierrez et al., 2021). Studies have also identified stressors that may be unique to, or more prevalent among, specific racial or ethnic minorities. Racial discrimination, for example, is a stressor that begins early in life and is linked to worse health outcomes (e.g., depression, anxiety, and psychological distress) (Lewis et al., 2015, Williams, 2018). A longitudinal study of black adolescents (ages 10 – 12, N=714) in rural Georgia showed racial discrimination was associated with conduct problems and depressive symptoms (Brody et al., 2006). It is also suggested that structural racism can contribute to a stress proliferation process (Pearlin et al., 2005) where an initial stressor can initiate or exacerbate additional stressors. More research is critically needed to understand risk factors that may be unique to a specific race or ethnicity to develop tailored intervention strategies.
3.2. Externalizing Psychopathology
There is also compelling evidence in support of an externalizing pathway from ELS to adolescent substance use and SUDs. Externalizing symptoms are typically characterized by high levels of behavioral disinhibition (Gorenstein and Newman 1980). Externalizing behaviors have been found to mediate the relationship between adolescent/young adult alcohol and cannabis use problems. In a 10-year prospective study, Handley and colleagues (2017) showed childhood conduct problems mediated the effect of childhood maltreatment on problematic alcohol use in emerging adulthood (Handley, Rogosch et al. 2017). Shin and colleagues (2019) found behavioral dysregulation mediated the association between childhood emotional abuse and problematic alcohol use in young adulthood (Shin, Jiskrova et al. 2019). Another study demonstrated a developmental pathway by which childhood maltreatment related to less adaptive childhood personality functioning, followed by externalizing problems in preadolescence, and the development of cannabis abuse and dependence symptoms in adolescence (Oshri, Rogosch et al. 2011). A prospective study following 1421 adolescents, from ages 10 to 22, found impulsivity-related externalizing problems (i.e., aggression, delinquency) partially mediated risk for SUDs following early life familial conflict (Skeer, McCormick et al. 2009). Externalizing psychopathology has also been linked to an earlier age of substance use initiation. For example, in a sample of 1,105 youth enrolled in the Longitudinal Studies of Child Abuse and Neglect, Proctor and colleagues (2017) found externalizing behavior problems in middle childhood (age 8) mediated the relationship between childhood neglect and/or sexual abuse (prior to age 6) and younger age of alcohol and marijuana initiation (Proctor, Lewis et al. 2017). Interestingly, Doan and colleagues’ (2019) recent longitudinal study found externalizing behaviors (i.e., aggression) buffered the detrimental effects of ELS on psychological dysregulation/allostatic load (measured through cortisol levels, urinary epinephrine and norepinephrine, resting systolic and diastolic blood pressure, and body mass index) at ages 9, 13, and 17 (Doan et al., 2019). The authors reasoned such externalizing behaviors that are typically considered maladaptive may actually, in some circumstances, be adaptive in that these behaviors can be an effective form of stress regulation. Another interpretation is that externalizing behaviors may be an adaptive response to ELS in the short term, but ultimately confer risk for adverse substance use and clinical outcomes later in life. One limitation is that the study did not examine substance use, which could directly impact these measures of allostatic load. This counterintuitive finding highlights the need for longitudinal studies with longer follow-up periods to clarify mechanisms of risk versus resiliency.
3.3. Interactions between Internalizing and Externalizing Symptoms: Impulsivity
Internalizing and externalizing pathways to adolescent substance use and SUDs are not mutually exclusive. Indeed, co-occurring internalizing and externalizing symptoms are commonly observed in adolescents, and symptoms may even bidirectionally interact to increase risk for substance use and ultimately SUDs. Impulsivity is a symptom characteristic of externalizing psychopathology and has been proposed as a common transdiagnostic marker observed across internalizing disorders (i.e., bipolar disorder, major depression, anxiety disorders) (Strakowski et al., 2010, Fields et al., 2021). ELS is associated with elevated levels of impulsivity (Elam, Wang et al. 2016), and impulsivity is a major risk factor for the initiation of substance use, heavy episodes of substance use, and addiction (Dawes 1997). The role of impulsivity may be especially salient in adolescence, as studies have found impulsivity traits peak at this developmental stage (Argyriou et al., 2018). Consequently, the role of impulsivity in the relationship between ELS and adolescent substance use and SUDs has received considerable attention (Shin, Lee et al. 2015, Proctor, Lewis et al. 2017, Kim, Hwang et al. 2018).
Negative urgency, or impaired self-control in the face of negative emotionality, is one facet of impulsivity that has consistently been related to adolescent substance use in individuals with a history of ELS (Stautz and Cooper 2014, Shin, McDonald et al. 2018). In a community sample of young adults, Shin and colleagues (2015) found negative urgency mediated the relationship between childhood emotional abuse and frequency of alcohol use, binge drinking, and alcohol use disorders in young adults (Shin, Lee et al. 2015). Similarly, Wardell and colleagues (2016) found the relationship between childhood maltreatment and alcohol and cannabis use problems in adolescence was mediated by negative urgency (Wardell, Strang et al. 2016). Additional facets of impulsivity, including positive urgency (impulsivity in the face of positive emotions) and sensation seeking also appear to mediate the relationship between childhood maltreatment and alcohol and cannabis use (Kim, Hwang et al. 2018, Oshri, Kogan et al. 2018). Co-occurring internalizing symptoms may exacerbate impulsive tendencies and consequently increase substance use. For example, Oshri and colleagues (2018) found the association between childhood maltreatment and alcohol use problems via impulsive decision making was modulated by acute stress response reactivity, measured by heart rate variability (Oshri, Liu et al. 2018). Another study found negative urgency mediated the relationship between depression and alcohol use problems in 143 college students (Gonzalez et al. 2011). Recently, studies have tested psychological interventions/techniques aimed at reducing impulsivity in healthy and substance using and dependent individuals (Martínez-Loredo and Fernández Hermida, 2019). While more work is needed, these techniques have garnered promising experimental support and may be especially relevant in minimizing substance use outcomes in adolescents with a history of ELS.
4. Hypothalamic-Pituitary-Adrenal (HPA) Axis Changes Associated with ELS and Substance Use
4.1. HPA Axis
ELS is associated with altered HPA axis basal functioning and stress reactivity (Heim and Nemeroff 2001, Lupien, McEwen et al. 2009). The prevailing view is that individuals with ELS show HPA axis hyperactivity in childhood (Danese and McEwen 2012), and consequent HPA axis hypoactivity in adolescence that persists throughout adulthood (Miller, Chen et al. 2007, Trickett, Noll et al. 2010, Doom, Cicchetti et al. 2014, Kaess, Whittle et al. 2018). Stress and stress reactivity play critical roles in the initiation and maintenance of substance use. HPA axis dysregulation is observed in individuals with SUDs, and studies have found cortisol levels influence drug self-administration, drug reinforcement (i.e., subjective “high”), withdrawal symptoms, and stress-induced craving and relapse (Sinha 2001, Cleck and Blendy 2008). Lower cortisol release has also been linked to enhanced motivation for substance use (Sinha 2001). Blaine and colleagues (2019) found lower cortisol response to stress and drug cues predicted greater alcohol consumption during an alcohol taste test in moderate and heavy social adult drinkers (Blaine, Nautiyal et al. 2019). Moss and colleagues (1999) observed a blunted cortisol response to stress in prepubertal sons of fathers with a SUD, with lower anticipatory cortisol response associated with cigarette smoking and marijuana use in adolescence (Moss, Vanyukov et al. 1999). Suppressed cortisol secretion in the face of stress is also associated with enhanced drug motivation and self-administration in individuals with and without a SUD (Blaine and Sinha 2017).
It is important to note findings with regards to HPA axis activity following ELS are mixed, with a number of studies documenting HPA axis hyperactivity in adolescents and adults with a history of ELS (Nemeroff 2016). Many of these studies, however, have focused on individuals with psychopathology, including major depression and borderline personality disorder, and a history of ELS (Heim et al., 2000, Heim et al., 2001, Heim et al., 2002, Carpenter et al., 2004, Fernando et al., 2012). Such psychopathology is associated with HPA axis dysregulation (particularly hyperactivity) (Varghese and Brown, 2001), and may confound findings. Interestingly, HPA axis hyperactivity has also been linked to heavier substance use (Piazza and Le Moal, 1996, Fahlke et al., 2000, de Wit et al., 2007, Huizink et al., 2009). This suggests multiple pathways to substance use following ELS, with HPA axis hyperactivity potentially driving substance use in individuals with psychopathology. Indeed, Andersen and Teicher (2008, 2009) theorized the link between ELS and SUDs may be mediated in part by a highly reactive HPA axis and using substances to cope with negative affect (Andersen and Teicher 2008, Andersen and Teicher 2009). While findings are mixed, this literature does converge to suggest HPA axis dysregulation—both hypo- and hyperactivity—serves as a biomarker of risk for substance use and development of SUDs in individuals with ELS. Substance use during adolescence and clinical factors (i.e., internalizing and externalizing symptoms) associated with ELS may contribute to dysregulation of HPA axis function (Alink, Cicchetti et al. 2012) and possibly discrepant findings.
Heavy substance use, particularly during adolescence, directly alters HPA axis functioning (Blaine and Sinha 2017). Alcohol, for example, stimulates the HPA axis, and heavy, chronic alcohol use is associated with changes in HPA negative feedback that typically result in a blunted response to acute stress (Lovallo 2006, Becker 2017). Despite the high rates of ELS reported in individuals with SUDs, few studies have directly examined the influence of ELS history. A recent study attempted to address this by investigating HPA axis functioning in alcohol dependent and healthy control adults with and without a history of childhood maltreatment (Muehlhan, Höcker et al. 2020). While alcohol dependent adults showed lower cortisol response to a Trier-Social-Stress-Test compared to healthy controls, childhood maltreatment was not associated with cortisol differences within the alcohol dependent group. Interestingly, healthy controls with a history of childhood maltreatment exhibited a lower cortisol response to stress, compared to controls without childhood maltreatment. Results could suggest alcohol dependence influences HPA axis functioning in a way that may mask differences in the HPA axis due to childhood maltreatment. It is also possible lower cortisol response to stress in healthy controls with a history of childhood maltreatment acts as a resiliency factor. Another study reported adrenocorticotropic hormone (ACTH) levels negatively correlated with childhood trauma questionnaire (CTQ) scores in alcohol dependent patients during acute withdrawal (day 14 after admission to a detoxification unit) (Schäfer et al., 2010), suggesting ELS and associated changes in HPA axis function may relate to differences in withdrawal and ultimately risk of relapse. ELS has been associated greater rates of relapse and poor treatment response (Heffner, Blom et al. 2011, Van Dam, Rando et al. 2014), and results could suggest differences in HPA axis activity following detoxification may be one mechanism contributing to this. Additional work investigating HPA axis functioning prior to the development of SUDs and across different phases of addiction is needed to delineate the role(s) of the HPA axis in risk for the development of SUDs following ELS, and interactions between adolescent substance use and ELS on HPA axis function and development and maintenance of SUDs.
5. Neurobiological Changes Associated with ELS and Substance Use
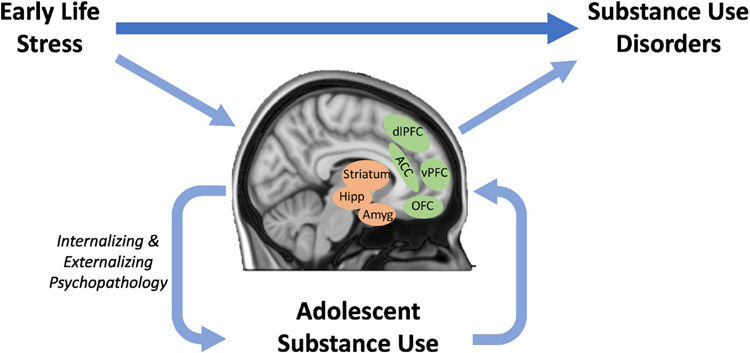
ELS is associated with brain changes that may heighten risk for the development of SUDs. These brain differences are strikingly similar to differences in the brain observed in individuals with SUDs. Specifically, ELS has been found to interfere with typical development of subcortical brain regions—including the amygdala and hippocampus—involved in the stress response, mesocorticolimbic brain system associated with reward processing and sensitivity (Teicher, Samson et al. 2016), and prefrontal cortical (PFC) regions that underlie executive control (Lupien, McEwen et al. 2009). These brain systems develop and mature throughout childhood and adolescence and are highly sensitive to stress; even brief periods of stress exposure can result in significant structural remodeling (Arnsten 2009, Hart and Rubia 2012). Preclinical work supports a causal relationship between ELS and neural alterations and has uncovered potential stress-induced remodeling processes (i.e., reduced dendritic length and branching, decreased spine density, and suppressed neurogenesis) (Arnsten 2009, Holmes and Wellman 2009, Lupien, McEwen et al. 2009). These neurobiological systems play critical roles in addiction-related behaviors, with differences in their structure and function enhancing risk for SUDs. In their stress-incubation/corticolimbic developmental cascade hypothesis, Andersen and Teicher (2009) proposed neurobiological changes associated with ELS predispose individuals to use drugs at an earlier age compared to the age of onset observed in populations without a history of ELS (Andersen and Teicher 2009). The brain continues to develop and mature during adolescence, rendering the adolescent brain highly susceptible to substance-related neurodegeneration (White and Scott Swartzwelder 2005, Crews and Nixon 2009, Jacobus and Tapert 2013). Thus, adolescent substance use may continue to shape these neurobiological systems in a way that further increases risk for SUDs (figure 1).
Figure 1.
Pathway from early life stress (ELS) to substance use disorders (SUDs) via adolescent substance use. ELS is associated with a large proportion of the population attributable risk for SUDs. While pathways to SUDs following ELS are complex and likely multifaceted, it is possible that ELS sculpts the developing brain in a way that increases risk for adolescent substance use, and substance use during this time results in further brain changes that either independently, additively, or interactively contribute to risk for SUDs. Internalizing and externalizing psychopathology may contribute to adolescent alcohol use following ELS. Both ELS and adolescent drug exposure have been related to differences in the structure and function of prefrontal and limbic-striatal regions, regions that are critically involved in the stress response, reward processing, and executive functions. dlPFC: dorsolateral prefrontal cortex; vPFC: ventral prefrontal cortex; OFC: orbitofrontal cortex; ACC: anterior cingulate cortex; Hipp: hippocampus; Amyg: Amygdala.
5.1. Stress Response: Amygdala and Hippocampus
ELS is associated with enduring alterations in brain regions that regulate the HPA axis and stress response. The amygdala and hippocampus mark two critical structures that bidirectionally interact with the HPA axis and are highly sensitive to ELS. Studies have consistently observed an effect of ELS on amygdala structure, though both increases and decreases in amygdala volume following ELS have been reported (Teicher, Samson et al. 2016). Oshri and colleagues (2019) attempted to address this discrepancy using high-resolution segmentation algorithms to assess volume of specific amygdala nuclei in emerging adults (Oshri, Gray et al. 2019). The group found higher ACE scores were associated with reduced right amygdala volume, and specifically basolateral and central-medial segments. While findings suggest ELS is associated with lower right amygdala volume, it is likely the influence of ELS on brain structure is complicated by additional factors like time and type of stress exposure. Teicher and colleagues proposed early exposure may result in amygdala enlargement, while later exposure may result in volume reduction and hypothesized this may stem from modifications to the developmental processes taking place at the time of exposure (i.e., overproduction of dendrites, axonal terminals, synapses, and receptors in early to middle childhood versus pruning of these processes during adolescence and early adulthood) (Teicher and Samson 2016, Teicher, Samson et al. 2016, Teicher and Khan 2019). Findings with respect to ELS and hippocampal morphometry are more consistent, with reduced hippocampal volume (Bremner et al., 1997, Vythilingam et al., 2002) and decreased adult hippocampal neurogenesis (Ming and Song, 2005) consistently observed in individuals with a history of ELS.
Studies have also shown amygdala and hippocampus hyperactivity in individuals with a history of ELS (Teicher, Samson et al. 2016, VanTieghem and Tottenham 2018), with recent work suggesting such hyperactivity may mediate the relationship between ELS and HPA axis dysfunction. Seo and colleagues (2019) found increased amygdala and hippocampal reactivity to acute stress mediated the relationship between lifetime trauma exposure and lower morning cortisol levels in healthy adults (Seo, Rabinowitz et al. 2019). Another study by Seo and colleagues (2014) found ELS was associated with stress induced hyperactivity of the amygdala and hippocampus, as well as of the insula and striatum, but hypoactivity of the orbitofrontal cortex (OFC) (Seo, Tsou et al. 2014). Lower PFC, including OFC, volume and activity is consistently observed in individuals with a history of ELS (Teicher, Samson et al. 2016, Kirsch, Tretyak et al. 2021). The PFC, particularly the OFC, shares reciprocal connections with the amygdala and hippocampus, and plays an integral role in regulating these regions’ response to stress (VanTieghem and Tottenham 2018). The PFC is highly sensitive to the effects of HPA dysregulation; the region has a high density of stress-susceptible glucocorticoid receptors, and both elevated and suppressed levels of glucocorticoids have been linked to disruptions in PFC development through mechanisms including apoptosis, myelination delays, inappropriate pruning, and decreases in brain growth factors (McEwen 2007, De Bellis and Zisk 2014). Lower PFC volume and activity could result in compromised top-down control of these subcortical regions, and ultimately a sensitized stress response. Indeed, a number of studies have observed differences amygdala-PFC and hippocampus-PFC functional connectivity in individuals with a history of ELS (Herringa, Birn et al. 2013, Marusak, Martin et al. 2015, Thomason, Marusak et al. 2015, VanTieghem and Tottenham 2018). For example, Peverill and colleagues (2019) found adolescents with a history of child abuse exhibited stronger negative amygdala - ventromedial PFC functional connectivity during an emotional regulation task compared to adolescents without this history (Peverill, Sheridan et al. 2019).
Amygdala, hippocampus, and PFC structural and functional differences in individuals with a history of ELS have been associated with adolescent/young adult substance use (Kirsch, Nemeroff et al. 2020). The aforementioned study by Oshiri and colleagues (2019) found reduced basolateral amygdala volume partially mediated the positive relationship between ACE scores and greater alcohol use, anxiety and depressive symptoms in young adults (Oshri, Gray et al. 2019). The insula, another region found to be highly sensitive to the effects of early life stress (Edmiston et al., 2011, Hart and Rubia, 2012, Lim et al., 2018), has also been associated with adolescent/young adult substance use. Kirsch and colleagues (2021) found childhood maltreatment correlated with lower insula gray matter volume, which was associated with greater frequency of cannabis use (Kirsch, Tretyak et al. 2021). Lower insula gray matter volume also related to greater quantity of alcohol use only in adolescents/young adults with familial risk for bipolar disorder, suggesting familial— possibly genetic—risk factors may influence substance use outcomes following ELS. Studies also indicate functional changes observed within these regions following ELS increase risk for substance use problems in adolescence/young adulthood. Sinha and colleagues (2016) found sustained ventromedial PFC hypoactivity to stress predicted higher levels of maladaptive coping behaviors, including binge alcohol intake, in young adults (Sinha, Lacadie et al. 2016). Peverill and colleagues (2019) found stronger negative amygdala - ventromedial PFC functional connectivity during emotional regulation in individuals with a history of ELS was associated with higher levels of concurrent internalizing and externalizing symptoms and with higher levels of externalizing symptoms two years later (Peverill, Sheridan et al. 2019). The authors conclude ELS may result in brain adaptations that allow for rapid identification of environmental threats, but ultimately result in greater emotional reactivity and difficulty modulating emotions. Acute alcohol administration attenuates amygdala and insula reactivity to emotional stimuli and weakens amygdala – ventral PFC functional connectivity during emotional processing and insula – ventral PFC functional connectivity during rest (Gilman, Ramchandani et al. 2008, Sripada, Angstadt et al. 2011, Gorka, Fitzgerald et al. 2013, Gorka, Phan et al. 2018). Thus, acute alcohol appears to alter activity within brain regions that show altered function following ELS. ELS-associated changes in function may interact with the effects of acute alcohol to contribute to motives for alcohol use. Several imaging studies have proposed functional connectivity changes following ELS may actually be adaptive and promote resiliency (Gee, Gabard-Durnam et al. 2013, Herringa, Burghy et al. 2016). For example, Kaiser and colleagues (2018) found women previously exposed to ELS showed increased variability in amygdala - subgenual anterior cingulate cortical (ACC) functional connectivity, with greater variability associated with improved mood and less blunting of the stress response (Kaiser, Clegg et al. 2018). Longitudinal studies are needed to disentangle mechanisms of risk versus resiliency. Taken together, work suggests ELS-related brain differences may predate substance use, and differences associated with greater substance use may occur through ELS-related alterations brain regions that subserve emotional and stress regulation. The role(s) of adolescent substance use on neurophysiological differences following ELS warrants more attention.
Adolescent substance use also has neurotoxic effects on the amygdala, hippocampus, and PFC. These brain regions may be uniquely vulnerable to substances during this time because the PFC, and connections between the PFC and the amygdala/hippocampus, continue to mature throughout adolescence and young adulthood (Silveri, 2012). Neuroimaging studies have consistently found adolescents who meet criteria for alcohol use disorders and who engage in sub-diagnostic heavy drinking show lower PFC and hippocampal volume and differences in functional activation of these regions, compared to non-using adolescents (De Bellis, Clark et al. 2000, Nagel, Schweinsburg et al. 2005, Medina, McQueeny et al. 2008, Jacobus and Tapert 2013). Additionally, a wealth of preclinical work suggests excessive alcohol consumption during adolescence compromises cortical and glial cell survival in the amygdala, hippocampus, and PFC, along with alterations in white matter tracts connecting these regions (White and Scott Swartzwelder 2005, Crews and Nixon 2009). For example, Taffe and colleagues (2010) found daily alcohol consumption reduced hippocampal neurogenesis and increased hippocampal neural degeneration in adolescent primates (Taffe, Kotzebue et al. 2010). These brain changes may further perpetuate a negative cycle, ultimately resulting in addiction (Jacobus, Squeglia et al. 2013). Adolescent psychopathology may also perpetuate this negative cycle. Whittle and colleagues (2013) found the presence of Axis I psychopathology during adolescence marks one mechanism by which ELS has continuing effects on amygdala and hippocampal brain development (Whittle, Dennison et al. 2013). Overall, data indicate both ELS and adolescent substance use influence development of the neurobiological systems underlying the stress response—in strikingly similar ways—and likely interact to contribute to addiction-related outcomes. Large scale longitudinal studies beginning prior to substance use are needed to disentangle mechanisms that contribute to risk for initiation of, and transition to, SUDs. The Adolescent Brain Cognitive Development (ABCD) study, for example, is an ongoing landmark study that is collecting neuroimaging data on 10,000 healthy children beginning at the age of 9-10 and following the cohort into early adulthood in order to track brain growth and investigate how environmental factors—including stressors (e.g., abuse, neglect, household challenges, parental substance use) and substance use—influence brain development and behavioral outcomes. The study collects stress-related measures that capture the domains included in the landmark ACE study (Dube et al., 2003, Hoffman et al., 2019) and investigates these experiences at the individual, family, peer, and community levels in youth participants as well as in their parents/guardians at each study visit. The ABCD study is uniquely positioned to investigate how stress and substance use prospectively relates to adolescent development.
Despite the high rates of ELS in SUDs, few studies have investigated the neural correlates of ELS in individuals with SUDs. Van Dam and colleagues (2014) found hippocampal gray matter volume deficits in individuals with SUDs were uniquely associated with childhood maltreatment (Van Dam et al., 2014). Bachi and colleagues (2018) observed decreased OFC volume in individuals with cocaine use disorders and a history of ELS compared to diagnostic controls with no ELS history (Bachi, Parvaz et al. 2018). Results suggest the possibility that hippocampal and PFC neural differences that distinguish clinically healthy controls from individuals with SUDs are more robust in, or possibly even restricted to, individuals with a history of ELS (Opel et al., 2014, Teicher et al., 2016). More work focused on understanding if and how the neural consequences of ELS confound and/or interact with those observed following heavy substance use is needed.
5.2. Reward Processing and Sensitivity: Mesocorticolimbic Brain Systems
ELS has been associated with altered reward sensitivity and differences in the mesocorticolimbic dopaminergic pathway, the brains’ reward system (Guyer, Kaufman et al. 2006). Altered behavioral and neural sensitivity to rewards is a well-established risk factor for the development of SUDs. While findings are mixed, studies suggest reduced reward sensitivity in individuals with a history of ELS. For example, Dillon and colleagues found childhood maltreatment was associated with a decreased positive subjective response to reward-predicting cues, as well as lower globus pallidus activation during a monetary incentive delay task (Dillon, Holmes et al. 2009). Additional fMRI studies have also supported blunted basal ganglia activity to anticipated rewards following ELS (Hanson, Albert et al. 2016, Teicher, Samson et al. 2016). Using a monetary incentive delay task, Boecker and colleagues (2014) observed an inverse relationship between ELS and ventral striatum and putamen activation during reward anticipation in young adults (Boecker, Holz et al. 2014). Mehta and colleagues similarly found blunted striatal responses during reward anticipation in adolescents who were adopted from Romanian institutions (Mehta, Gore-Langton et al. 2010). Imaging studies have also identified ELS-related structural differences in mesocorticolimbic regions involved in reward processing (Edmiston, Wang et al. 2011). Specifically, structural alterations in the OFC and ACC (Kelly, Viding et al. 2013, Kirsch, Tretyak et al. 2021), striatum (Cohen, Grieve et al. 2006, Edmiston, Wang et al. 2011), and in the white matter of fronto-striatal pathways (Behen, Muzik et al. 2009) have been reported in individuals with a history of ELS. Preclinical studies provide support for a causal role of ELS on reward hyposensitivity (Pryce, Dettling et al. 2004).
ELS is also associated with altered sensitivity to the subjective effects of substances, i.e., levels of drug-induced euphoria (Wand, Oswald et al. 2007). Studies have suggested a positive association between ELS and mesocorticolimbic dopamine release in response to acute drug administration. For example, Oswald and colleagues (2014) found amphetamine self-administration induced a stronger dopamine response in the ventral striatum of individuals with a history of childhood adversity compared to those without a history of adversity (Oswald, Wand et al. 2014). Stronger dopamine response to substance administration may enhance the reinforcing properties of drugs of abuse and increase risk for substance use (Piazza and Le Moal 1996). While the etiology underlying differences in sensitivity to substances following ELS is not entirely understood, neurobiological models have proposed interactions between stress and reward systems, such that neuroadaptations within the stress systems of the brain alter sensitivity to the rewarding properties of substances of abuse (Koob 2008). Cortisol, for example, has been found to influence reward sensitivity and drug self-administration through its interaction with the mesocorticolimbic reward system. Dopamine neurons in mesocorticolimbic brain regions express high levels of glucocorticoid receptors and are highly influenced by cortisol release in response to acute stress. In fact, increased HPA axis activity has been shown to mediate the relationship between ELS and greater mesocorticolimbic dopamine release following acute substance use (Meaney, Brake et al. 2002). For example, Pruessner and colleagues (2004) found healthy young adults with a history of low parental care exhibit a stronger cortisol response to a stress task, with levels of cortisol release positively associated with ventral striatum dopamine levels (Pruessner, Champagne et al. 2004). Another study found cortisol response following amphetamine administration was associated with dopamine binding in the ventral striatum and self-reported amphetamine-induced euphoria (Wand, Oswald et al. 2007). Aberrant amygdala reactivity during emotional processing may also contribute to differences in reward sensitivity in adolescents with a history of ELS (Marusak, Martin et al. 2015). Preclinical work supports a causal relationship; this work has shown suppression of corticosterone release decreases levels of dopamine under basal conditions and in response to stress and substance use (Piazza and Le Moal 1996, Barrot, Marinelli et al. 2000). While the aforementioned studies suggest greater cortisol release in response to acute stress may enhance dopamine release following acute drug administration, preclinical studies have also shown that chronic cortisol treatment may result in decreased sensitivity to the interceptive effects of drugs (Besheer, Fisher et al. 2012). Reward system functioning changes with age, and it is possible acute and chronic changes in cortisol differentially affect reward system functioning.
There appear to be important age-related differences in sensitivity to drugs of abuse. Specifically, studies support age-related increases in behavioral sensitivity to the effects of alcohol, including sedation, motor impairment, and hypothermia (Silveri, 2012). Adolescents exhibit reduced sensitivity to the effects of alcohol on these factors, which may result in greater levels of consumption. It is possible that adolescents with a history of ELS experience greater sensitivity to the euphoric effects of substances and reduced sensitivity to the sedative and motor impairing effects of substances, ultimately rendering these individuals at greater risk for heavy substance use. Experimental studies directly testing this hypothesis are needed. Despite their dampened behavioral sensitivity to substances, adolescents are highly susceptible to substance-related neurotoxicity. Therefore, serious efforts should be made to reduce heavy substance use during this critical developmental period. Cognitive behavioral alcohol intervention programs targeting an individual’s specific pre-existing vulnerability (i.e., level of response to alcohol) has shown some success in improving alcohol use outcomes in adolescents and young adults (Schuckit et al., 2012, Schuckit et al., 2015, Schuckit et al., 2016), although more research is needed. A better understanding of the pre-existing differences in substance sensitivity in adolescents with a history of ELS may offer new and fruitful directions for intervention programs.
5.3. Executive Function: Prefrontal Cortex
The PFC underlies a wide range of emotional and executive processes that are fundamental for proper neuropsychosocial functioning. Earlier, we discussed the PFC’s—particularly the OFC, ACC, and ventral PFC—critical role in regulating the stress response, and how disruptions in development of these prefrontal regions following ELS may contribute to a dysfunctional stress response and ultimately addiction liability. The PFC, including both ventral and dorsal prefrontal regions, also plays a critical role in a wide range of higher-order executive functions, including attentional and cognitive processes, behavioral control, impulsivity, working memory, and decision making (Goldstein and Volkow 2011, Girotti, Adler et al. 2018). There is a wealth of literature linking ELS with impaired executive functioning, including decreased working memory, attention, and inhibitory control (Holmes and Wellman 2009, Hart and Rubia 2012). These executive functions are also suggested to play a critical role in addiction onset and illness course, and deficits have been associated with a more severe clinical course characterized by increased craving, greater drug use, denial of illness, and increased risk of relapse (Goldstein and Volkow 2011, Blaine and Sinha 2017).
Preclinical studies have linked PFC dendritic changes—i.e., reduced dendritic length, branching, and spine density following ELS in preclinical models—with impairments in attentional and working memory processes (Arnsten 2009). HPA axis activity appears to influence executive function. Lyons and colleagues (2000) found excessive cortisol exposure impairs PFC function and inhibitory control in monkeys (Lyons et al. 2000), suggesting differences in stress response may also affect brain regions that underlie higher-order executive processes. Indeed, ELS is reported to be associated with greater stress-related cognitive impairments (Kuehl et al., 2020), and human fMRI studies have observed differences in PFC activation during tasks of executive functions. Mueller and colleagues observed greater PFC, including dorsal ACC and inferior PFC, activity during a cognitive (stop signal) task in adolescents with ELS compared to adolescents without a history of ELS (Mueller et al., 2010). Mueller and colleagues found correct, versus incorrect, responses recruited the inferior PFC more strongly in adolescents with a history of ELS compared to those without this history. These results may suggest inefficient processing and/or a compensatory mechanism in adolescents with ELS.
Neural and executive function consequences of ELS may also confound or interact with those observed in SUDs (Teicher and Khan 2019). In a recent study, De Bellis and colleagues (2019) aimed to test this hypothesis by examining how maltreatment history contributes to neurocognitive and brain structural differences in adolescent onset alcohol use disorders (De Bellis, Morey et al. 2019). Adolescents with alcohol use disorders and maltreatment scored lower in sustained attention, exhibited smaller anterior corpus callosum (the white matter tract that houses axons from the PFC and connects the left and right hemisphere of the brain) and larger PFC (specifically left pars triangularis) volumes, compared to adolescents with alcohol use disorders but no maltreatment. Results suggest maltreatment may independently or additively contribute neurocognitive and neurostructural differences associated with adolescent alcohol use. Other forms of co-occurring psychopathology may also influence PFC structure and cognitive functioning in individuals with ELS. For example, Saleh and colleagues (2017) found the relationship between childhood trauma exposure and cognitive function and brain structure differs between individuals who do and do not develop depression (Saleh et al., 2017). Specifically, depressed subjects exhibited an inverse association between ELS exposure and processing speed and OFC volume, whereas non-depressed subjects showed the opposite relationship between ELS and processing speed and OFC volume. Some researchers hypothesize individuals with SUDs (and other psychopathology) with and without history of ELS represent clinically and neurobiologically distinct subtypes of SUDs (Teicher and Samson 2013). Additional work aimed at understanding specific ELS-related consequences on neurophysiology—including within stress, reward, and executive systems—and role(s) in SUD risk, onset, and recurrence/treatment and interactions with adolescent substance use is critical for informing interventions and treatments best suited for this population.
6. Summary and Future Directions
ELS is associated with increased risk for SUDs—an association substantiated by many large-scale cross-sectional and longitudinal studies. ELS-related substance use outcomes emerge in adolescence and manifest in the form of earlier substance use initiation and heavy substance use. Substance use during this critical developmental period contributes to risk for SUDs, particularly for early onset SUDs. As a high proportion of individuals with ELS are exposed to substances of abuse during this vulnerable window, adolescent substance use may be an intermediate step in the pathway from ELS to SUDs. Clinical studies have identified internalizing and externalizing psychopathology as antecedents of adolescent substance use in youth with a history of ELS. Of course, SUDs can also precede internalizing and externalizing psychopathology and contribute to development of internalizing/externalizing psychopathology, as discussed in this special issue (Lippard and Nemeroff, this issue). Despite the complexity of examining neural processes in humans, clinical studies have also consistently demonstrated associations between ELS and altered development of the HPA axis, subcortical brain regions that underlie the stress response, mesocorticolimbic brain systems associated with reward sensitivity, and PFC regions that underlie executive control. It is possible that ELS sculpts the developing brain in a way that increases risk for early onset and heavy adolescent substance use. Indeed, longitudinal studies suggest differences in PFC structure and function may actually occur prior to and predict adolescent substance use. For example, lower ACC volume and activation during response inhibition in substance-naive adolescents predicts alcohol use initiation, heavy adolescent alcohol use, and alcohol-related problems in adolescence (Norman, Pulido et al. 2011, Cheetham, Allen et al. 2014, Squeglia, Rinker et al. 2014). Lower OFC gray matter volume has also been found to prospectively predict initiation of cannabis use (Cheetham, Allen et al. 2012). It is widely accepted that substance use alters neural structure and function of these systems. Adolescence marks a period of high brain plasticity, rendering the developing adolescent brain highly susceptible to the neurotoxic effects of drug exposure (Squeglia, Pulido et al. 2012, Pfefferbaum, Kwon et al. 2018). Substance use during this time may result in further brain changes that either independently, additively, or interactively contribute to risk for addiction in individuals with a history of ELS. Many of the large-scale studies substantiating the association between ELS, adolescent substance use, and SUDs relied on retrospective self-report measures of ELS and substance use. This is a considerable weakness in the literature, as the possibility of inaccurate recall and reporter bias cannot be excluded. Longitudinal studies with the power to disentangle temporal relations between ELS, substance use, adolescent brain development, and mechanisms that contribute to development and maintenance of SUDs have begun to emerge [e.g., ABCD, Environmental influences on Child Health Outcomes (ECHO) (Gillman and Blaisdell, 2018)]. These studies provide an unprecedented opportunity to further our understanding of the role of ELS on adolescent development, substance use, and interactions between brain development and substance use that may drive risk for SUDs.
6.1. Leveraging Protective Psychosocial Factors in Adolescence to Improve Outcomes
Adolescence marks a period of rapid development. While greater brain plasticity during this time may increase susceptibility to the effects of stress and substance use, it also makes it a critical window of opportunity for intervention (Romeo and McEwen, 2006). Studies have found psychosocial factors like living situation, parenting, environmental enrichment, social support all impact risk for SUDs in individuals with a history of ELS (Lansford, Malone et al. 2006, DuMont, Widom et al. 2007, Braithwaite, O'Connor et al. 2017, Cheong, Sinnott et al. 2017) and that this may occur, at least in part, through reversing ELS-related neurobiological changes. In a large sample (N=7047), Bellis and colleagues (2017) found following ELS, support from a trusted and always available adult during childhood decreased the prevalence of daily smoking and heavy alcohol use in adulthood (Bellis, Hardcastle et al. 2017). Shin and colleagues (2019) recently showed that high levels of paternal warmth weakened the association between childhood emotional abuse and alcohol-related problems (Shin, Wang et al. 2019). Supportive relationships with family members and peers teach healthy stress-coping strategies and foster healthy stress response systems. Improved childcare and alleviation of chronic stress is associated with a return to normal cortisol levels (Fries, Hesse et al. 2005, Gunnar and Quevedo 2008). Stress-induced morphological remodeling of the PFC and hippocampus also appear to be reversible following alleviation of chronic stress (Vyas, Pillai et al. 2004, Radley, Rocher et al. 2005). In a controlled study of Romanian orphans, Smyke and colleagues (2009) showed that children randomly assigned to a newly established system of higher-level foster care, as opposed to institutional care, exhibited lower levels of adverse clinical outcomes (Smyke et al., 2009) and a reversal of previously observed decreased white matter volume (Sheridan et al., 2012). Psychosocial interventions (i.e., trauma-focused cognitive behavioral therapy) have garnered empirical support and mark another avenue for further development (Cohen, Mannarino et al. 2006). Despite these promising results, more research is needed to investigate how psychosocial interventions and environmental enrichment can be most effective in reducing substance use outcomes in this population. For example, biological factors including genetics and sex influence substance use outcomes (box 1) and should be considered in intervention studies. Further, a focus on translating experimental findings into clinical practice is needed to improve adverse substance use outcomes following ELS. ECHO is a nationwide large-scale study aimed to understand the effects of early environmental influences on child health and development (Gillman and Blaisdell, 2018). The study includes pediatric cohorts recruited during pregnancy, allowing for prospective examination of prenatal conditions (i.e., maternal obesity, depression, and drug use). The study is also unique in that includes both observational and interventional research branches. The interventional branch of the study offers children in medically underserved and rural populations access to enroll in clinical trials. This unprecedented study has prioritized “solution-oriented” research questions that can readily inform programs, policies, and practices based on research in children from diverse populations across the United States.
Highlights.
Early life stress (ELS) is related to adolescent substance use/disorders (SUDs)
ELS and substance use are associated with brain changes that increase risk for SUDs
Adolescent substance use may be an intermediate step in the path from ELS to SUDs
Intervention during adolescence may effectively reduce substance use following ELS
Acknowledgements:
The authors were supported in part by research grants from NIAAA K01AA027573 (ETCL), R21AA027884 (ETCL), and Jones/Bruce Fellowship from the Waggoner Center on Alcohol and Addiction Research (DEK).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alink LR, Cicchetti D, Kim J, Rogosch FA, 2012. Longitudinal associations among child maltreatment, social functioning, and cortisol regulation. Dev. Psychol 48 (1), 224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anda RF, Felitti VJ, Bremner JD, Walker JD, Whitfield C, Perry BD, Dube SR, Giles WH, 2006. The enduring effects of abuse and related adverse experiences in childhood. Eur. Arch. Psychiatry Clin. Neurosci 174–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen SL, Teicher MH, 2008. Stress, sensitive periods and maturational events in adolescent depression. Trends Neurosci. 31 (4), 183–191. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Teicher MH, 2009. Desperately driven and no brakes: developmental stress exposure and subsequent risk for substance abuse. Neurosci. Biobehav. Rev 33 (4), 516–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argyriou E, Um M, Carron C, Cyders MA, 2018. Age and impulsive behavior in drug addiction: a review of past research and future directions. Pharmacol. Biochem. Behav 164, 106–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF, 2009. Stress signalling pathways that impair prefrontal cortex structure and function. Nat. Rev. Neurosci 10 (6), 410–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachi K, Parvaz MA, Moeller SJ, Gan G, Zilverstand A, Goldstein RZ, Alia-Klein N, 2018. Reduced orbitofrontal gray matter concentration as a marker of premorbid childhood trauma in cocaine use disorder. Front. Hum. Neurosci 12, 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey JA, McCloskey LA, 2005. Pathways to adolescent substance use among sexually abused girls. J. Abnorm. Child Psychol 33 (1), 39–53. [DOI] [PubMed] [Google Scholar]
- Barrot M, Marinelli M, Abrous DN, Rouge-Pont F, Le Moal M, Piazza PV, 2000. The dopaminergic hyper-responsiveness of the shell of the nucleus accumbens is hormone-dependent. Eur. J. Neurosci 12 (3), 973–979. [DOI] [PubMed] [Google Scholar]
- Becker HC, 2017. Influence of stress associated with chronic alcohol exposure on drinking. Neuropharmacology 122, 115–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behen ME, Muzik O, Saporta AS, Wilson BJ, Pai D, Hua J, Chugani HT, 2009. Abnormal fronto-striatal connectivity in children with histories of early deprivation: a diffusion tensor imaging study. Brain Imaging Behav. 3 (3), 292–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellis MA, Hardcastle K, Ford K, Hughes K, Ashton K, Quigg Z, Butler N, 2017. Does continuous trusted adult support in childhood impart life-course resilience against adverse childhood experiences - a retrospective study on adult health-harming behaviours and mental well-being. BMC Psychiatry 17 (1), 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensley LS, Spieker SJ, Van Eenwyk J, Schoder J, 1999. Self-reported abuse history and adolescent problem behaviors. II. Alcohol and drug use. J. Adolesc. Health 24 (3), 173–180. [DOI] [PubMed] [Google Scholar]
- Besheer J, Fisher KR, Grondin JJ, Cannady R, Hodge CW, 2012. The effects of repeated corticosterone exposure on the interoceptive effects of alcohol in rats. Psychopharmacology 220 (4), 809–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaine SK, Sinha R, 2017. Alcohol, stress, and glucocorticoids: from risk to dependence and relapse in alcohol use disorders. Neuropharmacology 122,136–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaine SK, Nautiyal N, Hart R, Guarnaccia JB, Sinha R, 2019. Craving, cortisol and behavioral alcohol motivation responses to stress and alcohol cue contexts and discrete cues in binge and non-binge drinkers. Addict. Biol 24 (5), 1096–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomeyer D, Treutlein J, Esser G, Schmidt MH, Schumann G, Laucht M, 2008. Interaction between CRHR1 gene and stressful life events predicts adolescent heavy alcohol use. Biol. Psychiatry 63 (2), 146–151. [DOI] [PubMed] [Google Scholar]
- Boecker R, Holz NE, Buchmann AF, Blomeyer D, Plichta MM, Wolf I, Baumeister S, Meyer-Lindenberg A, Banaschewski T, Brandeis D, Laucht M, 2014. Impact of early life adversity on reward processing in young adults: EEG-fMRI results from a prospective study over 25 years. PLoS One 9 (8), e104185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braithwaite EC, O'Connor RM, Degli-Esposti M, Luke N, Bowes L, 2017. Modifiable predictors of depression following childhood maltreatment: a systematic review and meta-analysis. Transl. Psychiatry 7 (7), e1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brajović M, Bellis M, Kukec A, Terzić N, Baban A, Sethi D, Zaletel-Kragelj L, 2019. Impact of adverse childhood experiences on alcohol use in emerging adults in Montenegro and Romania. Slov. J. Public Health 58 (3), 129–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Randall P, Vermetten E, Staib L, Bronen RA, Mazure C, Capelli S, McCarthy G, Innis RB, Charney DS, 1997. Magnetic resonance imaging-based measurement of hippocampal volume in posttraumatic stress disorder related to childhood physical and sexual abuse; a preliminary report. Biol. Psychiatry 41, 23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody GH, Chen YF, Murry VM, Ge X, Simons RL, Gibbons FX, Gerrard M, Cutrona CE, 2006. Perceived discrimination and the adjustment of african american youths: a five-year longitudinal analysis with contextual moderation effects. Child Dev. 77, 1170–1189. [DOI] [PubMed] [Google Scholar]
- Brown SA, McGue M, Maggs J, Schulenberg J, Hingson R, Swartzwelder S, Martin C, Chung T, Tapert SF, Sher K, Winters KC, Lowman C, Murphy S, 2008. A developmental perspective on alcohol and youths 16 to 20 years of age. Pediatrics 121 (Suppl. 4), S290–S310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter LL, Tyrka AR, McDougle CJ, Malison RT, Owens MJ, Nemeroff CB, Price LH, 2004. Cerebrospinal fluid corticotropin-releasing factor and perceived early-life stress in depressed patients and healthy control subjects. Neuropsychopharmacology 29, 777–784. [DOI] [PubMed] [Google Scholar]
- Chassin L, Sher KJ, Hussong A, Curran P, 2013. The developmental psychopathology of alcohol use and alcohol disorders: research achievements and future directions. Dev. Psychopathol 25 (4 0 2), 1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheetham A, Allen NB, Whittle S, Simmons JG, Yucel M, Lubman DI, 2012. Orbitofrontal volumes in early adolescence predict initiation of cannabis use: a 4-year longitudinal and prospective study. Biol. Psychiatry 71 (8), 684–692. [DOI] [PubMed] [Google Scholar]
- Cheetham A, Allen NB, Whittle S, Simmons J, Yucel M, Lubman DI, 2014. Volumetric differences in the anterior cingulate cortex prospectively predict alcohol-related problems in adolescence. Psychopharmacology 231 (8), 1731–1742. [DOI] [PubMed] [Google Scholar]
- Cheong EV, Sinnott C, Dahly D, Kearney PM, 2017. Adverse childhood experiences (ACEs) and later-life depression: perceived social support as a potential protective factor. BMJ Open 7 (9), e013228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang YC, Ennett ST, Bauman KE, Foshee VA, 2005. Neighborhood influences on adolescent cigarette and alcohol use: mediating effects through parent and peer behaviors. J. Health Soc. Behav 46 (2), 187–204. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA, 1999. Psychopathology as risk for adolescent substance use disorders: a developmental psychopathology perspective. J. Clin. Child Psychol 28 (3), 355–365. [DOI] [PubMed] [Google Scholar]
- Cleck JN, Blendy JA, 2008. Making a bad thing worse: adverse effects of stress on drug addiction. J. Clin. Invest 118 (2), 454–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JA, Mannarino AP, Murray LK, Igelman R, 2006. Psychosocial interventions for maltreated and violence-exposed children. J. Soc. Issues 62 (4), 737–766. [Google Scholar]
- Cohen RA, Grieve S, Hoth KF, Paul RH, Sweet L, Tate D, Gunstad J, Stroud L, McCaffery J, Hitsman B, Niaura R, Clark CR, McFarlane A, Bryant R, Gordon E, Williams LM, 2006. Early life stress and morphometry of the adult anterior cingulate cortex and caudate nuclei. Biol. Psychiatry 59 (10), 975–982. [DOI] [PubMed] [Google Scholar]
- Colich NL, Williams ES, Ho TC, King LS, Humphreys KL, Price AN, Ordaz SJ, Gotlib IH, 2017. The association between early life stress and prefrontal cortex activation during implicit emotion regulation is moderated by sex in early adolescence. Dev. Psychopathol 29, 1851–1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland WE, Angold A, Shanahan L, Dreyfuss J, Dlamini I, Costello EJ, 2012. Predicting persistent alcohol problems: a prospective analysis from the great Smoky Mountain study. Psychol. Med 42 (9), 1925–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello EJ, 2007. Psychiatric predictors of adolescent and young adult drug use and abuse: what have we learned? Drug Alcohol Depend. 88 (Suppl. 1), S97–S99. [DOI] [PubMed] [Google Scholar]
- Cowan CSM, Callaghan BL, Kan JM, Richardson R, 2016. The lasting impact of early-life adversity on individuals and their descendants: potential mechanisms and hope for intervention. Genes Brain Behav. 15, 155–168. [DOI] [PubMed] [Google Scholar]
- Crews FT, Nixon K, 2009. Mechanisms of neurodegeneration and regeneration in alcoholism. Alcohol 44 (2), 115–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese A, McEwen BS, 2012. Adverse childhood experiences, allostasis, allostatic load, and age-related disease. Physiol. Behav 106 (1), 29–39. [DOI] [PubMed] [Google Scholar]
- Dawes MA, Targer RE, Kirisci L, 1997. Behavioral self-regulation: correlates and 2 year follow-ups for boys at risk for substance abuse. Drug Alcohol Depend. 45, 165–176. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Zisk A, 2014. The biological effects of childhood trauma. Child Adolesc. Psychiatr. Clin. N. Am 23 (2), 185–222 vii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bellis MD, Clark DB, Beers SR, Soloff PH, Boring AM, Hall J, Kersh A, Keshavan MS, 2000. Hippocampal volume in adolescent-onset alcohol use disorders. Am. J. Psychiatry 157 (5), 737–744. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Morey RA, Nooner KB, Woolley DP, Haswell CC, Hooper SR, 2019. A pilot study of neurocognitive function and brain structures in adolescents with alcohol use disorders: does maltreatment history matter? Child Maltreat. 24 (4), 374–388. [DOI] [PubMed] [Google Scholar]
- de Wit H, Vicini L, Childs E, Sayla MA, Terner J, 2007. Does stress reactivity or response to amphetamine predict smoking progression in young adults? A preliminary study. Pharmacol. Biochem. Behav 86, 312–319. [DOI] [PubMed] [Google Scholar]
- DeWit DJ, Adlaf EM, Offord DR, Ogborne AC, 2000. Age at first alcohol use: a risk factor for the development of alcohol disorders. Am. J. Psychiatry 157 (5), 745–750. [DOI] [PubMed] [Google Scholar]
- Dias BG, Ressler KJ, 2014. Parental olfactory experience influences behavior and neural structure in subsequent generations. Nat. Neurosci 17, 89–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dienes KA, Hammen C, Henry RM, Cohen AN, Daley SE, 2006. The stress sensitization hypothesis: understanding the course of bipolar disorder. J. Affect. Disord 95, 43–49. [DOI] [PubMed] [Google Scholar]
- Dillon DG, Holmes AJ, Birk JL, Brooks N, Lyons-Ruth K, Pizzagalli DA, 2009. Childhood adversity is associated with left basal ganglia dysfunction during reward anticipation in adulthood. Biol. Psychiatry 66 (3), 206–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doan SN, Dich N, Fuller-Rowell TE, Evans GW, 2019. Externalizing behaviors buffer the effects of early life adversity on physiologic dysregulation. Sci. Rep 9, 13623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge KA, Malone PS, Lansford JE, Miller S, Pettit GS, Bates JE, 2009. A dynamic cascade model of the development of substance-use onset. Monogr. Soc. Res. Child Dev 74 (3), vii–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dom G, De Wilde B, Hulstijn W, Sabbe B, 2007. Traumatic experiences and posttraumatic stress disorders: differences between treatment-seeking early- and late-onset alcoholic patients. Compr. Psychiatry 48 (2), 178–185. [DOI] [PubMed] [Google Scholar]
- Doom JR, Cicchetti D, Rogosch FA, 2014. Longitudinal patterns of cortisol regulation differ in maltreated and nonmaltreated children. J. Am. Acad. Child Adolesc. Psychiatry 53 (11), 1206–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dube SR, Felitti VJ, Dong M, Chapman DP, Giles WH, Anda RF, 2003. Childhood abuse, neglect, and household dysfunction and the risk of illicit drug use: the adverse childhood experiences study. Pediatrics 111 (3), 564–572. [DOI] [PubMed] [Google Scholar]
- Dube SR, Miller JW, Brown DW, Giles WH, Felitti VJ, Dong M, Anda RF, 2006. Adverse childhood experiences and the association with ever using alcohol and initiating alcohol use during adolescence. J. Adolesc. Health 38 (4), 444.e441–410. [DOI] [PubMed] [Google Scholar]
- Dubowitz H, Thompson R, Arria AM, English D, Metzger R, Kotch JB, 2016. Characteristics of child maltreatment and adolescent marijuana use: a prospective study. Child Maltreat. 21 (1), 16–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuMont KA, Widom CS, Czaja SJ, 2007. Predictors of resilience in abused and neglected children grown-up: the role of individual and neighborhood characteristics. Child Abuse Negl. 31 (3), 255–274. [DOI] [PubMed] [Google Scholar]
- Edmiston EE, Wang F, Mazure CM, Guiney J, Sinha R, Mayes LC, Blumberg HP, 2011. Corticostriatal-limbic gray matter morphology in adolescents with self-reported exposure to childhood maltreatment. Arch. Pediatr. Adolesc. Med 165 (12), 1069–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elam KK, Wang FL, Bountress K, Chassin L, Pandika D, Lemery-Chalfant K, 2016. Predicting substance use in emerging adulthood: a genetically informed study of developmental transactions between impulsivity and family conflict. Dev. Psychopathol 28 (3), 673–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englund MM, Egeland B, Oliva EM, Collins WA, 2008. Childhood and adolescent predictors of heavy drinking and alcohol use disorders in early adulthood: a longitudinal developmental analysis. Addiction 103 (Suppl. 1), 23–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoch MA, 2011. The role of early life stress as a predictor for alcohol and drug dependence. Psychopharmacology 214 (1), 17–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahlke C, Lorenz JG, Long J, Champoux M, Suomi SJ, Higley JD, 2000. Rearing experiences and stress-induced plasma cortisol as early risk factors for excessive alcohol consumption in nonhuman primates. Alcohol. Clin. Exp. Res 24 (5), 644–650. [PubMed] [Google Scholar]
- Fairbanks LA, 1996. Individual differences in maternal style: causes and consequences for mothers and offspring. In: Advances in the Study of Behavior. Elsevier. [Google Scholar]
- Fernando SC, Beblo T, Schlosser N, Terfehr K, Otte C, Löwe B, Wolf OT, Spitzer C, Driessen M, Wingenfeld K, 2012. Associations of childhood trauma with hypothalamic-pituitary-adrenal function in borderline personality disorder and major depression. Psychoneuroendocrinology 37, 1659–1668. [DOI] [PubMed] [Google Scholar]
- Fields SA, Schueler J, Arthur KM, Harris B, 2021. The role of impulsivity in major depression: a systematic review. Curr. Behav. Neurosci. Rep 8, 38–50. [Google Scholar]
- Fries E, Hesse J, Hellhammer J, Hellhammer DH, 2005. A new view on hypocortisolism. Psychoneuroendocrinology 30 (10), 1010–1016. [DOI] [PubMed] [Google Scholar]
- Garami J, Valikhani A, Parkes D, Haber P, Mahlberg J, Misiak B, Frydecka D, Moustafa AA, 2019. Examining perceived stress, childhood trauma and interpersonal trauma in individuals with drug addiction. Psychol. Rep 122 (2), 433–450. [DOI] [PubMed] [Google Scholar]
- Gee DG, Gabard-Durnam LJ, Flannery J, Goff B, Humphreys KL, Telzer EH, Hare TA, Bookheimer SY, Tottenham N, 2013. Early developmental emergence of human amygdala-prefrontal connectivity after maternal deprivation. Proc. Natl. Acad. Sci 110 (39), 15638–15643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerra G, Zaimovic A, Castaldini L, Garofano L, Manfredini M, Somaini L, Leonardi C, Gerra ML, Donnini C, 2010. Relevance of perceived childhood neglect, 5-HTT gene variants and hypothalamus-pituitary-adrenal axis dysregulation to substance abuse susceptibility. Am. J. Med. Genet. B Neuropsychiatr. Genet 153b (3), 715–722. [DOI] [PubMed] [Google Scholar]
- Gillman MW, Blaisdell CJ, 2018. Environmental influences on child health outcomes, a research program of the National Institutes of Health. Curr. Opin. Pediatr 30, 260–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman JM, Ramchandani VA, Davis MB, Bjork JM, Hommer DW, 2008. Why we like to drink: a functional magnetic resonance imaging study of the rewarding and anxiolytic effects of alcohol. J. Neurosci 28 (18), 4583–4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girotti M, Adler SM, Bulin SE, Fucich EA, Paredes D, Morilak DA, 2018. Prefrontal cortex executive processes affected by stress in health and disease. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 85, 161–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND, 2011. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat. Rev. Neurosci 12 (11), 652–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein AL, Flett GL, Wekerle C, 2010. Child maltreatment, alcohol use and drinking consequences among male and female college students: an examination of drinking motives as mediators. Addict. Behav 35 (6), 636–639. [DOI] [PubMed] [Google Scholar]
- Gonzalez VM, Reynolds B, Skewes MC, 2011. Role of impulsivity in the relationship between depression and alcohol problems among emerging adult college drinkers. Exp. Clin. Psychopharmacol 19 (4), 303–313. [DOI] [PubMed] [Google Scholar]
- Gorenstein EE, Newman JP, 1980. Disinhibitory psychopathology: a new perspective and a model for research. Psychol. Rev 87 (3), 301–315. [PubMed] [Google Scholar]
- Gorka SM, Fitzgerald DA, King AC, Phan KL, 2013. Alcohol attenuates amygdala-frontal connectivity during processing social signals in heavy social drinkers: a preliminary pharmaco-fMRI study. Psychopharmacology 229 (1), 141–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorka SM, Phan KL, Childs E, 2018. Acute calming effects of alcohol are associated with disruption of the salience network. Addict. Biol 23 (3), 921–930. [DOI] [PubMed] [Google Scholar]
- Grant BF, Stinson FS, Harford TC, 2001. Age at onset of alcohol use and DSM-IV alcohol abuse and dependence: a 12-year follow-up. J. Subst. Abus 13 (4), 493–504. [DOI] [PubMed] [Google Scholar]
- Grayson CE, Nolen-Hoeksema S, 2005. Motives to drink as mediators between childhood sexual assault and alcohol problems in adult women. J. Trauma. Stress 18 (2), 137–145. [DOI] [PubMed] [Google Scholar]
- Green JG, McLaughlin KA, Berglund PA, Gruber MJ, Sampson NA, Zaslavsky AM, Kessler RC, 2010. Childhood adversities and adult psychiatric disorders in the national comorbidity survey replication I: associations with first onset of DSM-IV disorders. Arch. Gen. Psychiatry 67 (2), 113–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfield SF, Kolodziej ME, Sugarman DE, Muenz LR, Vagge LM, He DY, Weiss RD, 2002. History of abuse and drinking outcomes following inpatient alcohol treatment: a prospective study. Drug Alcohol Depend. 67 (3), 227–234. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Quevedo KM, 2008. Early care experiences and HPA axis regulation in children: a mechanism for later trauma vulnerability. Prog. Brain Res 167, 137–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE, Kaufman J, Hodgdon HB, Masten CL, Jazbec S, Pine DS, Ernst M, 2006. Behavioral alterations in reward system function: the role of childhood maltreatment and psychopathology. J. Am. Acad. Child Adolesc. Psychiatry 45 (9), 1059–1067. [DOI] [PubMed] [Google Scholar]
- Hamburger ME, Leeb RT, Swahn MH, 2008. Childhood maltreatment and early alcohol use among high-risk adolescents. J. Stud. Alcohol Drugs 69 (2), 291–295. [DOI] [PubMed] [Google Scholar]
- Handley ED, Rogosch FA, Cicchetti D, 2017. From child maltreatment to emerging adult problem drinking: identification of a multilevel internalizing pathway among african american youth. Dev. Psychopathol 29 (5), 1807–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannan SM, Orcutt HK, Miron LR, Thompson KL, 2015. Childhood sexual abuse and later alcohol-related problems: investigating the roles of revictimization, PTSD, and drinking motivations among college women. J. Interpers. Violence 32 (14), 2118–2138. [DOI] [PubMed] [Google Scholar]
- Hanson JL, Albert D, Iselin AM, Carre JM, Dodge KA, Hariri AR, 2016. Cumulative stress in childhood is associated with blunted reward-related brain activity in adulthood. Soc. Cogn. Affect. Neurosci 11 (3), 405–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison PA, Fulkerson JA, Beebe TJ, 1997. Multiple substance use among adolescent physical and sexual abuse victims. Child Abuse Negl. 21 (6), 529–539. [DOI] [PubMed] [Google Scholar]
- Hart H, Rubia K, 2012. Neuroimaging of child abuse: a critical review. Front. Hum. Neurosci 6, 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffner JL, Blom TJ, Anthenelli RM, 2011. Gender differences in trauma history and symptoms as predictors of relapse to alcohol and drug use. Am. J. Addict 20 (4), 307–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, Nemeroff CB, 2001. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol. Psychiatry 49 (12), 1023–1039. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Heit S, Graham YP, Wilcox M, Bonsall R, Miller AH, Nemeroff CB, 2000. Pituitary-adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. JAMA 284, 592–597. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Bonsall R, Miller AH, Nemeroff CB, 2001. Altered pituitary-adrenal axis responses to provocative challenge tests in adult survivors of childhood abuse. Am. J. Psychiatry 158, 575–581. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Wagner D, Wilcox MM, Miller AH, Nemeroff CB, 2002. The role of early adverse experience and adulthood stress in the prediction of neuroendocrine stress reactivity in women: a multiple regression analysis. Depress. Anxiety 15, 117–125. [DOI] [PubMed] [Google Scholar]
- Herringa RJ, Birn RM, Ruttle PL, Burghy CA, Stodola DE, Davidson RJ, Essex MJ, 2013. Childhood maltreatment is associated with altered fear circuitry and increased internalizing symptoms by late adolescence. Proc. Natl. Acad. Sci. U. S. A 110 (47), 19119–19124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herringa RJ, Burghy CA, Stodola DE, Fox ME, Davidson RJ, Essex MJ, 2016. Enhanced prefrontal-amygdala connectivity following childhood adversity as a protective mechanism against internalizing in adolescence. Biol. Psychiatry 1 (4), 326–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higley JD, Hasert MF, Suomi SJ, Linnoila M, 1991. Nonhuman primate model of alcohol abuse: effects of early experience, personality, and stress on alcohol consumption. Proc. Natl. Acad. Sci. U. S. A 88 (16), 7261–7265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hingson RW, Heeren T, Winter MR, 2006. Age of alcohol-dependence onset: associations with severity of dependence and seeking treatment. Pediatrics 118, e755–e763. [DOI] [PubMed] [Google Scholar]
- Hoffman EA, Clark DB, Orendain N, Hudziak J, Squeglia LM, Dowling GJ, 2019. Stress exposures, neurodevelopment and health measures in the ABCD study. Neurobiol. Stress 10, 100157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A, Wellman CL, 2009. Stress-induced prefrontal reorganization and executive dysfunction in rodents. Neurosci. Biobehav. Rev 33 (6), 773–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson A, Wekerle C, Goldstein AL, Ellenbogen S, Waechter R, Thompson K, Stewart SH, 2017. Gender differences in emotion-mediated pathways from childhood sexual abuse to problem drinking in adolescents in the child welfare system. J. Child Adolesc. Trauma 10 (1), 19–28. [Google Scholar]
- Huizink AC, Greaves-Lord K, Oldehinkel AJ, Ormel J, Verhulst FC, 2009. Hypothalamic-pituitary-adrenal axis and smoking and drinking onset among adolescents: the longitudinal cohort TRacking Adolescents' individual lives survey (TRAILS). Addiction 104, 1927–1936. [DOI] [PubMed] [Google Scholar]
- Huot RL, Thrivikraman KV, Meaney MJ, Plotsky PM, 2001. Development of adult ethanol preference and anxiety as a consequence of neonatal maternal separation in long Evans rats and reversal with antidepressant treatment. Psychopharmacology 158 (4), 366–373. [DOI] [PubMed] [Google Scholar]
- Hyman SM, Paliwal P, Chaplin TM, Mazure CM, Rounsaville BJ, Sinha R, 2008. Severity of childhood trauma is predictive of cocaine relapse outcomes in women but not men. Drug Alcohol Depend. 92 (1–3), 208–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irons DE, Iacono WG, McGue M, 2015. Tests of the effects of adolescent early alcohol exposures on adult outcomes. Addiction 110 (2), 269–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobus J, Tapert SF, 2013. Neurotoxic effects of alcohol in adolescence. Annu. Rev. Clin. Psychol 9, 703–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobus J, Squeglia LM, Bava S, Tapert SF, 2013. White matter characterization of adolescent binge drinking with and without co-occurring marijuana use: a 3-year investigation. Psychiatry Res. Neuroimaging 214 (3), 374–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaycox LH, Ebener P, Damesek L, Becker K, 2004. Trauma exposure and retention in adolescent substance abuse treatment. J. Trauma. Stress 17 (2), 113–121. [DOI] [PubMed] [Google Scholar]
- Kaess M, Whittle S, O'Brien-Simpson L, Allen NB, Simmons JG, 2018. Childhood maltreatment, pituitary volume and adolescent hypothalamic-pituitary-adrenal axis - evidence for a maltreatment-related attenuation. Psychoneuroendocrinology 98, 39–45. [DOI] [PubMed] [Google Scholar]
- Kaiser RH, Clegg R, Goer F, Pechtel P, Beltzer M, Vitaliano G, Olson DP, Teicher MH, Pizzagalli DA, 2018. Childhood stress, grown-up brain networks: corticolimbic correlates of threat-related early life stress and adult stress response. Psychol. Med 48 (7), 1157–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplow JB, Curran PJ, Dodge KA, 2002. Child, parent, and peer predictors of early-onset substance use: a multisite longitudinal study. J. Abnorm. Child Psychol 30 (3), 199–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Yang BZ, Douglas-Palumberi H, Crouse-Artus M, Lipschitz D, Krystal JH, Gelernter J, 2007. Genetic and environmental predictors of early alcohol use. Biol. Psychiatry 61 (11), 1228–1234. [DOI] [PubMed] [Google Scholar]
- Kelly PA, Viding E, Wallace GL, Schaer M, De Brito SA, Robustelli B, McCrory EJ, 2013. Cortical thickness, surface area, and gyrification abnormalities in children exposed to maltreatment: neural markers of vulnerability? Biol. Psychiatry 74 (11), 845–852. [DOI] [PubMed] [Google Scholar]
- Kessler RC, McLaughlin KA, Green JG, Gruber MJ, Sampson NA, Zaslavsky AM, Aguilar-Gaxiola S, Alhamzawi AO, Alonso J, Angermeyer M, Benjet C, Bromet E, Chatterji S, de Girolamo G, Demyttenaere K, Fayyad J, Florescu S, Gal G, Gureje O, Haro JM, Hu CY, Karam EG, Kawakami N, Lee S, Lepine JP, Ormel J, Posada-Villa J, Sagar R, Tsang A, Ustun TB, Vassilev S, Viana MC, Williams DR, 2010. Childhood adversities and adult psychopathology in the WHO world mental health surveys. Br. J. Psychiatry 197 (5), 378–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick DG, Acierno R, Saunders B, Resnick HS, Best CL, Schnurr PP, 2000. Risk factors for adolescent substance abuse and dependence: data from a national sample. J. Consult. Clin. Psychol 68 (1), 19–30. [DOI] [PubMed] [Google Scholar]
- Kim ST, Hwang SS, Kim HW, Hwang EH, Cho J, Kang JI, Kim SJ, 2018. Multidimensional impulsivity as a mediator of early life stress and alcohol dependence. Sci. Rep 8 (1), 4104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King KM, Chassin L, 2007. A prospective study of the effects of age of initiation of alcohol and drug use on young adult substance dependence. J. Stud. Alcohol Drugs 68 (2), 256–265. [DOI] [PubMed] [Google Scholar]
- King SM, Iacono WG, McGue M, 2004. Childhood externalizing and internalizing psychopathology in the prediction of early substance use. Addiction 99 (12), 1548–1559. [DOI] [PubMed] [Google Scholar]
- Kirsch DE, Nemeroff CB, Lippard ETC, 2020. Early life stress and substance use disorders: underlying neurobiology and pathways to adverse outcomes. Adversity Resilience Sci. 1, 29–47. [Google Scholar]
- Kirsch DE, Tretyak V, Radpour S, Weber WA, Nemeroff CB, Fromme K, Strakowski SM, Lippard ETC, 2021. Childhood maltreatment, prefrontal-paralimbic gray matter volume, and substance use in young adults and interactions with risk for bipolar disorder. Sci. Rep 11 (1), 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisely S, Mills R, Strathearn L, Najman JM, 2020. Does child maltreatment predict alcohol use disorders in young adulthood? A cohort study of linked notifications and survey data. Addiction 115 (1), 61–68. [DOI] [PubMed] [Google Scholar]
- Kisely S, Strathearn L, Najman J, 2020. The influence of child maltreatment on substance or alcohol use in 30-year-old adults: a birth cohort study. Drug Alcohol Rev. 40 (4), 673–680. [DOI] [PubMed] [Google Scholar]
- Klanecky AK, McChargue DE, Tuliao AP, 2016. Proposed pathways to problematic drinking via post-traumatic stress disorder symptoms, emotion dysregulation, and dissociative tendencies following child/adolescent sexual abuse. J. Addict. Dis 35 (3), 180–193. [DOI] [PubMed] [Google Scholar]
- Koob GF, 2008. A role for brain stress systems in addiction. Neuron 59 (1), 11–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten TA, Miserendino MJ, Kehoe P, 2000. Enhanced acquisition of cocaine self-administration in adult rats with neonatal isolation stress experience. Brain Res. 875 (1–2), 44–50. [DOI] [PubMed] [Google Scholar]
- Kosten TA, Sanchez H, Zhang XY, Kehoe P, 2004. Neonatal isolation enhances acquisition of cocaine self-administration and food responding in female rats. Behav. Brain Res 151 (1–2), 137–149. [DOI] [PubMed] [Google Scholar]
- Kosten TA, Zhang XY, Kehoe P, 2006. Heightened cocaine and food self-administration in female rats with neonatal isolation experience. Neuropsychopharmacology 31 (1), 70–76. [DOI] [PubMed] [Google Scholar]
- Kuehl LK, Schultebraucks K, Deuter CE, May A, Spitzer C, Otte C, Wingenfeld K, 2020. Stress effects on cognitive function in patients with major depressive disorder: does childhood trauma play a role? Dev. Psychopathol 32 (3), 1007–1016. [DOI] [PubMed] [Google Scholar]
- Kundakovic M, Champagne FA, 2015. Early-life experience, epigenetics, and the developing brain. Neuropsychopharmacology 40, 141–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansford JE, Malone PS, Stevens KI, Dodge KA, Bates JE, Pettit GS, 2006. Developmental trajectories of externalizing and internalizing behaviors: factors underlying resilience in physically abused children. Dev. Psychopathol 18 (1), 35–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansford JE, Dodge KA, Pettit GS, Bates JE, 2010. Does physical abuse in early childhood predict substance use in adolescence and early adulthood? Child Maltreat. 15 (2), 190–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larance B, Gisev N, Cama E, Nelson EC, Darke S, Larney S, Degenhardt L, 2018. Predictors of transitions across stages of heroin use and dependence prior to treatment-seeking among people in treatment for opioid dependence. Drug Alcohol Depend. 191, 145–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Moal M, Koob GF, 2007. Drug addiction: pathways to the disease and pathophysiological perspectives. Eur. Neuropsychopharmacol 17 (6–7), 377–393. [DOI] [PubMed] [Google Scholar]
- Le Strat Y, Ramoz N, Pickering P, Burger V, Boni C, Aubin HJ, Adès J, Batel P, Gorwood P, 2008. The 3' part of the dopamine transporter gene DAT1/SLC6A3 is associated with withdrawal seizures in patients with alcohol dependence. Alcohol. Clin. Exp. Res 32, 27–35. [DOI] [PubMed] [Google Scholar]
- Lewis TL, Kotch J, Wiley TR, Litrownik AJ, English DJ, Thompson R, Zolotor AJ, Block SD, Dubowitz H, 2011. Internalizing problems: a potential pathway from childhood maltreatment to adolescent smoking. J. Adolesc. Health 48 (3), 247–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis TT, Cogburn CD, Williams DR, 2015. Self-reported experiences of discrimination and health: scientific advances, ongoing controversies, and emerging issues. Annu. Rev. Clin. Psychol 11, 407–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim L, Hart H, Mehta M, Worker A, Simmons A, Mirza K, Rubia K, 2018. Grey matter volume and thickness abnormalities in young people with a history of childhood abuse. Psychol. Med 48, 1034–1046. [DOI] [PubMed] [Google Scholar]
- Lippard and Nemeroff, this issue, Lippard ETC Nemeroff CB. “Going Beyond Risk Factor: Childhood Maltreatment and Associated Modifiable Targets to Improve Life-long Outcomes in Mood Disorders.” This Issue. [DOI] [PubMed] [Google Scholar]
- Lippard ETC, Nemeroff CB, 2020. The devastating clinical consequences of child abuse and neglect: increased disease vulnerability and poor treatment response in mood disorders. Am. J. Psychiatry 177 (1), 20–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovallo WR, 2006. Cortisol secretion patterns in addiction and addiction risk. Int. J. Psychophysiol 59 (3), 195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C, 2009. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat. Rev. Neurosci 10 (6), 434–445. [DOI] [PubMed] [Google Scholar]
- Lyons DM, Lopez JM, Yang C, Schatzberg AF, 2000. Stress-level cortisol treatment impairs inhibitory control of behavior in monkeys. J. Neurosci 20 (20), 7816–7821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Loredo V, Fernández Hermida J, 2019. In: Impulsivity-targeted Selective Preventive Interventions and Treatments in Addictive Behaviors, p. 6. [Google Scholar]
- Martin-Gutierrez G, Wallander JL, Yang Y, Depaoli S, Elliott MN, Coker TR, Schuster MA, 2021. Racial/Ethnic differences in the relationship between stressful life events and quality of life in adolescents. J. Adolesc. Health 68, 292–299. [DOI] [PubMed] [Google Scholar]
- Marusak HA, Martin KR, Etkin A, Thomason ME, 2015. Childhood trauma exposure disrupts the automatic regulation of emotional processing. Neuropsychopharmacology 40 (5), 1250–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason A, Jean Russo M, Chmelka MB, Herrenkohl RC, Herrenkohl TI, 2017. Parent and peer pathways linking childhood experiences of abuse with marijuana use in adolescence and adulthood. Addict. Behav 66, 70–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald JL, Meyer TD, 2011. Self-report reasons for alcohol use in bipolar disorders: why drink despite the potential risks? Clin. Psychol. Psychother 18, 418–425. [DOI] [PubMed] [Google Scholar]
- McEwen BS, 2007. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol. Rev 87 (3), 873–904. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Brake W, Gratton A, 2002. Environmental regulation of the development of mesolimbic dopamine systems: a neurobiological mechanism for vulnerability to drug abuse? Psychoneuroendocrinology 27 (1–2), 127–138. [DOI] [PubMed] [Google Scholar]
- Medina KL, McQueeny T, Nagel BJ, Hanson KL, Schweinsburg AD, Tapert SF, 2008. Prefrontal cortex volumes in adolescents with alcohol use disorders: unique gender effects. Alcohol. Clin. Exp. Res 32 (3), 386–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta MA, Gore-Langton E, Golembo N, Colvert E, Williams SC, Sonuga-Barke E, 2010. Hyporesponsive reward anticipation in the basal ganglia following severe institutional deprivation early in life. J. Cogn. Neurosci 22 (10), 2316–2325. [DOI] [PubMed] [Google Scholar]
- Merrill JE, Wardell JD, Read JP, 2014. Drinking motives in the prospective prediction of unique alcohol-related consequences in college students. J. Stud. Alcohol Drugs 75, 93–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezquita L, Ibáñez MI, Moya J, Villa H, Ortet G, 2014. A longitudinal examination of different etiological pathways to alcohol use and misuse. Alcohol. Clin. Exp. Res 38 (6), 1770–1779. [DOI] [PubMed] [Google Scholar]
- Miller GE, Chen E, Zhou ES, 2007. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychol. Bull 133 (1), 25. [DOI] [PubMed] [Google Scholar]
- Mills R, Alati R, Strathearn L, Najman JM, 2014. Alcohol and tobacco use among maltreated and non-maltreated adolescents in a birth cohort. Addiction 109 (4), 672–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming GL, Song H, 2005. Adult neurogenesis in the mammalian central nervous system. Annu. Rev. Neurosci 28, 223–250. [DOI] [PubMed] [Google Scholar]
- Moffett MC, Vicentic A, Kozel M, Plotsky P, Francis DD, Kuhar MJ, 2007. Maternal separation alters drug intake patterns in adulthood in rats. Biochem. Pharmacol 73 (3), 321–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffitt TE, 2005. The new look of behavioral genetics in developmental psychopathology: gene-environment interplay in antisocial behaviors. Psychol. Bull 131 (4), 533–554. [DOI] [PubMed] [Google Scholar]
- Moss HB, Vanyukov M, Yao JK, Kirillova GP, 1999. Salivary cortisol responses in prepubertal boys: the effects of parental substance abuse and association with drug use behavior during adolescence. Biol. Psychiatry 45 (10), 1293–1299. [DOI] [PubMed] [Google Scholar]
- Muehlhan M, Höcker A, Miller R, Trautmann S, Wiedemann K, Lotzin A, Barnow S, Schäfer I, 2020. HPA axis stress reactivity and hair cortisol concentrations in recently detoxified alcoholics and healthy controls with and without childhood maltreatment. Addict. Biol 25 (1), e12681. [DOI] [PubMed] [Google Scholar]
- Mueller SC, Maheu FS, Dozier M, Peloso E, Mandell D, Leibenluft E, Pine DS, Ernst M, 2010. Early-life stress is associated with impairment in cognitive control in adolescence: an fMRI study. Neuropsychologia 48, 3037–3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel BJ, Schweinsburg AD, Phan V, Tapert SF, 2005. Reduced hippocampal volume among adolescents with alcohol use disorders without psychiatric comorbidity. Psychiatry Res. 139 (3), 181–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson EC, Agrawal A, Pergadia ML, Wang JC, Whitfield JB, Saccone FS, Kern J, Grant JD, Schrage AJ, Rice JP, Montgomery GW, Heath AC, Goate AM, Martin NG, Madden PA, 2010. H2 haplotype at chromosome 17q21.31 protects against childhood sexual abuse-associated risk for alcohol consumption and dependence. Addict. Biol 15 (1), 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeroff CB, 2016. Paradise lost: the neurobiological and clinical consequences of child abuse and neglect. Neuron 89 (5), 892–909. [DOI] [PubMed] [Google Scholar]
- Norberg MM, Norton AR, Olivier J, Zvolensky MJ, 2010. Social anxiety, reasons for drinking, and college students. Behav. Ther 41, 555–566. [DOI] [PubMed] [Google Scholar]
- Norman AL, Pulido C, Squeglia LM, Spadoni AD, Paulus MP, Tapert SF, 2011. Neural activation during inhibition predicts initiation of substance use in adolescence. Drug Alcohol Depend. 119 (3), 216–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ompad DC, Ikeda RM, Shah N, Fuller CM, Bailey S, Morse E, Kerndt P, Maslow C, Wu Y, Vlahov D, Garfein R, Strathdee SA, 2005. Childhood sexual abuse and age at initiation of injection drug use. Am. J. Public Health 95 (4), 703–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opel N, Redlich R, Zwanzger P, Grotegerd D, Arolt V, Heindel W, Konrad C, Kugel H, Dannlowski U, 2014. Hippocampal atrophy in major depression: a function of childhood maltreatment rather than diagnosis? Neuropsychopharmacology 39, 2723–2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlando M, Tucker JS, Ellickson PL, Klein DJ, 2004. Developmental trajectories of cigarette smoking and their correlates from early adolescence to young adulthood. J. Consult. Clin. Psychol 72 (3), 400–410. [DOI] [PubMed] [Google Scholar]
- Oshri A, Rogosch FA, Burnette ML, Cicchetti D, 2011. Developmental pathways to adolescent cannabis abuse and dependence: child maltreatment, emerging personality, and internalizing versus externalizing psychopathology. Psychol. Addict. Behav 25 (4), 634–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshri A, Kogan SM, Kwon JA, Wickrama KAS, Vanderbroek L, Palmer AA, MacKillop J, 2018. Impulsivity as a mechanism linking child abuse and neglect with substance use in adolescence and adulthood. Dev. Psychopathol 30 (2), 417–435. [DOI] [PubMed] [Google Scholar]
- Oshri A, Liu S, Duprey EB, MacKillop J, 2018. Child maltreatment, delayed reward discounting, and alcohol and other drug use problems: the moderating role of heart rate variability. Alcohol. Clin. Exp. Res 42 (10), 2033–2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshri A, Gray JC, Owens MM, Liu S, Duprey EB, Sweet LH, MacKillop J, 2019. Adverse childhood experiences and amygdalar reduction: high-resolution segmentation reveals associations with subnuclei and psychiatric outcomes. Child Maltreat. 24 (4), 400–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oswald LM, Wand GS, Kuwabara H, Wong DF, Zhu S, Brasic JR, 2014. History of childhood adversity is positively associated with ventral striatal dopamine responses to amphetamine. Psychopharmacology 231 (12), 2417–2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park T, Thompson K, Wekerle C, Al-Hamdani M, Smith S, Hudson A, Goldstein A, Stewart SH, 2019. Posttraumatic stress symptoms and coping motives mediate the association between childhood maltreatment and alcohol problems. J. Trauma. Stress 32 (6), 918–926. [DOI] [PubMed] [Google Scholar]
- Pearlin LI, Schieman S, Fazio EM, Meersman SC, 2005. Stress, health, and the life course: some conceptual perspectives. J. Health Soc. Behav 46, 205–219. [DOI] [PubMed] [Google Scholar]
- Peverill M, Sheridan MA, Busso DS, McLaughlin KA, 2019. Atypical prefrontal-amygdala circuitry following childhood exposure to abuse: links with adolescent psychopathology. Child Maltreat. 24 (4), 411–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Kwon D, Brumback T, Thompson WK, Cummins K, Tapert SF, Brown SA, Colrain IM, Baker FC, Prouty D, De Bellis MD, Clark DB, Nagel BJ, Chu W, Park SH, Pohl KM, Sullivan EV, 2018. Altered brain developmental trajectories in adolescents after initiating drinking. Am. J. Psychiatry 175 (4), 370–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza PV, Le Moal ML, 1996. Pathophysiological basis of vulnerability to drug abuse: role of an interaction between stress, glucocorticoids, and dopaminergic neurons. Annu. Rev. Pharmacol. Toxicol 36, 359–378. [DOI] [PubMed] [Google Scholar]
- Pilowsky DJ, Keyes KM, Hasin DS, 2009. Adverse childhood events and lifetime alcohol dependence. Am. J. Public Health 99 (2), 258–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor LJ, Lewis T, Roesch S, Thompson R, Litrownik AJ, English D, Arria AM, Isbell P, Dubowitz H, 2017. Child maltreatment and age of alcohol and marijuana initiation in high-risk youth. Addict. Behav 75, 64–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruessner JC, Champagne F, Meaney MJ, Dagher A, 2004. Dopamine release in response to a psychological stress in humans and its relationship to early life maternal care: a positron emission tomography study using [11C]raclopride. J. Neurosci 24 (11), 2825–2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryce CR, Dettling AC, Spengler M, Schnell CR, Feldon J, 2004. Deprivation of parenting disrupts development of homeostatic and reward systems in marmoset monkey offspring. Biol. Psychiatry 56 (2), 72–79. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Rocher AB, Janssen WG, Hof PR, McEwen BS, Morrison JH, 2005. Reversibility of apical dendritic retraction in the rat medial prefrontal cortex following repeated stress. Exp. Neurol 196 (1), 199–203. [DOI] [PubMed] [Google Scholar]
- Ramos-Olazagasti MA, Bird HR, Canino GJ, Duarte CS, 2017. Childhood adversity and early initiation of alcohol use in two representative samples of puerto rican youth. J. Youth Adolesc 46 (1), 28–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond-Rakerd LS, Slutske WS, Wood PK, 2017. Age of initiation and substance use progression: a multivariate latent growth analysis. Psychol. Addict. Behav 31 (6), 664–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo RD, McEwen BS, 2006. Stress and the adolescent brain. Ann. N. Y. Acad. Sci 1094, 202–214. [DOI] [PubMed] [Google Scholar]
- Rothman EF, Edwards EM, Heeren T, Hingson RW, 2008. Adverse childhood experiences predict earlier age of drinking onset: results from a representative US sample of current or former drinkers. Pediatrics 122 (2), e298–e304. [DOI] [PubMed] [Google Scholar]
- Saleh A, Potter GG, McQuoid DR, Boyd B, Turner R, MacFall JR, Taylor WD, 2017. Effects of early life stress on depression, cognitive performance and brain morphology. Psychol. Med 47, 171–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartor CE, Grant JD, Few LR, Werner KB, McCutcheon VV, Duncan AE, Nelson EC, Madden PAF, Bucholz KK, Heath AC, Agrawal A, 2018. Childhood trauma and two stages of alcohol use in African American and European American women: findings from a female twin sample. Prev. Sci 19 (6), 795–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäfer I, Teske L, Schulze-Thusing J, Homann K, Reimer J, Haasen C, Hissbach J, Wiedemann K, 2010. Impact of childhood trauma on hypothalamus-pituitary-adrenal axis activity in alcohol-dependent patients. Eur. Addict. Res 16, 108–114. [DOI] [PubMed] [Google Scholar]
- Schmid B, Blomeyer D, Treutlein J, Zimmermann US, Buchmann AF, Schmidt MH, Esser G, Rietschel M, Banaschewski T, Schumann G, Laucht M, 2010. Interacting effects of CRHR1 gene and stressful life events on drinking initiation and progression among 19-year-olds. Int. J. Neuropsychopharmacol 13 (6), 703–714. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Kalmijn JA, Smith TL, Saunders G, Fromme K, 2012. Structuring a college alcohol prevention program on the low level of response to alcohol model: a pilot study. Alcohol. Clin. Exp. Res 36, 1244–1252. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Kalmijn J, Skidmore J, Clausen P, Shafir A, Saunders G, Bystritsky H, Fromme K, 2015. The impact of focusing a program to prevent heavier drinking on a pre-existing phenotype, the low level of response to alcohol. Alcohol. Clin. Exp. Res 39, 308–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Clausen P, Fromme K, Skidmore J, Shafir A, Kalmijn J, 2016. The low level of response to alcohol-based heavy drinking prevention program: one-year follow-up. J. Stud. Alcohol Drugs 77, 25–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott KM, Smith DR, Ellis PM, 2010. Prospectively ascertained child maltreatment and its association with DSM-IV mental disorders in young adults. Arch. Gen. Psychiatry 67 (7), 712–719. [DOI] [PubMed] [Google Scholar]
- Seo D, Tsou KA, Ansell EB, Potenza MN, Sinha R, 2014. Cumulative adversity sensitizes neural response to acute stress: association with health symptoms. Neuropsychopharmacology 39 (3), 670–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo D, Rabinowitz AG, Douglas RJ, Sinha R, 2019. Limbic response to stress linking life trauma and hypothalamus-pituitary-adrenal axis function. Psychoneuroendocrinology 99, 38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan MA, Fox NA, Zeanah CH, McLaughlin KA, Nelson CA 3rd, 2012. Variation in neural development as a result of exposure to institutionalization early in childhood. Proc. Natl. Acad. Sci. U. S. A 109, 12927–12932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin SH, Edwards EM, Heeren T, 2009. Child abuse and neglect: relations to adolescent binge drinking in the national longitudinal study of adolescent health (AddHealth) study. Addict. Behav 34 (3), 277–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin SH, Hong HG, Hazen AL, 2010. Childhood sexual abuse and adolescent substance use: a latent class analysis. Drug Alcohol Depend. 109 (1–3), 226–235. [DOI] [PubMed] [Google Scholar]
- Shin SH, Miller DP, Teicher MH, 2013. Exposure to childhood neglect and physical abuse and developmental trajectories of heavy episodic drinking from early adolescence into young adulthood. Drug Alcohol Depend. 127 (1), 31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin SH, Lee S, Jeon SM, Wills TA, 2015. Childhood emotional abuse, negative emotion-driven impulsivity, and alcohol use in young adulthood. Child Abuse Negl. 50, 94–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin SH, McDonald SE, Conley D, 2018. Profiles of adverse childhood experiences and impulsivity. Child Abuse Negl. 85, 118–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin SH, Jiskrova GK, Wills TA, 2019. Childhood maltreatment and alcohol use in young adulthood: the role of self-regulation processes. Addict. Behav 90, 241–249. [DOI] [PubMed] [Google Scholar]
- Shin SH, Wang X, Yoon SH, Cage JL, Kobulsky JM, Montemayor BN, 2019. Childhood maltreatment and alcohol-related problems in young adulthood: the protective role of parental warmth. Child Abuse Negl. 98, 104238. [DOI] [PubMed] [Google Scholar]
- Shin SH, Jiskrova GK, Yoon SH, Kobulsky JM, 2020. Childhood maltreatment, motives to drink and alcohol-related problems in young adulthood. Child Abuse Negl. 108, 104657. [DOI] [PubMed] [Google Scholar]
- Shin SH, Ksinan Jiskrova G, Yoon SH, Kobulsky JM, 2020. Childhood maltreatment and problematic alcohol use in young adulthood: the roles of cognitive vulnerability to depression and depressive symptoms. Am. J. Drug Alcohol Abuse 46 (4), 438–446. [DOI] [PubMed] [Google Scholar]
- Silveri MM, 2012. Adolescent brain development and underage drinking in the United States: identifying risks of alcohol use in college populations. Harv. Rev. Psychiatry 20, 189–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, 2001. How does stress increase risk of drug abuse and relapse? Psychopharmacology 158 (4), 343–359. [DOI] [PubMed] [Google Scholar]
- Sinha R, 2008. Chronic stress, drug use, and vulnerability to addiction. Ann. N. Y. Acad. Sci 1141, 105–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Lacadie CM, Constable RT, Seo D, 2016. Dynamic neural activity during stress signals resilient coping. Proc. Natl. Acad. Sci. U. S. A 113 (31), 8837–8842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skeer M, McCormick MC, Normand SL, Buka SL, Gilman SE, 2009. A prospective study of familial conflict, psychological stress, and the development of substance use disorders in adolescence. Drug Alcohol Depend. 104 (1–2), 65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner ML, Hong S, Herrenkohl TI, Brown EC, Lee JO, Jung H, 2016. Longitudinal effects of early childhood maltreatment on co-occurring substance misuse and mental health problems in adulthood: the role of adolescent alcohol use and depression. J. Stud. Alcohol Drugs 77 (3), 464–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyke AT, Zeanah CH Jr., Fox NA, Nelson CA III, 2009. A new model of foster care for young children: the Bucharest early intervention project. Child Adolesc. Psychiatr. Clin. N. Am 18, 721–734. [DOI] [PubMed] [Google Scholar]
- Spear LP, 2000. The adolescent brain and age-related behavioral manifestations. Neurosci. Biobehav. Rev 24 (4), 417–463. [DOI] [PubMed] [Google Scholar]
- Squeglia LM, Pulido C, Wetherill RR, Jacobus J, Brown GG, Tapert SF, 2012. Brain response to working memory over three years of adolescence: influence of initiating heavy drinking. J. Stud. Alcohol Drugs 73 (5), 749–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia LM, Rinker DA, Bartsch H, Castro N, Chung Y, Dale AM, Jernigan TL, Tapert SF, 2014. Brain volume reductions in adolescent heavy drinkers. Dev. Cogn. Neurosci 9, 117–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripada CS, Angstadt M, McNamara P, King AC, Phan KL, 2011. Effects of alcohol on brain responses to social signals of threat in humans. NeuroImage 55 (1), 371–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stautz K, Cooper A, 2014. Urgency traits and problematic substance use in adolescence: direct effects and moderation of perceived peer use. Psychol. Addict. Behav 28 (2), 487–497. [DOI] [PubMed] [Google Scholar]
- Strakowski SM, Fleck DE, DelBello MP, Adler CM, Shear PK, Kotwal R, Arndt S, 2010. Impulsivity across the course of bipolar disorder. Bipolar Disord. 12, 285–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subodh BN, Sahoo S, Basu D, Mattoo SK, 2019. Age of onset of substance use in patients with dual diagnosis and its association with clinical characteristics, risk behaviors, course, and outcome: a retrospective study. Indian J. Psychiatry 61, 359–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administraction (SAMHSA), 1999. National Household Survey on Drug Abuse : 1997 Public Release Codebook. US Department of Health and Human Services, Rockville, MD. [Google Scholar]
- Taffe MA, Kotzebue RW, Crean RD, Crawford EF, Edwards S, Mandyam CD, 2010. Long-lasting reduction in hippocampal neurogenesis by alcohol consumption in adolescent nonhuman primates. Proc. Natl. Acad. Sci. U. S. A 107 (24), 11104–11109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarter RE, 2002. Etiology of adolescent substance abuse: a developmental perspective. Am. J. Addict 11 (3), 171–191. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Khan A, 2019. Childhood maltreatment, cortical and amygdala morphometry, functional connectivity, laterality, and psychopathology. Child Maltreat. 24 (4), 458–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher MH, Samson JA, 2013. Childhood maltreatment and psychopathology: a case for ecophenotypic variants as clinically and neurobiologically distinct subtypes. Am. J. Psychiatry 170 (10), 1114–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher MH, Samson JA, 2016. Annual research review: enduring neurobiological effects of childhood abuse and neglect. J. Child Psychol. Psychiatry 57 (3), 241–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher MH, Samson JA, Anderson CM, Ohashi K, 2016. The effects of childhood maltreatment on brain structure, function and connectivity. Nat. Rev. Neurosci 17 (10), 652–666. [DOI] [PubMed] [Google Scholar]
- Thomason ME, Marusak HA, Tocco MA, Vila AM, McGarragle O, Rosenberg DR, 2015. Altered amygdala connectivity in urban youth exposed to trauma. Soc. Cogn. Affect. Neurosci 10 (11), 1460–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonmyr L, Thornton T, Draca J, Wekerle C, 2010. A review of childhood maltreatment and adolescent substance use relationship. Curr. Psychiatr. Rev 6 (3), 223–234. [Google Scholar]
- Trickett PK, Noll JG, Susman EJ, Shenk CE, Putnam FW, 2010. Attenuation of cortisol across development for victims of sexual abuse. Dev. Psychopathol 22 (1), 165–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dam NT, Rando K, Potenza MN, Tuit K, Sinha R, 2014. Childhood maltreatment, altered limbic neurobiology, and substance use relapse severity via trauma-specific reductions in limbic gray matter volume. JAMA Psychiatry 71 (8), 917–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanTieghem MR, Tottenham N, 2018. Neurobiological programming of early life stress: functional development of amygdala-prefrontal circuitry and vulnerability for stress-related psychopathology. Curr. Top. Behav. Neurosci 38, 117–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varghese FP, Brown ES, 2001. The hypothalamic-pituitary-adrenal axis in major depressive disorder: a brief primer for primary care physicians. Prim. Care Companion J. Clin. Psychiatry 3, 151–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez V, Penit-Soria J, Durand C, Besson MJ, Giros B, Dauge V, 2006. Brief early handling increases morphine dependence in adult rats. Behav. Brain Res 170 (2), 211–218. [DOI] [PubMed] [Google Scholar]
- Vyas A, Pillai AG, Chattarji S, 2004. Recovery after chronic stress fails to reverse amygdaloid neuronal hypertrophy and enhanced anxiety-like behavior. Neuroscience 128 (4), 667–673. [DOI] [PubMed] [Google Scholar]
- Vythilingam M, Heim C, Newport J, Miller AH, Anderson E, Bronen R, Brummer M, Staib L, Vermetten E, Charney DS, Nemeroff CB, Bremner JD, 2002. Childhood trauma associated with smaller hippocampal volume in women with major depression. Am. J. Psychiatry 159, 2072–2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wand GS, Oswald LM, McCaul ME, Wong DF, Johnson E, Zhou Y, Kuwabara H, Kumar A, 2007. Association of amphetamine-induced striatal dopamine release and cortisol responses to psychological stress. Neuropsychopharmacology 32 (11), 2310–2320. [DOI] [PubMed] [Google Scholar]
- Wardell JD, Strang NM, Hendershot CS, 2016. Negative urgency mediates the relationship between childhood maltreatment and problems with alcohol and cannabis in late adolescence. Addict. Behav 56, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg NZ, Rahdert E, Colliver JD, Glantz MD, 1998. Adolescent substance abuse: a review of the past 10 years. J. Am. Acad. Child Adolesc. Psychiatry 37 (3), 252–261. [DOI] [PubMed] [Google Scholar]
- White AM, Swartzwelder H.Scott, 2005. Age-related effects of alcohol on memory and memory-related brain function in adolescents and adults. In: Galanter M, Lowman C, Boyd GM (Eds.), Recent Developments in Alcoholism: Alcohol Problems in Adolescents and Young Adults. Springer US, Boston, MA, pp. 161–176. [DOI] [PubMed] [Google Scholar]
- Whittle S, Dennison M, Vijayakumar N, Simmons JG, Yücel M, Lubman DI, Pantelis C, Allen NB, 2013. Childhood maltreatment and psychopathology affect brain development during adolescence. J. Am. Acad. Child Adolesc. Psychiatry 52 (9), 940–952.e941. [DOI] [PubMed] [Google Scholar]
- Widom CS, White HR, Czaja SJ, Marmorstein NR, 2007. Long-term effects of child abuse and neglect on alcohol use and excessive drinking in middle adulthood. J. Stud. Alcohol Drugs 68, 317–326. [DOI] [PubMed] [Google Scholar]
- Williams DR, 2018. Stress and the mental health of populations of color: advancing our understanding of race-related stressors. J. Health Soc. Behav 59, 466–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittchen H-U, Fröhlich C, Behrendt S, Günther A, Rehm J, Zimmermann P, Lieb R, Perkonigg A, 2007. Cannabis use and cannabis use disorders and their relationship to mental disorders: a 10-year prospective-longitudinal community study in adolescents. Drug Alcohol Depend. 88, S60–S70. [DOI] [PubMed] [Google Scholar]
- Wood MD, Read JP, Mitchell RE, Brand NH, 2004. Do parents still matter? Parent and peer influences on alcohol involvement among recent high school graduates. Psychol. Addict. Behav 18 (1), 19. [DOI] [PubMed] [Google Scholar]
- Wu NS, Schairer LC, Dellor E, Grella C, 2010. Childhood trauma and health outcomes in adults with comorbid substance abuse and mental health disorders. Addict. Behav 35 (1), 68–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda R, Engel SM, Brand SR, Seckl J, Marcus SM, Berkowitz GS, 2005. Transgenerational effects of posttraumatic stress disorder in babies of mothers exposed to the world trade center attacks during pregnancy. J. Clin. Endocrinol. Metab 90, 4115–4118. [DOI] [PubMed] [Google Scholar]
- Yoon S, Shi Y, Yoon D, Pei F, Schoppe-Sullivan S, Snyder SM, 2020. Child maltreatment, fathers, and adolescent alcohol and marijuana use trajectories. Subst. Use Misuse 55 (5), 721–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young-Wolff KC, Kendler KS, Prescott CA, 2012. Interactive effects of childhood maltreatment and recent stressful life events on alcohol consumption in adulthood. J. Stud. Alcohol Drugs 73 (4), 559–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker RA, Heitzeg MM, Nigg JT, 2011. Parsing the undercontrol–disinhibition pathway to substance use disorders: a multilevel developmental problem. Child Dev. Perspect 5 (4), 248–255. [DOI] [PMC free article] [PubMed] [Google Scholar]