Abstract
Background
The data we present consist of an inventory of exotic arthropods, potentially invasive, collected in exotic and mixed forests and disturbed native forest patches of the Azores Archipelago. The study was carried out between 2019 and 2020 in four islands: Corvo, Flores, Terceira and Santa Maria, where a total of 45 passive flight interception SLAM traps were deployed, during three to six consecutive months. This manuscript is the second contribution of the “SLAM Project - Long Term Ecological Study of the Impacts of Climate Change in the Natural Forest of Azores”.
New information
We provide an inventory of terrestrial arthropods belonging to Arachnida, Diplopoda, Chilopoda and Insecta classes from four Azorean islands. We identified a total of 21,175 specimens, belonging to 20 orders, 93 families and 249 species of arthropods. A total of 125 species are considered introduced, 89 native non-endemic and 35 endemic. We registered 34 new records (nine for Corvo, three for Flores, six for Terceira and 16 for Santa Maria), of which five are new for Azores, being all exotic possibly recently introduced: Dieckmanniellusnitidulus (Gyllenhal, 1838), Gronopsfasciatus Küster, 1851, Hadroplontustrimaculatus (Fabricius, 1775), Hypurusbertrandi (Perris, 1852) (all Coleoptera, Curculionidae) and Cardiocondylamauritanica Forel, 1890 (Hymenoptera, Formicidae). This publication highlights the importance of planted forests and disturbed native forest patches as reservoirs of potentially invasive arthropods and refuges for some rare relict endemic arthropod species.
Keywords: Arthropoda, Azores, endemic species, exotic species, exotic forest, inventory, Macaronesia, long-term sampling, SLAM traps
Introduction
Arthropod communities, particularly insects, are being affected by unprecedented and rapid population declines (Hallmann et al. 2017, Sánchez-Bayo and Wyckhuys 2019, Cardoso et al. 2020, Harvey et al. 2020,Cowie et al. 2022). The most important causes for this biodiversity loss are habitat loss, degradation and fragmentation, climate change and the introduction and spread of invasive species (Russell et al. 2017, Borges et al. 2019a). In this context, the biodiversity of oceanic islands has been especially and dramatically affected by these drivers as consequence of human colonisation, global trade and tourism (Triantis et al. 2010, Borges et al. 2019b, Cowie et al. 2022, Stüben 2022).
In the case of Azores islands, since Portuguese settlement in the 15th century, the original landscape was strongly altered by replacing pristine and native forest areas with exotic tree plantations, crops, pastures and urban areas (Triantis et al. 2010, Borges et al. 2019b, Norder et al. 2020). Currently, the remaining native forest covers only about 5% of the total surface of the Archipelago, being restricted to the higher elevation and inaccessible areas of the islands (Gaspar et al. 2008, Triantis et al. 2010, Stüben and Borges 2019, Norder et al. 2020).
Native forest destruction (Triantis et al. 2010) and the consequent lack of connectivity between forest patches (Aparício et al. 2018), climate change (Ferreira et al. 2016) and invasive species are the main factors that contribute to arthropod decline in Azores (Stüben 2003, Stüben 2004, Borges et al. 2019b). Previous studies demonstrated that endemic species of Azorean arthropods are restricted mainly to native vegetation dominated habitats, while introduced species usually occupy human-altered habitats (Cardoso et al. 2009, Florencio et al. 2015, Florencio et al. 2016). Additionally, the proportion of introduced arthropod species in Azores is higher than native (around 60%) and, due to the higher adaptability to environmental conditions of many introduced species, they represent one of the main threats to indigenous biota in the native forest areas (Borges et al. 2019b). Moreover, Tsafack et al. (2021) showed the importance of isolated and small native forest patches, as well exotic and mixed forests close to native areas, which can function as refuges for native and rare endemic species, playing a relevant role for conservation of native biota outside Azorean protected areas.
This publication is the second data paper of the project “SLAM Project - Long Term Ecological Study of the Impacts of Climate Change in the Natural Forest of Azores” (see first in Costa and Borges 2021) that aims to monitor the distribution and abundance of arthropods in native forests from Azores using SLAM traps (Sea, Land and Air Malaise traps). Additional publications, using data coming from this project, tested specific ecological questions, namely patterns of seasonal variation on species abundance (Borges et al. 2017), patterns of temporal beta diversity in native and exotic species (Matthews et al. 2018), the potential decline of endemic insects (Borges et al. 2020), patterns of arthropod diversity in Azorean urban gardens (Arteaga et al. 2020), patterns of species richness and beta diversity in a small elevational gradient (de Vries et al. 2021) and the investigation of the role of small lowland patches of exotic forests as refuges for rare endemic Azorean arthropods (Tsafack et al. 2021).
In this second data paper, we aim to: i) survey arthropods in exotic and mixed forests and small disturbed remnants of native forests; ii) investigate the occurrence and current distribution of exotic (potentially invasive) arthropods in those habitats; and also iii) investigate the occurrence of rare endemic arthropods in those habitats.
General description
Purpose
This publication provides an inventory of arthropods present in exotic and mixed forests of four Azores Islands (Corvo, Flores, Terceira and Santa Maria), as well as from small remnants of disturbed native forests in three Islands (Flores, Terceira and Santa Maria).
Additional information
The data we present are part of the long-term project SLAM (Long Term Ecological Study of the Impacts of Climate Change in the Natural Forest of Azores) that started in 2012 aiming to understand the impact of biodiversity erosion drivers on Azorean native forests (Azores, Macaronesia, Portugal). Passive flight interception SLAM traps (Sea, Land and Air Malaise traps) are being used to sample native forest plots in several Azorean islands (Costa and Borges 2021).
Project description
Title
SLAM Project II - A survey of exotic and endemic arthropods in Azorean disturbed Azorean forest habitats
Personnel
The project was conceived and led by Paulo A.V. Borges.
Fieldwork: Corvo Island - Alejandra Ros-Prieto, Maria Teresa Ferreira, Mário Boieiro, Paulo A. V. Borges, Rosalina Gabriel; Flores Island - Alejandra Ros-Prieto, Maria Teresa Ferreira, Mário Boieiro, Paulo A. V. Borges, Rosalina Gabriel; Terceira Island - Alejandra Ros-Prieto, Paulo A. V. Borges, Rosalina Gabriel; Santa Maria Island - Alejandra Ros-Prieto, Nelson Moura, Paulo A. V. Borges, Rosalina Gabriel.
Parataxonomists: Alejandra Ros-Prieto, Jonne Bonnet and Sébastien Lhoumeau.
Taxonomists: Paulo A. V. Borges, Mário Boieiro and Peter E. Stüben.
Voucher specimen management was mainly undertaken by Alejandra Ros-Prieto and Paulo A. V. Borges.
Study area description
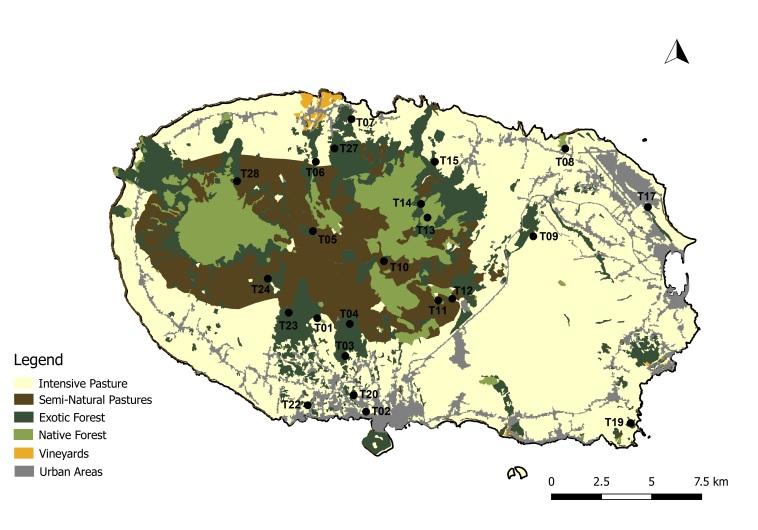
The study area comprises Corvo, Flores, Terceira and Santa Maria Islands, in the Azores Archipelago, located in the North Atlantic, roughly at 38°43'21"N 27°13'14"W and 38°27'30"N 28°19'22"W (Fig. 1). The climate is temperate oceanic, with regular and abundant rainfall, high levels of relative humidity and persistent winds, mainly during the winter and autumn seasons. The exotic forests are located at lower and mid-elevations and are dominated mainly by Pittosporumundulatum Vent., Eucalyptus spp., Cryptomeriajaponica D.Don, Acaciamelanoxylon R.Br. and Pinuspinaster Aiton. The studied native forests are located at several elevations and are mainly dominated by Ericaazorica Hochst. ex Seub., Laurusazorica (Seub.) Franco, Ilexazorica Gand. and Juniperusbrevifolia (Hochst. ex Seub.). Mixed forests included both exotic and native tree species.
Figure 1.
The Azores Archipelago location (Credit: Enésima Pereira, Azorean Biodiversity Group).
Design description
Passive flight interception SLAM traps (Sea, Land and Air Malaise traps) (Fig. 2) were used to sample 45 sites in the four study Islands (Corvo (n = 1), Flores (n = 5), Santa Maria (n =16) and Terceira (n = 23)) with one trap being set up at each plot. Although this protocol was originally developed to sample flying arthropods, by working as an extension of the tree, non-flying species can also crawl into the trap (Borges et al. 2017), enhancing the range of groups that can be sampled by this technique. Recent studies have used this sampling technique to study diversity and abundance variations in the communities of arthropod on Azorean native areas (Borges et al. 2017, Matthews et al. 2018, Borges et al. 2020, de Vries et al. 2021, Tsafack et al. 2021). The samples were collected every three or six months depending on sites. The collected specimens were sorted to morphospecies and posteriorly identified at species level by an expert taxonomist in laboratory.
Figure 2.
Passive flight interception SLAM trap (Sea, Land and Air Malaise traps) (Credit: Paulo A. V. Borges).
Funding
Portuguese National Funds, through FCT – Fundação para a Ciência e a Tecnologia, within the project UID/BIA/00329/2013-2023.
Direcção Regional do Ambiente - PRIBES (LIFE17 IPE/PT/000010) (2019-2020).
Direcção Regional do Ambiente – LIFE-BETTLES (LIFE18 NAT_PT_000864) (2020-2024).
AZORESBIOPORTAL –PORBIOTA (ACORES-01-0145-FEDER-000072) (2019-2022).
The database management and Open Access was funded by the project “MACRISK-Trait-based prediction of extinction risk and invasiveness for Northern Macaronesian arthropods” Fundação para a Ciência e Tecnologia FCT - PTDC/BIA-CBI/0625/2021 (2022-2024).
Sampling methods
Study extent
The study was conducted in four Islands of the Azores Archipelago, Corvo, Flores, Terceira and Santa Maria. The sampled habitats included exotic, mixed and disturbed native forest patches (Table 1).
Table 1.
List of the 45 sampled sites in the Corvo (n = 1), Flores (n = 5), Santa Maria (n =16) and Terceira (n = 23) Islands. Information about LocationID, Locality, decimal coordinates and elevation in metres are provided.
| Island | Habitat | Location ID | Locality | Latitude | Longitude | Elevation |
| Corvo | Mixed Forest - Picconia, Pittosporum | COR-CORO-Z-16 | Coroa do Pico | 39.68854 | -31.09191 | 248 |
| Flores | Exotic Forest - Cryptomeria | FLO-LAFLOR-T29 | Lajes- Estação Florestal | 39.39416 | -31.20682 | 315 |
| Flores | Exotic Forest - Cryptomeria | FLO-MAPS-TT25 | Criptomérias ao lado do T16 | 39.48697 | -31.18462 | 607 |
| Flores | Native Forest | FLO-NFFR-T-07 | Encosta Caldeira Funda | 39.40324 | -31.2175 | 381 |
| Flores | Native Forest | FLO-NFMA-T-08 | Morro Alto Este | 39.46003 | -31.20941 | 769 |
| Flores | Mixed Forest | FLO-PDEL-Z-11 | Ponta Delgada Km18_Mata das Acácias | 39.50744 | -31.2017 | 106 |
| Santa Maria | Native Forest - Erica, Picconia | SMR_PRIBS_T01 | Ponta do Pinheiro | 37.00336 | -25.12854 | 192 |
| Santa Maria | Mixed Forest - Erica, Picconia, Hedychium | SMR_PRIBS_T02 | Miradouro Pedra Rija | 36.97597 | -25.07578 | 355 |
| Santa Maria | Mixed Forest - Picconia, Pittosporum, Pinus | SMR_PRIBS_T03 | Piedade | 36.93424 | -25.0668 | 184 |
| Santa Maria | Mixed Forest - Laurus, Pittosporum, Picconia, Ilex | SMR_PRIBS_T04 | Setada | 36.95356 | -25.07398 | 374 |
| Santa Maria | Native Forest - Laurus, Erica, Ilex | SMR_PRIBS_T05 | Casas Velhas | 36.95375 | -25.07494 | 377 |
| Santa Maria | Mixed Forest - Picconia, Pittosporum, Erica, Hedychium, Vaccinium | SMR_PRIBS_T06 | Fontinhas Florestal | 36.96325 | -25.07505 | 406 |
| Santa Maria | Mixed Forest - Picconia, Pittosporum | SMR_PRIBS_T07 | Miradouro Espigão | 36.98215 | -25.0488 | 191 |
| Santa Maria | Mixed Forest - Erica, Picconia, Pittosporum | SMR_PRIBS_T08 | Estação Loran | 37.00931 | -25.05792 | 164 |
| Santa Maria | Mixed Forest - Erica, Cryptomeria, Hedychium | SMR_PRIBS_T10 | Piquinhos | 36.97206 | -25.08278 | 420 |
| Santa Maria | Mixed Forest - Picconia, Pittosporum | SMR_PRIBS_T11 | Lapa | 36.94849 | -25.02598 | 221 |
| Santa Maria | Mixed Forest - Acacia, Picconia | SMR_PRIBS_T12 | Monteiro | 36.97013 | -25.10942 | 191 |
| Santa Maria | Exotic Forest - Acacia | SMR_PRIBS_T13 | Aeroporto | 36.97048 | -25.1549 | 112 |
| Santa Maria | Exotic Forest - Pittosporum | SMR_PRIBS_T14 | Figueiral | 36.94919 | -25.12562 | 142 |
| Santa Maria | Mixed Forest | SMR_PRIBS_T15 | Ribeira dos Lemos | 37.00141 | -25.14769 | 61 |
| Santa Maria | Native Forest - Erica, Picconia | SMR_PRIBS_T16 | Caldeira | 36.99592 | -25.09864 | 304 |
| Santa Maria | Mixed Forest - Picconia, Erica, Laurus, Vaccinium, Hedychium, Myrcide | SMR-NFPA-T-01 (SMR_PRIBS_T09) | Pico Alto T01 | 36.97804 | -25.08756 | 460 |
| Terceira | Exotic Forest - Pittosporum | TER_PRIBS_T02 | Universidade | 38.65868 | -27.23262 | 43 |
| Terceira | Exotic Forest - Pittosporum | TER_PRIBS_T04 | Mata Estado Veredas | 38.69814 | -27.2421 | 450 |
| Terceira | Exotic Forest - Pittosporum | TER_PRIBS_T07 | Pittosporum Carpintaria dos Biscoitos | 38.79017 | -27.24136 | 93 |
| Terceira | Exotic Forest - Pittosporum | TER_PRIBS_T08 | Caldeira Lajes | 38.77705 | -27.11853 | 18 |
| Terceira | Native Forest - Juniperus | TER_PRIBS_T11 | Juniperal Trilho das Bestas | 38.7087 | -27.19133 | 521 |
| Terceira | Native Forest - Erica | TER_PRIBS_T12 | Erical Trilho das Bestas | 38.70957 | -27.18324 | 462 |
| Terceira | Native Forest - Juniperus | TER_PRIBS_T13 | Terra-Brava Rocha Cedrorum | 38.74598 | -27.19762 | 652 |
| Terceira | Exotic Forest - Cryptomeria, Calluna | TER_PRIBS_T14 | Pico Alto Cryptomeria_Calluna | 38.75212 | -27.20132 | 584 |
| Terceira | Exotic Forest - Pittosporum, Eucalyptus | TER_PRIBS_T15 | Eucaliptal Agualva | 38.77109 | -27.1934 | 344 |
| Terceira | Exotic Forest - Pittosporum | TER_PRIBS_T17 | Pittosporum_Eucalito Pizza-UT | 38.75087 | -27.07099 | 87 |
| Terceira | Exotic Forest - Pittosporum | TER_PRIBS_T19 | Pittosporum Maria Vieira | 38.65377 | -27.08076 | 102 |
| Terceira | Exotic Forest - Eucalyptus | TER_PRIBS_T20 | Ermida Penha França | 38.66603 | -27.23969 | 118 |
| Terceira | Exotic Forest - Eucalyptus, Acacia | TER_PRIBS_T22 | Eucalipto_Acacia_Canada Entre Picos | 38.6615 | -27.26605 | 78 |
| Terceira | Exotic Forest - Eucalyptus, Hedychium | TER_PRIBS_T23 | Eucalipto_Echinodium Escampador | 38.70309 | -27.27717 | 340 |
| Terceira | Exotic Forest - Pittosporum, Betula | TER_PRIBS_T24 | Betulas_Lagoa das Patas | 38.71833 | -27.28923 | 524 |
| Terceira | Exotic Forest - Eucalyptus | TER_PRIBS_T27 | Eucaliptal_Gruta Chocolate | 38.77696 | -27.25107 | 298 |
| Terceira | Exotic Forest - Pittosporum, Eucalyptus | TER_PRIBS_T28 | Eucaliptal_Pico Rachado_Altares | 38.76211 | -27.30704 | 522 |
| Terceira | Mixed Forest - Eucalyptus, Erica | TER-ACAR-T-25 (TER_PRIBS_T10) | Eucaliptal Algar do Carvão | 38.72638 | -27.22258 | 530 |
| Terceira | Exotic Forest - Pittosporum | TER-CABI-T166 (TER_PRIBS_T06) | Caparica - Biscoitos | 38.77094 | -27.26185 | 331 |
| Terceira | Exotic Forest - Acacia | TER-FTER-T-36 (TER_PRIBS_T09) | Fontinhas 1 | 38.73765 | -27.13681 | 245 |
| Terceira | Native Forest - Laurus, Erica | TER-MATE-T-13 (TER_PRIBS_T01) | Matela 1 | 38.70063 | -27.26074 | 392 |
| Terceira | Exotic Forest - Cryptomeria | TER-MNEG-T-62 (TER_PRIBS_T05) | Lagoa do Negro | 38.73977 | -27.26341 | 571 |
| Terceira | Exotic Forest - Pittosporum | TER-POSA-T172 (TER_PRIBS_T03) | Posto Santo | 38.68365 | -27.24457 | 246 |
Sampling description
A total of 45 passive flight interception SLAM traps (Sea, Land and Air Malaise traps) were used to sample the plots in the four study Islands, with one trap being set up at each plot. Trap size is of approximately 110 x 110 x 110 cm. The trap functions on the basis of intercepting arthropods that crawl up the mesh and then fall inside the sampling recipient, which is filled with propylene glycol (pure 1,2-propanodiol) (Borges et al. 2017). A total of 19 SLAM traps were deployed in exotic forest areas, eight on native forest patches and 18 on mixed forests. The trap samples were collected every three months in Flores and Corvo and six months in Terceira and Santa Maria. In Corvo Island, one trap was available in a mixed forest (Fig. 3; Table 1). In Flores Island, five traps were available in both exotic forests and native forests (Fig. 4; Table 1). In Santa Maria Island, a total of 16 traps were available with only three located in disturbed native forest patches (Fig. 5; Table 1). Finally, in Terceira Island, 23 traps were available with only four in disturbed native forest patches (Fig. 6; Table 1).
Figure 3.
Map with the location of the sampling sites in Corvo Island, Azores. Codes of sites as in Table 1 (Land-use data extracted from Cruz et al. 2007) (Credit: Enésima Pereira, Azorean Biodiversity Group).
Figure 4.
Map with the location of the sampling sites in Flores Island, Azores. Codes of sites as in Table 1 (Land-use data extracted from Cruz et al. 2007) (Credit: Enésima Pereira, Azorean Biodiversity Group).
Figure 5.
Map with the location of the sampling sites in Santa Maria Island, Azores. Codes of sites as in Table 1 (Land-use data extracted from Cruz et al. 2007) (Credit: Enésima Pereira, Azorean Biodiversity Group).
Figure 6.
Map with the location of the sampling sites in Terceira Island, Azores. Codes of sites as in Table 1 (Land-use data extracted from Cruz et al. 2007) (Credit: Enésima Pereira, Azorean Biodiversity Group).
Quality control
All sampled individuals were first sorted by trained paratoxonomists (see list above). All specimens were allocated to a taxonomic species by Paulo A. V. Borges. Despite the uncertainty of juvenile identification, juveniles are also included in the data presented in this paper, since the low diversity allowed a relatively precise identification of this life-stage in Azores.
Step description
At the laboratory, specimen sorting and arthropod identification followed standard procedures during the last 20 years or arthropod surveys in Azores. First, a combination of morphological and anatomical characters and reproductive structures was used for morphospecies creation. After, morphospecies were sent to experts for proper identification. With this procedure, a reference collection was made for all collected specimens by assigning them a morphospecies code number and respective taxonomic name and depositing them at the Dalberto Teixeira Pombo Insect Collection, University of Azores. Colonisation status of the species was obtained from the last updated checklist of Azorean arthropods (Borges et al. 2010).
Geographic coverage
Description
Corvo, Flores, Terceira and Santa Maria Islands, in the Azores Archipelago (Portugal).
Coordinates
36.90597988519294 and 39.740986355883564 Latitude; -31.2945556640625 and -24.949951171875 Longitude.
Taxonomic coverage
Description
The following Classes and Orders are covered:
Arachnida: Araneae; Opiliones; Pseudoscorpiones
Chilopoda: Geophilomorpha; Lithobiomorpha; Scolopendromorpha; Scutigeromorpha
Diplopoda: Julida; Polydesmida
Insecta: Archaeognatha; Blattodea; Coleoptera; Dermaptera; Hemiptera; Hymenoptera; Isoptera; Neuroptera; Orthoptera; Phasmatodea; Psocoptera; Thysanoptera; Trichoptera.
Taxa included
| Rank | Scientific Name | Common Name |
|---|---|---|
| order | Araneae | Spiders |
| order | Opiliones | Harvestmen |
| order | Pseudoscorpiones | Pseudoscorpions |
| class | Chilopoda | Centipedes |
| class | Diplopoda | Millipedes |
| order | Archaeognatha | Bristletails |
| order | Blattodea | Cockroaches |
| order | Coleoptera | Beetles |
| order | Dermaptera | Earwig |
| order | Hemiptera | Bugs |
| order | Hymenoptera | Ants |
| order | Isoptera | Termites |
| order | Neuroptera | Lacewings |
| order | Orthoptera | Grasshoppers, crickets |
| order | Phasmatodea | Stick insects |
| order | Psocodea | Booklice |
| order | Thysanoptera | Thrips |
| order | Trichoptera | Caddisflies |
Collection data
Collection name
Entomoteca Dalberto Teixeira Pombo (DTP); University of Azores
Collection identifier
DTP
Specimen preservation method
All specimens were preserved in 96% ethanol.
Curatorial unit
Curator: Paulo A. V. Borges
Usage licence
Usage licence
Creative Commons Public Domain Waiver (CC-Zero)
Data resources
Data package title
A survey of exotic arthropods in disturbed Azorean forest habitats using SLAM traps.
Resource link
Alternative identifiers
https://www.gbif.org/dataset/020231d8-39b6-478f-ac24-715bf97c8ef4
Number of data sets
2
Data set 1.
Data set name
Event Table
Data format
Darwin Core Archive format
Number of columns
24
Character set
UTF-8
Download URL
Data format version
version 1.5
Description
The dataset was published in Global Biodiversity Information Facility platform, GBIF (Borges et al. 2022). The following data table includes all the records for which a taxonomic identification of the species was possible. The dataset submitted to GBIF is structured as a sample event dataset that has been published as a Darwin Core Archive (DwCA), which is a standardised format for sharing biodiversity data as a set of one or more data tables. The core data file contains 45 records (eventID). This IPT (Integrated Publishing Toolkit) archives the data and thus serves as the data repository. The data and resource metadata are available for download in the Portuguese GBIF Portal IPT (Borges et al. 2022).
Data set 1.
| Column label | Column description |
|---|---|
| id | Unique identification code for sampling event data. |
| eventID | Identifier of the events, unique for the dataset. |
| samplingProtocol | The sampling protocol used to capture the species. |
| sampleSizeValue | The numeric amount of time spent in each sampling. |
| sampleSizeUnit | The unit of the sample size value. |
| eventDate | Date or date range the record was collected. |
| year | Year of the event. |
| minimumElevationInMetres | The lower limit of the range of elevation (altitude, usually above sea level), in metres. |
| verbatimEventDate | The verbatim original representation of the date and time information for an Event. In this case, we use the season and year. |
| habitat | The habitat of the sample. |
| locationID | Identifier of the location. |
| islandGroup | Name of archipelago. |
| island | Name of the island. |
| country | Country of the sampling site. |
| countryCode | ISO code of the country of the sampling site. |
| stateProvince | Name of the region of the sampling site. |
| municipality | Municipality of the sampling site. |
| locality | Name of the locality. |
| decimalLatitude | Approximate centre point decimal latitude of the field site in GPS coordinates. |
| decimalLongitude | Approximate centre point decimal longitude of the field site in GPS coordinates. |
| geodeticDatum | The ellipsoid, geodetic datum or spatial reference system (SRS) upon which the geographic coordinates given in decimalLatitude and decimalLongitude are based. |
| coordinateUncertaintyInMetres | Uncertainty of the coordinates of the centre of the sampling plot in metres. |
| coordinatePrecision | A decimal representation of the precision of the coordinates given in the decimalLatitude and decimalLongitude. |
| georeferenceSources | A list (concatenated and separated) of maps, gazetteers or other resources used to georeference the Location, described specifically enough to allow anyone in the future to use the same resources. |
Data set 2.
Data set name
Occurrence Table
Data format
Darwin Core Archive format
Number of columns
30
Character set
UTF-8
Download URL
https://www.gbif.org/dataset/020231d8-39b6-478f-ac24-715bf97c8ef4
Data format version
version 1.5
Description
The dataset was published in Global Biodiversity Information Facility platform, GBIF (Borges et al. 2022). The following data table includes all the records for which a taxonomic identification of the species was possible. The dataset submitted to GBIF is structured as an occurrence table that has been published as a Darwin Core Archive (DwCA), which is a standardised format for sharing biodiversity data as a set of one or more data tables. The core data file contains 2095 records (occurrenceID). This IPT (Integrated Publishing Toolkit) archives the data and thus serves as the data repository. The data and resource metadata are available for download in the Portuguese GBIF Portal IPT (Borges et al. 2022).
Data set 2.
| Column label | Column description |
|---|---|
| id | Unique identification code for sampling event data. |
| type | Type of the record, as defined by the Public Core standard. |
| licence | Reference to the licence under which the record is published. |
| institutionID | The identity of the institution publishing the data. |
| institutionCode | The code of the institution publishing the data. |
| collectionID | The identity of the collection publishing the data. |
| collectionCode | The code of the collection where the specimens are conserved. |
| basisOfRecord | The nature of the data record. |
| occurrenceID | Identifier of the record, coded as a global unique identifier. |
| recordedBy | A list (concatenated and separated) of names of people, groups or organisations who performed the sampling in the field. |
| organismQuantity | A number or enumeration value for the quantity of organisms. |
| organismQuantityType | The type of quantification system used for the quantity of organisms. |
| sex | The sex and quantity of the individuals captured. |
| lifeStage | The life stage of the organisms captured. |
| establishmentMeans | The process of establishment of the species in the location, using a controlled vocabulary: in the GBIF database, we used the Borges et al. (2010) original data: 'native', 'introduced', 'endemic'. |
| eventID | Identifier of the events, unique for the dataset. |
| identifiedBy | A list (concatenated and separated) of names of people, groups or organisations who assigned the Taxon to the subject. |
| dateIdentified | The date on which the subject was determined as representing the Taxon. |
| identificationRemarks | Information about morphospecies identification (code in Dalberto Teixeira Pombo Collection). |
| scientificName | Complete scientific name including author and year. |
| kingdom | Kingdom name. |
| phylum | Phylum name. |
| class | Class name. |
| order | Order name. |
| family | Family name. |
| genus | Genus name. |
| specificEpithet | Specific epithet. |
| infraspecificEpithet | Infrapecific epithet. |
| taxonRank | Lowest taxonomic rank of the record. |
| scientificNameAuthorship | Name of the author of the lowest taxon rank included in the record. |
Additional information
We collected a total of 27,958 specimens (Suppl. material 1; Borges et al. 2022) from which it was possible to identify to species level 76% of the specimens (21,175) (Table 2). These identified specimens belong to 20 orders, 93 families and 249 species of arthropods. A total of 125 species are considered introduced, 89 native non-endemic and 35 endemic (Table 2). Additionally, a total of 147 taxa were recorded at genus, family or order level (Suppl. material 1).
Table 2.
List of arthropod species collected in four islands of Azores, between 2019 and 2020 using SLAM traps. The list includes individuals identified at species-level. Scientific name, colonization status (CS: intr – introduced; nat - native non-endemic; end - endemic) and abundance per island (COR - Corvo; FLO - Flores; TER - Terceira; SMR - Santa Maria). Bold scientific names constitute new records for the Azores and bold numbers new records for a given island.
| Class | Order | Family | Scientific Name | CS | COR | FLO | TER | SMR |
| Arachnida | Araneae | Agelenidae | Tegenariapagana C.L. Koch, 1840 | intr | 118 | 1 | ||
| Arachnida | Araneae | Agelenidae | Textrixcaudata L. Koch, 1872 | intr | 3 | |||
| Arachnida | Araneae | Araneidae | Agalenatearedii (Scopoli, 1763) | intr | 1 | |||
| Arachnida | Araneae | Araneidae | Araneusangulatus Clerck, 1757 | intr | 1 | |||
| Arachnida | Araneae | Araneidae | Gibbaraneaoccidentalis Wunderlich, 1989 | end | 5 | 85 | 3 | |
| Arachnida | Araneae | Araneidae | Mangoraacalypha (Walckenaer, 1802) | intr | 1 | |||
| Arachnida | Araneae | Araneidae | Zygiellax-notata (Clerck, 1757) | intr | 4 | |||
| Arachnida | Araneae | Cheiracanthiidae | Cheiracanthiumerraticum (Walckenaer, 1802) | intr | 2 | 2 | ||
| Arachnida | Araneae | Cheiracanthiidae | Cheiracanthiumfloresense Wunderlich, 2008 | end | 5 | |||
| Arachnida | Araneae | Cheiracanthiidae | Cheiracanthiummildei L. Koch, 1864 | intr | 28 | 39 | ||
| Arachnida | Araneae | Clubionidae | Clubionaterrestris Westring, 1851 | intr | 6 | 1 | ||
| Arachnida | Araneae | Clubionidae | Porrhoclubionadecora (Blackwall, 1859) | nat | 158 | 43 | ||
| Arachnida | Araneae | Clubionidae | Porrhoclubionagenevensis (L. Koch, 1866) | intr | 15 | 19 | 41 | 47 |
| Arachnida | Araneae | Dictynidae | Emblynaacoreensis Wunderlich, 1992 | end | 3 | |||
| Arachnida | Araneae | Dictynidae | Lathysdentichelis (Simon, 1883) | nat | 2 | 16 | 7 | |
| Arachnida | Araneae | Dictynidae | Nigmapuella (Simon, 1870) | intr | 6 | 1 | ||
| Arachnida | Araneae | Dysderidae | Dysderacrocata C.L. Koch, 1838 | intr | 5 | 70 | 49 | |
| Arachnida | Araneae | Gnaphosidae | Marinarozeloteslyonneti (Audouin, 1826) | intr | 1 | |||
| Arachnida | Araneae | Linyphiidae | Acorigoneacoreensis (Wunderlich, 1992) | end | 1 | 35 | 1 | |
| Arachnida | Araneae | Linyphiidae | Agynetafuscipalpa (C. L. Koch, 1836) | intr | 11 | |||
| Arachnida | Araneae | Linyphiidae | Canariphantesacoreensis (Wunderlich, 1992) | end | 1 | |||
| Arachnida | Araneae | Linyphiidae | Canariphantesrelictus Crespo & Bosmans, 2014 | end | 2 | |||
| Arachnida | Araneae | Linyphiidae | Erigoneautumnalis Emerton, 1882 | intr | 12 | 3 | ||
| Arachnida | Araneae | Linyphiidae | Erigonedentipalpis (Wider, 1834) | intr | 2 | 1 | ||
| Arachnida | Araneae | Linyphiidae | Lessertiadentichelis (Simon, 1884) | intr | 3 | |||
| Arachnida | Araneae | Linyphiidae | Microlinyphiajohnsoni (Blackwall, 1859) | nat | 348 | |||
| Arachnida | Araneae | Linyphiidae | Miniciafloresensis Wunderlich, 1992 | end | 2 | |||
| Arachnida | Araneae | Linyphiidae | Nerieneclathrata (Sundevall, 1830) | intr | 2 | 1 | ||
| Arachnida | Araneae | Linyphiidae | Oedothoraxfuscus (Blackwall, 1834) | intr | 8 | |||
| Arachnida | Araneae | Linyphiidae | Palliduphantesschmitzi (Kulczynski, 1899) | nat | 2 | |||
| Arachnida | Araneae | Linyphiidae | Pelecopsisparallela (Wider, 1834) | intr | 2 | |||
| Arachnida | Araneae | Linyphiidae | Prinerigonevagans (Audouin, 1826) | intr | 1 | |||
| Arachnida | Araneae | Linyphiidae | Savigniorrhipisacoreensis Wunderlich, 1992 | end | 30 | 197 | 7 | |
| Arachnida | Araneae | Linyphiidae | Tenuiphantesmiguelensis (Wunderlich, 1992) | nat | 3 | 16 | ||
| Arachnida | Araneae | Linyphiidae | Tenuiphantestenuis (Blackwall, 1852) | intr | 6 | 17 | 346 | 21 |
| Arachnida | Araneae | Mimetidae | Erofurcata (Villers, 1789) | intr | 10 | |||
| Arachnida | Araneae | Oecobiidae | Oecobiusnavus Blackwall, 1859 | intr | 13 | |||
| Arachnida | Araneae | Pisauridae | Pisauraacoreensis Wunderlich, 1992 | end | 57 | |||
| Arachnida | Araneae | Salticidae | Macaroeriscata (Blackwall, 1867) | nat | 56 | |||
| Arachnida | Araneae | Salticidae | Macaroerisdiligens (Blackwall, 1867) | nat | 6 | 17 | 3 | |
| Arachnida | Araneae | Salticidae | Neonacoreensis Wunderlich, 2008 | end | 1 | |||
| Arachnida | Araneae | Salticidae | Phidippusaudax (Hentz, 1845) | intr | 3 | |||
| Arachnida | Araneae | Salticidae | Pseudeuophrysvafra (Blackwall, 1867) | intr | 1 | |||
| Arachnida | Araneae | Salticidae | Salticusmutabilis Lucas, 1846 | intr | 3 | |||
| Arachnida | Araneae | Segestriidae | Segestriaflorentina (Rossi, 1790) | intr | 10 | 1 | ||
| Arachnida | Araneae | Tetragnathidae | Metellinamerianae (Scopoli, 1763) | intr | 12 | |||
| Arachnida | Araneae | Tetragnathidae | Sancusacoreensis (Wunderlich, 1992) | end | 1 | 10 | ||
| Arachnida | Araneae | Theridiidae | Cryptachaeablattea (Urquhart, 1886) | intr | 31 | |||
| Arachnida | Araneae | Theridiidae | Lasaeolaoceanica Simon, 1883 | end | 2 | 1 | ||
| Arachnida | Araneae | Theridiidae | Rugathodesacoreensis Wunderlich, 1992 | end | 7 | 81 | 7 | |
| Arachnida | Araneae | Theridiidae | Steatodagrossa (C. L. Koch, 1838) | intr | 1 | 1 | ||
| Arachnida | Araneae | Theridiidae | Steatodanobilis (Thorell, 1875) | intr | 10 | 10 | ||
| Arachnida | Araneae | Theridiidae | Theridionmusivivum Schmidt, 1956 | nat | 9 | 3 | ||
| Arachnida | Araneae | Thomisidae | Xysticuscor Canestrini, 1873 | nat | 1 | |||
| Arachnida | Opiliones | Leiobunidae | Leiobunumblackwalli Meade, 1861 | nat | 13 | 857 | 3 | |
| Arachnida | Opiliones | Sclerosomatidae | Homalenotuscoriaceus (Simon, 1879) | nat | 1 | 24 | ||
| Arachnida | Pseudoscorpiones | Chthoniidae | Chthoniusischnocheles (Hermann, 1804) | intr | 7 | |||
| Arachnida | Pseudoscorpiones | Chthoniidae | Ephippiochthoniustetrachelatus (Preyssler, 1790) | intr | 11 | 37 | 29 | |
| Arachnida | Pseudoscorpiones | Neobisiidae | Neobisiummaroccanum Beier, 1930 | intr | 1 | 13 | ||
| Chilopoda | Geophilomorpha | Linotaeniidae | Strigamiacrassipes (C.L. Koch, 1835) | nat | 3 | |||
| Chilopoda | Lithobiomorpha | Lithobiidae | Lithobiuspilicornispilicornis Newport, 1844 | nat | 1 | 37 | ||
| Chilopoda | Scolopendromorpha | Cryptopidae | Cryptopshortensis (Donovan, 1810) | nat | 1 | |||
| Chilopoda | Scutigeromorpha | Scutigeridae | Scutigeracoleoptrata (Linnaeus, 1758) | intr | 1 | 4 | 27 | 148 |
| Diplopoda | Julida | Blaniulidae | Blaniulusguttulatus (Fabricius, 1798) | intr | 1 | |||
| Diplopoda | Julida | Blaniulidae | Nopoiuluskochii (Gervais, 1847) | intr | 4 | |||
| Diplopoda | Julida | Julidae | Ommatoiulusmoreleti (Lucas, 1860) | intr | 38 | 166 | 23 | |
| Diplopoda | Polydesmida | Paradoxosomatidae | Oxidusgracilis (C.L. Koch, 1847) | intr | 1 | 1 | 1 | |
| Diplopoda | Polydesmida | Polydesmidae | Polydesmuscoriaceus Porat, 1870 | intr | 5 | 1 | ||
| Insecta | Archaeognatha | Machilidae | Diltasaxicola (Womersley, 1930) | nat | 8 | 582 | 16 | |
| Insecta | Archaeognatha | Machilidae | Trigoniophthalmusborgesi Mendes, Gaju, Bach & Molero, 2000 | end | 15 | |||
| Insecta | Blattodea | Blattellidae | Lobopteradecipiens (Germar, 1817) | nat | 1 | |||
| Insecta | Blattodea | Corydiidae | Zethasimonyi (Krauss, 1892) | nat | 8 | 127 | 79 | |
| Insecta | Coleoptera | Anthicidae | Hirticollisquadriguttatus (Rossi, 1792) | nat | 18 | |||
| Insecta | Coleoptera | Apionidae | Aspidapionradiolus (Marsham, 1802) | nat | 6 | 25 | ||
| Insecta | Coleoptera | Apionidae | Kalcapionsemivittatumsemivittatum (Gyllenhal, 1833) | nat | 27 | 1 | ||
| Insecta | Coleoptera | Brentidae | Dieckmanniellusnitidulus (Gyllenhal, 1838) | intr | 5 | |||
| Insecta | Coleoptera | Carabidae | Anisodactylusbinotatus (Fabricius, 1787) | intr | 1 | |||
| Insecta | Coleoptera | Carabidae | Dromiusmeridionalis Dejean, 1825 | intr | 11 | 2 | ||
| Insecta | Coleoptera | Carabidae | Notiophilusquadripunctatus Dejean, 1826 | nat | 1 | |||
| Insecta | Coleoptera | Carabidae | Olisthopusinclavatus Israelson, 1983 | end | 1 | |||
| Insecta | Coleoptera | Carabidae | Stenolophusteutonus (Schrank, 1781) | nat | 2 | |||
| Insecta | Coleoptera | Cerambycidae | Monochamusgalloprovincialis (Olivier, 1795) | intr | 1 | |||
| Insecta | Coleoptera | Chrysomelidae | Chaetocnemahortensis (Fourcroy, 1785) | intr | 4 | 1 | ||
| Insecta | Coleoptera | Chrysomelidae | Chrysolinabankii (Fabricius, 1775) | nat | 1 | |||
| Insecta | Coleoptera | Chrysomelidae | Epitrixcucumeris (Harris, 1851) | intr | 7 | 1 | ||
| Insecta | Coleoptera | Chrysomelidae | Epitrixhirtipennis (Melsheimer, 1847) | intr | 1 | 4 | 1 | |
| Insecta | Coleoptera | Chrysomelidae | Longitarsuskutscherae (Rye, 1872) | intr | 1 | 1 | 38 | |
| Insecta | Coleoptera | Chrysomelidae | Psylliodeschrysocephalus (Linnaeus, 1758) | intr | 1 | 8 | ||
| Insecta | Coleoptera | Chrysomelidae | Psylliodesmarcida (Illiger, 1807) | nat | 1 | 3 | 40 | |
| Insecta | Coleoptera | Coccinellidae | Clitostethusarcuatus (Rossi, 1794) | intr | 1 | |||
| Insecta | Coleoptera | Coccinellidae | Noviuscardinalis (Mulsant, 1850) | intr | 9 | 3 | 2 | |
| Insecta | Coleoptera | Coccinellidae | Rhyzobiuslitura (Fabricius, 1787) | nat | 1 | |||
| Insecta | Coleoptera | Coccinellidae | Rhyzobiuslophanthae (Blaisdell, 1892) | intr | 1 | 1 | ||
| Insecta | Coleoptera | Coccinellidae | Scymnusinterruptus (Goeze, 1777) | nat | 5 | 2 | ||
| Insecta | Coleoptera | Coccinellidae | Stethoruspusillus (Herbst, 1797) | nat | 12 | |||
| Insecta | Coleoptera | Corylophidae | Sericoderuslateralis (Gyllenhal, 1827) | intr | 89 | 172 | ||
| Insecta | Coleoptera | Cryptophagidae | Cryptophaguscellaris (Scopoli, 1763) | intr | 1 | 3 | ||
| Insecta | Coleoptera | Curculionidae | Brachyperamultifida (Israelson, 1984) | end | 7 | |||
| Insecta | Coleoptera | Curculionidae | Brachytemnusporcatus (Germar, 1823) | intr | 2 | |||
| Insecta | Coleoptera | Curculionidae | Calacallessubcarinatus (Israelson, 1984) | end | 19 | 86 | 19 | |
| Insecta | Coleoptera | Curculionidae | Cathormioceruscurvipes (Wollaston, 1854) | nat | 4 | |||
| Insecta | Coleoptera | Curculionidae | Charagmusgressorius (Fabricius, 1792) | intr | 1 | 7 | ||
| Insecta | Coleoptera | Curculionidae | Coccotrypescarpophagus (Hornung, 1842) | intr | 7 | |||
| Insecta | Coleoptera | Curculionidae | Dichromacallesdromedarius (Boheman, 1844) | intr | 1 | |||
| Insecta | Coleoptera | Curculionidae | Gonipterusplatensis (Marelli, 1926) | intr | 47 | |||
| Insecta | Coleoptera | Curculionidae | Gronopsfasciatus Küster, 1851 | intr | 4 | |||
| Insecta | Coleoptera | Curculionidae | Hadroplontustrimaculatus (Fabricius, 1775) | intr | 1 | |||
| Insecta | Coleoptera | Curculionidae | Hypurusbertrandi (Perris, 1852) | intr | 1 | |||
| Insecta | Coleoptera | Curculionidae | Mecinuspascuorum (Gyllenhal, 1813) | intr | 17 | 4 | ||
| Insecta | Coleoptera | Curculionidae | Mogulonesgeographicus (Goeze, 1777) | intr | 2 | |||
| Insecta | Coleoptera | Curculionidae | Naupactuscervinus (Boheman, 1840) | intr | 49 | 23 | ||
| Insecta | Coleoptera | Curculionidae | Otiorhynchuscribricollis Gyllenhal, 1834 | intr | 19 | 4 | ||
| Insecta | Coleoptera | Curculionidae | Otiorhynchusrugosostriatus (Goeze, 1777) | intr | 1 | |||
| Insecta | Coleoptera | Curculionidae | Pseudophloeophagustenaxborgesi Stüben, 2022 | nat | 1 | 4 | 260 | 12 |
| Insecta | Coleoptera | Curculionidae | Rhopalomesitestardyi (Curtis, 1825) | nat | 2 | 4 | ||
| Insecta | Coleoptera | Curculionidae | Sitonadiscoideus Gyllenhal, 1834 | intr | 9 | |||
| Insecta | Coleoptera | Curculionidae | Sitonalineatus (Linnaeus, 1758) | intr | 1 | |||
| Insecta | Coleoptera | Curculionidae | Xyleborinusalni Nijima, 1909 | intr | 2 | |||
| Insecta | Coleoptera | Dryopidae | Dryopsalgiricus (Lucas, 1846) | nat | 2 | 1 | ||
| Insecta | Coleoptera | Dryopidae | Dryopsluridus (Erichson, 1847) | nat | 18 | |||
| Insecta | Coleoptera | Elateridae | Aeolusmelliculusmoreleti Tarnier, 1860 | intr | 1 | |||
| Insecta | Coleoptera | Elateridae | Athousazoricus Platia & Gudenzi, 2002 | end | 21 | |||
| Insecta | Coleoptera | Elateridae | Heteroderesazoricus (Tarnier, 1860) | end | 11 | 30 | 47 | |
| Insecta | Coleoptera | Elateridae | Heteroderesvagus Candèze, 1893 | intr | 2 | |||
| Insecta | Coleoptera | Elateridae | Melanotusdichrous (Erichson, 1841) | intr | 1 | |||
| Insecta | Coleoptera | Histeridae | Carcinopspumilio (Erichson, 1834) | intr | 1 | |||
| Insecta | Coleoptera | Hydrophilidae | Cercyonhaemorrhoidalis (Fabricius, 1775) | intr | 5 | |||
| Insecta | Coleoptera | Latridiidae | Cartoderebifasciata (Reitter, 1877) | intr | 21 | 1 | ||
| Insecta | Coleoptera | Latridiidae | Cartoderenodifer (Westwood, 1839) | intr | 16 | 1 | ||
| Insecta | Coleoptera | Latridiidae | Metophthalmusoccidentalis Israelson, 1984 | end | 15 | |||
| Insecta | Coleoptera | Leiodidae | Catopscoracinus Kellner, 1846 | nat | 80 | 33 | ||
| Insecta | Coleoptera | Malachiidae | Attaluslusitanicuslusitanicus Erichson, 1840 | nat | 4 | |||
| Insecta | Coleoptera | Mycetophagidae | Litargusbalteatus Le Conte, 1856 | intr | 3 | |||
| Insecta | Coleoptera | Mycetophagidae | Typhaeastercorea (Linnaeus, 1758) | intr | 3 | 1 | ||
| Insecta | Coleoptera | Nitidulidae | Brassicogethesaeneus (Fabricius, 1775) | intr | 6 | |||
| Insecta | Coleoptera | Nitidulidae | Carpophilusfumatus Boheman, 1851 | intr | 1 | |||
| Insecta | Coleoptera | Nitidulidae | Epuraeabiguttata (Thunberg, 1784) | intr | 1 | 3 | ||
| Insecta | Coleoptera | Nitidulidae | Phenolialimbatatibialis (Boheman, 1851) | intr | 2 | |||
| Insecta | Coleoptera | Nitidulidae | Stelidotageminata (Say, 1825) | intr | 19 | 106 | ||
| Insecta | Coleoptera | Phalacridae | Stilbustestaceus (Panzer, 1797) | nat | 16 | 25 | ||
| Insecta | Coleoptera | Ptiliidae | Ptenidiumpusillum (Gyllenhal, 1808) | intr | 3 | 47 | ||
| Insecta | Coleoptera | Ptinidae | Anobiumpunctatum (De Geer, 1774) | intr | 2 | 69 | ||
| Insecta | Coleoptera | Ptinidae | Calymmaderussolidus (Kiesenwetter, 1877) | intr | 1 | |||
| Insecta | Coleoptera | Rutelidae | Popilliajaponica Newman, 1838 | intr | 1 | 6 | ||
| Insecta | Coleoptera | Scraptiidae | Anaspisproteus Wollaston, 1854 | nat | 1 | 24 | 290 | 1 |
| Insecta | Coleoptera | Silvanidae | Cryptamorphadesjardinsii (Guérin-Méneville, 1844) | intr | 1 | |||
| Insecta | Coleoptera | Staphylinidae | Aleocharabipustulata (Linnaeus, 1760) | intr | 19 | 12 | ||
| Insecta | Coleoptera | Staphylinidae | Aloconotasulcifrons (Stephens, 1832) | nat | 38 | 1 | ||
| Insecta | Coleoptera | Staphylinidae | Amischaanalis (Gravenhorst, 1802) | intr | 1 | |||
| Insecta | Coleoptera | Staphylinidae | Astenuslyonessius (Joy, 1908) | nat | 2 | 3 | ||
| Insecta | Coleoptera | Staphylinidae | Athetaaeneicollis (Sharp, 1869) | intr | 17 | 9 | ||
| Insecta | Coleoptera | Staphylinidae | Athetafungi (Gravenhorst, 1806) | intr | 5 | 1 | 358 | 282 |
| Insecta | Coleoptera | Staphylinidae | Carpelimuscorticinus (Gravenhorst, 1806) | nat | 2 | 2 | ||
| Insecta | Coleoptera | Staphylinidae | Coproporuspulchellus (Erichson, 1839) | intr | 5 | |||
| Insecta | Coleoptera | Staphylinidae | Cordaliaobscura (Gravenhorst, 1802) | intr | 2 | |||
| Insecta | Coleoptera | Staphylinidae | Euconnusazoricus Franz, 1969 | end | 1 | 2 | ||
| Insecta | Coleoptera | Staphylinidae | Gyrohypnusfracticornis (Müller, 1776) | intr | 2 | |||
| Insecta | Coleoptera | Staphylinidae | Hypomedondebilicornis (Wollaston, 1857) | nat | 1 | |||
| Insecta | Coleoptera | Staphylinidae | Notothectadryochares (Israelson, 1985) | end | 1 | |||
| Insecta | Coleoptera | Staphylinidae | Ocypusaethiops (Waltl, 1835) | nat | 1 | |||
| Insecta | Coleoptera | Staphylinidae | Oligotapumilio Kiesenwetter, 1858 | nat | 5 | 4 | ||
| Insecta | Coleoptera | Staphylinidae | Phloeonomuspunctipennis Thomson, 1867 | nat | 1 | |||
| Insecta | Coleoptera | Staphylinidae | Proteinusatomarius Erichson, 1840 | nat | 4 | 2 | ||
| Insecta | Coleoptera | Staphylinidae | Rugilusorbiculatus (Paykull, 1789) | nat | 2 | 1 | ||
| Insecta | Coleoptera | Staphylinidae | Sepedophiluslusitanicus Hammond, 1973 | nat | 1 | |||
| Insecta | Coleoptera | Staphylinidae | Stenomastaxmadeirae Assing, 2003 | nat | 4 | |||
| Insecta | Coleoptera | Staphylinidae | Tachyporuschrysomelinus (Linnaeus, 1758) | intr | 1 | 22 | 14 | |
| Insecta | Coleoptera | Staphylinidae | Tachyporusnitidulus (Fabricius, 1781) | intr | 64 | 42 | ||
| Insecta | Coleoptera | Staphylinidae | Trichiusaimmigrata Lohse, 1984 | intr | 1 | |||
| Insecta | Coleoptera | Staphylinidae | Trichophyapilicornis (Gyllenhal, 1810) | nat | 1 | |||
| Insecta | Coleoptera | Staphylinidae | Xantholinuslongiventris Heer, 1839 | intr | 1 | |||
| Insecta | Coleoptera | Tenebrionidae | Lagriahirta (Linnaeus, 1758) | intr | 382 | |||
| Insecta | Coleoptera | Teredidae | Anommatusduodecimstriatus (Müller, 1821) | intr | 2 | |||
| Insecta | Coleoptera | Zopheridae | Tarphiusrufonodulosus Israelson, 1984 | end | 6 | |||
| Insecta | Dermaptera | Anisolabididae | Euborelliaannulipes (Lucas, 1847) | intr | 7 | |||
| Insecta | Dermaptera | Forficulidae | Forficulaauricularia Linnaeus, 1758 | intr | 58 | 17 | ||
| Insecta | Hemiptera | Anthocoridae | Anthocorisnemoralis (Fabricius, 1794) | nat | 1 | 3 | ||
| Insecta | Hemiptera | Anthocoridae | Brachystelesparvicornis (A. Costa, 1847) | nat | 2 | |||
| Insecta | Hemiptera | Anthocoridae | Buchananiellacontinua (White, 1880) | intr | 2 | |||
| Insecta | Hemiptera | Anthocoridae | Oriuslaevigatuslaevigatus (Fieber, 1860) | nat | 2 | 4 | 15 | |
| Insecta | Hemiptera | Aphididae | Cinarajuniperi (De Geer, 1773) | nat | 4 | 24 | 252 | |
| Insecta | Hemiptera | Cicadellidae | Aphrodeshamiltoni Quartau & Borges, 2003 | end | 4 | 1 | ||
| Insecta | Hemiptera | Cicadellidae | Eupteryxazorica Ribaut, 1941 | end | 14 | 64 | 107 | 23 |
| Insecta | Hemiptera | Cicadellidae | Eupteryxfilicum (Newman, 1853) | nat | 39 | 43 | ||
| Insecta | Hemiptera | Cicadellidae | Euscelidiusvariegatus (Kirschbaum, 1858) | nat | 2 | 3 | ||
| Insecta | Hemiptera | Cicadellidae | Sophoniaorientalis (Matsumura, 1912) | intr | 4 | |||
| Insecta | Hemiptera | Cixiidae | Cixiusazofloresi Remane & Asche, 1979 | end | 45 | |||
| Insecta | Hemiptera | Cixiidae | Cixiusazomariae Remane & Asche, 1979 | end | 278 | |||
| Insecta | Hemiptera | Cixiidae | Cixiusazoterceirae Remane & Asche, 1979 | end | 968 | |||
| Insecta | Hemiptera | Delphacidae | Kelisiaribauti Wagner, 1938 | nat | 21 | 8 | ||
| Insecta | Hemiptera | Flatidae | Cyphopterumadcendens (Herrich-Schäffer, 1835) | nat | 1 | 44 | 1613 | 49 |
| Insecta | Hemiptera | Flatidae | Siphantaacuta (Walker, 1851) | intr | 136 | 260 | ||
| Insecta | Hemiptera | Liviidae | Strophingiaharteni Hodkinson, 1981 | end | 340 | 192 | ||
| Insecta | Hemiptera | Lygaeidae | Kleidocerysericae (Horváth, 1908) | nat | 138 | 11 | ||
| Insecta | Hemiptera | Lygaeidae | Nysiusatlantidum Horváth, 1890 | end | 1 | 2 | ||
| Insecta | Hemiptera | Microphysidae | Loriculaelegantula (Bärensprung, 1858) | nat | 2 | 77 | 76 | |
| Insecta | Hemiptera | Miridae | Campyloneuravirgula (Herrich-Schaeffer, 1835) | nat | 1 | 128 | ||
| Insecta | Hemiptera | Miridae | Heterotomaplanicornis (Pallas, 1772) | nat | 1 | |||
| Insecta | Hemiptera | Miridae | Monalocorisfilicis (Linnaeus, 1758) | nat | 8 | 100 | 5 | |
| Insecta | Hemiptera | Miridae | Pilophorusconfusus (Kirschbaum, 1856) | nat | 13 | 3 | ||
| Insecta | Hemiptera | Miridae | Pilophorusperplexus Douglas & Scott, 1875 | nat | 1 | |||
| Insecta | Hemiptera | Miridae | Pinalitusoromii J. Ribes, 1992 | end | 1 | 18 | 35 | |
| Insecta | Hemiptera | Miridae | Taylorilygusapicalis (Fieber, 1861) | intr | 1 | |||
| Insecta | Hemiptera | Nabidae | Nabispseudoferusibericus Remane, 1962 | nat | 11 | 7 | ||
| Insecta | Hemiptera | Pentatomidae | Piezodoruslituratus (Fabricius, 1794) | nat | 1 | |||
| Insecta | Hemiptera | Psyllidae | Acizziauncatoides (Ferris & Klyver, 1932) | intr | 288 | 23 | ||
| Insecta | Hemiptera | Reduviidae | Empicorisrubromaculatus (Blackburn, 1889) | intr | 2 | 3 | ||
| Insecta | Hemiptera | Rhyparochromidae | Aphanusrolandri (Linnaeus, 1758) | nat | 1 | |||
| Insecta | Hemiptera | Rhyparochromidae | Beosusmaritimus (Scopoli, 1763) | nat | 2 | |||
| Insecta | Hemiptera | Rhyparochromidae | Emblethisdenticollis Horváth, 1878 | nat | 1 | |||
| Insecta | Hemiptera | Rhyparochromidae | Eremocorismaderensis (Wollaston, 1858) | nat | 1 | 13 | ||
| Insecta | Hemiptera | Rhyparochromidae | Plinthisusbrevipennis (Latreille, 1807) | nat | 2 | |||
| Insecta | Hemiptera | Rhyparochromidae | Plinthisusminutissimus Fieber, 1864 | nat | 1 | |||
| Insecta | Hemiptera | Rhyparochromidae | Scolopostethusdecoratus (Hahn, 1833) | nat | 2 | 3 | 71 | |
| Insecta | Hemiptera | Saldidae | Saldulapalustris (Douglas, 1874) | nat | 2 | |||
| Insecta | Hemiptera | Tingidae | Tingisauriculata (A. Costa, 1847) | intr | 1 | |||
| Insecta | Hemiptera | Triozidae | Triozalaurisilvae Hodkinson, 1990 | nat | 56 | 59 | ||
| Insecta | Hymenoptera | Apidae | Bombusruderatus (Fabricius, 1775) | intr | 6 | |||
| Insecta | Hymenoptera | Apidae | Bombusterrestris (Linnaeus, 1758) | intr | 1 | 61 | 2 | |
| Insecta | Hymenoptera | Formicidae | Cardiocondylamauritanica Forel, 1890 | intr | 1 | 4 | ||
| Insecta | Hymenoptera | Formicidae | Hypoponeraeduardi (Forel, 1894) | nat | 1 | 1 | 23 | 173 |
| Insecta | Hymenoptera | Formicidae | Hypoponerapunctatissima (Roger, 1859) | intr | 2 | |||
| Insecta | Hymenoptera | Formicidae | Lasiusgrandis Forel, 1909 | nat | 31 | 38 | 856 | 348 |
| Insecta | Hymenoptera | Formicidae | Linepithemahumile (Mayr, 1868) | intr | 55 | 199 | ||
| Insecta | Hymenoptera | Formicidae | Monomoriumcarbonarium (F. Smith, 1858) | nat | 786 | 310 | ||
| Insecta | Hymenoptera | Formicidae | Plagiolepisschmitzii Forel, 1895 | nat | 18 | 343 | ||
| Insecta | Hymenoptera | Formicidae | Temnothoraxunifasciatus (Latreille, 1798) | nat | 18 | |||
| Insecta | Hymenoptera | Formicidae | Tetramoriumbicarinatum (Nylander, 1846) | intr | 230 | |||
| Insecta | Hymenoptera | Formicidae | Tetramoriumcaespitum (Linnaeus, 1758) | nat | 52 | 4 | ||
| Insecta | Hymenoptera | Formicidae | Tetramoriumcaldarium (Roger, 1857) | intr | 121 | 2 | ||
| Insecta | Isoptera | Kalotermitidae | Kalotermesflavicollis (Fabricius, 1793) | intr | 10 | |||
| Insecta | Neuroptera | Hemerobiidae | Hemerobiusazoricus Tjeder, 1948 | end | 2 | 31 | 15 | |
| Insecta | Orthoptera | Gryllidae | Eumodicogryllusbordigalensis (Latreille, 1804) | intr | 1 | |||
| Insecta | Orthoptera | Tettigoniidae | Phaneropteranana Fieber, 1853 | nat | 1 | |||
| Insecta | Phasmatodea | Phasmatidae | Carausiusmorosus (Sinéty, 1901) | intr | 1 | 3 | ||
| Insecta | Psocodea | Caeciliusidae | Valenzuelaburmeisteri (Brauer, 1876) | nat | 136 | 35 | ||
| Insecta | Psocodea | Caeciliusidae | Valenzuelaflavidus (Stephens, 1836) | nat | 5 | 14 | 713 | 74 |
| Insecta | Psocodea | Ectopsocidae | Ectopsocusbriggsi McLachlan, 1899 | intr | 1 | 23 | 298 | 15 |
| Insecta | Psocodea | Ectopsocidae | Ectopsocusstrauchi Enderlein, 1906 | nat | 9 | 23 | 25 | |
| Insecta | Psocodea | Elipsocidae | Elipsocusazoricus Meinander, 1975 | end | 5 | 523 | 107 | |
| Insecta | Psocodea | Elipsocidae | Elipsocusbrincki Badonnel, 1963 | end | 12 | 627 | 1 | |
| Insecta | Psocodea | Epipsocidae | Bertkauialucifuga (Rambur, 1842) | nat | 23 | 9 | ||
| Insecta | Psocodea | Psocidae | Atlantopsocusadustus (Hagen, 1865) | nat | 3 | 74 | 51 | |
| Insecta | Psocodea | Trichopsocidae | Trichopsocusclarus (Banks, 1908) | nat | 8 | 56 | 618 | 143 |
| Insecta | Thysanoptera | Aeolothripidae | Aeolothripsgloriosus Bagnall, 1914 | nat | 1 | |||
| Insecta | Thysanoptera | Phlaeothripidae | Hoplothripscorticis (De Geer, 1773) | nat | 3 | 12 | ||
| Insecta | Thysanoptera | Thripidae | Heliothripshaemorrhoidalis (Bouché, 1833) | intr | 8 | 4 | ||
| Insecta | Thysanoptera | Thripidae | Hercinothripsbicinctus (Bagnall, 1919) | intr | 2 | |||
| Insecta | Trichoptera | Limnephilidae | Limnephilusatlanticus Nybom, 1948 | end | 3 | 1 |
Just considering the 249 arthropod species identified at archipelago level, the five most abundant species were the native bug Cyphopterumadcendens (Herrich-Schäffer, 1835) (n = 1707), the ants Lasiusgrandis Forel, 1909 (n = 1273) and Monomoriumcarbonarium (F. Smith, 1858) (n = 1096), the endemic cixiid Cixiusazoterceirae Remane & Asche, 1979 (n = 968) and the native harvestmen Leiobunumblackwalli Meade, 1861 (n = 873) (Table 2).
At island scale, the native ant Lasiusgrandis was also one of the most abundant arthropods in Corvo (n = 31) and Santa Maria (n = 348). Curiously, in both Islands, one of the two most abundant species represent a new island record, being the exotic spider Porrhoclubionagenevensis (L. Koch, 1866) (n = 15), new for Corvo and the exotic (possibly invasive) beetle Lagriahirta (Linnaeus, 1758) (n =382), new for Santa Maria.
In Flores, the native Trichopsocusclarus (Banks, 1908) (n = 143) and the endemic Eupteryxazorica Ribaut, 1941 (n = 23) were the most abundant arthropod species. Finally, in Terceira, the native and endemic Hemiptera, respectively Cyphopterumadcendens (n = 1613) and Cixiusazoterceirae (n = 968), were the most abundant species (Table 2).
New Azores species records
In this study, we registered a total of 34 new records for one or more islands of Azores (nine for Corvo, three for Flores, six for Terceira and 16 for Santa Maria), of which the curculionids Dieckmanniellusnitidulus (Gyllenhal, 1838), Gronopsfasciatus Küster, 1851, Hadroplontustrimaculatus (Fabricius, 1775) and Hypurusbertrandi (Perris, 1852) are new records for Azores. In addition, the ant Cardiocondylamauritanica Forel, 1890 (Hymenoptera, Formicidae) is also a new record for Azores. All these species are exotics, possibly recently introduced.
Dieckmanniellusnitidulus (Gyllenhal, 1838)
Dieckmanniellusnitidulus (Brentidae: Nanophyinae) is mainly widespread in the Mediterranean Region and lives monophagously on various Lythraceae (e.g. Lythrumsalicaria L.). The record on Santa Maria (Azores) probably refers to Lythrumborysthenicum (Schrank) Litv. or Lythrumjunceum Banks & Solander. The species was also introduced on five of the seven Canary Islands, where it lives on Lythrumhyssopifolia L., on which one of us (PS) was able to reliably detect it on La Gomera. Characteristic features: Head, funicle and club of antennae mainly black; elytra in front of the white, V-shaped transverse mark with a dark spot on the front third of the sutural strip (see Fig. 7); 1.4 – 2.1 mm (Stüben 2022).
Figure 7.
Dieckmanniellusnitidulus (Brentidae: Nanophyinae) (Credit: Peter E. Stüben).
Gronopscf.fasciatus Küster, 1851
The genus Gronops (Curculionidae: Cyclominae: Rhytirrhinini) includes about 20 Palaearctic species, mainly from the arid regions of North Africa and is only represented with certainty on the Canary Islands by the species Gronopsfasciatus. The determination of the specimen of Gronopscf.fasciatus, a male, recorded at the airfield of Santa Maria (Azores) in December 2019, must be checked again by a specialist of this group. The biology of these terricolous, flightless species is largely unknown, although they are often found near Caryophyllaceae and Amaranthaceae. Carry-over with soil is conceivable. Characteristic features of G.fasciatus compared to G.lunatus (Fabricius, 1775): elytra shorter, hardly narrowing towards the apex (subparallel); pronotum wider, strongly widened in the front third (see Fig. 8); length: 2.2–3.2 mm (Stüben 2022).
Figure 8.
Gronopscf.fasciatus Küster, 1851 (Curculionidae: Cyclominae: Rhytirrhinini) (Credit: Peter E. Stüben).
Hadroplontustrimaculatus (Fabricius, 1775)
Both European species, Hadroplontuslitura and H.trimaculatus (Curculionidae: Ceutorhynchinae) live on thistles. The latter lives on plants of the genus Carduus, mainly C.nutans and C.acanthoides. The single specimen (see Fig. 9) was sampled at Piquinhos, mixed forest with the presence of Carduustenuiflorus W.M.Curtis. H.trimaculatus differs from the very similar species H.litura by the beige to greyish-brown (not completely white) suture interval of the cruciform elytral spot (Stüben et al. 2014, Stüben 2022).
Figure 9.
Hadroplontustrimaculatus (Fabricius, 1775) (Curculionidae: Ceutorhynchinae) (Credit: Peter E. Stüben).
Hypurusbertrandi (Perris, 1852)
Hypurusbertrandi (Perris, 1852) (Curculionidae: Ceutorhynchinae) (see Fig. 10), originally a Mediterranean, now nearly cosmopolitan species, which lives monophagously on Portulacaoleracea L. The species was first reported from the Macaronesian Islands by García et al. (2016), collected in the Escuela de Capacitación Agraria near Tacoronte on Tenerife in 2015. The species also occurs in Cape Verde (São Tiago: S. Jorge; Colonnelli 1990) and on the Azores (Terceira: Caldeira Lajes). A characteristic feature is the strongly thickened hind femora (see Fig. 10; Stüben et al. 2012, Stüben 2022).
Figure 10.
Hypurusbertrandi (Perris, 1852) (Curculionidae: Ceutorhynchinae) (Credit: Peter E. Stüben). The scale refers to the insect. The photo of the plants refers to Portulacaoleracea L., in which the species lives as monophagous.
Cardiocondylamauritanica Forel, 1890
This ant species is native to northern Africa, Middle East, Afghanistan and Pakistan, but has been introduced in many other regions, including the United States of America, Mexico, Zimbabwe, several European countries and many islands worldwide (Wetterer 2012, Janicki et al. 2016, Seifert et al. 2017). In Macaronesia, C.mauritanica was perviously known to occur in Madeira and the Canary Islands (Espadaler 2008, Báez and Oromí 2010). These ants are small, inconspicuous and can be separated from other Cardiocondyla species using a combination of morphometric characters (Seifert 2003, Seifert et al. 2017) (see Fig. 11). They form polygynous colonies and mating occurs inside the nests (Seifert et al. 2017). These characteristics and their ability to co-exist with other aggressive invasive ant species, like the Argentine ant Linepithemahumile, are important to explain their ecological success and ongoing spread (Wetterer 2012). However, contrary to other exotic ant species, C.mauritanica does not seem to have significant ecological impacts on native biodiversity (Wetterer 2012).
Figure 11.
Cardiocondylamauritanica (Forel, 1890) (Formicidae). Specimen CASENT0746634 from AntWeb.org (Credit: Zach Lieberman).
Conservation remarks
This publication highlights the importance of exotic and mixed forest areas, as well as small native disturbed forest patches as potential reservoirs of both exotic potentially invasive species, as well as rare endemic species (see also Tsafack et al. 2021).
The high abundance of several native non-endemic (e.g. Cyphopterumadcendens; Lasiusgrandis, Monomoriumcarbonarium, Leiobunumblackwalli, Trichopsocusclarus) and endemic species (e.g. Cixiusazoterceirae, Elipsocusbrincki, Elipsocusazoricus, Strophingiaharteni) (Table 2) in these habitats is noteworthy.
Within endemics, we wish to comment on the recently-described subspecies Pseudophloeophagustenaxborgesi Stüben, 2022 (Curculionidae: Cossoninae) (Fig. 12). This subspecies, common in many islands of the Azores, was described only in 2022 (Stüben 2022). The nominotypic taxon occurs on Madeira. Accordingly, material of P.tenax from trap findings and in collections from the Azores must be assigned to this new subspecies. Type locality of P.tenaxborgesi is on São Jorge (Vigia da Baleia), but perhaps the subspecies occurs on all islands of the Azores. Apart from clear molecular differences in the mitochondrial COI gene (Stüben et al. 2012), this subspecies of the Azores differs from the nominotypic taxon on Madeira in the following characteristics: elytral striae more strongly and deeply punctured and the interstriae much narrower than in the sister taxon from Madeira; aedeagus narrower (see Fig. 12, Stüben and Borges 2019, Stüben 2022).
Figure 12.
Pseudophloeophagustenaxborgesi Stüben, 2022 (Curculionidae: Cossoninae) (Credit: Peter E. Stüben).
Several other rare endemic species were found in this study (see list below), which highlights the importance of expanding surveys in Azores to small isolated forest patches in order to find relict populations of rare endemic species:
- Canariphantesacoreensis (Wunderlich, 1992) (Araneae, Linyphiidae). This rare spider is usually found in pristine native forests and is considered Vulnerable (VU) by IUCN (Borges and Cardoso 2021b). In the current study, we sampled a single specimen in a disturbed mixed forest of Eucalyptus spp. and Pittospsorumundulatum at Terceira Island located near a native forest at Pico Rachado.
- Canariphantesrelictus Crespo & Bosmans, 2014 (Araneae, Linyphiidae). Another very rare species, classified as Critically Endangered (CR) (Borges and Cardoso 2021a). The species was found originally in high elevation at Santa Maria Island (Crespo et al. 2014), but in our study, two females were found at Piedade (PRIBS_T03_12_2019) at low elevation in a mixed forest of Picconiaazorica, Pittosporumundulatum and Pinus sp. The species possibly has a larger distribution than originally recorded.
-Olisthopusinclavatus Israelson, 1983 (Coleoptera, Carabidae). This is a very rare ground-beetle classified as Critically Endangered (CR) by IUCN (Borges 2018) and currently occurring only in exotic forests (dominated by Cryptomeriajaponica, Acacia spp.). In this study, the unique specimen was sampled in Monteiro (SMR_PRIBS_T12).
- Athousazoricus Platia & Gudenzi, 2002 (Coleoptera, Elateridae) (Fig. 13). This is a relatively rare species known from Flores, Graciosa, Terceira and S. Miguel Islands. Considered Endangered by the IUCN (Borges and Lamelas-López 2017a), this species tends to occur at low elevations in disturbed exotic forests. In the current study, we found the species in three places all at mid-elevations (300-500 m) and in three types of forest, one plantation of Cryptomeriajaponica (Mistérios Negros; TER-MNEG-T-62 corresponding also to code TER_PRIBS_T05), a mixed forest dominated by Eucalyptus spp. (Escampadouro; TER_PRIBS_T23) and a mixed forest dominated by Pittosporumundulatum (Mata do Estado; TER_PRIBS_T04). At least in Terceira Island, the species seems to be more widespread than previously assumed.
Figure 13.

Athousazoricus Platia & Gudenzi, 2002 (Coleoptera, Elateridae) (Credit: Enésima Pereira, Azorean Biodiversity Group).
- Brachyperamultifida (Israelson, 1984) (= Donusmultifudus (Israelson, 1984)) (Coleoptera, Curculionidae) (Fig. 14). This is a particularly rare curculionid beetle classified as Critically Endangered (CR) (Borges and Lamelas-López 2017b). Previously, it was sampled at high elevation at Pico Alto in Santa Maria Island. In the current survey, we sampled this species in three sites, Estação Loran (SMR_PRIBS_T08), Piquinhos (SMR_PRIBS_T10) and Figueiral (SMR_PRIBS_T14) expanding the range of the species to lower elevations and to different types of forest.
Figure 14.
Brachyperamultifida (Israelson, 1984) (= Donusmultifudus (Israelson, 1984)) (Coleoptera, Curculionidae) (Credit: Peter E. Stüben).
- Tarphiusrufonodulosus Israelson, 1984 (Coleoptera, Zopheridae) (Fig. 15). This is a rare ironclade beetle also Critically Endangered (CR) (Borges and Lamelas-López 2018) that is associated with the canopies of native trees (e.g. Picconiaazorica) and under-bark of dead trees, both in native and exotic forests (dominated by Acacia sp. and Cryptomeriajaponica). In the current study, one specimen was collected in mixed forests of Ericaazorica, Cryptomeriajaponica and Pittosporumundulatum at three locations at high elevation (Piquinhos and Fontinhas forest areas).
Figure 15.

Tarphiusrufonodulosus Israelson, 1984 (Coleoptera, Zopheridae) (Credit: Erno-Endre Gergely; Azorean Biodiverity Group).
Patterns of invasion
The main aim of this study was to investigate the importance of disturbed native forest patches and exotic vegetation areas as potential reservoirs of exotic potentially invasive arthropods. As expected, we found a large number of exotic species, some of them new for Azores, as listed above. In addition to the 125 species identified as introduced (Table 2), many more are waiting a proper identification (Suppl. material 1). In previous studies, we identified thirteen widespread exotic arthropods as new records for Azores (Borges et al. 2013) and some previously unknown exotic species in Azorean urban gardens (Arteaga et al. 2020). This clearly indicates that there is an ongoing continuous flux of new introductions in Azores.
Some of the introduced species found in the current study are a matter of concern for nature conservation in the Azores Archipelago and their populations should be monitored. For instance, Lagriahirta (Linnaeus, 1758) (Coleoptera, Tenebrionidae) that was recently recorded as new for Azores and found originally at Terceira Island (Borges et al. 2021), is expanding dramatically in Santa Maria. In Santa Maria, we found it everywhere at all elevations and habitats. This seems to be a recent introduction in Azores and the impact of this species is still unknown. The Australian exotic planthopper Siphantaacuta (Walker, 1851) (Hemiptera, Flatidae) was recorded originally for Azores in 2013 (Borges et al. 2013) and is expanding rapidly in several Azorean islands with potential impacts on agriculture. In our study, we found it quite abundant in many sites at Terceira and Santa Maria Islands. The expansion of the Eucalyptus snout beetle Gonipterusplatensis (Marelli, 1926) (Coleoptera, Curculionidae) that was found in several sites at Terceira Island is also of concern. This species was originally recorded for Azores by Borges et al. (2013) and is currently known also from São Miguel Island.
In potential expansion in Terceira (and also known from Pico) is the two-spotted leafhopper Sophoniaorientalis (Matsumura, 1912) (Hemiptera; Cicadellidae) (Tarantino et al. 2022). This species is native to south-east Asia and is a highly polyphagous pest, considered an invasive species that affects crops as well as endemic plants (Tarantino et al. 2022).
Several exotic ant species have been recorded in the Azores (Espadaler 2010) and, here, we report new findings at both island and archipelago levels that highlight their rapid spread. Jointly with the first record of Cardiocondylamauritanica for Azores, we found that two exotic Tetramorium species (T.bicarinatum and T.caldarium) are now present in Santa Maria. Both C.mauritanica and T.caldarium do not seem to have significant impacts on native biodiversity (Wetterer 2012, Wetterer and Hita Garcia 2015), but T.bicarinatum and, particularly, the Argentine ant L.humile, are serious threats to island native invertebrates and natural ecological processes and have also been reported as agricultural pests (Wetterer 2009, Wetterer et al. 2009). The severe consequences of Argentine ant invasion on local biodiversity have been reported from many areas around the world, including oceanic islands, but their effects remain poorly understood in Macaronesian archipelagos (Holway et al. 2002, Wetterer and Espadaler 2010, Boieiro et al. 2018a, Boieiro et al. 2018b).
Finally, it is important to highlight that, amongst the most abundant introduced species in our study, several are listed in the TOP100 worst invasive species of Azores and Macaronesia (Silva et al. 2008), namely the woodlouse spider Dysderacrocata C.L. Koch, 1838, the Argentine ant Linepithemahumile (Mayr, 1868) and the millipede Ommatoiulusmoreleti (Lucas, 1860).
Our study stresses the need for arthropod biodiversity monitoring in different habitats of oceanic islands as an important strategy for early detection of invasive species that may have severe impacts on the environment, economy and human well-being (see also Borges et al. 2018). It also allows us to assess changes on species abundance and distribution, thus providing valuable information to support decision-making by conservation managers.
Supplementary Material
Complete list of species and morphospecies.
Borges, P.A.V.
Data type
Excel
Brief description
Complete list of arthropod species collected in four islands of Azores, between 2019 and 2020 using SLAM traps. The list includes individuals identified at species-level and also Morphospecies. Abundance per island (COR - Corvo; FLO - Flores; TER - Terceira; SMR - Santa Maria) is provided.
File: oo_639564.txt
Acknowledgements
Many thanks to Enésima Pereira (Azorean Biodiversity Group, cE3c) for producing the maps. Trap acquisition and fieldwork were funded by the projects: Portuguese National Funds, through FCT – Fundação para a Ciência e a Tecnologia, within the project UID/BIA/00329/2013-2023; Direcção Regional do Ambiente - PRIBES (LIFE17 IPE/PT/000010) (2019-2020); Direcção Regional do Ambiente – LIFE-BETTLES (LIFE18 NAT_PT_000864) (2020-2024); AZORESBIOPORTAL – PORBIOTA (ACORES-01-0145-FEDER-000072) (2019-2022).
The database management and Open Access was funded by the project “MACRISK-Trait-based prediction of extinction risk and invasiveness for Northern Macaronesian arthropods” Fundação para a Ciência e a Tecnologia (FCT) - PTDC/BIA-CBI/0625/2021 (2022-2024).
MB was supported by FCT - DL57/2016/CP1375/CT0001. NT and MTF were supported by the project LIFE-BETTLES (LIFE18 NAT_PT_000864).
Funding Statement
Portuguese National Funds, through FCT – Fundação para a Ciência e a Tecnologia, within the project UID/BIA/00329/2013-2023; Direcção Regional do Ambiente - PRIBES (LIFE17 IPE/PT/000010) (2019-2020); Direcção Regional do Ambiente – LIFE-BETTLES (LIFE18 NAT_PT_000864) (2020-2024); AZORESBIOPORTAL –PORBIOTA (ACORES-01-0145-FEDER-000072) (2019-2022); The database management and Open Access was funded by the project “MACRISK-Trait-based prediction of extinction risk and invasiveness for Northern Macaronesian arthropods” Fundação para a Ciência e Tecnologia FCT - PTDC/BIA-CBI/0625/2021 (2022-2024)
Author contributions
PAVB and RG contributed to study conceptualisation. PAVB, ARP, RG, MB and MTF performed the fieldwork. PAVB, MB, PES and ARP performed the species sorting and identification. PAVB, ARP and LLL contributed to dataset preparation and data analysis. All authors contributed to manuscript writing.
References
- Aparício B. A., Cascalho José, Cruz M, J., Borges P. A. V., Azevedo E. B., Elias R. B., Ascensão F. Assessing the landscape functional connectivity using movement maps: a case study with endemic Azorean insects. Journal of Insect Conservation. 2018;22(2):257–265. doi: 10.1007/s10841-018-0059-7. [DOI] [Google Scholar]
- Arteaga Alba, Malumbres-Olarte Jagoba, Gabriel Rosalina, Ros-Prieto Alejandra, Casimiro Pedro, Sanchez Ana, Albergaria Isabel, Borges P. A. V. Arthropod diversity in two Historic Gardens in the Azores, Portugal. https://bdj.pensoft.net/article/54749/ Biodiversity Data Journal. 2020;8(e54749):1–27. doi: 10.3897/BDJ.8.e54749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Báez M., Oromí P. In: Lista de especies silvestres de Canarias. Hongos, plantas y animales terrestres. 1st. Arechavaleta M., Rodríguez S., Zurita N., García A., editors. Gobierno de Canarias; La Laguna: 2010. Hymenoptera .356-357 [Google Scholar]
- Boieiro M., Fagundes A. I., Gouveia C., Ramos J. A., Menezes D. Small but fierce: invasive ants kill Barolo Shearwater (Puffinuslherminieribaroli) nestling in Cima islet (Porto Santo, Madeira Archipelago). Airo. 2018;25:44–50. [Google Scholar]
- Boieiro M., Catry P., Jardim C. S., Menezes D., Silva I., Coelho N., Oliveira P., Gatt M. C., Pedro P., Granadeiro J. P. Invasive Argentine ants prey on Bulwer’s petrels nestlings on the Desertas Islands (Madeira) but do not depress seabird breeding success. Journal for Nature Conservation. 2018;43:35–38. doi: 10.1016/j.jnc.2018.02.013. [DOI] [Google Scholar]
- Borges P. A. V., Vieira V., Amorim I. R., Bicudo N., et al. In: A list of the terrestrial and marine biota from the Azores. 1st. Borges P. A. V., Costa A., Cunha R., Gabriel R., et al., editors. Princípia; Cascais: 2010. List of arthropods (Arthropoda)179-246. [Google Scholar]
- Borges P. A. V., Reut M., Ponte N. B., Quartau J. A., Fletcher M., Sousa A. B, Pollet M., Soares A. O., Marcelino J., Rego C., Cardoso P. New records of exotic spiders and insects to the Azores, and new data on recently introduced species. http://hdl.handle.net/10400.3/2079 Arquipelago Life and Marine Sciences. 2013;30:57–70. [Google Scholar]
- Borges P. A. V., Lamelas-López L. Athousazoricus . The IUCN Red List of Threatened Species. 2017 doi: 10.1119/1.4979653.2. [DOI]
- Borges P. A. V., Lamelas-López L. Donusmultifidus . The IUCN Red List of Threatened Species. 2017 doi: 10.1119/1.4979653.2. [DOI]
- Borges P. A. V., Pimentel R., Carvalho R., Nunes R., Wallon S., Ros Prieto A. Seasonal dynamics of arthropods in the humid native forests of Terceira Island (Azores) http://hdl.handle.net/10400.3/4470 Arquipelago Life and Marine Sciences. 2017;34:105–122. [Google Scholar]
- Borges P. A. V. Olisthopusinclavatus . IUCN Red List of Threatened Species. 2018 doi: 10.2305/iucn.uk.2018-1.rlts.t97114574a99166549.en. [DOI]
- Borges P. A. V., Lamelas-López L. Tarphiusrufonodulosus . IUCN Red List of Threatened Species. 2018 doi: 10.2305/iucn.uk.2018-1.rlts.t112215339a112215436.en. [DOI]
- Borges P. A. V., Cardoso Pedro, Kreft Holger, Whittaker R. J., Fattorini Simone, Emerson B. C., Gil Artur, Gillespie R. G., Matthews T. J., Santos A. M. C., Steinbauer M. J., Thébaud Christophe, Ah-Peng Claudine, Amorim I. R., Aranda S. C., Arroz A. M., Azevedo J. M. N., Boieiro Mário, Borda-de-Água Luís, Carvalho J. C., Elias R. B., Fernández-Palacios J. M., Florencio Margarita, González-Mancebo J. M., Heaney L. R., Hortal Joaquín, Kueffer Christoph, Lequette Benoit, Martín-Esquivel J. L., López Heriberto, Lamelas-López Lucas, Marcelino José, Nunes Rui, Oromí Pedro, Patiño Jairo, Pérez A. J., Rego Carla, Ribeiro S. P., Rigal François, Rodrigues Pedro, Rominger A. J., Santos-Reis Margarida, Schaefer Hanno, Sérgio Cecília, Serrano A. R. M., Sim-Sim Manuela, Stephenson P. J., Soares A. O., Strasberg Dominique, Vanderporten Alain, Vieira Virgílio, Gabriel Rosalina. Global Island Monitoring Scheme (GIMS): a proposal for the long-term coordinated survey and monitoring of native island forest biota. Biodiversity and Conservation. 2018;27(10):2567–2586. doi: 10.1007/s10531-018-1553-7. [DOI] [Google Scholar]
- Borges P. A. V., Gabriel R., Fattorini S. In: Life on Land. Encyclopedia of the UN Sustainable Development Goals. Leal Filho W., Azul A., Brandli L., Özuyar P, Wall T., editors. The Springer Nature; Switzerland: 2019. Biodiversity erosion: causes and consequences.1-10. [DOI] [Google Scholar]
- Borges P. A. V., Santos A. M. C., Elias R. B., Gabriel R. In: Encyclopedia of the world's biomes -Earth systems and environmental sciences. Anonymous, editor. Elsevier; Amsterdam: 2019. The Azores archipelago: biodiversity erosion and conservation biogeography.1-18. [DOI] [Google Scholar]
- Borges P. A. V., Rigal François, Ros‐Prieto Alejandra, Cardoso Pedro. Increase of insular exotic arthropod diversity is a fundamental dimension of the current biodiversity crisis. Insect Conservation and Diversity. 2020;13(5):508–518. doi: 10.1111/icad.12431. [DOI] [Google Scholar]
- Borges P. A. V., Cardoso P. Canariphantesrelictus . IUCN Red List of Threatened Species. 2021 doi: 10.2305/iucn.uk.2021-1.rlts.t58063699a58063710.en. [DOI]
- Borges P. A. V., Cardoso P. Canariphantesacoreensis . IUCN Red List of Threatened Species. 2021 doi: 10.2305/iucn.uk.2021-1.rlts.t58020645a58060882.en. [DOI]
- Borges P. A. V., Nunes Rui, Lamelas-López Lucas, Pereira Enésima, Costa Ricardo, Monjardino Paulo, Lopes D. H., Soares A. O., Gil Artur, Rigal François, Ferrante Marco, Lövei Gabor. Monitoring arthropods in Azorean agroecosystems: the project AGRO-ECOSERVICES. Biodiversity Data Journal. 2021;9(e77548) doi: 10.3897/bdj.9.e77548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borges P. A. V., Lamelas-López L., Ros-Prieto A. GBIF; 2022. A survey of exotic arthropods in disturbed Azorean forest habitats using SLAM traps. v.1.5. Universidade dos Açores. Dataset/Samplingevent. [DOI] [Google Scholar]
- Cardoso Pedro, Aranda S. C., Lobo J. M., Dinis Francisco, Gaspar Clara, Borges P. A. V. A spatial scale assessment of habitat effects on arthropod communities of an oceanic island. Acta Oecologica. 2009;35(5):590–597. doi: 10.1016/j.actao.2009.05.005. [DOI] [Google Scholar]
- Cardoso Pedro, Barton Philip S., Birkhofer Klaus, Chichorro Filipe, Deacon Charl, Fartmann Thomas, Fukushima C. S., Gaigher René, Habel J. C., Hallmann C. A., Hill M. J., Hochkirch Axel, Kwak M. L., Mammola Stefano, Ari Noriega Jorge, Orfinger A. B., Pedraza Fernando, Pryke J. S., Roque Fabio O., Settele Josef, Simaika J. P., Stork N. E., Suhling Frank, Vorster Carlien, Samways M. J. Scientists' warning to humanity on insect extinctions. Biological Conservation. 2020;242:108426. doi: 10.1016/j.biocon.2020.108426. [DOI] [Google Scholar]
- Colonnelli E. Curculionidae Ceutorrhynchinae from the Canaries and Macaronesia (Coleoptera). http://www.macaronesian.org/assets/files/file-9ea2a75b6ef51e.pdf Vieraea. 1990;18:317–337. [Google Scholar]
- Costa Ricardo, Borges P. A. V. SLAM Project - Long Term Ecological Study of the Impacts of Climate Change in the Natural Forest of Azores: I - the spiders from native forests of Terceira and Pico Islands (2012-2019) Biodiversity Data Journal. 2021;9 doi: 10.3897/bdj.9.e69924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowie RH., Bouchet P., Fontaine B. The Sixth Mass Extinction: fact, fiction or speculation? Biological Reviews. 2022 doi: 10.1111/brv.12816. [DOI] [PMC free article] [PubMed]
- Crespo L. C., Bosmans R., Cardoso P., Borges P. A. V. On three endemic species of the linyphiid spider genus Canariphantes Wunderlich, 1992 (Araneae, Linyphiidae) from the Azores archipelago. Zootaxa. 2014;3841(3):403–417. doi: 10.11646/zootaxa.3841.3.5. [DOI] [PubMed] [Google Scholar]
- Cruz J. V., Pereira R., Moreira A. Carta de ocupação do solo da região Autónoma dos Açores—projecto SUEMAC. Secretaria Regional do Ambiente. Direcção Regional do Ordenamento do território e dos Recursos Hídricos.; Horta: 2007. 56. [Google Scholar]
- de Vries J. P. R., van Loon Emiel, Borges P. A. V. A small-scale snalysis of elevational species richness and beta diversity patterns of arthropods on an oceanic island (Terceira, Azores) Insects. 2021;12(10) doi: 10.3390/insects12100936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espadaler X. In: A list of the terrestrial fungi, flora and fauna of Madeira and Selvagens archipelagos. 1st. Borges P. A. V., et al., editors. Direcção Regional do Ambiente da Madeira and Universidade dos Açores; Funchal and Angra do Heroísmo: 2008. Formicidae .352 [Google Scholar]
- Espadaler X. In: A list of the terrestrial and marine biota from the Azores. pp 245. Borges P. A.V., Costa A., Cunha R., Gabriel R., Gonçalves V., Martins A. F., Melo I., Parente M., Raposeiro P., Rodrigues P., Santos RS., Silva L., Vieira P., Vieira V., editors. Princípia; Cascais: 2010. Formicidae.432 [Google Scholar]
- Ferreira M. T., Cardoso Pedro, Borges P. A. V., Gabriel Rosalina, de Azevedo E. B., Reis Francisco, Araújo M. B., Elias R. B. Effects of climate change on the distribution of indigenous species in oceanic islands (Azores) Climatic Change. 2016;138:603–615. doi: 10.1007/s10584-016-1754-6. [DOI] [Google Scholar]
- Florencio Margarita, Lobo J. M., Cardoso Pedro, Almeida-Neto Mário, Borges P. A. V. The colonisation of exotic species does not have to trigger faunal homogenisation: lessons from the assembly patterns of arthropods on oceanic islands. PLOS One. 2015;10(5) doi: 10.1371/journal.pone.0128276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florencio Margarita, Rigal François, Borges P. A. V., Cardoso Pedro, Santos A. M. C., Lobo J. M. The role of plant fidelity and land-use changes on island exotic and indigenous canopy spiders at local and regional scales. Biological Invasions. 2016;18(8):2309–2324. doi: 10.1007/s10530-016-1162-x. [DOI] [Google Scholar]
- García R., García J., Sicilia P. Nuevas aportaciones al elenco de coleópteros (Insecta: Coleoptera) de Canarias. https://www.researchgate.net/publication/329542028_Nuevas_aportaciones_al_elenco_de_coleopteros_Insecta_Coleoptera_de_Canarias Revista de la Academia Canaria de Ciencias. 2016;28:79–88. [Google Scholar]
- Gaspar C., Borges P. A. V., Gaston K. J. Diversity and distribution of arthropods in native forests of the Azores archipelago. https://repositorio.uac.pt/bitstream/10400.3/249/1/pp_1_30_Gaspar_etal_25.pdf Arquipélago Life and Marine Sciences. 2008;25:1–30. [Google Scholar]
- Hallmann C. A., Sorg Martin, Jongejans Eelke, Siepel Henk, Hofland Nick, Schwan Heinz, Stenmans Werner, Müller Andreas, Sumser Hubert, Hörren Thomas, Goulson Dave, de Kroon Hans. More than 75 percent decline over 27 years in total flying insect biomass in protected areas. PLOS One. 2017;12(10) doi: 10.1371/journal.pone.0185809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey J. A., Heinen Robin, Armbrecht Inge, Basset Yves, Baxter-Gilbert J. H., Bezemer T. M., Böhm Monika, Bommarco Riccardo, Borges P. A. V., Cardoso Pedro, Clausnitzer Viola, Cornelisse Tara, Crone E. E., Dicke Marcel, Dijkstra Klaas-Douwe B., Dyer Lee, Ellers Jacintha, Fartmann Thomas, Forister M. L., Furlong M. J., Garcia-Aguayo Andres, Gerlach Justin, Gols Rieta, Goulson Dave, Habel Jan-Christian, Haddad N. M., Hallmann C. A., Henriques Sérgio, Herberstein M. E., Hochkirch Axel, Hughes A. C., Jepsen Sarina, Jones T. H., Kaydan B. M., Kleijn David, Klein Alexandra-Maria, Latty Tanya, Leather S. R., Lewis S. M., Lister B. C., Losey J. E., Lowe Elizabeth C., Macadam C. R., Montoya-Lerma James, Nagano C. D., Ogan Sophie, Orr M. C., Painting C. J., Pham Thai-Hong, Potts S. G., Rauf Aunu, Roslin T. L., Samways M. J., Sanchez-Bayo Francisco, Sar S. A., Schultz C. B., Soares A. O., Thancharoen Anchana, Tscharntke Teja, Tylianakis J. M., Umbers K. D. L., Vet L. E. M., Visser Marcel E., Vujic Ante, Wagner D. L., WallisDeVries M. F., Westphal Catrin, White T. E., Wilkins Vicky L., Williams P. H., Wyckhuys K. A. G., Zhu Zeng-Rong, de Kroon Hans. International scientists formulate a roadmap for insect conservation and recovery. Nature Ecology & Evolution. 2020;4(2):174–176. doi: 10.1038/s41559-019-1079-8. [DOI] [PubMed] [Google Scholar]
- Holway D. A., Lach L., Suarez A. V., Tsutsui N. D, Case T. J. The causes and consequences of ant invasions. Annual Review of Ecology, Evolution, and Systematics. 2002;33:181–233. doi: 10.1146/annurev.ecolsys.33.010802.150444. [DOI] [Google Scholar]
- Janicki Julia, Narula Nitish, Ziegler Matt, Guénard Benoit, Economo E. P. Visualizing and interacting with large-volume biodiversity data using client–server web-mapping applications: The design and implementation of antmaps.org. Ecological Informatics. 2016;32:185–193. doi: 10.1016/j.ecoinf.2016.02.006. [DOI] [Google Scholar]
- Matthews T. J., Sadler Jon, Carvalho Rui, Nunes Rui, Borges P. A. V. Differential temporal beta‐diversity patterns of native and non‐native arthropod species in a fragmented native forest landscape. Ecography. 2018;42(1):45–54. doi: 10.1111/ecog.03812. [DOI] [Google Scholar]
- Norder S. J., de Lima R. F., de Nascimento Lea, Lim J. Y., Fernández-Palacios J. M., Romeiras M. M., Elias R. B., Cabezas F. J., Catarino Luís, Ceríaco L. M. P., Castilla-Beltrán Alvaro, Gabriel Rosalina, de Sequeira M M, Rijsdijk K. F., Nogué Sandra, Kissling W. D., van Loon E. E., Hall Marcus, Matos Margarida, Borges P. A. V. Global change in microcosms: Environmental and societal predictors of land cover change on the Atlantic Ocean Islands. Anthropocene. 2020;30(100242):1–9. doi: 10.1016/j.ancene.2020.100242. [DOI] [Google Scholar]
- Russell J. C., Meyer J. Y., Holmes N. D., Pagad S. Invasive alien species on islands: impacts, distribution, interactions and management. Environmental Conservation. 2017;44(4):359–370. doi: 10.1017/s0376892917000297. [DOI] [Google Scholar]
- Sánchez-Bayo Francisco, Wyckhuys K. A. G. Worldwide decline of the entomofauna: A review of its drivers. Biological Conservation. 2019;232:8–27. doi: 10.1016/j.biocon.2019.01.020. [DOI] [Google Scholar]
- Seifert B. The ant genus Cardiocondyla (Insecta: Hymenoptera: Formicidae) - a taxonomic revision of the C.elegans, C.bulgarica, C.batesii, C.nuda, C.shuckardi, C.stambuloffii, C.wroughtonii, C.emeryi and C.minutior species groups. https://www.zobodat.at/pdf/ANNA_104B_0203-0338.pdf Annals of the Natural History Museum Wien. 2003;104:203–338. [Google Scholar]
- Seifert B., Okita I., Heinze J. A taxonomic revision of the Cardiocondyla nuda group (Hymenoptera: Formicidae) Zootaxa. 2017;4290(2) doi: 10.11646/zootaxa.4290.2.4. [DOI] [Google Scholar]
- Silva L., Ojeda Land E., Rodriguez Luengo J. L., Borges P. A. V., Oliveira P., Jardim R. In: Invasive Terrestrial Flora & Fauna of Macaronesia. TOP 100 in Azores, Madeira and Canaries. Silva L., Ojeda Land E., Rodriguez Luengo J. L., editors. ARENA; Ponta Delgada: 2008. Invasive alien species in Macaronesia.159-165. [Google Scholar]
- Stüben P. E. The Rediscovery of Acallesdroueti Crotch 1867 and Curculionoidea collected on an excursion on the Azores: A Report. (Coleoptera: Curculionidae: Cryptorhynchinae). Weevil News. 2003;16:1–10. [Google Scholar]
- Stüben P. E. Die Cryptorhynchinae der Azoren (Coleoptera: Curculionidae) SNUDEBILLER, Studies on taxonomy, biology and ecology of Curculionoidea. 2004;5(53):34–59. [Google Scholar]
- Stüben P. E., Sprick P., Müller G., Bayer C., Behne L., Krátký J. Digital-Weevil-Determination for Curculionoidea of West Palaearctic: Transalpina: Ceutorhynchinae (1. Part). (Mononychini, Phytobiini, Hypurini, Cnemogonini, Scleropterini & Amalini) SNUDEBILLER: Studies on taxonomy, biology and ecology of Curculionoidea. 2012;13(192):18–33. [Google Scholar]
- Stüben P. E., Müller G., Müller U., Krátký J., Bayer Ch., Sprick P., Behne L. Digital-Weevil-Determination for Curculionoidea of the West Palearctic: Transalpina: Ceutorhynchinae (3 part). (Ceutorhynchini: Datonychus, Ethelcus, Glocianus, Hadroplontus, Microplontus, Mogulones, Mogulonoides, Neoglocianus, Oprohinus, Parethelcus, Prisistus, Ranunculiphilus) SNUDEBILLER: Studies on taxonomy, biology and ecology of Curculionoidea. 2014;15(222):1–25. [Google Scholar]
- Stüben P. E., Borges P. A. V. Die Curculionoidea (Coleoptera) von den Inseln der Azoren. https://www.researchgate.net/publication/340600093_Die_Curculionoidea_Coleoptera_von_den_Inseln_der_Azoren SNUDEBILLER: Studies on taxonomy, biology and ecology of Curculionoidea. 2019;20(279):1–59. [Google Scholar]
- Stüben P. E. Weevils of Macaronesia – Canary Islands, Madeira, Azores (Coleoptera: Curculionoidea) 1st. CURCULIO-Institute; Mönchengladbach: 2022. 784 [Google Scholar]
- Tarantino Elisa, Ros-Prieto A., Lopes D. J. H., Borges P. A. V. First finding of Sophoniaorientalis (Matsumura) in the Azores. EPPO Bulletin. 2022 doi: 10.1111/epp.12812. [DOI]
- Triantis K. A., Borges P. A. V., Ladle R. J., Hortal Joaquín, Cardoso Pedro, Gaspar Clara, Dinis Francisco, Mendonça Enésima, Silveira L. M. A., Gabriel Rosalina, Melo Catarina, Santos A. M. C., Amorim I. R., Ribeiro S. P., Serrano A. R. M., Quartau J. A., Whittaker R. J. Extinction debt on oceanic islands. Ecography. 2010;33:285–294. doi: 10.1111/j.1600-0587.2010.06203.x. [DOI] [Google Scholar]
- Tsafack Noelline, Fattorini Simone, Boieiro Mário, Rigal François, Ros-Prieto Alejandra, Ferreira M. T., Borges P. A. V. The role of small lowland patches of exotic forests as refuges of rare endemic Azorean arthropods. Diversity. 2021;13(9):1–1. doi: 10.3390/d13090443. [DOI] [Google Scholar]
- Wetterer J. K. Worldwide spread of the Penny ant, Tetramoriumbicarinatum (Hymenoptera: Formicidae). Sociobiology. 2009;54:811–830. [Google Scholar]
- Wetterer J. K., Wild A. L., Suarez A. V., Roura-Pascual N., Espadaler X. Worldwide spread of the Argentine ant, Linepithemahumile (Hymenoptera: Formicidae). Myrmecol News. 2009;12:187–194. [Google Scholar]
- Wetterer J. K., Espadaler X. In: Terrestrial arthropods of Macaronesia. Biodiversity, Ecology and Evolution. pp 133-143. Serrano A. R.M., Borges P. A.V., Boieiro M., Oromí P., editors. Sociedade Portuguesa de Entomologia; Portugal: 2010. Invasive ants of Macaronesia. [Google Scholar]
- Wetterer J. K. Worldwide spread of the Moorish sneaking ant, Cardiocondylamauritanica (Hymenoptera: Formicidae) Sociobiology. 2012;59:985–997. doi: 10.13102/sociobiology.v59i3.561. [DOI] [Google Scholar]
- Wetterer J. K., Hita Garcia F. Worldwide spread of Tetramoriumcaldarium (Hymenoptera: Formicidae). Myrmecological News. 2015;21:93–99. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Complete list of species and morphospecies.
Borges, P.A.V.
Data type
Excel
Brief description
Complete list of arthropod species collected in four islands of Azores, between 2019 and 2020 using SLAM traps. The list includes individuals identified at species-level and also Morphospecies. Abundance per island (COR - Corvo; FLO - Flores; TER - Terceira; SMR - Santa Maria) is provided.
File: oo_639564.txt