Abstract
Background:
Oncotype DX recurrence score (ODX- RS) is a prognostic biomarker for early-stage, node-negative, estrogen receptor-positive (ER+) breast cancer. Whether test uptake, associated factors and test’s prognostic values differ by race/ethnicity is unknown.
Methods:
From the National Cancer Database, 2010–2014, we identified 227,259 early-stage ER+, node-negative breast cancer cases. Logistic regression was used to examine ODX RS uptake and associated factors among non-Hispanic White (White), non-Hispanic Black (Black), Hispanic, and Asian American patients. Cox regression was used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) for overall mortality with ODX RS by race/ethnicity.
Results:
White patients were more likely to receive an ODX RS test compared with Black, Hispanic and Asian American patients (36.7%, 32.8%, 31.6% and 35.5%, respectively; P< 0.001). Disparities persisted after adjustments for demographics, clinical characteristics and access-to-care, with rate ratios of 0.87 (95%CI=0.85–0.88), 0.82 (95%CI= 0.80–0.85) and 0.89 (95%CI= 0.87–0.92), respectively, for Black, Hispanic and Asian American compared with White patients. Black patients had higher proportions of high-risk scores (≥26) compared with White, Hispanic and Asian American patients (19.1%, 14.0%, 14.2% and 15.6%, respectively; P<0.0001). ODX RS was predictive for total mortality across all races/ethnicities, particularly younger patients (<50). No significant race/ethnicity interactions were observed.
Conclusions:
Although ODX RS uptake and risk distribution varied by race/ethnicity, ODX RS was prognostic for mortality across groups.
Impact:
These findings emphasize the importance of developing strategies to increase ODX RS uptake among racial/ethnic minorities and call for more investigations on potential racial/ethnic differences in breast cancer biology.
Introduction
Breast cancer is the most common cancer and second leading cause of cancer death among women in the United States (U.S.).1 Disparities in screening, referral rate, receipt of surgery, type of treatment, and time to treatment have been reported for racial and ethnic minorities and women of lower socioeconomic status,2–4 contributing to differences in breast cancer survival, with 10-year survival rates highest among White (80%) and lowest among Black (66%) women.5–8 The majority (68%−73%) of breast cancer across all racial/ethnic groups is Luminal A cancer subtype (hormone receptor-positive [HR+]/human epidermal growth factor receptor 2-negative [HER2−]).9,10
Historically, treatment for HR+/HER2− tumors included surgery followed by adjuvant systemic therapy, including endocrine therapy with or without chemotherapy. However, among women treated with chemotherapy, side effects can impede their quality of life.3,11 The Oncotype DX Risk Recurrence Score (ODX RS) is a 21-gene marker based on expressions of 15 tumor-associated genes and five reference genes and is utilized as a predictive and prognostic tool among clinicians.12 Traditional ODX RS groups patients into low- (score <18), intermediate- (18–30), and high-risk (>30) categories. Studies have shown that traditional ODX RS categorization correlates with 10-year risk of distant recurrence and predicts the benefit of adjuvant chemotherapy on reducing recurrence risk among early-stage, estrogen receptor-positive (ER+) breast cancer.2 A recent randomized trial, Trial Assigning Individualized Options for Treatment (TAILORx), used revised ODX RS cutoffs of low-risk (<11), intermediate-risk (11–25), and high-risk (>25) groups12 and confirmed the efficacy of the ODX test to guide chemotherapy among women with HR+/HER2−, axillary node–negative breast cancer, particularly those with midrange (11–25) recurrence scores.12 Researchers also observed some survival benefit of chemotherapy among women younger than 50 years with recurrence scores of 16–25.12 Despite the findings from the TAILORx trial, little is known about whether uptake rates and prognostic values of the ODX RS test differ across racial/ethnic groups.
To address this knowledge gap, we conducted this study with the following aims: to 1) examine ODX RS assay uptake by racial/ethnic group among patients who met National Comprehensive Cancer Network (NCCN) guidelines to receive the test; 2) compare distributions of ODX RS categories across racial/ethnic groups; 3) evaluate whether ODX RS was differentially associated with total mortality across racial/ethnic groups; and 4) evaluate whether ODX RS predicted a chemotherapy benefit among intermediate- and high-risk ODX RS groups by racial/ethnic group.
Materials and Methods
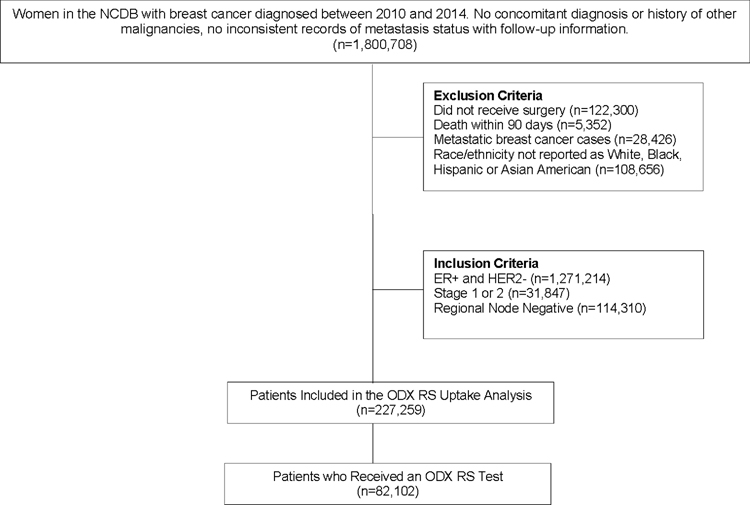
This study used data from the clinical oncology National Cancer Database (NCDB), sourced from hospital registry data provided by more than 1,500 Commission on Cancer (CoC)-accredited facilities.13 NCDB data represent over 70% of newly diagnosed cancer cases in the U.S. Women with self-reported race/ethnicity as non-Hispanic White (White), non-Hispanic Black (Black), Hispanic and Asian American, with a primary diagnosis of breast cancer between 2010–2014, were identified from the NCDB. Patients with ER+/HER2−, lymph node-negative, stage I or II invasive breast cancers who received surgery were eligible for the study (n=227,259) (Fig. 1). Deidentified information was provided by the NCDB; therefore, this study was approved by Vanderbilt University Medical Center’s Institutional Review Board as human subject exempt.
Figure 1.
Study Flowchart. Study data were drawn from the National Cancer Database (NCDB). Exclusion Criteria: no surgery, death within 90 days, metastatic breast cancer cases, race/ethnicity other than White, Black, Hispanic or Asian American. Inclusion Criteria: ER+ and HER2−, stage 1 or 2, regional node negative. Uptake analyses included all patients eligible to receive an ODX RS test.
Information on demographic characteristics available from the NCDB included race/ethnicity, age at diagnosis, year of diagnosis (2010 to 2014), urban/rural residence, neighborhood median household income (≤$30,000, $30,000-$34,999, $35,000-$45,999, $46,000+, unknown) and educational attainment (≥29.0%, 20.0%−28.9%, 14.0%−19.9%, <14.0%, unknown), patients’ insurance status (not insured, private insurance, Medicaid, Medicare, other government insurance, unknown), treatment facility type (community center, comprehensive community cancer center, academic/research, integrated network cancer program), region (Northeast, Midwest, South, West, unknown) and distance to care. In the NCDB, individual-level data on education attainment and income were not available; thus, they were estimated at the neighborhood level by matching patient zip codes recorded at time of diagnoses against Year 2000 U.S. Census data and 2012 American Community Survey data13 and provided to researchers. The NCDB does not provide individual zip code data to researchers for patient privacy protection. Clinical characteristics included tumor size, nodal status, progesterone receptor (PR) status, ER status, HER2 status, histology type, Nottingham combined histological grade (grade), lymphovascular invasion (LVI), and Charlson/Deyo score, with a score of zero indicating no comorbidity at cancer diagnosis. Treatment (chemotherapy, radiotherapy, endocrine therapy) and surgery (lumpectomy, mastectomy) data were also included.
Statistical Analysis
Primary outcomes were ODX RS test uptake and overall survival (OS). Disease-free survival and breast cancer-specific survival could not be evaluated because cancer recurrence/progression events and cause of death are not available in the NCDB. Test uptake rates among eligible patients were calculated, and the differences across White, Black, Hispanic and Asian American patients were compared using ꭕ2 tests for categorical and Student t tests for continuous variables. Robust Poisson regression analyses were carried out to evaluate the influence of patient, clinical and demographic characteristics on racial/ethnic differences in rate ratios (RR) of ODX RS test uptake. Among patients receiving ODX RS tests, demographic, clinical and treatment characteristics by race/ethnicity were compared using the ꭕ2 tests or Student t tests. For survival analysis, time scale was defined as months from cancer diagnosis to death from any cause or last contact; patients lost to follow-up were censored at last contact. Patients were categorized into low-, intermediate-, and high-risk groups based on traditional ODX RS (<18, 18–30, >30) and TAILORx ODX RS (<11, 11–25, >25) cutoffs. Cox proportional hazard models were used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) for associations of ODX RS groups with overall mortality by race/ethnicity. HRs and 95% CIs were derived with (i) adjustments for demographic and clinical characteristics and treatments, except chemotherapy, and (ii) further adjustment for chemotherapy. As TAILORx results suggested the chemotherapy benefit varied across age group (<50 vs ≥50 years), Cox regression analyses were also conducted with stratification by age group. A heterogeneity test was conducted to assess whether ODX RS with OS associations differed across racial/ethnic group and by pairwise comparison to White patients (Black vs. White, Hispanic vs. White, Asian American vs. White). All statistical tests were based on two-sided probability, with significance levels set at p<0.05, and were performed using SAS Office Analytics version 7.4.
Results
Of 227,259 breast cancer patients eligible for receiving the ODX RS test, 188,157 (82.8%) were White, 18,635 (8.2%) were Black, 10,712 (4.7%) were Hispanic, and 9,755 (4.3%) were Asian American women. Among them, 84,867 received an ODX RS test order, and 82,102 (93%) took the test. ODX RS test uptake, primarily reflecting physician ordering of an ODX RS test, varied significantly by race/ethnicity. White patients were more likely to receive ODX RS tests compared with Black, Hispanic and Asian American patients (36.7% vs. 32.8%, 31.6%, 35.5%; P<0.0001, respectively). The age- and comorbidity-adjusted RRs for ODX RS uptake were 0.85 (95% CI= 0.83–0.87), 0.78 (95% CI= 0.76–0.80) and 0.87 (95% CI= 0.85–0.89), respectively, for Black, Hispanic and Asian American compared with White patients. Adjustments for additional covariates showed that factors potentially related to access-to-care, including education, income, region, location, treatment facility, insurance and year of diagnosis, accounted for approximately one-fifth to one-fourth of racial/ethnic disparities in ODX RS test uptake (Table 1). The racial/ethnic differences in test uptake persisted in a fully-adjusted model; RRs were 0.87 (95% CI=0.85–0.88) for Black, 0.82 (95% CI= 0.80–0.85) for Hispanic, and 0.89 (95% CI= 0.87–0.92) for Asian American compared with White patients (Table 1).
Table 1.
Rate Ratio (RR) and the Percent Change in RR for ODX RS Uptake by Race/Ethnicity with Adjustments for Various Covariates
| Black vs. White | Hispanic vs. White | Asian American vs. White | ||||
|---|---|---|---|---|---|---|
| RR (95% CI) | Proportion of Change in RR* | RR (95% CI) | Proportion of Change in RR* | RR (95% CI) | Proportion of Change in RR* | |
| Model1 | 0.85(0.83–0.87) | ref | 0.78(0.76–0.80) | ref | 0.87(0.85–0.89) | ref |
| Model2 | 0.84(0.82–0.85) | 6.7 | 0.77(0.75–0.79) | 4.5 | 0.86(0.83–0.88) | 15.4 |
| Model3 | 0.85(0.83–0.87) | 0 | 0.78(0.76–0.80) | 0 | 0.87(0.85–0.90) | 0 |
| Model4 | 0.88(0.86–0.90) | −20.0 | 0.84(0.81–0.86) | −27.3 | 0.90(0.88–0.93) | −23.1 |
| Model5 | 0.87(0.85–0.89) | −13.3 | 0.82(0.80–0.85) | −18.2 | 0.89(0.87–0.92) | −8.7 |
| Model6 | 0.87(0.85–0.88) | −13.3 | 0.82(0.80–0.85) | −18.2 | 0.89(0.87–0.92) | −8.7 |
Proportion of change in RR was calculated by (RR1-RRi)/(RR1–1). RR1 is the RR for model 1, and RRi is the RR derived from I model with additional adjustments.
Model1: Adjusted for age and comorbidity.
Model2: Adjusted for age, comorbidity and clinical factors (PR status, Grade, lymphovascular invasion status, tumor size and tumor type).
Model3: Adjusted for age, comorbidity and clinical treatment (type of surgery, radiotherapy and chemotherapy).
Model4: Adjusted for age, comorbidity, economic status and access to care (education, income, region, location, treatment facility, insurance and year of diagnosis).
Model5: Adjusted for age, comorbidity, clinical factors (model2), economic and access to care (model4).
Model6: Adjusted for all above covariates.
Table 2 shows demographic and clinical characteristics of patients who received an ODX RS test (n=82,102). Overall, there were 69,125 (84.2%) White, 6,118 (7.4%) Black, 3,391 (4.1%) Hispanic, and 3,468 (4.2%) Asian American patients. ODX RS distributions varied significantly by race (P<0.001) for both traditional and TAILORx cutoffs. White patients had the highest proportion (59.8%) of traditional low ODX RS compared with Black, Hispanic and Asian American patients (55.0%, 59.2%, 58.5%, respectively), while Black patients had the highest proportion (11.4%) of traditional high ODX RS compared with White, Hispanic and Asian American women (7.5%, 7.9%, 7.9%, respectively). When using TAILORx cutoffs, the majority of women were categorized with intermediate (11–25) scores. Similar to traditional ODX RS cutoff patterns, Black patients had the highest proportion of falling to the high-risk group compared with White, Hispanic and Asian American patients (19.1% vs. 14.0%, 14.2%, 15.6%, respectively).
Table 2.
Characteristics of Patients with Breast Cancer and Received an Oncotype DX RS Test by Race/Ethnicity
| White | Black | Hispanic | Asian American | p | |
|---|---|---|---|---|---|
| n | 69125 | 6118 | 3391 | 3468 | |
|
| |||||
| Age (mean (SD)) | 58.5 (10.2) | 57.1 (10.4) | 55.2 (10.7) | 54.6 (10.7) | <0.001 |
| Age Group | <0.001 | ||||
| 18–34 | 0.9 | 1.4 | 1.6 | 2.0 | |
| 35–44 | 8.1 | 10.4 | 15.1 | 17.1 | |
| 45–54 | 26.8 | 28.1 | 32.1 | 32.3 | |
| 55–64 | 33.5 | 34.4 | 30.0 | 28.3 | |
| 65–74 | 25.6 | 21.4 | 17.1 | 17.6 | |
| >75 | 5.0 | 4.3 | 4.0 | 2.7 | |
| Year of Diagnosis | <0.001 | ||||
| 2010 | 13.6 | 13.4 | 12.3 | 13.0 | |
| 2011 | 17.8 | 17.5 | 17.2 | 16.1 | |
| 2012 | 20.3 | 19.2 | 19.2 | 19.1 | |
| 2013 | 23.1 | 24.5 | 23.4 | 24.8 | |
| 2014 | 25.3 | 25.5 | 27.8 | 27.0 | |
| ODX Risk Recurrence Score (Median [IQR]) | 16.0 [11.0, 21.0] | 16.0 [11.0, 23.0] | 16.0 [11.0, 21.0] | 16.0 [11.0, 22.0] | <0.001 |
| Traditional Risk Recurrence Score | <0.001 | ||||
| 0–17 | 59.8 | 55.0 | 59.2 | 58.5 | |
| 18–30 | 32.8 | 33.6 | 32.9 | 33.6 | |
| 31–100 | 7.5 | 11.4 | 7.9 | 7.9 | |
| TAILORx Risk Recurrence Score | |||||
| 0–10 | 23.4 | 22.0 | 23.0 | 23.0 | |
| 11–25 | 62.6 | 59.0 | 62.8 | 61.4 | |
| 25–100 | 14.0 | 19.1 | 14.2 | 15.6 | |
| Region | <0.001 | ||||
| Northeast | 25.0 | 21.0 | 26.0 | 26.0 | |
| Midwest | 28.4 | 19.9 | 10.3 | 13.1 | |
| South | 29.1 | 50.0 | 31.5 | 19.4 | |
| West | 14.8 | 4.6 | 26.6 | 34.8 | |
| Unknown | 2.7 | 4.4 | 5.6 | 6.7 | |
| Geographic Location | <0.001 | ||||
| Metro | 82.5 | 90.8 | 94.1 | 92.3 | |
| Urban | 13.4 | 6.6 | 3.6 | 4.2 | |
| Rural | 1.8 | 0.7 | 0.3 | 0.5 | |
| Unknown | 2.4 | 1.9 | 2.0 | 2.9 | |
| Income Quartile1 | <0.001 | ||||
| <$30000 | 8.3 | 30.2 | 16.7 | 5.4 | |
| $30000–$34999 | 17.3 | 21.1 | 20.9 | 10.1 | |
| $35000–$45999 | 26.7 | 22.9 | 27.6 | 21.0 | |
| >$46000 | 46.5 | 24.9 | 34.0 | 62.4 | |
| Unknown | 1.1 | 0.9 | 0.8 | 1.1 | |
| High School Education Quartile1 | <0.001 | ||||
| ≥29% | 7.9 | 25.7 | 34.9 | 12.0 | |
| 20%–28.9% | 18.9 | 33.6 | 23.3 | 16.6 | |
| 14%–19.9% | 31.9 | 23.7 | 21.3 | 27.8 | |
| <14% | 40.2 | 16.1 | 19.7 | 42.3 | |
| Unknown | 1.1 | 0.9 | 0.7 | 1.2 | |
| Facility Type | <0.001 | ||||
| Community | 8.9 | 6.0 | 7.1 | 8.2 | |
| Comprehensive Community | 48.1 | 38.7 | 37.3 | 39.3 | |
| Academic/Research Program | 31.9 | 39.6 | 39.2 | 43.2 | |
| Integrated Network | 11.1 | 15.8 | 16.4 | 9.3 | |
| Insurance | <0.001 | ||||
| No Insurance | 1.0 | 2.5 | 6.0 | 2.0 | |
| Private Insurance | 65.1 | 57.8 | 54.8 | 69.1 | |
| Government Insurance | 33.0 | 38.7 | 38.1 | 28.1 | |
| Unknown | 0.9 | 1.0 | 1.1 | 0.8 | |
| Tumor Size (median [IQR]) | 15.0 [11.0, 20.0] | 15.0 [11.0, 21.0] | 15.0 [11.0,21.0] | 15.0 [11.0, 21.0] | <0.001 |
| Tumor Size (mm) | <0.001 | ||||
| ≤10 | 24.4 | 21.8 | 21.8 | 21.9 | |
| 11–20 | 52.1 | 50.7 | 51.5 | 51.3 | |
| >20 | 23.0 | 26.8 | 25.9 | 26.2 | |
| Unknown | 0.5 | 0.7 | 0.7 | 0.6 | |
| Histologic Grade | <0.001 | ||||
| I | 31.0 | 25.8 | 26.5 | 24.7 | |
| II | 50.2 | 49.7 | 52.4 | 52.2 | |
| III | 13.4 | 19.1 | 15.3 | 15.4 | |
| Unknown | 5.2 | 5.4 | 5.8 | 7.6 | |
| Histologic Type | <0.001 | ||||
| Ductal | 73.6 | 71.8 | 73.8 | 76.3 | |
| Lobular | 12.2 | 11.6 | 10.6 | 7.5 | |
| Other | 14.2 | 16.5 | 15.7 | 16.3 | |
| PR Status | <0.001 | ||||
| Positive | 91.1 | 87.9 | 92.0 | 91.0 | |
| Negative | 8.8 | 12.0 | 7.8 | 8.9 | |
| Borderline | 0.05 | 0.05 | 0.1 | 0.03 | |
| Unknown | 0.02 | 0.00 | 0.1 | 0.1 | |
| Lymph Vascular Invasion | 0.003 | ||||
| Negative | 80.0 | 80.0 | 78.0 | 80.4 | |
| Positive | 9.7 | 9.4 | 11.1 | 10.7 | |
| Unknown | 10.2 | 10.6 | 10.9 | 9.0 | |
| Comorbidity | <0.001 | ||||
| No | 86.3 | 78.1 | 82.3 | 87.2 | |
| Yes | 11.7 | 18.0 | 14.6 | 11.4 | |
| Unknown | 2.0 | 3.9 | 3.1 | 1.5 | |
| Surgery | <0.001 | ||||
| Lumpectomy | 69.4 | 70.6 | 66.0 | 63.1 | |
| Mastectomy | 30.6 | 29.4 | 34.0 | 36.8 | |
| Radiation | <0.001 | ||||
| No | 30.7 | 29.7 | 33.8 | 36.2 | |
| Yes | 69.2 | 70.1 | 66.1 | 63.7 | |
| Unknown | 0.1 | 0.2 | 0.1 | 0.1 | |
| Chemotherapy | <0.001 | ||||
| No | 78.3 | 73.4 | 74.7 | 75.1 | |
| Yes | 20.3 | 24.9 | 23.3 | 23.0 | |
| Unknown | 1.4 | 1.6 | 2.0 | 1.8 | |
| Follow-up (median [IQR]) | 40.4 [27.8, 55.6] | 40.1 [27.9, 55.1] | 37.9 [25.5,53.0] | 38.5 [26.7, 54.1] | <0.001 |
Median annual household income and educational attainment were estimated by matching patients’ zip codes against American Community Survey data from 2000 or 2012, depending on the time of diagnosis.
Among patients who received ODX RS tests, White patients were older compared with Black, Hispanic and Asian American patients (58.4 vs. 57.1, 55.2, 54.6 years; P<0.0001, respectively). Differences in geographic residence existed by race/ethnicity, with larger proportions of White patients residing in rural areas compared with Black, Hispanic and Asian American patients (1.8% vs. 0.7%, 0.3%, 0.5%; P<0.001, respectively). Black patients were substantially more likely to reside in areas with an income quartile <$30,000 compared with White, Hispanic and Asian American patients (30.2% vs. 8.3%, 16.7%, 5.4%; P<0.001, respectively), while Hispanic patients were more likely to live in areas with a high school education quartile ≥29.0% compared with White, Black and Asian American patients (34.9% vs. 7.9%, 25.7%, 12.0%; P<0.001, respectively). Hispanic patients were more likely to report not having health insurance, compared with White, Black and Asian American patients (6.0% vs.1.0%, 2.5%, 2.0%; P<0.001, respectively). Black patients were more likely to have large tumor sizes (>20mm: 26.8% vs. 23.0%, 25.9%, 26.2%; P<0.0001) and higher tumor grades (grade III: 19.1% vs. 13.4%, 15.3%, 15.4%; P<0.0001) compared with White, Hispanic and Asian American patients, respectively.
The median follow-up times were 40.4 months for White, 40.1 months for Black, 37.9 months for Hispanic and 38.5 months for Asian American patients. After adjustments for clinical and demographic characteristics and treatment (besides chemotherapy), traditional ODX RS was associated with significant increases in overall mortality risk in all racial/ethnic groups. There were no statistically significant interactions between ODX RS and racial/ethnic group in association with total mortality (Pinteraction=0.77) (Table 3). Among White women, those in intermediate- and high-risk traditional ODX RS categories had respective 20% (HR: 1.20; 95% CI, 1.06–1.36) and 135% (HR: 2.35; 95% CI, 1.99–2.78) higher mortality risk than their White counterparts with low risk. Among Black women, intermediate- and high-risk traditional ODX RS groups had 13% (HR: 1.13; 95% CI, 0.77–1.65) and 218% (HR: 3.18; 95% CI, 2.00–5.03) higher mortality risk compared with their Black counterparts with low risk. However, Among Asian American women only high-risk traditional ODX RS group had 335% (HR: 4.35; 95% CI, 1.53–12.4) higher mortality risk; notably, the point estimate for the high-risk group had a very wide confidence interval. Among Hispanic women in the high-risk traditional ODX RS category, non-significantly elevated mortality (HR: 2.67; 95% CI, 0.93–7.66) was observed compared with those in the low-risk category. Chemotherapy utilization increased with higher ODX RS categories in all racial/ethnic groups; among patients with RS >25, 84.8% of White, 87.4% of Black, 83.6% of Hispanic, and 85.1% of Asian American patients received chemotherapy (Table 3.) After additional adjustment for chemotherapy, association patterns between RS and mortality did not change; however, the magnitude of estimates increased after adjustment for chemotherapy, with the exception for black patients.
Table 3.
HRs (95% CI) for Total Mortality in Association with ODX RS by Race/Ethnicity
| White | Black | Hispanic | Asian American | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Event | %Chemo | HR (95%CI) | Event | %Chemo | HR (95%CI) | Event | %Chemo | HR (95%CI) | Event | %Chemo | HR (95%CI) | |
|
| ||||||||||||
| Traditional RS | ||||||||||||
|
| ||||||||||||
| Model 1 | ||||||||||||
| Low | 635/41307 | 3.5 | 1.0 | 73/3363 | 3.9 | 1.0 | 17/2006 | 5.1 | 1.0 | 6/1606 | 4.8 | 1.0 |
| Intermediate | 457/22640 | 36.1 | 1.20 (1.06–1.36) | 47/2059 | 38.1 | 1.13 (0.77–1.65) | 9/1117 | 41.4 | 0.91 (0.40–2.11) | 7/940 | 40.1 | 0.95 (0.37–2.45) |
| High | 262/5178 | 84.8 | 2.35 (1.99 –2.78) | 39/696 | 87.4 | 3.18 (2.00–5.03) | 6/268 | 83.6 | 2.67 (0.93–7.66) | 6/227 | 85.1 | 4.35 (1.53–12.4) |
| Model 2 | ||||||||||||
| Low | 635/41307 | 3.5 | 1.0 | 73/3363 | 3.9 | 1.0 | 17/2006 | 5.1 | 1.0 | 6/1606 | 4.8 | 1.0 |
| Intermediate | 457/22640 | 36.1 | 1.24 (1.09–1.41) | 47/2059 | 38.1 | 1.13 (0.76–1.67) | 9/1117 | 41.4 | 0.97 (0.41–2.27) | 7/940 | 40.1 | 1.22 (0.47–3.18) |
| High | 262/5178 | 84.8 | 2.58 (2.14–3.12) | 39/696 | 87.4 | 3.15 (1.85–5.36) | 6/268 | 83.6 | 3.19 (0.99–10.3) | 6/227 | 85.1 | 9.59 (2.90–31.8) |
|
| ||||||||||||
| TAILORx RS | ||||||||||||
|
| ||||||||||||
| Model 1 | ||||||||||||
| Low | 263/16201 | 1.69 | 1.0 | 35/1341 | 2.2 | 1.0 | 9/779 | 3.5 | 1.0 | 2/628 | 2.5 | 1.0 |
| Intermediate | 699/43282 | 15.0 | 1.05 (0.91–1.21) | 76/3608 | 16.5 | 0.87 (0.58–1.31) | 16/2130 | 18.3 | 0.80 (0.34–1.88) | 10/1707 | 16.8 | 1.14 (0.40–3.19) |
| High | 392/9642 | 75.1 | 1.99 (1.68–2.36) | 48/1169 | 76.9 | 1.71 (1.05–2.80) | 7/482 | 77.2 | 1.28 (0.41–4.04) | 7/438 | 77.7 | 2.41 (0.73–7.97) |
| Model 2 | ||||||||||||
| Low | 263/16201 | 1.69 | 1.0 | 35/1341 | 2.2 | 1.0 | 9/779 | 3.5 | 1.0 | 2/628 | 2.5 | 1.0 |
| Intermediate | 699/43282 | 15.0 | 1.06 (0.92–1.22) | 76/3608 | 16.5 | 0.86 (0.57–1.29) | 16/2130 | 18.3 | 0.81 (0.35–1.89) | 10/1707 | 16.8 | 1.22 (0.43–3.43) |
| High | 392/9642 | 75.1 | 2.14 (1.77–2.57) | 48/1169 | 76.9 | 1.55 (0.91–2.66) | 7/482 | 77.2 | 1.38 (0.41–4.70) | 7/438 | 77.7 | 4.07 (1.11–15.0) |
Model 1: adjusted for age (continuous) grade, histology type, progesterone status, tumor size, lymph vascular invasion, comorbidity, surgery, endocrine therapy, radiotherapy, neighborhood-level education, neighborhood-level income, insurance, and year of diagnosis.
Model 2: Adjusted for all of the variables listed above in addition to chemotherapy.
Traditional RS: 0–17 Low, 18–30 Intermediate, 31–100 High, Pinteraction, between RS and race was 0.77.
TAILORx RS: 0–10 Low, 11–25 Intermediate, 26–100 High, Pinteraction, between RS and race was 0.83.
When associations were evaluated using TAILORx cutoffs, White (HR: 1.99; 95% CI, 1.68–2.36), Black (HR: 1.71; 95% CI,1.05–2.80), Hispanic (HR: 1.28; 95% CI, 0.41–4.04) and Asian American (HR: 2.41; 95% CI, 0.73–7.97) patients in the high-risk group had elevated mortality risk compared with their counterparts in the low-risk group, although associations for Hispanic and Asian American patients were not statistically significant. Association patterns and point estimates were not materially changed after further adjustment for chemotherapy with an exception in Asian American patients. Intermediate TAILORx score was not associated with total mortality in all racial/ethnic groups. No significant interactions were observed between race/ethnicity and RS group for TAILORx cutoffs (Pinteraction=0.83).
We conducted additional analyses stratified by race/ethnicity and age group (<50, ≥50 years) (Table 4). Due to small sample sizes, this sub-analysis was only conducted in White and Black patients, among whom, mortality associations for high-risk traditional and TAILORx groups were substantially stronger among women <50 years of age. After additional adjustment for chemotherapy, association patterns did not materially change. Younger (<50 years of age) White patients in the high-risk groups had a higher risk of mortality compared with their low-risk counterparts for traditional (HR: 5.78; 95% CI, 3.15–10.6) and TAILORx (HR: 7.72; 95% CI, 3.45–17.4). Similar results were observed among younger Black women for traditional (HR: 5.89; 95% CI, 1.34–25.9), while associations were not significant for TAILORx (HR: 1.76; 95% CI, 0.43–7.25) cutoffs.
Table 4.
HRs (95% CI) for Total Mortality in Association with ODX RS by Race among Patients by Age
| Patients ≥50 years | ||||||||
| White | Black | |||||||
|
| ||||||||
| %Chemo | Deaths/Patients | HR (95%CI) | P-Value | %Chemo | Deaths/Patients | HR (95%CI) | P-Value | |
|
| ||||||||
| Traditional RS | ||||||||
|
| ||||||||
| Model 1 | ||||||||
| Low | 2.3 | 599/32419 | 1.0 | 1.00 | 3.1 | 68/2593 | 1.0 | 1.00 |
| Intermediate | 31.2 | 412/17841 | 1.14 (1.00–1.30) | 0.04 | 32.3 | 38/1519 | 1.03 (0.68–1.56) | 0.88 |
| High | 82.9 | 227/4147 | 2.09 (1.75–2.49) | <0.001 | 85.5 | 31/504 | 3.07 (1.86–5.05) | <0.001 |
| Model 2 | ||||||||
| Low | 2.3 | 599/32419 | 1.0 | 1.00 | 3.1 | 68/2593 | 1.0 | 1.00 |
| Intermediate | 31.2 | 412/17841 | 1.18 (1.04–1.35) | 0.02 | 32.3 | 38/1519 | 1.02 (0.67–1.56) | 0.91 |
| High | 82.9 | 227/4147 | 2.37 (1.94–2.90) | <0.001 | 85.5 | 31/504 | 2.96 (1.66–5.28) | <0.001 |
|
| ||||||||
| TAILORx RS | ||||||||
| Model 1 | ||||||||
|
| ||||||||
| Low | 1.2 | 254/13267 | 1.00 | 1.00 | 1.4 | 32/1087 | 1.00 | 1.00 |
| Intermediate | 12.1 | 649/33399 | 1.02 (0.89–1.20) | 0.63 | 13.7 | 68/2680 | 0.90 (0.59–1.38) | 0.63 |
| High | 72.0 | 335/7741 | 1.73 (1.45–2.06) | <0.001 | 72.7 | 37/849 | 1.63 (0.96–2.77) | 0.07 |
| Model 2 | ||||||||
| Low | 1.2 | 254/13267 | 1.00 | 1.00 | 1.4 | 32/1087 | 1.00 | 1.00 |
| Intermediate | 12.1 | 649/33399 | 1.04 (0.89–1.20) | 0.51 | 13.7 | 68/2680 | 0.88 (0.57–1.36) | 0.57 |
| High | 72.0 | 335/7741 | 1.88 (1.55–2.29) | <0.001 | 72.7 | 37/849 | 1.44 (0.81–2.56) | 0.22 |
|
| ||||||||
| Patients <50 years of age | ||||||||
| White | Black | |||||||
| %Chemo | Deaths/Patients | HR (95%CI) | P-Value | %Chemo | Deaths/Patients | HR (95%CI) | P-Value | |
|
| ||||||||
| Traditional RS | ||||||||
|
| ||||||||
| Model 1 | ||||||||
| Low | 7.6 | 36/8888 | 1.0 | 1.00 | 6.7 | 5/770 | 1.0 | 1.00 |
| Intermediate | 54.4 | 45/4799 | 2.12 (1.36–3.31) | <0.001 | 54.6 | 9/540 | 2.01 (0.63–6.38) | 0.24 |
| High | 92.4 | 35/1031 | 6.88 (4.06–11.7) | <0.001 | 92.2 | 8/192 | 4.77 (1.29–17.6) | 0.02 |
| Model 2 | ||||||||
| Low | 7.6 | 36/8888 | 1.0 | 1.00 | 6.7 | 5/770 | 1.0 | 1.00 |
| Intermediate | 54.4 | 45/4799 | 1.90 (1.17–3.09) | 0.00 | 54.6 | 9/540 | 2.29 (0.67–7.84) | 0.19 |
| High | 92.4 | 35/1031 | 5.78 (3.15–10.6) | <0.001 | 92.2 | 8/192 | 5.89 (1.34–25.9) | 0.01 |
|
| ||||||||
| TAILORx RS | ||||||||
|
| ||||||||
| Model 1 | ||||||||
| Low | 4.0 | 9/2934 | 1.00 | 1.00 | 5.5 | 3/254 | 1.00 | 1.00 |
| Intermediate | 24.5 | 50/9883 | 1.55 (0.76–3.15) | 0.23 | 24.6 | 8/928 | 0.53 (0.13–2.06) | 0.36 |
| High | 87.9 | 57/1901 | 8.23 (3.95–17.1) | <0.001 | 88.1 | 11/320 | 1.78 (0.43–7.33) | 0.42 |
| Model 2 | ||||||||
| Low | 4.0 | 9/2934 | 1.00 | 1.00 | 5.5 | 3/254 | 1.00 | 1.00 |
| Intermediate | 24.5 | 50/9883 | 1.52 (0.74–3.11) | 0.25 | 24.6 | 8/928 | 0.53 (0.14–2.07) | 0.53 |
| High | 87.9 | 57/1901 | 7.72 (3.45–17.4) | <0.001 | 88.1 | 11/320 | 1.76 (0.43–7.25) | 0.43 |
Model 1: adjusted for age (continuous) grade, histology type, progesterone status, tumor size, lymph vascular invasion, comorbidity, surgery, endocrine therapy, radiotherapy, education, income, insurance, and year of diagnosis.
Model 2: Adjusted for all of the variables listed above in addition to chemotherapy.
Traditional RS: 0–17 Low, 18–30 Intermediate, 31–100 High.
TAILORx RS: 0–10 Low, 11–25 Intermediate, 26–100 High.
Discussion
To our knowledge, this is one of the first and largest registry-based studies examining overall mortality according to both traditional and TAILORx risk groups by race/ethnicity and age. We observed racial differences in ODX RS test uptake rates and distributions of ODX RS categories. Among ODX RS-eligible women, minority groups, particularly Black and Hispanic women, were less likely to receive ODX RS tests compared with White patients. Access-to-care accounted for approximately one-third of racial/ethnic disparities in test uptake. When factors related to access-to-care, patient and clinical characteristics were adjusted for, Black, Hispanic and Asian American breast cancer patients were still about 17–23% less likely to take ODX RS tests than their White counterparts. In addition, Black women had the highest proportion of the high-risk ODX RS category. Nevertheless, association patterns for ODX RS categories with overall mortality did not vary significantly across White, Black, Hispanic and Asian American women, irrespective of how ODX RS category was defined (traditional vs. TAILORx cutoff). In all groups, women in high-risk score categories had substantially higher mortality compared with women in low-risk categories.
Consistent with prior observations, in our study, Black and Hispanic women eligible to receive ODX RS tests were less likely than White women to have received a test order and have a test performed. Among Black women, research suggests that uptake differences could be associated with types of cancer center (academic vs. non-academic), hospital ownership and location (urban vs. rural);14 however, in our study, disparities persisted after adjustment for relevant access-to-care and other factors. Previous studies on decision-making styles of patients demonstrated that the most common reasons women eligible for ODX RS tests did not receive it was because their doctors did not offer it to them (80%), which was confirmed in our study, or they had not heard of it (65%).14 Studies by Reeder-Hayes et al. showed that both provider volume and specialty were significant predictors of gene expression profiles, such as the ODX RS test.15 Among Hispanic women, age, insurance status, and area-level socioeconomic status have been shown to contribute to unequal access to genetic testing.16 Appropriate programs and measures should be developed to increase ODX RS uptake to minimize disparities in receiving standardized care.
It is worth mentioning, despite the lower uptake rate of the ODX RS test among racial/ethnic minorities, the receipt of chemotherapy after this test was largely consistent across racial/ethnic groups, particularly within each strata of risk categories. This finding is in line with the study by Press et al.17 but differs from that of Han et al., which used Surveillance, Epidemiology, and End Results Program (SEER) data and showed lower odds of chemotherapy use among Blacks with high RS risk in comparison to their White counterparts.18 Additional studies are needed to better characterize the potential racial disparity in receiving chemotherapy after the ODX RS test and to identify possible barriers to chemotherapy among Black patients. The ODX RS algorithm was developed on the basis of multigene profiles to quantify the risk of distant recurrence. Studies, including ours, have consistently shown that high proportions of Black patients had high-risk ODX RS.3,18–22 The observed differences in RS distributions between racial/ethnic groups suggest that distinct biological etiologies may exist. For example, it has been reported that Black women have higher expressions of suspected poor prognosis genes (GSTT2, PSPHL, SQLE, and TYMS) compared with White women.23 Additional reports also indicate that Black women with ER+ tumors have higher PAM50 risk of recurrence assay scores.19 However, this hypothesis was not supported by the subgroup analysis of TAILORx trial data, which showed no substantial differences in RS across randomized racial/ethnic populations.24 While selection bias can exist in both clinical trials and real-world studies, the totality of data appears to suggest tumor biological differences may play a role in breast cancer disparities. Further investigations into the biological characteristics and genomic variations in different racial/ethnic patients are warranted.
Among women with breast cancer, prognostic values of ODX RS on patient outcome have been well-validated in both clinical trials and routine practice settings,25–28 which included very few racial/ethnic minorities. A previous study by Press et al., which also used NCDB data, showed racial/ethnic disparities in receipt of ODX RS but did not investigate disparities in its prognostic values.17 In our study, we observed that among all racial/ethnic groups, women in high-risk score categories, according to both traditional and TAILORx categorizations, had substantially higher mortality compared with women in low-risk categories. Additional analyses stratified by age <50 and ≥50 years showed that both traditional and TAILORx ODX RS were more strongly associated with mortality among younger patients.
Collin et al. conducted a mortality study using data from the Georgia Cancer Registry and reported that Black women were more likely to have high-risk recurrence scores and die of axillary node-negative breast cancer compared with White women with comparable RS. In addition, this study suggested that the ODX RS test had lower prognostic accuracy in Black women, suggesting that genomic assays used to identify candidates for adjuvant chemotherapy may require model calibration in populations with greater racial/ethnic diversity.3 Similar results were observed in a study which evaluated associations of RS and breast cancer-specific mortality by race/ethnicity using SEER data,19 as well as another study using NCDB data.29 Both of these studies employed c-index to compare prognostic values of the ODX RS test across racial/ethnic groups and showed slightly lower prognostic accuracy in Black compared with White women, although statistical significance was not provided in the latter. Results from these studies are, in general, similar to ours, indicating higher proportions of high-risk ODX RS among Black women; however, we did not observe significant effect modifications by race/ethnicity for associations between ODX RS and mortality. It is noteworthy that our study included only stage I and II, node negative breast cancer patients, while the SEER-based and early NCDB-based studies included stage I-III cancers. In addition, factors related to access-to-care were not adjusted in these two previous studies. On the other hand, the SEER study focused on breast cancer-specific mortality, which may be more appropriate than the total mortality outcome evaluated in our study. Nonetheless, in our study, despite non-significant interactions between race/ethnicity and RS, the point estimates of HR for overall mortality in association with the high RS category were lower for Black patients in comparison to their White counterparts when TAILORx cutoffs were applied in overall and age stratified analyses. Considering that patients with an ODX RS test tended to have similar rates of chemotherapy use across racial/ethnic groups, our findings emphasize the importance of increasing ODX RS test uptake among minority groups to promote equity of cancer care for ER+, node-negative breast cancer.
Strengths of our study include the large sample size and generalizability. The NCDB registry captures approximately 70% of all US cancer cases, and racial/ethnic proportions are similar to that of SEER. This study allowed for a comprehensive evaluation of racial/ethnic disparities in mortality, while adjusting for numerous clinical and demographic covariates. However, this study also has some limitations. The follow-up period of our study was relatively short, with a median follow-up of 40.0 months. Information on cancer recurrence and cause of death is unavailable in the NCDB, which prevented a direct assessment of cancer-specific mortality. However, given the relative younger age of breast cancer patients included in the current study, we can be reasonably confident that the differences between RS groups in overall mortality largely reflect cancer-driven deaths. This assumption is supported by the fact that our findings were similar to the studies using Georgia Cancer Registry and SEER data, which evaluated breast cancer-specific mortality. Clinical variables, such as pathological information and patient records, were not reviewed systematically; therefore, information bias could exist. Information on other genetic tests and downstream information on the cancer care continuum is not available. Our study also has limited statistical power for some subgroup analyses, and those findings should be interpreted with caution. In addition, because individual level of education, income and zip code information is unavailable, we had to use neighborhood-level education and income as surrogate measures of socioeconomic status in our study, so misclassification cannot be ruled out. Another notable limitation of our study is the possible selection bias, as the NCDB is a hospital-based rather than a population-based registry.
In conclusion, among routine oncology care populations with early-stage breast cancer, we observed that Black and Hispanic women had lower ODX RS test uptakes but higher proportions of patients with high ODX RS compared with White and Asian American women. ODX RS had similar prognostic values for total mortality among White, Black, Hispanic and Asian American women. Additional research is needed to disentangle the reason(s) for higher RS among Black patients and lower ODX RS test uptake among minorities, as well as to understand the complex multifactorial intersection of social determinants of health, tumor biology and health services utilization.
Acknowledgements
All information was derived from the American College of Surgeons’ National Cancer Database. The American College of Surgeons and the Commission on Cancer are not responsible for conclusions drawn from the data. We thank Dr. Mary Shannon Byers for her editorial assistance.
This work was supported by the National Institutes of Health, Vanderbilt Training Program in the Molecular and Genetic Epidemiology of Cancer (grant number T32 CA160056; Dr. Jaleesa Moore). Dr. Fei Wang was supported by the program of China Scholarships Council and Ingram Cancer Professorship fund to Dr. XO Shu. The funders had no role in the data interpretation.
Footnotes
Conflict of Interest Disclosures: The authors declare no conflict of interest.
References
- 1.U.S. Cancer Statistics Working Group. USCS Data Visualizations [Internet]. U.S. Cancer Statistics Data Visualizations Tool, based on November 2017 submission data (1999–2015). 2018. [cited 2019 Jan 9]. Available from: https://gis.cdc.gov/grasp/USCS/DataViz.html [Google Scholar]
- 2.Davis BA, Aminawung JA, Abu-Khalaf MM, Evans SB, Su K, Mehta R, et al. Racial and Ethnic Disparities in Oncotype DX Test Receipt in a Statewide Population-Based Study. Journal of the National Comprehensive Cancer Network. 2017. Mar 1;15(3):346–54. [DOI] [PubMed] [Google Scholar]
- 3.Collin LJ, Yan M, Jiang R, Ward KC, Crawford B, Torres MA, et al. Oncotype DX recurrence score implications for disparities in chemotherapy and breast cancer mortality in Georgia. npj Breast Cancer. 2019. Sep 26;5(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Newman LA, Griffith KA, Jatoi I, Simon MS, Crowe JP, Colditz GA. Meta-analysis of survival in African American and white American patients with breast cancer: ethnicity compared with socioeconomic status. J Clin Oncol. 2006. Mar 20;24(9):1342–9. [DOI] [PubMed] [Google Scholar]
- 5.Iqbal J, Ginsburg O, Rochon PA, Sun P, Narod SA. Differences in Breast Cancer Stage at Diagnosis and Cancer-Specific Survival by Race and Ethnicity in the United States. JAMA. 2015. Jan 13;313(2):165–73. [DOI] [PubMed] [Google Scholar]
- 6.DeSantis CE, Fedewa SA, Sauer AG, Kramer JL, Smith RA, Jemal A. Breast cancer statistics, 2015: Convergence of incidence rates between black and white women. CA: A Cancer Journal for Clinicians. 2016;66(1):31–42. [DOI] [PubMed] [Google Scholar]
- 7.Schinkel JK, Zahm SH, Jatoi I, McGlynn KA, Gallagher C, Schairer C, et al. Racial/ethnic differences in breast cancer survival by inflammatory status and hormonal receptor status: an analysis of the Surveillance, Epidemiology, and End Results data. Cancer Causes Control. 2014. Aug 1;25(8):959–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Curtis E, Quale C, Haggstrom D, Smith-Bindman R. Racial and Ethnic Differences in Breast Cancer Survival. Cancer. 2008. Jan 1;112(1):171–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kohler BA, Sherman RL, Howlader N, Jemal A, Ryerson AB, Henry KA, et al. Annual Report to the Nation on the Status of Cancer, 1975–2011, Featuring Incidence of Breast Cancer Subtypes by Race/Ethnicity, Poverty, and State. JNCI: Journal of the National Cancer Institute [Internet]. 2015. Jun [cited 2020 Sep 24];107(6). Available from: 10.1093/jnci/djv048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Female Breast Cancer Subtypes - Cancer Stat Facts [Internet]. SEER. [cited 2020 Sep 24]. Available from: https://seer.cancer.gov/statfacts/html/breast-subtypes.html [Google Scholar]
- 11.Tao JJ, Visvanathan K, Wolff AC. Long term side effects of adjuvant chemotherapy in patients with early breast cancer. Breast. 2015. Nov;24(0 2):S149–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sparano JA, Gray RJ, Makower DF, Pritchard KI, Albain KS, Hayes DF, et al. Adjuvant Chemotherapy Guided by a 21-Gene Expression Assay in Breast Cancer. New England Journal of Medicine. 2018. Jul 12;379(2):111–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.NCDB. National Cancer Database [Internet]. American College of Surgeons. [cited 2020 Feb 17]. Available from: https://www.facs.org/quality-programs/cancer/ncdb [Google Scholar]
- 14.Ricks-Santi LJ, McDonald JT. Low utility of Oncotype DX® in the clinic. Cancer Med. 2017. Feb 1;6(3):501–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Katherine ER, Stephanie BW, Christopher B, Xi Z, Ke M, Megan R, et al. Influence of provider factors and race on uptake of breast cancer gene expression profiling. Cancer. 2018;124(8):1743–1751. [DOI] [PubMed] [Google Scholar]
- 16.Acuna N, Plascak JJ, Tsui J, Stroup AM, Llanos AAM. Oncotype DX Test Receipt among Latina/Hispanic Women with Early Invasive Breast Cancer in New Jersey: A Registry-Based Study. International Journal of Environmental Research and Public Health. 2021. Jan;18(10):5116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.David JP, Abiola I, M ED, Kathleen HG, Suzanne C, Dezheng H. Racial disparities in omission of oncotype DX but no racial disparities in chemotherapy receipt following completed oncotype DX test results. Breast Cancer Res Treat. 2018;168(1):207–220. [DOI] [PubMed] [Google Scholar]
- 18.Han Y, Miao Z-F, Lian M, Peterson LL, Colditz GA, Liu Y. Racial and ethnic disparities in 21-gene recurrence scores, chemotherapy, and survival among women with hormone receptor-positive, node-negative breast cancer. Breast Cancer Res Treat. 2020. Dec 1;184(3):915–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoskins KF, Danciu OC, Ko NY, Calip GS. Association of Race/Ethnicity and the 21-Gene Recurrence Score With Breast Cancer–Specific Mortality Among US Women. JAMA Oncology. 2021. Mar 1;7(3):370–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holowatyj AN, Cote ML, Ruterbusch JJ, Ghanem K, Schwartz AG, Vigneau FD, et al. Racial Differences in 21-Gene Recurrence Scores Among Patients With Hormone Receptor–Positive, Node-Negative Breast Cancer. JCO. 2018. Jan 17;36(7):652–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lund MJ, Mosunjac M, Davis KM, Gabram-Mendola S, Rizzo M, Bumpers HL, et al. 21-Gene recurrence scores. Cancer. 2012;118(3):788–96. [DOI] [PubMed] [Google Scholar]
- 22.Ibraheem AF, Press DJ, Olopade OI, Huo D. Community Clinical Practice Patterns and Mortality in Patients with Oncotype DX Intermediate Score: Who benefits from Chemotherapy? Cancer. 2019. Jan 15;125(2):213–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parada H, Sun X, Fleming JM, Williams-DeVane CR, Kirk EL, Olsson LT, et al. Race-associated biological differences among luminal A and basal-like breast cancers in the Carolina Breast Cancer Study. Breast Cancer Res. 2017. Dec 11;19(1):131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kathy SA, Robert JG, Della FM, Amir F, Daniel FH, Charles EG, et al. Race, Ethnicity, and Clinical Outcomes in Hormone Receptor-Positive, HER2-Negative, Node-Negative Breast Cancer in the Randomized TAILORx Trial. J Natl Cancer Inst. 2021;113(4):390–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paik S, Shak S, Tang G, Kim C, Baker J, Cronin M, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004. Dec 30;351(27):2817–26. [DOI] [PubMed] [Google Scholar]
- 26.Wang F, Reid S, Zheng W, Pal T, Meszoely I, Mayer IA, et al. Sex Disparity Observed for Oncotype DX Breast Recurrence Score in Predicting Mortality Among Patients with Early Stage ER-Positive Breast Cancer. Clin Cancer Res. 2020. Jan 1;26(1):101–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mamounas EP, Tang G, Fisher B, Paik S, Shak S, Costantino JP, et al. Association between the 21-gene recurrence score assay and risk of locoregional recurrence in node-negative, estrogen receptor-positive breast cancer: results from NSABP B-14 and NSABP B-20. J Clin Oncol. 2010. Apr 1;28(10):1677–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dowsett M, Cuzick J, Wale C, Forbes J, Mallon EA, Salter J, et al. Prediction of risk of distant recurrence using the 21-gene recurrence score in node-negative and node-positive postmenopausal patients with breast cancer treated with anastrozole or tamoxifen: a TransATAC study. J Clin Oncol. 2010. Apr 10;28(11):1829–34. [DOI] [PubMed] [Google Scholar]
- 29.Abiola I, Olufunmilayo IO, Dezheng H. Propensity score analysis of the prognostic value of genomic assays for breast cancer in diverse populations using the National Cancer Data Base. Cancer. 2020;126(17):4013–4022. [DOI] [PMC free article] [PubMed] [Google Scholar]