Abstract
Objective:
To determine the dose-dependent effect of contemporary delta-9-tetrahydrocannabinol (THC) exposure on male testes and reproductive health in a non-human primate model (NHP).
Design:
Research animal study.
Setting:
Research institute environment.
Patient(s):
Adult male rhesus macaques (8–10 years of age; n=6).
Intervention(s):
Daily THC edible at medically and recreationally relevant contemporary doses.
Main Outcome Measure(s):
Testicular volume and epididymal head width, serum inhibin B, albumin, total testosterone, prolactin (PRL), follicle stimulating hormone (FSH), estradiol (E2), and luteinizing hormone (LH); semen volume, and sperm motility, morphology, and concentration.
Result(s):
For every mg/7kg/day increase in THC dosing, there was a marked loss in total testicular volume bilaterally by 11.8 cm3 (95% CI: 8.3–15.4, p<0.001). In total, average bilateral testicular volume decreased by 58%. A dose-response significant decrease in mean total testosterone by 1.49 ng/ml (95% CI: 0.83–2.15, p<0.001) and estradiol by 3.8 pg/ml (95% CI: 2.2–5.4, p<0.001), but a significant increase in FSH by 0.06 ng/ml (95% CI: 0.02–0.10, p=0.001), LH by 0.16 ng/ml (95% CI: 0.08–0.25, p<0.001), and prolactin by 7.4 ng/ml (95%CI: 3.4–11.3, p<0.001) were also observed. There were no statistically significant changes in semen parameters present.
Conclusion(s):
In rhesus macaques, chronic THC exposure resulted in significant dose-response testicular atrophy, increased gonadotropins, and decreased serum sex steroids, suggestive of primary testicular failure. Further studies are needed to determine if reversal of these observed adverse effects would occur if THC was discontinued and for validation of findings in a human cohort.
Keywords: marijuana, delta-9-tetrahydrocannabinol, testicular volume, male reproductive health, cannabis, sperm, testosterone
Capsule:
Increasing doses of chronic delta-9-tetrahydrocannabinol results in a dose-response relationship of decreased testicular volume and an adverse impact on male reproductive health.
INTRODUCTION
Marijuana is the most commonly used federally illegal drug in the United States (US) and worldwide with increasing popularity as both a recreational and medicinal drug, especially among men of reproductive age (1,2). In 2015, the estimated prevalence of past year use amongst 18- to 25-year-old men in the US was 36% (or about 7.4 million) (3). This high prevalence is due in part to the recent legalization trend, which has increased the availability of marijuana products and its perceived safety. Marijuana’s main active ingredient, delta-9-tetrahydrocannabinol (THC) is mediated through cannabinoid receptors 1 (CB1) and 2 (CB2). The available published studies on the effect of marijuana exposure on male infertility are inconsistent. Previous studies have demonstrated the presence of cannabinoid receptors in the male reproductive tract (4) and on sperm, and that the endocannabinoid system has a role in regulating male reproduction, suggesting the potential for marijuana to disrupt sperm function (5,6). Given the rising prevalence of marijuana use, it is critical to determine whether chronic marijuana use, a modifiable risk factor, adversely impacts male reproductive health.
The hypothalamic-pituitary-gonadal (HPG) axis plays a critical role in both spermatogenesis and testosterone production. This axis regulates the release of two vital gonadotropin hormones for reproduction, follicle-stimulating hormone (FSH) and luteinizing hormone (LH), which act on cells in the testes including Leydig cells, which produce testosterone. Previous animal studies suggest the impact of the endocannabinoid system on spermatogenesis (7) includes inhibition of Leydig cell function and steroidogenesis, reduction in gonadotropins9−11 testicular atrophy (12–16), and abnormal sperm morphology (17,18) following acute exposure to THC. The effect of chronic marijuana use in men is uncertain with some studies reporting an association with lower testosterone and LH levels (19,20) and poorer semen quality (21–24), while other studies could not replicate these findings (21,23,25–27). A large population-based study of healthy, young Danish men reported an increase in testosterone levels, but lower sperm count, among regular marijuana smokers (23). However, the direct effects of regular contemporary marijuana exposure on sperm concentration, motility and function have not been well studied.
Prior human studies assessing the effects of marijuana exposure on the male reproductive system have focused largely on male subjects typically being recruited from assisted reproductive centers or with polysubstance abuse histories, limiting the generalizability of the findings and precluding determination of a causal effect specific to marijuana (17,28–31). The majority of these studies are also observational or retrospective. Additionally, unlike alcohol where a shot, a glass of wine, and a beer have similar alcohol content based on the volume they contain, no such equivalency for marijuana exists because the different strains of marijuana plants and delivery mechanisms vary in potency and there are not consistent labeling and formulation (32). This heterogeneity in marijuana potency has also resulted in limited knowledge to counsel patients who are unable to abstain, regarding a dose-response effect from marijuana use.
To better understand the impact of chronic marijuana use on male fertility and reproductive health, a relevant, translational animal model such as the non-human primate (NHP) can overcome the obstacles and limitations of human studies. Compared with other animal models, the NHP offers many advantages, including similar plasma THC disposition (33,34), physiologic, genetic, anatomical and endocrine properties to humans resulting in observations that are directly translatable to humans (35,36). Additionally, the NHP model allows for minimization of inter-subject variability and potential confounders, to determine the direct effects of THC only. The objective of our study was to determine the dose-dependent effect of contemporary THC exposure on male testes and reproductive health in a NHP model.
MATERIALS AND METHODS
Experimental Design
A cohort of sexually mature, adult male rhesus macaques (Macaca mulatta) (n=6) ages 8–10 years old and weighing 9.3–12.7kg, with prior proven paternity, were used in this study. The animals were socially-housed and all procedures were approved by the Oregon National Primate Research Center (ONPRC) Institutional Animal Care and Use Committee (IACUC) and conformed to all applicable regulations (IP0001389). Animals were maintained on a standard chow diet (TestDiet, St. Louis, Missouri) with a daily cookie containing THC (THC edible) made using research-grade THC obtained directly from the National Institute of Drug Administration (NIDA) Drug Supply Program as previously published (37). Animals were fed a diet of fresh chow and produce enrichment, and only water was available ad libitum. All cookies were administered prior to the animal’s morning chow to ensure they were consumed on an empty stomach and to confirm complete ingestion. Animals were slowly titrated up to 2.5mg/7kg/day of THC with a dose increase every 70 days (the NHP sperm life cycle is approximately 64 days) over approximately a 7 month time period to model published medical marijuana acclimation recommendations (38). Specifically, animals were initially maintained on a dose of 0.5mg/7kg/day of THC for days 1 to 70, 1mg/7kg/day (moderate THC dose) for days 71–140, and 2.5mg/7kg/day (heavy THC dose) for days 141 to 210. This THC dosage was calculated from the recommended THC starting dose of 5mg (NIDA’s designated standard unit of THC for research) for a 68kg adult, followed by titration to 10mg for moderate users, and 20–30mg for heavy users (38–40). Most states with legalized marijuana consider 10mg of THC a single serving of marijuana (38). To minimize potential confounders and inter-animal variability, each individual animal served as its own control during the study.
Blood was sampled (2mL) at each dose adjustment time point during THC induction, three hours (38,40) following edible consumption, to determine peak THC concentrations with each increase in THC dosage. Immediately prior to each dosing increase, animal weight, scrotal ultrasound, and semen analysis were performed.
Scrotal Ultrasound
All animals underwent scrotal ultrasounds before THC was initiated and at the end of each THC dosing period. Testicular volume was calculated by the measurement of the maximal length (longitudinal diameter), width (transverse diameter) and height (anterior posterior diameter) of the testes. The maximal length and width of the epididymal head was measured in the longitudinal plane. These scans were performed with animals non-sedated in a sitting position by a single, ultrasound-trained practitioner (J.O.L), using image-directed pulsed and color Doppler equipment with a 5- to 9 MHz sector probe (GE Voluson) per standard human clinical practice protocols. Testicular volume was calculated using the standard clinical formula of: length (L) × width (W) × height (H). Color Doppler was used to evaluate for symmetry of flow to both testes, the testicular vasculature and the presence of varicoceles, which can also affect sperm quality and male fertility.
Semen Collection and Processing
Prior to THC initiation, all males were trained by the ONPRC Behavioral Services Unit for collaborative semen collection by non-sedated electro-ejaculations (41). Collections were performed on three separate occasions pre-THC for baseline semen measurements and at the end of each THC dosing timepoint. Semen samples were collected and allowed to liquefy at 37°C for 30 min before evaluation. The rhesus macaque ejaculate is comprised of liquid and solid (coagulum) fractions that were both measured. The volume of the liquid fraction was measured with a pipette and transferred into a sterile 15-ml conical tube. The coagulum was rinsed with 2–3 ml of warm HEPES-buffered Tyrode albumin lactate pyruvate (TALP-Hepes) with bovine serum albumin (BSA) supplemented at 3 mg/ml to recover additional sperm and the rinse was combined with the liquid fraction, before adding TALP-Hepes Q.S. to 12 ml. The sample was washed at 300 × g for 7 minutes. After the first wash, the supernatant (11 ml) was aspirated and the sperm pellet was resuspended in the remaining 1 ml. An aliquot was further diluted 1:20 into TALP-Hepes and analyzed by a computer assisted sperm analysis (CASA) system (IVOS II-Animal Motility software, version 1.11, Hamilton Thorne, Beverly, MA), programmed specifically for macaque sperm physiology to determine sperm motility parameters and concentration. The remaining sample was centrifuged again at 300 × g for 7 minutes to obtain a pellet. The supernatant was discarded and another aliquot was taken and diluted (1:60 to 1:100) for assessment of plasma membrane integrity (Live/dead Sperm Viability Kit, Catalog #L7011, Molecular Probes, Eugene, OR), mitochondrial membrane potential/mitochondrial activity (MitoTracker Orange, Catalog #M7510, Molecular Probes, Eugene, Oregon), and gross sperm morphology (10% formalin solution). To assess plasma membrane integrity, the washed sperm were mixed with the Live/dead stain, incubated at 37°C for 10 min, and a smear was prepared. A total of 100 spermatozoa were classified as live (intact plasma membrane) or dead (injured plasma membrane) using a fluorescence microscope. Assessment of mitochondrial membrane potential was performed by mixing washed sperm with MitoTracker orange and incubating at 37°C for 20 min before a smear was prepared. A total of 100 spermatozoa were classified as having active (stained) or inactive (non-stained) mitochondria using a fluorescence microscope. For assessment of gross sperm morphology, sperm were diluted in a pre-warmed (37°C) 10% formalin solution, resuspended and 10 μl of fixed spermatozoa were placed on a microscope slide under a coverslip and analyzed per standard clinical protocol per World Health Organization (WHO) criteria (42). A total of 100 spermatozoa were assessed using a phase contrast microscope at 1000 X magnification under oil immersion. Spermatozoa was classified under five main categories: abnormal head, abnormal midpiece, sharp bend/coiled tail, double head/tail or normal, and the percentage of normal sperm was compared across treatment groups.
Serum Hormones
Peripheral blood samples (2 mL collected pre-THC and at each THC dose) were obtained for total testosterone (T), follicle stimulating hormone (FSH), luteinizing hormone (LH) concentrations, estradiol (E2), prolactin (PRL), inhibin, and albumin by detailed assays below that were performed by the Endocrine Technologies Core (ETC) at the ONPRC. Total testosterone, FSH, LH, estradiol, prolactin, inhibin and albumin were tested to measure the effect of THC on the HPG axis and their critical role in regulating spermatogenesis.
Total Testosterone
Total testosterone (T) concentrations were measured by automatic immunoassay on a Roche Cobas e411 instrument (Roche Diagnostics, Indianapolis, IN). The assay range was 0.025–15 ng/ml. Intra-assay coefficient of variation (CV) using an in-house NHP serum quality control (QC) pool was 4.2% (n=1 assay).
FSH and LH
FSH and LH concentrations were measured using a double-antibody RIA procedure similar to that described by Niswender and Spies (43). The LH and FSH RIA kits were purchased from Dr. Albert Parlow (NHPP, Harbor-UCLA Medical Center, Los Angeles). These are homologous cynomolgus macaque assays with recombinant cynomolgus FSH (AFP-6940A) or LH (AFP-6936A) or for both iodination and standards. Rabbit anti-cynomolgus FSH (AFP-782594) or LH (AFP-342994) were used at final dilutions of 1:1,038,462 and 1:750,000 for FSH and LH, respectively. The standard curves ranged between 0.005 and 10 ng/tube for both assays. The detection limit of each assay was 0.005 – 0.02 ng/tube. Intra-assay variations were 6.5% and 4.7% for FSH and LH, respectively (n=1 assay).
Estradiol
Estradiol (E2) concentrations were determined by automatic immunoassay on a Roche Cobas e411 instrument (Roche Diagnostics, Indianapolis, IN). The assay range was 5–4300 pg/ml. Intra-assay CV using an in-house nonhuman primate serum QC pool was 0.7% (n=1 assay).
Prolactin
Serum prolactin (PRL) concentrations were determined by automatic immunoassay on a Roche Cobas e411 system (Roche Diagnostics, Indianapolis, IN). The assay range was 0.047–470 ng/ml. Intra-assay CV using an in-house nonhuman primate serum QC pool was 4.6%.
Inhibin B
Inhibin B concentrations were measured by ELISA following the manufacturer’s instructions (Beckman-Coulter, Pasadena, CA). The assay range was 11.5–1100 pg/ml. Intra-assay CV was 4.3% and inter-assay CV was 6.9% (n=2 assays).
Albumin
Albumin concentrations were determined by enzyme-linked immunoassay (ELISA) following the manufacturer’s instructions (MilliporeSigma, St. Louis, MO). Samples were diluted 1:50,000–1:1,000,000 for analysis. The assay range was 4.915–1200 ng/ml. Intra-assay CV was 2.3%; inter-assay CV was 9.0% (n=2 assays).
Tetrahydrocannabinol (THC) testing
Chemicals and Reagents
The Strata Impact Protein precipitation plate and 2 ml collection plates were from Phenomenex (Torrance, CA). Oasis Prime elution plates and 1 ml round collection plates were from Waters (Milford, MA). THC and metabolites as well as their deuterated internal standards were purchased from Cerilliant (Round Rock, TX). Acetonitrile, methanol and water were purchased from Honeywell (Mexico City, Mexico) and formic acid along with sample vials and other high performance liquid chromatography supplies were purchased from Fisher Scientific (Rockwood, TN). Human EDTA Plasma for standards was purchased from Innovative research (Novi, Michigan) with a voluntary drug free affidavit, though multiple samples were tested before a drug free matrix was obtained. Control rhesus macaque plasma was used for initial testing and quality controls. Preparation of plasma and calibration was performed per previously published protocol (37).
LC-MS/MS Analysis of Cannabinoid Metabolites
THC and metabolites were analyzed using a 5500 Q-TRAP hybrid/triple quadrupole linear ion trap mass spectrometer (SCIEX, Framingham, MA) with electrospray ionization (ESI) in positive mode per previously published protocol (37).
Statistical Analysis
We assessed the average association between THC dose and plasma THC levels, testicular measures, hormone levels, and semen parameters using linear mixed effects modeling with random intercepts by animal. We generated scatter plots of individual animal measurements with the predicted marginal changes from the mixed effects models for all outcomes. All statistical tests were two-sided and used an alpha of 0.05. All analyses were performed using Stata® version 15.1 (StataCorp, College Station, TX).
RESULTS
Of the six rhesus macaque males in this study, all were of reproductive age (mean of 9.1 years, SD=0.6) with prior proven paternity and no previous exposure to THC or any other significant known environmental exposures. The average baseline weight of all animals was 11.6kg (SD=1.4) and was 11.9kg (SD=1.3) at the highest THC dose; all animals gained weight after starting the THC treatment. For every mg/7kg/day increase in THC, there was an increase in average weight of all animals by 0.1kg (p=0.095) that was not significant. The animals’ behavior post-THC treatment was not noted to be grossly different per veterinary and animal support staff.
During the THC induction, average plasma THC concentrations increased by 2.54 ng/mol for each mg/7kg/day increase in THC (95% CI: 1.35–3.73 ng/mol, p<0.001) (Figure 1). With increasing THC edible dosing, a marked decrease in testicular volume was observed; average total bilateral testicular volume decreased by 58%. The average bilateral total testicular volume decreased by 12.6cm3 for each mg/7kg/day of THC (95% CI: 10.4–14.9, p<0.001) (Figure 2). A similar decrease was also observed in the left epididymal head width by 0.15cm (95% CI: 0.10–0.20, p<0.001) and right epididymal head width by 0.13cm (95% CI: 0.08–0.19, p<0.001) (Figure 2). No scrotal masses or varicoceles were noted on ultrasound or physical examination.
Figure 1. Plasma THC concentrations with increasing THC dosing.

Individual (dots) and average fixed effect (line) plasma THC concentration (ng/mol) in response to increasing oral THC dosage (0 to 2.5 mg/7kg/day) among 6 male rhesus macaques. *p-value < 0.001.
Figure 2. Significantly decreased testicular volume and epididymal head width with increasing THC dosing.
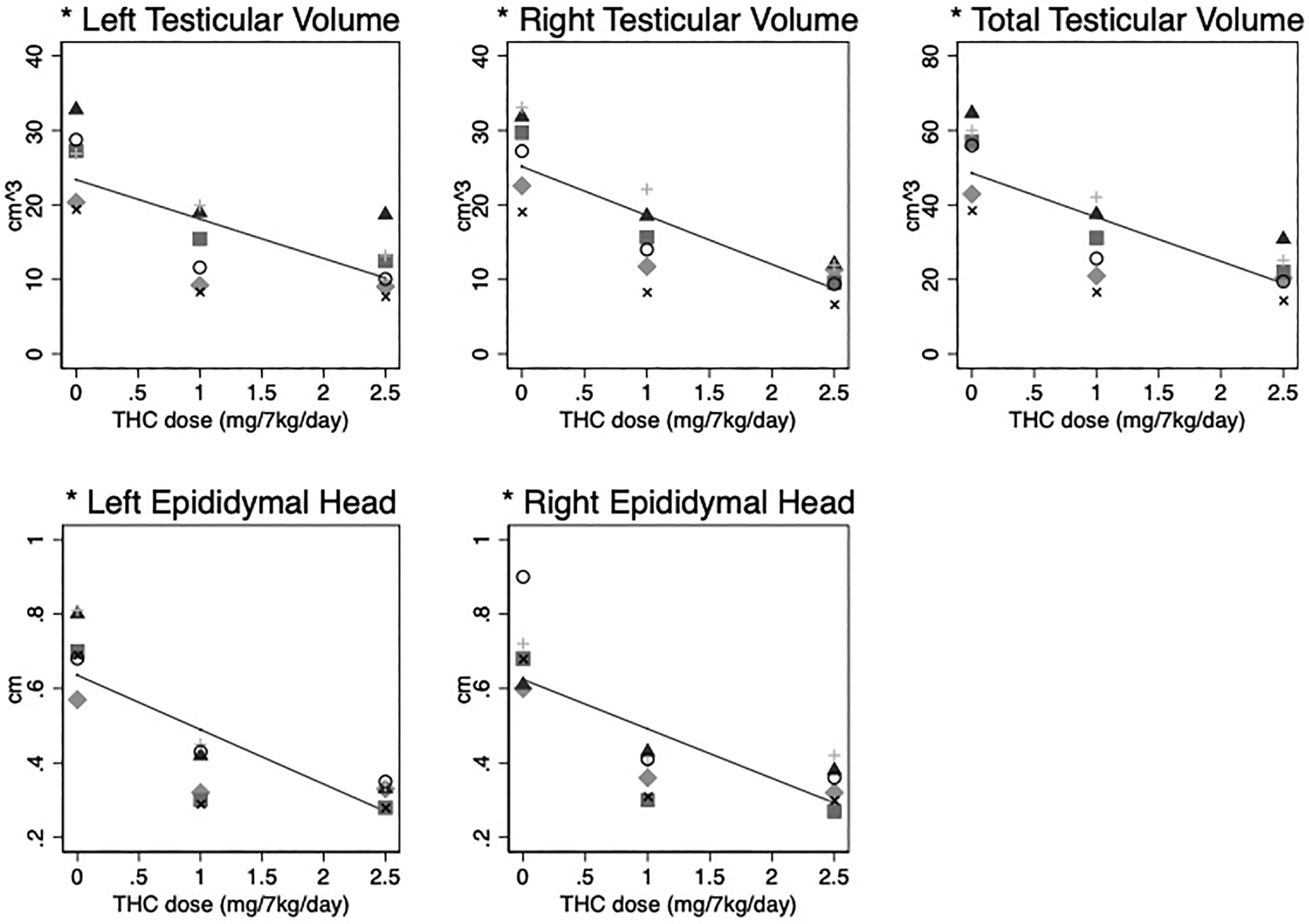
Individual (dots) and average fixed effect (lines) testicular volume (cm3) and epididymal head width (cm) in response to increasing oral THC dosage (0 to 2.5 mg/7kg/day) among 6 male rhesus macaques. *p-value < 0.001.
In terms of the reproductive endocrine axis, serum FSH, LH and prolactin concentrations rose significantly with increasing THC dose. FSH increased by 0.06 ng/ml (95% CI: 0.02–0.10, p=0.001), LH by 0.16 ng/ml (95% CI: 0.08–0.25, p<0.001), and prolactin by 7.4 ng/ml (95% CI: 3.4–11.3, p<0.001) for each mg/7kg/day THC (Figure 3). In contrast, a significant decreased dose-response was observed for total testosterone by 1.49 ng/ml (95% CI: 0.83–2.15, p<0.001) and estradiol by 3.8 pg/ml (95% CI: 2.2–5.4, p<0.001) for every mg/7kg/day THC increased (Figure 3). As anticipated, there was no statistically significant change in albumin concentration (p=0.735).
Figure 3. Follicle stimulating hormone (FSH), luteinizing hormone (LH), total testosterone, albumin, estradiol, prolactin, and inhibin concentrations with increasing THC dosing.
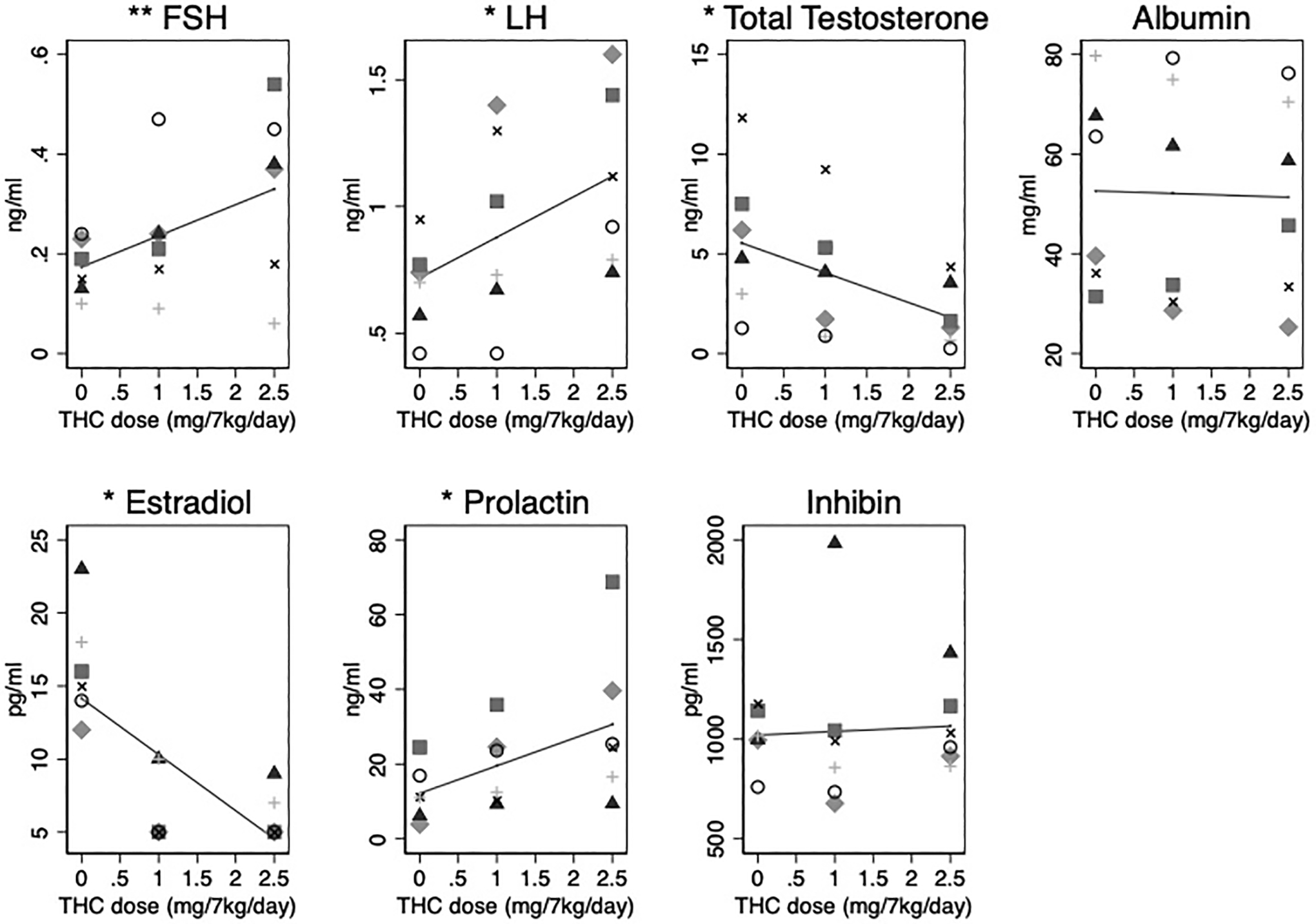
Individual (dots) and average fixed effect (lines) FSH (ng/ml), LH (ng/ml), total testosterone (ng/ml), albumin (μg/ml), estradiol (pg/ml), prolactin (ng/ml), and inhibin (pg/ml) concentration in response to increasing oral THC dosage (0 to 2.5 mg/7kg/day) among 6 male rhesus macaques. *p-value < 0.001, **p-value=0.001. All other hormone associations are not significant at the 0.05 level.
There were no statistically significant changes in any semen characteristics (Table 1) including weight of coagulum (p=0.084), liquid fraction volume (p=0.177), sperm concentration (p=0.187), total sperm count (p=0.128), motility (p=0.394) and morphology (p=0.438) with increasing THC dose.
Table 1:
Average semen characteristics (± standard deviation) of 6 rhesus macaques at 3 doses of oral THC (0 to 2.5 mg/7kg/day) and change with 1 mg/7kg/day increase in THC dose, with 95% confidence interval and associated p-value, from random intercept mixed effects model.
| 0mg/7kg/day THC | 1 mg/7kg/day THC | 2.5 mg/7kg/day THC | Change per 1mg/7kg/day THC dose | 95% CI | p-value | |
|---|---|---|---|---|---|---|
| Weight of coagulum (g) | 0.80±0.31 | 0.47±0.13 | 0.54±0.16 | −0.09 | −0.20–0.01 | 0.084 |
| Liquid fraction volume (ml) | 0.45±0.14 | 0.26±0.16 | 0.32±0.18 | −0.05 | −0.12–0.02 | 0.177 |
| Sperm conc. (million/ml) | 795±326 | 1483±1005 | 1388±1464 | 214 | −104–531 | 0.187 |
| Total sperm count (million) | 338±135 | 274±166 | 269±189 | −26 | −59–7 | 0.128 |
| Motility (%) | 88.9±6.2 | 86.3±8.4 | 87.0±7.9 | −0.7 | −2.3–0.9 | 0.394 |
| Morphology (% normal) | 57.8±26.0 | 55.0±18.8 | 51.2±22.1 | −3.0 | −10.6–4.6 | 0.438 |
DISCUSSION
This is the first study using a NHP model to examine the impact of chronic THC use on male reproductive health. Our study found a positive correlation between oral THC dosage and plasma concentration, as previously demonstrated (37), that confirms the success of our NHP oral THC dosing regimen. Average male plasma THC concentrations on the highest oral THC edible dose were also within the expected contemporary dosing range reported in humans 3 hours after consuming a similar oral THC dose (40,44). As observed in our prior study (37) and consistent with the existing literature, we did not find a significant THC effect on weight gain (45).
A significant dose-response impact of chronic THC use on testicular volume and epididymal head width was also observed. Average bilateral total testicular volume decreased by 58%. Males were exposed for a total of approximately seven months (e.g. three sperm lifecycles) with only the last two and a half months at an equivalent, heavy medical marijuana dose. Prior animal studies have also suggested a dose-dependent association between chronic marijuana exposure and decreased prostate and seminal vesicle weight (13,15,16,37,46) in mice and rats as well as testicular atrophy in dogs (12). The underlying mechanism for these observed testicular effects is unclear, but is likely a result from THC exposure given cannabinoid receptor expression has been demonstrated throughout the male reproductive tract including the testis, specifically the Sertoli cells, epididymis, seminal vesicle and prostate (4). Prior studies have suggested that THC exposure is associated with oxidative stress and decreased antioxidant enzymes in the affected testicles (47,48). Further histologic studies examining THC-exposed testicular tissue for morphologic regional changes are needed to better understand the mechanisms involved.
We observed a significant impact of THC on male reproductive hormones. The rise in LH and FSH with increased THC exposure, coupled with decreased testosterone and estradiol, suggests that primary testicular failure is the mechanism for hormonal dysregulation by THC. The few studies (49) that have investigated the effect of acute or chronic marijuana exposure on FSH levels in males have found minimal effect, while there is consistent evidence to support the inhibitory effect of marijuana on LH. Plasma LH has been demonstrated to be significantly acutely and chronically depressed after smoking marijuana (49), and there is a weaker LH response to exogenous gonadotropin releasing hormone (GnRH) in marijuana smokers compared to non-smokers (50). In a study comparing two groups of chronic THC users, no difference in LH was noted between those who smoked five to nine cigarettes weekly versus more than ten cigarettes weekly, suggesting an upper limit from the dose-dependent effect of THC on LH (51). The human and animal literature describing the effects of THC on testosterone have mixed findings (20,52). Gundersen et al. reported 7% higher testosterone levels in male THC users compared with nonusers (23), while a subsequent study using the National Health and Nutrition Examination Survey (NHANES) database found no difference in testosterone levels between marijuana ever-users (53) and never users and found that the effect of THC on testosterone may be acute and transient. In comparison, our study results indicate that direct, dose-dependent THC changes, not confounded by smoking marijuana, to the HPG axis are significant and follow a pattern of primary testicular failure. Additionally, although serum prolactin concentrations were increased with higher THC doses, prolactin levels were not elevated enough to inhibit the release of gonadotropins from the anterior pituitary gland and affect spermatogenesis. In contrast, other existing animal and human studies have found no change in prolactin levels between marijuana users and controls (50,54).
We did not observe clear changes in semen characteristics with THC exposure. The lack of statistically significant change seen in our study may be due to the inter-animal variability observed, consistent with the reported high variability of semen analysis results taken from the same human subject (55). This is present even with strict abstinence intervals, which is why two semen analyses are utilized in the evaluation of infertile men. Other animal and human studies have reported an impact of marijuana on semen parameters, including changes to motility, morphology, and sperm count and concentration (23,56–58), but their study design utilized a single ejaculate collection. Given the degree if variation observed between multiple semen analyses in a single subject, caution should be taken when comparing studies using single versus multiple ejaculates to assess changes or trends in semen characteristics. Additionally, the absence of change in semen characteristics, despite a significant decrease in total testosterone, may be because a critical level of total testosterone was still produced by the Leydig cells to interact with Androgen Binding Protein secreted by the Sertoli cells to maintain adequate total testosterone concentrations in the seminiferous tubules to support sperm production and development. While the semen parameters did not change appreciably, we do not know the direct effects of THC on the ability of the sperm to undergo capacitation, traverse the female reproductive tract, and fertilize an egg to form a normal embryo. This would be important to pursue in a future study.
Our study had many strengths, it is the first NHP study examining a dose-response effect from THC on male reproductive characteristics. This animal model provides precise control over experimental variables such as age, weight, prior proven paternity, and quantity of THC administered. To ensure rigor and reproducibility, the THC administered in this study was in an edible form to avoid toxins from smoke, from a single source, the NIDA Drug Supply Program, and utilized the standardized THC unit for research per recent NIDA guidelines. To also minimize inter-animal variability, each male was their own control with similar environmental exposures, including diet and housing. These variables are often inconsistent in humans and confound human studies. Compared to other smaller animal studies, this NHP study also provides further understanding on the impact of increasing THC dose on male testes and reproductive health that is translatable and applicable to humans.
Limitations of this study were the animal cohort size, but this was addressed by using a single-case experimental design where each male rhesus macaque served as its own control. Additionally, seasonal changes on male semen parameters have been reported in the NHP of mostly animals that are outdoor housed, unlike our study where all males were housed indoors. The observed significant impact on male reproductive hormones and testicular volume was suggestive of a dose-response effect. However, because the THC dosing was increased after 70 days (e.g. one sperm lifecycle), it is possible that the changes seen were in part due to the duration of THC exposure rather than THC dose.
In summary, our study indicates a significant, adverse, dose-response effect on male testes and reproductive health from chronic contemporary THC exposure. Further studies are needed to determine the impact of a longer duration of exposure and whether these observed effects are permanent, or if they would be reversed with abstinence from THC. As marijuana use is becoming more commonplace with significantly increased potency, our study provides important insight regarding the potential consequences of marijuana use that would help providers guide couples interested in conception or are affected by male infertility.
Conclusions
These data suggest that increasing doses of chronic THC consumption, even at a moderate dose, has an adverse impact on male reproductive health and results in a significant dose-response relationship of testicular atrophy, increased gonadotropins, and decreased serum sex steroids.
Acknowledgements
Financial Support: All Oregon National Primate Research Center (ONPRC) cores and units were supported by the National Institutes of Health (NIH) Grant P51 OD011092. Research reported in this publication was supported by the Reproductive Scientist Development Program (K12 HD000849 to J.L.), Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), American Society for Reproductive Medicine (ASRM), National Institute of Drug Abuse (NIDA), Oregon Health & Science University (OHSU) Medical Research Foundation Award, and Silver Family Innovation Award. The contents of this study are solely the responsibility of the authors and do not necessarily represent the official view of NIH NICHD and NIDA.
We would also like to thank the veterinary, husbandry and Behavioral Services Unit staff who provided excellent care for the animals used in this study, in particular Dr. Lauren Drew Martin, Travis Hodge, Lisa Houser and Breanna Kolwitz. Additionally, we would like to thank the Endocrine Technologies Core and Assisted Reproductive Core at the ONPRC as well as the Bioanalytical Shared Resource/Pharmacokinetics Core at OHSU. Lastly, we would like to thank Thomas O’Leary, director of the OHSU Andrology Lab.
Footnotes
Presented as an oral presentation at the 77th American Society for Reproductive Medicine Annual Meeting in Baltimore, Maryland, October 17–20, 2021.
Conflict of Interest Statement: None of the authors have financial or other relationships that could result in a conflict of interest.
References
- 1.NIDA. 2020, April 8. What is the scope of marijuana use in the United States? Retrieved from https://www.drugabuse.gov/publications/research-reports/marijuana/what-scope-marijuana-use-in-united-states. Last accessed November 20, 2021.
- 2.United Nations Office on Drugs and Crime (UNODC). World Drug Report 2017 – United Nations Office on Drugs and Crime. 2017. [Google Scholar]
- 3.Center for Behavioral Health Statistics and Quality: 2016 National Survey on Drug Use and Health: Detailed Tables. Rockville, Maryland: Substance Abuse and Mental Health Services Administration; 2017. [Google Scholar]
- 4.Uhlen M, Fagerberg L, Hallstrom BM, Lindskog C, Oksvold P, Mardinoglu A, et al. Proteomics. Tissue-based map of the human proteome. Science. 2015; 347:1260419. [DOI] [PubMed] [Google Scholar]
- 5.Aquila S, Guido C, Santoro A, Perrotta I, Laezza C, Bifulco M. Human sperm anatomy: ultrastructural localization of the cannabinoid1 receptor and a potential role of anandamide in sperm survival and acrosome reaction. Anat Rec. 2010;293:298.−309. [DOI] [PubMed] [Google Scholar]
- 6.Jensen B, Chen J, Furnish T, Wallace M. Medical marijuana and chronic pain: a review of basic science and clinical evidence. Curr Pain Headache Rep. 2015;19:50. [DOI] [PubMed] [Google Scholar]
- 7.Grimaldi P, Orlando P, Di Siena S, Lolicato F, Petrosino S, Bisogno T, Geremia R, De Petrocellis L, Di Marzo V. The endocannabinoid system and pivotal role of the CB2 receptor in mouse spermatogenesis. Proc Natl Acad Sci USA. 2009;106;11131–11136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dalterio S, Bartke A, Burstein S. Cannabinoids inhibit testosterone secretion by mouse testes in vitro. Science. 1977;196:14772–1473. [DOI] [PubMed] [Google Scholar]
- 9.Symons AM, Teale JD, Marks V. Proceedings: Effect of delta9-tetrahydrocannabinol on the hypothalamic-pituitary-gonadal system in the maturing male rat. J Endocrinol. 1976;68:43P–4. [PubMed] [Google Scholar]
- 10.Smith CG, Moore CE, Besch NF, Kaufman RH. The effect of marijuana (delta-9-tetrahydrocannabinol) on the secretion of sex hormones in the mature male rhesus monkey. Clin Chem. 1976;22:1184. [Google Scholar]
- 11.Murphy LL, Gher J, Steger RW, Bartke A. Effects of delta 9-tetrahydrocannabinol on copulatory behavior and neuroendocrine responses of male rats to female conspecifics. Pharmcol Biochem Behav. 1994;48:1011–1017. [DOI] [PubMed] [Google Scholar]
- 12.Dixit VP, Gupta CL, Agrawal M. Testicular degeneration and necrosis induced by chronic administration of cannabis extract in dogs. Endokrinologie. 1977;69:299–305. [PubMed] [Google Scholar]
- 13.Dixit VP, Sharma VN, Lohlya NK. The effect of chronically administered cannabis extract on the testicular function of mice. Eur J Pharmacol. 1974;26:111–114. [DOI] [PubMed] [Google Scholar]
- 14.Thompson GR, Mason MM, Rosenkrantz H, Braude MC. Chronic oral toxicity of cannabinoids in rats. Toxicol Appl Pharmacol. 1973;25:373–90. [DOI] [PubMed] [Google Scholar]
- 15.Banerjee A, Singh A, Srivastava P, Turner H, Krishna A. Effects of chronic bhang (cannabis) administration on the reproductive system of male mice. Birth defects Res B Dev Reprod Toxicol. 2011;92:195–205. [DOI] [PubMed] [Google Scholar]
- 16.Harclerode J Endocrine effects of marijuana in the male: preclinical studies. NIDA Res Monogr. 1984;44:46. [PubMed] [Google Scholar]
- 17.Hembree WC 3rd, Nahas GG, Zedenberg P, Huang HF. Changes in human spermatozoa associated with high dose marijuana smoking. Adv Biosci. 1978;22–23:429–439. [DOI] [PubMed] [Google Scholar]
- 18.Zimmerman AM, Bruce WR, Zimmerman S. Effects of cannabinoids on sperm morphology. Pharmacology. 1979;18:143–148. [DOI] [PubMed] [Google Scholar]
- 19.Dalterio S, Badr F, Bartke A, Mayfield D. Cannabinoids in male mice: effects on fertility and spermatogenesis. Science. 1982;216:315–316. [DOI] [PubMed] [Google Scholar]
- 20.Kolodny RC, Masters WH, Kolodner RM, Toro G. Depression of plasma testosterone levels after chronic intensive marihuana use. N Engl J Med. 1974;290:872–4. [DOI] [PubMed] [Google Scholar]
- 21.Hembree WC, Zeidenberg P, Nahas GG. Marijuana’s effect on human gonadal function. In: Nahas GG, ed. Marihuana: chemistry, biochemistry, and cellular effects. New York: Springer-Verlag, 1976:52. [Google Scholar]
- 22.Hembree WC, Zeidenberg P, Nahas GG. Changes in human spermatozoa associated with high dose marijuana smoking. In: Nahas GG, Paton W, eds. Marihuana: biological effects. New York: Pergamon, 1979:429–439. [DOI] [PubMed] [Google Scholar]
- 23.Gundersen TD, Jørgensen N, Andersson AM, Bang AK, Nordkap L, Skakkebaek NE, et al. Association between use of marijuana and male reproductive hormones and semen quality: a study among 1,215 healthy young men. Am J Epidemiol 2015;182:473–81. [DOI] [PubMed] [Google Scholar]
- 24.Issidorides MR. Observations in chronic hashish users: nuclear aberrations in blood and sperm and abnormal acrosomes in spermatozoa. In: Nahas GG, Paton W, eds. Marihuana: biological effects. New York: Pergamon, 1979:377–88. [DOI] [PubMed] [Google Scholar]
- 25.Mendelson JH, Kuehnle J, Elingboe J, Babor TF. Plasma testosterone levels before, during and after chronic marijhuana smoking. N Engl J Med. 1974;291:1051. [DOI] [PubMed] [Google Scholar]
- 26.Block RI, Farinpour R, Schlechte JA. Effects of chronic marijuana use on testosterone, luteinizing hormone, follicle stimulating hormone, prolactin and cortisol in men and women. Drug Alcohol Depend. 1991;28:121–128. [DOI] [PubMed] [Google Scholar]
- 27.Schaefer CF, gunn CG, Dubowski KM. Letter: Normal plasma testosterone concentrations after marihuana smoking. N Engl J Med. 1975;292:867–868. [DOI] [PubMed] [Google Scholar]
- 28.Wise LA, Wesselink AK, Hatch EE, Rothman KJ, Mikkelsen EM, Sorensen HT. Marijuana use and fecundability in a North American preconception cohort study. J Epidemiol Community Health. 2018;72:208–215. [DOI] [PubMed] [Google Scholar]
- 29.Close CE, Roberts PL, Berger RE. Cigarettes, alcohol and marijuana are related to pyospermia in infertile men. J Urol. 1990;144:900–903. [DOI] [PubMed] [Google Scholar]
- 30.El-Gothamy Z, el Samahy M. Ultrastructure sperm defects in addicts. Fertil Steril. 1992;57:699–702. [DOI] [PubMed] [Google Scholar]
- 31.Nassan FL, Arvizu M, Minguez-Alarcon L, Williams PL, Attaman J, Petrozza J, et al. Marijuana smoking and markers of testicular function among men from a fertility centre. Hum Reprod. 2019;34:715–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Elsohly MA, Mehmedic Z, Foster S, Gon C, Chandra S, Church JC. Changes in cannabis potency over the last 2 decades (1995–2014): analysis of current data in the united states. Biol Psychiatry. 2016;79:613–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grant KS, Petroff R, Isoherranen N, Stella N, Burbacher TM. Cannabis use during pregnancy: pharmacokinetics and effects on child development. Pharmacol Ther. 2018;182:133–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huestis MA. Human cannabinoid pharmacokinetics. Chemical Biodiversity. 2007;4(8):1770–1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wolf DP, Thomson JA, Zelinski-Wooten MB, Stouffer RL. In vitro fertilization-embryo transfer in nonhuman primates: the technique and its applications. Mol Reprod Dev. 1990;27: 261–80. [DOI] [PubMed] [Google Scholar]
- 36.Sibal LR, Samson KJ. Nonhuman primates: a critical role in current disease research. ILAR Journal. 2001;42: 74–84. [DOI] [PubMed] [Google Scholar]
- 37.Ryan KS, Mahalingaiah S, Campbell LR, Roberts VHJ, Terrobias JJD, Naito CS, et al. The effects of delta-9-tetrahydrocannabinol exposure on female menstrual cyclicity and reproductive health in rhesus macaques. F&S Science. 2021;2:287–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cannabis Clinicians Colorado. Medical marijuana new patient success guide. Available from: http://coscc.org/wp-content/uploads/2016/07/CCC-General-CannabisAsMedicine-2017DRAFT.pdf Last accessed November 20, 2021.
- 39.Colorado Department of Public Health and Environment: Retail Marijuana Public Health Advisory Committee’s Monitoring Health Concerns Related to Marijuana in Colorado. 2017. Available from: https://www.colorado.gov/pacific/marijuana/safety-edibles Last accessed November 20, 2021.
- 40.Karschner EL, Darwin WD, Goodwin RS, Wright S, Huestis MA. Plasma cannabinoid pharmacokinetics following controlled oral delta9-tetrahydrocannabinol and oromucosal cannabis extract administration. Clin Chem. 2011;57:66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Houser LA, Ramsey C, de Carvalho FM, Kolwitz B, Naito C, Coleman K, Hanna CB. Improved training and semen collection outcomes using the closed box chair for macaques. Animals. 2021;11:2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.World Health Organization, WHO laboratory manual for the examination and processing of human semen, 2021. 6th edn. WHO Press, Geneva. [Google Scholar]
- 43.Niswender GD, Spies HG. Serum levels of luteinizing hormone, follicle-stimulating hormone and progesterone throughout the menstrual cycle of rhesus monkeys. J. Clin. Endocrinol. Metab 1973;37:326–28. [DOI] [PubMed] [Google Scholar]
- 44.Ohlsson A, Lindgren JE, Wahlen A, Agurell S, Hollister LE, Gillespie HK. Plasma delta-9 tetrahydrocannabinol concentrations and clinical effects after oral and intravenous administration and smoking. Clin Pharmacol ther. 1980;28:409–16. [DOI] [PubMed] [Google Scholar]
- 45.Sansone RA, Sansone LA. Marijuana and body weight. Innov Clin Neurosci. 2014;11:50–54. [PMC free article] [PubMed] [Google Scholar]
- 46.Fujimoto GI, Morrill GA, O’Connell ME, Kostellow AB, Retura G. Effects of cannabinoids given orally and reduced appetite on the male rat reproductive system. Pharmacology. 1982;24: 303–313. [DOI] [PubMed] [Google Scholar]
- 47.Mandal TK, Das NS. Testicular toxicity in cannabis extract treated mice: association with oxidative stress and role of antioxidant enzyme systems. Toxicol Ind Health. 2010;26:11–23. [DOI] [PubMed] [Google Scholar]
- 48.Alagbonsi IA, Olayaki LA, Salman TM. Melatonin and vitamin C exacerbate Cannabis sativa-induced testicular damage when administered separately but ameliorate it when combined in rats. J Basic Clin Physiol Pharmacol. 2016; 27: 277–287. [DOI] [PubMed] [Google Scholar]
- 49.Cone EJ, Johnson RE, Moore JD, Roache JD. Acute effects of smoking marijuana on hormones, subjective effects and performance in male human subjects. Pharmacol Biochem Behav. 1986; 24:1749–1754. [DOI] [PubMed] [Google Scholar]
- 50.Vescovi PP, Pedrazzoni M, Michelini M, Maninetti L, Bernardelli F, Passeri M. Chronic effects of marihuana smoking on luteinizing hormone, follicle-stimulating hormone and prolactin levels in human males. Drug Alcohol Depend. 1992; 30:59–63. [DOI] [PubMed] [Google Scholar]
- 51.Kolodny RC, Lessin P, toro G, Masters WH, Cohen S. Depression of plasma testosterone with acute marihuana administration. In: The Pharmacology of marijuana. Braude MC, Szara S, eds. New York: Raven, 1976:217–225. [Google Scholar]
- 52.Rajanahally S, Raheem O, Rogers M, Brisbane W, Ostrowski K, Lendvay T, et al. The relationship between marijuana and male infertility, sexual health, and neoplasm: a systematic review. Andrology. 2019;7:139–147. [DOI] [PubMed] [Google Scholar]
- 53.Thistle JE, Graubard BI, Braunlin M, Vesper H, Trabert B, Cook MB, et al. Marijuana use and serum testosterone concentrations among U.S. males. Andrology. 2017;5:732–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Murphy LL, Steger RW, Smith MS, Bartke A. Effects of delta-9-tetrahydrocannabinol, cannabinol and cannabidiol, alone and in combinations, on luteinizing hormone and prolactin release and on hypothalamic neurotransmitters in the male rate. Neuroendocrinology. 1990;52:316–321. [DOI] [PubMed] [Google Scholar]
- 55.Oshio S, Ashizawa Y, Yotsukura M, Tohyama Y, Iwabuchi M, Adachi Y, et al. Individual variation in semen parameters of health young volunteers. Arch Androl. 2004;50:417–425. [DOI] [PubMed] [Google Scholar]
- 56.Carroll K, Pottinger AM, Wynter S, DaCosta V. Marijuana use and its influence on sperm morphology and motility: identified risk for fertility among Jamaican men. Andrology. 2020;8:136–142. [DOI] [PubMed] [Google Scholar]
- 57.Barbonetti A, Vassallo MR, Fortunato D, Francavilla S, Maccarrone M, Francavilla F. Energetic metabolism and human sperm motility: impact of CB1 receptor activation. Endocrinology. 2010;151:5882–5892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Murphy SK, Itchon-Ramos N, Visco Z, Huang Z, Grenier C, Schrott R, et al. Cannabinoid exposure and altered DNA methylation in rat and human sperm. Epigenetics. 2018;13:1208–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]


